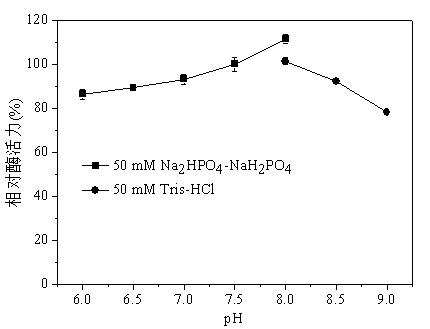
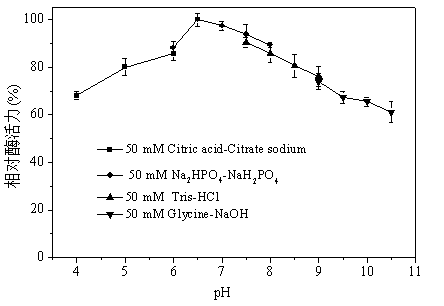
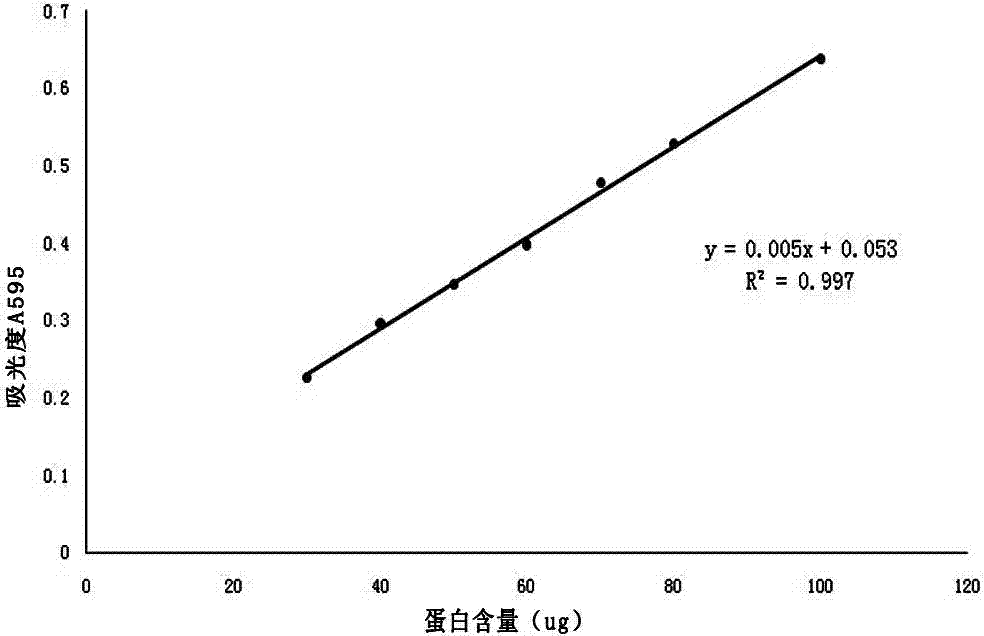
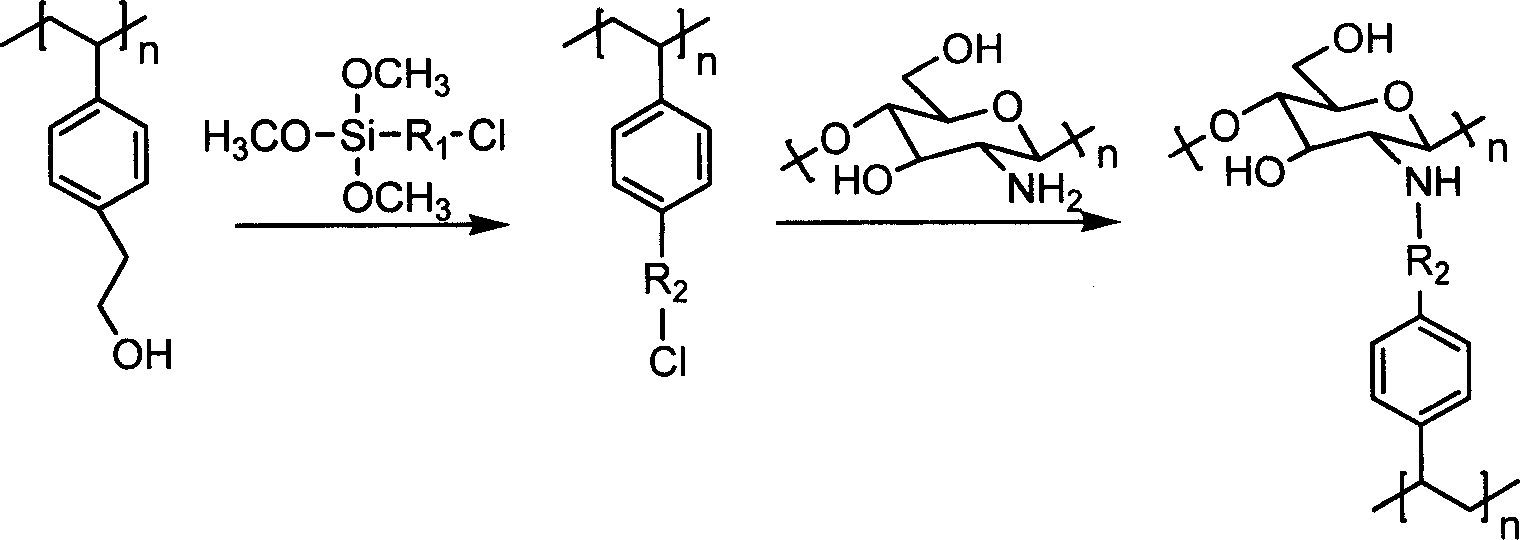
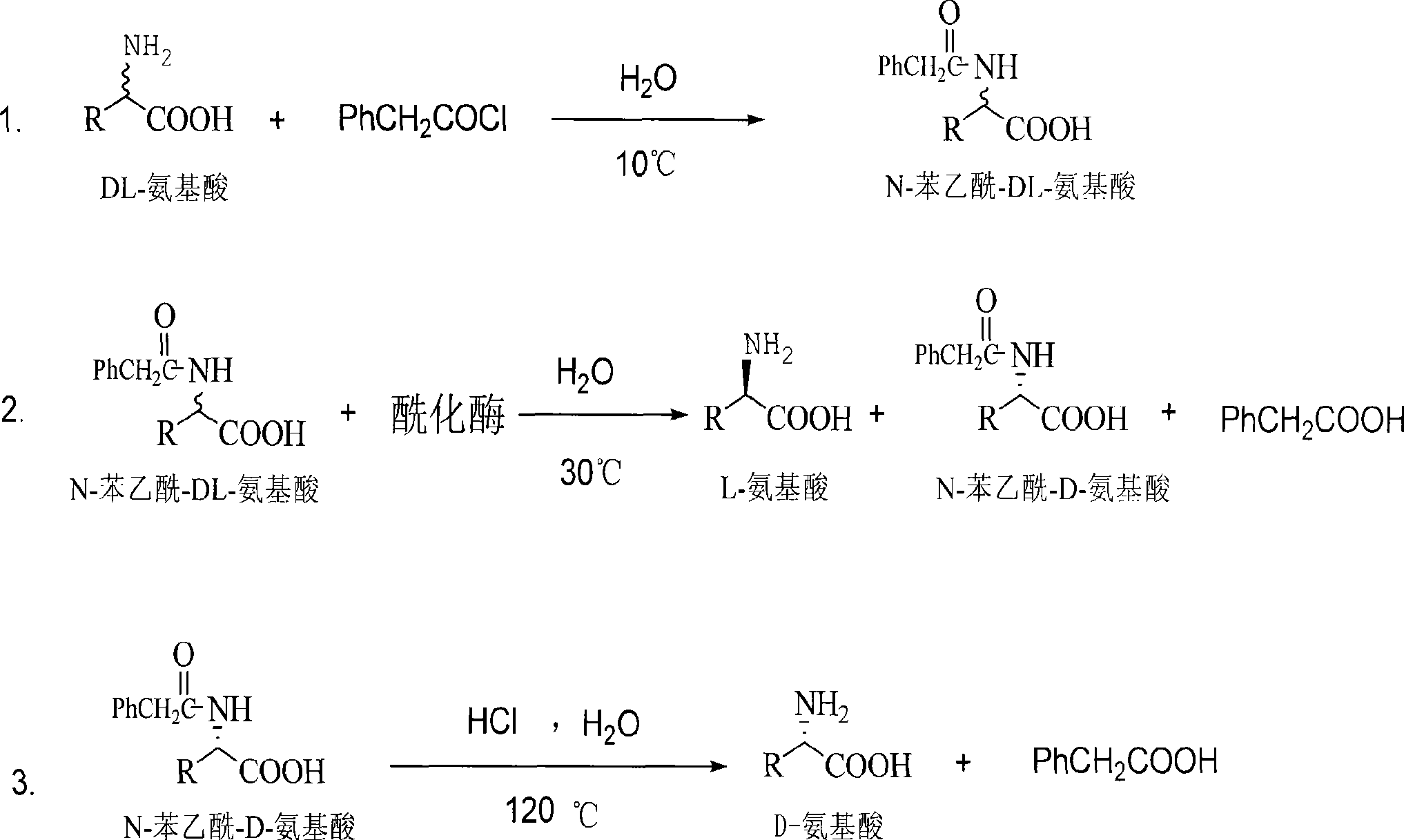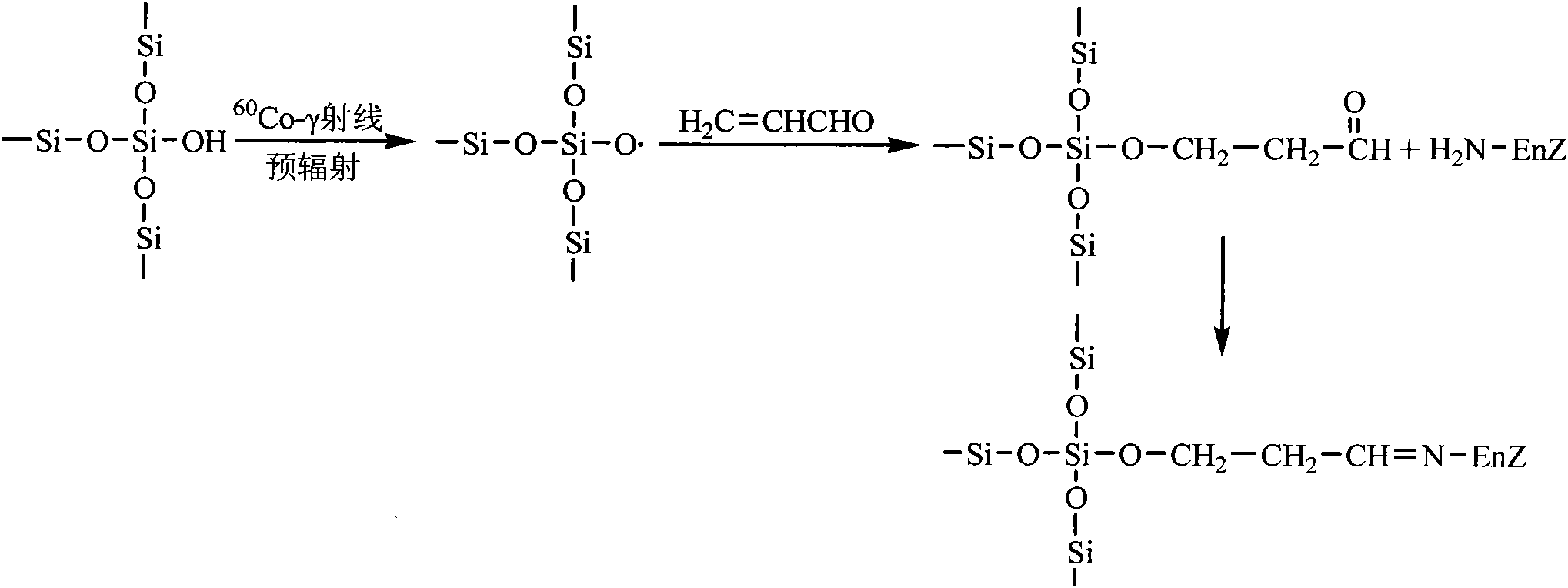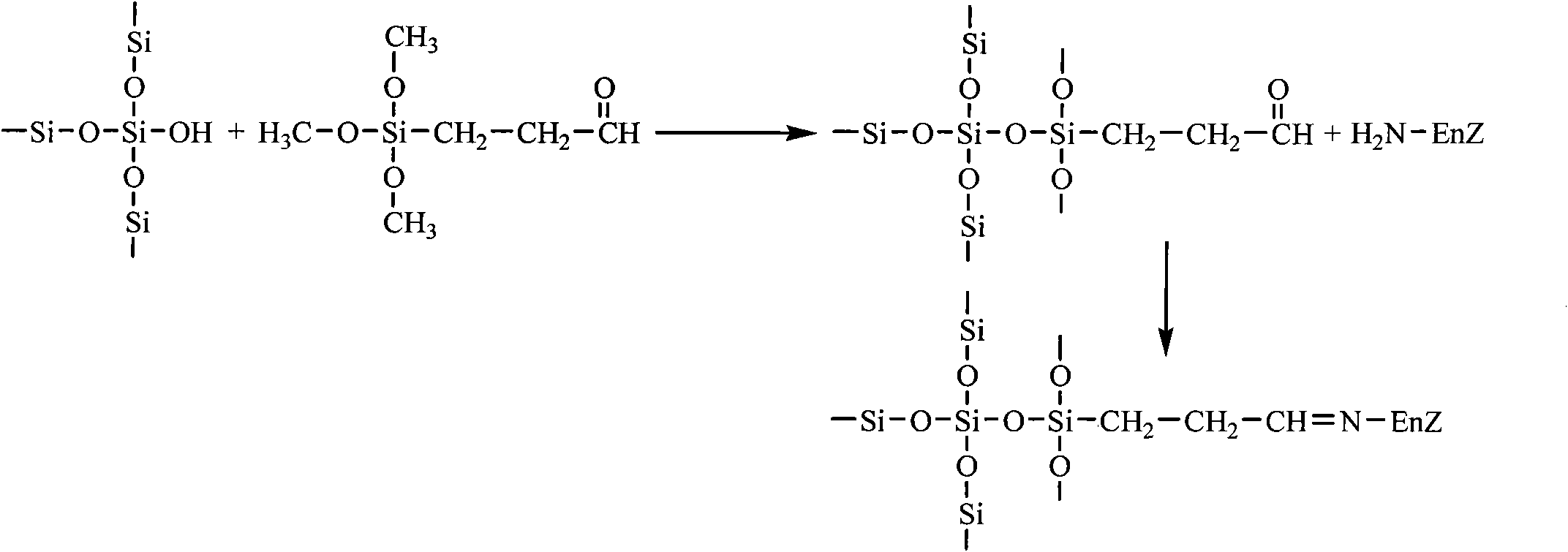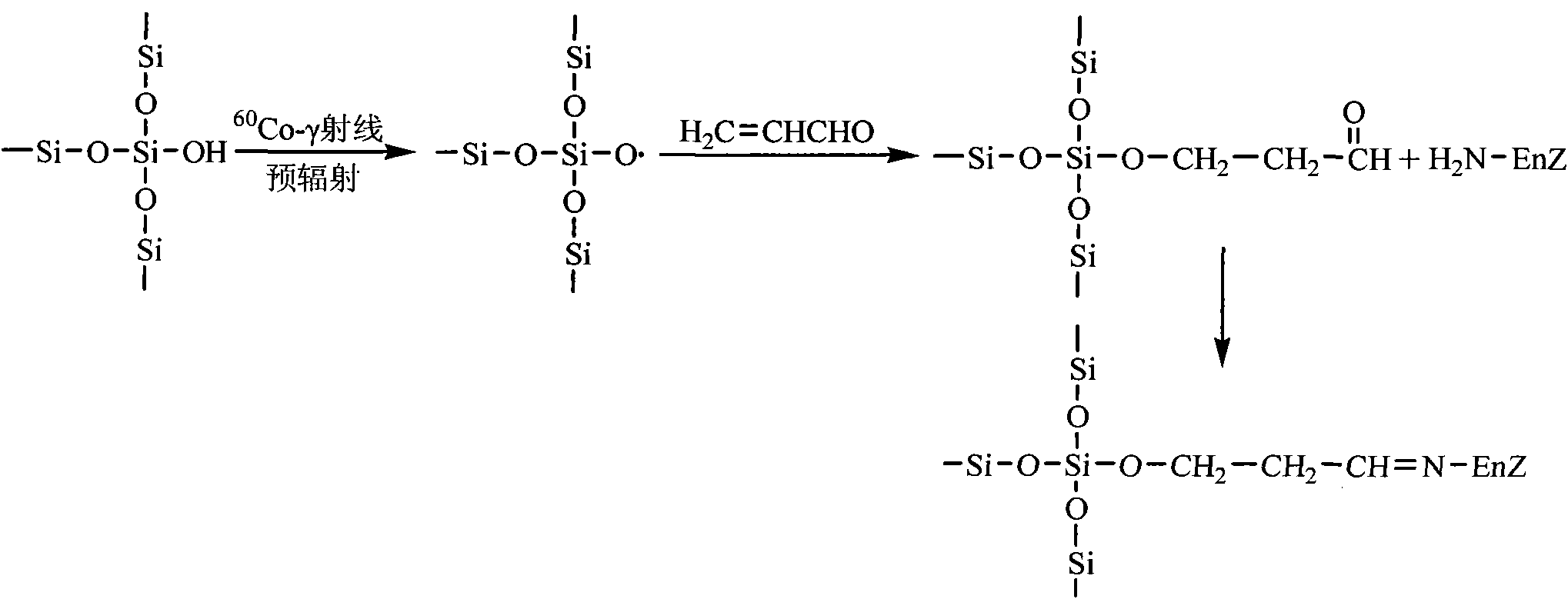Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Penicillin Acylase" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Also called penicillin acylase. In enzymology, a penicillin amidase (EC 3.5.1.11) is an enzyme that catalyzes the chemical reaction. Thus, the two substrates of this enzyme are penicillin and H2O, whereas its two products are carboxylate and 6-aminopenicillanate.
Process for preparing cefixime
The invention provides a method for preparing cefixime, which belongs to the medicine synthesis technical field and comprises the steps of preparation of 7-AVCA, preparation of cefixime methyl ester, preparation of the cefixime and so on. The method aims at the prior amino de-protection to make an improvement, and uses penicillin acylase to substitute trifluoroacetic acid and so on in the prior process to ensure that an amino de-protection reaction is greatly improved, the use of organic solvents can be completely avoided, and acylase can also be reused at the same time. Methylene dichloride and tetrahydrofuran solvents in the prior process are substituted by adopting alcohol, ketone or ester solvents, so that an amidation reaction is greatly improved, which ensures that reaction solvents are easy to recover and reuse, can reduce production cost, reduce the discharge of waste liquid, reduce the pollution to the environment, can completely recover a byproduct, namely mercaptobenzothiazole produced by the reaction, improve the atom utilization rate of reaction raw-materials, and greatly improve the hydrolyzation and crystallization of subsequent products and the quality of a finished product.
Owner:ZHEJIANG ANGLIKANG PHARMA
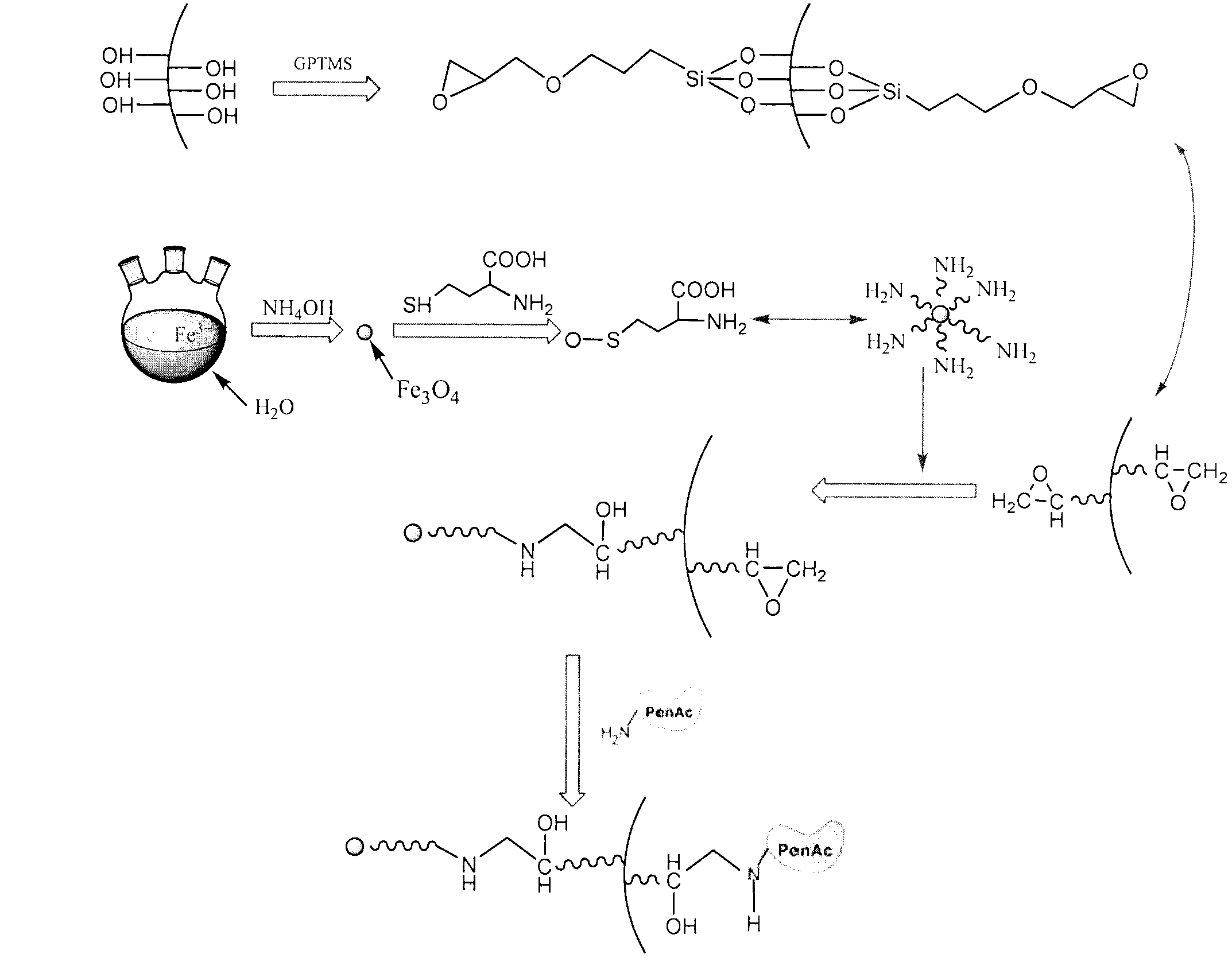
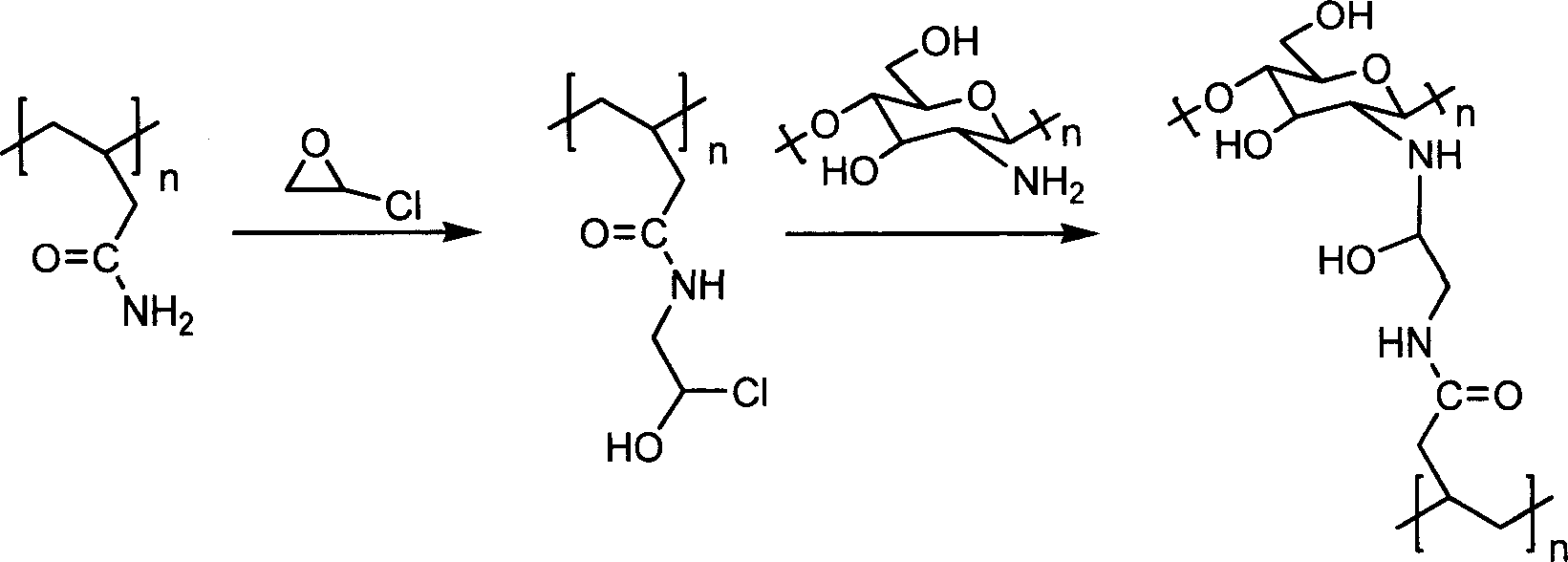
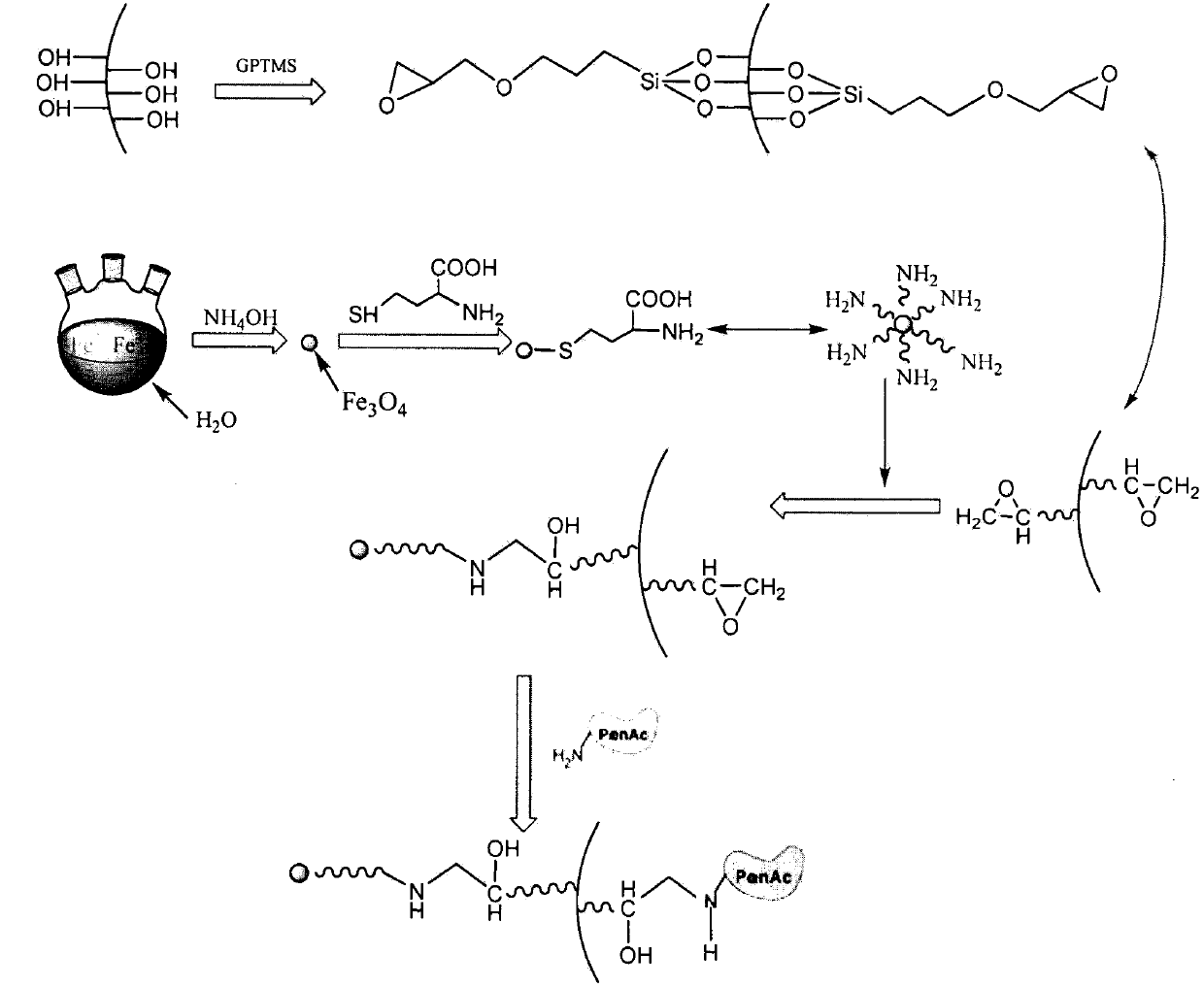
Aramagnetic epoxy group mesoporous molecular sieve for immobilized biological enzymes, and preparation method thereof
ActiveCN102703411AReduce cloggingEasy to separateImmobilised enzymesMolecular-sieve and base-exchange compoundsEpoxyMolecular sieve
