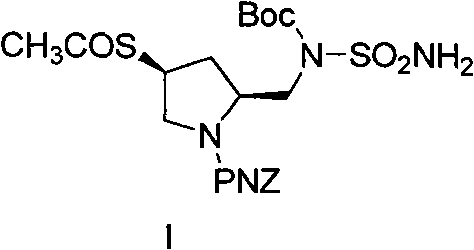
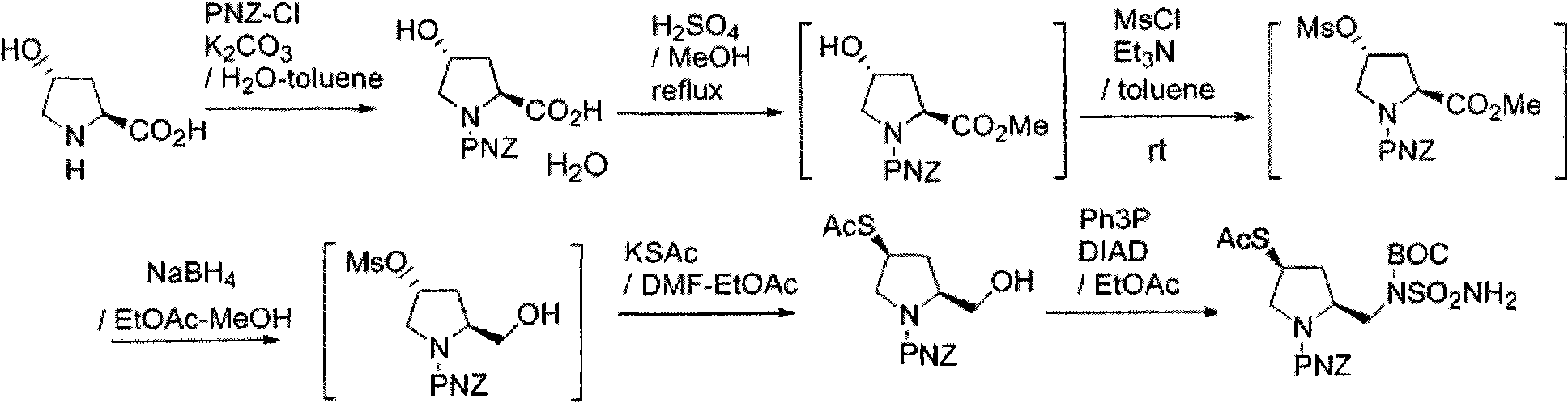
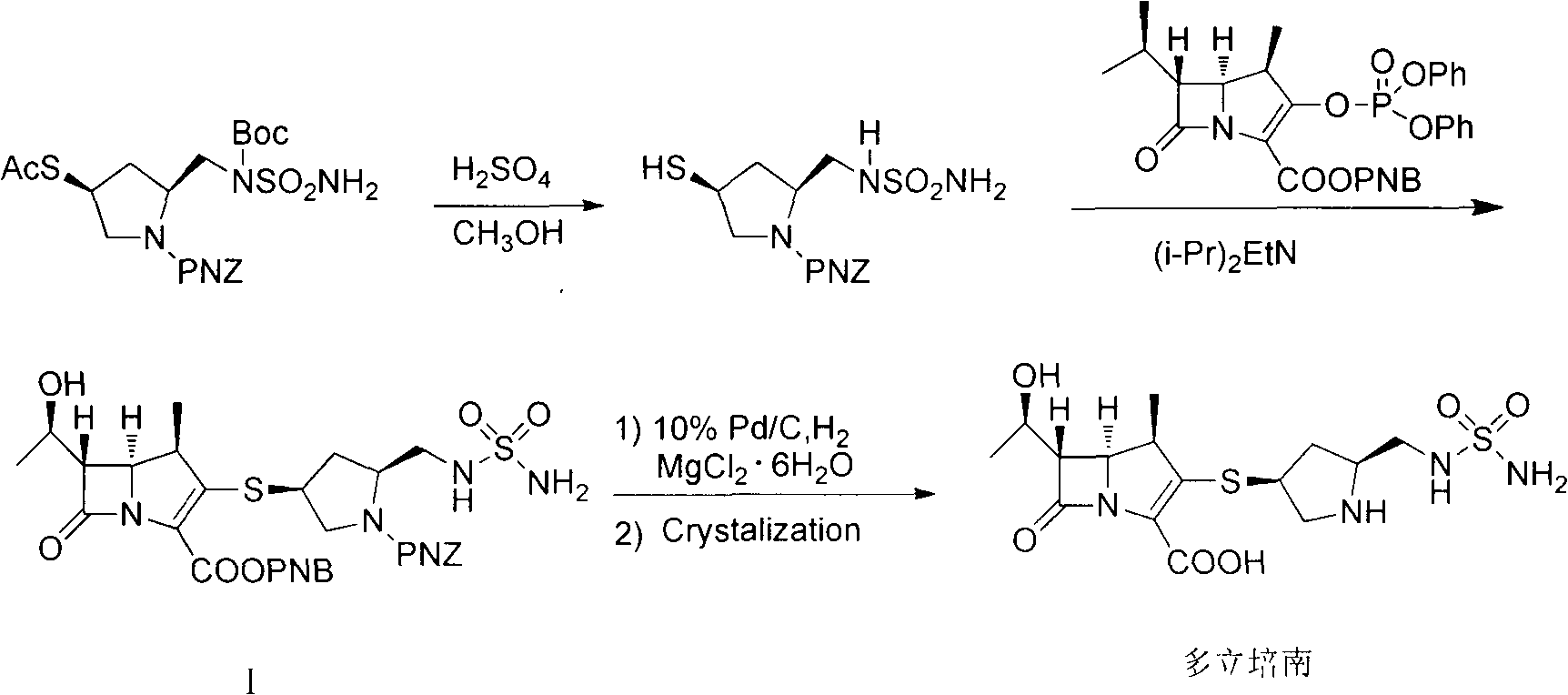
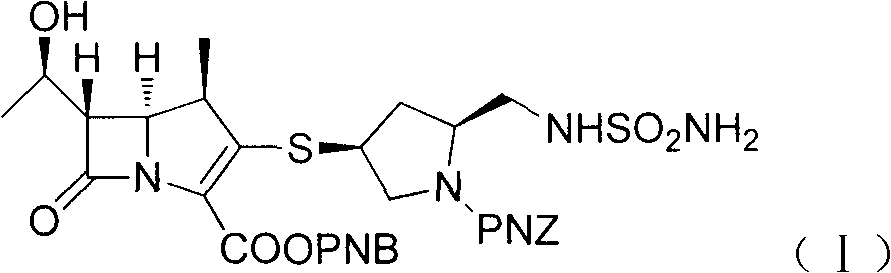
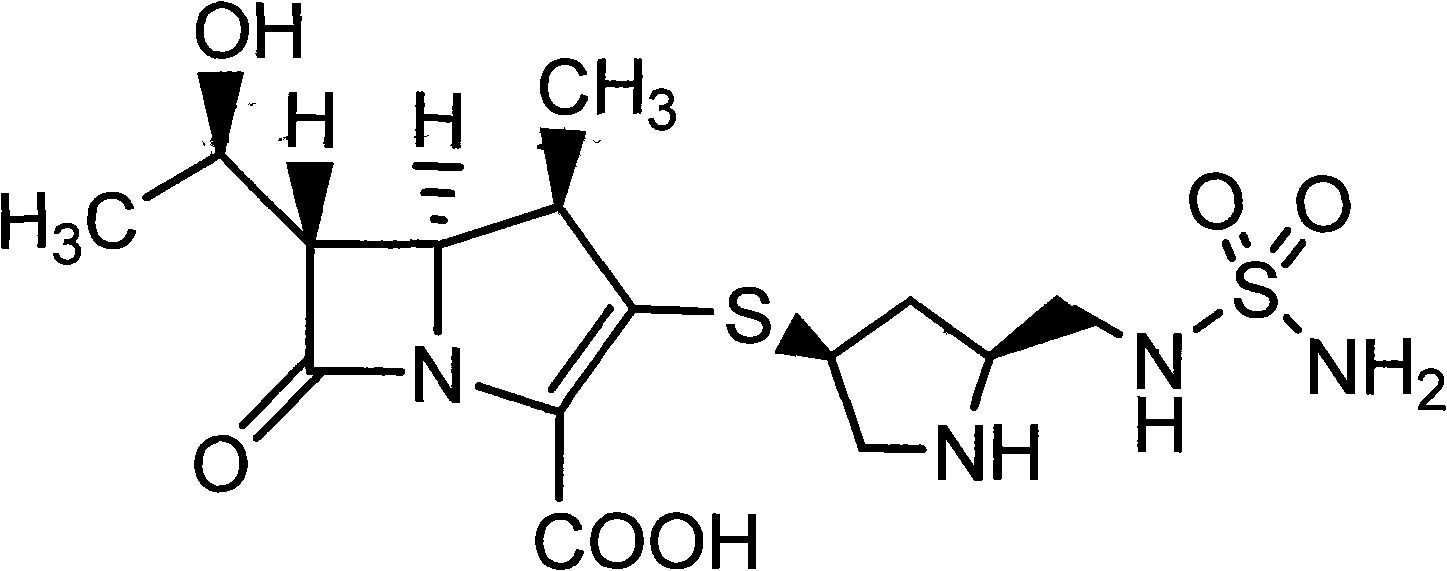
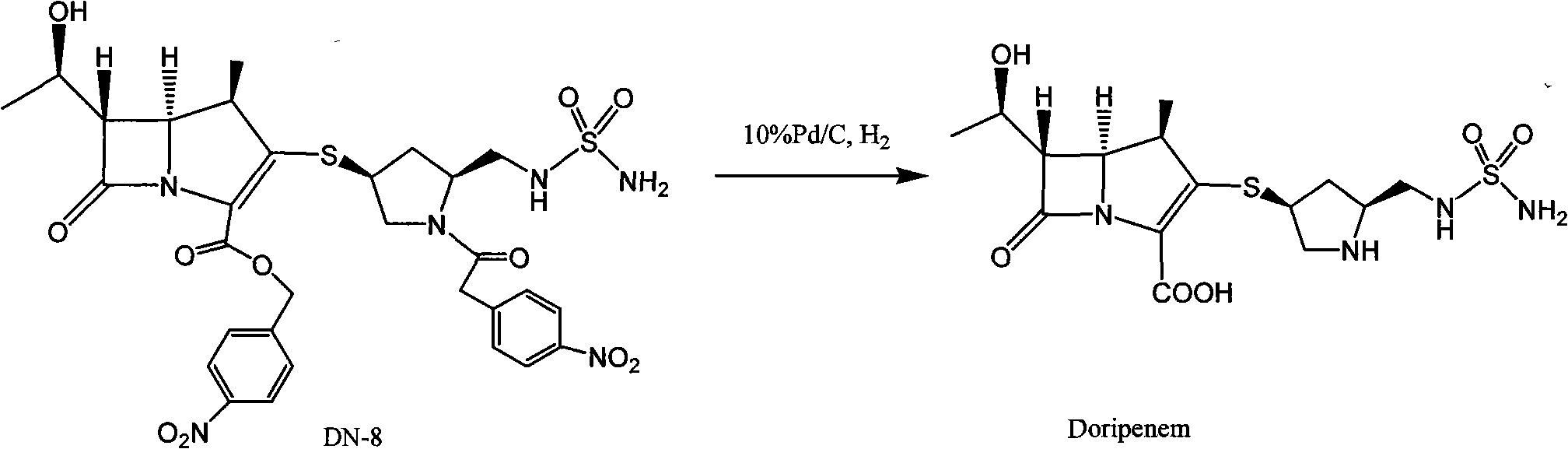
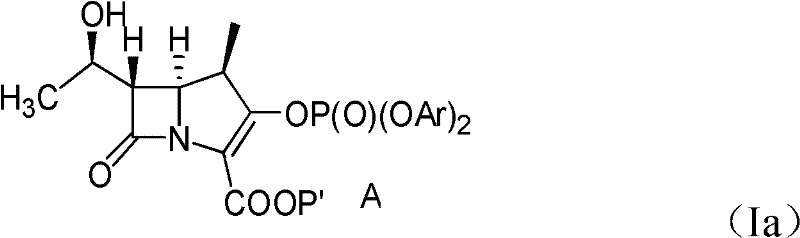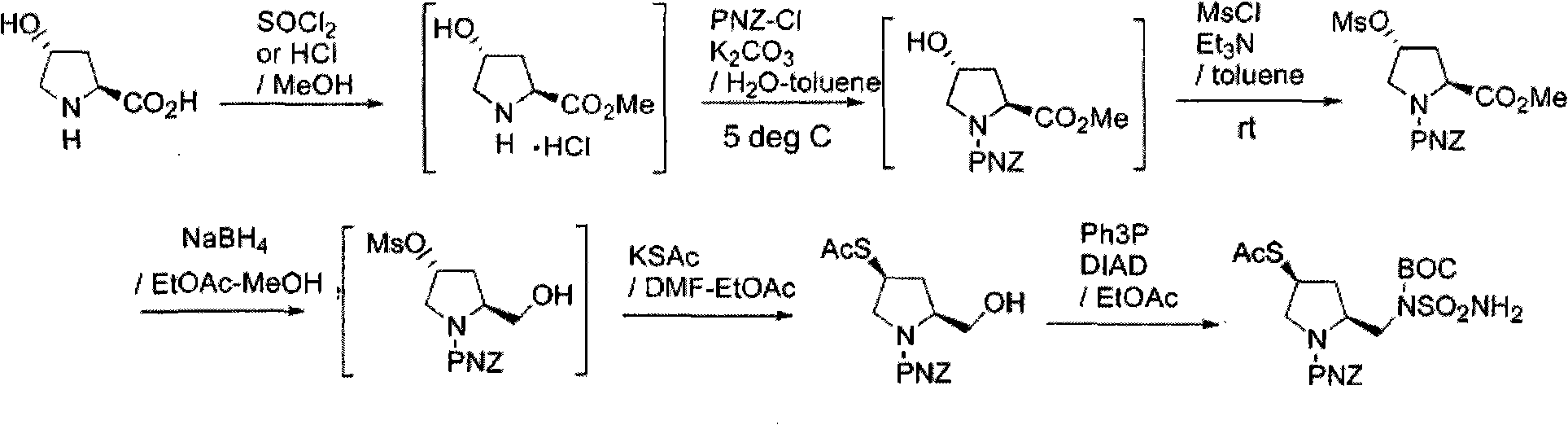Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
57 results about "Doripenem" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
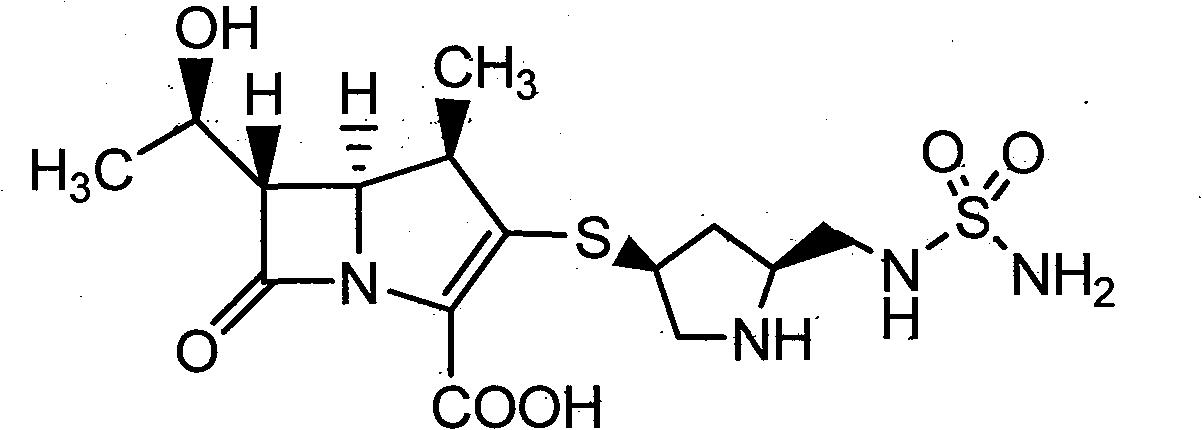
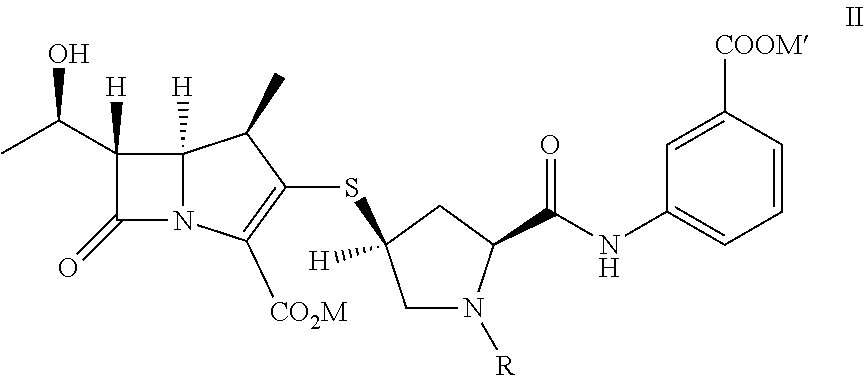
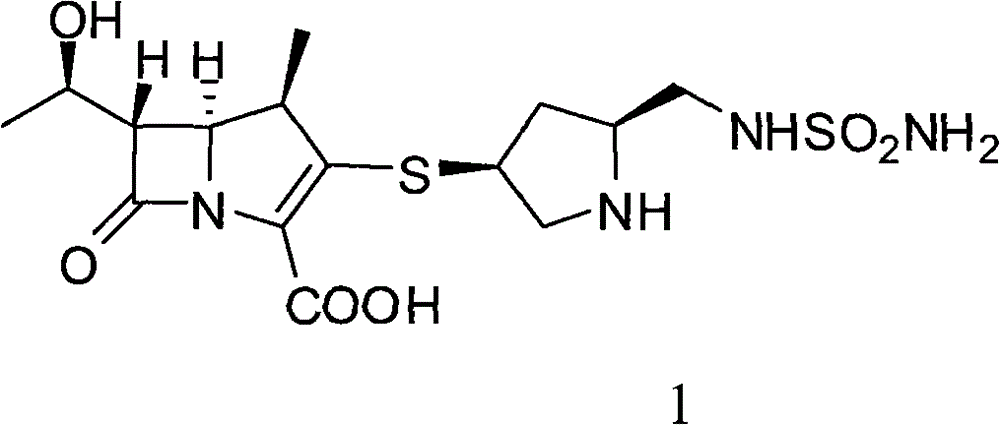
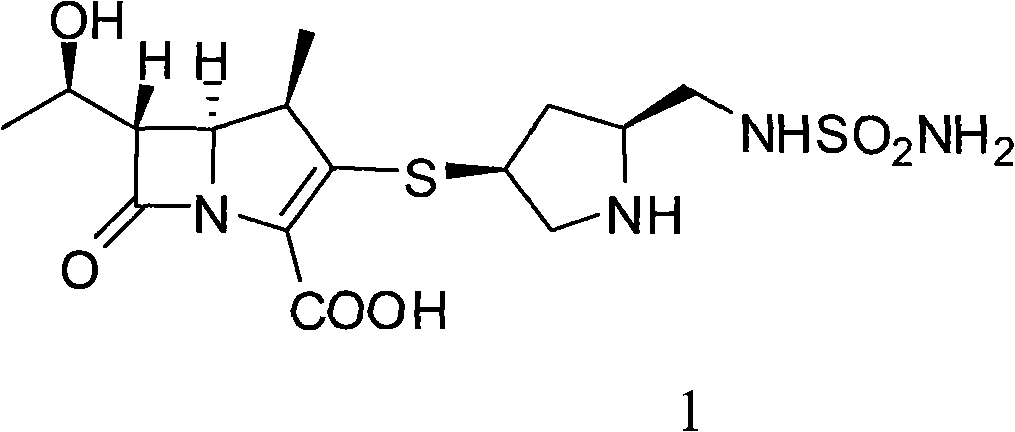
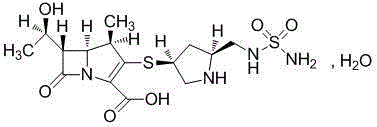
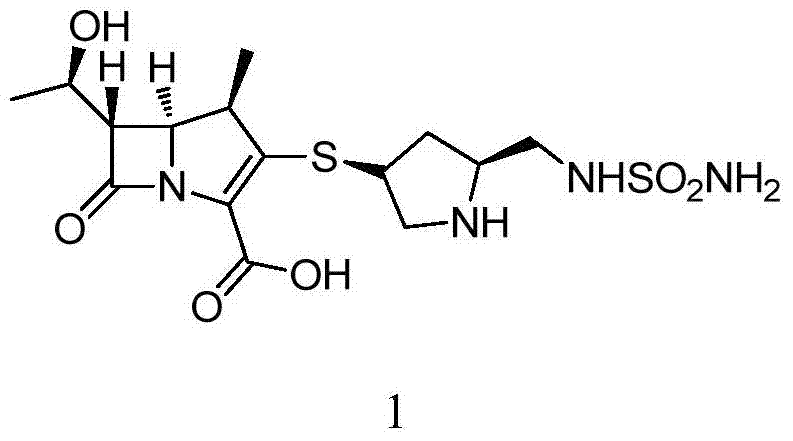
Doripenem is used to treat a wide variety of bacterial infections.
