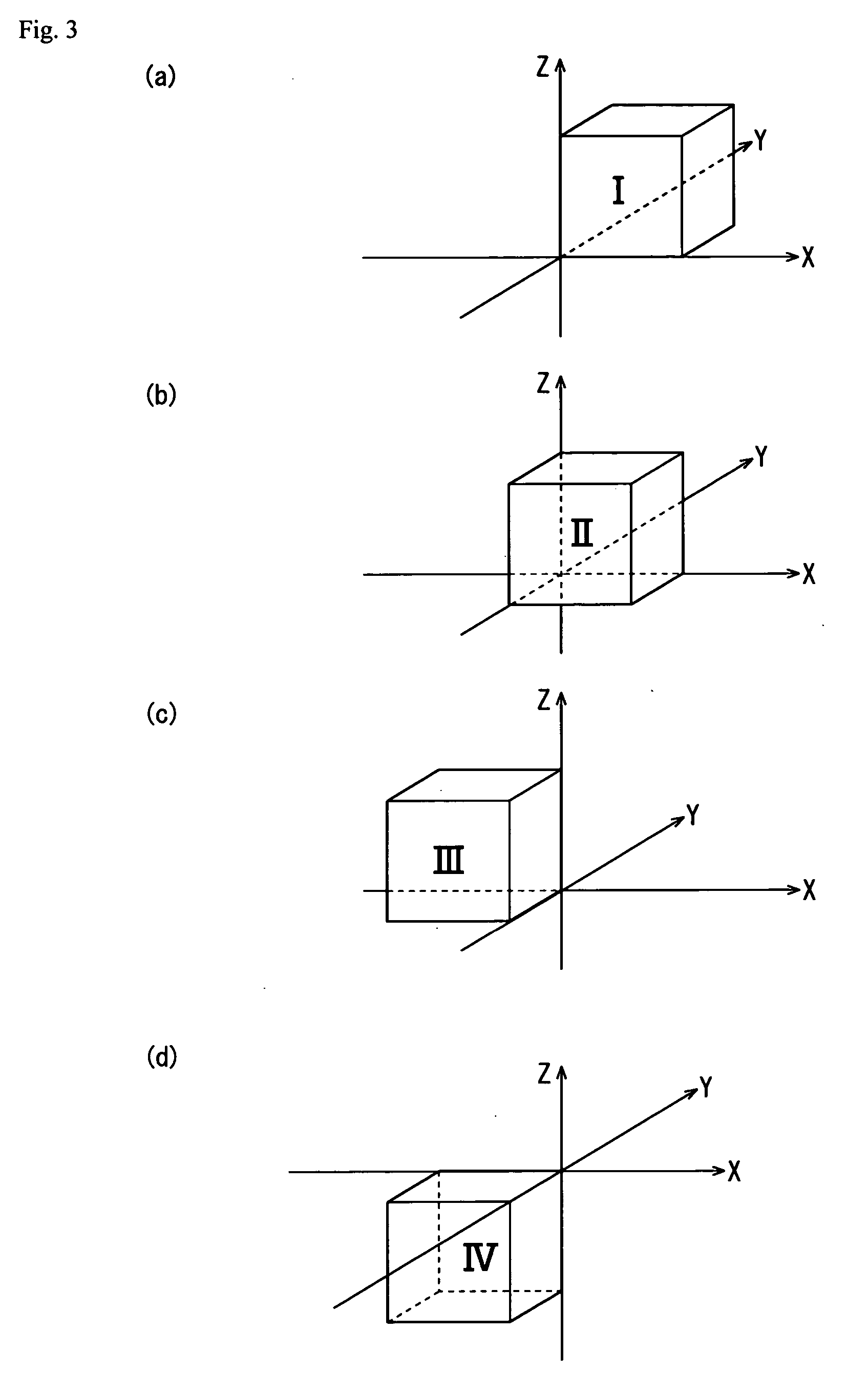
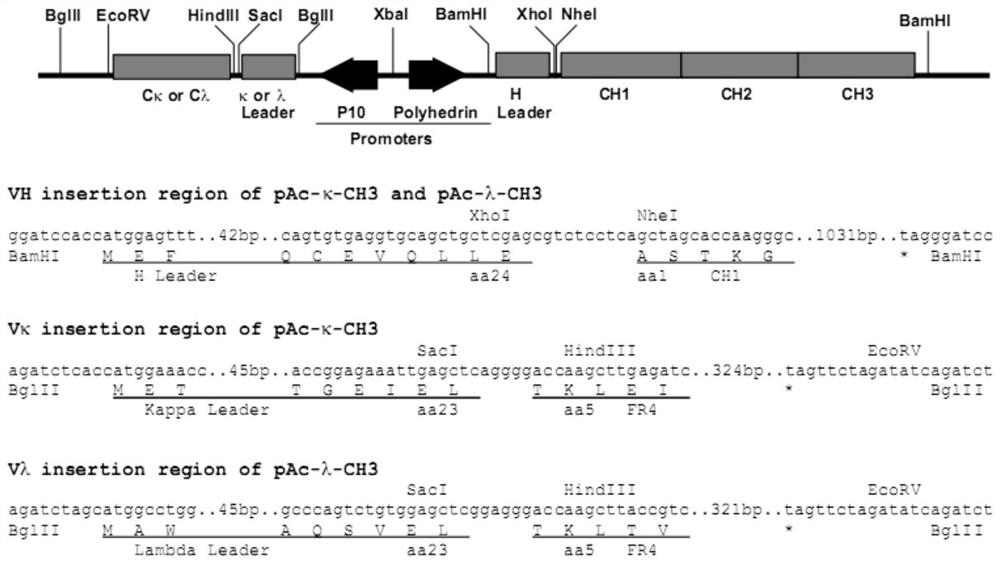
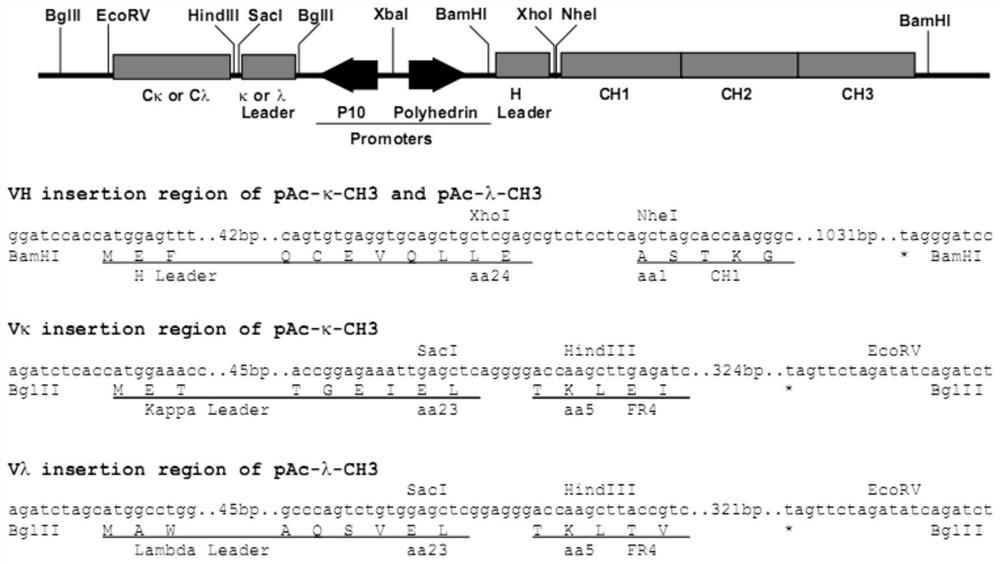
