Improved preparation method of ampicillin
An ampicillin and enzymatic preparation technology, applied in directions such as fermentation, can solve problems such as unfavorable economy and environmental protection, large amount of hydrochloride, inappropriate proportion, etc., and achieve the effects of good product quality, high quality, and reducing the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
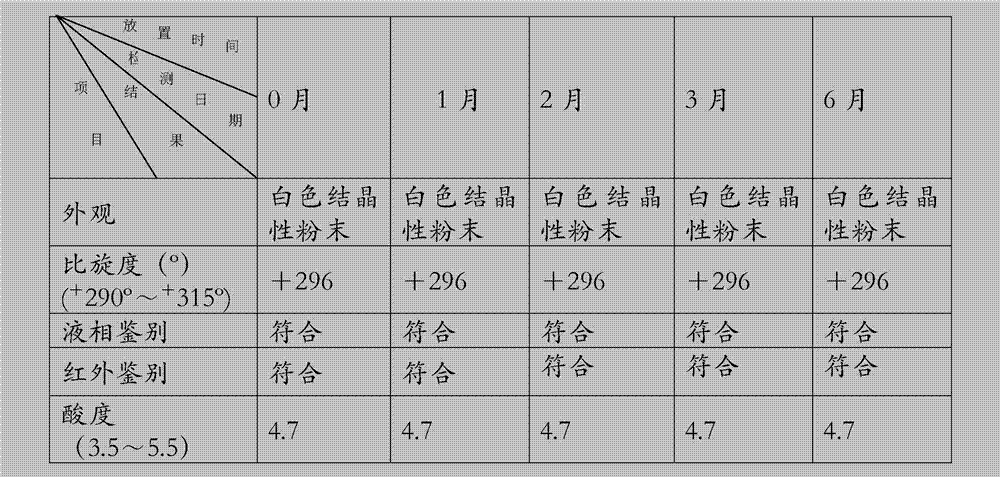
Image
Examples
Embodiment 1
[0039] Material preparation:
[0040] 6-APA35g; D-phenylglycine methyl ester 29g; ampicillin synthase 1.0KU / L; 98% acetone 35ml; EDTA-2Na0.35g; 10% hydrochloric acid 56ml; ; Purified water 585ml.
[0041] making process:
[0042] Add 350ml of purified water into the reactor, add 35g of 6-APA and 29g of D-phenylglycine methyl ester under stirring and stir evenly.
[0043] Cool down to 10°C, add 1.0KU / L of ampicillin synthase, use 3mol / L ammonia water to control pH 6.4-6.5 throughout the reaction, temperature 10±0.5°C, and stop the reaction when the residual concentration of 6-APA is less than 2mg / ml. The dosage of 3mol / L ammonia water is 2ml.
[0044] Ampicillin synthase is separated to obtain ampicillin crude product.
[0045] Put the crude ampicillin into the beaker, add 200ml of purified water, raise the temperature to 20°C, add 56ml of 10% hydrochloric acid dropwise, then the ampicillin dissolves, pH = 1.01, add 0.35g of EDTA-2Na, stir for 10min, and filter to obtain th...
Embodiment 2
[0048] Material preparation:
[0049] 6-APA35g; D-phenylglycine methyl ester hydrochloride 35g; ampicillin synthase 1.0KU / L; 80% acetone 35ml; EDTA-2Na0.35g; 15% hydrochloric acid 37ml; Sodium aqueous solution 78ml; purified water 585ml.
[0050] making process:
[0051] Add 350ml of purified water into the reactor, add 35g of 6-APA and 35g of D-phenylglycine methyl ester hydrochloride under stirring, and stir evenly.
[0052] Cool down to 11°C, add 1.3KU / L of ampicillin synthase, use 3mol / L ammonia water to control pH 6.3-6.4 throughout the reaction, the temperature is 11±0.5°C, stop the reaction when the residual concentration of 6-APA is less than 2mg / ml, The dosage of 3mol / L ammonia water is 55ml.
[0053] Ampicillin synthase is separated to obtain ampicillin crude product.
[0054] Put crude ampicillin into the beaker, add 200ml of purified water, heat up to 21°C, add 37ml of 15% hydrochloric acid dropwise, and then ampicillin dissolves, pH = 1.04.
[0055] Transfer ...
Embodiment 3
[0057] Material preparation:
[0058] 6-APA35g; D-phenylglycine methyl ester hydrochloride aqueous solution 88ml (concentration 400mg / ml); ampicillin synthase 1.2KU / L; 70% methanol 35ml; EDTA-2Na0.35g; 20% hydrochloric acid 28ml; 3mol / L Ammonia water 46ml; 15% sodium hydroxide aqueous solution 51ml; purified water 497ml.
[0059] making process:
[0060] Add 262ml of purified water into the reactor, add 35g of 6-APA and 88ml of D-phenylglycine methyl ester hydrochloride aqueous solution under stirring, and stir evenly.
[0061] Cool down to 12°C, add ampicillin synthase 1.2KU / L, use 3mol / L ammonia water to control pH 6.3-6.4 throughout the reaction, temperature 12±0.5°C, stop the reaction when the residual concentration of 6-APA is less than 2mg / ml, The dosage of 3mol / L ammonia water is 46ml.
[0062] Ampicillin synthase is separated to obtain ampicillin crude product.
[0063] In a beaker, put crude ampicillin, add 200ml of purified water, heat up to 22°C, add 28ml of 20%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com