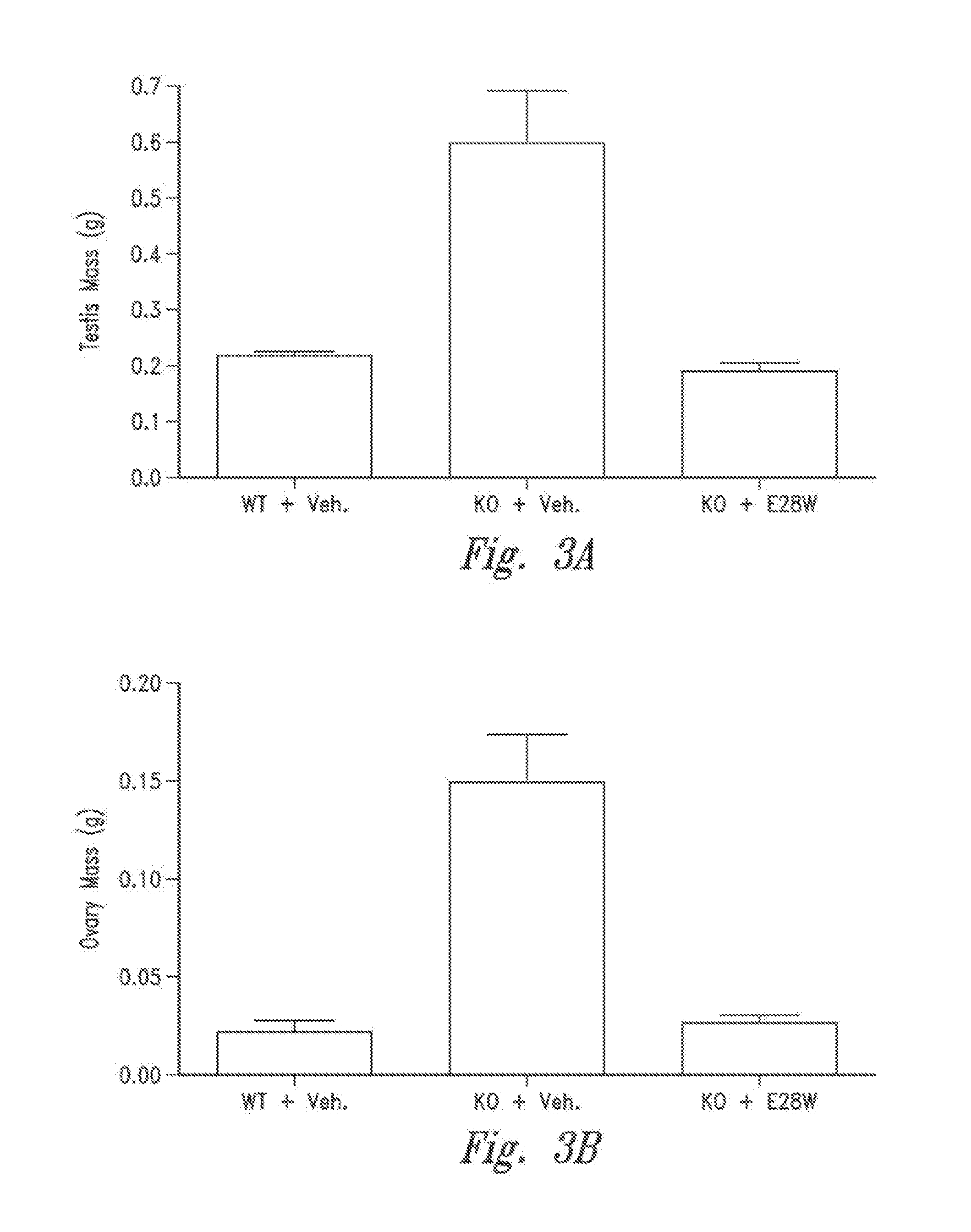
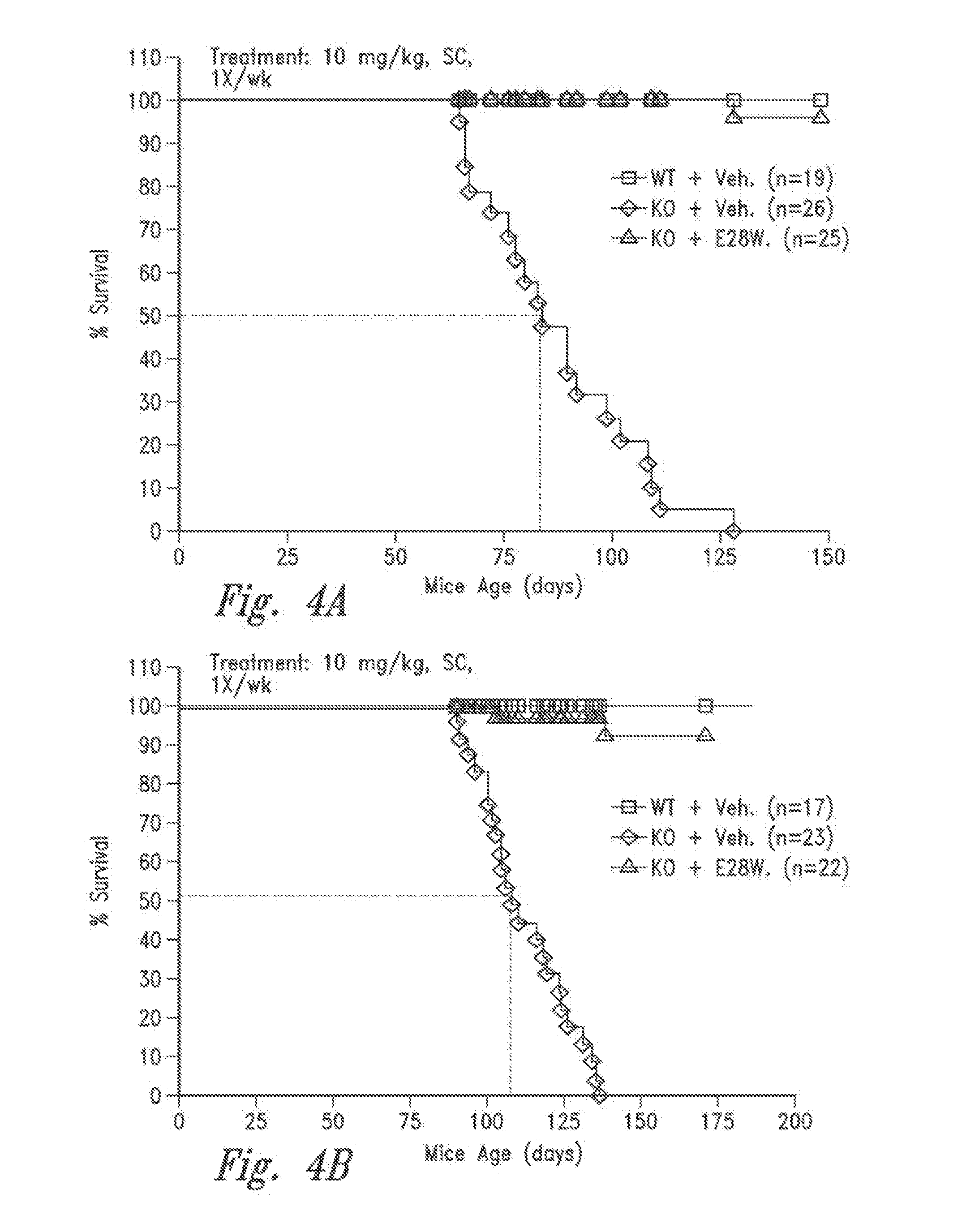
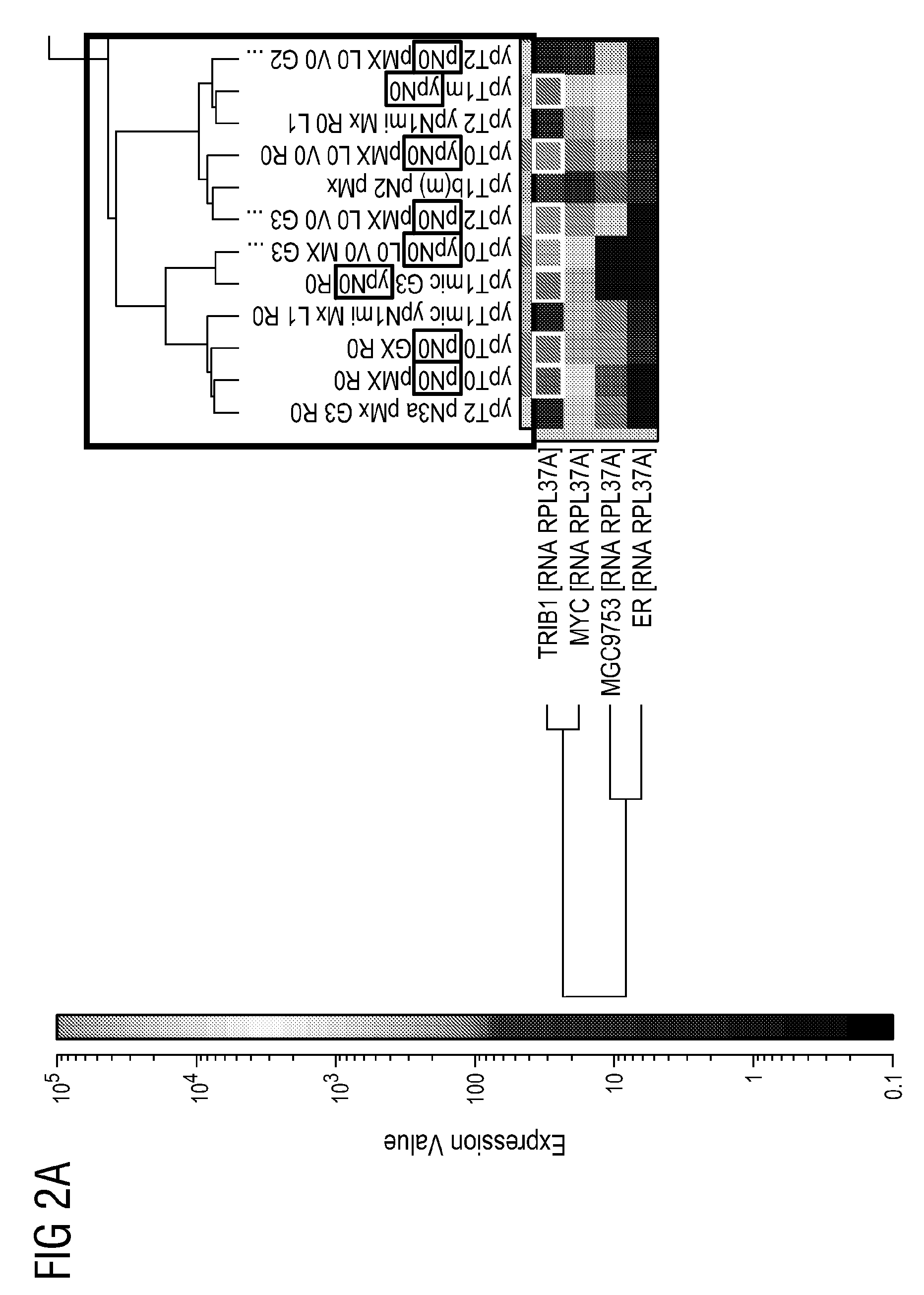
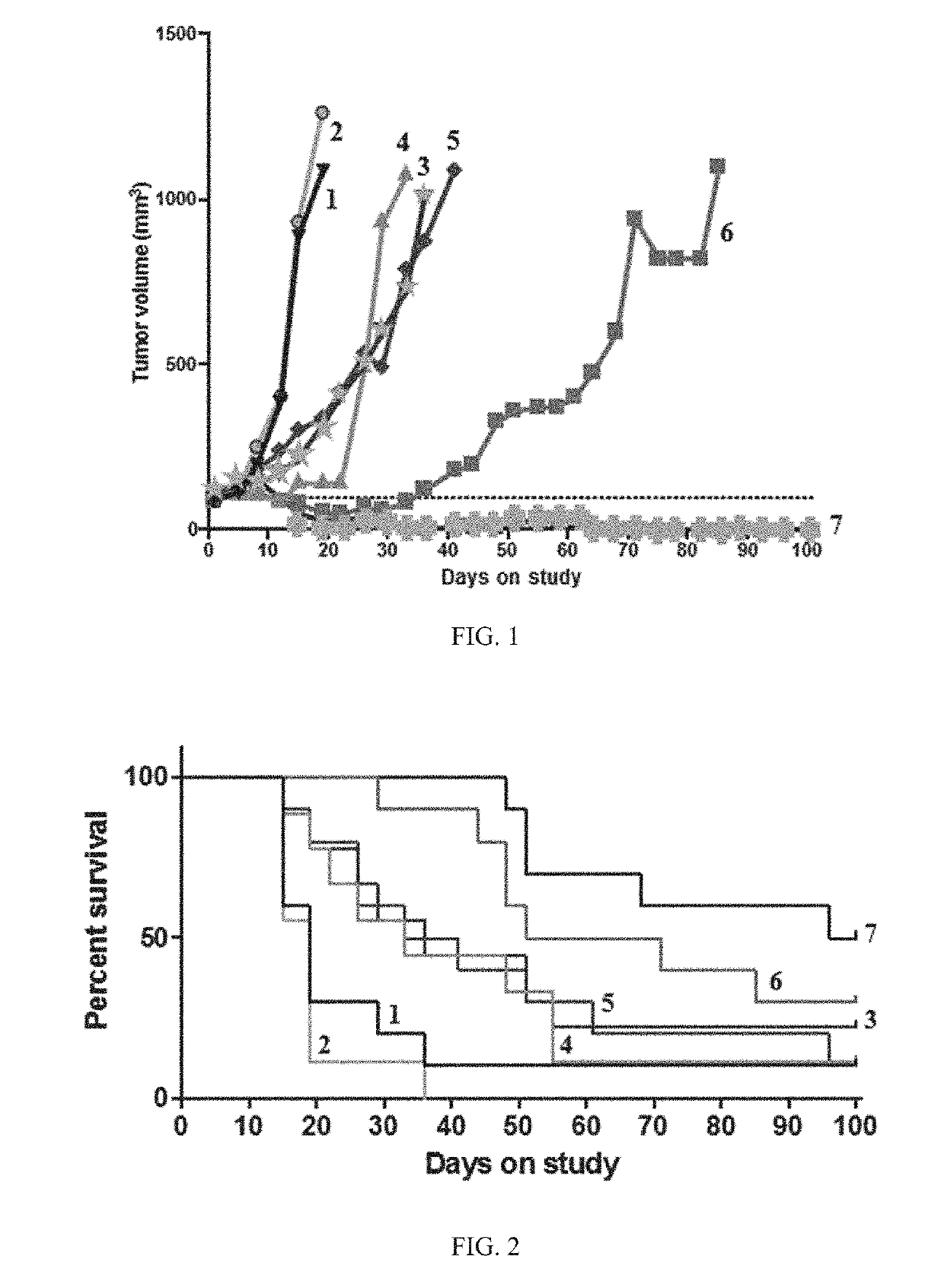
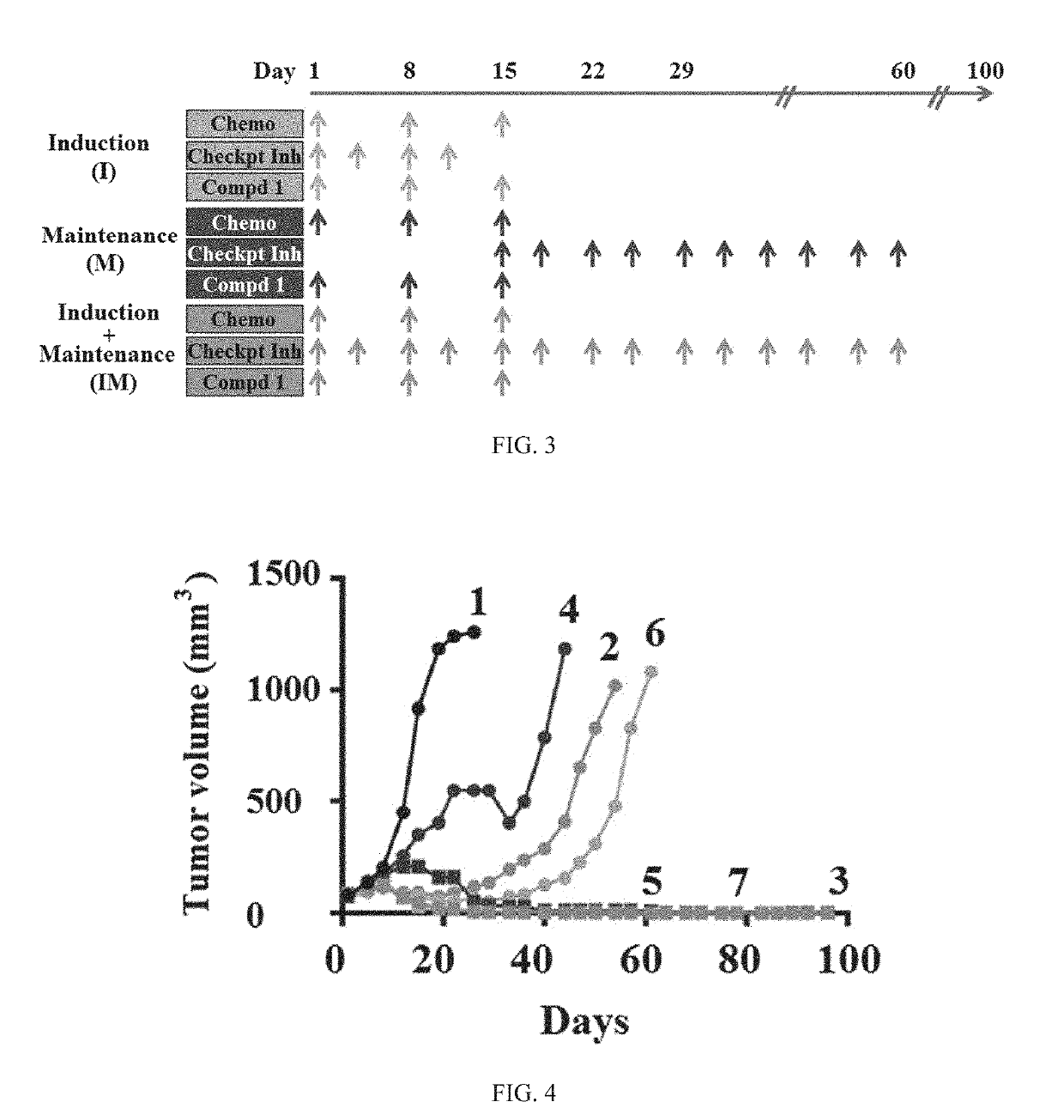
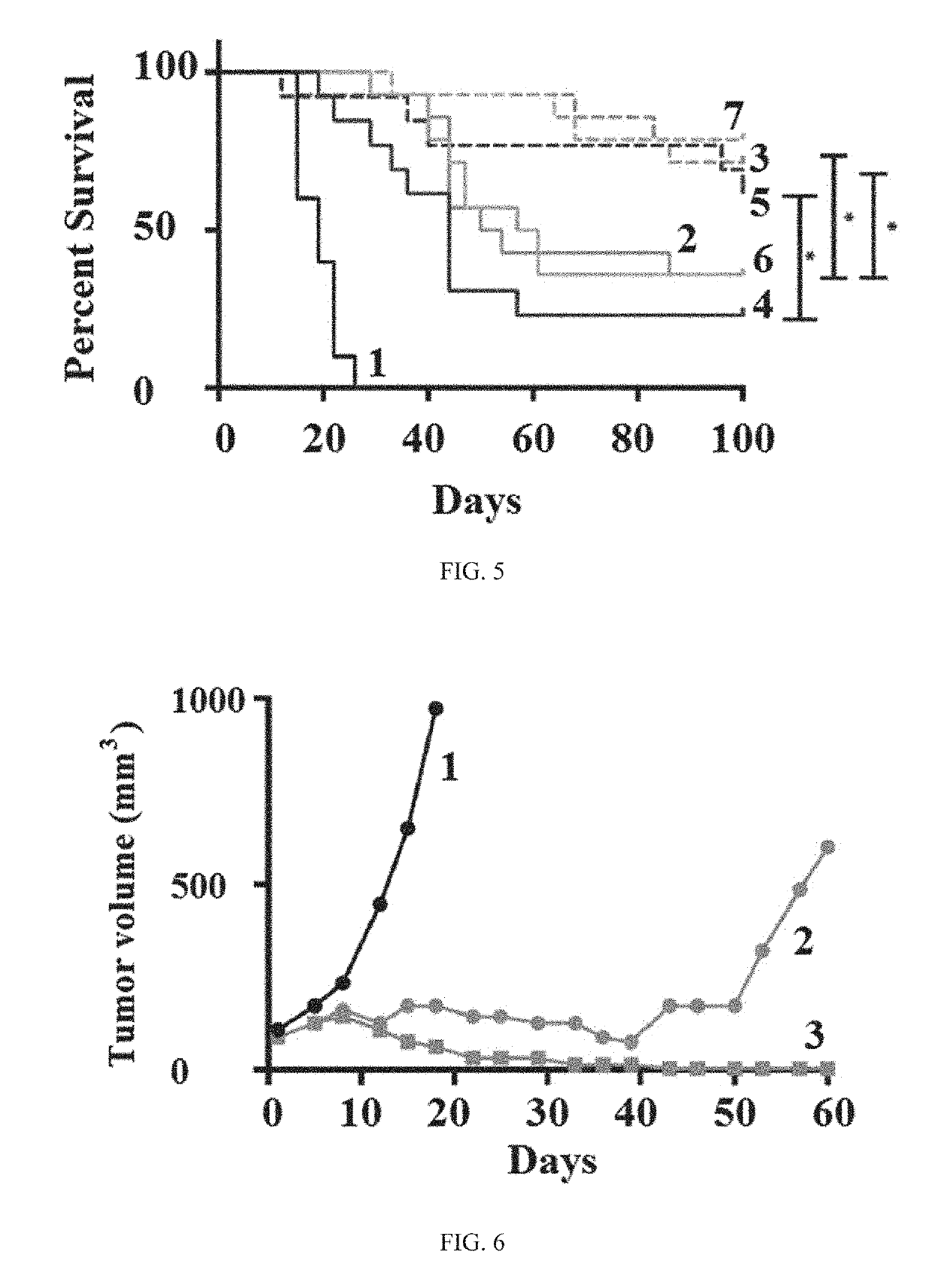
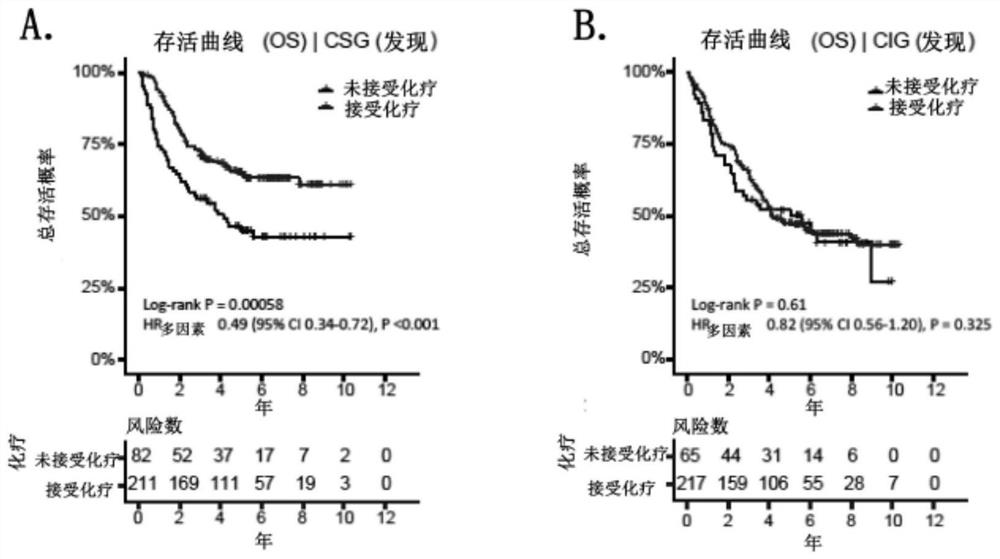
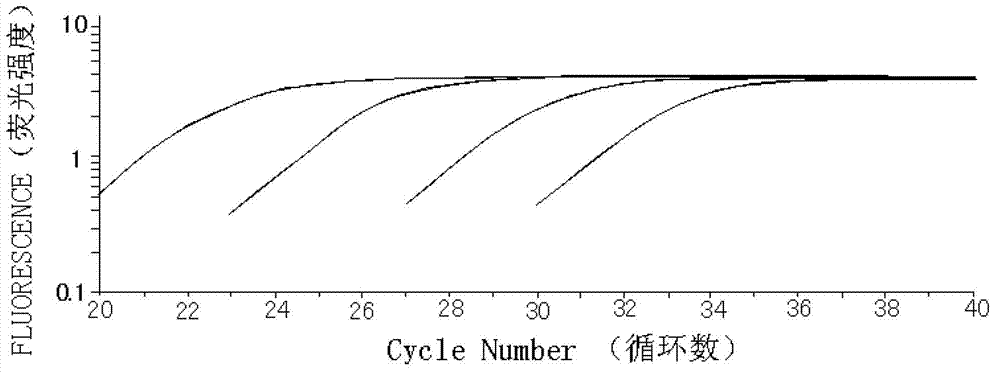
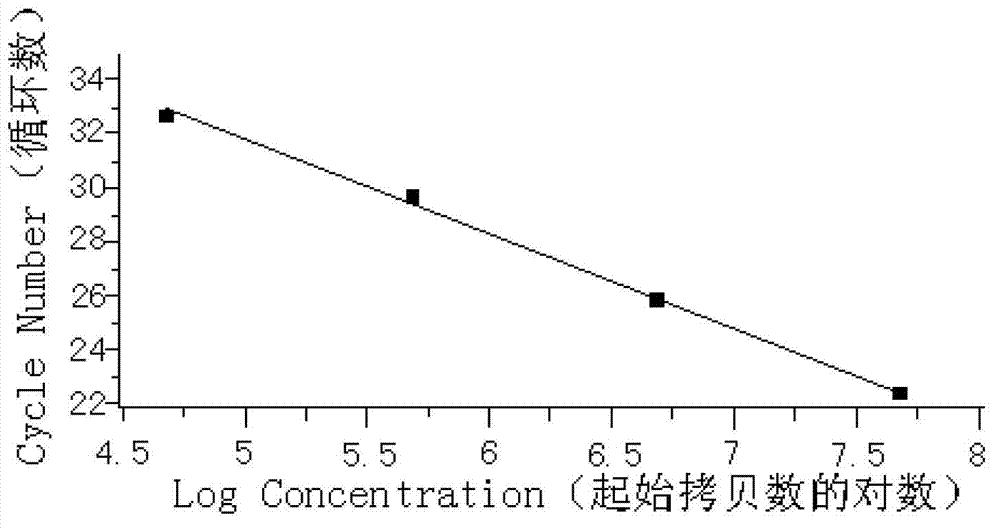
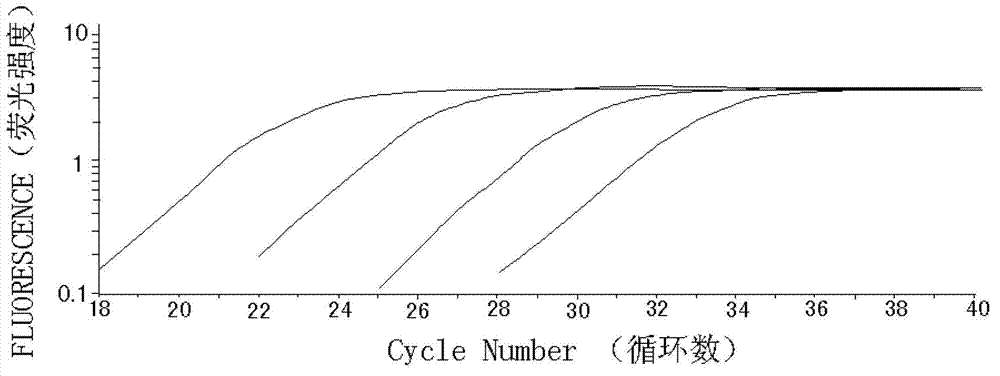
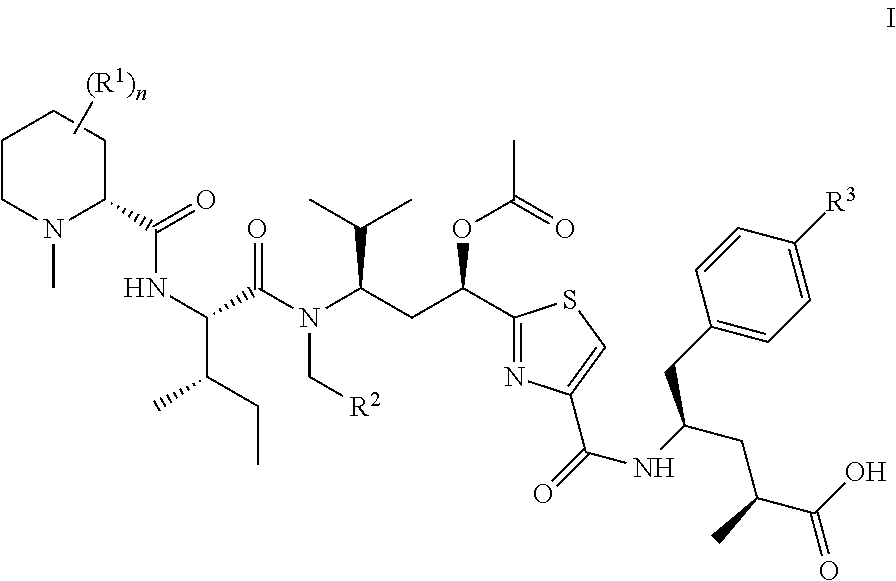
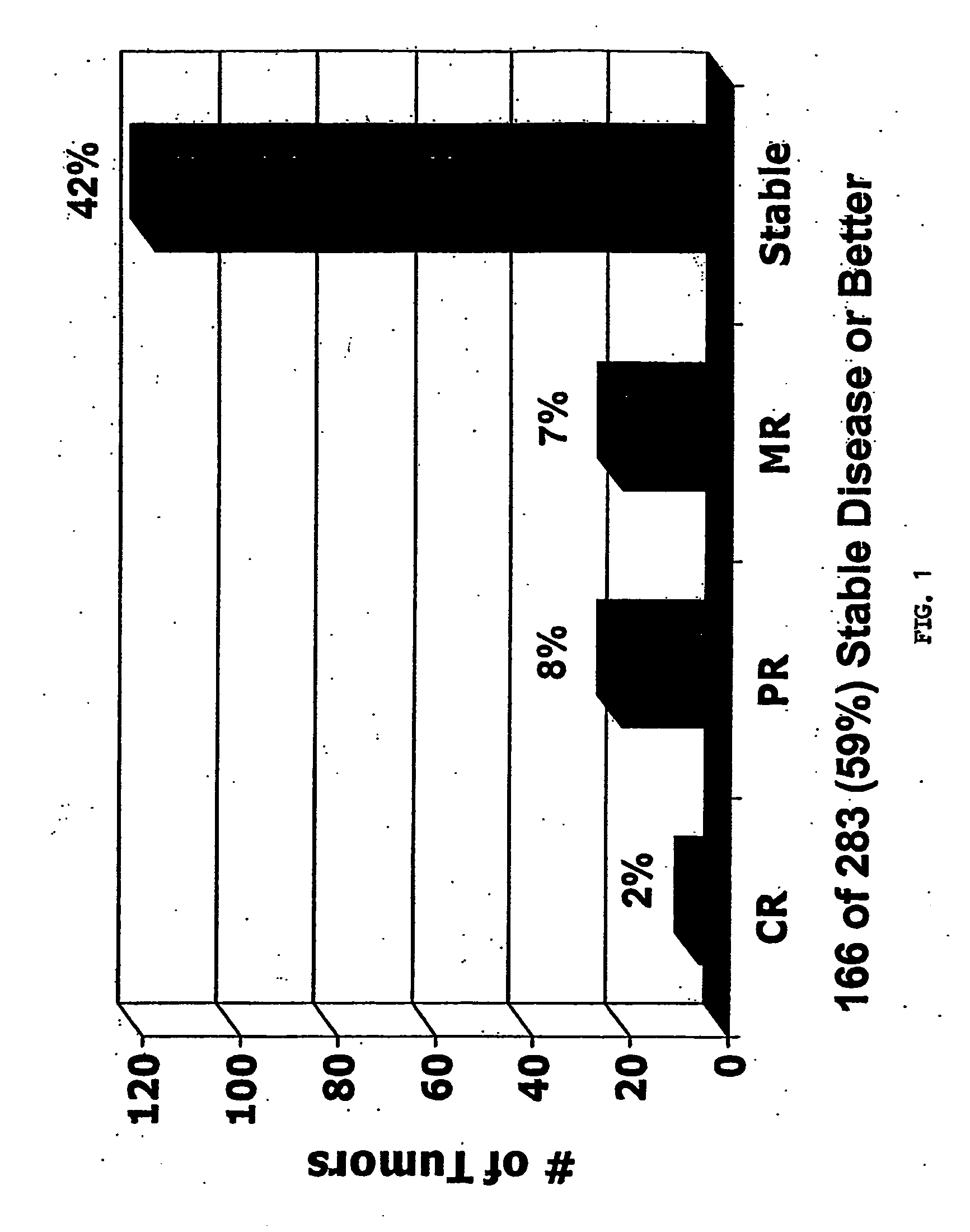
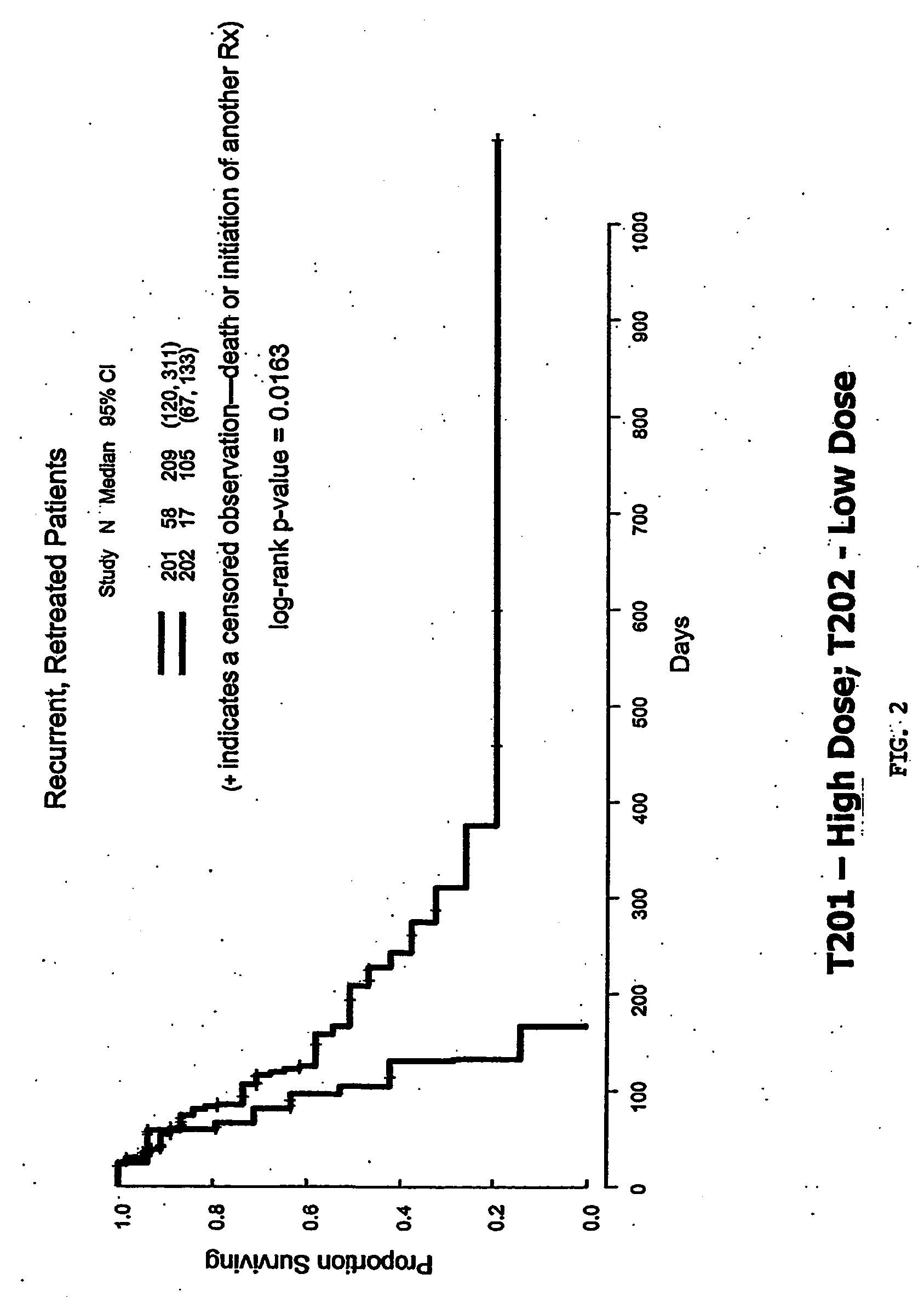
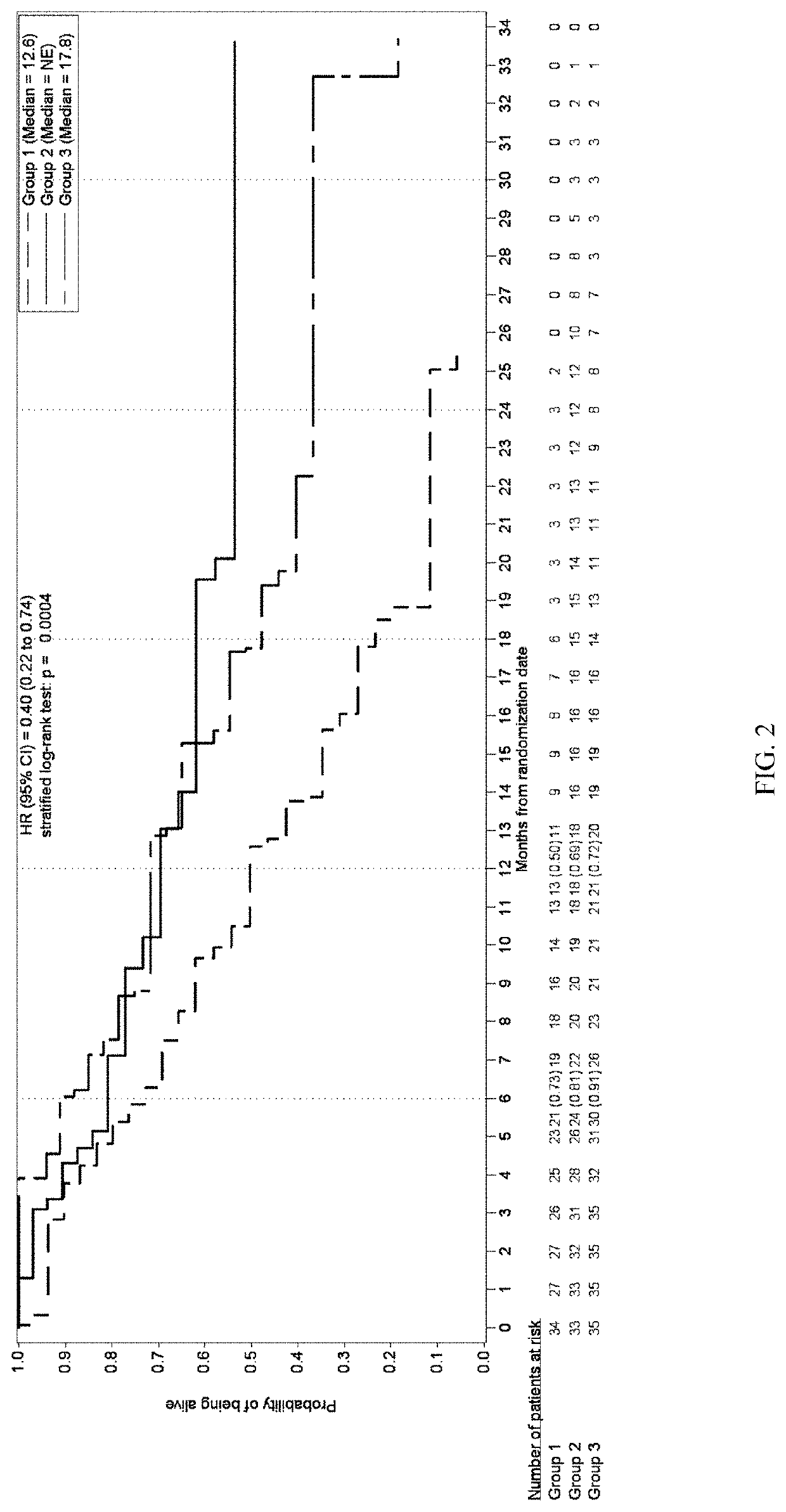
