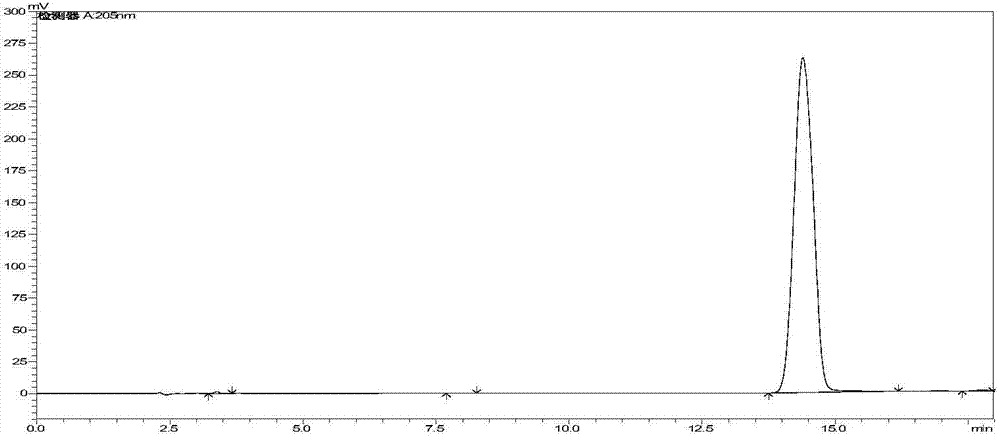
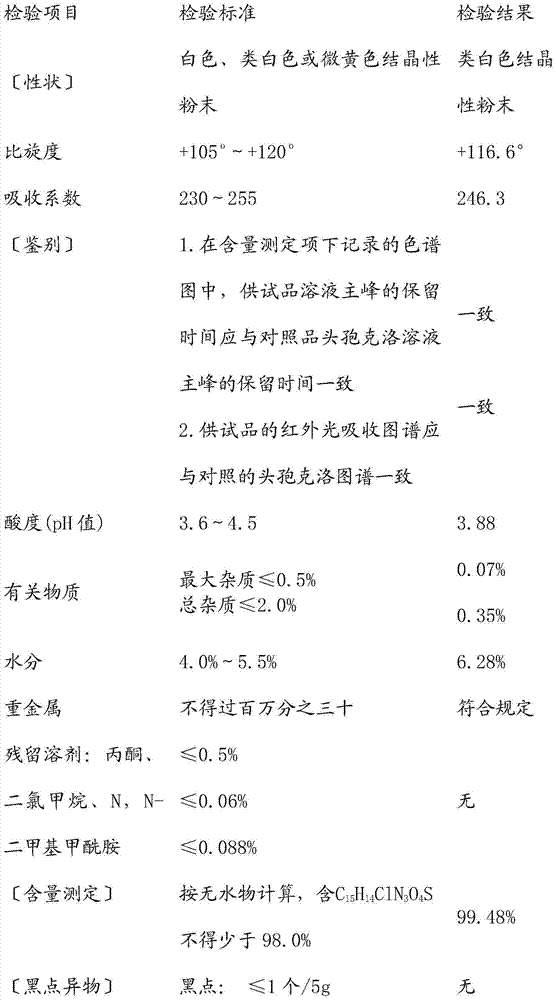
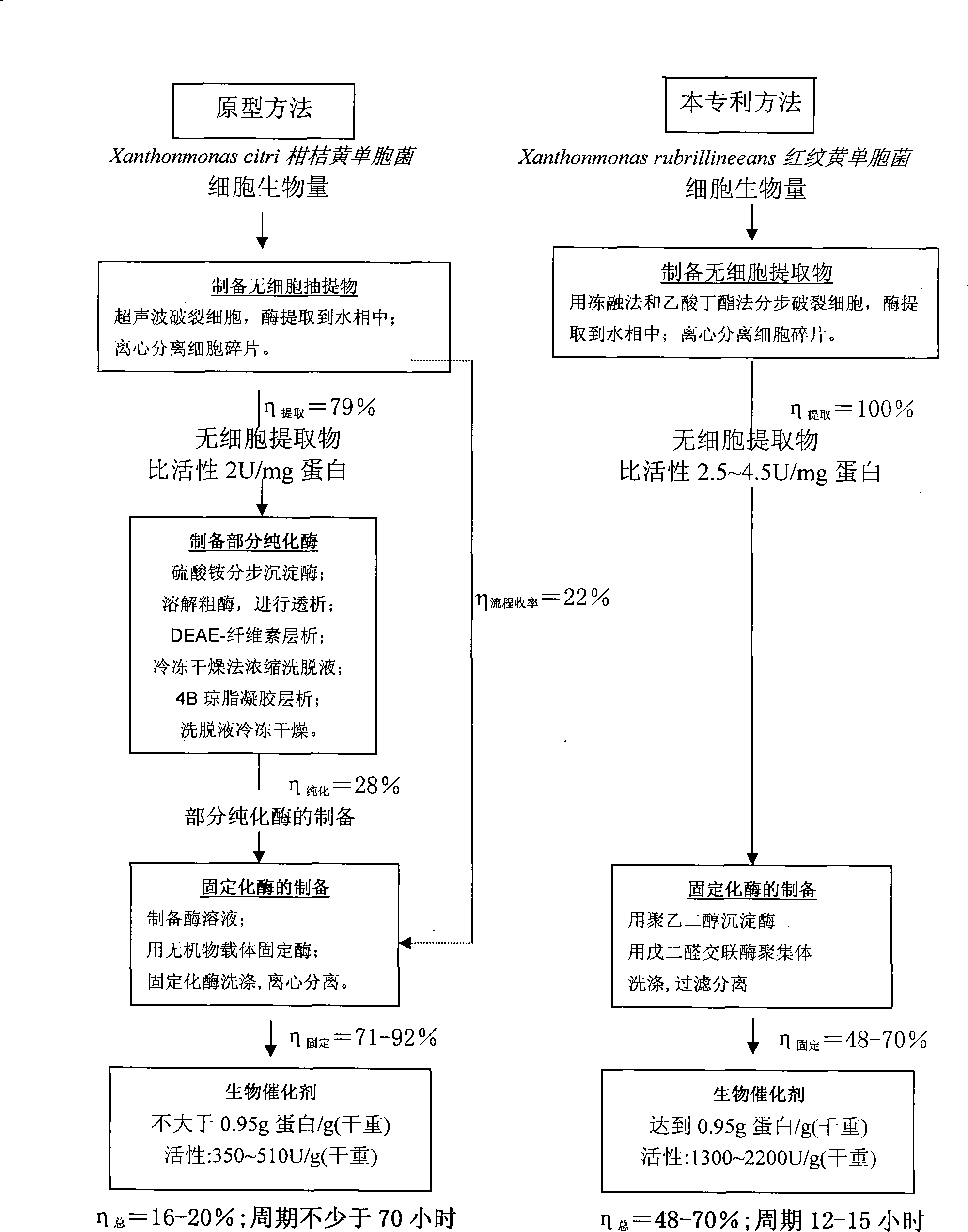
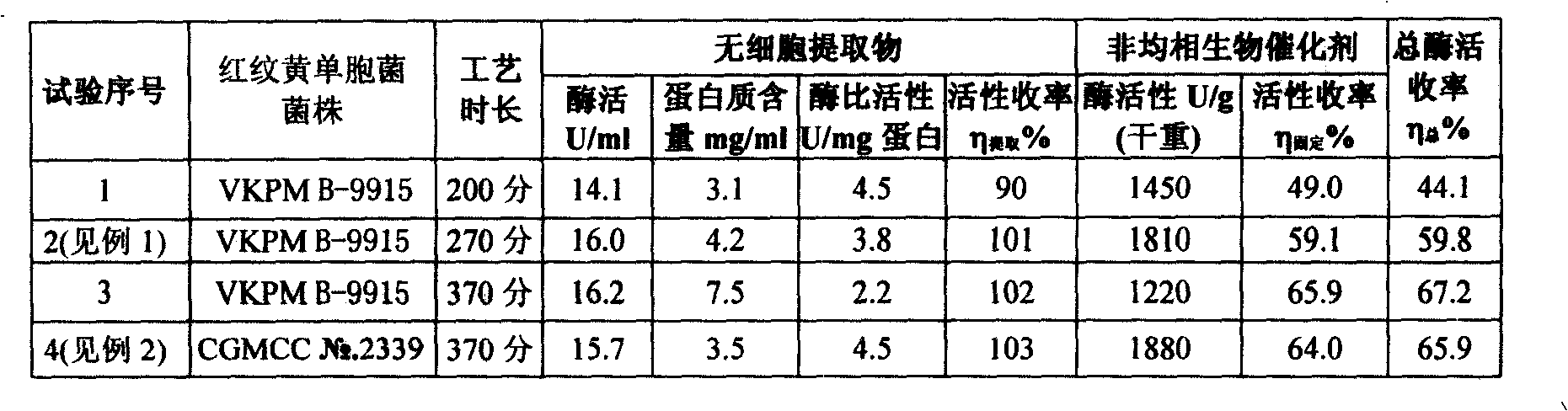
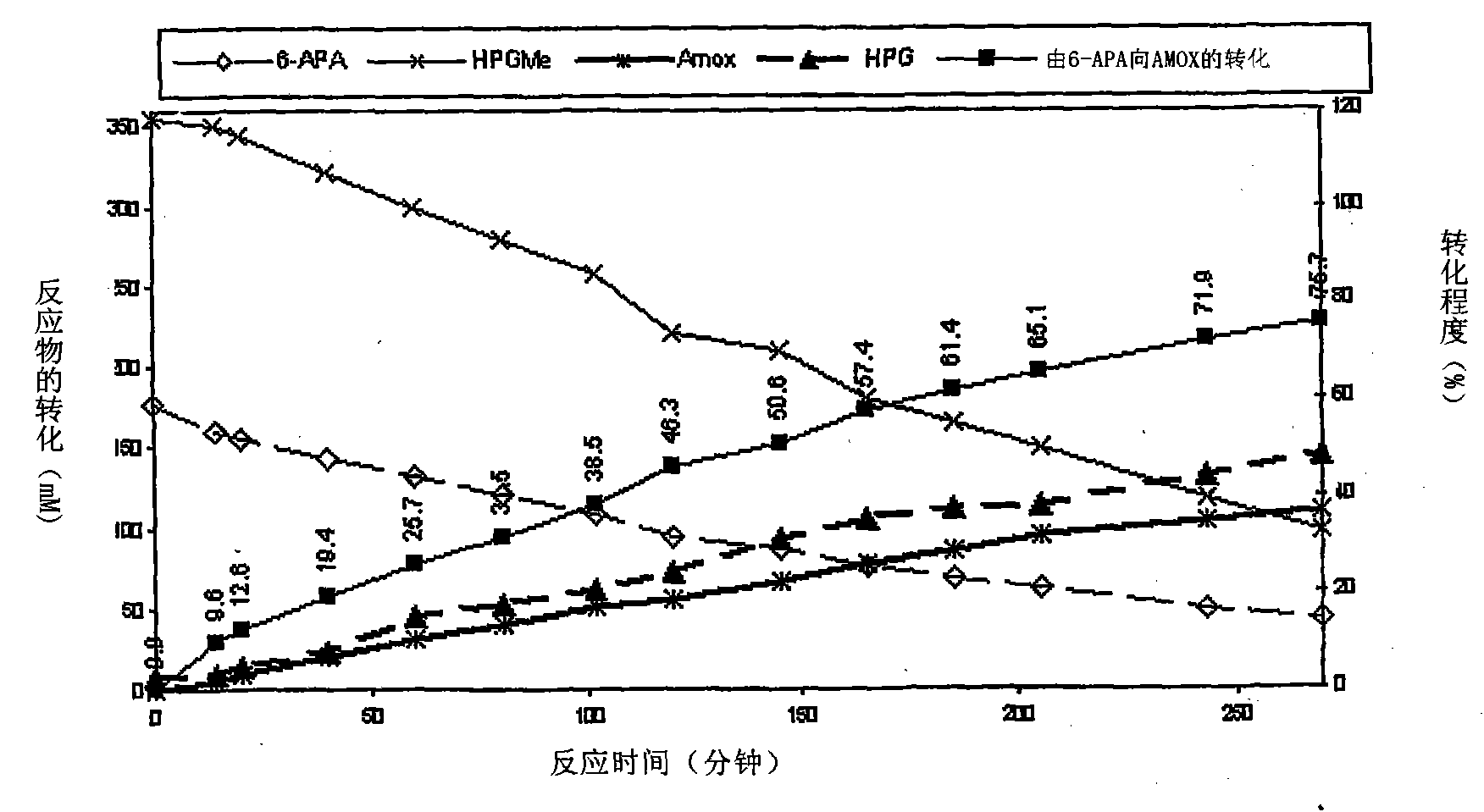
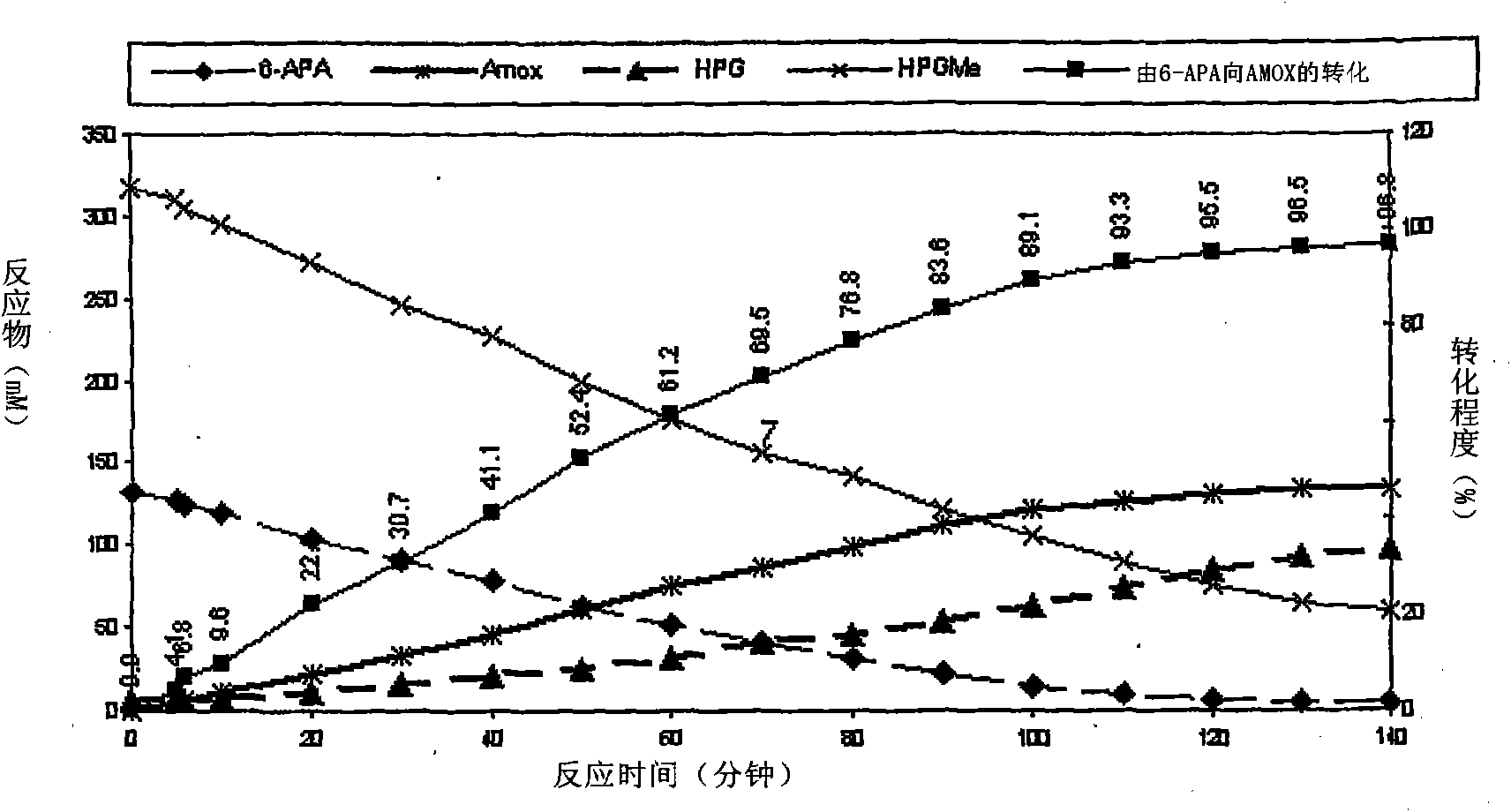
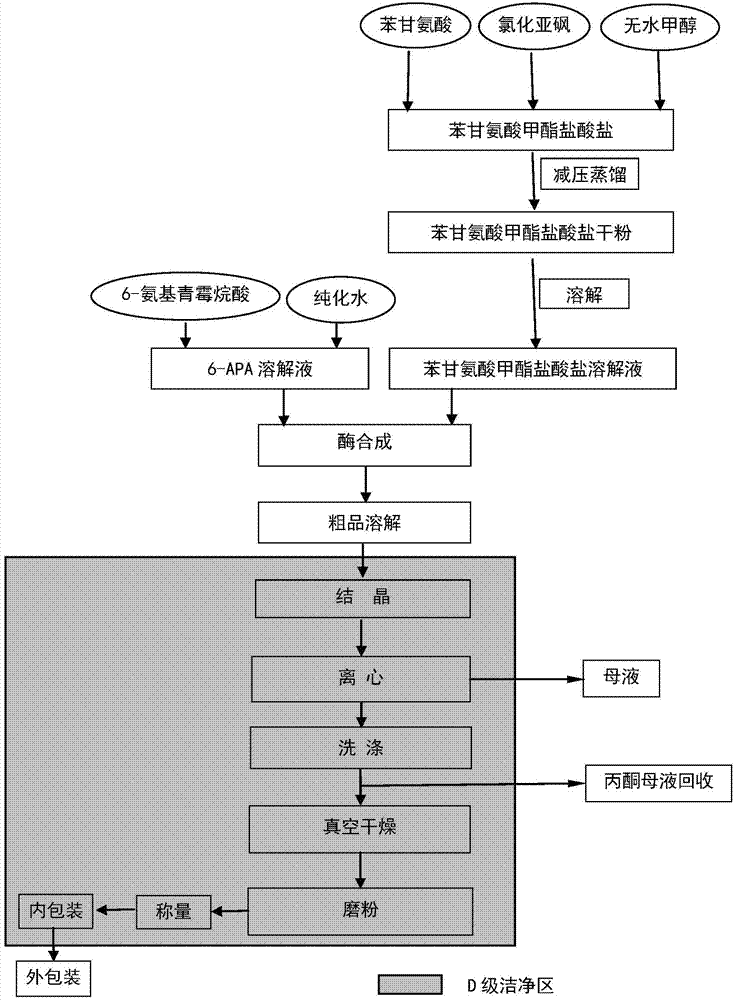
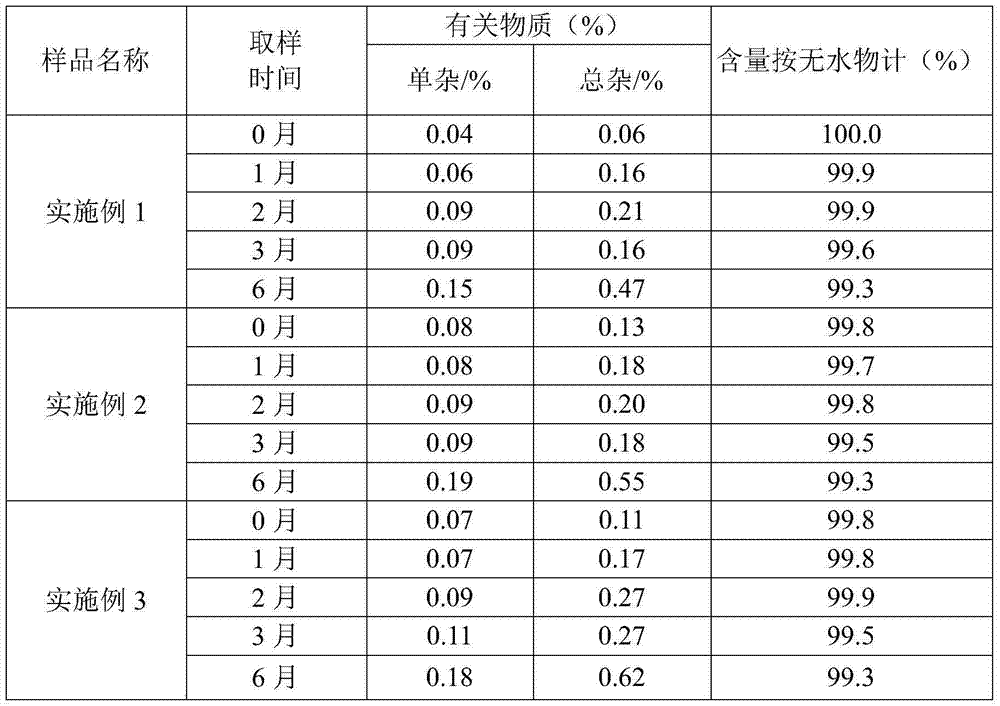
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
39 results about "Phenylglycine methyl ester" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
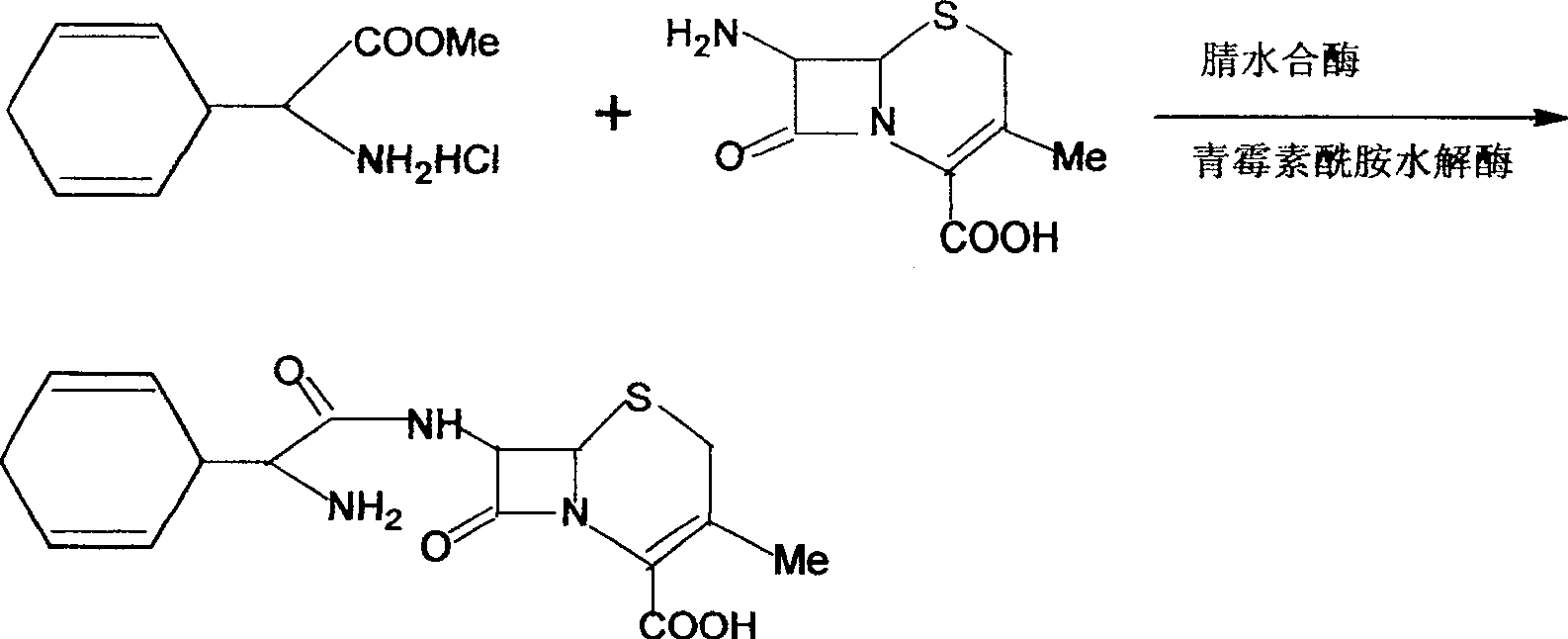
Cefaclor and synthetic method thereof
ActiveCN103757085AHigh purityImprove conversion rateOrganic chemistryFermentationPhenylglycine methyl esterMedicinal chemistry
The invention provides cefaclor and a synthetic method thereof. The synthetic method of cefaclor comprises that in the presence of an enzyme, cefaclor is generated by forming a reaction mixed solution of 7-ACCA and a D-phenylglycine methyl ester salt derivative and performing reaction, wherein cefaclor crystal seeds are added into the reaction mixed solution, and the crystal seeds are added before cefaclor generated in the reaction is precipitated. The method helps to improve the reaction efficiency, shorten the synthetic period, improve the conversion rate of 7-ACCA and improve the purity of obtained cefaclor.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
Separation and purification method for cefaclor by enzymatic synthesis
ActiveCN103571907AResolve separabilitySolution concentrationOrganic chemistryFermentationPurification methodsPhenylglycine methyl ester
The invention discloses a separation and purification method for cefaclor by enzymatic synthesis. The separation and purification method comprises the following steps: dissolving 7-ACCA by ammonia water in a water phase, thereafter adding immobilized enzyme; keeping the temperature within 10-35 DEG C; dripping phenylglycine methyl ester hydrochloride or phenylglycine methyl ester mesylate; continuously maintaining the pH value of the reaction within 5.5-7.5; continuously making the generated cefaclor be precipitated from the system with occurrence of enzyme digestion; continuously separating the immobilized enzyme