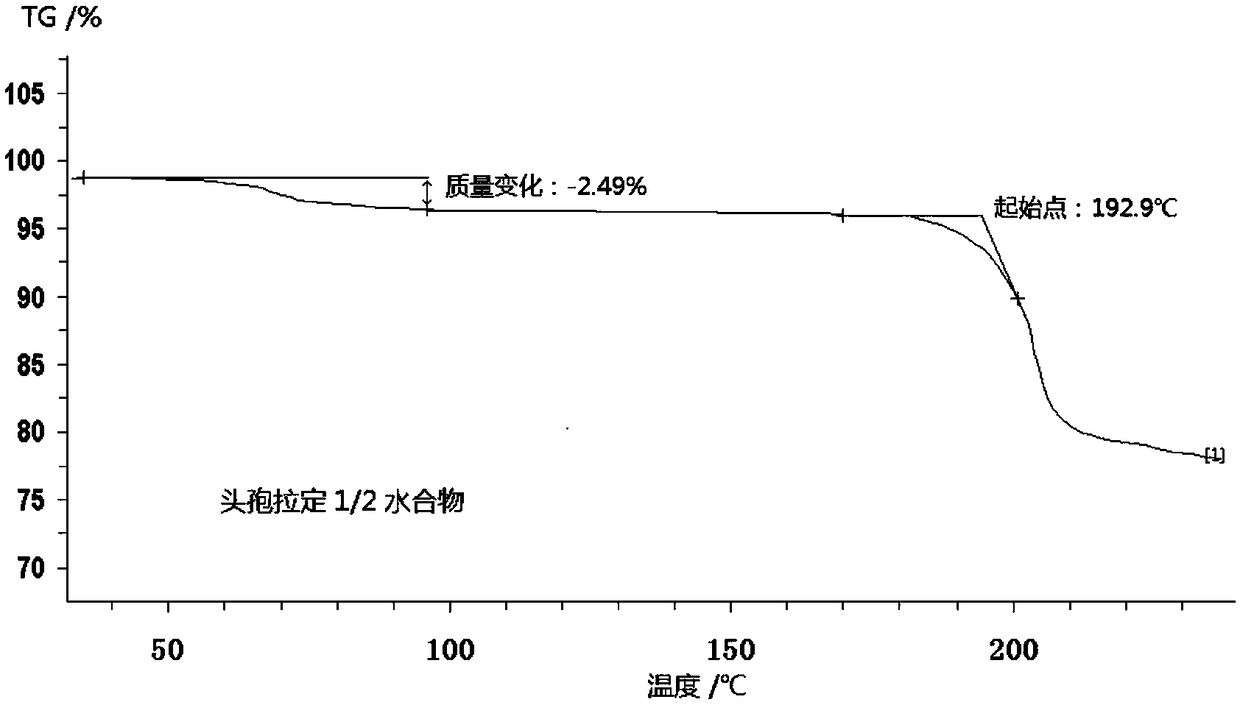
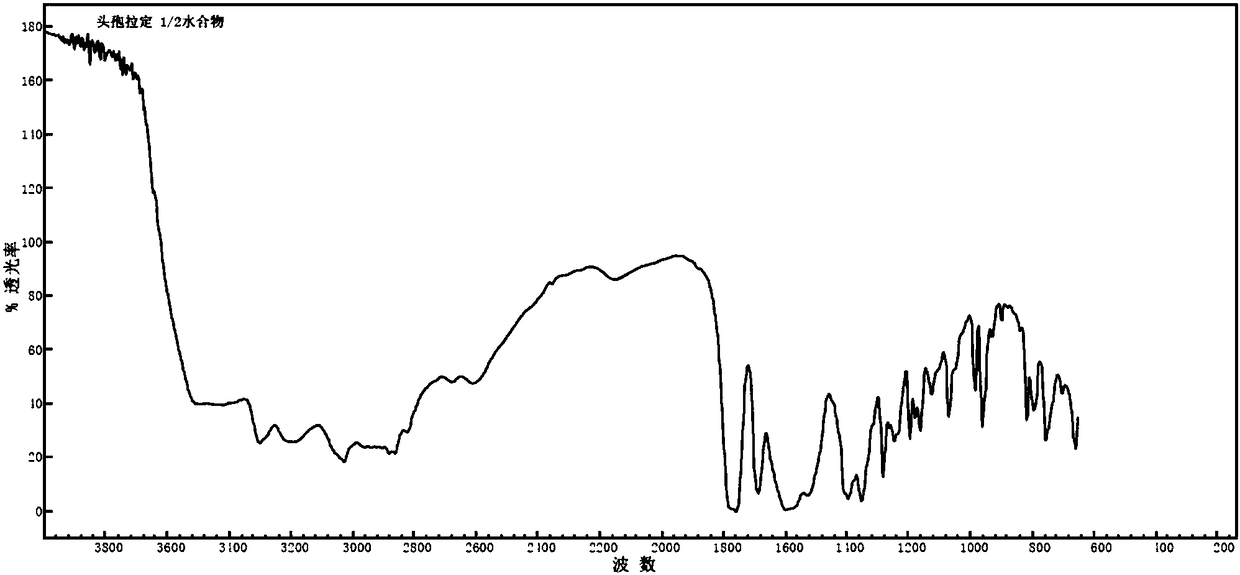
Cefradine compound containing half water
A cefradine and compound technology, which is applied in the field of 1/2 water cefradine compound and its preparation, can solve the problems of backward preparation method, instability between batches and high impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the preparation of 1 / 2 water cephradine compound
[0033] Dissolve 10.1 g of crude cephradine in 100.4 g of water, control the temperature in a water bath at 35 ° C, add 0.5 g of activated carbon, stir and absorb for 30 min, and filter; adjust the pH value of the filtrate to 6.0 with hydrochloric acid; slowly add the dissolving agent dichloride to the above solution Methane, filter, wash the filter cake with ethanol 30mL×2; dissolve the filter cake in a mixed solution of 700mL water and methanol (6:1), stir to dissolve; add seed crystals, control the temperature at 8°C, and stand for crystallization for 3h , filtered; the filtrate was washed with 30 mL of ethanol × 2, and dried in vacuum at 25° C. for 40 min to obtain 9.78 g of 1 / 2 water cephradine compound.
[0034] The X-ray powder diffraction pattern has characteristic diffraction peaks at diffraction angles 2θ of 7.25°, 10.98°, 14.53°, 16.28°, 17.80°, 19.38°, 20.21°, 22.06°, and 22.90°. The relative di...
Embodiment 2
[0037] Embodiment 2: the preparation of 1 / 2 water cephradine compound
[0038] Dissolve 15.3 g of crude cephradine in 150.1 g of water, control the temperature in a water bath at 30 ° C, add 0.7 g of activated carbon, stir and adsorb for 30 min, and filter; adjust the pH value of the filtrate to 6.5 with hydrochloric acid; slowly add the