D-phenylglycine methyl ester phosphate crystal, preparation method and solution
A technology of phenylglycine methyl ester and phenylglycine, which is applied in the field of D-phenylglycine methyl ester phosphate crystallization and preparation method, and solution field, can solve the problems of equipment corrosion, low synthesis conversion rate, etc., and achieves the benefit of production amplification and improved reaction effect. , the effect of significant economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
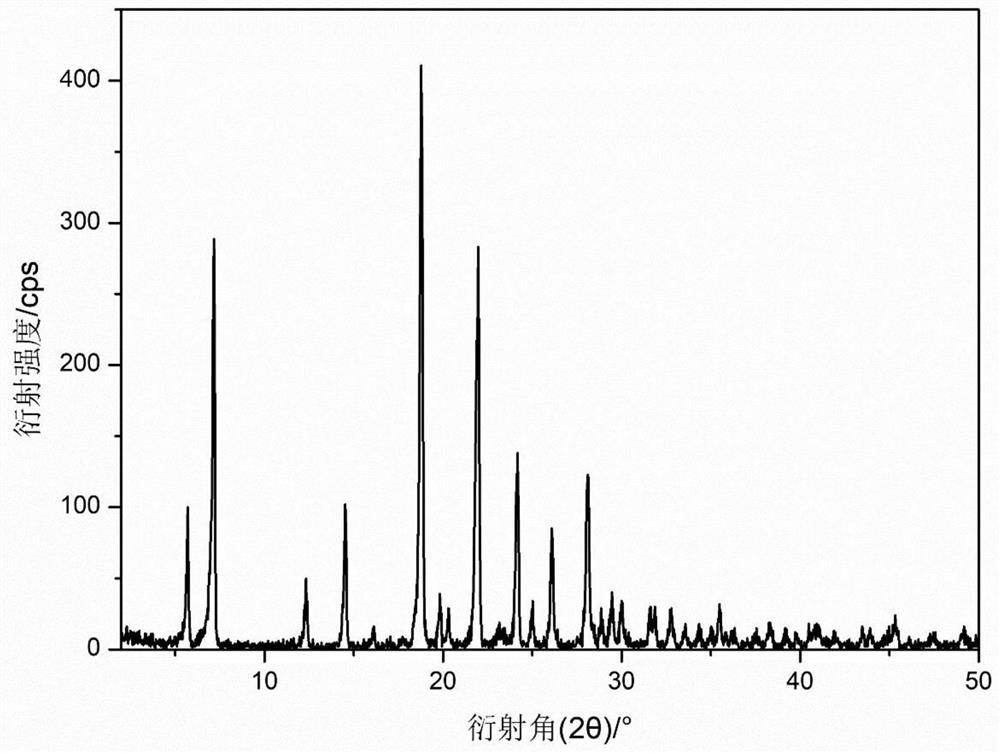
[0042] The preparation of embodiment 1D-phenylglycine methyl ester phosphate crystal
[0043]In a 200L reaction tank, add 80L of methanol, add 20Kg of D-phenylglycine while stirring, after stirring evenly, add 20Kg of phosphoric acid, and control the temperature below 40°C. After the feeding was completed, the temperature in the reaction tank was maintained at 75° C., and the reflux reaction was carried out for 2 hours, and the reflux reaction was completed. Then carry out vacuum dehydration, adopt the mode of vacuum distillation, specifically, the vacuum degree in the reaction tank is maintained at 0.04~0.06MPa, after the mass content of methanol in the solution in the reaction tank reaches 2%, stop the vacuum distillation. Then add methanol 60L, continue the reflux reaction for 1h, after the reflux reaction ends, distill under reduced pressure, and cool down to crystallize after the mass content of methanol in the solution in the reaction tank reaches 2%, repeat this 5 times...
Embodiment 2
[0049] The preparation of embodiment 2 cephalexin
[0050] Suspend 20 g of 7-aminodeacetoxycephalosporanic acid (7-ADCA) in 100 mL of water, and control the temperature at 25°C. The mixture was stirred for 5 minutes while maintaining the pH at 7.0 by adding 15% aqueous ammonia solution, and 10 g of immobilized enzyme was added. Next, 25 g of D-phenylglycine methyl ester phosphate crystals were added at a constant rate within 90 min. Once all the D-phenylglycine methyl ester phosphate crystallization was added, the pH was maintained at 7.0 by adding 15% aqueous ammonia or by adding 30% aqueous sulfuric acid. After 230 min, the pH was adjusted by adding 30% aqueous sulfuric acid to 5.8. During the reaction, samples were taken and analyzed by high performance liquid chromatography (High Performance Liquid Chromatography, HPLC). The analysis results are shown in Table 2.
[0051] Table 2 Sampling analysis results for the preparation of cephalexin based on D-phenylglycine methyl...
Embodiment 3
[0064] The preparation of embodiment 3 ampicillin
[0065] Suspend 20 g of 6-aminopenicillanic acid (6-APA) in 100 mL of water, and control the temperature at 25°C. The mixture was stirred for 5 minutes while maintaining the pH at 7.0 by adding 15% aqueous ammonia solution, and 10 g of immobilized enzyme was added. Next, 25 g of D-phenylglycine methyl ester phosphate crystals were added at a constant rate within 90 min. Once all of the D-phenylglycine methyl ester phosphate crystallization was added, the pH was maintained at 7.0 by the addition of 15% aqueous ammonia or by the addition of 30% aqueous sulfuric acid. After 230 minutes, the pH was adjusted to 5.8 by the addition of 30% aqueous sulfuric acid . During the reaction, samples were taken and analyzed by HPLC, and the analysis results are shown in Table 5.
[0066] Table 5 Sampling analysis results for the preparation of ampicillin based on the crystallization of D-phenylglycine methyl ester phosphate
[0067]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com