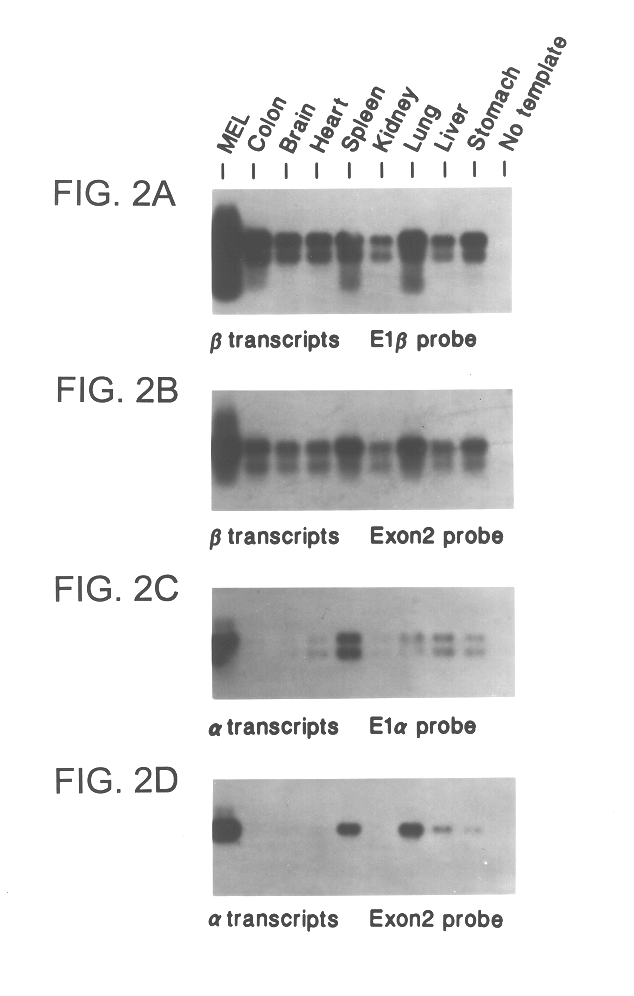
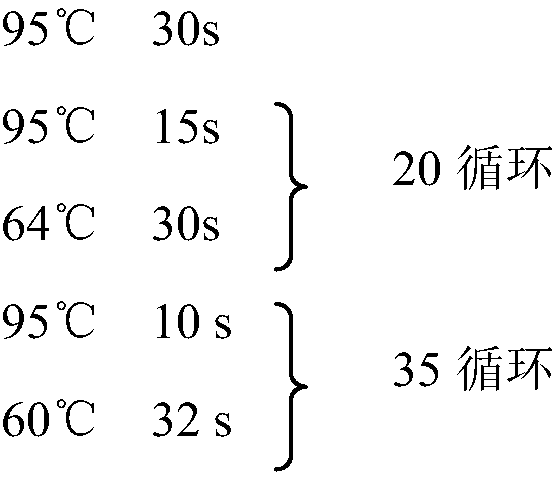
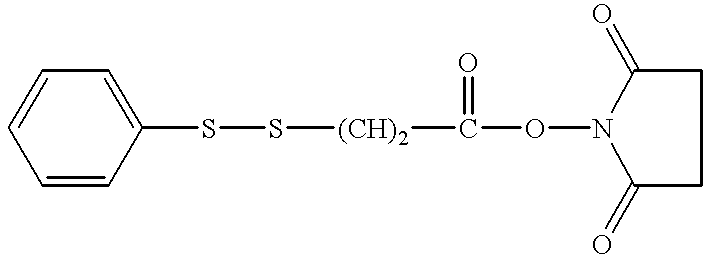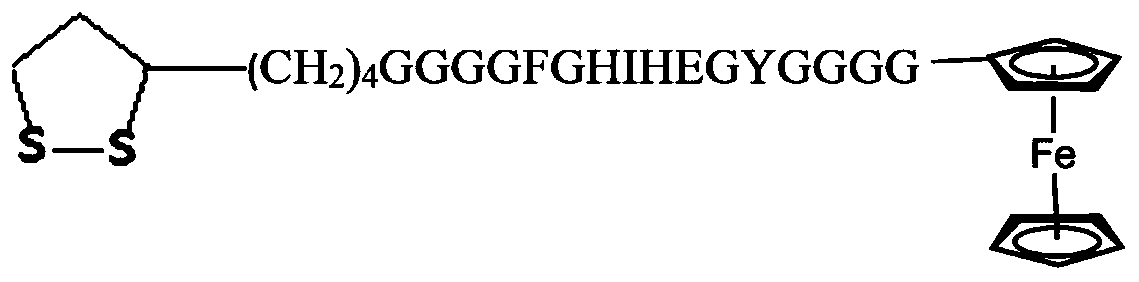Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
71 results about "Multiple Tumor Suppressor-1" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
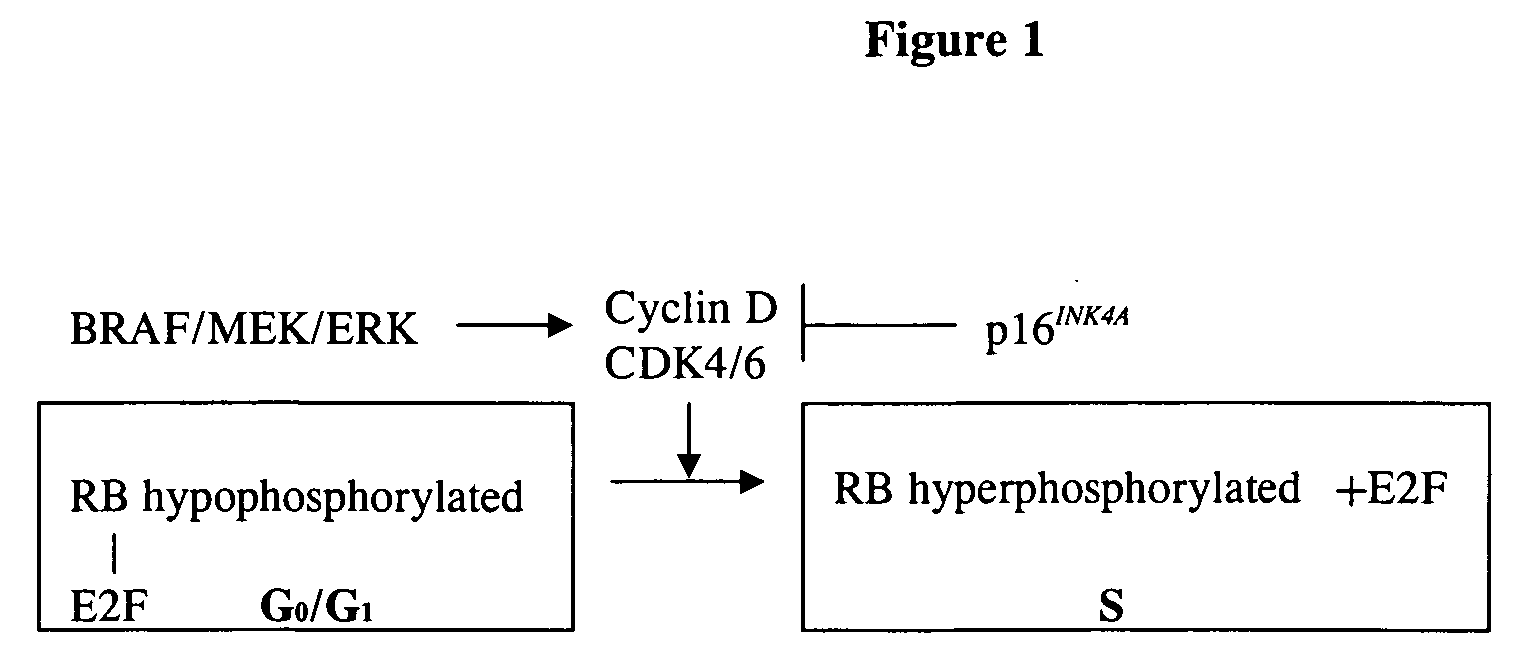
P16 (also known as p16ᴵᴺᴷ⁴ᵃ, cyclin-dependent kinase inhibitor 2A, multiple tumor suppressor 1 and as several other synonyms), is a tumor suppressor protein, that in humans is encoded by the CDKN2A gene. p16 plays an important role in cell cycle regulation by decelerating the cell's progression from G1 phase to S phase, and therefore acts as a tumor suppressor that is implicated in the prevention of cancers, notably melanoma, oropharyngeal squamous cell carcinoma, cervical cancer, and esophageal cancer. p16 can be used as a biomarker to improve the histological diagnostic accuracy of CIN3. Expression of the CDKN2A gene is frequently changed in a wide variety of tumors.
ARF-P19, a novel regulator of the mammalian cell cycle
InactiveUS6407062B1Peptide/protein ingredientsAntibody mimetics/scaffoldsAbnormal tissue growthCancer cell
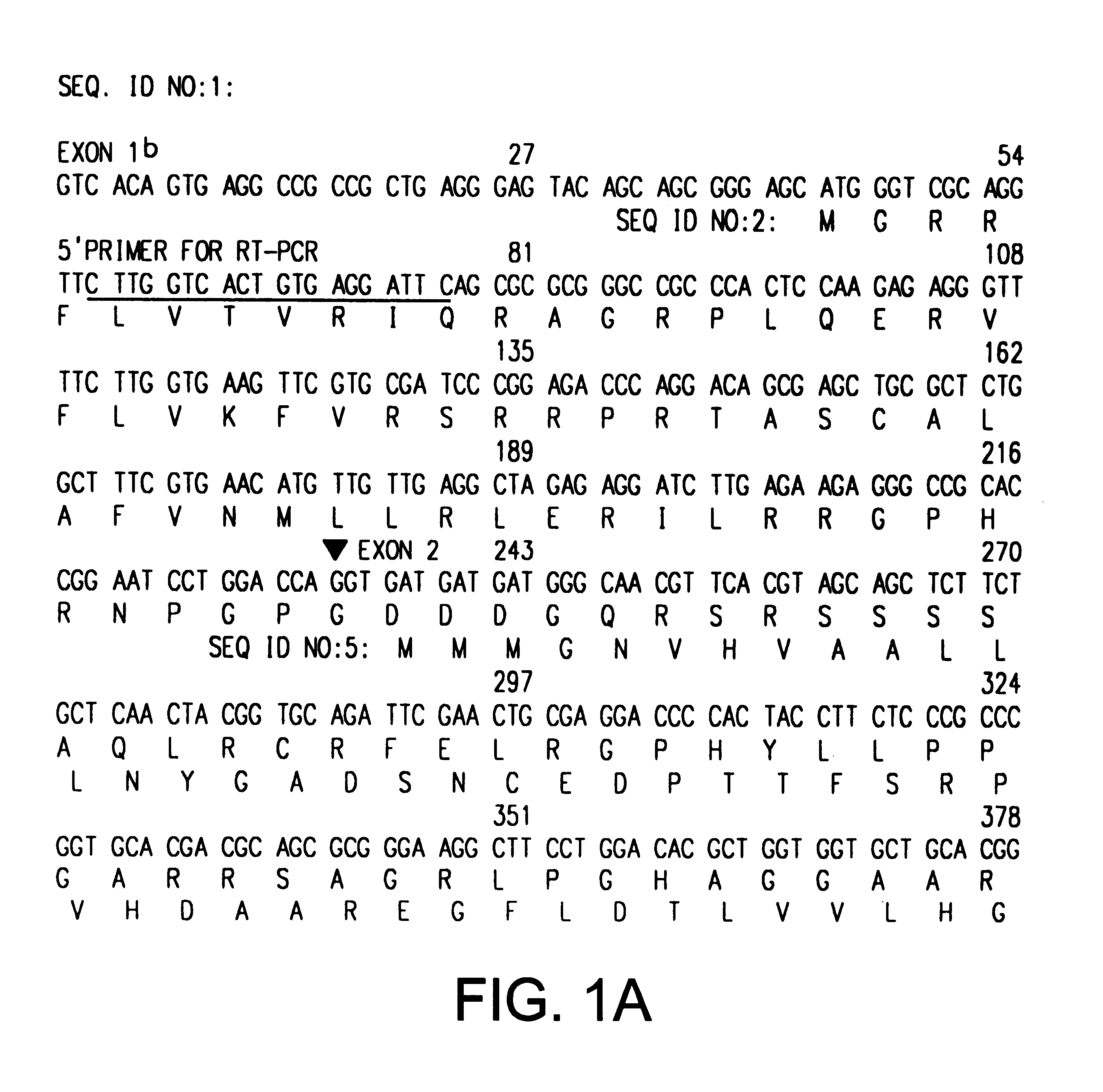
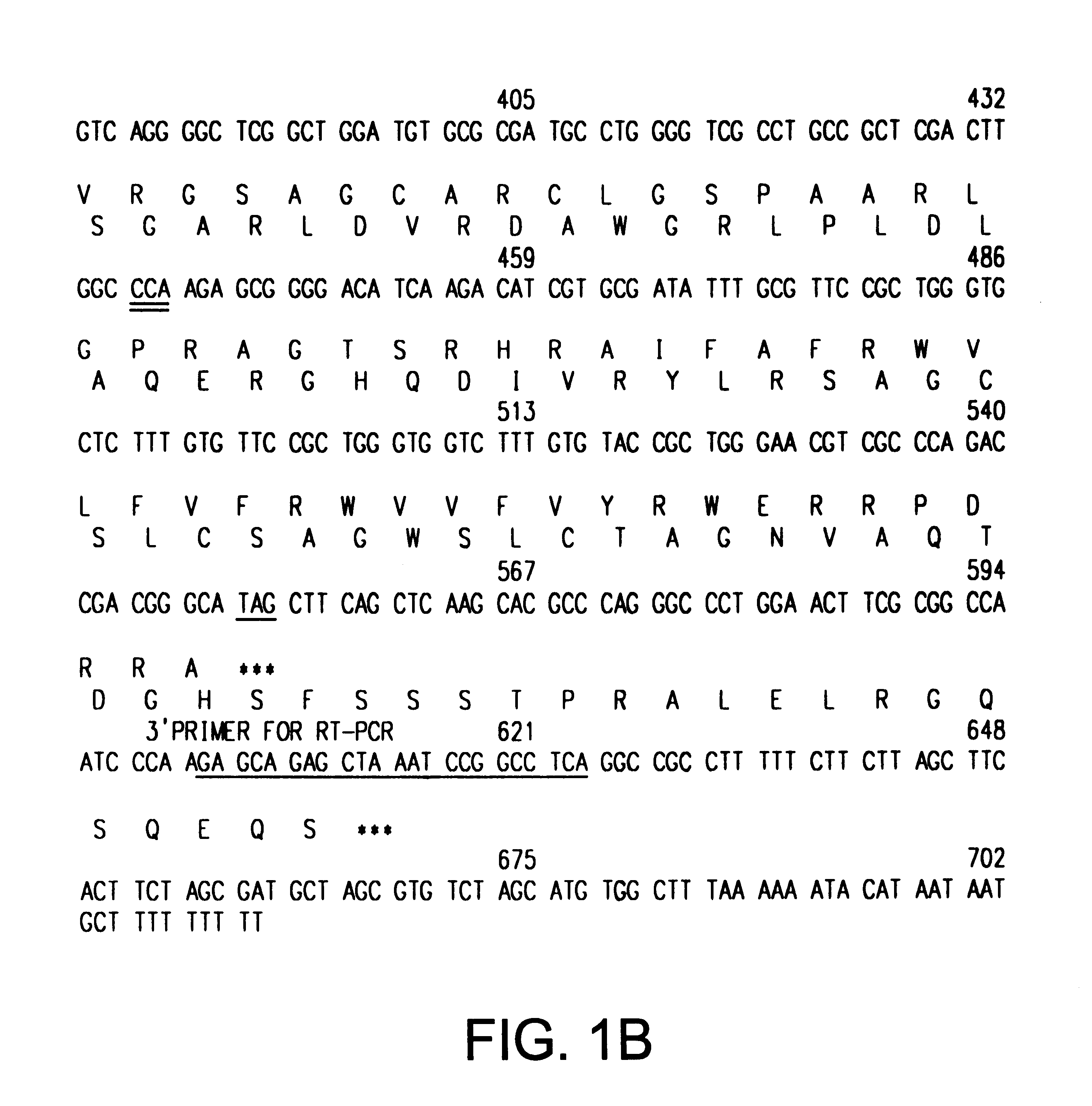
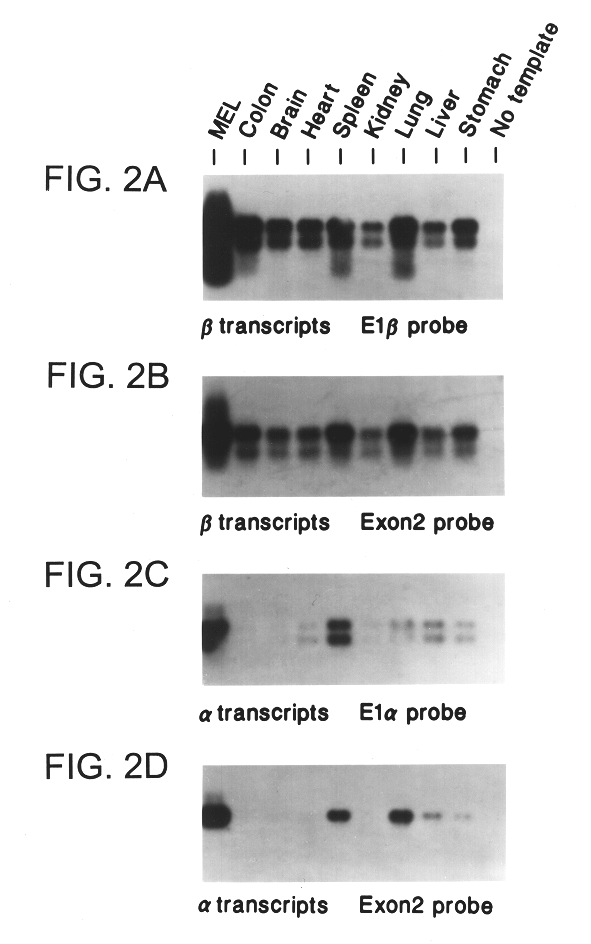
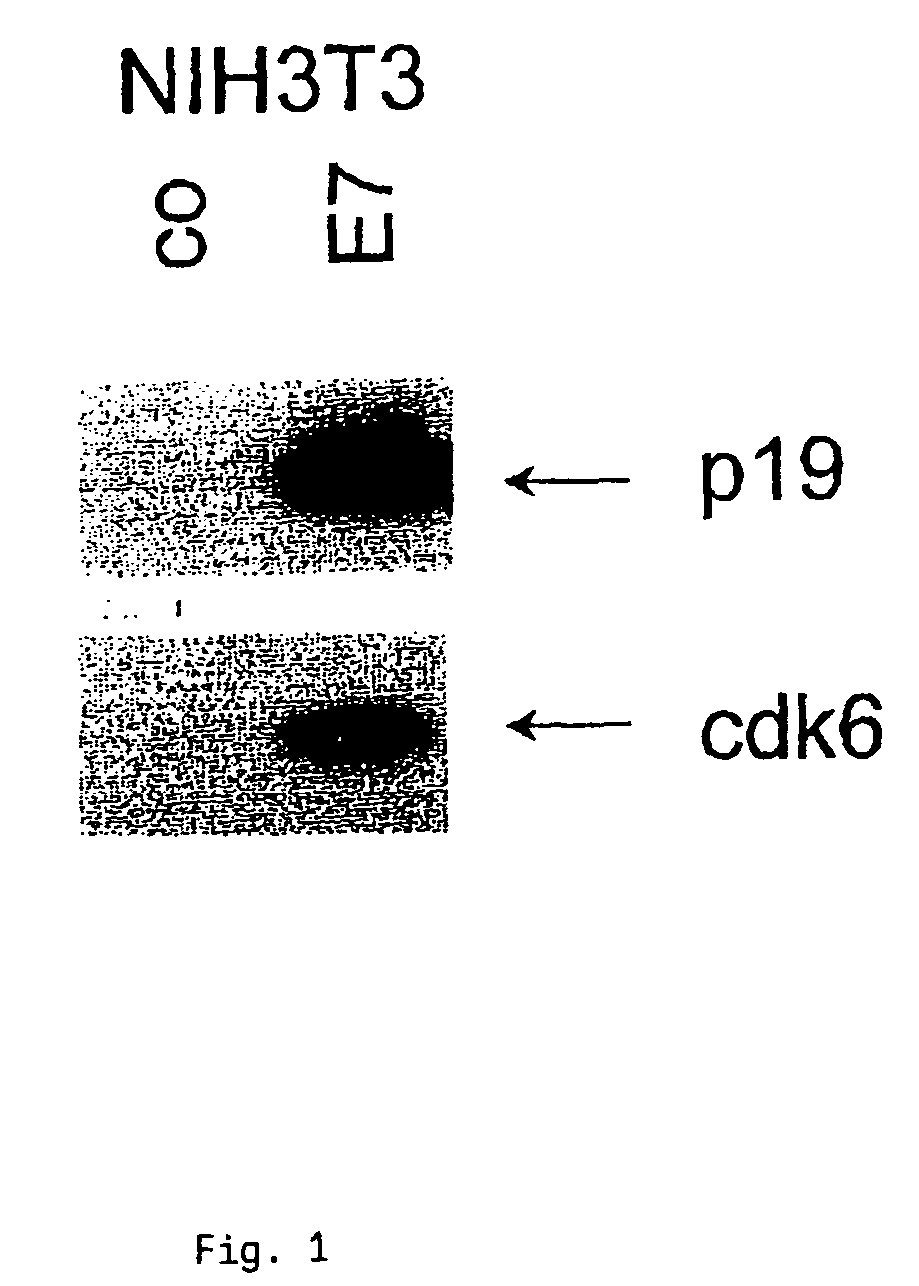
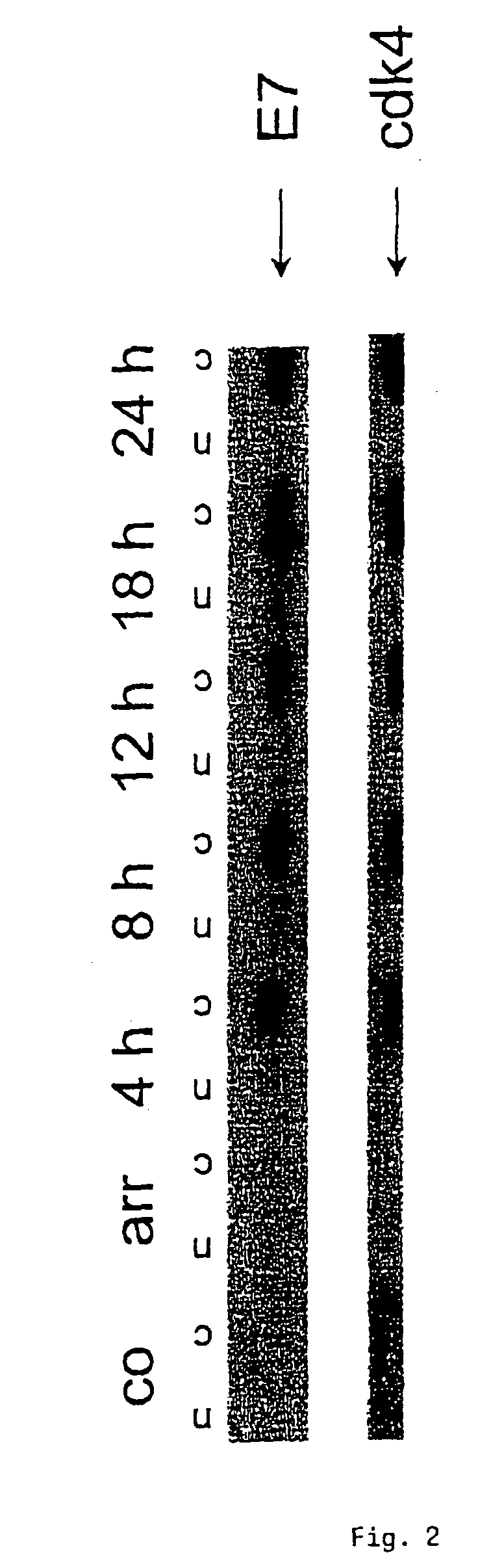
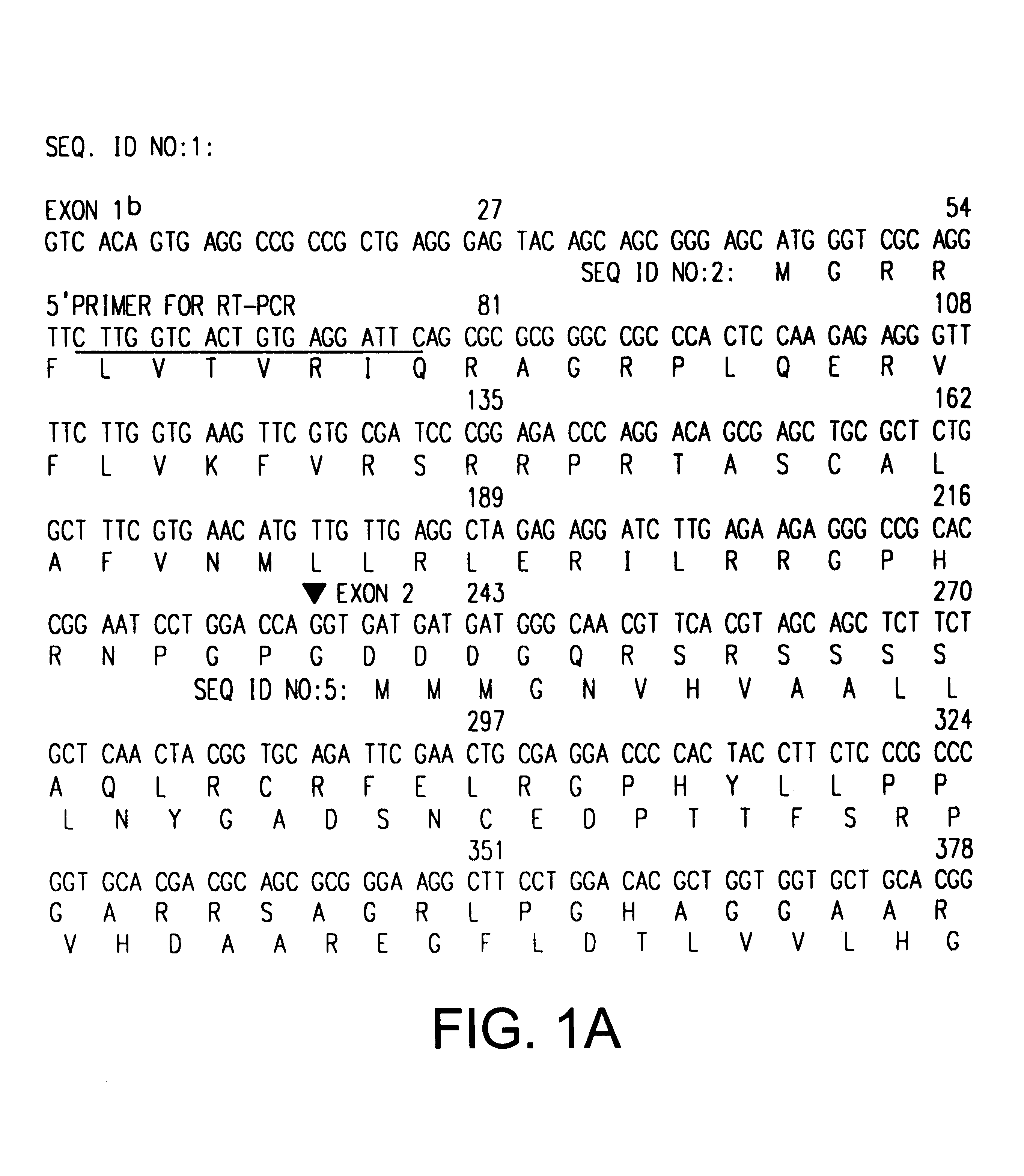
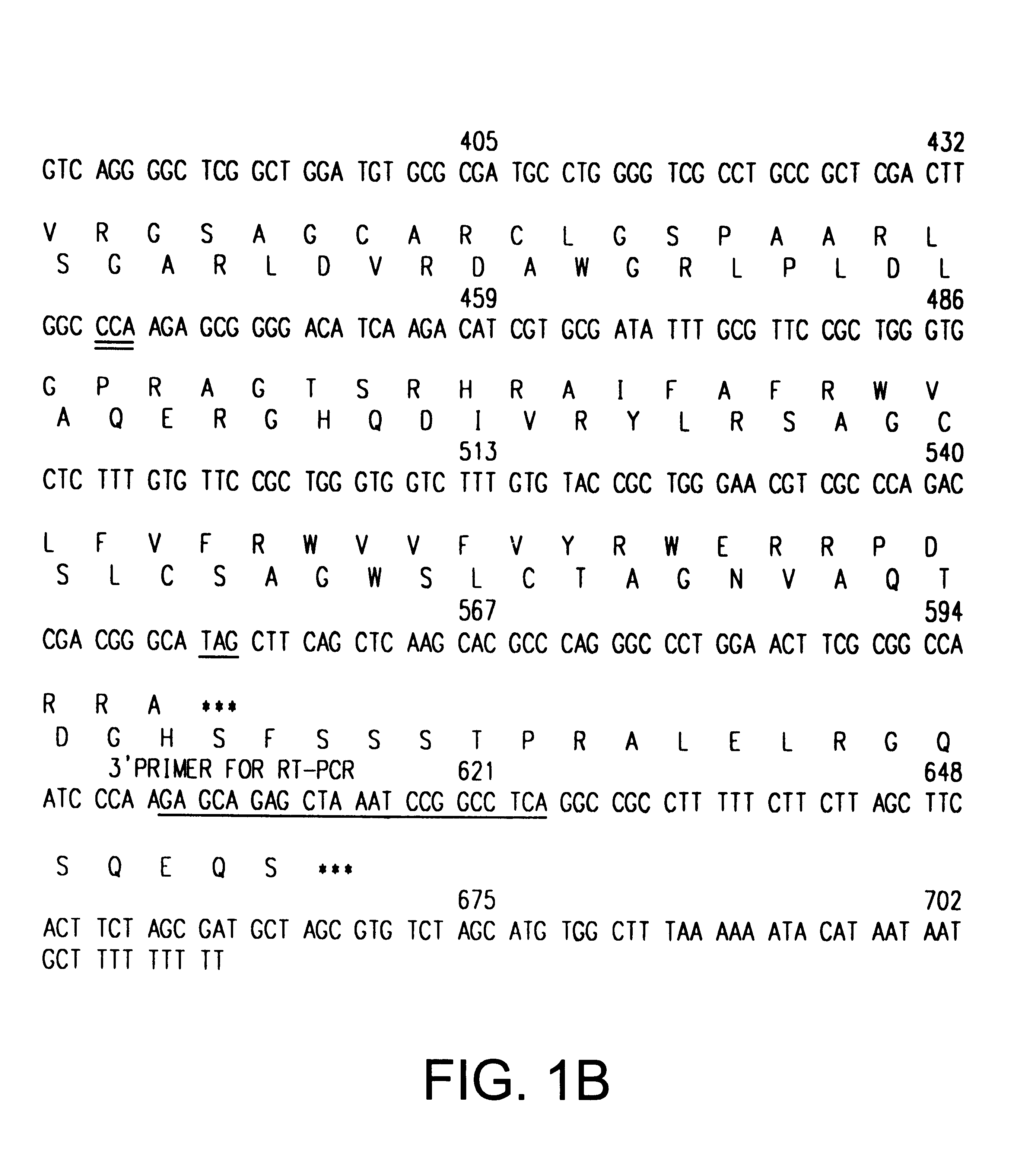
The INK4A (MTS1, CDKN2) gene encodes a specific inhibitor (InK4a-p16) of the cyclin D-dependent kinases CDK4 and CDK6. InK4a-p16 can block these kinase from phosphorylating the retinoblastoma protein (pRb), preventing exit from the G1 phase of the cell cycle. Deletions and mutations involving the gene encoding InK4a-p16, INK4A, occur frequently in cancer cells, implying that INK4a-p16, like pRb, suppresses tumor