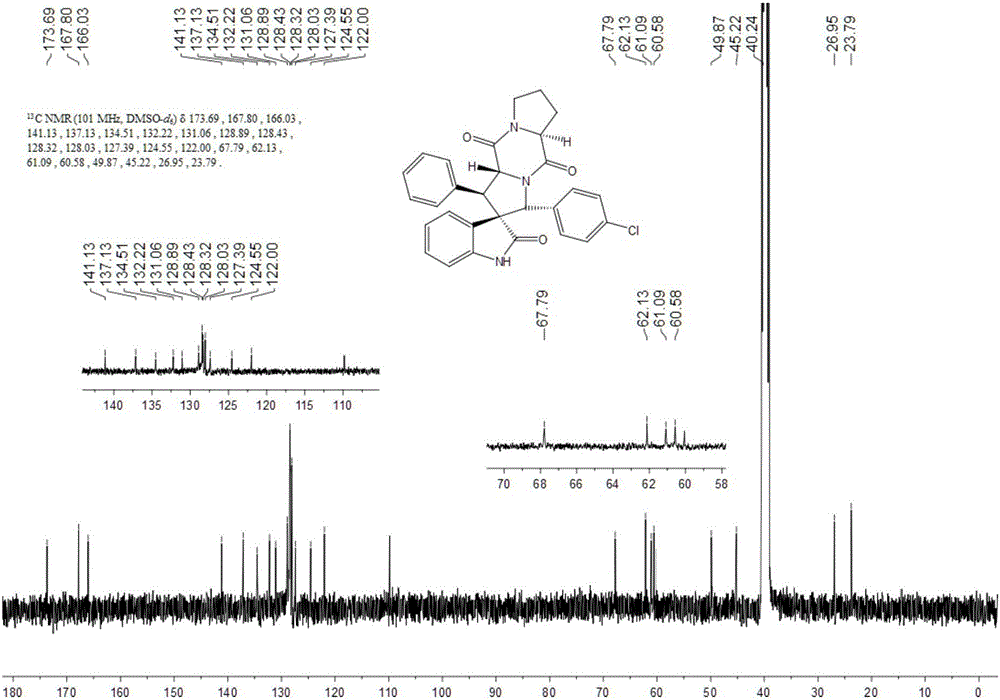
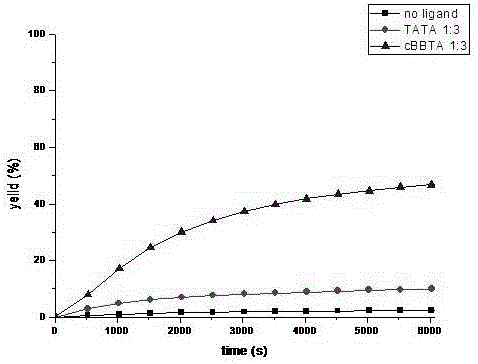
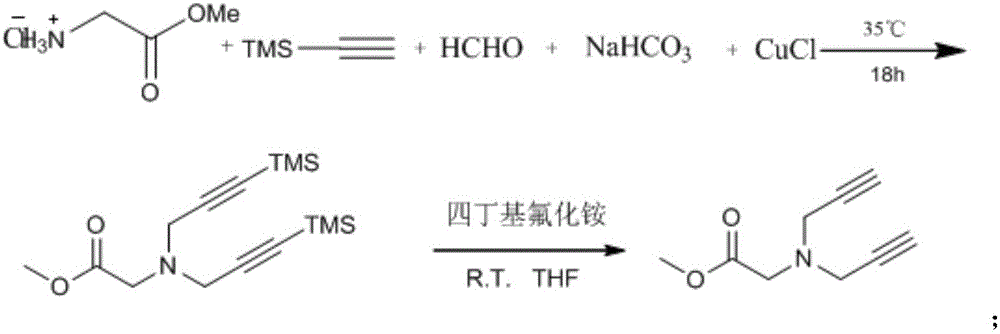
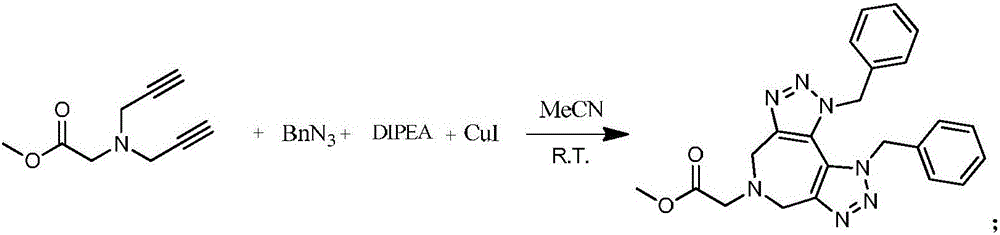
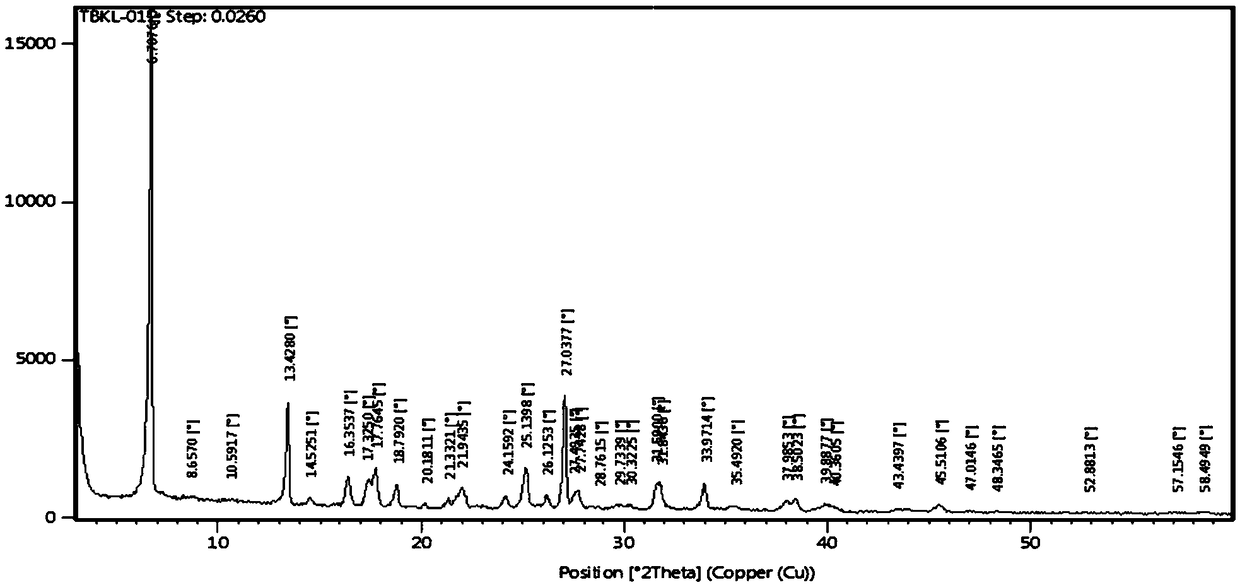
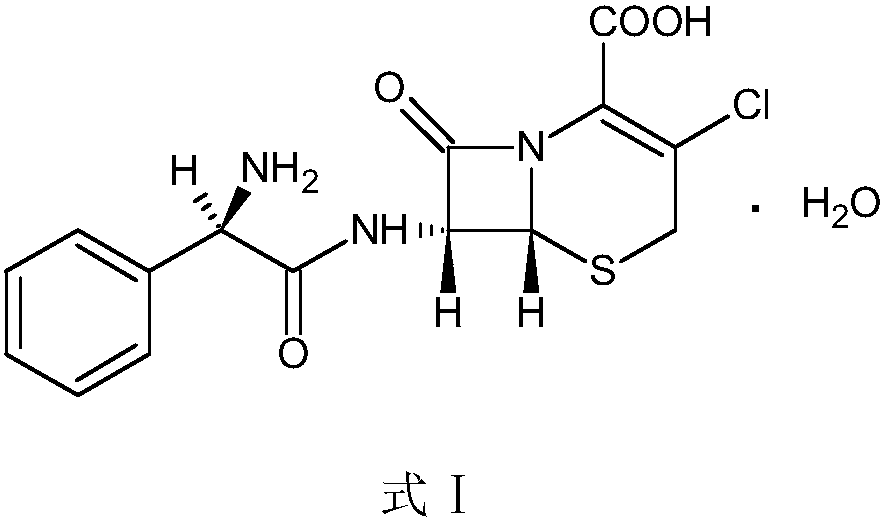
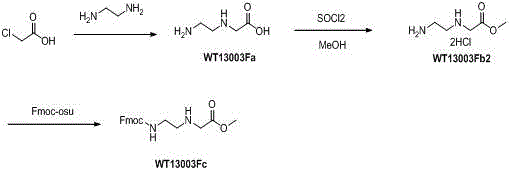
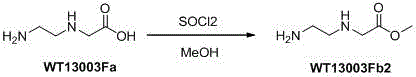
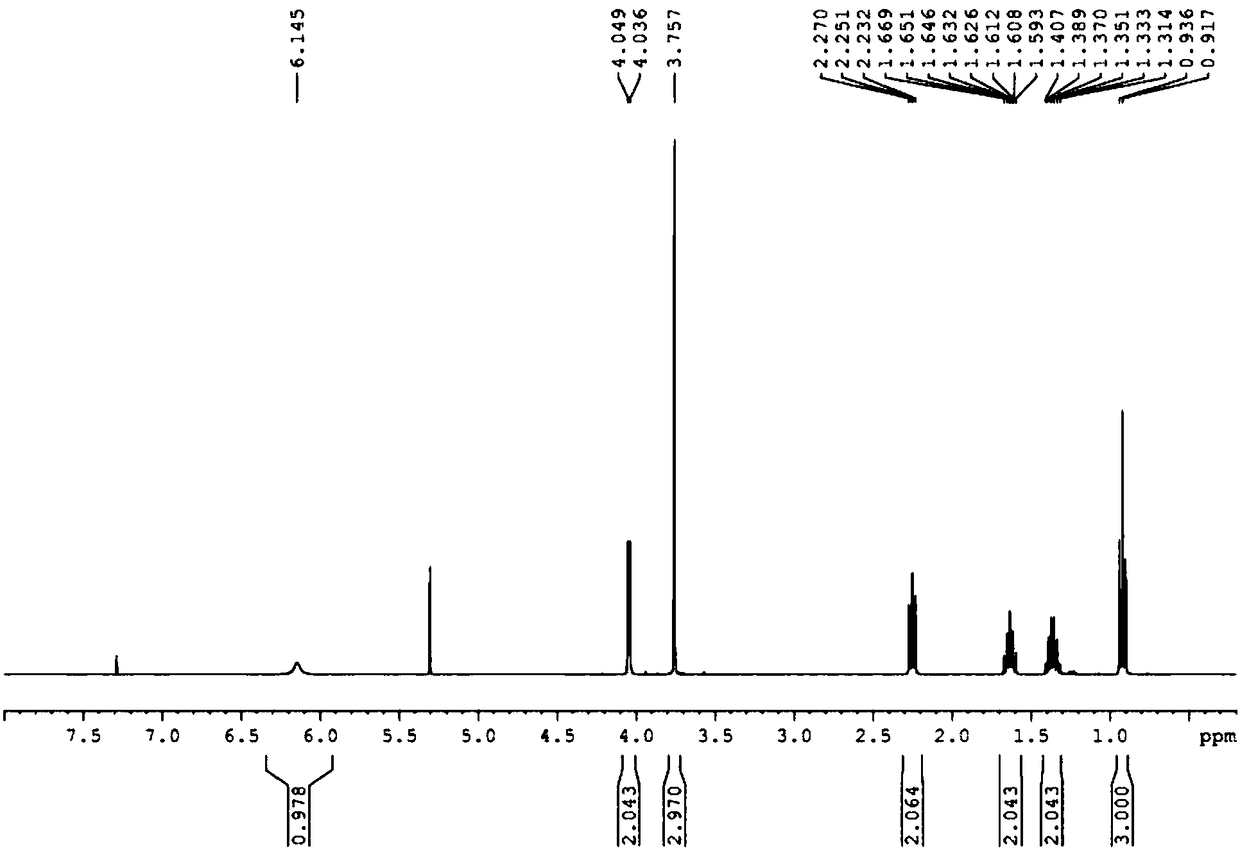
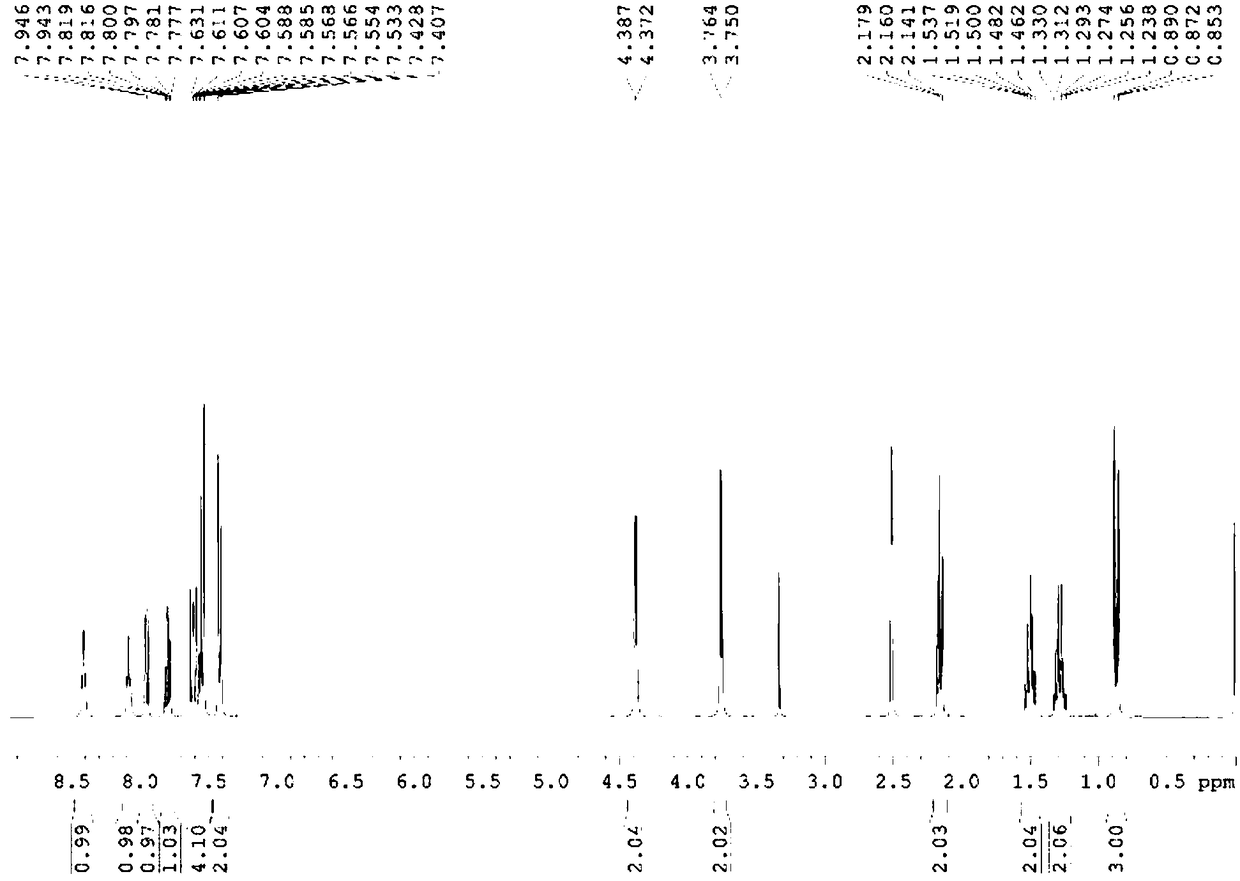
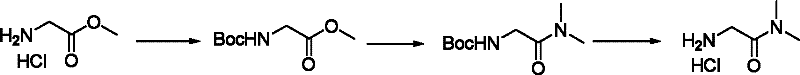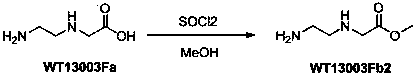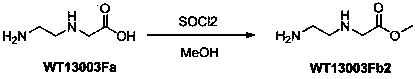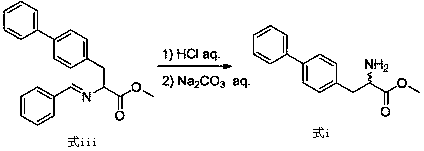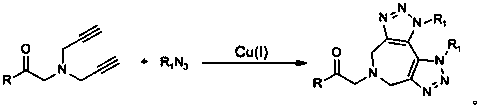Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Glycine methyl ester hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
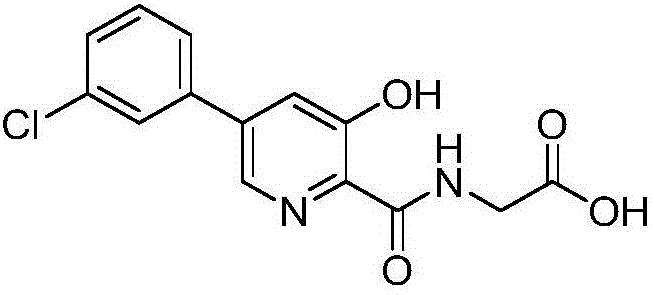
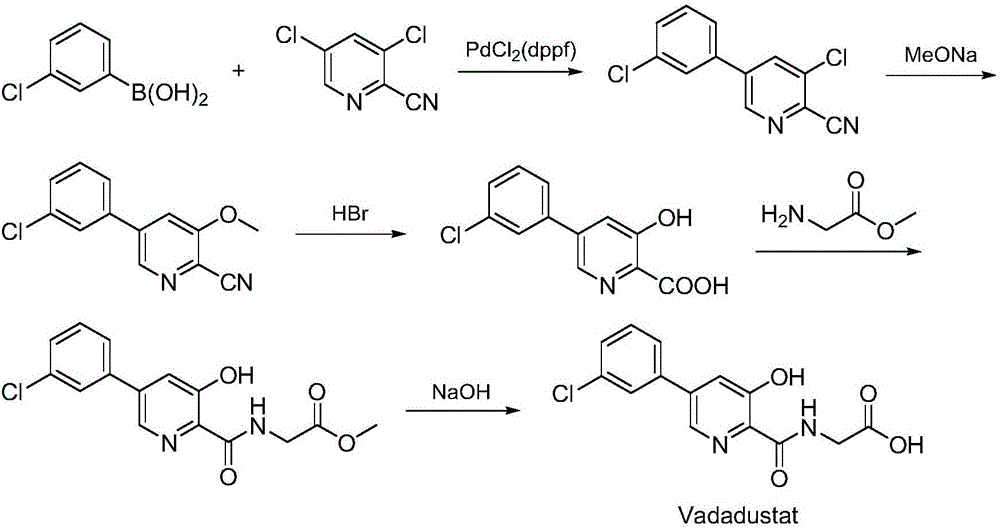
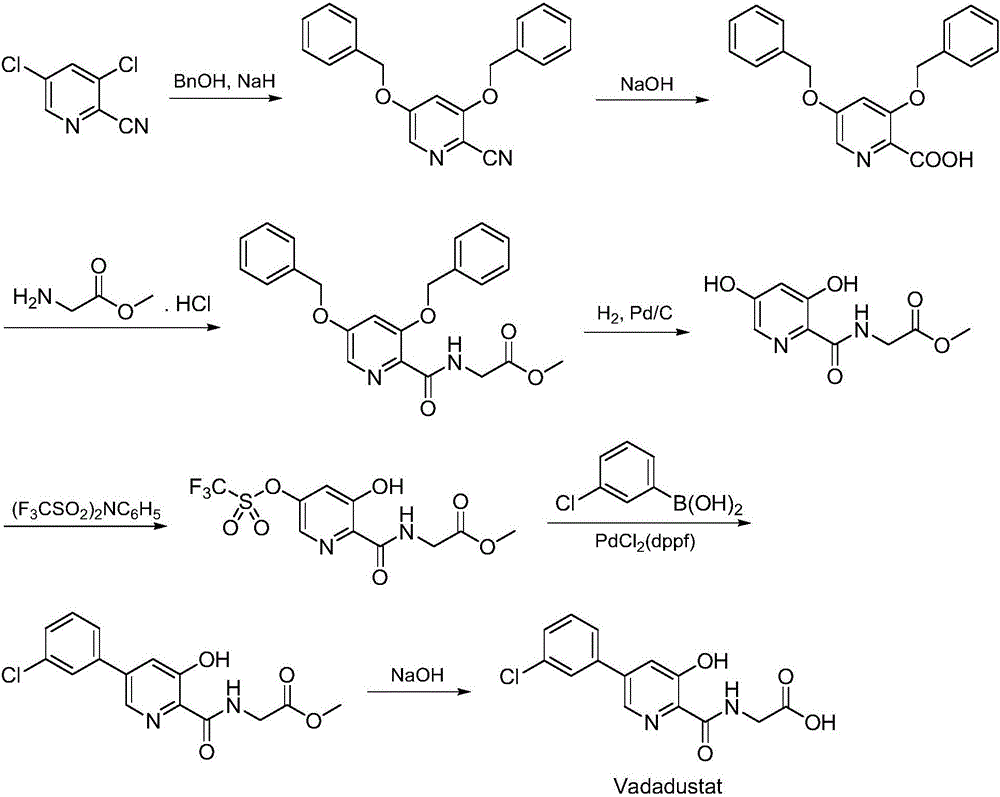
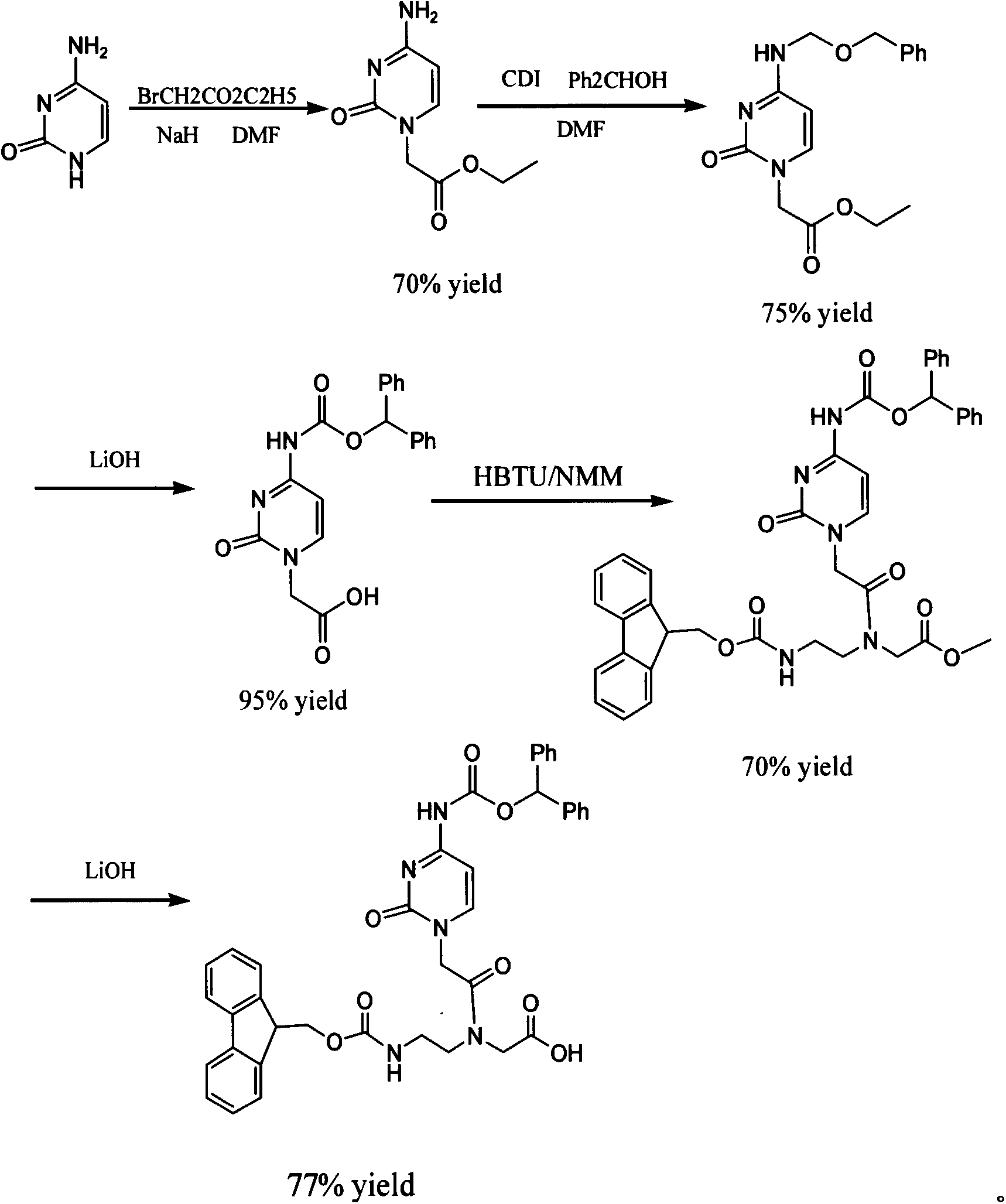
Synthesis method of Vadadustat
InactiveCN105837502AMeet the needs of useLess impuritiesOrganic chemistrySodium methoxideGlycine methyl ester hydrochloride
The invention discloses a synthesis method of Vadadustat. The method 3,5-dichloro-2-picolinic acid and glycine methyl ester hydrochloride carry out condensation reaction; the obtained N-(3,5-dichloropyridine-2-carbonyl) glycine methyl ester and 3-chlorobenzene Boronic acid is carried out catalytic coupling reaction; The N-[5-(3-chlorophenyl)-3-chloropyridine-2-carbonyl] glycine methyl ester obtained and sodium methylate are carried out methoxy substitution reaction; The obtained N- [5-(3-chlorophenyl)-3-methoxypyridine-2-carbonyl]glycine is hydrolyzed to obtain finished product Vadadustat. The synthesis method has short route steps, simplified operation and low cost, is a green and environment-friendly method, and is suitable for industrial production.