M-1 from the cefaclor by a filtering separation method in an enzymatic liquid; circulating the mother liquid back to a reactor until the 7-ACCA has complete reaction; purifying the obtained wet cefaclor product, and then obtaining the cefaclor. According to the separation and purification method disclosed by the invention, during precipitation of the cefaclor, the cefaclor is separated from the enzymatic reaction liquid, so that the problems of separation and reaction concentration of the enzymic catalytic reaction are solved.
Owner:苏州盛达药业有限公司
Immobilized alpha-amino-acid ester hydrolase, preparation and application thereof
InactiveCN101525603AMany preparation stepsShort manufacturing cycleHydrolasesChemical industryCross-linkPenicillin
The invention discloses a heterogeneous biocatalyst taking alpha-amino-acid ester hydrolase as basis, a preparation method thereof and application in synthesizing amino beta-lactams. The activity of catalyst synthetase is 2,200 U / g by dry weight, and the content of protein is 0.95 g / g by dry weight; and xanthomonas rubrilineans cell biomass is processed in an organic solvent under a pH gradient through low temperature effect to extract enzyme, and enzyme aggregates are deposited and cross-linked to obtain the hydrolase. The alpha-amino-acid ester hydrolase as a catalyst can be synthesized into amino penicillin and amino cephalosporin drugs through acylating a beta-lactam compound by D-phenylglycine methyl ester derivatives in water or in a mixed medium of water and an organic solvent.
Owner:SICHUAN INDAL INST OF ANTIBIOTICS CHINA NAT PHARMA GROUP CORP +1
Preparation process of D-phenylglycine methyl ester hydrochloride crystals
ActiveCN104829478AReasonable useAvoid applyingOrganic compound preparationAmino-carboxyl compound preparationChemical industryPhenylglycine methyl ester
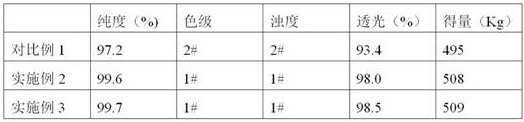
