Ampicillin preparation technology by direct method
A technology for preparing ampicillin and ampicillin, which is applied in the field of pharmaceutical preparation, can solve the problems of high cost, achieve high conversion rate, reduce energy consumption, and increase profits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
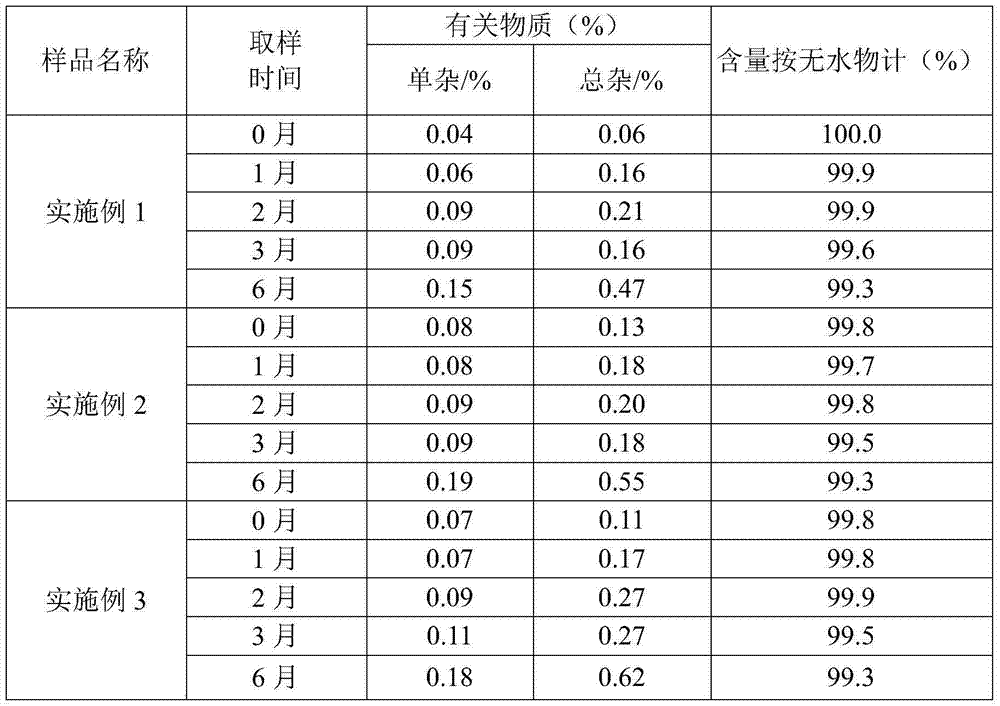
Embodiment 1
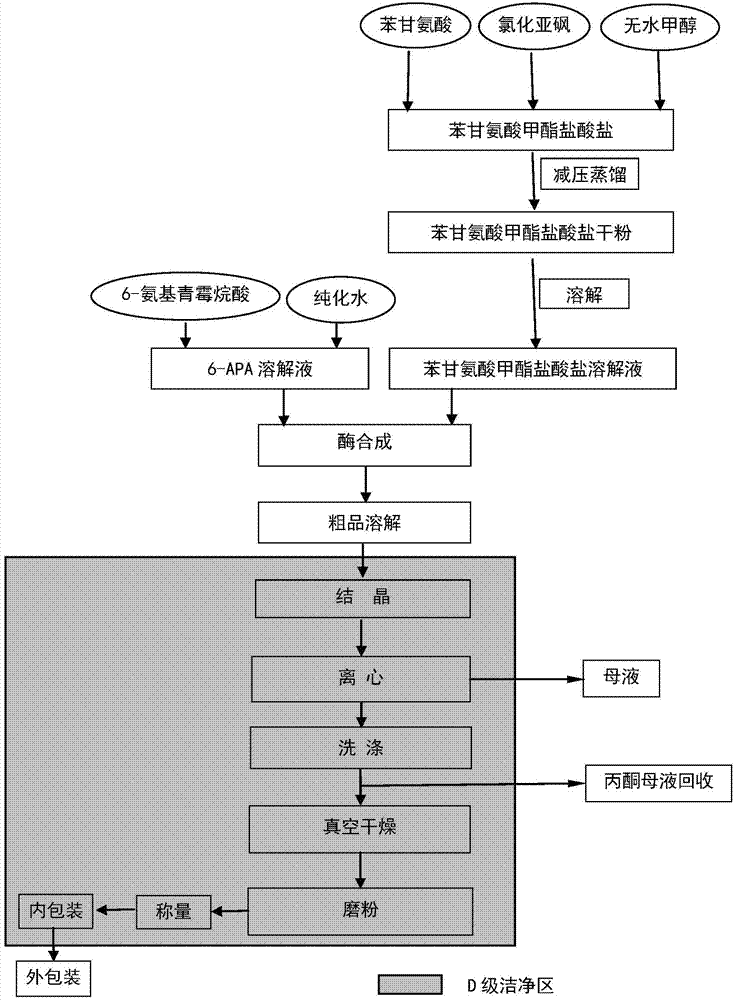
[0017] Embodiment 1 Straight-through method prepares ampicillin
[0018] (1) Dissolve 0.4mol L-phenylglycine in 4.8mol anhydrous methanol, add thionyl chloride dropwise under ice-water bath cooling and stir, wherein, the molar ratio of L-phenylglycine, anhydrous methanol and thionyl chloride is 1 :12:1; after the dropwise addition of thionyl chloride, heat and reflux in a 65°C water bath for 1.8h, the rate of dropping thionyl chloride is 4mL / min; when the purity of phenylglycine methyl ester hydrochloride is ≥97%, Stop the reaction, distill the unreacted methanol under reduced pressure at a temperature of 60°C and a vacuum of ≥0.070Mp, add water to the obtained white solid until the water is dissolved and clear, then adjust the pH to 3.0 with 10% ammonia water, The solution of L-phenylglycine methyl ester hydrochloride was obtained;
[0019] (2) 0.36mol6-APA is dissolved in water and adjusted to pH 8.0 with 10% ammonia water to clarify the solution, and put into the reactor t...
Embodiment 2
[0021] Embodiment 2 Straight-through method prepares ampicillin
