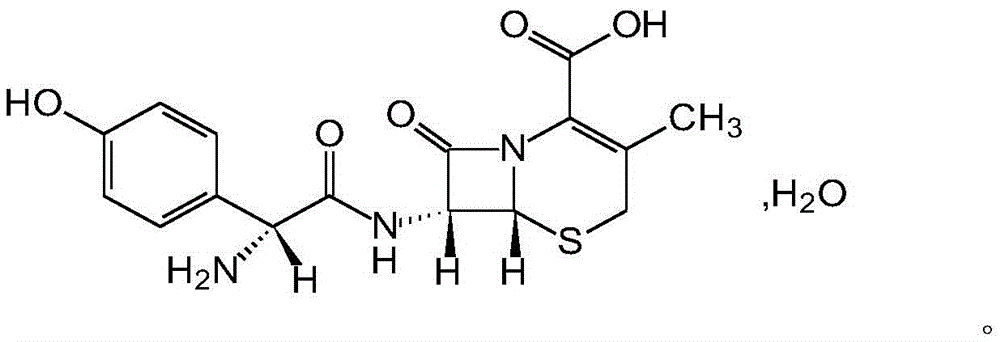
Cefadroxil tablet and preparation method thereof
A technology of cefadroxil tablet and padroxil tablet, which is applied in the field of medicine, can solve the problems of easy hygroscopic degradation, unfavorable long-term storage, safety and effectiveness hidden dangers, etc., and achieve the goal of improving bioavailability and stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 cefadroxil of the present invention
[0044] (1) 100 g of cefadroxil crude product is dissolved in 800 ml of mixed solvent A of tetrahydrofuran and water with a volume ratio of 1:2, and ammonia water is added dropwise to adjust the pH value to 8.0;
[0045] (2) Filter, add dropwise acetic acid to the filtrate to adjust the pH value to 6.8, then slowly add 320ml dropwise of a mixed solvent B of 2-butanone and chloroform with a volume ratio of 1:0.3, stir while adding, and the stirring speed is 20 rpm / Minute;
[0046] (3) Cool down to 5°C, crystallize, and grow crystals for 4 hours;
[0047] (4) Filtrate to obtain a solid, and dry it under reduced pressure at 35° C. for 3 hours to obtain 97.92 g of cefadroxil.
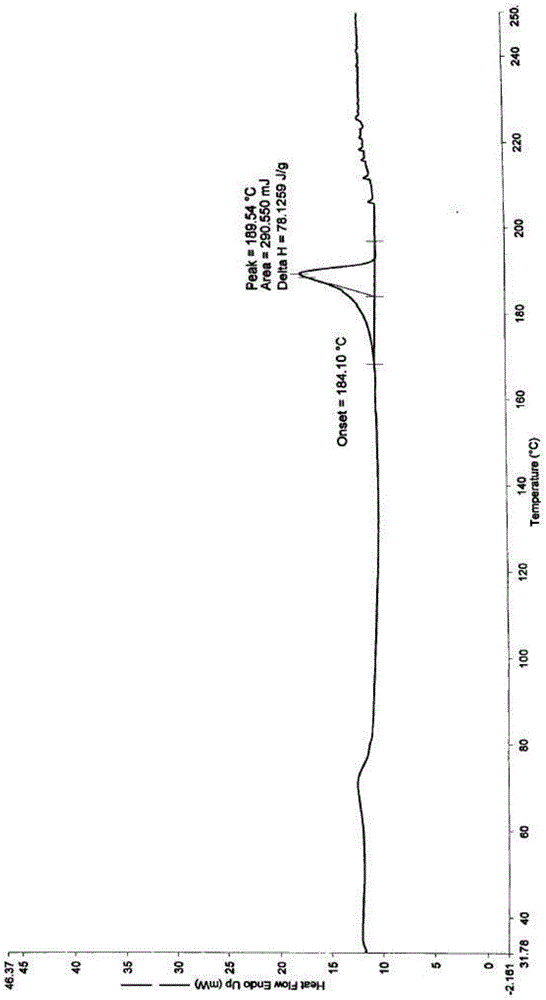
[0048] The cefadroxil prepared in this example has an X-ray powder diffraction pattern measured by Cu-Kα rays at 2θ of 4.27±0.2°, 8.52±0.2°, 17.13±0.2°, 19.92±0.2°, 20.52±0.2° , 21.44±0.2°, 23.87±0.2°, 25.09±0.2°, 25.85±0.2°, 29...
Embodiment 2
[0049] The preparation of embodiment 2 cefadroxil of the present invention
[0050] (1) Dissolve 100 g of cefadroxil crude product in 600 mL of mixed solvent A of tetrahydrofuran and water with a volume ratio of 1:2, and add ammonia water dropwise to adjust the pH value to 8.2;
[0051] (2) Filter, add dropwise acetic acid to the filtrate to adjust the pH value to 7.0, then slowly add 240ml dropwise of a mixed solvent B of 2-butanone and chloroform with a volume ratio of 1:0.3, stir while adding, and the stirring speed is 15 rpm / Minute;
[0052] (3) Cool down to -10°C, crystallize, and grow crystals for 2 hours;
[0053](4) Filtrate to obtain a solid, and dry it under reduced pressure at 30° C. for 2 hours to obtain 97.31 mg of cefadroxil.
[0054] The X-ray powder diffraction pattern and differential scanning calorimetry pattern of the cefadroxil prepared in this embodiment are the same as those in Example 1, and its purity as determined by high performance liquid chromato...
Embodiment 3
[0055] The preparation of embodiment 3 cefadroxil of the present invention
[0056] (1) Dissolve 100 g of cefadroxil crude product in 1000 mL of mixed solvent A of tetrahydrofuran and water at a volume ratio of 1:2, and add ammonia water dropwise to adjust the pH value to 7.6;
[0057] (2) Filter, add dropwise acetic acid to the filtrate to adjust the pH value to 6.5, then slowly add 400ml dropwise of a mixed solvent B of 2-butanone and chloroform with a volume ratio of 1:0.3, stir while adding, and the stirring speed is 25 rpm / Minute;
[0058] (3) Cool down to 0°C, crystallize, and grow crystals for 6 hours;
[0059] (4) Filtrate to obtain a solid, and dry under reduced pressure at 40°C for 4 hours to obtain 96.27 mg of cefadroxil.
[0060] The X-ray powder diffraction pattern and differential scanning calorimetry pattern of the cefadroxil prepared in this embodiment are the same as those in Example 1, and its purity as determined by high performance liquid chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com