Preparation method of D-phenylglycine methyl ester hydrochloride/D-dihydrophenylglycine methyl ester hydrochloride
A technology of dihydrophenylglycine methyl ester and dihydrophenylglycine, which is applied in the field of preparation of D-phenylglycine methyl ester hydrochloride/D-dihydrophenylglycine methyl ester hydrochloride, can solve the problems affecting production , low product quality and other issues, to achieve the effect of benefiting industrial production, high purity and increasing profits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] in 2M 3 In the reaction tank, add 1200L of methanol, and add 400Kg of D-phenylglycine while stirring. After stirring evenly, add 460Kg of thionyl chloride, and control the temperature below 50°C. 4h, the reaction ended. Then carry out vacuum distillation, and the vacuum degree in the reactor is maintained at 0.03MPa. After the mass content of methanol in the solution in the reaction tank reaches 15%, stop the vacuum distillation, cool down and crystallize, drop to 0°C, stir for 30-60min, then centrifuge, and vacuum-dry at 50°C for 8h to obtain D-phenylglycine methyl ester salt Salt 480Kg.
[0029] The volume of the mother liquor was 250L, and then vacuum concentrated to 62.5L, cooled to 0°C, and then centrifuged to obtain a yellow recovered product; the recovered product was rinsed with 4Kg of methyl acetate, and vacuum-dried at 50-60°C to obtain a white recovered product of 26.0Kg ( The content of D-phenylglycine is 31.0%); the recovered product is applied mechanica...
Embodiment 2
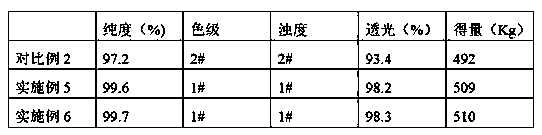
[0031] in 2M 3 Add 1223.4L of methanol to the reaction tank, add 400Kg of D-phenylglycine and 26Kg of recovered product (containing 31.0% phenylglycine) under stirring, after stirring evenly, add 469Kg of thionyl chloride, control the temperature below 50°C, and complete the dropwise addition Afterwards, the temperature in the reactor was maintained at 59° C., and the reaction was completed by reflux for 4 hours. Then carry out vacuum distillation, and the vacuum degree in the reactor is maintained at 0.03MPa. After the mass content of methanol in the solution in the reaction tank reaches 15%, stop the vacuum distillation, cool down and crystallize, drop to 0°C, stir for 30-60min, then centrifuge, and vacuum-dry at 50°C for 8h to obtain D-phenylglycine methyl ester salt Salt 508Kg (mol yield: 95.49%), purity 99.6%, color grade and turbidity both 1#, light transmission 98.4%, see Table 1 for details.
[0032] The volume of the mother liquor is 250L, then vacuum concentrated t...
Embodiment 3
[0034] in 2M 3 Add 1223.4L of methanol to the reaction tank, add 400Kg of D-phenylglycine and 26Kg of recovered product (containing 31.4% of phenylglycine) under stirring, after stirring evenly, add 469Kg of thionyl chloride, control the temperature below 50°C, and complete the dropwise addition Afterwards, the temperature in the reactor was maintained at 60° C., refluxed for 4 hours, and the reaction was completed. Then carry out vacuum distillation, and the vacuum degree in the reactor is maintained at 0.03MPa. After the mass content of methanol in the solution in the reaction tank reaches 16%, stop the vacuum distillation, cool down and crystallize, drop to 0°C, stir for 30-60min, then centrifuge, and vacuum-dry at 50°C for 8h to obtain D-phenylglycine methyl ester salt Salt 509Kg (mol yield: 95.68%), purity 99.7%, color grade and turbidity both 1#, light transmission 98.5%, see Table 1 for details.
[0035] The volume of the mother liquor is 250L, then concentrated in va...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com