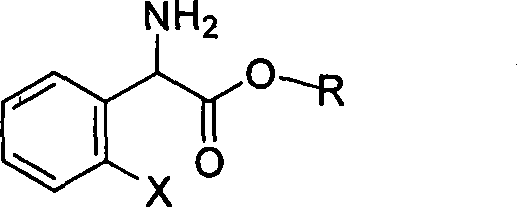
Method for splitting S-(+)-o-chlorobenzene glycine methyl ester
A technology of phenylglycine methyl ester and process, which is applied in the field of splitting 2-substituted phenylglycine methyl ester, can solve the problems of large solvent usage, potential danger, and unfavorable industrialization, and achieve high raw material utilization rate and short operation cycle The effect of shortening and saving the use of solvent and resolving agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0037] Add L-(+)-tartaric acid (6.6 g, 0.044 mol) and anhydrous methanol (64 ml) into a 100 ml three-necked flask, and stir at room temperature until they are completely dissolved. A mixed solution of 2-chlorophenylglycine methyl ester (8.0 g, 0.04 mol) and acetone (16 ml) was added dropwise into the reactor, and solids were precipitated during the dropwise addition, which took about one hour. After the dropwise addition, react at 28-32°C for 15-20 hours. After the reaction was cooled, stirring was continued for one hour. Suction filtration and filter cake drying to obtain dry solid (S)-(+)-2-chlorophenylglycine methyl ester tartrate, yield 86%, melting point 164-166°C (document: 163-167°C) optical rotation [α] D +86 0 (C=1, CH 3 OH) (Document: [α] D +88 0 (C=1, CH 3 OH)).
example 2
[0039] Add L-(+)-tartaric acid (6.6 g, 0.044 mol) and anhydrous methanol (64 ml) into a 100 ml three-necked flask, and stir at room temperature until they are completely dissolved. After adding a catalytic amount of acetophenone, a mixed solution of 2-chlorophenylglycine methyl ester (8.0g, 0.04mol) and acetone (16ml) was added dropwise to the reactor. During the dropwise addition, solids were precipitated, which took about One hour. After the dropwise addition, react at 28-32°C for 5-6 hours. After the reaction was cooled, stirring was continued for one hour. Suction filtration and drying of the filter cake gave dry solid (S)-(+)-2-chlorophenylglycine methyl ester tartrate. Yield 92%, melting point 164-166°C (document: 163-167°C) optical rotation [α] D +88 0 (C=1, CH 3 OH) (Document: [α] D +88 0 (C=1, CH 3 OH)).
example 3
[0041]Add L-(+)-tartaric acid (6.6 g, 0.044 mol) and anhydrous methanol (64 ml) into a 100 ml three-necked flask, and stir at room temperature until they are completely dissolved. After adding a catalytic amount of formaldehyde, a mixed solution of 2-chlorophenylglycine methyl ester (8.0g, 0.04mol) and acetone (16ml) was added dropwise into the reactor, and solids were precipitated during the dropwise addition, which took about one hour . After the dropwise addition, react at 28-32° C. for 10-20 hours. After the reaction was cooled, stirring was continued for one hour. Suction filtration and drying of the filter cake gave dry solid (S)-(+)-2-chlorophenylglycine methyl ester tartrate. Yield 85%, melting point 164-166°C (document: 163-167°C) optical rotation [α] D +88 0 (C=1, CH 3 OH) (Document: [α] D +88 0 (C=1, CH 3 OH)).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com