The invention belongs to the technical field of biology, and discloses an aramagnetic epoxy group mesoporous molecular sieve for immobilizing biological enzymes, and a preparation method thereof. According to the invention, gamma glycidoxy propyl trimethoxy silane is utilized to introduce aramagnetic epoxy groups onto the surface of the mesoporous molecular sieve, and then a covalent bond is utilized to immobilize aramagnetic Fe3O4 nano particles and biological enzymes onto the outer surface and the inner surface of the mesoporous molecular sieve respectively, wherein the aramagnetic Fe3O4 nano particles are subjected to L-cysteine surface modification and have the particle sizes larger than the pore diameter of the mesoporous molecular sieve, as a result, specially-structured aramagnetic immobilized enzymes can be prepared and are separated from the liquid phase system easily under the action of an external magnetic field, and further, the property and separation efficiency of the immobilized enzymes are improved. The aramagnetic epoxy group mesoporous molecular sieve can be used for immobilization of water-soluble biological enzymes such as penicillin acylase, glucose isomerase, glucosylase, trypsin and amylase, the activity of the prepared aramagnetic immobilized penicillin acylase is 8800 U / g, and 94.5% of the initial activity is retained after ten times of cycle use.
Owner:EAST CHINA UNIV OF SCI & TECH
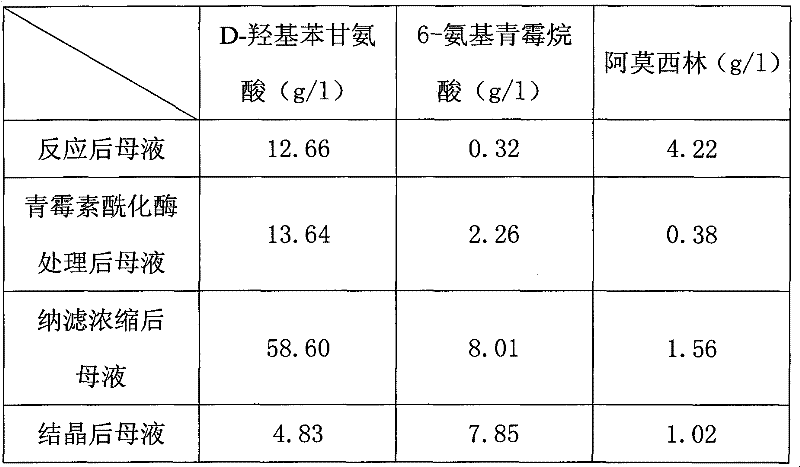
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
ActiveCN102392060AAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisIsoelectric point
The invention discloses a recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration. The method comprises the following steps: (1) regulating pH of the mother liquor; (2) hydrolyzing amoxicillin in the mother liquor by utilizing penicillin acylase, thus only D-hydroxyphenylglycine and 6-amino-penicillanic acid (6-amino-penicillanic acid) exist in the other liquor; (3) preparing concentrated mother liquor by nanofiltration; and (4) separating and recovering the D-hydroxyphenylglycine and the 6-amino-penicillanic acid (6-amino-penicillanic acid) by isoelectric point crystallization. The method has the advantages that: the recycling rate of an inactive lateral chain can be improved regarding certain amoxicillin synthetase with highhydrolysis activity, so that the enzymatic synthesis of amoxicillin is more economical.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Preparation method for 6-amino penicillanic acid
The invention discloses a preparation method for 6-amino penicillanic acid, which comprises the following steps: a, performing ultrafiltration membrane separation and nanofiltration membrane concentration on a penicillin fermentation liquor to obtain a concentrated filter liquor; b, placing the concentrated filter liquor into a reaction tank, adding an immobilized penicillin acylase 4MU / m<3> concentrated filter liquor and performing conversion reaction to obtain a 6-amino penicillanic acid