Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
32 results about "Hematopoietic Tumor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions for detecting non-hematopoietic cells from a blood sample
InactiveUS20060252054A1Aid in diagnosis and prognosisPromote recoveryMembranesOther blood circulation devicesRare cellHematopoietic cell
The present invention recognizes that diagnosis and prognosis of many conditions can depend on the enrichment of rare cells, especially tumor cells, from a complex fluid sample such as a blood sample. In particular, the present invention is directed to methods and compositions for detecting a non-hematopoietic cell, e.g., a non-hematopoietic tumor cell, in a blood sample via, inter alia, removing red blood cells (RBCs) from a blood sample using a non-centrifugation procedure, removing white blood cells (WBCs) from said blood sample to enrich a non-hematopoietic cell, if any, from said blood sample; and assessing the presence, absence and / or amount of said enriched non-hematopoietic cell.
Owner:AVIVA BIOSCI
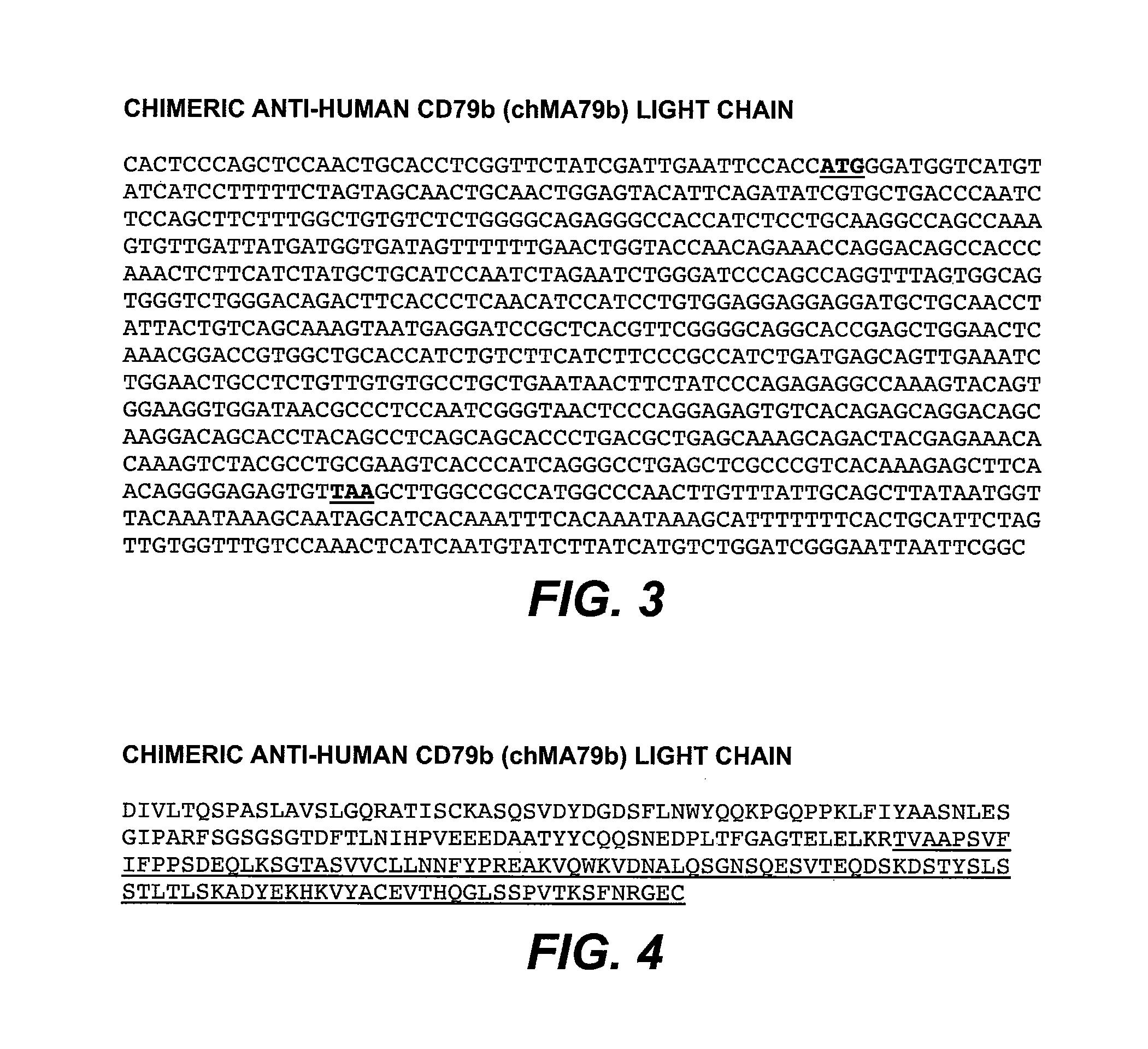
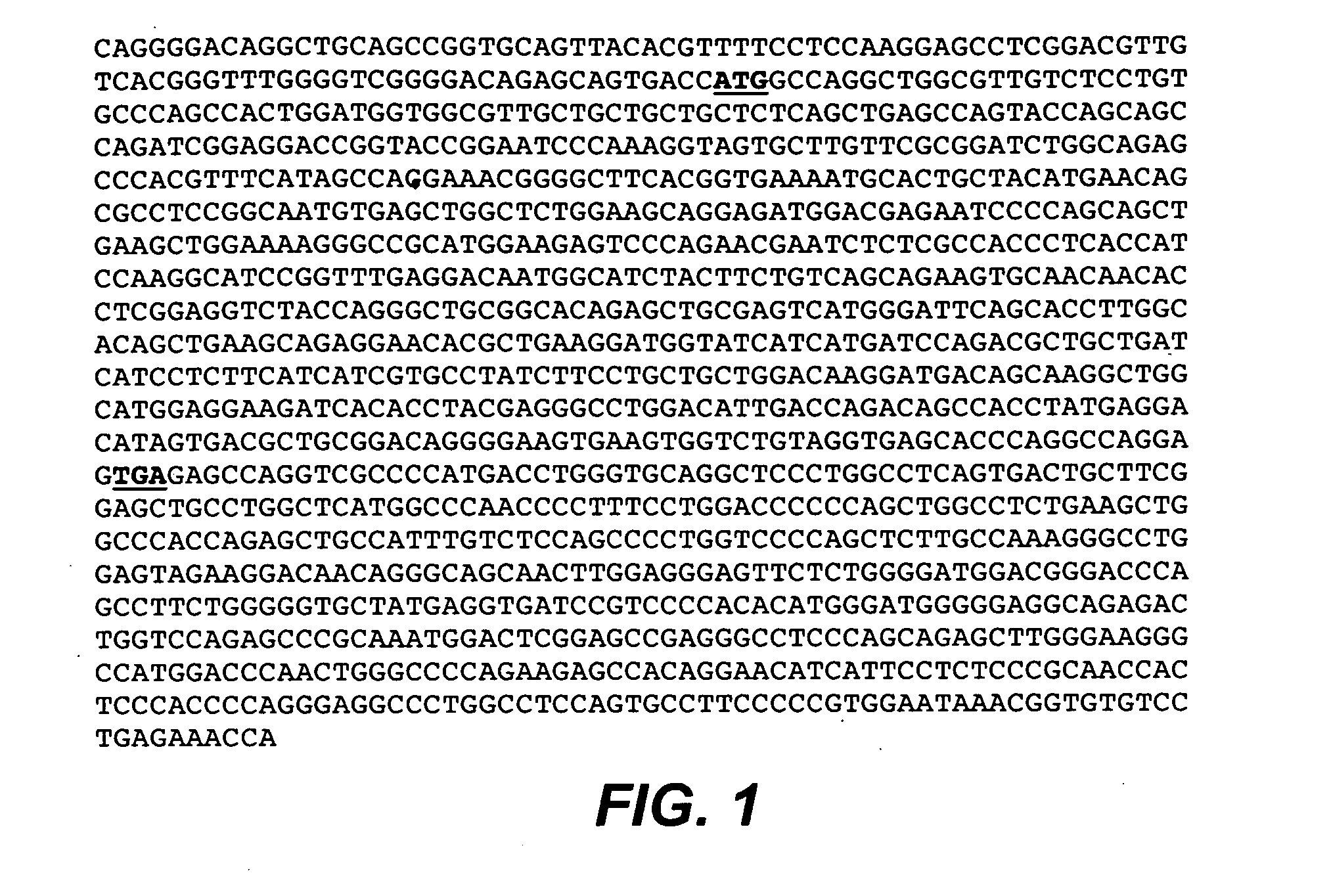
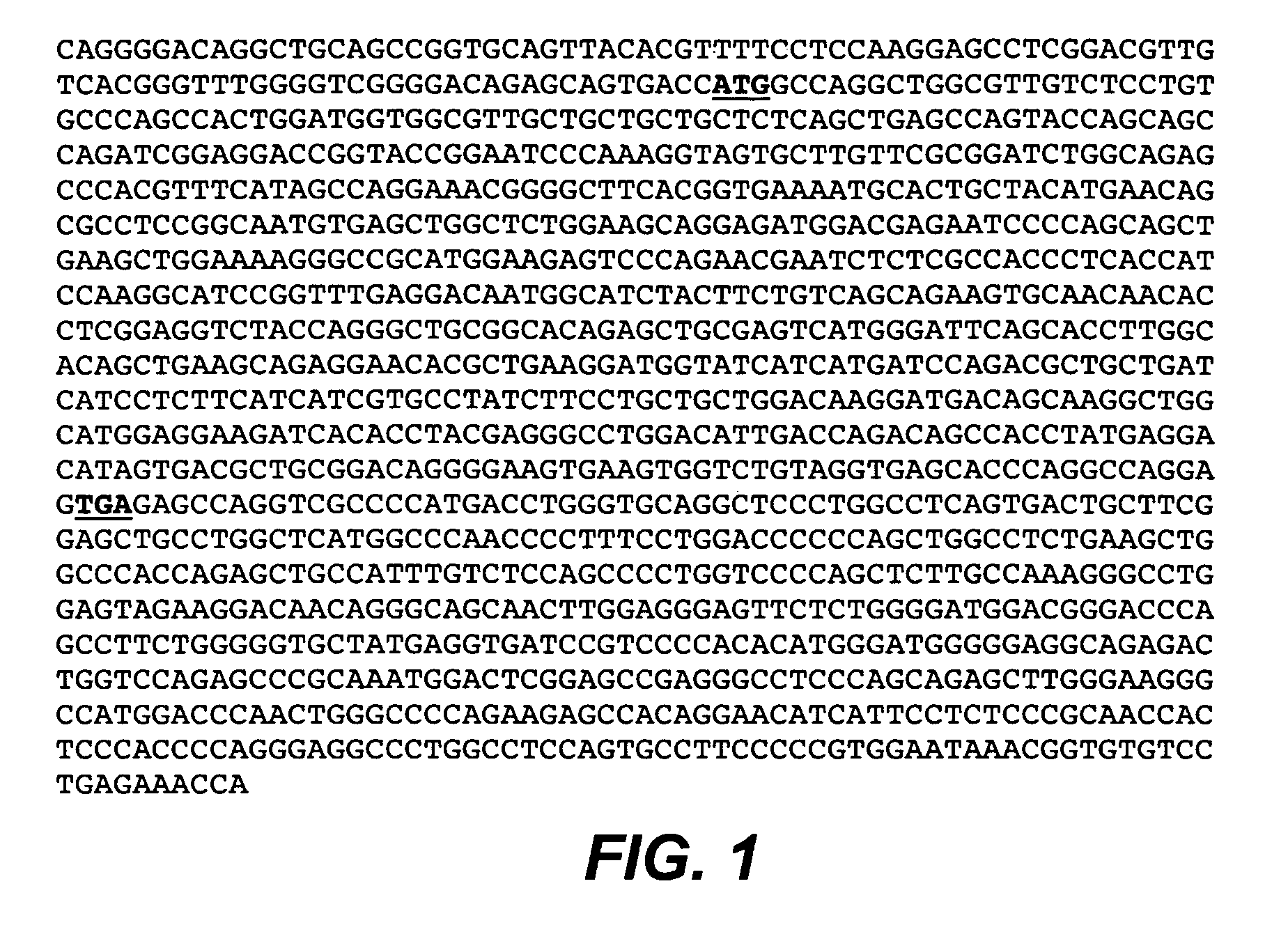
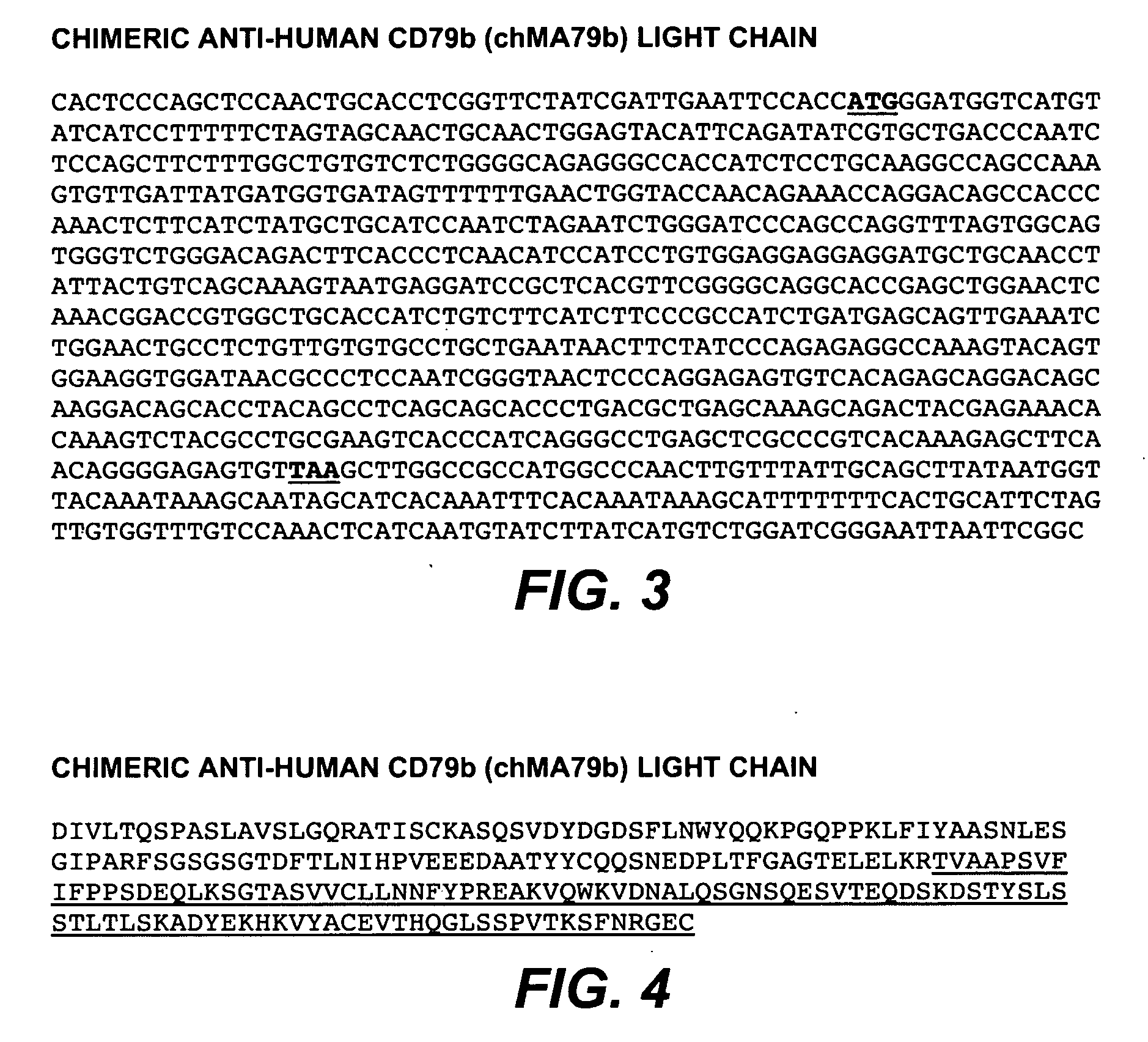
Anti-CD79B Antibodies and Immunoconjugates and Methods of Use
ActiveUS20090028856A1Effectively preventingEffectively treatingOrganic active ingredientsBacteriaMammalHematopoietic Tumor
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
Anti-CD79B antibodies and immunoconjugates and methods of use
The present invention is directed to anti-CD79b antibody, huMA79b.v28, and compositions of matter thereof useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
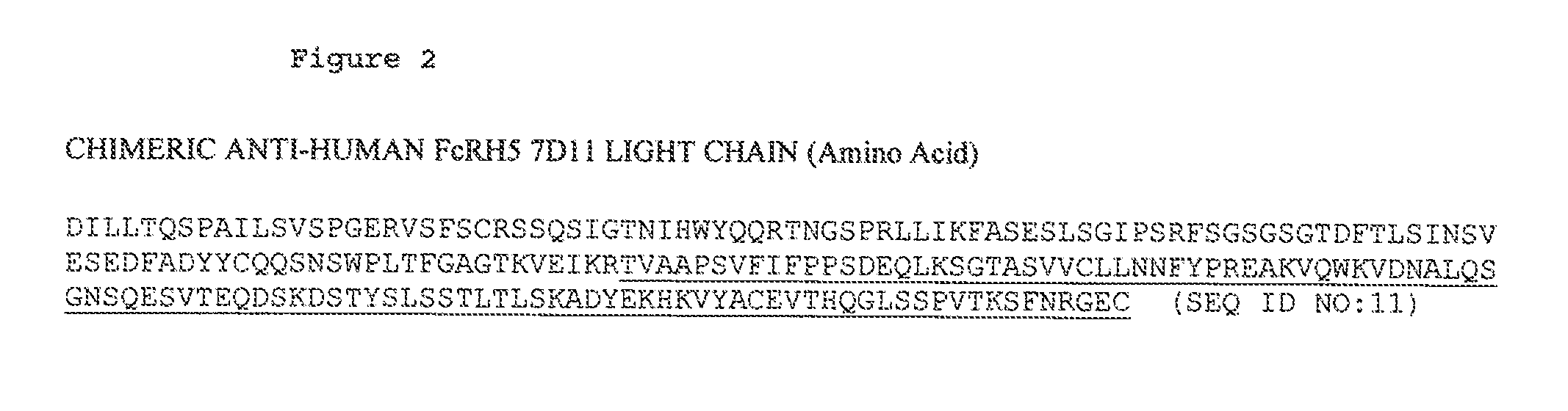
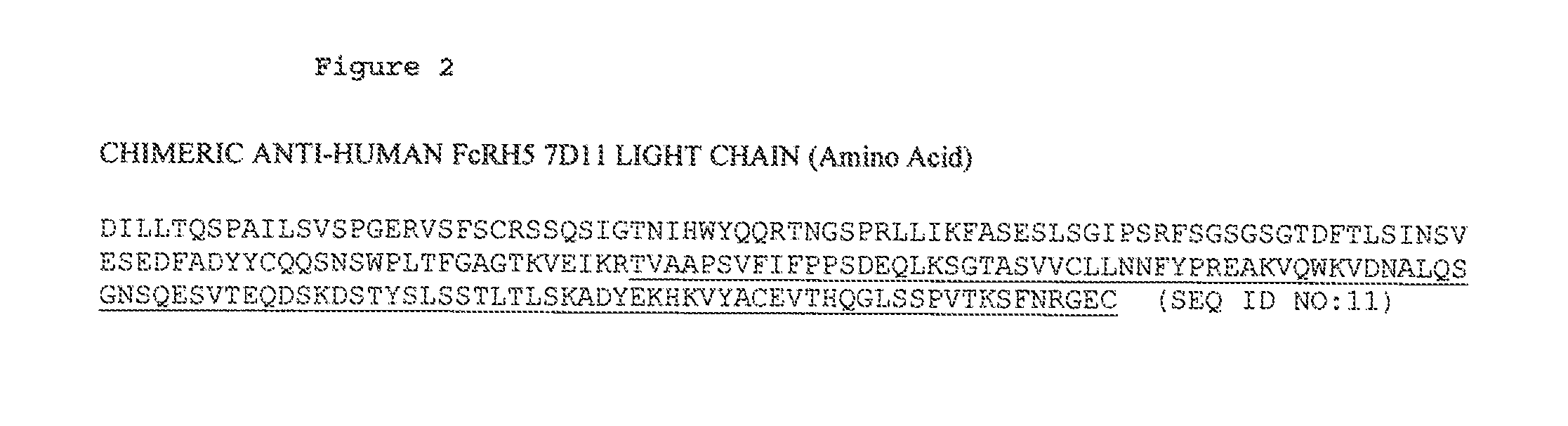
ANTI-FcRH5 ANTIBODIES AND IMMUNOCONJUGATES AND METHODS OF USE
