Method for detecting macromolecular substances in perhexiline injection
A technology of macromolecular substances and detection methods, which is applied in the field of detection of macromolecular substances in Guanxinning injection, can solve the problems of irregularity and achieve the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
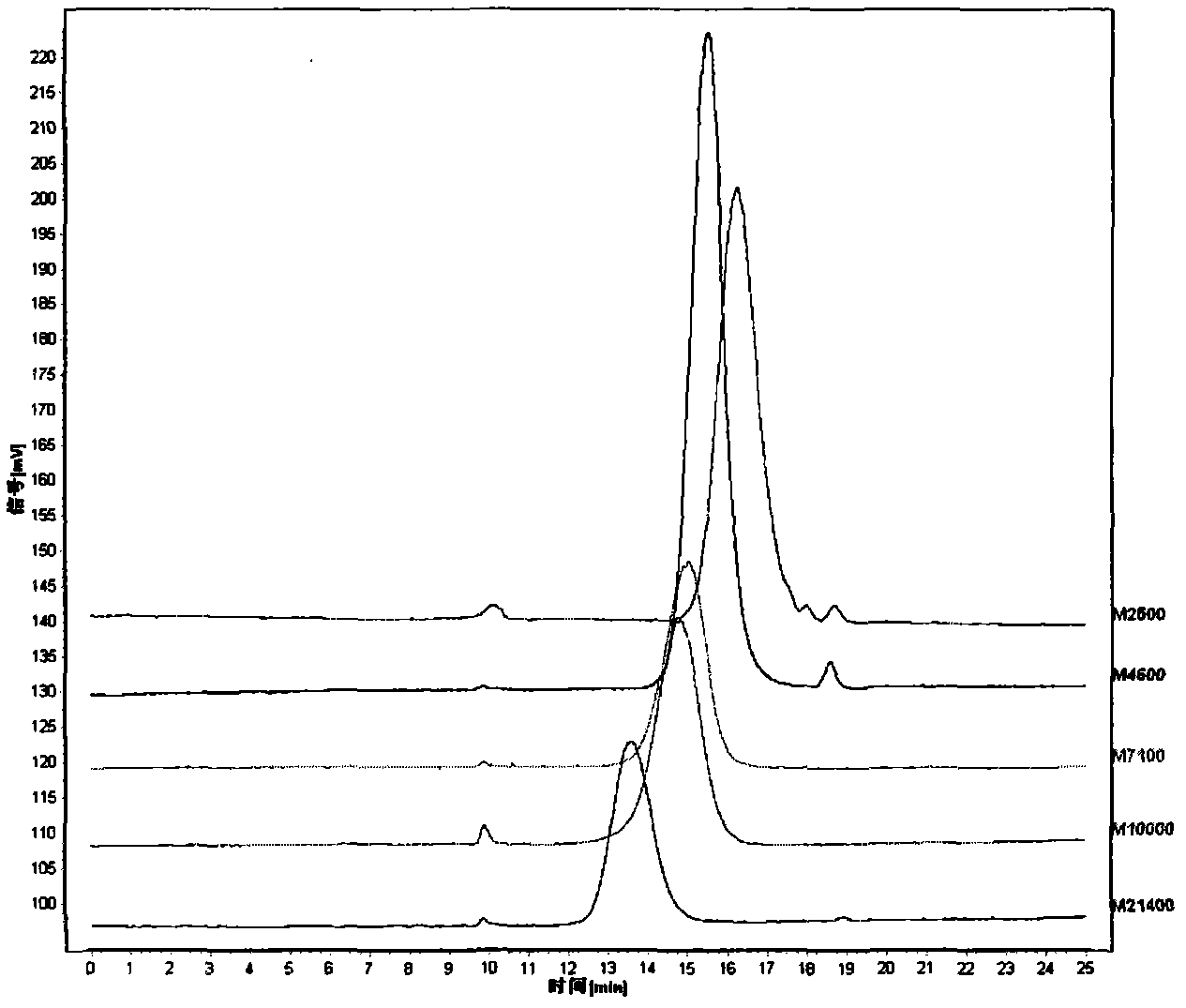
[0068] Chromatographic conditions: the chromatographic column is TSKgel G3000PWXL (7.8mm×300mm); the mobile phase is aqueous solution; the flow rate is 0.8ml / min; the column temperature is 30°C; the injection volume is 5μl;
[0069] Evaporative light scattering detector conditions Drift tube temperature: 110°C; carrier gas (air) flow rate: 3.0ml / min;
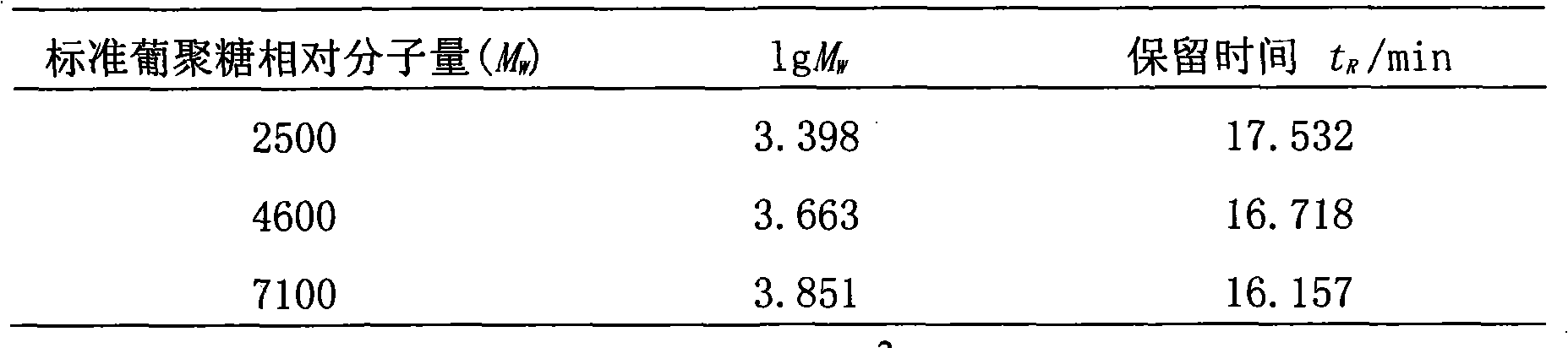
[0070] Accurately weigh 5 parts of dextran reference substances whose relative molecular weights are respectively 2500, 4600, 7100, 10000, 21400, and prepare each 1mg / ml solution with an aqueous solution to obtain the reference substance solution I. Meanwhile, the aqueous solution is taken as a blank control solution. Injection test, with retention time t R For the abscissa, the lgM of the molecular mass of dextran W Perform regression processing for the ordinate;