conversion solution; c, performing actived carbon decoloration and filtering on the conversion solution to obtain a 6-amino penicillanic acid filter liquor; and d, adding seed grain into the 6-amino penicillanic acid filter liquor obtained through the procedures in the step c, growing the grain, crystallizing, filtering, washing and drying. The preparation method has the advantages of simple process flow, easiness for operation, safety, environmental protection, and capabilities of effectively improving the yield of 6-APA, reducing the production cost and improving the labor productivity.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing cefalexin
The invention discloses a method for preparing cefalexin. The method comprises the steps of charging a D-2-phenylglycine ester derivative and 7-ADCA (amino desacetoxy cephalosporanic acid) in a molar ratio of (1.15-1.6):1, and adding a catalyst penicillin acylase with amount of 1-2 times of amount of the 7-ADCA for acylation reaction, wherein the coded gene sequence of the penicillin acylase is shown as SEQIDNO:3. The method effectively overcomes reverse reaction during enzyme condensation reaction, so as to greatly reduce the using amount of side chains, avoid the phenomenon that more impurities are generated when high side chains are consumed, and solve the problem that the objective product is difficult to purify; and besides, the 7-ADCA conversion rate can be greatly improved to be up to more than 98%, the product quality is further improved and the production cost is reduced.
Owner:NORTH CHINA PHARMA COMPANY
Magnetic polymer microsphere for enzyme immobilization and preparation method thereof
InactiveCN101250247AAvoid performance impactImprove apparent activityOrganic/organic-metallic materials magnetismOn/in organic carrierFunctional monomerSuperparamagnetism
The invention discloses a magnetic polymer microsphere for enzyme immobilization and a relative preparation method, which uses hydrophilic nanometer magnetic particles as magnetic material, uses vinyl compound as functional monomer, uses composite surface activator as disperser and uses inverse suspension polymerization technique to prepare the macromolecule polymer pear carrier with narrow grain distribution and superparamagnetic and hydrophilic expoy group. The immobilization penicillin acylase prepared by the magnetic carrier has high apparent activity as 330IU / g (humidity), and the immobilization enzyme can be recovered easily to be circulated by external magnetic field, thereby improving the utilization of immobilization. The preparation method has simple process, easy operation, low production cost and support for large-scale production.
