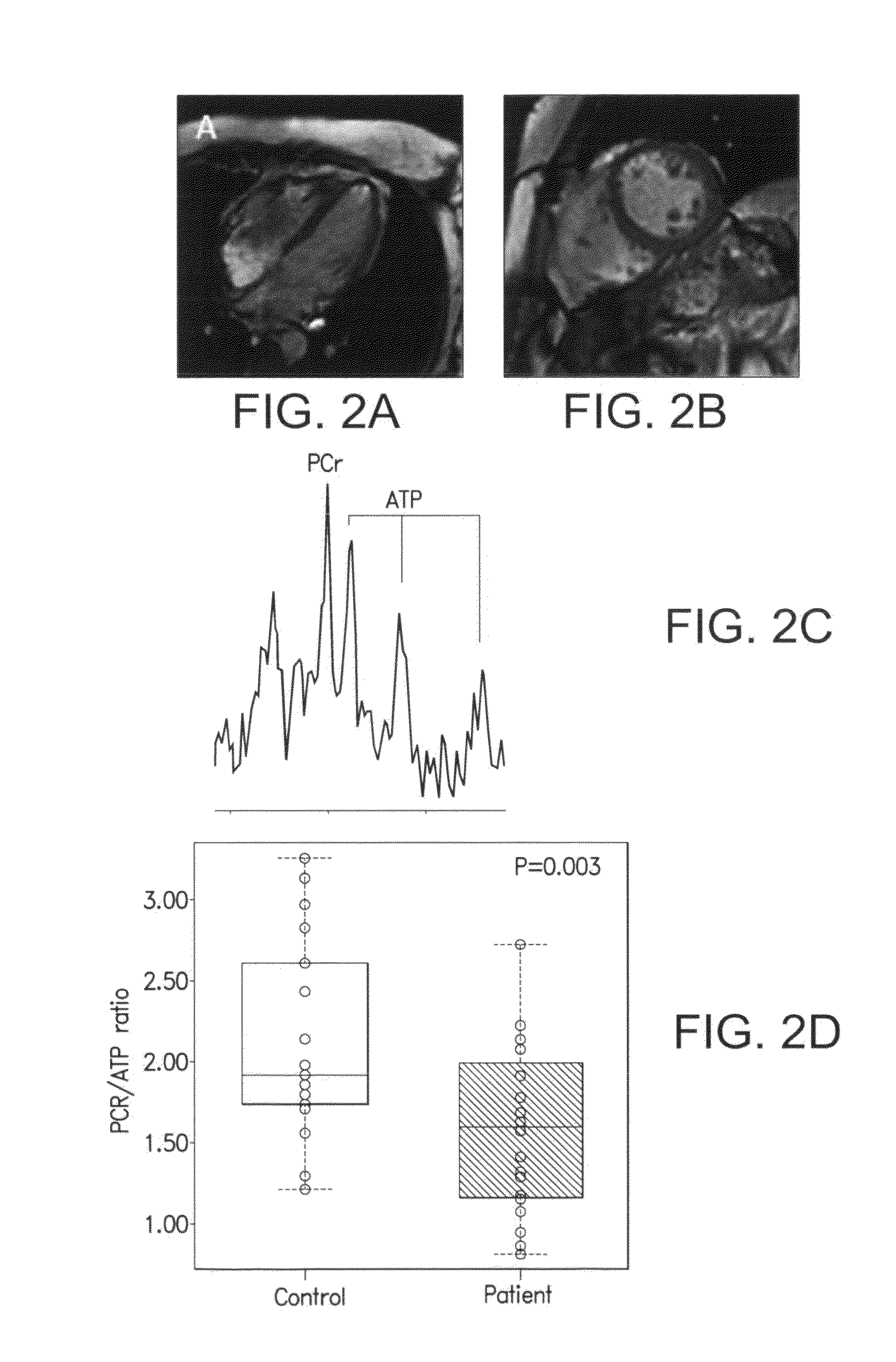
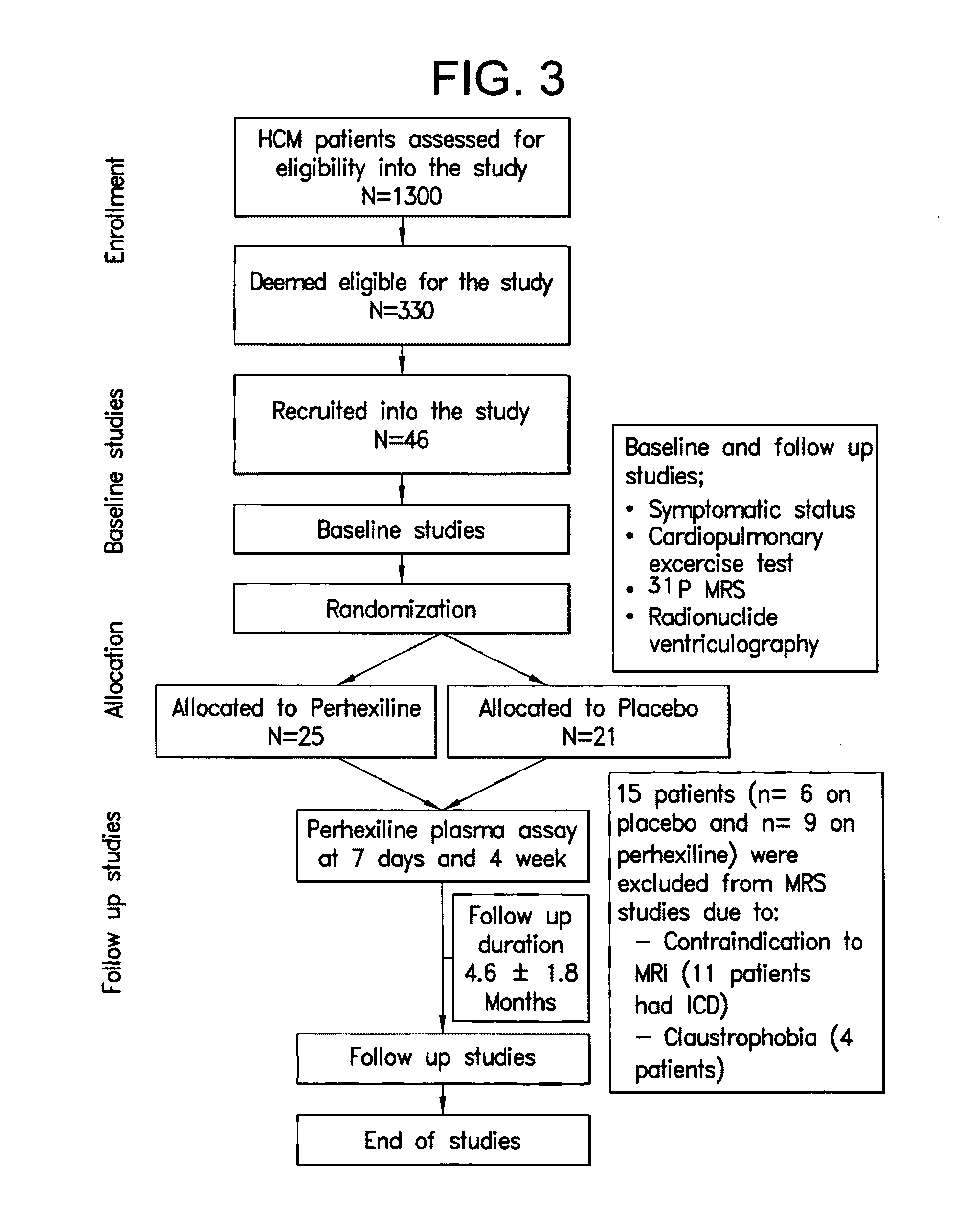
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Perhexiline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
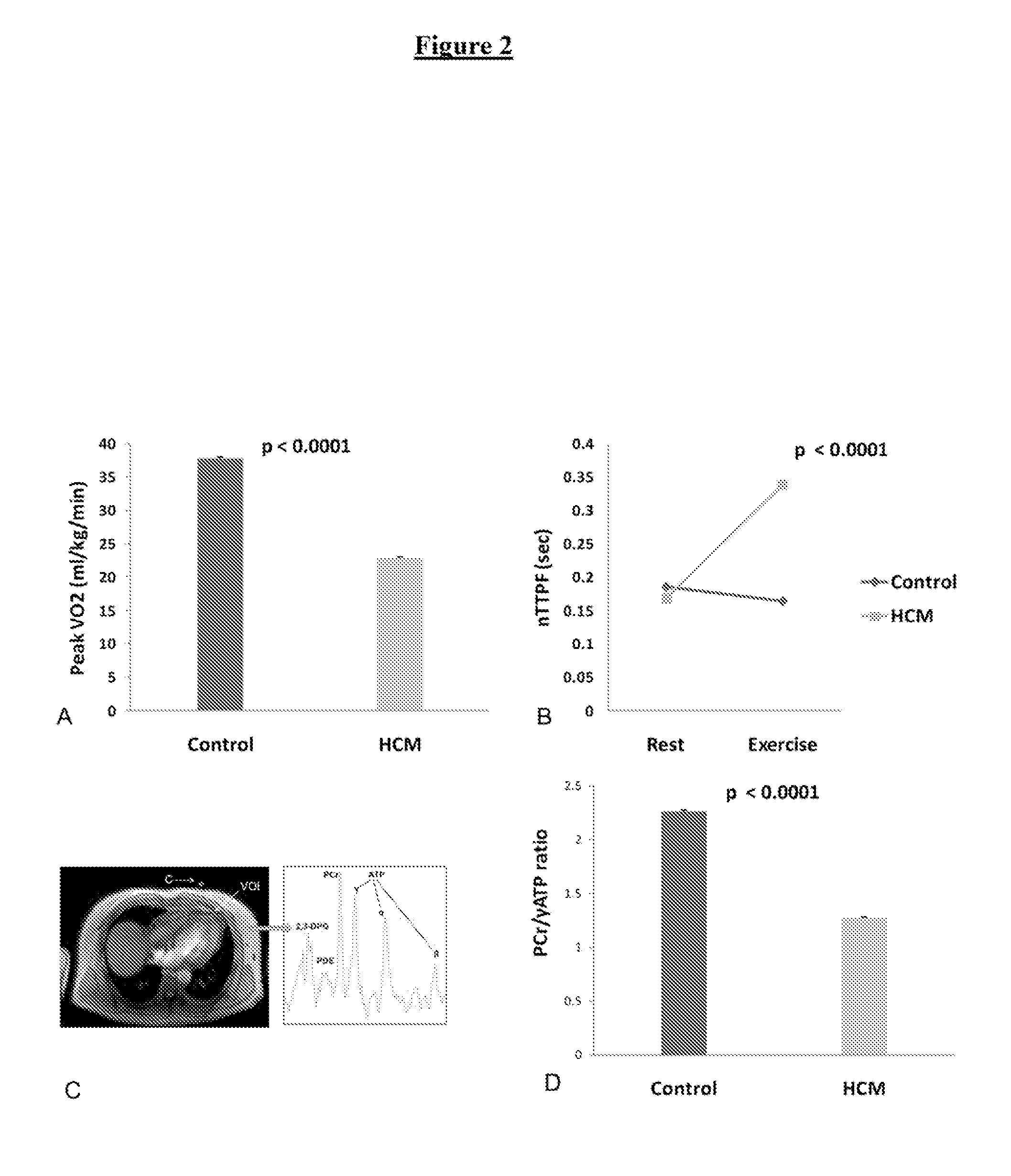
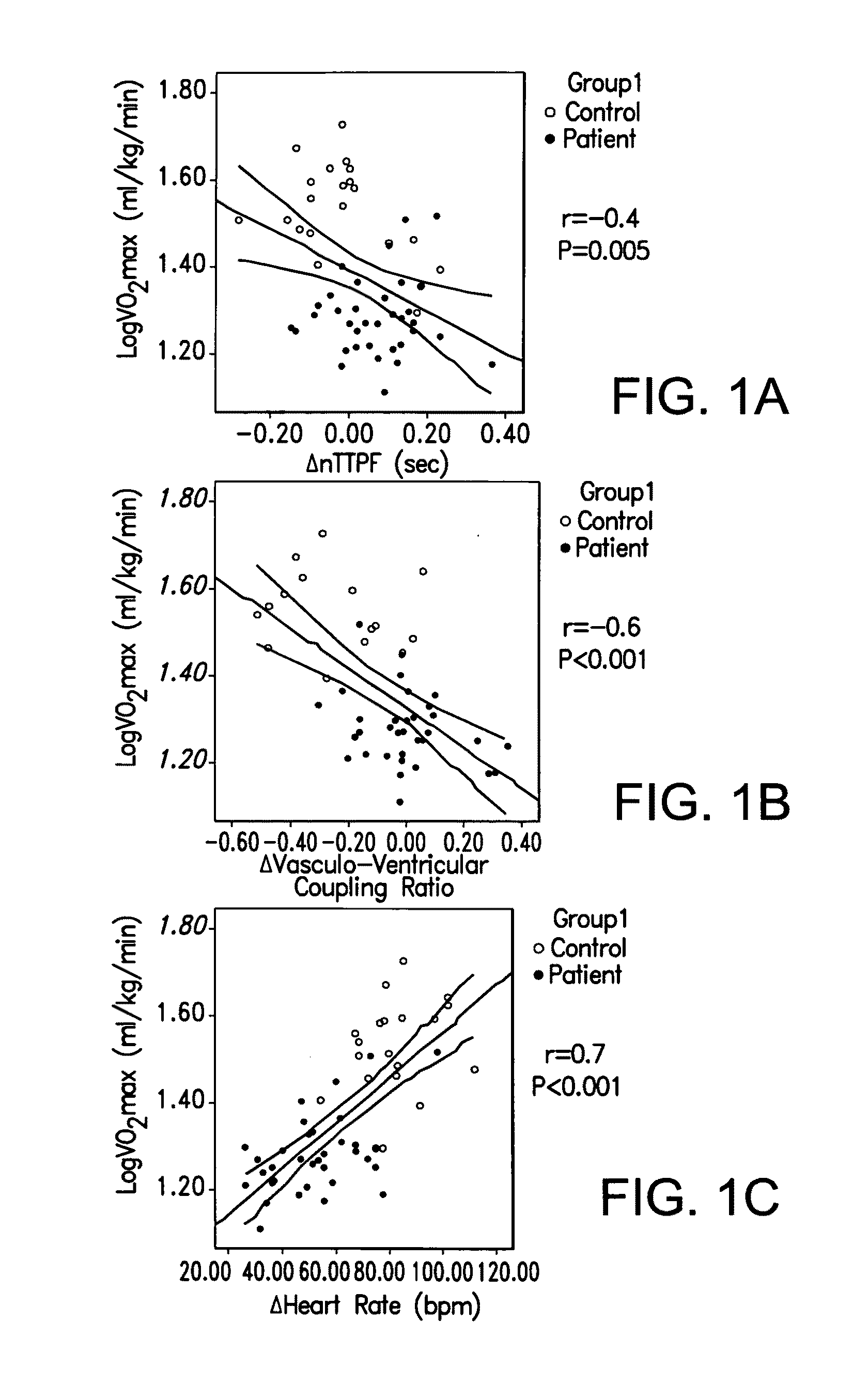
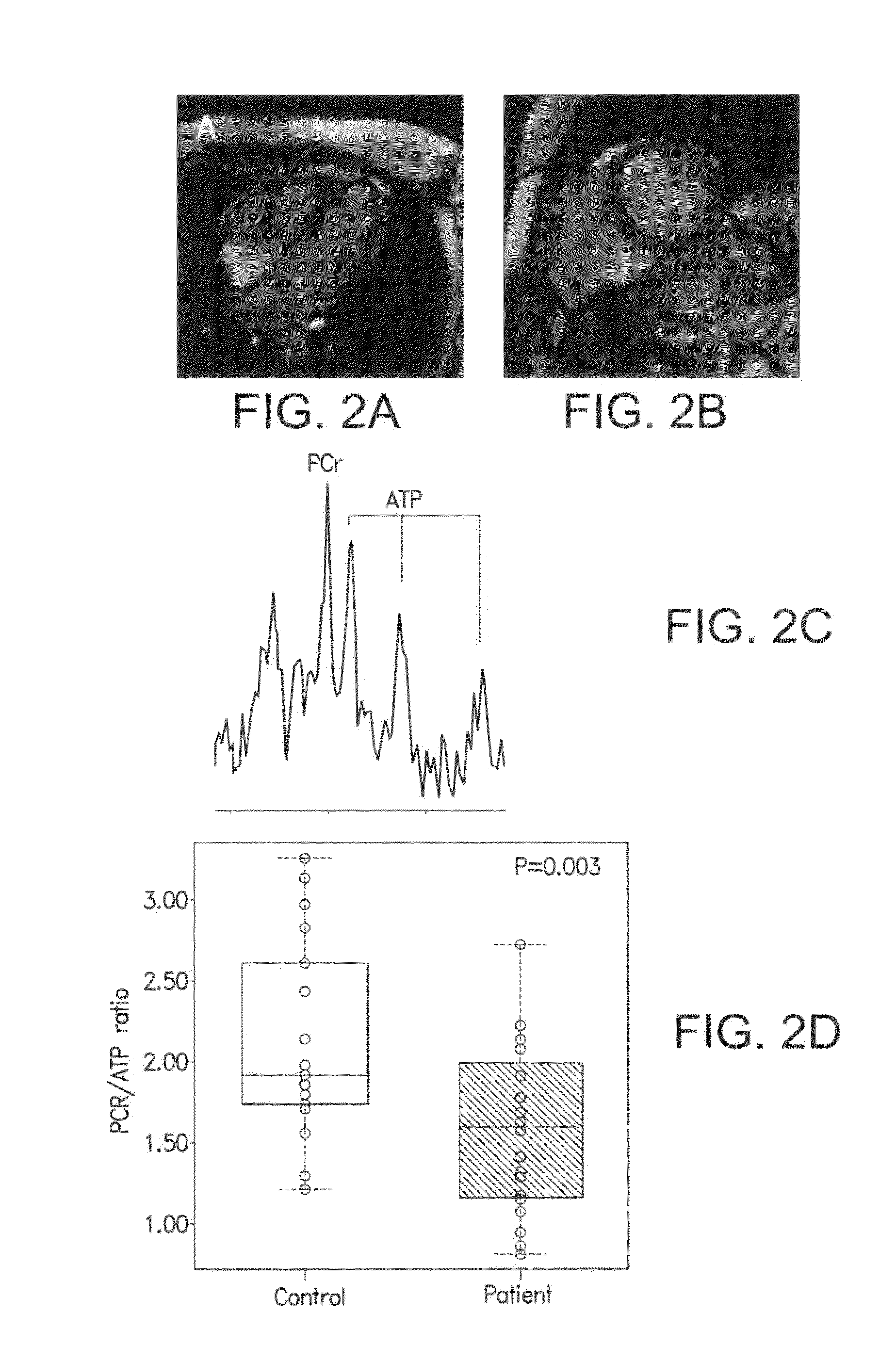
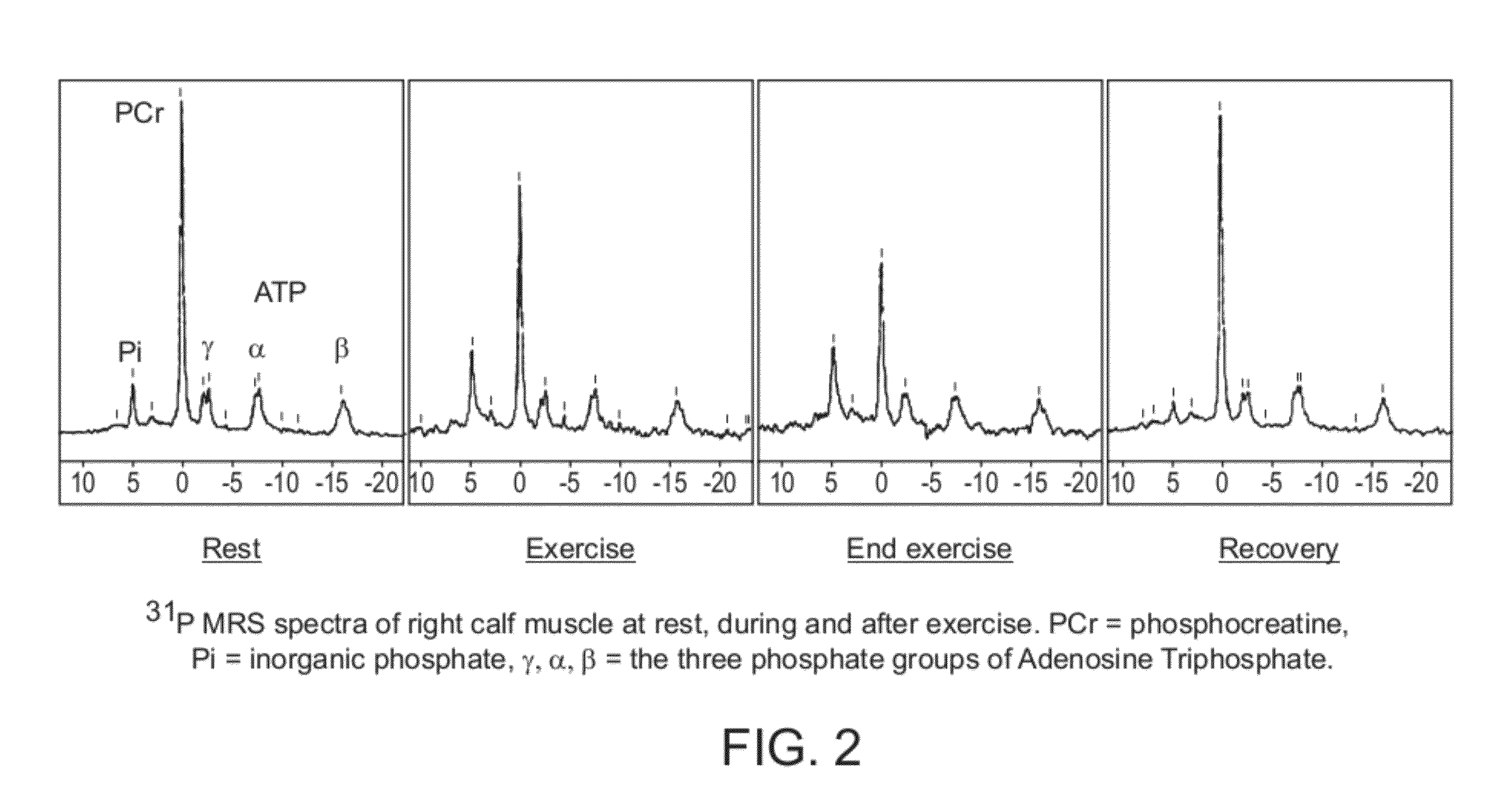
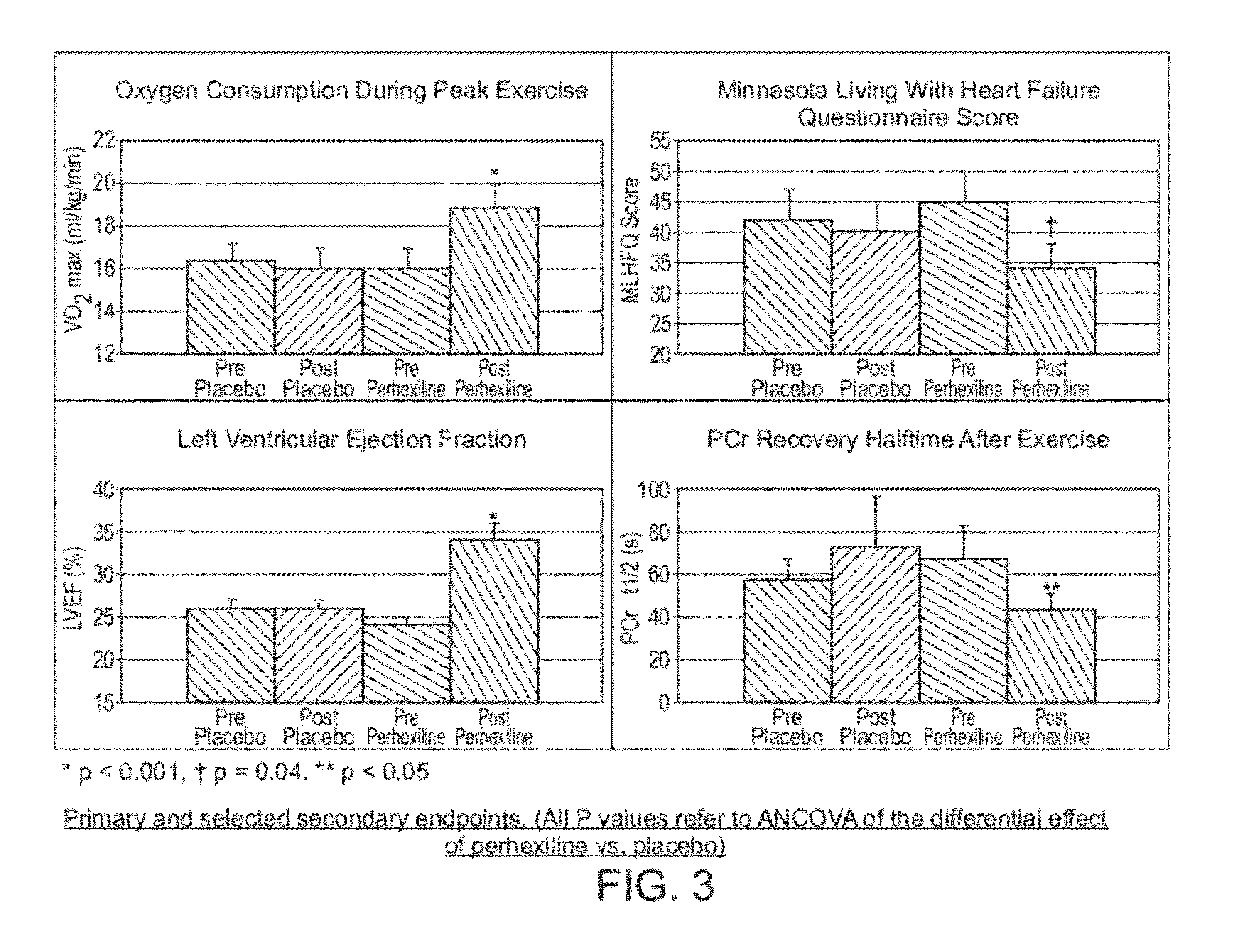
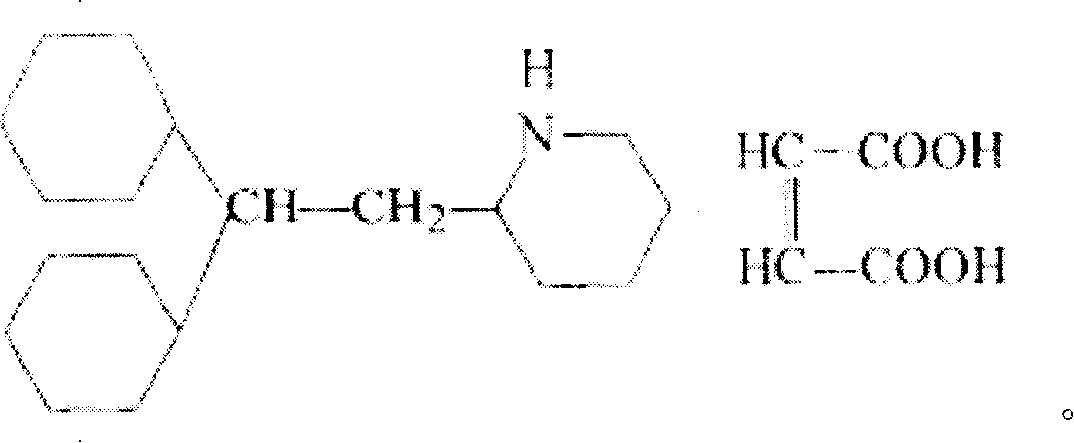
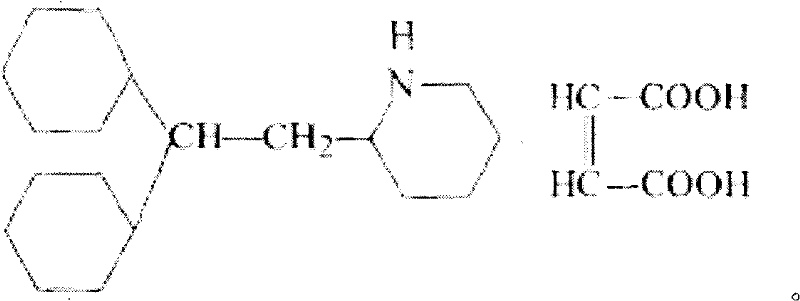
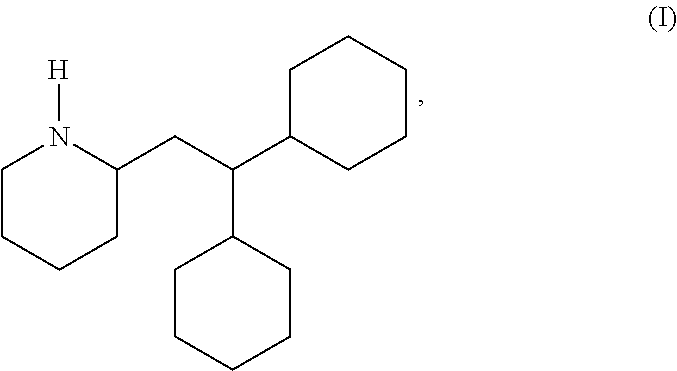
Perhexiline (Pexsig) is a prophylactic antianginal agent used primarily in Australia and New Zealand. Perhexiline is thought to act by inhibiting mitochondrial carnitine palmitoyltransferase-1. This shifts myocardial metabolism from fatty acid to glucose utilisation which results in increased ATP production for the same O₂ consumption and consequently increases myocardial efficiency. Its clinical use has been limited by its narrow therapeutic index and high inter- and intra-individual pharmacokinetic variability. It was outlawed in many countries due to its adverse effects on poor metabolisers (PM). The product has been reintroduced for patients who have contraindications, or have not responded to other treatments for angina.
Method for preparing perhexiline pharmaceutical preparation
ActiveCN102048821AGood curative effectIncrease contentCardiovascular disorderPlant ingredientsSalvia miltiorrhizaDrug carrier
The invention discloses a preparation method of a perhexiline pharmaceutical preparation, comprising the following steps of: (a) preparing pharmaceutical components salvia and szechuan lovage rhizome, wherein the weight ratio of salvia to szechuan lovage rhizome 1-3: 1-3 by weight; (b) decocting the salvia, injecting concentrated liquor in a macroporous absorption resin column, and concentrating and drying eluent to obtain salvia extractive; (c) decocting the szechuan lovage rhizome, injecting concentrated liquor in a macroporous absorption resin column, and concentrating and drying eluent to obtain szechuan lovage rhizome extractive; and (d) adding pharmacologically allowed pharmaceutical carriers in the prepared extractives uses as active ingredients to prepare various forms of pharmaceutical preparation. The perhexiline pharmaceutical preparation prepared by the method disclosed in the invention has effect superior to that of the perhexiline pharmaceuticals prepared according to the prior art.