formulation. However, a completely unrelated protein (ARF-p19) arises in major part from an alternative reading frame of the mouse INK4A gene. Expression of an ARF-p19 cDNA (SEQ ID NO:1) in rodent fibroblasts induces both G1 and G2 phase arrest. Economical reutilization of protein coding sequences in this manner is without precedent in mammalian genomes, and the unitary inheritance of INK4a-p16 and ARF-p19 may reflect a dual requirement for both proteins in cell cycle control.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Method for screening cervical cancer
InactiveUS20050221399A1Effective diagnosisEfficient screeningBiological testingSquamous CarcinomasScreening method
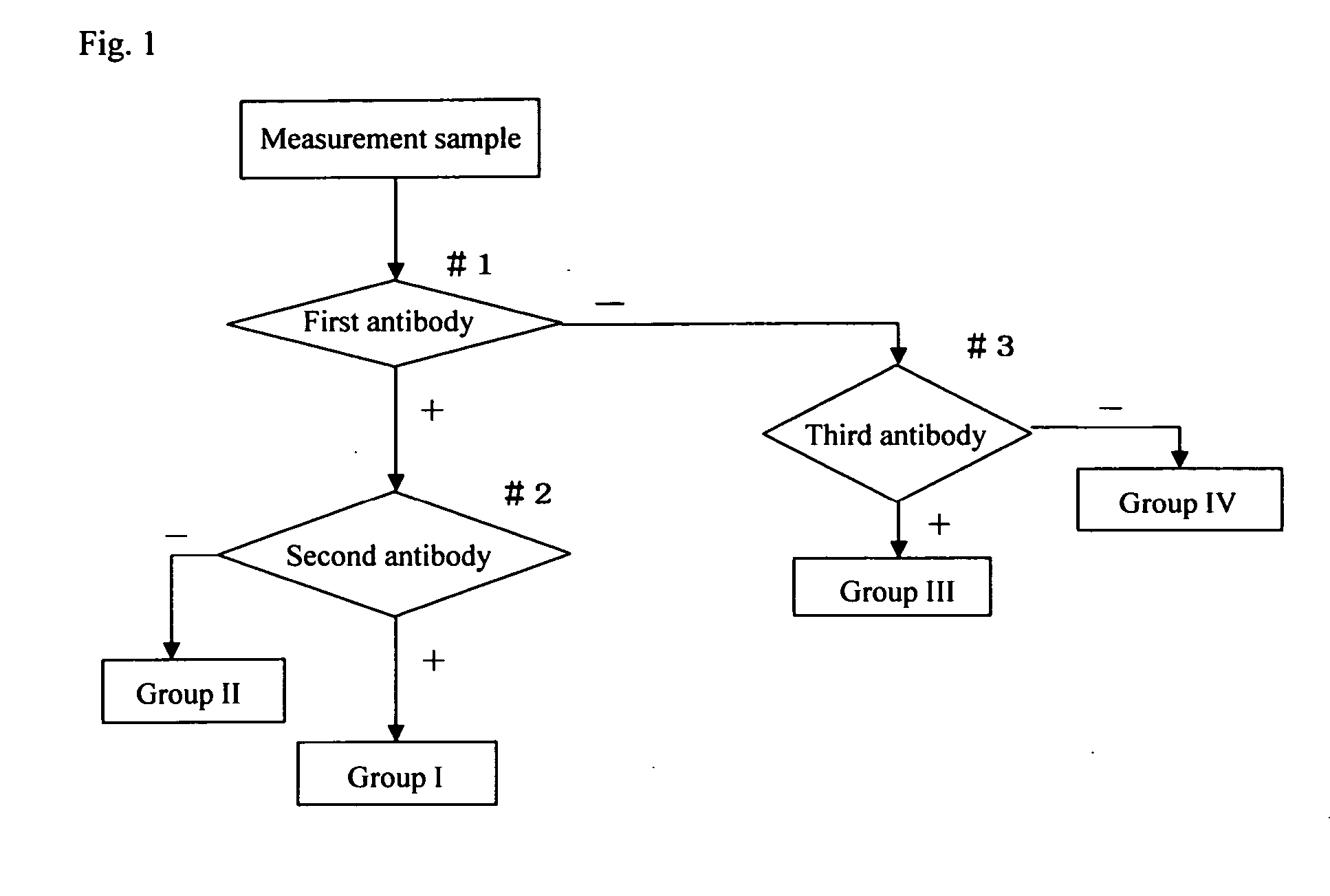
The invention provides a reagent for diagnosing cervical cancer, which can not only detect the presence or absence of cervical cancer but can also distinguish squamous cell carcinoma and adenocarcinoma from each other, a method for screening cervical cancer by using the reagent, particularly a screening method capable high speed processing by utilizing flow cytometry. The reagent comprises a first labeled antibody reacting with gland cells, a second labeled antibody reacting with adenocarcinoma cells and a third labeled antibody reacting with atypical cervical squamous cells, the antibodies being labeled with mutually distinguishable labels respectively. Preferably, at least one selected from the group consisting of MUC1 antibody, cytokeratin 7 antibody, and cytokeratin 18 antibody is used as the first labeled antibody, at least one selected from the group consisting of cytokeratin 8 antibody and HIK1083 antibody is used as the second labeled antibody, and at least one member selected from the group consisting of NMP179 antibody, p16INK4A antibody, Ki-67 antibody, p53 antibody, p21 antibody, EMA antibody, CEA antibody and MIB-1 antibody is used as the third labeled antibody.