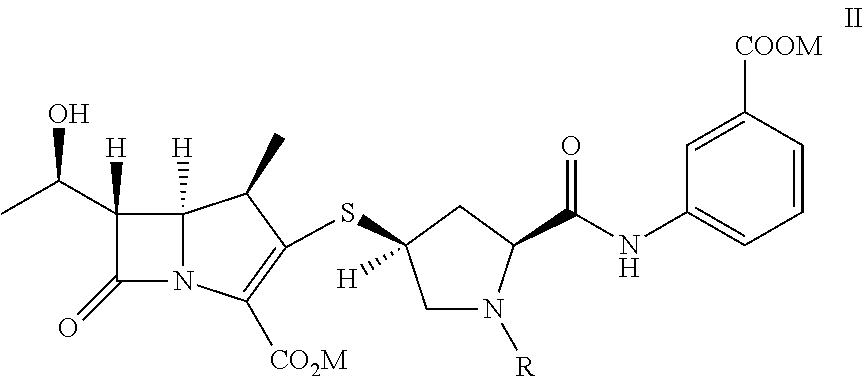
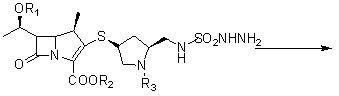
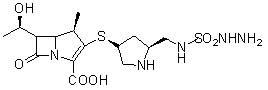
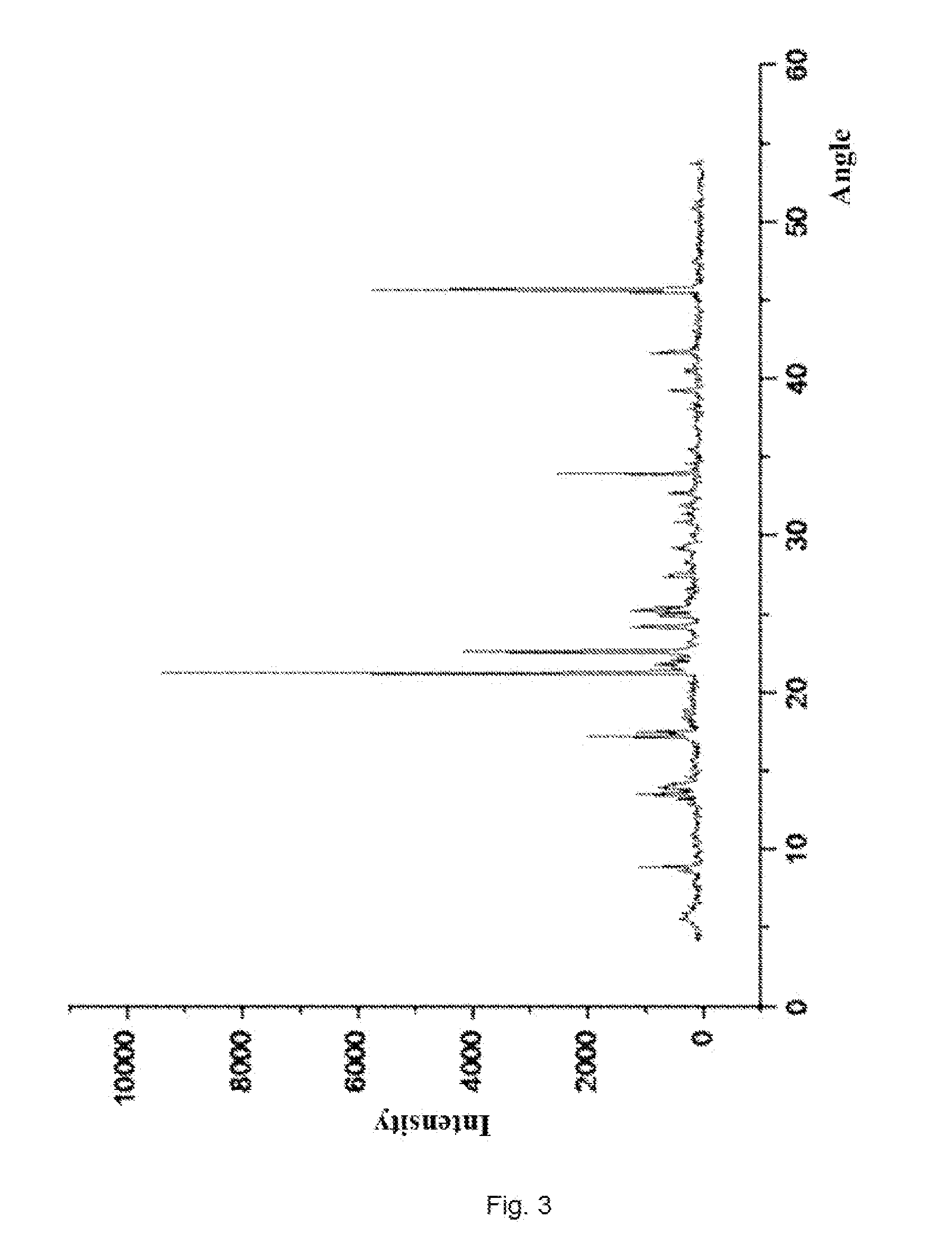
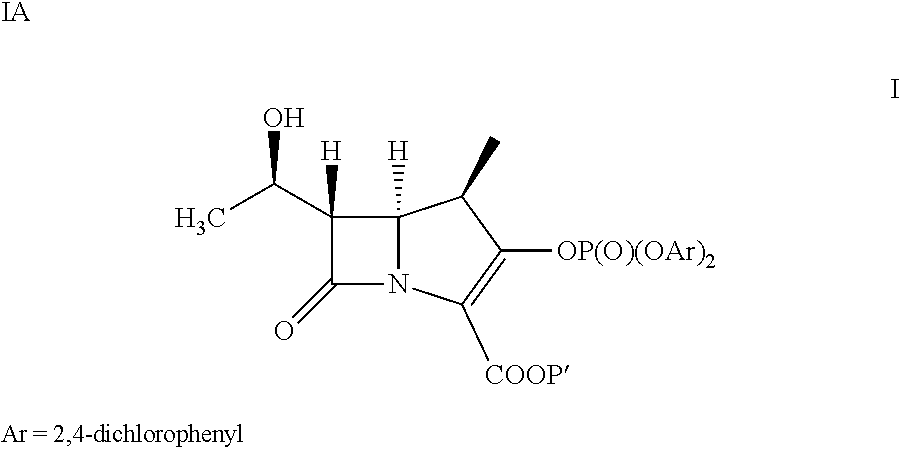
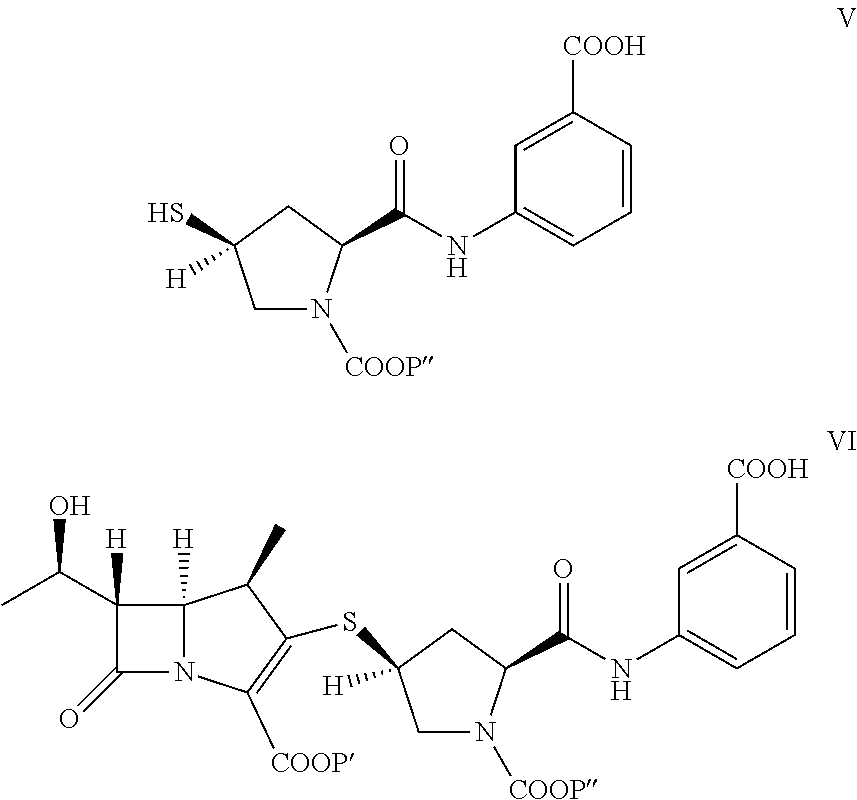
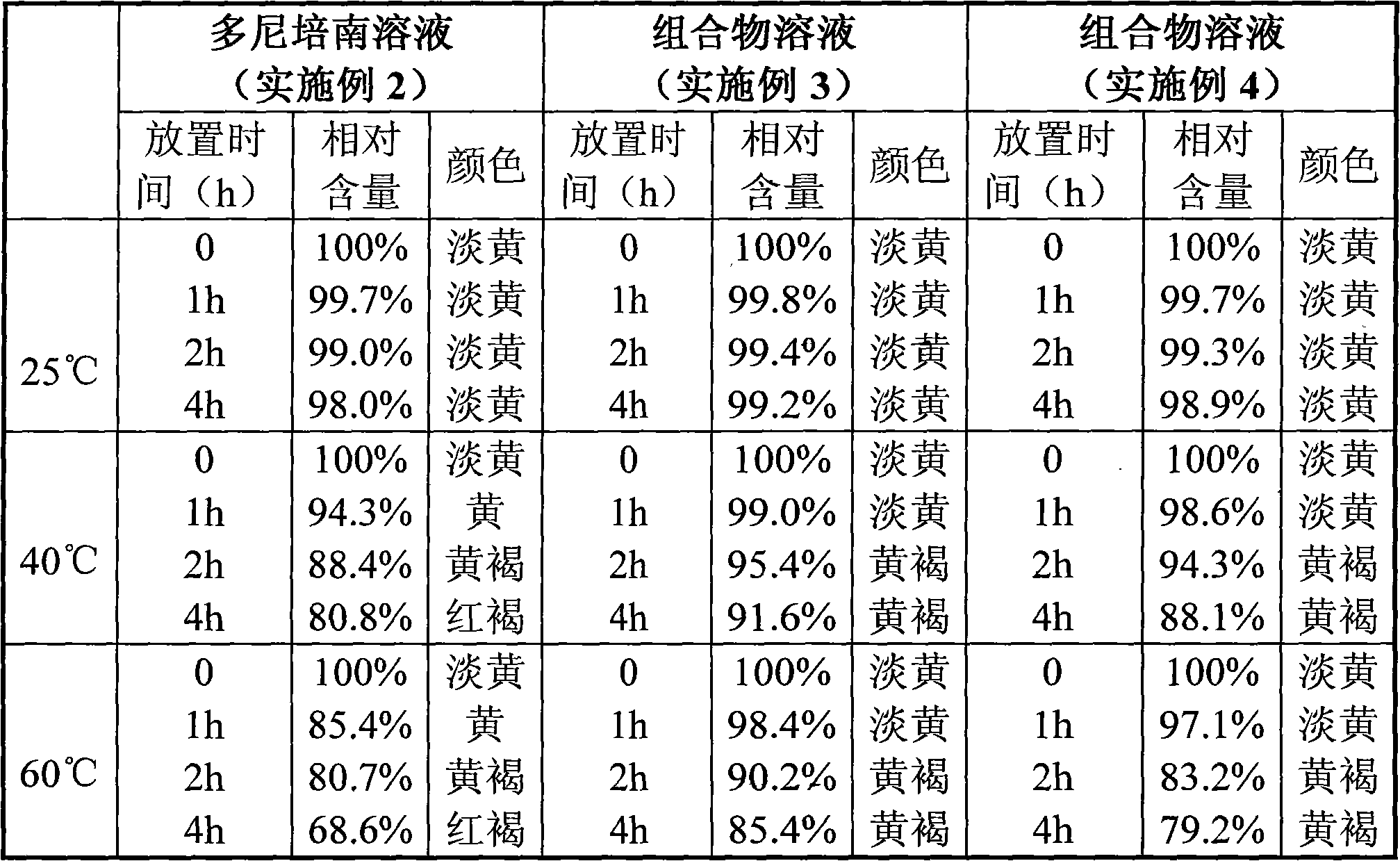
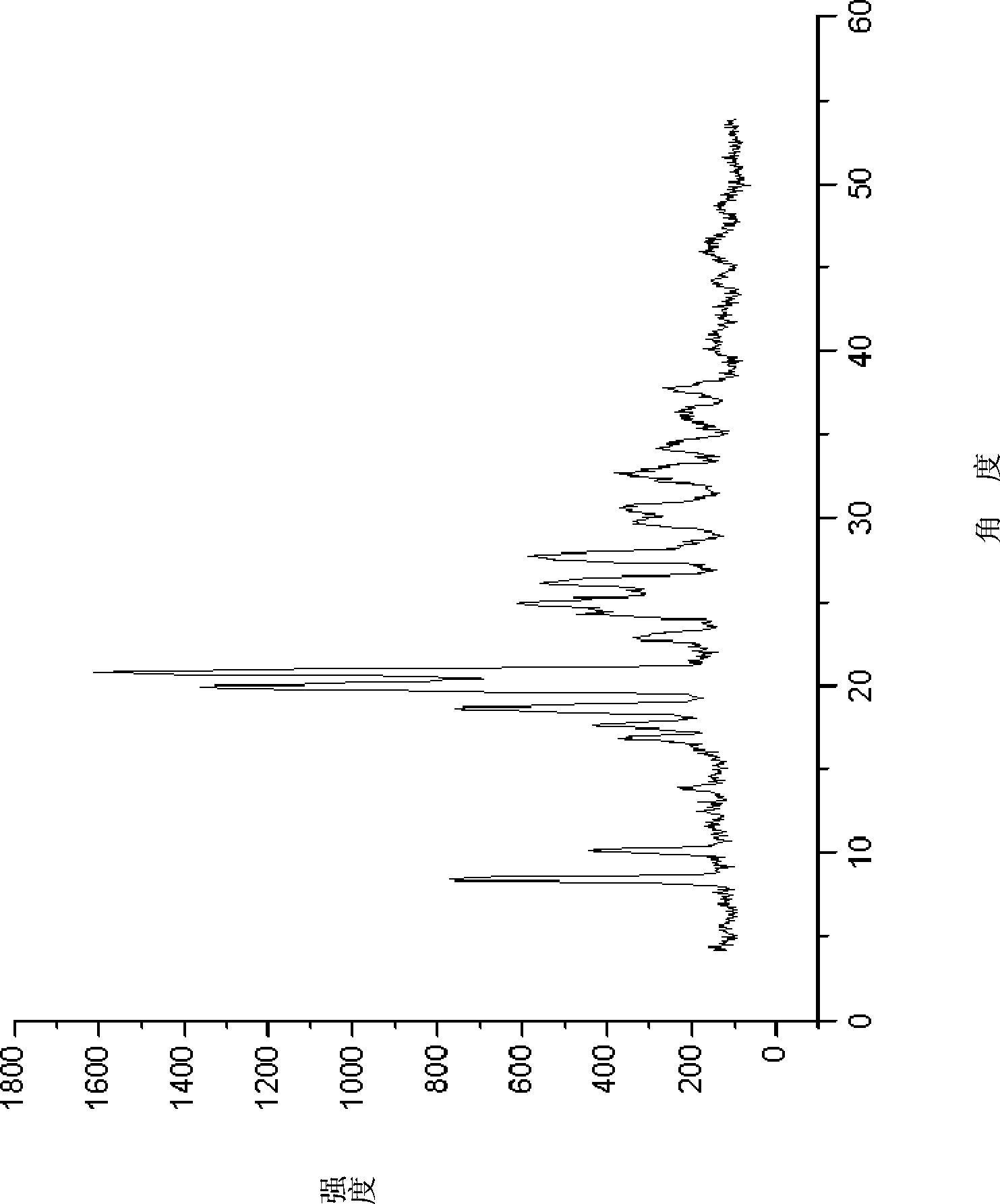
A kind of doripenem hydrate crystal and preparation method thereof
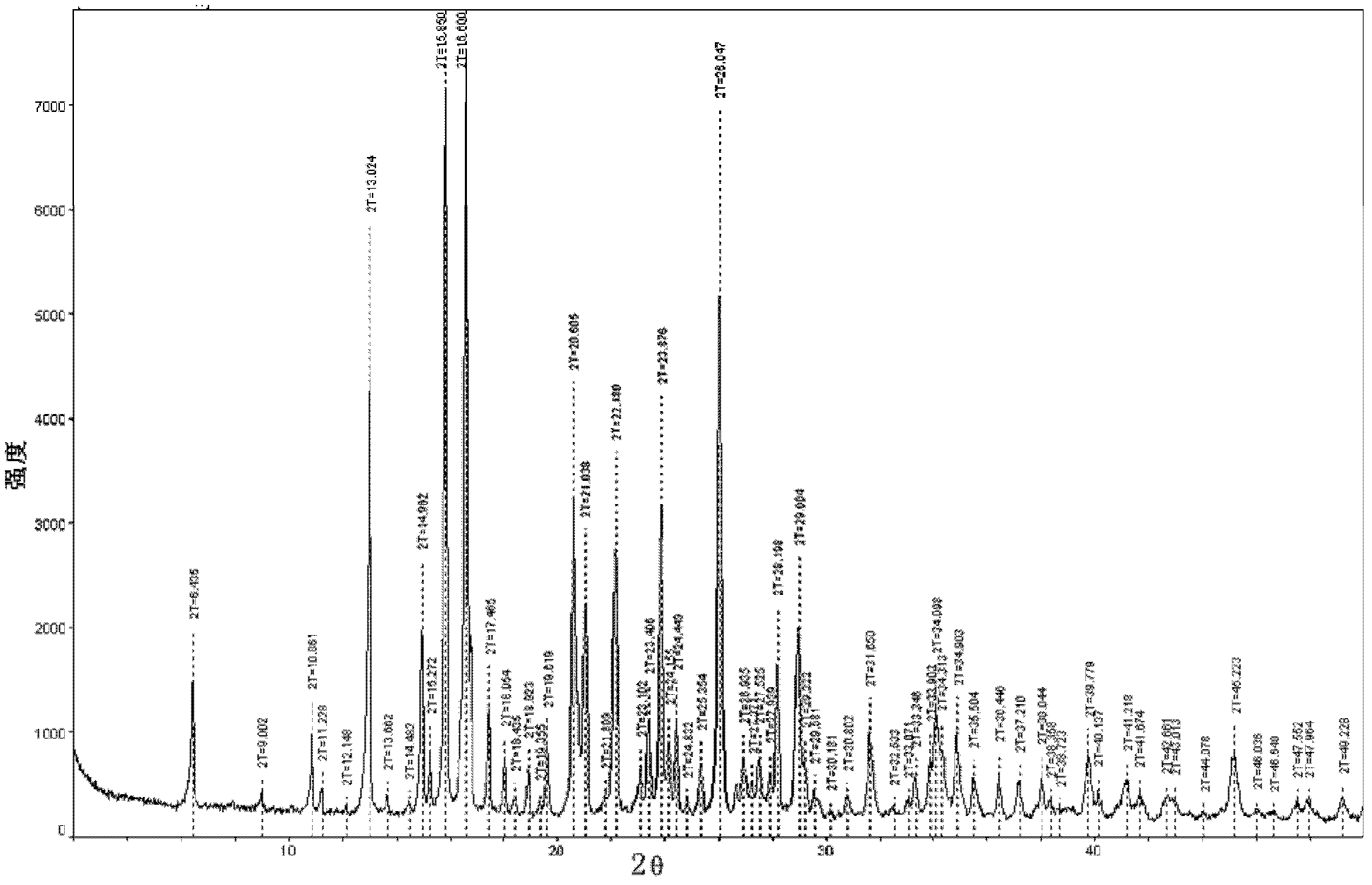
ActiveCN102285988AHigh puritySimple processAntibacterial agentsOrganic active ingredientsPhysical chemistryAnalytical chemistry
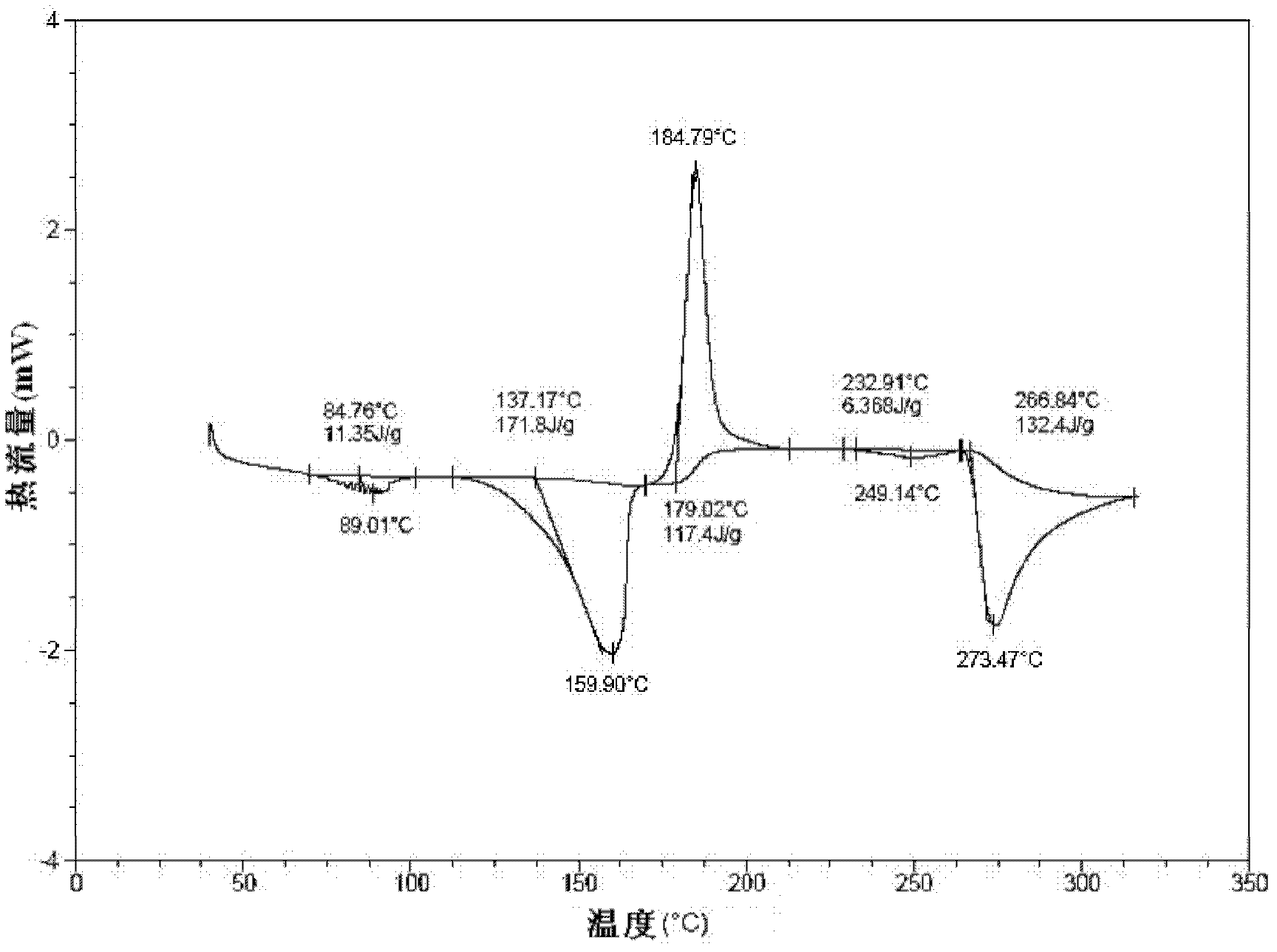
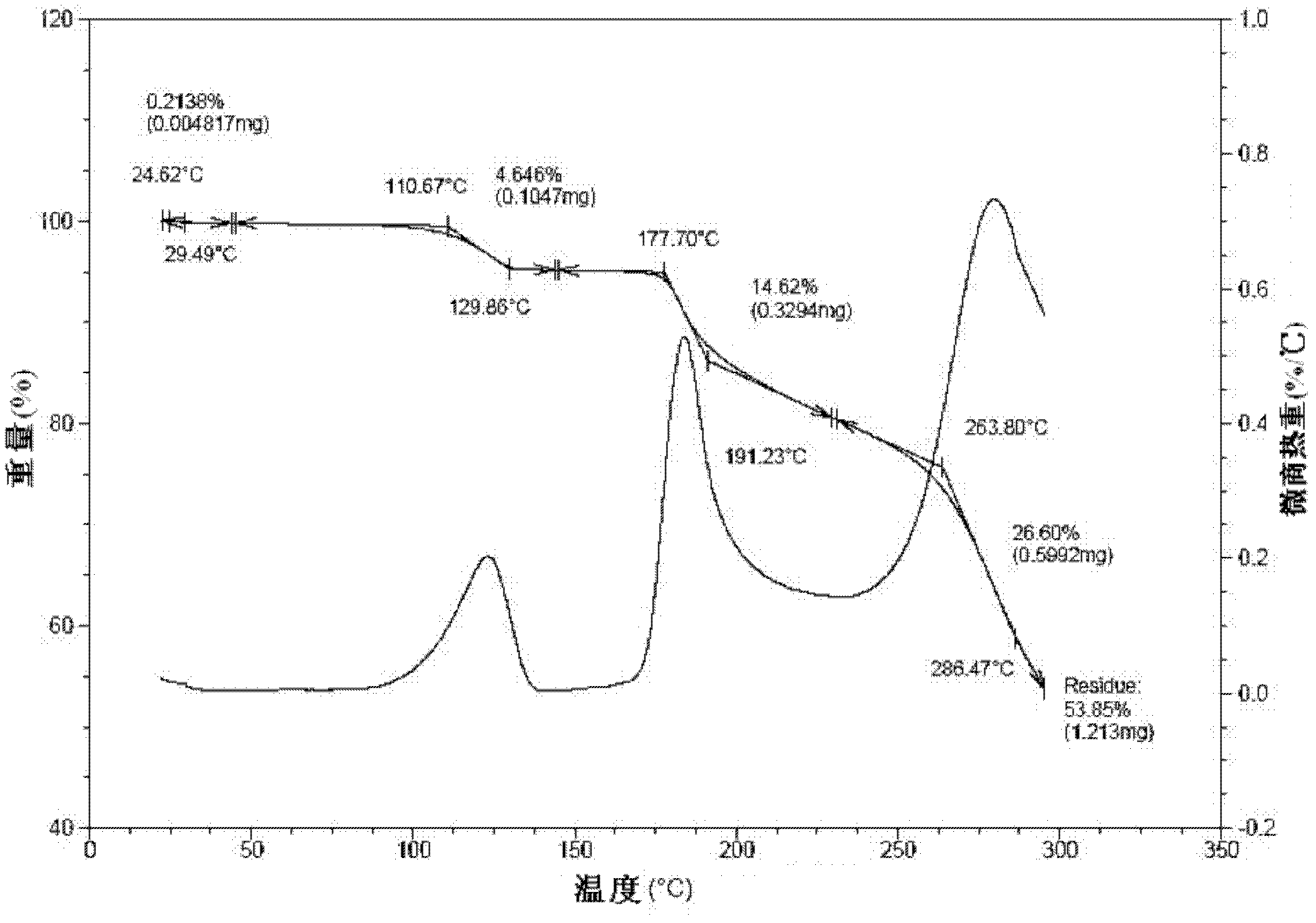
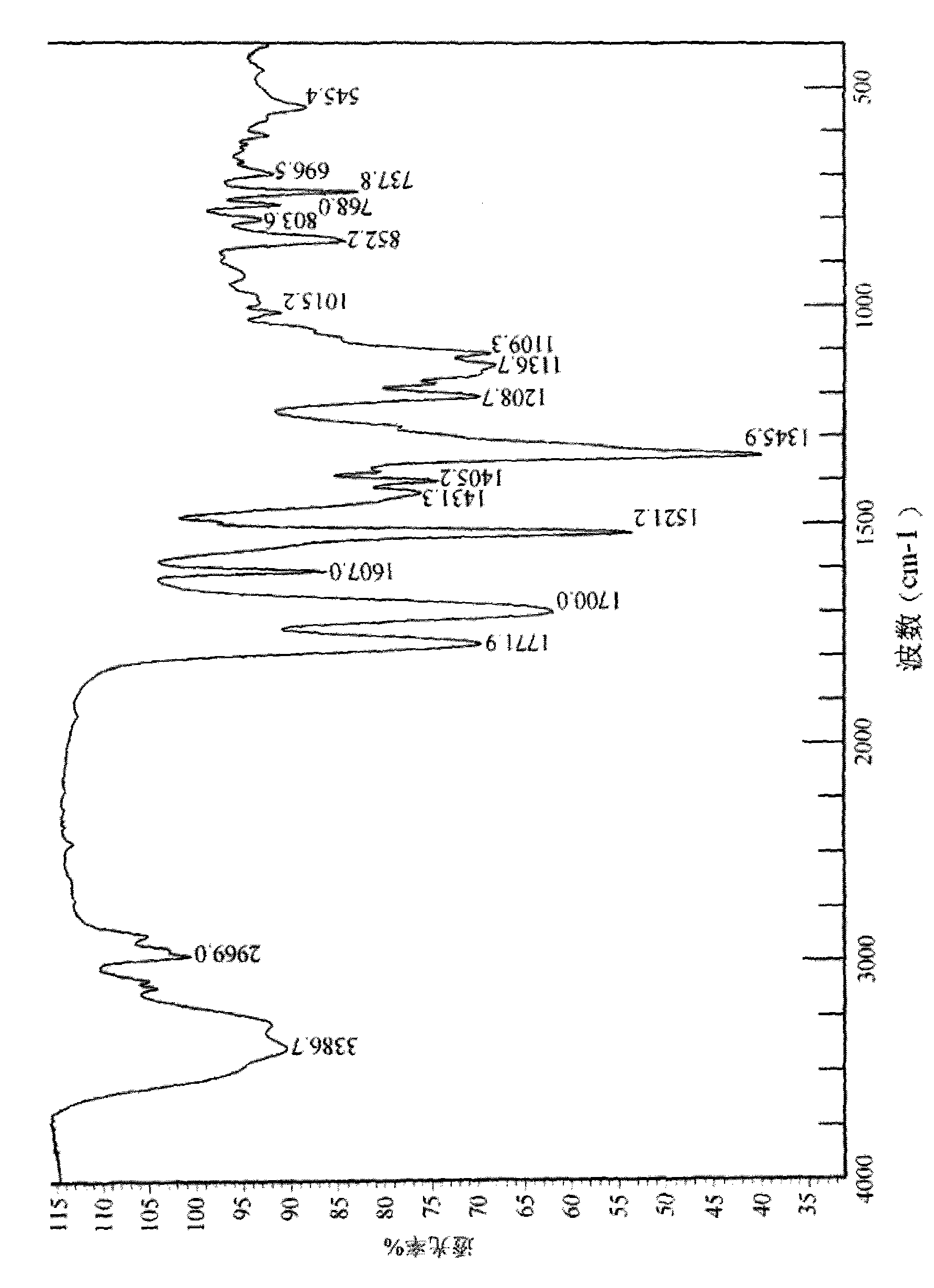
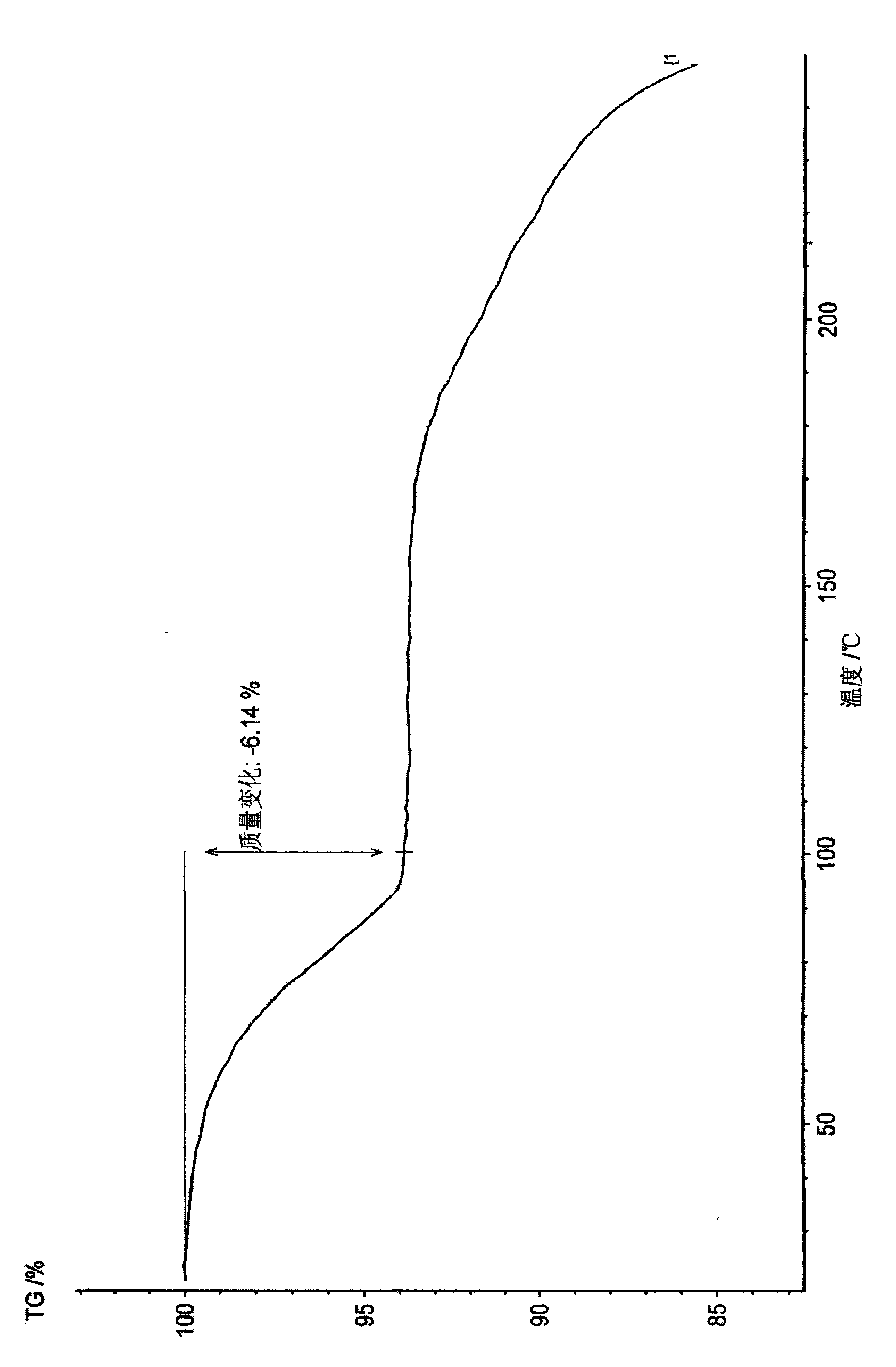
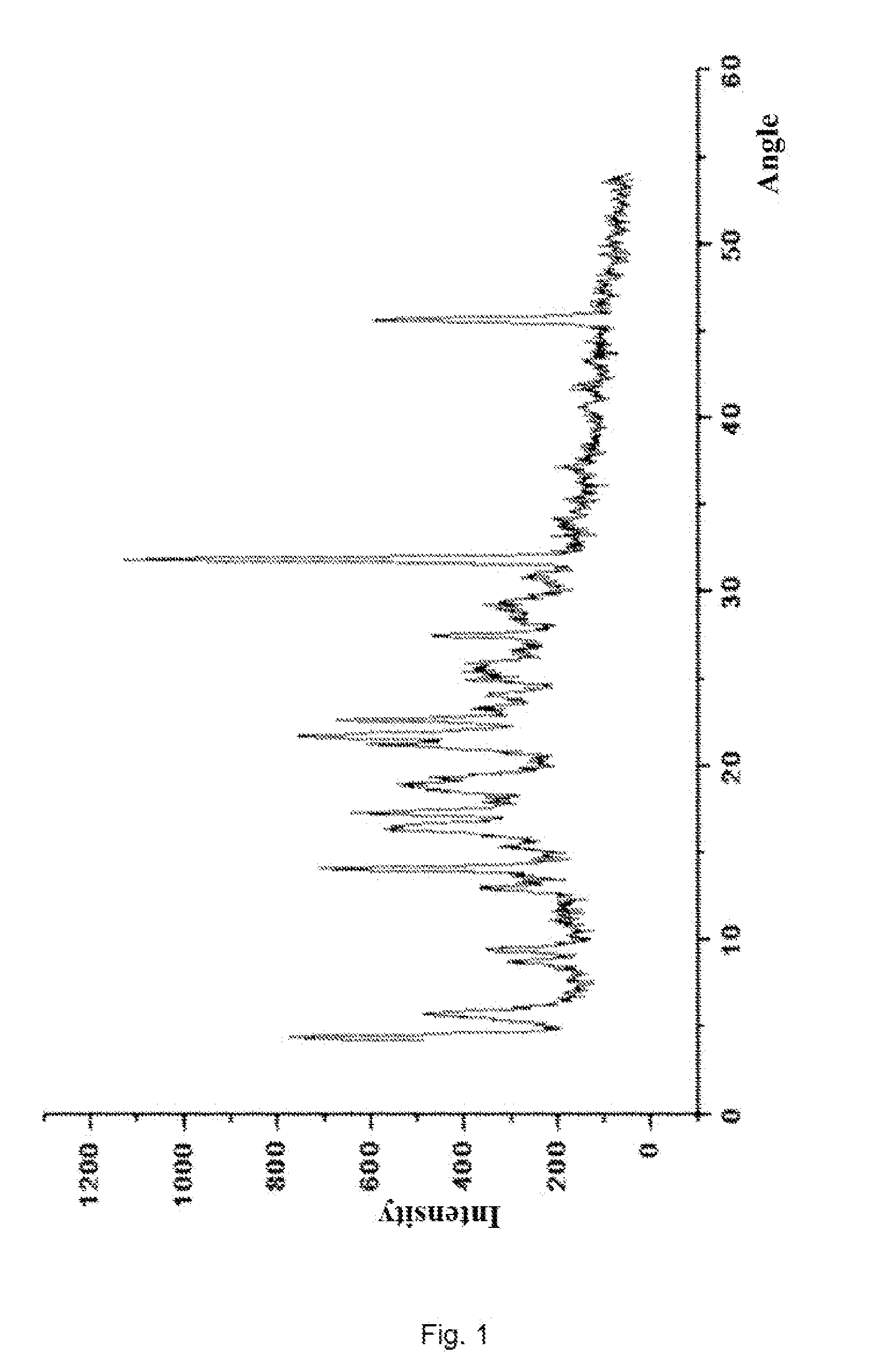
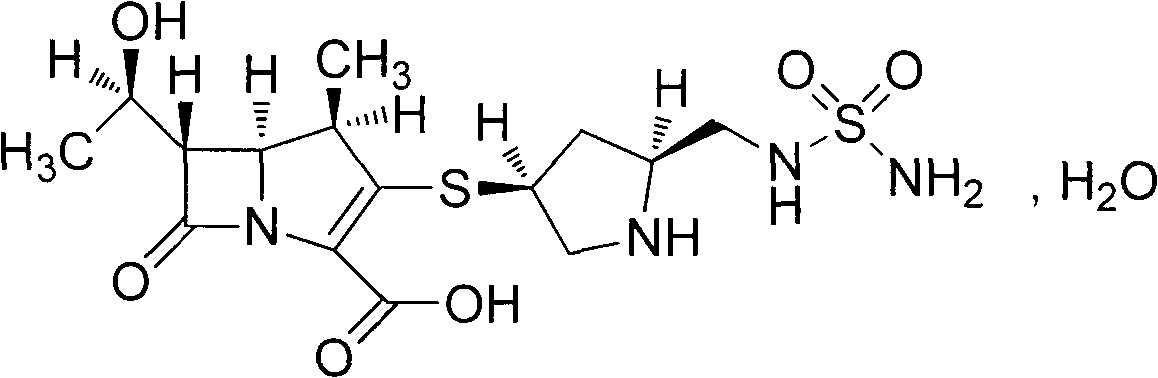
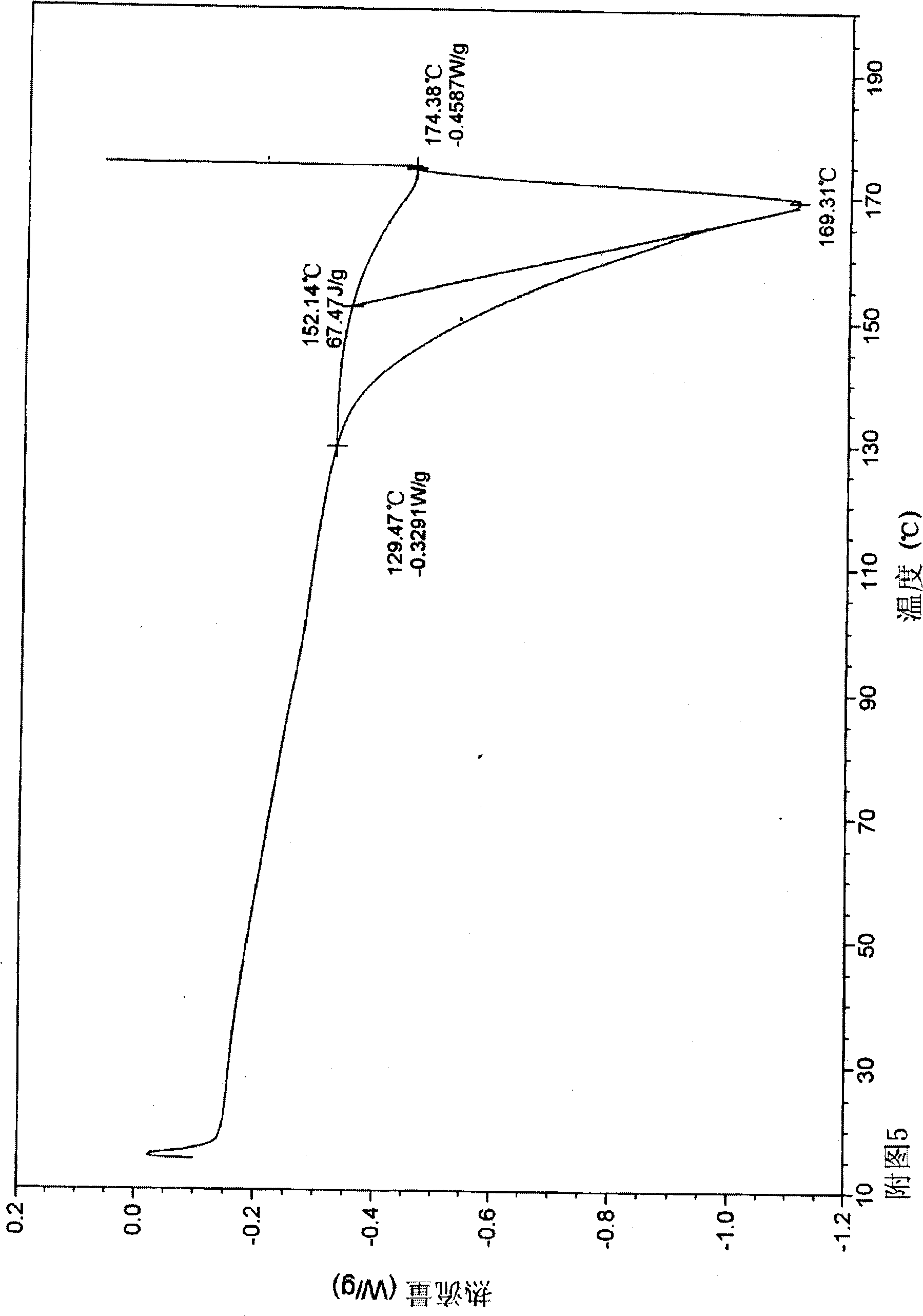
Disclosed are a doripenem hydrate crystal and preparation method therefor. The X-ray diffraction spectrogram of the crystal powder is basically as represented in figure 1, and the measured water content is 4.4 to 5.5%. The doripenem hydrate crystal of the present invention has high purity, low residual solvent, good stability, and application safety. Additionally, the preparation method for the doripenem hydrate crystal of the present invention has simple techniques and low preparation cost, and is suitable for industrial production.
Owner:SHANGHAI ACEBRIGHT PHARMA CO LTD +1
Process for the preparation of carbapenem using cabapenem intermediates and recovery of cabapenem
InactiveCN102250145AReduce generationNovel preparation methodOrganic active ingredientsGroup 5/15 element organic compoundsErtapenemMedicinal chemistry
The present invention aims to prepare carbapenem intermediates which can effectivly produce Ertapenem, Meropenem and Doripenem; and provides an effective process for recovering ertapenem compounds.
