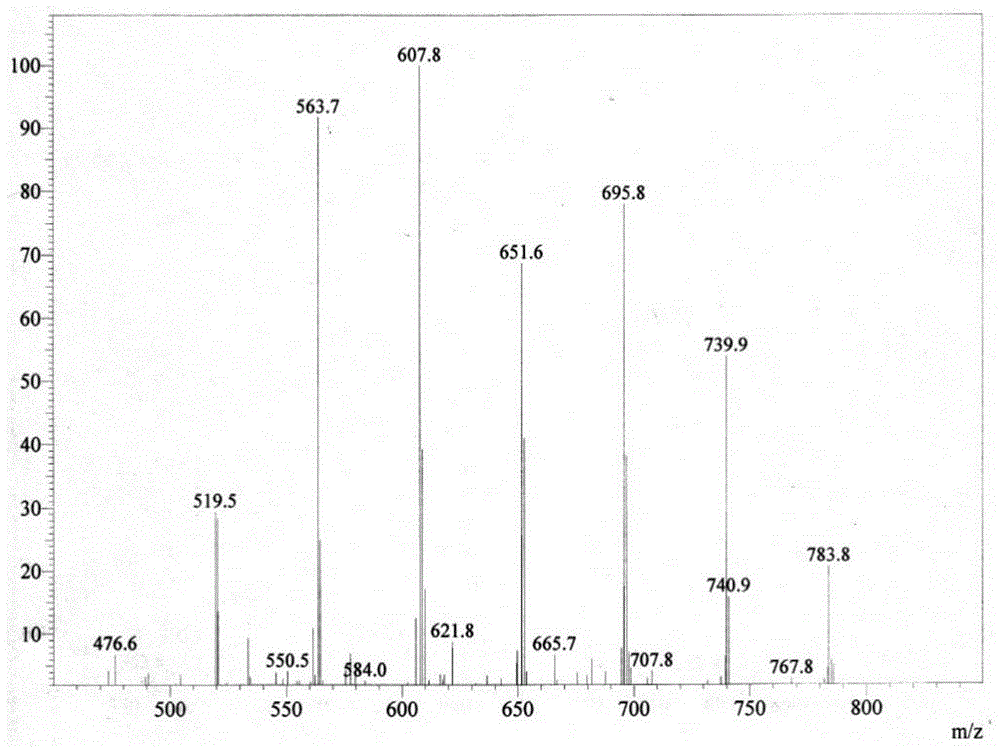
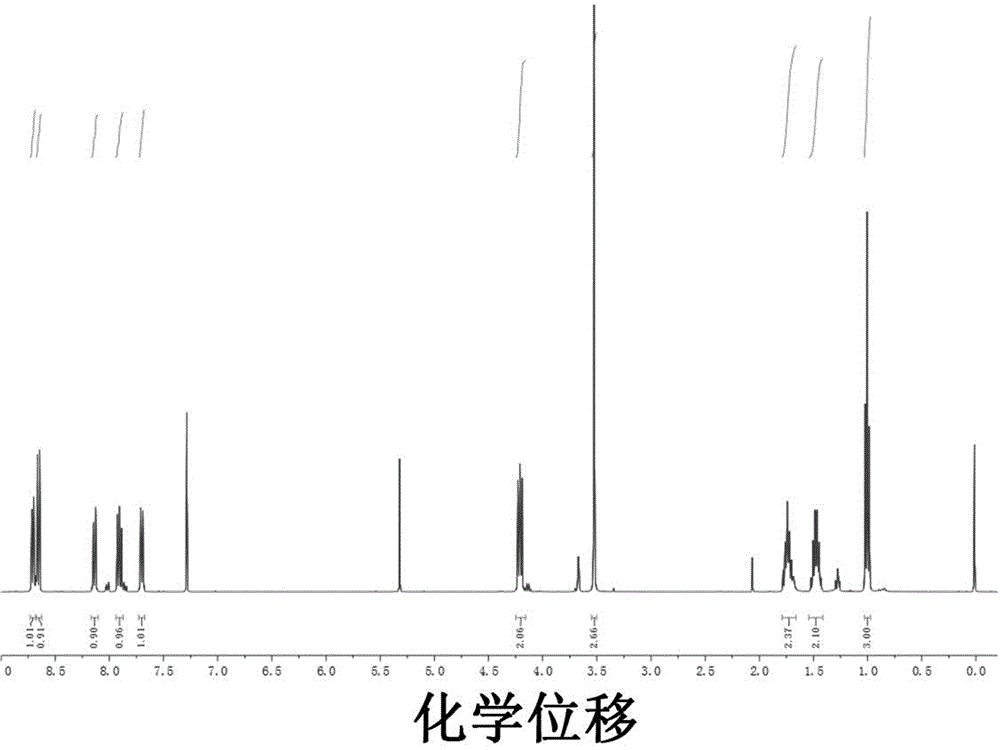
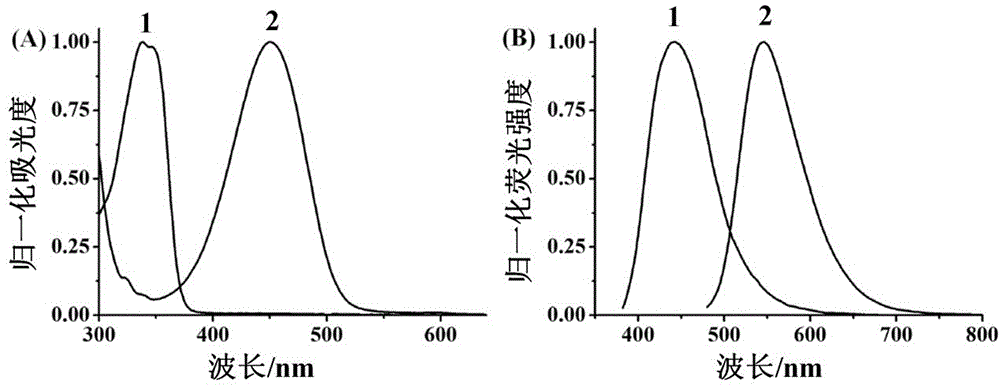
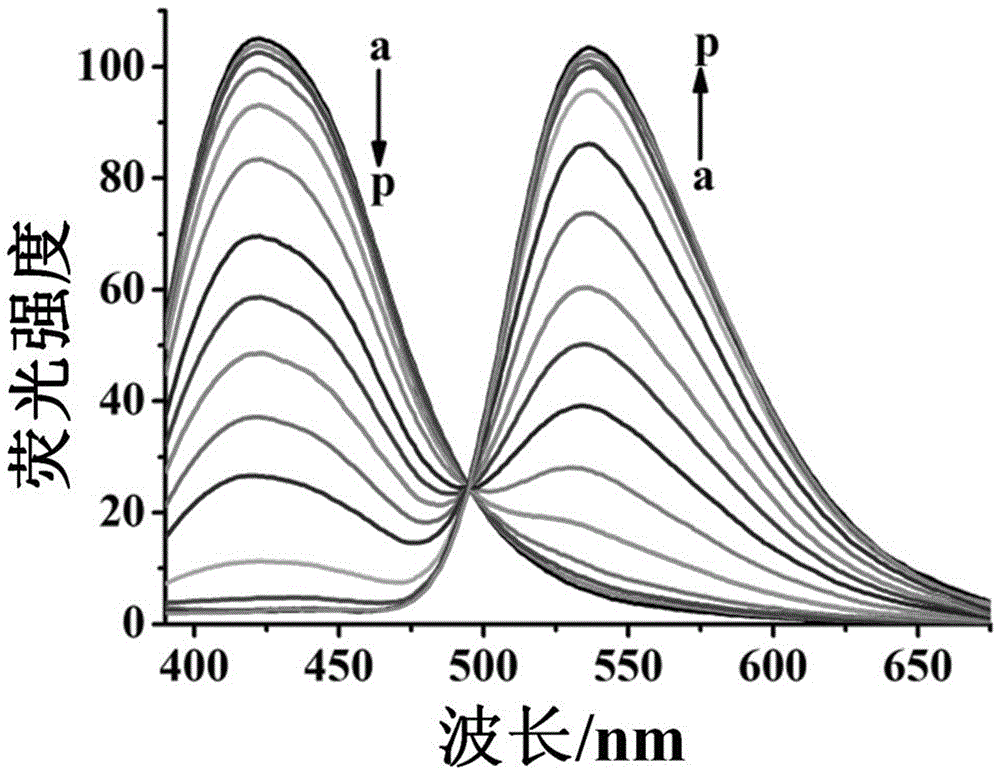
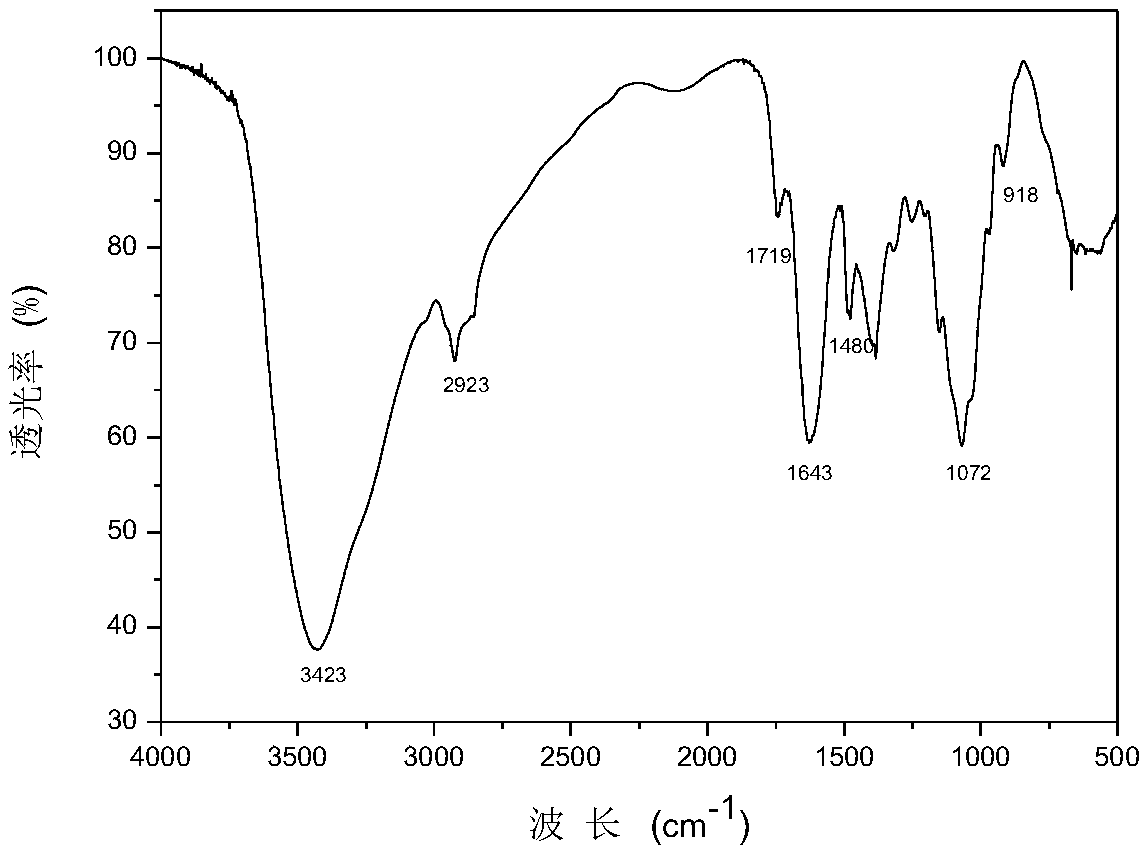
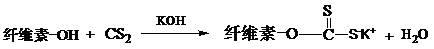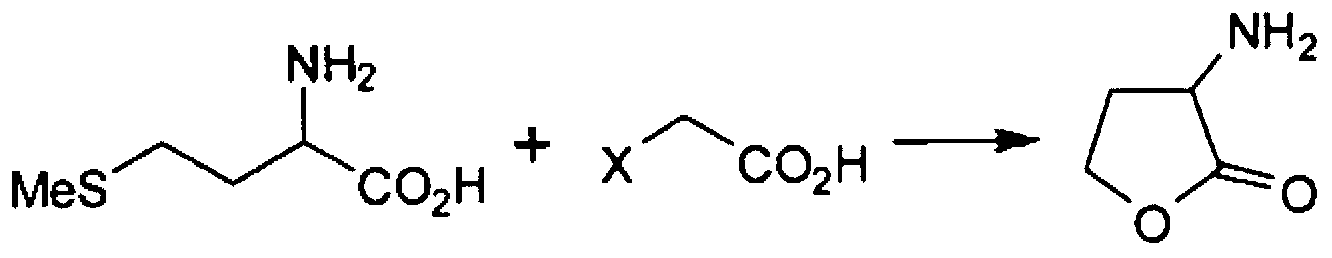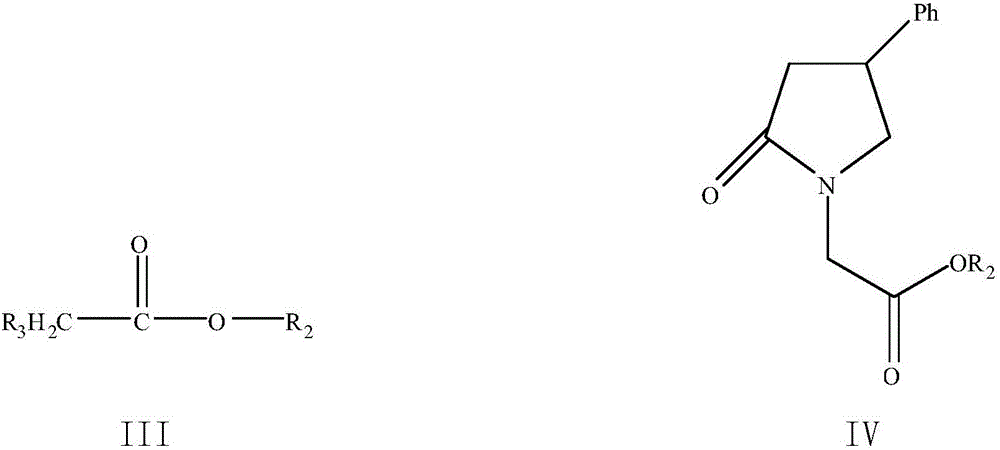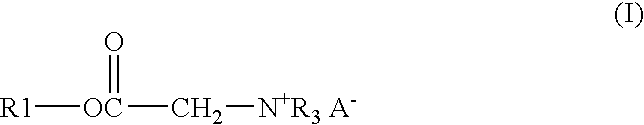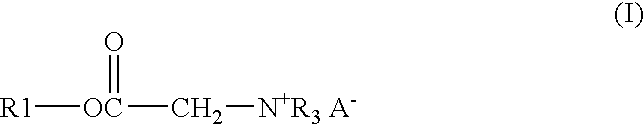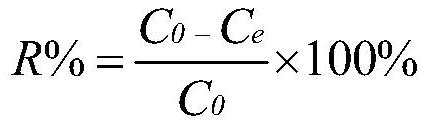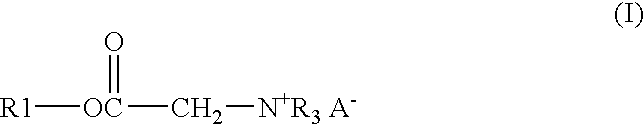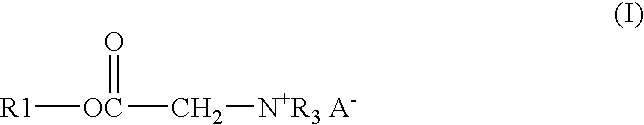Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
109 results about "Haloacetic acids" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Haloacetic acids are carboxylic acids in which a halogen atom takes the place of a hydrogen atom in acetic acid. Thus, in a monohaloacetic acid, a single halogen would replace a hydrogen atom. For example, chloroacetic acid would have the structural formula CH₂ClCO₂H. In the same manner, in dichloroacetic acid two chlorine atoms would take the place of two hydrogen atoms (CHCl₂CO₂H). The inductive effect caused by the electronegative halogens often result in the higher acidity of these compounds by stabilising the negative charge of the conjugate base.