Owner:SYSMEX CORP
Kit for identifying lung nodules and/or lung cancer status and application thereof
InactiveCN109022567AHigh sensitivityImprove featuresMicrobiological testing/measurementTrue positive rateFHIT
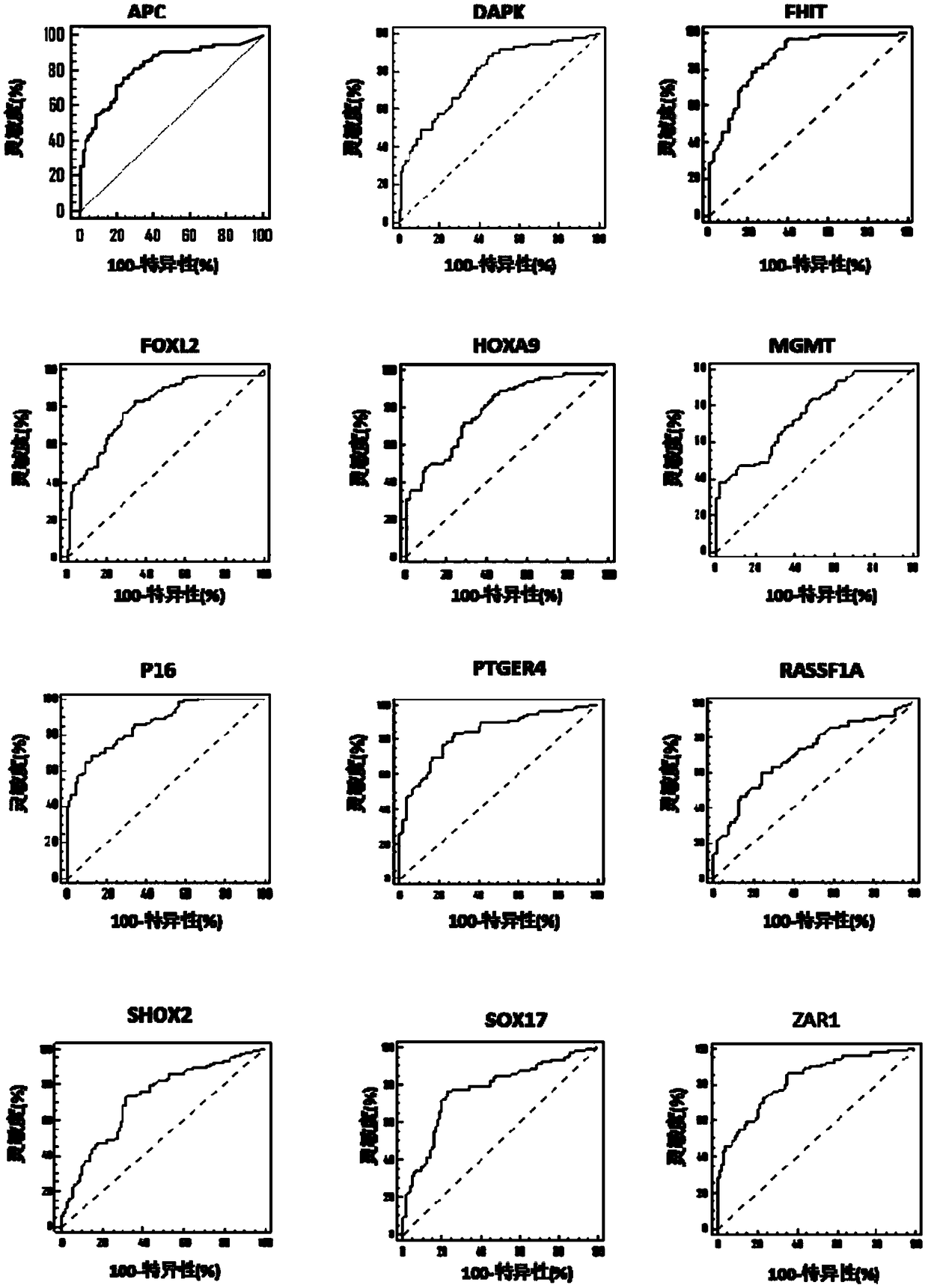
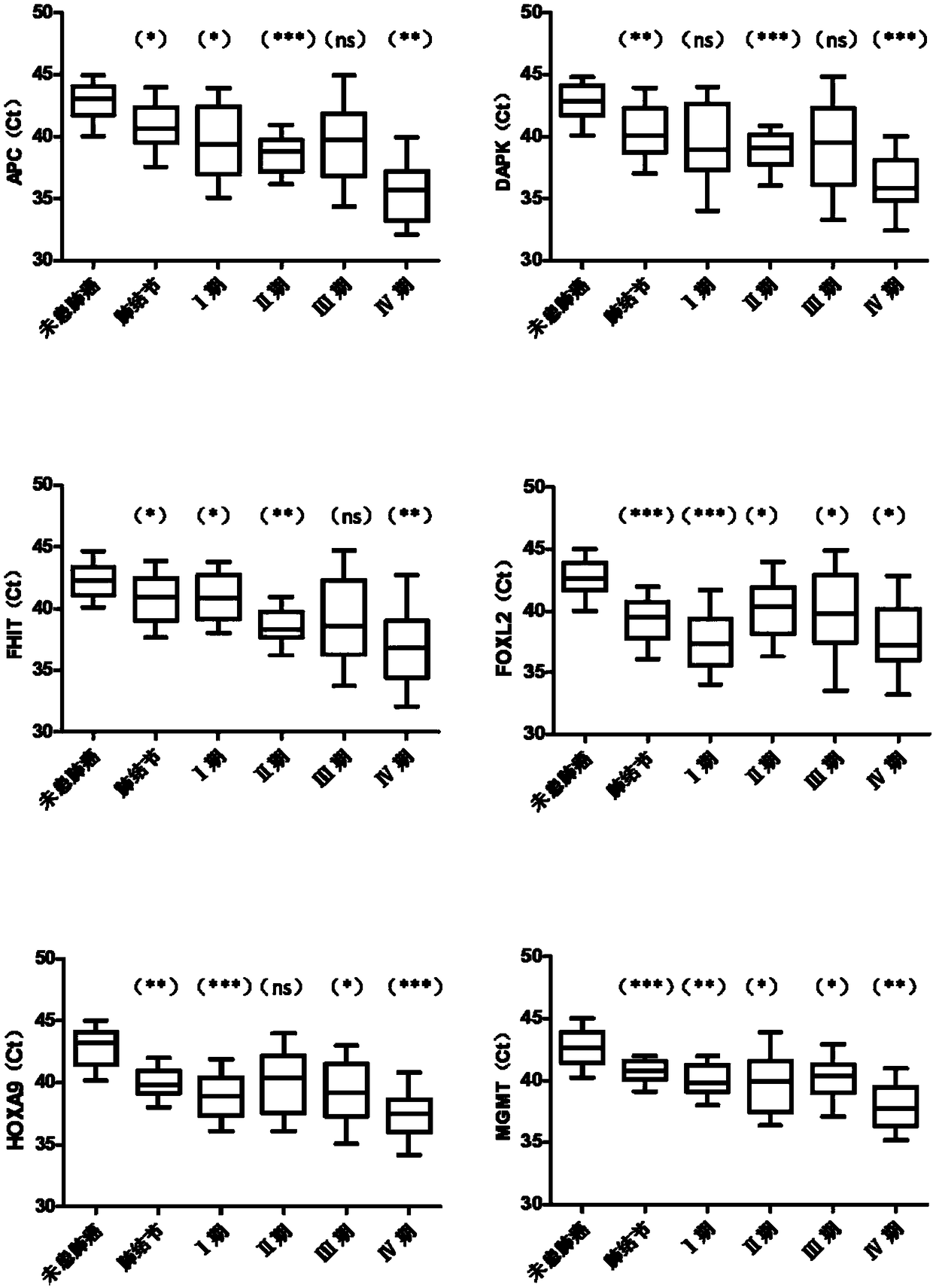
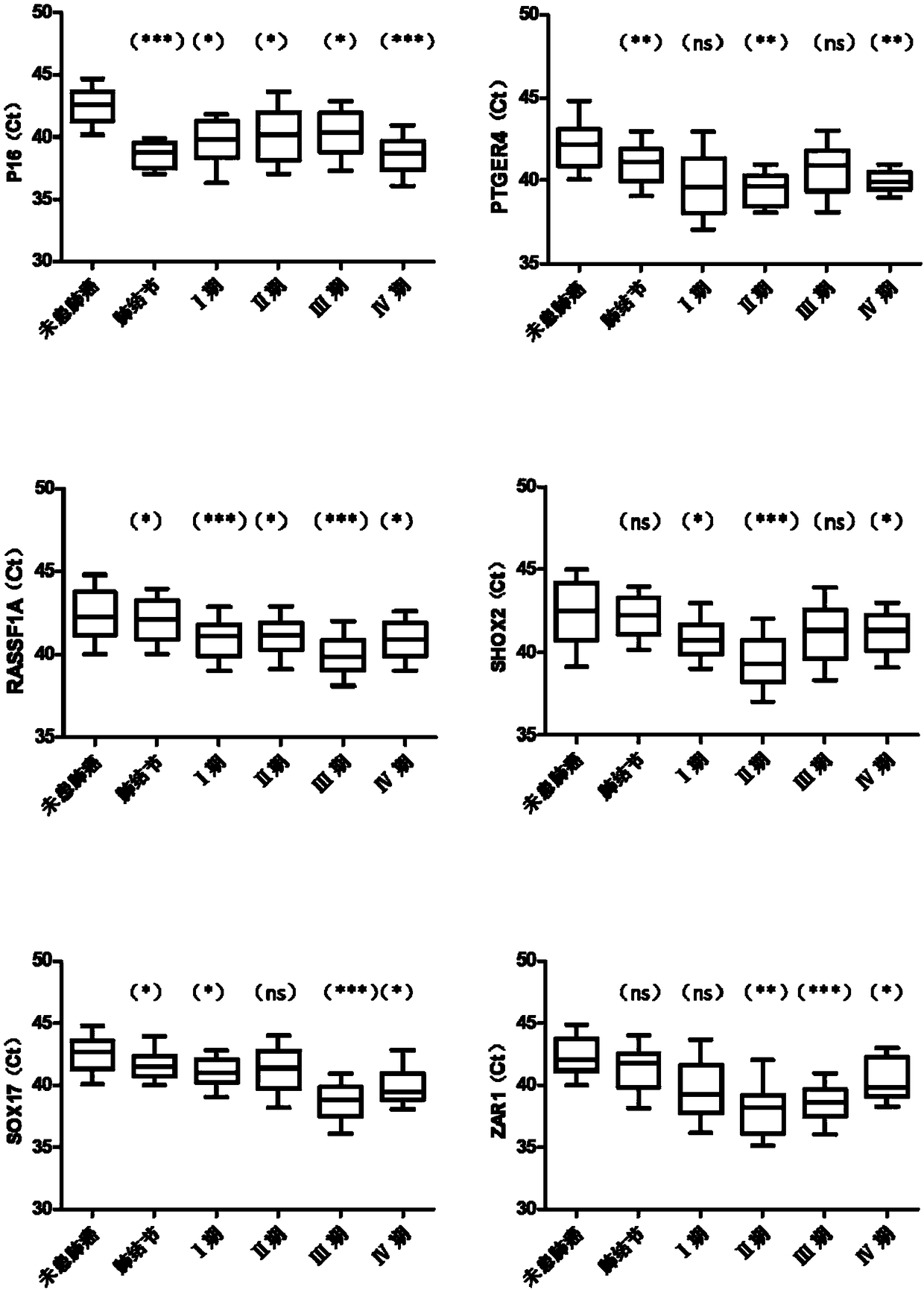
The present invention relates to a kit for identifying lung nodules and / or lung cancer status and application thereof. The kit includes a primer pair for detecting methylation levels of biomarker genes or corresponding fragments in a biological sample from a subject; the primer pair is used for PCR amplification by use of the biomarker genes or the fragments treated with hydrosulphite as templates; and the biomarker genes are selected from one or more of APC, DAPK, FHIT, FOXL2, HOXA9, MGMT, P16, PTGER4, RASSF1A, SHOX2, SOX17 and ZAR1. The method improves the detection sensitivity and specificity of the lung nodules and lung cancer by jointly detecting the methylation levels of the biomarker genes or the corresponding fragments, thus ensuring the correctness and reliability of detection results, and provides a fast, reliable and accurate new way for the prediction, diagnosis and evaluation of the lung nodules and the lung cancer.
Owner:BEIJING EXELLON MEDICAL TECH CO LTD
Method for detecting carcinomas in a solubilized cervical body sample
ActiveUS7306926B2Biological material analysisPeptide preparation methodsCervical intraepithelial neoplasiaMultiple Tumor Suppressor-1
The present invention relates to a method for the early diagnosis of carcinomas and their preliminary stages, which comprises determining the overexpression of a cell cycle regulatory protein in a solubilized body sample. The present invention is particularly directed to a method for detecting cervical carcinomas, cervical intraepithelial neoplasias, or cervical carcinomas in-situ from a solubilized cervical body sample of a human subject, by solubilizing the cervical body sample in a lysis buffer, and determining the overexpression of cyclin dependent kinase inhibitor p16 in the solubilized cervical sample. The invention also concerns a test kit usable for this purpose as well as an in-vitro diagnostic device.
Owner:VENTANA MEDICAL SYST INC
Receptor-mediated gene transfer system for targeting tumor gene therapy
InactiveUS6339139B1Efficient targetingGrowth inhibitionOrganic active ingredientsPeptide/protein ingredientsMultiple Tumor Suppressor-1Oligopeptide
The invention relates to a gene transfer system binding to a growth factor receptor, comprising 4-element complex gene transfer system consisting of ligand oligopeptide / polycationic polypeptide / endosome release oligopeptide / exogenous DNA or 3-element complex consisting of ligand oligopeptide / polycationic polypeptide / exogenous DNA. The invention exemplifies E5, GE7, GV1 and GV2 systems and they can be targeted for the introduction of exogenous genes into malignant tumor cells or tumor vascular endotheliocytes. They are also able to highly inhibit the growth of tumor cells in animals while p15, p16 or p21WAF-1 was used as exogenous genes. The system according to the invention is a new introduction system in tumor gene therapy.
