Method for measuring Doripenem and/or relevant substances by utilizing high performance liquid chromatography
A technology of high performance liquid chromatography and related substances, applied in the field of drug analysis, can solve the problem of unstable doripenem peak time, unfavorable impurity research, poor reproducibility between batches and between columns and columns, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Example 1 Selection of mobile phase-stationary phase system
[0098] Referring to the prior art, the effects of different mobile phase-stationary phase systems on the detection of doripenem and related substances were investigated
[0099] 1.1 MOPS (3-(N-morpholino)propanesulfonic acid)
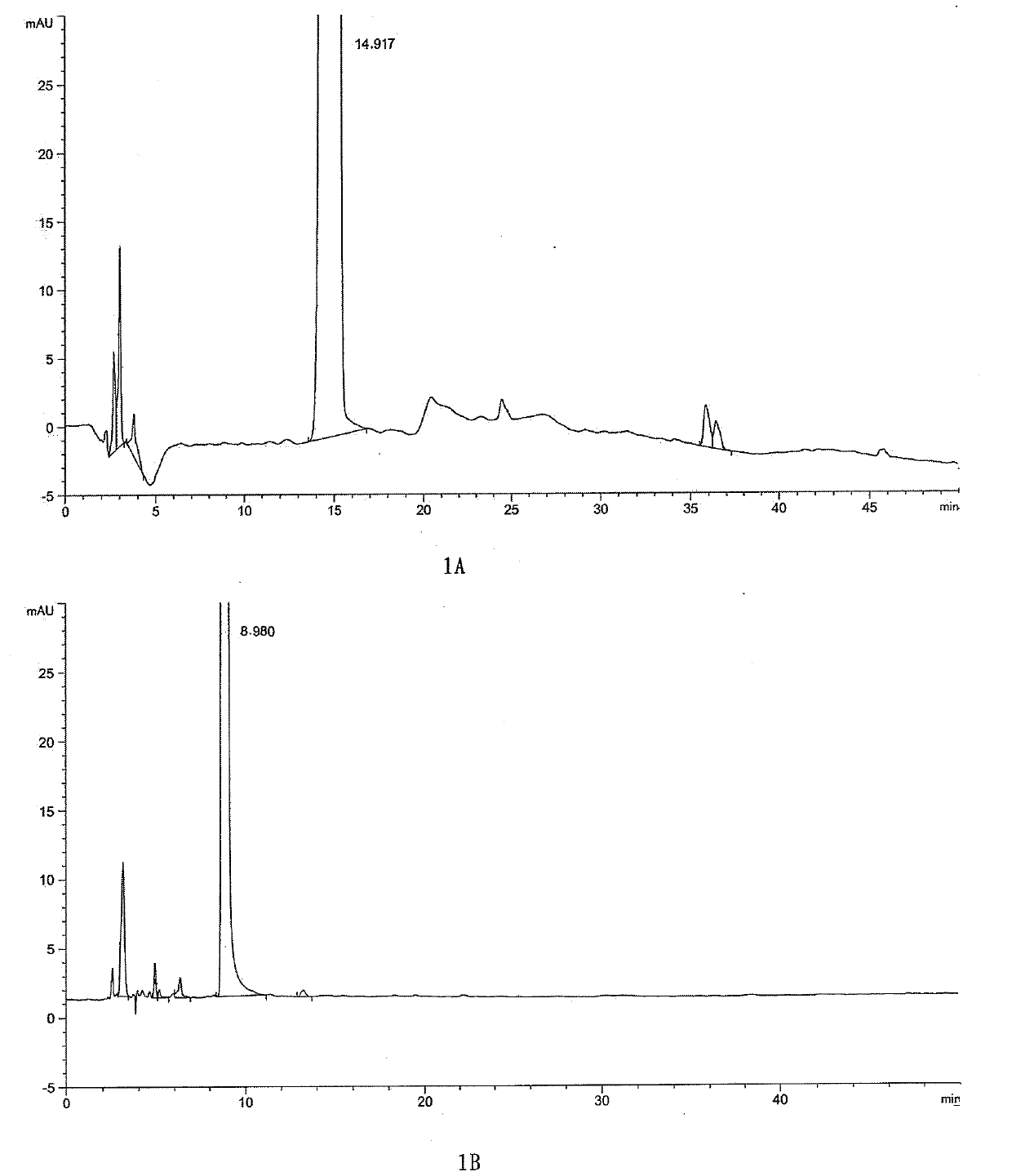
[0100] Chromatographic column: general-purpose C18 column (4.6×250, 5 μm); column temperature: 25°C; mobile phase: 10mmol / L MOPS (adjust pH=5.7 with acetic acid) as mobile phase A, acetonitrile as mobile phase B; flow rate : 1.0ml / min, detection wavelength: 230nm; using the gradient elution program in Table 1, see the detection results figure 1 A and Table 4.