Owner:SHINEWAY PHARMA GRP LTD
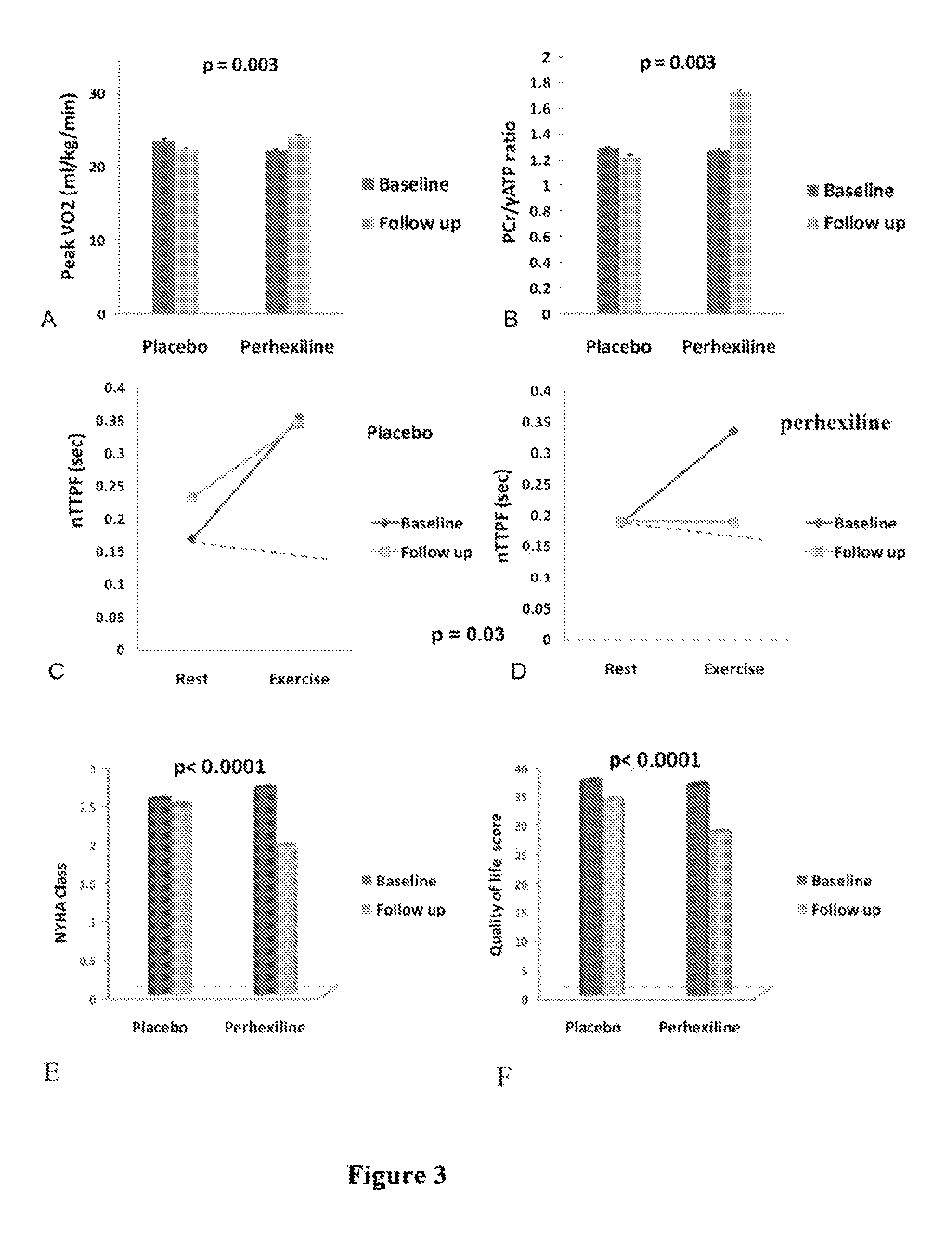
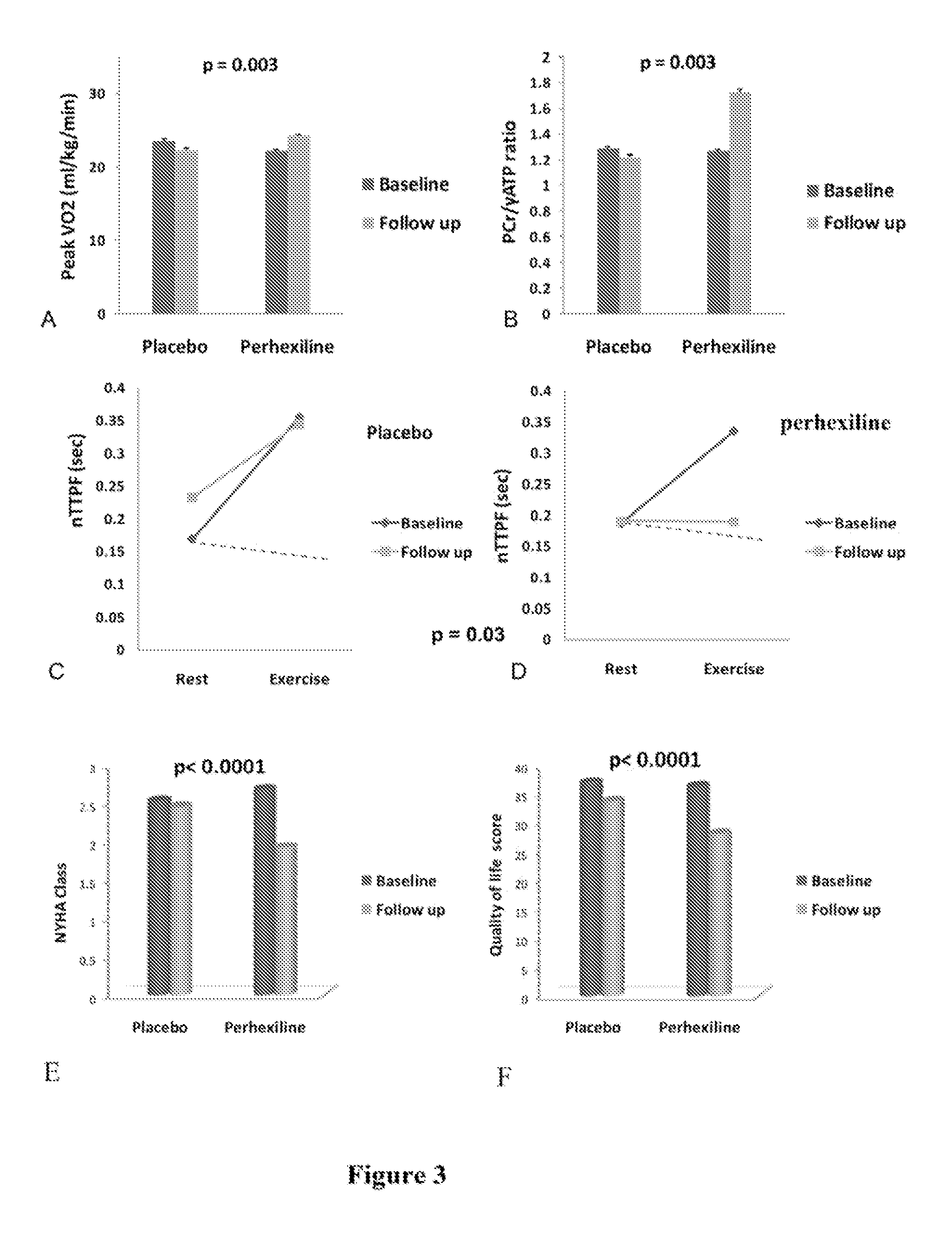
Perhexiline for use in the treatment of hypertrophic cardiomyopathy (HCM)
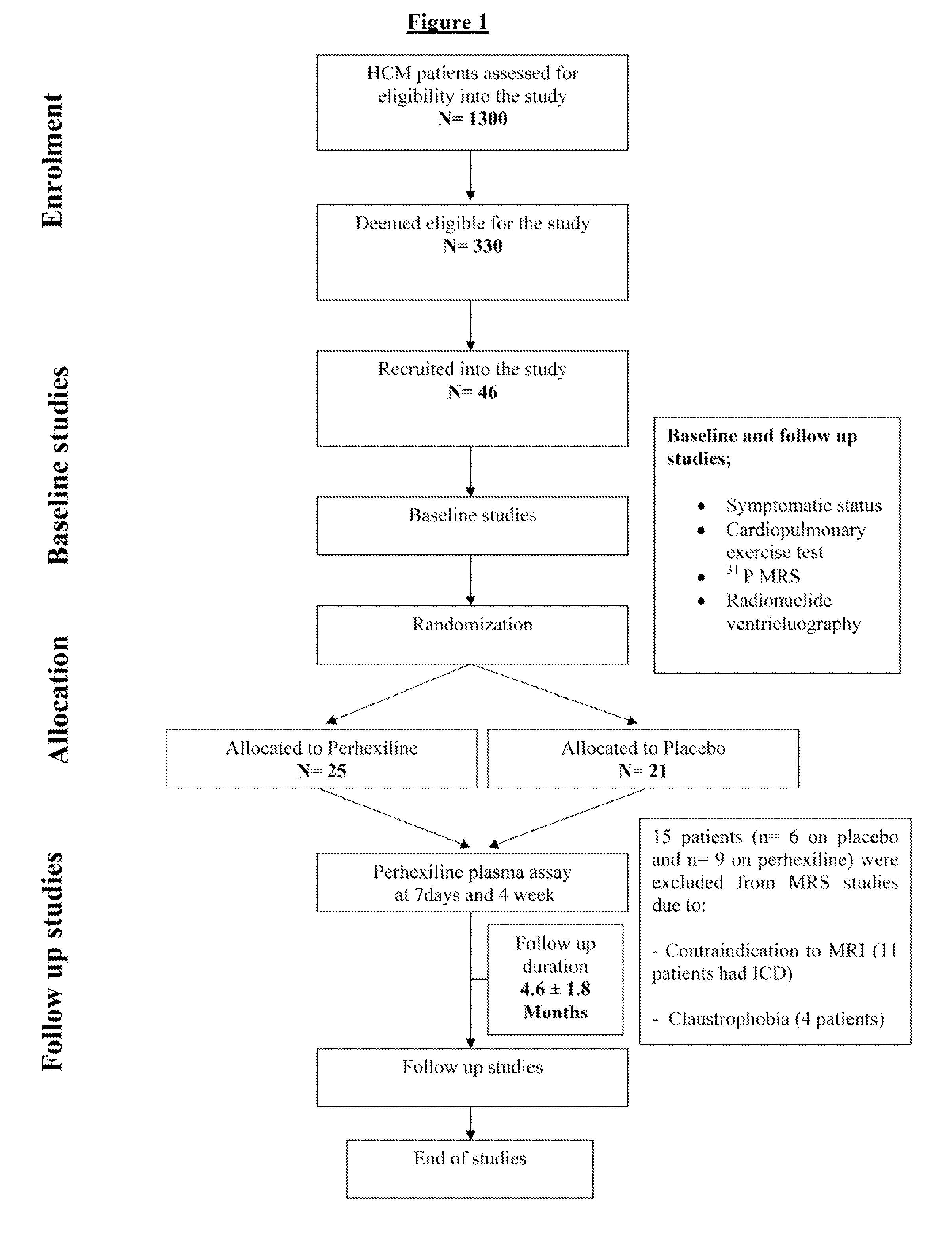
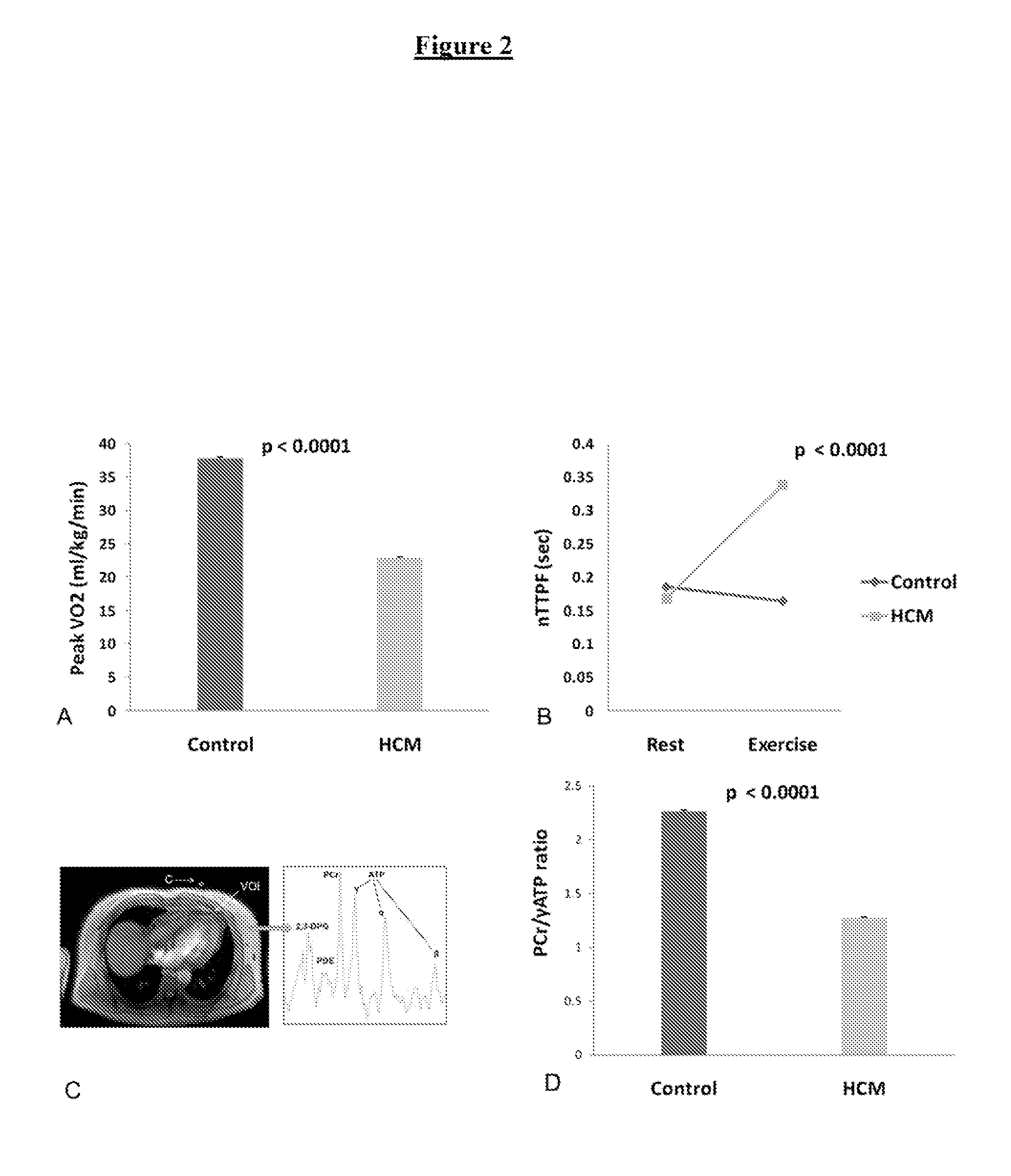
The invention relates to perhexiline, or a pharmaceutically acceptable salt thereof, for use in the treatment of hypertrophic cardiomyopathy, as well as to a method of treating HCM, which comprises administering to an animal in need thereof an effective amount of perhexiline, or a pharmaceutically acceptable salt thereof, to treat said HCM. The invention further relates to a treatment programme for treating HCM, which involves the co-use or co-administration of perhexiline with one or more other compounds that are advantageous in treating HCM or the symptoms thereof.
Owner:HEART METABOLICS
Perhexiline for use in the treatment of hypertrophic cardiomyopathy (HCM)
The invention relates to perhexiline, or a pharmaceutically acceptable salt thereof, for use in the treatment of hypertrophic cardiomyopathy, as well as to a method of treating HCM, which comprises administering to an animal in need thereof an effective amount of perhexiline, or a pharmaceutically acceptable salt thereof, to treat said HCM. The invention further relates to a treatment programme for treating HCM, which involves the co-use or co-administration of perhexiline with one or more other compounds that are advantageous in treating HCM or the symptoms thereof.