Owner:SHANGHAI INST OF ONCOLOGY
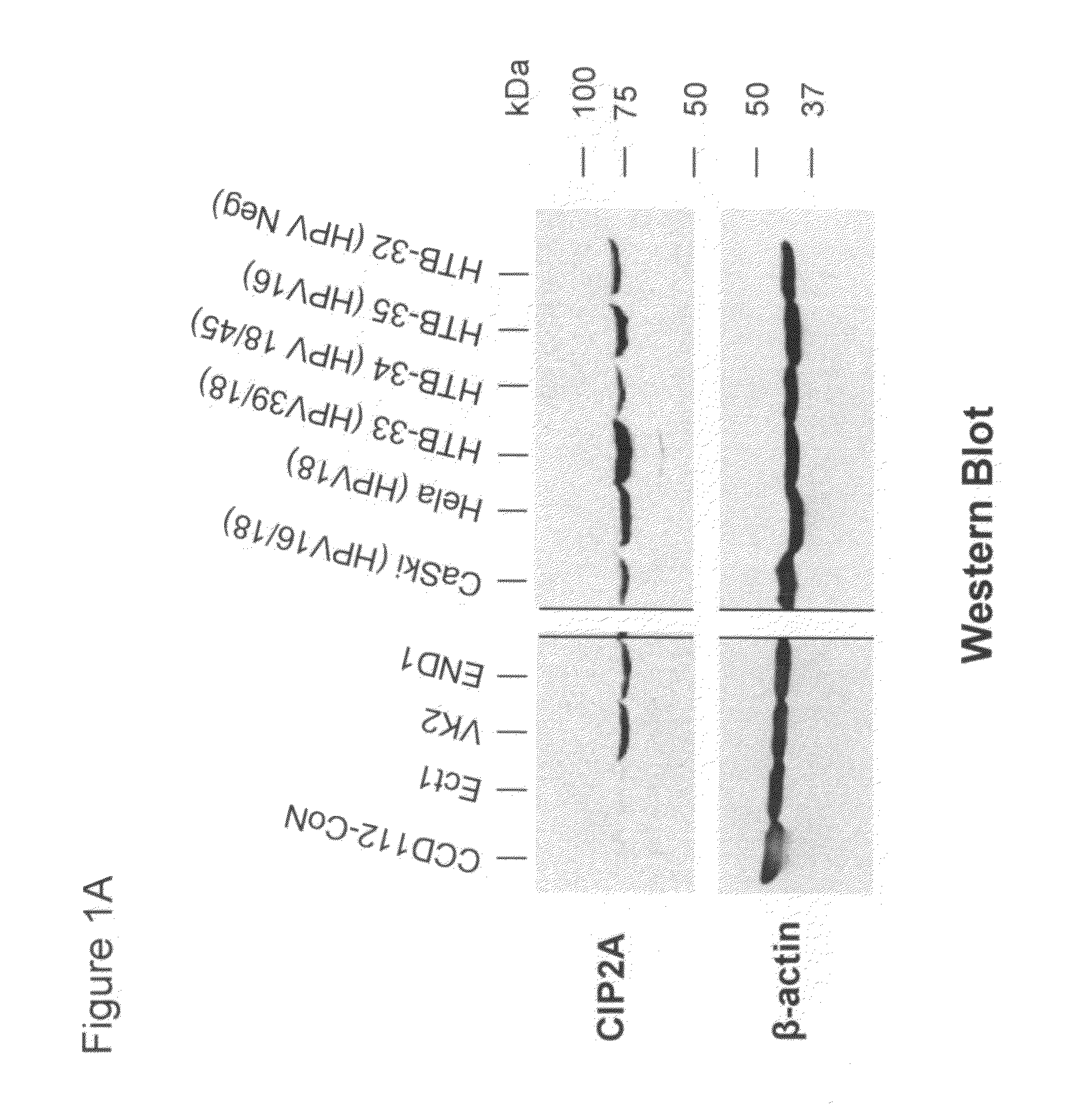
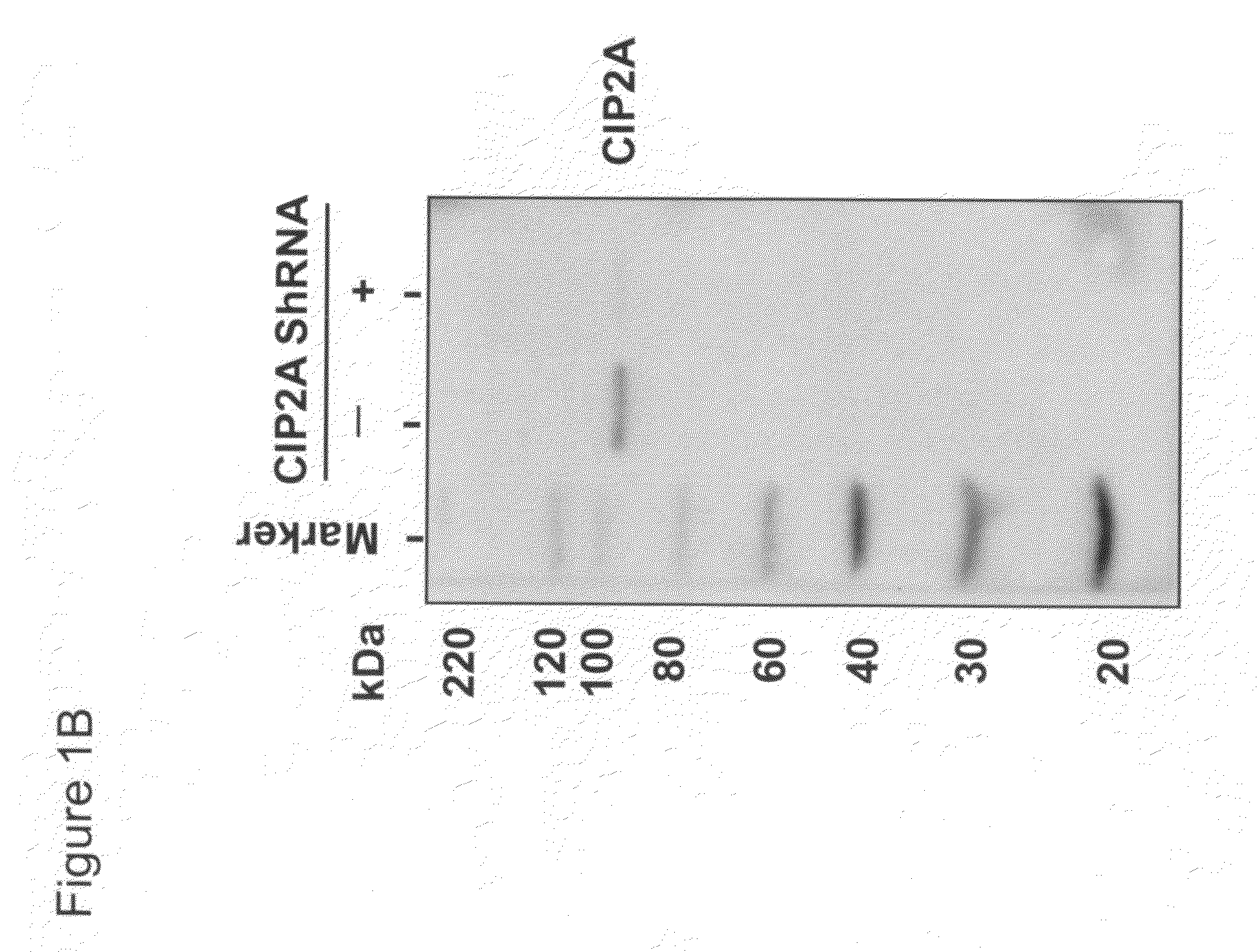
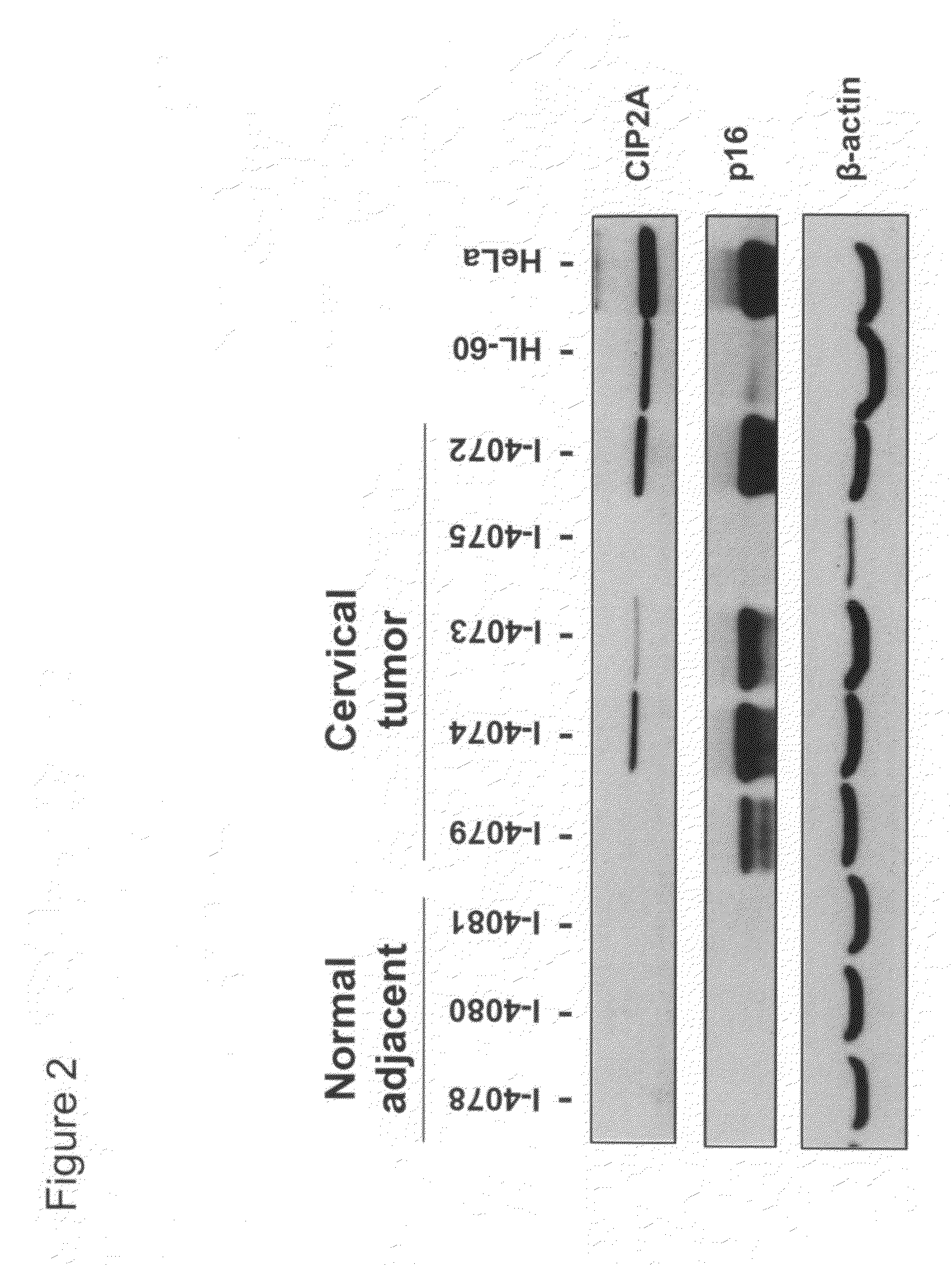
CIP2A as a biomarker in detection of cervical cancer
InactiveUS20120070837A1Microbiological testing/measurementBiological testingCombined useTrue positive rate
The present invention provides methods and compositions for detecting cervical cancer in a human. The present invention provides CIP2A as a biomarker detectable in a biological sample from cervix of an individual, an increased expression of same is an indication of cervical cancer in the individual. CIP2A mRNA or protein can both serve as reliable biomarkers. In one embodiment, the present invention provides a combined use of CIP2A with at least one additional biomarker selected from the group consisting of Ki67, TOP2A, MCM2, MCM5, p14ARF and p16INK4a, to better serve as a detection means for cervical cancer. The present invention provides a reliable and accurate assay methods and compositions which may be used for detecting cervical cancer in an individual with a high sensitivity and specificity. The present assay provides a novel use of CIP2A as a tool for early detection of cervical cancer in humans.
Owner:MEDICAL DIAGNOSTIC LAB
Transgenic mouse having a transgene that converts a prodrug into a cytotoxic compound in senescent cells
ActiveUS9901080B2Reduce the possibilityInhibit cellular senescenceCompounds screening/testingVectorsAge related diseaseCytotoxicity
This invention provides a transgenic mouse for studying the role of senescent cells on an age-related disorder or an age-sensitive trait. The transgene contains a p16 promoter sequence that controls expression of an enzyme so as to cause the enzyme to be expressed in senescent cells in the mouse. The enzyme converts a prodrug to a cytotoxic agent, so that treating the mouse with the prodrug results in the prodrug selectively killing the senescent cells. As a result, progression of an age-related disorder or an age-sensitive trait is delayed. Included is the 3MR mouse model, which also expresses bioluminescent and fluorescent markers under control of the p16 promoter so that senescent cells in the mice can be visualized.
Owner:ERASMUS UNIV MEDICAL CENT ROTTERDAM ERASMUS MC +2
Non-invasive detection method and kit for early screening bladder cancer by multi-gene combination
PendingCN108531594AHigh acceptanceImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationMultiple Tumor Suppressor-1Non invasive
The invention discloses a non-invasive detection method and a kit for early screening a bladder cancer by multi-gene combination. The combined genes include APC, NID2 and p16, and specific methylationprimers are designed to detect three nucleotide sequences of target gene fragment methylation of the APC, the NID2 and the p16, so that early screening and auxiliary diagnosis of the bladder cancer are achieved. Methylation levels of three genetic markers of the APC, the NID2 and the p16 in urinary sediments are analyzed by a three-channel fluorescent quantitative PCR (polymerase chain reaction)method, and a positive or negative result of a sample is judged according to a reported CT (computed tomography) value. The non-invasive detection method can detect 90% of early bladder cancers, 64% of precancerous adenomas (larger than or equal to 1 centimeter) in preclinical study, specificity reaches 100%, invasiveness is completely omitted, the method only needs to collect urine of 50mL, and acceptability of throngs wearing no symptoms is high.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
P16<INK4a> double antibody sandwich ELISA kit
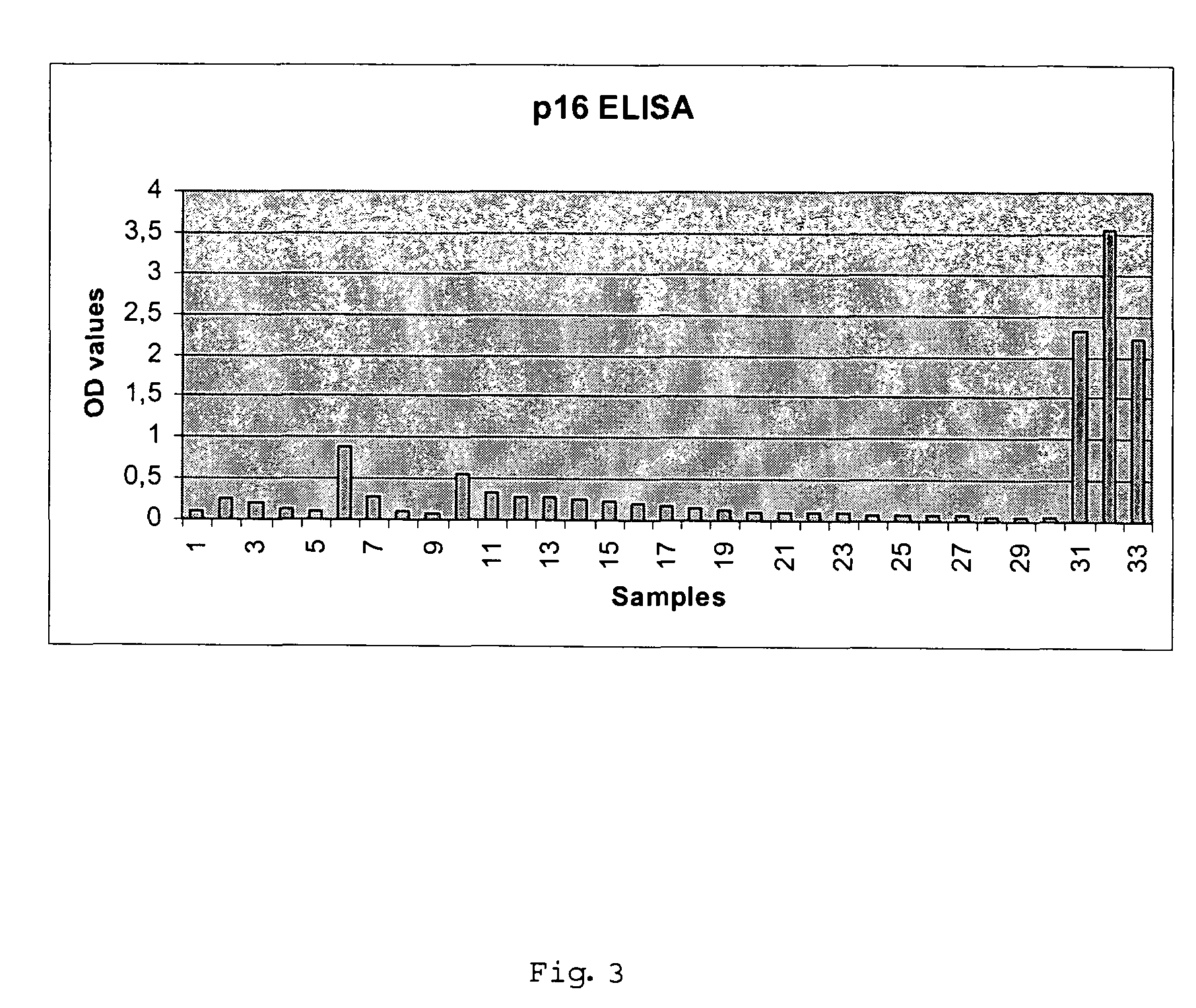
P16<INK4a> double antibody sandwich ELISA kit comprises avidin, peroxidase compound, coloration liquid, reaction stop solution, positive reference substance, antibody diluent and concentrated cleaning solution and is characterized in that the kit also comprises ELIAS plate coated by P16<INK4a> polyclonal antibody and P16<INK4a> monoclonal antibody marked by biotin. The invention has the advantages that the double antibody sandwich ELISA kit is used for screening cervical carcinoma, wherein, the sensibility is 87.80%, the specificity is 92.43%, and the general coincidence rate is 91.59% and the negative predictive value is 97.16%. The kit is simple to be used, the detection result is obtained fast and is correct and objective; a plurality of samples can be detected simultaneously; in addition, the kit is applicable for the cancer screening for a large sample population, and the detection cost is lower than the cost of the traditional caner screening method.
Owner:邹先进
Method for discrimination of metaplasias from neoplastic or preneoplastic lesions
ActiveUS7422859B2High riskMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementGene productMultiple Tumor Suppressor-1
The present invention relates to a method for discrimination of p16INK4a overexpressing metaplasias from neoplastic or preneoplastic p16INK4a overexpressing lesions by determination of the level of high risk HPV encoded gene-products such as e.g. HPV E2 and / or HPV E7 molecules in biological samples in the course of cytological testing procedures. The method thus enables for reduction of false positive results in the p16INK4a based detection of anogenital lesions in cytological testing procedures.
Owner:VENTANA MEDICAL SYST INC
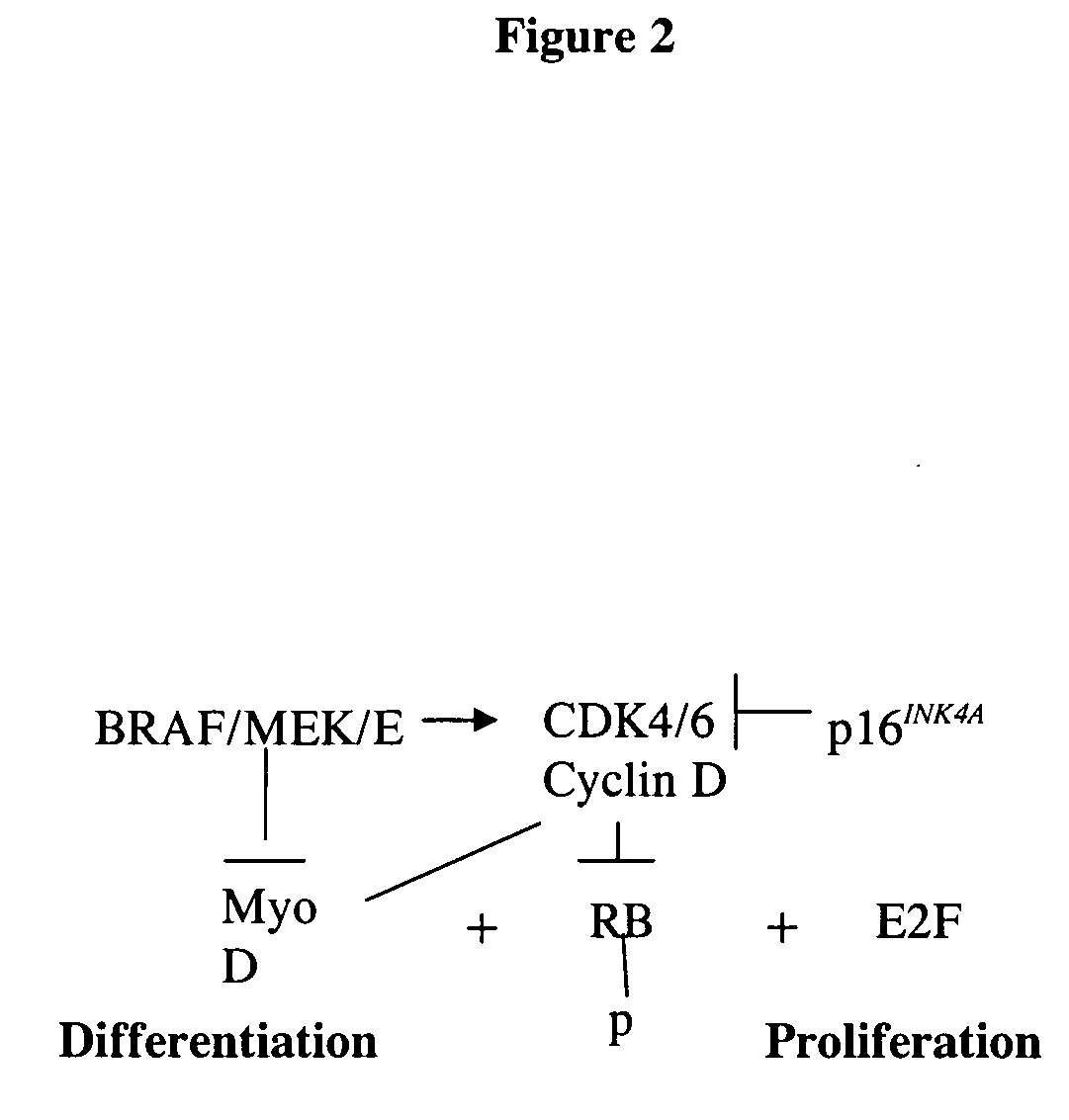
Treatment of cancer by simultaneous inhibiton of BRAF and restoration or mimicry of p16INK4A activity
InactiveUS20060134068A1Growth inhibitionHigh activityBiocideOrganic active ingredientsMelanomaWild type
Provided is a method for inhibiting growth of a tumor cell which comprises oncogenically activated BRAF and defective p16INK4A by inhibiting activated BRAF and restoring functional p16INK4A. Also provided is a method for sensitizing the foregoing tumor cell to cytotoxic or cytostatic effect of a chemotherapeutic agent or radiation by further contacting the cell with such an agent. Also provided is a method for treating cancer, especially melanoma, by simultaneously inhibiting expression or activity of activated BRAF and restoring or mimicking the activity of wild-type p16INK4A.