[0101] Table 1 MOPS gradient elution program
[0102] t (min)
0
15
30
35
45
50
51
56
A(%)
96
96
90
90
85
85
96
96
B(%)
4
4
10
10
15
15
4
4
[0103] 1.2 Ammonium acetate
[0104] Chromatographic col...
Embodiment 2
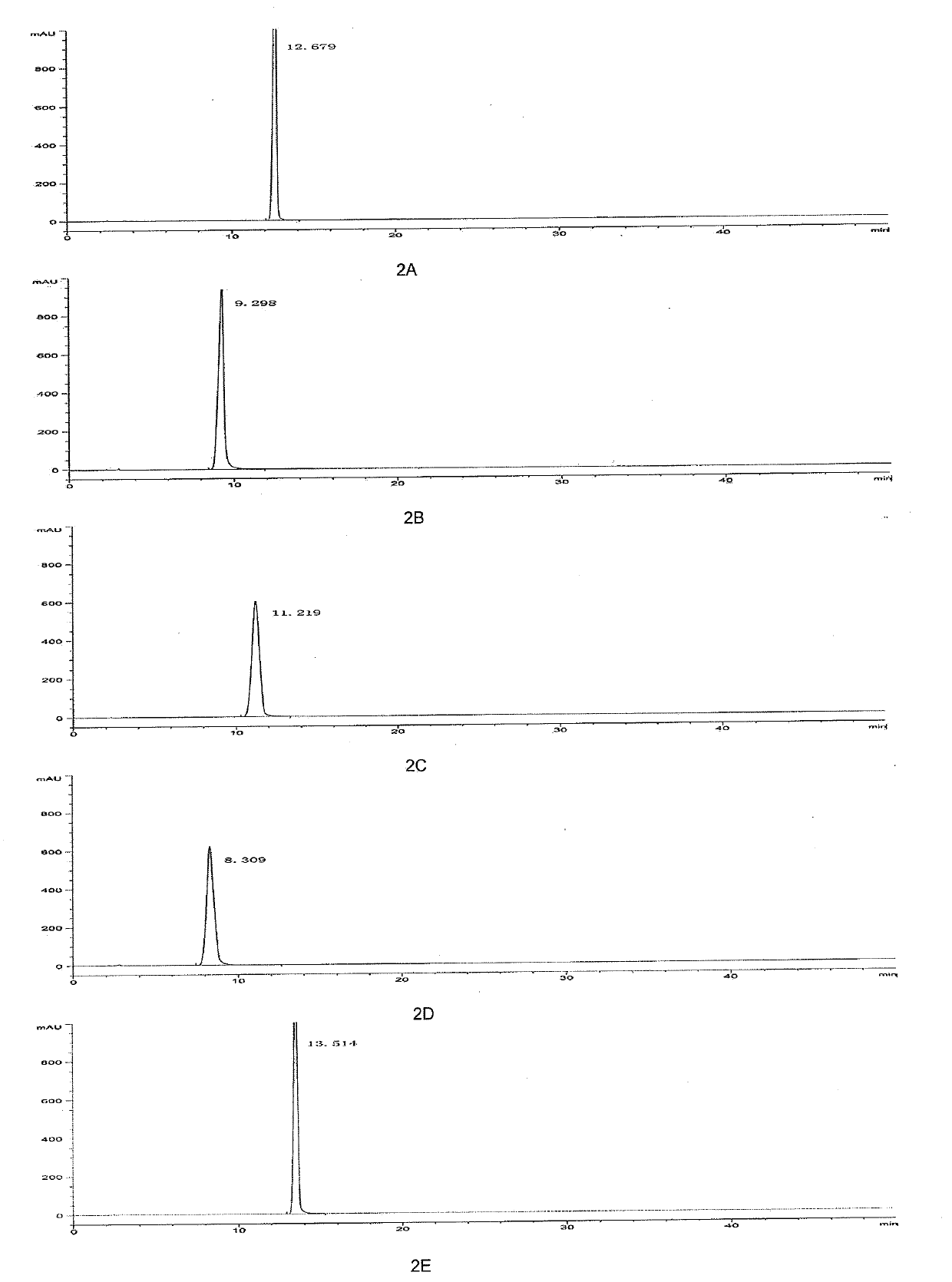
[0115] Example 2 Validation of mobile phase-stationary phase systems
[0116] In order to exclude the influence of the mobile phase on the separation effect, phosphate solution-acetonitrile was used as the mobile phase uniformly to investigate the separation effect of different immobilized phases on doripenem and related substances.
[0117] 2.1 Test object
[0118] Universal C8 column: Agilent ZORBAX Eclipse plus C8 (4.6×250mm, 5μm);
[0119] Universal C18 column: Merck LP C18 (4.6×250mm, 5μm);
[0120] Octadecylsilane bonded silica gel column with polar groups (aqueous C18 column): Welch Polar-RP (4.6×250mm, 5μm).