InactiveUS20100260748A1Heavy metal active ingredientsPeptide/protein ingredientsAntiendomysial antibodiesBiochemistry
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:F HOFFMANN LA ROCHE & CO AG
Humanized Anti-CD79B Antibodies and Immunoconjugates and Methods of Use
ActiveUS20090068202A1Immunoglobulins against cell receptors/antigens/surface-determinantsTissue cultureAntiendomysial antibodiesOncology
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
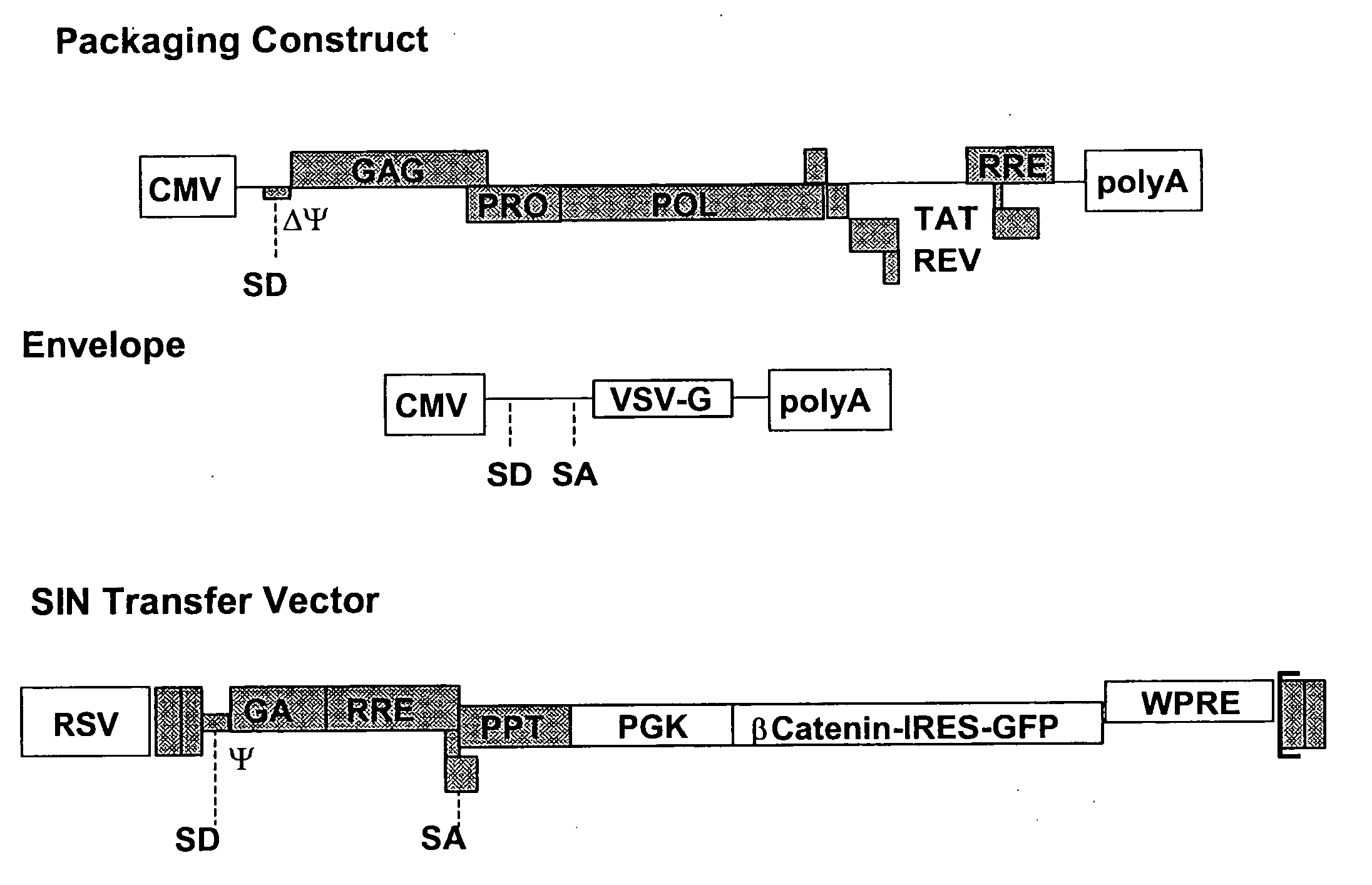
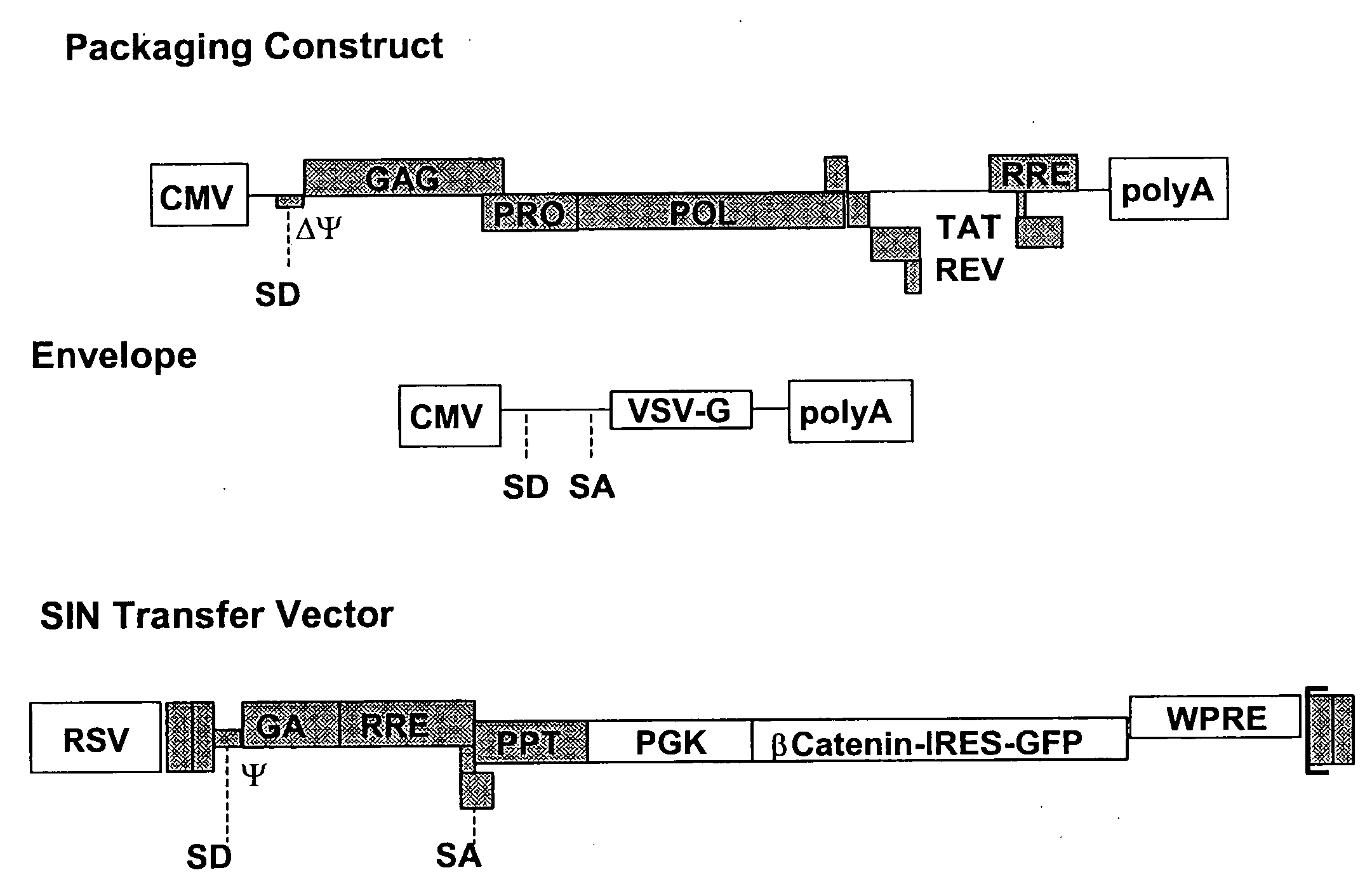
Methods of identifying and isolating stem cells and cancer stem cells
InactiveUS7217568B2AlterationEasy to analyze and useMicrobiological testing/measurementArtificial cell constructsHematopoietic cellCancer cell
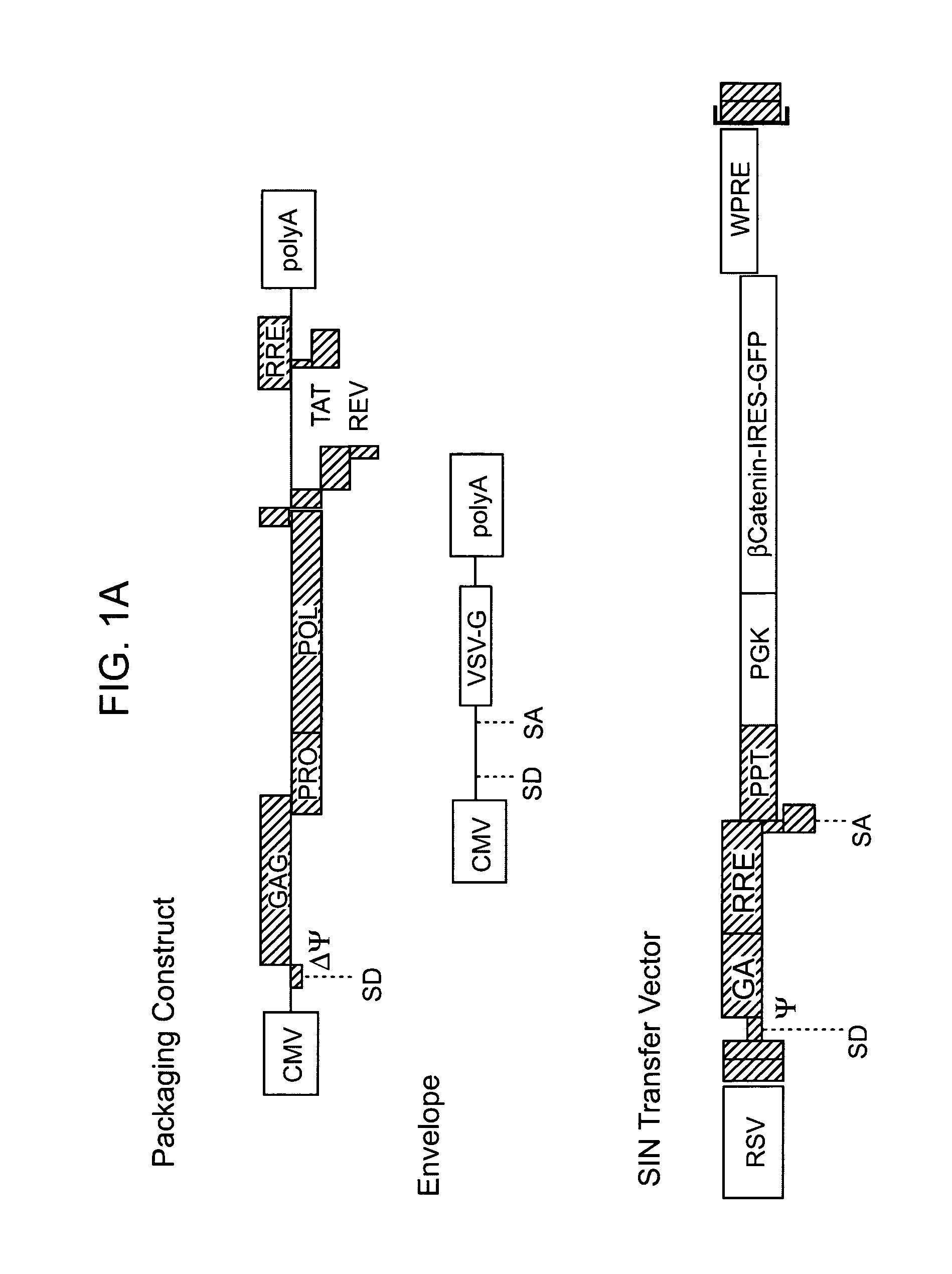
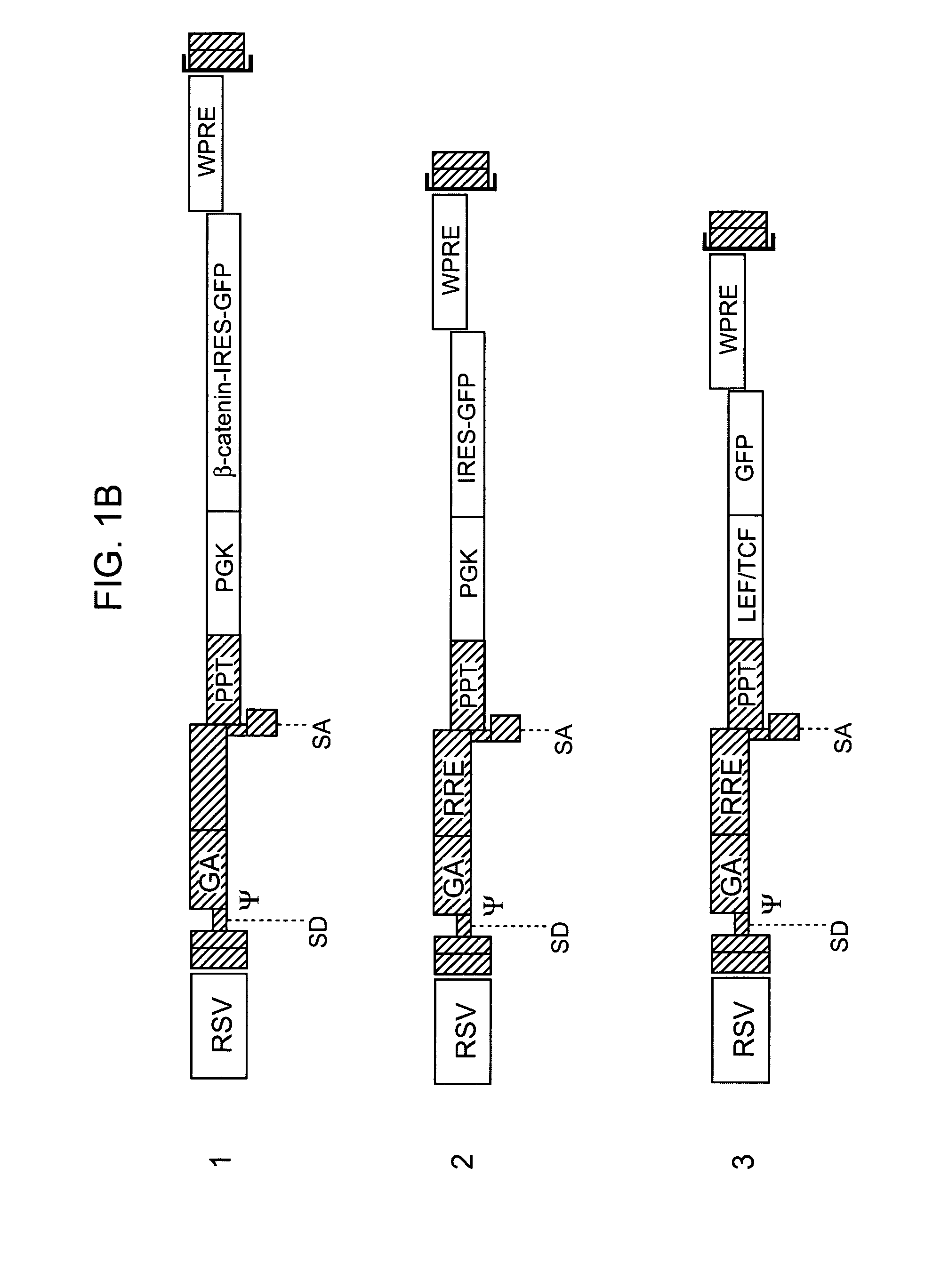
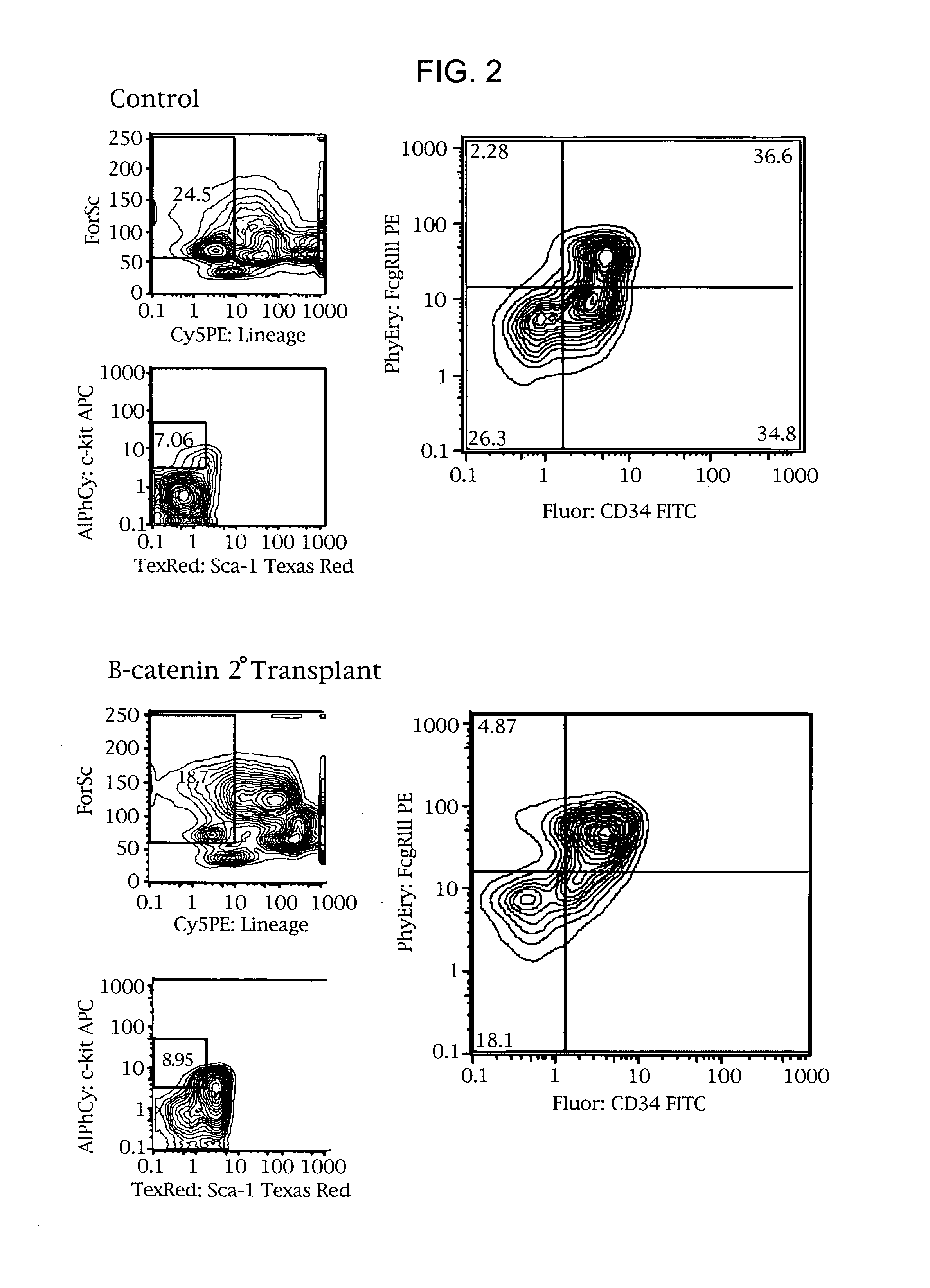
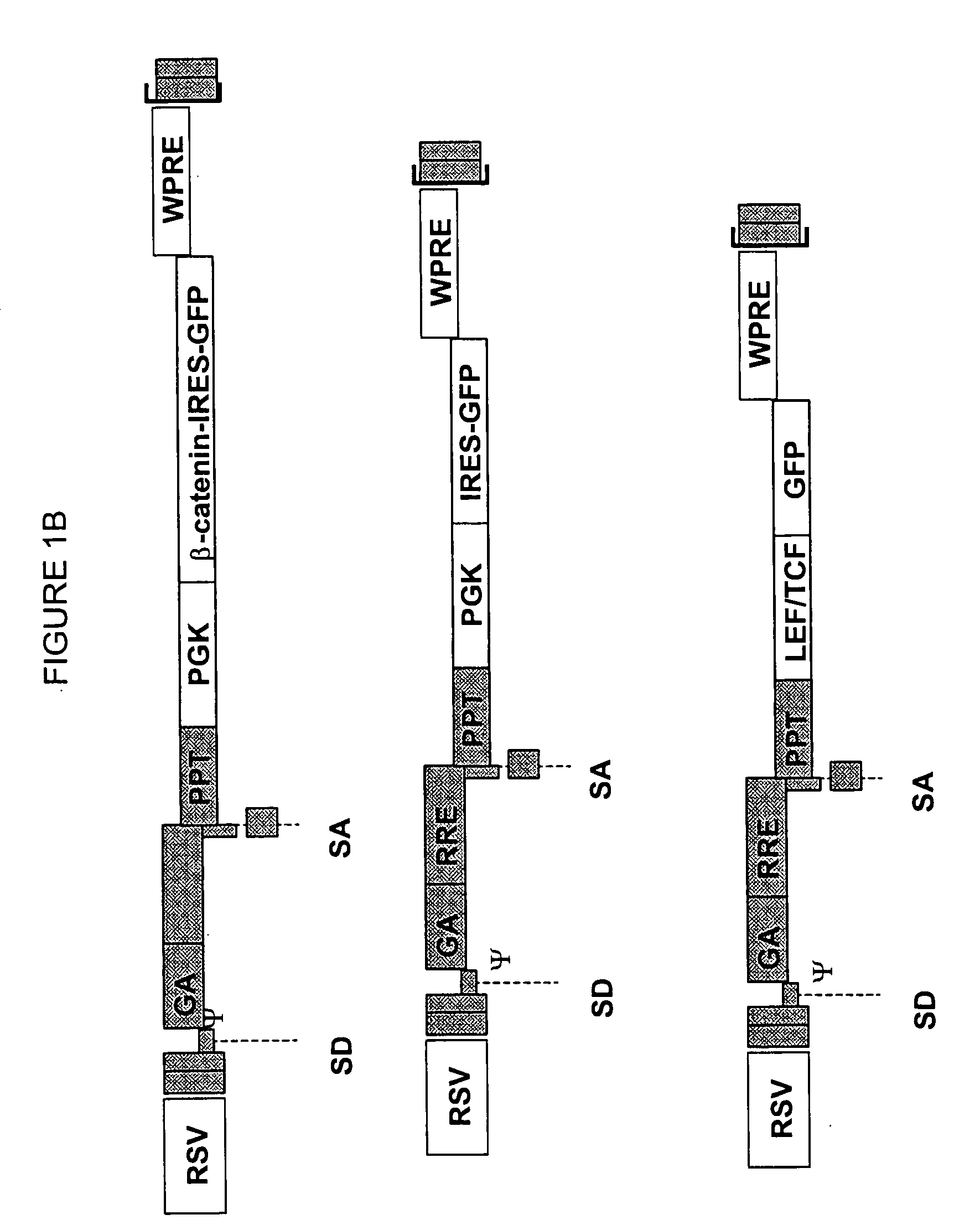
Methods and compositions are provided for the identification of stem cells and cancer stem cells. β-catenin is also identified as a target for the development of therapeutic moieties against hematopoietic tumors, i.e. leukemia and lymphoma cells, which may include screening assays directed at β-catenin, or members of the β-catenin signaling pathway. Cellular proliferation in hematopoietic cells can be altered by introducing stabilized β-catenin into a hematopoietic cell that is altered in its ability to undergo apoptosis but which is not fully transformed. The immortalized cells are useful in screening assays, and in the analysis of pathways by which hematopoietic cells undergo transformation.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Compositions and Methods for the Treatment of Tumor of Hematopoietic Origin
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