Owner:湖南欧亚药业有限公司
Chemical-enzyme method for preparing (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate
InactiveCN101864464AHigh optical activityEliminate the recrystallization stepFermentationOrganic acidGlycine methyl ester hydrochloride
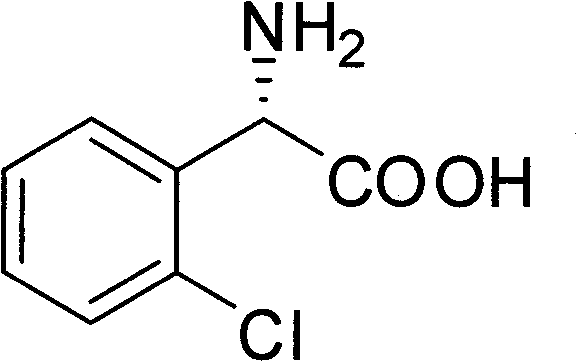
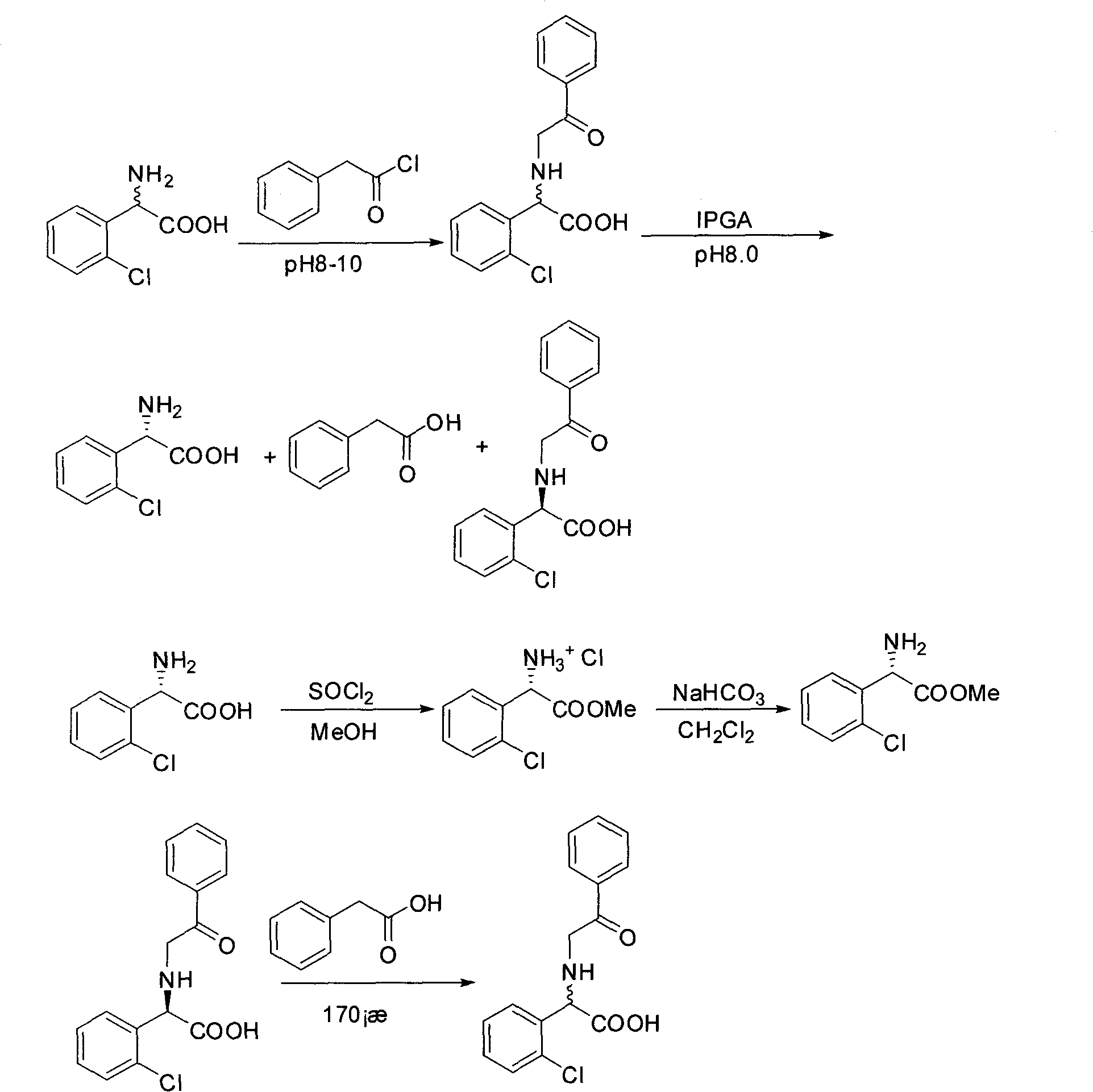
The invention provides a chemical-enzyme method for preparing an (S)-2-chlorophenyl glycine methyl ester clopidogrel chiral intermediate. In the method, (R,S)-2-chlorophenyl glycine is used as a raw material, and the (R,S)-2-chlorophenyl glycine is converted to (R,S)-N-phenylacetyl-2-chlorophenyl glycine by a deacylating agent; an immobilized penicillin acylase is used as a biocatalyst to catalyze a reaction for selectively converting the (R,S)-N-phenylacetyl-2-chlorophenyl glycine into (S)-2-chlorophenyl glycine, phenylacetic acid and (R)-N-phenylacetyl-2-chlorophenyl glycine in a water medium correspondingly; the (S)-2-chlorophenyl glycine is converted into (S)-2-chlorophenyl glycine methyl ester hydrochloride, and the (S)-2-chlorophenyl glycine methyl ester hydrochloride is desalinized to form the (S)-2-chlorophenyl glycine methyl ester; and the (R)-N-phenylacetyl-2-chlorophenyl glycine is mutually resolved with an organic acid and is racemized to form the (R,S)-N-phenylacetyl-2-chlorophenyl glycine which is used for resolution circularly. The method has the characteristics of high yield, high optical purity and environmental friendliness.