Owner:SAVIOR LIFETEC CORP
Preparation process for intermediate of doripenem
The invention relates to the technical field of medicines and particularly provides a preparation process of the intermediate I of doripenem. In the process, a reagent which is environmentally-friendly and low in cost is used in place of a high-cost and high-toxicity reagent in the prior art, the synthesis route is optimized, the product purity is improved, and the preparation method is simple, reliable, easy to operate, high in yield and low in cost, reduces emission to the environment to the lowest degree and contributes to industrial production. The structure of the intermediate I of the doripenem is shown below.
Owner:BEIJING LUNARSUN PHARMA
Methanol solvate of doripenem intermediate and preparation method thereof
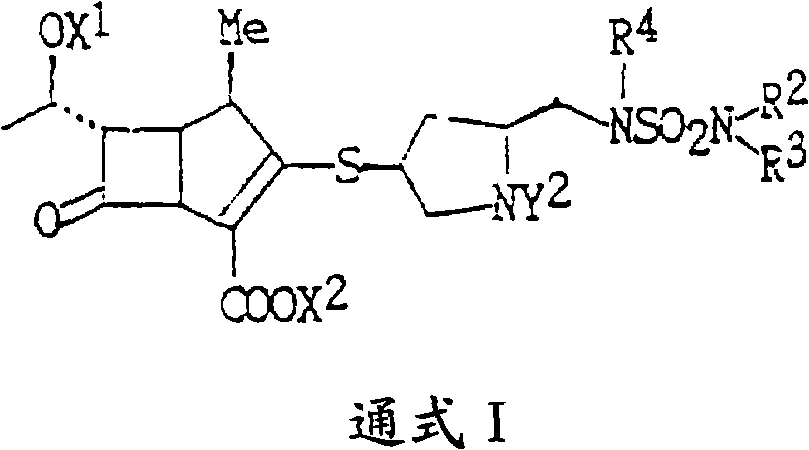
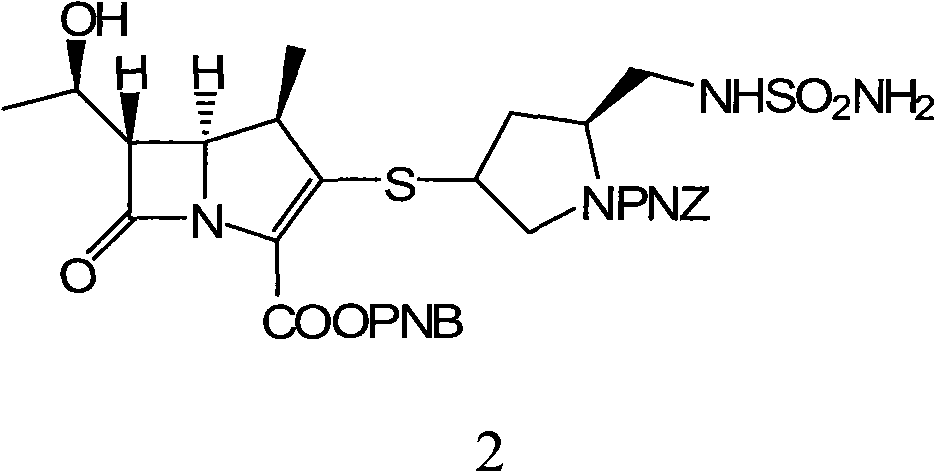
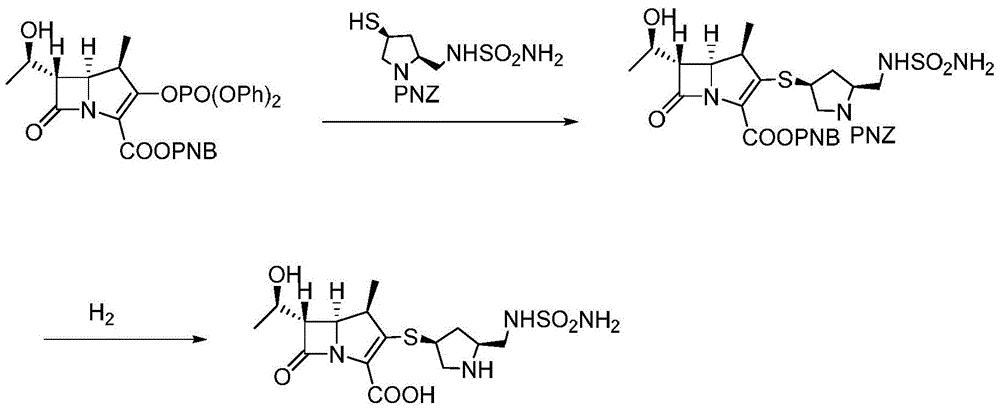
The invention discloses a methanol solvate of a doripenem intermediate as shown by a formula I and a preparation method thereof, wherein PNB in an intermediate I represents para-nitrobenzyl, and PNZ represents para-nitro-carbobenzoxygroup. The methanol solvate has the advantages of high purity, good stability, and easy operation and storage; and the preparation method is simple and reliable, and is suitable for industrial implementation.
Owner:SHANGHAI INST OF PHARMA IND +1
Process for synthesizing doripenem lateral chain
InactiveCN102249970ABlocking will notUniform crystal particlesOrganic chemistryVulcanizationBiological activation
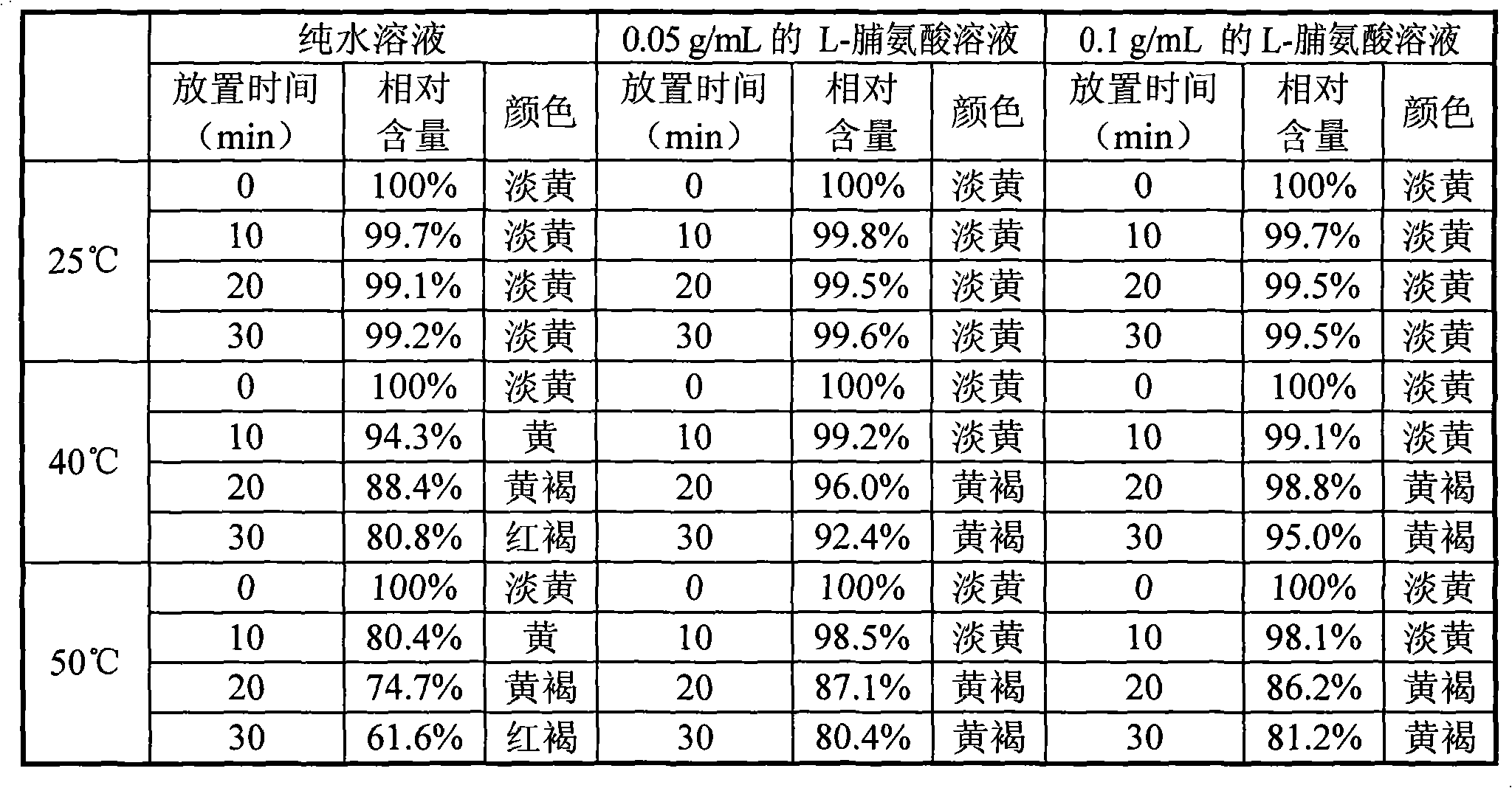
