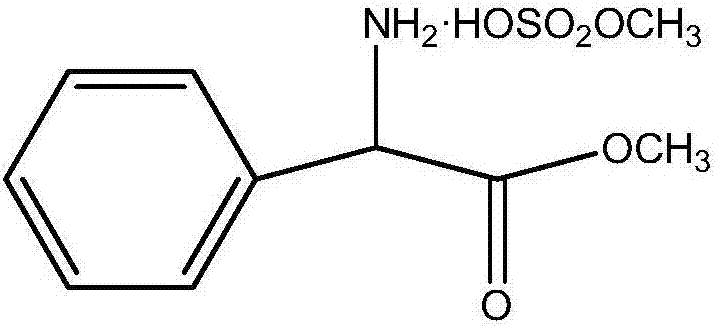
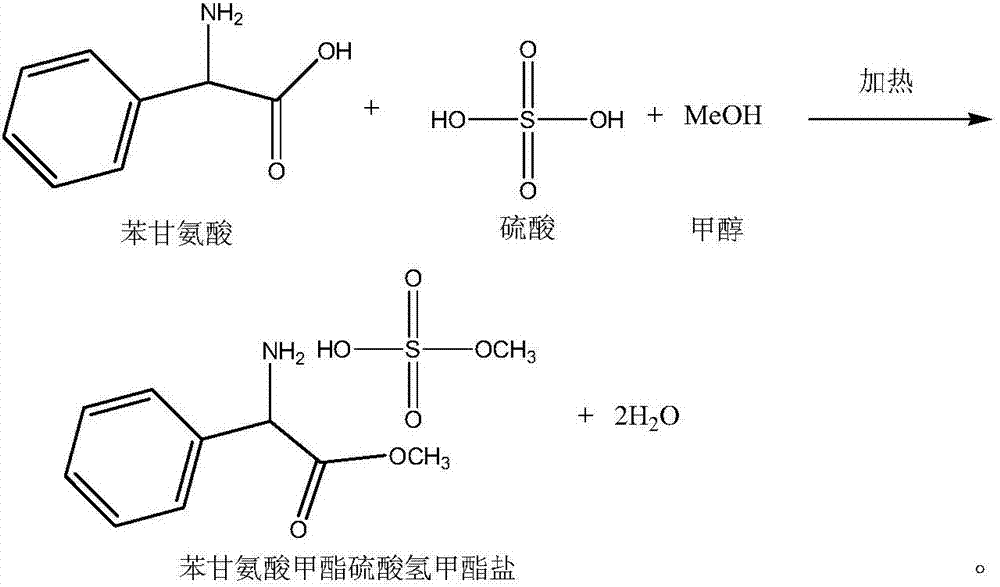
Preparation method of phenylglycine methyl ester methyl hydrogen sulfate
A technology of phenylglycine methyl ester and methyl hydrogen sulfate, applied in the field of pharmaceutical synthesis, can solve problems such as difficulty in waste gas treatment, and achieve the effects of high esterification reaction yield, reduced production cost, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Test device: 500ml glass reaction bottle, equipped with a vacuum-insulated glass split column with a length of 150cm, an inner diameter of 2.5cm, and a built-in glass spring packing; a water distributor (water outlet from the upper end) and a glass condenser at the top of the fractionation column.
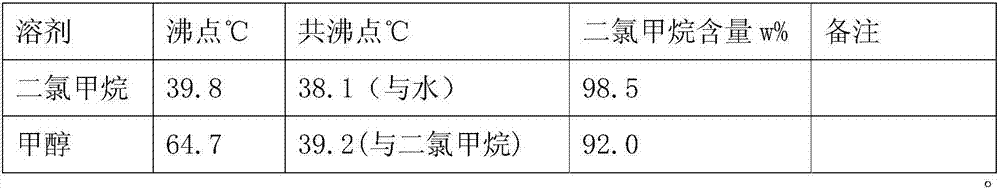
[0049]Test operation: Add 100g of L-phenylglycine and 250ml of methanol into the reaction bottle, add 80g of sulfuric acid dropwise under stirring, heat up to 70-75°C, reflux for 1 hour, cool slightly, add 100ml of dichloromethane, heat up to reflux, and reach the top temperature When the temperature is stable at 38.1-38.2°C, start to fractionate with water, the fractionated methylene chloride / water azeotrope is condensed and layered in the water separator, the water is in the upper layer, which can be separated from the upper end, and the lower layer is dichloromethane , back into the fractionation column. After refluxing with water for about 6 hours, when almost no water c...
Embodiment 2
[0060] Test device: 500ml glass reaction bottle, equipped with a vacuum-insulated glass split column with a length of 150cm, an inner diameter of 2.5cm, and a built-in glass spring packing; a water distributor (water outlet from the upper end) and a glass condenser at the top of the fractionation column.
[0061] Test operation: Add 100g of L-phenylglycine and 300ml of recovered methanol into the reaction flask, add 80g of sulfuric acid dropwise under stirring, heat up to 70-75°C, reflux for 1 hour, cool slightly, add 120ml of recovered dichloromethane, heat up to reflux, until When the top temperature is stable at 38.1-38.2°C, fractional distillation begins with water, and the dichloromethane / water azeotropic mixture obtained by fractionation is condensed and layered in the water separator. The water is in the upper layer and can be separated from the upper end, and the lower layer is two Chloromethane, back into the fractionation column. After refluxing with water for about ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com