Owner:HEART METABOLICS
Perhexiline high-dose injection and its preparing method
InactiveCN1813891APharmaceutical delivery mechanismCardiovascular disorderSalvia miltiorrhizaHigh doses
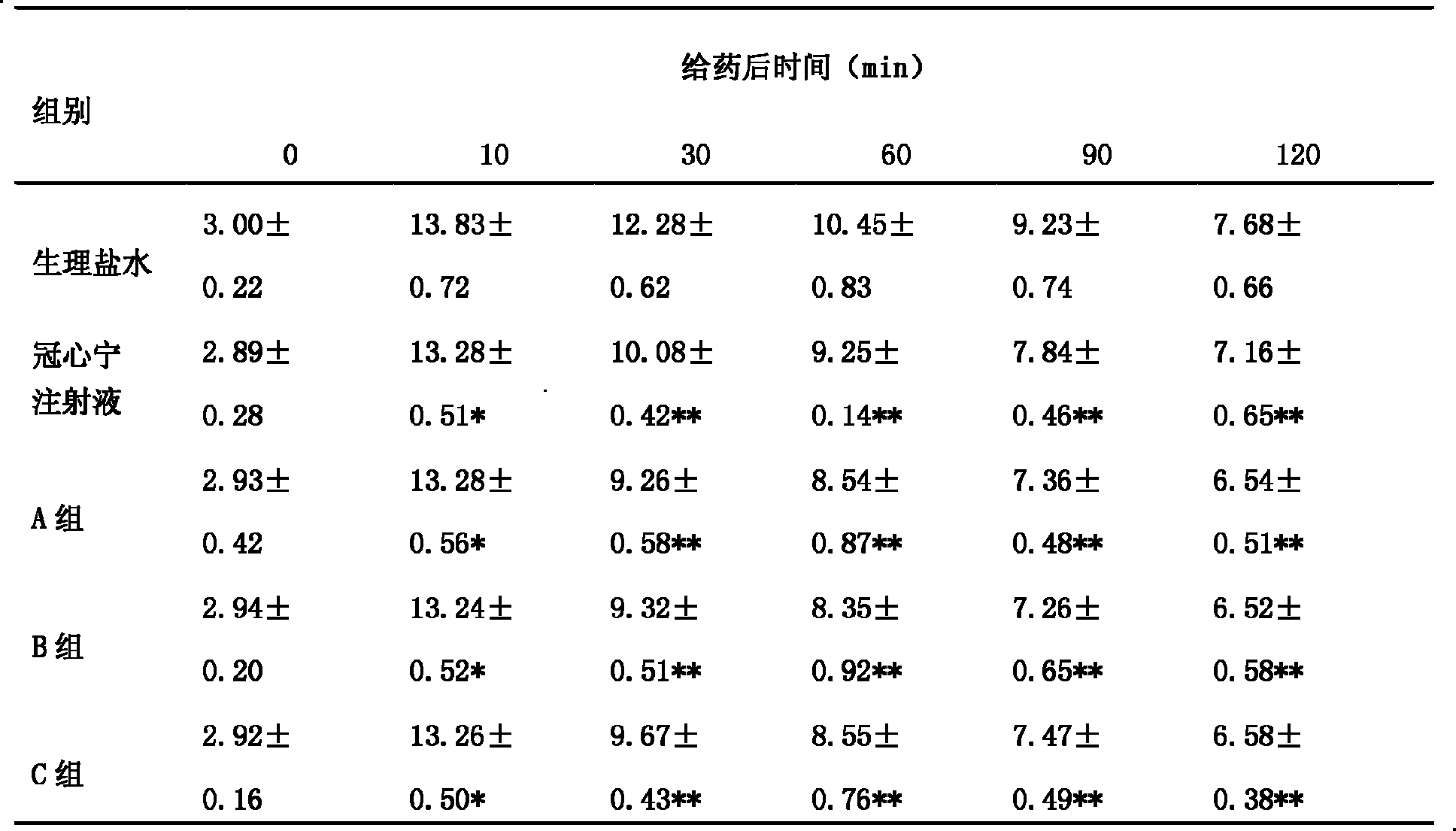
The present invention discloses a Guanxinning large-volume injection and its preparation method. It is characterized by that after the Chinese medicinal materials of salvia root and ligusticum root are extracted and purified, their purified extracts are combined with medicinal auxiliary material so as to obtain the invented large-volume injection.
Owner:孟繁浩
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
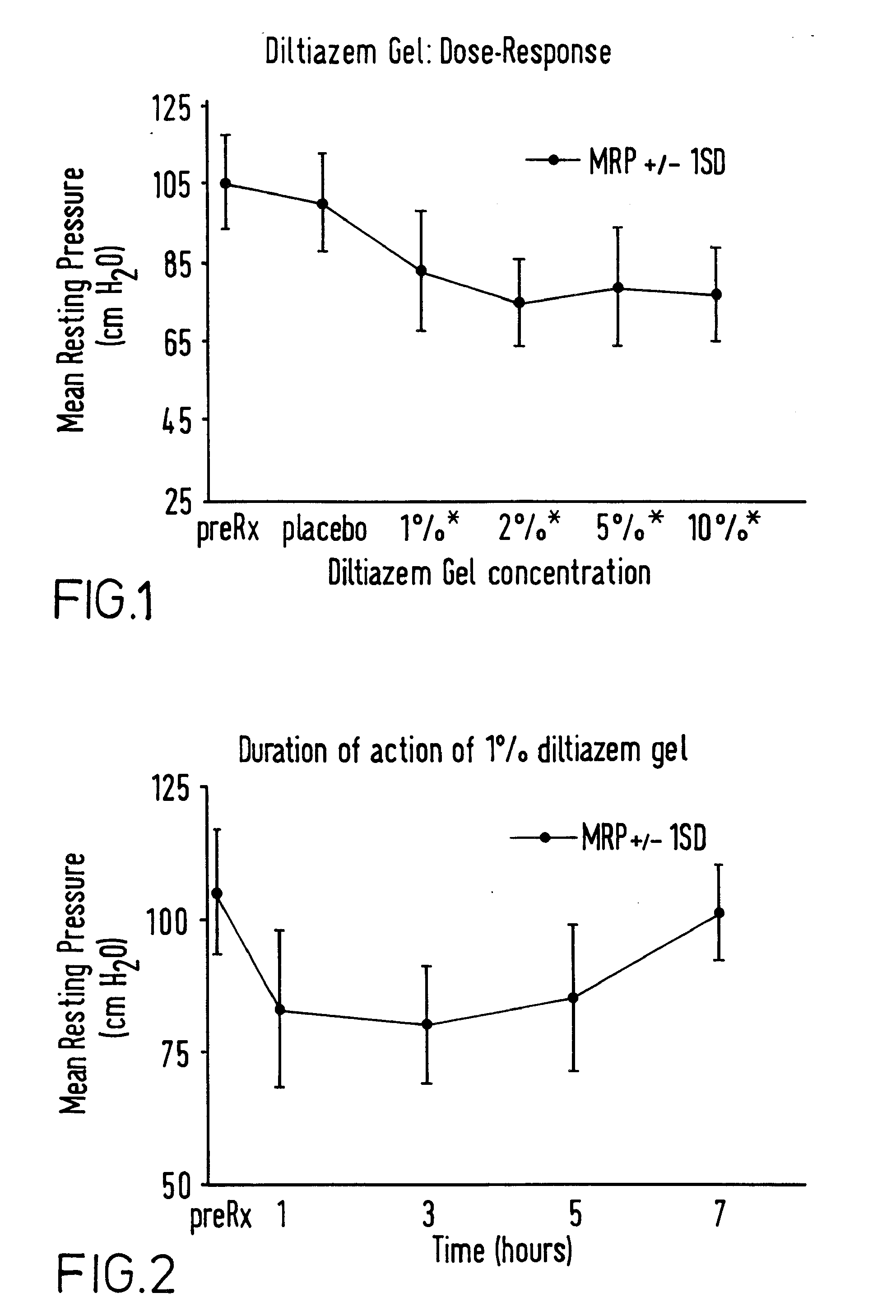
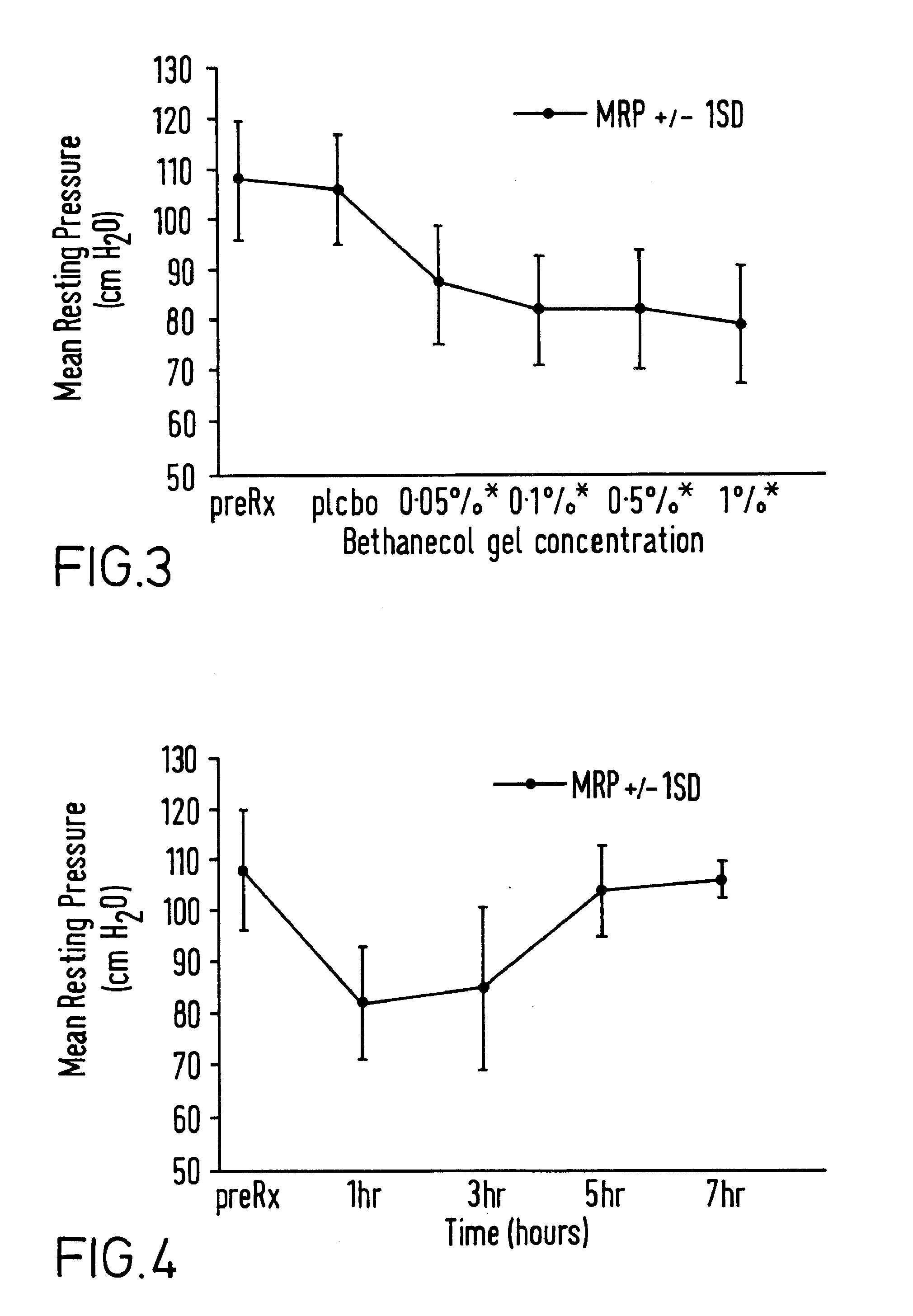
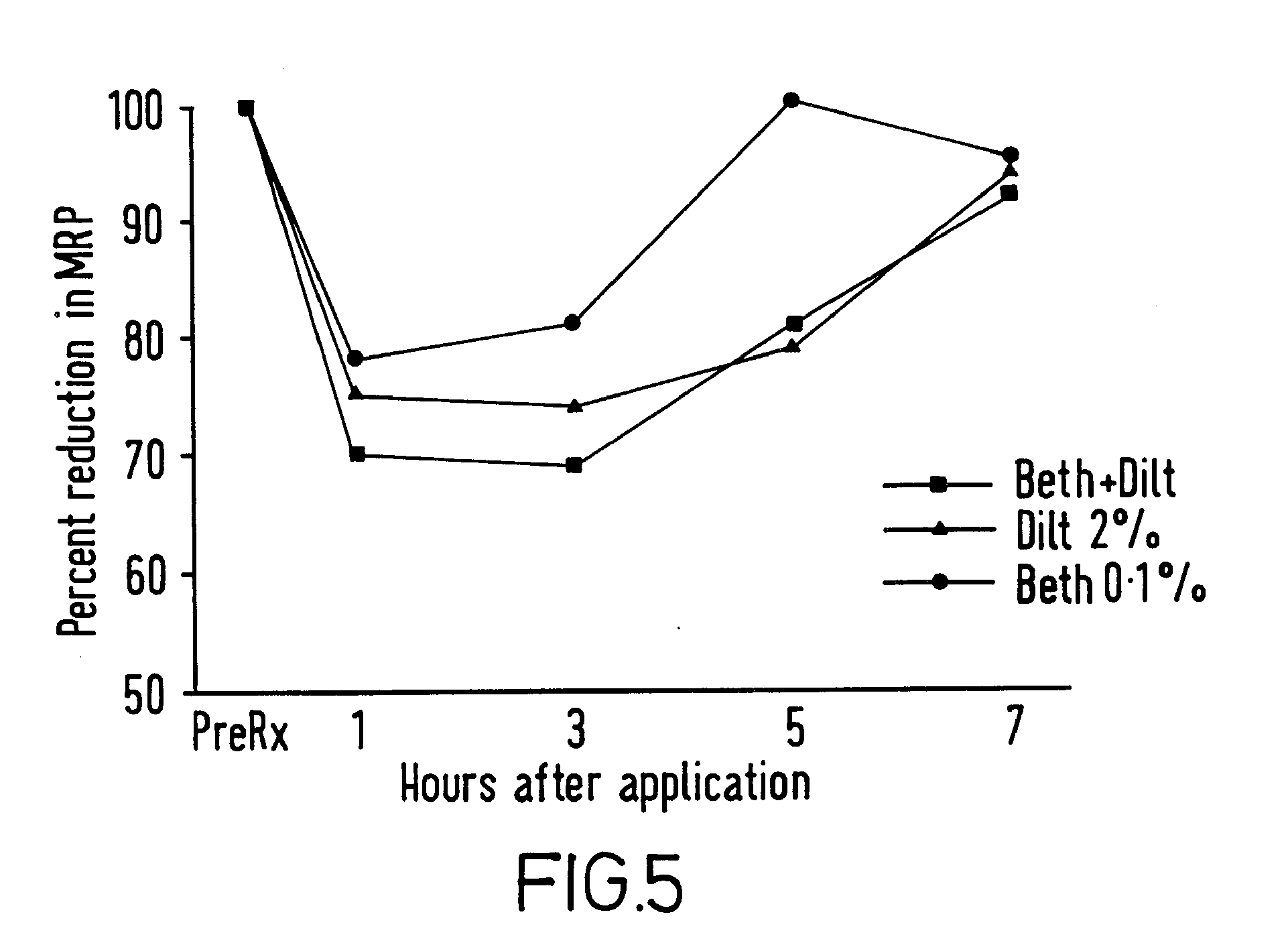
A method and composition are provided for the treatment of an anorectal disorder and for controlling the pain associated therewith. The method comprises administering to a subject in need of such treatment therapeutically effective amounts of a calcium channel blocker either alone or together with a nitric oxide donor. Amlodipine, anipamil, barnidipine, benidipine, bepridil, darodipine, diltiazem, efonidipine, felodipine, isradipine, lacidipine, lercanidipine, lidoflazine, manidipine, mepirodipine, nicardipine, nifedipine, niludipine, nilvadipine, nimodipine, nisoldipine, nitrendipine, perhexiline, tiapamil, verapamil and pharmaceutically acceptable salts thereof, are suitable calcium channel blockers.
Owner:SLA PHARMA AG
TREATMENT OF HFnEF
The invention relates to perhexiline, or a pharmaceutically acceptable salt thereof, for use in the treatment of HfnEF, as well as to a method of treating HfnEF, which comprises administering to an animal in need thereof an effective amount of perhexiline, or a pharmaceutically acceptable salt thereof, to treat said HFnEF. The invention further relates to a treatment programme for treating HFnEF, which involves the co-use or co-administration of perhexiline with one or more other compounds that are advantageous in treating HFnEF or the symptoms thereof.