Owner:MT SINAI SCHOOL OF MEDICINE
ARF-P19, a novel regulator of the mammalian cell cycle
The INK4A (MTS1, CDKN2) gene encodes a specific inhibitor (InK4a-p16) of the cyclin D-dependent kinases CDK4 and CDK6. InK4a-p16 can block these kinase from phosphorylating the retinoblastoma protein (pRb), preventing exit from the G1 phase of the cell cycle. Deletions and mutations involving the gene encoding InK4a-p16, INK4A, occur frequently in cancer cells, implying that INK4a-p16, like pRb, suppresses tumor formulation. However, a completely unrelated protein (ARF-p19) arises in major part from an alternative reading frame of the mouse INK4A gene. Expression of an ARF-p19 cDNA (SEQ ID NO:1) in rodent fibroblasts induces both G1 and G2 phase arrest. Economical reutilization of protein coding sequences in this manner is without precedent in mammalian genomes, and the unitary inheritance of INK4a-p16 and ARF-p19 may reflect a dual requirement for both proteins in cell cycle control.
Owner:ST JUDE CHILDRENS RES HOSPITAL INC
Cervical cancer screening detection kit
InactiveCN108717120AIncrease connection forceAvoid it happening againMaterial analysisWater basedStaining
The invention provides a cervical cancer screening detection kit. The kit comprises a p16 / Ki-67 hybrid primary antibody, a hybrid enzyme-labeled secondary antibody and a staining solution, wherein thestaining solution comprises an AP-Red staining solution and a DAB staining solution, and the preparation method of the AP-Red staining solution comprises chromogen preparation, substrate preparationand staining solution preparation. A novel cervical cancer screening detection kit is provided in the technical scheme. The AP-Red staining solution of a novel formula is used in the kit, the chromogen in the staining solution possibly has multiple reactive groups, and the groups can react with groups on tissues so as to form covalent bonds, so that cervical cell slices using the kit can directlypass through organic reagents after staining, a water-based sealing agent does not need to be used for sealing the slices, bubbles are avoided, the thickness is controlled, the time and labor cost canbe saved, observation of staining results is prevented from being influenced, and the detection efficiency and detection quality are improved.
Owner:FUZHOU MAIXIN BIOTECH CO LTD
Kit for early screening and detecting carcinoma of urinary bladder by demethylation fluorescent quantitation PCR method
InactiveCN108441561AStrong specificityHigh detection sensitivityMicrobiological testing/measurementDNA/RNA fragmentationBladder cancerForward primer
The invention belongs to the field of molecular biology, and relates to a kit for early screening and detecting carcinoma of urinary bladder by a demethylation fluorescent quantitation PCR method. Thekit comprises primers and probes of the abovementioned two target genes p16 and RASSF1A, a forward primer and a probe of GAPDH, PCR Master Mix, a cracking solution, protease K, a washing solution, ahydrosulphite solution and ddH2O. According to the kit, two target genes which are p16 and RASSF1A are combined to detect the carcinoma of urinary bladder; the detection sensitivity is high; and the specificity is high.
Owner:ANHUI DAJIAN MEDICAL TECH CO LTD
Antihypertensive peptide P16 as well as preparation method and application thereof
InactiveCN104059126AClear structureInhibitory activityPeptide/protein ingredientsPeptide preparation methodsMultiple Tumor Suppressor-1Combinatorial chemistry
The invention discloses an antihypertensive peptide P16 which has the amino acid sequence shown as SEQ IDNo.1. The antihypertensive peptide P16 is clear and definite in structure and good in antihypertensive activity. The invention further discloses a preparation method of the antihypertensive peptide P16. The preparation method has a compact process and realizes the standardization of the quality of the antihypertensive peptide P16. The invention further discloses an application of the antihypertensive peptide P16 in preparation of a medicine or a food with an antihypertensive function.
Owner:INFINITUS (CHINA) CO LTD
Assessment of disease risk by quantitative determination of epimutation in normal tissues
