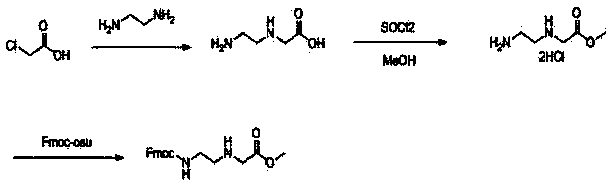
Synthesis method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride
The technology of a glycine methyl ester and a synthetic method is applied in the synthesis field of N-glycine methyl ester hydrochloride, and can solve the problems of high cost and low yield, and achieve the effects of reducing usage, reducing cost and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A kind of synthetic method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride, comprises the following steps:
[0021] The first step: at room temperature, add chloroacetic acid (800g, 8.47 mol) to ethylenediamine (5.5 L ) in batches within 3 hours, stir overnight after completion, and remove excess ethylenediamine by distillation under reduced pressure with an oil pump to obtain light Yellow viscous oil, dimethyl sulfoxide was added overnight, a white solid was precipitated, filtered, washed twice, and dried to obtain 700g of crude product N-(2-aminoethyl)glycine, yield: 54.5%, measured by TLC Purity is 60%, directly used in the next step;
[0022] Step 2: Suspend N-(2-aminoethyl)glycine in methanol (1.5L), cool to 0°C, add thionyl chloride (230mL, 3.2mol) dropwise, heat to reflux for 4h after completion, and use a water pump The solvent was removed under reduced pressure to obtain 84 g of white product N-(2-aminoethyl) glycine methyl ester, yield: 100%, whic...
Embodiment 2
[0025] A kind of synthetic method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride, comprises the following steps:
[0026] The first step: at room temperature, add chloroacetic acid (800g, 8.47 mol) to ethylenediamine (5.5 L ) in batches within 3 hours, stir overnight after completion, and remove excess ethylenediamine by distillation under reduced pressure with an oil pump to obtain light Yellow viscous oil, dimethyl sulfoxide was added overnight, a white solid was precipitated, filtered, washed twice, and dried to obtain 700g of crude product N-(2-aminoethyl)glycine, yield: 54.5%, measured by TLC Purity is 60%, directly used in the next step;
[0027] Step 2: Suspend N-(2-aminoethyl)glycine in methanol (1.5L), cool to 0°C, add thionyl chloride (230mL, 3.2mol) dropwise, heat to reflux for 4h after completion, and use a water pump The solvent was removed under reduced pressure to obtain 84 g of white product N-(2-aminoethyl) glycine methyl ester, yield: 100%, whic...
Embodiment 3
[0030] A kind of synthetic method of N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride, comprises the following steps:
[0031] The first step: at room temperature, add chloroacetic acid (800g, 8.47 mol) to ethylenediamine (5.5 L ) in batches within 3 hours, stir overnight after completion, and remove excess ethylenediamine by distillation under reduced pressure with an oil pump to obtain light Yellow viscous oil, dimethyl sulfoxide was added overnight, a white solid was precipitated, filtered, washed twice, and dried to obtain 700g of crude product N-(2-aminoethyl)glycine, yield: 54.5%, measured by TLC Purity is 60%, directly used in the next step;
[0032] Step 2: Suspend N-(2-aminoethyl)glycine in methanol (1.5L), cool to 0°C, add thionyl chloride (230mL, 3.2mol) dropwise, heat to reflux for 4h after completion, and use a water pump The solvent was removed under reduced pressure to obtain 84 g of white product N-(2-aminoethyl) glycine methyl ester, yield: 100%, whic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com