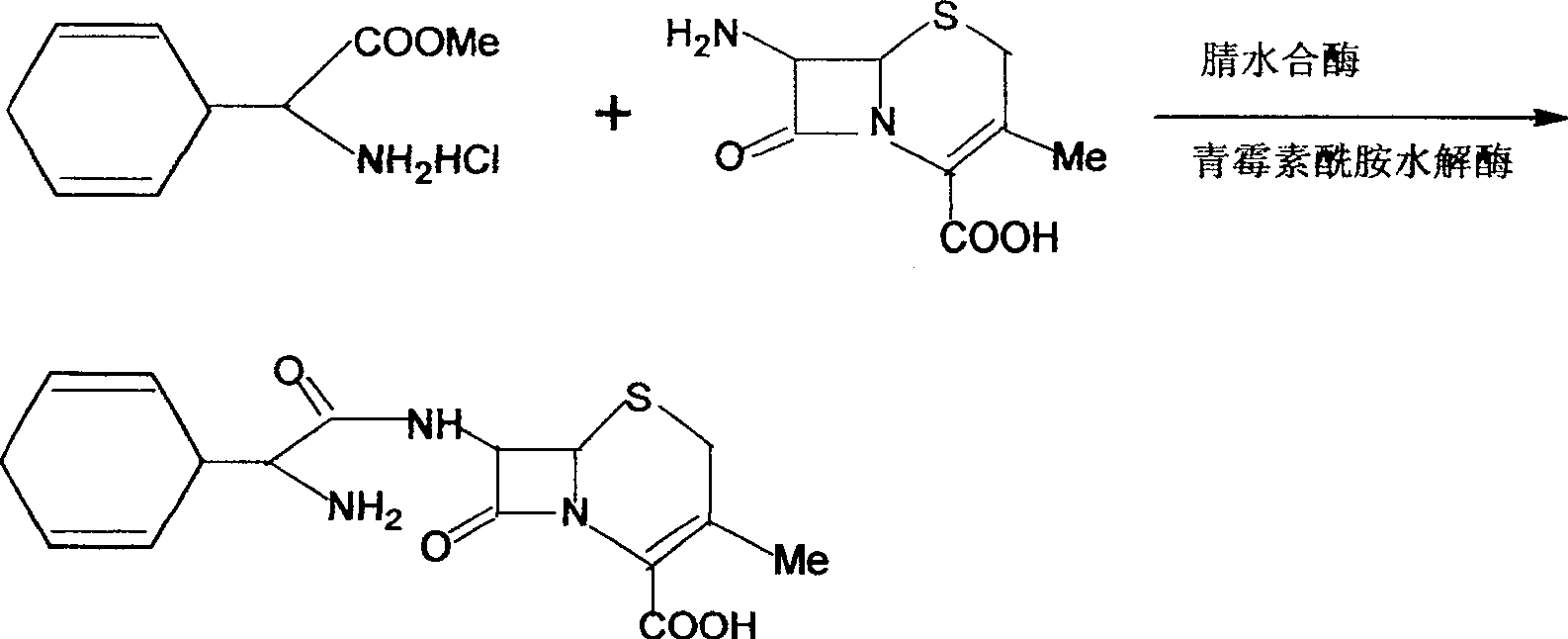
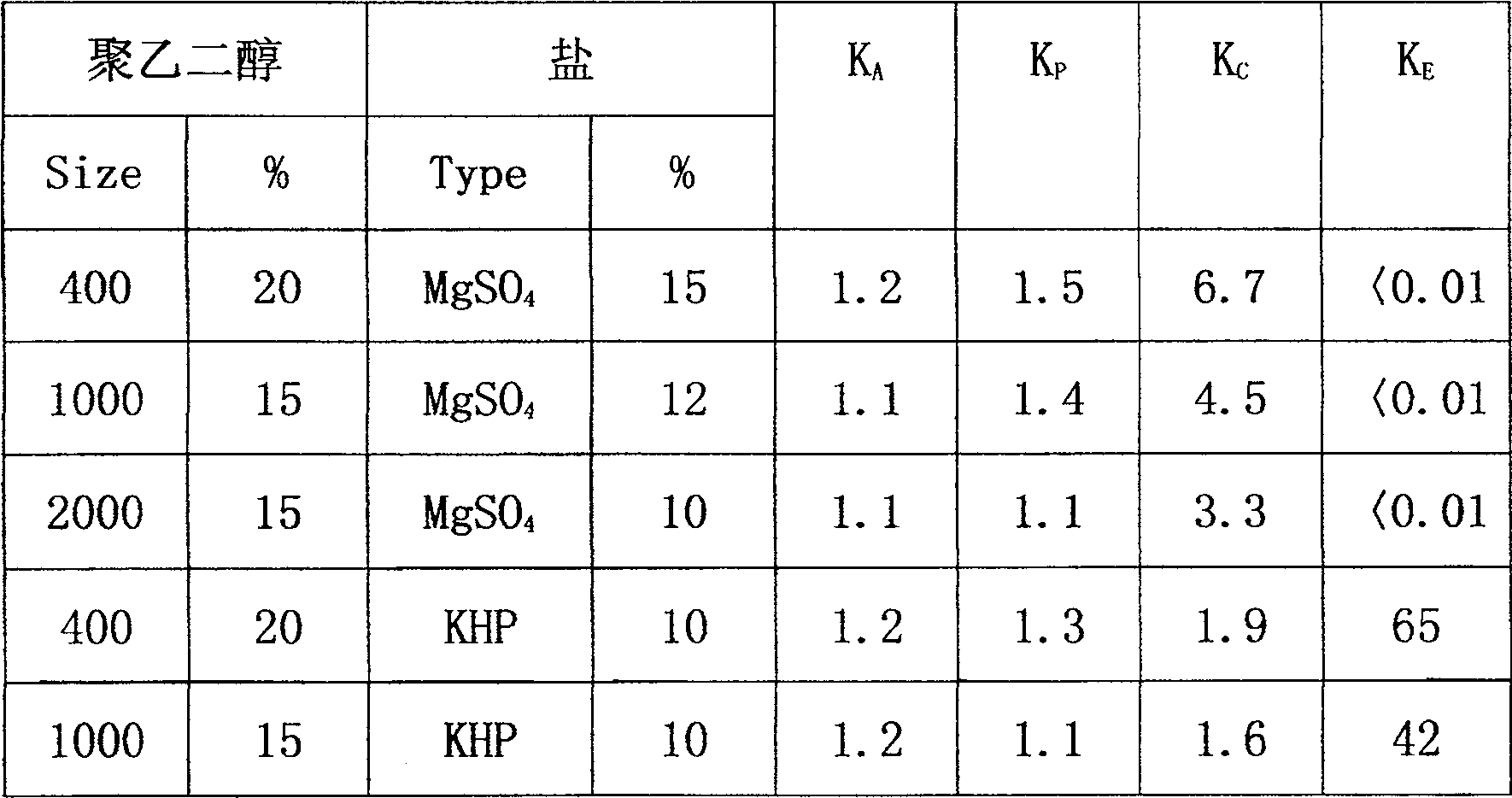
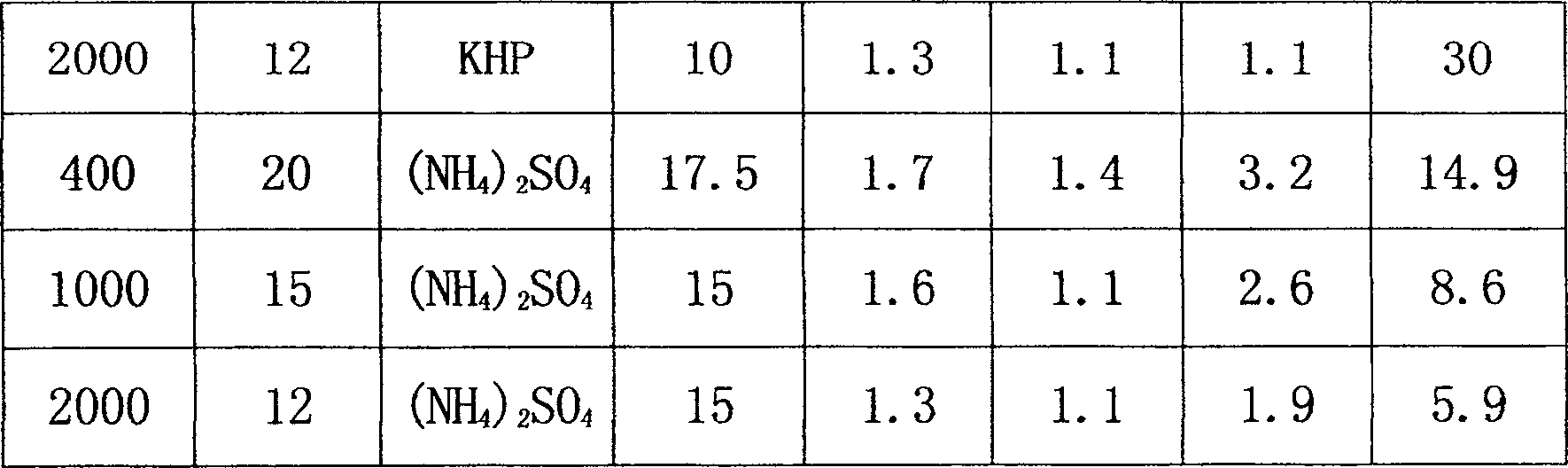
Cefradine preparing process
A technology of cephradine and polyethylene glycol, applied in the field of biopharmaceuticals, can solve the problems that the catalytic performance is easily affected by pH value, temperature, ionic strength and organic solvents, the catalyst performance cannot be maximized, and the cost of biocatalysts is expensive. , to achieve the effects of continuous and automatic control, easy separation, and improved conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a reaction vessel equipped with a two-phase aqueous system at a ratio of 1:1, add 4mol / L sulfuric acid to adjust the pH of the system to 5.0, and use a temperature control device to control the reaction liquid The temperature is controlled at T=5℃, and the immobilized penicillin acylase (carrier is polypropylene fiber, acetate fiber, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T=15°C, PH=6.5, continuous reaction for about 3 hours, proofing analysis result is that 65% of 7-ADCA has been converted