The invention belongs to the technical field of the chemical industry, and in particular relates to a preparation process of D-phenylglycine methyl ester hydrochloride crystals. The preparation process comprises the following steps: sequentially adding methanol and D-phenylglycine into a reactor, stirring uniformly, and slowly adding sulfoxide chloride; controlling the temperature in the reactor below 55DEG C, and controlling the temperature in the reactor to be 55-65DEG C after adding sulfoxide chloride; performing reflux reaction, then performing vacuum azeotropic distillation, and finally performing temperature controlled cooling crystallization; and filtering, washing and drying to obtain a D-phenylglycine methyl ester hydrochloride crystal product. The preparation process provided by the invention is high in one-pass yield, the prepared product is high in purity, good in color grade and stable in quality, the preparation process is low in production cost, and the operation is easy to control.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
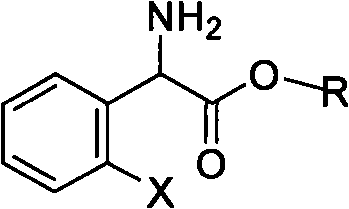
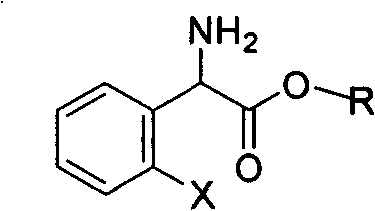
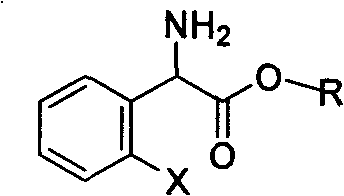
Method for splitting S-(+)-o-chlorobenzene glycine methyl ester
InactiveCN101497575AHigh yieldShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationAlkaneChlorobenzene
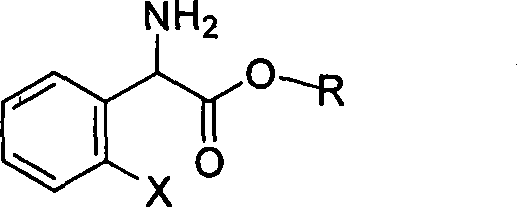
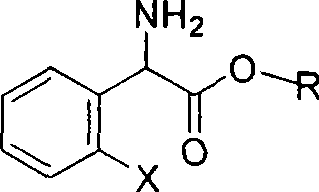
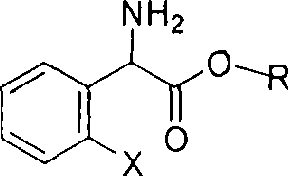
The invention relates to division of a clopidogrel intermediate 2-substituted phenylglycine methyl ester (or acid), which is a medicine for resisting platelet aggregation. The 2-substituted phenylglycine methyl ester (or acid) has the structure shown in a structural formula I. By utilizing the method, the raw materials and the reagents are low-priced and easily obtained, the reaction conditions are mild and the yield is satisfactory, reaching 98 percent. Therefore, the method for dividing the 2-substituted phenylglycine methyl ester (or acid) is easily industrialized. In the structural formula I, R is equal to H, alkyl (C1-C4, benzene)X is equal to a halogen, and for OR1 and SR1, R1 is equal to H or alkane(C1-C3) is equal to a benzene or the benzene for free substitution.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD +1
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212AImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
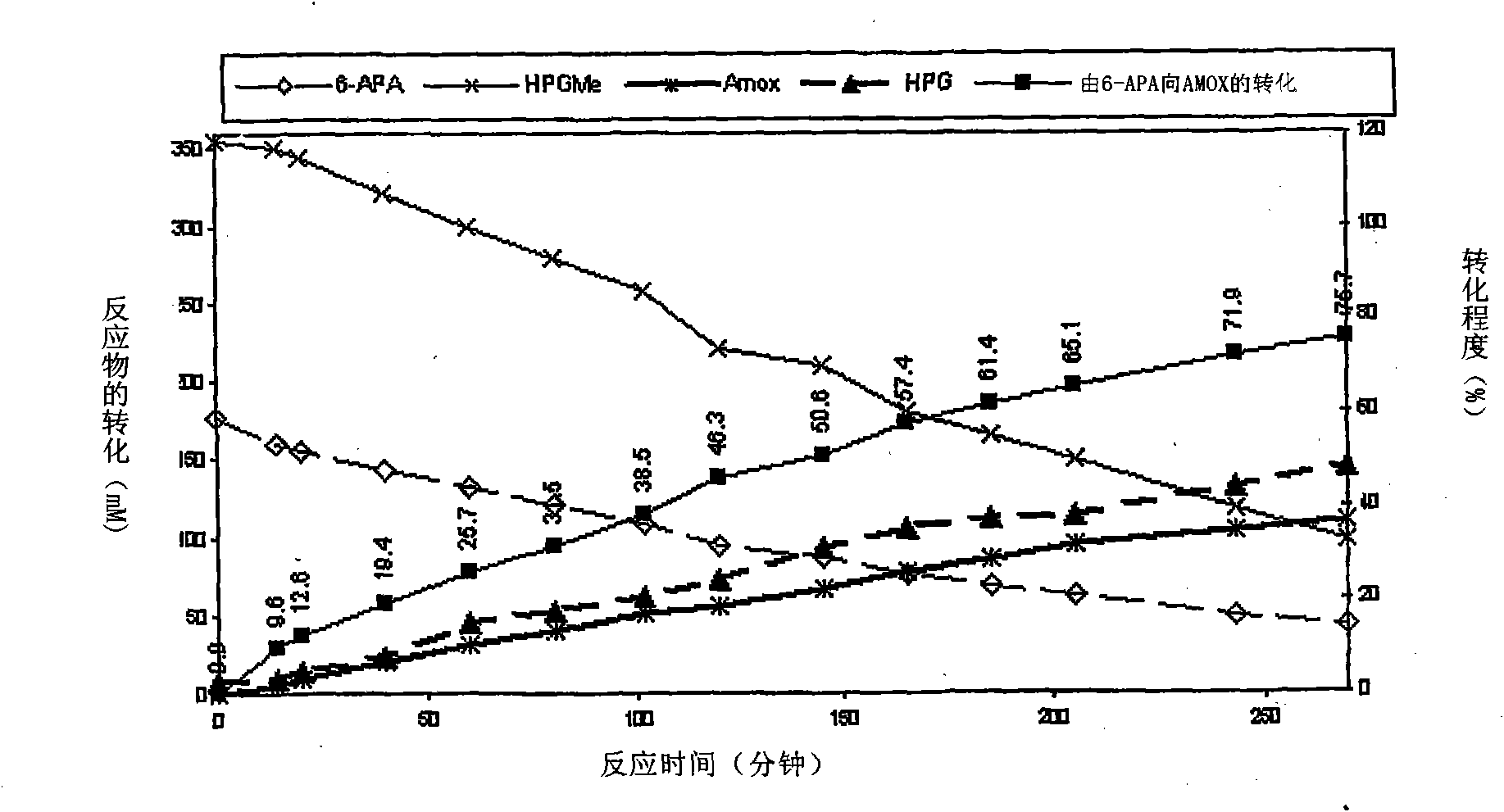