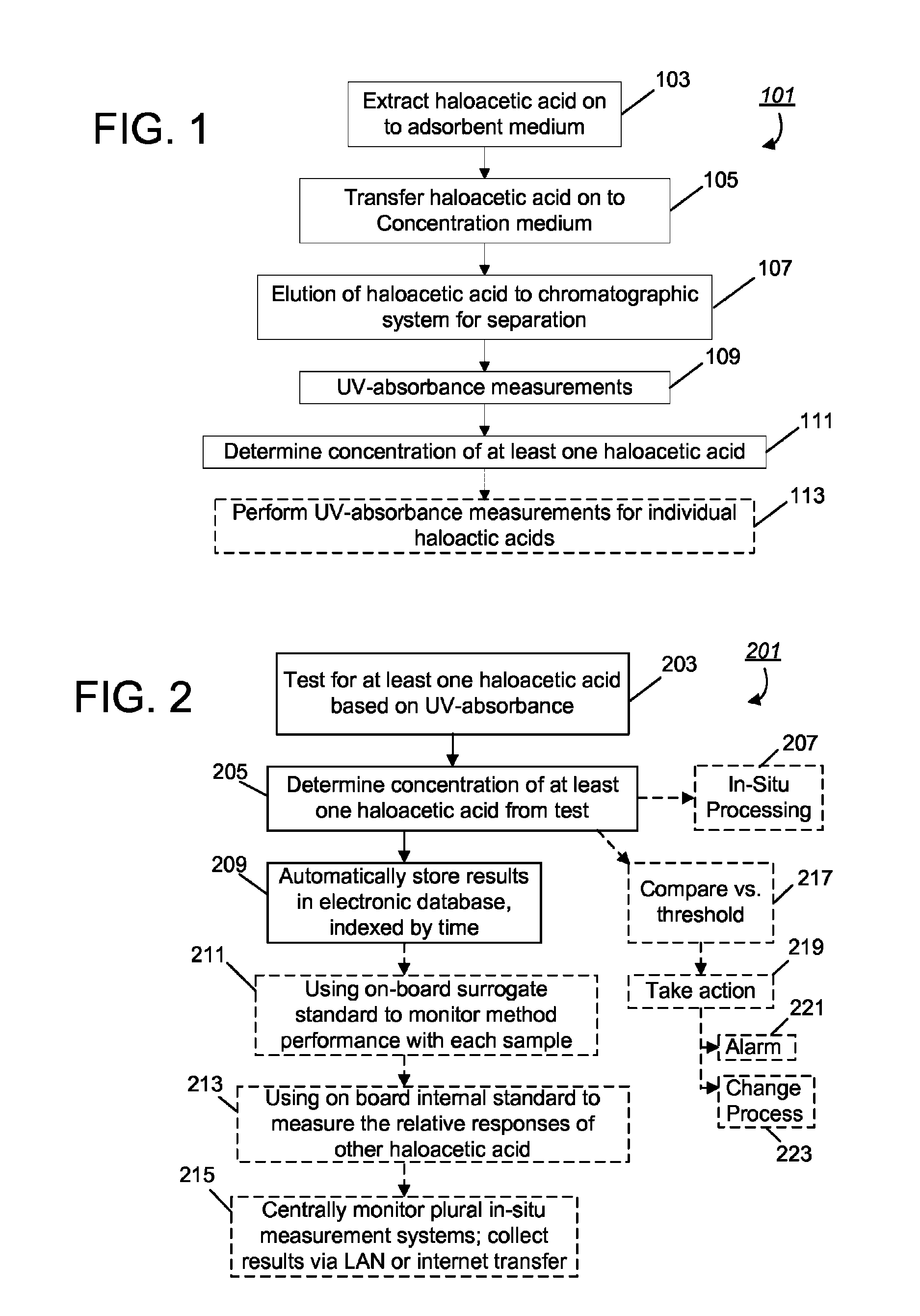
Method for quickly detecting haloacetic acids serving as disinfection byproducts in drinking water
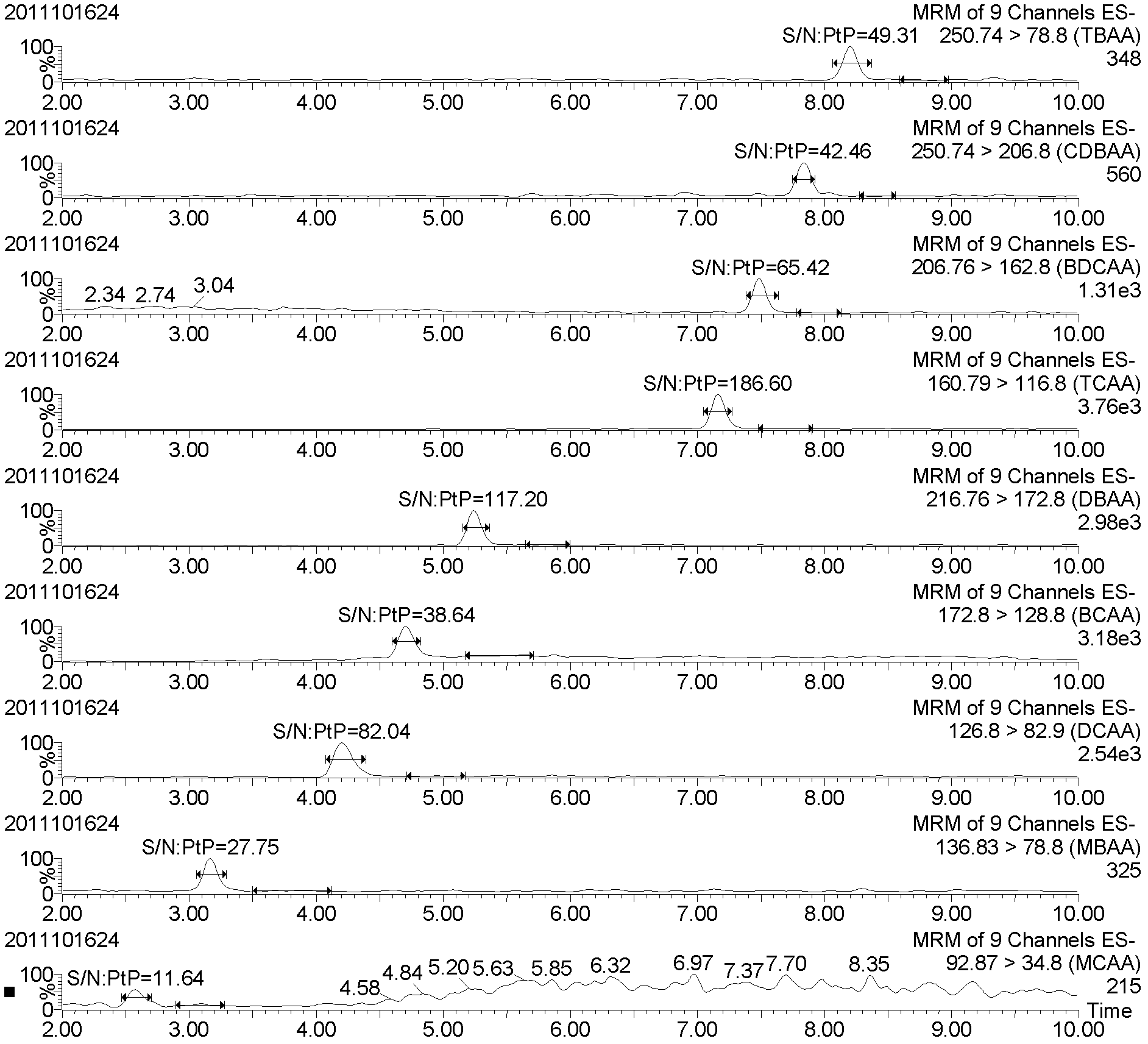
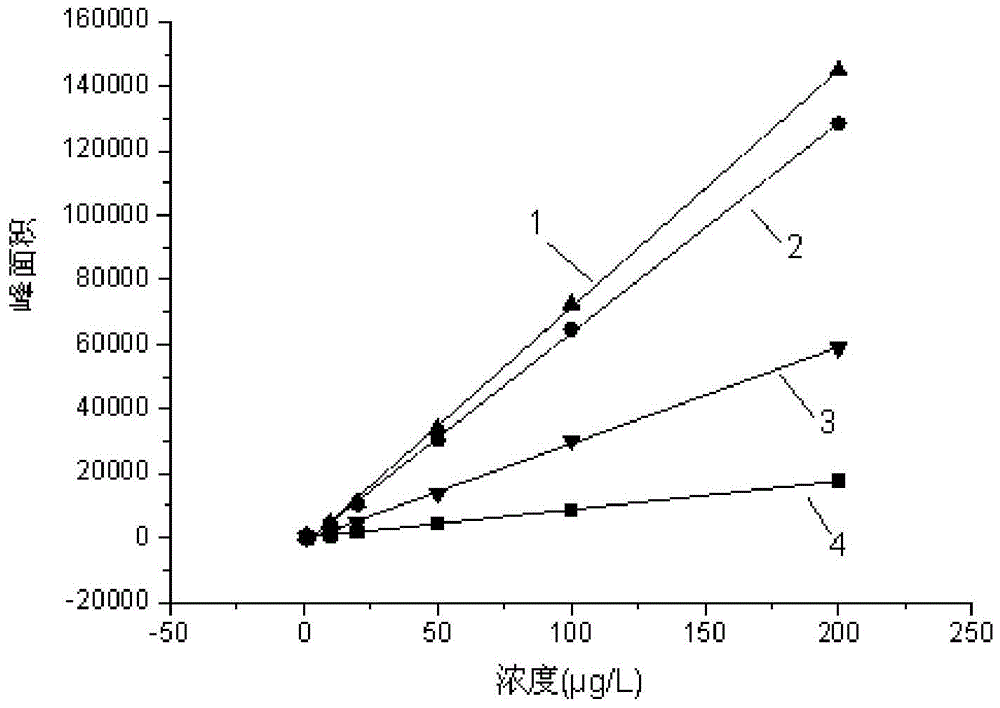
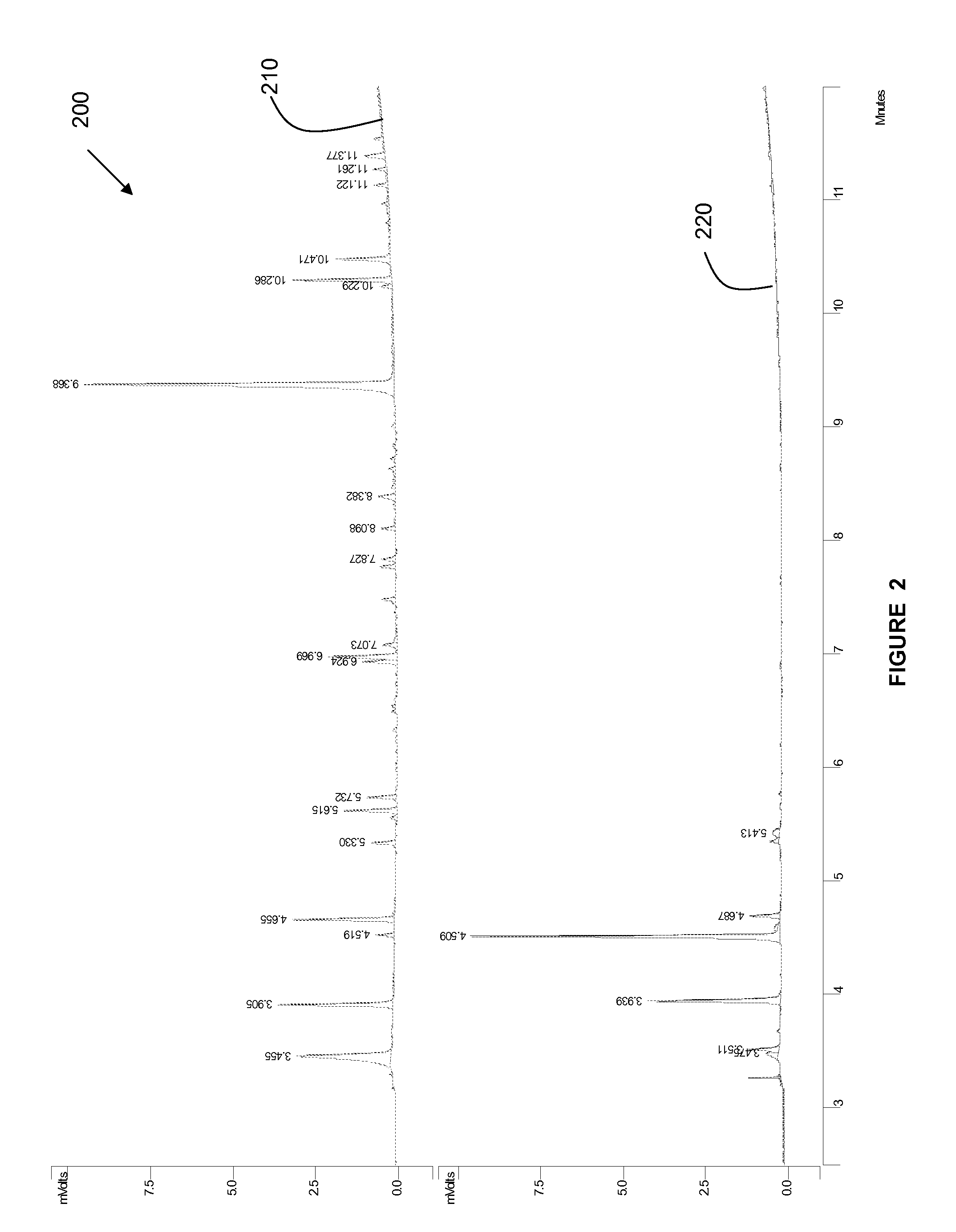
The invention discloses a method for quickly detecting haloacetic acids serving as disinfection byproducts in drinking water. Chromatographic methanol and super pure water containing methanoic acid with volume ratio of between 0.005 and 0.0001 percent are used as flow phases; corresponding mass spectrum conditions and chromatograph conditions are set by using an ultra performance liquid chromatogram-electrospray ionization-series four-level-rod mass spectrometer (UPLC-ESI-MS / MS) and an UPLC hierarchical service system (HSS) T3 chromatographic column (the column length* the inner diameter= 100mm* 2.1 mm) in the Waters company; a standard curve is constructed; and the PH of a sample is adjusted to be 2.0 to 3.0, so that a matrix effect is eliminated. According to the method, nine haloacetic acids can be totally separately measured within 13.5 minutes; the method has the advantages of high sensitivity, precision, accuracy and the like, and toxic chemical residual substance cannot be produced; and the method is applied to quickly detecting the haloacetic acids in the drinking water.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Method for preparing water-retention fertilizer from aquatic plants
ActiveCN101962308ASimple structurePrevent compaction degradationFertilizer mixturesCellulosePotamogeton crispus
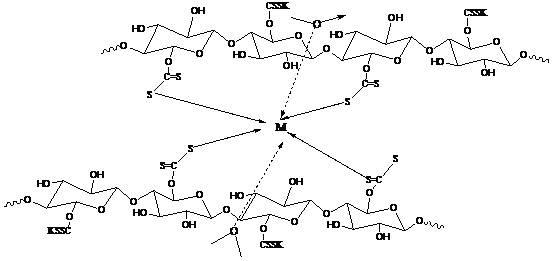
The invention relates to a method for preparing water-retention fertilizer from aquatic plants, comprising the followings steps: using aquatic plants of water hyacinth, Potamogeton crispus L, and alternanthera philoxeroides as raw materials, using haloacetic acid and potassium salt thereof or carbon disulfide in alkaline solution containing potassium ion as chemical modifier, and using FeCl3 or AlCl3 as cross-linking agent so as to obtain aquatic plant fiber with water absorption and water retention property. In the invention, a large amount of aquatic plant fiber generated by ecosystem restoration of water and pollution prevention engineering is used so as to ensure long time operation of ecosystem restoration engineering. The water-retention fertilizer prepared by the invention can regulate water in soil, supply potassium fertilizer and nitrogen fertilizer in soil after applying since containing the potassium ion, ammonia water, and urea. The natural aquatic plant fiber is used as basis material to prepare water-retention fertilizer which can be completely biodegraded, can improve soil structure and can prevent hardening of tilled land.