Owner:CHONGQING CHIRAL BIOCATALYSIS TECH
Process for synthesizing lornoxicam intermediate against inflammation and pain
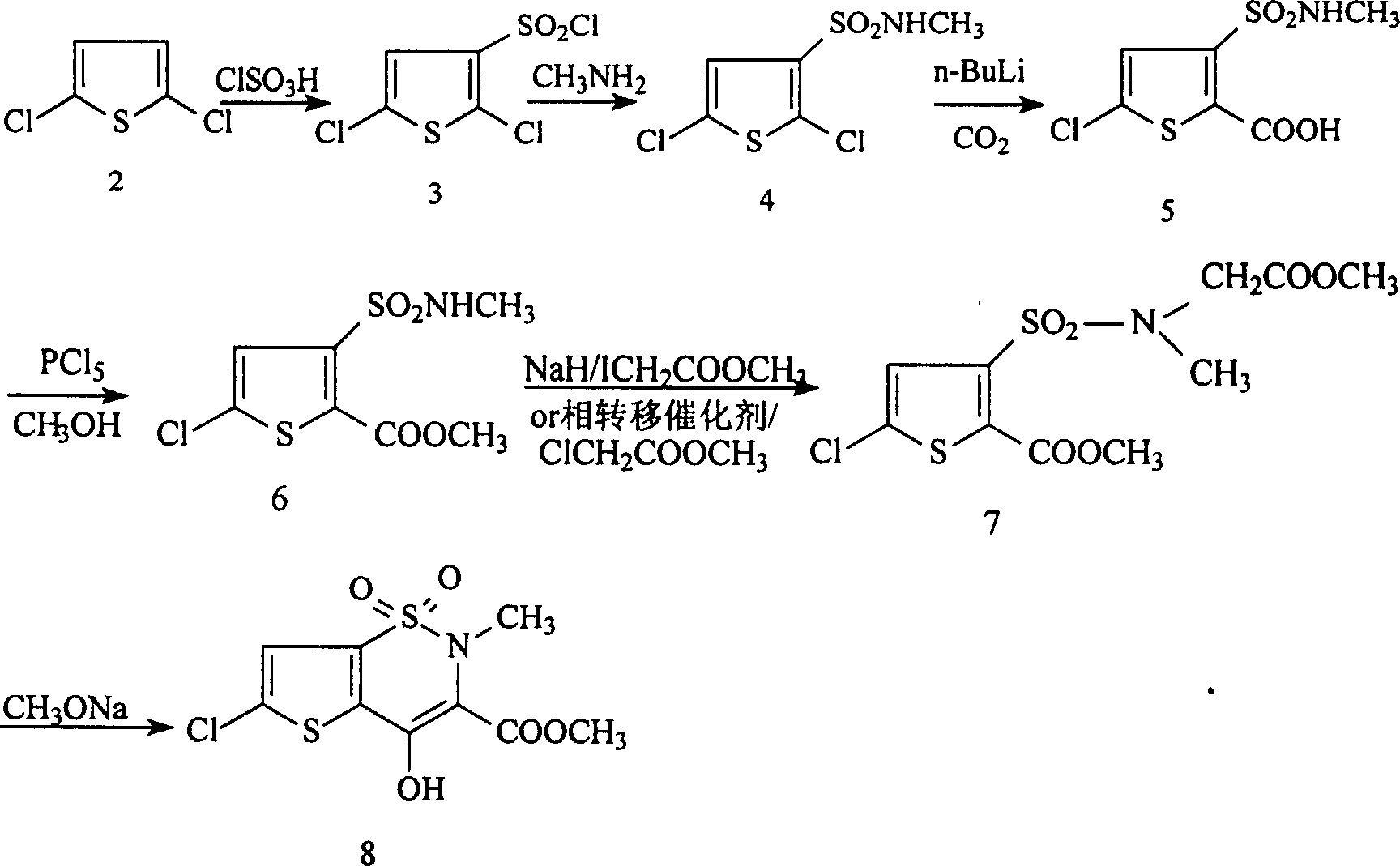
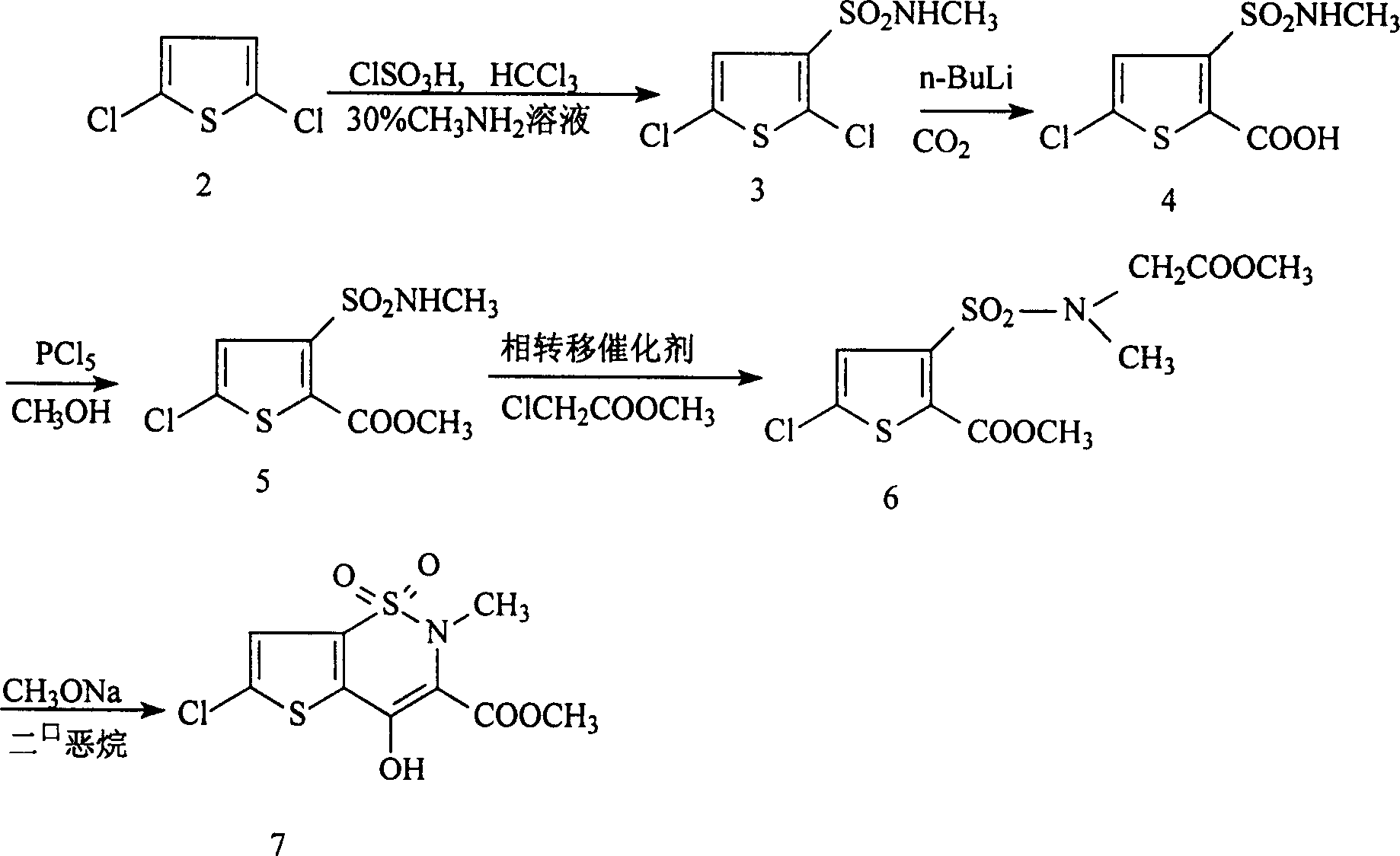
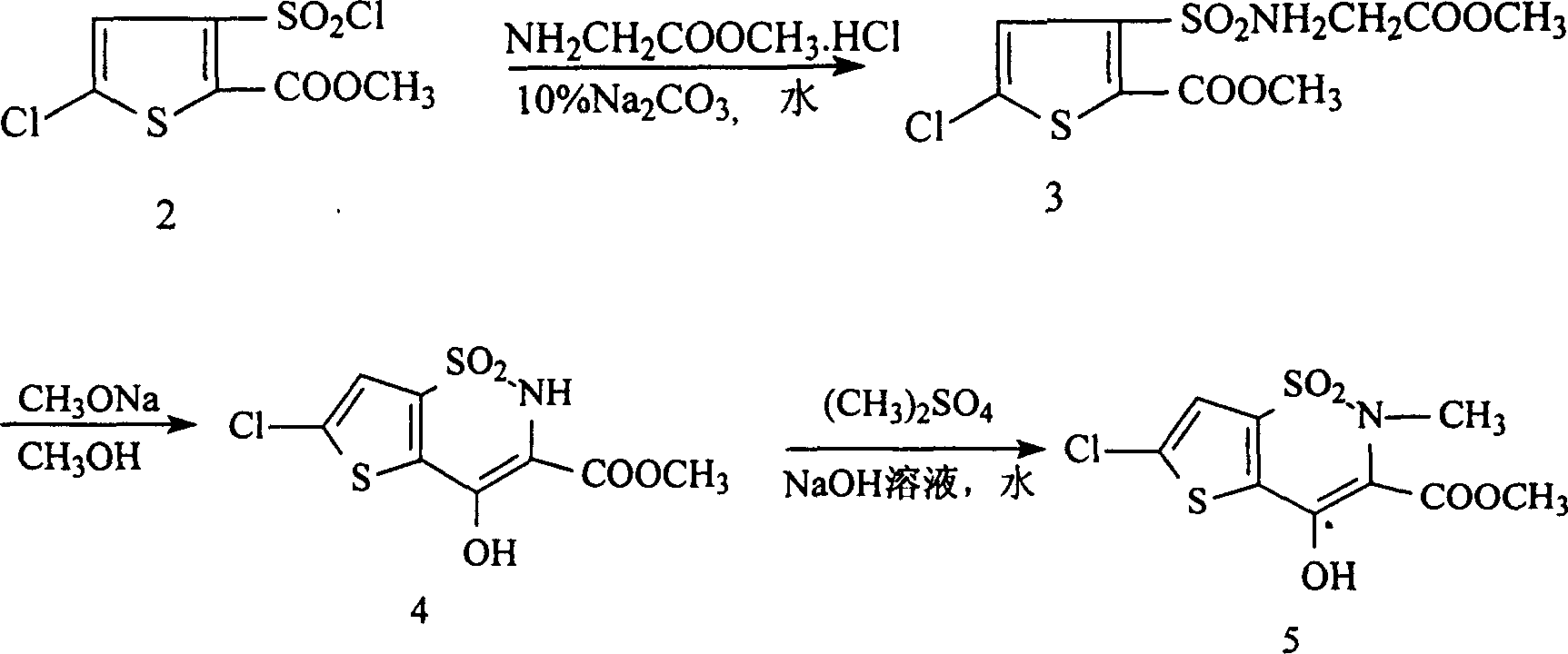
Disclosed is a process for synthesizing lornoxicam intermediate against inflammation and pain, which comprises using 5-chloro-3-thiophene sulfonyl chloride-2-carboxylate as raw material, charging 2-10% of sodium carbonate aqueous solution and 5-30% of glycine methylester hydrochloride simultaneously into water and methanol phases, thermal insulating 6-26 hours at 10-60 deg. C, then filtering and drying to obtain 6-chloro-3-sulfamoyl amido methyl acetate thiophene-2 methyl formate, reacting it with 5-27% of sodium methylate methanol solution 1-15 hours at 30-75 deg. C, filtering to obtain 6-chloro-4-hydroxyl-2H-thione[2,3-e]-1 and 2-thiazine-3-methyl formate-1,1-dioxide, then reacting the filtered substance with dimethyl sulfate in 1-10% aqueous solution of sodium-hydroxide.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
Glycine and application of acid salt thereof in preparation of glyphosate
InactiveCN102161678AGroup 5/15 element organic compoundsGlycine methyl ester hydrochlorideGlycine methyl ester
The invention relates to a glycine and an application of an acid salt thereof in preparation of herbicide-glyphosate, and in particular relates to an application of glycine hydrochloride and glycine methyl ester hydrochloride in preparation of the herbicide-glyphosate.
Owner:李坚
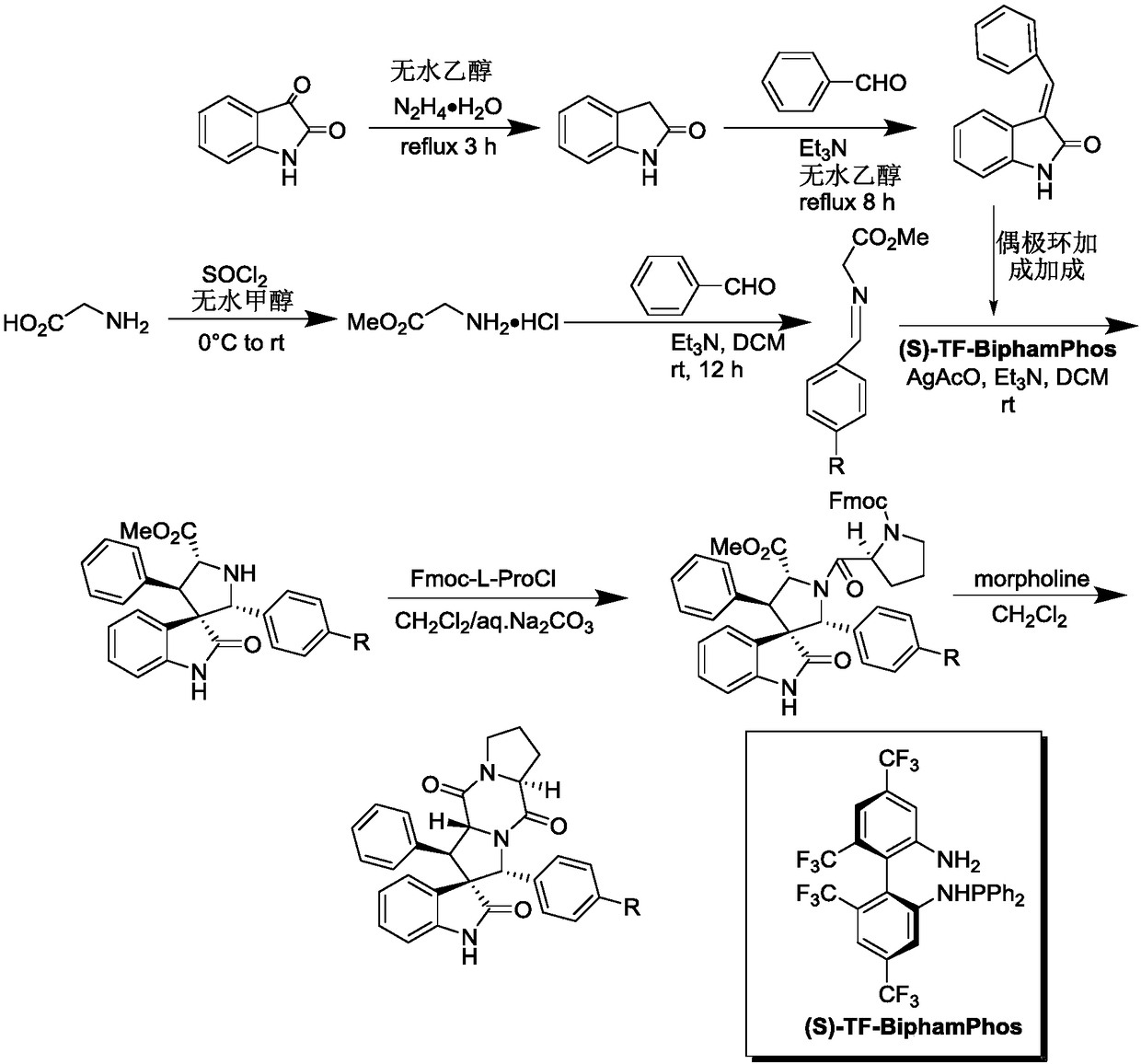
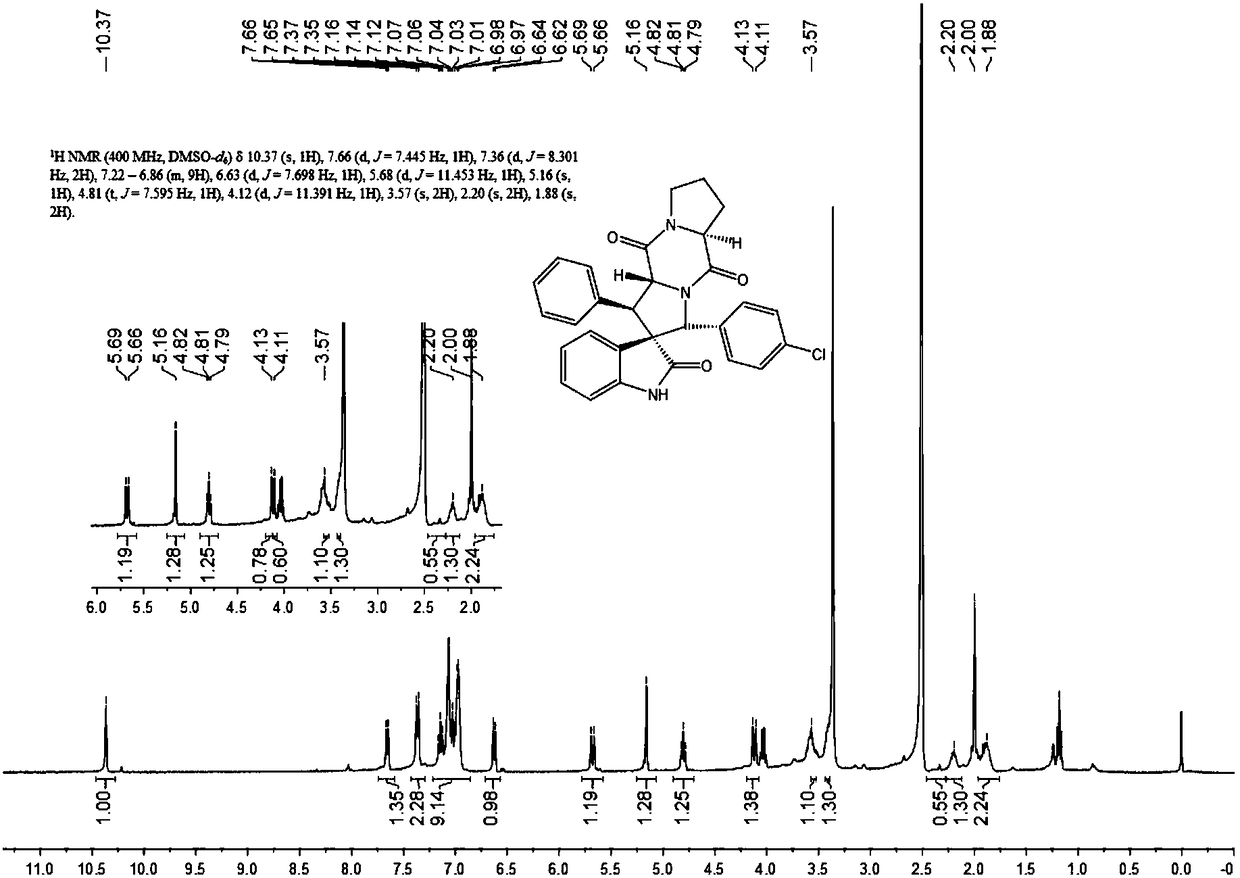
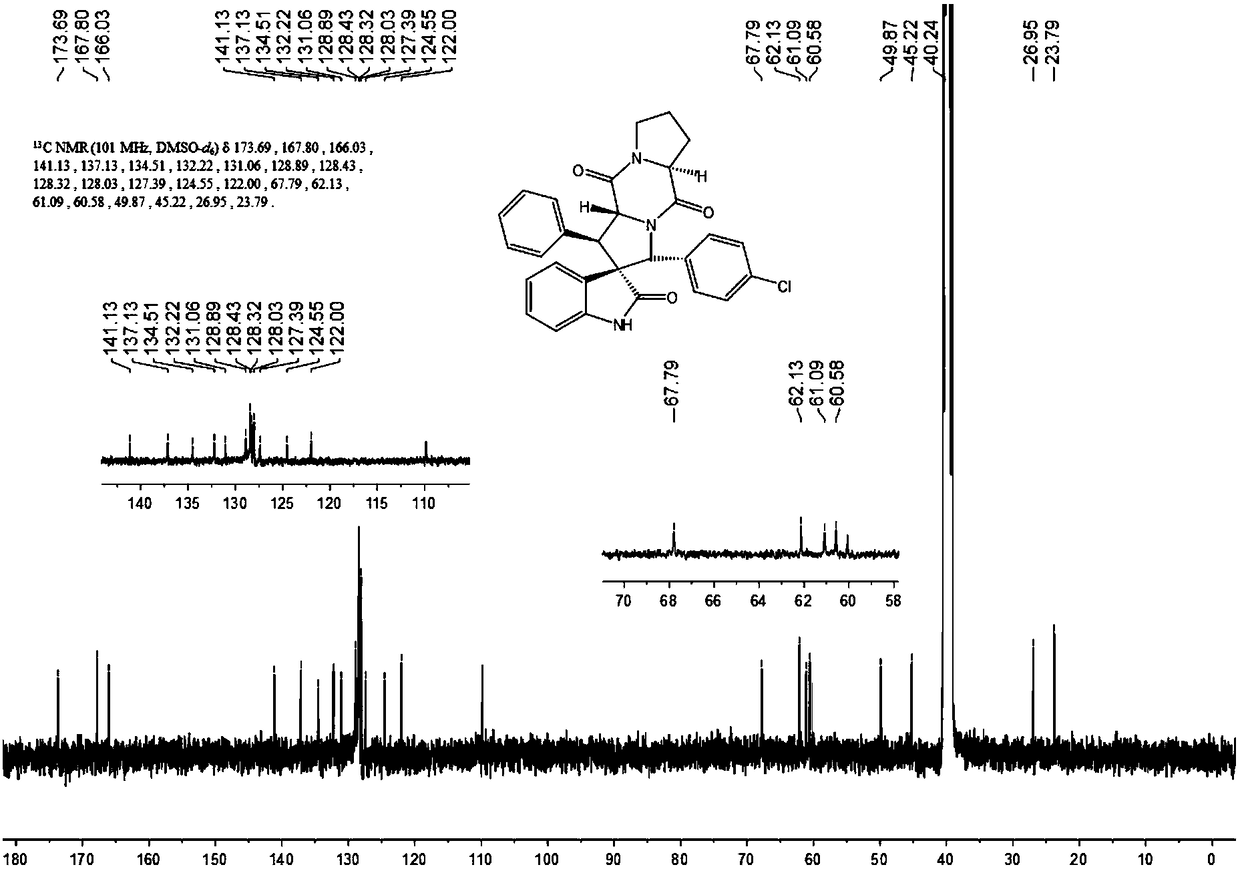
Aromatic substitution spiro indolyl diketopiperazine compound and synthesis method thereof
ActiveCN106478645AGuaranteed singularityHigh conformational specificityOrganic chemistryBulk chemical productionBenzaldehydeCycloaddition
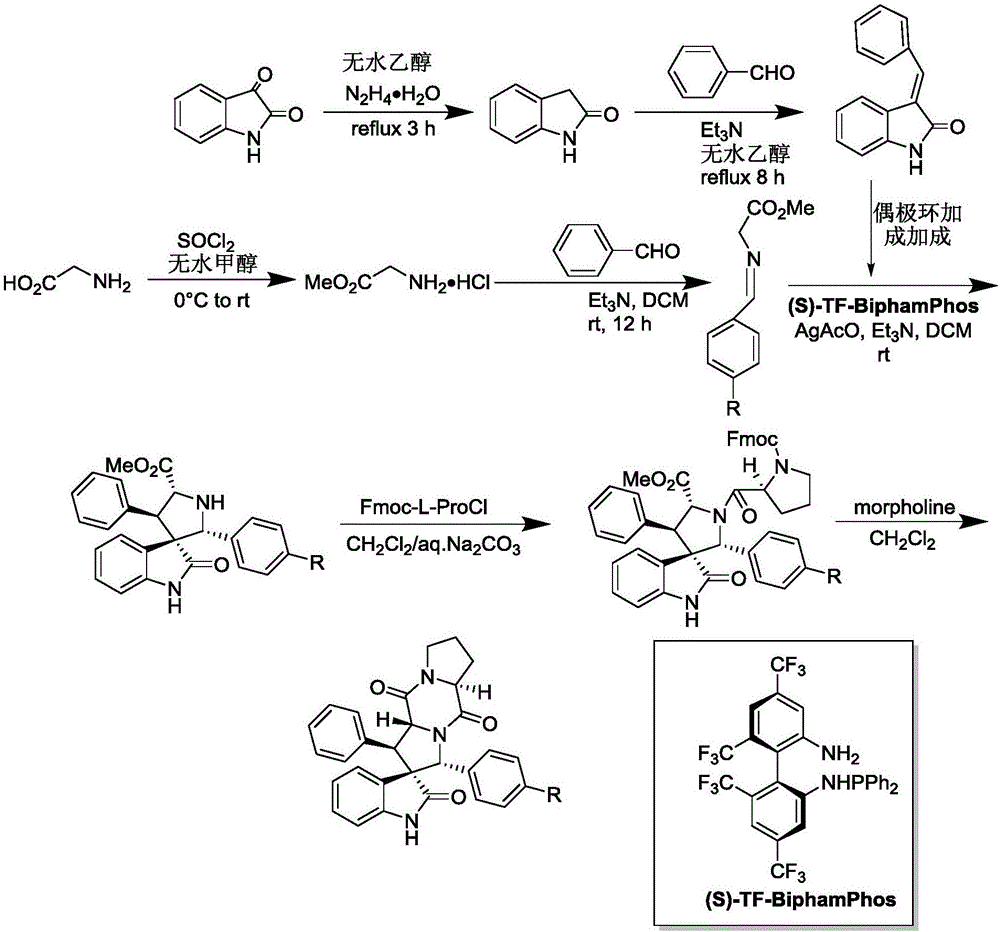
The invention discloses an aromatic substitution spiro indolyl diketopiperazine compound and a synthesis method thereof. Glycine is used as a raw material and subjected to esterification reaction with methyl alcohol and thionyl chloride to obtain methyl glycinate hydrochloride, methyl glycinate hydrochloride and aromatic aldehyde are subjected to condensation reaction to obtain Schiff base, isatin is used as a raw material and subjected to reduction reaction under the action of hydrazine hydrate to obtain indoline-2-ketone, indoline-2-ketone and benzaldehyde are subjected to Knoevenagel reaction under piperidine catalysis to obtain 3-phenylidene-1,3-dihydro-2H-indol-2 ketone, 3-phenylidene-1,3-dihydro-2H-indol-2 ketone and Schiff base are subjected to 1,3-dipole cycloaddition reaction under catalysis of chiral ligand (S)-TF-BiphamPhos / AgoAc to obtain spiro pyrrolidine, and protecting group removal and ring closure are carried out on spiro pyrrolidine and N-(9-fluorene methoxycarbonyl)-L-prolyl chloride through base catalysis to obtain the target product. The method has the advantages of being simple in path, high in yield, high in diastereoselectivity and single in product spatial configuration, and the raw materials are low in price and easy to obtain.