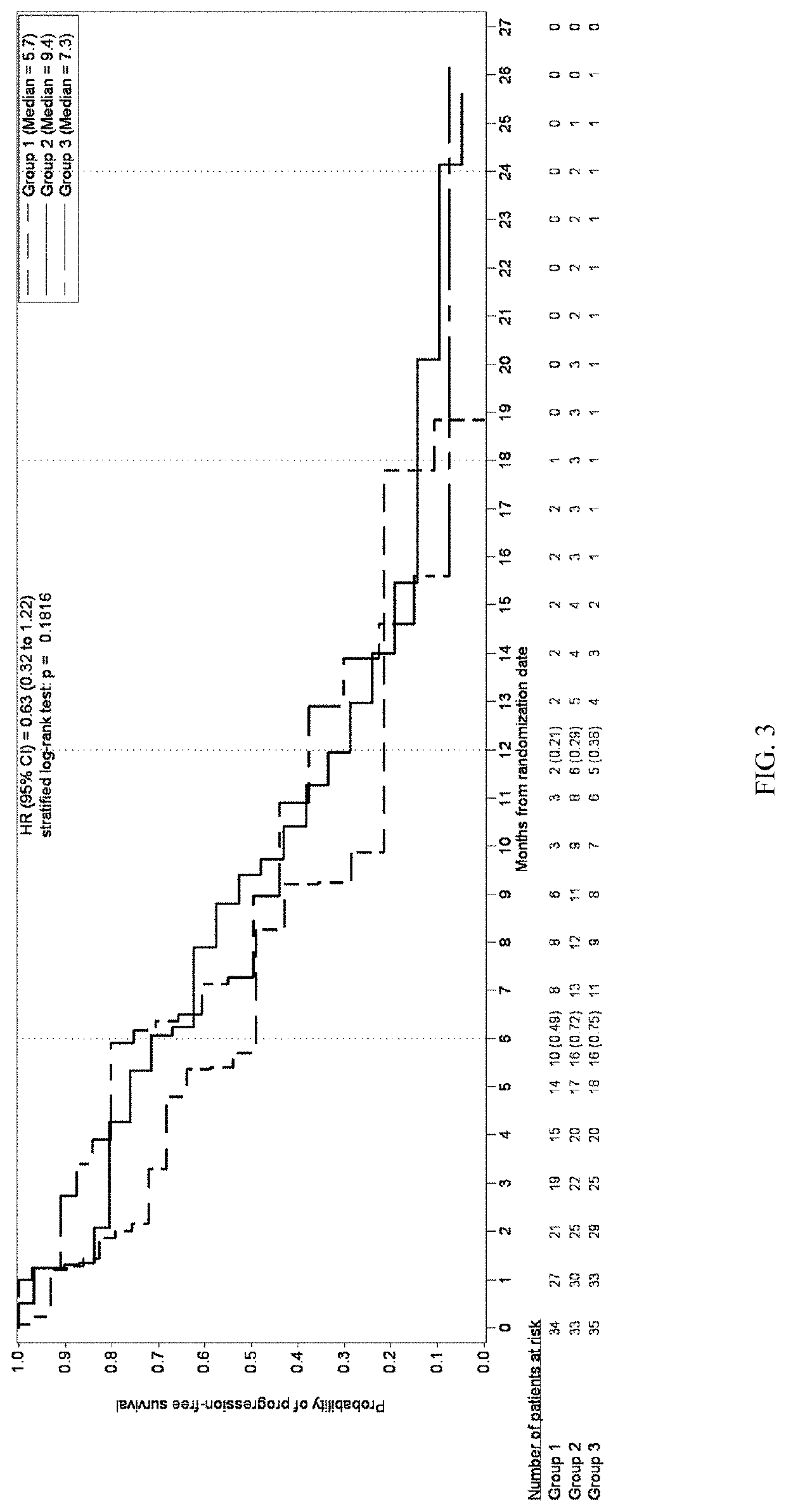
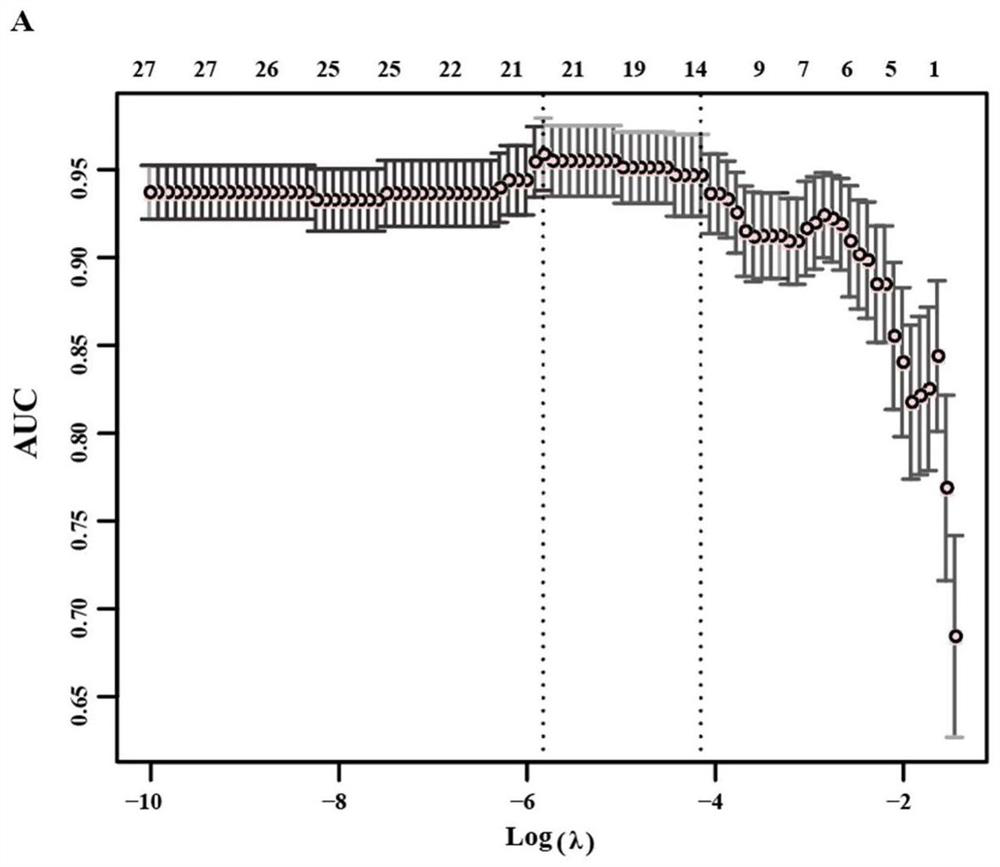
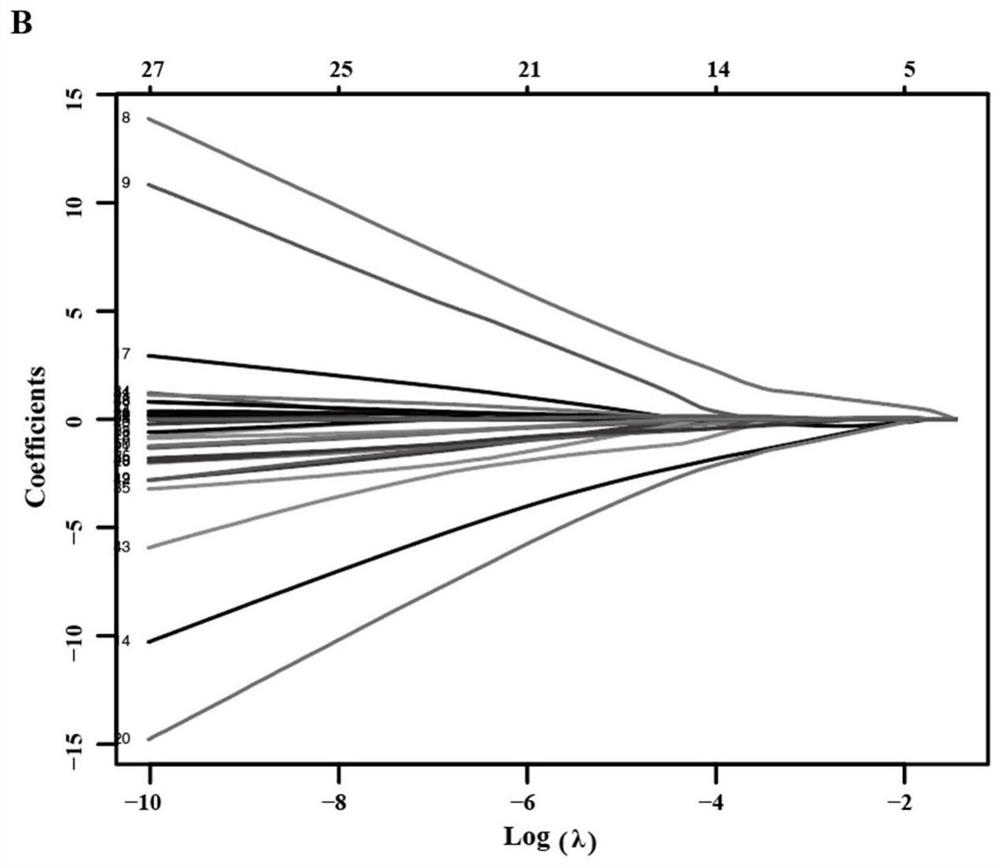
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
106 results about "Chemotherapy combinations" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chemotherapy regimen is a regimen for chemotherapy, defining the drugs to be used, their dosage, the frequency and duration of treatments, and other considerations. In modern oncology, many regimens combine several chemotherapy drugs in combination chemotherapy.