An assay for assessing the risk of disease in an individual, wherein said assay comprises the steps of isolating a population of cells from normal tissue of said individual, and quantitatively determining the frequency of epimutation of a particular gene in said population of cells, wherein the epimutation of said gene is associated with said disease and said gene is other than one that is subject to normal parent of origin-specific expression. Preferably, the epimutation is DNA methylation of a tumour suprressor gene such as hMLH1, hMSH2, APC 1A, APC 1B and p16.
Owner:ST VINCENTS HOSPITAL SYDNEY +1
Detection method of L-arginine and sensor
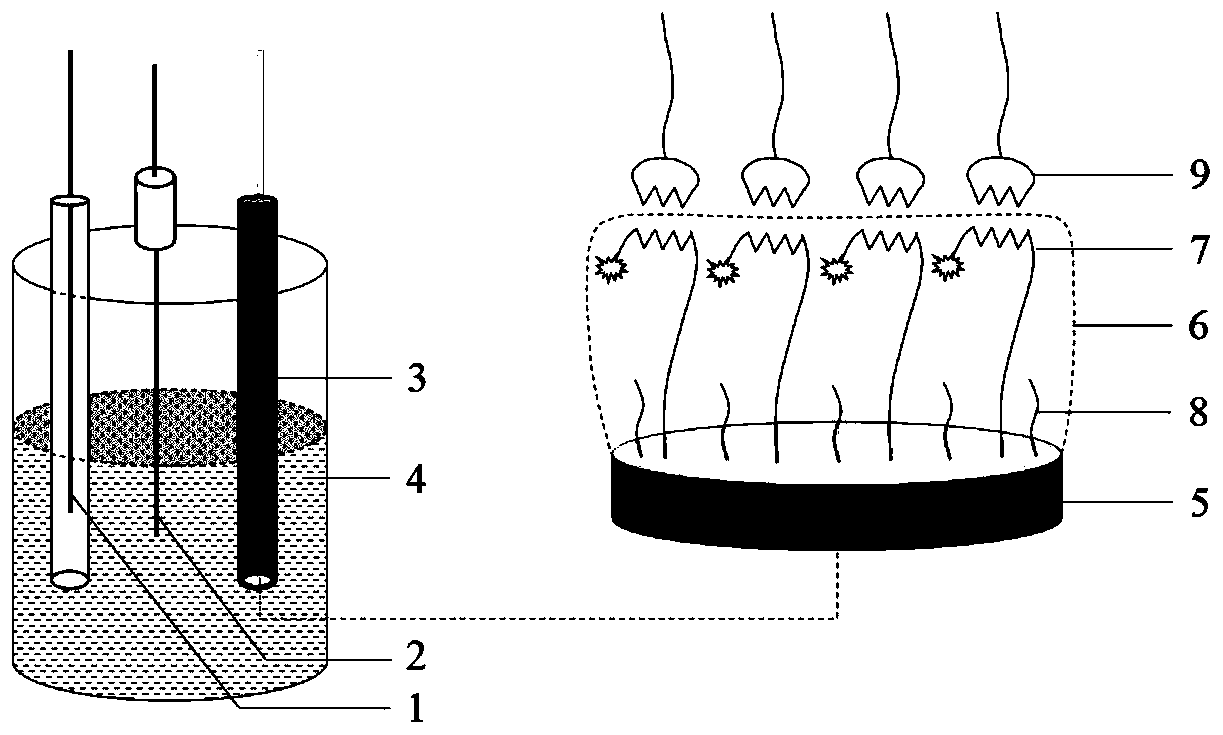
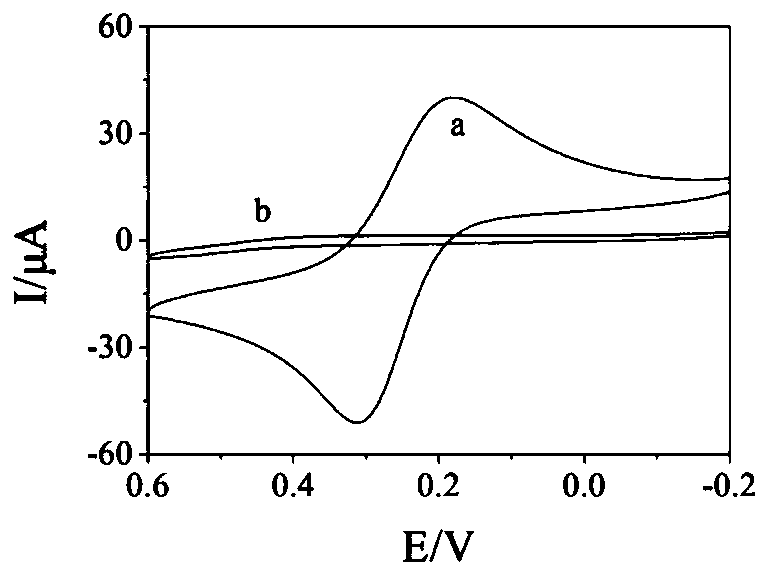
PendingCN110220960AGood reproducibilityGood repeatabilityPeptide preparation methodsMaterial electrochemical variablesElectrochemical responsePhosphate
The invention discloses a detection method of L-arginine (L-Arg) and a sensor. The method comprises the steps of: synthesizing ferrocene functional hexadeceptide dithio cyclopentane (FC-P16 Peptide);preparing a FC-P16 Peptide / AuE polypeptide composite membrane modified electrode; carrying out L-Arg detection; and the like. A result shows that the FC-P16 Peptide / AuE polypeptide composite membranemodified electrode shows the excellent electrochemical response characteristic for the L-Arg. In 10mmol / L PBS (Phosphate Buffered Solution) (pH is 7.4), a DPV response peak current of the polypeptidecomposite membrane modified electrode and concentration of the L-Arg has an excellent linear relationship within a range of 1.0*10<-13> to 1.0*10<-7> mol / L; and a detection limit is 1.0*10<-13> mol / L.The modified electrode has excellent reproducibility, repeatability and selectivity, and has potential application value in the aspects of life sciences and nutrition and health.
Owner:CHANGSHA UNIVERSITY OF SCIENCE AND TECHNOLOGY
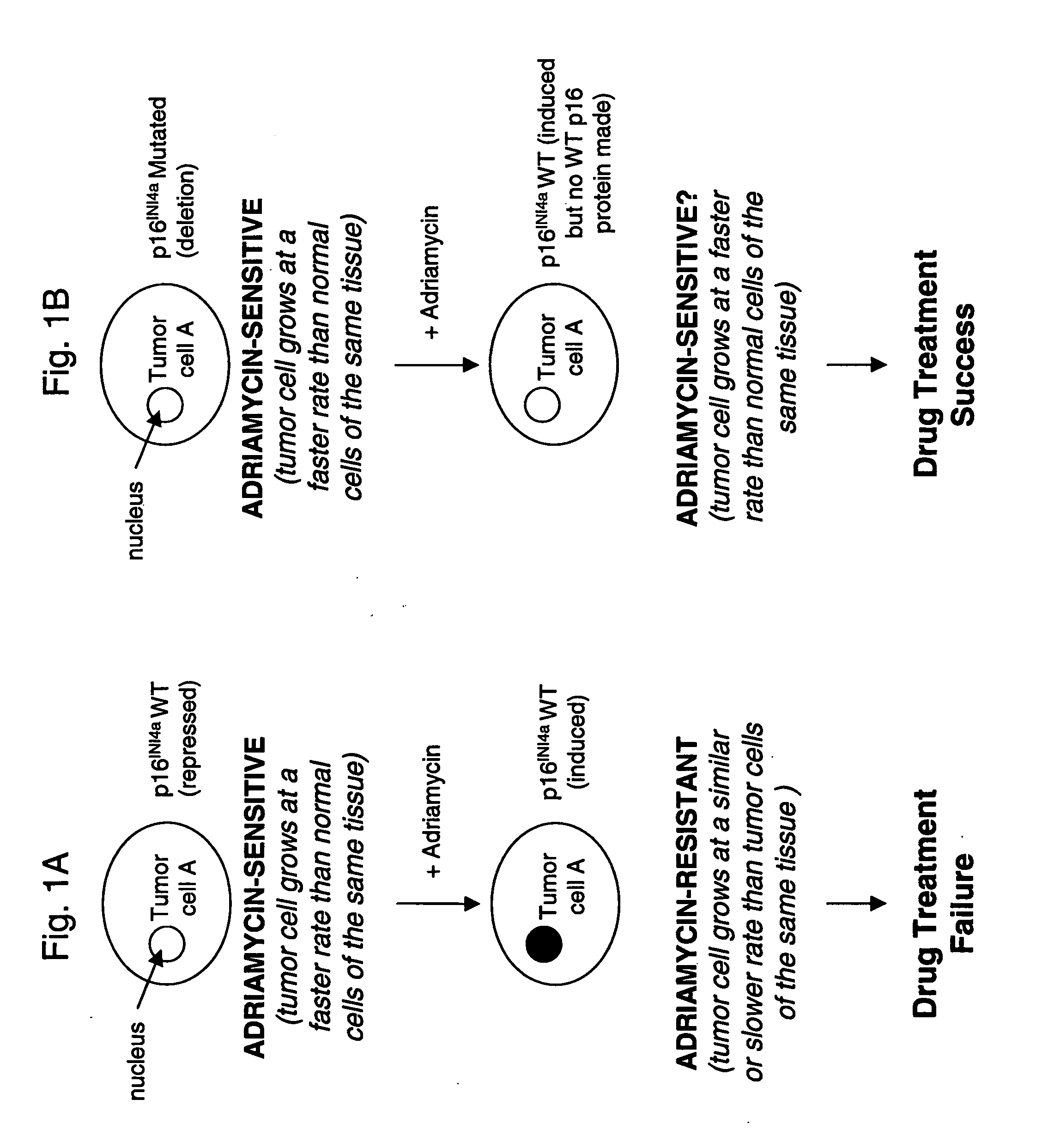
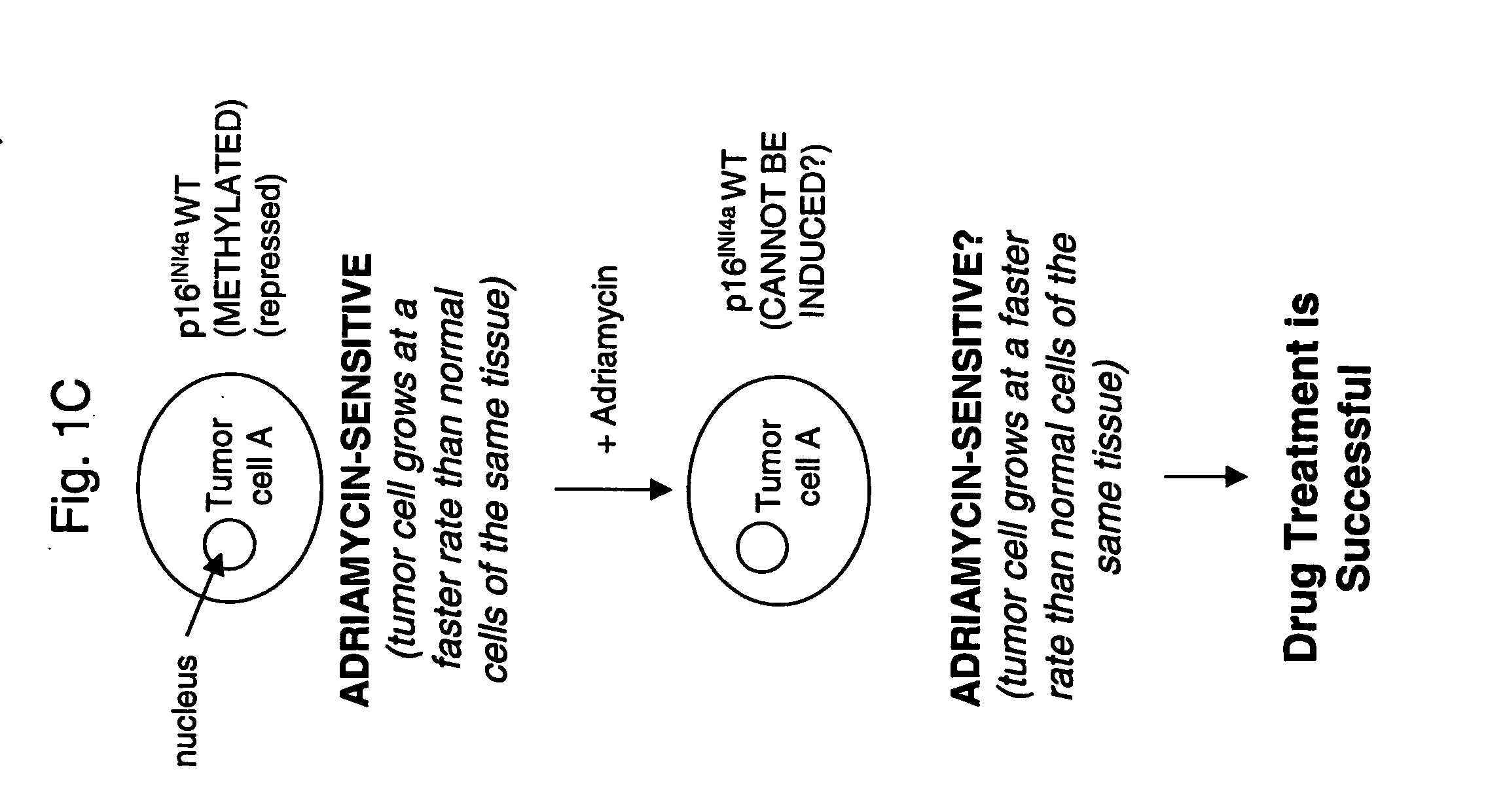
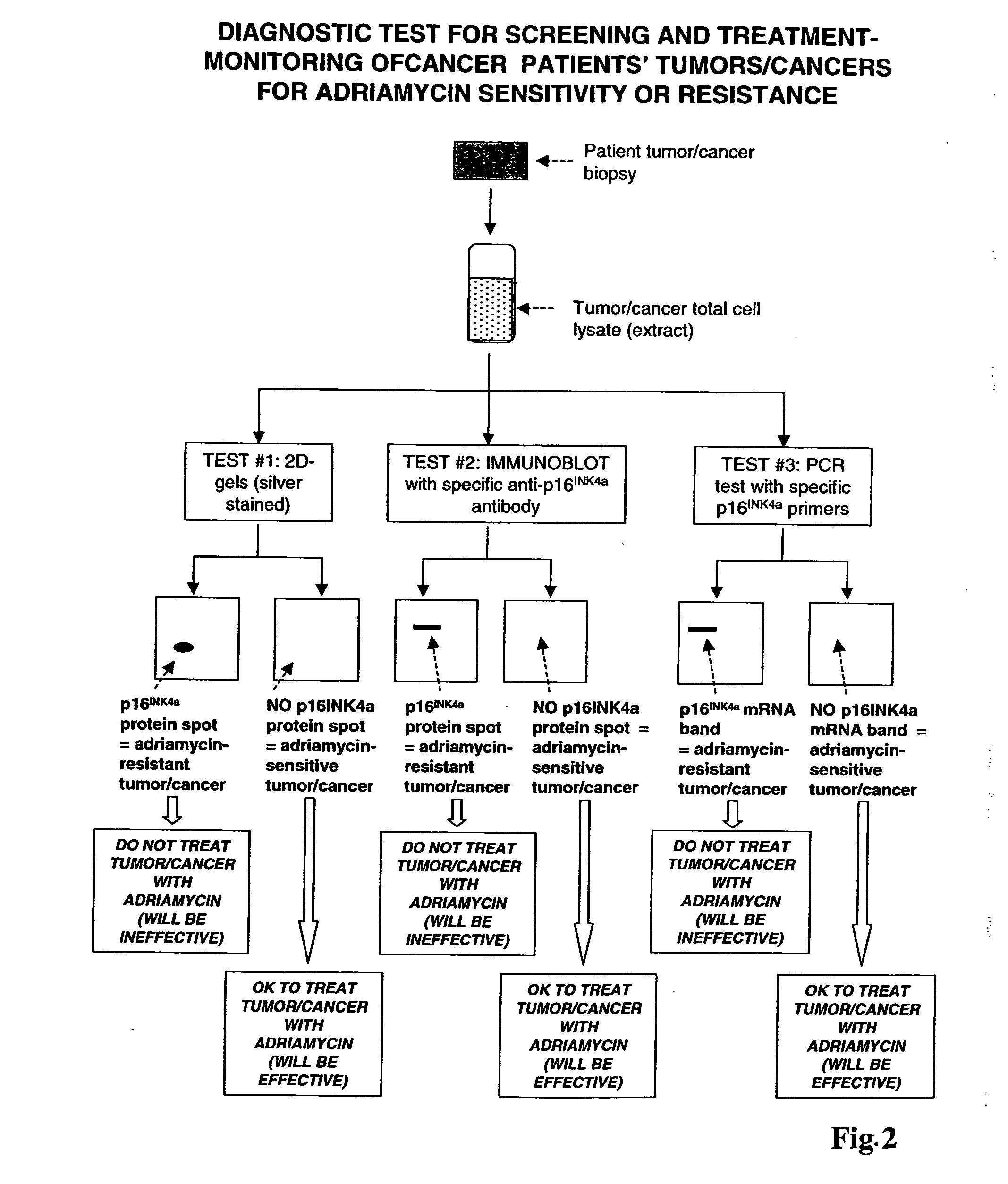
Methods for identifying chemotherapeutic resistance in non-hematopoietic tumors
InactiveUS20060188507A1Increase drug sensitivitySilencing of p16 expressionBiocideGenetic material ingredientsMultiple Tumor Suppressor-1Hematopoietic Tumor
Disclosed are methods for detecting adriamycin resistance in a test neoplastic cell from a non-hematological cancer. The methods include detecting a level of p16 expression in the test neoplastic cell of a given origin or cell type, and comparing the level of p16 expression detected in the test neoplastic cell to the level of p16 expression in a nonresistant neoplastic cell of the same origin or cell type, wherein the test neoplastic cell is adriamycin resistant if the level of p16 expression is greater than the level of p16 expression in the nonresistant neoplastic cell of the same origin or cell type. Also disclosed are therapeutic compositions comprising an agent that inhibits p16 and a pharmaceutically acceptable carrier.
Owner:AURELIUM BIOPHARMA
Immune p16<INK4a> antigen preserving liquid used for thin prep liquid-based cytology test and preparation method of same
ActiveCN107271246AStrong ability to break down mucusEffective maintenance of osmotic pressurePreparing sample for investigationLiquid base cytologyAntigen
The invention belongs to the technical field of clinical medicine pathology and particularly relates to an immune p16<INK4a> antigen preserving liquid used for thin prep liquid-based cytology test and a preparation method of same. The antigen preserving liquid includes: 60-100 mmol / L of NaCl, 2-10 mmol / L of KCl, 1-5 mmol / L of EDTA disodium, 30-50 mmol / L of urea, 1-5 mmol / L of sodium citrate, 5-20 mmol / L of sucrose, 4-10 mmol / L of PEG-400, 2-3% of paraformaldehyde, 2-10 mmol / L of glycerol and 10-20 mmol / L of dithiothreitol. The preserving liquid is not only suitable for traditional slide preparation and staining of liquid-based cells, but also is suitable for cervical liquid-based cell p16<INK4a> protein immuno-labeling and staining, thus avoiding defects in conventional non-specific Papanicolaou staining.
Owner:GUANGZHOU JIANGYUAN MEDICAL SCI & TECH CO LTD
Combination therapy