dissolution agent trichloride to the above solution Methane, filter, wash the filter cake with ethanol 30mL×2; dissolve the filter cake in a mixed solution of 1000mL water and methanol (7:1), stir to dissolve; add seed crystals, control the temperature at 10°C, and stand for crystallization for 3h , filtered; the filtrate was washed with 30 mL of ethanol × 2, and dried in vacuum at 35° C. for 30 min to obtain 15.02 g of cephradine compound in 1 / 5 water.
[0039] The X-ray powder diffraction pattern has characteristic diffraction peaks at diffraction angles 2θ of 7.26°, 10.99°, 14.55°, 16.27°, 17.82°, 19.36°, 20.20°, 22.05°, and 22.92°. The rel...
Embodiment 3
[0042] Embodiment 3: the preparation of 1 / 2 water cephradine compound
[0043] Dissolve 10.5 g of crude cephradine in 100.5 g of water, control the temperature in a water bath at 40 ° C, add 0.5 g of activated carbon, stir and adsorb for 30 min, and filter; adjust the pH value of the filtrate to 6.2 with sulfuric acid; slowly add the dissolution agent trichloride to the above solution Methane, filter, wash the filter cake with ethanol 30mL×2; dissolve the filter cake in a mixed solution of 700mL water and methanol (6:1), stir and dissolve; add seed crystals, control the temperature at 10°C, and stand for crystallization for 3h , filtered; the filtrate was washed with 30 mL of ethanol × 2, and dried in vacuum at 30° C. for 35 min to obtain 10.01 g of cephradine compound in 1 / 5 water.
[0044] The X-ray powder diffraction pattern has characteristic diffraction peaks at diffraction angles 2θ of 7.23°, 10.97°, 14.54°, 16.26°, 17.82°, 19.36°, 20.20°, 22.03°, and 22.92°. The relativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com