Owner:溧阳常大技术转移中心有限公司
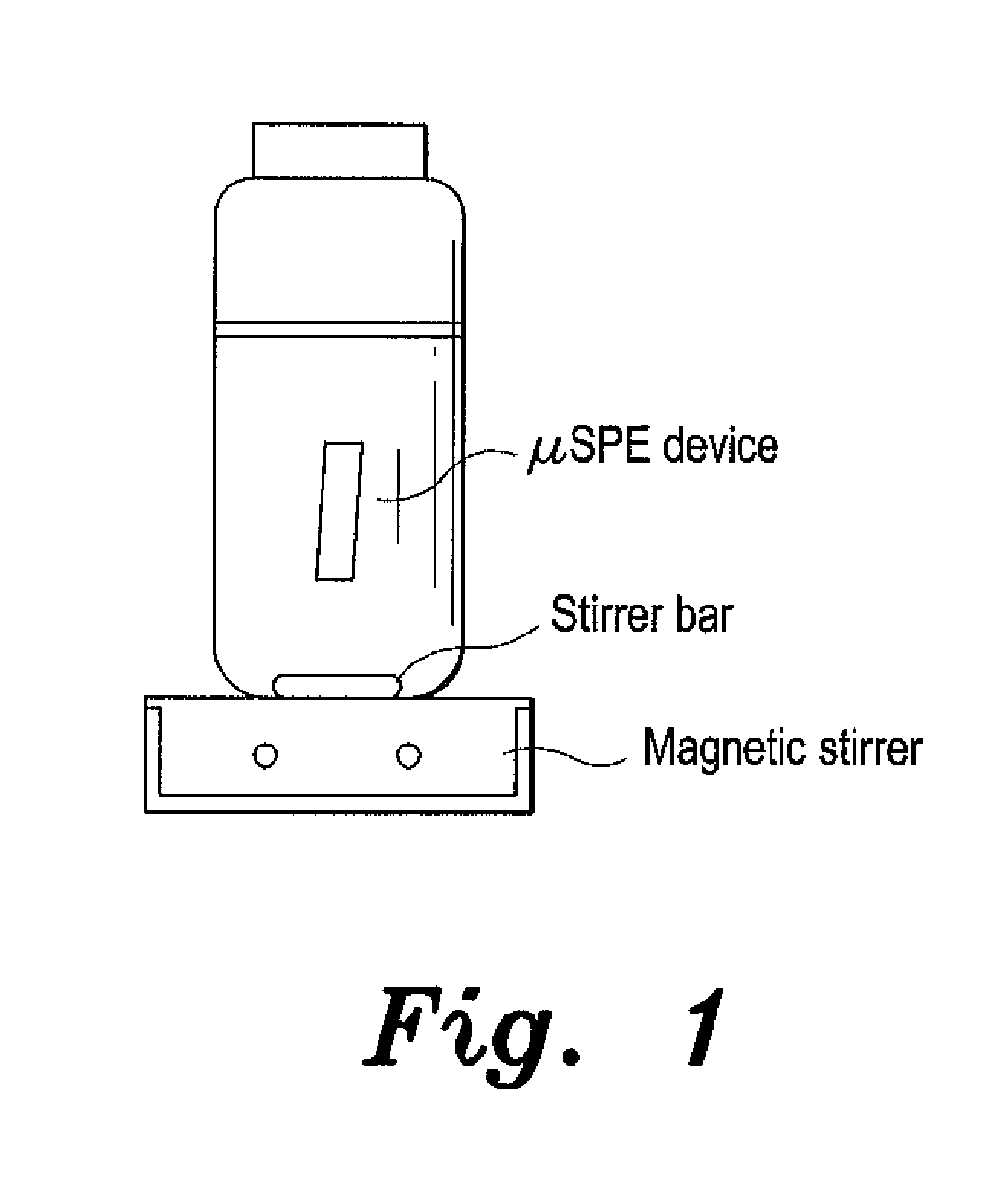
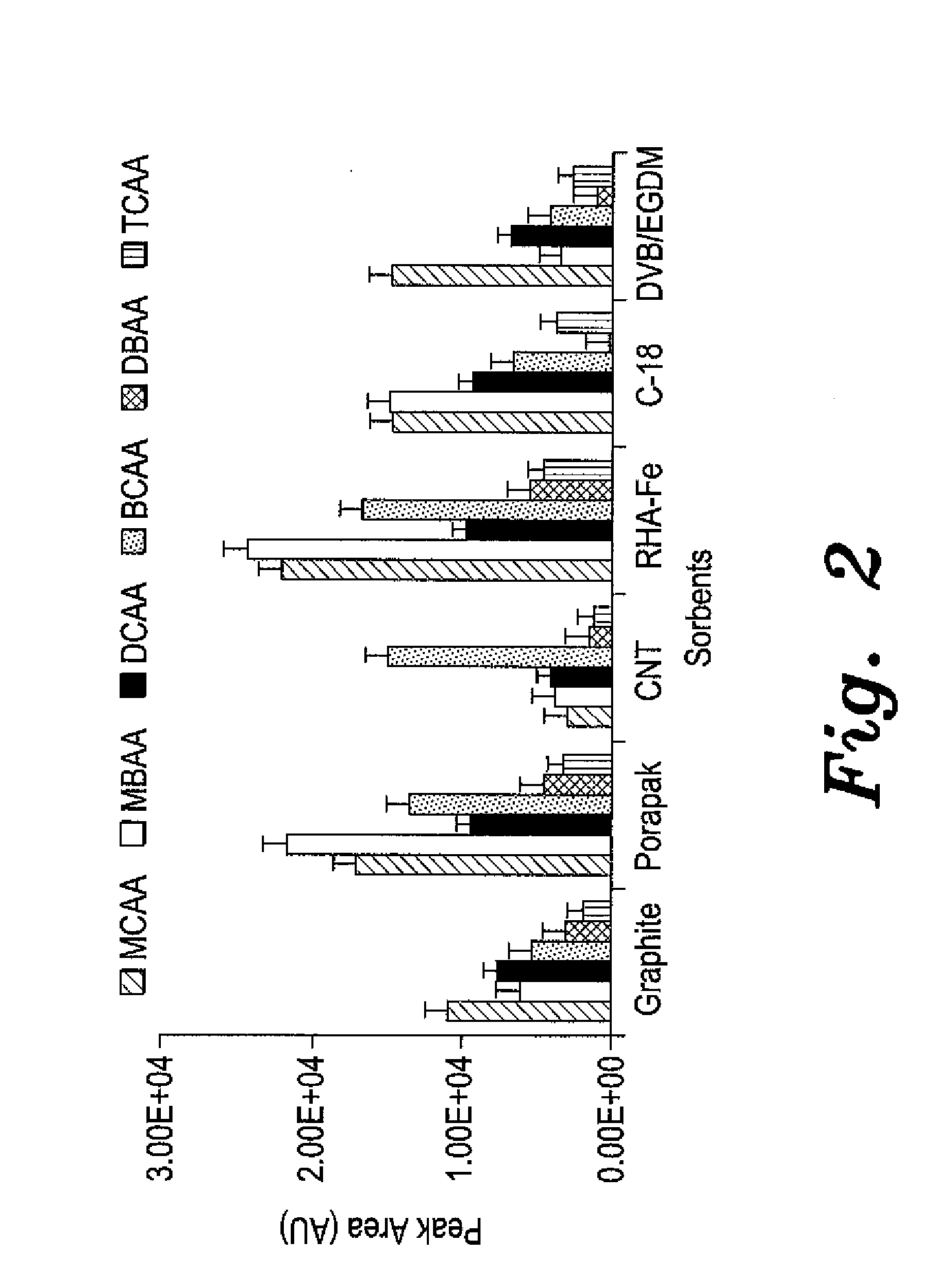
Micro-solid phase extraction of haloacetic acids
ActiveUS20150068291A1Sufficient supplyReadily apparentSamplingComponent separationSorbentSolid phase extraction
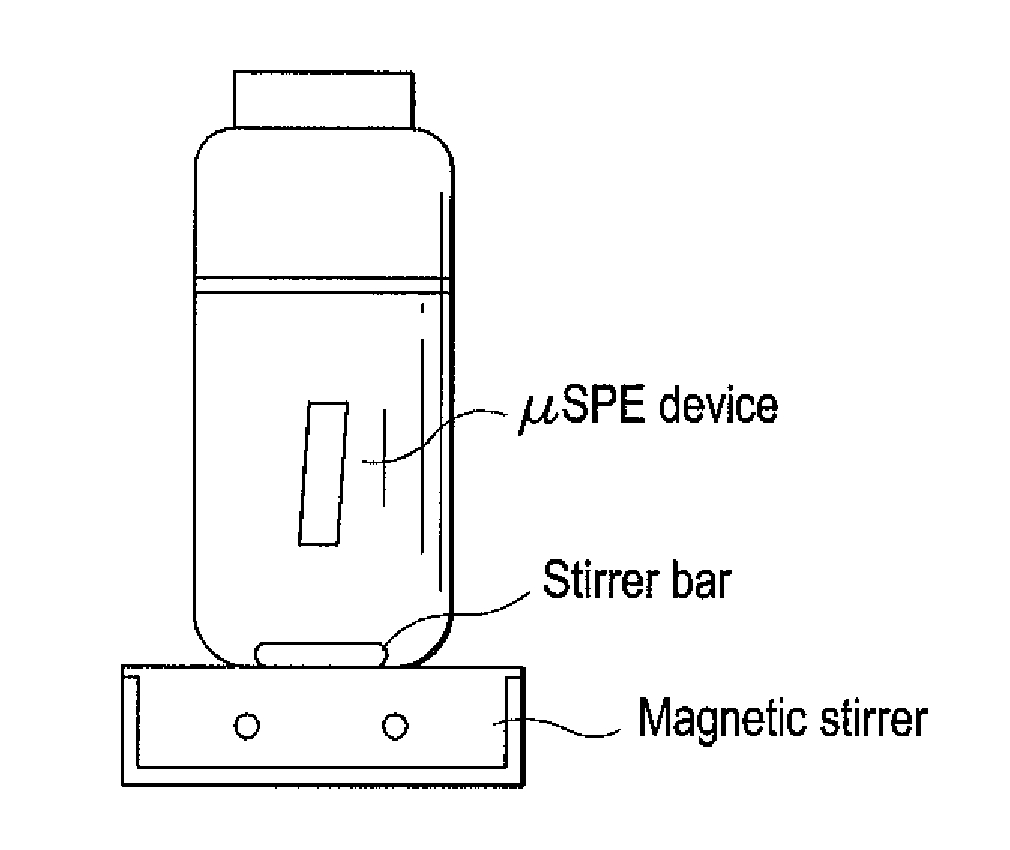
The micro-solid phase extraction of haloacetic acids is a procedure that extracts haloacetic acids from aqueous solution using iron-modified rice husk silica as the stationary phase or sorbent. Rice husks provide an excellent source of silica. The sorbent is prepared by incinerating the husks to produce a powder that is treated with 1.0 M nitric acid for 24 hours to produce rice husk silica. The silica is washed with base, cetyltrimethylammonium bromide is added, and then titrated with a 10% Fe3+ solution to pH 5, which forms a gel. The gel is aged, filtered, dried, and calcined to produce a nitrate-free iron-modified rice husk sorbent. The sorbent is then packaged in porous, heat-sealed polypropylene membrane envelopes and used for extraction of HAAs from water. The HAA analytes are desorbed by ultrasonication in methanol for analysis and quantification.
Owner:KING FAHD UNIVERSITY OF PETROLEUM AND MINERALS +1
Method for synchronously determining content of trihalomethane and haloacetic acid in drinking water
The invention discloses a method for synchronously determining the content of trihalomethane and haloacetic acid in drinking water, relates to a method for determining the content of trihalomethane and haloacetic acid in the drinking water, and aims at solving the problems that in the prior art, synchronous pretreatment cannot be performed on trihalomethane and haloacetic acid in the drinking water, and time and labor are consumed and the cost is high when trihalomethane and haloacetic acid in the drinking water are synchronously determined and separately determined. The method comprises the steps that 1, a water sample to be determined is acidized; 2, the polarity of the water sample to be determined is enhanced; 3, extracting is performed; 4, deriving is performed; 5, neutralizing is performed; 6, the peak area of trihalomethane in the water sample to be determined is determined; 7, the peak area of haloacetic acid in the water sample to be determined is determined; 8, a standard curve is drawn; 9, the concentration of trihalomethane in the water sample to be determined is calculated; 10, the concentration of haloacetic acid in the water sample to be determined is calculated. The method for synchronously determining the content of trihalomethane and haloacetic acid in the drinking water can be achieved.
Owner:黑龙江省工研龙创环境产业集团有限公司
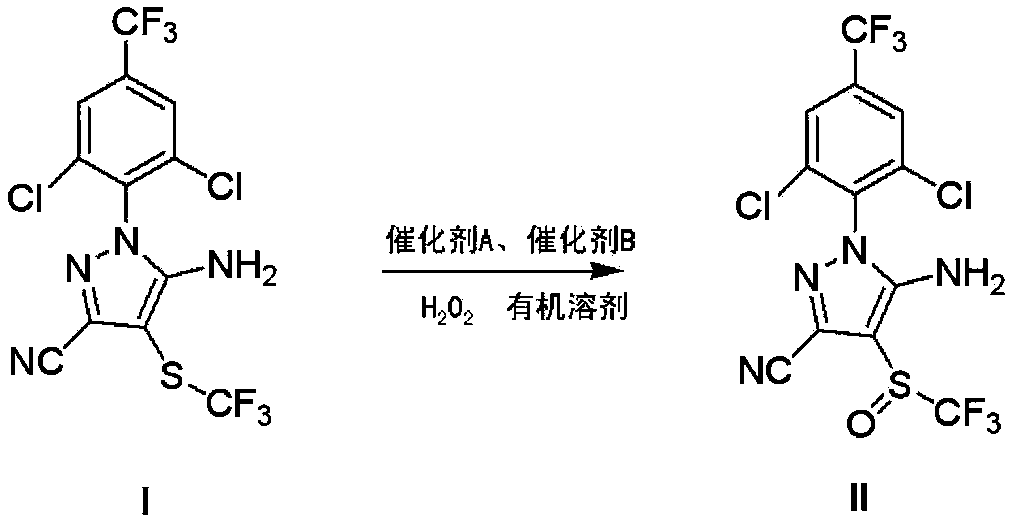
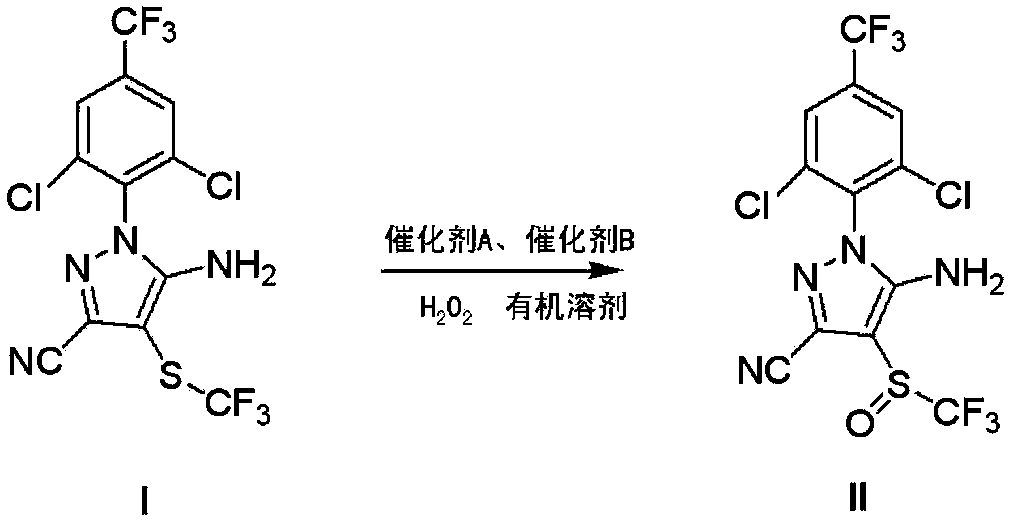
Preparation method of fipronil
The invention discloses a preparation method of fipronil. The preparation method comprises the following step of: obtaining fipronil (II) by control and oxidization of 5-amino-3-cyan-1-(2, 6-dichloro-4-trifluoromethyl phenyl)-4-trifluoromethylmercaptoparazole (I) in the presence of an organic solvent, hydrogen peroxide, a catalyst A and a catalyst B at 5-35 DEG C, wherein the catalyst A is selected from a haloacetic acid; the catalyst B is selected from one of sulfonic acids or mixture of different sulfonic acids, one of inorganic acids or mixture of different inorganic acids, or mixture of sulfonic acid and inorganic acid by any ratio; and the control on the oxidization condition refers to cancellation treatment when the reaction transformation rate is 60%-80%. According to the preparation method disclosed by the invention, the preparation process of the fipronil can be stably performed within 5-35 DEG C, the usage of haloacetic acid is greatly reduced, high-purity products and few byproducts are obtained, the production cost is reduced, and the yield of the fipronil in preparation is increased.
Owner:HAIZHENG CHEM NANTONG CO LTD
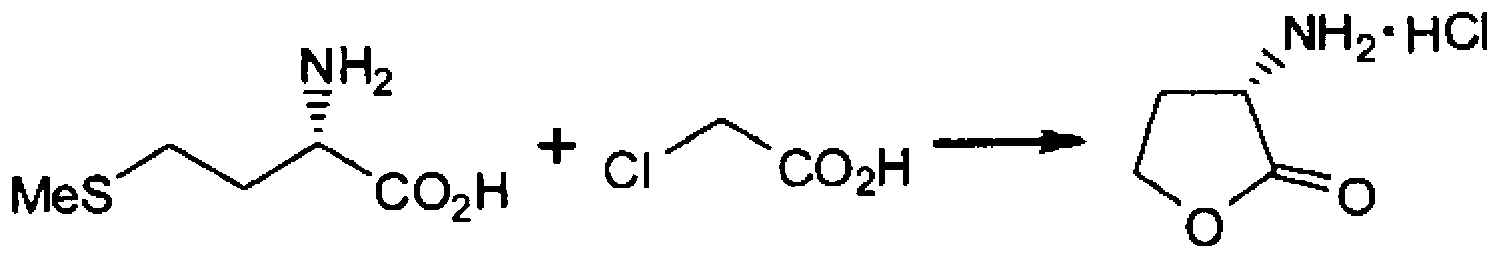
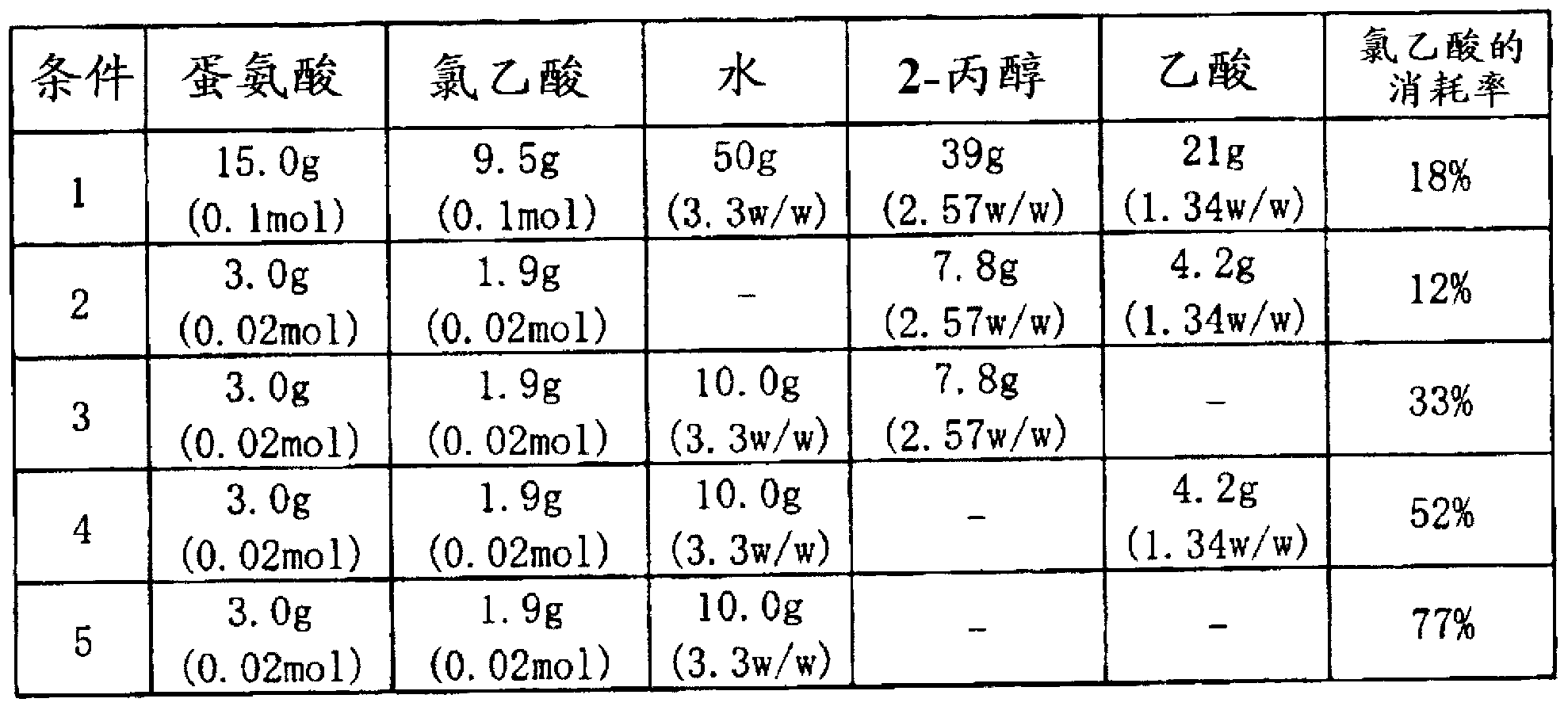
Method for producing alpha -mino-gamma-butyrolactone
A method for producing alpha -mino-gamma -butyrolactone or a salt thereof, which comprises a step wherein methionine and a haloacetic acid, namely chloroacetic acid or bromoacetic acid, are caused to react with each other in a solvent, and which is characterized in that the solvent contains 60% by weight or more of water relative to the total weight of the solvent. By this method, a-amino-?-butyrolactone or a salt thereof can be produced with high yield.