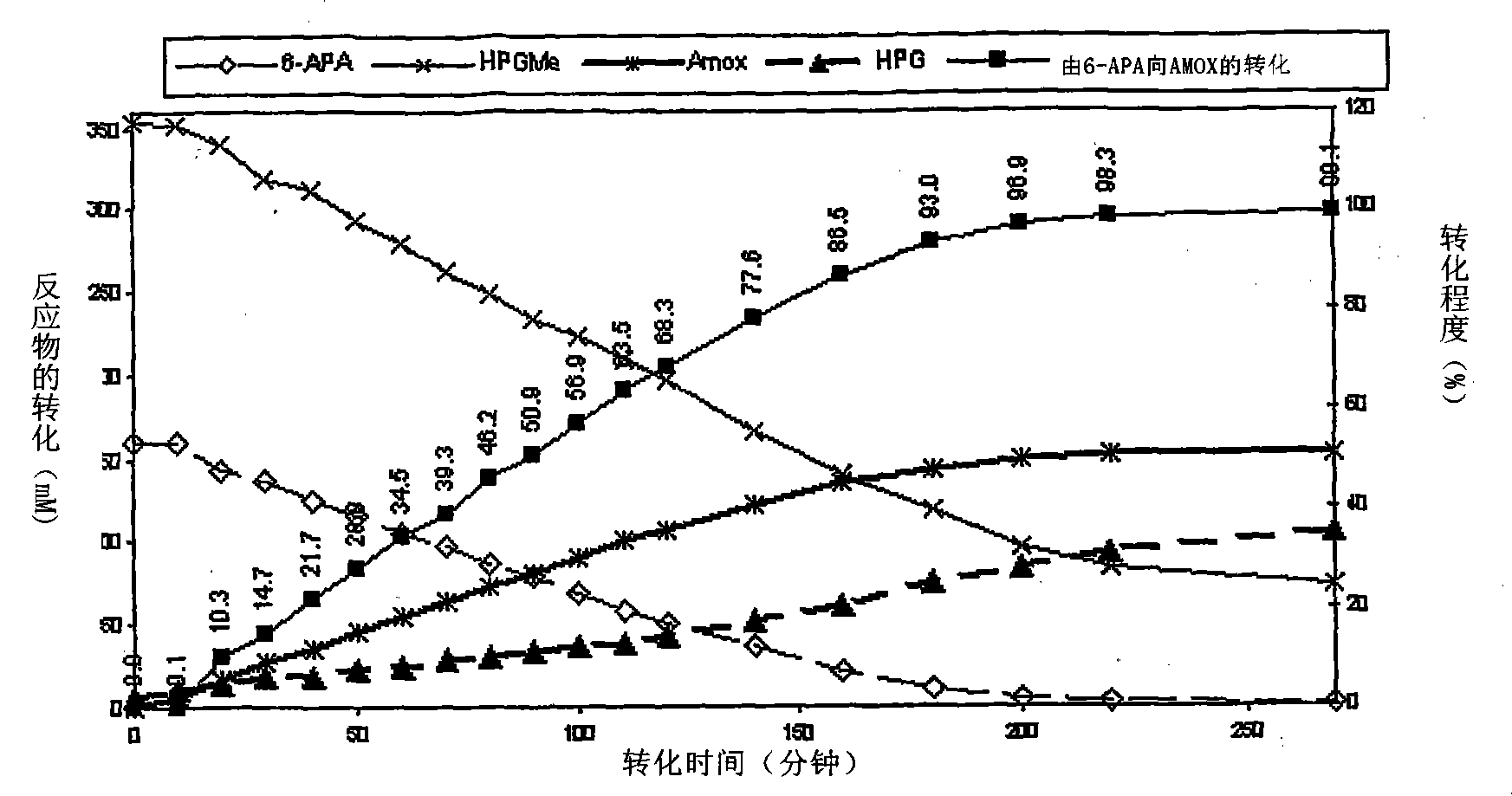
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic [beta]-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
2-sulfohydantoin as well as preparation method and application thereof
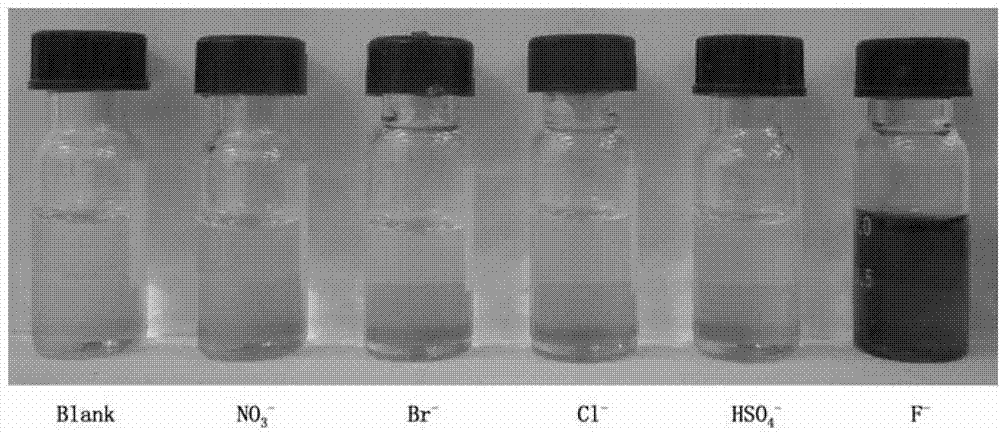
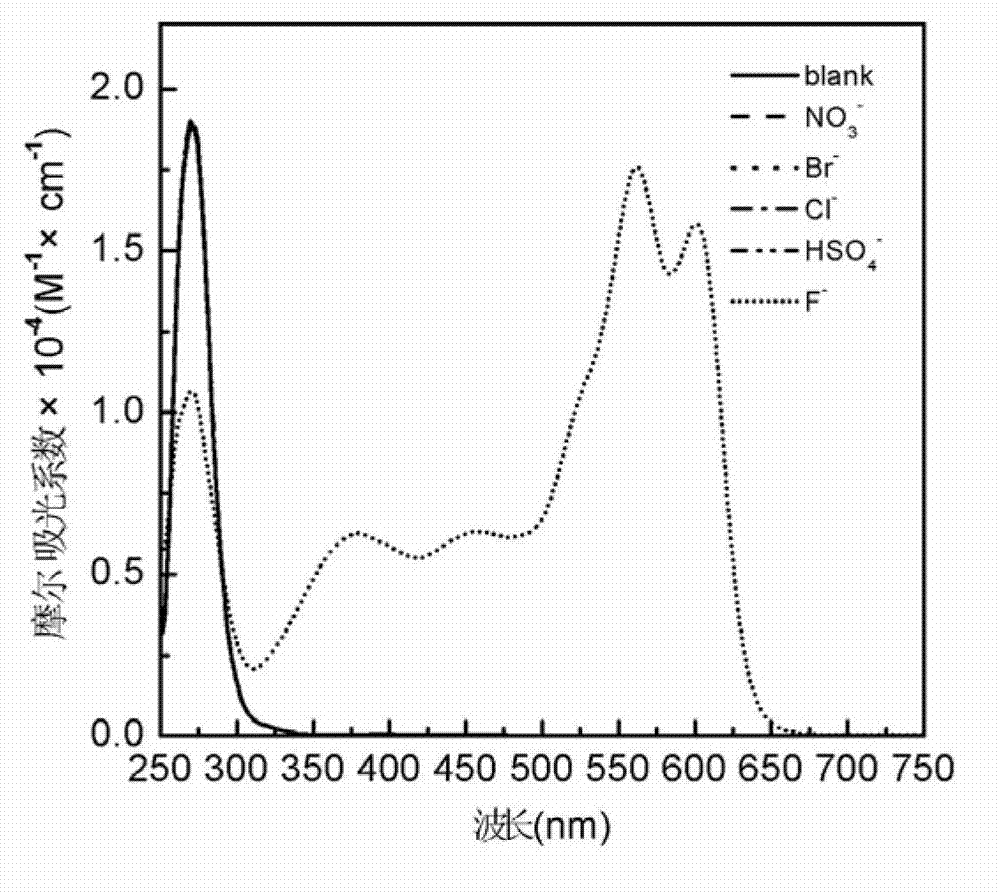
InactiveCN102924383AEasy to carryQuick checkOrganic chemistryMaterial analysis by observing effect on chemical indicatorChemical structurePhenylglycine methyl ester
The invention relates to a sulfur-containing heterocyclic compound. The compound is 2-sulfohydantoin; a chemical structure of the 2-sulfohydantoin is shown in a formula (I) in the specification; and in the formula (I), R is one of C1-12 alkyl, p-nitrophenyl, p-cyano phenyl, p-methoxyphenyl, p-butyl phenyl, 3,5-di(trifluoromethyl)phenyl and phenyl. The 2-sulfohydantoin is obtained by reacting isothiocyanate with para hydroxy phenylglycine methyl ester, has an anion recognition performance, and can be used for detecting fluorinion.
Owner:SOUTHERN MEDICAL UNIVERSITY
Improved preparation method of ampicillin
The invention relates to the pharmaceutical field and provides a method for preparing ampicillin and a product obtained by the method. The method comprises the following steps: 1) mixing 6-APA and D-phenylglycine methyl ester or salt thereof in water and adding into ampicillin synthase at the temperature of 0 DEG C-30 DEG C, and reacting at the pH value of 5.5-7.5 and the temperature of 10 DEG C-30 DEG C; 2) adjusting the product obtained in the step 1) with acid till solution is clear, and keeping the pH value at 0.5-2.0 and the temperature at 10 DEG C-30 DEG C; 3) cooling the solution obtained in the step 2) to 12 DEG C-15 DEG C, then regulating the pH value to 3.00-3.50, further cooling to 5 DEG C-6 DEG C, and maintaining the pH value at 3.50; then, adjusting the pH value to 4.9-5.0, cooling to 1 DEG C-2 DEG C, and keeping so as to obtain ampicillin crystals. By adopting the method provided by the invention, the yield and quality of ampicillin products are greatly upgraded, the production efficiency is improved, and the medication safety of the ampicillin products is further ensured.
