Immobilized alpha-amino-acid ester hydrolase, preparation and application thereof
An amino acid ester, hydrolase technology, applied in hydrolase, immobilized on/in organic carriers, chemical industry, etc., can solve the problem of desorption of enzymes and other proteins, poor thermal stability of immobilized enzymes, and inability to ensure that enzymes and Problems such as the firmness of the carrier to achieve a wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
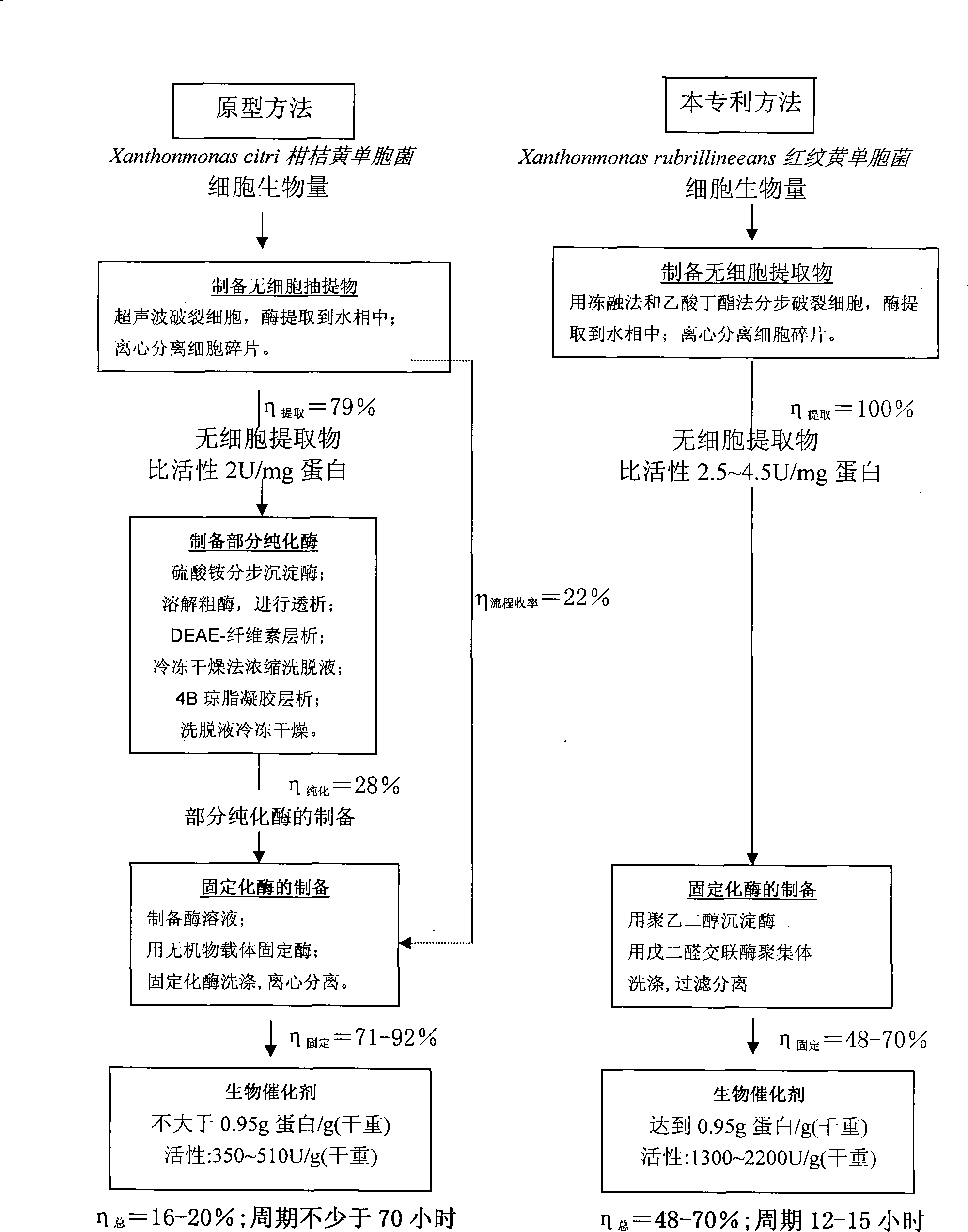
[0056] The preparation process of the heterogeneous biocatalyst is illustrated by the following examples and the usage of the enzyme product. In the following examples, the activity of the enzyme preparations is calculated relative to cephalexin synthesis. The initial velocity of target product formation is measured under the following conditions: pH6.0-6.2, 40±1°C, 7-ADCA concentration 0.040±0.002mole / l, phenylglycine methyl ester (MethylEster Phenyl Glycine, MEPhGl) 0.080±0.004mole / l l.