Owner:EAST CHINA UNIV OF SCI & TECH
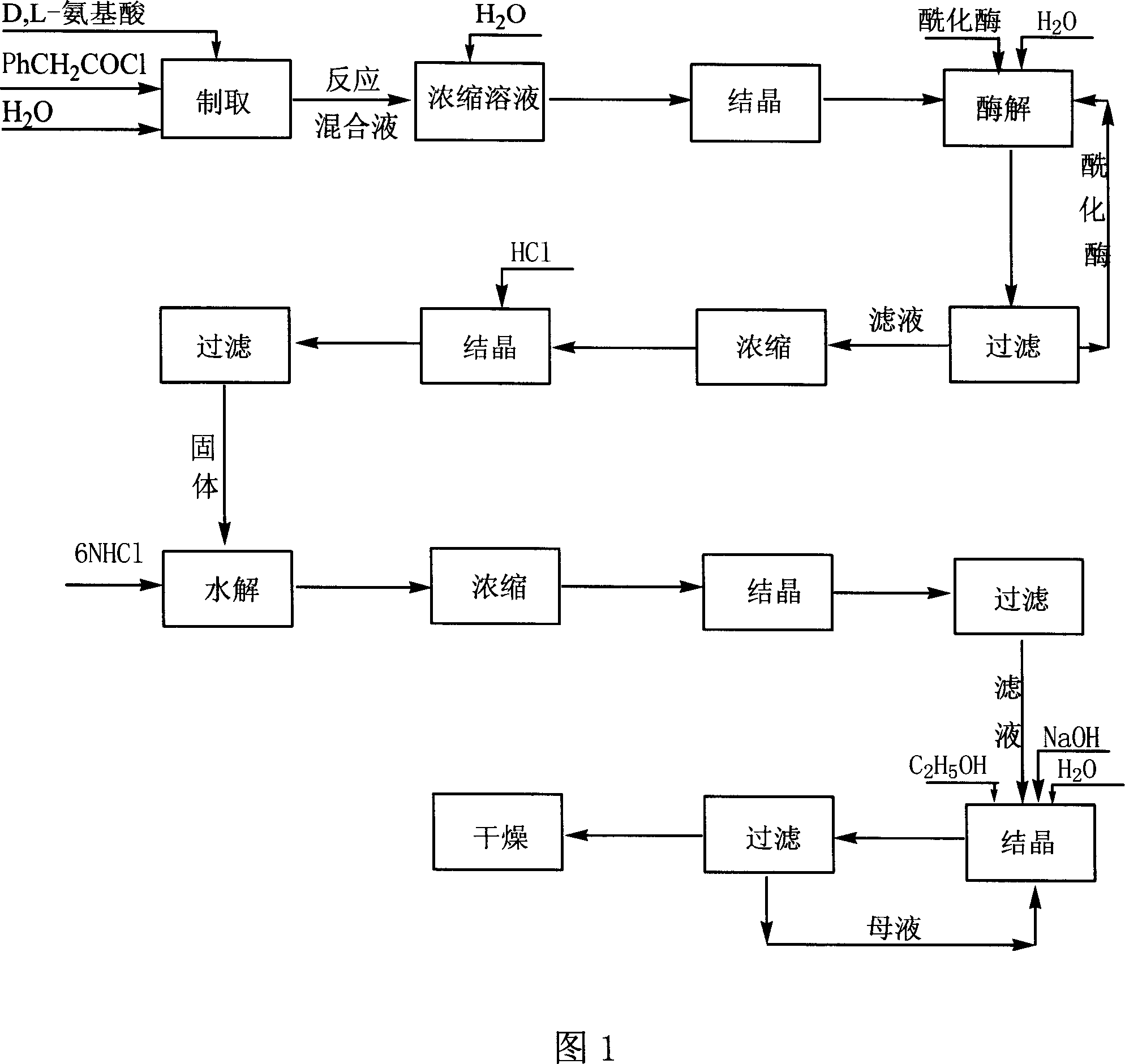
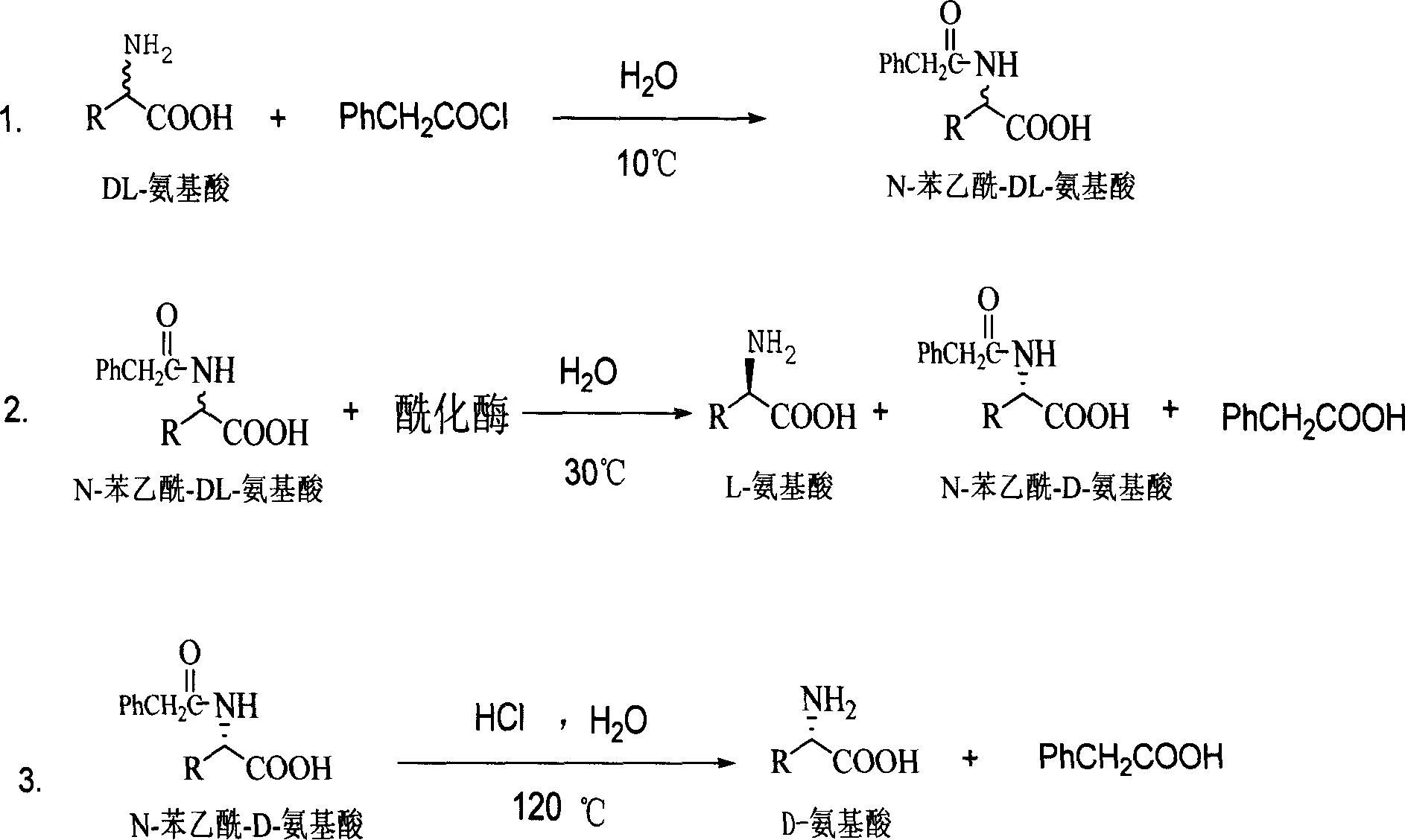
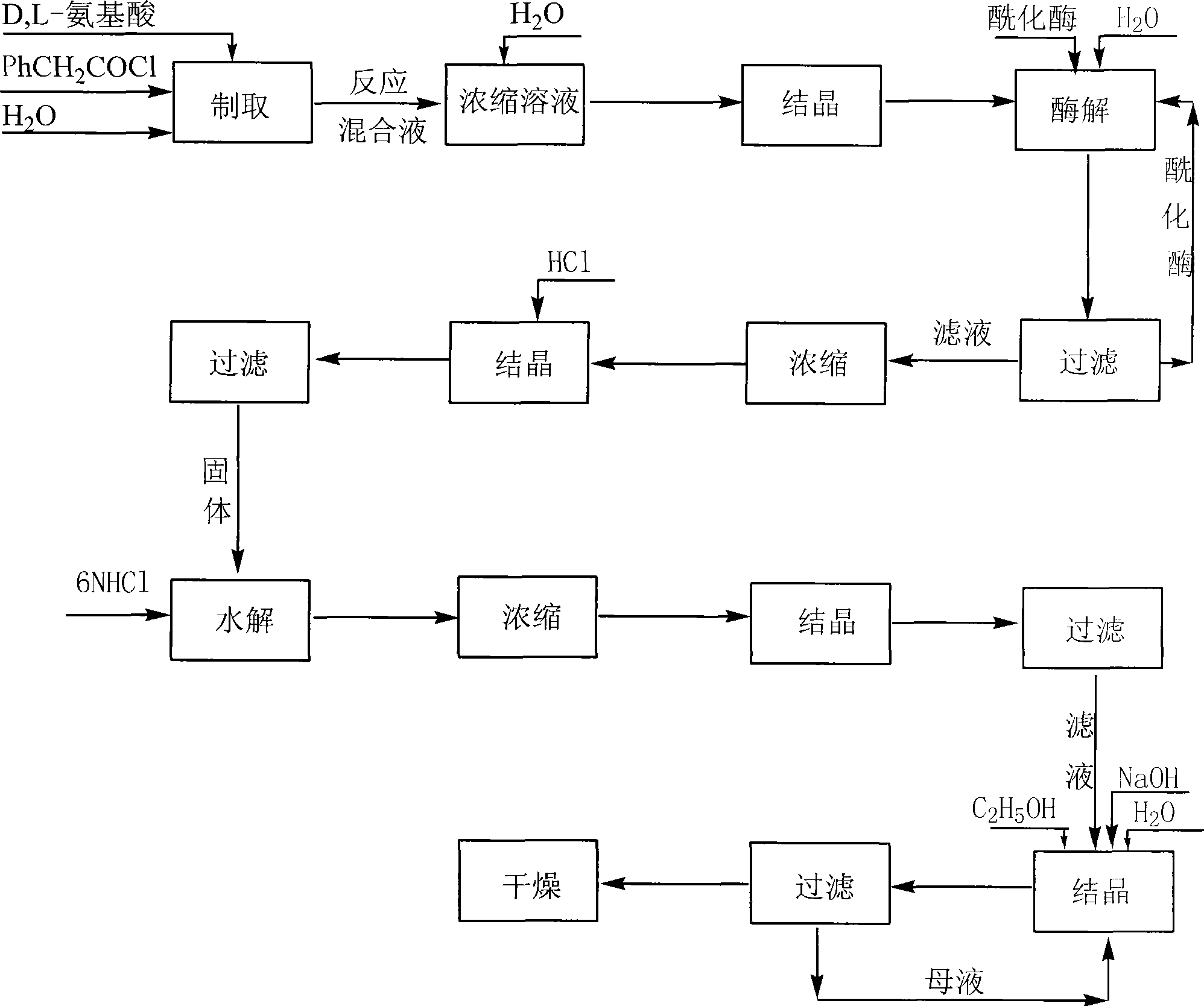
Method for producing D amino acid by immobilizing acylation enzyme of penicillin
This invention discloses a method for preparing D-amino acid. The method comprises: derivatizing DL-amino acids to obtain N-phenylacetyl-DL-amino acids, performing enzyme-catalyzed asymmetric hydrolysis in aqueous solution to obtain N-phenylacetyl-D-amino acid, and performing chemical hydrolysis and crystallization to obtain D-amino acid. The enzyme used is immobilized penicillin acylase, which can be used for more than 100 times. The method has such advantages as high yield and high product optical purity, and is suitable for the majority of DL-amino acids resolution.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by enzyme process
ActiveCN104357528AImprove product qualityCreate economic growthOrganic compound preparationAmino-carboxyl compound preparationUltrafiltrationChemistry
The invention relates to a method for comprehensively recovering effective ingredients in amoxicillin mother liquid prepared by an enzyme process. The method comprises the following steps: (1) concentrating the amoxicillin mother liquid, namely adjusting the pH value of the amoxicillin mother liquid prepared by the enzyme process to be 8.0-9.5, and performing nanofiltration and concentration to obtain concentrated mother liquid; (2) synthesizing amoxicillin under enzyme catalysis, namely adjusting the pH value of the concentrated mother liquid to be 5.8-7.0, and converting 6-APA (6-amino penicillanic acid) and D-methyl hydroxyphenyl glycinate into amoxicillin in the presence of immobilized penicillin acylase for synthesis; (3) preparing D-hydroxyphenyl glycine concentrated liquid by ultrafiltration and nanofiltration, namely separating after the enzyme catalysis reaction is ended to obtain amoxicillin crystals and secondary amoxicillin mother liquid, and performing ultrafiltration and nanofiltration on the secondary mother liquid to obtain the D-hydroxyphenyl glycine concentrated liquid; (4) crystallizing D-hydroxyphenyl glycine. According to the method, 6-APA and D-methyl hydroxyphenyl glycinate remained in the mother liquid are consumed through an indirect process of synthesizing amoxicillin under enzyme catalysis, the product quality of D-hydroxyphenyl glycine is improved, and the yield of D-hydroxyphenyl glycine is increased.
Owner:SHANXI WEIQIDA PHARMA IND
Method for preparing D-aminophenol with immobilization penicillin acylated enzyme catalysis
The invention relates to a preparation method of D-amino acid; DL-amino acid is used as the material and then N-phenylacetyl-DL-amino acid is obtained through derivation; under an aqueous liquid, the N-phenylacetyl-DL-amino acid obtained is unsymmetrically hydrolyzed by enzyme; then the D-amino acid is obtained through carrying out chemical hydrolyzing and crystallizing on the N-phenylacetyl-DL-amino acid. The enzyme related to the invention is immobilized penicillin acylase the repeated use batches of which achieves more than 100 times. The method has the advantages of short dismounting route, stable technique, simple operation, good separating effect, high product purity and low production cost. Besides, only an organic solvent of ethanol is used during the production process of the method; therefore, the whole process is environmental-friendly..
