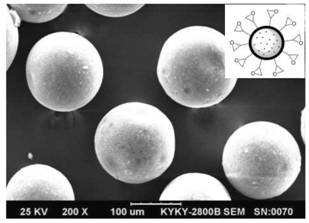
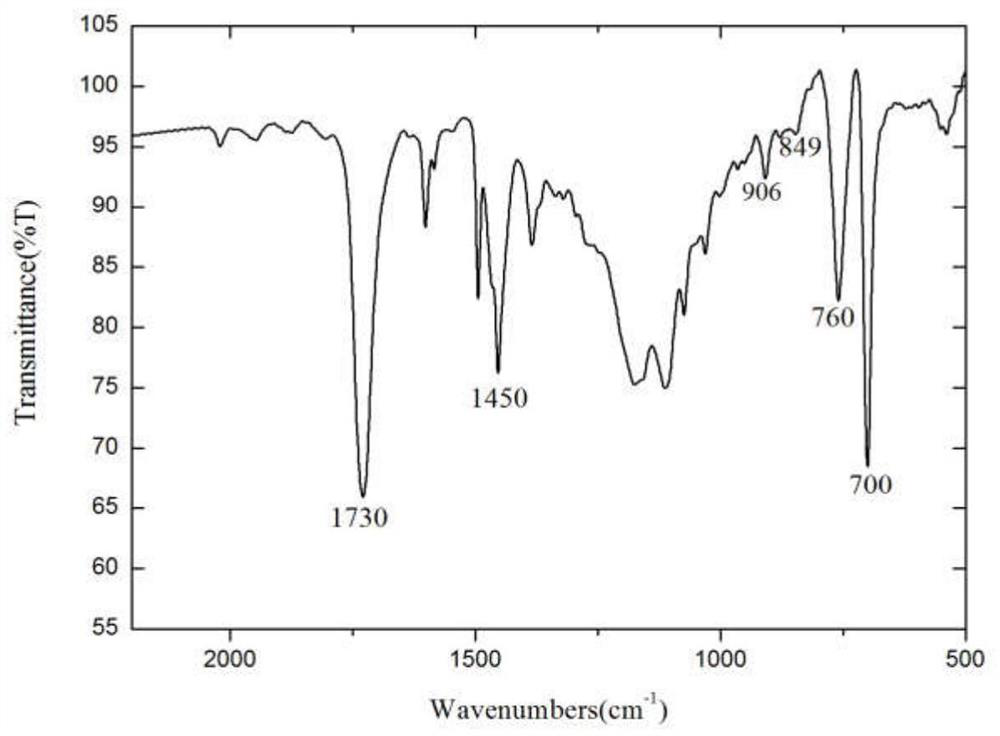
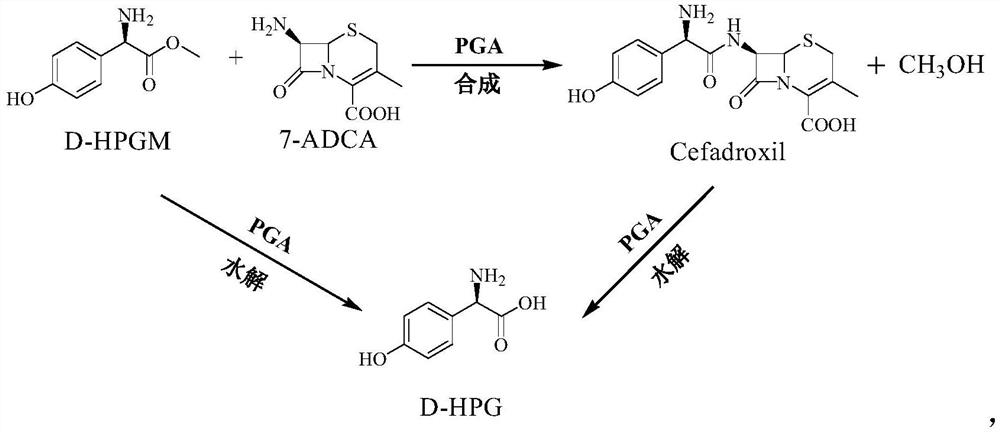
Preparation method of cefadroxil
A cefadroxil and magnetic technology, applied in the field of medicine, can solve the problems of decreased catalytic activity of penicillin acylase, harm to ecological environment due to volatile organic solvent, low yield of target product and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] A method for preparing cefadroxil provided by the invention comprises the following steps: adding a predetermined volume of ionic liquid and a phosphate buffer solution to a reactor with a constant temperature in a water bath to form a co-solvent; adding a predetermined molar ratio of D to the co-solvent -HPGM and 7-ADCA and stirring them, the molar ratio of D-HPGM and 7-ADCA is 1:1~1:2.0; the concentration of 7-ADCA is 0.1~1.0mol / L; The magnetically immobilized penicillin G acylase was added to the co-solvent of ADCA, and the mass ratio of the magnetically immobilized penicillin G acylase to 7-ADCA was 1:10 to 1:50, and the magnetically immobilized penicillin G acylase was continuously stirred. Fully contact with D-HPGM and 7-ADCA until the end of the reaction, wherein the stirring speed is controlled to be 150 to 250r / min; the reaction system liquid after the reaction ends is layered and left to stand, so that the upper layer of phosphate buffered with cefadroxil is di...
Embodiment 1
[0036] Embodiment 1, add 60mL ionic liquid 1-butyl-3-methylimidazole bis-trifluoromethanesulfonimide salt and 40mL sodium phosphate buffer solution (0.10mol / L, pH 7.0) in the reactor, after mixing evenly 0.1 mol of 7-ADCA and 0.15 mol of D-HPGM were added, and the concentration of 7-ADCA at this time was 214 g / L. Then add 0.42g magnetic immobilized penicillin G acylase, at this time the mass ratio of the immobilized enzyme to 7-ADCA is 1:50, the temperature of the water bath is controlled at 30°C, the stirring speed is controlled to be 150r / min, and the reaction is performed for 10 hours. The immobilized enzyme was isolated under the action. The pH value of the upper layer solution of the separated reaction system was adjusted to 5.2, and crystals of cefadroxil were obtained by crystallization. The reactor can be a batch reactor commonly used in the industry.
Embodiment 2
[0037] Embodiment 2, add 60mL ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate and 40mL sodium phosphate buffer solution (0.10mol / L, pH 7.0) in the reactor, add 0.1mol 7- ADCA and 0.15mol D-HPGM, the concentration of 7-ADCA at this time was 214g / L. Then 0.42g of magnetically immobilized penicillin G acylase was added, the mass ratio of the immobilized enzyme and 7-ADCA was 1:50, the temperature of the water bath was controlled at 30°C, the stirring speed was controlled to be 150r / min, and the reaction was performed for 10 hours. The immobilized enzyme was isolated. The pH value of the upper layer solution of the separated reaction system was adjusted to 5.0, and crystals of cefadroxil were obtained by crystallization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com