[0022] (1) Dissolve 0.4mol L-phenylglycine in 4.0mol anhydrous methanol, add thionyl chloride dropwise under ice-water bath cooling and stir, wherein, the molar ratio of L-phenylglycine, anhydrous methanol and thionyl chloride is 1 :10:1; after the dropwise addition of thionyl chloride, heat and reflux in a 65°C water bath for 1.5h, and dropwise add thionyl chloride at a speed of 3mL / min; when the purity of phenylglycine methyl ester hydrochloride is ≥97%, Stop the reaction, distill the unreacted methanol under reduced pressure at a temperature of 55°C and a vacuum degree of ≥0.070Mp, add water to the obtained white solid until the water is dissolved and clear, adjust the pH to 2.0 with 5% ammonia water, The solution of L-phenylglycine methyl ester hydrochloride was obtained;
[0023] (2) 0.37mol6-APA is dissolved in water and adjusted to pH 7.5 with 5% ammonia water to clarify the solution, and put into the re...
Embodiment 3
[0025] Embodiment 3 Straight-through method prepares ampicillin
[0026] (1) Dissolve 0.4mol L-phenylglycine in 5.6mol anhydrous methanol, add thionyl chloride dropwise under ice-water bath cooling and stir, wherein, the molar ratio of L-phenylglycine, anhydrous methanol and thionyl chloride is 1 :14:1; after the dropwise addition of thionyl chloride, heat and reflux in a 65°C water bath for 1.5h, the speed of dropping thionyl chloride is 5mL / min; when the purity of phenylglycine methyl ester hydrochloride is ≥97%, Stop the reaction, distill the unreacted methanol under reduced pressure at a temperature of 65°C and a vacuum degree of ≥0.070Mp, add water to the obtained white solid until the water is dissolved and clear, adjust the pH to 4.0 with 15% ammonia water, The solution of L-phenylglycine methyl ester hydrochloride was obtained;
[0027] (2) 0.34mol6-APA is dissolved in water and adjusted to pH 8.5 with 15% ammonia water to clarify the solution, and put into the reacto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com