Owner:SHAANXI UNIV OF SCI & TECH
Preparation method of 1,2,3-bis-triazole ligands and application of 1,2,3-bis-triazole ligands in CuAAC reaction
ActiveCN105968116AObvious categoryExpand categoryEsterified saccharide compoundsSugar derivativesBis triazoleGlycine methyl ester hydrochloride
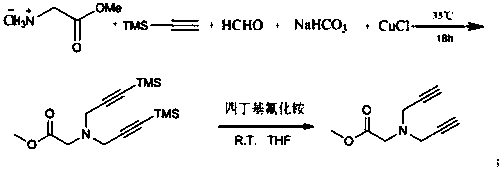
The invention discloses a preparation method of 1,2,3-bis-triazole ligands and an application of the 1,2,3-bis-triazole ligands in a CuAAC reaction. With cheap and easily available glycine methyl ester hydrochloride as a base raw material, and through derivation and conversion of functional groups, a diyne compound with glycine as a structure is obtained; then under a condition of room temperature and with organic azide as a raw material and copper iodide as a catalyst, 1,2,3-bis-triazole seven-membered-ring compounds are synthesized; and with the bis-triazole compounds and derivatives thereof as substrates, a series of methods for synthesis of the 1,2,3-bis-triazole derivatives are developed. A variety of novel, highly efficient, simple and easily available ligand catalysts are provided for a click reaction, and the 1,2,3-bis-triazole ligands have obvious effect on accelerating the reaction when used as the ligands and have quite practical application.
Owner:HENAN NORMAL UNIV
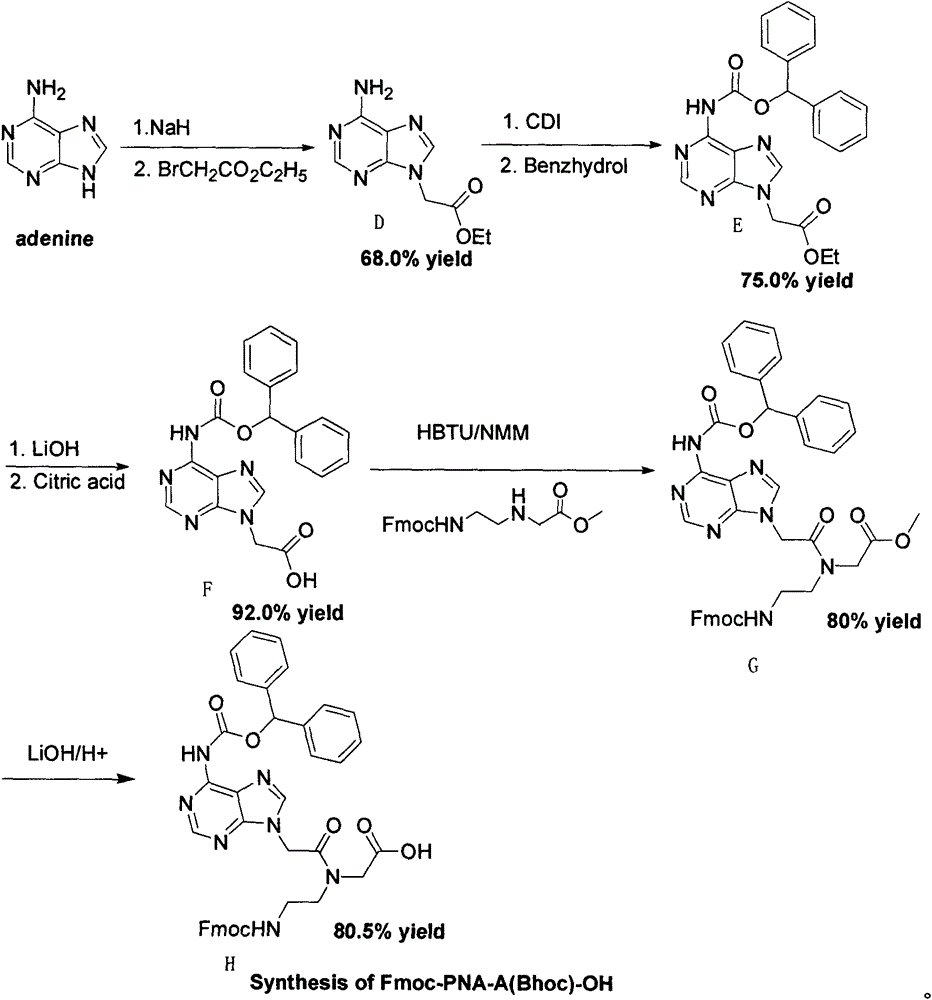
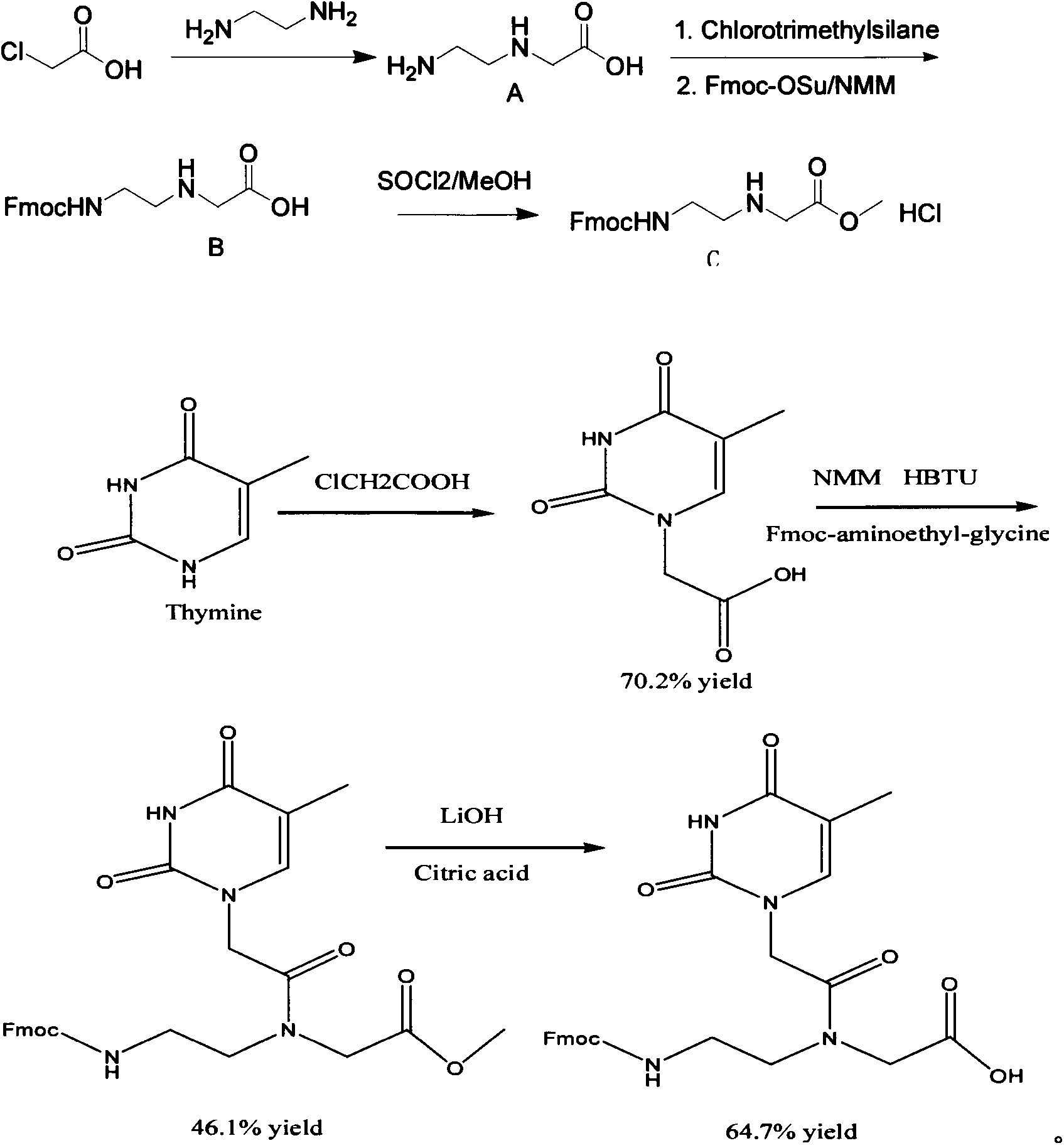
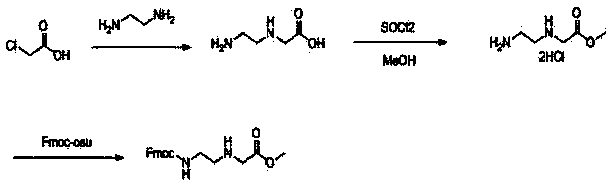
Feather weight PNA (pentose nucleic acid) synthesis method
The invention discloses a feather weight PNA (pentose nucleic acid) synthesis method. The method comprises the following steps: reacting ethylene diamine with chloroacetic acid, and recrystallizing with DMSO to obtain N-(2-aminoethyl)glycine; by taking the N-(2-aminoethyl)glycine as a reactant, adding raw materials, and reacting to obtain N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride; by taking adenine as a reactant, adding raw materials, and reacting to obtain 9-ehtyl acetate adenine; by taking the 9-ehtyl acetate adenine and carbonyl diimidazole as reactants, adding raw materials, and reacting to obtain 6-N-(benzhydryloxycarbonyl)adenine-9-acetic acid; by taking the 6-N-(benzhydryloxycarbonyl)adenine-9-acetic acid as a reactant, adding raw materials, and reacting to obtain the PNA. The feather weight PNA synthesis method belongs to large-scale production technology, does material preparation for the large-scale research of the PNA, as well as technological preparation for the future large-scale application.