The invention discloses a process for synthesizing a doripenem lateral chain. According to the process disclosed by the invention, L-hydroxyproline is taken as a starting raw material, and the doripenem lateral chain is obtained through the following steps of: amino protection, carboxyl protection, hydroxyl activation, reduction reaction, acetyl vulcanization reaction and condensation reaction. The process provided by the invention has the advantages of simple and easily-obtained raw materials, no high-temperature and high-pressure reactions and no super-low-temperature reactions; the 'three wastes' generated in the production process mainly comprise waste liquid and the wastewater in the waste liquid is neutralized by appropriate acid and alkali and then is discharged after achieving the discharging standard; and the organic solvents in the waste liquid comprises dichloromethane, dichloromethane and alcohols and can be recycled to be reutilized so that the pollution on the environment is small.
Owner:HUANGSHAN SHEXIAN HONGHUI CHEM
Purification method of pyrrolidine carbapenem antibiotics
ActiveCN101880282AImprove stabilityHigh purityOrganic active ingredientsOrganic chemistryPurification methodsAntibiotic Y
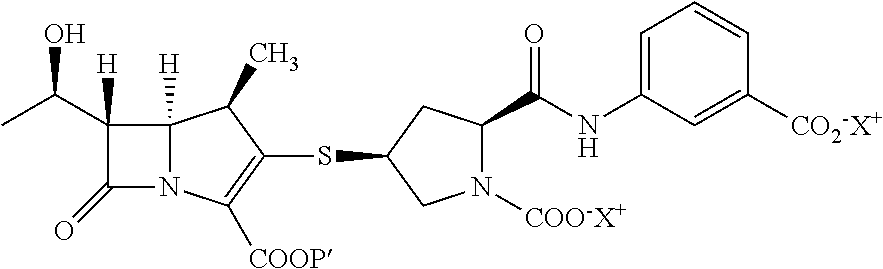
The invention relates to a purification method of pyrrolidine carbapenem antibiotics, and in particular to a method for purifying Doripenem through crystallization or re-crystallization. In the method, the Doripenem is separated out from aqueous solution containing Doripenem and proline.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Preparation of Carbapenem Intermediate and Their Use
InactiveUS20110288289A1Simple and commercially viableGood yieldOrganic chemistryErtapenemMedicinal chemistry
The present invention relates to preparing carbapenem intermediates that are useful to produce Ertapenem, Meropenem and Doripenem.