Owner:UNITED LAB INNER MONGOLIA CO LTD
Comprehensive recovery method of effective components in enzymatic synthesized ampicillin crystal mother liquor
ActiveCN107698607AImprove solution qualityEfficient ConcentrationOrganic compound preparationAmino-carboxyl compound preparationHigh concentrationRecovery method
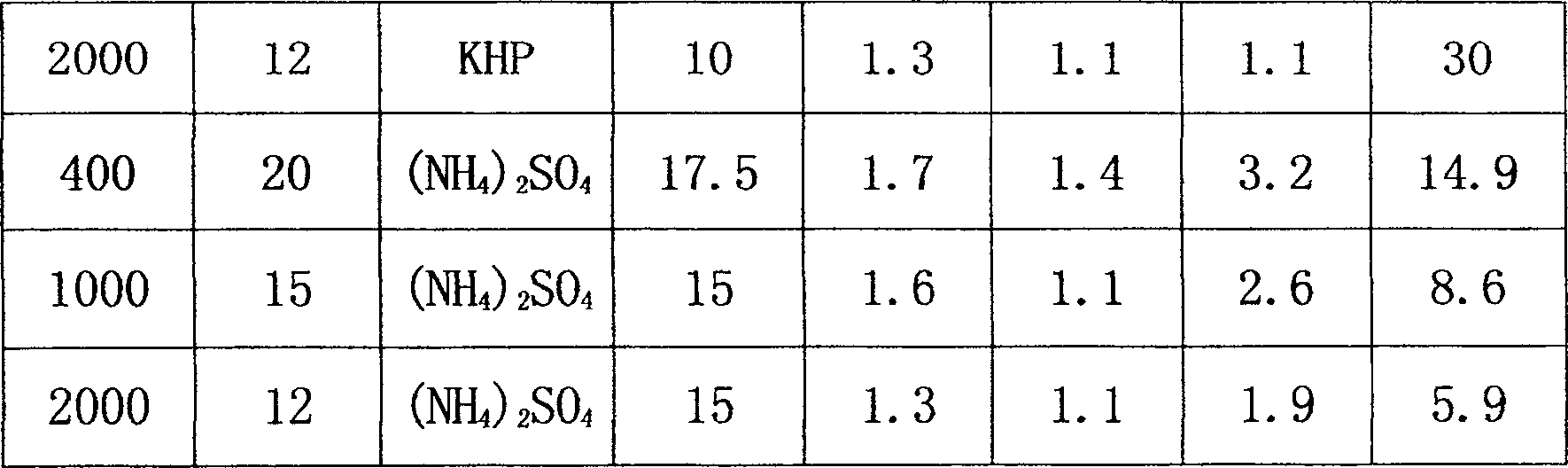
The invention belongs to the pharmaceutical technical field, and relates to a comprehensive recovery method of effective components in enzymatic synthesized ampicillin crystal mother liquor. D-phenylglycine is separated with ampicillin, 6-APA and D-phenylglycine methyl ester by a separation method of macroporous adsorption resin, D-phenylglycine is not adsorbed by the resin and enters an adsorption residual liquid, the adsorption residual liquid is subjected to electrodialysis, reverse osmosis concentration and crystallization, and then high-quality D-phenyglycine is recycled; ampicillin, 6-APA and D-phenylglycine methyl ester adsorbed on the resin are desorbed and are subjected to nanofiltration concentration, the obtained nanofiltration concentrate can be directly used as reaction bottomwater in an ampicillin enzymatic synthesis process so as to be recycled and reused. The effective components of D-phenylglycine, ampicillin, 6-APA and D-phenylglycine methyl ester in the enzymatic ampicillin crystal mother liquor are comprehensively recycled, economic benefits are created, at the same time, the discharge of high-concentration wastewater is reduced, and the environmental-protection, clean and green production is realized.
Owner:SHANXI WEIQIDA PHARMA IND
Method for preparing cefaclor from enzyme process
ActiveCN107523603AHigh purityHigh yieldOrganic chemistryFermentationFiltrationPhenylglycine methyl ester
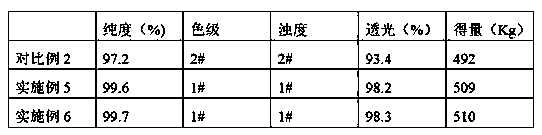
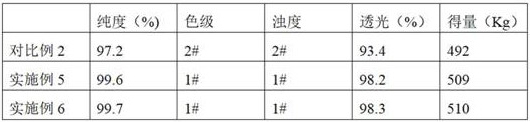
The invention discloses a method for preparing cefaclor from an enzyme process. The method comprises the following steps: preparing cefaclor coarse powder from 7-amino-3-chloro-cephem acid, D-o-phenylglycine methyl ester hydrochloride, immobilized cefaclor synthetase and cefaclor crystal seed; dissolving and discoloring the cefaclor coarse powder, adding the cefaclor crystal seed to regulate the pH value, stirring for a first time to grow the grains, and stirring for a second time to grow the grains after regulating the pH value, thereby preparing a crystal mixed solution; performing the suction filtration on the crystal mixed solution to obtain a crystal product and crystallized mother liquor; and sequentially performing washing with water, soaking washing, suction filtration and vacuum drying on the crystal product to obtain cefaclor. The method for preparing cefaclor from the enzyme process has twice stirring grain-growing operation, and the cefaclor slowly separates out in a re-crystallizing process, and the prepared crystal is uniform and is better in crystalline form; and the prepared cefaclor has very high purity greater than 99.7%.
Owner:长沙凯晓生物科技有限公司
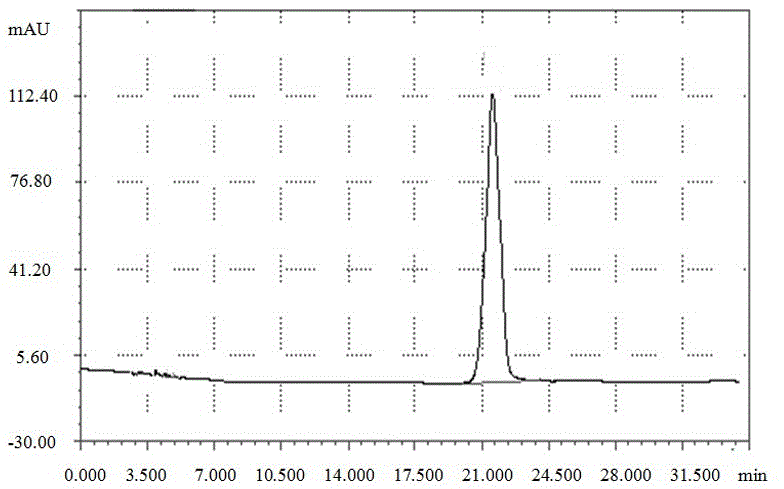
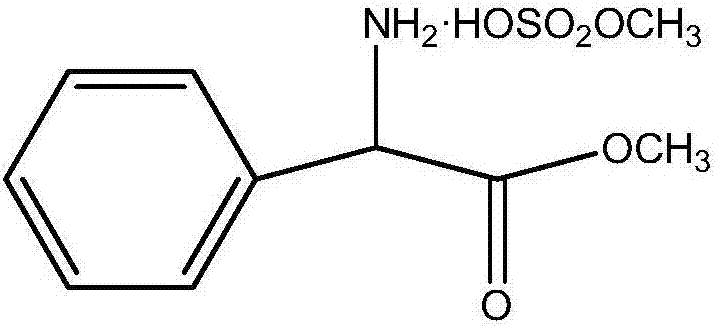
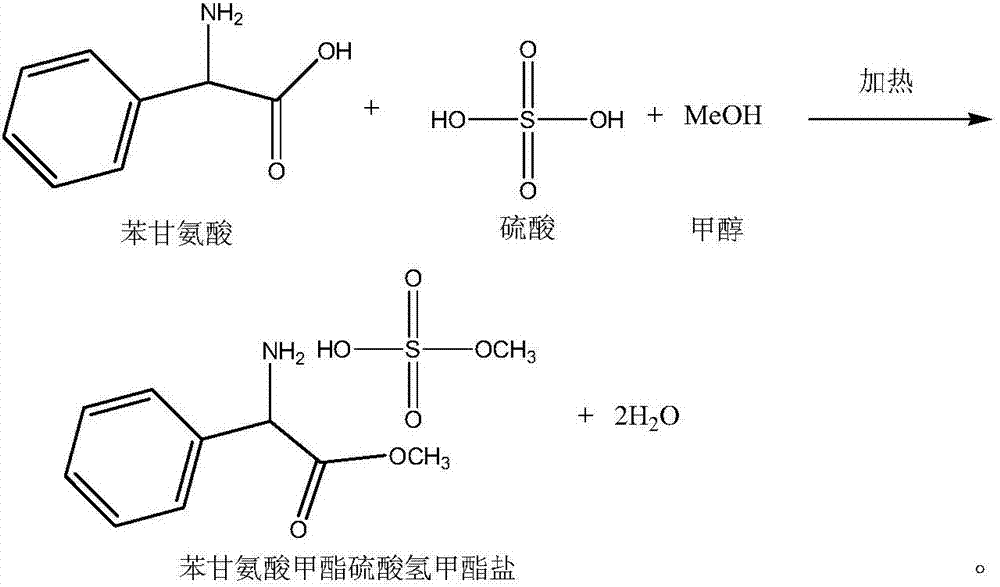
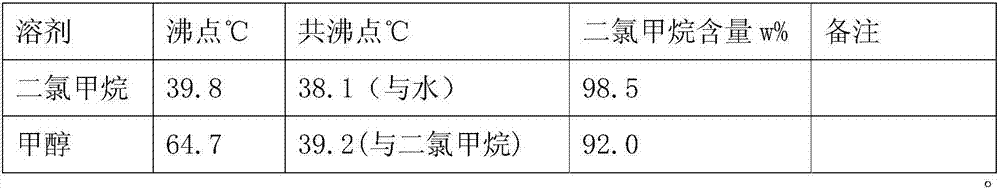
Preparation method of phenylglycine methyl ester methyl hydrogen sulfate
InactiveCN106957236AQuick responseEasy to removeOrganic compound preparationAmino-carboxyl compound preparationChemical synthesisHydrogen Sulfate
The invention discloses a preparation method of phenylglycine methyl ester methyl hydrogen sulfate and belongs to the technical field of chemical synthesis. The preparation method is characterized by comprising the following steps: adding phenylglycine and methanol into a reactor containing a rectifying and water separating device, and adding excessive amount of concentrated sulfuric acid, wherein the mol ratio of phenylglycine to the concentrated sulfuric acid in usage amount is 1:(1-2); then heating, refluxing, carrying out esterification reaction, then adding an azeotropic water-carrying agent dichloromethane, and continuously separating out water which is carried out by azeotrope of dichloromethane and water and is produced by the esterification reaction by virtue of the top of a rectifying tower, wherein the esterification reaction of the phenylglycine is basically complete after refluxing and water carrying are carried out for a period of time. The preparation method disclosed by the invention has the advantages of simplicity in operation, short working procedure time and high product yield.
Owner:ZHEJIANG ANGLIKANG PHARMA
Enzymatic synthesis method of cefaclor
ActiveCN111394415AReduce solubilityAvoid hydrolysisOrganic chemistryFermentationGlycineCephalosporanic Acids
The invention provides an enzymatic synthesis method of cefaclor. The enzymatic synthesis method of the cefaclor comprises the following steps: adding 7-amino-3-chloro cephalosporanic acid into a mixed solvent, adjusting a pH value to be 8.1-8.3, adding an immobilized cefaclor synthetase, adding D-p-phenylglycine methyl ester hydrochloride and methyl (R)-aminophenylacetate, carrying out an enzymatic synthesis reaction, separating out reaction liquid and the immobilized cefaclor synthetase after the reaction is completed to obtain a crude product of the cefaclor, and conducting recrystallization to obtain a cefaclor product, wherein the mixed solvent comprises methanol, glutaraldehyde and a soluble phosphate. According to the provided production method, a pH value of the reaction process does not need to be controlled, the number of times of recycling of the recoverable synthetase in the reaction is significantly increased, the purity of the produced cefaclor product can reach 99% or above, the bulk density can reach 6.2 g / mL or above, the number of times of recycling of the recoverable synthetase can reach 200, the production technology is simplified, the production cost is reduced, and the enzymatic synthesis method of the cefaclor is suitable for large-scale industrial production.