Reversing resistance to a B-Raf inhibitor for the treatment of a proliferative disease by obtaining a tumor sample from the patient and testing it for genetic alterations in a panel of genes comprising BRAF, CRAF, CCND1, CDK4, HER2, IGF-1R, cMET, FGFR1, FGFR2, FGFR3 EGFR, MAP2K1, MAP2K2, NRAS, KRAS HRAS, PTEN, PIK3CA, and P16 and administering a drug combination therapy comprising the B-Raf inhibitor and a second inhibitor which overcomes resistance to the B-Raf inhibitor, which second inhibitor is selected based on genetic alterations discovered in the tumor sample.
Owner:ARRAY BIOPHARMA
Liver cancer early warning method
The invention relates to a kit for judging whether a subject suffers from liver cancer or predicting the risk of liver cancer of the subject. The kit includes a reagent capable of identifying the methylation status of characteristic genes and / or a regulatory region thereof. The characteristic genes include P16, SFRP1 and RASSF1A. The invention also provides a method for judging whether the subjectsuffers from liver cancer or predicting the risk of liver cancer of the subject.
Owner:江苏吉睿生物技术研究院有限公司
Identification and isolation of neural stem cells and neurosphere initiating cells
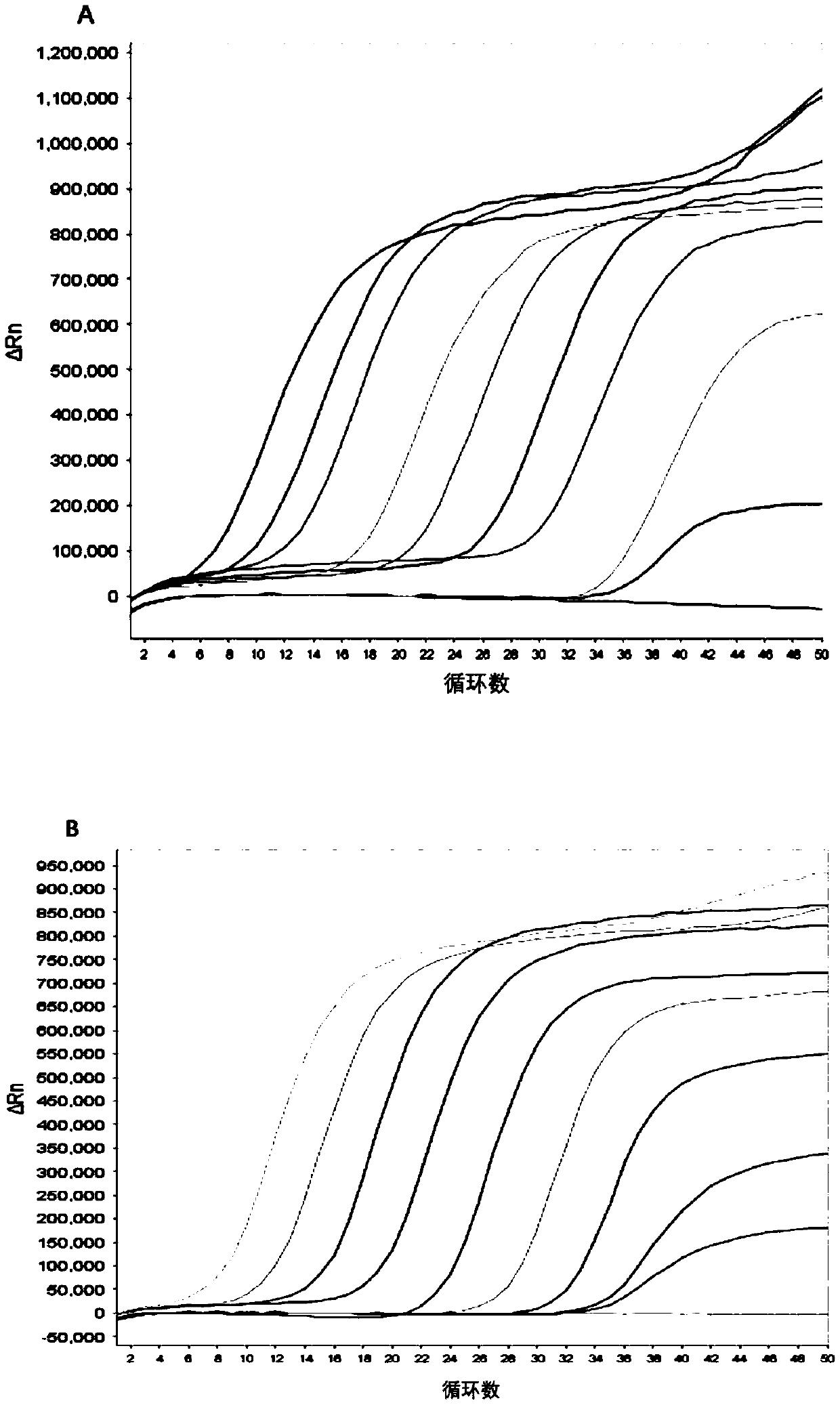
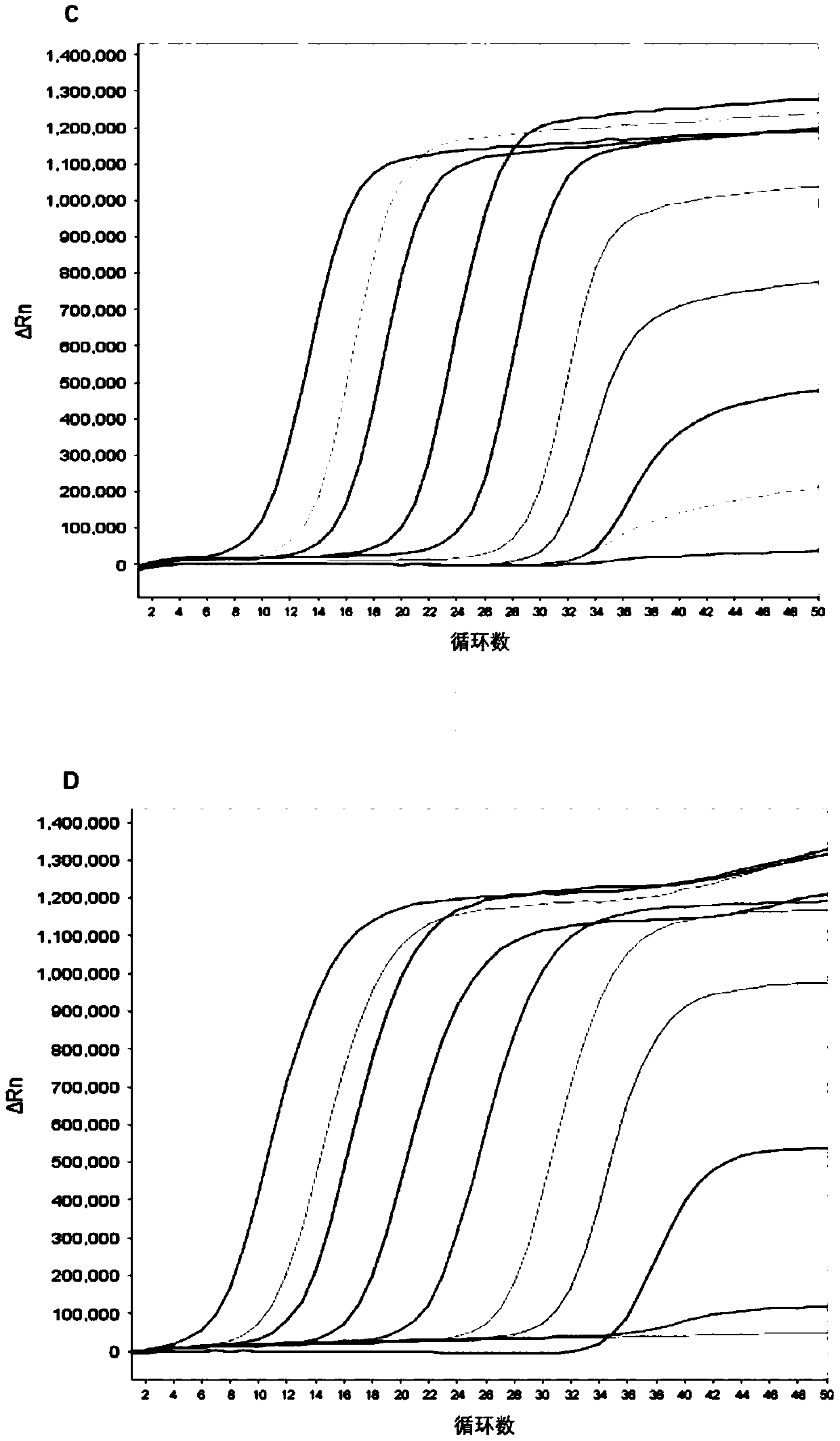
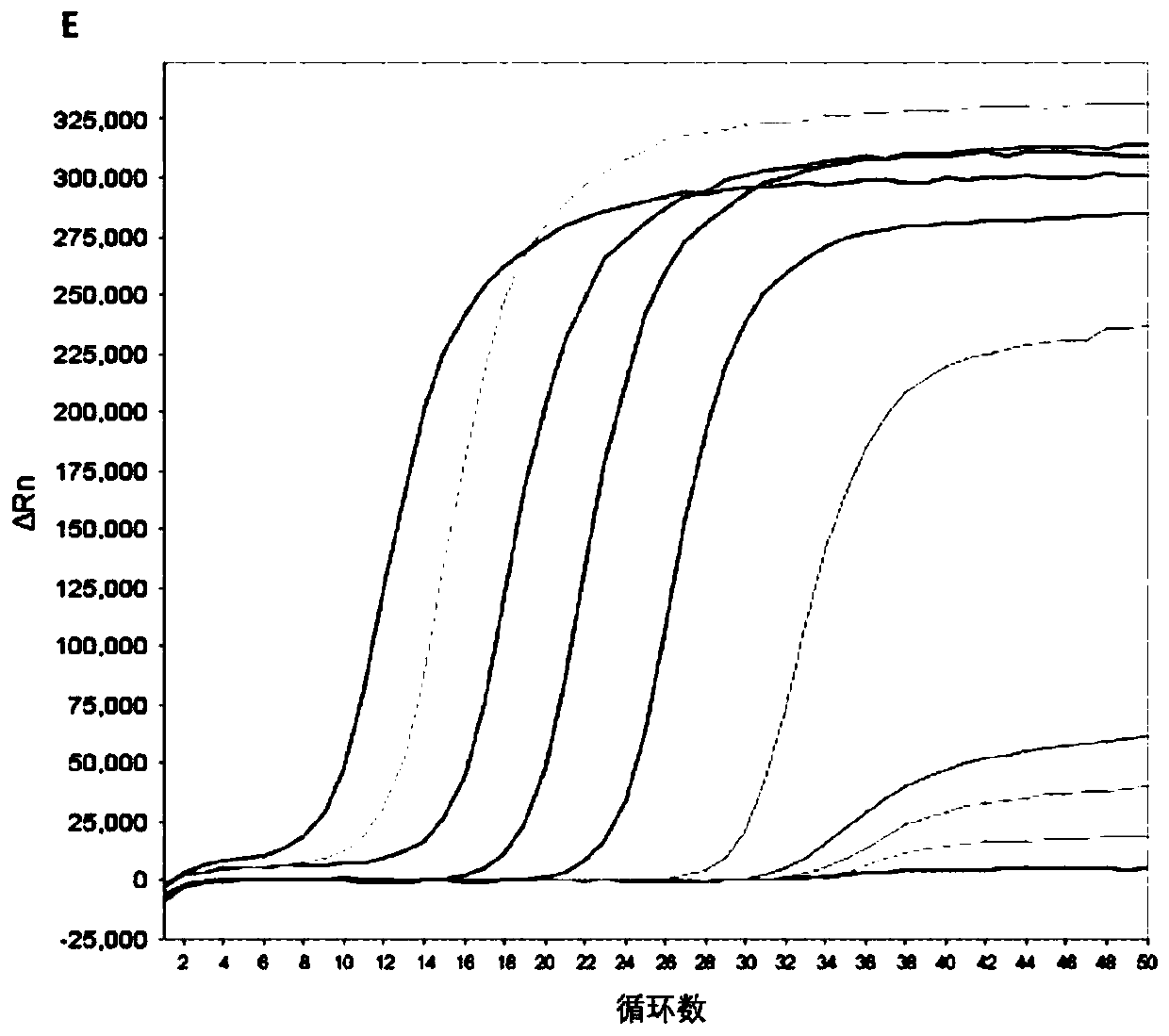
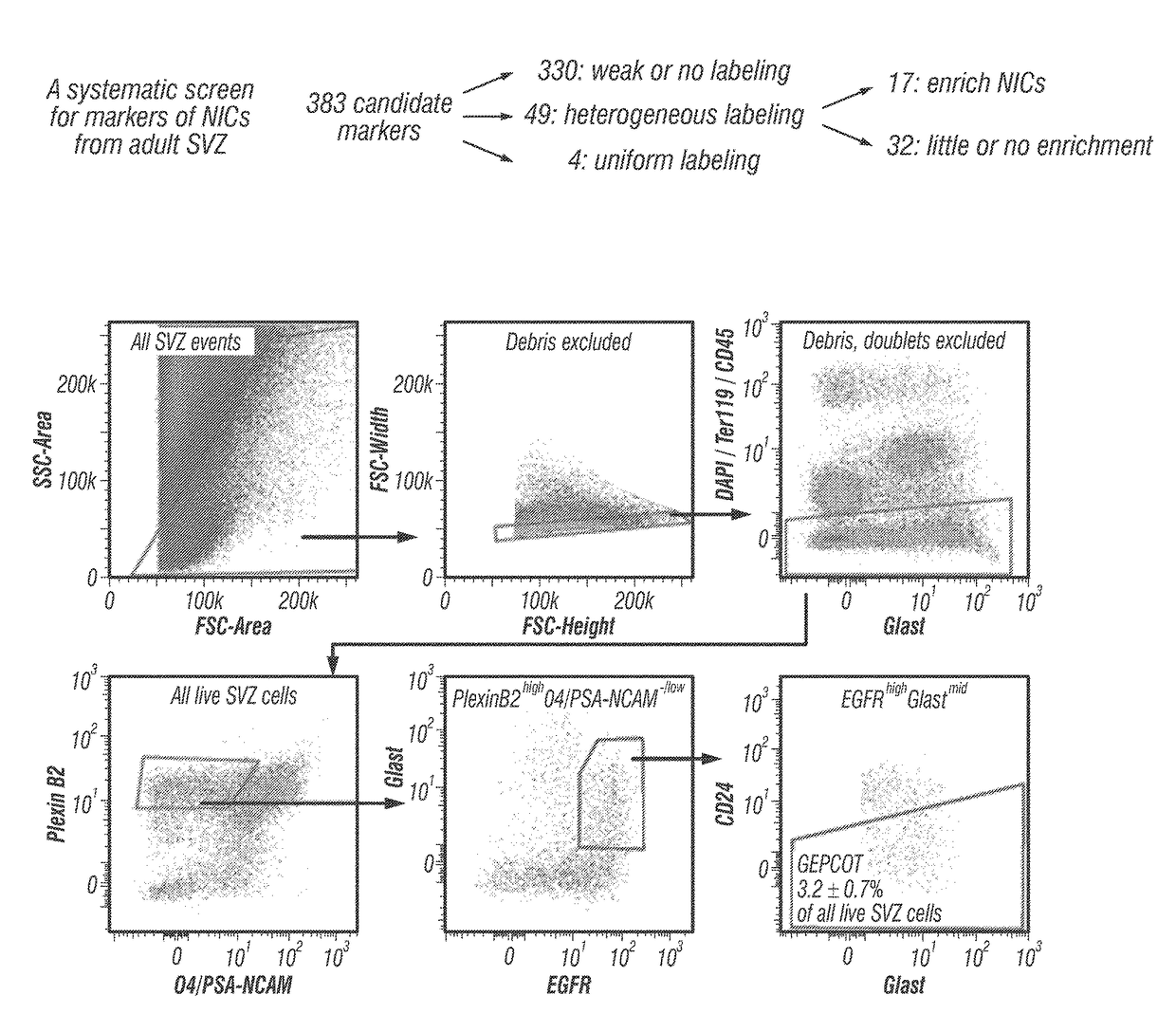
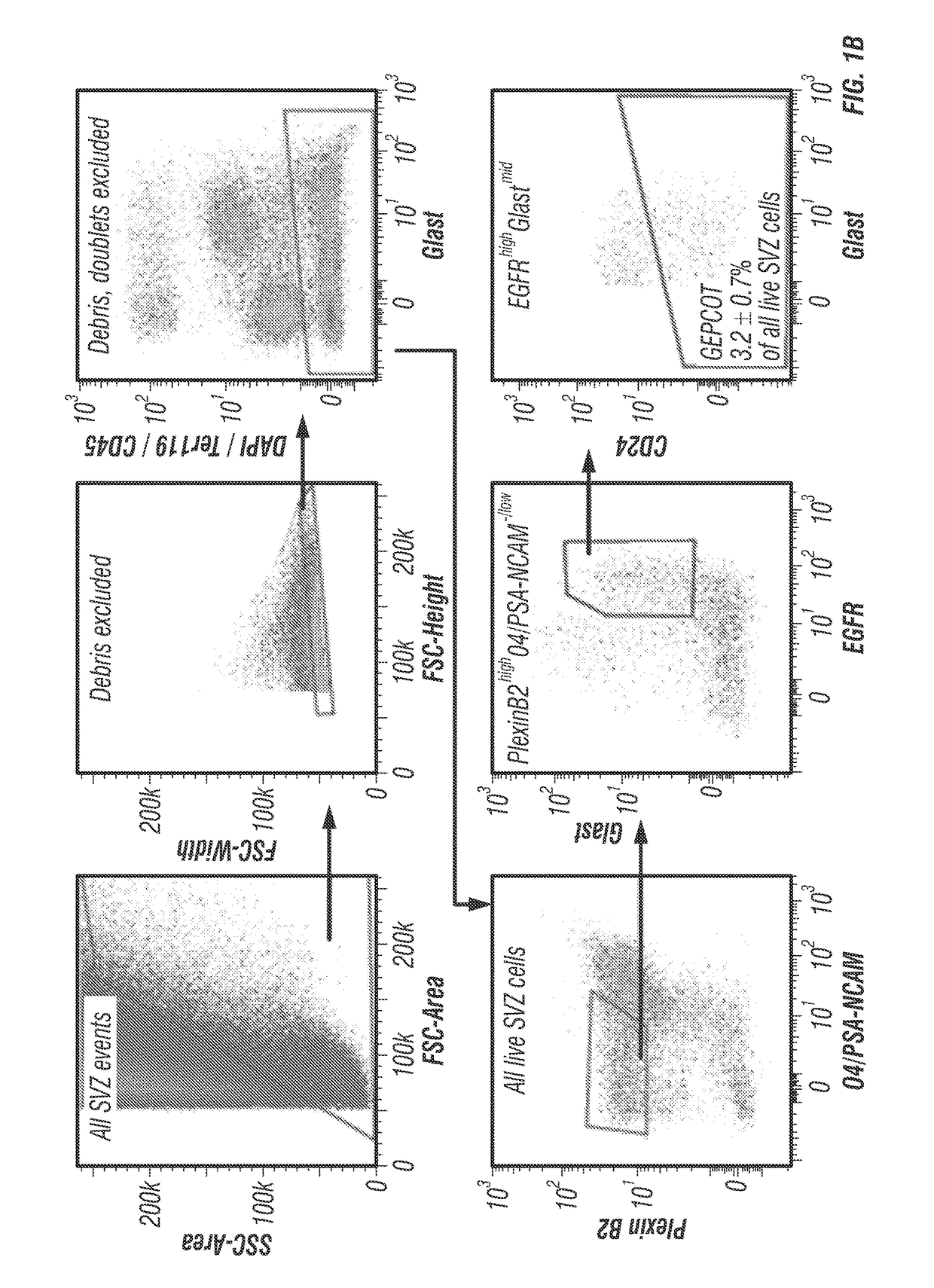
The disclosure reports on the identification and isolation of adult mouse lateral ventricle subventricular zone (SVZ) neurosphere initiating cells (NICs) by flow cytometry on the basis of GlastmidEGFRhighPlexinB2highCD24− / lowO4 / PSA-NCAM− / lowTer-119 / CD45− markers (GEPCOT cells). These cells are highly mitotic and short-lived in vivo based on fate-mapping with Ascl1CreERT2 and Dlx1CreERT2. In contrast, pre-GEPCOT cells were quiescent, expressed higher Glast, and lower EGFR and PlexinB2. Pre-GEP-COT cells could not form neurospheres but expressed the stem cell markers Glast-CreERT, GFAP-CreERT2, Sox2CreERT2, and Gli1C-reERT2 and were long-lived in vivo. While GEPCOT NICs were ablated by temozolomide, pre-GEPCOT cells survived and repopulated the SVZ. Conditional deletion of the Bmi-1 polycomb protein depleted pre-GEPCOT and GEPCOT cells, though pre-GEPCOT cells were more dependent upon Bmi-1 for p16Ink4a repression. These data distinguish quiescent NSCs from NICs and make it possible to study their properties in vivo.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
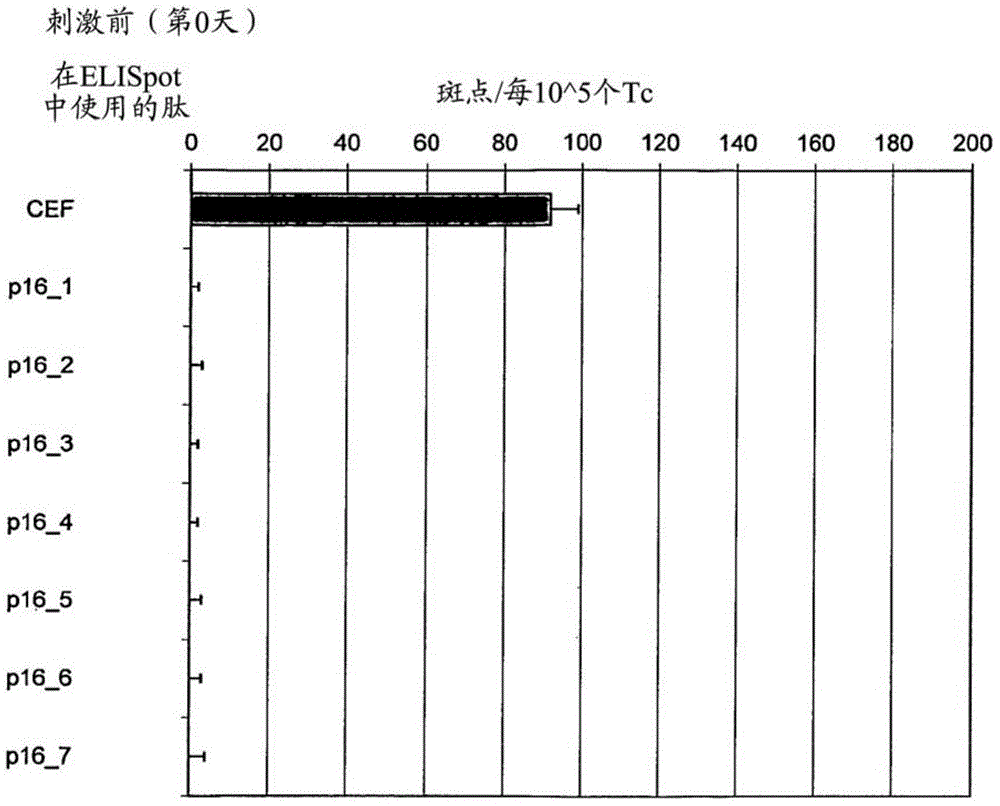
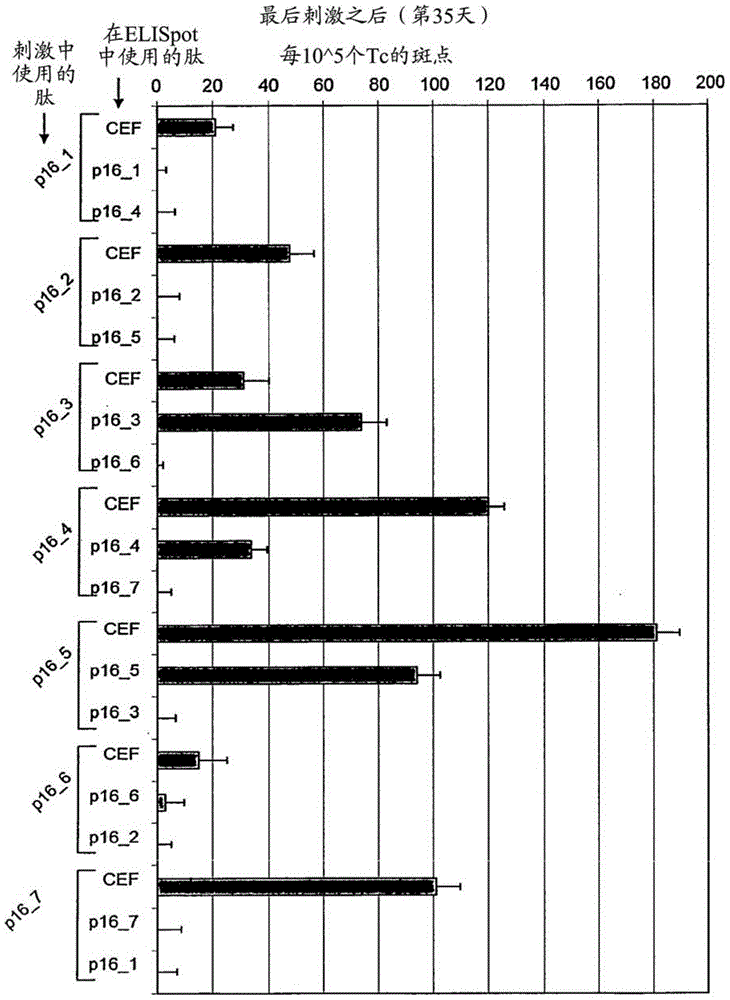
P16INK4A derived peptides for prophylaxis and therapy of HPV-associated tumors and other p16 expressing tumors
ActiveCN104837497APeptide/protein ingredientsCancer antigen ingredientsMultiple Tumor Suppressor-1T cell
Described are particular fragments of the cyclin-dependent kinase inhibitor p16 capable of increasing IFN-ϒ secretion of T cells or inducing proliferation of T cells and the use of said fragments for immunizing an individual against HPV-associated or other p16INK4a expressing carcinomas, preferably advanced carcinomas.
Owner:UNIVERSITY OF HEIDELBERG
Method for increasing carbon source utilization rate in aspergillus oryzae L-malic acid synthesizing process
InactiveCN105969790AImprove conversion rateLow cost of industrializationFungiMicroorganism based processesEscherichia coliBiotechnology
The invention discloses a method for increasing the carbon source utilization rate in the aspergillus oryzae L-malic acid synthesizing process and belongs to the field of genetic engineering. According to the method, Aspergillus oryzae NRRL 3488 serves as an original strain, metabolic flux for synthesizing malic acid from oxaloacetic acid is enhanced by expressing pyruvic carboxylase and malic dehydrogenase in aspergillus oryzae cytoplasm, and the shake flask yield of L-malic acid is increased to 50.5+ / -1.06 g / L and is increased by 38.4%; then, the synthesizing efficiency of oxaloacetic acid from phosphoenolpyruvic acid in aspergillus oryzae cytoplasm is improved by expressing pyruvic carboxylase and pyruvate carboxykinase of Escherichia coli in the converter Aspergillus oryzae p16, finally, the yield of L-malic acid reaches 63.2+ / -1.32 g / L, the concentration of succinic acid is 8.88+ / -0.45 g / L, the production intensity is also improved to 0.7 g / (L.h) from original 0.24 g / (L.h) of Aspergillus oryzae NRRL 3488, and a foundation is laid for further metabolic engineering reform of Aspergillus oryzae for producing L-malic acid.