Owner:SUMITOMO CHEM CO LTD
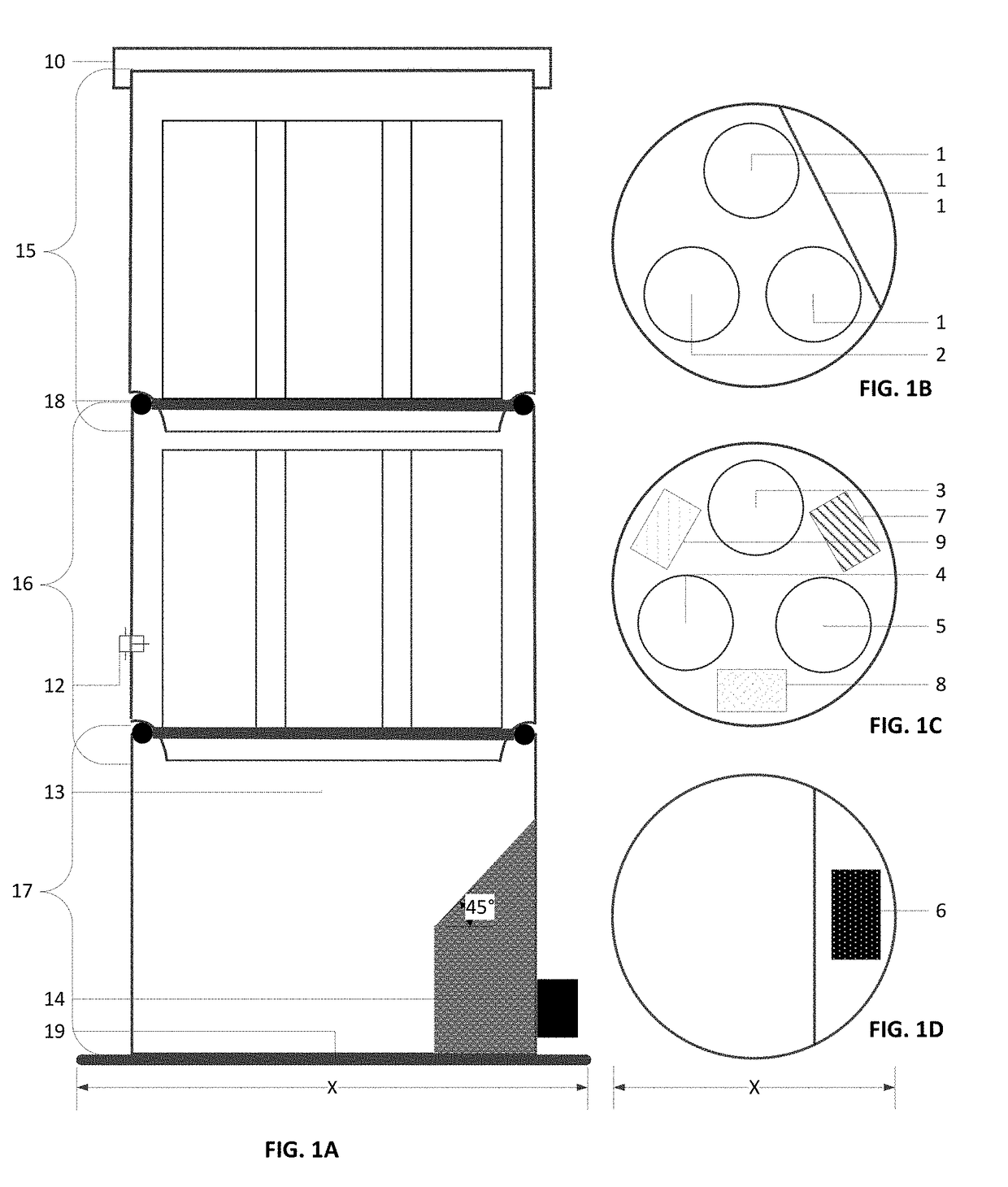
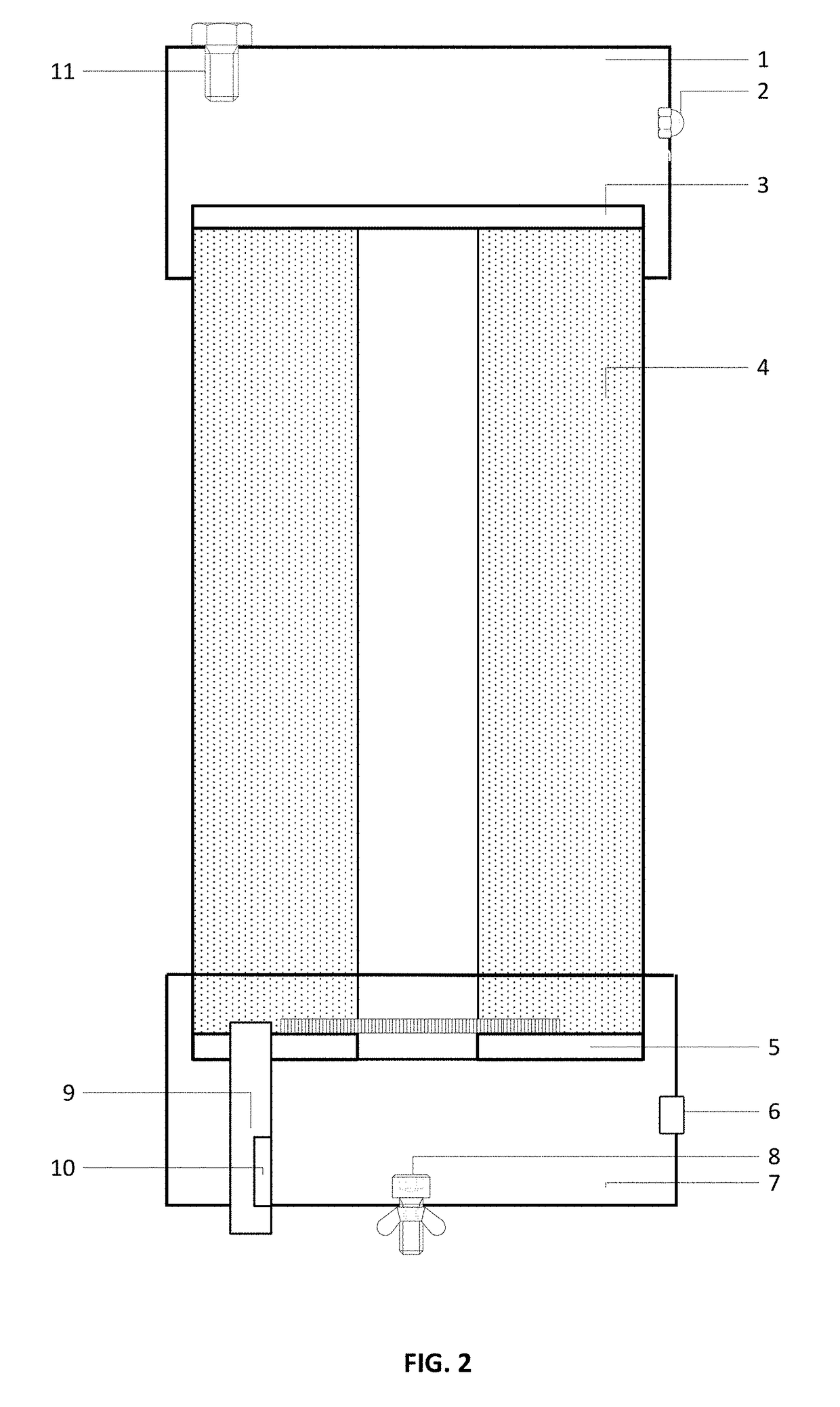
Filtering medium used for eliminating haloacetic acids in water and preparation method thereof and filter element made of the filtering medium
InactiveCN101125270AEfficient removalPurify waterOther chemical processesAluminium silicatesAcetic acidHalogen
The present invention relates to a filtering medium preparation method. The method comprises the steps as follows; fully mix and press 100 to 300 weight ratio of ultra-high molecular weight polyethylene, 50 to 200 weight ratio of active carbon, 10 to 50 weight ratio of modified zeolete, 5 to 35 weight ratio of diatom fine soil, 20 to 80 weight ratio of hole generating agent, 0 to 25 weight ratio of calcium sulfite; sinter the mixture for 80 to 150 minutes under 180 to 250 DEG C; and then cool the mixture. The filtering medium and the filtering element prepared by above method can be used for eliminating the halogen acetic acid in water. The present invention has the advantages of no secondary pollution and high efficiency of eliminating halogen acetic acid, and easy usage and low cost of the filter element.
Owner:QIDI ELECTRIC GROUP
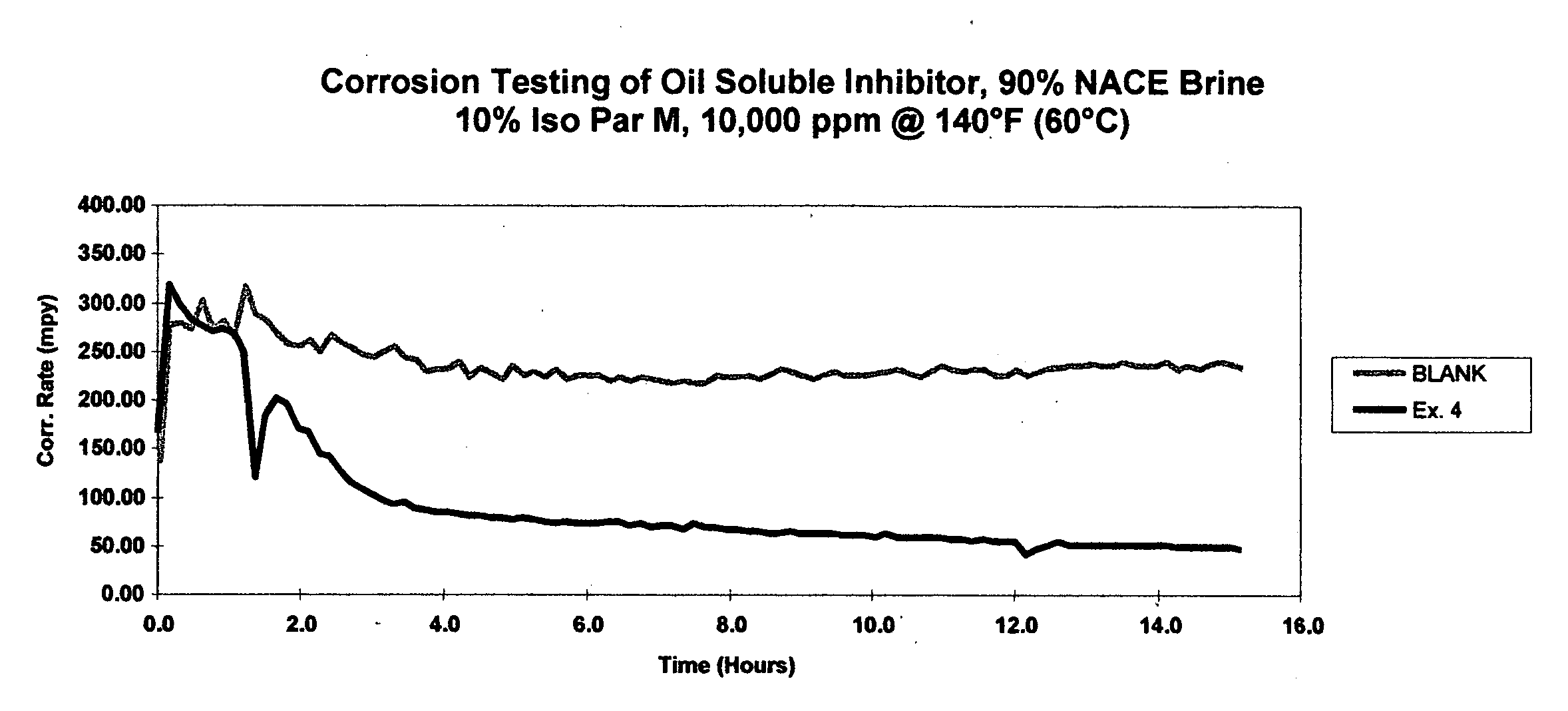
Acidification corrosion inhibitor based on interpolymer indolizine derivative as well as preparation method and application thereof
InactiveCN108440527AEfficient corrosion inhibitionGood corrosion inhibitionOrganic chemistryDrilling compositionAcetic acidQuaternary ammonium cation
The invention discloses an acidification corrosion inhibitor based on an interpolymer indolizine derivative as well as a preparation method and application thereof. The acidification corrosion inhibitor contains the interpolymer indolizine derivative; the interpolymer indolizine derivative is prepared by carrying out decarboxylation on heterocyclic alkali including (substituted) quinoline, (substituted) pyridine and the like, and carboxymethyl heterocyclic alkali quaternary ammonium salt obtained by alpha-haloacetic acid, and then carrying out intermolecular addition polymerization reaction onquaternary ammonium salt of the heterocyclic alkali including the (substituted) quinoline, the (substituted) pyridine and the like. The acidification corrosion inhibitor disclosed by the invention has relatively good corrosion inhibition performance under the condition that common corrosion inhibition synergists including alkynol and the like do not need to be compounded; the use amount of the acidification corrosion inhibitor is less and the acidification corrosion inhibitor can reach, even be better than the requirements of an acidification corrosion inhibitor performance testing method andfirst-grade to third-grade standards in evaluation indexes SY / T 5405-1996 when being independently used.
Owner:CHINA UNIV OF PETROLEUM (EAST CHINA)
Aryl alkyl alcohol polyoxyethylene ether carboxylate and preparation and application thereof
ActiveCN104529756AGood anti-adsorption performanceGood water solubilityOrganic compound preparationDrilling compositionSolubilityAryl
Owner:PETROCHINA CO LTD
High-selectivity fluorescence probe for detecting cyanide ions in ratio mode and synthesis method and application thereof
ActiveCN106518763ARaw materials are easy to getHigh synthetic yieldOrganic chemistryMaterial analysis by observing effect on chemical indicatorAcetic acidSynthesis methods
The invention discloses a high-selectivity fluorescence probe for detecting cyanide ions in a ratio mode and a synthesis method and application thereof, and belongs to the technical field of chemical analysis and detection. A 4-amino-1,8-naphthalimide skeleton and haloacetic acid or a derivative thereof are subjected to condensation reaction to obtain the probe, and the probe has the following structure (the structure is defined in the description). The fluorophore of the probe is of a naphthalimide skeleton structure, and the response group for the cyanide ions is a haloacetic acid unit. The probe molecule has high selectivity and sensitivity to the cyanide ions, the detection range is 1.0-80.0 micromol / L, and the detection limit is 0.23 micromol / L. The probe can be used for qualitatively and quantitatively detecting the cyanide ions in the water body and a practical sample.