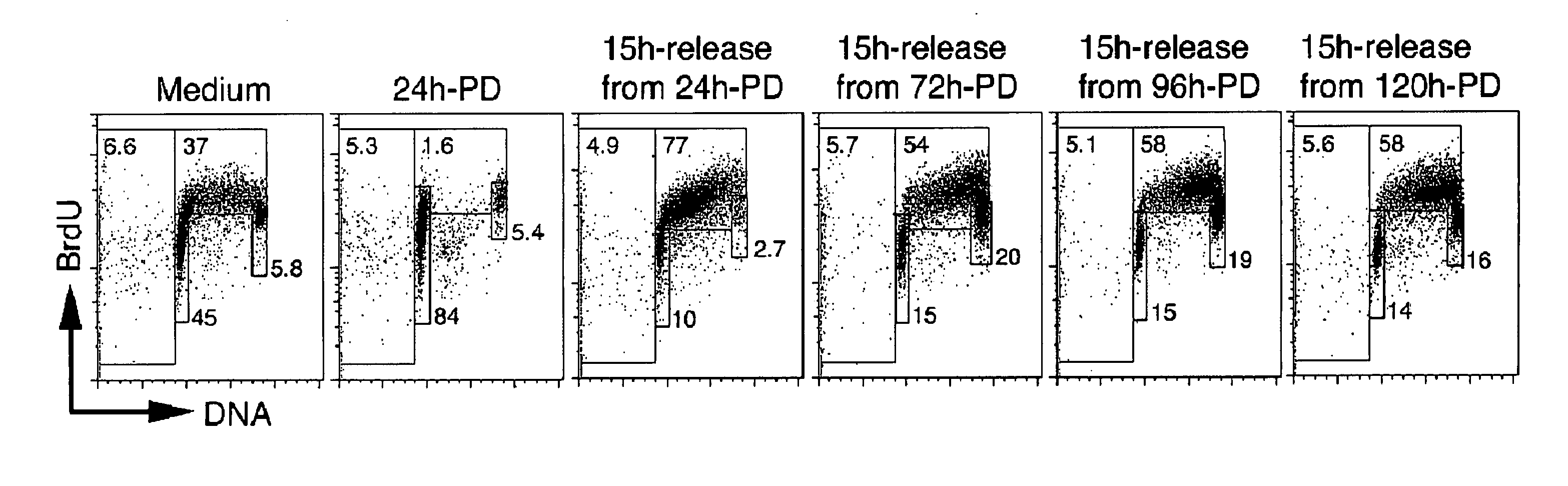
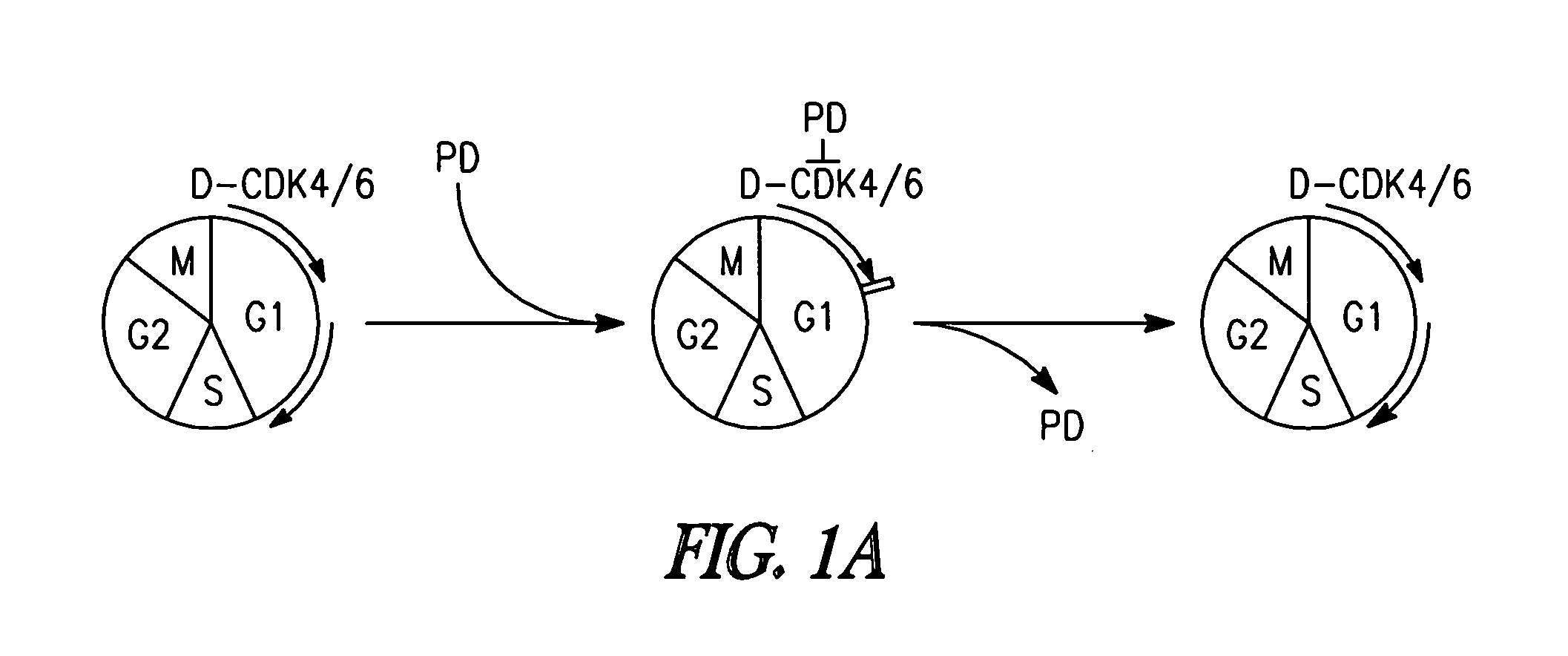
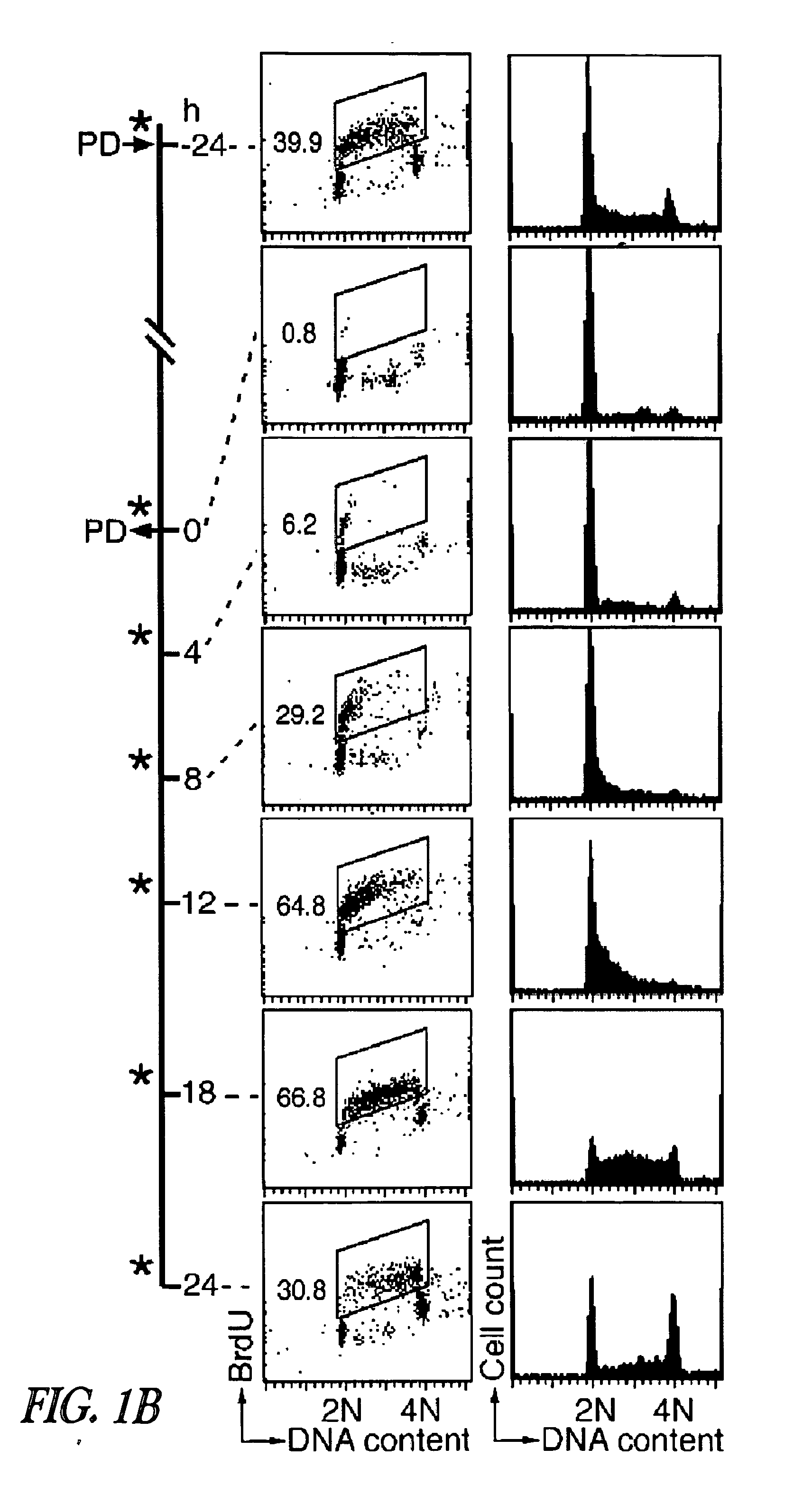
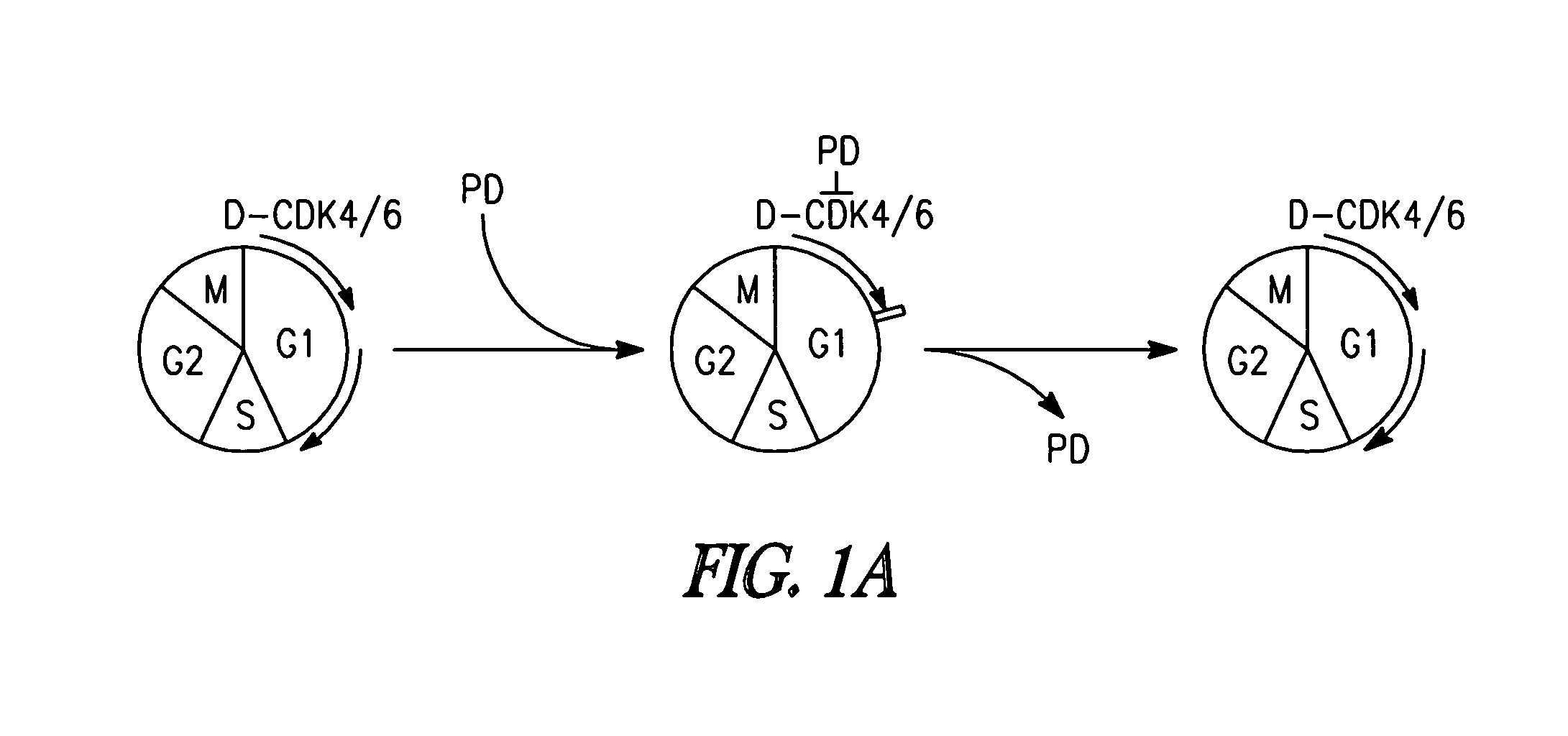
Targeting cdk4 and cdk6 in cancer therapy
ActiveUS20110009353A1Decreased cell growthGreat susceptibilityBiocideSugar derivativesCancer cellCell Cycle Inhibition
The invention involves methods of inhibiting the cancer cell cycle to make cancer cells more susceptible to chemotherapeutic agents. In particular, inhibition of CDK4 and / or CDK6 inhibits cell cycle progression in cancer cells. When combined with chemotherapy such cell cycle inhibition can effectively treat even aggressive cancer types that are drug-resistant and intractable to most chemotherapies.
Owner:CORNELL UNIVERSITY
Variant activin receptor polypeptides, alone or in combination with chemotherapy, and uses thereof
ActiveUS20140348827A1Size of tumor massOrganic active ingredientsPeptide/protein ingredientsMyostatinDisease
The present invention provides variant activin IIB soluble receptor polypeptides and proteins capable of binding and inhibiting the activities of activin A, myostatin, or GDF-11. The present invention also provides polynucleotides, vectors and host cells capable of producing the variant polypeptides and proteins. Compositions and methods for treating muscle-wasting and other diseases and disorders are also provided.
Owner:AMGEN INC
Methods and Compositions for the Prediction of Response to Trastuzumab Containing Chemotherapy Regimen in Malignant Neoplasia
InactiveUS20090280493A1Large capacityEasy to explainMicrobiological testing/measurementMaterial analysisDiseaseNeoplastic tissue
The invention relates to methods and compositions for the prediction, diagnosis, prognosis, prevention and treatment of neoplastic disease. Neoplastic disease is often caused by chromosomal rearrangements which lead to over- or underexpression of the rearranged genes. The invention discloses genes which are overexpressed in neoplastic tissue and are useful as diagnostic markers and targets for treatment. Methods are disclosed for predicting, diagnosing and prognosing as well as preventing and treating neoplastic disease.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Preparing method of hybridization metal organic framework compound for drug release
InactiveCN109985247ANo wasteEasy to makeOrganic active ingredientsEnergy modified materialsIn situ polymerizationPh control
The invention provides a preparing method of a hybridization metal organic framework compound for drug release. According to the preparing method, synthesis of a zeolite imidazole framework-8 (ZIF-8)and loading of polydopamine (PDA) and doxorubicin (DOX) are achieved through one-step-method in-situ polymerization, and the metal organic framework-polydopamine-doxorubicin hybridization metal organic framework compound is prepared. The PDA and the DOX are encapsulated in a ZIF-8 crystal, pH and near-infrared double-response drug release and photo-thermal therapy and chemotherapy combination areachieved, the preparing method has excellent pH control drug release performance, and the disease treatment efficiency is improved.
Owner:HENAN INST OF SCI & TECH
Preservation of immune response during chemotherapy regimens
ActiveUS20190321370A1Promote resultsProfound effectOrganic active ingredientsPeptide/protein ingredientsRegimenHalf-life
The addition of a selective, fast-acting, short half-life CDK 4 / 6 inhibitor in a very specific dosage regimen to the combination of chemotherapy with a checkpoint inhibitor provides superior results in the treatment of a tumor or cancer. The unexpected discovery is that the short pulsatile specifically-timed administration of a selective, fast-acting, short half-life CDK 4 / 6 inhibitor during administration of the chemotherapy portion of the triple combination therapy has a profound effect on the immune cells in the cancer microenvironment.