Owner:SUZHOU VIVOTIDE BIOTECH
Feather weight preparation method of cytosine containing PNA (pentose nucleic acid) monomer
The invention discloses a feather weight preparation method of a cytosine containing PNA (pentose nucleic acid) monomer. The feather weight preparation method comprises the following steps: reacting ethanediamine with chloroacetic acid and recrystallizing by using DMSO (dimethylsulfoxide) so as to obtain N-(2-aminoethyl) glycine; using N-(2-aminoethyl) glycine as a reactant, adding raw materials, and reacting so as to obtain N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride which serves as an intermediate 1; using cytosine as a reactant, adding raw materials, and reacting so as to obtain 4-N-(benzhydryloxycarbonyl) cytosine-acetic acid; and using the 4-N-(benzhydryloxycarbonyl) cytosine-acetic acid as a reactant, and adding the raw materials, thus obtaining the product. The feather weight preparation method is simple in operation and mild in reaction conditions, belongs to the large-scale production technology and provides the material guarantee for the large-scale research of the PNA and the technology guarantee for the subsequent large-scale application.
Owner:SUZHOU VIVOTIDE BIOTECH
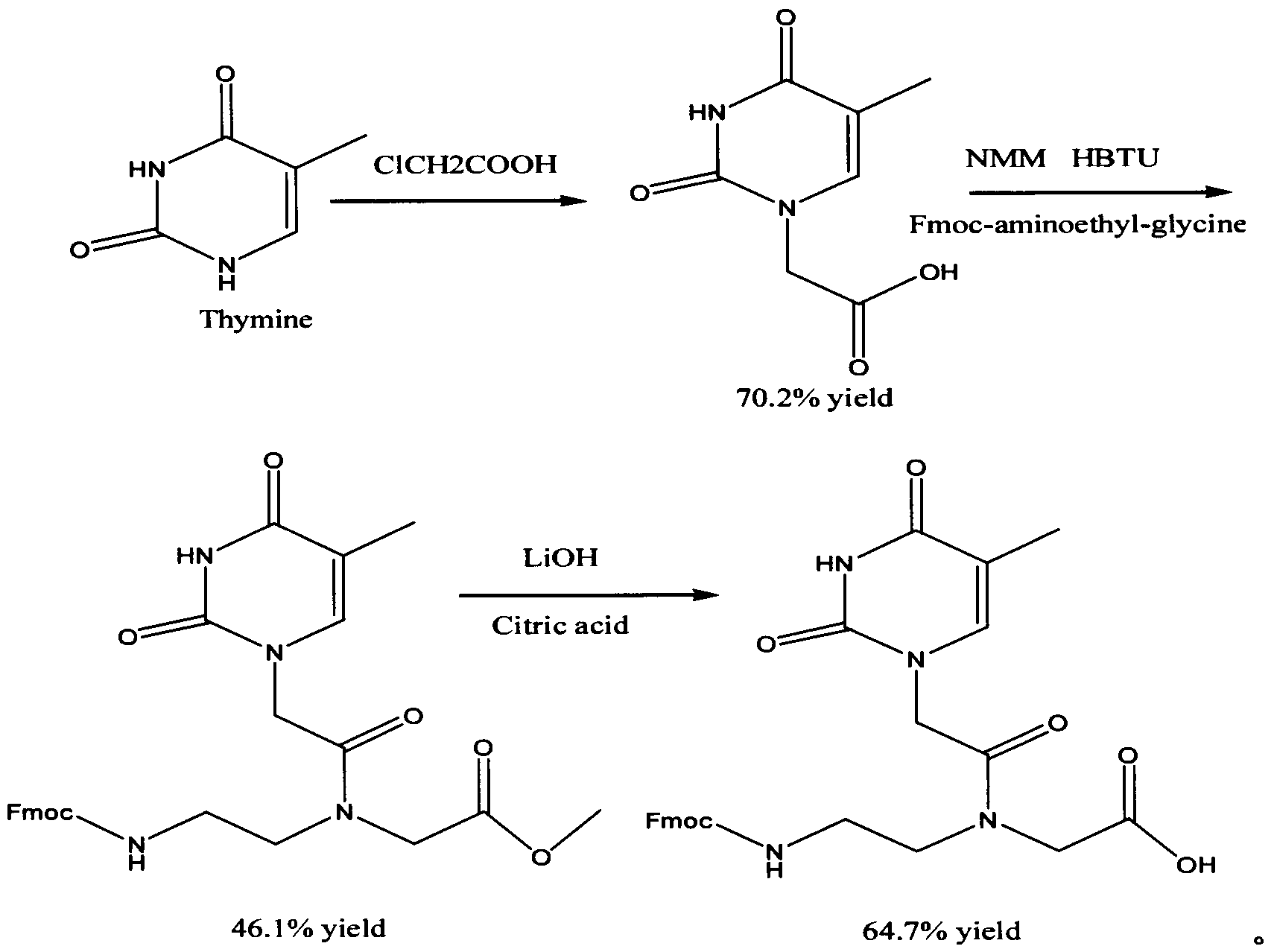
Feather weight synthesis method of Fmoc-PNA-T-OH
The invention discloses a feather weight synthesis method of Fmoc-PNA-T-OH. The feather weight synthesis method comprises the following steps: reacting ethanediamine serving as a raw material with haloacetic acid, and recrystallizing by using DMSO (dimethylsulfoxide) so as to obtain N-(2-aminoethyl) glycine; reacting the N-(2-aminoethyl) glycine serving as a raw material with Fmoc-osu so as to obtain N-(2-Fmoc-aminoethyl) glycine, and then reacting with methyl alcohol so as to obtain N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride; using thymine as a raw material, adding raw materials, and reacting so as to obtain 1-carbethoxy thymine; reacting the 1-carbethoxy thymine serving as the raw material with the N-(2-Fmoc-aminoethyl) glycine methyl ester so as to obtain Fmoc-PNA-T-OMe; and hydrolyzing the Fmoc-PNA-T-OMe so as to obtain the product.
Owner:SUZHOU VIVOTIDE BIOTECH
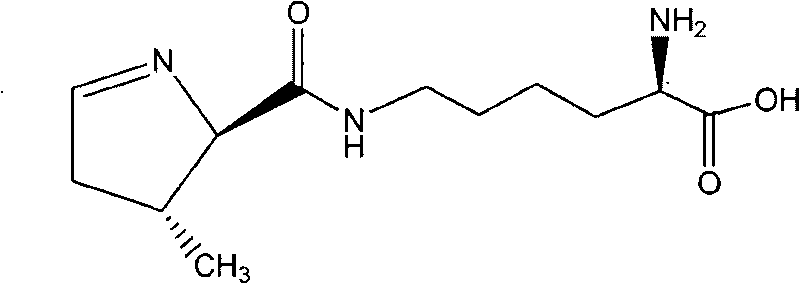
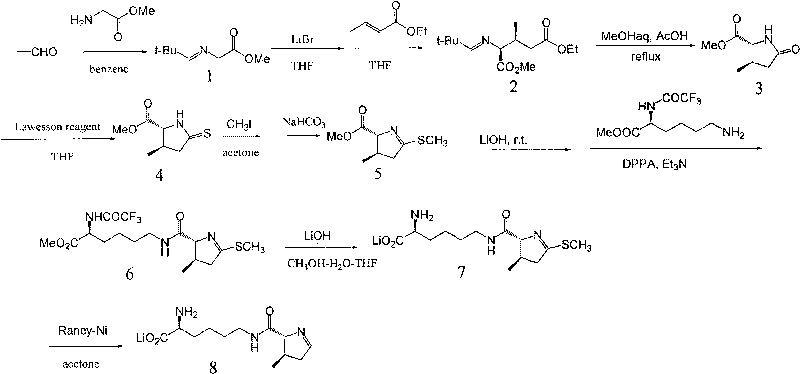
Chemical complete synthesis method for 22nd natural amino acid-pyrrolysine
InactiveCN101709047AWide variety of sourcesLow priceOrganic chemistryBulk chemical productionPyrrolysineSynthesis methods
The invention relates to a chemical complete synthesis method for 22nd natural amino acid-pyrrolysine. 2,2-dimethyl propionaldehyde reacts with glycine methyl ester hydrochloride to form (E)-2-(2,2-dimethyl propylidene-amino) methylacetate, and the product generates Michael addition with olefine aldehyde by the catalysis of DBU and then carries out hydrolysis to obtain a pyrrole ring. After being vulcanized and methylated, the pyrrole ring is condensed with a derivative of lysine, and finally sulfomethyl is removed to obtain the pyrrolysine. The invention has cheap and easily acquired chemical raw materials, wide source, low cost, mild and easily controlled reaction condition, simple reaction device and low operation cost; by adopting various protecting groups, all intermediate compounds are more stable and are difficult for modification; and all reactions are mature and are in fixed quantity, and the yield is higher.
Owner:EAST CHINA UNIV OF SCI & TECH
Method for preparing 2-amino-dimethyl acetamide hydrochloride
ActiveCN102351733ARaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationGlycine methyl ester hydrochlorideAminolysis
A method for preparing 2-amino-dimethyl acetamide hydrochloride. An initial raw material is selected from commercialized raw material or easily prepared glycine methyl ester hydrochloride and is prepared into 2-amino-dimethyl acetamide hydrochloride by amino protection, aminolysis and deprotection. The invention has advantages of cheap and easily available raw material, mild reaction conditions, stable technology, a stable overall yield at 78-84%, a stable object product purity higher than 99%, and intermediates obtained by direct separation with a purity higher than 98%. Meanwhile operations of the whole production process are simple and without generation of three wastes (waste gas, waste water and industrial residue) so as to save production costs substantially. The method is suitable for large scale production and provides a new thinking and approach for preparation of 2-amino-dimethyl acetamide hydrochloride.