Owner:SAVIOR LIFETEC CORP
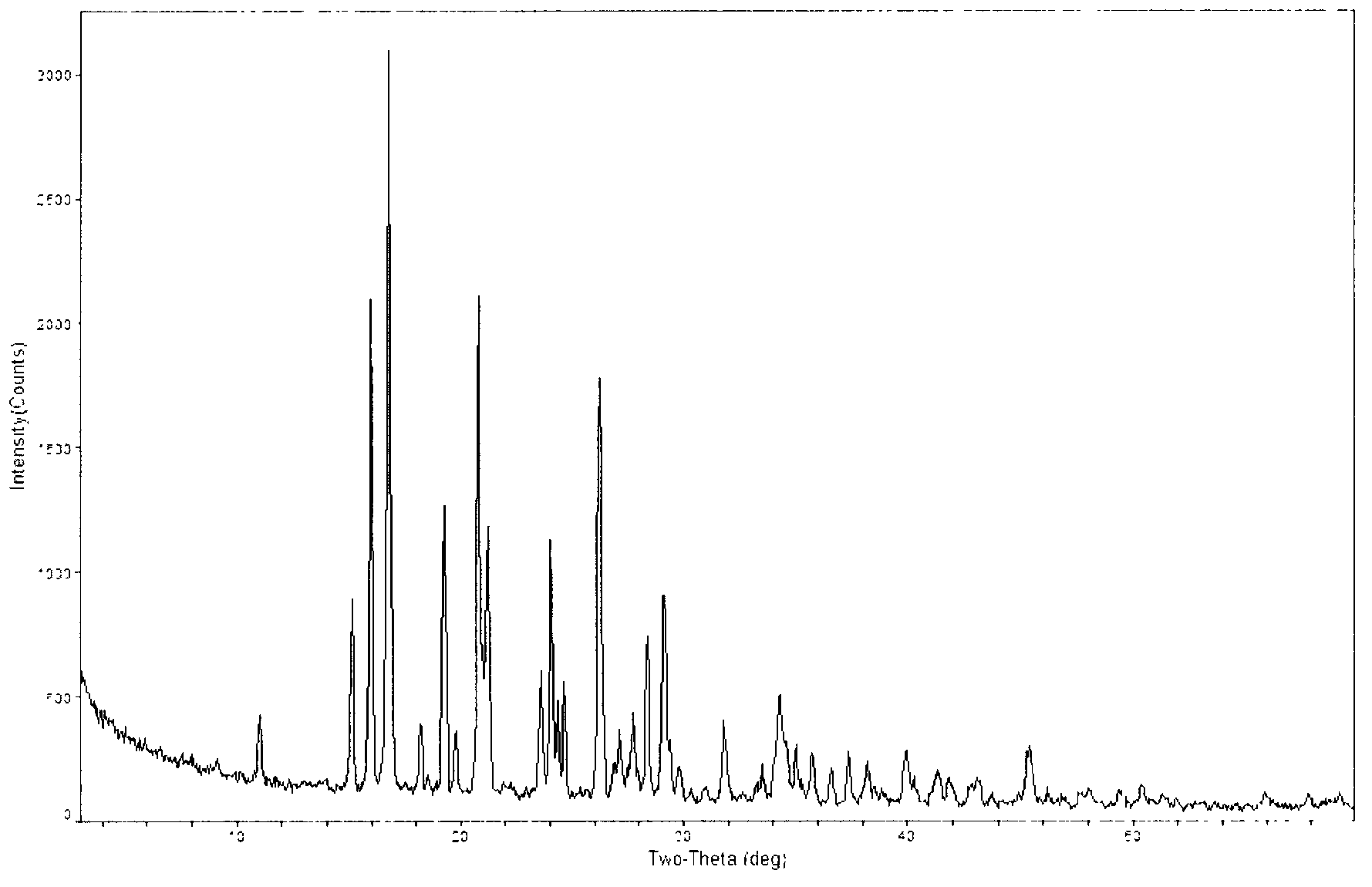
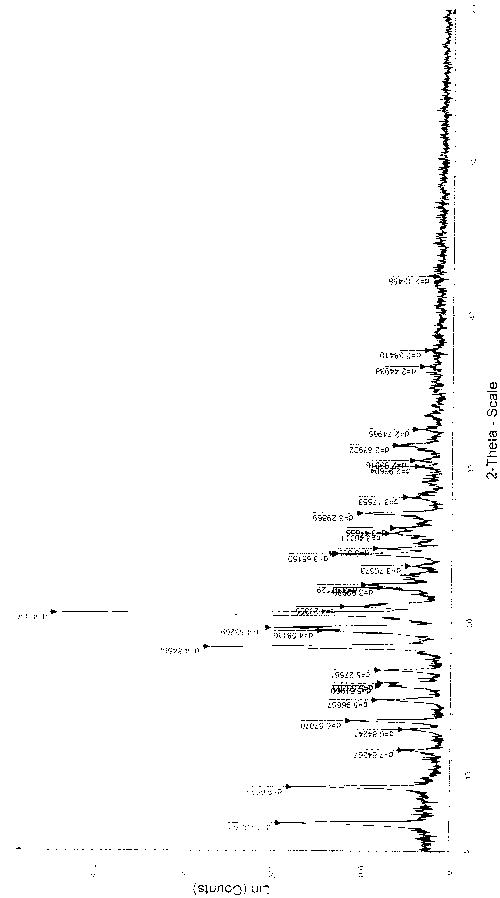
Novel crystal form of carbapenem antimicrobial medicament and preparation method of novel crystal form
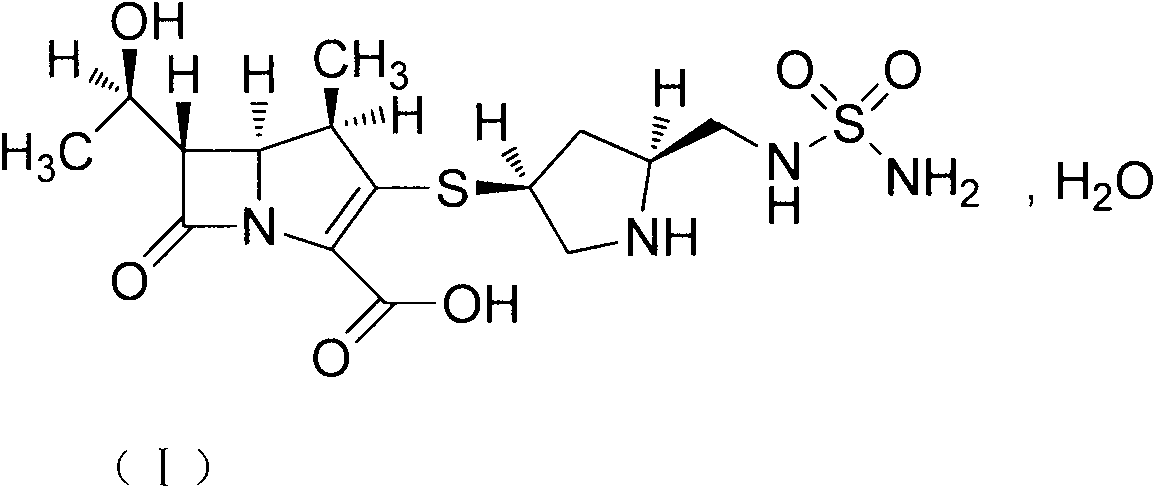
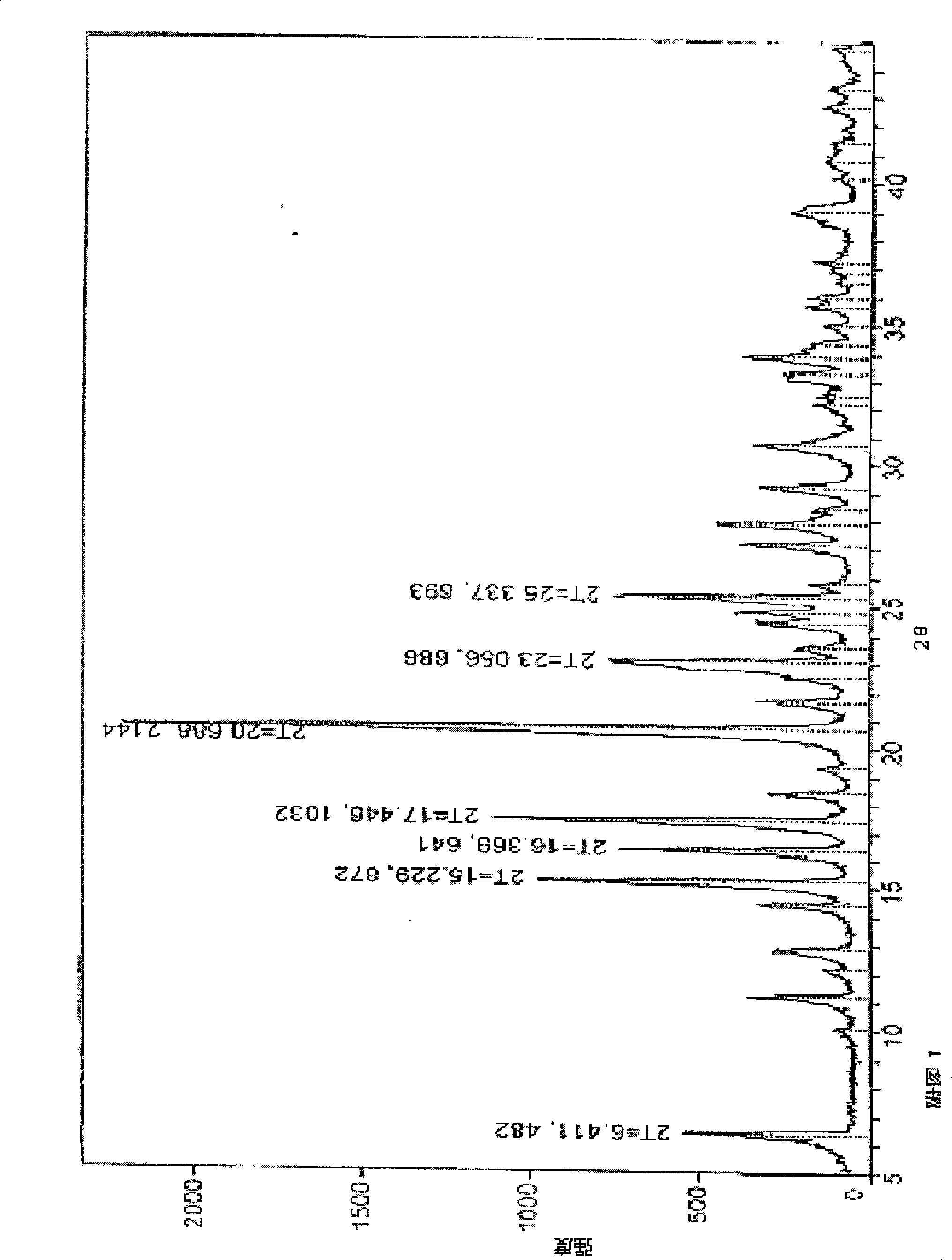
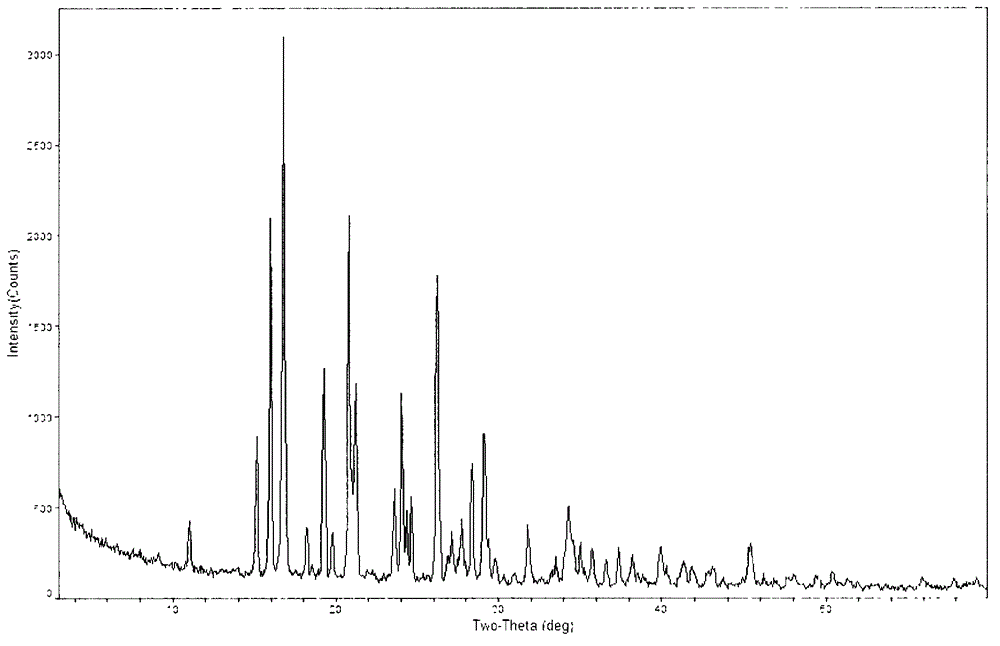
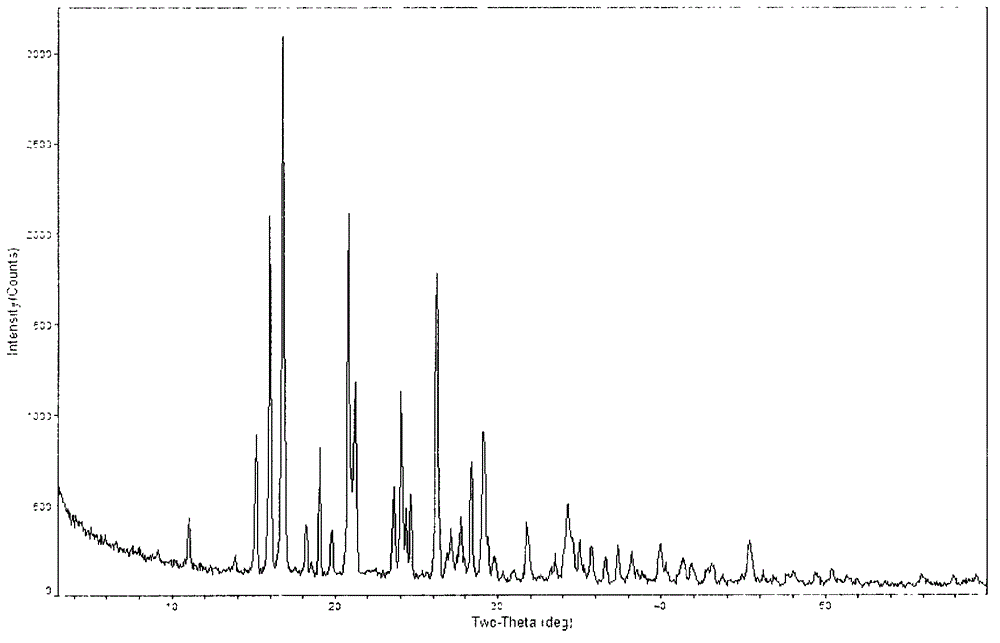
The invention provides a novel crystal form of a carbapenem antimicrobial medicament, namely, (+)-(4R,5S,6S)-3-[[(3S,5S)-5-(aminosulfonyl)aminomethyl]-3-pyrrolidine]sulfur]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclic[3,2,0]heptyl-2-ene-2-carboxylic acid-hydrate (doripenem). The X-ray powder diffraction pattern of the crystal powder shows main peaks when 2theta is equal to 15.20 degrees, 16.06 degrees, 16.83 degrees, 19.40 degrees, 21.29 degrees, 23.68 degrees, 24.08 degrees, 24.70 degrees, 26.28 degrees, 28.43 degrees, 29.17 degrees and 34.35 degrees. The novel crystal form has the advantages of easiness in preparation and industrial production, low cost, high solubility and high stability.
Owner:SHANDONG FREDA PHARMA GRP CO LTD +1
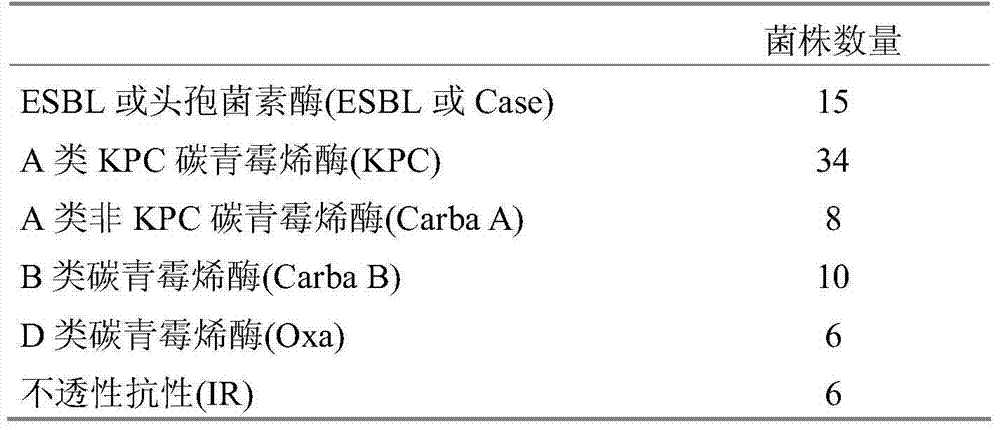
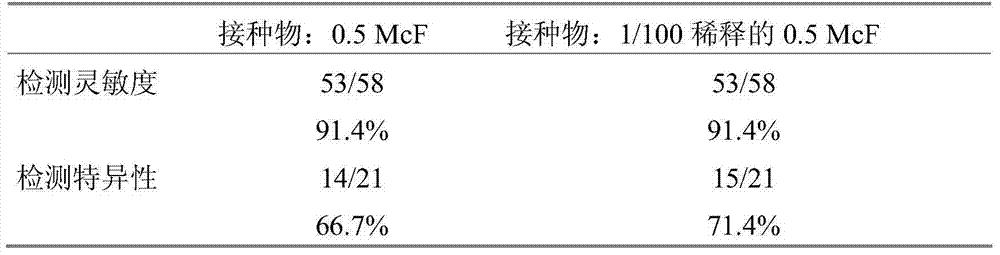
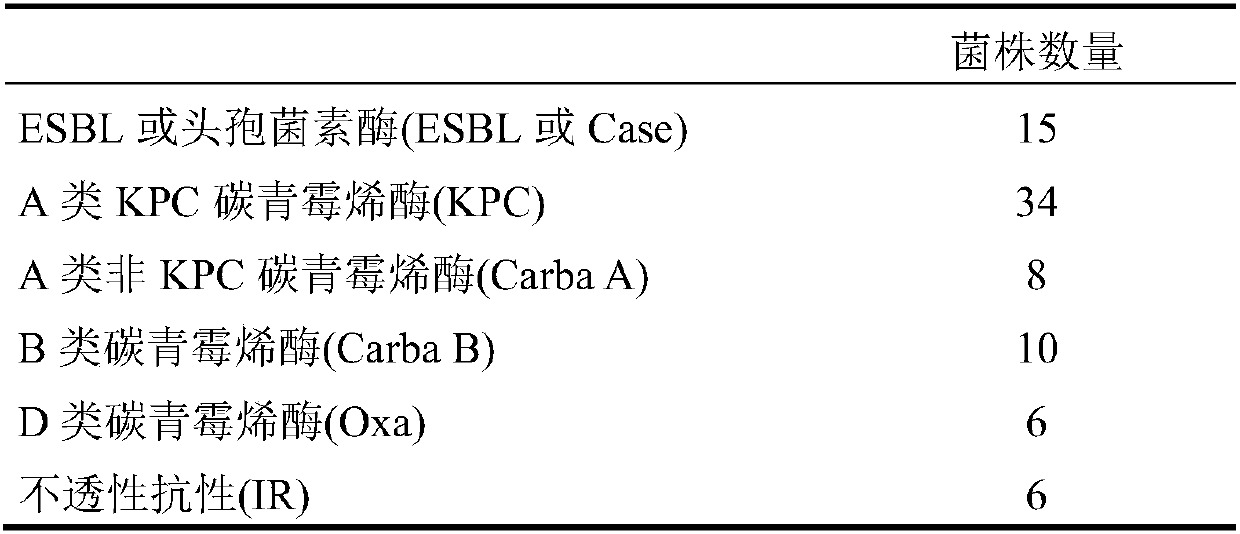
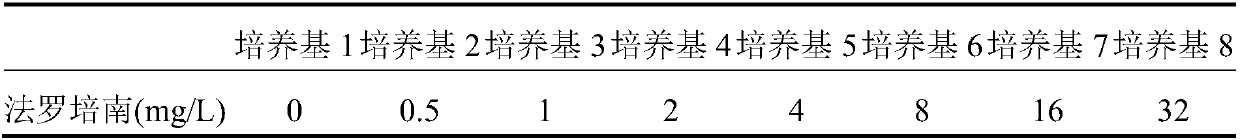
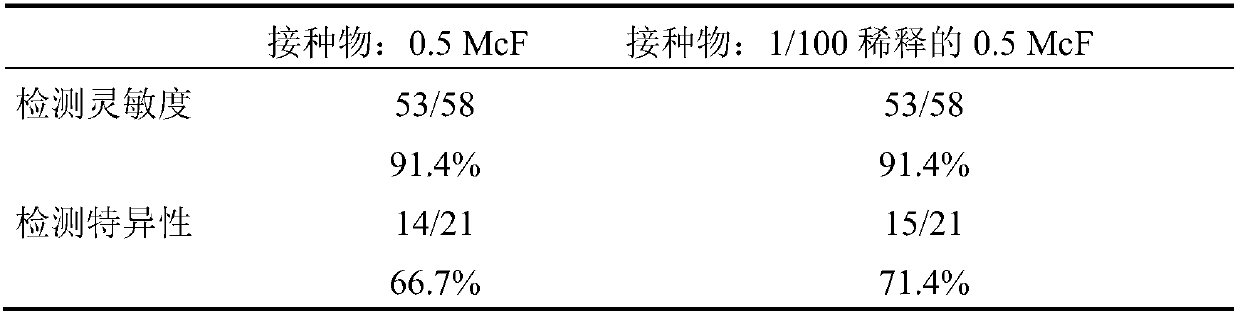
Detection of bacteria having a resistance to carbapenems
Disclosed is a process for detecting and / or identifying, in a biological sample, bacteria exhibiting a resistance to carbapenems, including: a) contacting said sample with a reaction medium including at least one chromogenic agent and faropenem and / or doripenem; b) incubating the whole so as to allow the bacteria to grow; and c) detecting the strains exhibiting a resistance to carbapenems. The medium employed in step a) also contains cloxacillin and / or a combination of cloxacillin and PAbetaN.