Owner:G1 THERAPEUTICS INC
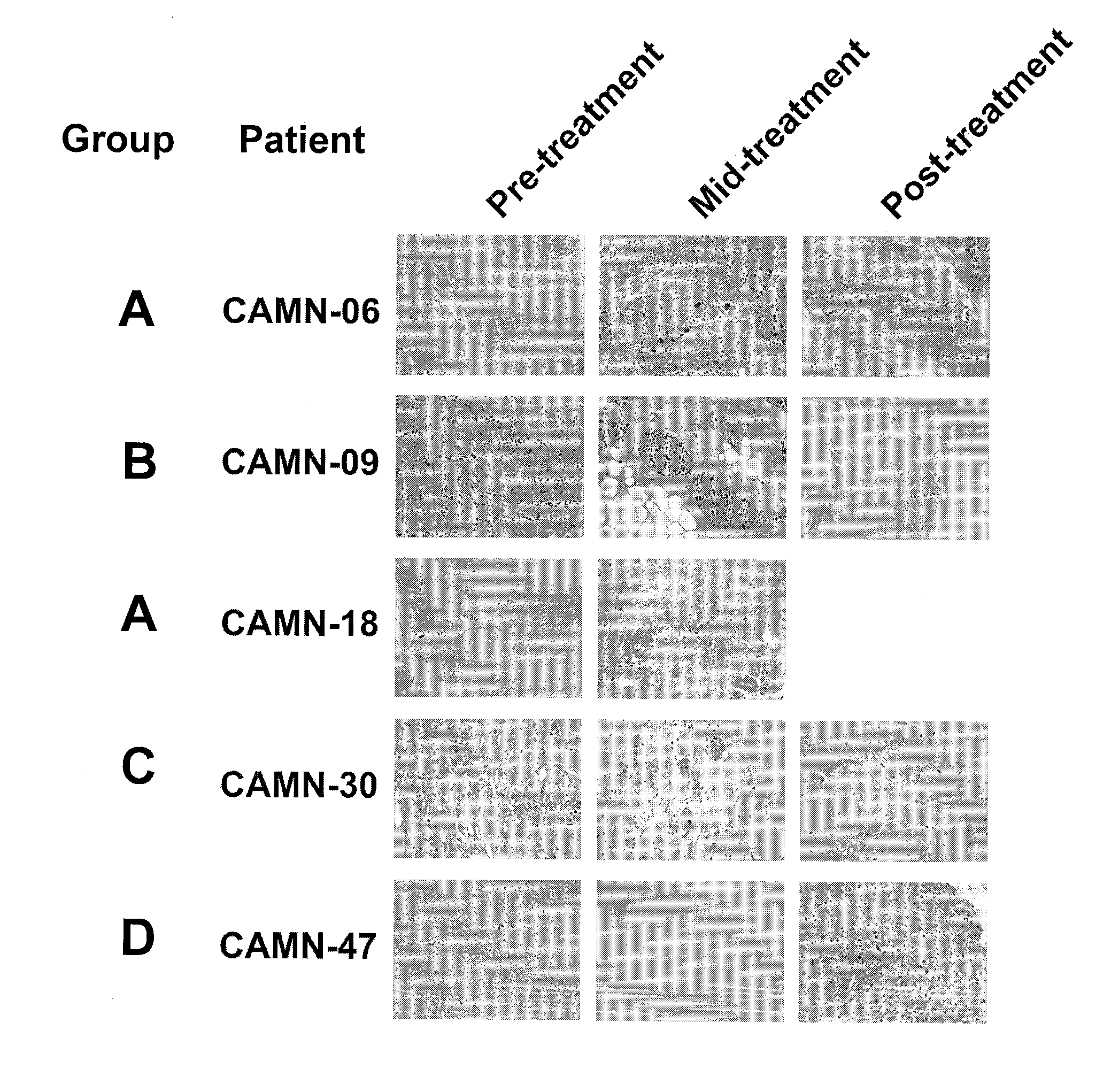
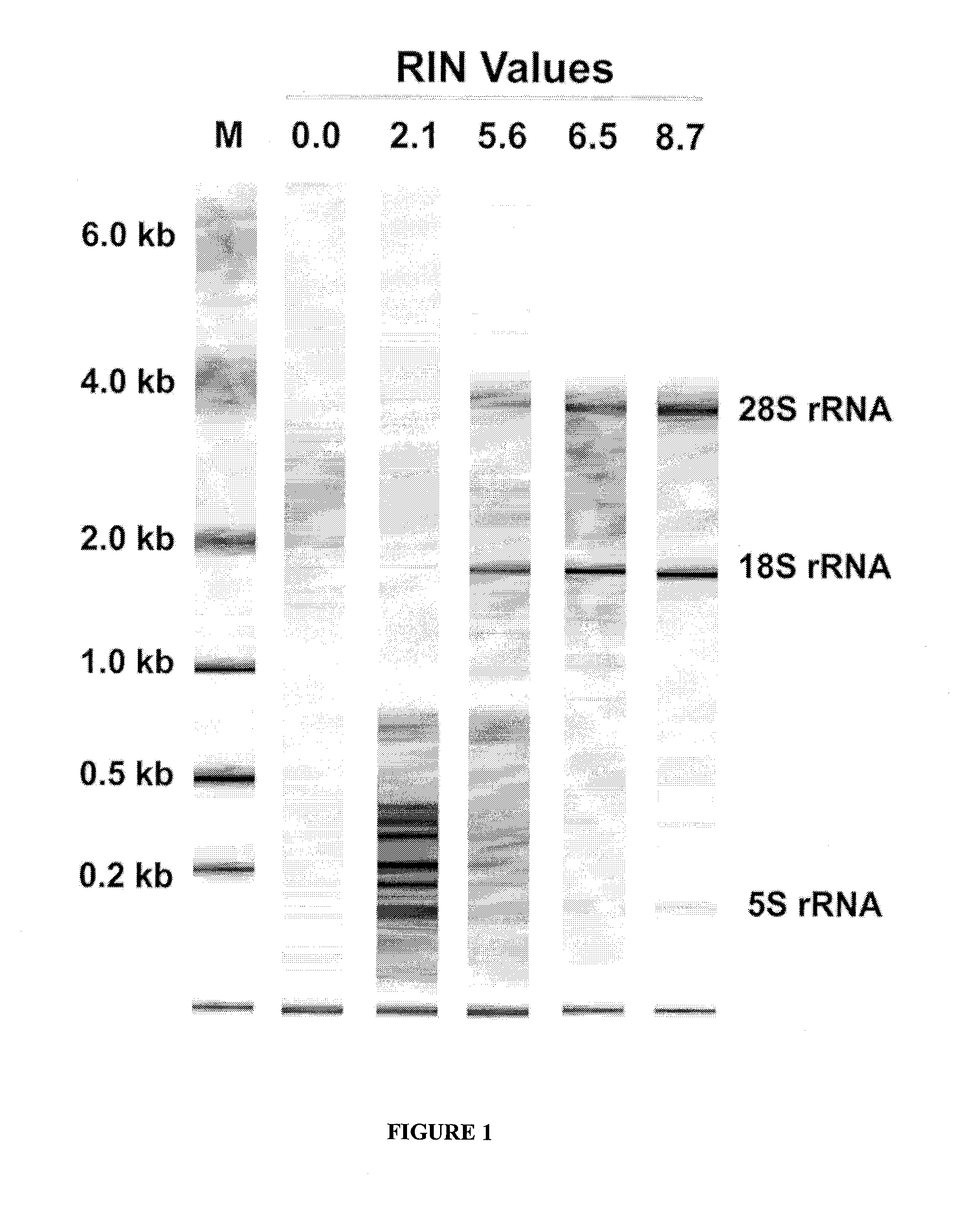
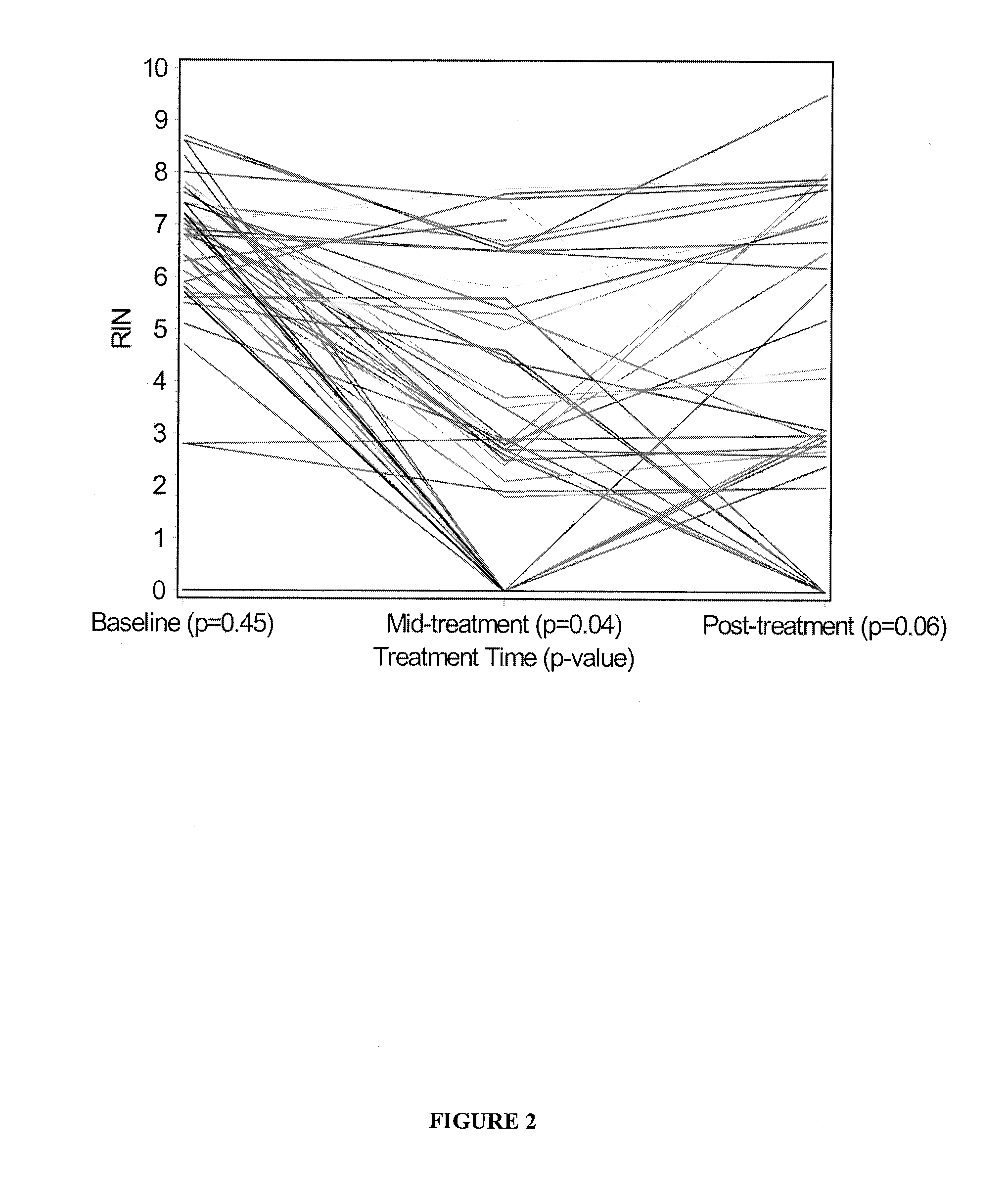
Method of using tumour RNA integrity to measure response to chemotherapy in cancer patients
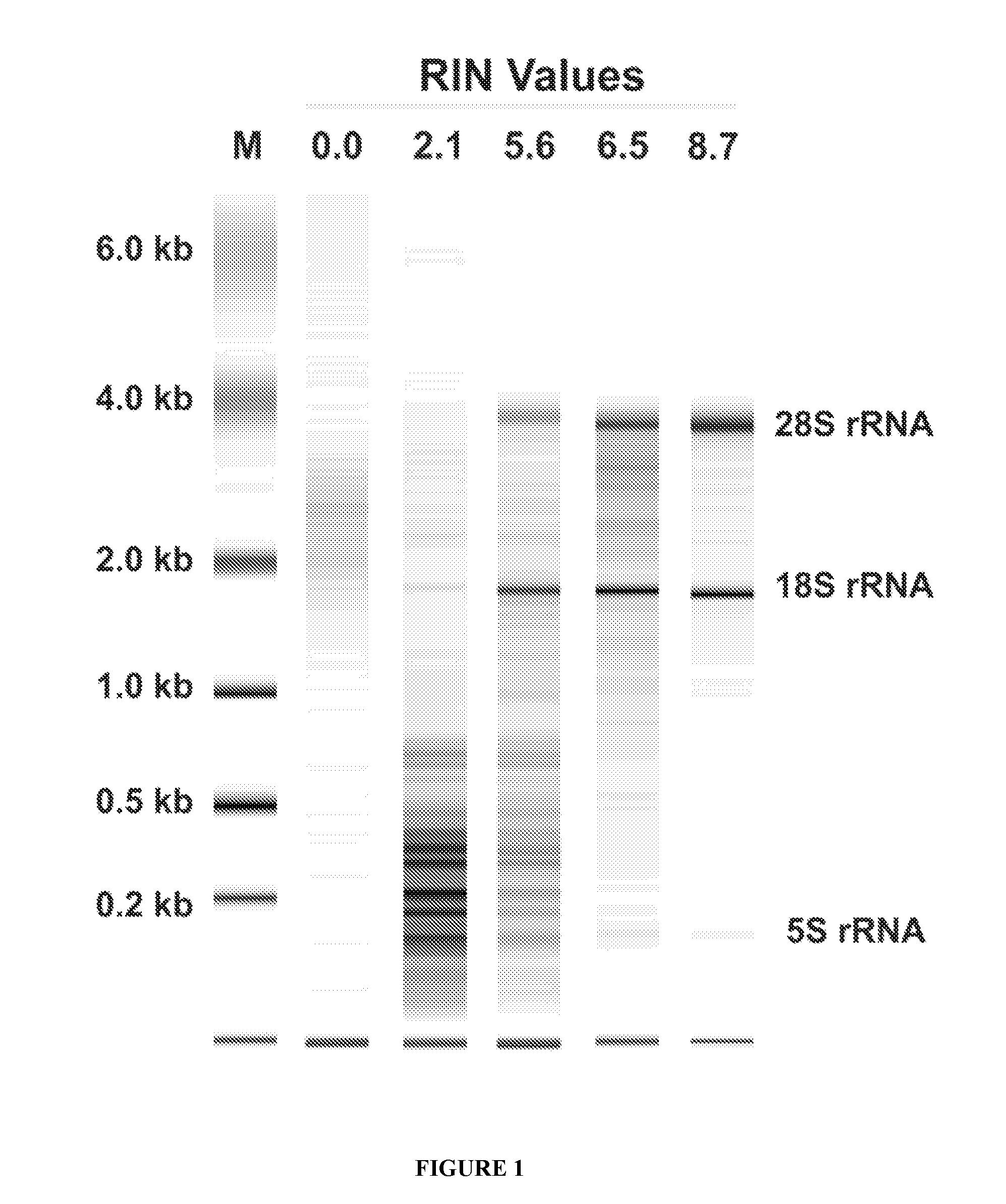
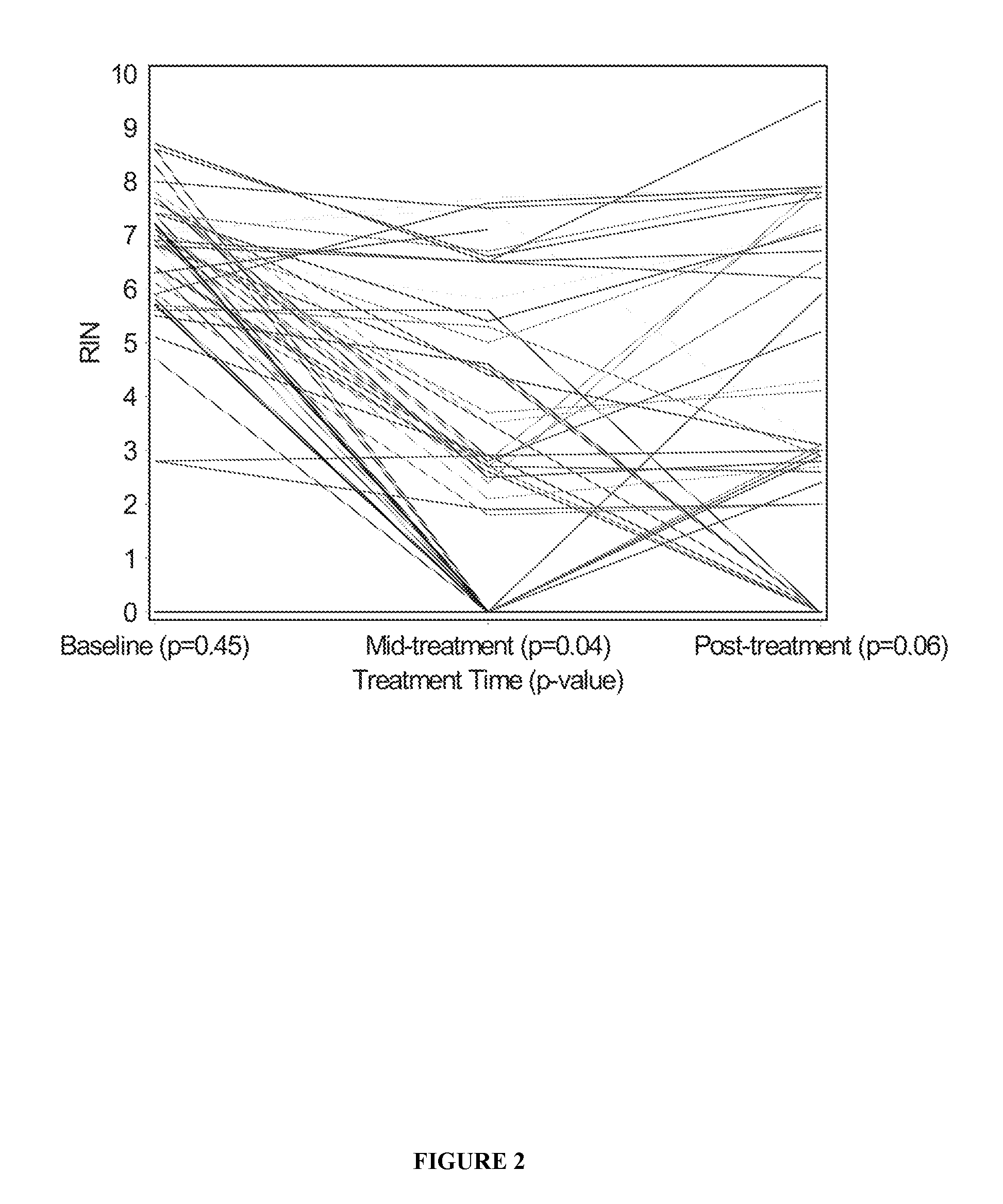
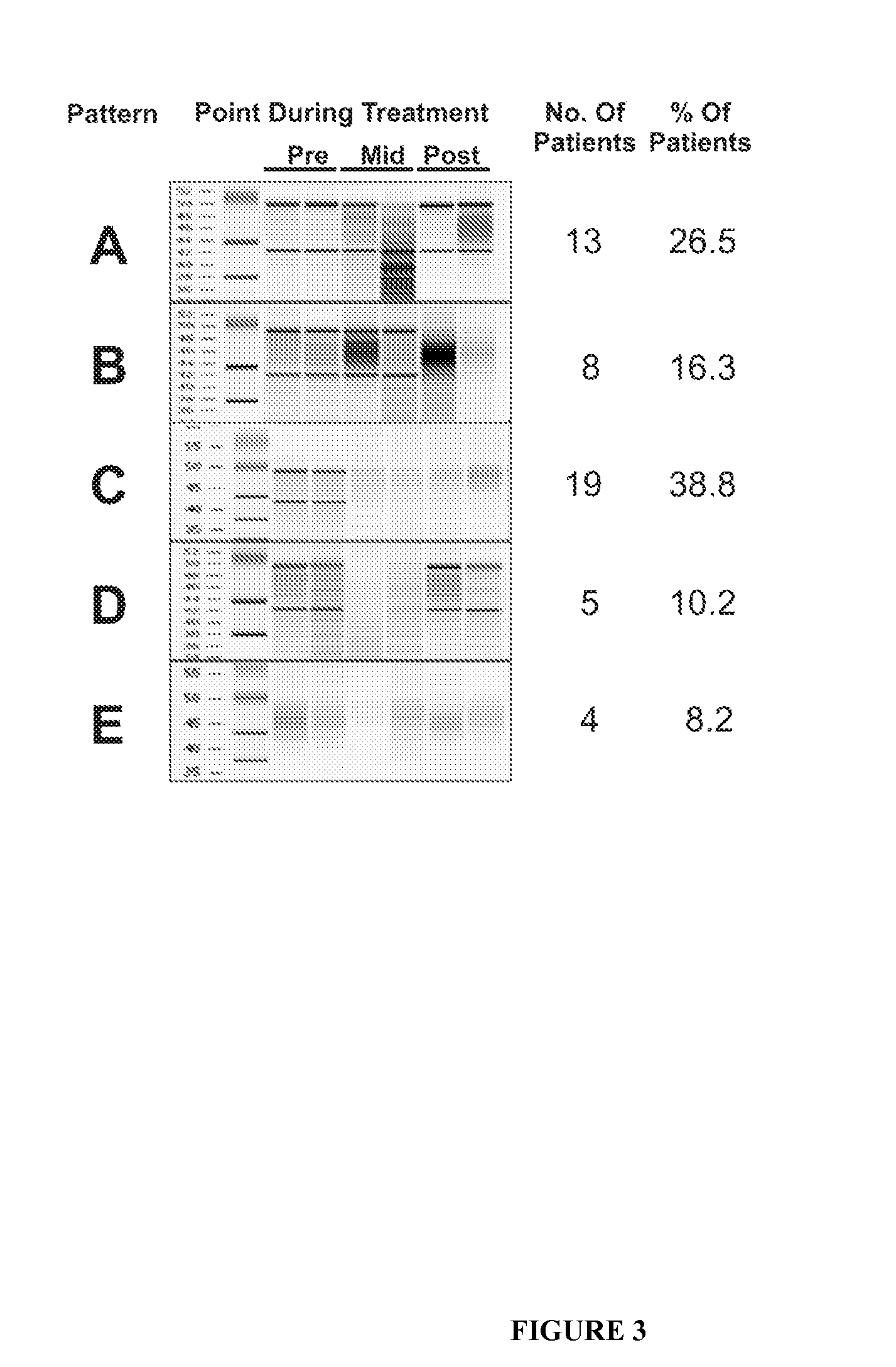
InactiveUS20100317001A1Easy accessRapid assessmentOrganic active ingredientsMicrobiological testing/measurementRegimenChemotherapy combinations
Cancerous tumours vary significantly in their response to chemotherapy agents. Currently, it is difficult to reliably assess the level of tumour responsiveness to a chemotherapy regimen during or post-administration. Biomarkers of tumour sensitivity to chemotherapy agents have hitherto been unknown. Such a biomarker would expedite identification of nonresponsive patients, who may then switch to other, possibly more effective regimens. The present invention provides a method for determining tumour responsiveness to a chemotherapy agent, wherein RNA is isolated from tumour cells of a patient before, during, and after chemotherapy. The quality of the RNA can be determined by capillary electrophoresis and assignment of an RNA integrity number (RIN). RIN values during and / or after chemotherapy are inversely proportionate to the level of tumour responsiveness. The tumour RIN is an easily accessed biomarker of tumour responsiveness to chemotherapy. The tumour RIN may also be used to assess the efficacy of a chemotherapy regimen.