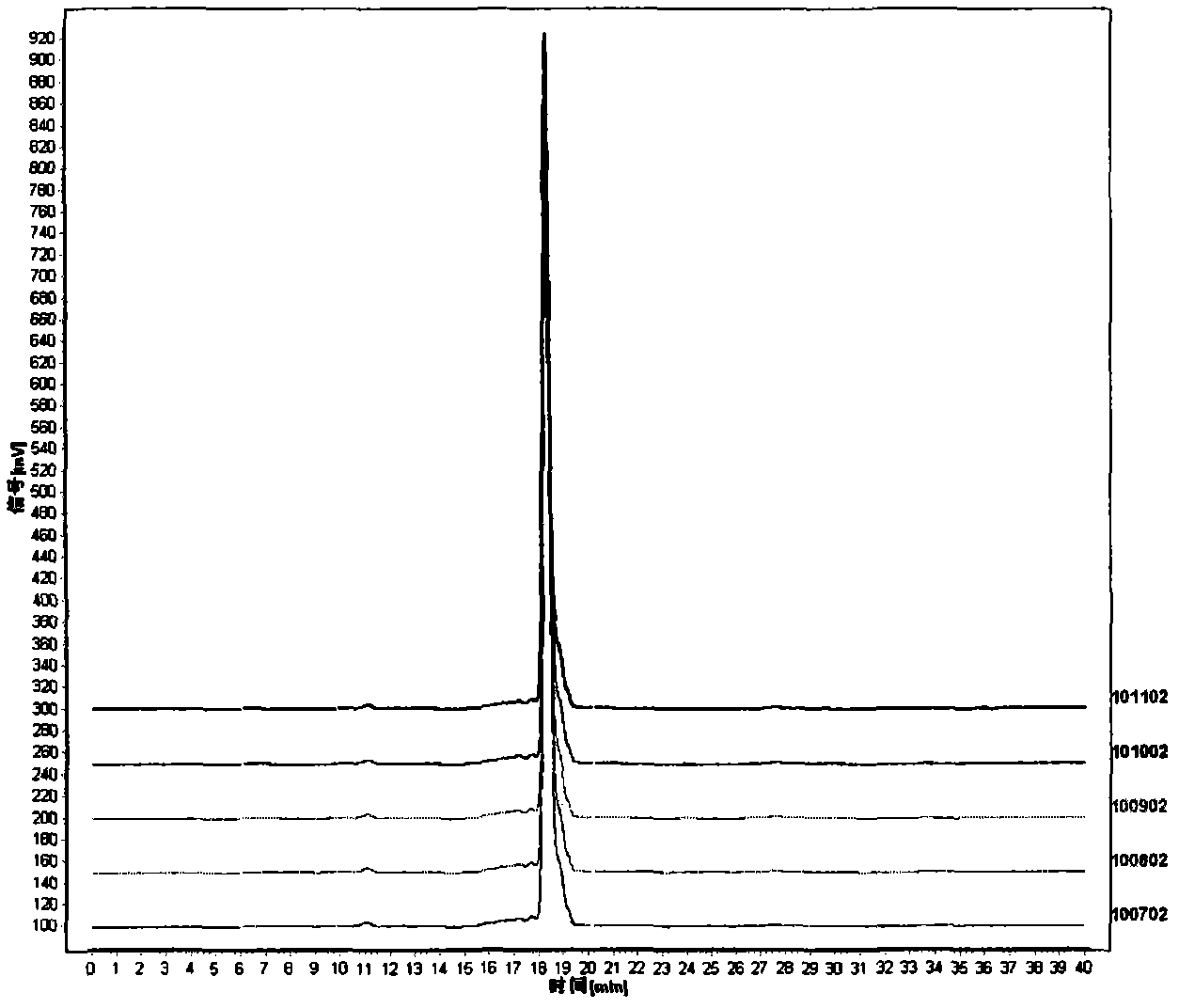
[0071] Accurately weigh the dextran reference substances with relative molecular masses of 4600 and 10000 respectively, dissolve them in aqueous solution to obtain...
Embodiment 2
[0076] Chromatographic conditions: the chromatographic column is TSKgel G3000PWXL (7.8mm×300mm); the mobile phase is aqueous solution; the flow rate is 0.5ml / min; the column temperature is 35°C; the injection volume is 8μl;
[0077] Evaporative light scattering detector conditions Drift tube temperature: 108°C; carrier gas (air) flow rate: 2.5ml / min;
[0078] Accurately weigh 5 parts of dextran reference substances with relative molecular masses of 2500, 4600, 7100, 10000, and 21400, and prepare 1.2 mg / ml solutions with aqueous solution to obtain the reference substance solution I. At the same time, take the aqueous solution as a blank control solution , injection test, with retention time t R For the abscissa, the lgM of the molecular mass of dextran W Perform regression processing for the ordinate;
[0079] Accurately weigh the dextran reference substances with relative molecular masses of 4600 and 10000 respectively, dissolve them in aqueous solution to obtain solutions w...
Embodiment 3
[0084] Chromatographic conditions: the chromatographic column is TSKgel G3000PWXL (7.8mm×300mm); the mobile phase is aqueous solution; the flow rate is 0.12ml / min; the column temperature is 25°C; the injection volume is 3μl;
[0085] Evaporative light scattering detector conditions Drift tube temperature: 112°C; carrier gas (air) flow rate: 3.5ml / min;
[0086] Accurately weigh 5 parts of dextran reference substances with relative molecular masses of 2500, 4600, 7100, 10000, and 21400 respectively, and prepare 0.8 mg / ml solutions with aqueous solution to obtain the reference substance solution I. At the same time, take the aqueous solution as a blank control solution , injection test, with retention time t R For the abscissa, the lgM of the molecular mass of dextran W Perform regression processing for the ordinate;
[0087] Accurately weigh the dextran reference substances with relative molecular masses of 4600 and 10000 respectively, dissolve and constant volume with aqueous s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com