into cefradine. At this stage, samples are taken from the upper and lower phases every half an hour, and the concentration of reactants and products are analyzed by HPLC. After the final detection, the centrifuge device is rotated to separate the upper and lower phases again, and the upper phase sol...
Embodiment 2
[0034] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a reaction vessel equipped with a two-phase aqueous system at a ratio of 1:1, add 4mol / L sulfuric acid to adjust the pH of the system to 6.5, and use a temperature control device to control the reaction liquid The temperature is controlled at T=20℃, and the immobilized penicillin acylase (carrier is polypropylene fiber, acetate fiber, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T=15°C, PH=6.5, continuous reaction for about 3 hours, proofing analysis result shows that 70% of 7-ADCA has been converted into cefradine. At this stage, samples are taken from the upper and lower phases every half an hour, and the concentration of reactants and products are analyzed by HPLC. After the final detection, the centrifuge device is rotated to separate the upper and lower phases again, and the upper phase...
Embodiment 3
[0036] Dissolve dihydrophenylglycine methyl ester and 7-ADCA in a reaction vessel equipped with a two-phase aqueous system at a ratio of 1.5:1, add 4mol / L sulfuric acid to adjust the pH of the system to 6.5, and use a temperature control device to control the reaction liquid The temperature is controlled at T=10℃, and the immobilized penicillin acylase (carrier is polypropylene fiber, cellulose acetate, amino silica gel, resin, diatomaceous earth) and nitrile hydratase are added for about 1 hour, and they are carried out under the following conditions Acylation: temperature T=15°C, PH=6.5, continuous reaction for about 3 hours, proofing analysis result shows that 75% of 7-ADCA has been converted into cefradine. At this stage, samples are taken from the upper and lower phases every half an hour, and the concentration of reactants and products are analyzed by HPLC. After the final detection, the centrifuge device is rotated to separate the upper and lower phases again, and the upper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com