Method for preparing ring-opening impurity of carbapenems
A carbapenem and antibiotic technology, applied in the field of preparation of ring-opening impurities, can solve problems such as rare reports, and achieve the effects of controlling product quality, short reaction time and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
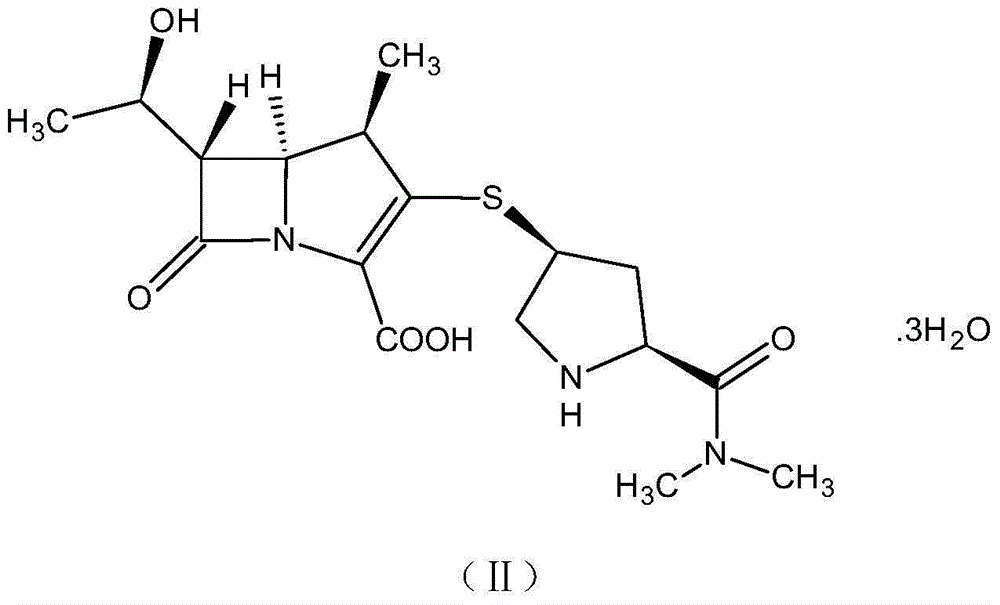
[0056] A method for preparing a ring-opening impurity of meropenem, the ring-opening impurity has the following structure:
[0057]
[0058] Including the following steps:
[0059] Dissolve 0.4g of sodium hydroxide in 100ml of water, cool down to 0-5°C, add meropenem with a net content of 2.0g, keep warm and stir for 2 hours, adjust the pH to 7.0-8.0 with 0.5mol / L hydrochloric acid after the reaction is completed, and freeze-dry. 2.3 g of the ring-opening impurity of meropenem was obtained as a light yellow solid with a purity of 97.4% and a water content of 2.6% by HPLC.
[0060] Wherein, the freeze-drying temperature curve is as follows:
[0061] temperature(°C) Heating time (h) Constant temperature time (h) Vacuum (mtorr) -20 / 40 80~120 10 1 5 80~120 40 1 5 80~120 50 0.5 5 80~120
[0062] The ring-opening impurity of meropenem obtained in Example 1 was detected by NMR, and the results are as follows:
[0063] 1 HNMR (CD...
Embodiment 2
[0065] The preparation method of the ring-opening impurity of meropenem comprises the following steps:
[0066] Dissolve 0.3g of potassium hydroxide in 100ml of water, cool down to 0-5°C, add meropenem with a net content of 2.0g, fully insulate and stir for 2 hours, adjust the pH to 7.0-8.0 with 0.5mol / L hydrochloric acid after the reaction is completed, and freeze-dry. 2.2 g of the ring-opening impurity of meropenem was obtained as a light yellow solid with a purity of 97.5% and a water content of 2.1% by HPLC.
[0067] Wherein, the freeze-drying temperature curve is as follows:
[0068] temperature(°C) Heating time (h) Constant temperature time (h) Vacuum (mtorr) -10 / 20 80~120 10 1 5 80~120 40 1 5 80~120 50 0.5 5 80~120
Embodiment 3
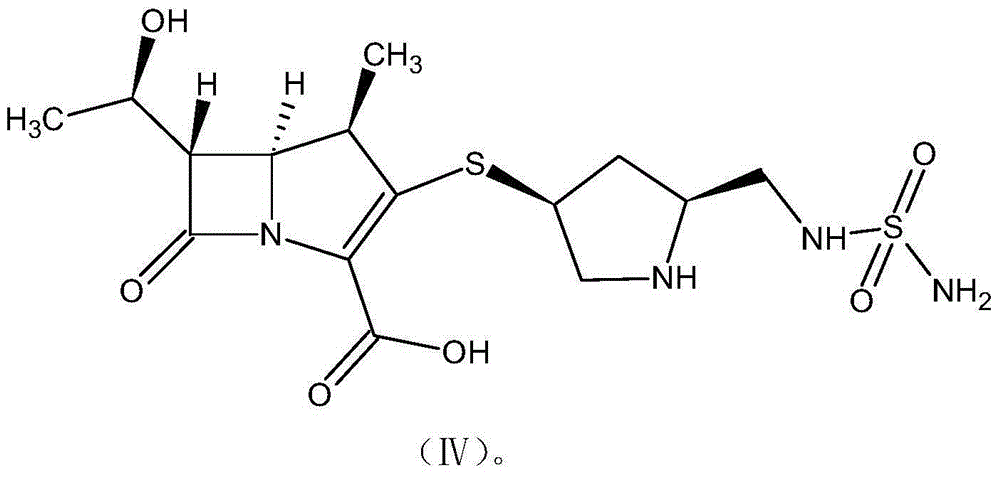
[0070] A method for preparing a ring-opening impurity of ertapenem, the ring-opening impurity has the following structure:
[0071]
[0072] Including the following steps:
[0073]Dissolve 0.4g of sodium hydroxide in 100ml of water, lower the temperature to 0-5°C, add ertapenem with a net content of 2.3g, fully insulate and stir for 2 hours, adjust the pH to 7.0-8.0 with 0.5mol / L hydrochloric acid after the reaction is completed, freeze After drying, 2.6 g of the ring-opening impurity of ertapenem was obtained as a yellow solid with a purity of 96.8% and a water content of 2.8% as detected by HPLC.
[0074] Wherein, the freeze-drying temperature curve is as follows:
[0075] temperature(°C) Heating time (h) Constant temperature time (h) Vacuum (mtorr) -10 / 24 80~120 10 1 5 80~120 40 1 5 80~120 50 0.5 5 80~120
[0076] The ertapenem ring-opening impurity obtained in Example 3 was subjected to nuclear magnetic detection, and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com