Immunoglobulin and/or Toll-Like Receptor Proteins Associated with Myelogenous Haematological Proliferative Disorders and Uses Thereof
InactiveUS20110044894A1Reduced expression levelAddress bad outcomesIn-vivo radioactive preparationsMicrobiological testing/measurementReceptorMyelogenous
The disclosure relates to methods and compositions effective in the diagnosis, prognosis and treatment of human hematopoietic cancers. In particular, the disclosure provides tumor-associated genes that encode for members of the immunoglobulin (Ig) and / or toll-like receptor superfamilies that are differentially expressed in hematopoietic tumor cells of myeloid origin compared with other cells, e.g., normal stem cells.
Owner:CELLERANT THERAPEUTICS INC
Anti-cd79b antibodies and immunoconjugates and methods of use
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
Compositions and methods for the treatment of tumor of hematopoietic
InactiveUS20110070243A1Cell receptors/surface-antigens/surface-determinantsSugar derivativesMammalHematopoietic Tumor
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:CROWLEY CRAIG +11
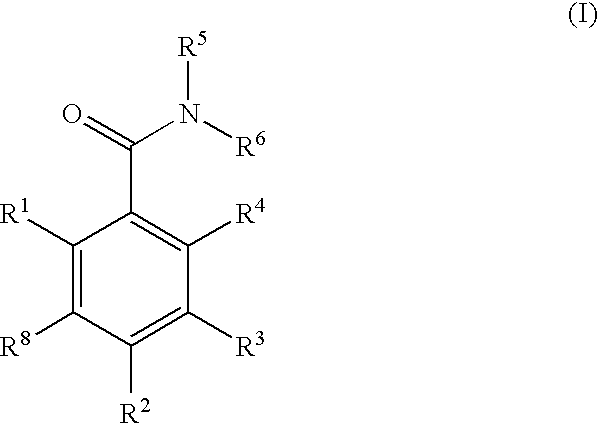
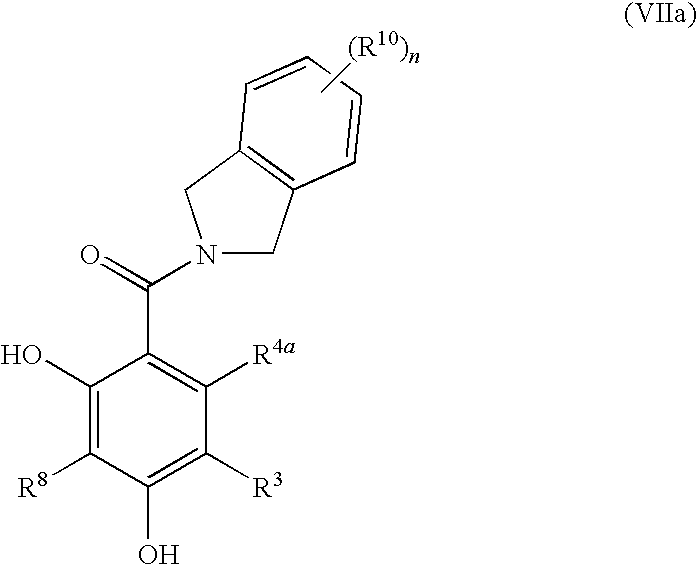
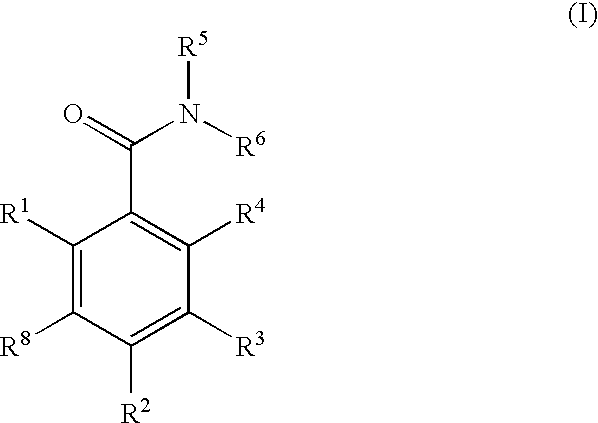
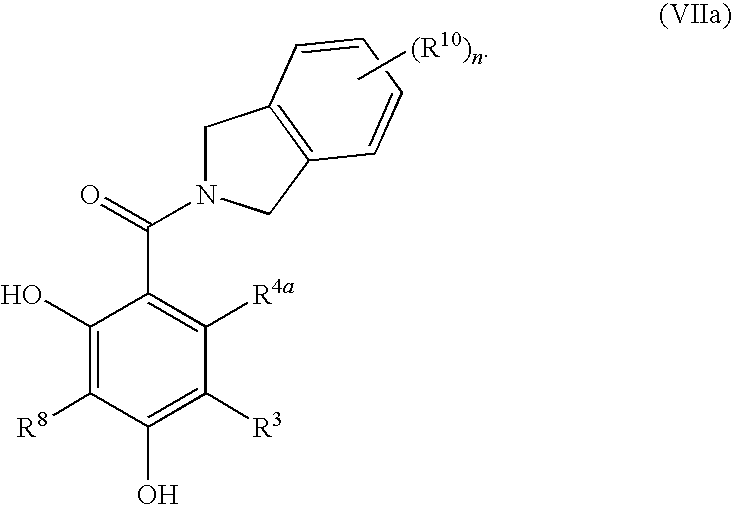
Dihydroxyphenyl isoindolymethanones
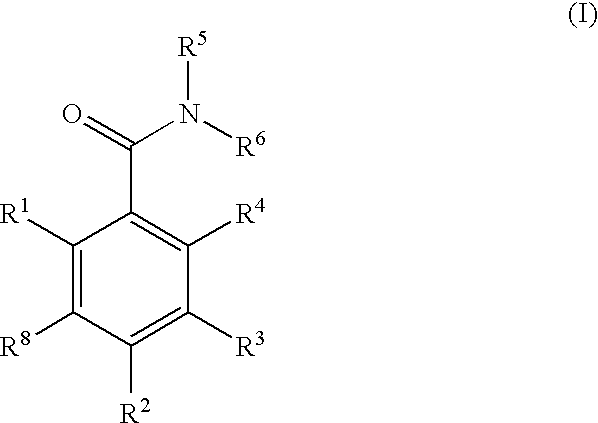
InactiveUS7754725B2Preventing huntington protein aggregationFast rebuildBiocideOrganic chemistryStromal tumorMelanoma
Compounds having the formula (I):and in particular, those of subgenus VIIaare disclosed as inhibits or modulators of the activity of the heat shock protein Hsp90. As such they are useful for treating cancer, particularly hematopoietic tumors of lymphoid or myeloid lineage, prostate cancer, lung cancer, gastrointestinal stromal tumor, breast cancer and melanoma.