Owner:BIOMERIEUX SA
Novel process for preparing doripenem
InactiveCN102174047AReduce pollutionImprove protectionOrganic chemistryBulk chemical productionTert-Butyloxycarbonyl protecting groupDiphenylphosphine oxide
The invention discloses a process for preparing (2S, 4S)-1-p-nitro benzyloxycarbonyl-2-(N-tert-butoxycarbonyl amine-N-sulfonyl amino) methyl-4-mercapto-pyrrolidine which is an important intermediate used during preparation of doripenem and further discloses a novel process for preparing the doripenem by carrying out reaction on the intermediate which is used as a starting raw material and (1R, 5S, 6S)-6-[(1R)-1-hydroxy ethyl]-2-diphenylphosphine oxide acyloxy-1-methyl-1-carbon substituted-2-penem-3-carboxylic acid p-nitro benzyl ester with diisopropylethylamine used as the alkaline and removing protecting groups by adopting Pd / C. in the method, the readily available raw materials are adopted, other reagents are adopted to replace the traditional volatile reagents and the recrystallization method is adopted to purify the important intermediate, the high yield is ensured and the process is suitable for industrial production.
Owner:湖南欧亚药业有限公司
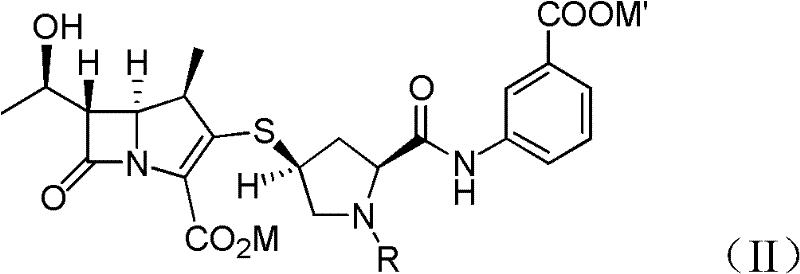
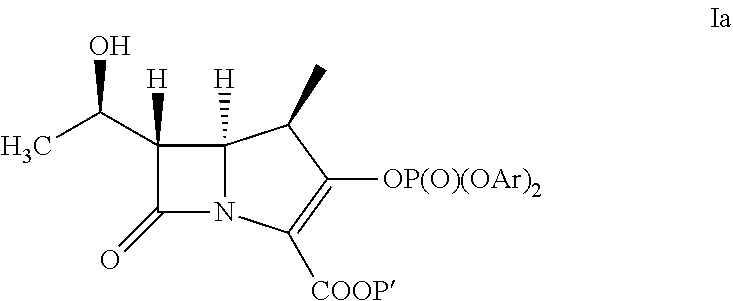
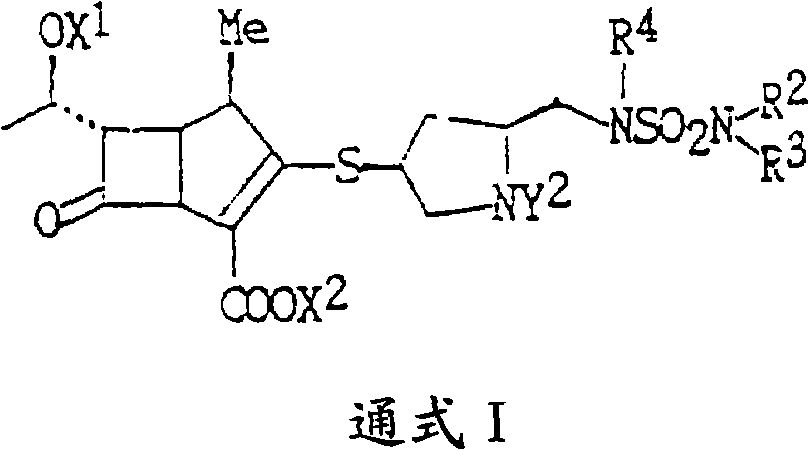
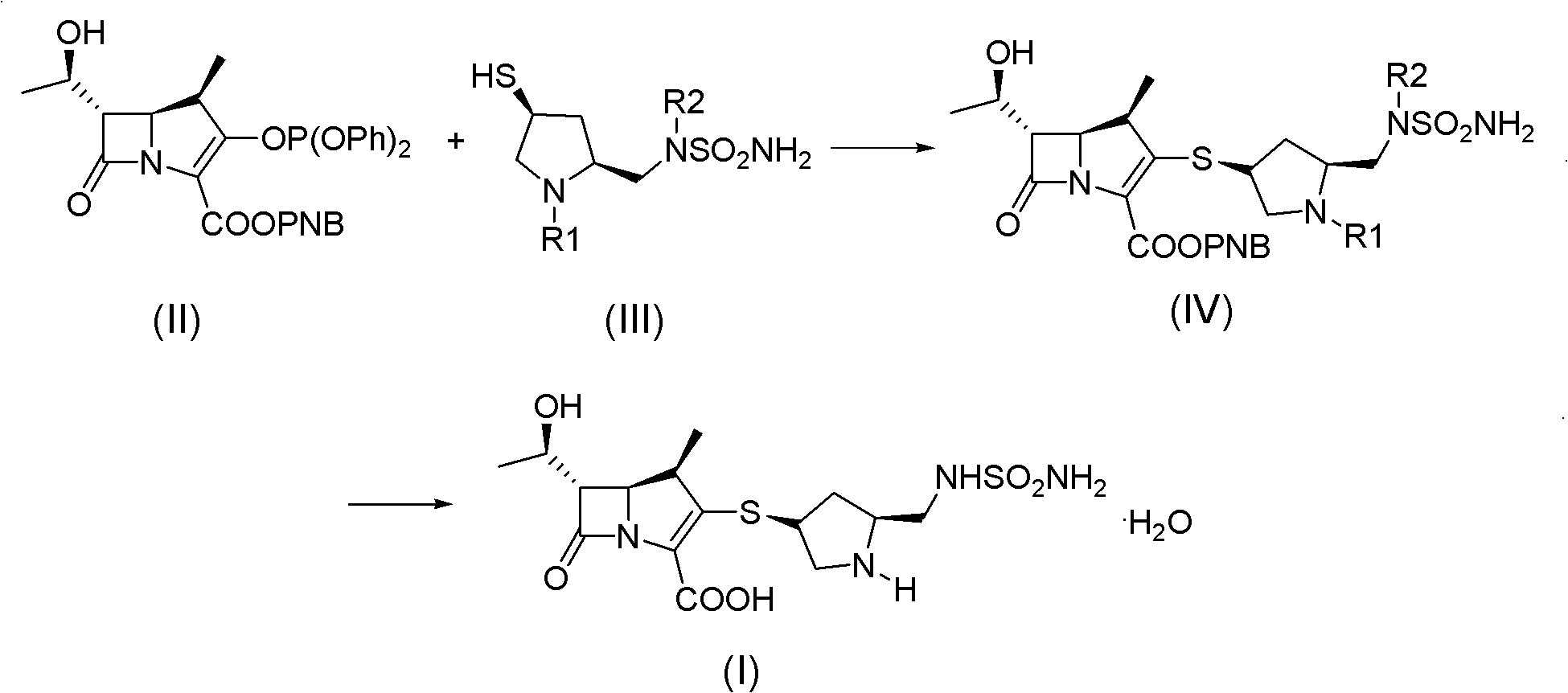
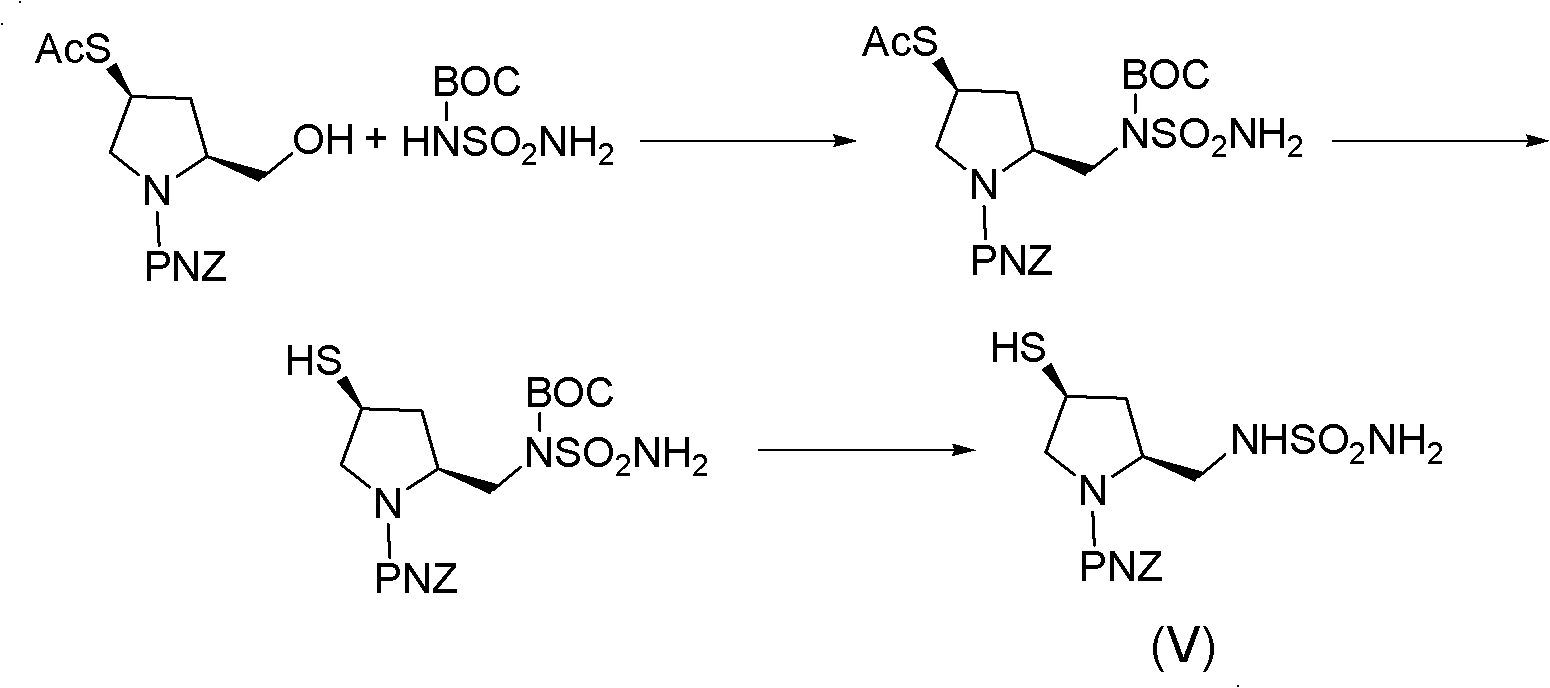
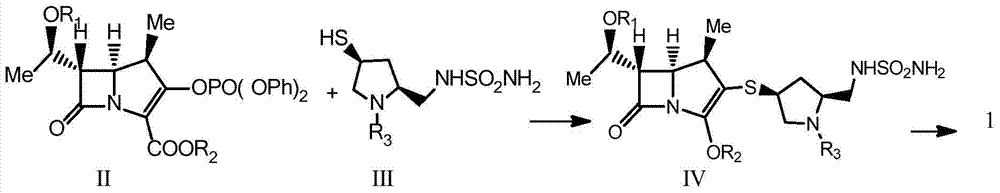
Doripenem intermediate compound, preparation process therefor and use thereof, and preparation process for doripenem
ActiveUS20150038700A1Reduction in yieldIncrease production costOrganic chemistryBulk chemical productionProtecting groupMonobasic acid
The present invention provides a doripenem intermediate compound shown by formula (XIV), wherein PNB is p-nitrobenzyl, and HX is an acid; and when HX is a monobasic acid, n=1; and when HX is a polybasic acid, n=2. The present invention also provides a process for preparing the doripenem intermediate compound (XIV). In addition, the present invention provides a process for preparing doripenem (I) from the doripenem intermediate compound (XIV) in a simple manner, with a high yield and low production costs. The new mono-protected doripenem intermediate compound provided in the present invention contains only one protecting group, reducing the difficulty and complexity in the subsequent de-protection step by catalytic hydrogenation, increasing the yield of the catalytic hydrogenation reaction, and thus reducing the production cost of the final product. The process is easy to operate and suitable for industrialized production.
Owner:SHENZHEN HAIBIN PHARMA
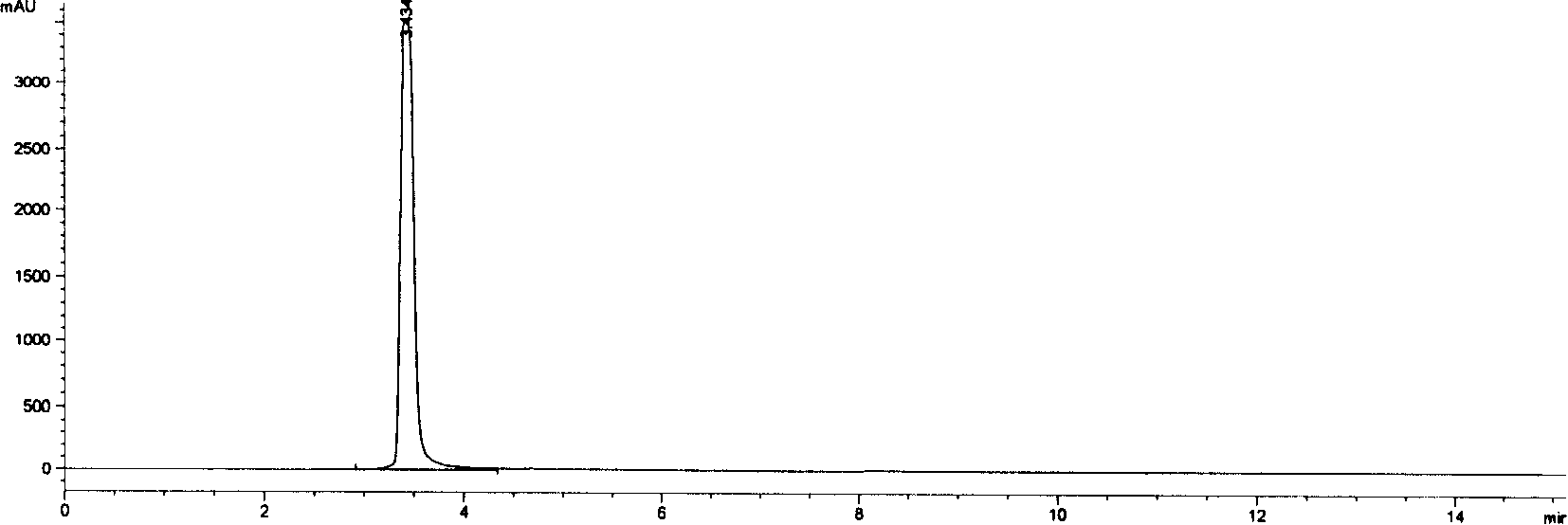
Method for measuring Doripenem and/or relevant substances by utilizing high performance liquid chromatography
ActiveCN103389347AGood peak shapeEfficient separationComponent separationStationary phaseUltraviolet detectors
The invention provides a method for measuring Doripenem and / or relevant substances by utilizing a high performance liquid chromatography. The method comprises the following steps of: measuring by utilizing an ultraviolet detector with the detection wavelength of 230nm; with silane-bonded silica gel bonded with polar groups as a stationary phase, with a mixture compounded of a sodium salt or sylvite aqueous solution of phosphoric acid with the pH of 4.7-6.0 and the concentration of 0.01-0.2mol / L and acetonitrile as a mobile phase, carrying out isocratic or gradient elution. Compared with the prior art, the method provided by the invention has the advantages that more impurities can be detected, good separation degree and peak shape of the Doripenem and / or relevant substances can be realized, and good base line separation can be realized between the Doripenem and the impurities as well as between the impurities and the impurities; and the method has good reproducibility between batches and between columns and is suitable for industrial production.
Owner:SHENZHEN HAIBIN PHARMA
Process for the preparation of carbapenem using cabapenem intermediates and recovery of cabapenem
ActiveUS20110288290A1Increase effective yieldGeneration of impurity can be reducedOrganic active ingredientsGroup 5/15 element organic compoundsErtapenemOrganic chemistry
Owner:SAVIOR LIFETEC CORP
High performance liquid chromatography method for measuring doripenem content
InactiveCN101191787AGood peak shapeHigh sensitivityComponent separationTesting medicinal preparationsUltraviolet detectorsPhosphoric acid
The invention discloses a method of detecting the content of doripenem with high performance liquid chromatography, which adopts an ultraviolet detector for detecting. The invention is characterized in that: the method adopts an octyl chemically bonded silica chromatographic column as the chromatographic column, and acetonitrile and solium salt aqueous solution of phosphoric acid as the mobile phase, and the pH of the solium salt aqueous solution of phosphoric acid is 5.0-6.0. The invention has simple operation, high sensitivity and good peak form, and can detect the doripenem in the range of pH 5.0-6.0.
Owner:SHANGHAI INST OF PHARMA IND CO LTD
Method for preparing ring-opening impurity of carbapenems
The invention relates to a method for preparing a ring-opening impurity of carbapenems. The ring-opening impurity is of a structure represented by formula I. In the formula I, R represents a meropenem side chain, an ertapenem side chain or a doripenem side chain. The method includes the following steps: (1) adding carbapenems into an alkaline solution, stirring and reacting for 1-4 hours; (2) adjusting the pH to 6.0-9.0; (3) freeze-drying the materials to obtain the ring-opening impurity of carbapenems. The method provided by the invention is simple to operate and short in reaction time; the product has the purity of 95% or above and can be directly used as a reference substance for quantitative and qualitative research on the ring-opening impurity of carbapenem products, so that the product quality can be effectively controlled. The formula I is shown in the description.
