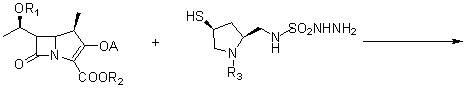
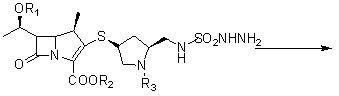
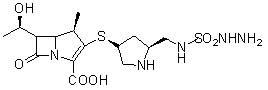
Novel process for preparing doripenem
A preparation process, the technology of doripenem, which is applied in the field of new preparation process of doripenem, can solve the problems affecting the content of doripenem, the purification method of compound III and the compound V are not reported, and achieve quality assurance , reduce impurities, and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0069] In order to make the technical means, creative features, work flow, and use methods of the present invention achieve the purpose and effect easily understood, the present invention will be further described below in conjunction with specific embodiments.
[0070] A new preparation process of doripenem, first of all, (2S,4S)-1-p-nitrobenzyloxycarbonyl-2-(N-tert-butoxycarbonyl-N-sulfamylaminosulfonyl)methyl-4 -Mercaptopyrrolidine is prepared as follows:
[0071]
[0072] (1) (2)
[0073]
[0074] (3) (4)
[0075]
[0076] (5) (6)
[0077]
[0078] (Ⅲ)
[0079]
[0080] (Ⅳ)
[0081]
[0082] (I)
[0083] Compound Ⅱ, namely (1R, 5S, 6S)-6-[(1R)-1-hydroxyethyl]-2-diphenoxyphosphoryloxy-1-methyl-1-carbo-2-penicillium P-nitrobenzyl alkene-3-carboxylate is available in the market and can be purchased directly.
[0084] Synthesis of compound (2):
[0085] Add 820 ml of 5N sodium hydroxide aqueous solution into the reaction flask, and add 262 g of trans-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com