Owner:OXFORD FINANCE
Anti-cd79b antibodies and immunoconjugates and methods of use
ActiveUS20110135667A1Organic active ingredientsDipeptide ingredientsHematopoietic TumorCancer research
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
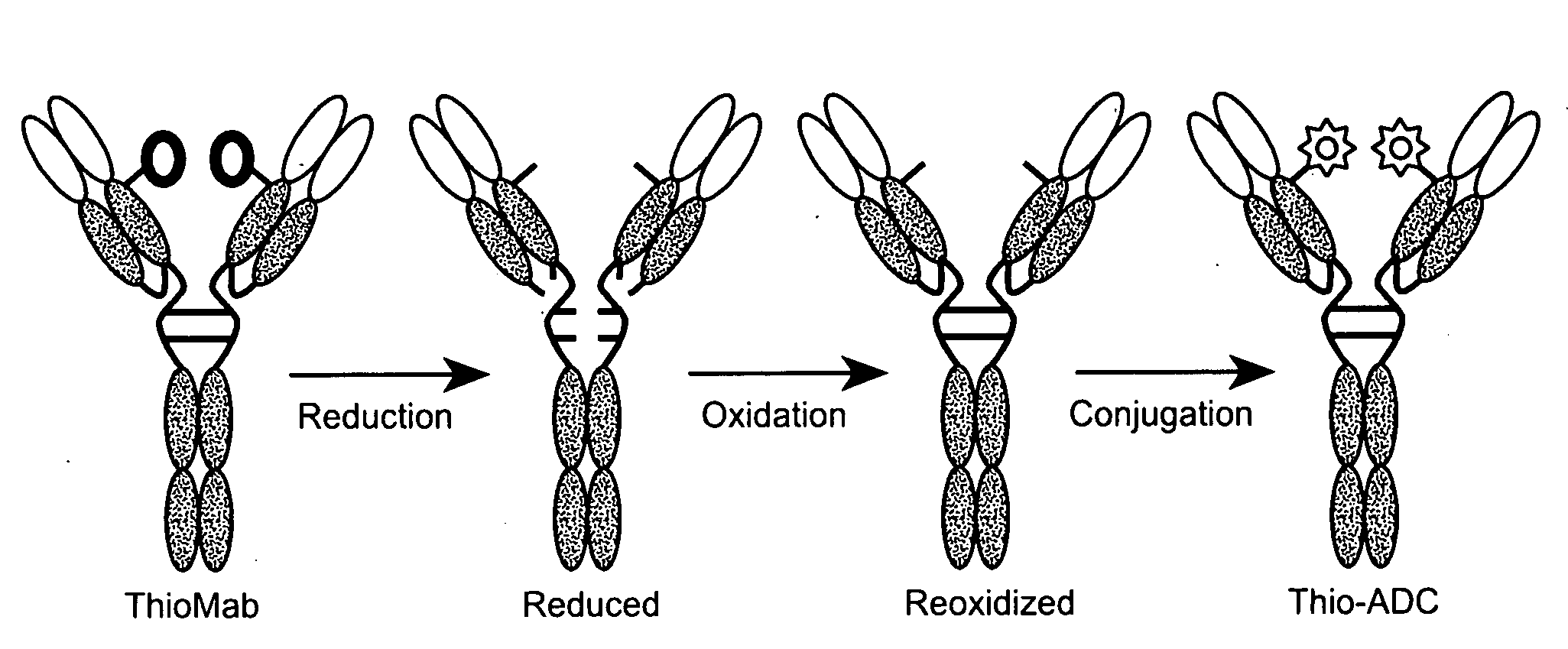
Anti-CD79B antibodies and immunoconjugates and methods of use
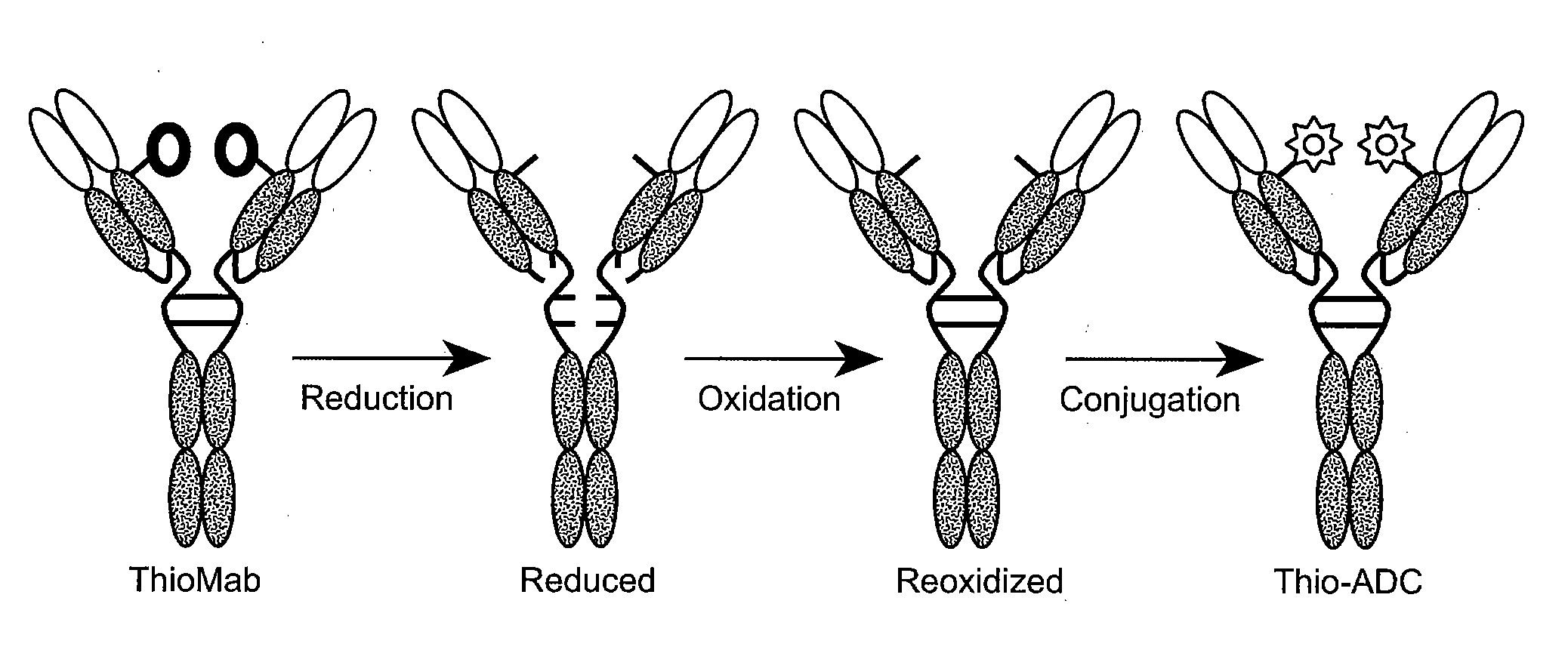
The present invention is directed to cysteine-engineered anti-CD79b antibody, huMA79b.v28, and compositions of matter thereof useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
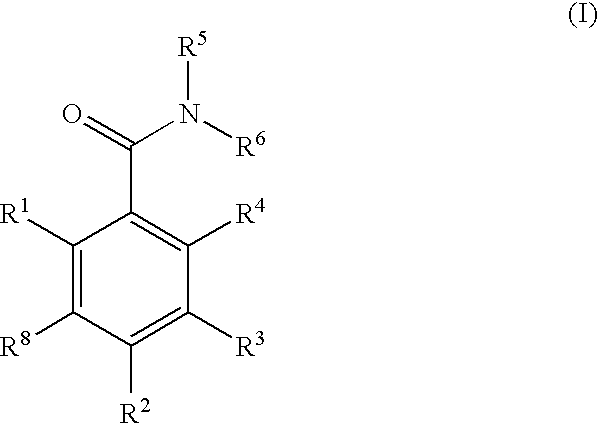
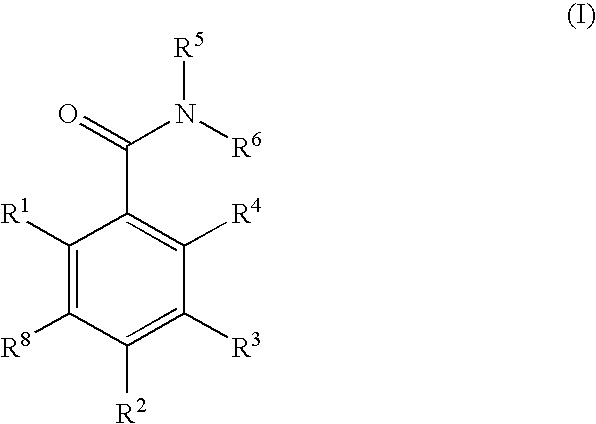
Dihydroxyphenyl isoindolylmethanones
InactiveUS20070259886A1Preventing huntington protein aggregationEffective treatmentBiocideOrganic chemistryStromal tumorMelanoma
Compounds having the formula (I): and in particular, those of subgenus VIIa are disclosed as inhibits or modulators of the activity of the heat shock protein Hsp90. As such they are useful for treating cancer, particularly hematopoietic tumors of lymphoid or myeloid lineage, prostate cancer, lung cancer, gastrointestinal stromal tumor, breast cancer and melanoma.
Owner:OXFORD FINANCE
ANTI-FcRH5 ANTIBODIES AND IMMUNOCONJUGATES AND METHODS OF USE