Owner:JIANGNAN UNIV
Genetic therapy breast cancer drug preparation method based on microfluidic chip
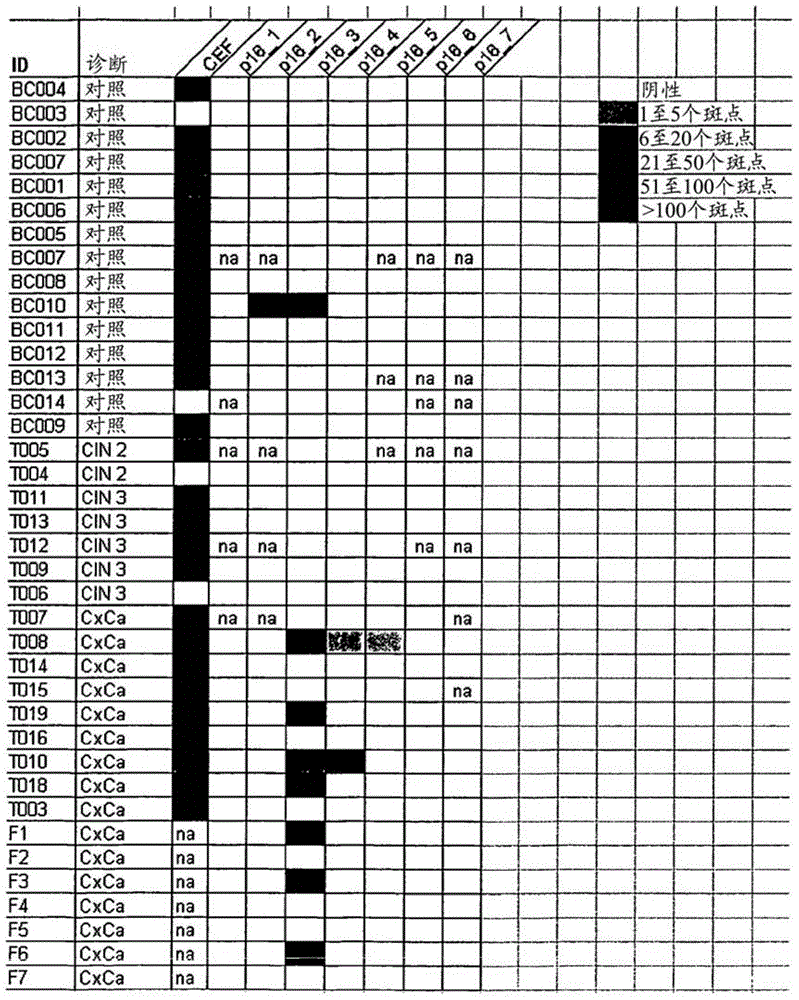
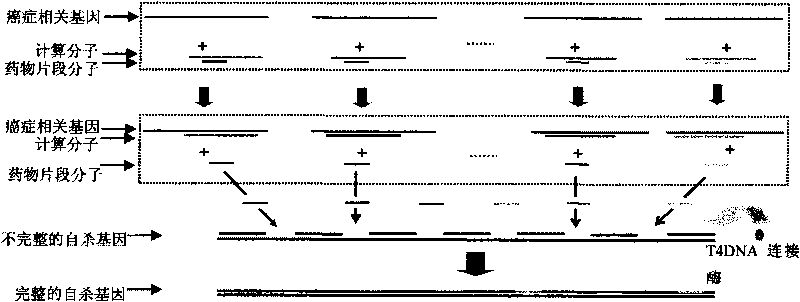
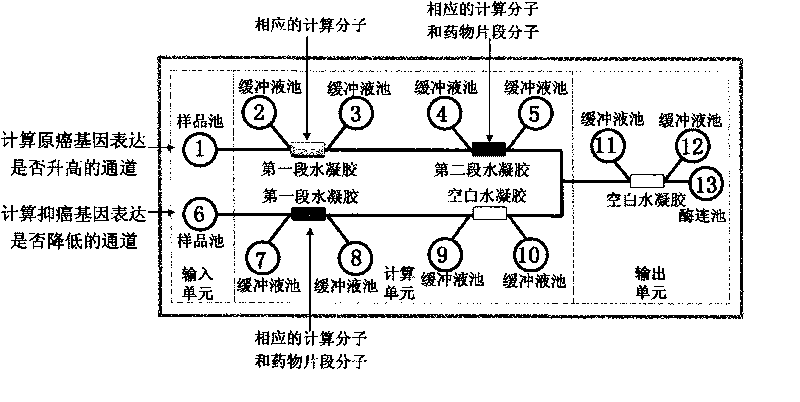
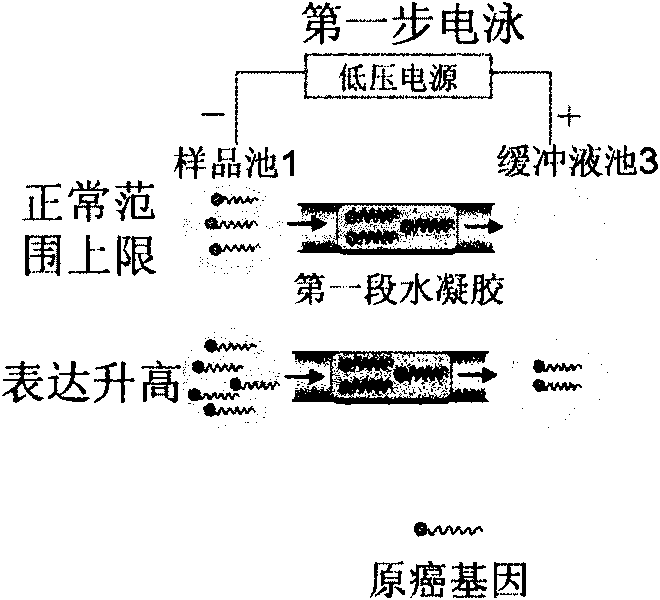
InactiveCN101745121AMicrobiological testing/measurementGenetic material ingredientsTherapeutic effectC erbb 2
The invention discloses a genetic therapy breast cancer drug preparation method based on microfluidic chip, which includes that: the method adopts biological molecules as computation medium to compute the cancer-related gene expression through biochemical reaction; when the result meets the requirement, the anti-cancer drug is automatically synthesized and released in targeted manner; the anti-cancer drug is complete suicide gene; the biological molecules used as computation medium are DNA molecules and / or enzyme; the oncogene related to breast cancer includes: C-erbB-2, EGFR, c-myc, ras, int-2, bcl-2, BAG-1, BCSG-2 and survivin; the cancer suppressor gene related to breast cancer includes P53, nm23, PTEN, Rb, P16, P21, CHEK2, BRCA1 and BRCA2. The invention can synthesize and release anti-cancer drugs for breast cancer on specified conditions; and the anti-cancer drugs are complete suicide gene. The method can be combined with the nanometer technology and micromachining technology to help improve the treatment effect.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Drug screening method based on p16 promoters
InactiveCN102250941AMicrobiological testing/measurementFluorescence/phosphorescenceScreening methodDrugs preparations
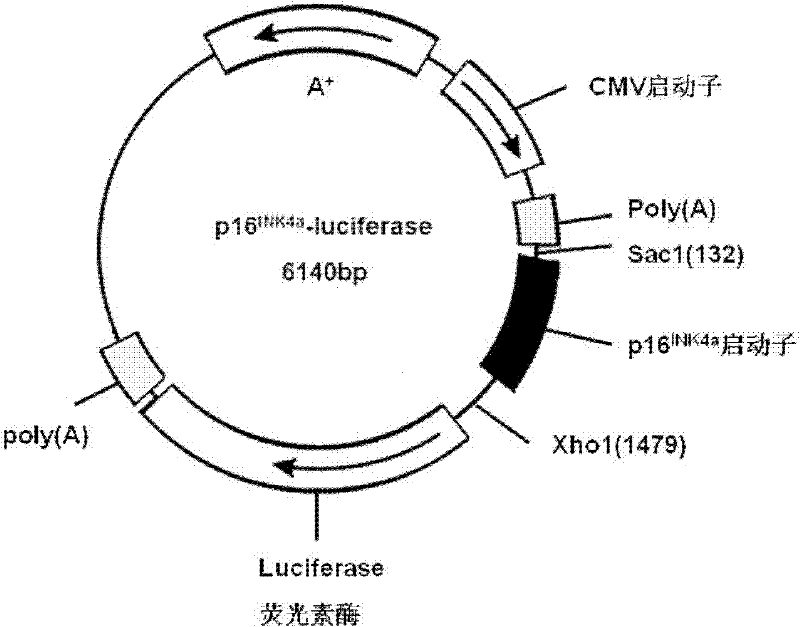
The invention relates to a drug screening method based on p16 promoters, belonging to the technical field of drug preparation. The sequence of the p16<INK4a> promoter-luciferase eukaryon expression vector is disclosed as Seq ID NO.1; the full length of the sequence is 3300bp, wherein 138th-1478th base pairs are p16<INK4a> promoters, and 1535th-3187th base pairs are luciferase reading frames; and the eukaryon expression vector can be used for detecting the anticancer activity of a compound.
Owner:乐清市康诺生物医药公共服务平台有限公司
Application of gastrin in inhibiting gastrointestinal tumor tumors
ActiveCN101890157AHas therapeutic effectSignificant negative correlationPeptide/protein ingredientsDigestive systemMultiple Tumor Suppressor-1Stomach cancer
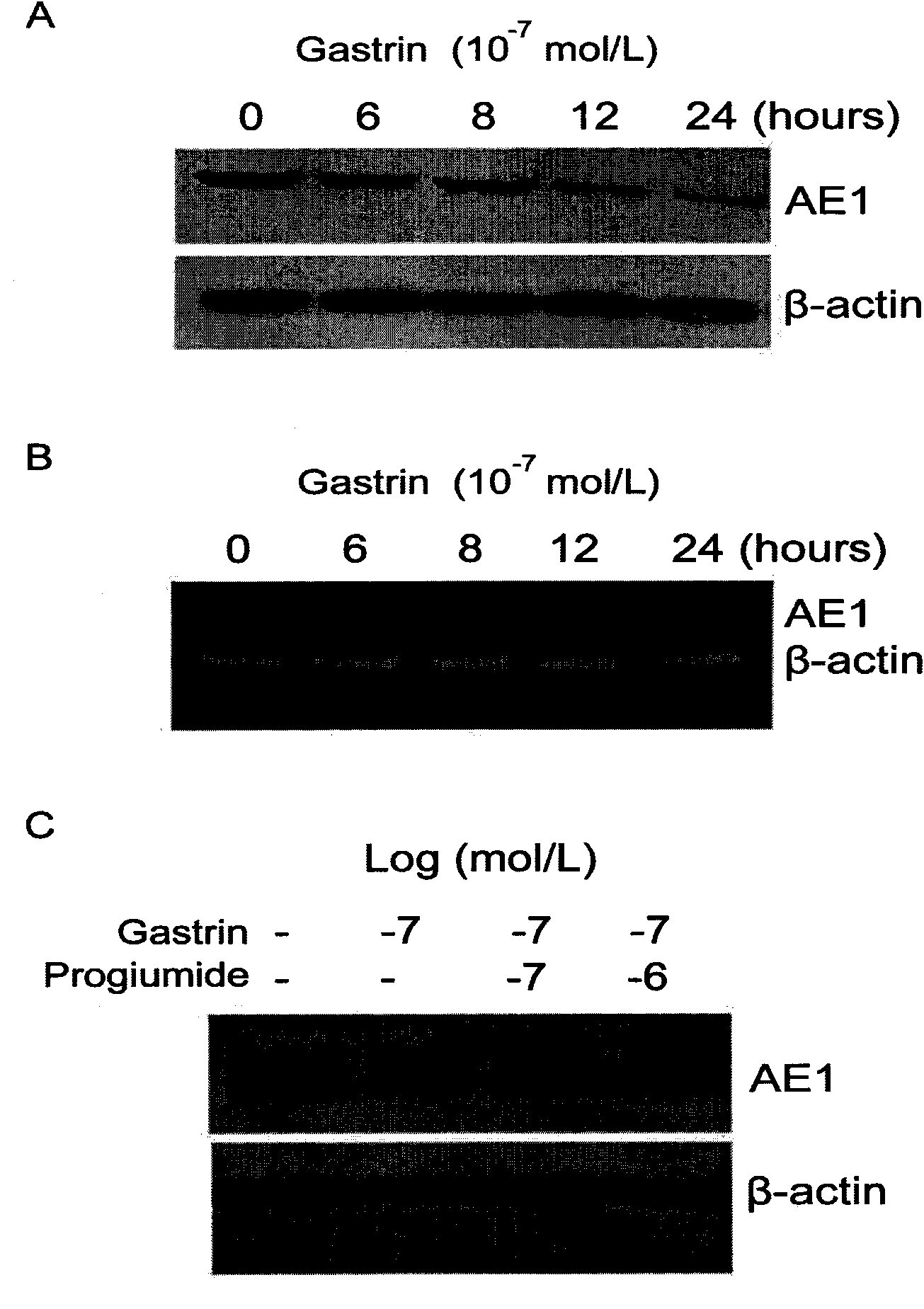
The invention discloses a novel application of gastrin in inhibiting gastrointestinal tumor tumors. Gastric cancer target gene anion exchange protein 1 is interacted with tumor suppressor protein p16 so as to induce gastric cancer, however, 17 peptide gastrin (H-pGlu-Gly-Pro-Trp-Leu-Glu-Glu-Glu-Glu-Glu-Ala-Tyr-Gly-Trp-Met-Asp-Phe-NH2) can effectively inhibit expression of gastric cancer target gene AE1 and p16 and breaks through the traditional view that gastrin induces tumors, and clinical pathologies show that the gastrin has obvious negative correlation with AE1 and p16. The invention alsodiscloses that the gastrin with the concentration of 10-10mol / L-10-70mol / L has obvious inhibition effect on gastric cancer cell activity after a certain time, thus prompting that the gastrin has curing effect on gastric antrum cancer and providing a new direction for curing gastric cancer based on the test results.
Owner:SHANGHAI JIAOTONG UNIV SCHOOL OF MEDICINE
Antibodies to cell-cycle regulatory protein p16, and uses related thereto
The present invention relates to the discovery in eukaryotic cells, particularly mammalian cells, of a family of cell-cycle regulatory proteins (“CCR-proteins”). As described herein, this family of proteins includes a polypeptide having an apparent molecular weight of 16 kDa, and a polypeptide having an apparent molecular weight of approximately 15 kDa, each of which can function as an inhibitor of cell-cycle progression, and therefore ultimately of cell growth. The present invention comprises antibodies directed to such CCR-proteins. The present invention is directed to a kit for detecting the level of cyclin-dependent kinase inhibitor p16 gene expression comprising antibodies directed to a p16 protein.
Owner:COLD SPRING HARBOR LAB INC
Biological chip for detecting control of related gene expression by pharmaceutical active component
InactiveCN101134983AOmit experimentSpeed up the processMicrobiological testing/measurementDNA/RNA fragmentationMultiple Tumor Suppressor-1CDC25A
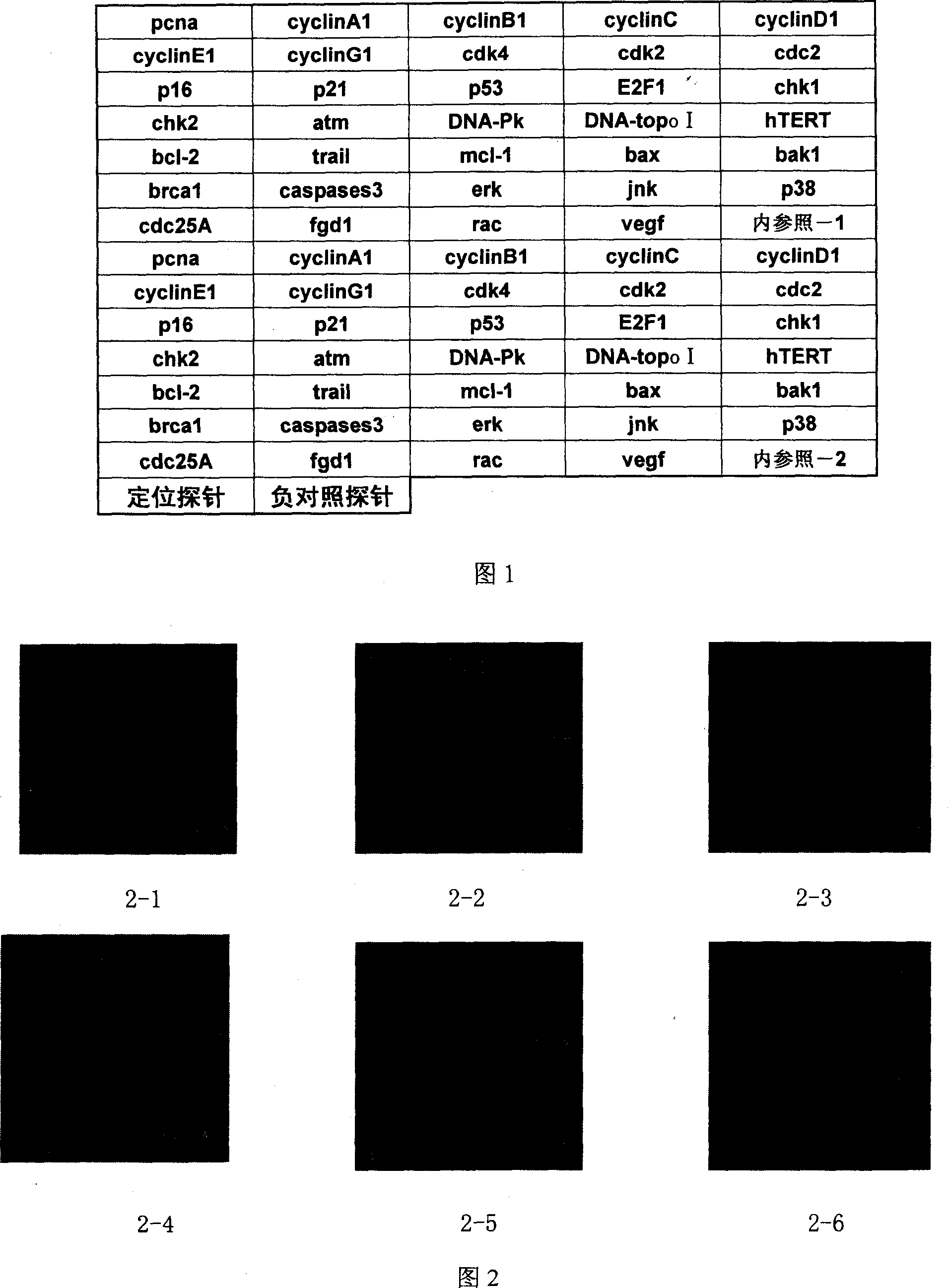
The present invention discloses one kind of biochip for detecting the regulation and control of active medicine components on the expression of relevant genes. On one glass chip, one silicon chip or polymer chip, are fixed gene DNA probes for detecting PCNA, CyclinA1, CyclinB1, CyclinC, CyclinD1, CyclinD1, CyclinE1, CyclinG1, Cdk4, Cdk2, Cdc2, p16, p21, p53, E2F1, Chk1, Chk2, ATM, DNA-PK, DNA-Topo I, hTERT, Bcl-2, Trail, Mcl-1, Bax, Bak1, Brca1, Caspase3, Erk-3, Jnk, p38, Cdc25A, Fgd1, Rac and VEGF. The present invention can detect the regulation and control of active medicine components on the expression of relevant genes, and has the features of real-time, high sensitivity, high accuracy, high efficiency, etc.
Owner:LOGISTICS UNIV OF CAPF
Assessment of disease risk by quantitative determination of epimutation in normal tissues
An assay for assessing the risk of disease in an individual, wherein said assay comprises the steps of isolating a population of cells from normal tissue of said individual, and quantitatively determining the frequency of epimutation of a particular gene in said population of cells, wherein the epimutation of said gene is associated with said disease and said gene is other than one that is subject to normal parent of origin-specific expression. Preferably, the epimutation is DNA methylation of a tumour suppressor gene such as hMLH1, hMSH2, APC 1A, APC 1B and p16.
Owner:VICTOR CHANG CARIDAC RES INST LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com