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Preparation method of D-para hydroxybenzene glycine methyl ester
InactiveCN103113250AHigh yieldHigh purityOrganic compound preparationAmino-carboxyl compound preparationSynthesis methodsDistillation
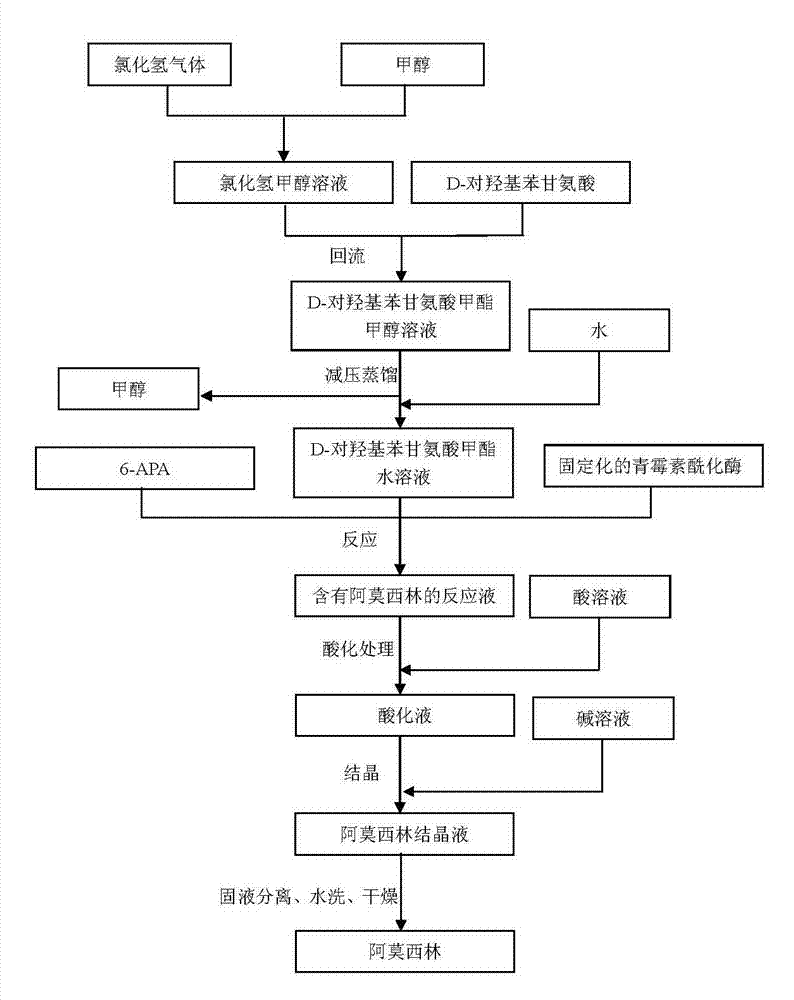
The invention provides a preparation method of D-para hydroxybenzene glycine methyl ester. The method comprises the following steps of: firstly, preparing a hydrochloric acid methanol solution; adding D-para hydroxybenzene glycine into the hydrochloric acid methanol solution to perform reflux reaction for 2-4 hours at 65-80 DEG C; performing pressure reduction distillation and removing methanol; and adding water to obtain a D-para hydroxybenzene glycine methyl ester aqueous solution. Based on that, the invention also provides anenzymatic synthesis method of amoxicillin. The method comprises the following steps of: adding 6-APA and immobilized penicillin acylase into the D-para hydroxybenzene glycine methyl ester aqueous solution to react for 1-8 hours at 10-30 DEG C; regulating the pH value of a reaction liquid to 0.8-1.0 by using hydrochloric acid or sulfuric acid aqueous solution; regulating the pH value to 4.5-6.0 by using ammonia water or sodium hydroxide aqueous solution to crystallize for 1-5 hours at 0-30 DEG C; separating solid from liquid; collecting a solid; and washing and drying to obtain amoxicillin. The method is simple in steps, and low in cost; and the obtained -para hydroxybenzene glycine methyl ester is high in yield and less in impurities and can be directly applied to anenzymatic synthesis of amoxicillin.
Owner:NORTH CHINA PHARM GRP SEMISYNTECH CO LTD
Immobilized penicillin acylated enzyme with silicon gel rubber as carrier and preparation method
InactiveCN101508986ALarge specific surface areaNot easy to infect bacteriaOn/in inorganic carrierSilicone GelsPenicillin
The invention relates to an immobilized penicillin acylase using silicone gel as a carrier and a preparation method thereof. The immobilized penicillin acylase is porous immobilized penicillin acylase with grain diameter between 13 and 15mum and aperture between 14 and 30nm, which is prepared by covalent binding between porous silicone gel with a functional group on the surface obtained by copolymerization of silicone sol and a silane coupling agent and penicillin acylase. The preparation method comprises the following steps: first, preparing the carrier; then, activating the carrier; and preparing the immobilized penicillin acylase using the silicone gel as the carrier. The immobilized penicillin acylase using the silicone gel as the carrier prepared by the method has proper grain diameter and aperture, has larger specific surface area (300 to 600m2 / g), can not be contaminated by bacteria easily, has strength superior to agarose 6B microballons and acrylic resin microballs commonly adopted overseas, has the advantages of low price of raw materials, good stability, simple preparation method and stable reaction process, and is applicable to industrial mass production.
Owner:TIANJIN UNIV
Method of synthesizing cefadroxil by enzyme process
The invention relates to a method of synthesizing cefadroxil by an enzyme process. The method comprises the following steps of: by taking 7-ADCA as an initial raw material, performing a reaction on D-tyrosine methyl ester or D-p-hydroxyl phenylglycine ethyl ester and 7-ADCA in water by directly inputting the solid in the presence of penicillin acylase at 10-25 DEG C; after reaction, separating a cefadroxil coarse product and enzyme reaction mother liquor; further purifying the cefadroxil coarse product to obtain a white cefadroxil product; and adding beta-naphthol or 2,7-dioxynaphthalene into the enzyme reaction mother liquor to obtain a cefadroxil compound. Cefadroxil can be further treated and recovered from the cefadroxil compound, so that the recovery rate of cefadroxil is increased. The product obtained by the method is high in yield and purity. The product is white in appearance, multi-step reaction of a chemical process and various solvents and auxiliary materials are not needed, and green synthesis of cefadroxil is realized.
Owner:苏州盛达药业有限公司 +1
Immobilized penicillin amidase carrier and its preparing method
The present invention discloses a kind of immobilized penicillin amidase carrier and its preparation process. The immobilized pencillin amidase carrier is prepared with surfactant as dispersant and vinyl compound as monomer and through crosslinking polymerization in reverse suspension technology. The carrier immobilized pencillin amidase has an apparat enzyme activity in preparing 6-APA with benzyl pencillin potassium as high as 125 IU / g. The polymer bead carrier containing epoxy group of the present invention has high apparent enzyme activity, simple technological process, easy operation andcheap material and may be used in industrial production.
Owner:EAST CHINA UNIV OF SCI & TECH
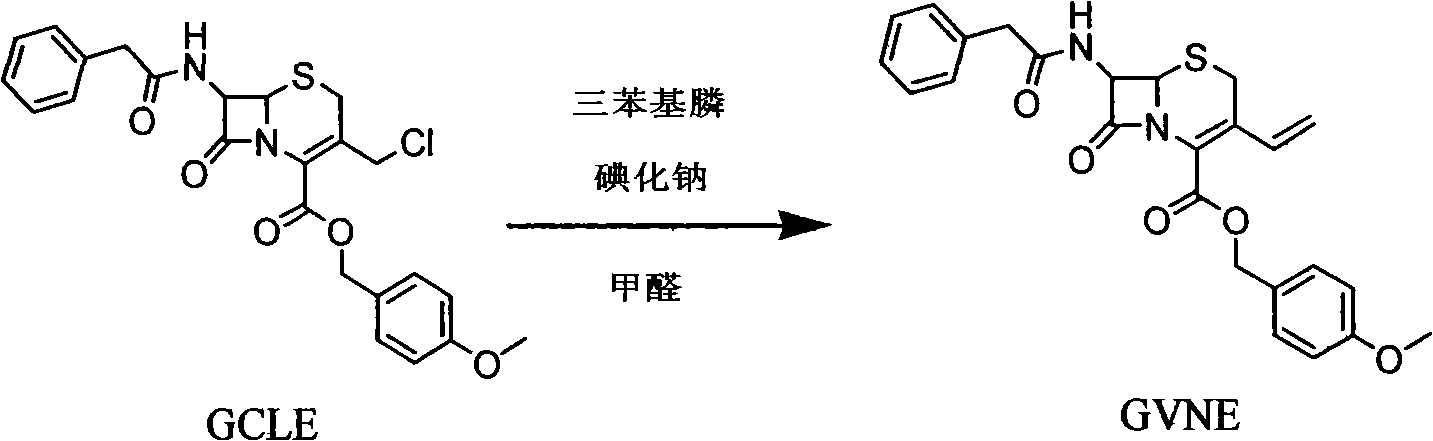
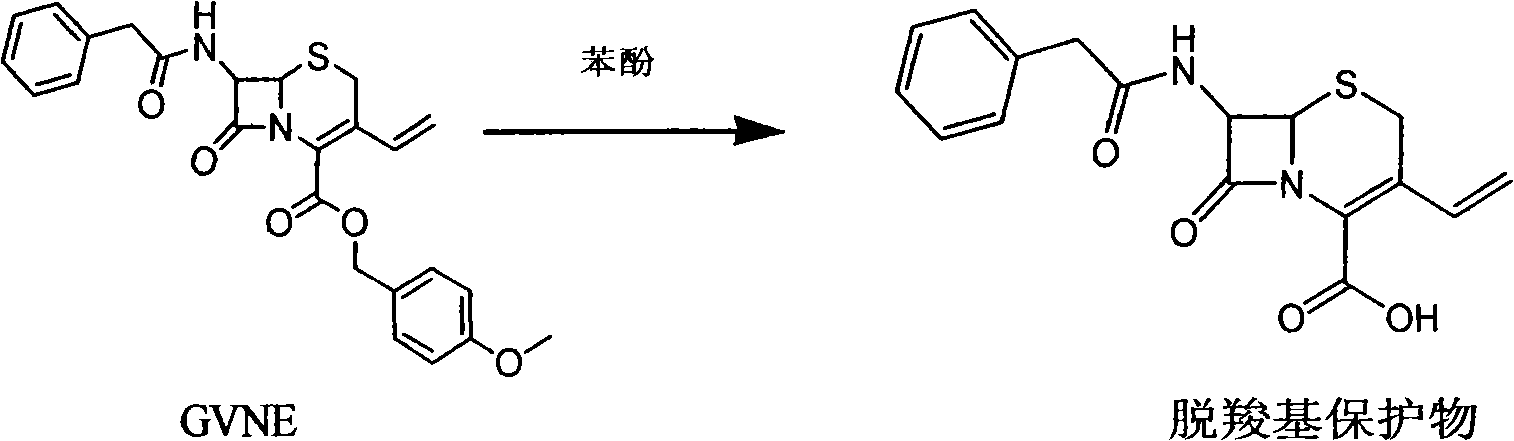
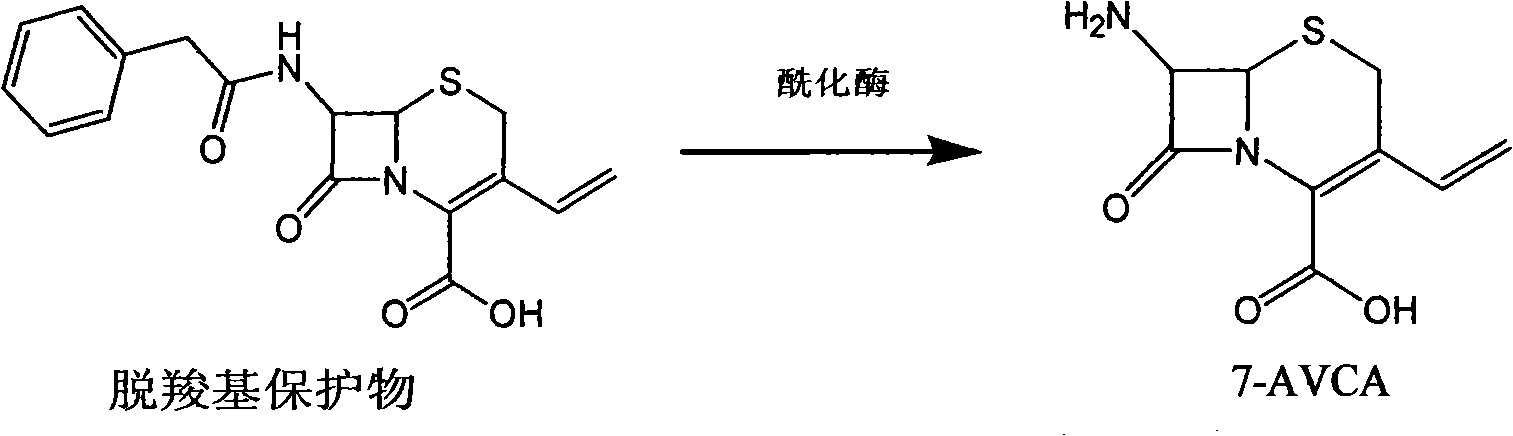
Method for synthetizing 7-amino-3-vinyl-cephalosporin ring-4-carboxylic acid
InactiveCN104073543AImprove conversion rateHigh yieldOrganic chemistryFermentationReaction temperatureCarboxylic acid
The invention discloses a method for synthetizing 7-amino-3-vinyl-cephalosporin ring-4-carboxylic acid. According to the technical scheme, the method comprises the following steps: reacting 7-phenylacetamido-3-chloromethyl-4-cephalosporanic acid p-methoxy benzyl ester (GCLE) serving as a raw material with triphenylphosphine under the action of strong alkali to generate 7-phenylacetamido-3-vinyl-4-cephalosporanic acid p-methoxy benzyl ester (GVNE), carrying out hydrolysis reaction on the GVNE in a phenol solution to remove the carboxyl protecting group, adjusting the PH to alkaline without separating, and adding immobilized penicillin acylase to carry out enzymolysis reaction to remove the carboxyl protecting group to obtain the 7-amino-3-vinyl-4-cephalosporanic acid. The method has the advantages that the product conversion ratio is high; the extracting solvent is low in toxicity and easy to recycle; the reaction temperature is moderate and easy to control; moreover, the immobilized penicillin acylase can be recycled; the production cost is low; the environmental pollution is little; and the harm to the health of operators is hardly caused.