Owner:HEART METABOLICS
Perhexiline injection liquid and preparation method thereof
InactiveCN104415091AQuality improvementImprove clinical drug safetyInorganic non-active ingredientsPharmaceutical delivery mechanismEthanol precipitationBiology
The invention discloses a perhexiline injection liquid and a preparation method thereof, wherein the perhexiline injection liquid is prepared from radix salviae miltiorrhizae, ligusticum chuanxiong, sodium metabisulfite, sodium calcium edetate, HS-15 and water for injection. The preparation method of the perhexiline injection liquid comprises the steps: adding water to radix salviae miltiorrhizae and ligusticum chuanxiong, decocting, filtering the decoction, concentrating, carrying out precipitation treatment for 2 times with ethanol, filtering, concentrating the filtrate, diluting with water for injection, regulating the pH value with dilute hydrochloric acid, refrigerating, filtering, regulating the pH value with a sodium hydroxide solution, concentrating the filtrate, refrigerating, and filtering to obtain a solution 1; taking 100 ml of water for injection, adding sodium calcium edetate and sodium metabisulfite, stirring until being dissolved, and filtering with activated carbon to obtain a solution 2; adding the HS-15 into 100 ml of water for injection, evenly stirring to obtain a solution 3; and merging the three solutions, filtering, regulating the pH value, adding water for injection to the full amount, filling and sealing, and thus obtaining the product. With adopting of the new prescription and process, the perhexiline injection liquid has an outstanding advantage of greatly enhancing the medication security of the perhexiline injection liquid.
Owner:CHENGDU LIST PHARMA
Perhexiline for treating chronic heart failure
InactiveUS20120101128A1Compounds screening/testingSalicyclic acid active ingredientsPerhexilinePhysical therapy
Disclosed are methods for the treatment of chronic heart failure, comprising administering to an animal in need thereof an effective amount of perhexiline, or a pharmaceutically acceptable salt thereof, to treat said chronic heart failure. The chronic heart failure may be non-ischaemic or ischaemic. Also disclosed is the use of perhexiline in the manufacture of a medicament to treat chronic heart failure, including chronic heart failure of a non-ischaemic origin and chronic heart failure of an ischaemic origin.
Owner:HEART METABOLICS
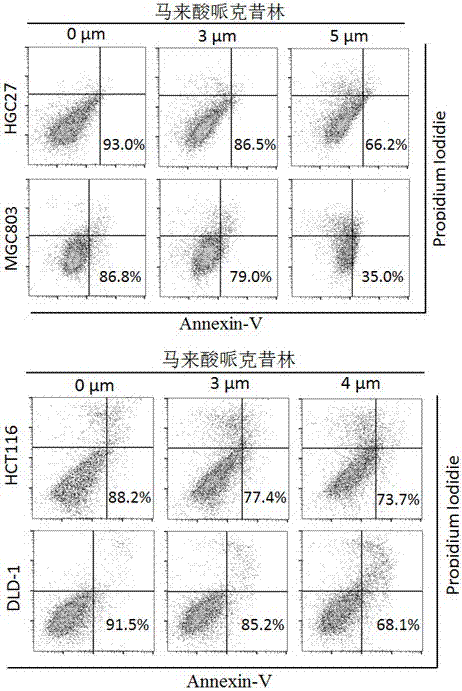
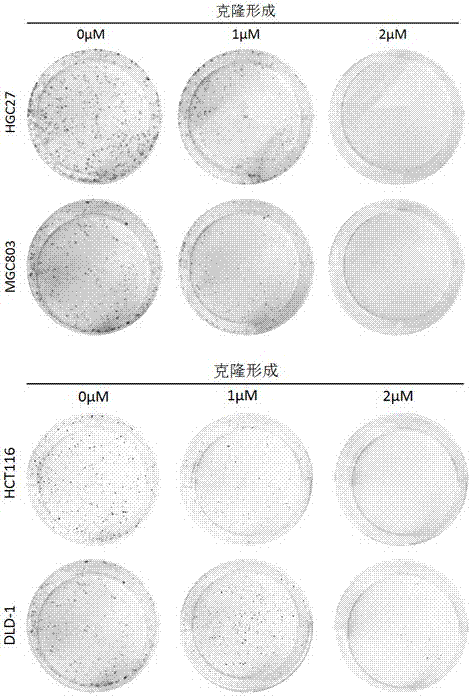
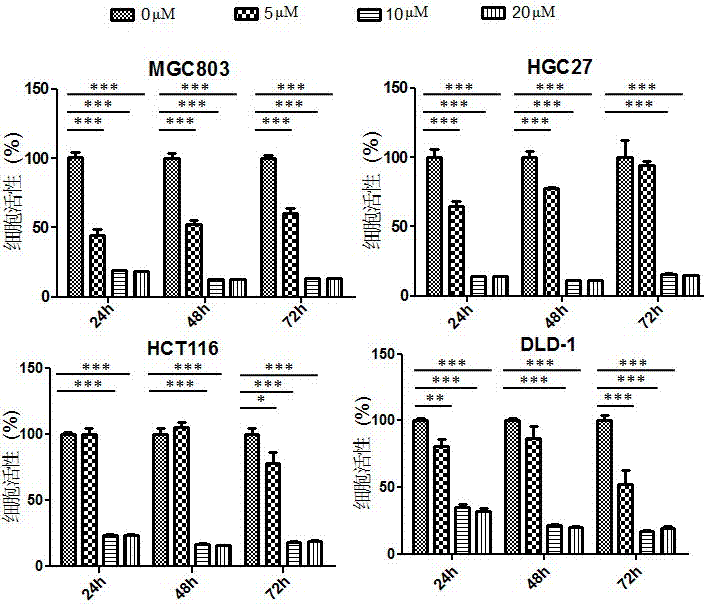
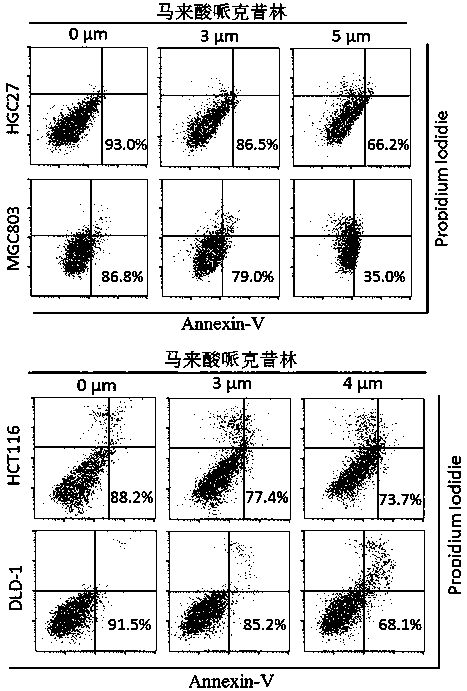
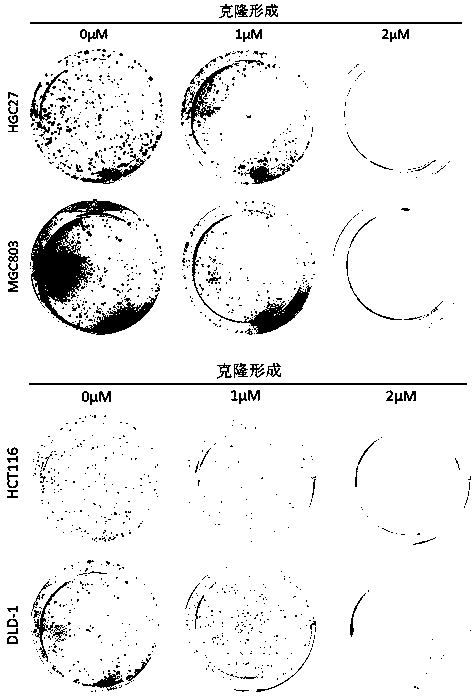
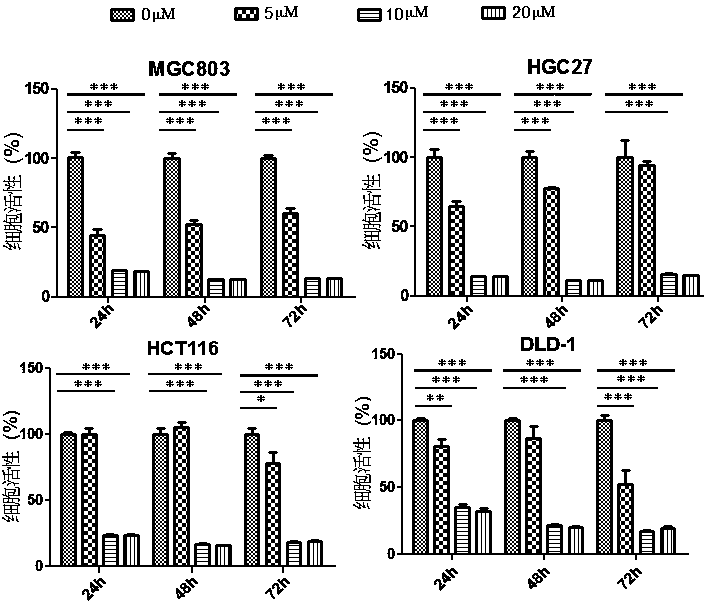
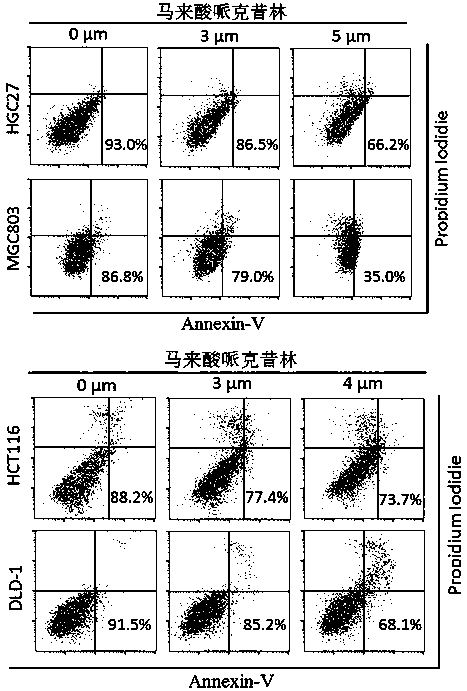
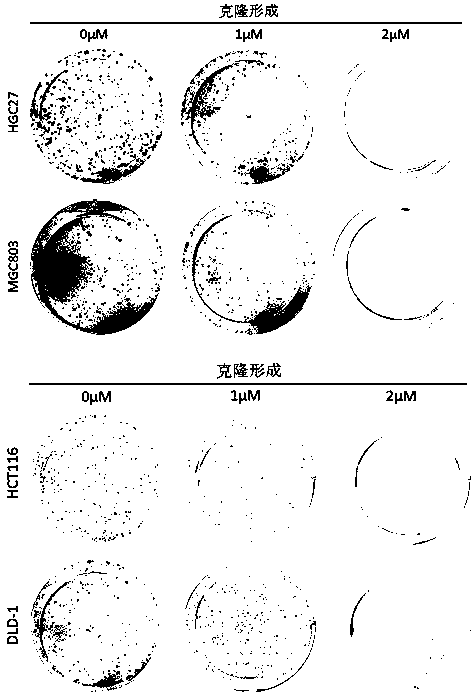
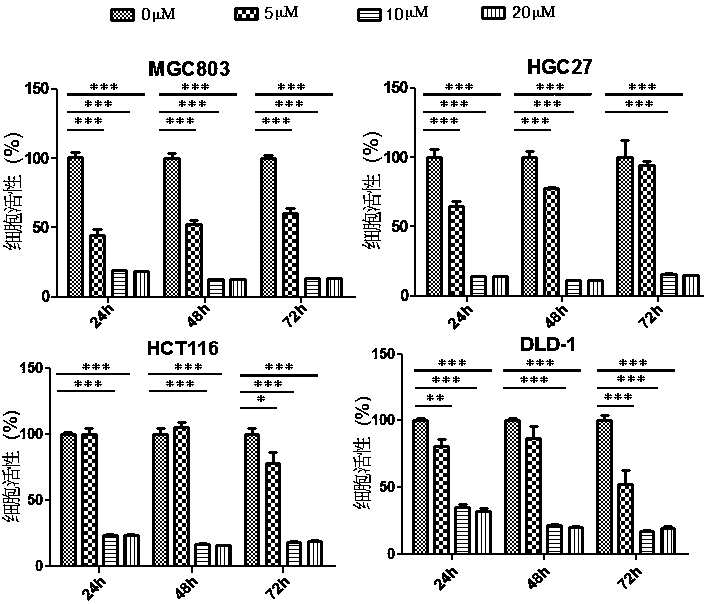
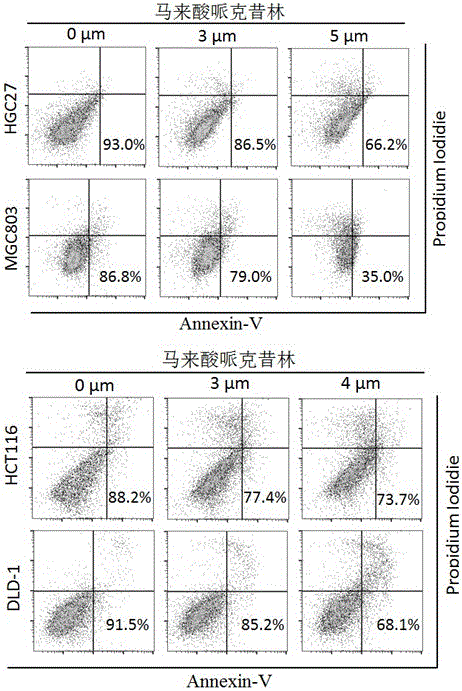
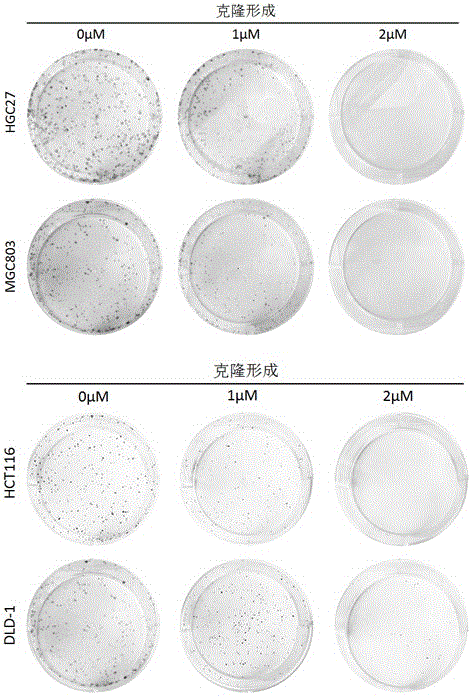
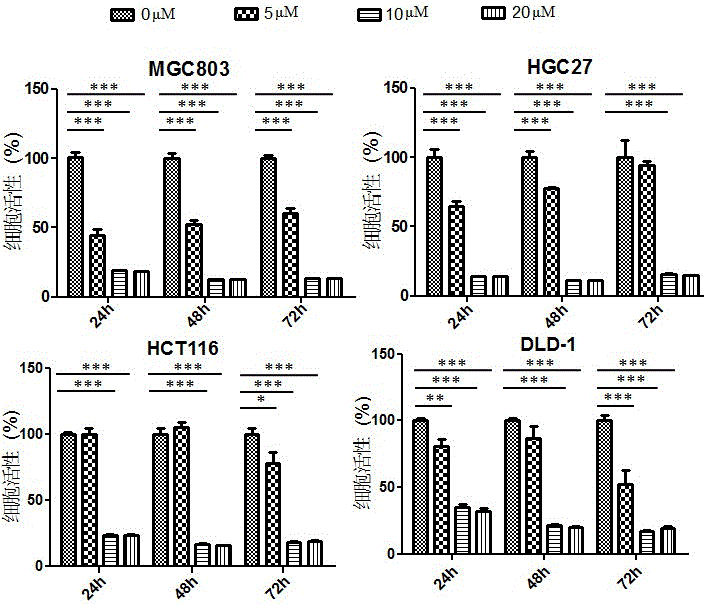
Application of perhexiline/oxaliplatin drug combination in aspect of treatment of stomach cancer and colorectal cancer
ActiveCN106860462AGood treatment effectSignificant effectOrganic active ingredientsDigestive systemIntestinal CancerTreatment effect
The invention discloses application of perhexiline / oxaliplatin drug combination in the aspect of treatment of stomach cancer and colorectal cancer. The research shows that the perhexiline is capable of obviously inhibiting the growth, proliferation and clone formation capacities of the stomach cancer and colorectal cancer cells, inducing the apoptosis of the stomach cancer and colorectal cancer cells and inhibiting the invasion and transfer capacities of the stomach cancer and colorectal cancer cells, has obvious treatment effects on stomach cancer and colorectal cancer, and therefore, can be used in drugs for treating stomach cancer and intestinal cancer. The invention provides new application of perhexiline, thereby widening the clinical application fields of perhexiline; and the invention also provides a new scheme of perhexiline / oxaliplatin drug combination in treating stomach cancer and colorectal cancer, thereby increasing the options for chemotherapeutic schemes of stomach cancer and colorectal cancer, and having favorable clinical application prospects.