Owner:TIANJIN UNIV +1
Preparation method of D-phenylglycine methyl ester hydrochloride/D-dihydrophenylglycine methyl ester hydrochloride
ActiveCN110128285AReduce dosageHigh yieldOrganic compound preparationOrganic chemistry methodsGlycine methyl ester hydrochlorideCentrifugation
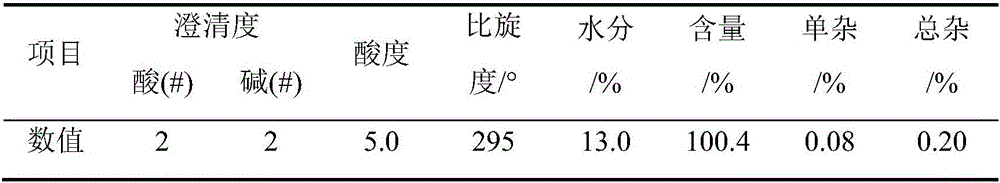
The invention provides a preparation method of D-phenylglycine methyl ester hydrochloride / D-dihydrophenylglycine methyl ester hydrochloride. The method comprises the following steps: (a) adding D-phenylglycine or D-dihydrophenylglycine and methanol into a reaction tank according to a ratio of 1 g:3-5 mL, performing uniform stirring, and slowly adding thionyl chloride; (b) after the thionyl chloride is added, performing a reflux reaction; (c) performing vacuum distillation, performing cooling crystallization, performing centrifugation, and performing drying to obtain a white product; (d) recovering the reaction mother liquid, performing vacuum concentration on the mother liquid, performing cooling crystallization, and performing centrifugation to obtain a yellow recovered product; (e) washing the yellow recovered product by using an organic reagent, and performing vacuum drying to obtain a white recovered product; and (f) recycling the white recovered product in the reaction tank, and preparing latter batch products according to operation of steps (a) to (c). The method provided by the invention proposes a novel way of recycling the mother liquid, and the product has more stable quality, high purity, and excellent color grade and turbidity.
Owner:NORTH CHINA PHARMA COMPANY
D-phenylglycine methyl ester phosphate crystal, preparation method and solution
PendingCN111909046AHigh synthetic conversion rateImprove buffering effectOrganic compound preparationOrganic chemistry methodsO-Phosphoric AcidPhosphate crystals
The invention discloses a D-phenylglycine methyl ester phosphate crystal, a preparation method and a solution, and the preparation method comprises the following steps: step a, adding Dphenylglycine and methanol into a reaction tank, uniformly stirring, and feeding phosphoric acid; step b, after the phosphoric acid is fed, carrying out reflux reaction; c, after the reflux reaction in the step b isfinished, carrying out vacuum dehydration, and then repeating the following operations for n times: adding methanol, continuously carrying out the reflux reaction, and carrying out vacuum dehydrationafter the reflux reaction is finished; and d, adjusting the pH value to be acidic, adding the Dphenylglycine methyl ester phosphate seed crystal for crystallization, and drying to obtain the Dphenylglycine methyl ester phosphate crystal. The problems of low subsequent enzymatic synthesis conversion rate and severe corrosion to equipment in the related art are solved.
Owner:TIANJIN UNIV
Process for the preparation of immobilized recombinant penicillin acylase catalyst from Achromobacter sp. CCM 4824 expressed in E. coli BL 21 CCM 7394 and its use for the synthesis of beta-lactam antibiotics
ActiveCN101802212BImprove synthesis abilityExtended active timeFermentationAmpicillinBeta lactam antibiotic
The present invention discloses isolation of Penicillin Acylase (PA) from Achromobacter sp CCM 4824 expressed in recombinant strain E. coli BL21 CCM 7394 bearing the recombinant plasmid pKXIP1 and processing of PA into biocatalyst useful for the industrial synthesis of antibiotics. More particularly the invention discloses a synthesis of semi-synthetic &bgr;-lactam antibiotics in the reaction mixture consisting of activated acyl-donor (D-p-hydroxyphenylglycine methyl ester or amide for Amoxicillin and Cefadroxil; D-phenylglycine methyl ester or amide for Ampicillin and Cephalexin) and nucleophile (6-APA or 7-ADCA) catalyzed by PA obtained from recombinant E. coli BL21 CCM 7394 as the biocatalyst.
Owner:FERMENTA BIOTECH
Ampicillin preparation technology by direct method
ActiveCN104726528ASave post-dryingImprove conversion rateFermentationAmpicillinPhenylglycine methyl ester
The invention relates to an ampicillin preparation technology by a direct method and belongs to the technical field of medicine preparation. The ampicillin preparation technology includes that phenylglycine is adopted as a starting material and synthesized into ampicillin finally. Compared with a chemical method and other enzymic methods, the ampicillin preparation technology has the advantages that subsequent steps including a drying step and the like for phenylglycine methyl ester hydrochloride are omitted, so that yield coefficient is increased obviously, investment of fixed assets is reduced, energy loss, equipment loss and cost are lowered, efficiency is improved, benefit is increased, and the ampicillin preparation technology is worth being popularized in production.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Synthesis of clopidogrel impurity intermediate
InactiveCN101747219AGood effectFew stepsOrganic compound preparationAmino-carboxyl compound preparationReaction temperatureSolvent
The invention relates to a process for resolving 2-substituted phenylglycine methyl ester (or acid) in high yield. S-(+)-o-chlorophenylglycine methyl ester is an important intermediate for preparing antiplatelet clopidogrel with high activity. R-(-)-o-chlorophenylglycine methyl ester is an important intermediate for preparing a clopidogrel related compound after liquid phase positioning. The process has the advantages of high utilization ratio of the raw material, higher purity of the product, short reaction time, low reaction temperature, higher yield, cyclic utilization of the solvent and the resolution reagent, fully utilization of the resolution reagent and the solvent, no three wastes discharge, environmental protection and the like.
Owner:SHANGHAI ECUST BIOMEDICINE CO LTD
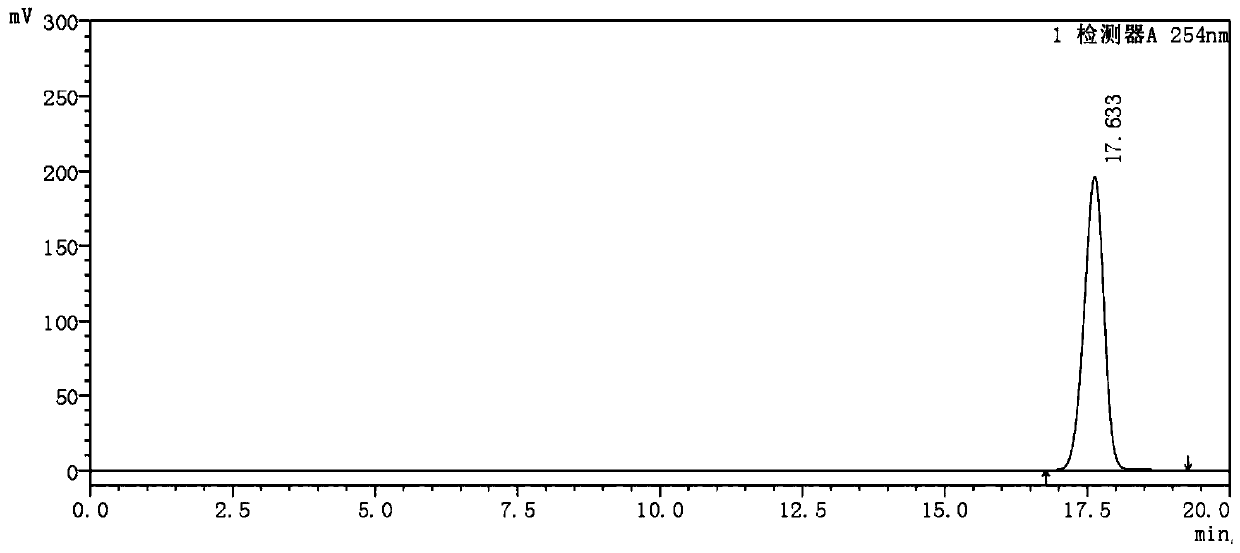
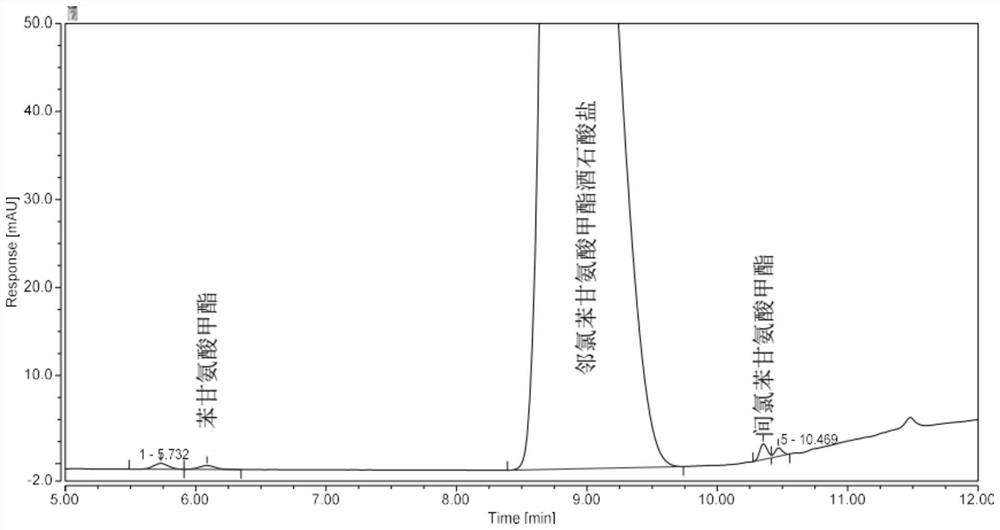
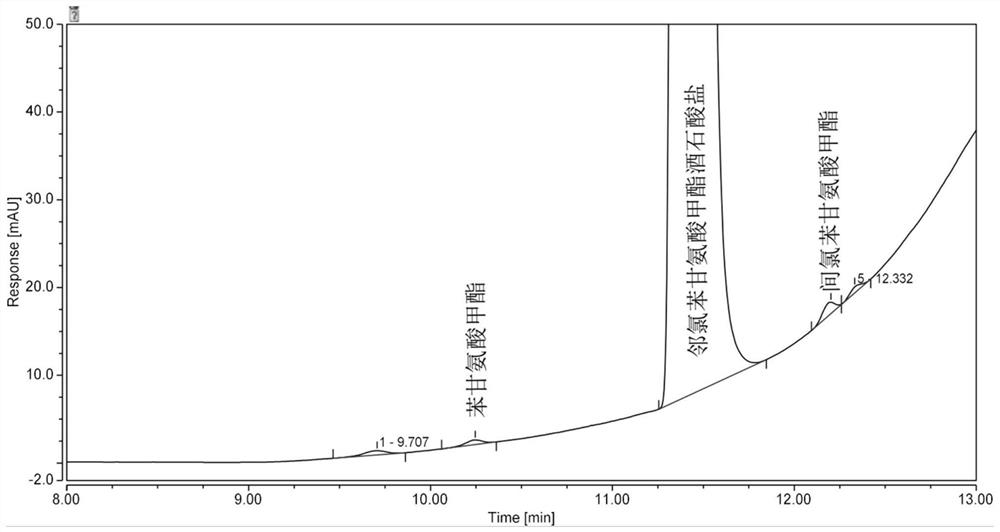
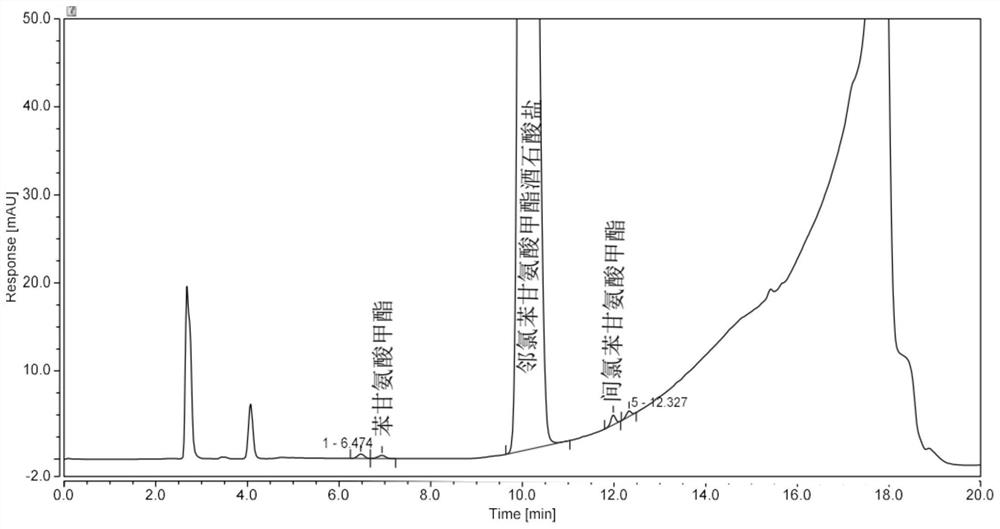
Analysis method for determining 2-chlorophenylglycine methyl ester tartrate and impurities
The invention discloses a method for analyzing 2-chlorophenylglycine methyl ester tartrate and impurities. The main impurities are phenylglycine methyl ester and m-chlorophenylglycine methyl ester. A high performance liquid chromatography method is adopted, an acid aqueous solution and an organic solvent are used as mobile phases, gradient elution is carried out on a sample solution of the chlorophenylglycine methyl ester, and the method is rapid, simple, accurate, good in repeatability and suitable for control and impurity research of the chlorophenylglycine methyl ester tartrate and impurities.