Owner:GUANGDONG LIGUO PHARMACY
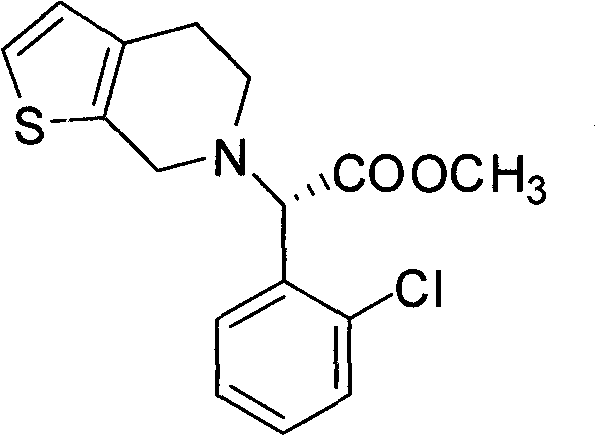
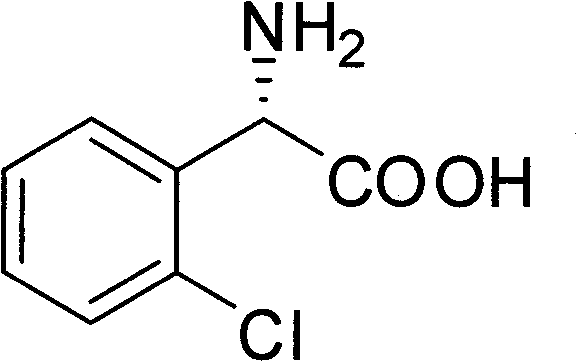
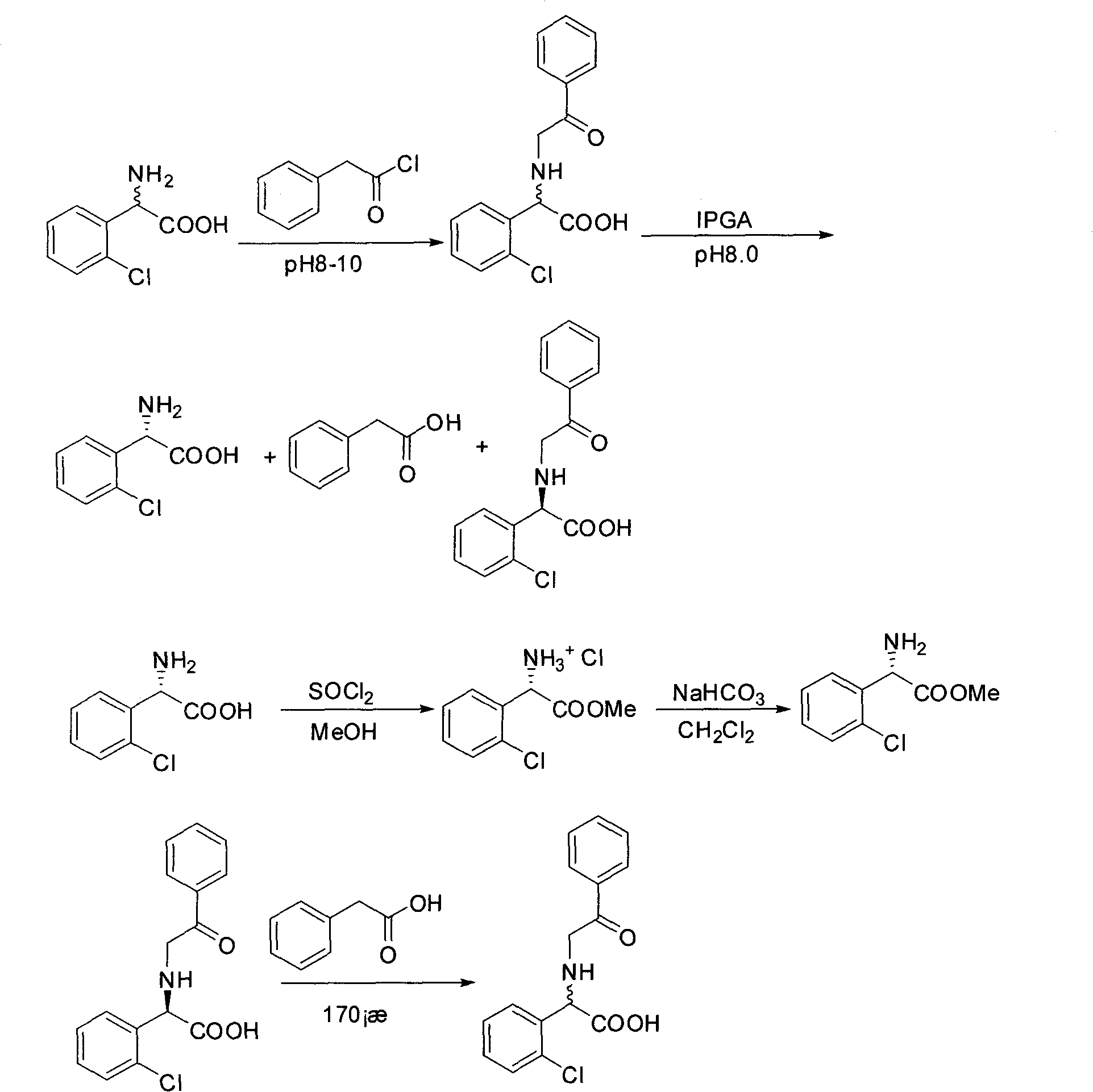
Chemical-enzyme method for preparing (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate
InactiveCN101864464AHigh optical activityEliminate the recrystallization stepFermentationOrganic acidGlycine methyl ester hydrochloride
The invention provides a chemical-enzyme method for preparing an (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate. In the method, (R,S)-2-chlorophenyl glycine is used as a raw material, and the (R,S)-2-chlorophenyl glycine is converted to (R,S)-N-phenylacetyl-2-chlorophenyl glycine by a deacylating agent; an immobilized penicillin acylase is used as a biocatalyst to catalyze a reaction for selectively converting the (R,S)-N-phenylacetyl-2-chlorophenyl glycine into (S)-2-chlorophenyl glycine, phenylacetic acid and (R)-N-phenylacetyl-2-chlorophenyl glycine in a water medium correspondingly; the (S)-2-chlorophenyl glycine is converted into (S)-2-chlorophenyl glycine methyl ester hydrochloride, and the (S)-2-chlorophenyl glycine methyl ester hydrochloride is desalinized to form the (S)-2-chlorophenyl glycine methyl ester; and the (R)-N-phenylacetyl-2-chlorophenyl glycine is mutually resolved with an organic acid and is racemized to form the (R,S)-N-phenylacetyl-2-chlorophenyl glycine which is used for resolution circularly. The method has the characteristics of high yield, high optical purity and environmental friendliness.
Owner:CHONGQING CHIRAL BIOCATALYSIS TECH
Method for enzymatic synthesis of cefprozil in recyclable aqueous two-phase system by using immobilized penicillin acylase
The invention discloses a method for the enzymatic synthesis of cefprozil in a recyclable aqueous two-phase system by using immobilized penicillin acylase. The method comprises the following steps: dissolving a cefprozil parent nucleus and an acylation reagent in an aqueous two-phase polymer solution, wherein the molar ratio of the parent nucleus to the acylation reagent is 1: (1-4); and adding immobilized penicillin G acylase, then adding a two-phase distribution conditioning agent, adjusting the pH value to 4.5-7, and reacting for 1 to 80 hours at the temperature of 5-30 DEG C, wherein the concentration of enzyme in a reaction system is 50-150U / ml. By adopting the method, the hydrolytic activity of the penicillin acylase can be effectively inhibited, thus the hydrolysis degrees of the acylation reagent and the cefprozil product can be reduced. Compared with a method using water as a medium, the method disclosed by the invention has the advantages that the product and the enzyme are distributed in two different water phases, a reactant is fully in contact with the enzyme, so that a synthesis / hydrolysis specific value is greatly increased, the yield of the cefprozil can be increased by 20%, and the highest yield can reach 78%; the adopted novel recyclable aqueous two-phase system can be recycled, so that the production cost is reduced.
Owner:南通康鑫药业有限公司 +1
Recovering method of effective components in amoxicillin enzymatic synthesis mother liquor by utilizing nanofiltration
ActiveCN102392060BAvoid destructionImprove hydrolysis efficiencyOrganic compound preparationAmino-carboxyl compound preparationEnzymatic synthesisNanofiltration
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Penicillin acylase, as well as high-yield strain and application thereof
InactiveCN103184182AIncrease vitalityIncreased substrate specificityBacteriaHydrolasesAchromobacter xylosoxidansPenicillin
The invention provides penicillin acylase, as well as a high-yield strain and an application thereof, belonging to the field of bio-pharmaceuticals. The strain is Achromobacter xylosoxidans K18 with the collection number of CCTCC NO: M2012541. The penicillin acylase is obtained by fermenting the strain K18. The amino acid sequence of the penicillin acylase is as shown in SEQ ID NO: 2, and the gene sequence of the penicillin acylase is as shown in SEQ ID NO: 1. The invention further provides the application of the penicillin acylase in synthesis of beta-lactam type antibiotics. The strain K18 is the high-yield strain of the penicillin acylase, and when fermentation is performed through a culture medium containing an inducing agent, the yield of the penicillin acylase can be above 200U / L. The penicillin acylase produced by the strain K18 has the advantages of high temperature resistance, capability of keeping higher activity under an acidic environment, higher specificity of a substrate, strong organic solvent tolerance and the like.
Owner:NANJING UNIV OF TECH
Novel beta-lactam antibiotic synthetase production method
ActiveCN103695405AIncrease enzyme activityImprove stabilityHydrolasesVector-based foreign material introductionNucleotideAntibiotic Y
The present invention discloses a method for producing novel lactam antibiotics by using specific engineered bacteria transformant BL21(DE3) / PET28-ASPGA. According to the method, a penicillin acylase amino acid sequence represented by SEQIDNO:1 is adopted as basis, a mutation site is introduced, reverse design is performed to obtain a nucleotide sequence, optimization is performed, complete gene synthesis and recombinant vector construction are sequentially performed to construct a recombinant strain, and the constructed recombinant strain is treated through steps of fermentation, cell disruption, separation, purification, decoloration and immobilization to finally obtain the novel lactam antibiotic immobilized enzyme finished product capable of being directly used for industrial production. According to the present invention, the immobilized enzyme prepared by using the method has characteristics of high enzyme activity, good stability and excellent repeated use effect, and can be provided for catalyzing synthesis of a variety of lactam antibiotics such as amoxicillin, cephalexin and cefaclor.
Owner:NORTH CHINA PHARMA HEBEI HUAMIN PHARMA
Method for synthesizing cefprozil through green enzymatic method
The invention relates to a method for synthesizing cefprozil through a green enzymatic method. The method includes following steps: S1, adding parent nucleus 7-APRA or hydrochloride thereof into a buffer solution, and adding D-para hydroxybenzene glycinate derivative and / or D-para hydroxybenzene glycine amide under the condition that pH is 5-8; S2, adding cefprozil synthetase into the S1, reacting at temperature of 15-30 DEG C and pH of 6.5-7.8 for 1-3h, separating out separation liquid after reaction finishes, and immobilizing cefprozil synthetase to obtain a cefprozil crude product; S3, subjecting the crude product obtained in the S2 to acidolysis dissolved clarification, filtering and re-crystallizing to obtain cefprozil. Raw materials are cheap and easy to obtain, reaction is simple, hydrolysis activity of penicillin acylase can be effectively inhibited, and production cost is low; compared with conventional chemical synthesis methods, the method is simple and convenient to operate and low in cost, synthesis period is shortened, production efficiency is improved, total yield is high, controllability is high, and needs on industrial production are met.
Owner:GUANGZHOU BAIYUNSHAN PHARM CO LTD
Penicillin acylation enzyme-fixing carrier preparation method and carrying method
The invention discloses a making method of fixed penicillin acylated enzyme, which comprises the following steps: (1) grinding the porous resin with lateral functional group; sieving; drying; activating; blending with chitose solution; drying; obtaining the solid; (2) adding solid in the step (1) in the polar hydrophilic solvent; adding adjuvant to suck after decorating; washing; drying to obtain the porous resin carrier; (3) loading acylated penicillin on the porous resin carrier; obtaining the product.