Owner:LAURENTIAN UNIV OF SUDBURY
Targeting CDK4 and CDK6 in cancer therapy
Owner:CORNELL UNIVERSITY
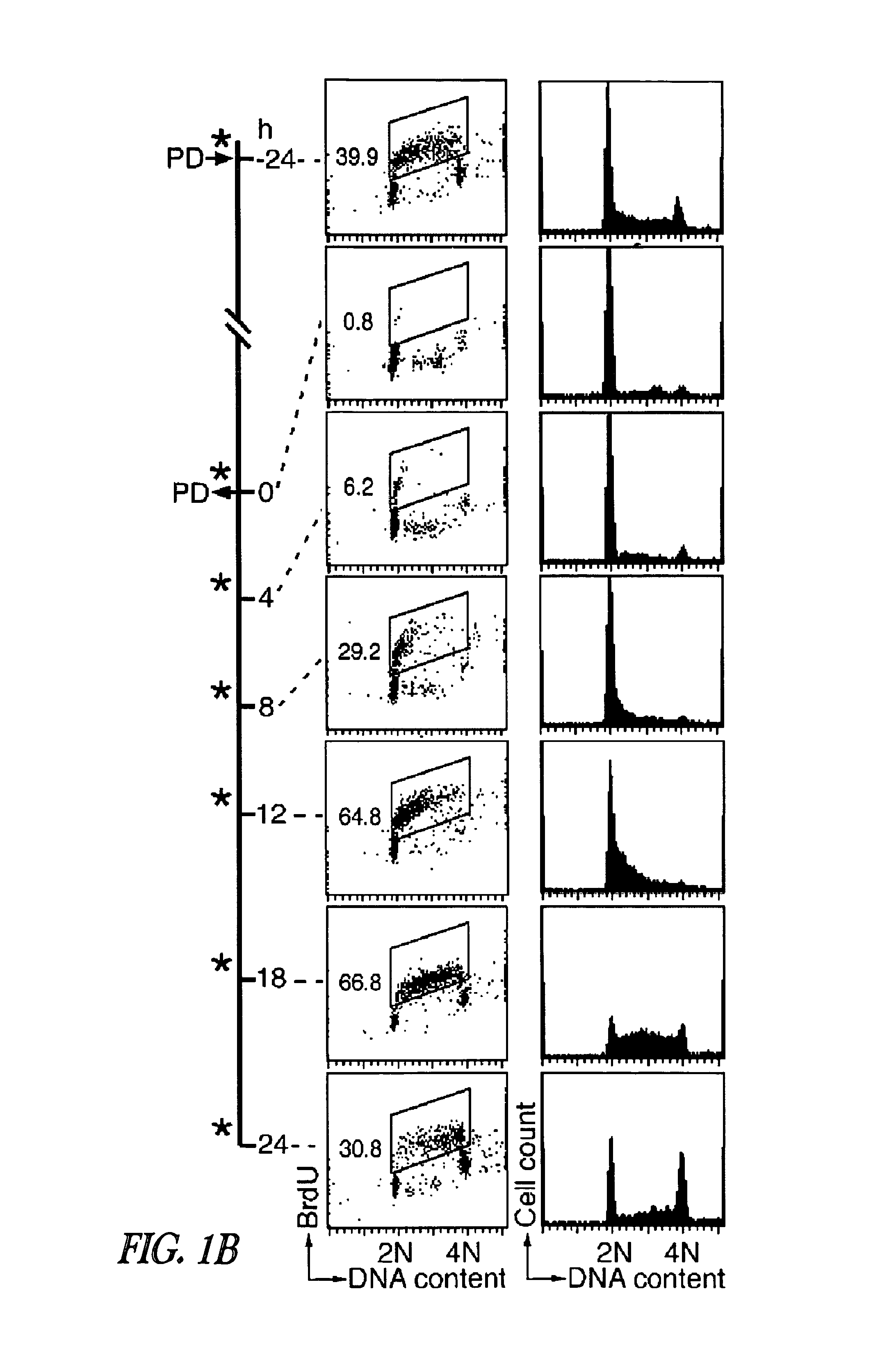
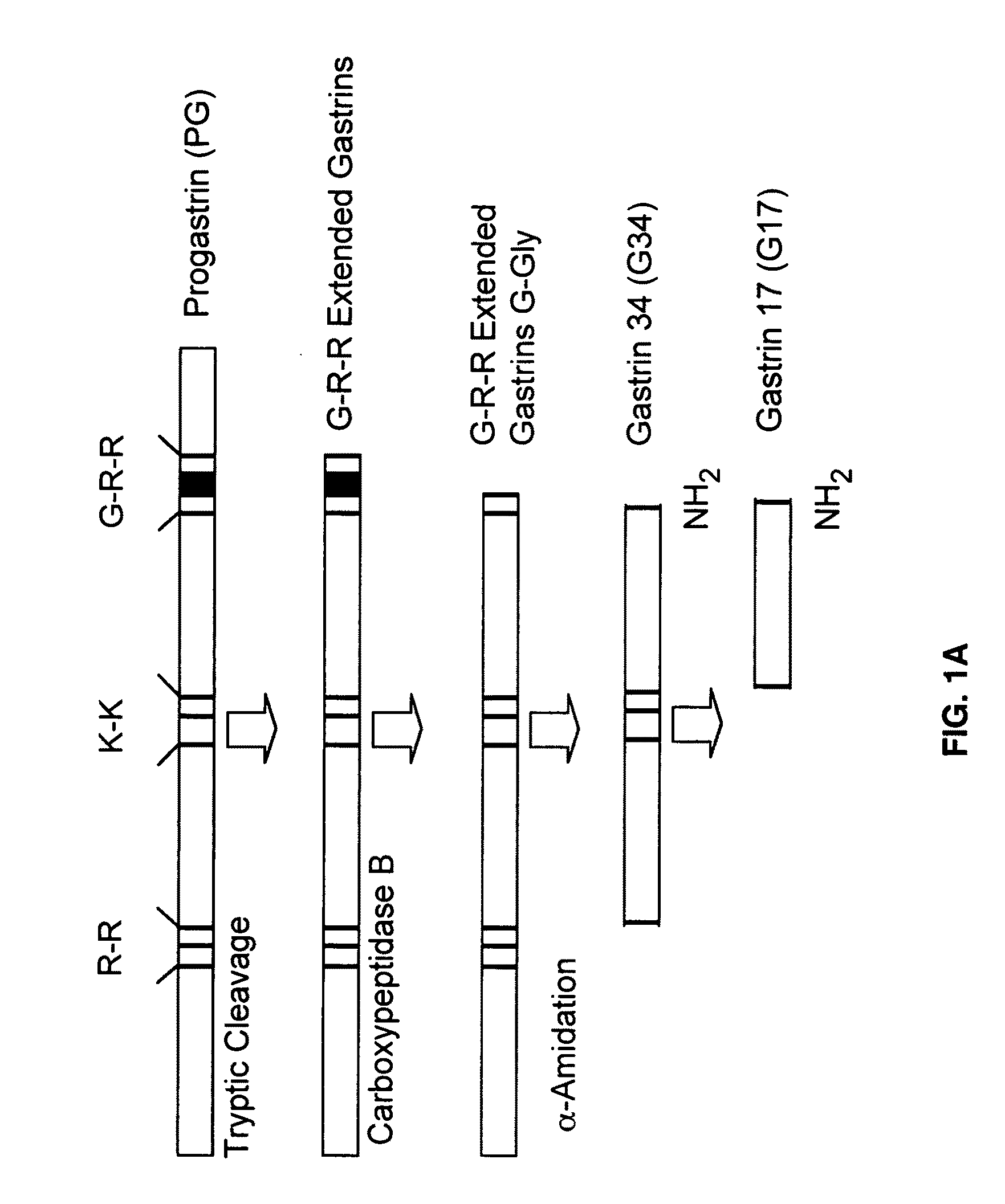
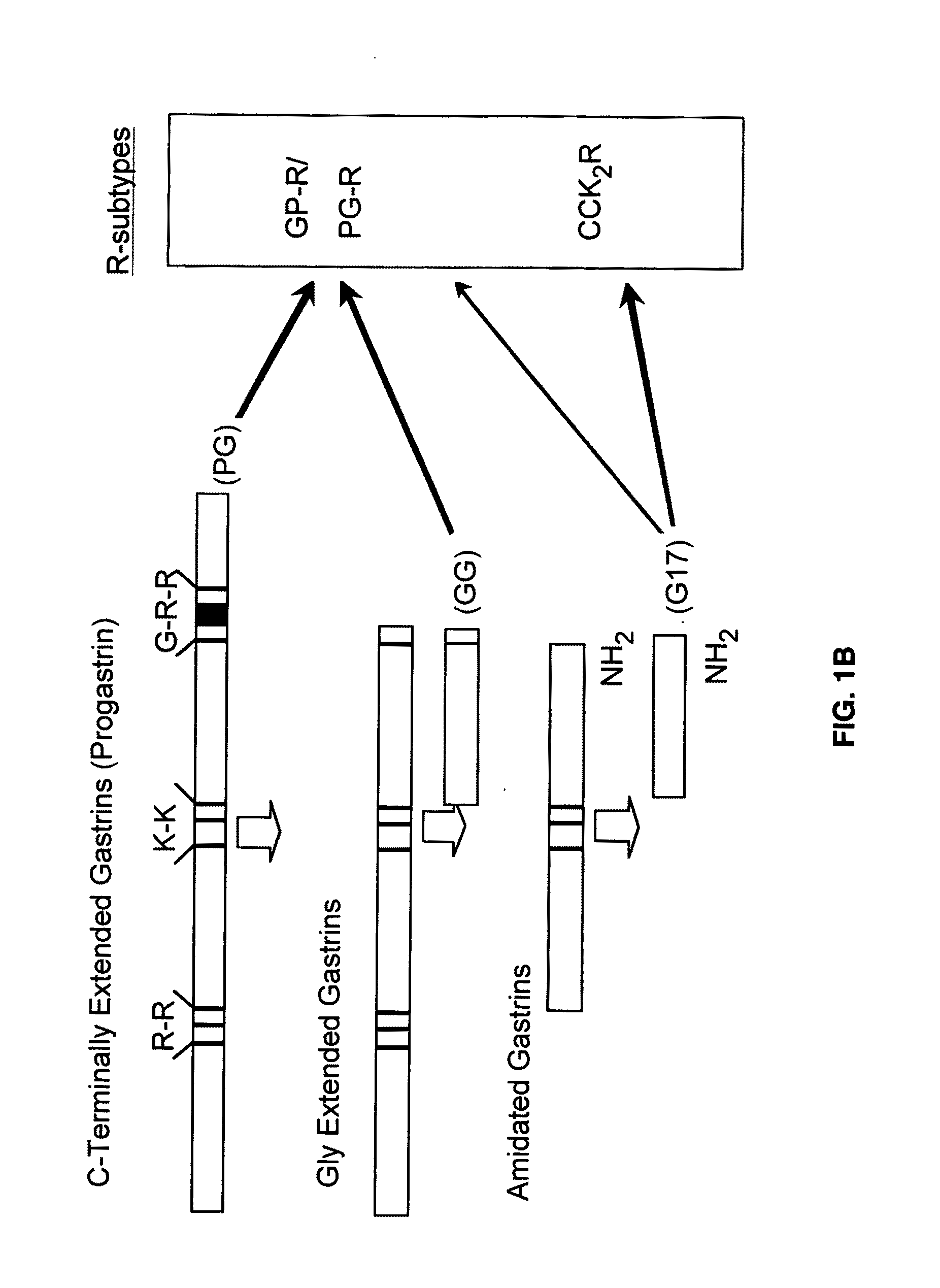
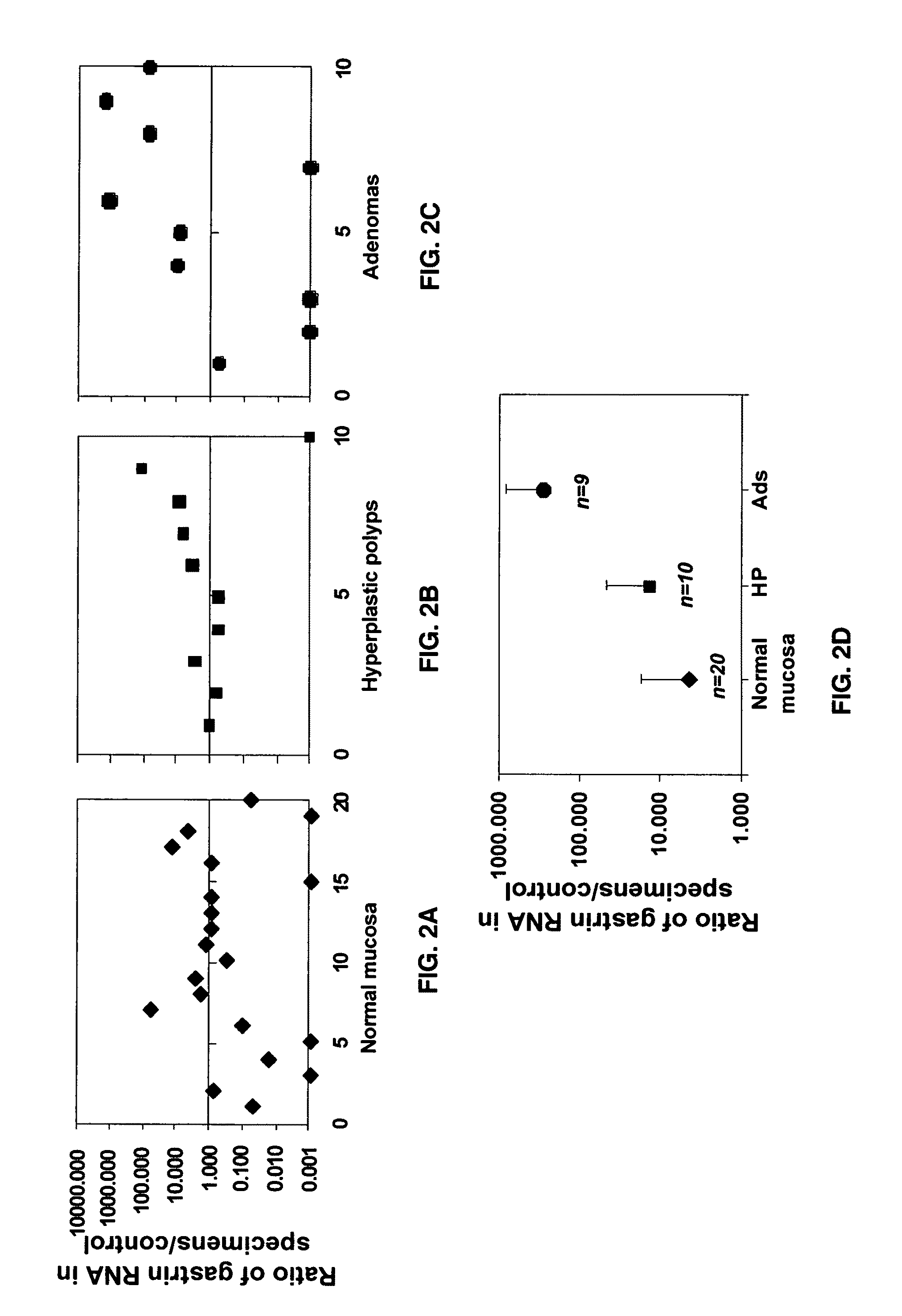
Immunogenic compositions comprising progastrin and uses thereof
ActiveUS20100291193A1Inhibits gastrin-induced proliferationTreat cancerPowder deliveryOrganic active ingredientsChemotherapy combinationsOncology
The present invention is drawn to immunotherapeutic methods to treat tumors / cancers that produce progastrin ectopically or are dependent on progastrin for their growth. Disclosed herein are immunogenic compositions comprising agents that target progastrin, agents that target the progastrin receptor, annexin II, or both. Such a composition may be administered in combination with chemotherapy or to an individual who had been previously subjected to chemotherapy or radiation therapy. The cancers that may be treated using such a composition may include but are not limited to colon cancer, breast cancer, lung cancer or pancreatic cancer.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Coumarin-modified BODIPY (boron dipyrromethene) derivative as well as preparation and application thereof
ActiveCN108440586AGood anticancer effectSingle structureEnergy modified materialsGroup 3/13 element organic compoundsResearch ObjectSynthesis methods
The invention discloses a coumarin-modified BODIPY (boron dipyrromethene) derivative as well as preparation and an application thereof. Chemotherapy drug coumarin covalent bonds with clear curative effects are linked to a BODIPY photosensitizer for PDT (photon dynamic treatment), so that a PDT and chemotherapy combined anti-tumor drug, namely, the coumarin-modified BODIPY derivative, is obtained.Meanwhile, the coumarin-modified BODIPY derivative is taken as a research object, a human gastric carcinoma cell BGC-823 is taken as a tested cell line, the in-vitro anti-cancer activity research is carried out, an efficient anti-cancer prodrug is screened out, and a foundation is laid for PDT and chemotherapy combination of the coumarin-modified BODIPY derivative. The synthesis method of the compound is simpler, raw materials are easy to obtain, the cost is low, few side reactions exist, the yield is higher, purification is easy, and industrial production is facilitated.
Owner:FUZHOU UNIV
Cancer precise chemotherapy typing marker screening method, chemotherapy sensitivity molecular typing method and application
PendingCN111933211AA large amountSolve pain pointsMicrobiological testing/measurementBiostatisticsMedicineChemotherapy combinations
The invention relates to a cancer precise chemotherapy typing marker screening method, a chemotherapy sensitivity molecular typing method and application. According to the method, a typing marker is obtained from a multi-center and large sample queue to discover molecular typing, and a classifier is constructed by using the typing marker or differential protein obtained based on a typing label asa selection characteristic; when the classifier carries out classification application, expression profile data of a collected sample is input; and after expression spectrum data preprocessing, classifier feature matching and logarithm conversion are performed, prediction is performed by the classifier constructed through a machine learning classification algorithm or an artificial intelligence model; and finally an output label of a chemotherapy sensitive group or a chemotherapy insensitive group is obtained, and molecular typing of accurate chemotherapy is performed, so that the problem of pain spots in the field of tumor medical treatment is solved, wherein the problems comprise: accurately judging whether chemotherapy is beneficial to people, providing first-line medication regimen optimal combination recommendation and providing chemotherapy regimen optimal period recommendation.
Owner:北京谷海天目生物医学科技有限公司
Kit for detecting mRNA expression level of PML-RARa fusion gene
ActiveCN102925558AStrong specificityImprove accuracyMicrobiological testing/measurementReference genesFluorescence
The invention relates to a kit for the mRNA expression level of a PML-RARa fusion gene, and belongs to the field of biotechnology. The kit comprises a PML-RARa L fusion gene system, a PML-RARa V fusion gene system or a PML-RARa s fusion gene system and a reference gene system ABL, wherein each system comprises an upstream primer and a downstream primer and Taqman fluorescence probe. The PML-RARa fusion protein, as a variant retinoic acid receptor, has different DNA properties with a wild type RARa protein and is an intrinsic and effective repressor for retinoic acid (RA) signal which is the transcription factor of an intrinsic RARa target gene. Therefore, when the fluorescence quantitative polymerase chain reaction (PCR) method is used for detecting the mRNA expression level of the PML-RARa fusion gene, the detection result is more specific and sensible. The kit provides a novel quick and simple genetic diagnosis technology for predicting the prognosis of acute promyelocytic leukemia and determining chemotherapy regimens.
Owner:广州市宝创生物技术有限公司
Multiple-gene detecting kit related to antitumor drugs
ActiveCN103882138AEasy to analyzeReliable analysisMicrobiological testing/measurementDPYDReference genes
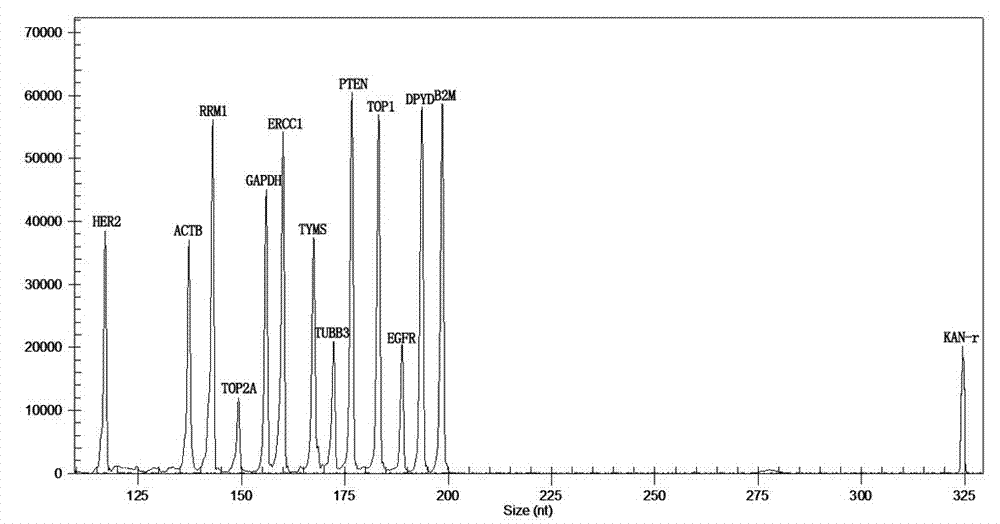
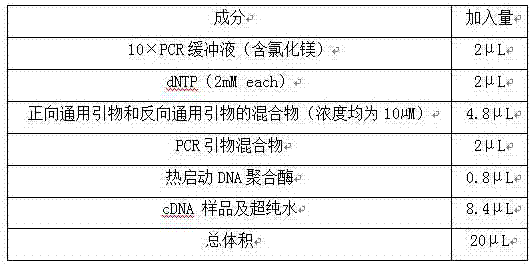
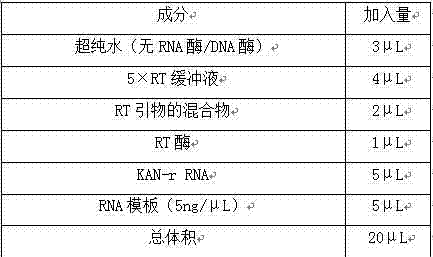
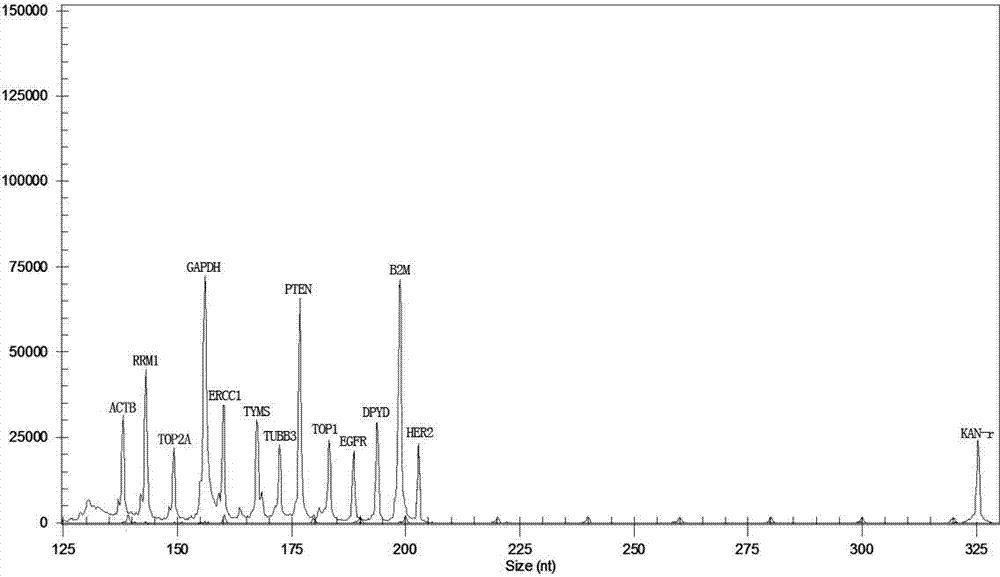
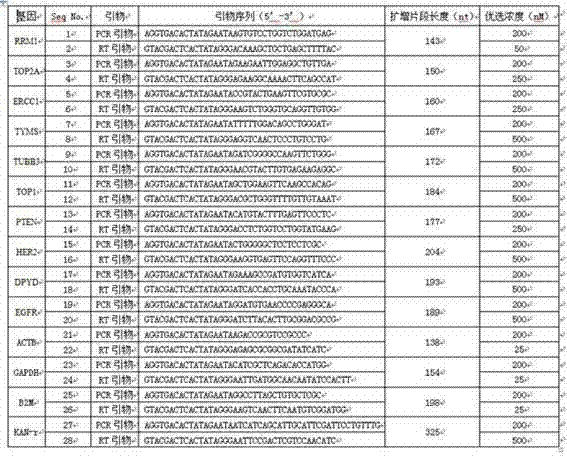
The invention relates to a multiple-gene detecting kit related to antitumor drugs. The multiple-gene detecting kit comprises a mixture of RT (reverse transcription) primers and a mixture of PCR (polymerase chain reaction) amplification primers, wherein each RT primer and each PCR amplification primer based on genetic groups, reference genes and a reaction internal label are respectively contained in each of the mixture of the RT primers and the mixture of the PCR amplification primers; the multiple-gene detecting kit is characterized in that the genetic groups comprise PTEN, EGFR, DPYD, HER2, RRM1, ERCC1, TUBB3, TOP1, TYMS and TOP2A; the reference genes comprise ACTB, GAPDH and B2M; the reaction internal label is KAN-rRNA (ribosomal RNA). The multiple-gene detecting kit related to antitumor drugs disclosed by the invention can be used for systemically detecting the expression level of a plurality of genes closely related to the antitumor drugs by one step, is simple and convenient to use, good in accuracy and high in detecting efficiency, and can be used for guiding chemotherapy drugs and selecting chemotherapy regimens very well.