Owner:SHANGQIU NORMAL UNIVERSITY
Method and apparatus for determination of haloacetic acid (“HAA”) presence in aqueous solution
InactiveUS9222921B2Low costEliminate needGeneral water supply conservationComponent separationUv absorbanceAcetic acid
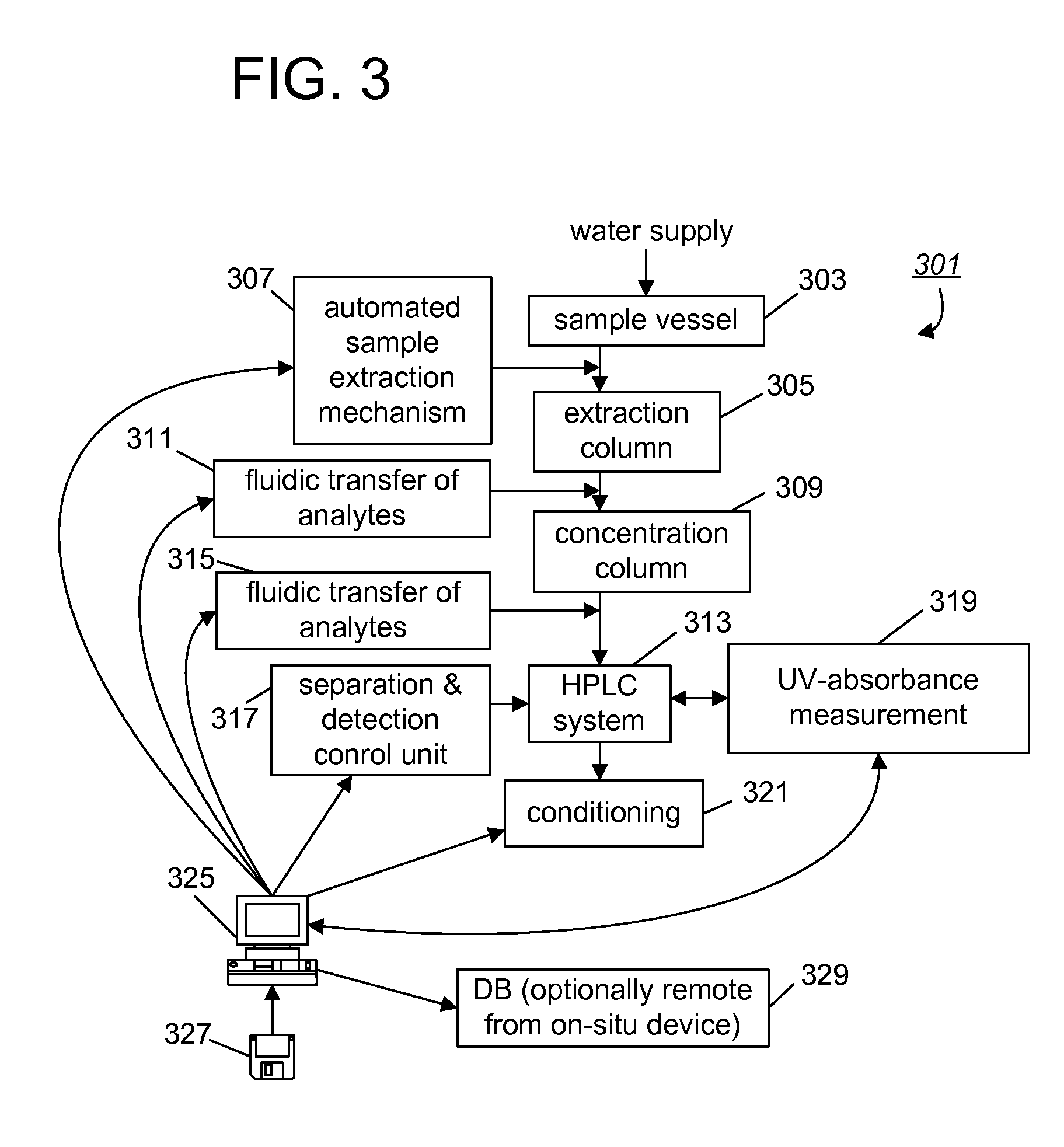
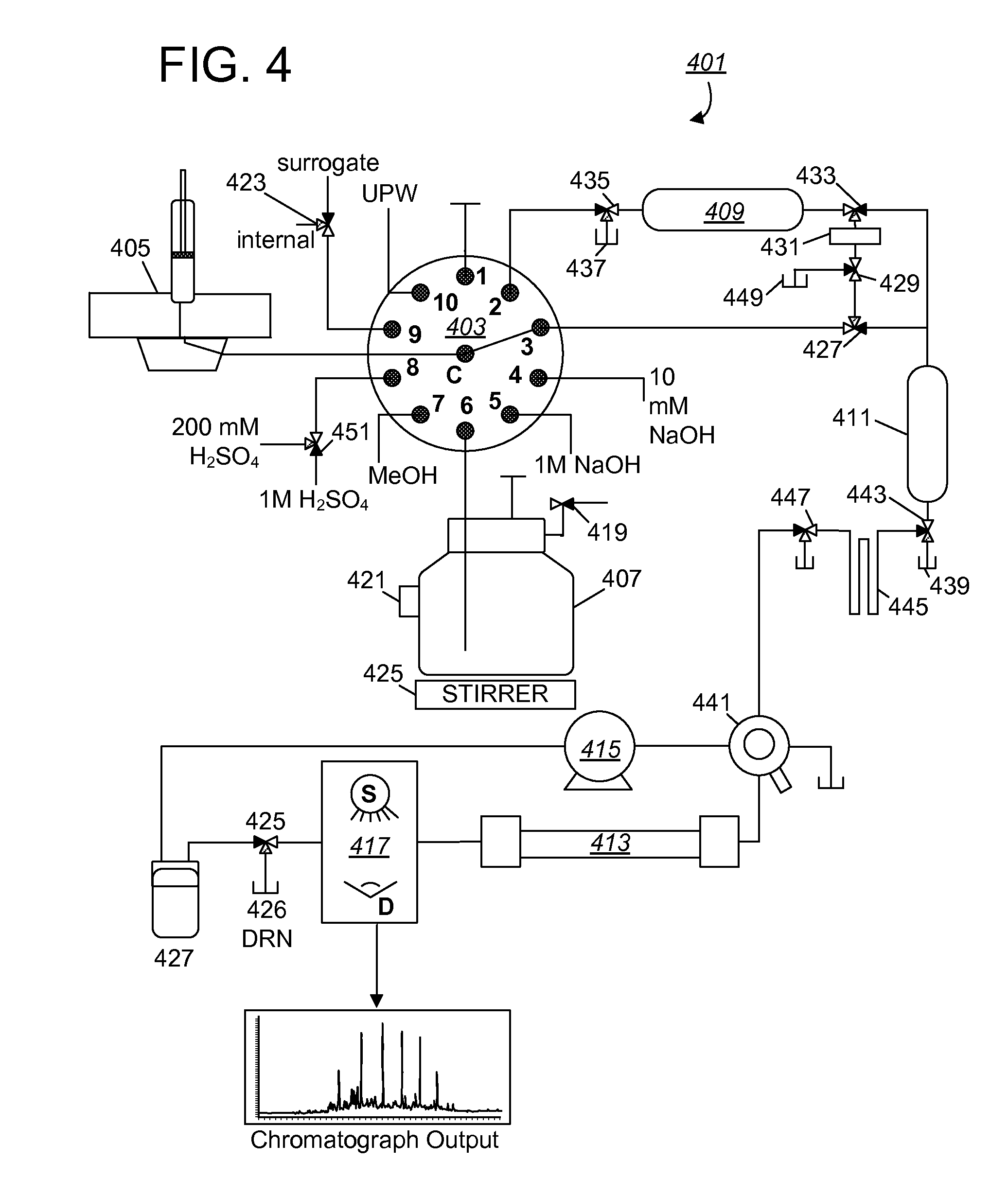
A real-time method and analytical system for determining haloacetic acids in water which operate by: (1) extracting samples on ion-exchange absorbent medium; (2) concentrating haloacetic acids on hyper-crosslinked medium; (3) eluting the analytes from the concentration medium for injection into an HPLC system; (4) separating individual haloacetic acid in reverse-phase chromatography performed by the HPLC system; and (5) measuring optical characteristics (UV-absorbance) of haloacetic acids, to determine concentration. The entire process can be performed using a completely self-contained, in-situ mechanism that sits at a water distribution point for 24 / 7 testing, with automated control, monitoring, reporting, and employment of remedial measures (e.g., automated adjustment of the water treatment process).
Owner:AMS TRACE METALS INC
Adaptive catalytic technology water treatment system
ActiveUS20170313611A1Improve efficiencyReduce and eliminate organic compound contaminantTreatment involving filtrationEnergy based wastewater treatmentWaste streamHandling system
This Adaptive Catalytic Technology (ACT) water treatment invention uses a series of integrated sequential modular advanced technologies to treat and eliminate or reduce suspended solids, hardness, heavy metals, organic compounds and microorganisms and to provide good tasting chlorine-free sanitized drinking water. The advanced technologies used herein are specifically designed to provide synergistic benefits that minimizes power consumption while improving the overall treatment effectiveness, making it possible to provide a cost effective and sustainable ACT water treatment for point of use drinking water supply for remote or developing areas, as well as residential, commercial, and industrial applications. The advanced technologies employed are environmentally friendly and safe. Specifically, the ACT water treatment invention does not require hazardous chemicals that need special handling to operate or maintain and it does not produce a waste stream or generates disinfection by-products (DBPs), such as, trihalomethanes (THMs) or haloacetic acids (HAAs).
Owner:ADAPTIVE CATALYTIC TECH LLC
Haloacetic acid-containing chitosan quaternary ammonium salt as well as preparation method and application thereof
The invention relate to the technical field of marine chemical engineering, in particular to haloacetic acid-containing chitosan quaternary ammonium salt as well as a preparation method and application thereof. The haloacetic acid-containing chitosan quaternary ammonium salt is prepared from the raw materials such as chitosan, 3-chlorine-2-hydroxypropyl trimethyl ammonium chloride, sodium hydroxide, isopropanol and haloacetic acid compounds through the steps of dispersing the chitosa into the isopropanol, swelling, stirring at room temperature for 4 hours, adding the 3-chlorine-2-hydroxypropyltrimethyl ammonium chloride and a sodium hydroxide solution, continuously reacting for 12 hours, precipitating by using a proper amount of ethanol to obtain chitosan quaternary ammonium salt, dissolving the chitosan quaternary ammonium salt into water, and performing ion exchange on the solution and haloacetate to obtain a final product. The haloacetic acid-containing chitosan quaternary ammoniumsalt as well as the preparation method and the application thereof have the following advantages: the preparation process is simple; the used materials have low cost; and research proves that the haloacetic acid-containing chitosan quaternary ammonium salt has high derivative water solubility, has high bacteriostatic activity and can be widely applied in the fields of medicine, pesticide and thelike.
Owner:YANTAI INST OF COASTAL ZONE RES CHINESE ACAD OF SCI
Method for preparing 2,4-dichlorphenoxyacetic acid
InactiveCN106892808AGood choiceHigh yieldPreparation from carboxylic acid saltsOrganic compound preparationAlcoholPhenoxyacetates
The invention provides a method for preparing 2,4-dichlorphenoxyacetic acid. The method comprises the step S1 of causing haloacetic acid and alkali metal alkoxide react in an alcohol solvent toobtain halogenated acetate, the step S2 of causing the halogenated acetate and phenoxide to react in the alcohol solvent to obtain phenoxyacetic acid salt, the step S3 of causing the phenoxyacetic acid salt to undergo chlorination in the alcohol solvent, and obtaining 2,4-dichlorobenzene acetate, and the step S4 of acidizing 2,4-dichlorobenzene acetate to obtain 2,4-dichlorphenoxyacetic acid. Compared with the prior art, the method enables chlorination to be performed in an anhydrous system, reaction selectivity is good, by-products are fewer, the yield is high, the 2,4-dichlorphenoxyacetic acid can be obtained after acidification, and the 2,4-dichlorphenoxyacetic acid is simple to prepare.
Owner:SHANDONG RUNBO BIOTECH CO LTD
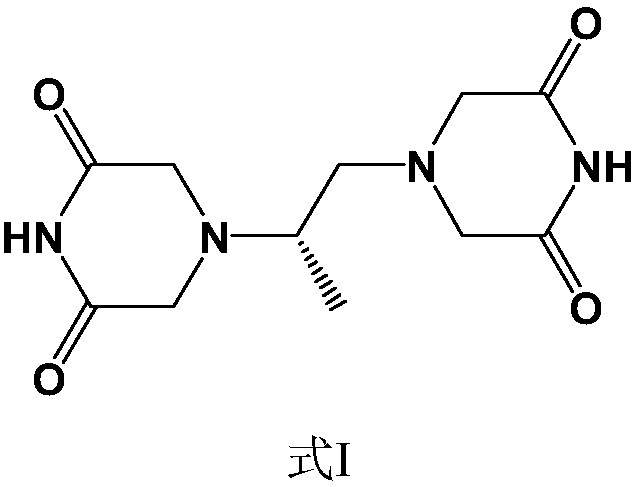
Preparation method of piperazine diketone type compound
ActiveCN108250094AHigh yieldHigh purityOrganic compound preparationOrganic chemistry methodsDiketoneAlcohol
The invention discloses a method for preparing a piperazine diketone type compound and specifically relates to a method for preparing a compound shown as a formula (I) by cyclization after substitution. The method takes (S)-1,2-diaminopropane tartrate, haloacetic acid and low-carbon alcohol as raw materials to prepare the compound shown as the formula (I). Compared with the prior art, a pluralityof disadvantages are improved and overcome by the preparation method; the preparation method has the advantages of simple steps, low cost, convenience for post-treatment, cleanness, high yield and purity, low toxicity and less pollution, and industrial production is facilitated.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
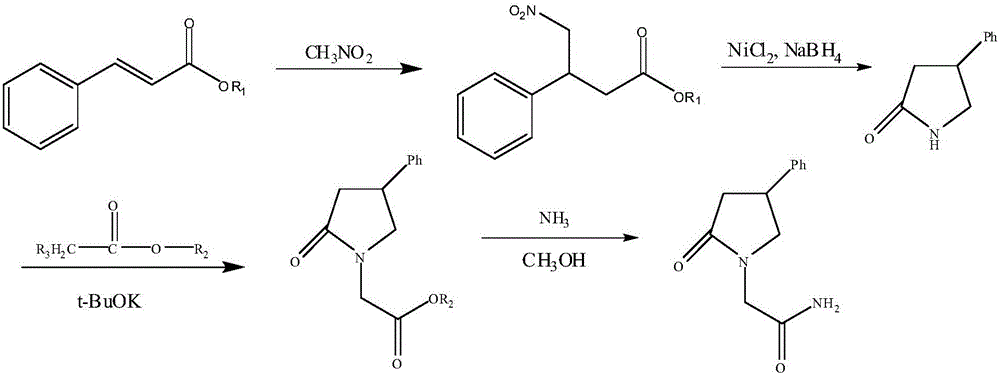
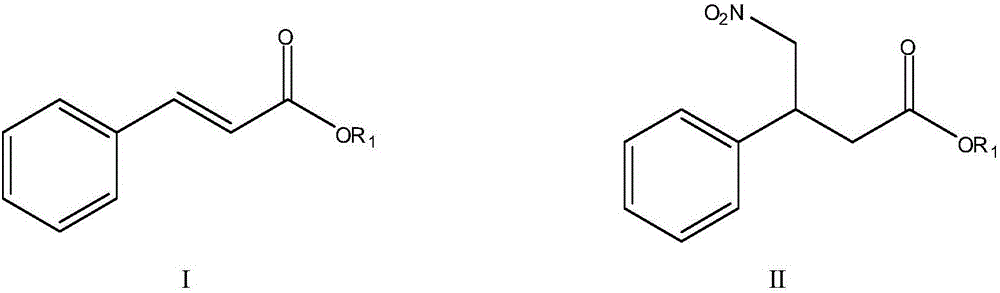
Method for preparing phenyl piracetam
The invention discloses a method for preparing phenyl piracetam, and belongs to the field of compound preparing. The method includes the following steps that alkali, cinnamic acid alkyl ester and nitromethane are subjected to an addition reaction to obtain 4-nitryl-3-phenylbutyric acid alkyl ester; after nitryl of the 4-nitryl-3-phenylbutyric acid alkyl ester is reduced with a reducing agent, the reduced nitryl and carbonyl are cyclized, and 4-phenyl-2-pyrrolidone is obtained; alkali, haloacetic acid alkyl ester and the 4-phenyl-2-pyrrolidone are subjected to an alkylation reaction to obtain 4-phenyl-2-pyrrolidone-1-acetic acid alkyl ester; the 4-phenyl-2-pyrrolidone-1-acetic acid alkyl ester and ammonia gas are reacted to obtain the phenyl piracetam. The method has the advantages of being short in reaction step, high in atom utilization, more environmentally friendly, safe in operation, high in reaction yield and purity, beneficial for achieving industrialization an the like.
