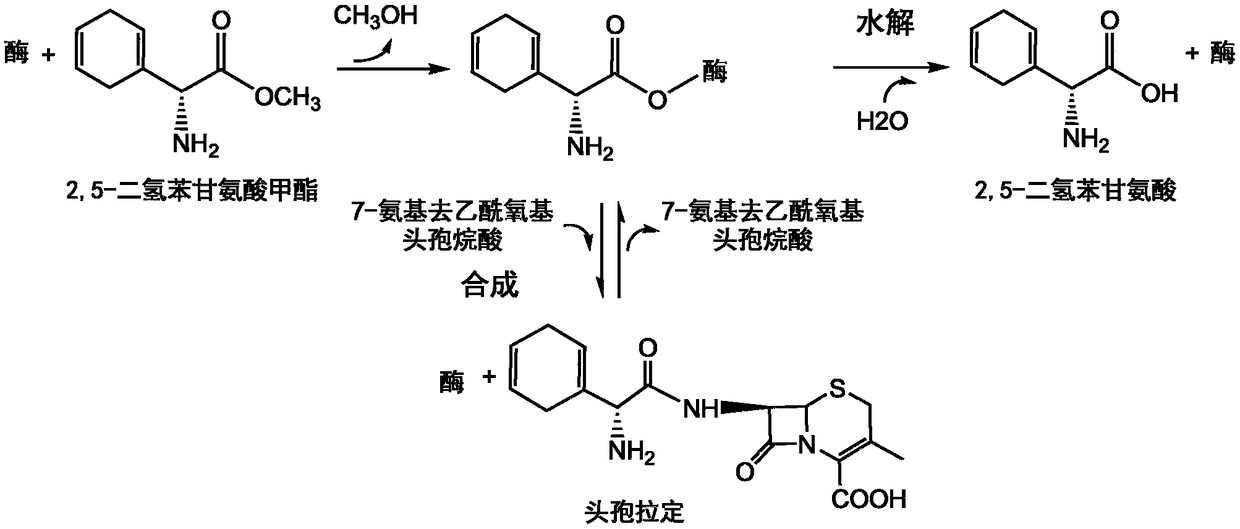
High-selectivity mutant of cefradine synthesizing enzyme and coding gene thereof
A cephradine and encoding technology, applied in the field of biochemistry, to achieve high activity and high expression level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, preparation and purification of penicillin G acylase mutant
[0053] 1. Construction of gene encoding penicillin G acylase mutant and recombinant expression vector
[0054] The wild Escherichia coli penicillin G acylase gene was obtained according to literature. Sequence The pET28a-PGA_M3 gene of the present invention was amplified by Overlapping PCR (see SEQ ID No.1 for the sequence). Histidine tag His 6 -tag is added at the end of the carbon-terminus of the gene sequence to facilitate subsequent purification steps. After being double-digested and purified with NcoI and XhoI, it was ligated overnight with the expression vector pET28a(+) (containing the kanamycin resistance gene) that was double-cut with the same endonuclease, and then transformed into competent cells BL21( DE3), the recombinant expression plasmid pET28a-PGA_M3 was obtained.
[0055] After sequencing, the sequence of pET28a-PGA_M3 was correct.
[0056] Description of the structure of ...
Embodiment 2
[0070] Embodiment 2, the hydrolysis DHME catalytic activity assay of enzyme mutant
[0071] The DHME conversion product was analyzed by HPLC (LC-20AT, Shimadzu Corporation), so as to determine the catalytic activity and kinetic parameters of the enzyme mutant PGA_M3 prepared in Example 1 to hydrolyze DHME. The chromatographic column selected was an Inertsil C18 reverse-phase column (GL Sciences, 5 μm, 150×4.6 mm). During the reaction, the ratio of the enzyme mutant to the substrate was as follows: 0.5mL of the purified target protein (sufficient, stored in 100mM potassium phosphate buffer, pH 7.0) and 0.5mL of DHME solution (pH 7.0, the concentration was a gradient change, the highest Concentration is 1g / 100mL), react at 22°C for 8min. After the reaction was completed, 1 mL of methanol was added to terminate the reaction. The mobile phase ratio of HPLC is: 75% potassium phosphate buffer solution (30mM, pH 4.5): 25% methanol (v / v), flow rate 0.8mL / min, detection wavelength is...
Embodiment 3
[0076] Embodiment 3, the hydrolysis cephradine catalytic activity determination of enzyme mutant
[0077] The conversion product of cephradine was analyzed by HPLC (LC-20AT, Shimadzu Corporation), thereby determining the catalytic activity and kinetic parameters of the hydrolysis of cephradine by the enzyme mutant PGA_M3 prepared in Example 1. The selected chromatographic column is an Inertsil C18 reverse-phase column (GL Sciences, 5 μm, 150×4.6 mm). During the reaction process, the ratio of enzyme mutant to substrate is: 0.5mL purified target protein (sufficient, stored in 100mM potassium phosphate buffer, pH 7.0) and 0.5mL cephradine solution (pH 7.0, the concentration is a gradient change, The highest concentration is 2g / 100mL), react at 22°C for 8min. After the reaction was completed, 1 mL of methanol was added to terminate the reaction. The mobile phase ratio of HPLC is: 75% potassium phosphate buffer (30mM, pH 4.5), 25% methanol (v / v), flow rate 0.8mL / min, detection wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com