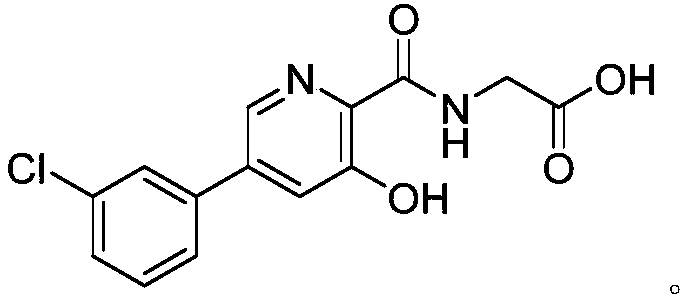
Preparation method and application of picolinamide
A technology of pyridine amide and picolinamide group, applied in the field of preparation of pyridine amide, can solve the problems of high requirements on reaction vessel, inability to enlarge, inconvenient operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
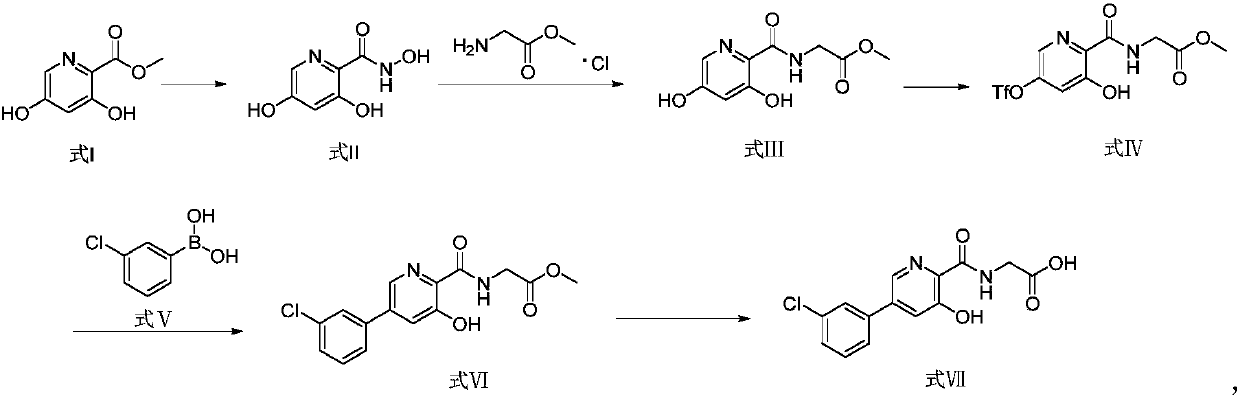
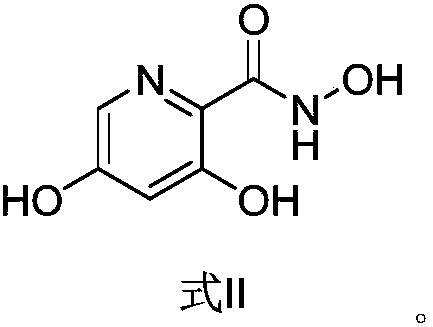
[0049] Synthesis of 3,5-dihydroxy-2-pyridinecarboylhydroxamic acid
[0050] Add methyl 3,5-dihydroxy-2-pyridinecarboxylate (6.0g), hydroxylamine hydrochloride (4.8g), KOH solid (9.9g) and MeOH (methanol, 120mL) into the reaction flask at room temperature. After the reaction was completed, the pH was adjusted to 5-6 with 20% acetic acid aqueous solution, and the organic phase was extracted and separated with 240 mL ethyl acetate, and concentrated to dryness to obtain 5.16 g of solid product, with a yield of 85.50% and a purity of 91.80%.
[0051] MS: [M+1] = 171.1;
[0052]1H NMR (400MHz, DMSO) δ7.67(d, J=2.3Hz, 1H), 6.63(d, J=2.3Hz, 1H), 13CNMR (101MHz, DMSO) δ166.18(s), 158.96(s ), 158.91(s), 130.51(s), 123.05(s), 109.75(s).
Embodiment 2
[0054] Synthesis of (3,5-dihydroxy-2-pyridinecarboxamido)methyl acetate
[0055] Add 3,5-dihydroxy-2-pyridineformylhydroxamic acid (2.0g), iodine element (6.0g), glycine methyl ester hydrochloride (3.0g), triethylamine (3.6g) into the reaction flask at room temperature ) and dimethyl sulfoxide (20.0mL), add and stir the reaction at room temperature. After completion, use 80mL of ethyl acetate to extract and separate liquids to obtain an organic phase. %.
[0056] MS: [M+1] = 227.1;
[0057] 1H NMR (400MHz, DMSO) δ12.27(s, 1H), 10.82(s, 1H), 9.11(t, J=5.9Hz, 1H), 7.77(d, J=2.2Hz, 1H), 6.69( d, J=2.2Hz, 1H), 4.05(d, J=6.1Hz, 2H), 3.66(s, 3H).
Embodiment 3
[0059] Synthesis of Methyl (3-Hydroxy-5-trifluoromethanesulfonyloxy-2-pyridinecarboxamido)acetate
[0060] Add methyl (3,5-dihydroxy-2-pyridinecarboxamido)acetate (1.8g), methanol (54.0mL) and N,N-dimethylformamide (27.0mL) into the reaction flask at room temperature, add After completion, the temperature was lowered to 0°C under the protection of nitrogen, and then N-phenylbis(trifluoromethanesulfonyl)imide (2.8g) and DIPEA (N,N'-diisopropylethylamine, 1.0g) were added, and the After the reaction was completed, 80 mL of ethyl acetate was used to extract and separate the liquid to obtain an organic phase, which was concentrated to dryness to obtain 2.19 g of solid, with a yield of 76.77% and a purity of 94.50%.
[0061] MS: [M+1] = 358.9;
[0062] 1H NMR (600MHz, DMSO) δ12.64(s, 1H), 9.61(s, 1H), 8.43(d, J=2.3Hz, 1H), 7.87(d, J=2.4Hz, 1H), 4.11( d, J=6.2Hz, 2H), 3.68(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com