Owner:苏州正济医药研究有限公司
A kind of synthetic method of amoxicillin production intermediate
ActiveCN114105795BOvercoming serious pollution defectsHigh yieldPhysical/chemical process catalystsOrganic compound preparationO-Phosphoric AcidPtru catalyst
The present invention relates to a kind of synthesis method of amoxicillin production intermediate, use DL-p-hydroxyphenylglycine and methanol as raw materials, and use solid phosphoric acid as catalyst to synthesize D-p-hydroxyphenylglycine methyl ester, including preparation of solid phosphoric acid catalyst, DL-p-hydroxyphenylglycine Preparation of p-hydroxyphenylglycine methyl ester, hydrolysis of solid phosphoric acid catalyst, crystallization of D-p-hydroxyphenylglycine methyl ester and racemization of crystallization mother liquor in 5 parts. In the present invention, the solid phosphoric acid catalyst can be hydrolyzed into phosphoric acid after the esterification is completed, and forms a phosphoric acid double salt with DL-p-hydroxyphenylglycine methyl ester. ‑Resolving agent for methyl p-hydroxyphenylglycine. In the present invention, the synthesis and resolution of DL-p-hydroxyphenylglycine methyl ester is carried out in methanol aqueous solution, and the crystallization mother liquor is recycled, which greatly reduces the generation of waste liquid, and is a clean production process of D-p-hydroxyphenylglycine methyl ester.
Owner:TIANJIN HANRUI PHARMA +1
Cefradine preparing process
ActiveCN100519564CEmission reductionReduce pollutionOrganic chemistryFermentationHydrochlorothiazideEnzyme catalysis
Owner:ZHEJIANG ANGLIKANG PHARMA
Penicillin g Acylase Mutant and Its Application in the Synthesis of Cephalosporin Antibiotics
Owner:ZHEJIANG APELOA TOSPO PHARMA +1
A method for enzymatically synthesizing cefaclor
The invention provides a method for enzymatically synthesizing cefaclor, comprising the following steps: adding 7-amino-3-chloro-cephemic acid to a mixed solvent, adjusting the pH to 8.1-8.3, and adding immobilized cefaclor synthase , adding D-p-phenylglycine methyl ester hydrochloride and D-phenylglycine methyl ester to carry out the enzyme-catalyzed synthesis reaction. After the reaction, the reaction solution and immobilized cefaclor synthetase were separated to obtain cefaclor crude product and recrystallized , to obtain the cefaclor product; wherein, the mixed solvent includes methanol, glutaraldehyde and soluble phosphate. The preparation method provided by the invention does not need to control the pH value of the reaction process, and significantly improves the number of recycling enzymes recovered in the reaction, and the purity of the prepared cefaclor product can reach more than 99%, and the bulk density can reach 6.2g More than / mL, the recycled enzyme can be used up to 200 times, which simplifies the production process, reduces the production cost, and is suitable for large-scale industrial production.
Owner:TIANJIN UNIV +1
Method for preparing ampicillin from benzylpenicillin potassium
ActiveCN106520893AAvoid inhibitionIncrease concentrationOrganic chemistryFermentationAmpicillinPhenylacetic acid
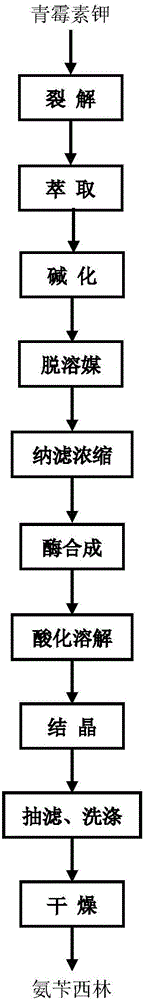
The invention relates to a method for preparing ampicillin from benzylpenicillin potassium and belongs to the technical field of pharmacy. According to the method for preparing the ampicillin from the benzylpenicillin potassium, the benzylpenicillin potassium is split into 6-APA and phenylacetic acid under the action of penicillin acylase, a lysis solution is extracted through dichloromethane, and the 6-APA is separated from the phenylacetic acid. Ammonia water is added into an extracted water phase, the pH of a feed solution is adjusted to the neutral level, the dichloromethane is removed, the obtained feed solution is subjected to concentration by nanofiltration, and the ampicillin is synthesized by a concentrated solution and D-phenylglycine methyl ester hydrochloride under the action of amoxicillin synthetase. The inhibiting effect of the phenylacetic acid remaining in the 6-APA solution obtained after extracting the lysis solution on the enzyme activity of the synthetase is overcome, the concentration of the 6-APA in the 6-APA solution is high, few colored impurities exist in the extraction and do not influence the quality of ampicillin products, and the method for preparing the ampicillin from the benzylpenicillin potassium is easily applied to production.
Owner:INNER MONGOLIA CHANGSHENG PHARMA
Reaction equipment for synthesizing D-phenylglycine methyl ester hydrochloride
InactiveCN106268592AFast samplingAccurate measurementChemical/physical/physico-chemical stationary reactorsPhenylglycine methyl esterD-phenylglycine
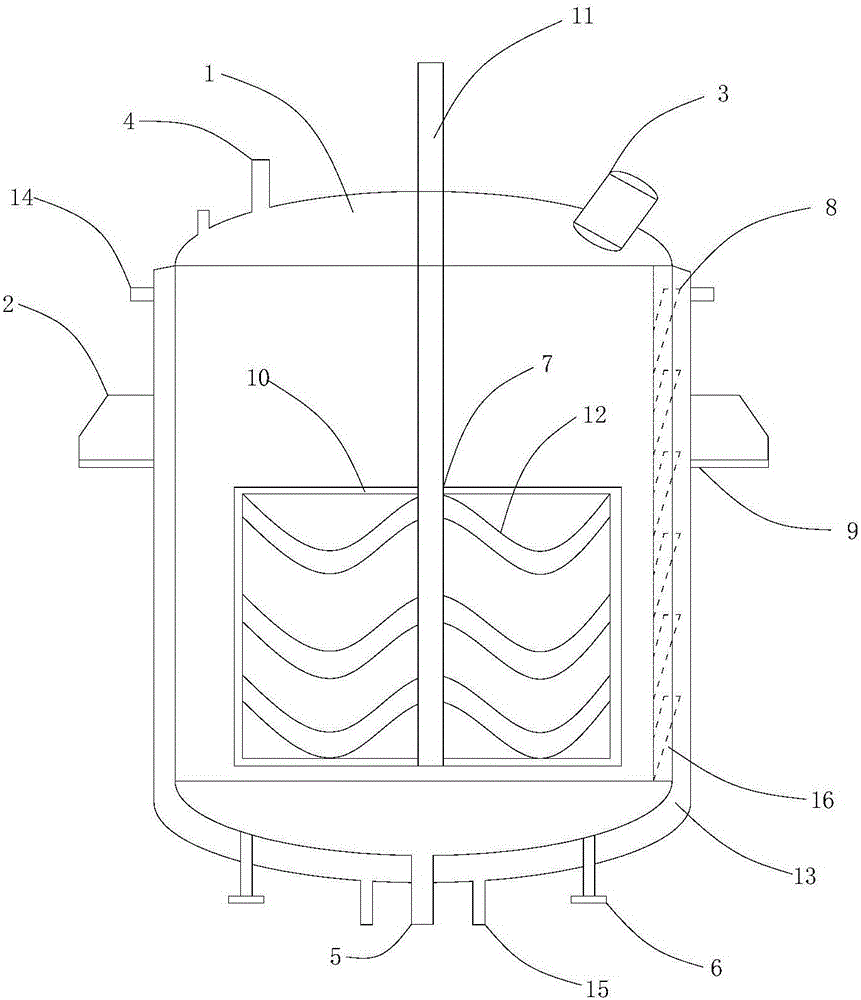
The invention discloses reaction equipment for synthesizing D-phenylglycine methyl ester hydrochloride, belongs to the technical field of reaction equipment for synthesizing the D-phenylglycine methyl ester hydrochloride, and solves the problems that reaction substances in a reaction kettle are especially hard to sample, for example, equipment is excessively large, the reaction substances are located at the bottom of the equipment during primary reaction, and accordingly the reaction substances are hard to sample, and the like in the prior art. The reaction equipment comprises a kettle, wherein the kettle is provided with lugs, an observation port, a feeding port, a discharge port and heating pipes, a stirring device is arranged in the kettle, a plurality of sampling pipes are arranged on the outer wall of the kettle, the sampling pipes incline 15-45 degrees outwardly, and the sampling outlet of each sampling pipe is higher than the connection part of the previous sampling pipe and the kettle.
