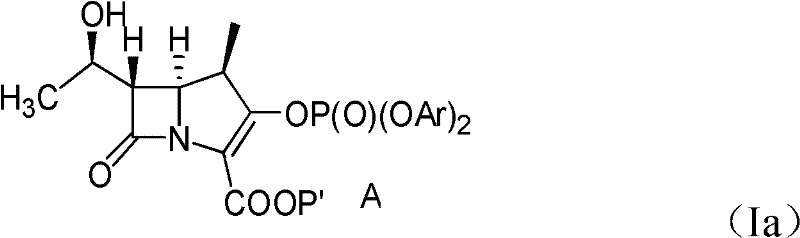Process for the preparation of carbapenem using cabapenem intermediates and recovery of cabapenem
A technology for compounds and analogs, applied in the field of preparing carbapenem intermediates, can solve the problems of difficult recovery and purification, low concentration and difficult crystallization, product time damage, etc., and achieves shortened reaction process time, long service life, and reduced manufacturing process. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] A. Preparation of compounds of formula (Ia)
[0060] After 43.2 grams of compounds having the formula (III) structural formula were placed in 777ml of dichloromethane and stirred and dissolved, then 145mg of Rh was added in this solution. 2 October 4 , and after heating the mixture at reflux for 7 hours, 388 ml of dichloromethane were removed by distilling the solution. A dichloromethane (dichloromethane) solution containing a compound of formula (IV) is cooled to a temperature lower than -35°C, and at a temperature lower than -35°C, 71.3 grams of chlorophosphoric acid bis(2, 4-dichlorophenyl) ester (bis(2,4-dichlorophenyl)-chlorophosphate, DDCP) and 17.63 grams of diisopropylethylamine (diisopropylethylamine, DIPEA) dissolved in 43ml of methylene chloride and 40mg of 4 - a mixture of dimethylaminopyridine (4-dimethylamino pyridine), added to the reaction solution, and reacted for 2 hours. Then, at a temperature of 0-5°C, mix 100ml of 1% hydrochloric acid (HCl, liqui...
Embodiment 2
[0064] A. Prepare the compound with formula (VI) structural formula by the compound with formula (Ia) structural formula
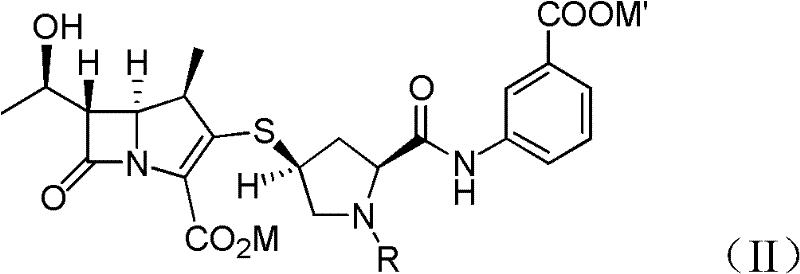
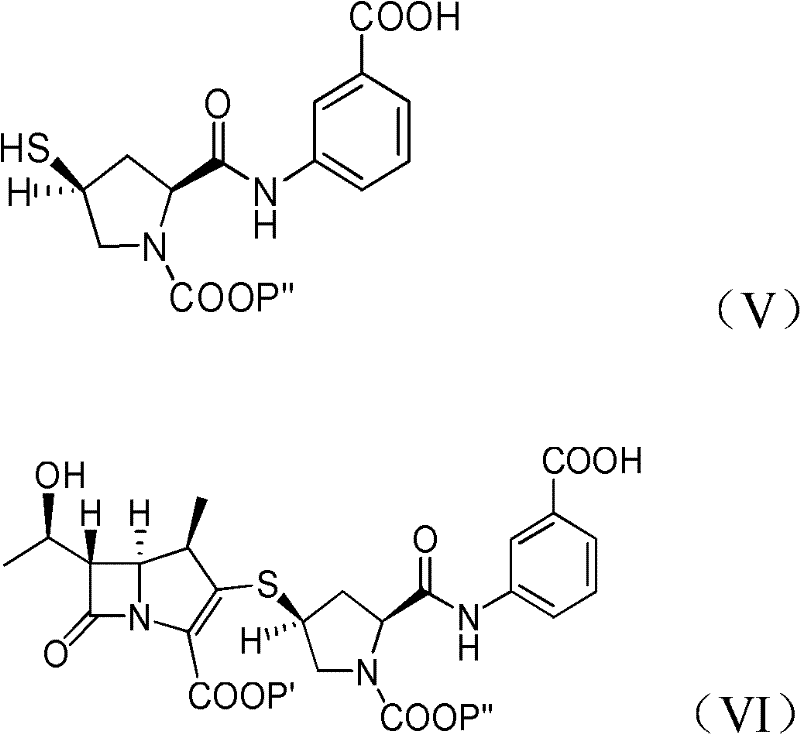
[0065] Provide the compound of formula (Ia) obtained in Example 1A at -30°C dissolved in 500 ml of dichloromethane: p-nitrobenzyl (1R, 5S, 6S)-6-[(IR)-1-hydroxyethyl ]-2-[(bis(2,4-dichlorophenyl)phosphoryloxy)]-1-carbapenem-2-ene-3-carboxylic acid (p-nitrobenzyl(1R,5S,6S) -6-[(IR)-1-hydroxyethyl]-2-[(bis(2,4-dichlorophenyl)phosphono)oxy]-1-methylcarbapen-2-en-3-carboxylate), add 44.7 grams of 3-( [[(2S,4S)-sulfhydryl-2-pyrrolidinyl-1-(4-nitrobenzyloxy)formyl]formyl]amino)benzoic acid (3-([[(2S,4S) -mercapto-2-pyrrolidinyl-1-(4-nitrobenzoic acid)carbonyl]carbonyl]amino)benzoic acid). To react the mixture, 41.0 g of diisopropylethylamine (DIPEA) was added and stirred at -30°C. After the reaction was completed, 500ml of water was added to the reacted mixture, stirred and separated, and the organic layer obtained could generate a compound of formula (VI), a...
Embodiment 3
[0071] A. Preparation of Compound (R=H) with Formula (II) Structural Formula
[0072] At 20° C., in the compound having the structural formula (VI) dissolved in dichloromethane prepared by Example 2A, add 648 ml of purified water containing 48 grams of 10% palladium carbon (Palladium on carbon, Pd / C) and 37.2 grams of sodium bicarbonate. The nitrogen in the reactor was replaced twice by introducing hydrogen gas, and then within the first hour under the hydrogen atmosphere, the pressure was adjusted to 25-80 psi, and the reaction temperature was controlled at 20°C and maintained for 4 After ~5 hours, lower the temperature to below 10°C, then adjust the pH value (pH) to about 5.0 with 5% hydrochloric acid (HCl), filter off 10% palladium carbon (Pd / C) and separate the organic layer . Adjust the pH value of the liquid layer to about 6.5, and then add about 1 kg of dichloromethane for extraction. At a temperature of 0-5°C, the liquid solution produced in the above steps was extr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com