Owner:ASYMCHEM LAB FUXIN
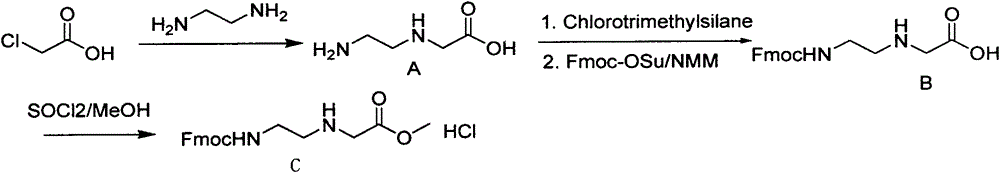
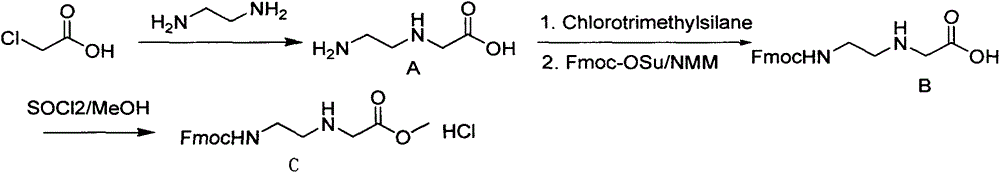
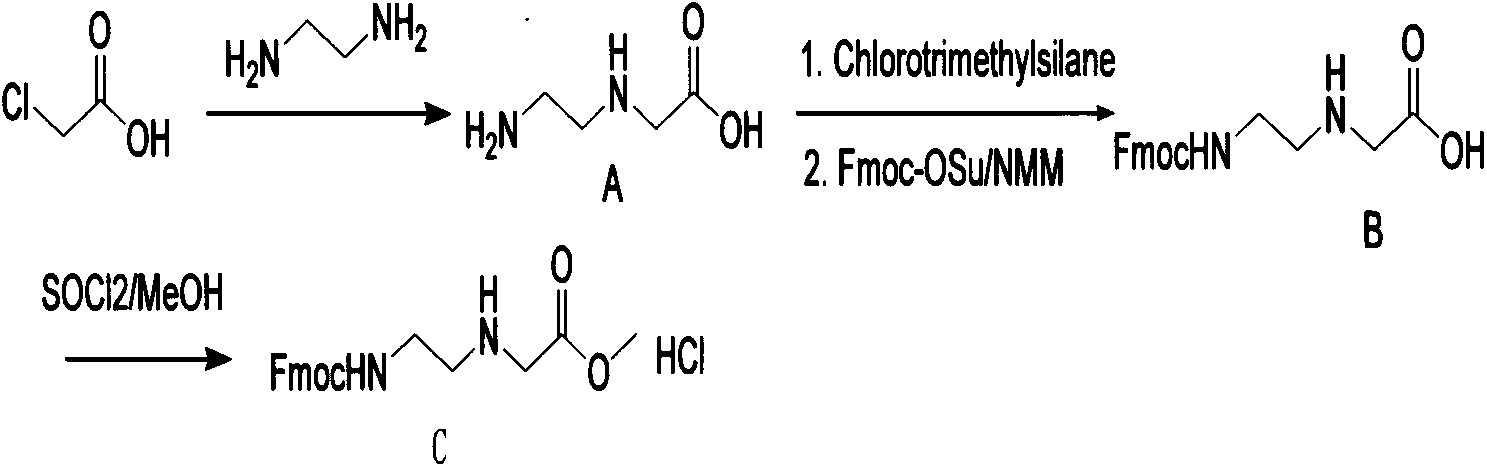
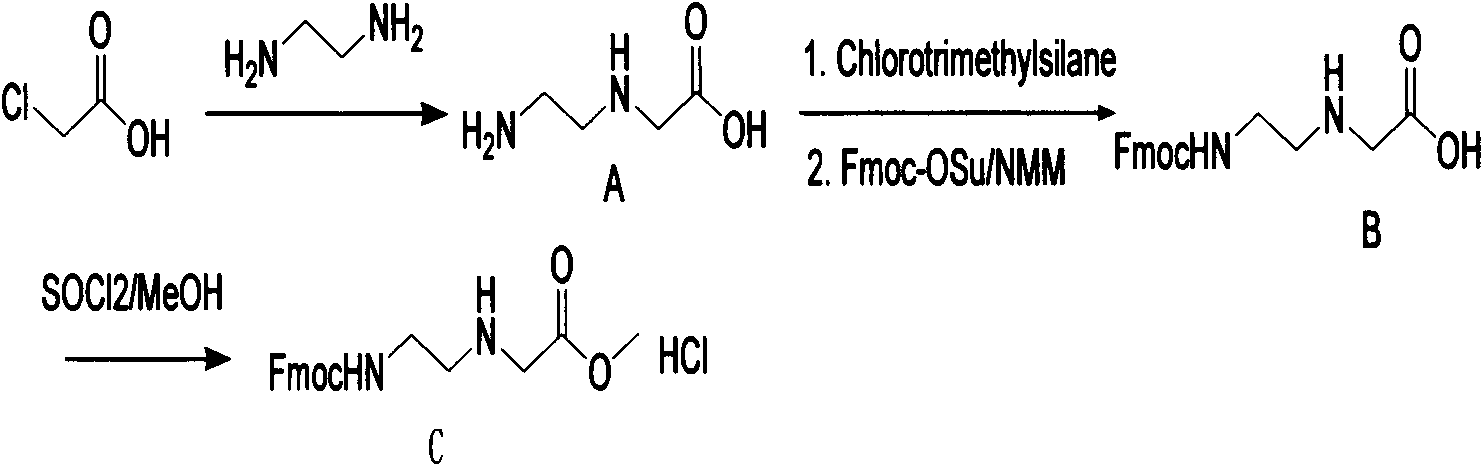
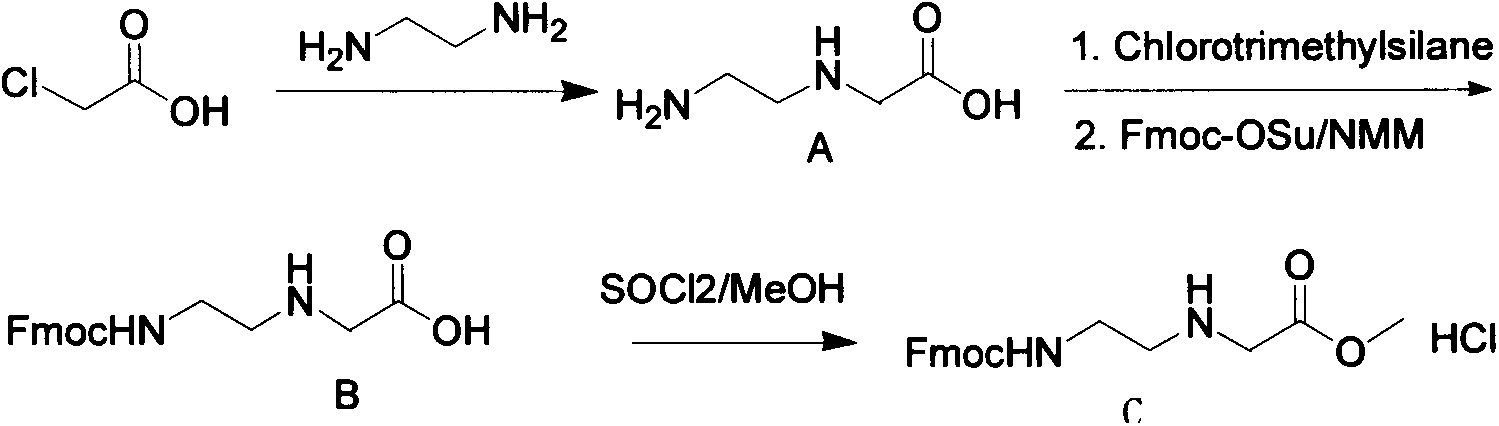
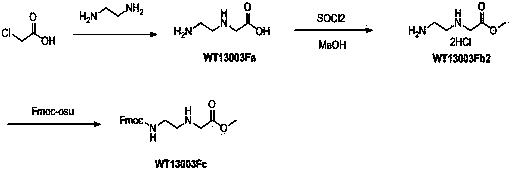
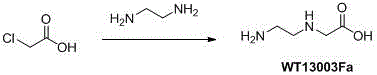
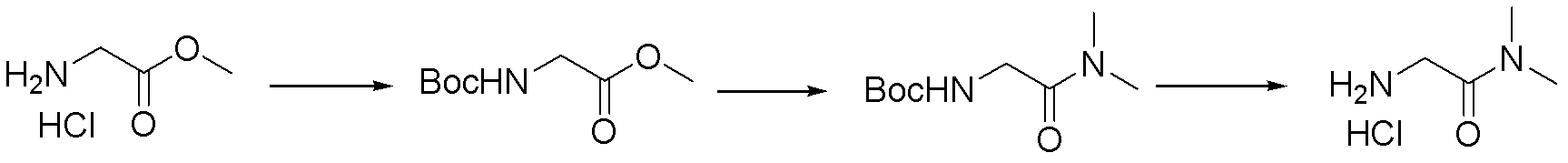
Synthetic method for N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride
ActiveCN104211619AReduce usageLow costCarbamic acid derivatives preparationOrganic compound preparationAcetic acidGlycine methyl ester hydrochloride
A disclosed synthetic method for N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride comprises the following steps: reacting ethylenediamine with a halogenated acetic acid, using DMSO for recrystalization and further for obtaining N-(2-aminoethyl)glycine (WT13003Fa); taking N-(2-aminoethyl)glycine as a raw material, reacting with methanol with the participation of thionyl chloride , so as to prepare N-(2-aminoethyl)glycine methyl ester (WT13003Fb2); and then reacting N-(2-aminoethyl)glycine methyl ester with Fmoc-osu to prepare N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride (WT13003Fc). The N-(2-Fmoc-aminoethyl)glycine methyl ester hydrochloride prepared by employing the synthetic method has the purity of 97% and the yield of 47%.
Owner:SUZHOU VIVOTIDE BIOTECH
Preparation method of D-phenylglycine methyl ester hydrochloride/D-dihydrophenylglycine methyl ester hydrochloride
ActiveCN110128285AReduce dosageHigh yieldOrganic compound preparationOrganic chemistry methodsGlycine methyl ester hydrochlorideCentrifugation
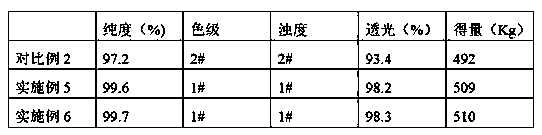
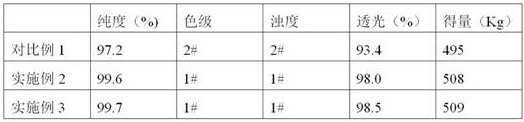
The invention provides a preparation method of D-phenylglycine methyl ester hydrochloride / D-dihydrophenylglycine methyl ester hydrochloride. The method comprises the following steps: (a) adding D-phenylglycine or D-dihydrophenylglycine and methanol into a reaction tank according to a ratio of 1 g:3-5 mL, performing uniform stirring, and slowly adding thionyl chloride; (b) after the thionyl chloride is added, performing a reflux reaction; (c) performing vacuum distillation, performing cooling crystallization, performing centrifugation, and performing drying to obtain a white product; (d) recovering the reaction mother liquid, performing vacuum concentration on the mother liquid, performing cooling crystallization, and performing centrifugation to obtain a yellow recovered product; (e) washing the yellow recovered product by using an organic reagent, and performing vacuum drying to obtain a white recovered product; and (f) recycling the white recovered product in the reaction tank, and preparing latter batch products according to operation of steps (a) to (c). The method provided by the invention proposes a novel way of recycling the mother liquid, and the product has more stable quality, high purity, and excellent color grade and turbidity.
Owner:NORTH CHINA PHARMA COMPANY
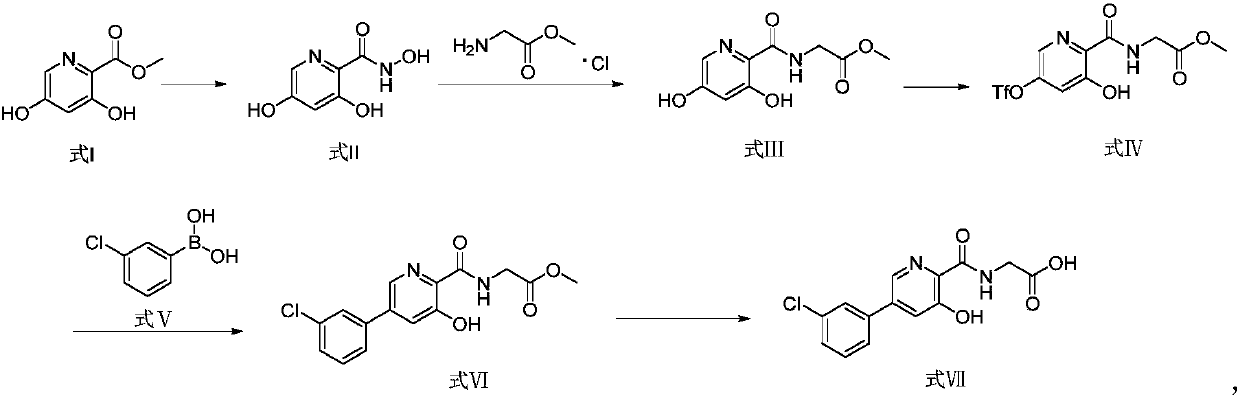
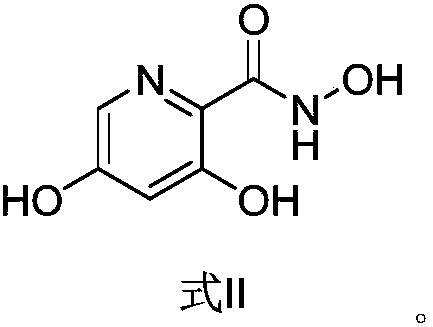
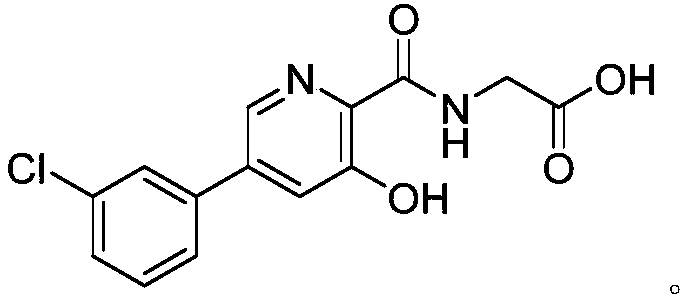
Preparation method and application of picolinamide
ActiveCN111320577AHigh purityHigh yieldOrganic chemistryBulk chemical productionChemical industryHydroxylamine
The invention provides a preparation method of a picolinamide compound, and belongs to the field of pharmaceutical and chemical industry. According to the preparation method, 3,5-dihydroxypicolinic acid methyl ester, hydroxylamine hydrochloride, an alkali and an organic solvent are mixed, ammonolysis is performed to obtain 3,5-dihydroxyl-2-pyridine formyl hydroxamic acid; and 3,5-dihydroxyl-2-pyridine formyl hydroxamic acid and glycine methyl ester hydrochloride sequentially carry out a reaction, upper protection, coupling and hydrolysis to obtain {[5-(3-chlorphenyl)-3-hydroxypyridine-2-yl]amino} acetic acid. The product produced by the method is high in purity, and high in yield. The process cost is low, the operation is simple, and the process is stable.
Owner:SUNSHINE LAKE PHARM CO LTD
Preparation method of 3-(tert-butoxycarbonyl-methoxycarbonylmethyl-amino)-methyl propionate and intermediate of 3-(tert-butoxycarbonyl-methoxycarbonylmethyl-amino)-methyl propionate
PendingCN114380717AHigh yieldCarbamic acid derivatives preparationOrganic compound preparationMeth-Propanoic acid
The invention relates to a preparation method of 3-(tert-butoxycarbonyl-methoxycarbonyl methyl-amino)-methyl propionate and an intermediate thereof, and the preparation method of the 3-(methoxycarbonyl-methyl-amino)-methyl propionate comprises the following steps: dissolving glycine methyl ester hydrochloride in a solvent, adding potassium trimethylsilanolate, stirring, reacting, filtering, washing, and drying to obtain the 3-(tert-butoxycarbonyl-methoxycarbonyl methyl-amino)-methyl propionate. And then adding methyl acrylate and reacting at 40-65 DEG C for 1-3 hours. When 3-(methoxycarbonyl-methyl-amino)-methyl propionate is prepared, potassium trimethylsilanolate is used as a catalyst, so that the product yield can be increased.
Owner:郑州猫眼农业科技有限公司
Preparation method of cefaclor suitable for industrial production
InactiveCN109266713AReduce usageEasy to separateOrganic chemistryFermentationGlycine methyl ester hydrochlorideDrugs synthesis
The invention belongs to the technical field of pharmaceutical synthesis and relates to a preparation method of cefaclor suitable for industrial production. The preparation method uses 7 amino-3-chloro-cephem acid as a starting material, and reacts with L-phenylglycine methyl ester hydrochloride under the action of an immobilized penicillin acylase for synthesis to obtain cefaclor. The invention solves the difficult problem of filtering enzyme in the prior art, reduces the production man-hour, has high product yield, produces less three wastes, is green and environmentally friendly, and is suitable for large-scale industrial production.
Owner:QILU ANTIBIOTICS PHARMA
Preparation method of high-purity glycine methyl ester hydrochloride
InactiveCN110003028AAddress reactivitySolve problems such as \"locking\" of the systemOrganic compound preparationAmino-carboxyl compound preparationFiltrationSolvent free
A preparation method of high-purity glycine methyl ester hydrochloride specifically comprises the steps that 1, glycine and absolute methanol are added into a reaction kettle and uniformly stirred andmixed; 2, hydrogen chloride gas is introduced, and the reaction temperature of a system is increased to be 40-60 DEG C; 3, hydrogen chloride gas is continuously introduced for 0-20 minutes when crystal is separated out in the system due to a thermal reaction; 4, the stirring rate is controlled and gradiently reduced until the temperature of the system is lower than or equal to 10 DEG C, and suction filtration and drying are carried out to obtain the white crystalline powder glycine methyl ester hydrochloride. According to the method, the glycine and the absolute methanol serve as raw materials, the introducing amount and introducing stage of the hydrogen chloride gas are controlled, and amino acid methyl ester hydrochloride with the purity higher than 99% is prepared at a time through a gradient cooling crystallization method, so that the problems of instant crystallization of the solvent-free reaction system, equipment stirring locking, high filtration energy consumption and the likeare effectively solved. According to the method, the reaction equipment is simple, reaction conditions are mild, crystallization is easy to control, and the product yield can reach 95%.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
A kind of synthetic method of n-(2-fmoc-aminoethyl) glycine methyl ester hydrochloride
ActiveCN104211619BReduce usageLow costCarbamic acid derivatives preparationOrganic compound preparationEthylenediamineAcetic acid
Owner:SUZHOU VIVOTIDE BIOTECH
The preparation method of d-phenylglycine methyl ester hydrochloride/d-dihydrophenylglycine methyl ester hydrochloride
ActiveCN110128285BReduce dosageHigh yieldOrganic compound preparationOrganic chemistry methodsPhenyl groupMethanol
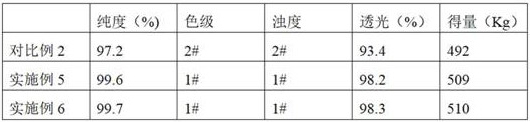
The invention provides a preparation method of D-phenylglycine methyl ester hydrochloride / D-dihydrophenylglycine methyl ester hydrochloride, comprising the following steps: (a) mixing D-phenylglycine or D-dihydrobenzene Glycine and methanol were added to the reaction tank at a ratio of 1g: 3~5mL, and after stirring evenly, slowly added thionyl chloride; (b) reflux reaction after the addition of thionyl chloride; (c) vacuum distillation, Then cool down and crystallize, centrifuge and dry to obtain a white product; (d) recover the reaction mother liquor, concentrate the mother liquor in a vacuum, cool down and crystallize, and centrifuge to obtain a yellow recovered product; (e) wash the yellow recovered product with an organic reagent, and dry it in a vacuum to obtain White recycled product; (f) Apply the white recycled product to the reaction tank, and then follow steps (a)~(c) to prepare the next batch of products. The invention proposes a new way of applying the mother liquor, so that the product quality is more stable, the purity is high, and the color grade and turbidity are excellent.
Owner:NORTH CHINA PHARMA COMPANY
Method for preparing 2-amino-dimethyl acetamide hydrochloride
ActiveCN102351733BRaw materials are cheap and easy to getMild reaction conditionsOrganic compound preparationCarboxylic acid amides preparationGlycine methyl ester hydrochlorideAminolysis
A method for preparing 2-amino-dimethyl acetamide hydrochloride. An initial raw material is selected from commercialized raw material or easily prepared glycine methyl ester hydrochloride and is prepared into 2-amino-dimethyl acetamide hydrochloride by amino protection, aminolysis and deprotection. The invention has advantages of cheap and easily available raw material, mild reaction conditions, stable technology, a stable overall yield at 78-84%, a stable object product purity higher than 99%, and intermediates obtained by direct separation with a purity higher than 98%. Meanwhile operations of the whole production process are simple and without generation of three wastes (waste gas, waste water and industrial residue) so as to save production costs substantially. The method is suitable for large scale production and provides a new thinking and approach for preparation of 2-amino-dimethyl acetamide hydrochloride.