Owner:山东安弘制药有限公司
Method for preparing doripenem intermediate compound
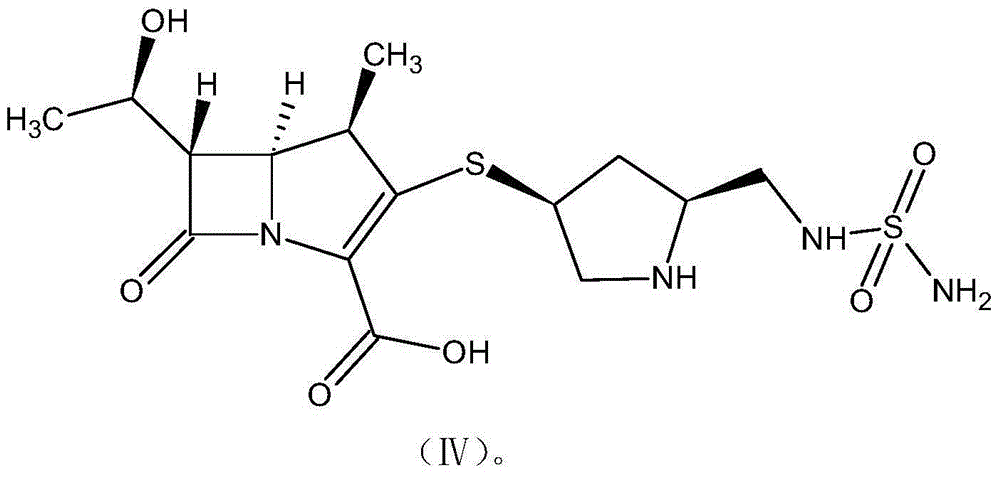
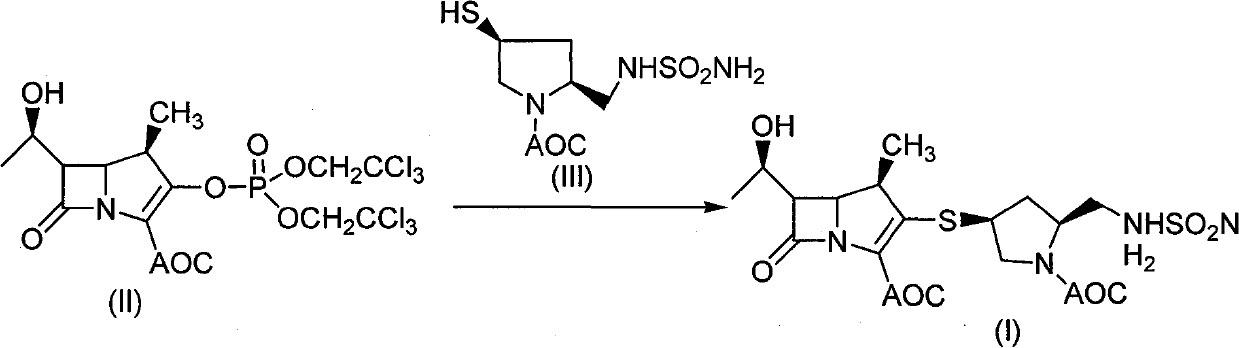
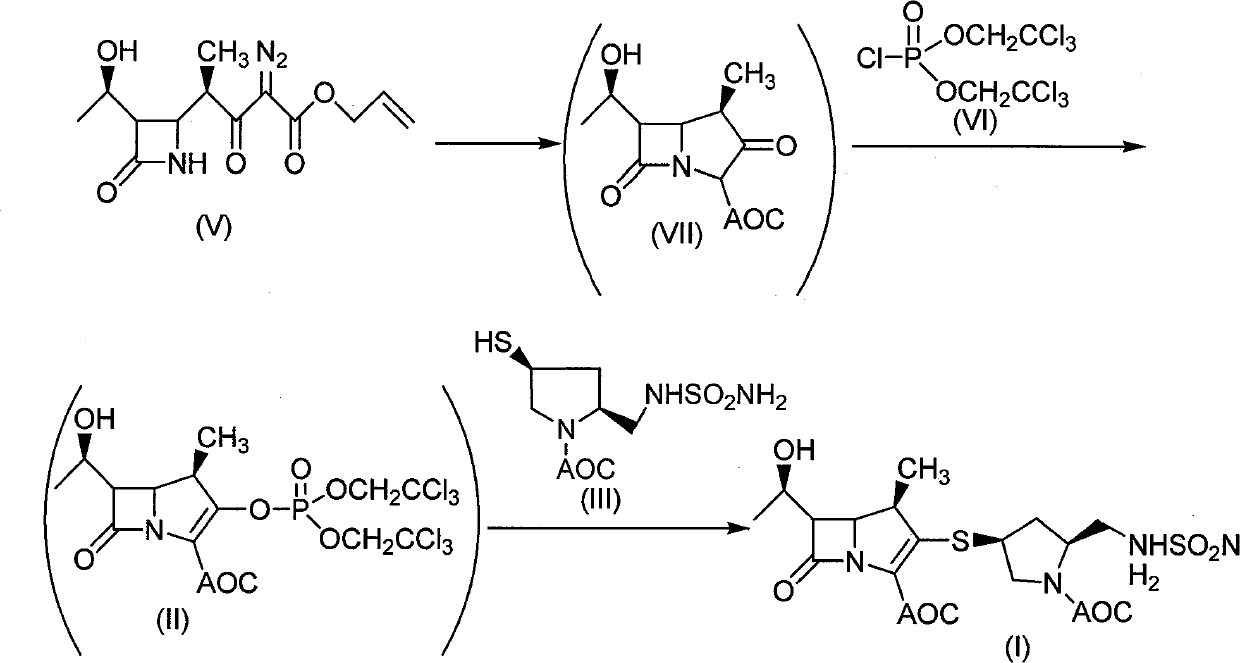
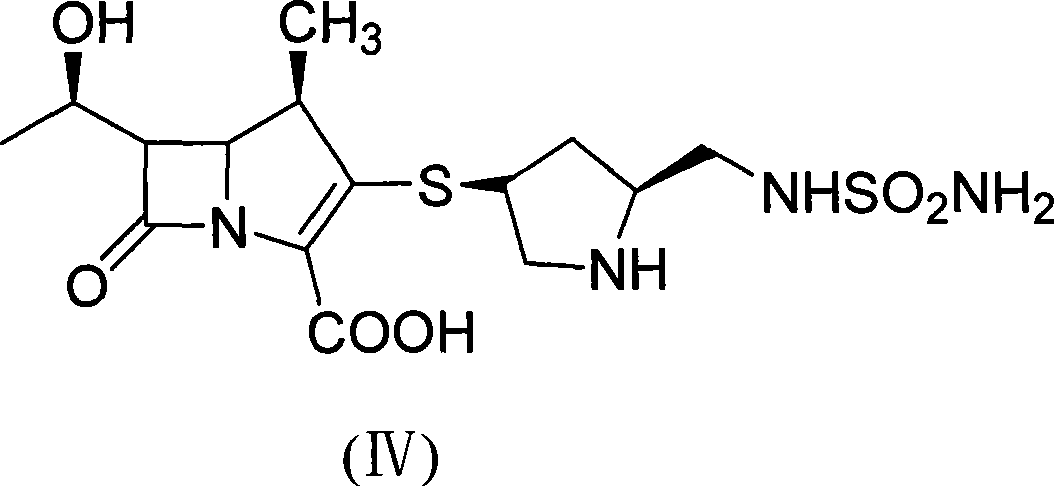
The invention discloses a method for preparing a doripenem intermediate compound (I), which comprises the following step: under the protection of inert gas, in a polar aprotic solvent, reacting a compound (II) with a compound (III) at the temperature of between -60 and 25 DEG C under the action of organic base, wherein AOC is allyloxycarbonyl group. The preparation method has the advantages of high yield and high purity of the product, simple operation, simple posttreatment, cost conservation and easy realization of industrialization.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
A kind of preparation method of doripenem
ActiveCN102731505BSolve dissolveReduce degradationOrganic chemistryBulk chemical productionOrganic solventSolvent
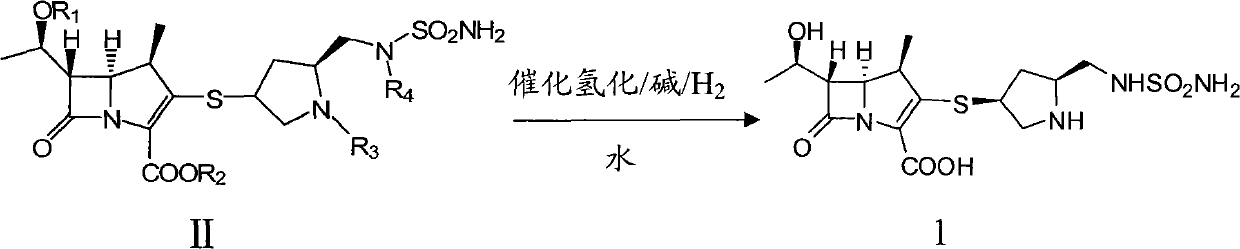
The invention relates to a preparation method of doripenem as shown in the formula 1. The method comprises the following steps of: using a doripenem intermediate II as a raw material, using a single solvent--water as a reaction solvent and conducting a hydrogenated deprotection reaction in the presence of alkali and a catalyst. The single solvent water is used as a reaction solvent, thus solving a series of problems caused by the use of an organic solvent in the reaction solvent, reducing product degradation and raising product purity. In addition, the preparation method is economical, safe and environmentally friendly, and is more suitable for industrial operation at large scale.
Owner:SHANGHAI RUNSHI MEDICAL TECH CO LTD
Industrial synthesis method of doripenem
InactiveCN103664946AHigh puritySuitable for industrial productionOrganic chemistrySynthesis methodsTwo phase composite
The invention relates to a method for preparing a carbapenem antibacterial medicament doripenem. The method comprises the following steps: dispersing a compound of a formula (I) as shown in the specification in a two-phase composite solvent so as to be subjected to hydrogenation deprotection, layering so as to obtain an enriched doripenem phase, and adjusting the pH value by using acid so as to obtain doripenem crystal. PNZ is p-nitryl benzyl oxygroup carbonyl; PNB is p-nitryl benzyl.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Composition of Doripenem and amino acid
ActiveCN101933921AImprove stabilityGood storage stabilityAntibacterial agentsPowder deliveryThreonineAminocaproic acid
The invention relates to a composition containing Doripenem, in particular to a composition containing the Doripenem and amino acid, wherein the amino acid is selected from one ore some of aminopropionic acid, phenylalanine, aminocaproic acid and threonine.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD +1
Process for preparing doripenem
The invention relates to the technical field of pharmacy, in particular to a process for preparing doripenem. The process comprises the following steps: taking tetrahydrofuran, a doripenem condensation compound, magnesium chloride hexahydrate, 10% Pd / C and distilled water as raw materials, carrying out a series of reactions; extracting an aqueous phase by using ethyl acetate and n-butyl alcohol; washing with propyl alcohol to obtain a crude product of doripenem, treating with activated carbon and propyl alcohol, thus obtaining sterile doripenem. The operation is simple, is favorable for the environment protection and health of workers, and the raw materials are readily available and low in price; therefore, the process is more suitable for industrial production of doripenem.
Owner:ANHUI YOUCARE KAIYUE PHARMA
Preparation method of doripenem
InactiveCN105254632AHigh yieldLow costOrganic chemistryTert-Butyloxycarbonyl protecting groupSolvent
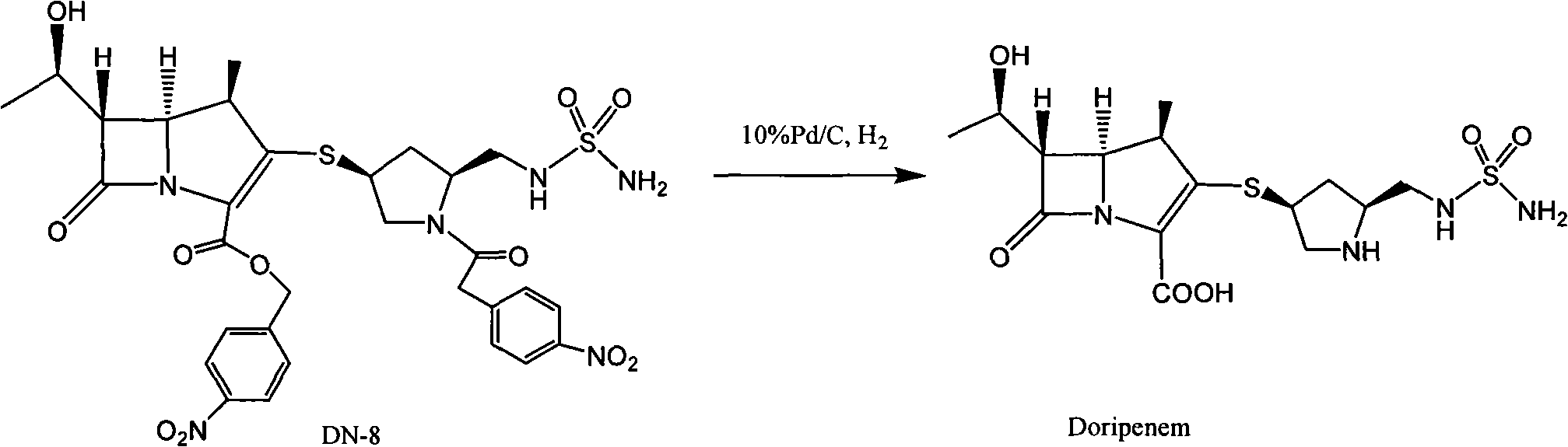
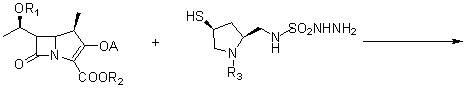
The invention relates to a preparation method of doripenem. The method comprises the following steps: removing an acetyl group and a tert-butyloxycarbonyl group from an acetylthiopyrrolidine derivative (SM-1) used as an initial raw material to prepare a thiopyrrolidine derivative (DN-1), carrying out a nucleophilic substitution reaction on the DN-1 and a doripenem parent nucleus (SM-2, MAP) to prepare protected doripenem, carrying out catalytic hydrogenation to remove PNZ and PNB protection, and purifying to obtain final doripenem. A solvent and a catalyst used in the method can be recycled, so the production cost is greatly saved, and the method is suitable for large-scale industrial production.
Owner:双鹤药业(海南)有限责任公司
Doripenem intermediate solvate
The invention relates to a Doripenem intermediate alcoholate shown as a formula 2 and a preparation method and application of the Doripenem intermediate alcoholate. Doripenem intermediate alcoholate is stable in property, easy to preserve, and high in purity, and establishes a foundation for a following hydrogenating deprotection reaction. The preparation method of the alcoholate is simple in operation, and a used crystallization reagent is ethanol which is less harmful to human health and environment, and the preparation method is suitable for industrial production.
Owner:SHANGHAI RUNSHI MEDICAL TECH CO LTD
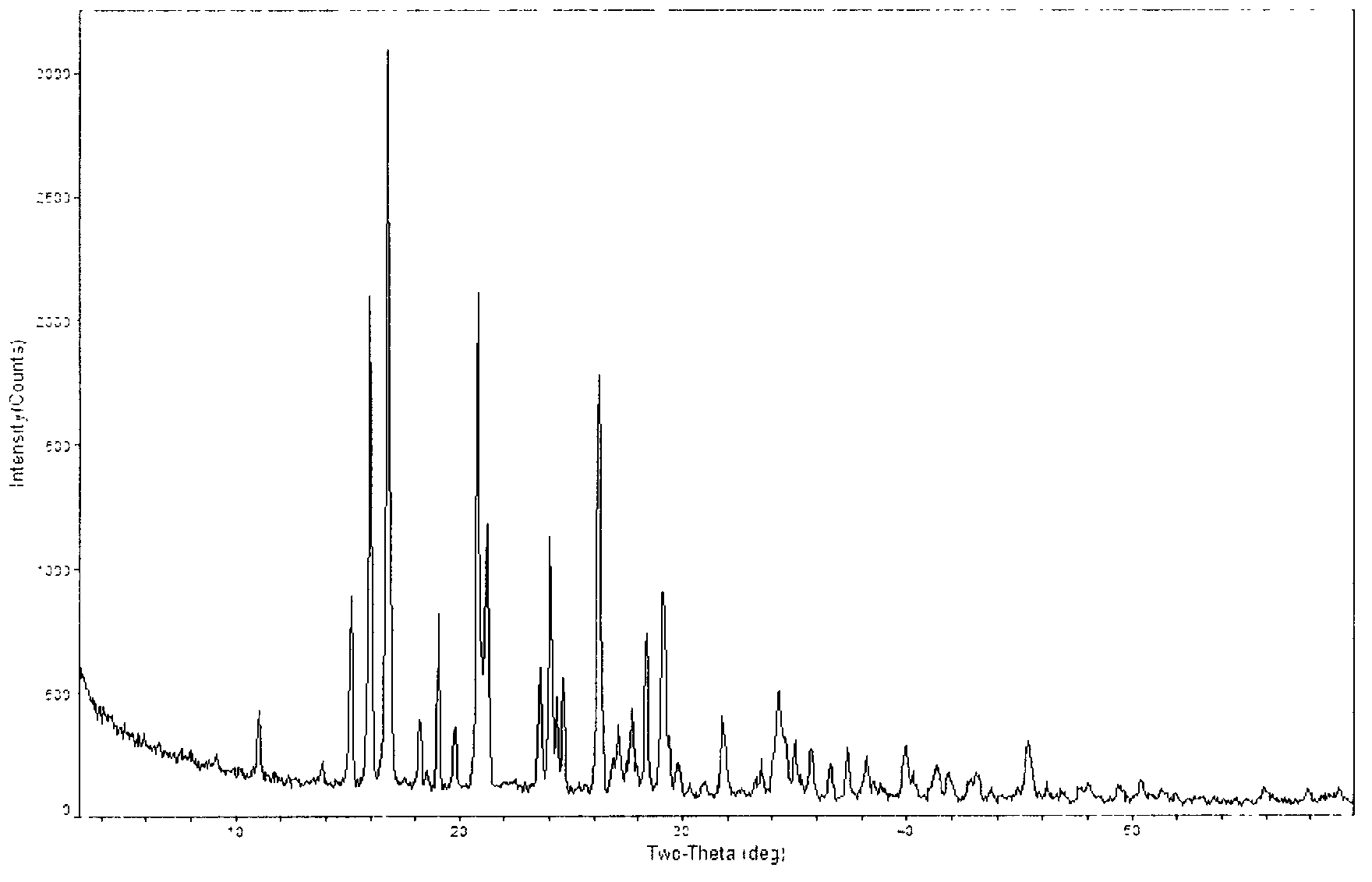
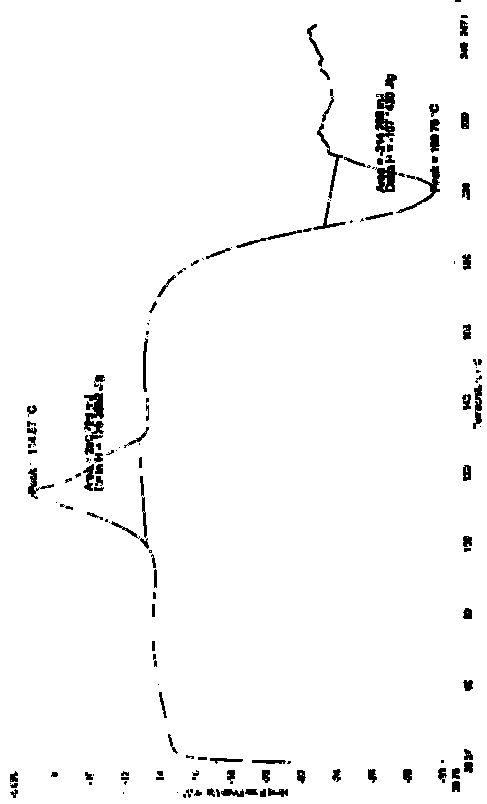
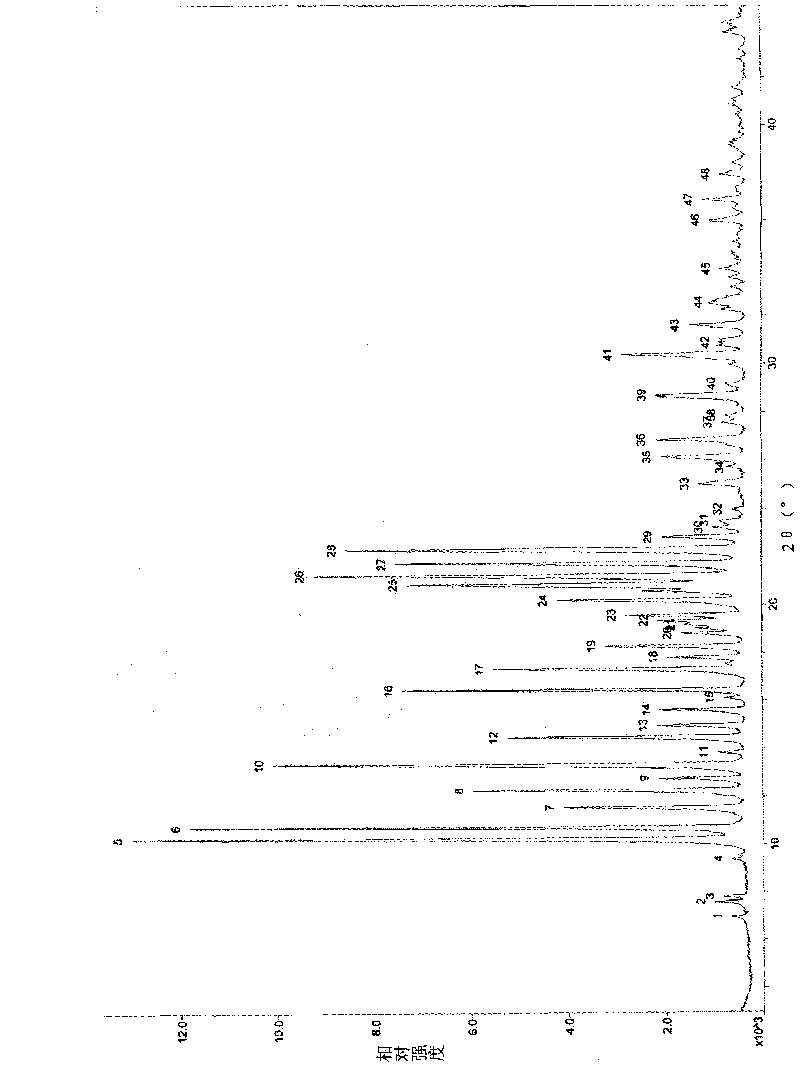
Novel crystal of doripenem, preparation method and use thereof
ActiveCN101100469BImprove stabilityAntibacterial agentsOrganic active ingredientsCarboxylic acidPyrrolidine
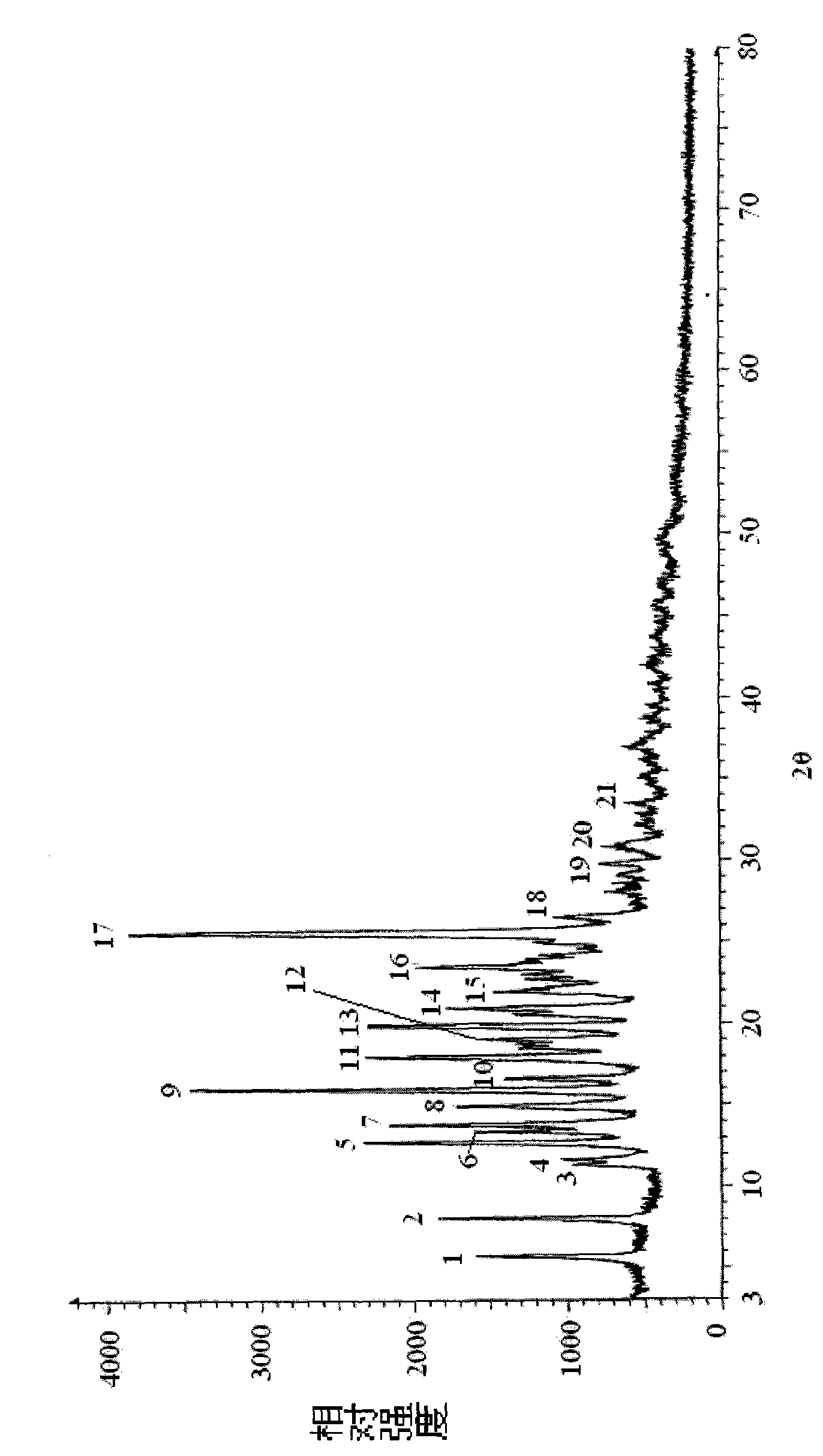
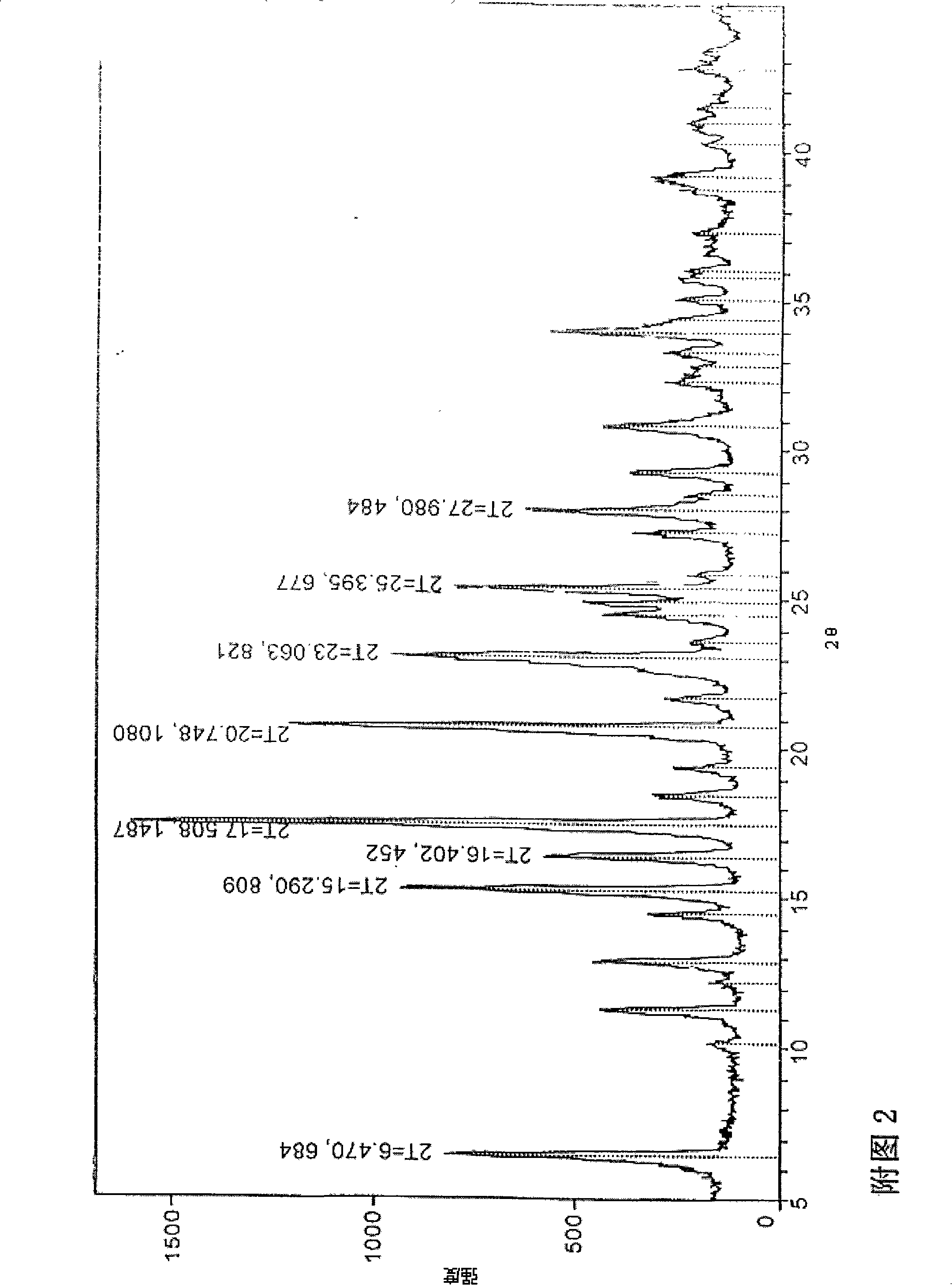
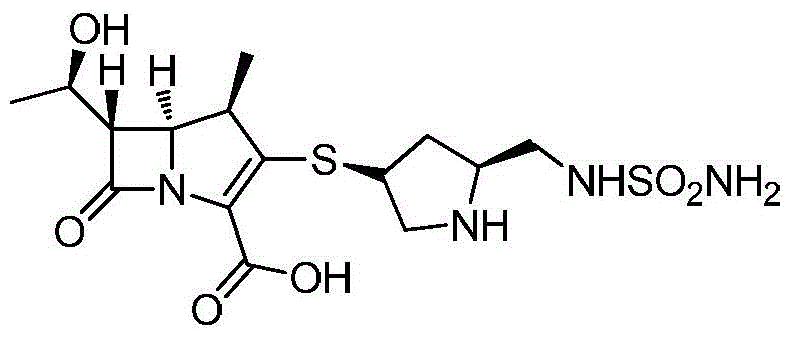
A (1R, 5S, 6S)-6-((1R)-1-ethoxyl)-2-((3S, 5S)-5-sulfonamide-amino-methyl-pentazane-3-base) sulfur-1-methyl-1-carbon-2-penicillic vinyl-3-carboxylic acid or its hydrate, its medicinal composite containing multi-nipenan crystal, its production and use in preparation of anti-infectious medicine are disclosed. In the crystallizing powdery X-ray diagram, it has main peak at diffraction angle (2 theta)=6.46, 15.27, 16.41, 17.49, 20.72, 23.05 and 25.38 deg. minus or plus 0.1. It's simple and cheap, and has excellent thermodynamic stability and dissolubility.
Owner:CHENGDU DIAO JIU HONG PHARMACEUTICAL FACTORY
A kind of preparation method of high-purity doripenem
