Immunoglobulin and/or Toll-Like Receptor Proteins Associated with Myelogenous Haematological Proliferative Disorders and Uses Thereof
a technology of immunoglobulin and toll-like receptors, which is applied in the direction of immunoglobulins against animals/humans, drug compositions, peptides, etc., can solve the problems of serious side effects, cytogenetic defects may not be solely responsible for proliferative traits, and toxicity to dividing cells, so as to achieve favorable outcomes and lower expression levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
10.1 Example 1
Identification of Members of the Immunoglobulin (Ig) Superfamily or Toll-Like Receptor (TLR) Superfamily Associated with HTCs
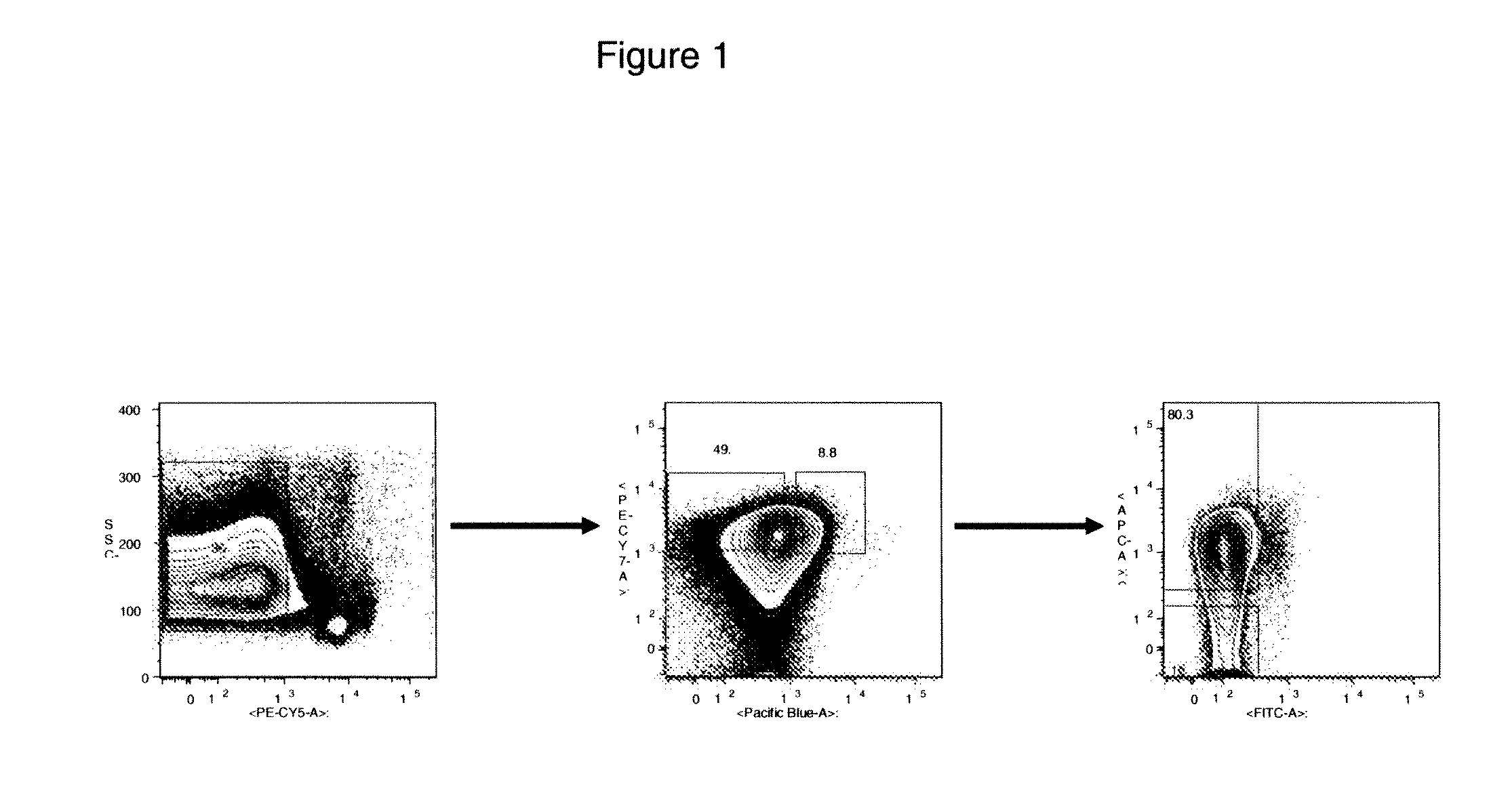
[0215]HTC markers were identified by comparing RNA transcript levels in normal HSCs and in AML CSCs for a variety of genes using microarrays. Specifically, data was obtained for test samples comprising AML Lin−CD34+CD38− cells, where three AML samples were taken from peripheral blood; Lin−CD34+CD38− and Lin−CD34+CD38+ cells were double sorted. The sorting strategy produced cells which were also over 90% CD90−. The sorting strategy produced purities greater than 98%.
[0216]To allow for direct comparison, data was then obtained from control samples comprising normal HSCs. A sample was taken from mobilized peripheral blood (MPB) of each of three individual donors and Lin−CD34+CD90+CD45RA−CD38− and Lin− CD34+CD90+CD45RA−CD38+ cells were double sorted. The sorting strategy produced a purity of over 99%.
[0217]For both sorting strategies, the Lineage (Li...
example 2
10.2 Example 2
Production and In Vivo Efficacy of Monoclonal Antibodies
10.2.1. Preparation of Monoclonal Antibodies that Specifically Bind a Member of the Immunoglobulin (Ig) Superfamily or Toll-Like Receptor (TLR) Superfamily Associated with HTCs
[0224]Techniques for producing the monoclonal antibodies are known in the art and are described, for instance, in Goding, Monoclonal Antibodies: Principles and Practice, pp. 59-103 (Academic Press, 1986). Immunogens that may be employed include a purified polypeptide corresponding to CD84; lymphocyte antigen 86 (Ly86); CD180 (RP105); HAVCR2; LILRA1; LILRA2, NEGR1; or TLR2, as well as fusion proteins containing the same. Alternatively, cells expressing recombinant an AML-expressed isoform of CD84; Ly86; CD180; HAVCR2; LILRA1; LILRA2, NEGR1; or TLR2, on the cell surface, may be used. Selection of the immunogen can be made by the skilled artisan without undue experimentation.
[0225]Mice, such as Balb / c, are immunized with the selected immunogen,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Cytotoxicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com