Owner:ASYMCHEM LAB FUXIN
A kind of preparation method of biphenylalanine derivative
ActiveCN104725256BRaw materials are easy to getLow equipment requirementsOrganic compound preparationAmino-carboxyl compound preparationGlycine methyl ester hydrochlorideBenzaldehyde
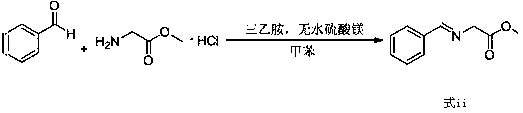
The invention relates to a preparation method of a compound of formula (i). The compound of formula i is prepared by using glycine methyl ester or its hydrochloride as a starting material, through protection of benzaldehyde, alkylation of benzyl chloride, and deprotection. The starting material of the invention is easy to obtain and low in price, and the intermediates involved in the reaction process do not need to be separated and purified, the operation is simple, the reaction conditions are mild, and the method is suitable for large-scale industrial production.
Owner:迪嘉药业集团股份有限公司
Preparation method of sivelestat sodium hydrate intermediate
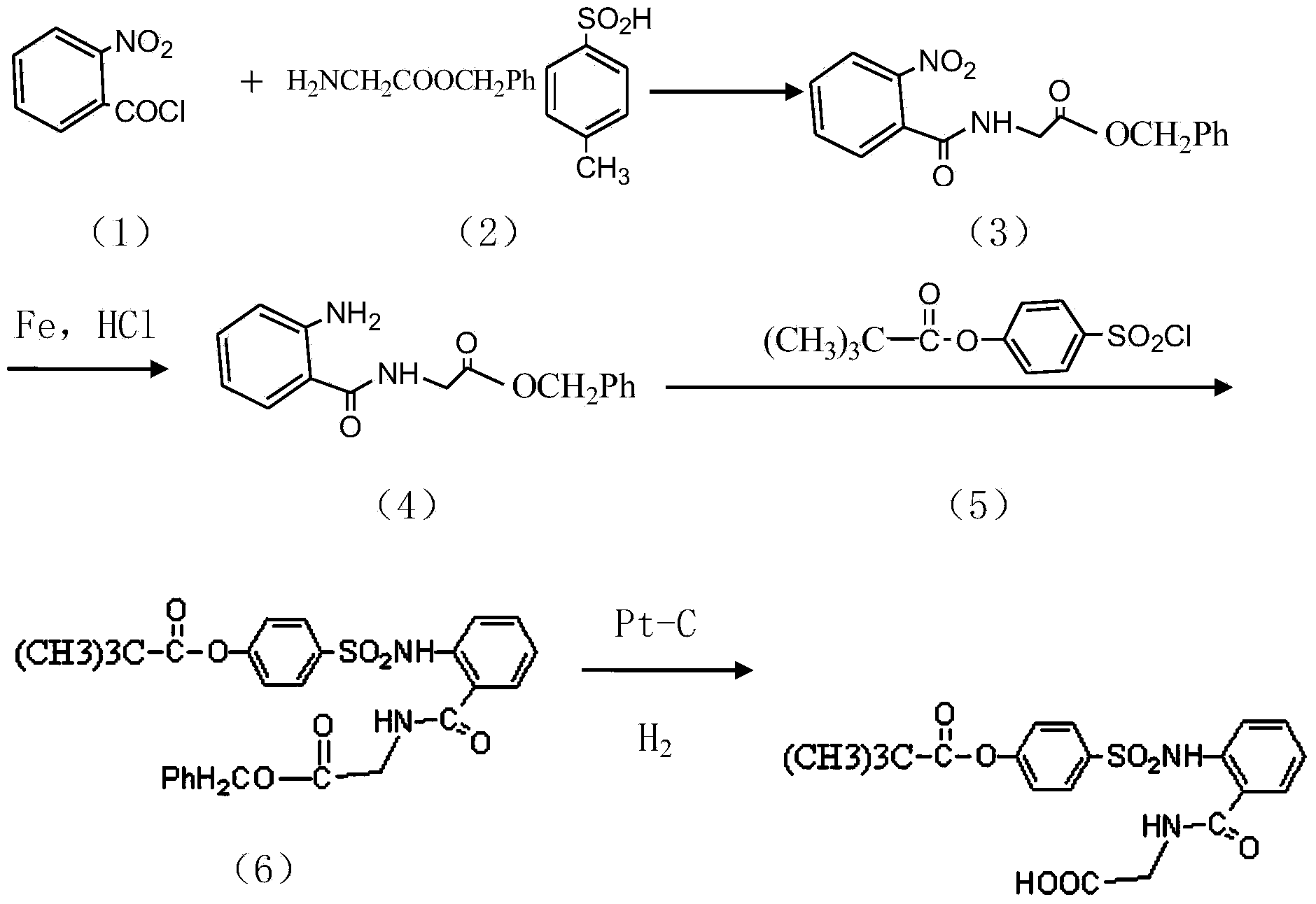
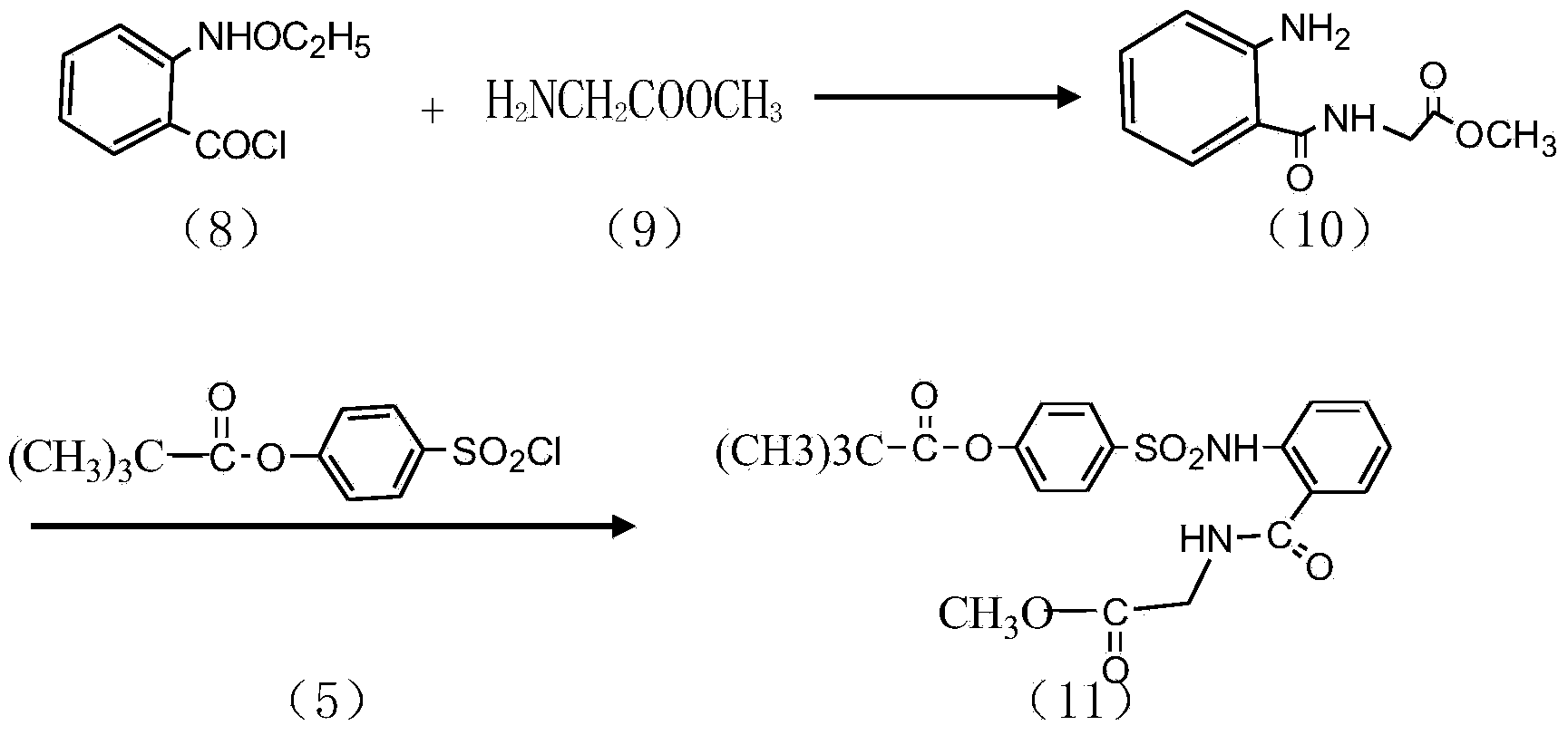
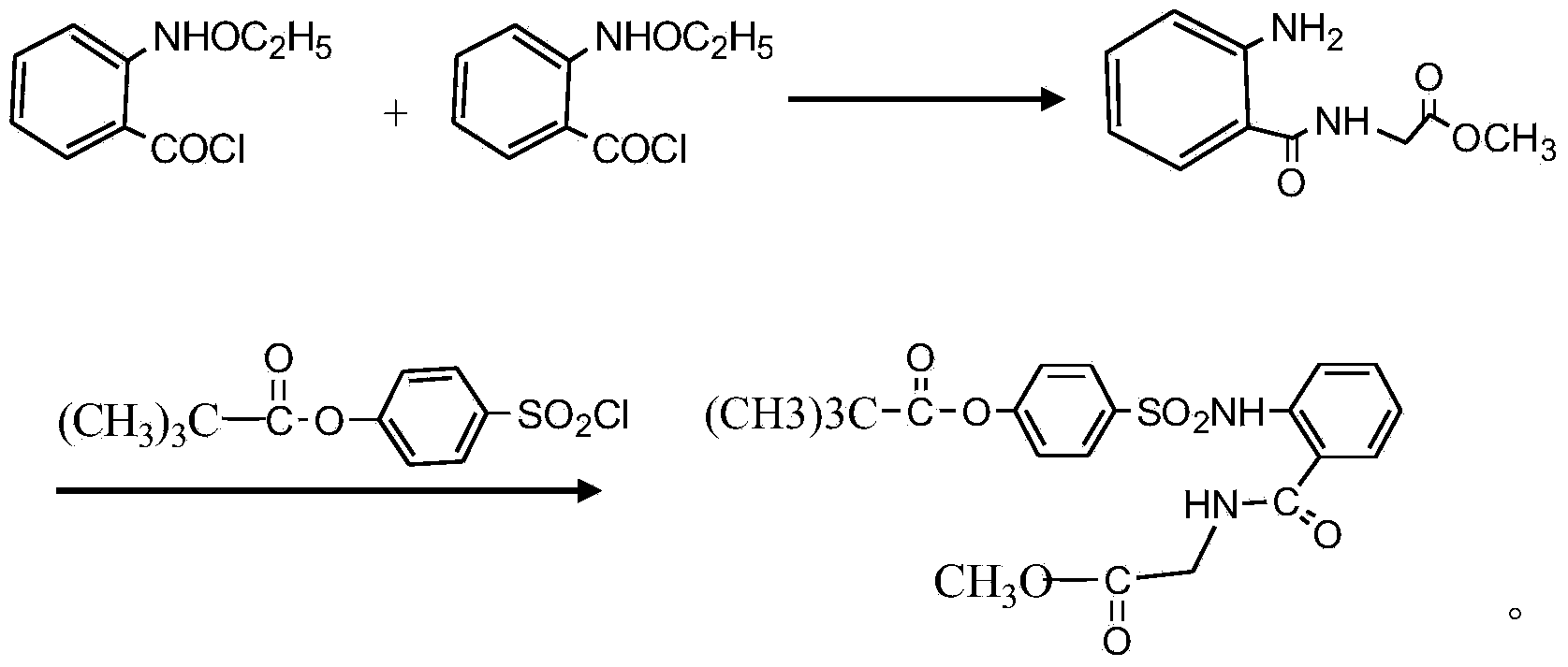
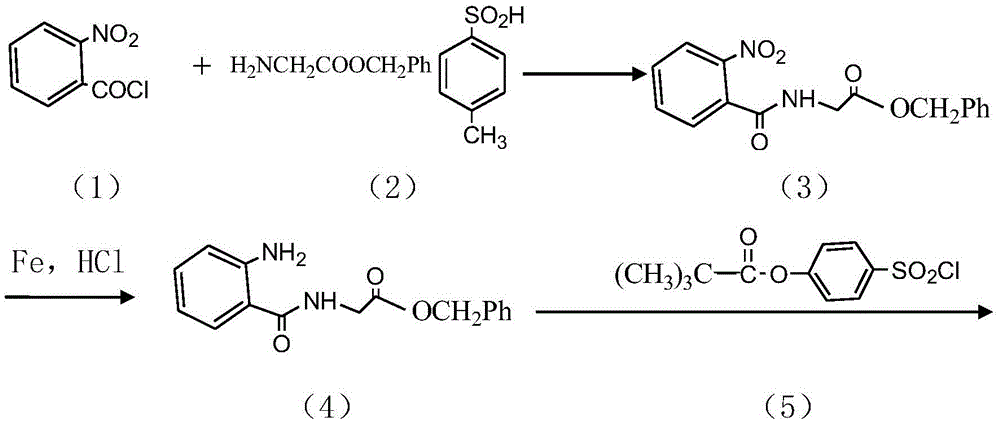
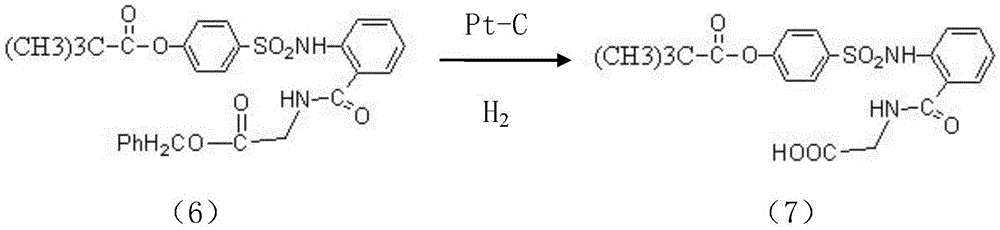
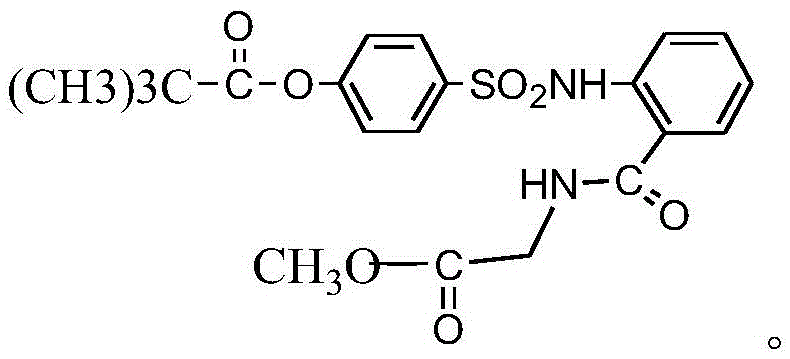
InactiveCN104045586ASuitable for mass productionShort synthetic routeSulfonic acid amide preparationOrganic layerBenzenesulfonyl chloride
The invention discloses a preparation method of a sivelestat sodium hydrate intermediate, comprising the following steps: successively pouring acyl chloride, a nonpolar organic solvent, an alkaline acid-binding agent, glycine methyl ester hydrochloride and an amide-type catalyst into a reaction vessel to react, pouring reaction products into dilute hydrochloric acid, standing and removing an organic layer, adjusting pH value of a water layer to neutral with diluted alkali, filtering, drying, recrystalizing, drying to obtain N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine, successively pouring N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine, 4-pivaloyloxybenzenesulfonyl choride, the nonpolar organic solvent, the alkaline acid-binding agent and the amide-type catalyst into the reaction vessel to react, and pouring reaction products into dilute hydrochloric acid to react so as to obtain the sivelestat sodium hydrate intermediate. By the preparation method of the sivelestat sodium hydrate intermediate, synthetic process route is greatly shortened. The preparation method is easy to operate; reaction conditions are mild; and industrial production safety coefficient is raised.
Owner:ANHUI HERYI CHEM
Preparation method of 1,2,3-bistriazole ligand and its application in cuaac reaction
ActiveCN105968116BObvious categoryExpand categoryEsterified saccharide compoundsSugar derivativesGlycine methyl ester hydrochlorideRoom temperature
The invention discloses a preparation method of 1,2,3-bis-triazole ligands and an application of the 1,2,3-bis-triazole ligands in a CuAAC reaction. With cheap and easily available glycine methyl ester hydrochloride as a base raw material, and through derivation and conversion of functional groups, a diyne compound with glycine as a structure is obtained; then under a condition of room temperature and with organic azide as a raw material and copper iodide as a catalyst, 1,2,3-bis-triazole seven-membered-ring compounds are synthesized; and with the bis-triazole compounds and derivatives thereof as substrates, a series of methods for synthesis of the 1,2,3-bis-triazole derivatives are developed. A variety of novel, highly efficient, simple and easily available ligand catalysts are provided for a click reaction, and the 1,2,3-bis-triazole ligands have obvious effect on accelerating the reaction when used as the ligands and have quite practical application.