Owner:南昌市赛尔医药科技有限公司
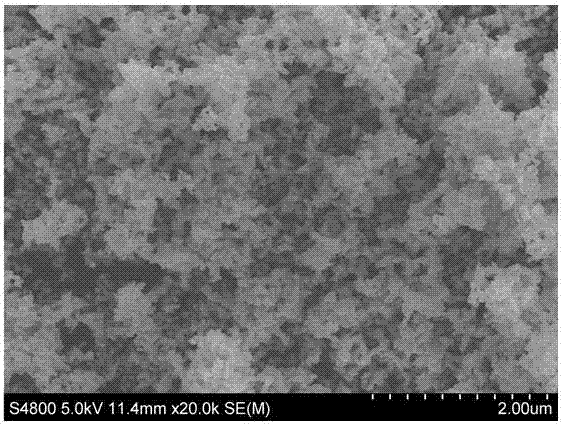
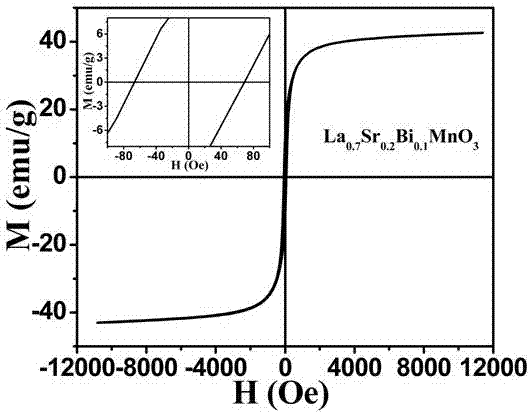
Magnetic nanoparticle, preparation method and application of magnetic nanoparticle
InactiveCN107452457AImprove performanceHigh purityEnergy modified materialsPharmaceutical non-active ingredientsMagnetic heatingCancer cell
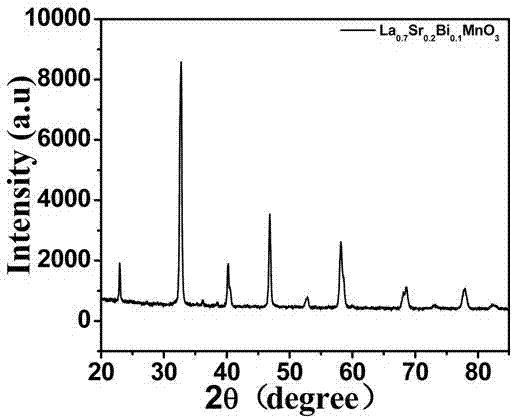
The invention belongs to the field of biomedical materials, and particularly relates to a La0.7Sr0.2Bi0.1MnO3 magnetic nanoparticle, a preparation method and an application of the magnetic nanoparticle. The sodium hyaluronate modified La0.7Sr0.2Bi0.1MnO3 magnetic nanoparticle serves as a magnetic thermal therapy medium, a magnetic fluid takes the La0.7Sr0.2Bi0.1MnO3 magnetic nanoparticle as a core, and the surface of the magnetic nanoparticle is modified by the sodium hyaluronate to form stable magnetic hydrosol. The magnetic thermal therapy medium has good biocompatibility and chemical stability and high magnetic heating performance, can effectively kill cancer cells and has a wide application prospect in magnetic thermal therapy and thermo-chemotherapy combination and anti-tumor diagnosis and treatment integration.
Owner:LANZHOU UNIVERSITY
Chemotherapy regimen selection
The present invention provides, inter alia, kits for selecting a chemotherapy regimen for a subject. The kits comprise one or more components for detecting the expression of at least one gene from the group of SLC12A7, GZMB, TAF6L, NFIB, METRN, ROPN1B, TTK, CCND1, PTTG1, H2AFZ, WDR45L, DEK, MCM2, USP1, CDT1, TMEM97, RER1, MCM6, LZTFL1, C11orf17, CCL5, XCL1, XCL2, MELK, CTSL2, TPX2, AURKA, CDKN2C, BRP44, PNP, SMC4, NR4A2, C3orf37, MTPAP, CDC25B, ABCF1, MTAP, SNAPC3, RANBP9, COIL, FAM86B1, ITGA6, S100P, RANBP1, PRSS16, SMARCA2, STK24, TSPYL5, SRI, LRP12, CENPF, TUBD1, KIAA1324, DBF4, CCNA2, DLGAP5, FHL1, SIRT3, GTSE1, PCNA, CCNE2, CHD3, CAP1, GPM6B, GUSBP3, GNAI3, LMO4, PSRC1, USP1, STK38, BAT2L1, PMP22, NME5, CENPA, BANK1, and derivatives thereof. Methods for selecting a chemotherapy regimen for a subject are also provided.
Owner:INNOMEDICINE LLC
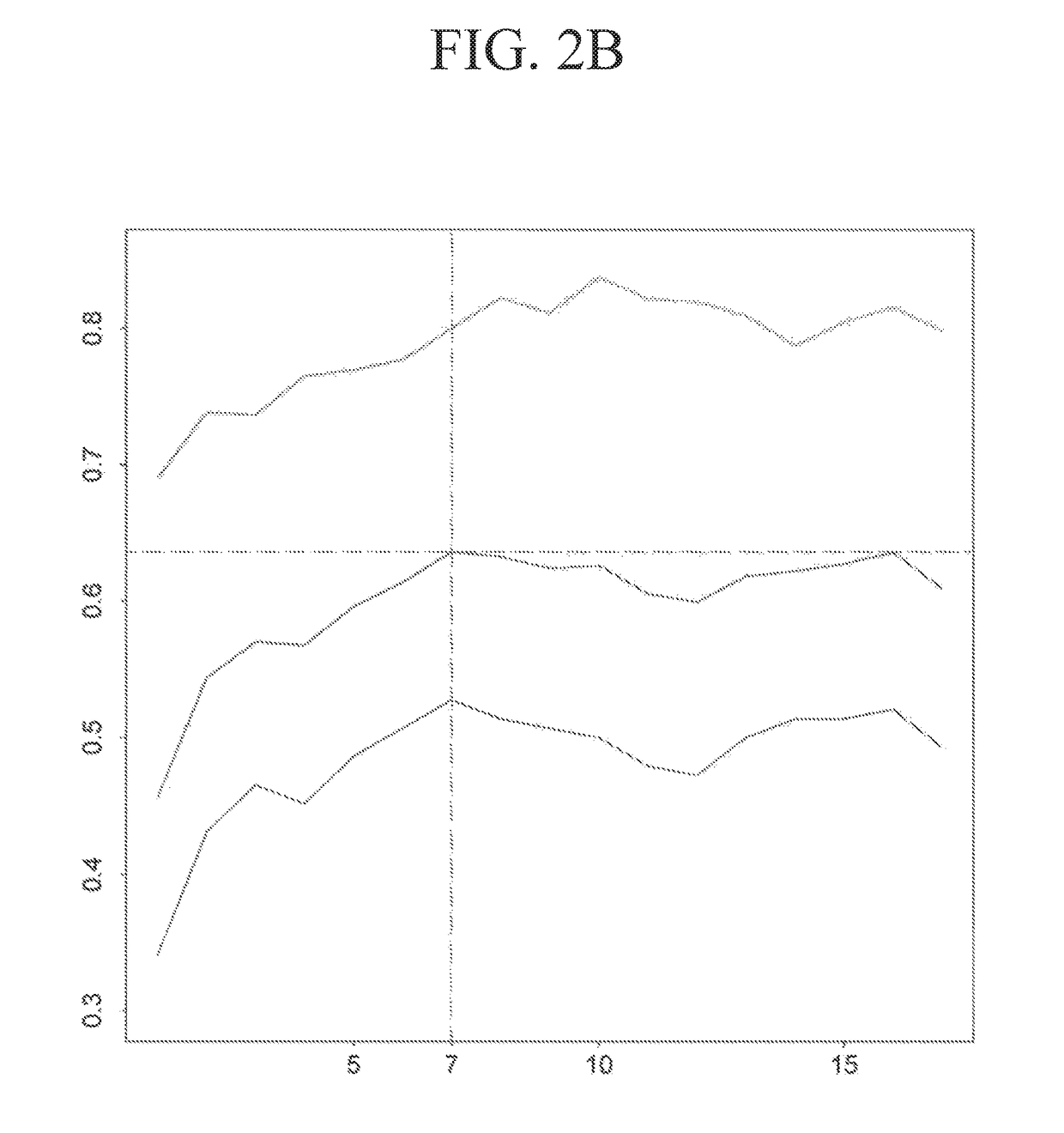
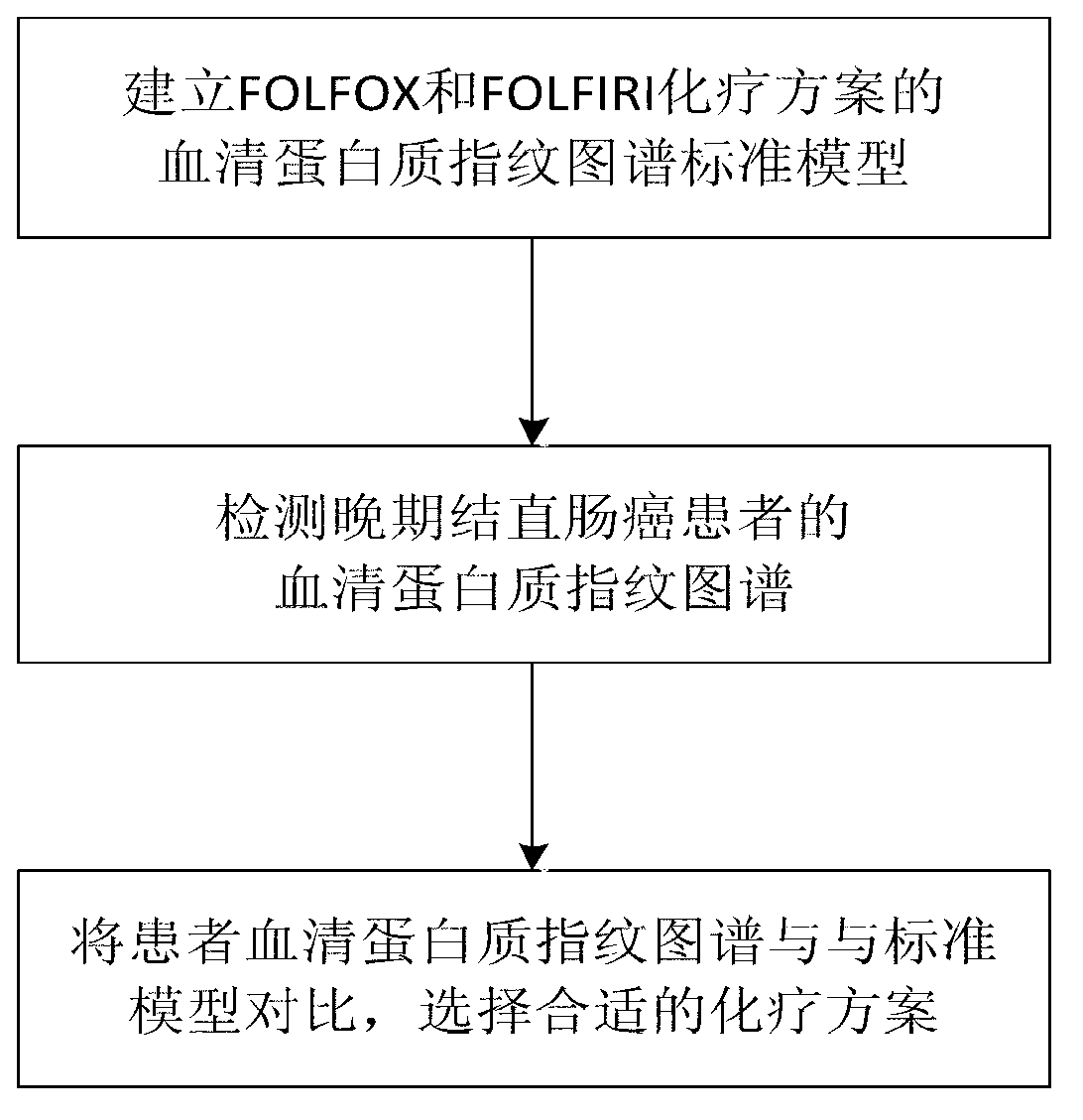
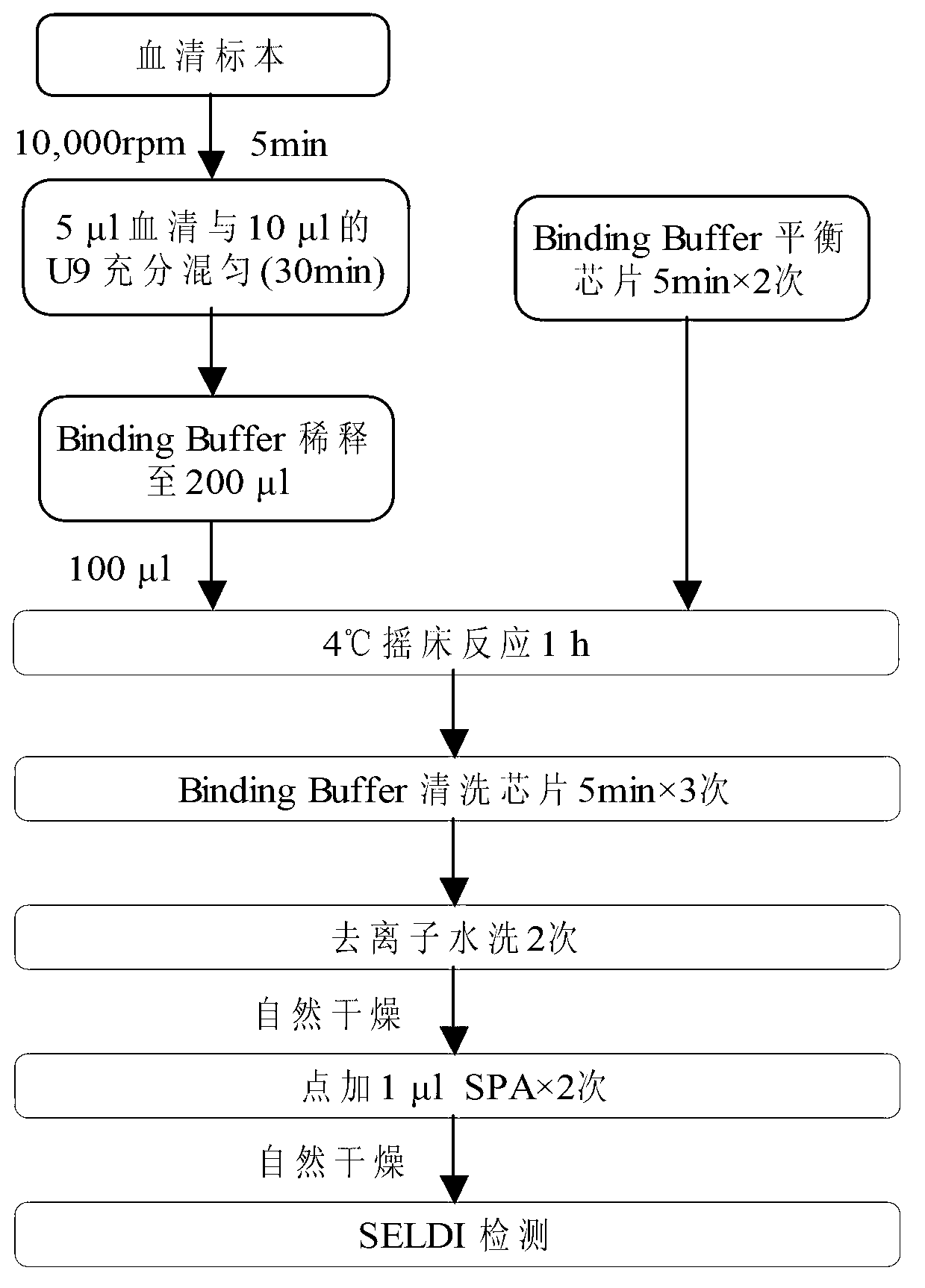
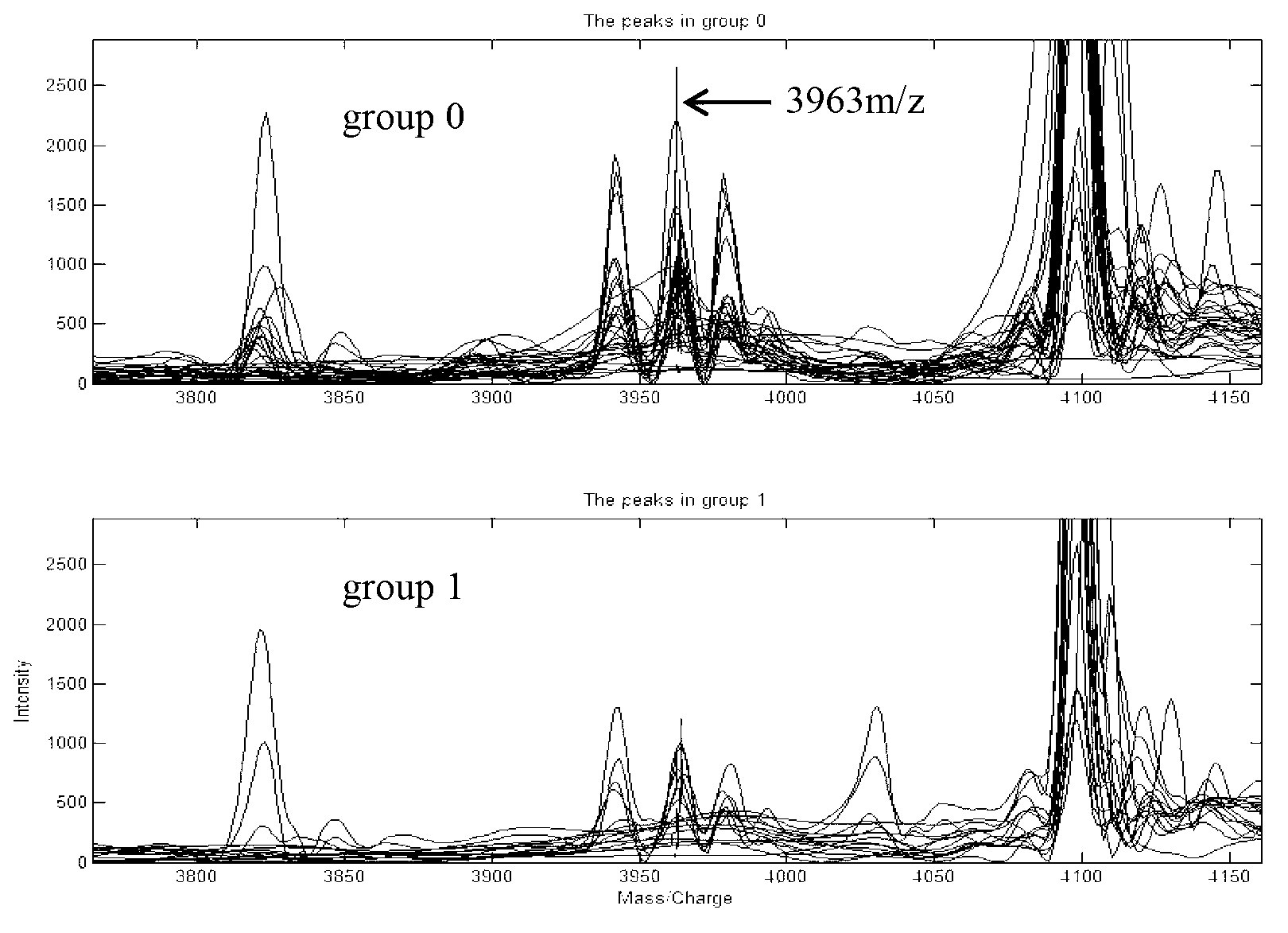
Method for modeling and analyzing protein fingerprinting of advanced colorectal cancer and kit
InactiveCN103323518ASolve blindnessReduce medical costsMaterial analysis by electric/magnetic meansFOLFOX RegimenRegimen
The invention discloses a method for modeling and analyzing protein fingerprinting of advanced colorectal cancer and a kit. The steps comprises: (1) respectively establishing serum protein fingerprinting for patient samples with advanced colorectal cancer, who suffer from a chemotherapy regimen; (2) extracting serum protein fingerprint characteristics in the serum protein fingerprinting of the obtained samples, and respectively establishing standard models based on the chemotherapy regimen; (3) establishing serum protein fingerprinting for patients with advanced colorectal cancer, who do not suffer from the chemotherapy regimen, and extracting the serum protein fingerprint characteristics; and (4) contrasting and analyzing the serum protein fingerprint characteristics of the patients who do not suffer from the chemotherapy regimen, with the standard models established in the step (2). The chemotherapy regimen may be FOLFOX or FOLFIRI. The method overcomes a blindness problem of a therapeutic regimen for advanced colorectal cancer. Before chemotherapy, the serum protein fingerprinting of the patients with advanced colorectal cancer is detected, to analyze the patient is a beneficiary of the FOLFOX regimen or the FOLFIRI regimen.