Owner:NORTHEAST PHARMA GRP
Method for efficiently synthesizing beta-benzyl butyrolactone having specific configuration
ActiveCN105175365ARaw materials are cheap and easy to getShort stepsOrganic chemistry3-phenylpropanoic acidChemical synthesis
The present invention discloses a method for efficiently synthesizing beta-benzyl butyrolactone having a specific configuration. The method is characterized in that phenylpropionic acid or a derivative thereof is adopted as a starting material, the phenylpropionic acid or the derivative thereof and an oxazolidone chiral prosthetic group are subjected to condensation, a haloacetic acid ester attacks the carbonyl ortho position carbon of the phenylpropionic acid under the effect of a large steric hindrance organic alkali, the prosthetic group is hydrolyzed and recovered after the specific chiral center is successfully constructed, and the corresponding product is subjected to an intramolecular ester exchange reaction to generate the beta-benzyl butyrolactone having the specific configuration. According to the present invention, the prepared beta-benzyl butyrolactone can be used for chemical synthesis of a lot of dibenzyl type lignins having potential medicinal values, and has characteristics of cheap and readily available raw materials, short step, high yield, and high optical purity.
Owner:LIAONING UNIV OF TRADITIONAL CHINESE MEDICINE
Electrode for removal of disinfection byproducts in water and preparation method thereof
InactiveCN103420453AImprove electrocatalytic activityEasy to prepareWater/sewage treatmentElectrodesDisinfection by-productIron nanoparticle
The invention belongs to the technical field of water treatment, relates to an electrode for removal of disinfection byproducts in water and a preparation method thereof, and particularly relates to the electrode obtained by successively and respectively electrodepositing a noble metal and iron on the surface of carbon paper. The preparation method of the electrode for removal of the disinfection byproducts in water comprises the steps: with the carbon paper as a carrier, through an electrodeposition method, the in-situ generated noble metal and iron are successively and respectively electrodeposited on the surface of the carbon paper, and the electrode for removal of the disinfection byproducts in water can be obtained. In a case of energizing, the electrode can reduce and remove the disinfection by-products such as haloacetic acids, bromates and the like having stronger polarity and more stable in water. The preparation method of the electrode is simple, and is easy to operate; and the electrode prepared by electrodepositing in-situ generated noble metal nanoparticles and iron nanoparticles on the surface of the carbon paper has low cost, and has the excellent ability of reducing removal of the disinfection byproducts in water.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method for removing haloacetic acids from water
InactiveCN102557340AEasy to handleImprove processing efficiencyWater contaminantsMultistage water/sewage treatmentActivated carbonAcetic acid
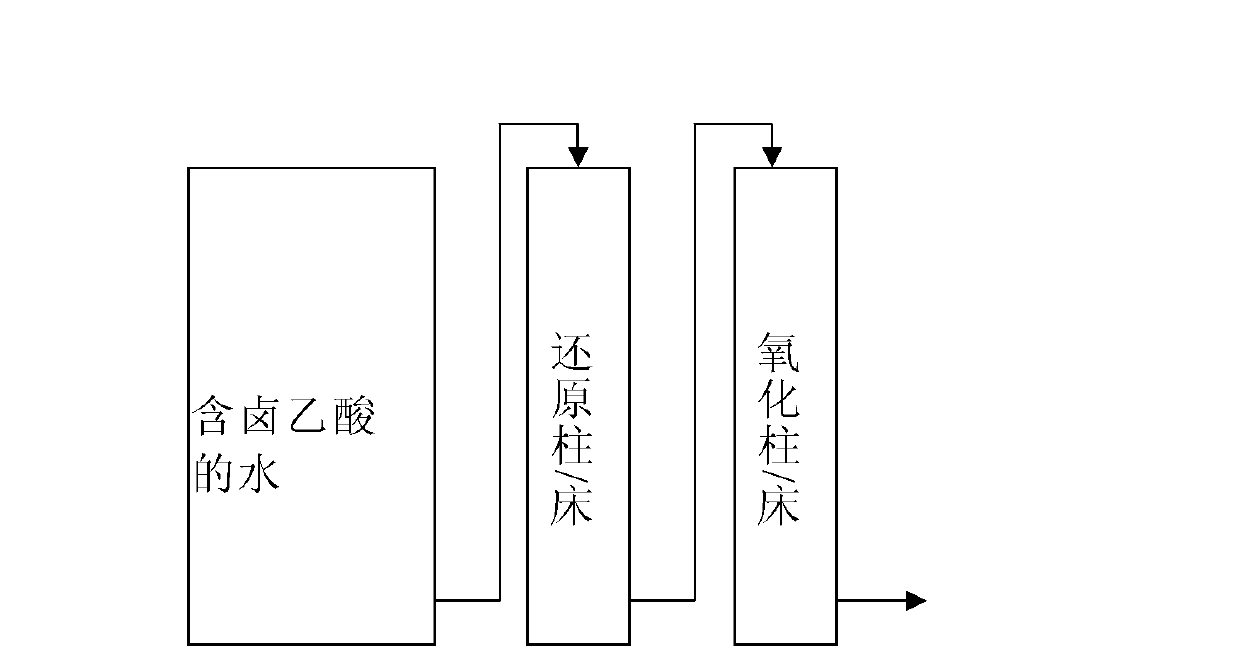
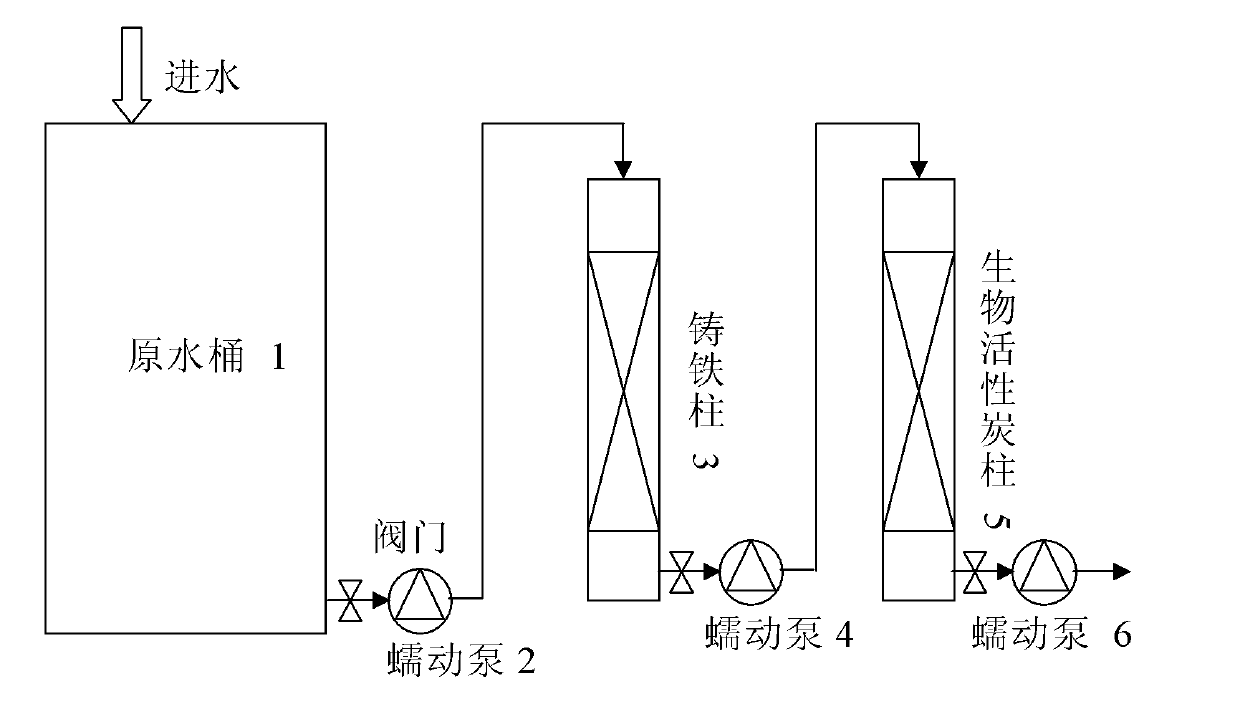
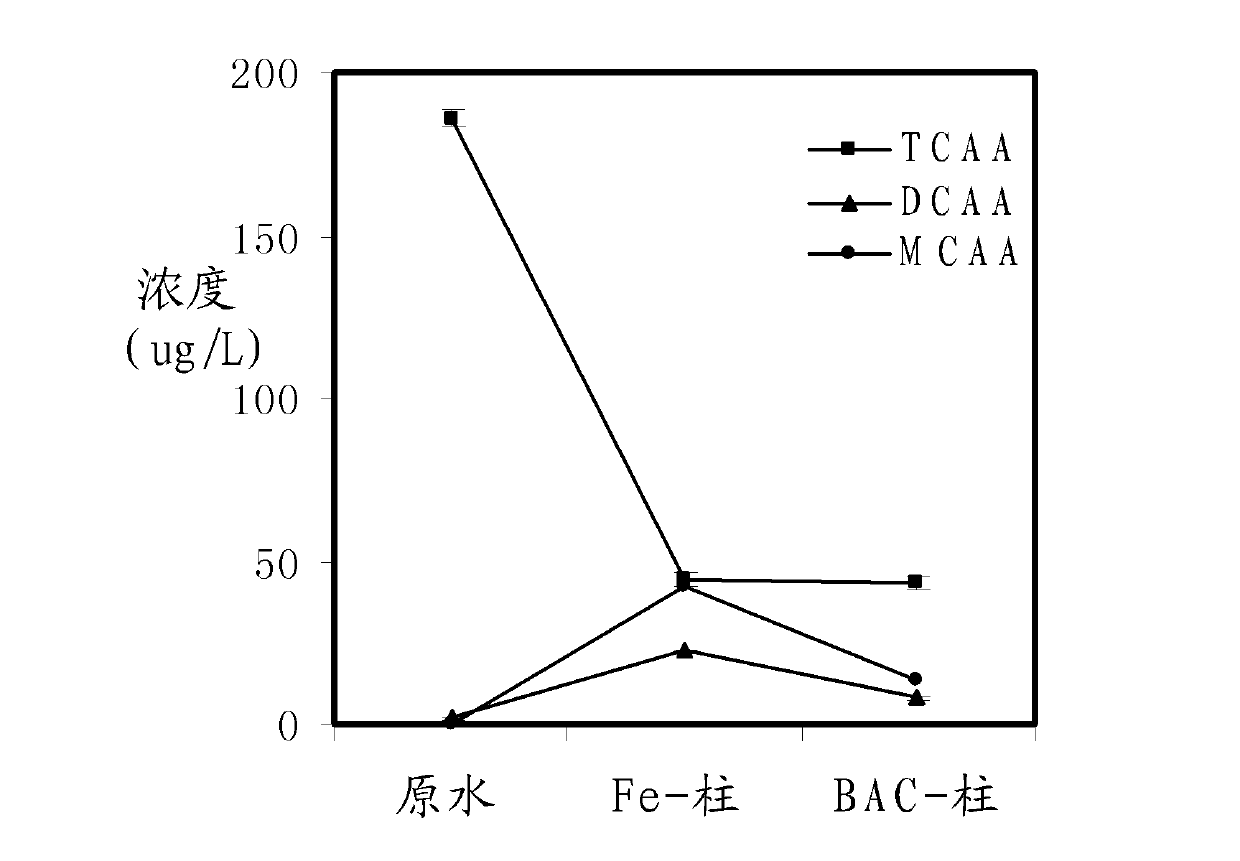
The invention relates to a method for removing haloacetic acids from water. The water containing the haloacetic acids flows through a reduction column / bed and an oxidation column / bed in turn; a filler in the reduction column / bed at least contains zero-valent iron; a filler in the oxidation column / bed at least contains a filler suitable for the growth of a biological film; the shape of the filler containing the zero-valent iron is one or more of a granular shape, a strip shape and a net shape; the filler is one or more of pure iron, cast iron and rusted iron; and the filler suitable for the growth of the biological film is one or more of activated carbon, ceramsite, anthracite, rubber and plastic. By the method, the treated water can meet the requirement of the drinking water quality standard on the concentration of the haloacetic acids; and the method has the characteristics of simplicity, reliability and high efficiency.
Owner:TSINGHUA UNIV
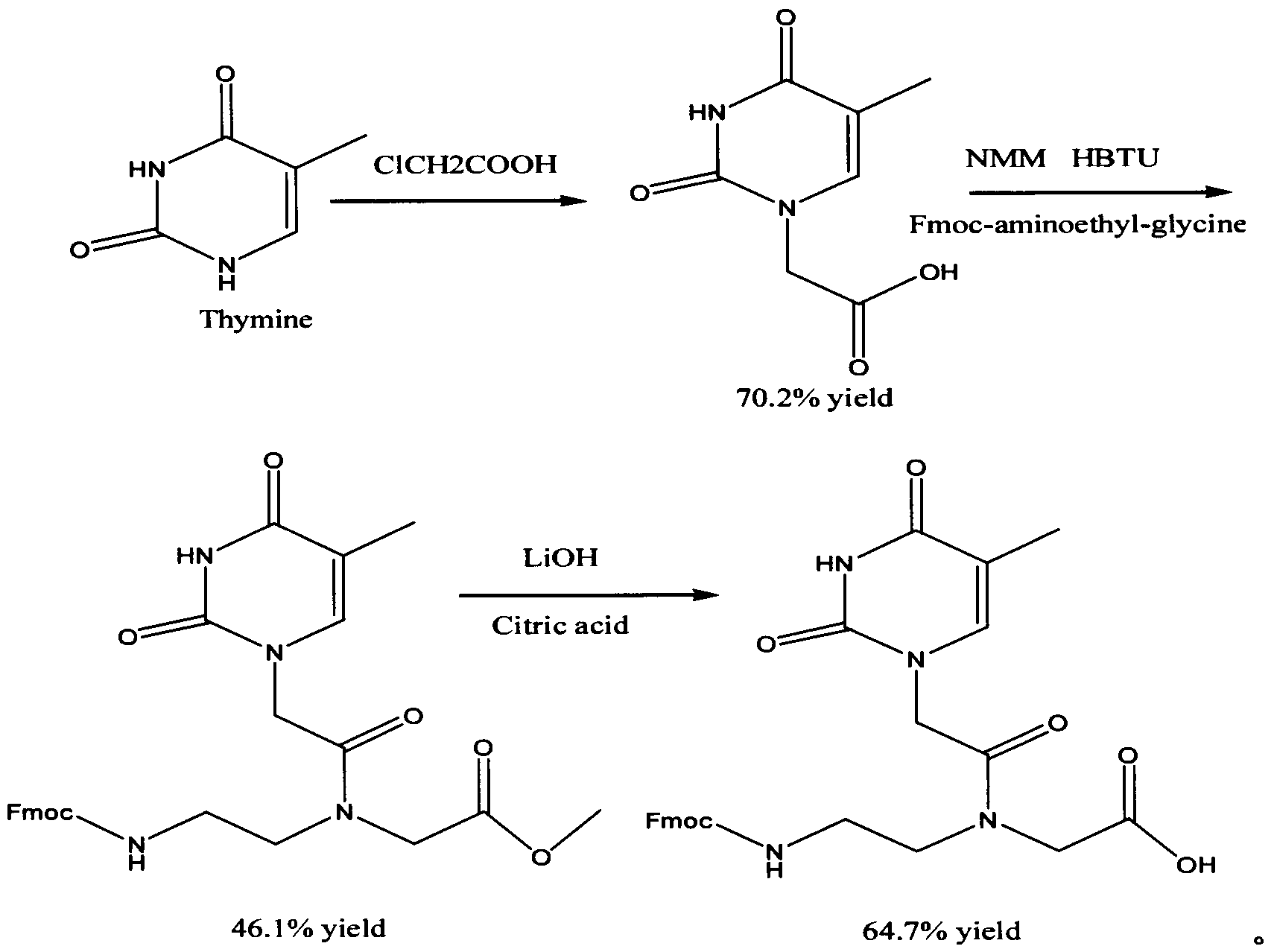