Owner:HENAN NORMAL UNIV
New synthetic method of sivelestat sodium hydrate
ActiveCN104045587AEasy to recycleSuitable for mass productionSulfonic acid amide preparationOrganic layerBenzenesulfonyl chloride
The invention discloses a new synthetic method of sivelestat sodium hydrate, comprising the following steps: successively pouring acyl chloride, a nonpolar organic solvent, an alkaline acid-binding agent, glycine methyl ester hydrochloride and an amide-type catalyst into a reactor so as to react, pouring reaction products into diluted hydrochloric acid, removing an organic layer, adjusting pH of a water layer to neutral with diluted alkali, filtering, drying, recystalizing, drying to obtain N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine; successively pouring N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine, 4-pivaloyloxybenzenesulfonyl choride, the nonpolar organic solvent, the alkaline acid-binding agent and the amide-type catalyst into a reaction vessel to react, pouring reaction products into diluted hydrochloric acid, standing and removing an organic layer, pouring sodium hydroxide into a water layer, cooling reaction products after the end of the reaction, filtering out crystals, dissolving and decolouring to obtain sivelestat sodium hydrate. By the new synthetic method of sivelestat sodium hydrate, synthetic process route is greatly shortened. The synthetic method is easy to operate. Reaction conditions are mild, and industrial production safety coefficient is raised.
Owner:ANHUI HERYI CHEM
Continuous synthesis method of glycine methyl ester hydrochloride
InactiveCN110003027ARealize continuous synthesisShort reaction cycleOrganic compound preparationOrganic chemistry methodsSynthesis methodsMixed materials
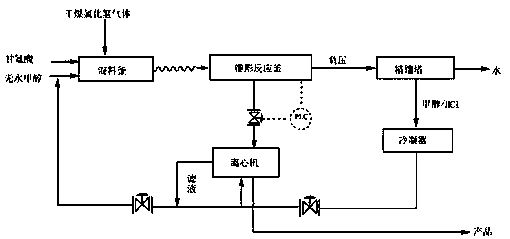
A continuous synthesis method of glycine methyl ester hydrochloride specifically includes the steps that glycine and anhydrous methanol are added into a mixing kettle, and under the stirring condition, hydrogen chloride gas is introduced until the glycine is completely dissolved; the uniformly mixed material is continuously pumped into a reaction kettle, the temperature of a reaction system is maintained to be 55-60 DEG C, a seed crystal heat preservation reaction is added, the stirring speed is controlled, and continuously separated crystals are deposited at the bottom of the reaction kettle;then the crystals are subjected to centrifugation, washing and drying to obtain white crystal powder, and centrifugal mother liquor is pumped into the mixing kettle for cyclic utilization; while thecontinuous feeding reaction is carried out, a negative pressure system of 0.02-0.04 MPa is built in the reaction kettle, and water generated by the reaction is discharged by excessive methanol in thesystem during the reaction. According to the method, the process route is simple, the utilization rate of the reaction equipment is high, the product yield can reach 93% or above, the purity is higherthan 98%, no waste is generated, and automatic industrial production is easy to realize.
Owner:SHANDONG TAIHE WATER TREATMENT TECH CO LTD
A kind of synthetic method of sivelestat sodium
ActiveCN104045587BEasy to recycleSuitable for mass productionSulfonic acid amide preparationSynthesis methodsOrganic layer
The invention discloses a new synthetic method of sivelestat sodium hydrate, comprising the following steps: successively pouring acyl chloride, a nonpolar organic solvent, an alkaline acid-binding agent, glycine methyl ester hydrochloride and an amide-type catalyst into a reactor so as to react, pouring reaction products into diluted hydrochloric acid, removing an organic layer, adjusting pH of a water layer to neutral with diluted alkali, filtering, drying, recystalizing, drying to obtain N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine; successively pouring N-benzoyloxycarbonylmethyl-2-aminobenzamideglycine, 4-pivaloyloxybenzenesulfonyl choride, the nonpolar organic solvent, the alkaline acid-binding agent and the amide-type catalyst into a reaction vessel to react, pouring reaction products into diluted hydrochloric acid, standing and removing an organic layer, pouring sodium hydroxide into a water layer, cooling reaction products after the end of the reaction, filtering out crystals, dissolving and decolouring to obtain sivelestat sodium hydrate. By the new synthetic method of sivelestat sodium hydrate, synthetic process route is greatly shortened. The synthetic method is easy to operate. Reaction conditions are mild, and industrial production safety coefficient is raised.
Owner:ANHUI HERYI CHEM
Synthesis method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride
InactiveCN108424376AReduce usageHigh reaction yieldCarbamic acid derivatives preparationOrganic compound preparationEthylenediaminePGI2 methyl ester
The invention provides a synthesis method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride. The preparation method is as follows: firstly, reacting ethylenediamine with haloacetic acid, andthen, carrying out recrystallization by using dimethyl sulfoxide to obtain N-(2-aminoethyl) glycine; secondly, mixing N-(2-aminoethyl) glycine with methanol, carrying out a reaction under the participation of thionyl chloride to prepare N-(2-aminoethyl) glycine methyl ester; and thirdly, mixing N-(2-aminoethyl) glycine methyl ester with an organic solvent and alkali, slowly dropwise adding Fmoc-osu, and adding a hydrochloric acid solution after ending the reaction to obtain N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride. By using the synthesis method of N-(2-Fmoc-aminoethyl) glycinemethyl ester hydrochloride, provided by the invention, the yield of the product can be greatly increased and reaches up to 81% or above, and the purity of the product reaches up to 98%.
Owner:苏州凌科特新材料有限公司
A kind of aromatic ring substituted spiro ring indole diketopiperazine compound and its synthetic method
ActiveCN106478645BGuaranteed singularityHigh conformational specificityOrganic chemistryBulk chemical productionBenzaldehydeCycloaddition
The invention discloses an aromatic substitution spiro indolyl diketopiperazine compound and a synthesis method thereof. Glycine is used as a raw material and subjected to esterification reaction with methyl alcohol and thionyl chloride to obtain methyl glycinate hydrochloride, methyl glycinate hydrochloride and aromatic aldehyde are subjected to condensation reaction to obtain Schiff base, isatin is used as a raw material and subjected to reduction reaction under the action of hydrazine hydrate to obtain indoline-2-ketone, indoline-2-ketone and benzaldehyde are subjected to Knoevenagel reaction under piperidine catalysis to obtain 3-phenylidene-1,3-dihydro-2H-indol-2 ketone, 3-phenylidene-1,3-dihydro-2H-indol-2 ketone and Schiff base are subjected to 1,3-dipole cycloaddition reaction under catalysis of chiral ligand (S)-TF-BiphamPhos / AgoAc to obtain spiro pyrrolidine, and protecting group removal and ring closure are carried out on spiro pyrrolidine and N-(9-fluorene methoxycarbonyl)-L-prolyl chloride through base catalysis to obtain the target product. The method has the advantages of being simple in path, high in yield, high in diastereoselectivity and single in product spatial configuration, and the raw materials are low in price and easy to obtain.
Owner:SHAANXI UNIV OF SCI & TECH
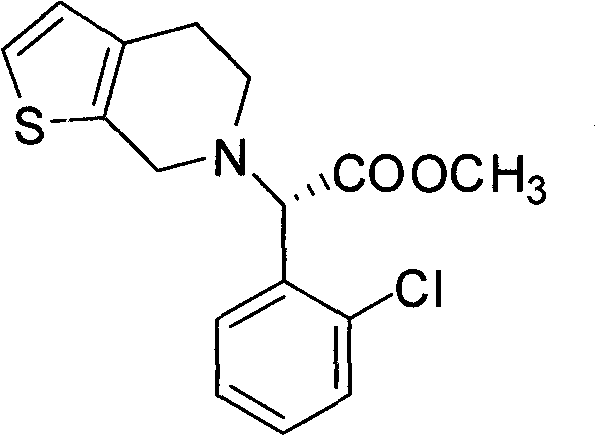
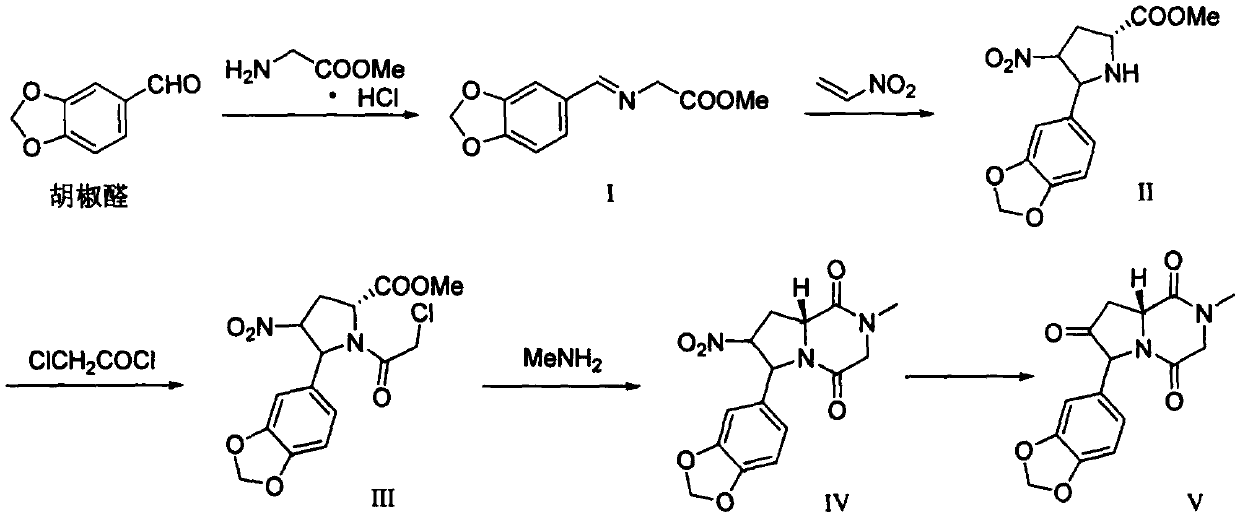
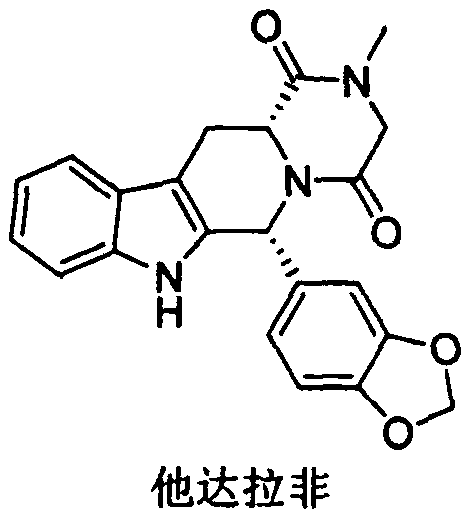
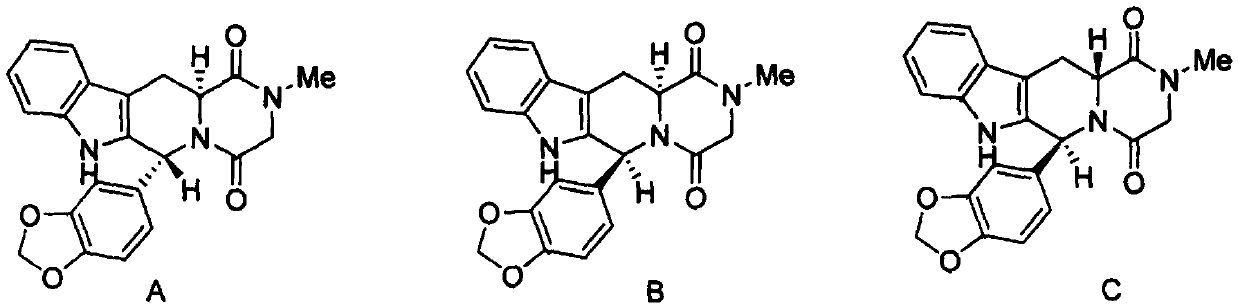
A kind of preparation method of tadalafil related substance f
Owner:迪嘉药业集团股份有限公司
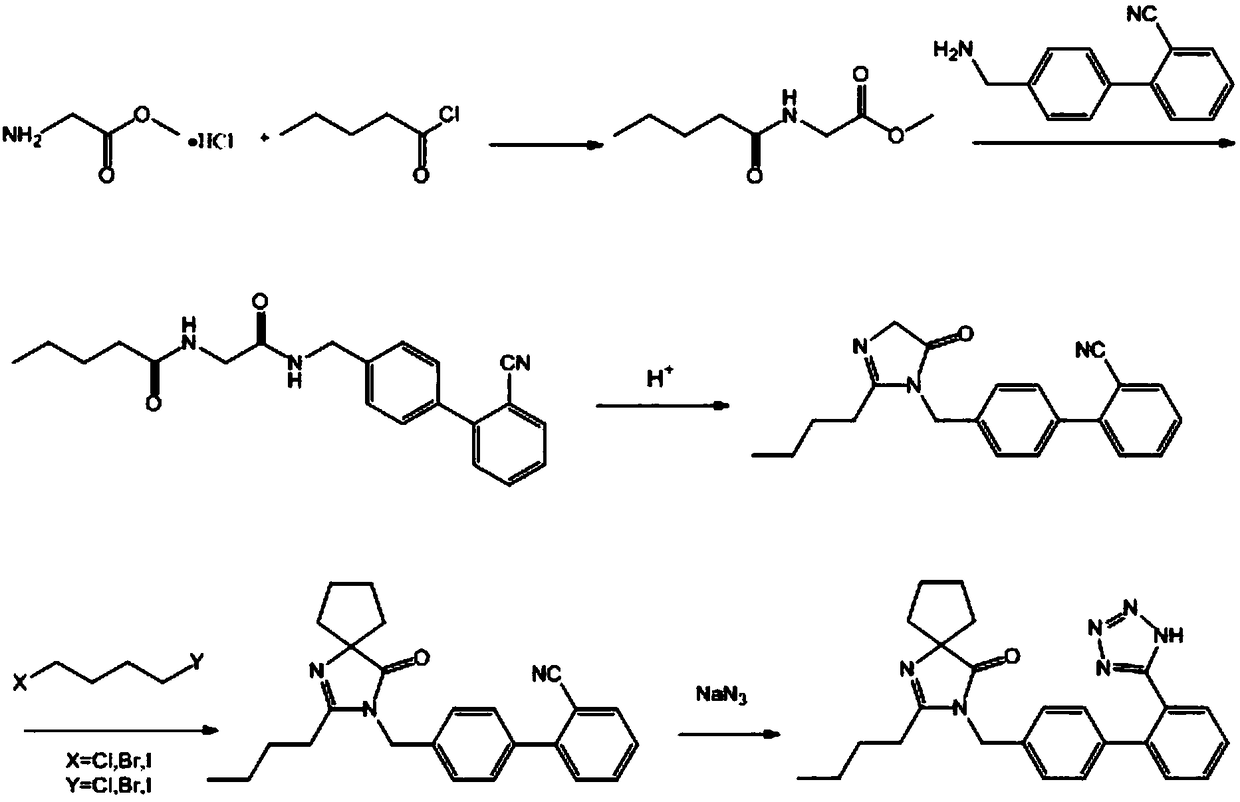
Synthetic method of irbesartan
ActiveCN108276389AEasy to getEasy to operateOrganic chemistryGlycine methyl ester hydrochloridePotassium cyanide
The invention discloses a synthetic method of irbesartan. The method comprises the following steps: by using glycine methyl ester hydrochloride as an initial raw material, performing the acylation reaction on the glycine methyl ester hydrochloride and valeryl chloride to obtain N-pentanoyl glycine methyl ester, performing the aminolysis reaction of ester on the N-pentanoyl glycine methyl ester and4-aminomethyl-2'-cyanobiphenyl, thus obtaining a bisamide intermediate, condensing the bisamide intermediateunder the catalysis of acid to establish a five-membered ring, reacting with 1,4-dihalogenated butane in the presence of alkali to obtain irbesartan hydrocarbon, and finally performing the cyclization reaction with sodium azide to obtain irbesartan. According to the synthetic method of theirbesartan, the glycine methyl ester hydrochloride is first used as the initial raw material, so that the use of sodium cyanide or potassium cyanide in an existing production process is avoided, and the production operation is safer and more convenient. The novel synthetic method has the advantages that the raw materials are low in price and easy to obtain, no toxic cyanide is used, the environment friendliness is achieved, the cost is low and the like, and is suitable for the industrialized application.
Owner:山东大医精诚药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com