[0057] The amount of dry matter contained in the preparation is determined after drying the preparation to constant weight at 105±1°C.
Embodiment 1
[0058] Embodiment 1 prepares biocatalyst method with Xanthomonas rhodoprint strain VKPM B-9915
[0059] The same bacterial strain used in the present invention has been submitted to the "China Microbiological Culture Collection Management Committee General Microbiology Center (CGMCC)" and "All-Russia Industrial Microbiology Preservation Department (VKPM)" for preservation, and the bacterial strains given by CGMCC and VKPM have been obtained. No. CGMCC №.2339 and VKPM B-9915, the proposed taxonomic designations are Xanthomonas rubrilineans and Xanthomonas rubrilineans K40, respectively.
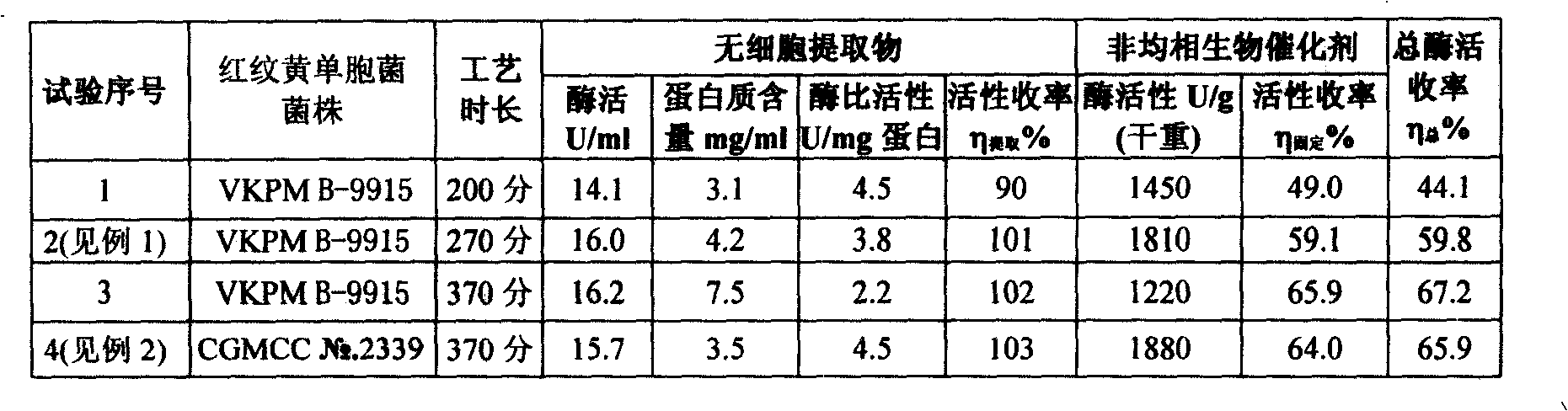
[0060] Get 273g (wet weight) Xanthomonas rhodoprint strain VKPM B-9915 cell biomass as the starting material of heterogeneous biocatalyst, its content is 18.5% (dry weight), synthase activity 83.3U / g (wet weight), 450U / g (dry weight).
[0061] Put the cell biomass into a plastic container, freeze at -28°C for 3 hours, take out the frozen plastic container, let the cell biomass thaw at room te...
Embodiment 2
[0067] Embodiment 2 prepares the method for biocatalyst with Xanthomonas rhodophylla bacterial strain CGMCC №.2339
[0068] Get 91.2g (wet weight) Xanthomonas rhodoprint strain CGMCC No. 2339 cell biomass as the starting material of heterogeneous biocatalyst, its dry weight content 19.5%, synthase activity 70.2U / g (wet weight) , 360U / g (dry weight).
[0069] The cell biomass was treated according to the procedure in Example 1 and kept at -30°C for 5 hours.
[0070] The process of treating the cells with butyl acetate and extracting the enzyme into the aqueous phase is carried out according to the method of Example 1, the conditions are: the suspended cell biomass concentration is 50 mg (dry weight) / ml, the concentration of butyl acetate is 4.0% (by volume), and 28 °C, pH gradient, continuous stirring. The amount of reagents used: buffer 50ml, mercaptoethanol 50ml, butyl acetate 14ml, mixed to obtain 355ml of suspension. The enzyme extraction process takes 6 hours and 10 min...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com