Owner:SUN YAT SEN UNIV CANCER CENT
Method for detecting macromolecular substances in perhexiline injection
ActiveCN102590408AEasy to operateDetection is simple and fastComponent separationMacromolecular SubstancesPerhexiline
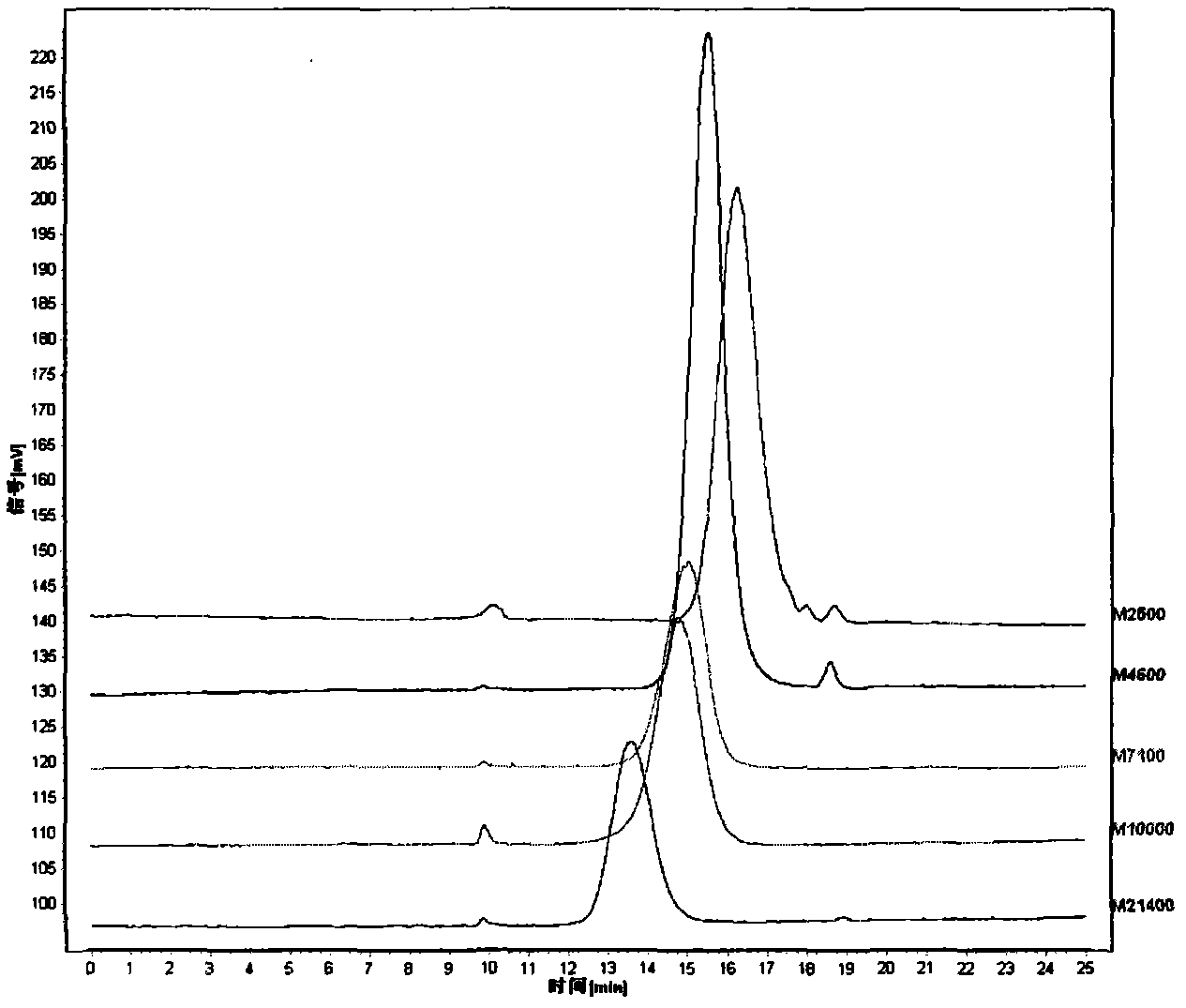
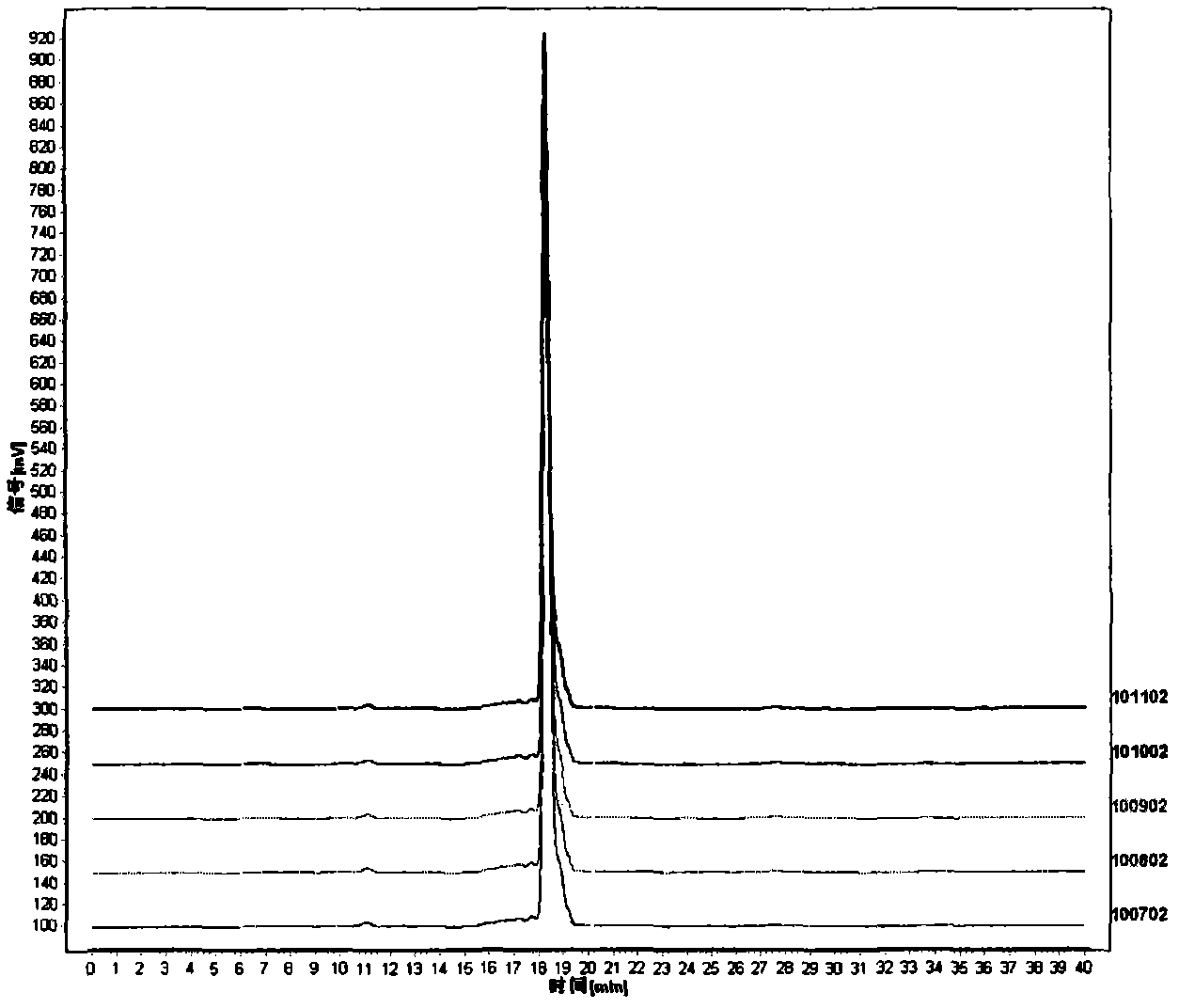
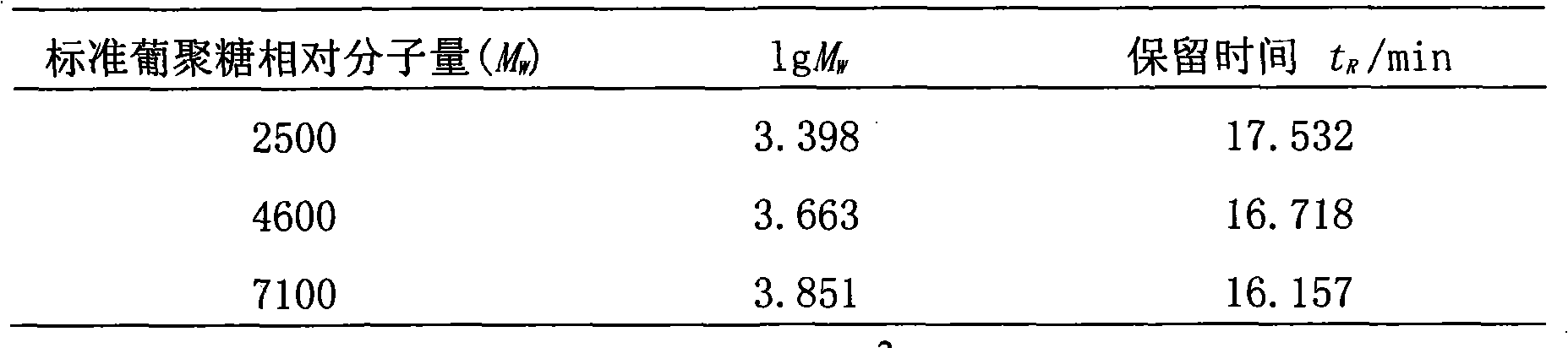
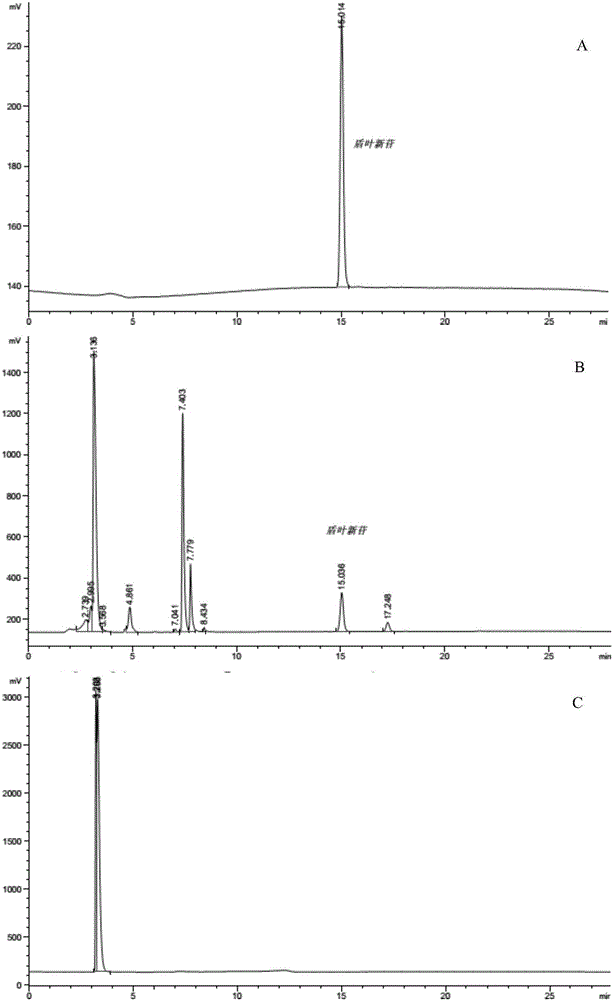
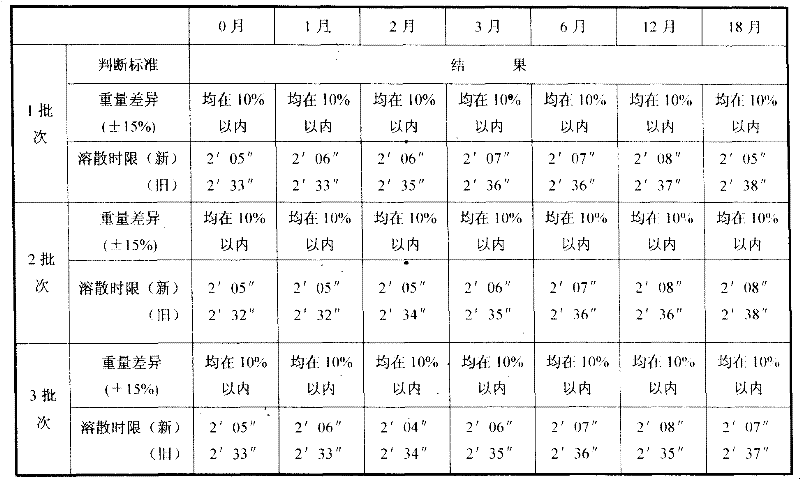
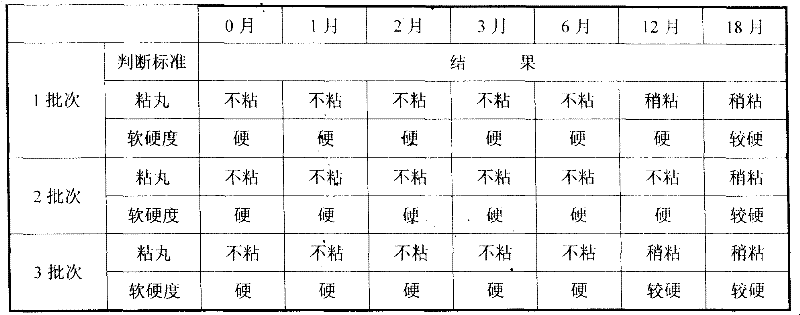
The invention discloses a method for detecting macromolecular substances in a perhexiline injection. The method after being subjected to a series of investigation, testing and verification of specificity and linearity investigation, degree of precision, stability and the like, can detect the macromolecular substances in the perhexiline injection easily, conveniently and quickly. From an HPLC (High Performance Liquid Chromatography) spectrum, macromolecular substances with molecular weight greater than 2500 are not detected in five batches of detected perhexiline injection samples.
Owner:YABAO PHARMA GRP CO LTD
Topical pharmaceutical composition comprising a cholinergic agent or a calcium channel blocker
Owner:SLA PHARMA AG
Treatment of heart failure
The invention relates to perhexiline, or a pharmaceutically acceptable salt thereof, for use in the treatment of HfnEF, as well as to a method of treating HfnEF, which comprises administering to an animal in need thereof an effective amount of perhexiline, or a pharmaceutically acceptable salt thereof, to treat said HFnEF. The invention further relates to a treatment program for treating HFnEF, which involves the co-use or co-administration of perhexiline with one or more other compounds that are advantageous in treating HFnEF or the symptoms thereof.