Owner:ZHEJIANG UNIV
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212AImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic [beta]-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
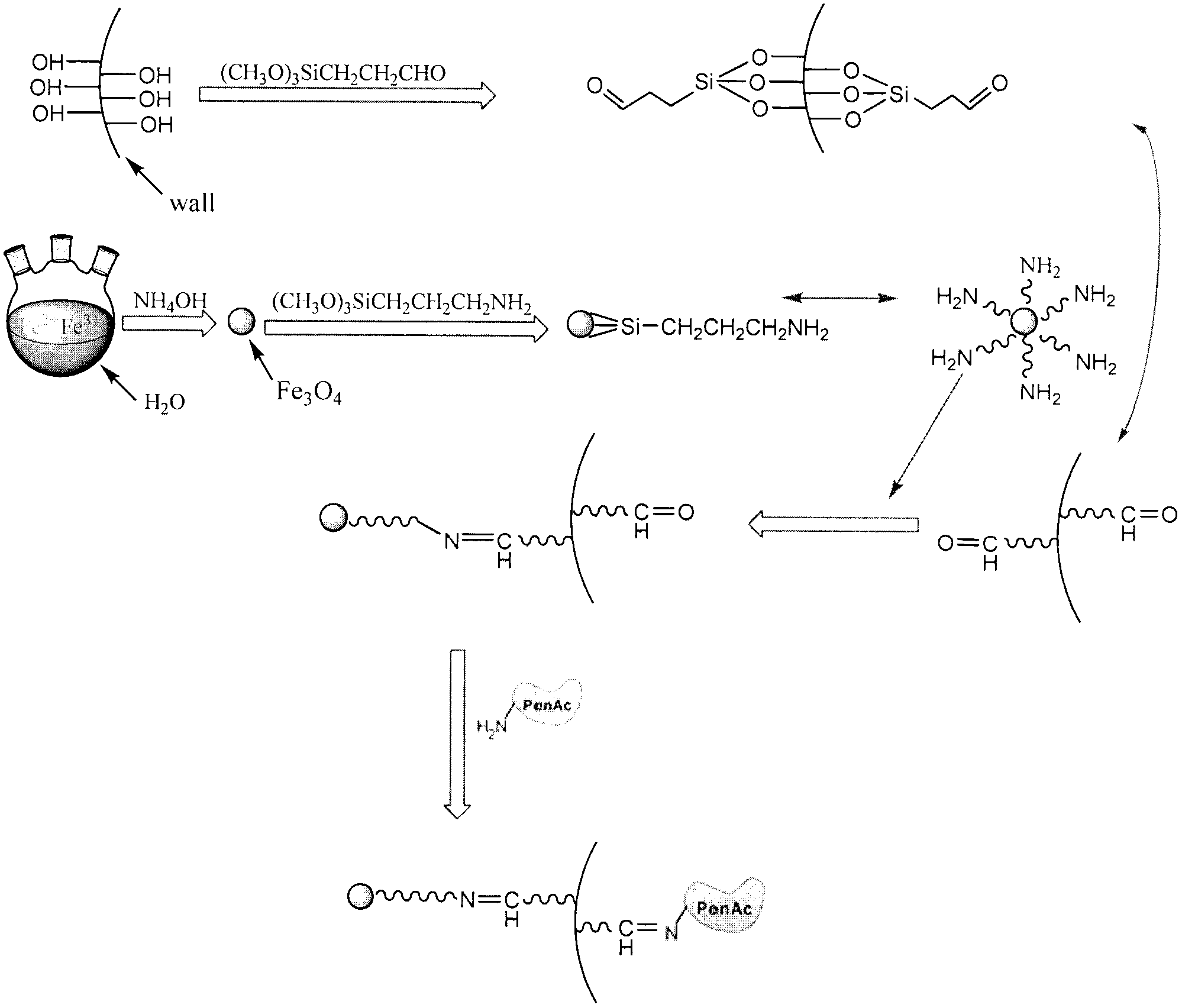
Aramagnetic aldehyde group mesoporous molecular sieve for immobilized biological enzymes, and preparation method thereof
InactiveCN102703412AReduce cloggingEasy to separateImmobilised enzymesMolecular-sieve and base-exchange compoundsSilanesTrypsin
The invention belongs to the technical field of biology, and discloses an aramagnetic aldehyde group mesoporous molecular sieve for immobilizing biological enzymes, and a preparation method thereof. According to the invention, gamma aldehyde glycidoxy propyl trimethoxy silane is utilized to introduce aldehyde groups onto the surface of the mesoporous molecular sieve, and then a covalent bond is utilized to immobilize aramagnetic Fe3O4 nano particles and biological enzymes onto the outer surface and the inner surface of the mesoporous molecular sieve respectively, wherein the aramagnetic Fe3O4 nano particles are subjected to gamma-amino propyl triethoxy silane surface modification and have the particle sizes larger than the pore diameter of the mesoporous molecular sieve, as a result, specially-structured aramagnetic immobilized enzymes can be prepared and are separated from the liquid phase system easily under the action of an external magnetic field, and further, the property and the separation efficiency of the immobilized enzymes are improved. The aramagnetic aldehyde group mesoporous molecular sieve can be used for immobilization of water-soluble biological enzymes such as penicillin acylase, glucose isomerase, glucosylase, trypsin and amylase, the activity of the prepared aramagnetic immobilized penicillin acylase is 9901 U / g, and 91.2% of the initial activity is retained after ten times of cycle use.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing cefprozil in pH responsive regenerative double aqueous phase system
PendingCN106939327AIncreased molar yieldEasy to separateFermentationChemical synthesisCentrifugation
The invention relates to a method for preparing cefprozil in a pH responsive regenerative double aqueous phase system. The method comprises (1) preparing two double-aqueous phase systems, (2) orderly adding 7-APRA, D-p-hydroxyphenylglycine methyl ester hydrochloride into the systems, adjusting solution pH to 5.00-6.50 and controlling a solution temperature in a range of 10-30 DEG C, (3) adding immobilized penicillin acylase into the solution obtained by the step (2), and (4) standing the mixed solution, removing the immobilized penicillin acylase, adjusting the pH of the reaction solution, recovering P<ADBA> / <PMDB> and P<ADB> / P<MDB> polymers of the double-aqueous phase systems, feeding the supernatant to a crystallization section, carrying out crystallization, and carrying out centrifugation, washing and drying to obtain a product. The method reduces a chemical synthesis cost, improves a low product conversion rate of the monohydrolase catalytic reaction, effectively improves a yield, simplifies the operation, reduces a cost and realizes easy recovery of the double-aqueous phase systems.
Owner:EAST CHINA UNIV OF SCI & TECH
Chemical-enzymatic method for preparing D-serine
InactiveCN102321695AConvenient sourceEfficient separationOrganic compound preparationAmino-carboxyl compound preparationPenicillinPhenylacetic acid
The present invention provides a chemical-enzymatic method for preparing D-serine. According to the method, DL-serine is adopted as a raw material and is derived into DL-N-phenylacetyl serine by using an acylating agent; immobilized penicillin acylase is adopted as a biocatalyst, correspondingly and selectively catalyze the DL-N-phenylacetyl serine in an aqueous medium to obtain L-serine, phenylacetic acid and D-N-phenylacetyl serine; a metal complexation method and an isoelectric point crystallization method are adopted to carry out separation to obtain optically pure D-phenylacetyl serine; the D-N-phenylacetyl serine is subjected to acid hydrolysis, concentration and crystallization to obtain the D-serine, wherein the yield of the D-serine is 45%, ee of the D-serine is 99.6%. The method provided by the present invention has characteristics of high yield, high chemical purity, high optical purity, environment-friendly property, and is suitable for the large-scale production of the D-serine.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Epoxy mesoporous molecular sieve for use in bio-enzyme immobilization and preparation method thereof
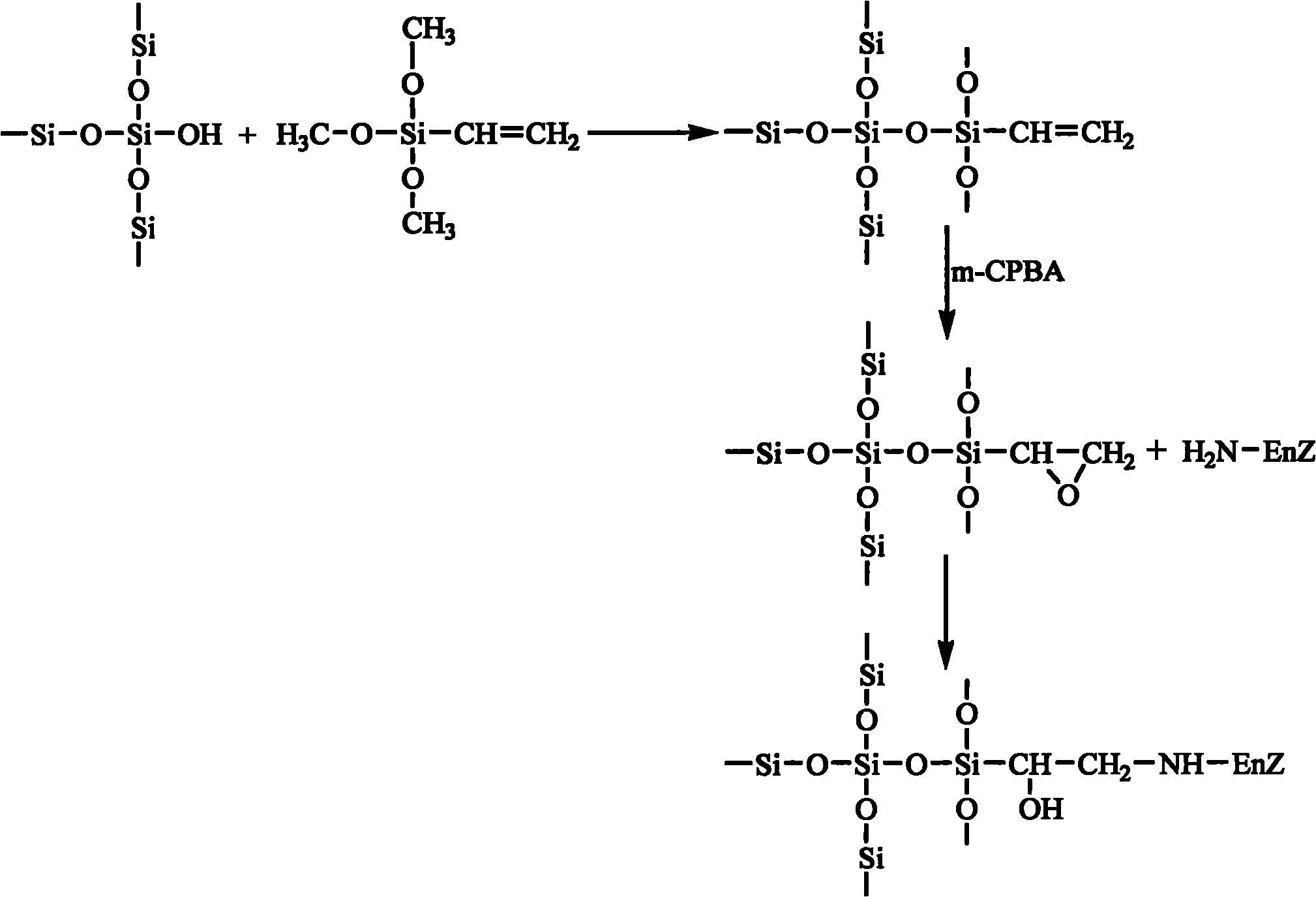
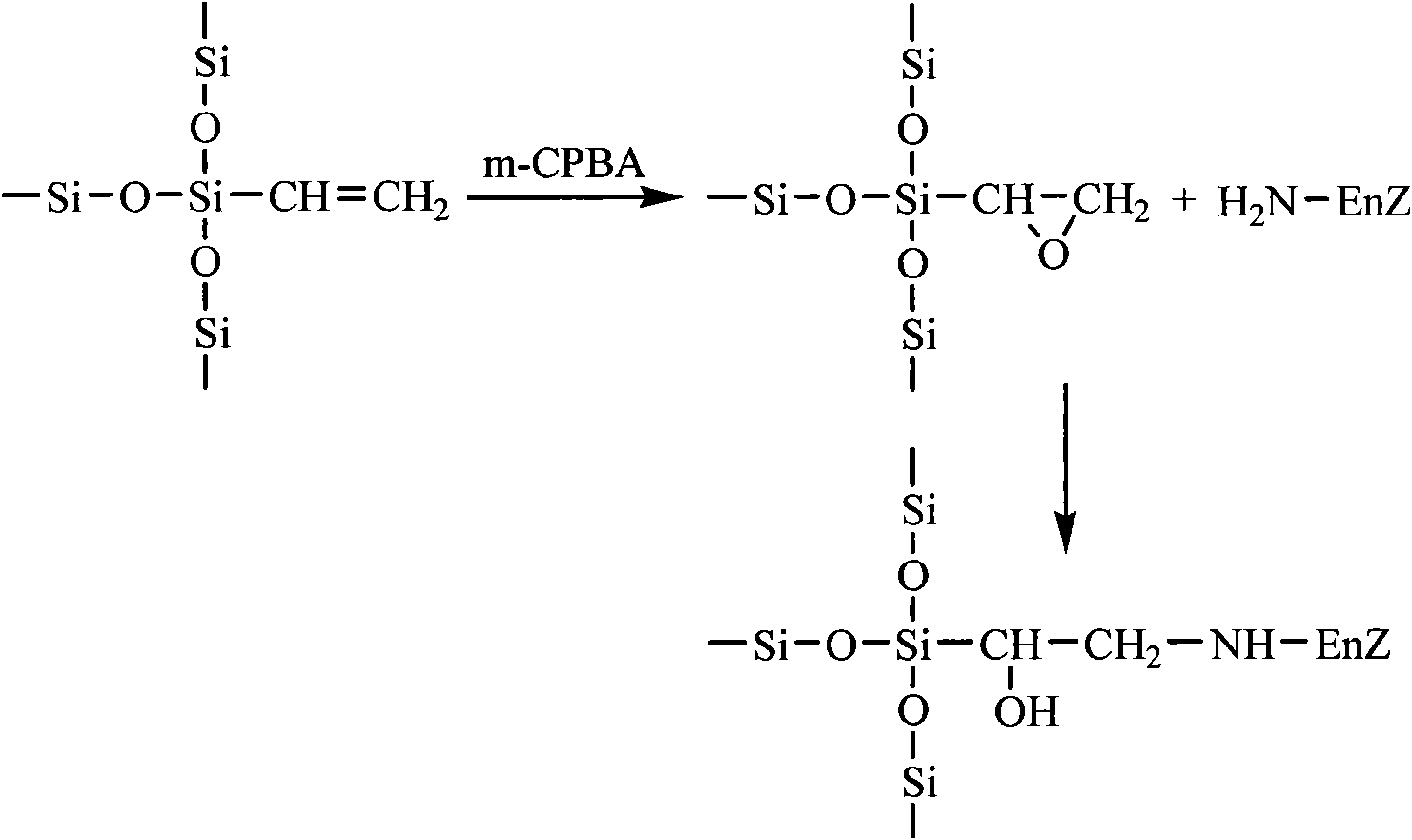
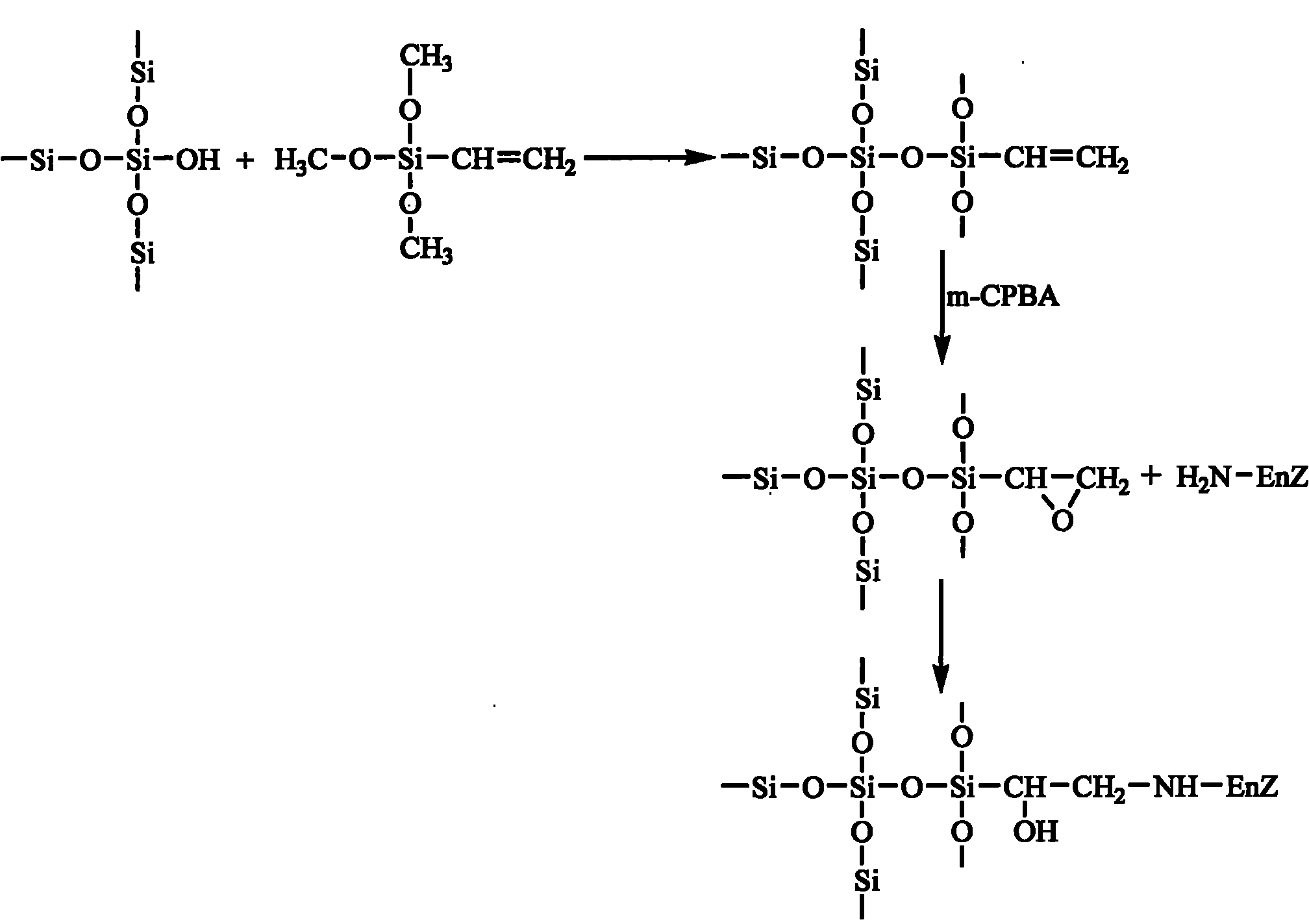
The invention discloses an epoxy mesoporous molecular sieve for use in bio-enzyme immobilization and a preparation method thereof. In the invention, cyclo groups of the mesoporous molecular sieve which contains vinyl and has a cubic phase Ia3d structure and the mesoporous molecular sieve which has a surface vinyl function are oxidized by using metachloroperbenzoic acid to introduce epoxy functional groups with a shorter chain length onto the surface of the mesoporous molecular sieve while the influences of a surface functionization process on the aperture, the specific surface area and the pore volume of the mesoporous molecular sieve are reduced as much as possible, so the bio-enzyme can be directly immobilized on the surface of the mesoporous molecular sieve in a covalent bonding form without further activation, and the performance of the immobilized enzyme can be improved. The epoxy mesoporous molecular sieve can be used for the immobilization of water soluble bio-enzymes such as penicillin acylase, glucose isomerase, glucosidetransferase, parenzyme and diastase, in particular for the immobilization of the penicillin acylase; and the activity of the obtained immobilized enzyme is 8416U / g, and 90 percent of original activity of the immobilized enzyme is retained after 10 times of recycling.
Owner:EAST CHINA UNIV OF SCI & TECH
