Method for preparing doripenem intermediate compound
A technology for doripenem and compounds, applied in the field of preparation of doripenem intermediate compounds, can solve the problems of cumbersome operation, high cost, and many products, and achieve simple operation, high product purity and yield, and product separation easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
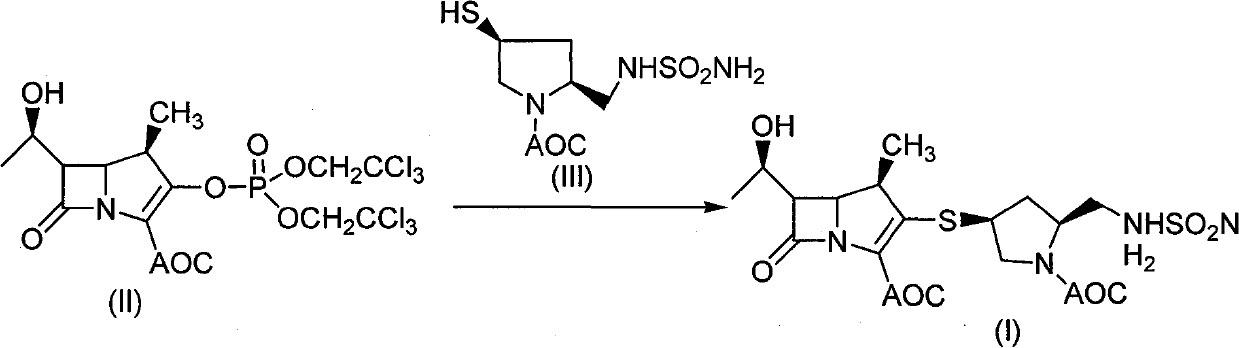
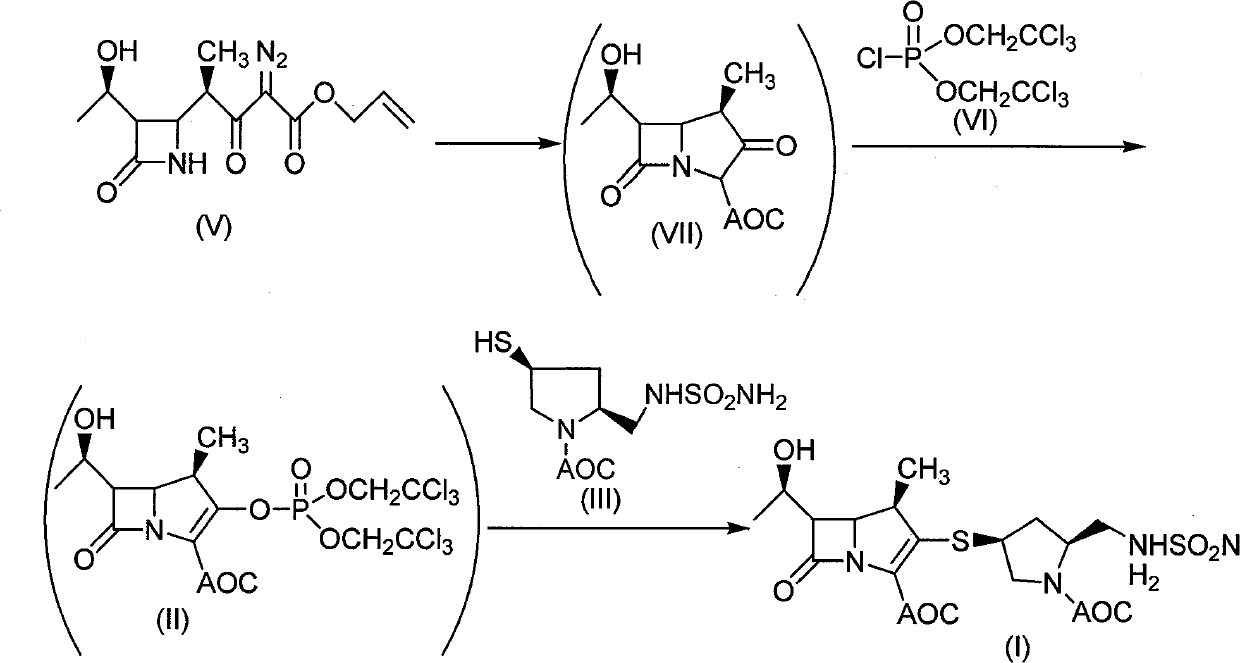
[0039] Embodiment 1: the synthesis of compound (I)
[0040] Under the protection of nitrogen, add white powder compound of formula (II) (10.9g, 0.0179mol), add about 80ml of DMF to dissolve and mix, cool in ice bath to about 0°C, add compound (III) (5.28g, 0.0179mol) in DMF (15ml) solution, cooled to 0°C, gradually added triethylamine (2.54g, 0.025mol) dropwise, after the addition, stirred at -5°C to 0°C for 10 hours to complete the reaction, then added ethyl acetate Ethyl acetate, extracted with water, washed the ethyl acetate layer with one equivalent of dilute hydrochloric acid, adjusted the pH to about 3, added saturated aqueous sodium bicarbonate solution to wash, adjusted the pH value to 7-8, washed twice with saturated saline, and separated the ethyl acetate Layers were evaporated to dryness under reduced pressure to obtain off-white foamy solid (I), dried under reduced pressure, about 6.57g, yield: 67.5%.
[0041] The property determination result of compound (I): 1 ...
Embodiment 2
[0042] Embodiment 2: the synthesis of compound (I)
[0043]Under the protection of nitrogen, add white powder compound of formula (II) (10.9g, 0.0179mol), add about 54.5ml of DMF to dissolve and mix, cool to about 0°C in an ice bath, add compound (III) (5.28g , 0.0179mol) in DMF (15ml) solution, cooled to 0°C, gradually added dropwise N-methylmorpholine (2.53g, 0.025mol), after adding, -5°C~0°C, stirred for 10 hours and the reaction was complete , add ethyl acetate, extract with water, wash the ethyl acetate layer with one equivalent of dilute hydrochloric acid, adjust the pH to about 3, add saturated aqueous sodium bicarbonate solution to wash, adjust the pH to 7-8, wash twice with saturated saline, divide The ethyl acetate layer was removed and evaporated to dryness under reduced pressure to obtain off-white foamy solid (I), which was dried under reduced pressure, about 5.86 g, yield: 60.2%.
[0044] The property determination result of compound (I): 1 H-NMR δ 1.16(d, 3H),...
Embodiment 3
[0045] Embodiment 3: the synthesis of compound (I)
[0046] Under the protection of nitrogen, add white powder compound of formula (II) (10.9g, 0.0179mol), add about 163.5ml of acetonitrile to dissolve and mix, cool in ice bath to about 0°C, add compound (III) (5.28g , 0.0179mol) in acetonitrile (15ml) solution, cooled to 0°C, gradually added diisopropylethylamine (3.23g, 0.025mol) dropwise, after adding, stirred at -5°C to 0°C for 10 hours to react Complete, add ethyl acetate, extract with water, wash the ethyl acetate layer with one equivalent of dilute hydrochloric acid, adjust the pH to about 3, add saturated aqueous sodium bicarbonate solution to wash, adjust the pH to 7-8, wash twice with saturated saline, The ethyl acetate layer was separated and evaporated to dryness under reduced pressure to obtain off-white foamy solid (I), dried under reduced pressure, about 5.85 g, yield: 60.1%.
[0047] The property determination result of compound (I): 1 H-NMR δ 1.16(d, 3H), 1....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com