InactiveUS20110171125A1Effectively preventingEffectively treatingBlood/immune system cellsImmunoglobulinsMammalHematopoietic Tumor
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:F HOFFMANN LA ROCHE & CO AG
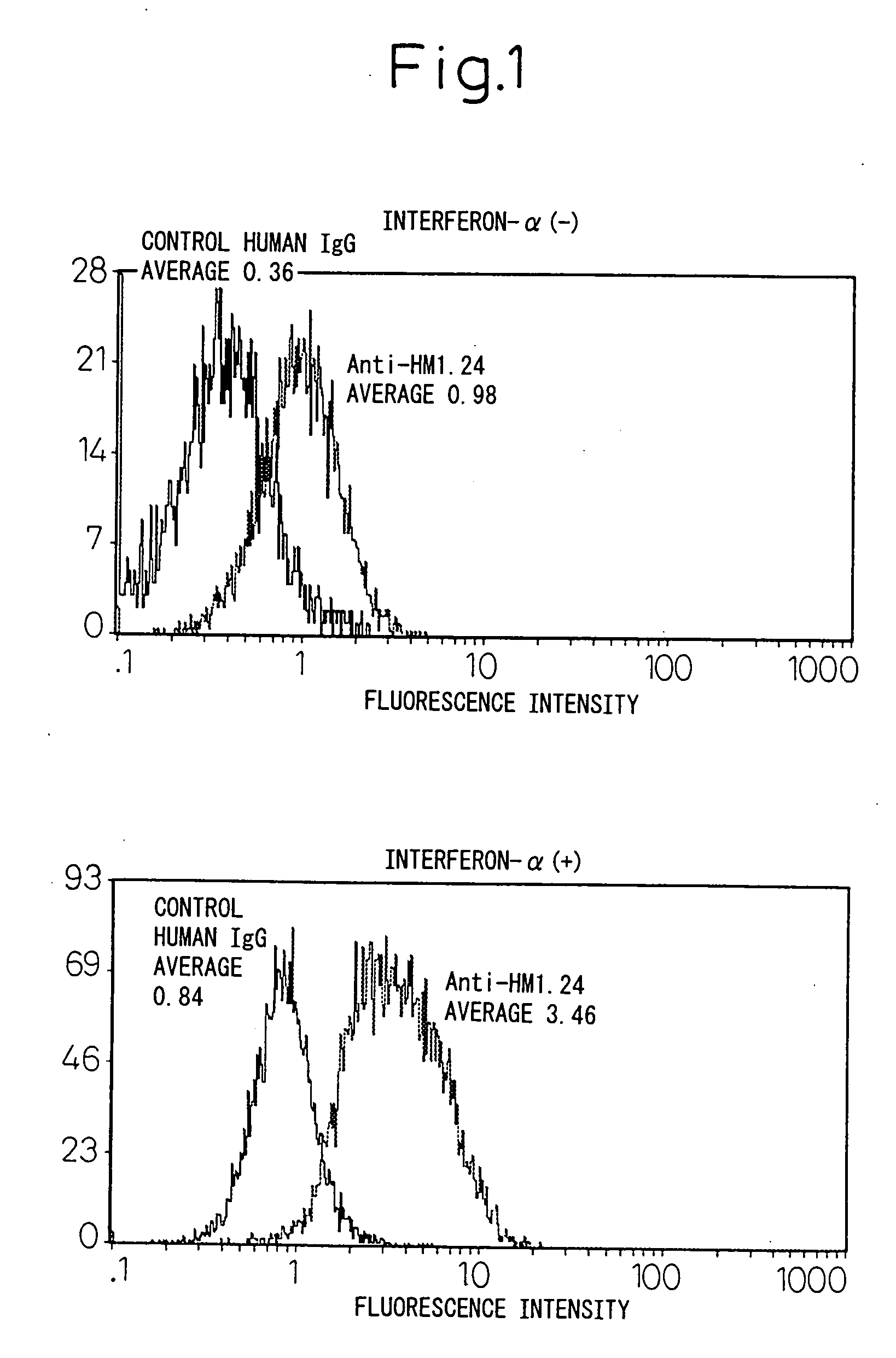
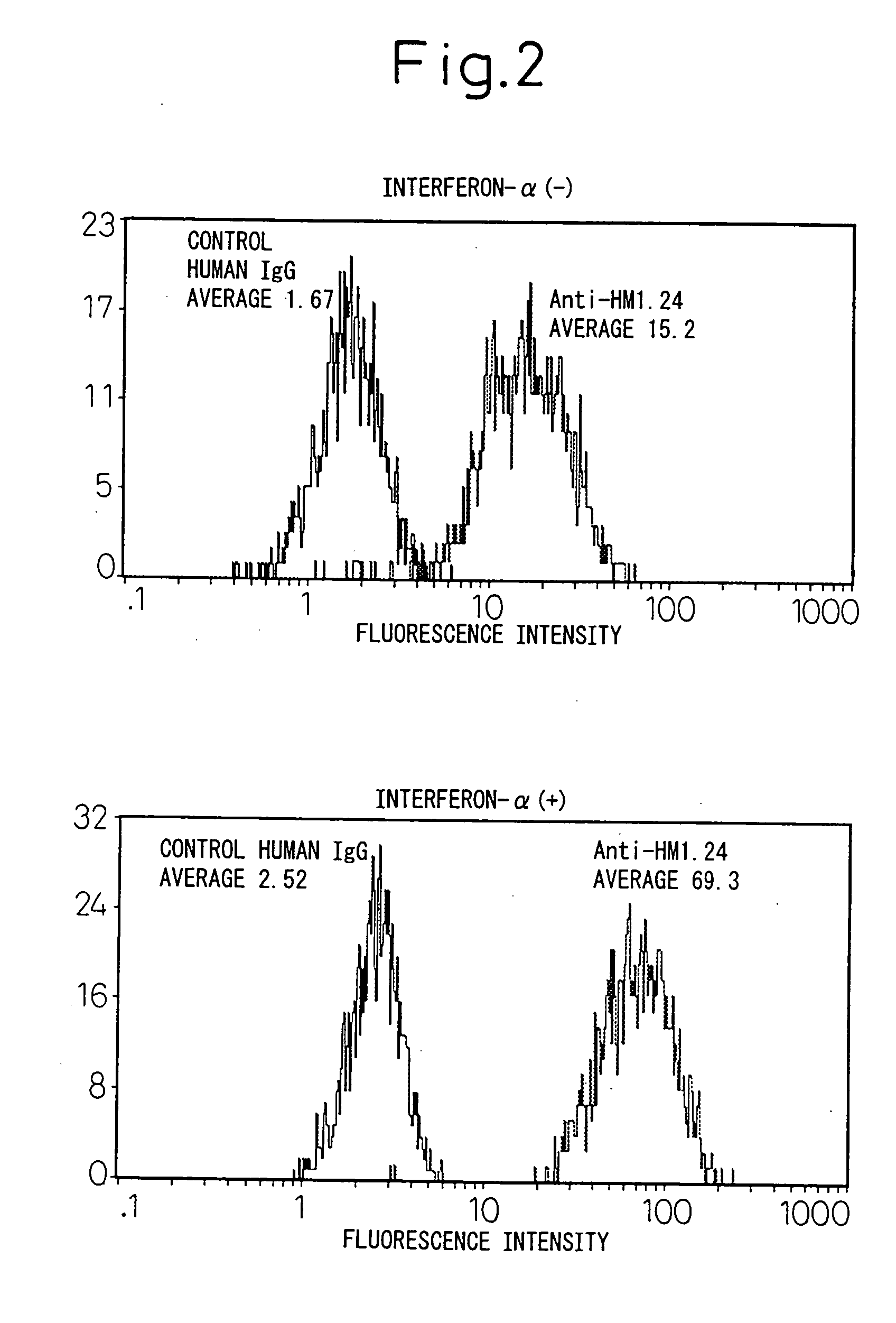
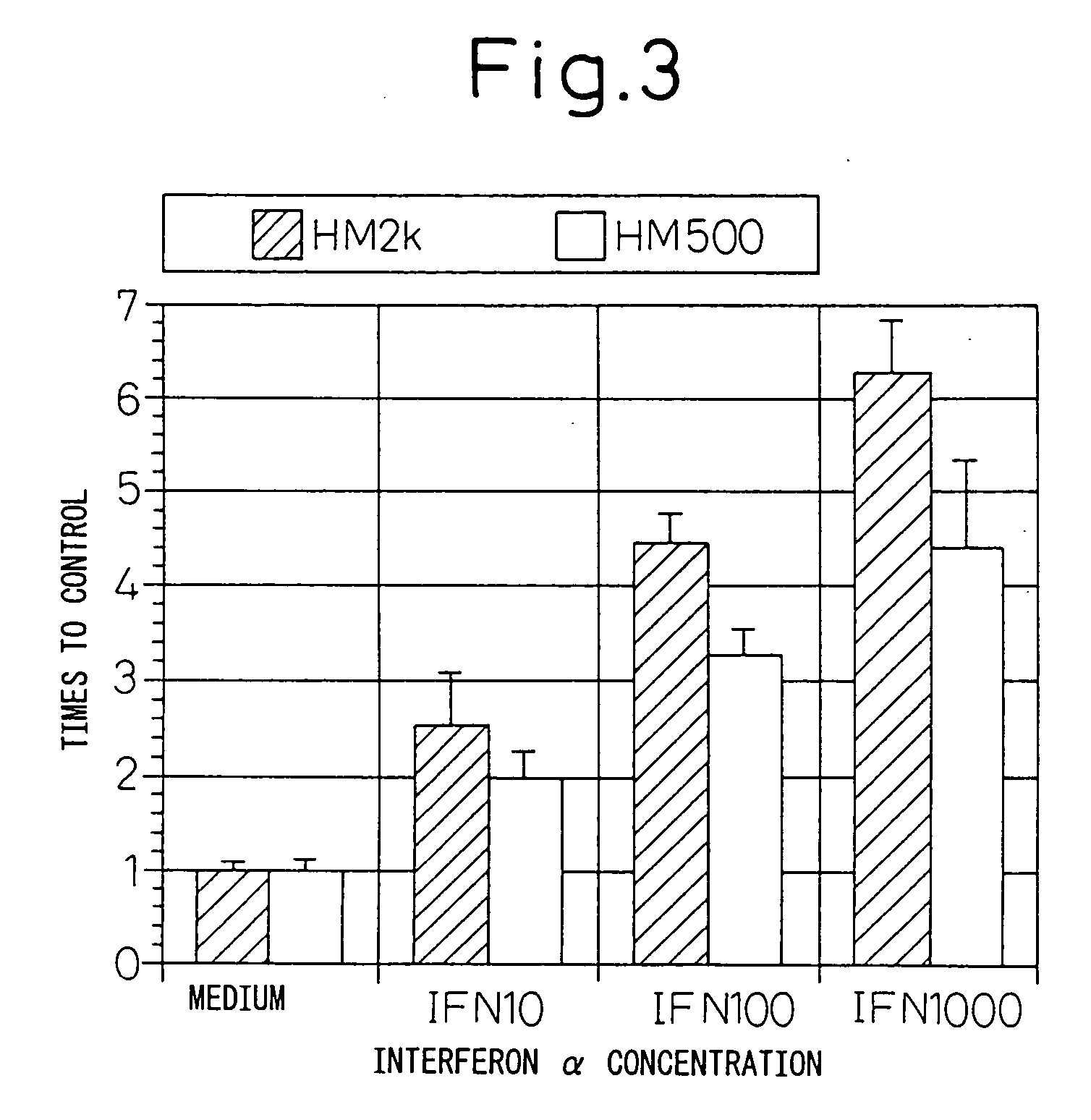
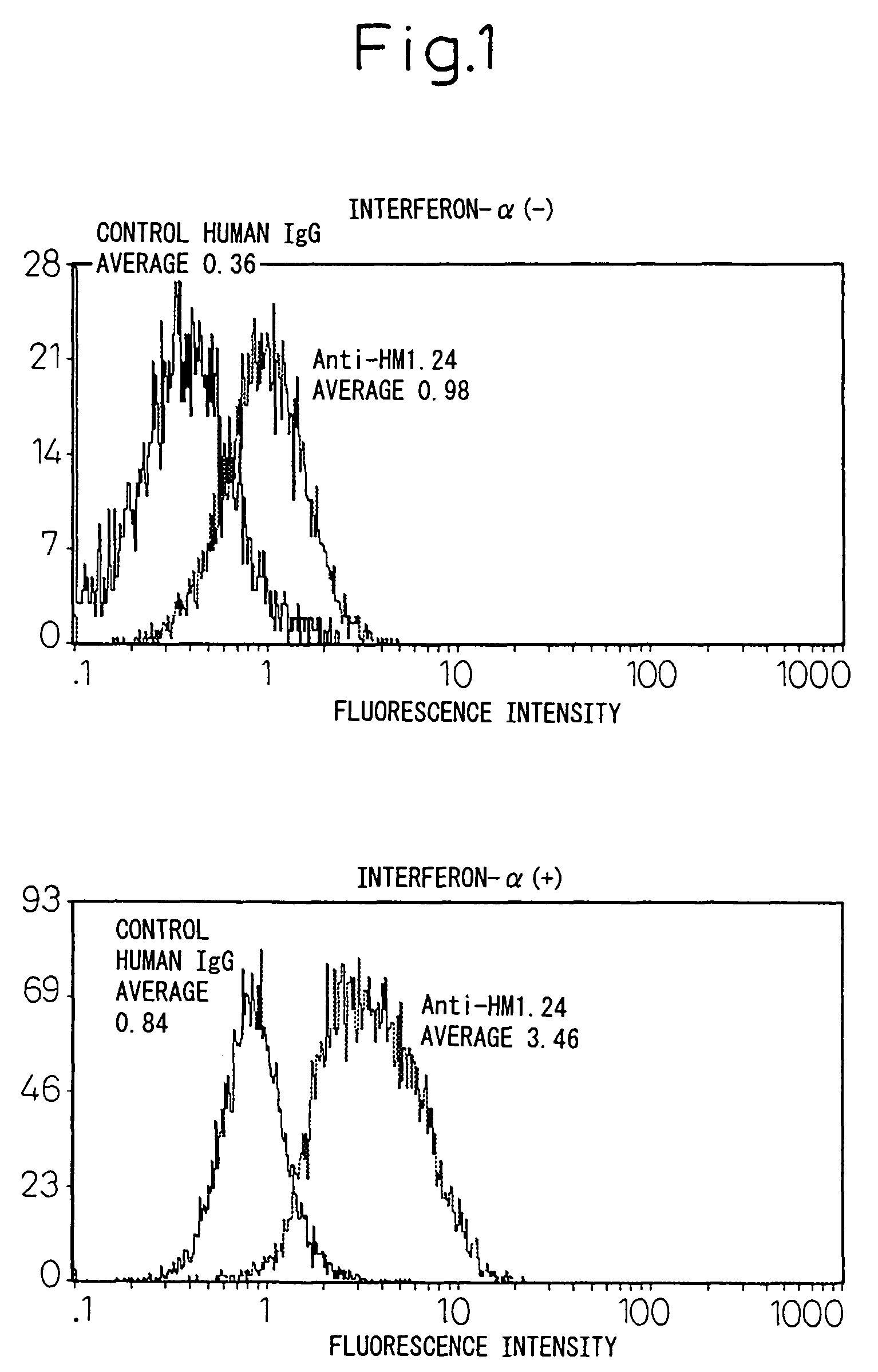
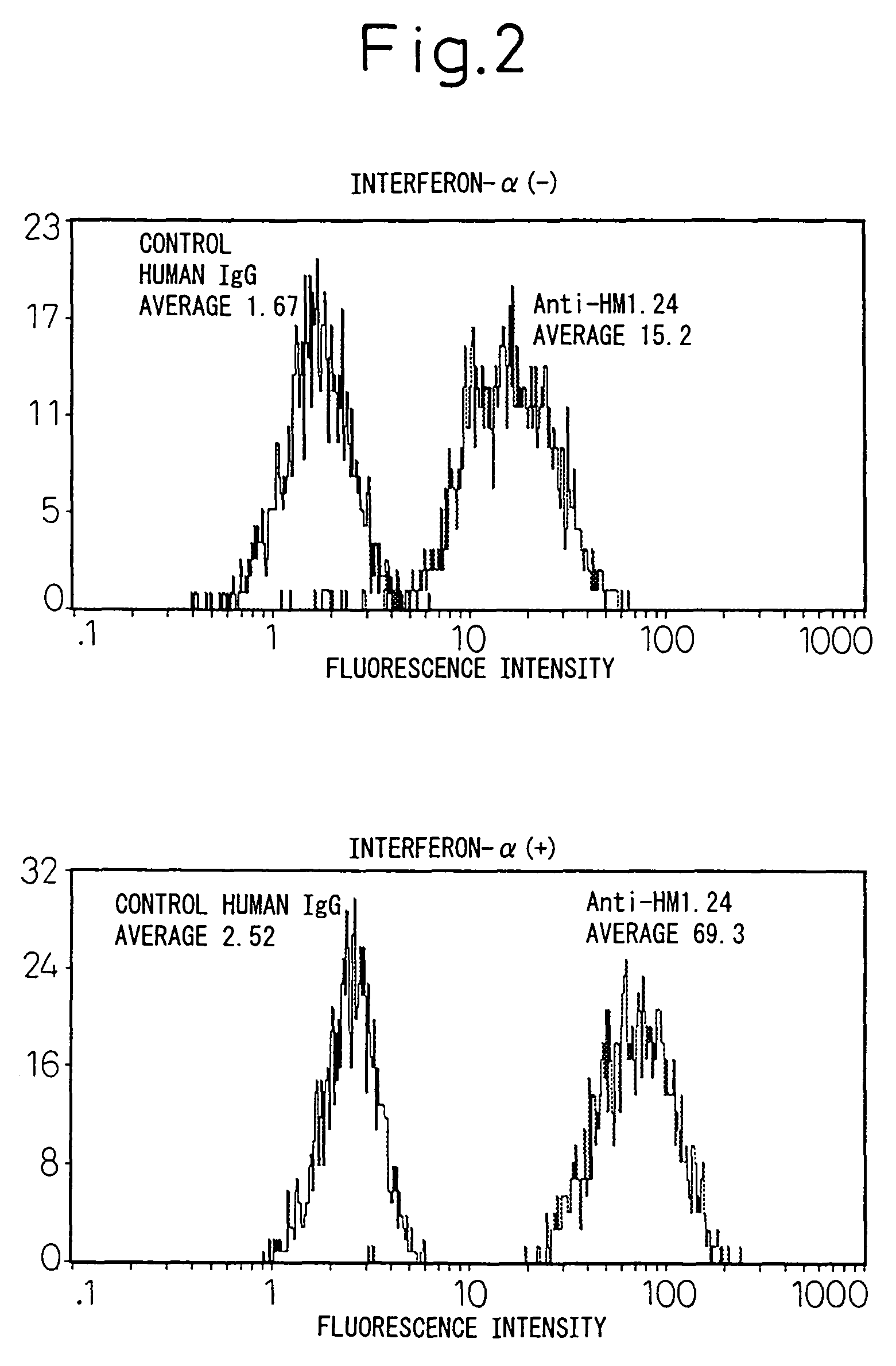
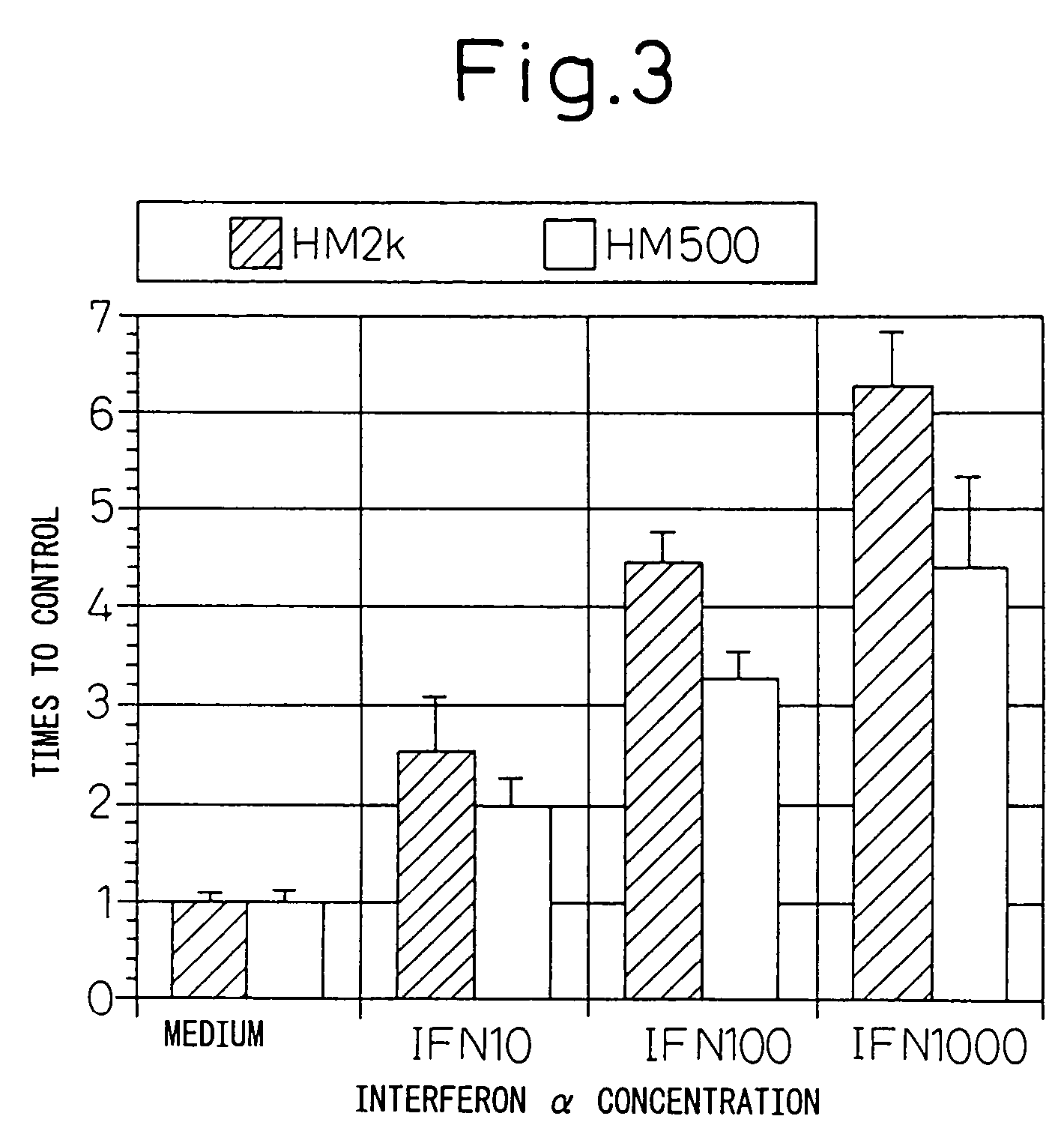
Remedies for tumor in hematopoietic organs
InactiveUS20060078539A1Improve survivalExhibit some effectPeptide/protein ingredientsSkeletal disorderAntigenAbnormal tissue growth
An inducing agent or enhancing agent, for the expression of HM1.24 antigen in hematopoietic tumor cells, comprising interferon α, interferon γ, or the IRF-2 protein as an active ingredient, as well as an anti-tumor agent for hematopoietic tumors which comprises a combination of said inducing agent or enhancing agent and an antibody against HM1.24.
Owner:CHUGAI PHARMA CO LTD
ANTI-FcRH5 ANTIBODIES AND IMMUNOCONJUGATES AND METHODS OF USE
InactiveUS20130089555A1Heavy metal active ingredientsPeptide/protein ingredientsHematopoietic TumorCancer research
The present invention is directed to compositions of matter useful for the treatment of hematopoietic tumor in mammals and to methods of using those compositions of matter for the same.
