Cefaclor and synthetic method thereof
A technology of cefaclor and a synthesis method, applied in the field of cefaclor synthesis and biological synthesis of cefaclor, can solve the problems of complicated separation and purification methods of cefaclor, low purity of cefaclor, long reaction period and the like, and achieves shortening The effect of synthesis cycle, high product yield and simple circuit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Take 30g (128mmol) of 7-ACCA, add 200ml of pure water and dissolve it with 6mol / L ammonia water, the pH after dissolving is 7.8~8.1, add 2000U penicillin acyltransferase, add D-phenylglycine methyl ester hydrochloride at a constant speed within 0~10 minutes Solution (solution preparation method 28.4g (141mmol) solid was added to 60ml pure water). Five minutes after the reaction started, before 0.2% (w / v) cefaclor was precipitated in the reaction solution, cefaclor seed crystals (purity greater than 90%) were added in one go. The synthetic pH is 6.4 and the temperature is 15°C.
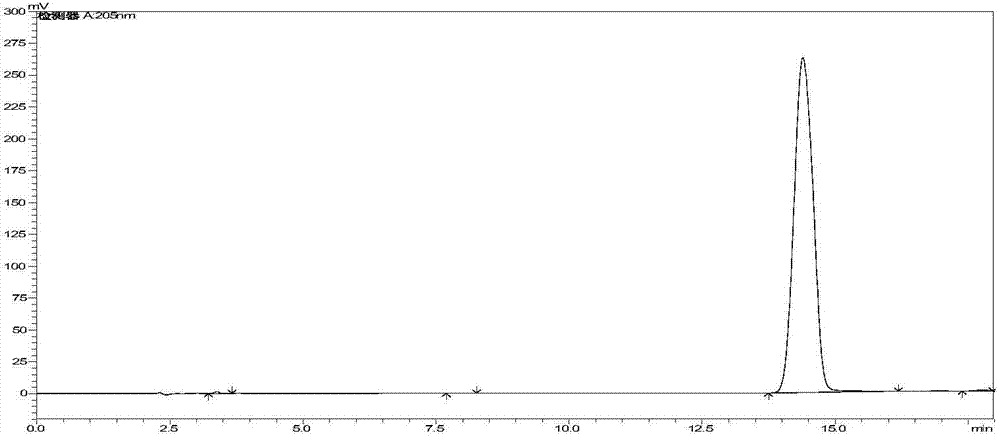
[0049] After reacting for 120 minutes, sample HPLC detection, the 7-ACCA concentration at this time was 0.27mg / ml (see figure 1 ), 7-ACCA conversion rate 99.6%, sieve separation penicillin acylase and cefaclor suspension, cefaclor suspension weight after refining is 43.6g, and use 2-naphthol to reclaim cephalosporin from crystallization mother liquor Clo, obtain 2.9g of reclaimed cefaclor, and...
Embodiment 2
[0051] Take 30g (128mmol) 7-ACCA, add 200ml pure water, add 6mol / L ammonia water to dissolve, add 2000U penicillin acyltransferase, add D-phenylglycine methyl ester hydrochloride solution at a uniform speed within 0-10 minutes (solution preparation method 28.4g (141mmol) solid adds 60ml pure water), after reaction begins 15 minutes, before cefaclor does not separate out in the reaction solution, the cefaclor 2.9g crystal seed (purity is greater than 90%) that 2-naphthol reclaims among the example 1 is added disposablely ), synthesis pH6.4, temperature 15°C.
[0052] After 120 minutes of reaction, samples were taken for HPLC detection. At this time, the concentration of 7-ACCA was 0.38 mg / ml, and the conversion rate of 7-ACCA was 99.5%. The penicillin acylase and cefaclor suspension were separated by screen, and the cefaclor suspension was refined. After weight is 44.50g, cefaclor weight yield is 1.48.
Embodiment 3
[0054] Take 7-ACCA and D-phenylglycine methyl ester hydrochloride at a molar ratio of 1:1.05. Add pure water to 7-ACCA and dissolve it with 6mol / L ammonia water, add 12000U penicillin acyltransferase, add D-phenylglycine methyl ester hydrochloride aqueous solution at a constant speed within 0-30 minutes, and add 5% ( w / v) Cefaclor as seed crystal (purity greater than 50%), reaction pH 5.8, temperature 5°C.
[0055] Samples were taken at 60 minutes for HPLC detection. At this time, the concentration of 7-ACCA was 0.18 mg / ml, the conversion rate of 7-ACCA was 99.7%, and the total weight yield of cefaclor was 1.49.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com