Chemical-enzyme method for preparing D-basic amino acid hydrochloride
InactiveCN102392061AGuaranteed high optical activitySimpler than ion exchange separationOrganic compound preparationAmino-carboxyl compound preparationPenicillinPhenylacetic acid
The invention provides a chemical-enzyme method for preparing D-basic amino acid hydrochloride. The method comprises the following steps of: derivatizing DL-basic amino acid serving as a raw material to obtain DL-N-diphenyl acetyl basic amino acid by utilizing an acylating agent, hydrolyzing the DL-N-diphenyl acetyl basic amino acid by using immobilized penicillin acylase as a biocatalyst through non-stereo selectivity and enantioselectivity to obtain L-basic amino acid, phenylacetic acid and D-alpha-N-phenylacetyl basic amino acid; and performing acidolysis on the D-alpha-N-phenylacetyl basic amino acid to obtain phenylacetic acid and D-basic amino acid dihydrochloride, and performing treatment of epoxypropane to obtain the D-basic amino acid hydrochloride. The D-lysine hydrochloride and D-ornithine hydrochloride which are prepared by the method have the yield of more than 40 percent, and ee is more than 99 percent. The method has the characteristics of high yield and optical purity, simple process and the like, and is suitable for the large-scale production of the D-basic amino acid hydrochloride.
Owner:CHONGQING UNIV OF POSTS & TELECOMM
Aldehyde group mesoporous molecular sieve used for immobilization of biological enzyme and preparation method thereof
The invention discloses an aldehyde group mesoporous molecular sieve used for immobilization of a biological enzyme and a preparation method thereof. In the preparation method, a 60 Co-gamma ray pre-irradiation grafting acrolein technique or gamma-aldehyde propyl trimethoxy silane is used for performing functionalized modification on the surface of the mesoporous molecular sieve, an aldehyde propyl functional group with a shorter chain length is introduced to the surface of the mesoporous molecular sieve, the influences, caused by the surface functionalizing process, on the aperture, the specific area and the pore volume of the mesoporous molecular sieve are reduced as much as possible, and further activation is not needed to ensure that the biological enzyme is directly immobilized on the surface of the mesoporous molecular sieve in a covalent bonding mode, and the performance of the immobilized enzyme is improved. The aldehyde group mesoporous molecular sieve can be applied to the immobilization of water-soluble enzymes such as penicillin acylase, glucose isomerase, transglucosidase, trypsase, amylase and the like, and is particularly suitable for the immobilization of the penicillin acylase; and the obtained immobilized enzyme has the activity of 8895 U / g, and after 10 times of recycling, the immobilized enzyme maintains 92 percent of the initial activity.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for synthesizing amoxicillin and generating byproduct of sodium phenylacetate solution by using semi-direct method
InactiveCN107988306AHigh yieldImprove product qualityOrganic compound preparationCarboxylic acid salt preparationPhenylacetic acidBenzylpenicillin potassium
The invention relates to a method for synthesizing amoxicillin and generating a byproduct of a sodium phenylacetate solution by using a semi-direct method and belongs to the technical field of medicine preparation. The method comprises the following steps: splitting benzylpenicillin potassium into 6-APA (Amino Penicillanic Acid) and phenylacetic acid under the action of penicillin acylase, extracting a splitting solution with dichloromethane to separate 6-APA from the phenylacetic acid, putting ammonia water into an extraction water phase, adjusting the pH value of a material liquid to be neutral, removing the dichloromethane, putting the solid 6-APA into the obtained 6-APA dissolving liquid, and synthesizing the amoxicillin together with D-methyl p-hydroxyphenylglycinate under the actionof amoxicillin synthetase. A sodium phenylacetate solution can be prepared by alkalizing the dichloromethane obtained by extracting the splitting solution, the sodium phenylacetate solution can be directly applied to fermentation production of penicillin, the step of recycling the phenylacetic acid is avoided, and the production cost is reduced.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Aramagnetic epoxy group mesoporous molecular sieve for immobilized biological enzymes, and preparation method thereof
ActiveCN102703411BReduce cloggingEasy to separateImmobilised enzymesMolecular-sieve and base-exchange compoundsEpoxySilanes
The invention belongs to the technical field of biology, and discloses an aramagnetic epoxy group mesoporous molecular sieve for immobilizing biological enzymes, and a preparation method thereof. According to the invention, gamma glycidoxy propyl trimethoxy silane is utilized to introduce aramagnetic epoxy groups onto the surface of the mesoporous molecular sieve, and then a covalent bond is utilized to immobilize aramagnetic Fe3O4 nano particles and biological enzymes onto the outer surface and the inner surface of the mesoporous molecular sieve respectively, wherein the aramagnetic Fe3O4 nano particles are subjected to L-cysteine surface modification and have the particle sizes larger than the pore diameter of the mesoporous molecular sieve, as a result, specially-structured aramagnetic immobilized enzymes can be prepared and are separated from the liquid phase system easily under the action of an external magnetic field, and further, the property and separation efficiency of the immobilized enzymes are improved. The aramagnetic epoxy group mesoporous molecular sieve can be used for immobilization of water-soluble biological enzymes such as penicillin acylase, glucose isomerase, glucosylase, trypsin and amylase, the activity of the prepared aramagnetic immobilized penicillin acylase is 8800 U / g, and 94.5% of the initial activity is retained after ten times of cycle use.
Owner:EAST CHINA UNIV OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com