Owner:GENENTECH INC
Methods of identifying and isolating stem cells and cancer stem cells
InactiveUS20070238127A1Microbiological testing/measurementBlood/immune system cellsHematopoietic cellΒ catenin signaling
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Kit for detecting expression index of mRNA (messager Ribose Nucleic Acid) of WT1 (Wilms Tumor 1) gene
ActiveCN102912018AStrong specificityImprove accuracyMicrobiological testing/measurementFluorescence/phosphorescenceNewly diagnosedDisease monitoring
The invention relates to a kit for detecting an expression index of mRNA (messager Ribose Nucleic Acid) of a WT1 (Wilms Tumor 1) gene, and belongs to the field of biotechnology. The kit comprises detection primers, a fluorescent probe, a cDNA (complementary Deoxyribose Nucleic Acid) first strand synthesis reagent, a fluorescent quantitative PCR (Polymerase Chain Reaction) mixed solution, negative reference and positive reference, wherein the detection primers and the fluorescent probe comprise a WT1 gene primer, an internal reference gene ABL primer and a Taqman fluorescent probe. The WT1 gene is related with hematopoietic tumor incidence, is of over-expression in about 80% of patients with newly diagnosed acute myelocytic leukemia and acute lymphocytic leukemia, is recognized as a leukemia marker gene, and can serve as an independent minimal residue disease monitoring and prognosis prompting index. The level of the mRNA of the WT1 gene is detected by adopting a fluorescent quantitative PCR technology with higher sensitivity and specificity, and both the specificity and the sensitivity of a detection result are remarkably improved. The kit provides a brand-new quick, simple and convenient gene diagnosis technology for prognosing the acute myelocytic leukemia and the acute lymphocytic leukemia and confirming chemotherapy regimens.
Owner:童永清 +1
Methods and compositions for detecting non-hematopoietic cells from a blood sample
InactiveUS20140073536A1Aid in diagnosis and prognosisPromote recoveryOther blood circulation devicesUltrafiltrationHematopoietic cellRare cell
The present invention recognizes that diagnosis and prognosis of many conditions can depend on the enrichment of rare cells, especially tumor cells, from a complex fluid sample such as a blood sample. In particular, the present invention is directed to methods and compositions for detecting a non-hematopoietic cell, e.g., a non-hematopoietic tumor cell, in a blood sample via, inter alia, removing red blood cells (RBCs) from a blood sample using a non-centrifugation procedure, removing white blood cells (WBCs) from said blood sample to enrich a non-hematopoietic cell, if any, from said blood sample; and assessing the presence, absence and / or amount of said enriched non-hematopoietic cell.
Owner:AVIVA BIOSCI
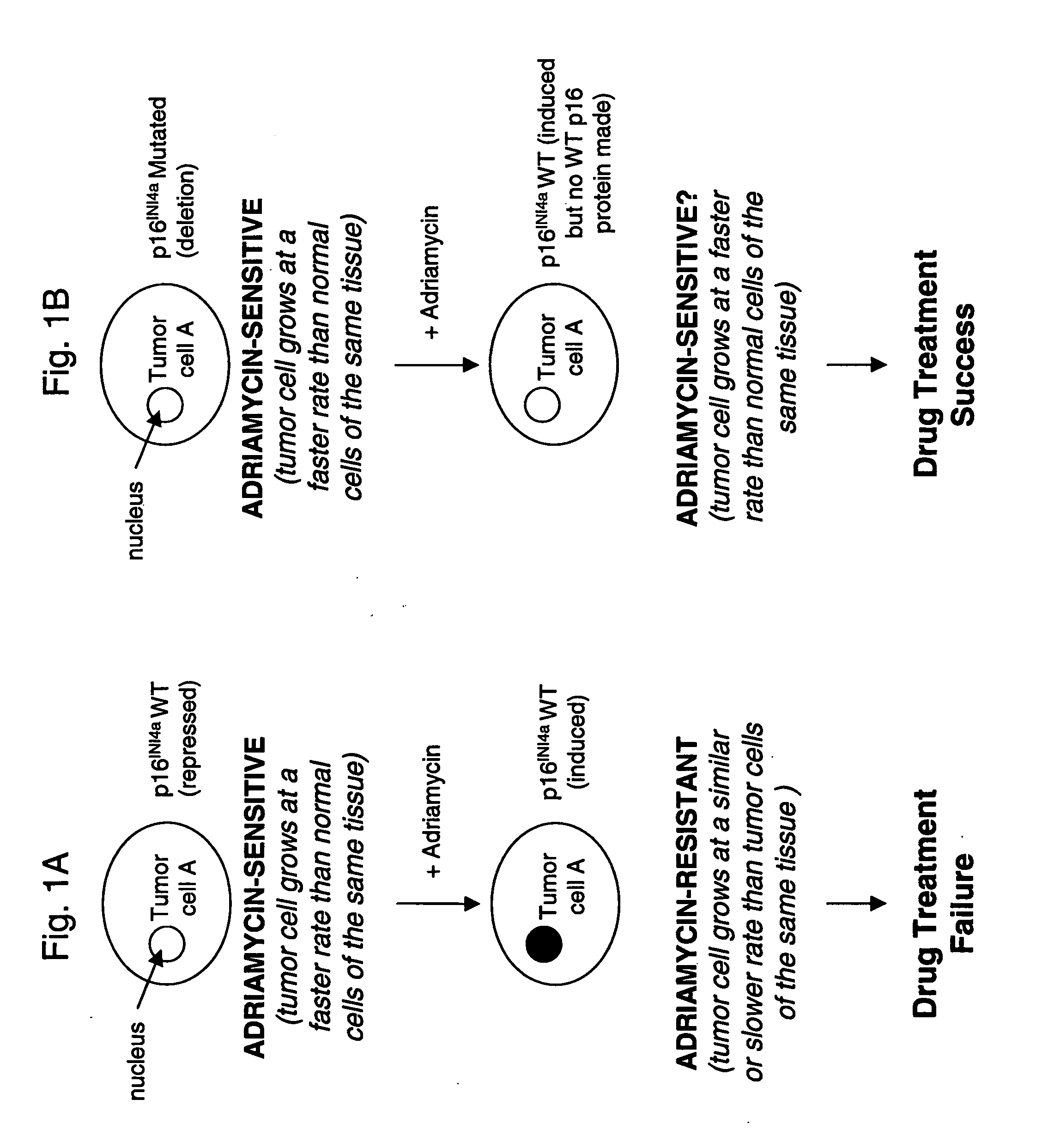
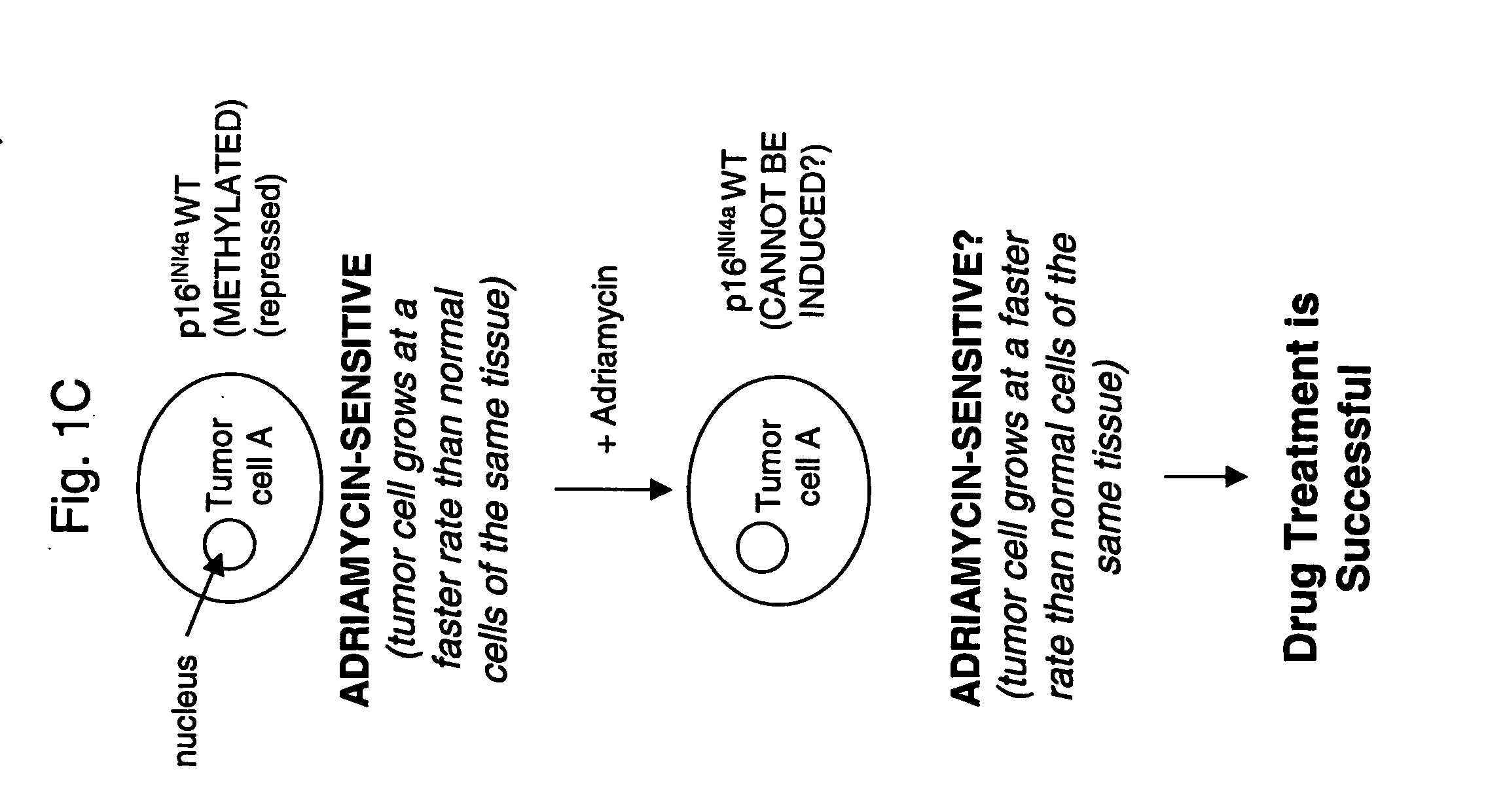
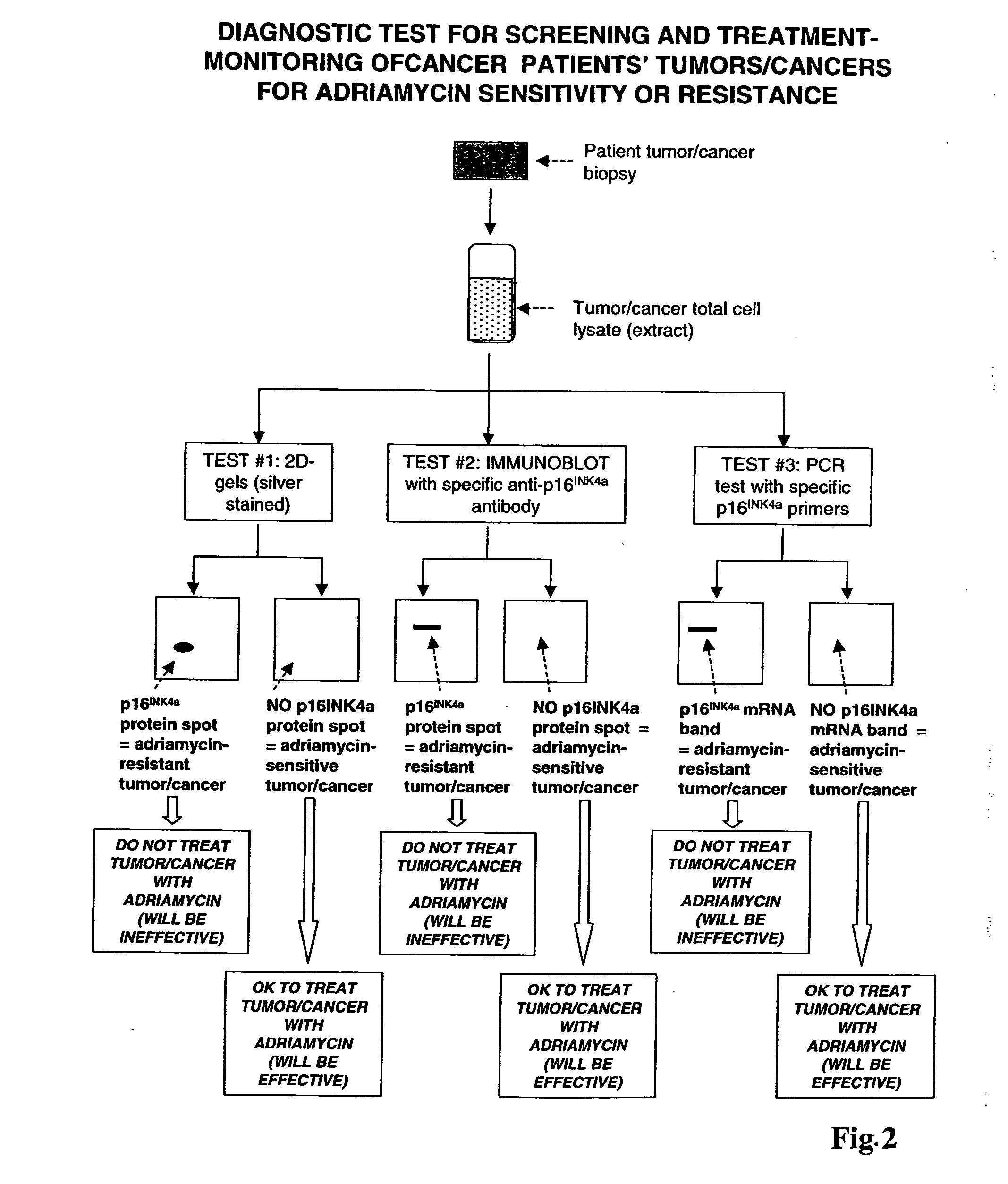
Methods for identifying chemotherapeutic resistance in non-hematopoietic tumors
InactiveUS20060188507A1Increase drug sensitivitySilencing of p16 expressionBiocideGenetic material ingredientsMultiple Tumor Suppressor-1Hematopoietic Tumor
Disclosed are methods for detecting adriamycin resistance in a test neoplastic cell from a non-hematological cancer. The methods include detecting a level of p16 expression in the test neoplastic cell of a given origin or cell type, and comparing the level of p16 expression detected in the test neoplastic cell to the level of p16 expression in a nonresistant neoplastic cell of the same origin or cell type, wherein the test neoplastic cell is adriamycin resistant if the level of p16 expression is greater than the level of p16 expression in the nonresistant neoplastic cell of the same origin or cell type. Also disclosed are therapeutic compositions comprising an agent that inhibits p16 and a pharmaceutically acceptable carrier.
Owner:AURELIUM BIOPHARMA
Therapeutic agent for hematopoietic tumors
InactiveUS7931897B2Good effectIncrease volumePeptide/protein ingredientsSkeletal disorderAntigenHematopoietic Tumor
An inducing agent or enhancing agent, for the expression of HM1.24 antigen in hematopoietic tumor cells, comprising interferon α, interferon γ, or the IRF-2 protein as an active ingredient, as well as an anti-tumor agent for hematopoietic tumors which comprises a combination of said inducing agent or enhancing agent and an antibody against HM1.24.