Owner:SICHUAN TONGSHENG BIOTECH
The preparation method of d-phenylglycine methyl ester hydrochloride/d-dihydrophenylglycine methyl ester hydrochloride
ActiveCN110128285BReduce dosageHigh yieldOrganic compound preparationOrganic chemistry methodsPhenyl groupMethanol
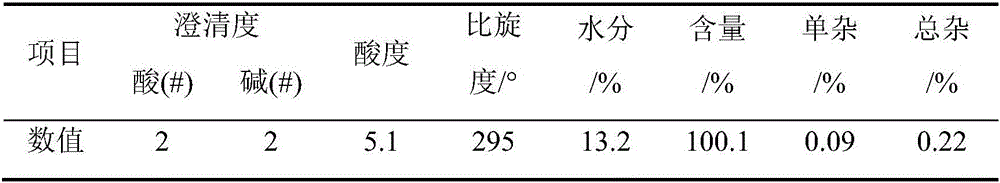
The invention provides a preparation method of D-phenylglycine methyl ester hydrochloride / D-dihydrophenylglycine methyl ester hydrochloride, comprising the following steps: (a) mixing D-phenylglycine or D-dihydrobenzene Glycine and methanol were added to the reaction tank at a ratio of 1g: 3~5mL, and after stirring evenly, slowly added thionyl chloride; (b) reflux reaction after the addition of thionyl chloride; (c) vacuum distillation, Then cool down and crystallize, centrifuge and dry to obtain a white product; (d) recover the reaction mother liquor, concentrate the mother liquor in a vacuum, cool down and crystallize, and centrifuge to obtain a yellow recovered product; (e) wash the yellow recovered product with an organic reagent, and dry it in a vacuum to obtain White recycled product; (f) Apply the white recycled product to the reaction tank, and then follow steps (a)~(c) to prepare the next batch of products. The invention proposes a new way of applying the mother liquor, so that the product quality is more stable, the purity is high, and the color grade and turbidity are excellent.
Owner:NORTH CHINA PHARMA COMPANY
Synthetic method of amoxicillin production intermediate
ActiveCN114105795AOvercoming serious pollution defectsHigh yieldPhysical/chemical process catalystsOrganic compound preparationO-Phosphoric AcidPtru catalyst
The invention relates to a synthesis method of an amoxicillin production intermediate, which comprises the following steps of: synthesizing D-p-hydroxyphenylglycine methyl ester by taking DL-p-hydroxyphenylglycine and methanol as raw materials and solid phosphoric acid as a catalyst; comprising the following five parts: preparation of a solid phosphoric acid catalyst, preparation of DL-p-hydroxyphenylglycine methyl ester, hydrolysis of the solid phosphoric acid catalyst, crystallization of D-p-hydroxyphenylglycine methyl ester and racemization of crystallization mother liquor. The solid phosphoric acid catalyst can be hydrolyzed into phosphoric acid after esterification is completed, phosphoric acid and DL-p-hydroxyphenylglycine methyl ester form phosphoric acid double salt, and phosphoric acid serves as an esterification catalyst for synthesis of DL-p-hydroxyphenylglycine methyl ester and also serves as a resolving agent of DL-p-hydroxyphenylglycine methyl ester. The synthesis and resolution of the DL-p-hydroxyphenylglycine methyl ester are carried out in the methanol aqueous solution, and the crystallization mother liquor is recycled, so that the generation of waste liquid is greatly reduced, and the method is a clean production process of the D-p-hydroxyphenylglycine methyl ester.
Owner:TIANJIN HANRUI PHARMA +1
Method for preparing amoxicillin or ampicillin through full water phase
ActiveCN105132513BHigh vitality coefficientEasy to synthesizeFermentationPenicillin VKHigh concentration
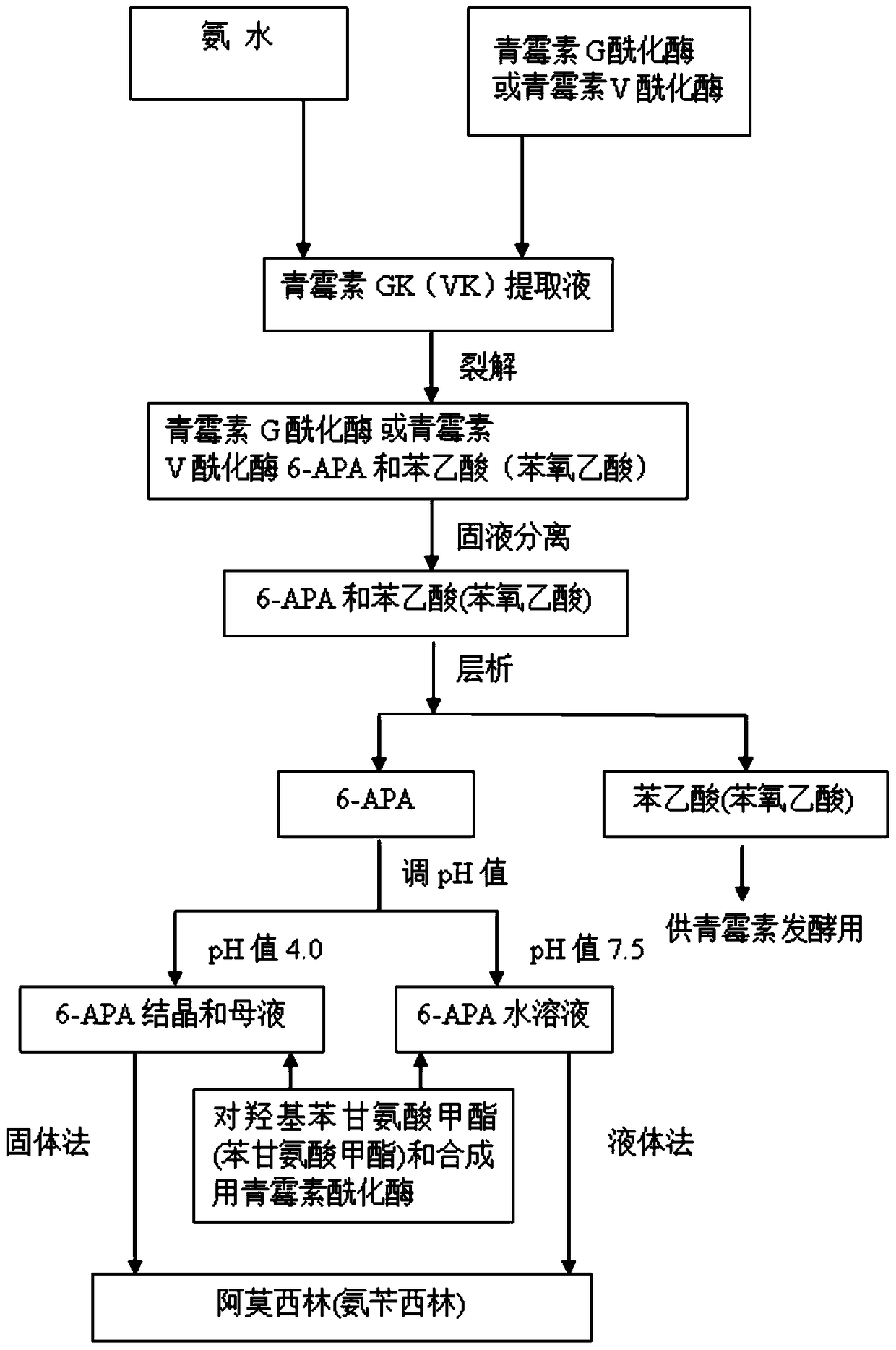
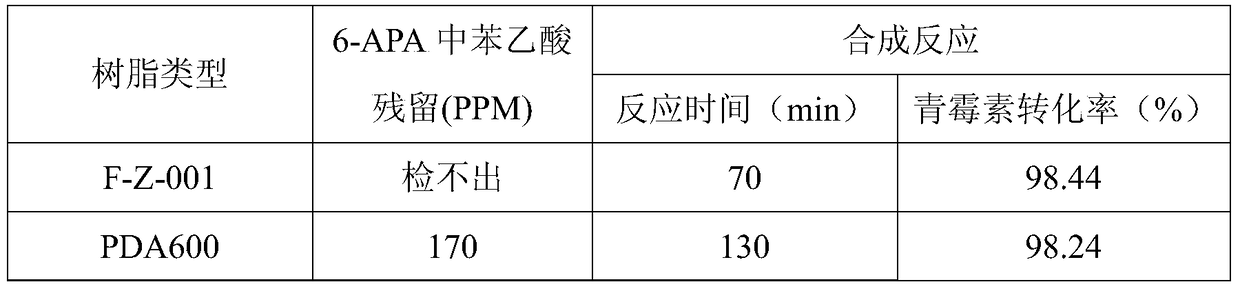
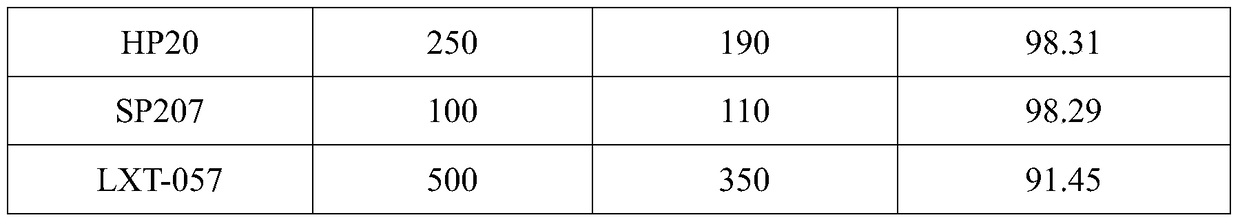
The invention discloses a method for preparing amoxicillin or ampicillin in a full-water-phase through mode. The method includes the steps that a high-concentration penicillin GK or penicillin VK extracting solution is used as a raw material, immobilized penicillin G acylase or penicillin V acylase is used as an enzyme catalyst, a high-concentration 6-APA solution or crystal is obtained through full-water-phase operations such as catalytic cracking, separating, acidization, filtering, chromatography and concentration by nanofiltration, and then the amoxicillin or the ampicillin is synthesized by the solution or crystal and HPGME or phenylglycine methyl ester under the catalytic action of the immobilized penicillin synthetase. According to the method, water-phase reacting is conducted in the whole process, no organic solvent is used, and environmental pollution is reduced; besides, the special immobilized penicillin G acylase and the penicillin V acylase are used for cracking the high-concentration penicillin GK or VK extracting solution, and special macroporous absorption resin is used for separating and cracking products, so that the processing steps are greatly simplified, the production cost is lowered, the product yield is increased, and the industrial production requirement is met.
Owner:HUNAN FLAG BIOTECHNOLOGY CO LTD
High-purity D-dihydrophenylglycine methyl ester and preparation method thereof
PendingCN113387824ALow costSimple production processOrganic compound preparationOrganic chemistry methodsBiochemical engineeringPhenylglycine methyl ester
The invention discloses high-purity D-dihydrophenylglycine methyl ester and a preparation method thereof, and belongs to the field of pharmaceutical chemicals, and the preparation method comprises the following steps: uniformly mixing and stirring D-dihydrophenylglycine and methanol, carrying out heating reflux, adding a reaction reagent concentrated sulfuric acid or thionyl chloride, and carrying out reflux reaction to obtain the high-purity D-dihydrophenylglycine methyl ester. According to the preparation method, the existing preparation process is changed, the content of phenylglycine methyl ester in the product is less than 0.67%, and due to the innovative preparation mode, the product cost is reduced. In addition, the production process is simple and easy to operate, low in production cost and beneficial to industrial production.
Owner:NORTH CHINA PHARMA COMPANY
A kind of method for preparing cefaclor by enzymatic method
ActiveCN107523603BHigh purityHigh yieldOrganic chemistryFermentationGlycinePhenylglycine methyl ester
Owner:长沙凯晓生物科技有限公司
A kind of preparation technology of d-phenylglycine methyl ester hydrochloride crystallization
ActiveCN104829478BReasonable useAvoid applyingOrganic compound preparationAmino-carboxyl compound preparationChemical industryOne pass
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com