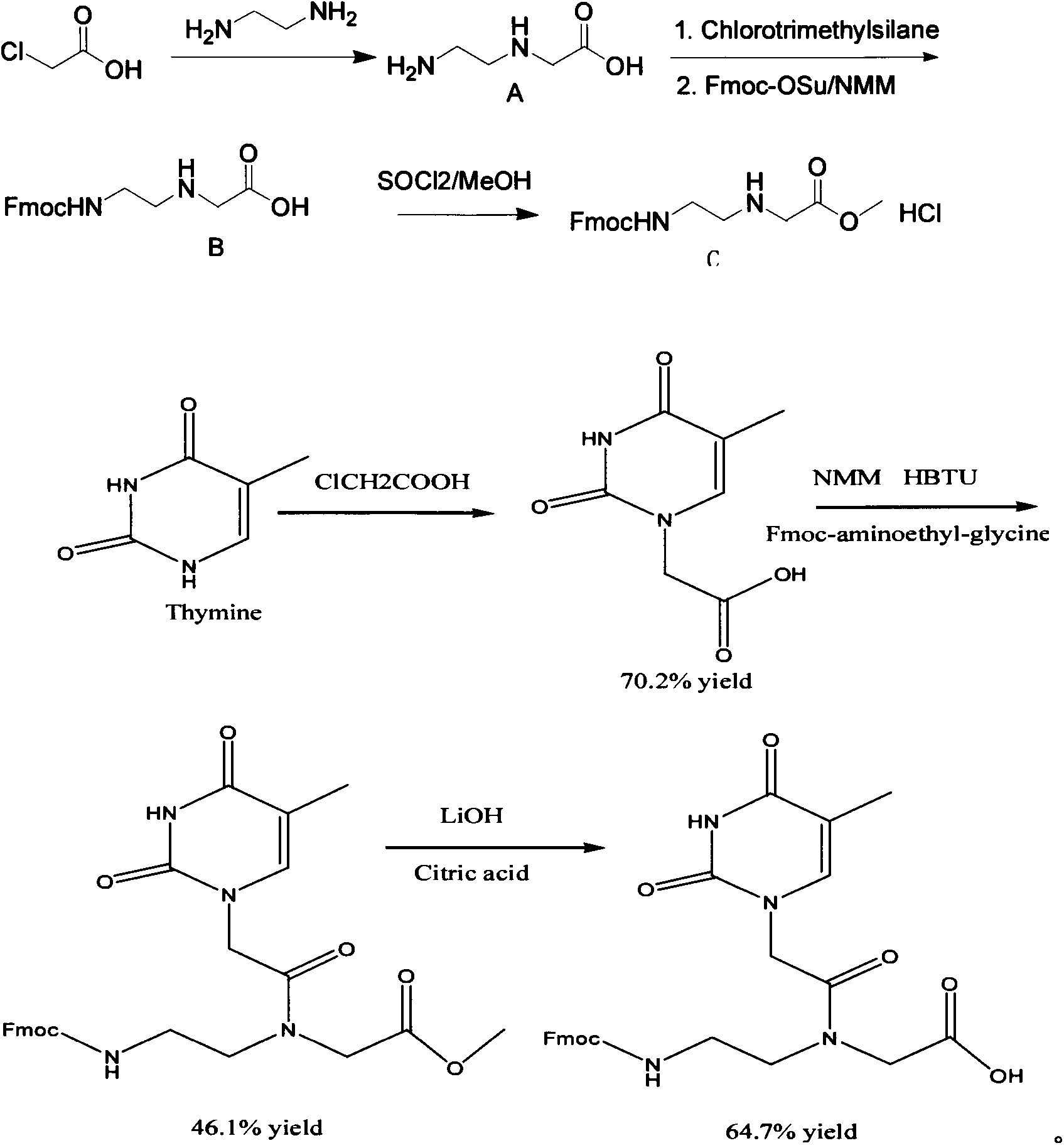
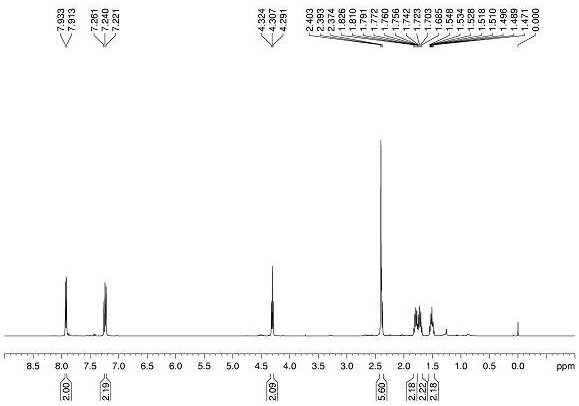
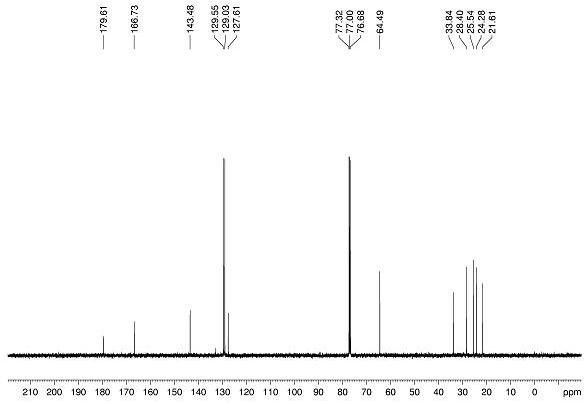
Feather weight synthesis method of Fmoc-PNA-T-OH
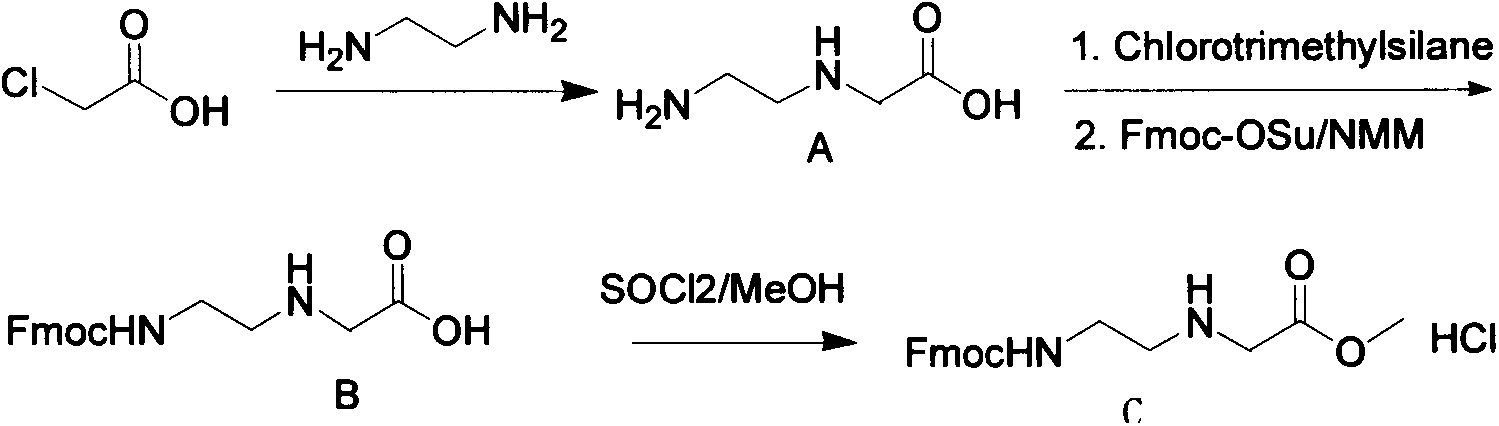
The invention discloses a feather weight synthesis method of Fmoc-PNA-T-OH. The feather weight synthesis method comprises the following steps: reacting ethanediamine serving as a raw material with haloacetic acid, and recrystallizing by using DMSO (dimethylsulfoxide) so as to obtain N-(2-aminoethyl) glycine; reacting the N-(2-aminoethyl) glycine serving as a raw material with Fmoc-osu so as to obtain N-(2-Fmoc-aminoethyl) glycine, and then reacting with methyl alcohol so as to obtain N-(2-Fmoc-aminoethyl) glycine methyl ester hydrochloride; using thymine as a raw material, adding raw materials, and reacting so as to obtain 1-carbethoxy thymine; reacting the 1-carbethoxy thymine serving as the raw material with the N-(2-Fmoc-aminoethyl) glycine methyl ester so as to obtain Fmoc-PNA-T-OMe; and hydrolyzing the Fmoc-PNA-T-OMe so as to obtain the product.
Owner:SUZHOU VIVOTIDE BIOTECH
A method for one-step synthesis of carboxylic acids with two extended carbon chains from olefins
ActiveCN111718228AAvoid interconversionEasy to operateOrganic compound preparationCarboxylic acid esters preparationDistillationCarboxylic acid
The invention relates to a method for one-step synthesis of carboxylic acid with two extended carbon chains from olefin, which comprises the following steps: under the protection of inert gas, sequentially adding an olefin substrate, a photocatalyst, a hydrogen atom transfer reagent, alpha-haloacetic acid, a reducing agent, a solvent and protonic acid into a reactor, and reacting at normal temperature under the irradiation of 25W blue light to obtain a reaction product; diluting, alkalizing, washing, acidifying and extracting the reaction product to obtain an organic phase; and finally, carrying out reduced pressure distillation and column chromatography on the organic phase to obtain a carboxylic acid product with two extended carbon chains; or carrying out reduced pressure distillation and column chromatography on the reaction product to obtain a carboxylic acid product with two extended carbon chains. The invention is simple to operate, direct synthesis conditions are mild, mutual conversion among various functional groups in the traditional carboxylic acid compound synthesis process is avoided, and the atom and step economy of the reaction is improved. Meanwhile, the method disclosed by the invention can also be applied to the simplified synthesis of the medicines cinacarbazide and tirofiban.
Owner:LANZHOU UNIVERSITY
Method for predicting concentration of disinfection by-product haloacetic acid in water supply system
PendingCN110287651AGood forecastImprove the detection rateGeneral water supply conservationComponent separationWater qualityNetwork model
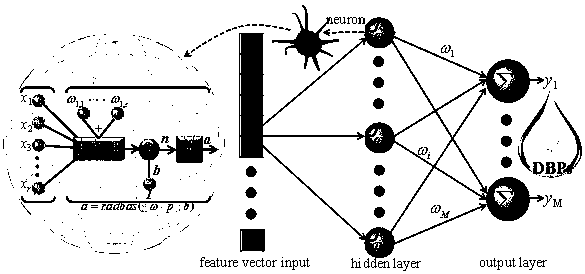
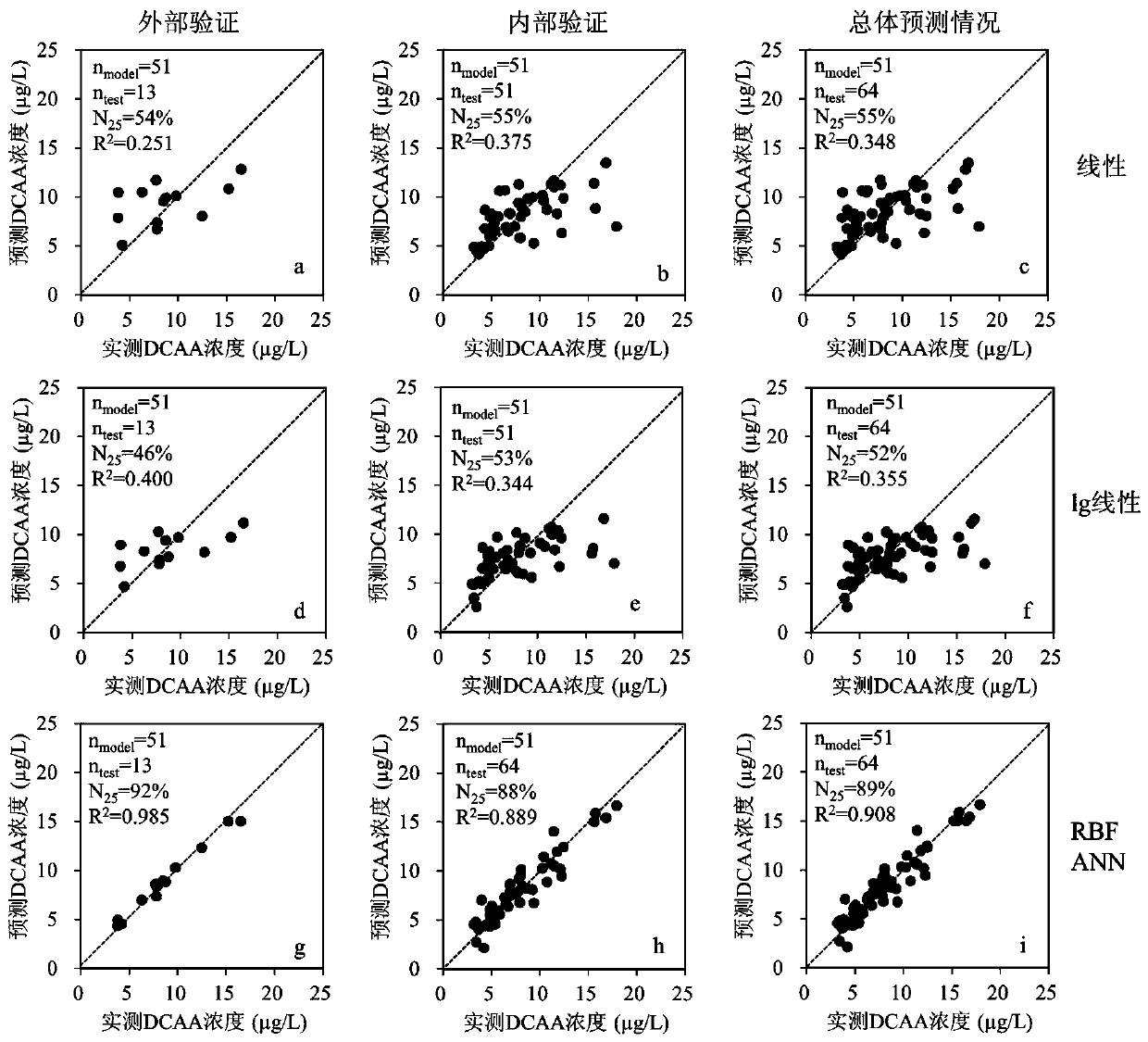
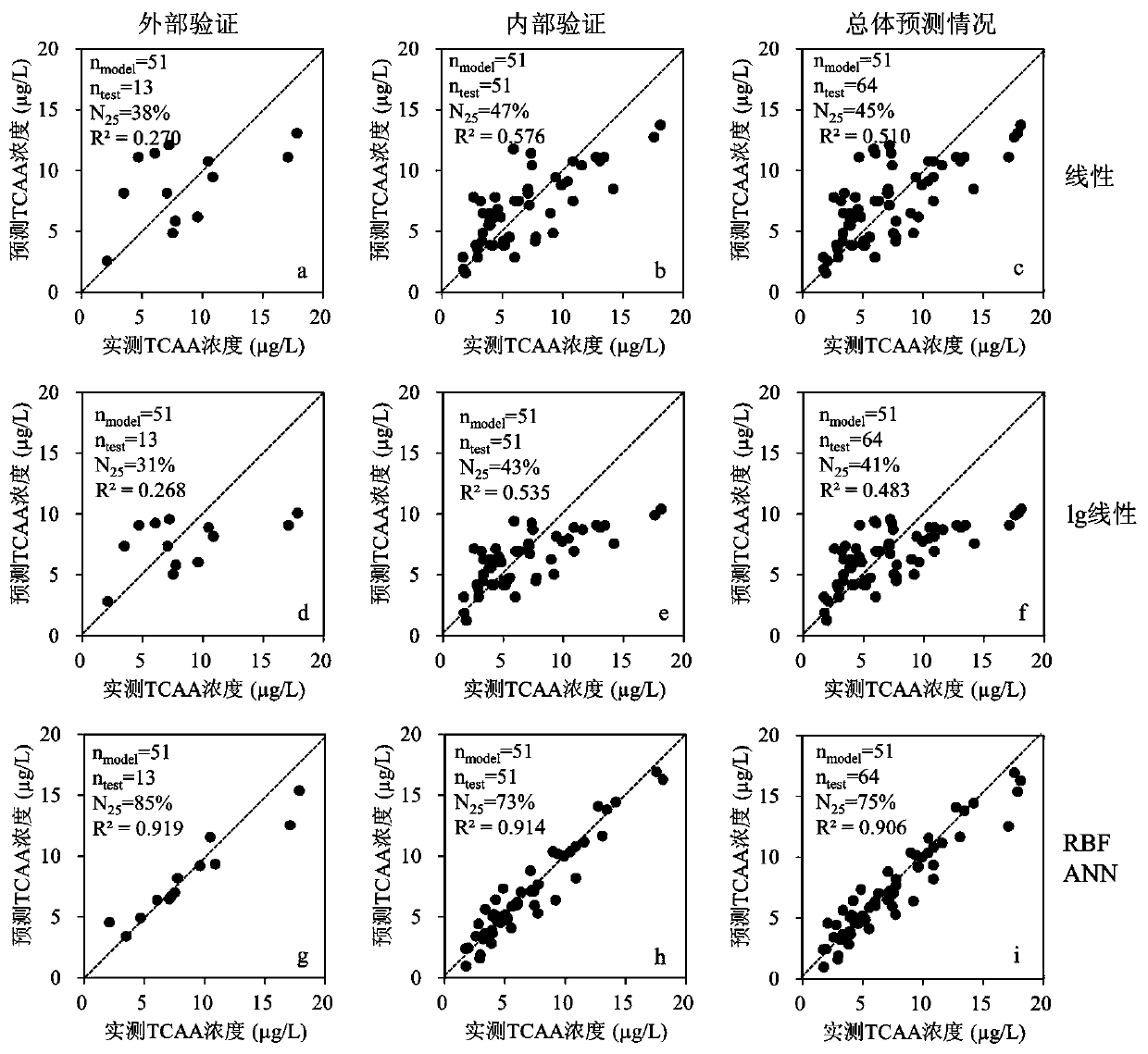
The invention discloses a method for predicting the concentration of a disinfection byproduct haloacetic acid in a water supply system. The method comprises the following steps: S1, dividing a haloacetic acid and water quality parameter database into a training set and a prediction set; S2, establishing a neural network model by using a neural network newrb function in MATLAB (Matrix Laboratory), wherein the format of the neural network model is net = newrb (P, T, GOAL, SPREAD, MN, DF); S3, training the built neural network model, verifying and checking the prediction performance of the neural network model through the model, directly entering the step S5 when the correct rate is high, and performing the step S4 when the correct rate is low; S4, when the test accuracy is low, adjusting the number of SPREAD and neurons, and repeating the step S2 until a satisfactory result is obtained; S5, stopping. The prediction effect of the RBF neural network model is much better than that of a linear model or an lg linear model, the time consumed in the RBF neural network operation process is very short, and the operation time of each neural network model is generally 10-13s.