Owner:ZHEJIANG UNIV
Kit for detecting expression index of mRNA (messager Ribose Nucleic Acid) of WT1 (Wilms Tumor 1) gene
ActiveCN102912018AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceNewly diagnosedDisease monitoring
The invention relates to a kit for detecting an expression index of mRNA (messager Ribose Nucleic Acid) of a WT1 (Wilms Tumor 1) gene, and belongs to the field of biotechnology. The kit comprises detection primers, a fluorescent probe, a cDNA (complementary Deoxyribose Nucleic Acid) first strand synthesis reagent, a fluorescent quantitative PCR (Polymerase Chain Reaction) mixed solution, negative reference and positive reference, wherein the detection primers and the fluorescent probe comprise a WT1 gene primer, an internal reference gene ABL primer and a Taqman fluorescent probe. The WT1 gene is related with hematopoietic tumor incidence, is of over-expression in about 80% of patients with newly diagnosed acute myelocytic leukemia and acute lymphocytic leukemia, is recognized as a leukemia marker gene, and can serve as an independent minimal residue disease monitoring and prognosis prompting index. The level of the mRNA of the WT1 gene is detected by adopting a fluorescent quantitative PCR technology with higher sensitivity and specificity, and both the specificity and the sensitivity of a detection result are remarkably improved. The kit provides a brand-new quick, simple and convenient gene diagnosis technology for prognosing the acute myelocytic leukemia and the acute lymphocytic leukemia and confirming chemotherapy regimens.
Owner:童永清 +1
Erbb receptor methods and kits for monitoring chemotherapy resistance
InactiveUS20060281093A1Improved treatment of cancerMicrobiological testing/measurementDisease diagnosisAbnormal tissue growthChemotherapy combinations
The present invention relates to monitoring of ErbB receptor levels in methods and kits for determining the prognosis of cancer in a subject or improving the effectiveness of a cancer treatment. The invention also provides a method for predicting the recurrence of clinical signs of a cancer in a subject. In some embodiments, the invention provides methods for predicting the development of resistance to a chemotherapy regimen. In other embodiments, the invention provides methods for improving the effectiveness of a cancer treatment in a subject by monitoring levels of ErbB-2, ErbB-3 and / or ErbB-4. Preferably, the subject in the methods of the invention has been previously treated with a chemotherapy regimen for an ErbB-1 positive tumor.
Owner:EURO-CELTIQUE SA
Tubulysin derivatives
Novel tubulysin derivatives which may be useful as cytotoxic agents to provide therapeutic benefits in the treatment of various types of cancers, alone, as drug conjugates or in combination with other chemotherapies are provided.
Owner:MEDIMMUNE LLC
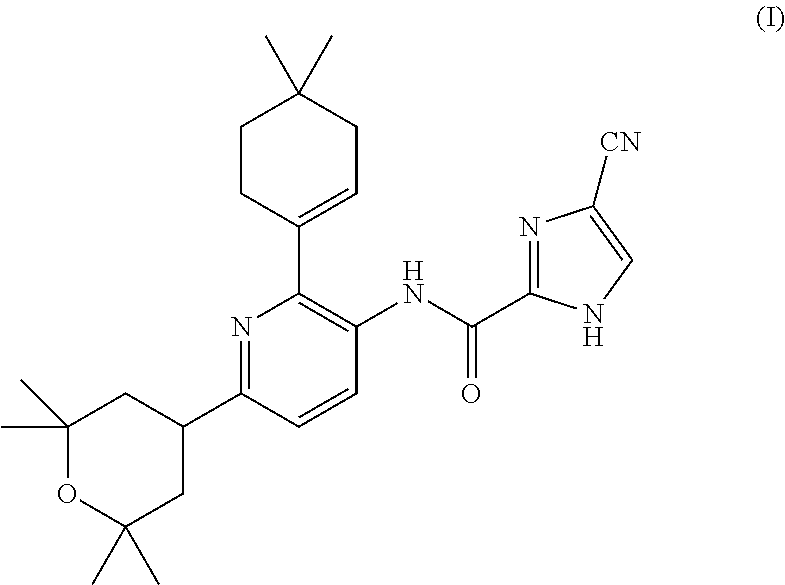
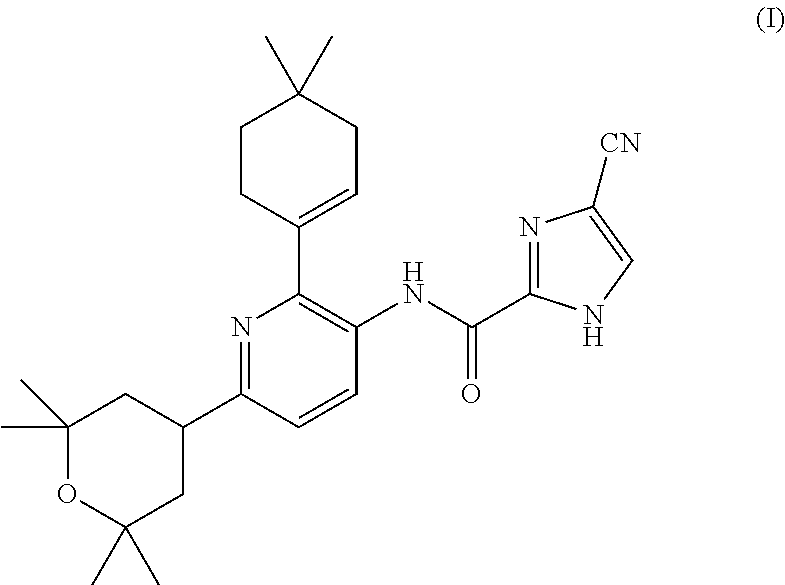
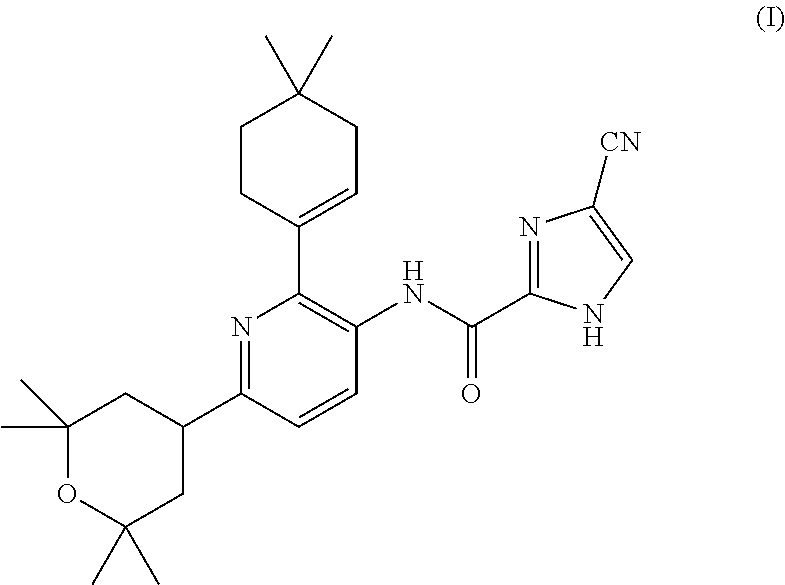
4-cyano-n-(2-(4,4-dimethylcyclohex-1-en-1-yl)-6-(2,2,6,6-tetramethyltetrahydro-2h-pyran-4-yl)pyridin-3-yl)-1h-imidazole-2-carboxamide for the treatment of hodgkin's lymphoma
InactiveUS20160015700A1BiocidePhosphorous compound active ingredientsChemotherapy regimenChemotherapy combinations
The present invention is directed to methods for the treatment of Hodgkin's lymphoma comprising administering to a patient in need thereof, a therapeutically effective amount of 4-cyano-N-(2-(4,4-dimethylcyclohex-1-en-1-yl)-6 -(2,2,6,6-tetramethyltetrahydro-2H-pyran-4-yl)pyridin-3-yl)-1H-imidazole-2-carboxamide as mono-therapy or as combination or co-therapy with one or more chemotherapeutic agent or chemotherapy regimens.
Owner:JANSSEN PHARMA NV
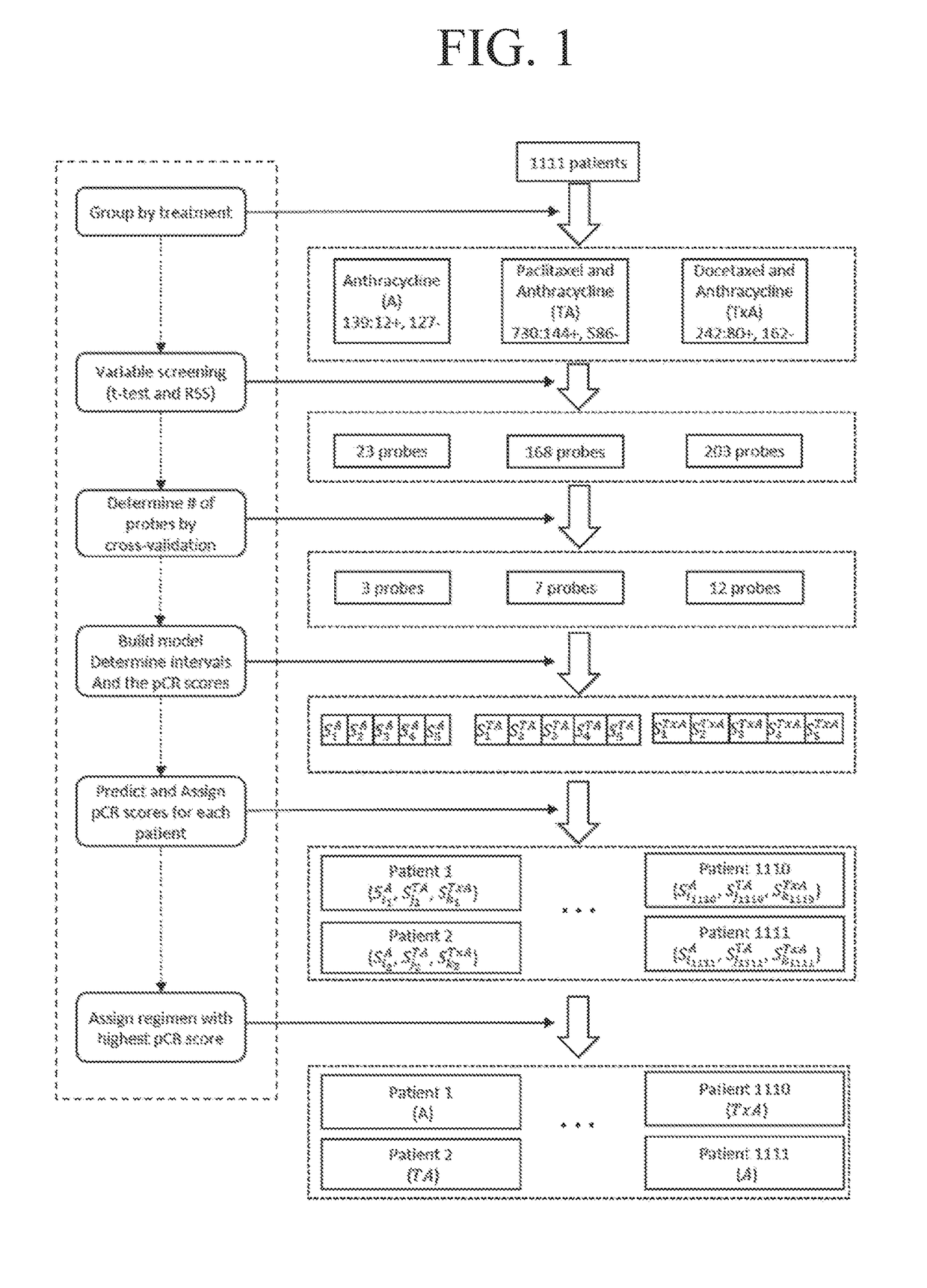
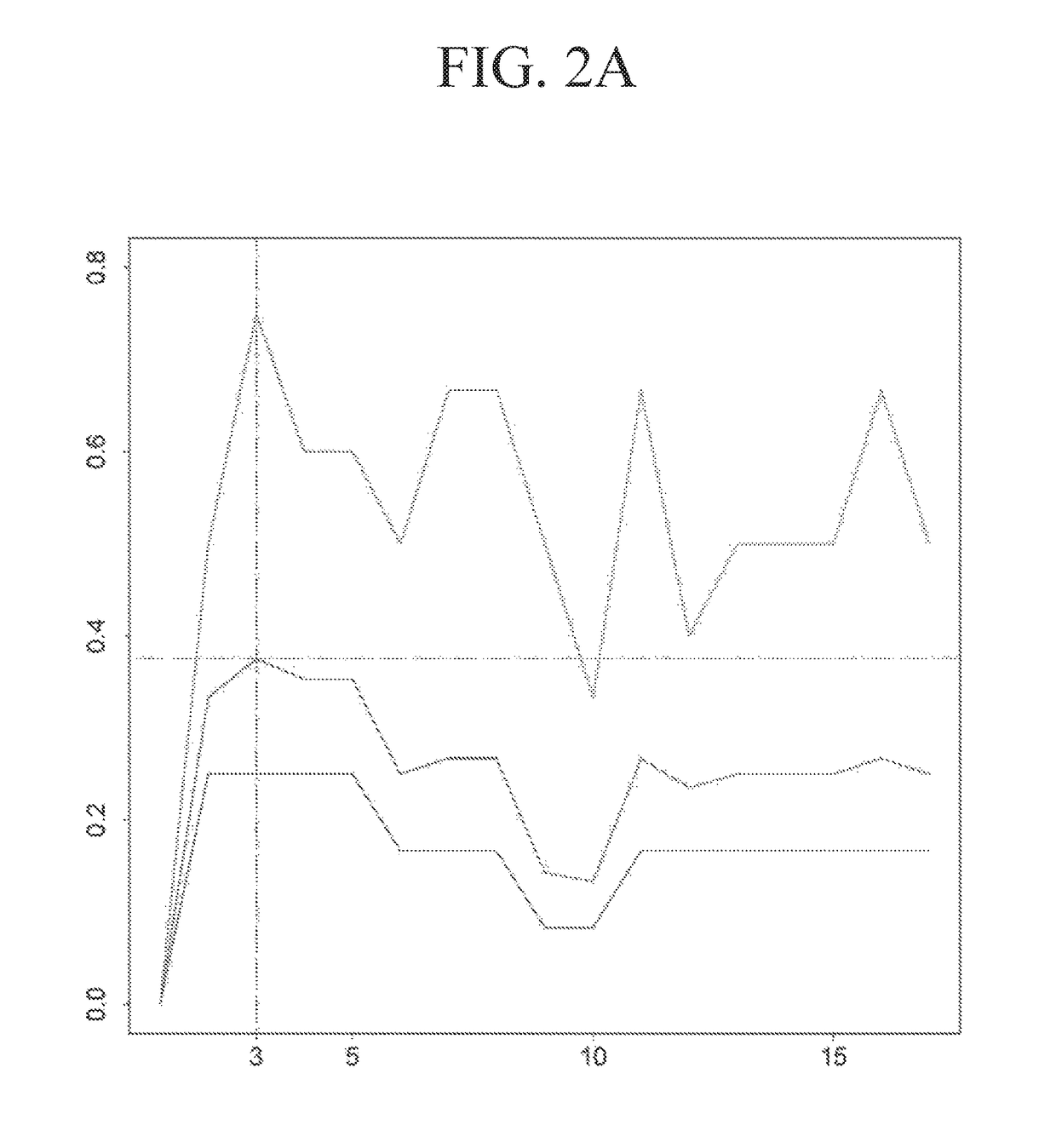
Application of reagent for detecting gene expression level and construction method of NAC (neoadjuvant chemotherapy) curative effect prediction model for breast cancer patients
ActiveCN109439753AChemotherapy is effectiveImprove survival rateMicrobiological testing/measurementChemotherapy combinationsGene expression level
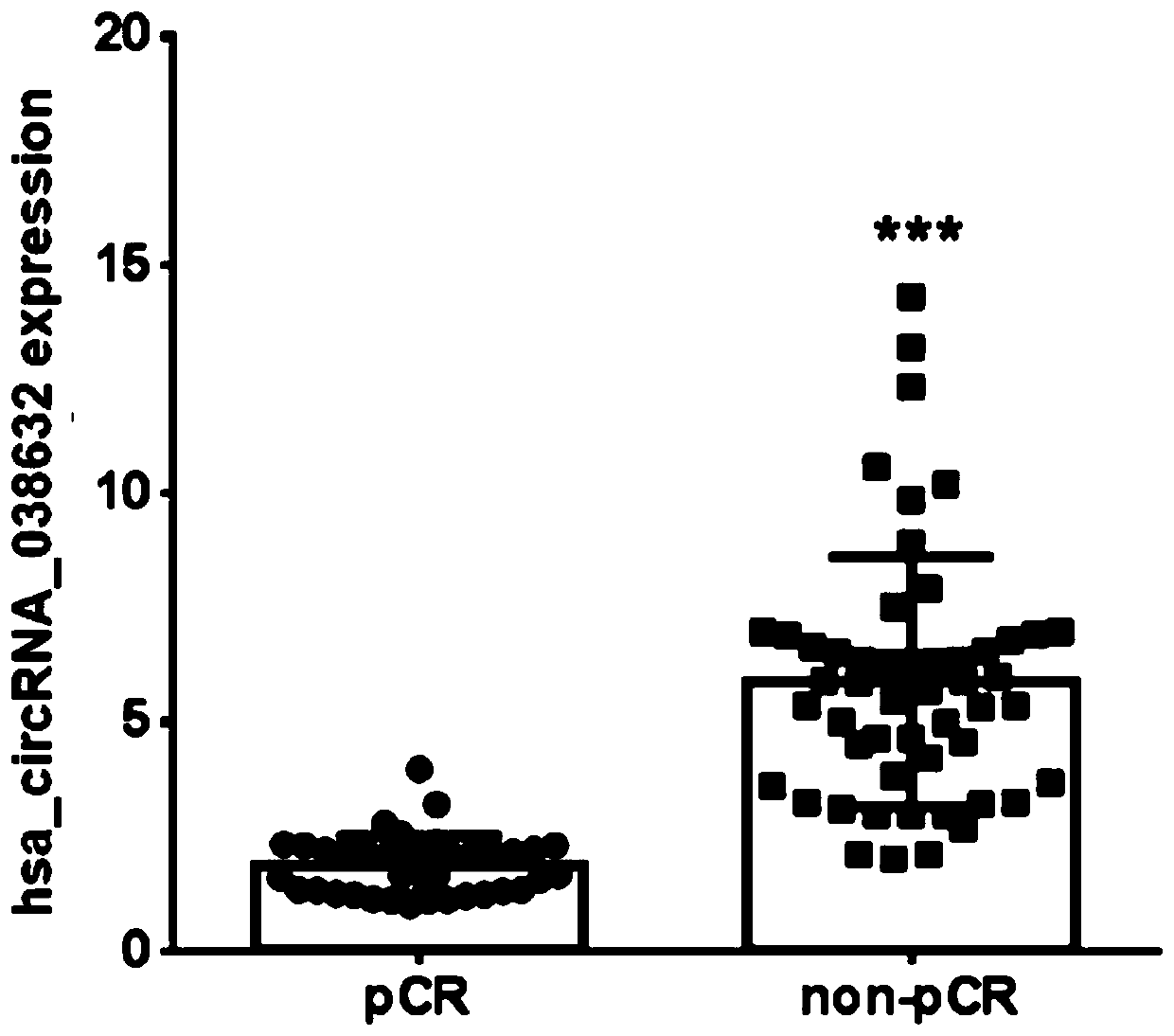
The invention discloses an application of a reagent for detecting gene expression level and a construction method of an NAC (neoadjuvant chemotherapy) curative effect prediction model for breast cancer patients, and relates to the technical field of breast cancer. Particularly, the prediction model can predict the curative effect of anthracyclines and taxus drugs in NAC, so as to provide a basis for whether the breast cancer patients accept NAC regimens or choose which type of NAC regimens. The patients who are sensitive to chemotherapy regimens and may reach pCR can seize the opportunity of NAC by predicting the curative effect, so that effective NAC is conducted, and the breast-conserving rate or survival rate is increased; however, the patients who are insensitive to chemotherapy regimens or may cause tumor progression can choose other effective treatments in time.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Dendritic cell tumor injection (DCTI) therapy
The invention relates to a method of treating tumor cells within a patient wherein immature dendritic cells developed from the patient's monocyte cells and an adjuvant are introduced into the patient directly into the patient's tumor cells. The immature dendritic cells and adjuvant combine with the antigens in the tumor cells to form a cancer vaccine, thereby immediately treating the tumor cells of the patient. The invention also provides a precursor treatment step of treating the patient with a radiation therapy or chemotherapy regimen.
Owner:HASUMI KENICHIRO
Radiosensitizer formulations and methods for use
Owner:MATTHEWS RICHARD H DR
Combination of Ad-P53 and Chemotherapy for the Treatment of Tumours
InactiveUS20080293652A1Treat cancerOrganic active ingredientsPeptide/protein ingredientsRecurrent CancerChemotherapy combinations
The present invention relates to the use of p53 gene therapy to treat recurrent cancers in combination with a radio- or chemotherapy. Patients with recurring cancers are treated with a p53 expression construct followed by subsequent radio- or chemotherapy treatment. Viral and non-viral delivery systems, as well as various radio- and chemotherapy regimens are disclosed.
Owner:INTROGEN THERAPEUTICS INC
Multi-gene detection kit for selection of chemotherapy regimens
ActiveCN103882139AEasy to analyzeReliable analysisMicrobiological testing/measurementDPYDReference genes
The invention relates to a multi-gene detection kit for the selection of chemotherapy regimens. The multi-gene detection kit comprises a mixture of RT (reverse transcriptase primer) primers and a mixture of PCR (Polymerase Chain Reaction) amplification primers, wherein the mixture of RT primers and the mixture of PCR amplification primers comprise each RT primer and each PCR amplification primer based on genetic groups, reference genes and an internal standard substance of the reaction respectively, also comprise random primers, the genetic groups comprise RRM1, TOP2A, ERCC1, TYMS, TUBB3, TOP1, PTEN, HER2, DPYD and EGFR; the reference genes comprise ACTB, GAPDH and B2M; the internal standard substance of the reaction is KAN-rRNA. The multi-gene detection kit for the selection of chemotherapy regimens, which is disclosed by the invention, can be used for simultaneously detecting the expression levels of multiple genes closely related to anti-tumor medicines systematically, and is easy to use, good in accuracy, high in detection efficiency and can be well used to guide chemotherapy medication and select chemotherapy regimens.