[0121] 2.2 Experimental method
[0122] A 0.02 mol / L potassium dihydrogen phosphate buffer solution adjusted to pH=5.7 with freshly prepared KOH was used as the mobile phase A, and acetonitrile was used as the mobile phase B; the gradient elution program shown in Table 5 was used. Column temperature: 25°C, flow rate: 1ml / min, detection wavelength: 230nm.
...
Embodiment 3
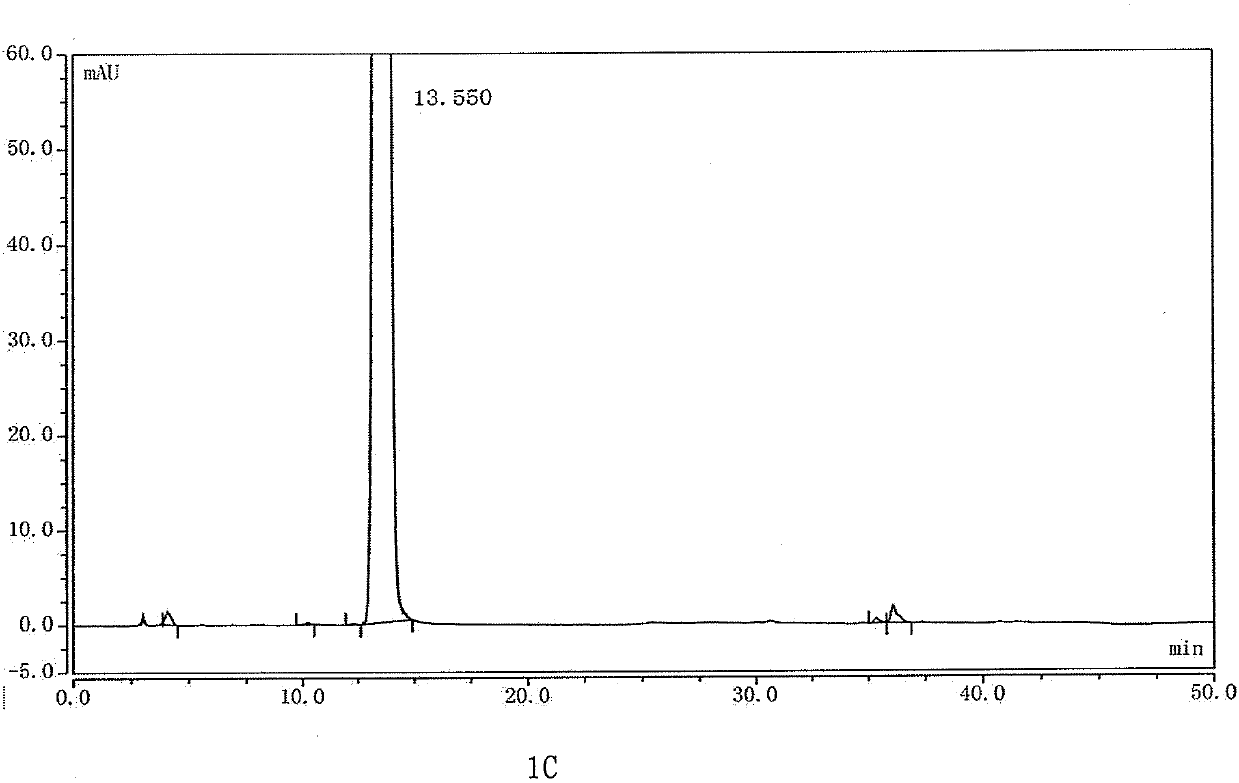
[0135] Example 3 Choice of detection wavelength
[0136] Chromatographic column: Octadecylsilane bonded silica gel chromatographic column Welch Polar-RP (5μm, φ4.6×250mm) with bonded polar groups;
[0137] Sample: 2.5mg / mL doripenem aqueous solution;
[0138] Injection volume: 10μl;
[0139] Flow rate: 1.0ml / min;
[0140] Column temperature: 25°C;
[0141] Mobile phase A is KH 2 PO 4 Aqueous solution (0.02mol / L, adjusted to pH 5.7 with KOH), mobile phase B is acetonitrile, gradient elution according to Table 5;
[0142] The same sample was detected at wavelengths of 210nm, 220nm, 230nm, 240nm, 250nm and 298nm.
[0143] Experimental results: see Figure 5 . The peak area of each impurity peak in the spectrum was integrated, and the percentage content of each impurity was calculated using the normalization method. The results are shown in Table 8.
[0144] Table 8 The status of impurities detected at different wavelengths
[0145]
[0146] The maximum ultraviole...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com