Owner:ZHEJIANG NORMAL UNIVERSITY
Method capable of determining nine haloacetic acids in air or waste gas at the same time in one time
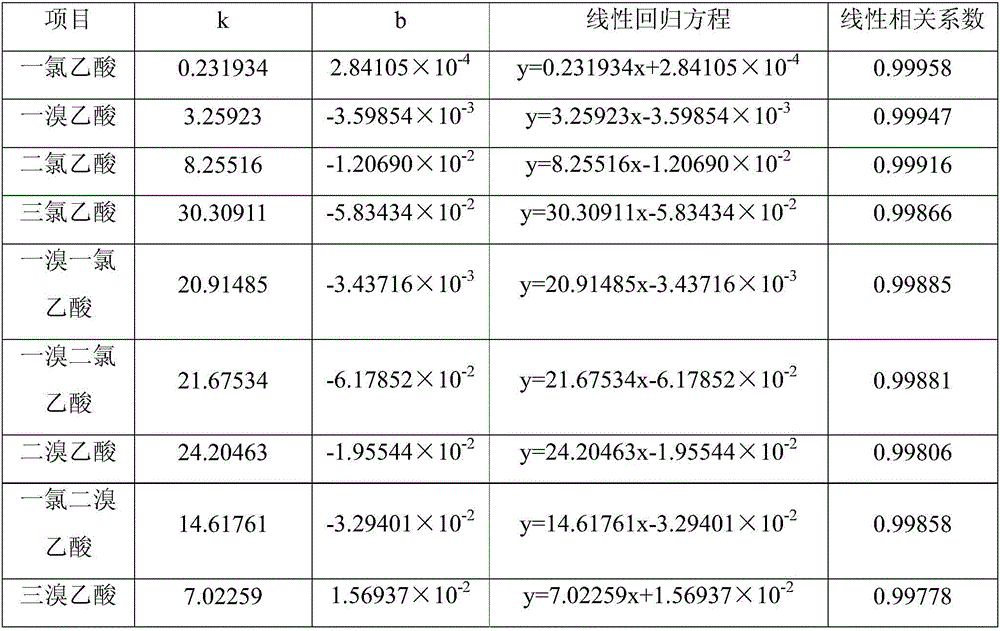
The invention belongs to the technical field of environment detection and particularly relates to a method capable of determining nine haloacetic acids in air or waste gas at the same time in one time. According to the method, the haloacetic acids in the air or the waste gas are sampled through an adsorption tube filled with filler, water is utilized as a desorption regent, the haloacetic acids adsorbed by the filler in the adsorption tube is desorbed and transferred into water, then methyl tertiary butyl ether containing an internal standard substance is used for extracting, the methyl tertiary butyl ether is utilized as a carrier solvent to generate esterification reaction with methyl alcohol, and a peak area is determined through a gas chromatograph with an electronic catcher to perform result determination. The method has the advantages of high accuracy, low detection limit and good determined result repeatability.
Owner:肖洋
Production method of (r)-3- hydroxy-3-(2-phenylethyl)hexanoic acid and intermediate thereof
InactiveCN1617846AOrganic compound preparationCarboxylic acid esters preparationEthyl groupCombinatorial chemistry
A production method of a racemic 3-hydroxy-3-(2-phenylethyl)hexanoic acid ester, which comprises reacting magnesium, haloacetic acid ester and 1-phenyl-3-hexanone.
Owner:SUMITOMO CHEM CO LTD
Process and composition for lower toxicity quaternary ammonium compounds
ActiveUS20050069515A1Low toxicityEasy to implementCationic surface-active compoundsOrganic compound preparationBetaineAmmonium compounds
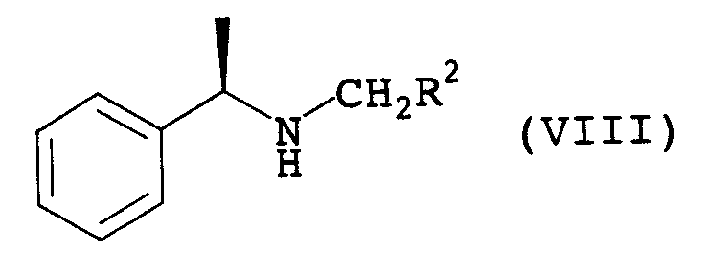
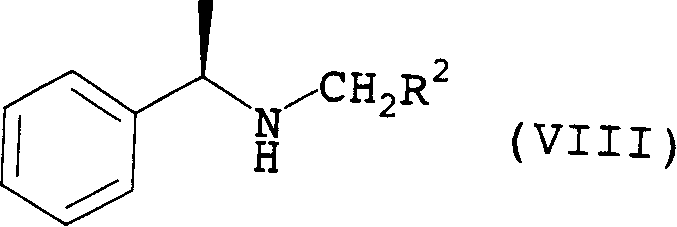
Betaine ester quaternary ammonium compounds with reduced toxicity and improved biodegradability are formed by esterification of a haloacetic acid with an alcohol containing at least 4 carbon atoms, followed by quaternization of the halo-acetate with a tertiary amine containing at least 4 carbon atoms. In one non-limiting embodiment the alkyl substituents on the nitrogen of the tertiary amine have at least 2 carbon atoms, and in another non-limiting embodiment are each n-butyl.
Owner:BAKER HUGHES INC
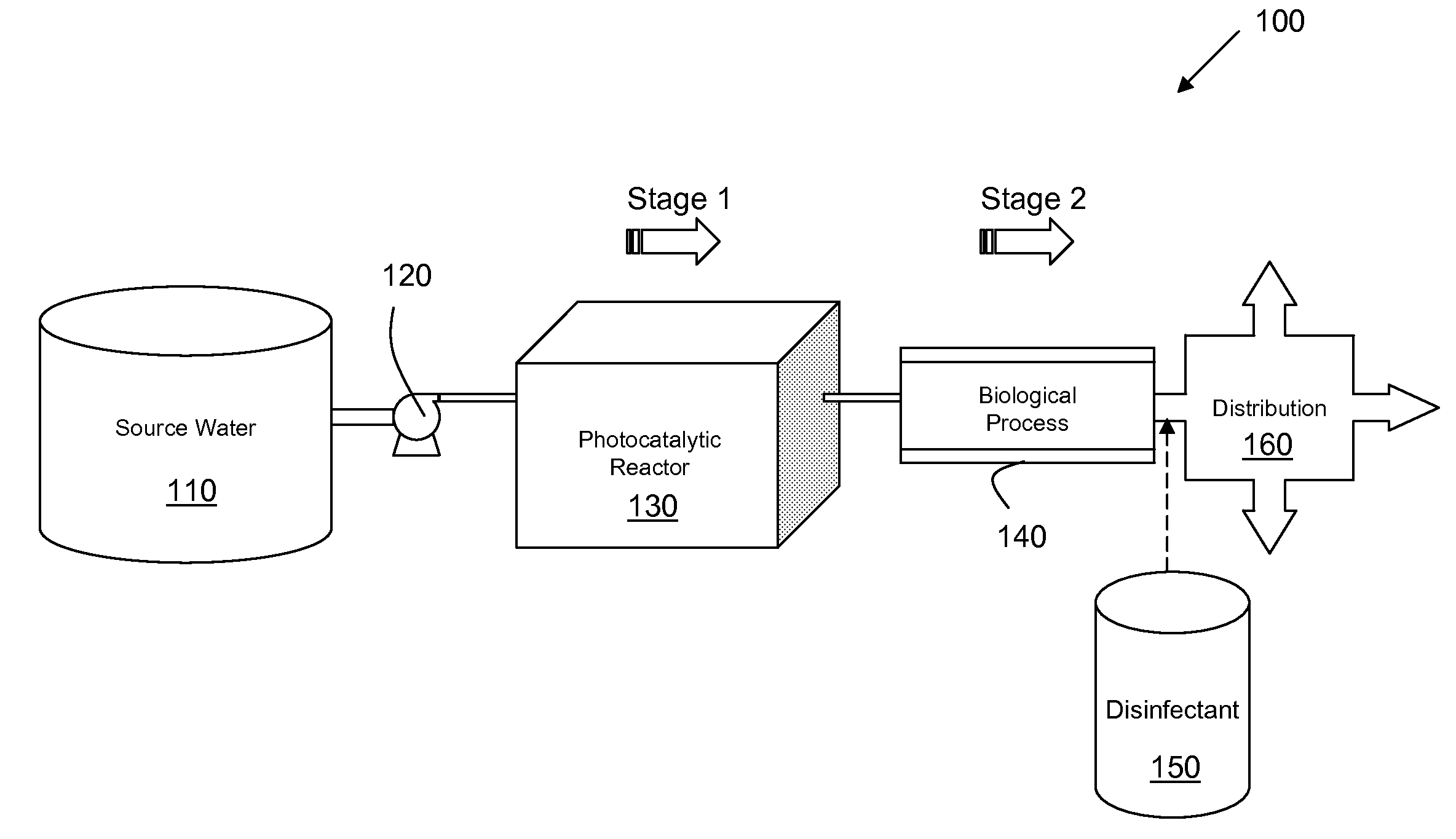
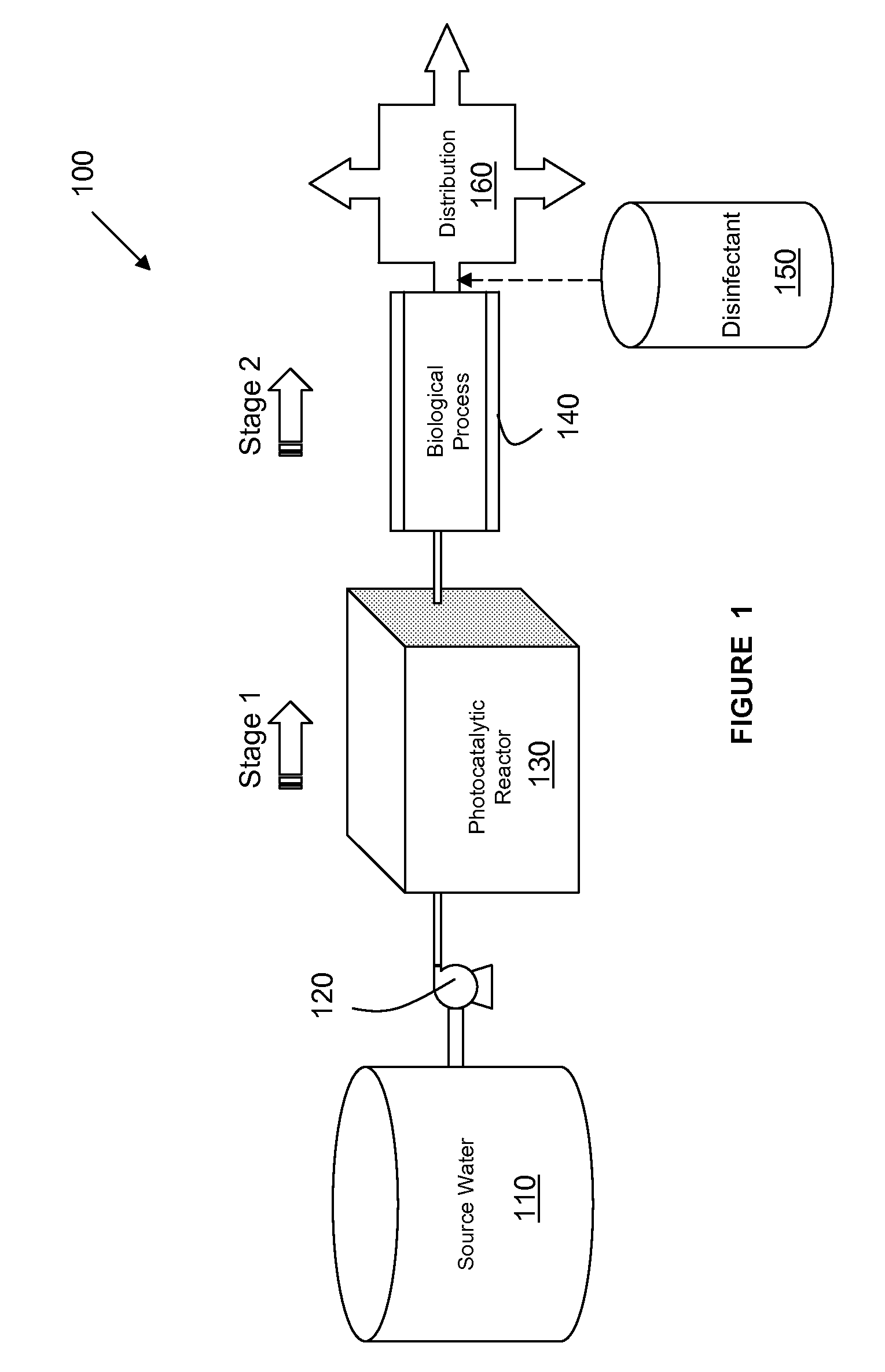
Water Treatment Process for the Reduction of THM & HAA Formation
ActiveUS20110024352A1Inhibition formationFully removedTreatment using aerobic processesWater/sewage treatment by irradiationChemical physicsNatural organic matter
Disclosed herein are systems and process integrating the chemical-free oxidation of a photocatalytic decontamination process with a biological decontamination system to eliminate the THM and HAA precursors in drinking water. In one embodiment, a system may comprise a source providing fluid media contaminated with toxic natural organic matter, and a photocatalytic decontamination subsystem configured to oxidize the toxic natural organic matter via a photocatalytic process into non-toxic natural organic matter having a molecular weight low enough for biodegrading said non-toxic matter. The system may include a biological decontamination subsystem configured to immediately receive the contaminated fluid output from the photocatalytic decontamination subsystem, and employing a biological agent to biologically degrade the low-molecular weight non-toxic natural organic matter in the contaminated fluid to a concentration sufficient to prevent the formation of trihalomethanes or haloacetic acids. Also, such a system may include a disinfectant sub-system configured to disinfect the fluid output from the biological decontamination subsystem.
Owner:BUTTERS BRIAN E +1
Cloquintocet-mexyl synthesizing method
InactiveCN101293870AHigh yieldRaw materials are easy to getBiocideOrganic chemistryEsterification reactionToluene
The invention relates to a method for synthesizing mefenpyr-diethyl, sequentially comprising the following steps of: allowing esterification reaction of haloacetic acid and 2-heptanol in the presence of catalyst to obtain haloacetic acid-2-heptanol, and allowing condensation reaction of the haloacetic acid-2-heptanol, 5-chloro-8-hydroxyquinoline and potassium carbonate at a molar ratio of 1:(1-1.2):(1-4) in a solvent by heating, stirring and refluxing for 2.5-8 hours to obtain mefenpyr-diethyl. The solvent is toluene or a mixture of toluene and dimethyl formamide. The synthetic method has the advantages of short synthetic route, high yield, and low cost.
Owner:SUZHOU CHIEN SHIUNG INST OF TECH
Amino acid type chelating agent as well as large-scale preparation method and application thereof
InactiveCN109912440ARaw materials are easy to getNo emissionsOrganic compound preparationAmino-carboxyl compound preparationIodideReaction temperature
The invention provides degradable amino acid type chelating agent composition suitable for large-scale industrial production and a preparation method of the degradable amino acid type chelating agentcomposition. Amino acid, haloacetic acid and alkali in an aqueous solution are subjected to a reaction at reaction temperature of 40-120 DEG C in a regulated pH range of 8-13 under the action of an iodide catalyst, and after the reaction, the amino acid type chelating agent composition is obtained. The method has the characteristics of being safe, efficient and free of amplification effect, containing fewer impurities and facilitating realization of large-scale production; the raw materials are easily available, and reaction yield is high; an obtained product has better application performance. The invention further provides an application of the composition as a chelating agent in the fields of daily chemicals, food, agriculture, forestry, papermaking, textile, printing and dyeing, watertreatment, coal and the like.
Owner:NANJING HUASHI NEW MATERIAL
Straw-based amphoteric dye adsorbent as well as preparation method and application thereof
InactiveCN112934188AShort selectivityShort adsorption timeOther chemical processesWater contaminantsCelluloseEthyl ester
The invention relates to the technical field of dye adsorbents, in particular to a straw-based amphoteric dye adsorbent as well as a preparation method and an application thereof. The preparation method comprises the following steps: by taking straw as a polymer skeleton, firstly reacting with haloacetic acid in an ethanol-water mixed system, grafting carboxyl on a cellulose molecular chain of the straw to prepare carboxymethyl straw cellulose, then carrying out graft copolymerization on the carboxymethyl straw cellulose and dialkylaminoethyl methacrylate, and grafting tertiary amino on a cellulose molecular chain of the straw, and forming the straw-based amphoteric adsorbent with both anions and cations. The method is simple to operate, mild in reaction condition and short in synthesis path, the used main raw material is waste straw, and the cost is low, so that the obtained product has good environmental friendliness, can be used as an adsorbent for treating anionic dyes and cationic dyes, has the characteristics of strong adsorption capacity, high decolorization rate, good selectivity, short adsorption time, wide application range and the like, is beneficial to industrial practical application, and has important significance in the field of environmental protection.
Owner:QINGDAO TECHNOLOGICAL UNIVERSITY
Process and composition for lower toxicity quaternary ammonium compounds
ActiveUS7314951B2Low toxicityEasy to implementOrganic compound preparationAmino-carboxyl compound preparationAmmonium compoundsBetaine
Betaine ester quaternary ammonium compounds with reduced toxicity and improved biodegradability are formed by esterification of a haloacetic acid with an alcohol containing at least 4 carbon atoms, followed by quaternization of the halo-acetate with a tertiary amine containing at least 4 carbon atoms. In one non-limiting embodiment the alkyl substituents on the nitrogen of the tertiary amine have at least 2 carbon atoms, and in another non-limiting embodiment are each n-butyl.
Owner:BAKER HUGHES HLDG LLC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com