Owner:HEART METABOLICS
Freeze dried perhexiline powder for injection and its prepn process
InactiveCN1810269AGood resolubilityReduce moisturePowder deliveryLyophilised deliveryDiseaseSalvia miltiorrhiza
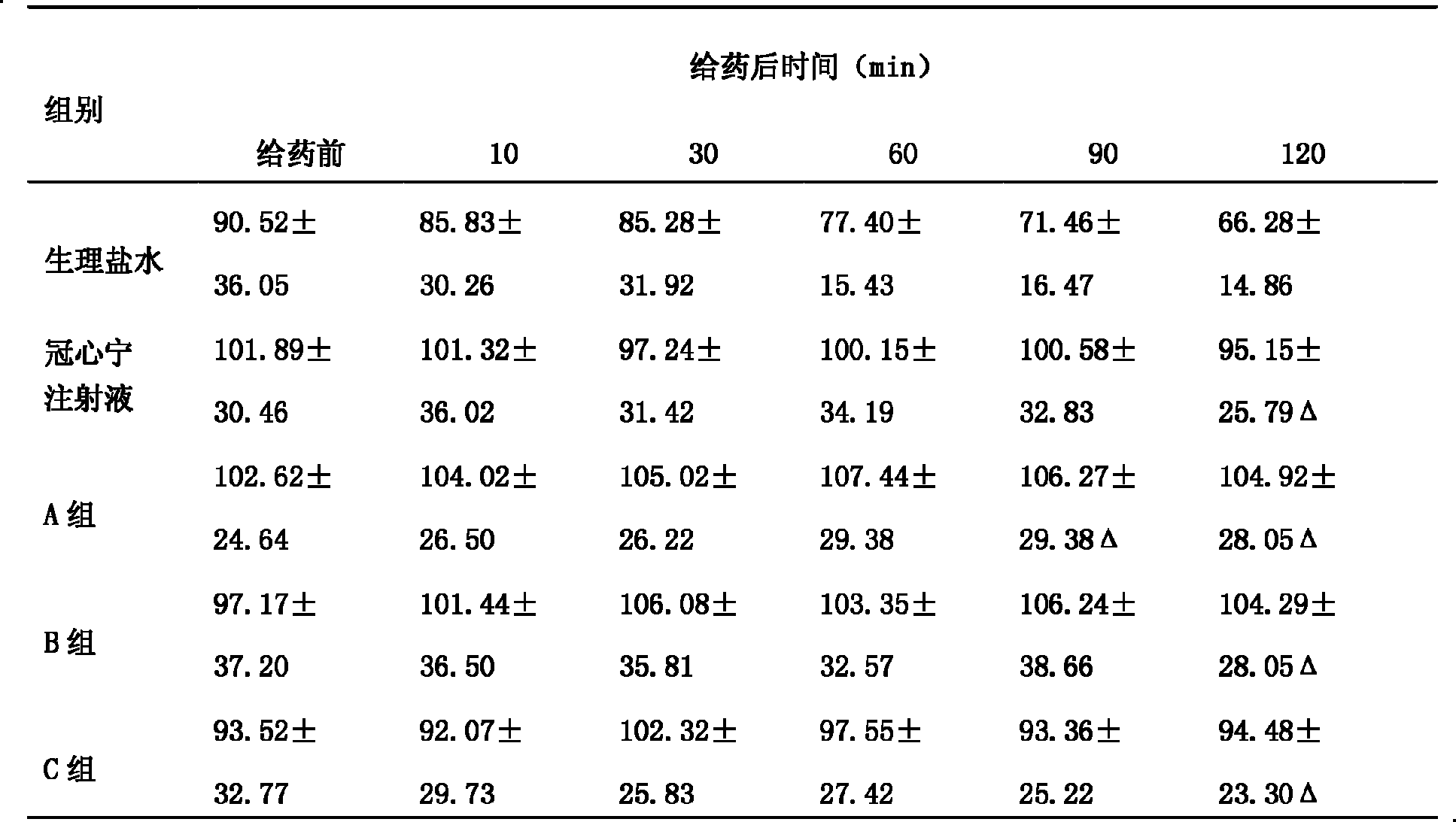
The present invention provides one kind of freeze- dried powder, named as Guanxinning, for injection and its preparation process. It consists of red sage and Chuanxiong rhizome extract 50-100 weight portions and excipient 0-50 weight portions. The Guanxinning injection has the features of quick acting, high effect, high solubility and high stability, and is suitable for treatment of cardiac cerebral vascular diseases.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Peltate leaf perhexiline oral cavity disintegrating tablet and preparation method thereof
InactiveCN101647930ADissolution rate is fastQuick effectPill deliveryCardiovascular disorderAnginaTableting
The invention relates to a peltate leaf perhexiline oral cavity disintegrating tablet which is prepared by the following raw materials in parts by weight: 100-800 peltate leaf dioscorea rhizome extract, 50-300 filling agent, 20-150 disintegrating agent, 1-15 glidant and 1-8 flavoring agent. A preparation method of the peltate leaf perhexiline oral cavity disintegrating tablet comprises the following steps: respectively pulverizing the peltate leaf dioscorea rhizome extract, the filling agent, the disintegrating agent, the glidant and the flavoring agent and screening by a sieve in 50-100 meshes; then mixing all raw material powders; tabletting and preparing a finished product. The peltate leaf perhexiline oral cavity disintegrating tablet has the advantages of rapid disintegration and absorption, good taste, high bioavailability, quite convenient taking and simple and convenient preparation process and more rapid disintegration and dissolution of main medicine components, is more suitable for the emergency treatment of an acute patient, such as an angina patient and enters the gastrointestinal tract to rapidly disperse and widely cover the gastrointestinal mucosal after being disintegrated.
Owner:石庆平
Dripping pills of perhexiline maleate and its preparation
InactiveCN1546026ADisintegration and dissolution fastQuality improvementOrganic active ingredientsPill deliveryPerhexilineBiomedical engineering
The invention relates to a maleic acid perhexiline drop pill prepared by utilizing ultramicro disintegration and drop pill manufacturing process, which has the advantages of improving collapse and dissolving speed, quick effect, increased medicament stability, reduced adjuvant consumption, lowered production costs, and easiness in carrying and use. It has good compliance, thus is especially suitable for children, the elderly, bedridden patients and dysphagia patients.
Owner:HONGYI SCI & TECH CO LTD NANCHANG
Perhexiline injection
InactiveCN106361690AImprove stabilityInorganic non-active ingredientsPharmaceutical delivery mechanismAnginaCoronary heart disease
The invention discloses a perhexiline injection which is prepared from the following raw materials in parts by weight: 1900-2100 parts of radix salviae miltiorrhizae, 1900-2100 parts of hemlock parsley, 0.4-3.8 parts of cosolvent, 0.3-1.8 parts of stabilizer and 4000-6000 parts of water for injection. The perhexiline injection has the effects of activating blood circulation to dissipate blood stasis, promoting circulation of qi and nourishing the heart, is used for coronary heart disease and angina pectoris, has favorable stability, and thus, has favorable application prospects.
Owner:上海辰松新材料科技有限公司
Detection method for content of peltate leaf perhexiline preparation
InactiveCN105866286AImprove quality controllabilityWith promoting blood circulation and removing blood stasisComponent separationCoronary heart diseaseEvaporation
The invention belongs to the field of traditional Chinese medicine preparations and specifically discloses a detection method for content of a peltate leaf perhexiline preparation. The method is a content detection method established on the basis of taking zingiberensis newsaponin as an index component. The peltate leaf perhexiline preparation is a traditional Chinese medicine preparation taking peltate yam rhizome extract as a prescription and has the effects of promoting circulation and removing stasis, promoting qi circulation to relieve pain and nourishing the blood and soothing the nerves. The peltate leaf perhexiline preparation is used for treating patients suffering the symptoms of thoracic obstruction, cardiodynia Qi depression blood stasis, hyperlipemia, coronary heart disease and stenocardia. According to the content detection method disclosed by the invention, an evaporation light scattering detector is used for detecting and a high performance liquid chromatography is used for detecting the content of zingiberensis newsaponin. The method is accurate, the stability is high, the quality controllability of the peltate leaf perhexiline preparation is increased, the effectiveness of the medicine is ensured, and standardized production of the medicine can be conveniently realized.
Owner:江苏黄河药业股份有限公司
Dropping pills containing perhexiline maleic acid and method for preparing the same
InactiveCN101194904AHigh degree of naturalSmall toxicityOrganic active ingredientsPharmaceutical product form changeChemical synthesisFood additive
The invention provides a dripping pill which contains maleic perhexiline and a process for preparation. The medicament is mainly used for anti-arrhythmia. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
Dropping pills containing perhexiline maleic acid and method for preparing the same
InactiveCN101194904BImprove directionGreat tasteOrganic active ingredientsPharmaceutical product form changeChemical synthesisFood additive
The invention provides a dripping pill which contains maleic perhexiline and a process for preparation. The medicament is mainly used for anti-arrhythmia. The invention overcomes the shortcomings of the prior dripping pills that pure natural degrees of findings which are used by the prior dripping pills are not high, and chemosynthesis findings which are commonly used are not in food additives catalogs of some countries, and tastes of the dripping pills are worse. The invention is a pharmaceutical preparation whose natural degree is higher, safety is stronger, and side effect is lower.
Owner:TIANJIN TASLY PHARMA CO LTD
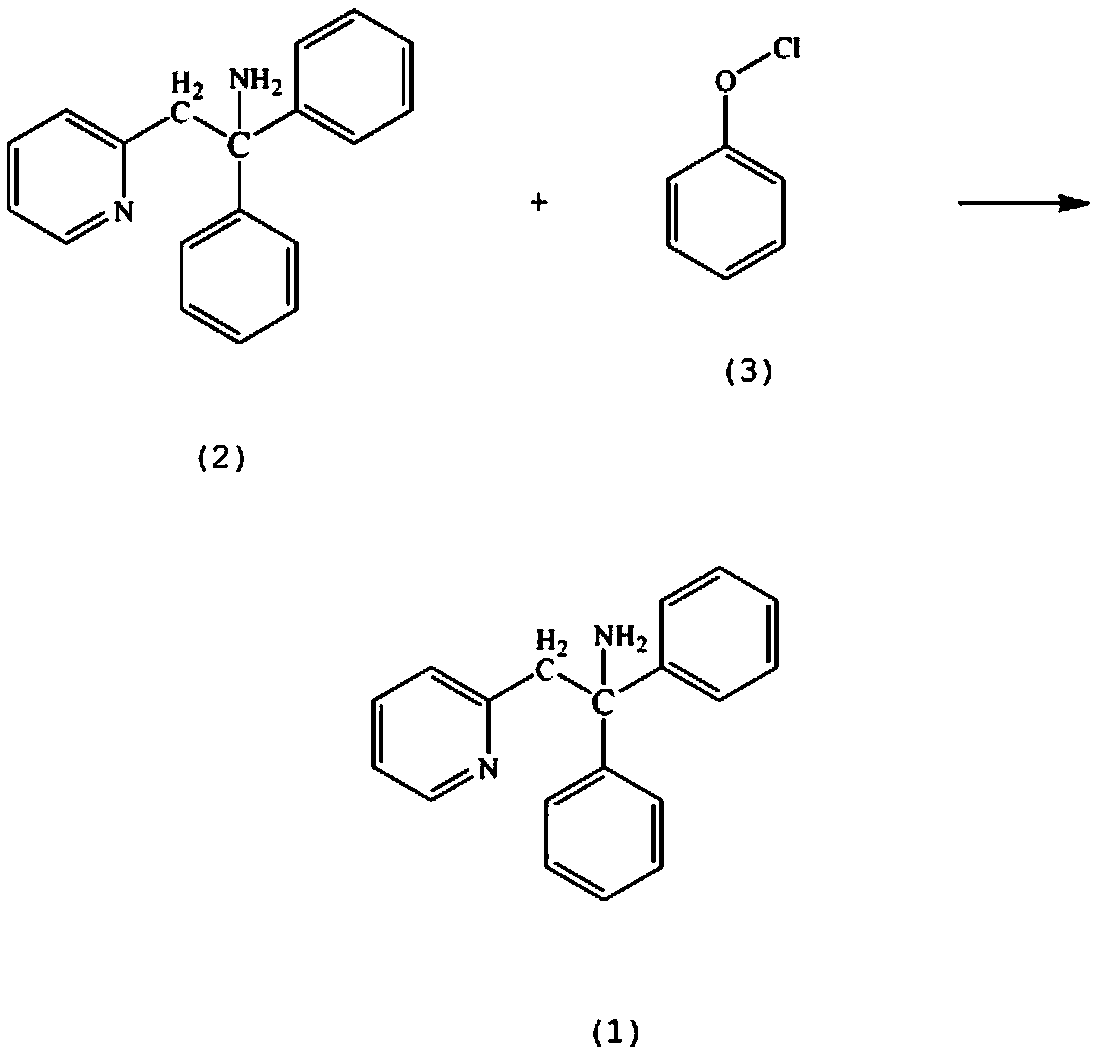
Synthesis method of perhexiline drug intermediate alpha-(2,2-diphenyl vinyl) pyridine
InactiveCN105503702AReduce intermediate linksLow reaction temperatureOrganic chemistryChlorobenzeneFiltration
Owner:CHENGDU QIANYE LONGHUA PETROLEUM ENG TECH CONSULTING
Prevention and/or treatment of contrast-induced acute kidney injury
PendingUS20210401832A1Preventing contrast-induced acute kidney injuryReduce and preventOrganic active ingredientsUrinary disorderOXFENICINETrimetazidine
Methods are provided for preventing, reducing, and / or treating contrast-induced acute kidney injury which include administering an inhibitor of fatty acid oxidation to a patient in need thereof. Also provided are methods involving use of trimetazidine or pharmaceutically acceptable salts thereof for the prevention and / or treatment of contrast-induced acute kidney injury. Methods are also provided for preventing and / or treating contrast-induced acute kidney injury which include administration of one or more of trimetazidine, etomoxir, oxfenicine, perhexiline, mildronate, or ranolazine, or pharmaceutically acceptable salts of any of the preceding.
Owner:SAGHMOS THERAPEUTICS INC
Application of perhexiline in the treatment of gastric cancer and intestinal cancer
ActiveCN106727553BSignificant effectGrowth inhibitionOrganic active ingredientsAntineoplastic agentsIntestinal CancerChemotherapeutic drugs
The invention discloses an application of perhexiline in treating stomach cancers and intestinal cancers. After proofing by study, the perhxiline can obviously inhibit the growth, proliferation and coloning formation abilities of stomach cancer and colorectal cancer cells, induce the withering of the stomach cancer and colorectal cancer cells, inhibit the invasion and transfer abilities of the stomach cancer and colorectal cancer cells, and have obvious treatment effect on the stomach cancer and colorectal cancer, so that the perhxiline can be used as a medicine for treating the stomach cancer and intestinal cancer. The application has the advantages that a new application of the perhxiline is provided, the clinical application field of the perhxiline is widened, and a new chemical therapy medicine and a therapy path are provided for the therapy of stomach cancer and colorectal cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
Perhexiline injection and its preparing method
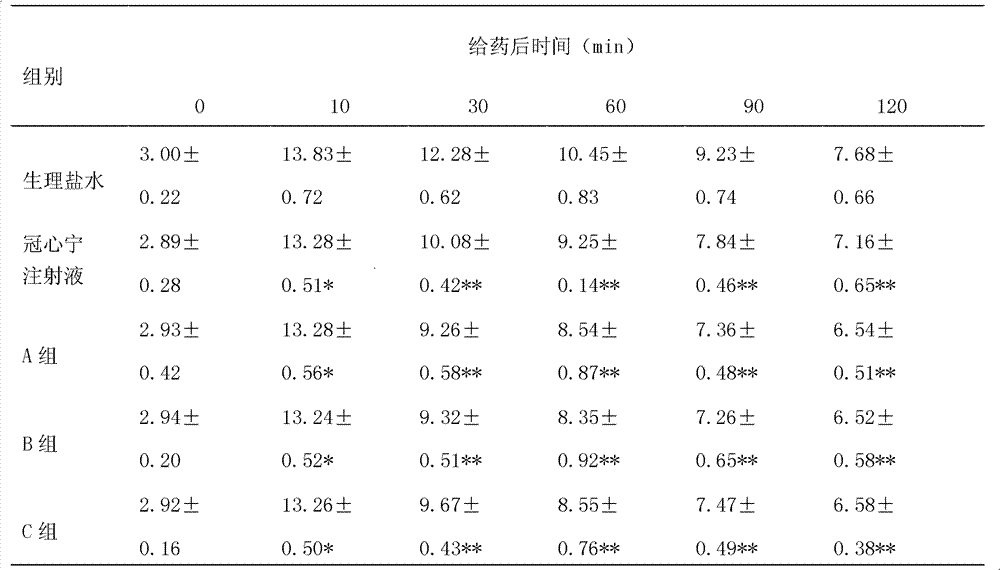
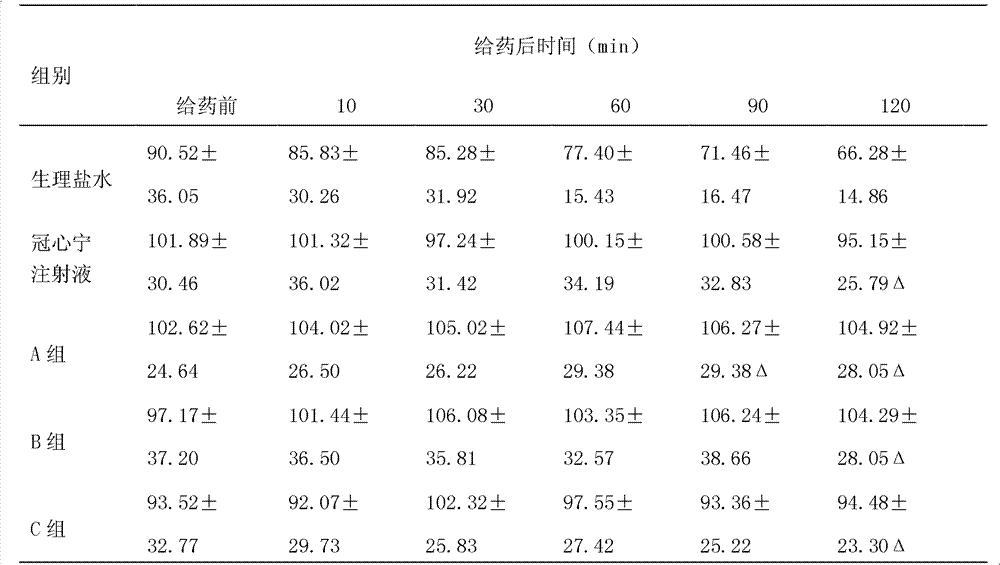
InactiveCN100462077CEfficient removalDefinite curative effectKetone active ingredientsAnhydride/acid/halide active ingredientsDiseaseMedicine
The present invention discloses a Guanxinning injection for effectively curing coronary heart disease and ischemic cerebrovascular disease. It is characterized by that said injection is basically formed from tanshinone, salvia phenol acid matter, liguistrazine and ligusticum pherol acid ester matter. Besides, said invention also provides its preparation method by utilizing fresh salvia root and ligusticum root and its concrete steps. It is characterized by that the invented injection not only contains tanshinone II A and ligustrazine, but also contains cryptotanshinone, tanshinone I, salvia phenol acid matter and ligusticum phenol acid ester matter.