The invention discloses a method for preparing high purity doripenem. The method comprises the following steps: (1) conducting a contact reaction on carbapenem bicyclic nucleus and (2S,4S)-1-p-nitrocarbobenzoxy-4-sulfenyl-2-(N-sulfamoyl amino)methylpyrrolidine in a water and 1,4-dioxane mixed solvent in the presence of copper salt and triethylamine; adding water and ethyl acetate, stirring, standing for layering, concentrating the ethyl acetate layer; recrystallizing a dichloromethane and petroleum ether mixed solvent to obtain (1R,5S,6S)-2-[(3S,5S)-1-nitrophenyl formate-5-sulfamoyl amino methylpyrrolidine-3-sulfenyl]-6-[(1R)-1-ethoxyl]-1-methyl-1-carba-2-penem-3-p-nitrobenzyl carboxylate; and (2) adding the product obtained in the step (1), tetrabutyl ammonium chloride and 0.2M of phosphate buffered solution having a pH value of 8 into a reaction kettle which is filled with water, replacing three times with hydrogen, adding hydrogen to react, filtering, concentrating the filtrate, and recrystallizing in methanol to obtain doripenem.
Owner:高霞 +3
Preparation method of doripenem
ActiveCN102731505ASolve dissolveReduce degradationOrganic chemistryBulk chemical productionOrganic solventSolvent
The invention relates to a preparation method of doripenem as shown in the formula 1. The method comprises the following steps of: using a doripenem intermediate II as a raw material, using a single solvent--water as a reaction solvent and conducting a hydrogenated deprotection reaction in the presence of alkali and a catalyst. The single solvent water is used as a reaction solvent, thus solving a series of problems caused by the use of an organic solvent in the reaction solvent, reducing product degradation and raising product purity. In addition, the preparation method is economical, safe and environmentally friendly, and is more suitable for industrial operation at large scale.
Owner:SHANGHAI RUNSHI MEDICAL TECH CO LTD
Doripenem side-chain compound and preparation method and application thereof
ActiveCN102452969BImprove utilization efficiencyImprove the yield of hydrogenation reactionOrganic chemistryBulk chemical productionHydrogenation reactionSide chain
Owner:SHENZHEN HAIBIN PHARMA +1
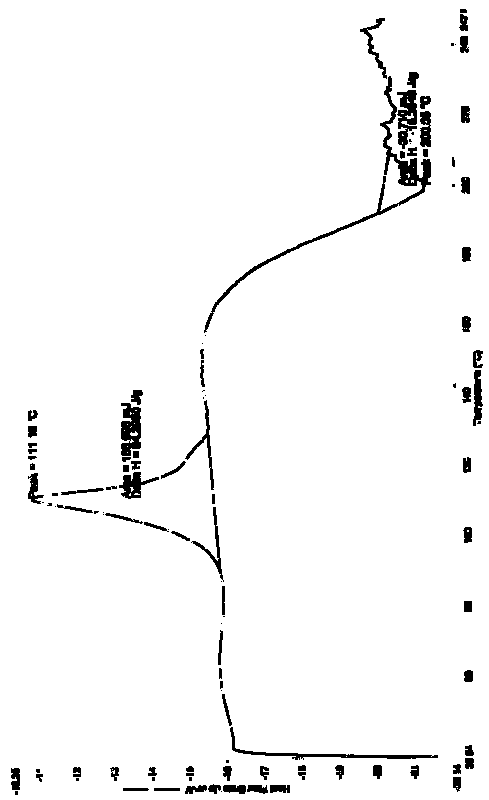
Crystal of doripenem intermediate and preparation method thereof
ActiveCN101747251AEasy to storeEasy to operateOrganic chemistryTert-Butyloxycarbonyl protecting groupX-ray
The invention discloses a crystal of a doripenem intermediate compound (I). In an X-ray diffraction pattern of powder of the crystal, main peaks are present at a diffraction angle 2 theta which is equal to 10.27 degrees, 10.75 degrees, 12.28 degrees, 13.35 degrees, 17.33 degrees, 20.85 degrees, 21.24 degrees, 21.75 degrees or 22.31 degrees and an error range of a value of the 2 theta is + / -0.2 degree; and BOC is tert-butoxycarbonyl group. The invention also discloses two preparation methods for the crystal, which comprise the following steps: (1) dissolving a mixture of the compound (1) and triphenylphosphine oxide in a soluble solvent, adding water, and stirring to separate out the crystal of the compound (1); and (2) dissolving a mixture of the compound (1) and triphenylphosphine oxide in methanol, and stirring to separate out the crystal of the compound (I), wherein the volume / mass ratio of the methanol to the mixture is 0.5 to 2.0ml / g. The crystal has the advantages of easy storage, easy operation, good stability and high purity; and the preparation methods have the advantages of simpleness, reliability, cost conservation, simple separation, and suitability for industrial production.
Owner:SHANGHAI INST OF PHARMA IND +1
A kind of crystal form of carbapenem antibacterial drug and preparation method thereof
The invention provides a novel crystal form of a carbapenem antimicrobial medicament, namely, (+)-(4R,5S,6S)-3-[[(3S,5S)-5-(aminosulfonyl)aminomethyl]-3-pyrrolidine]sulfur]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclic[3,2,0]heptyl-2-ene-2-carboxylic acid-hydrate (doripenem). The X-ray powder diffraction pattern of the crystal powder shows main peaks when 2theta is equal to 15.20 degrees, 16.06 degrees, 16.83 degrees, 19.40 degrees, 21.29 degrees, 23.68 degrees, 24.08 degrees, 24.70 degrees, 26.28 degrees, 28.43 degrees, 29.17 degrees and 34.35 degrees. The novel crystal form has the advantages of easiness in preparation and industrial production, low cost, high solubility and high stability.
Owner:SHANDONG FREDA PHARMA GRP CO LTD +1
Detection of bacteria having a resistance to carbapenems
The invention relates to a method for detecting and / or identifying, in a biological sample, bacteria having a resistance to carbapenems. The method comprises the following steps consisting in: a) bringing the sample into contact with a reaction medium containing at least one chromogenic agent and faropenem and / or doripenem; b) incubating the sample and reaction medium brought into contact in step(a), such as to allow the growth of bacteria; and c) detecting the strains having a resistance to carbapenems. The medium used in step (a) also contains cloxacillin and / or a combination of cloxacillinand PAbetaN.
Owner:BIOMERIEUX SA
A kind of preparation method of doripenem
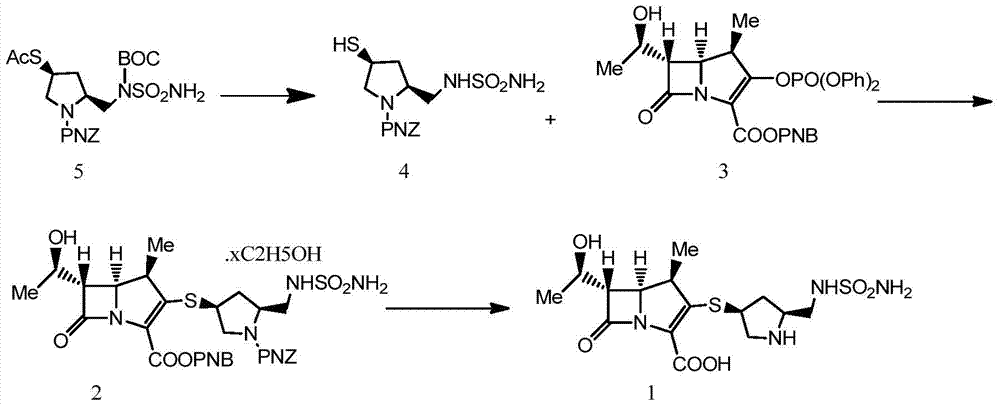
The invention belongs to the field of medicine synthesis and particularly relates to a doripenem preparation method. The method includes the steps that a compound 5 reacts with concentrated sulfuric acid in methanol to obtain a compound 4; the compound 4 and p-Nitrobenzyl-6-(1-hydroxyethyl)-1-azabicyclo(3.2.0)heptane-3,7-dione-2-carboxylate (a compound 3) are subjected to a condensation reaction under the condition that N,N-diisopropylethylamine exists, water and ethyl acetate are added and stirred after the reaction, an ethyl acetate layer is collected, alcohol is added in ethyl acetate collection liquid, crystallization is carried out, and a compound 2 is obtained; the product is dissolved through ethyl acetate; after a monopotassium phosphate solution and a phase transfer reagent of triethylbenzylammonium chloride are added, zinc powder is added into the ethyl acetate / monopotassium phosphate solution in batches to react and obtain doripenem. According to the method, reaction conditions are moderate, the technology is simple, and the conversion rate and the yield are high. Two-phase reaction is used in deprotection reaction, and the after-treatment process is simplified. The zinc powder which is cheap is used, so that the method is economical, and a new concept and a new method are provided for doripenem preparation.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com