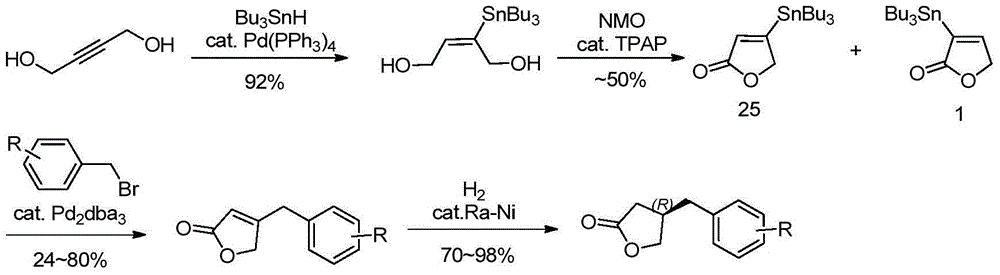
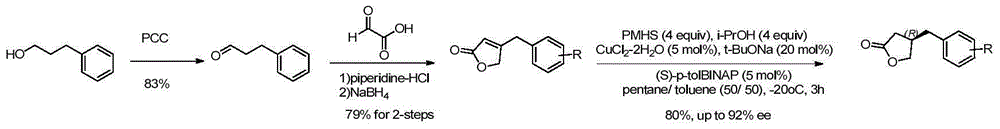
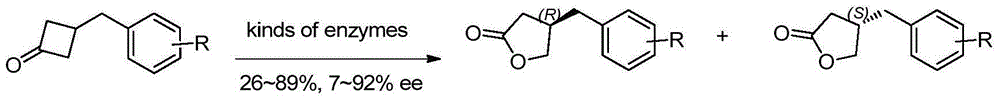
Method for efficiently synthesizing beta-benzyl butyrolactone having specific configuration
A technology of benzylbutyrolactone and configuration, applied in the field of medicine, can solve the problems of poor substrate applicability, difficult preparation of raw materials, and high preparation cost, and achieve the effects of short steps, cheap raw materials and high optical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0042] 1. Preparation of (R)-β-(3,4-dimethoxybenzyl)butyrolactone
[0043]
[0044] 3,4-dimethoxyphenylpropionic acid (2.1g, 10mmol, 1.0eq.) was dissolved in 60mL of anhydrous THF, pivaloyl chloride (1.2mL, 10mmol, 1.0eq.) was added dropwise at -20°C and Triethylamine (4.2mL, 30mmol, 3.0eq.), continue stirring for 20min. A THF solution (20 mL) of (S)-4-benzyl-2-oxazolidinone (1a, 1.6 g, 9.0 mmol, 0.9 eq.) was added dropwise, and LiCl (420 mg, 10 mmol, 1.0 eq.) was added in one portion , Stirring was continued at this temperature for 20 min. Then it was raised to room temperature and stirred for 2h. TLC showed the starting material was completely reacted. The reaction solution was concentrated under reduced pressure to about 20 mL, diluted with ethyl acetate, washed successively with 5% aqueous potassium bisulfate solution, 10% aqueous sodium bicarbonate solution, and saturated sodium chloride, dried over anhydrous sodium sulfate, filtered with suction, and the filtrate w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com