Owner:王锋
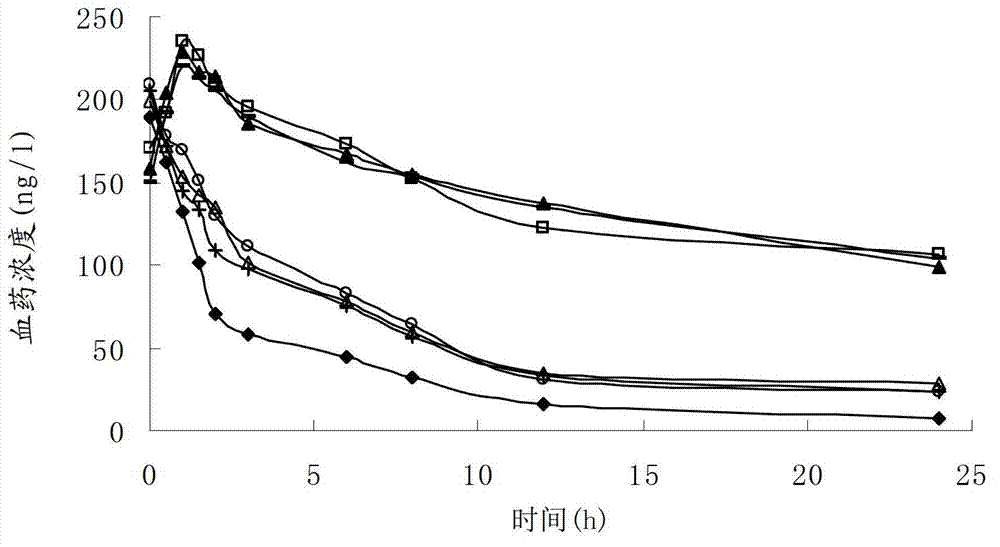
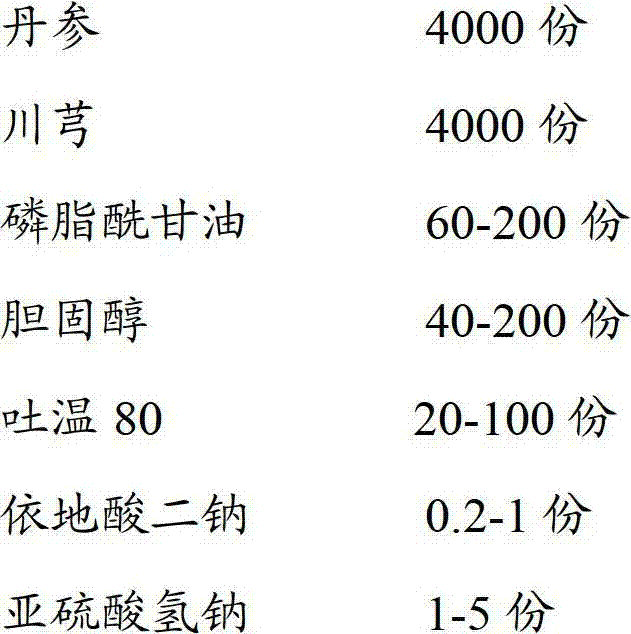
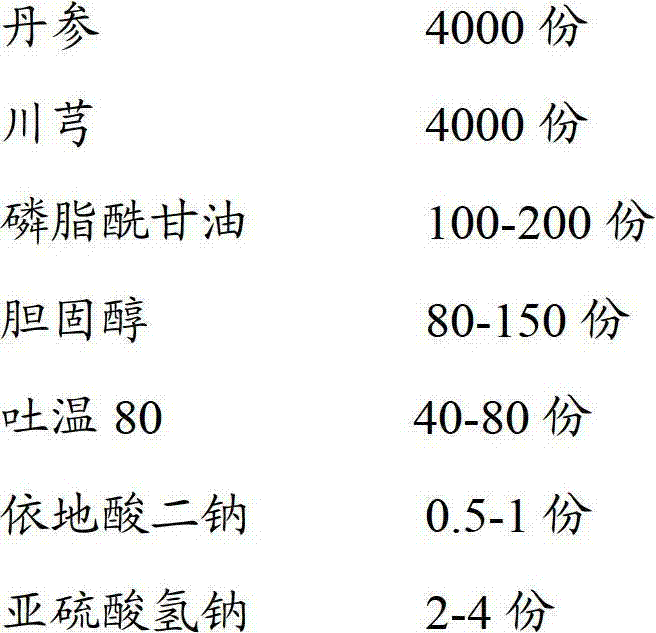
Perhexiline lipidosome injection
InactiveCN103040937BQuality improvementImprove bioavailabilityNervous disorderMetabolism disorderSalvia miltiorrhizaSide effect
The invention provides perhexiline lipidosome injection which is mainly prepared from salvia miltiorrhiza, ligusticum wallichii, phosphatidyl glycerol, cholesterol, tween 80, edetate disodium and sodium hydrogen sulfite. Compared with the existing preparation, the injection largely improves the stability and the bioavailability of the preparation, smoothly releases drugs, improves the preparation product quality, reduces the poison and side effects, and has more remarkable curative effect.
Owner:海南路易丹尼生物科技有限公司
Use of perhexiline
InactiveUS20170157102A1Solve the real problemOrganic active ingredientsAntiparasitic agentsPerhexilinePathology
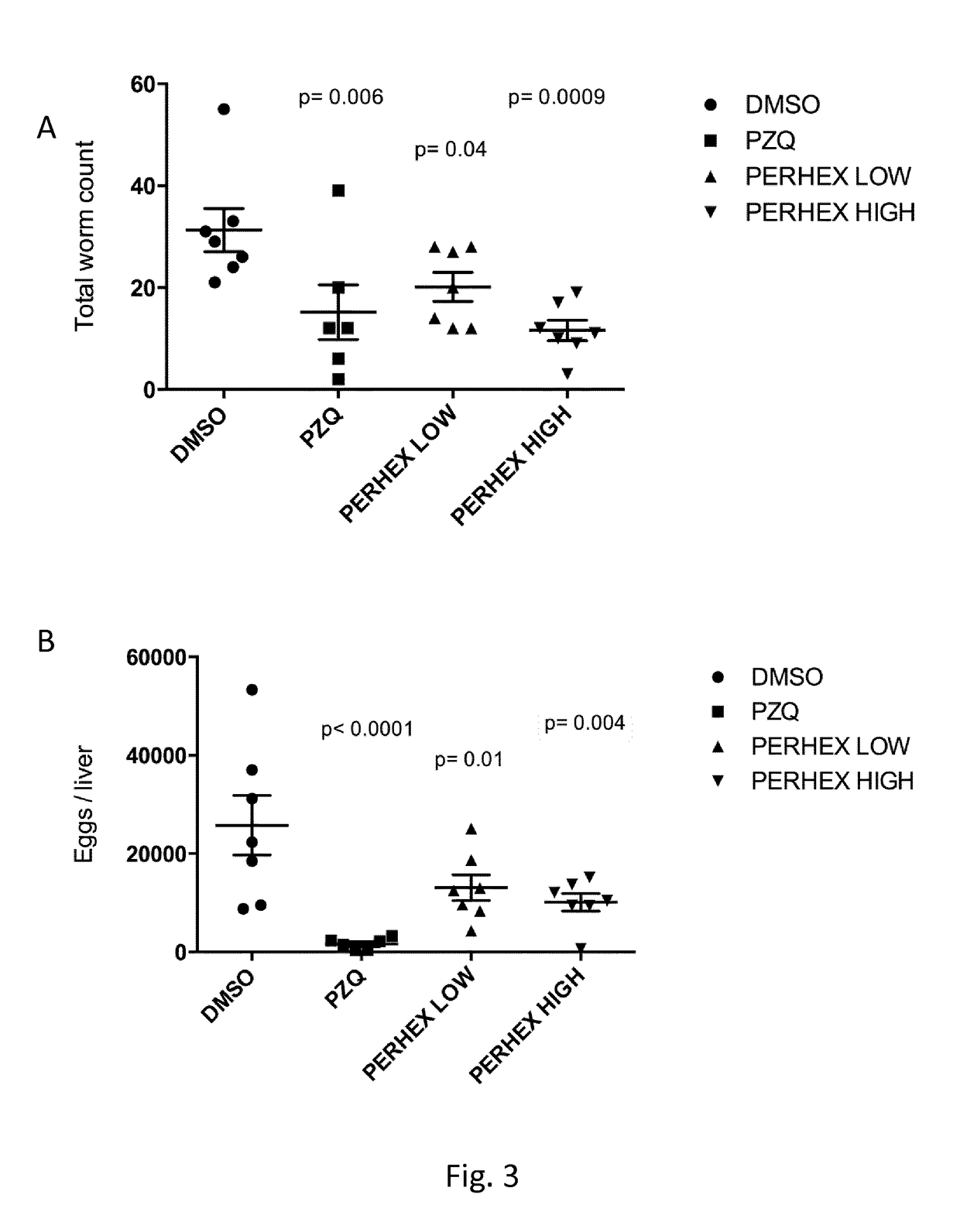
The present invention relates to Perhexiline, or a pharmaceutically acceptable salt thereof, for use in the treatment of a pathology caused by trematodes.
Owner:CONSIGLIO NAT DELLE RICERCHE +2
Method for preparing perhexiline pharmaceutical preparation
ActiveCN102048821BGood curative effectIncrease contentCardiovascular disorderPlant ingredientsSalvia miltiorrhizaDrug carrier
The invention discloses a preparation method of a perhexiline pharmaceutical preparation, comprising the following steps of: (a) preparing pharmaceutical components salvia and szechuan lovage rhizome, wherein the weight ratio of salvia to szechuan lovage rhizome 1-3: 1-3 by weight; (b) decocting the salvia, injecting concentrated liquor in a macroporous absorption resin column, and concentrating and drying eluent to obtain salvia extractive; (c) decocting the szechuan lovage rhizome, injecting concentrated liquor in a macroporous absorption resin column, and concentrating and drying eluent toobtain szechuan lovage rhizome extractive; and (d) adding pharmacologically allowed pharmaceutical carriers in the prepared extractives uses as active ingredients to prepare various forms of pharmaceutical preparation. The perhexiline pharmaceutical preparation prepared by the method disclosed in the invention has effect superior to that of the perhexiline pharmaceuticals prepared according to the prior art.
Owner:SHINEWAY PHARMA GRP LTD
Sustained release preparation of perhexiline
InactiveCN101015535AOrganic active ingredientsInorganic non-active ingredientsBlood concentrationCurative effect
The invention discloses a slow release preparation of Perhexiline and its preparing process, wherein the raw materials of the preparation include Perhexiline of a predetermined proportion, slow release matrix material and medicinal material, the preparation can be prepared into solid dispersing agent, wherein the medicament can be released slowly and continuously after being administrated, the effective concentration in blood can be maintained, and long action can be achieved. The advantages of the invention include decreased frequency of medicinal administration, improved patient's adaptability, lowered blood concentration peak-valley, increased medicinal effect and safety, and reduced total medicinal dose, thereby optimum curative effect can be achieved through minimum dose, thus the preparation is more suitable for patients.
Owner:刘凤鸣
Perhexiline capsule and preparation method thereof
The invention discloses a perhexiline capsule and a preparation method thereof. The perhexiline capsule is characterized in that 155 g of nutmeg, 77.5 g of eupatorium, 1080 g of pubescent holly roots, 540 g of ginkgo leaves, 330 g of motherwort and 330 g of herba siegesbeckiae are taken, a carbon dioxide supercritical extraction method is adopted for extraction, drying is performed under reduced pressure, the materials are smashed into nano dry paste through a high-energy nano impacting mill, functional auxiliaries are added, the perhexiline capsule is prepared, the disintegrating time is remarkably shortened, the curative effect is remarkably superior to that of perhexiline capsules sold on the market, and a positive effect is achieved.
Owner:HEILONGJIANG JIANGHENG PHARMA TECH CO LTD
The application of combination of perhexiline and oxaliplatin in the treatment of gastric cancer and colorectal cancer
ActiveCN106860462BGood treatment effectSignificant effectOrganic active ingredientsDigestive systemIntestinal CancerChemotherapy combinations
The invention discloses application of perhexiline / oxaliplatin drug combination in the aspect of treatment of stomach cancer and colorectal cancer. The research shows that the perhexiline is capable of obviously inhibiting the growth, proliferation and clone formation capacities of the stomach cancer and colorectal cancer cells, inducing the apoptosis of the stomach cancer and colorectal cancer cells and inhibiting the invasion and transfer capacities of the stomach cancer and colorectal cancer cells, has obvious treatment effects on stomach cancer and colorectal cancer, and therefore, can be used in drugs for treating stomach cancer and intestinal cancer. The invention provides new application of perhexiline, thereby widening the clinical application fields of perhexiline; and the invention also provides a new scheme of perhexiline / oxaliplatin drug combination in treating stomach cancer and colorectal cancer, thereby increasing the options for chemotherapeutic schemes of stomach cancer and colorectal cancer, and having favorable clinical application prospects.
Owner:SUN YAT SEN UNIV CANCER CENT
Application of perhexiline in treating stomach cancers and intestinal cancers
ActiveCN106727553ASignificant effectGrowth inhibitionOrganic active ingredientsAntineoplastic agentsIntestinal CancerChemotherapeutic drugs
The invention discloses an application of perhexiline in treating stomach cancers and intestinal cancers. After proofing by study, the perhxiline can obviously inhibit the growth, proliferation and coloning formation abilities of stomach cancer and colorectal cancer cells, induce the withering of the stomach cancer and colorectal cancer cells, inhibit the invasion and transfer abilities of the stomach cancer and colorectal cancer cells, and have obvious treatment effect on the stomach cancer and colorectal cancer, so that the perhxiline can be used as a medicine for treating the stomach cancer and intestinal cancer. The application has the advantages that a new application of the perhxiline is provided, the clinical application field of the perhxiline is widened, and a new chemical therapy medicine and a therapy path are provided for the therapy of stomach cancer and colorectal cancer.
Owner:SUN YAT SEN UNIV CANCER CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com