Owner:CHUGAI PHARMA CO LTD
Dihydroxyphenyl isoindolylmethanones
Owner:ASTEX THERAPEUTICS LTD
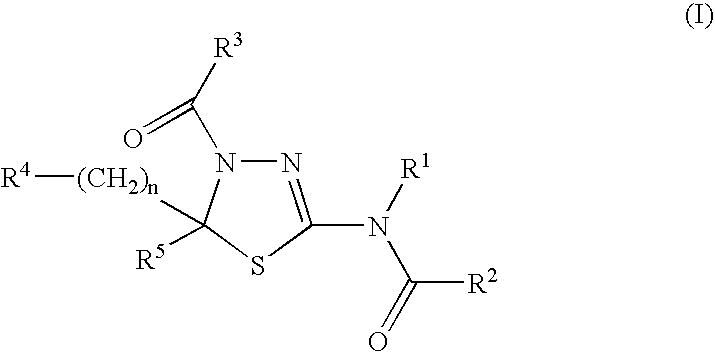
Therapeutic Agent for Hematopoietic Tumor
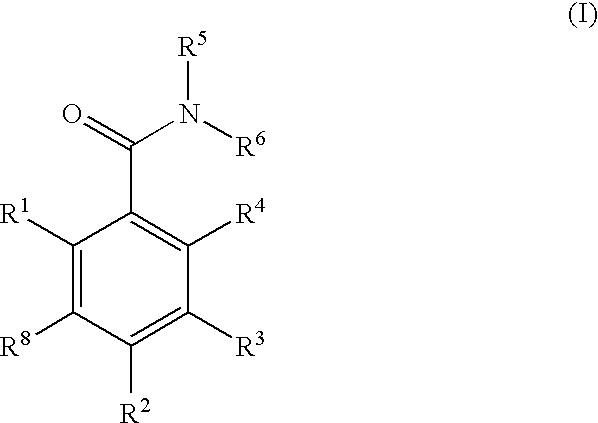
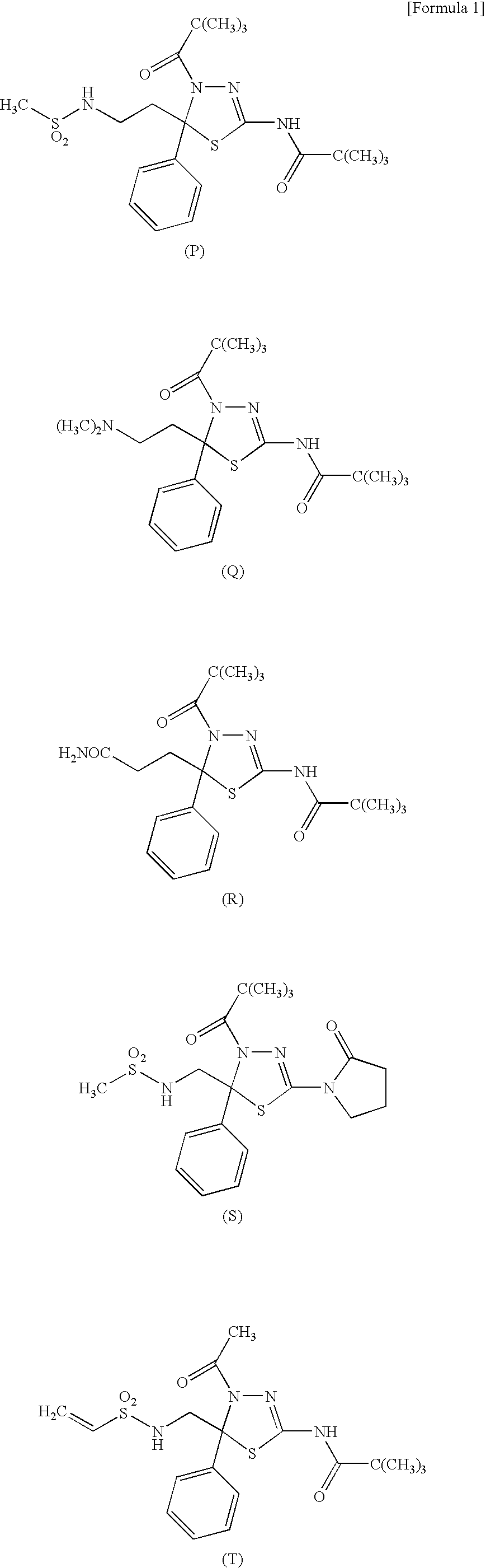
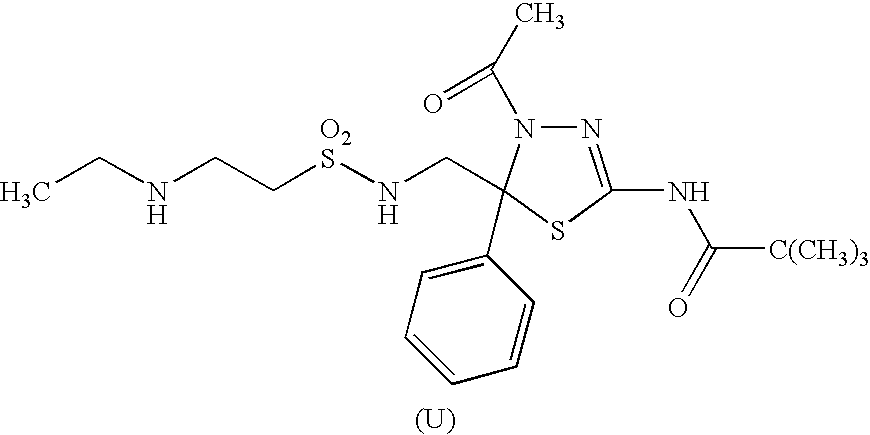
A therapeutic and / or prophylactic agent for a hematopoietic tumor, which comprises a thiadiazoline derivative represented by the general formula (I), or a pharmaceutically acceptable salt thereof:[Formula 1][wherein, n represents an integer of 1 to 3, R1 represents a hydrogen atom, R2 represents lower alkyl, or R1 and R2 are combined together to represent alkylene, R3 represents lower alkyl, R4 represents NHSO2R6 (wherein R6 represents hydroxy or the like) or the like, and R5 represents aryl or the like] and the like are provided.
Owner:KYOWA HAKKO KIRIN CO LTD +1
Diagnosis marker for rare hematopoietic tumor, test method, therapeutic agent, and screening method
PendingUS20190017125A1Microbiological testing/measurementDisease diagnosisScreening methodTherapeutic effect
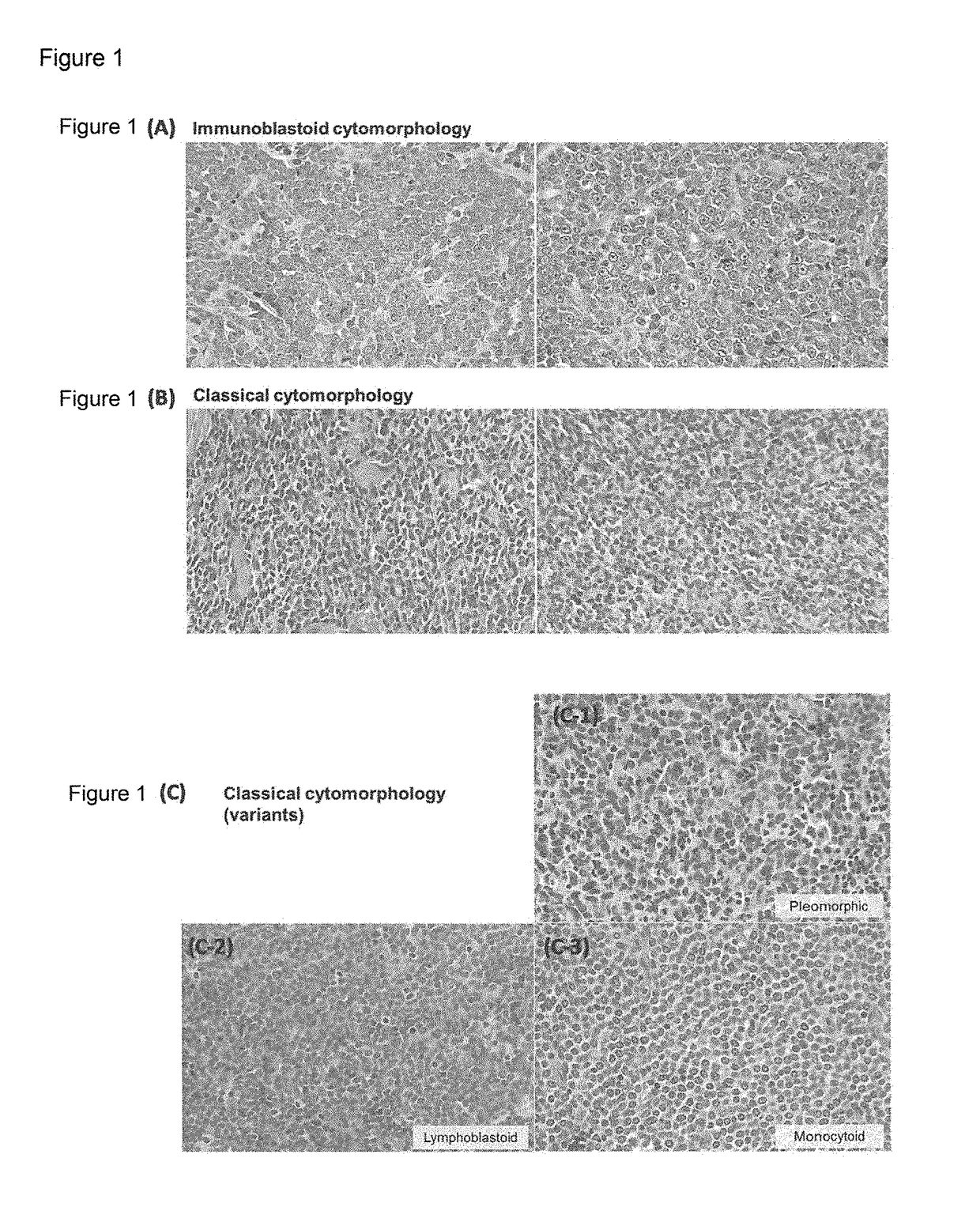
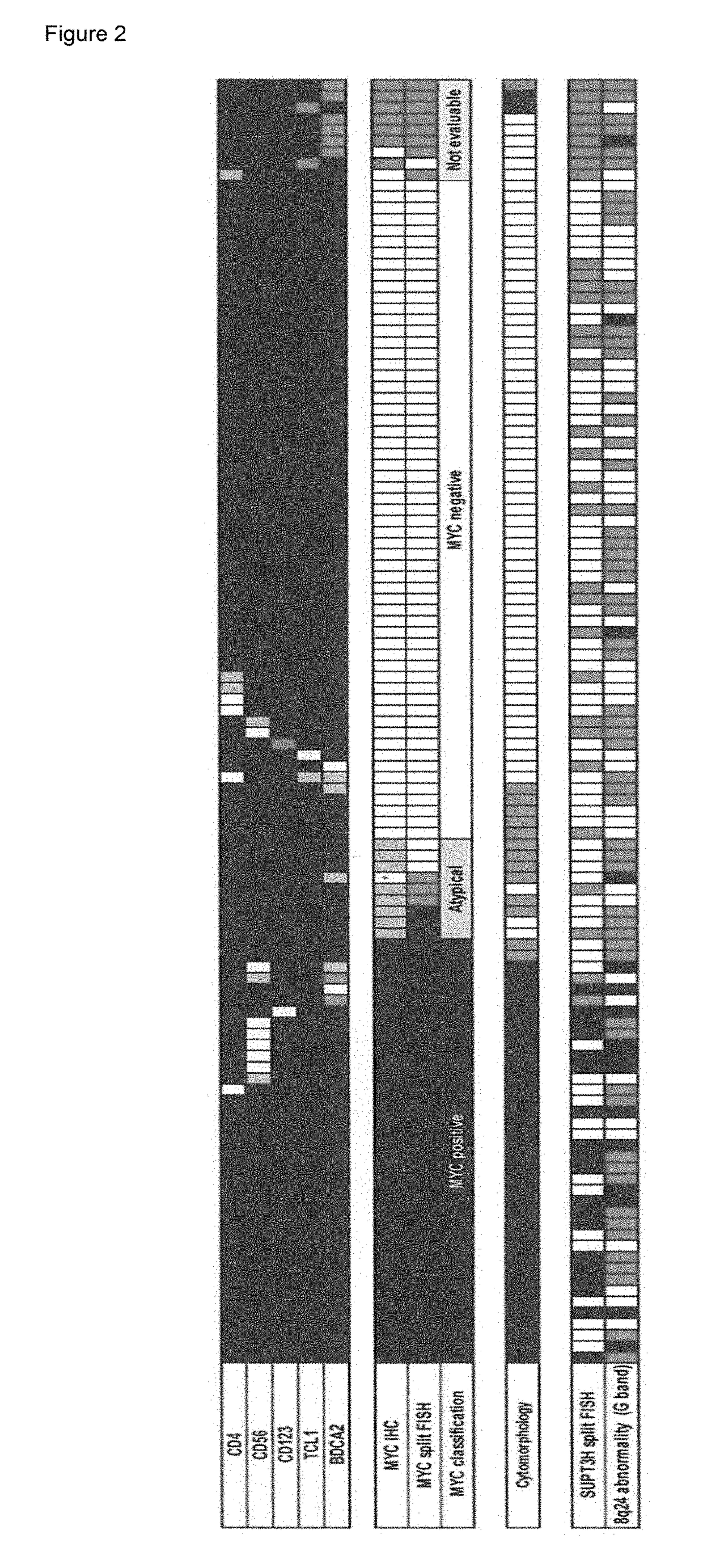
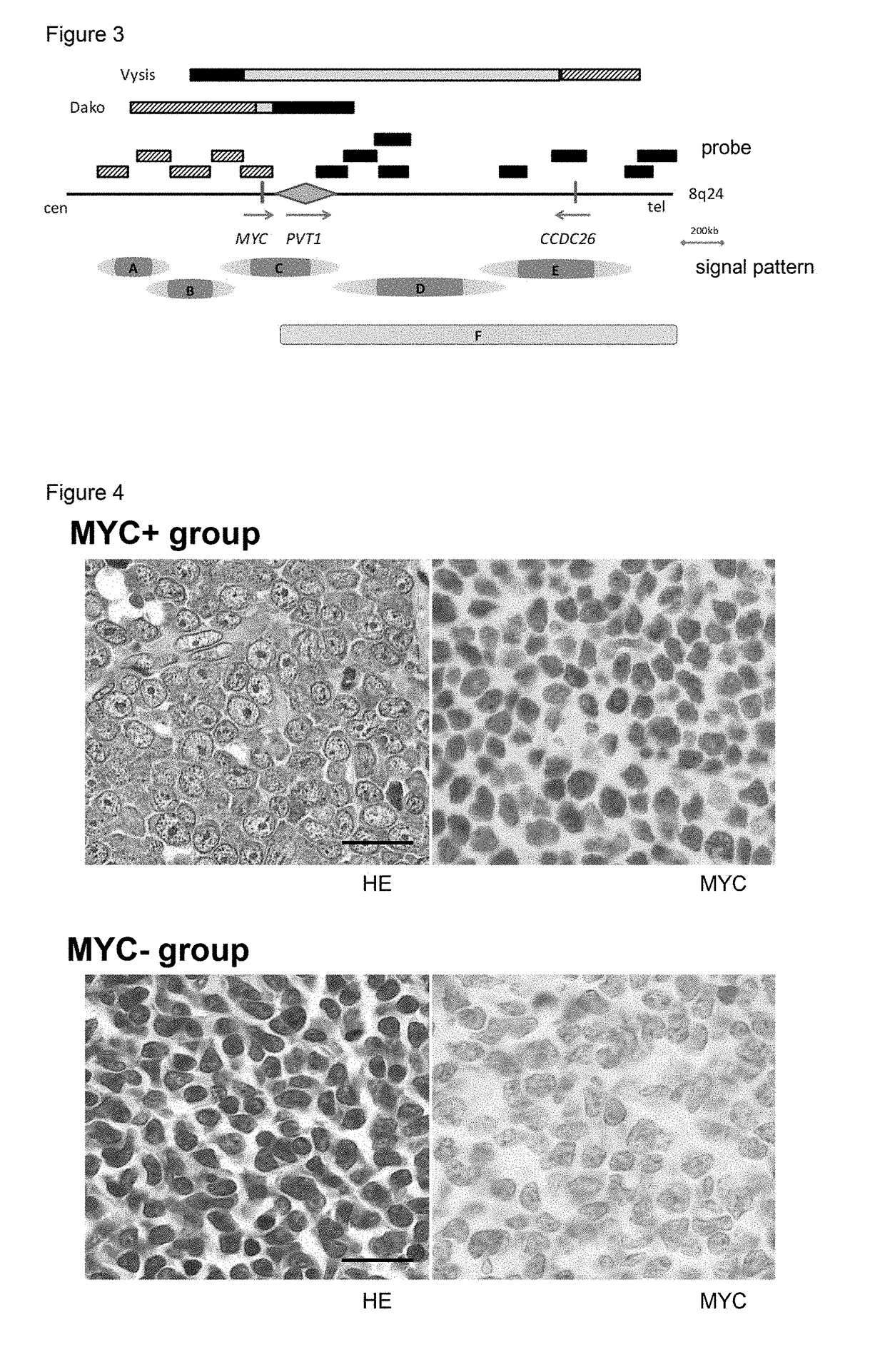
The diagnostic markers that provide novel diagnostic criteria to blastic plasmacytoid dendritic cell neoplasm (BPDCN) has been searched, and the presence of immunoblastoid cytomorphology, 8q24 rearrangement, and MYC expression were established as novel markers for subtyping BPDCN. It has been further found that the inhibitors which directly or indirectly inhibit the expression, functions, or signaling pathways of MYC, such as BET bromodomain-selective inhibitors or aurora kinase inhibitors, are effective in MYC-positive BPDCN, and HDAC inhibitors or BCL2 family protein inhibitors are effective as therapeutic drugs for BPDCN.
Owner:JAPANESE FOUND FOR CANCER RES
Method for the prognosis and/or treatment of acute promyelocytic leukemia
InactiveUS20190284635A1Lower Level RequirementsReducing methylationOrganic active ingredientsMicrobiological testing/measurementEZH2Acetylation
The present invention relates to a method for the diagnosis of low overall survival acute promyelocytic leukemia and / or of predicting and / or monitoring the response and / or the efficacy of a therapy for acute promyelocytic leukemia or to identify a subject to be treated with an inhibitor of HAT and / or an inhibitor of EZH2 by determining the acetylation or methylation status of specific relevant regions and relative kit and microarray. The invention also refers to histone acetyl transferase (HAT) inhibitor for use in the treatment of a solid or hematopoietic tumor.