Owner:南昌市赛尔医药科技有限公司
Targeted Therapies for Cancer
Methods of selecting a chemotherapy regimen for treatment of cancer in a patient are disclosed. A patient genetic sample from a bilary cancer such as cholangiocarcinoma is analyzed for a mutation in ERRFI1 and a chemotherapeutic agent is selected as a result of the analysis. If a mutation in ERRFI1 is present, treatment with an inhibitor of Epidermal Growth Factor Receptor (EGFR) is shown to have inhibitory effects on tumor growth. In this manner, the chemotherapy regimen is targeted to a given mutation in a patient's cancer.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES +1
Patient selection for enhancement of Anti-tumor immunity in cancer patients
PendingUS20220175787A1Enhance immune activationPromote antitumor immunityMicrobiological testing/measurementAntibody ingredientsChemotherapy combinationsPharmaceutical medicine
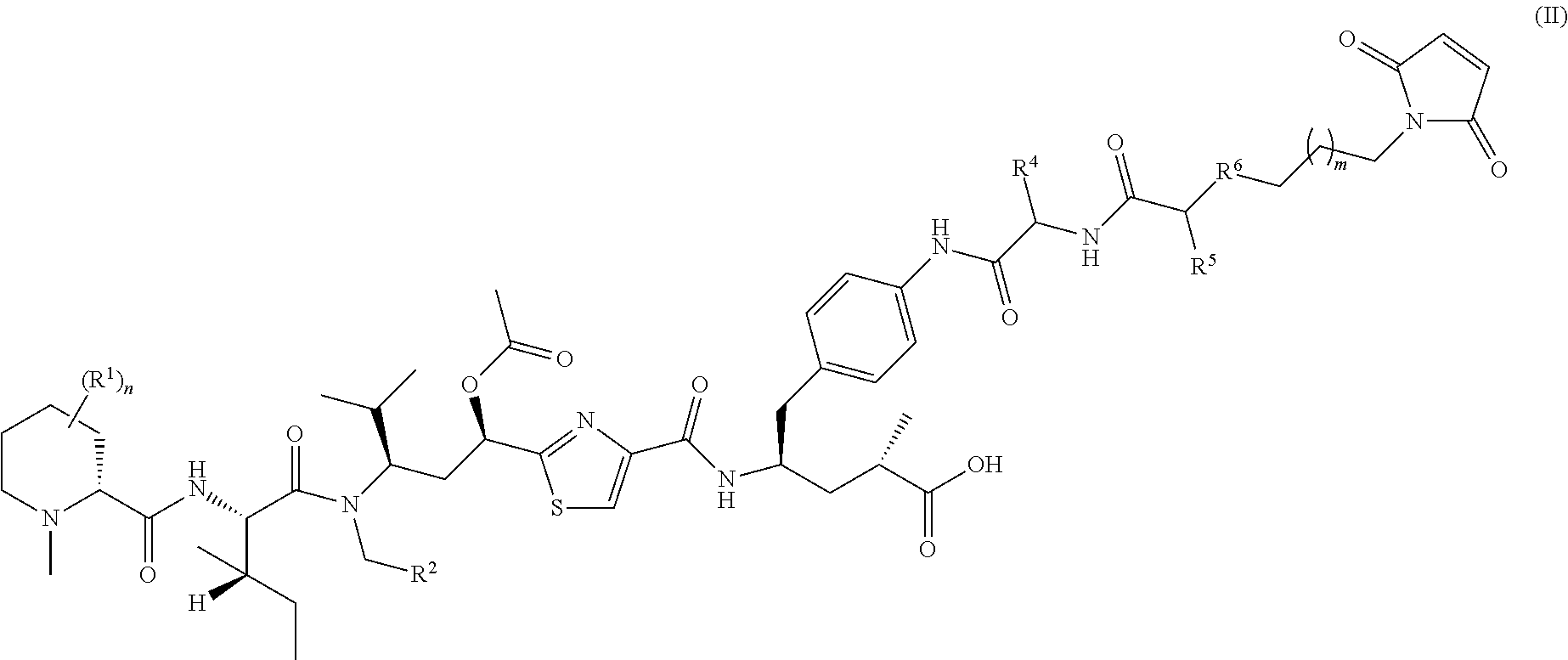
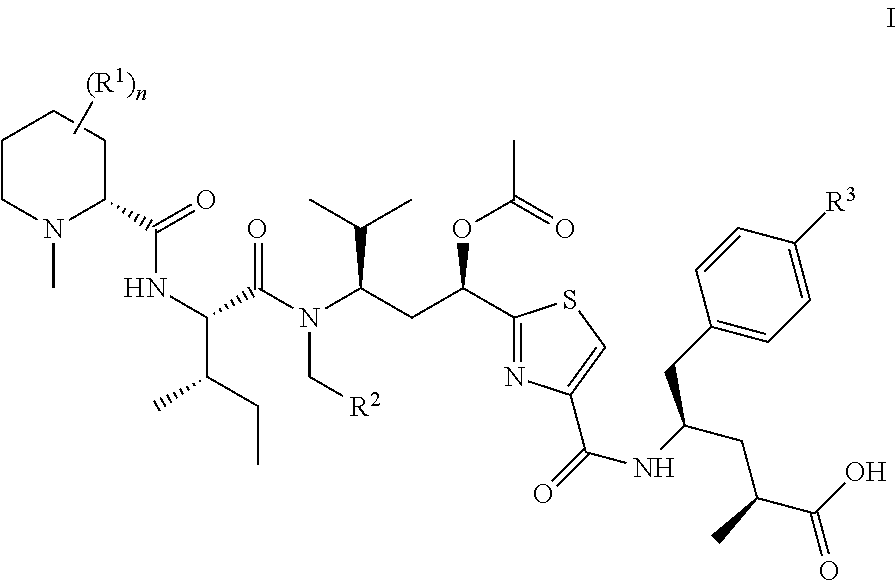
A method for increasing the progression free survival or overall survival of a patient with cancer comprising: determining if the cancer has a surrounding microenvironment that is favorable to immune modulation; determining if the chemotherapy regimen induces immunogenic cell death, and if both are yes, administering an effective amount of a CDK 4 / 6 inhibitor selected from Compounds I, II, III, IV, or V, or a pharmaceutically acceptable salt thereof, wherein the CDK4 / 6 inhibitor is administered prior to the administration of the chemotherapy or optionally prior to and concurrently with chemotherapy; and, wherein the increase in progression free survival or overall survival is in comparison to the progression free survival or overall survival based on administration of the chemotherapy alone, either based on literature or otherwise publicly available evidence, a comparative during preclinical or clinical trials, or other means accepted by persons skilled in the field.
Owner:G1 THERAPEUTICS INC
Gene tag for predicting breast cancer neoadjuvant chemotherapy sensitivity and application thereof
ActiveCN113061655AGood predictive abilityGood forecastMicrobiological testing/measurementProteomicsChemotherapy combinationsLogistische regression
The invention belongs to the technical field of tumor gene detection, and particularly relates to a gene tag for predicting breast cancer paclitaxel and anthracycline neoadjuvant chemotherapy sensitivity and application thereof. In the invention, based on LASSO logistic regression, a gene tag consisting of 25 genes related to breast cancer neoadjuvant chemotherapy sensitivity is obtained, and a score containing a gene expression quantity is calculated and predicted, so that the sensitivity of a breast cancer patient using paclitaxel and anthracycline neoadjuvant chemotherapy can be accurately predicted, the response of the patient to treatment is predicted, and whether the patient benefits from chemotherapy is discriminated, and thus selection of neoadjuvant chemotherapy regimens for breast cancer is guided, excessive treatment is avoided, and the medical cost is reduced.
Owner:HEFEI INSTITUTES OF PHYSICAL SCIENCE - CHINESE ACAD OF SCI +1
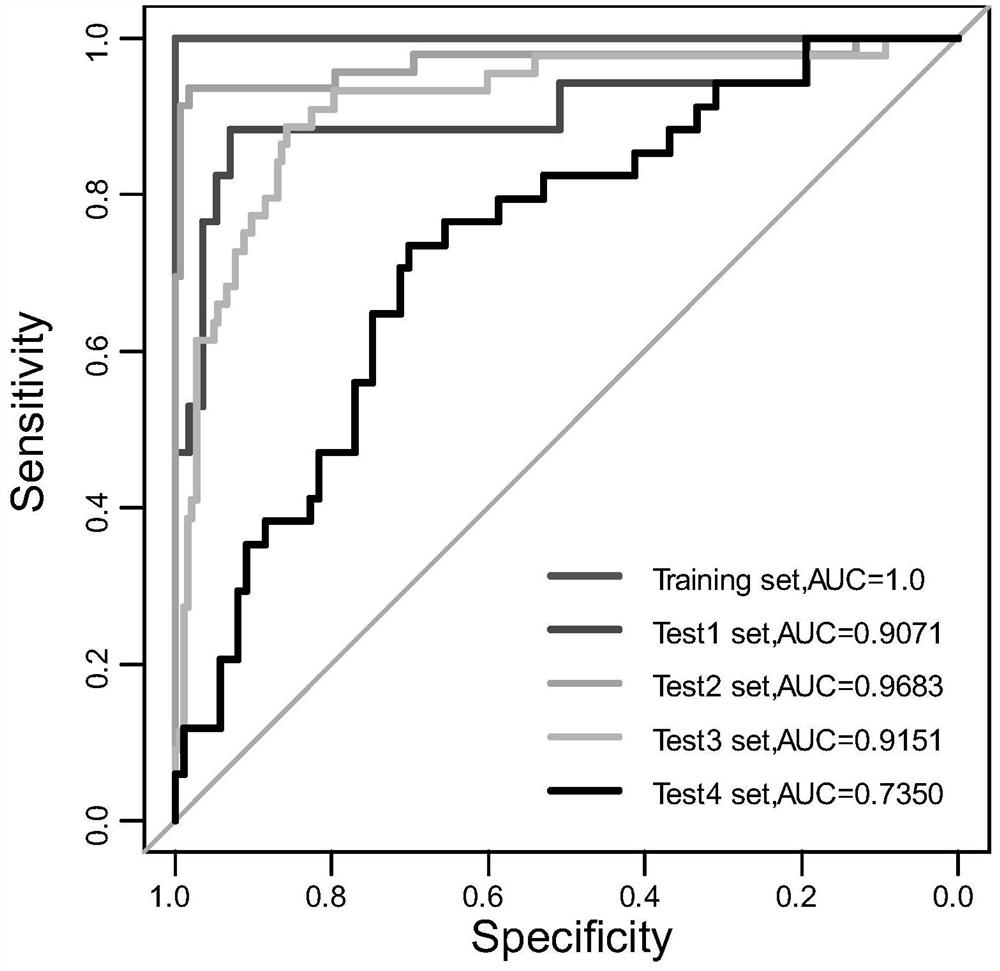
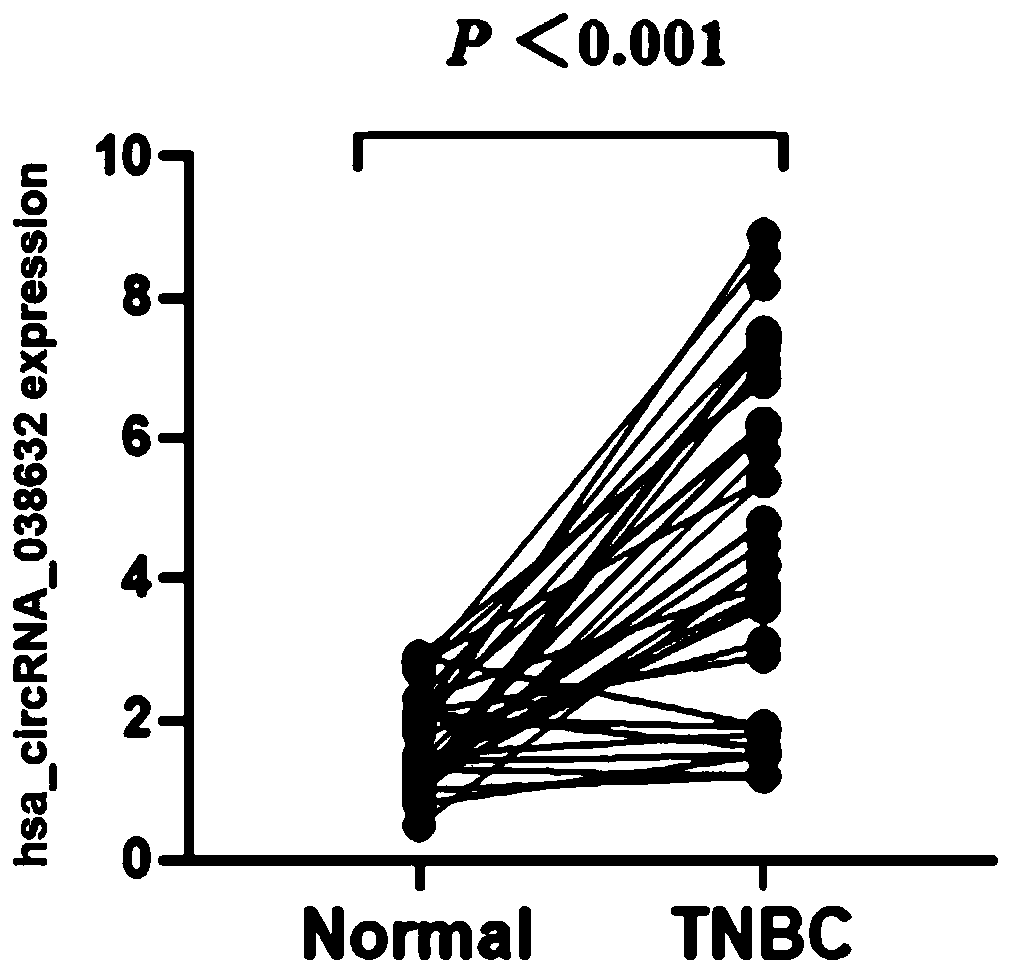
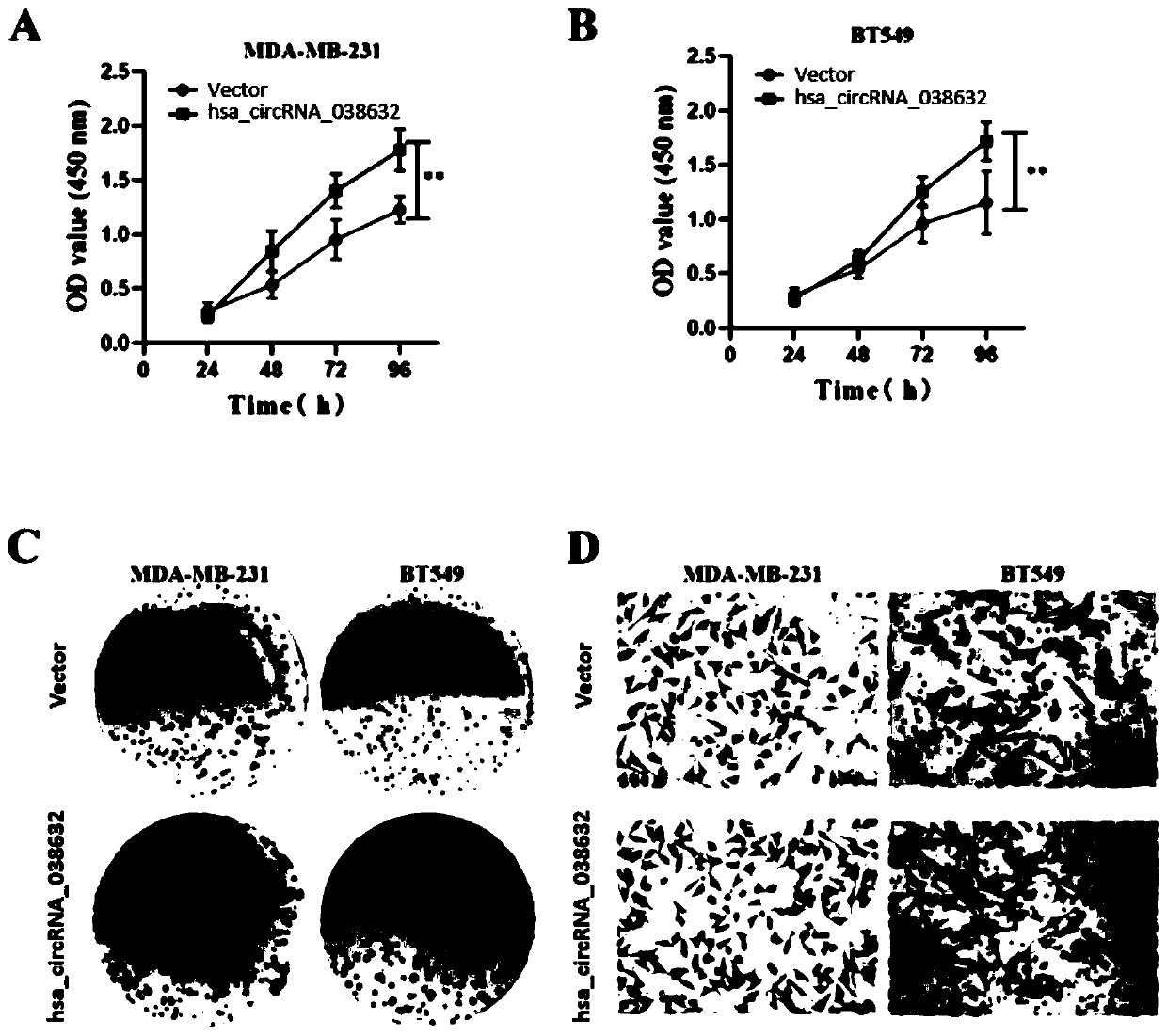
CircRNA detection kit for predicting neoadjuvant chemotherapy reactivity of triple-negative breast cancer (TNBC)
ActiveCN110923321AResponsive PredictionHelp determineMicrobiological testing/measurementDNA/RNA fragmentationNucleotideTnbc patient
The invention provides circRNA for predicting neoadjuvant chemotherapy sensitivity of triple-negative breast cancer (TNBC). The circRNA is hsa_circRNA_038632, with a nucleotide sequence shown in SEQ ID NO: 1. The invention further provides a kit for predicting neoadjuvant chemotherapy sensitivity of TNBC. The invention finds the correlation between the hsa_circRNA_038632 and reactivity of TNBC patients to neoadjuvant chemotherapy for the first time, and the reactivity of the TNBC patients to the neoadjuvant chemotherapy can be predicted by detecting the expression level of the hsa_circRNA_038632, so that determination of chemotherapy regimens for the TNBC patients is facilitated.
Owner:GUANGDONG GENERAL HOSPITAL
Method of using tumour RNA integrity to measure response to chemotherapy in cancer patients
InactiveUS20160077051A1Easy accessRapid assessmentOrganic active ingredientsElectrolysis componentsRegimenCapillary electrophoresis
Cancerous tumours vary significantly in their response to chemotherapy agents. Currently, it is difficult to reliably assess the level of tumour responsiveness to a chemotherapy regimen during or post-administration. Biomarkers of tumour sensitivity to chemotherapy agents have hitherto been unknown. Such a biomarker would expedite identification of nonresponsive patients, who may then switch to other, possibly more effective regimens. The present invention provides a method for determining tumour responsiveness to a chemotherapy agent, wherein RNA is isolated from tumour cells of a patient before, during, and after chemotherapy. The quality of the RNA can be determined by capillary electrophoresis and assignment of an RNA integrity number (RIN). RIN values during and / or after chemotherapy are inversely proportionate to the level of tumour responsiveness. The tumour RIN is an easily accessed biomarker of tumour responsiveness to chemotherapy. The tumour RIN may also be used to assess the efficacy of a chemotherapy regimen.
Owner:LAURENTIAN UNIV OF SUDBURY
Compound Chinese medicine for treating multidrug-resistant tuberculosis
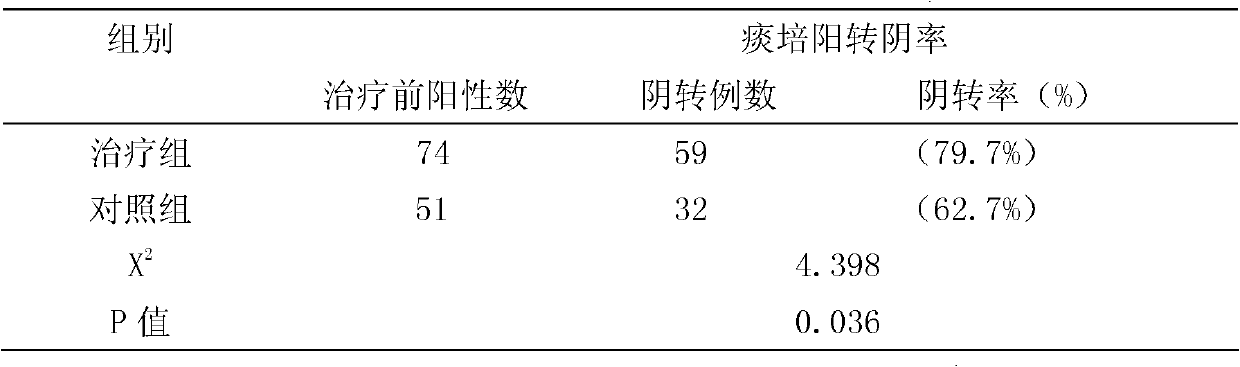
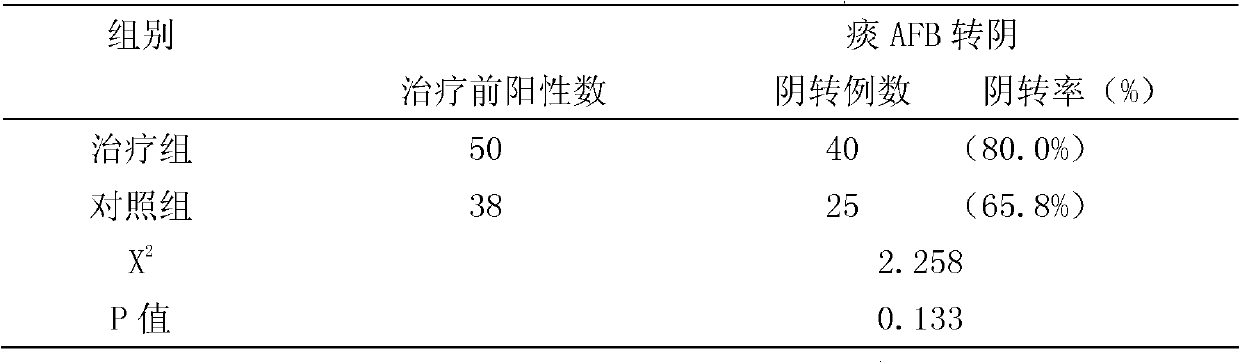
InactiveCN102166304AGood treatment effectEasy to controlAntibacterial agentsUnknown materialsResistant tuberculosisChemotherapy combinations
The invention relates to a compound Chinese medicine for treating multidrug-resistant tuberculosis, which is prepared from following raw medicinal materials: 15 to 20 grams of manyflower solomonseal rhizome, 10 to 20 grams of common bletilla tuber, 15 to 20 grams of heterophylly falsestarwort root, 10 to 15 grams of sessile stemona root, 15 to 20 grams of Japanese ardisia herb, 10 to 15 grams of common coltsfoot flower, 15 to 20 grams of Phippine violet herb, 10 to 12 grams of Japanese thistle herb or root, 15 to 20 grams of cochinchinese asparagus root, and 15 to 20 grams of turtle shell. The compound Chinese medicine capable of treating multidrug-resistant tuberculosis can enhance a curative effect, and has functions of nourishing Yi, moistening lung, killing parasites, eliminating phlegm, removing blood stasis and promoting tissue regeneration. The compound Chinese medicine provided by the invention integrates a western chemotherapy regimen to treat multidrug-resistant tuberculosis, and also can accelerate sputum conversion, promote the absorption of focus, improve immunity, and accelerate the recovery of tuberculosistoxic symptoms.
Owner:长沙市中心医院 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com