Owner:EPI C SRL
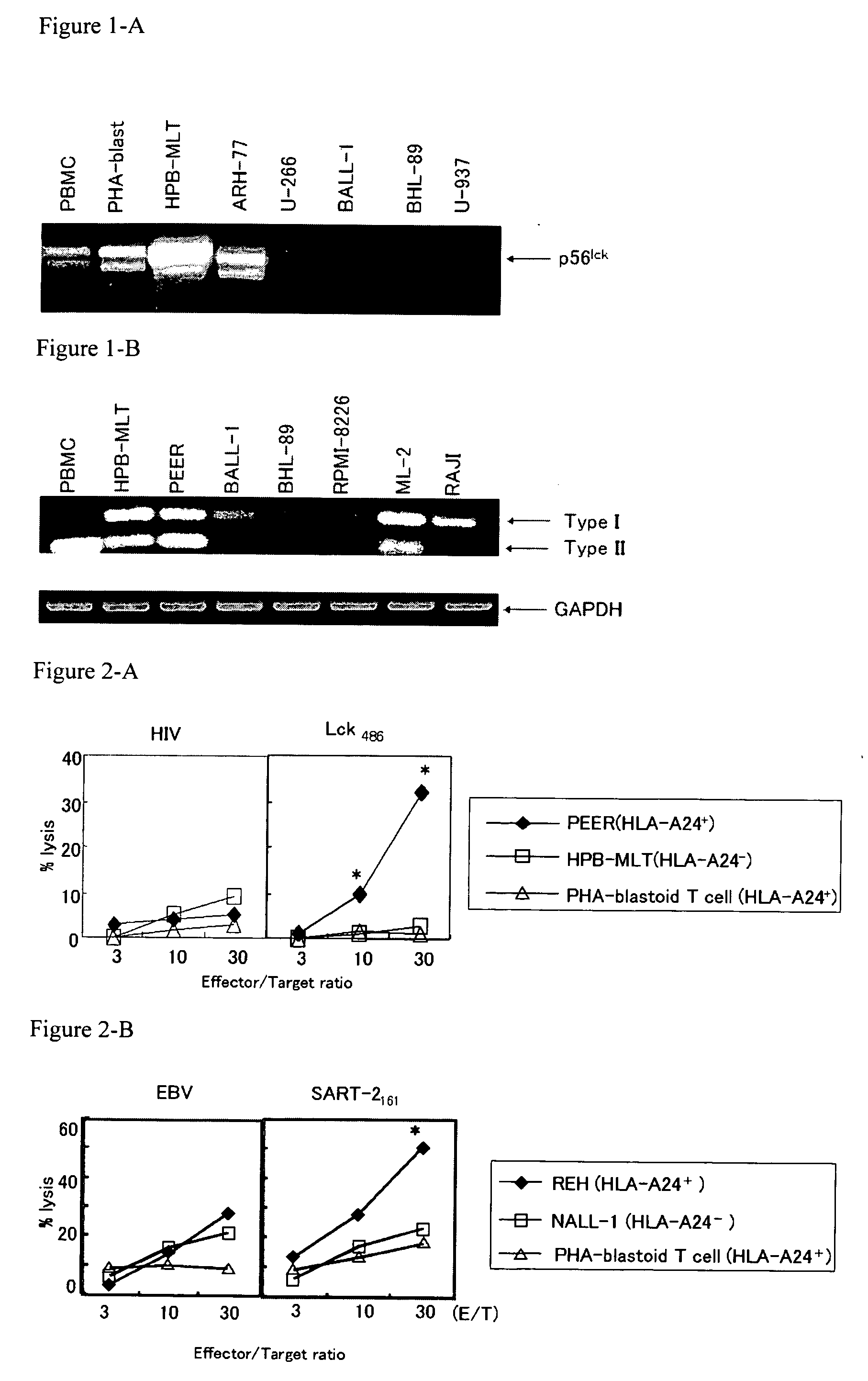
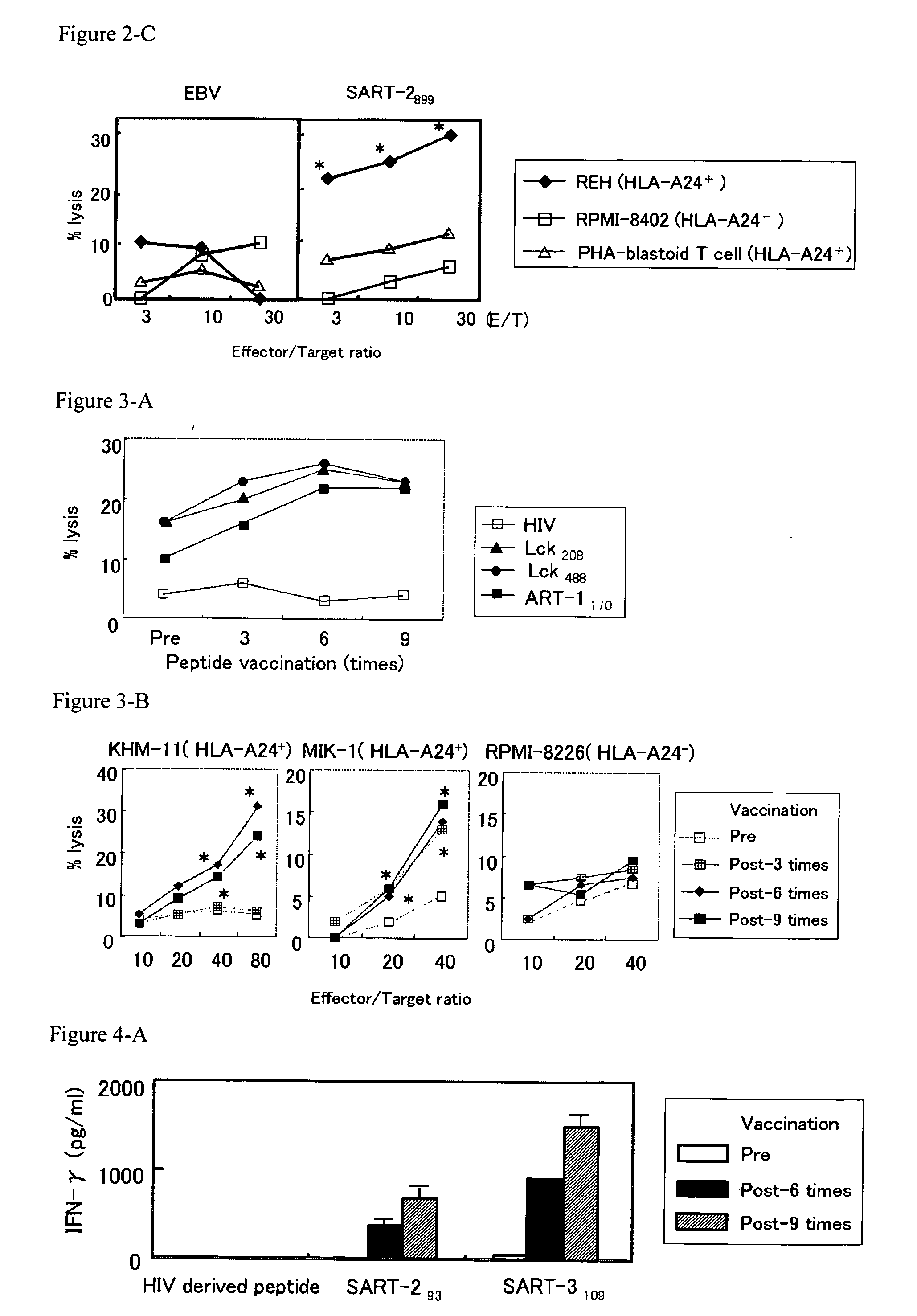
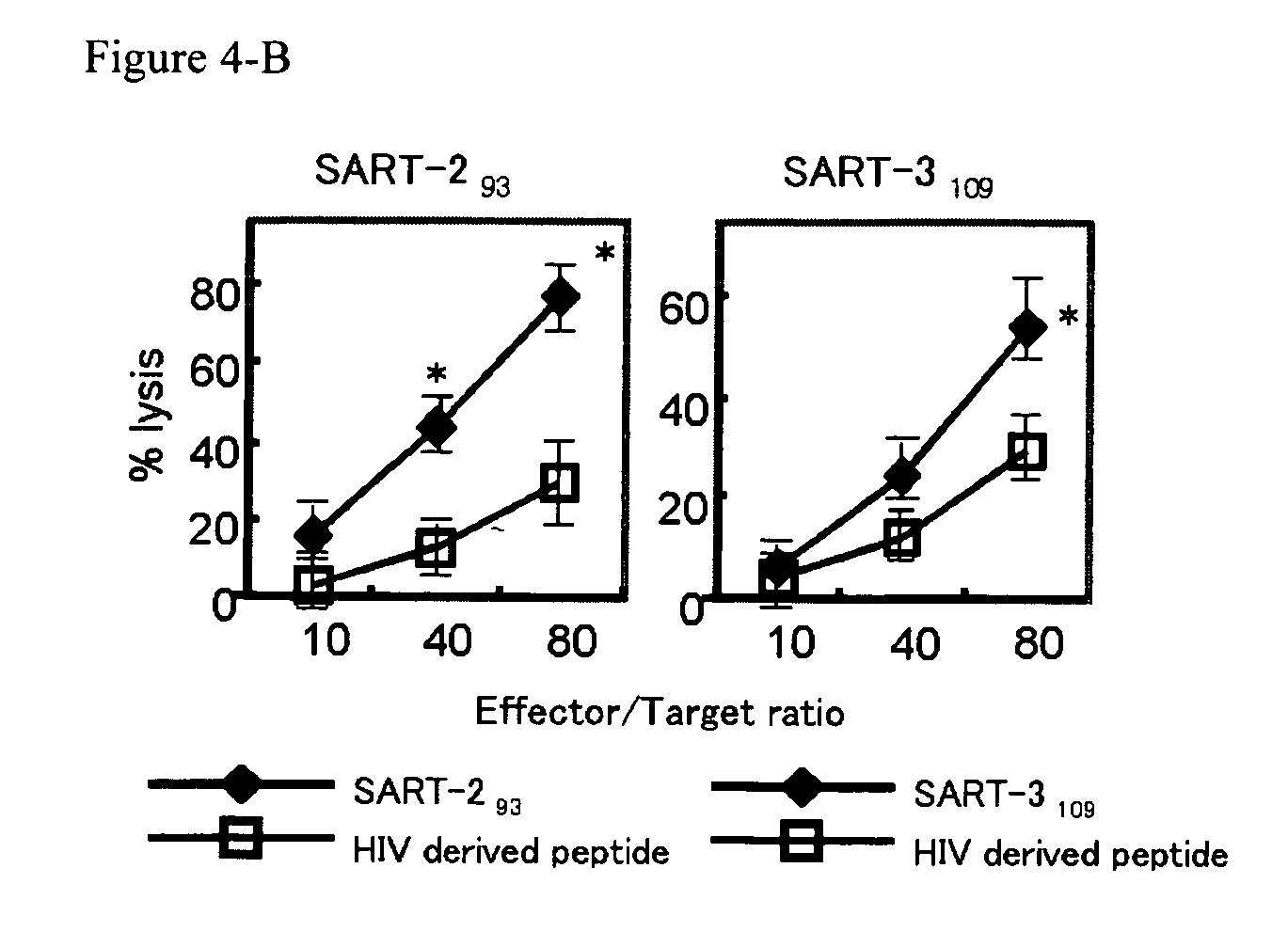
Preventing and/or remedy hematopoietic tumor
InactiveUS20060240026A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsAdjuvantHematopoietic Tumor
In the present invention, a useful molecular target for a specific immunotherapy of hematologic malignancies was found, and a means capable of preventing and / or treating hematologic malignancies was provided. Specifically, provided were the followings: an agent for preventing and / or treating hematologic malignancies comprising as an active ingredient at least one peptide having an amino acid sequence selected from any one of SEQ ID NOs: 1 to 10 in the sequence listing; the agent for preventing and / or treating hematologic malignancies further comprising an adjuvant; and the preventing and / or treating agent to use as a cancer vaccine for hematologic malignancies, which allowed prevention and / or treatment of hematologic malignancies such as an HLA-A24 positive hematologic malignancies or the hematologic malignancies that are HLA-A24 positive and express a protein containing the peptide.
Owner:GREEN PEPTIDE CO LTD
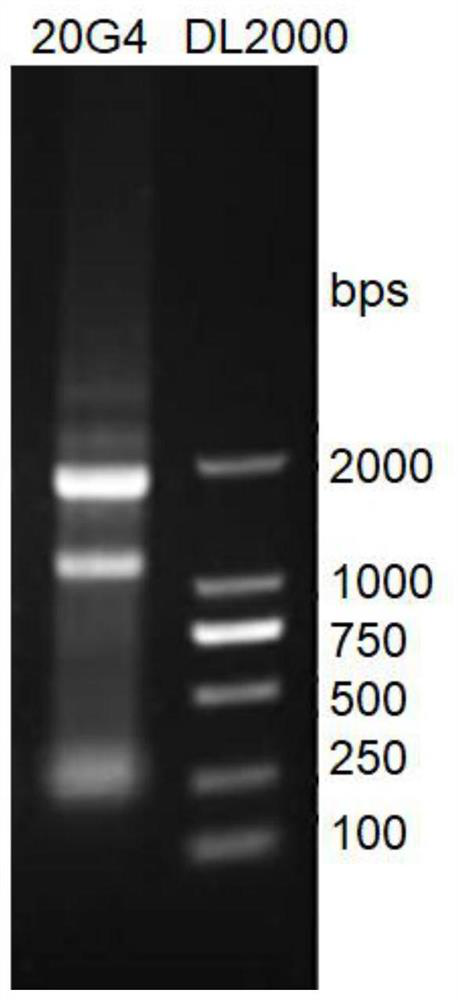
Anti-pax-5 protein monoclonal antibody and its cell line, preparation method and application
ActiveCN108623683BStrong characteristicIncreased sensitivityImmunoglobulins against animals/humansMicroorganism based processesAntiendomysial antibodiesHematopoietic Tumor
The invention relates to a monoclonal antibody 20G4 capable of recognizing human B cell-specific activation protein Pax-5, a secreting cell line, a preparation method of an immunogen and an application in immunoassay. Pax‑5, also known as B cell-specific activation protein, is expressed in the early stage of B cell differentiation, and Pax‑5 is expressed in 97% of Hodgkin's lymphoma Reed‑Sternberg cells, and it has been It has been used as a marker of hematopoietic tumor cells of B cell origin. The present invention provides an immunogen of Pax-5 protein for immunizing animals, the monoclonal antibody cell line 20G4 obtained by screening the immunogen after immunizing mice, and discloses the DNA of the variable region of the heavy chain and the light chain and amino acid sequence.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Application of lappaconitine or 12-epi-lappaconitine in preparation of medicine for treating leukemia
ActiveCN110974826APromote apoptosisPrevent proliferationOrganic active ingredientsAntineoplastic agentsEpitopeMyeloid leukemia
The invention provides an application of lappaconitine or 12-epi-lappaconitine in preparation of a medicine for treating leukemia, which belongs to the field of medicines. Researches of the inventor and find that both lappaconitine and 12-epitope-lappaconitine can effectively inhibit the proliferation of leukemia cells, can also promote the apoptosis of leukemia cells, and particularly have a moreremarkable effect on myeloid leukemia cells. Therefore, both lappaconitine and 12-epi-lappaconitine have anti-leukemia and anti-hematopoietic tumor activity, can be independently or jointly used as amedicine for treating anti-leukemia and anti-hematopoietic tumors, and are wide in application prospect.
Owner:川北医学院
HA-1 epitopes and uses thereof
InactiveUS20080206268A1Improve anti-tumor effectPeptide/protein ingredientsMammal material medical ingredientsImmune therapyHematopoietic Tumor
Peptide sequences constituting T-cell epitopes of minor Histocompatibility antigen, HA-1. HA-1 is associated with Graft versus Host Disease. The peptides and their derivatives find many uses, for instance, in bone marrow transplantation, organ transplantation and in treatment of leukemia and non-hematopoietic tumors. The peptide and / or its derivatives can be incorporated in vaccines, in pharmaceutical formulations and they can be used in diagnostic test kits. HA-1 is expressed by non-hematopoietic tumor cells. While absent in normal epithelial cells, tumor cells and tumor cell lines, particularly from epithelial origin, express HA-1 and are recognized by HA-1 cytotoxic T-cells. The invention provides means and methods for HA-1 specific immunotherapy for HA-1-positive patients with non-hematopoietic tumor cells.
Owner:GOULMY ELSA A J M +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com