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
48 results about "CEA Antibody" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
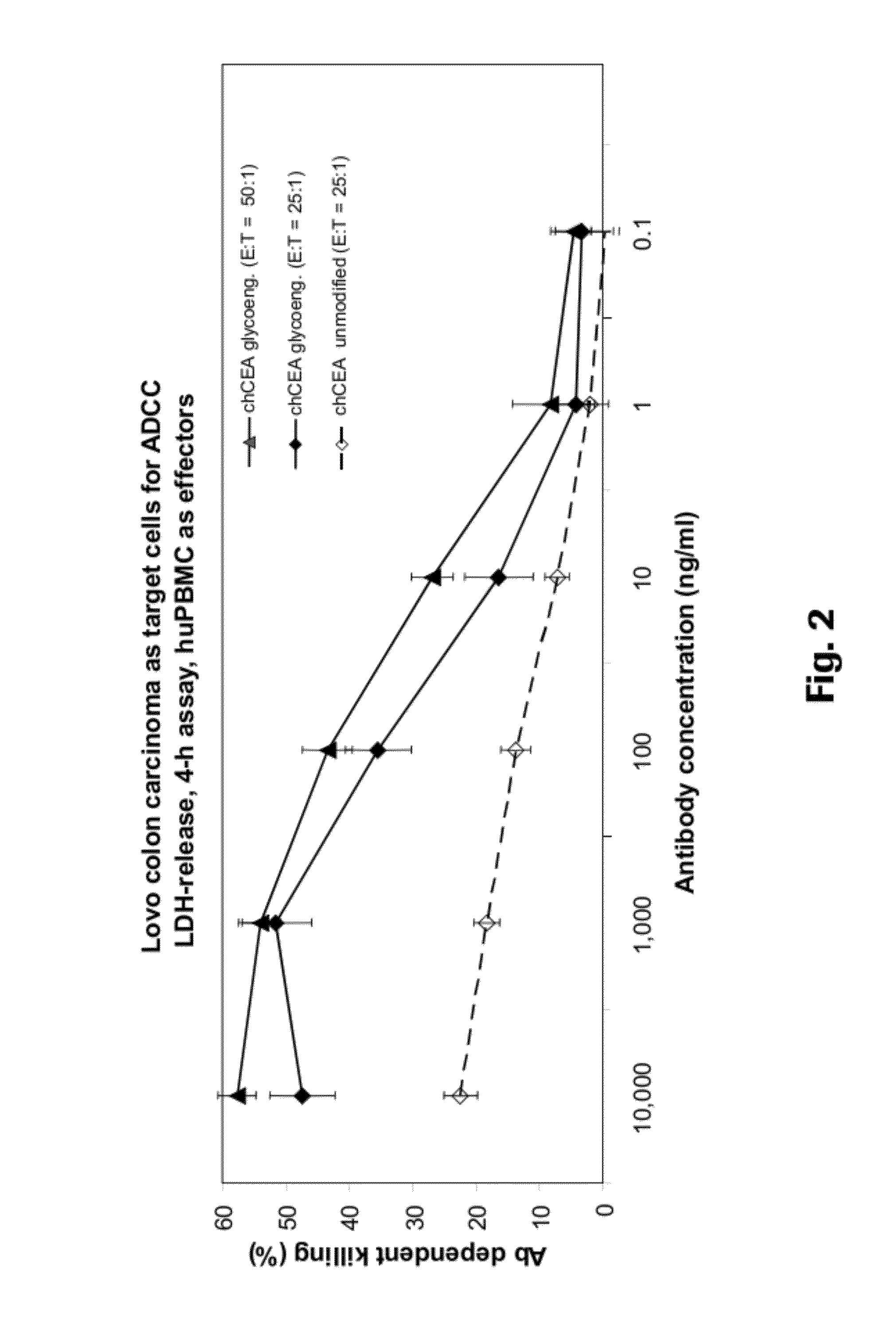
An anti-CEA antibody is an antibody against the carcinoembryonic antigen (CEA), a protein present on certain types of cancer cells.
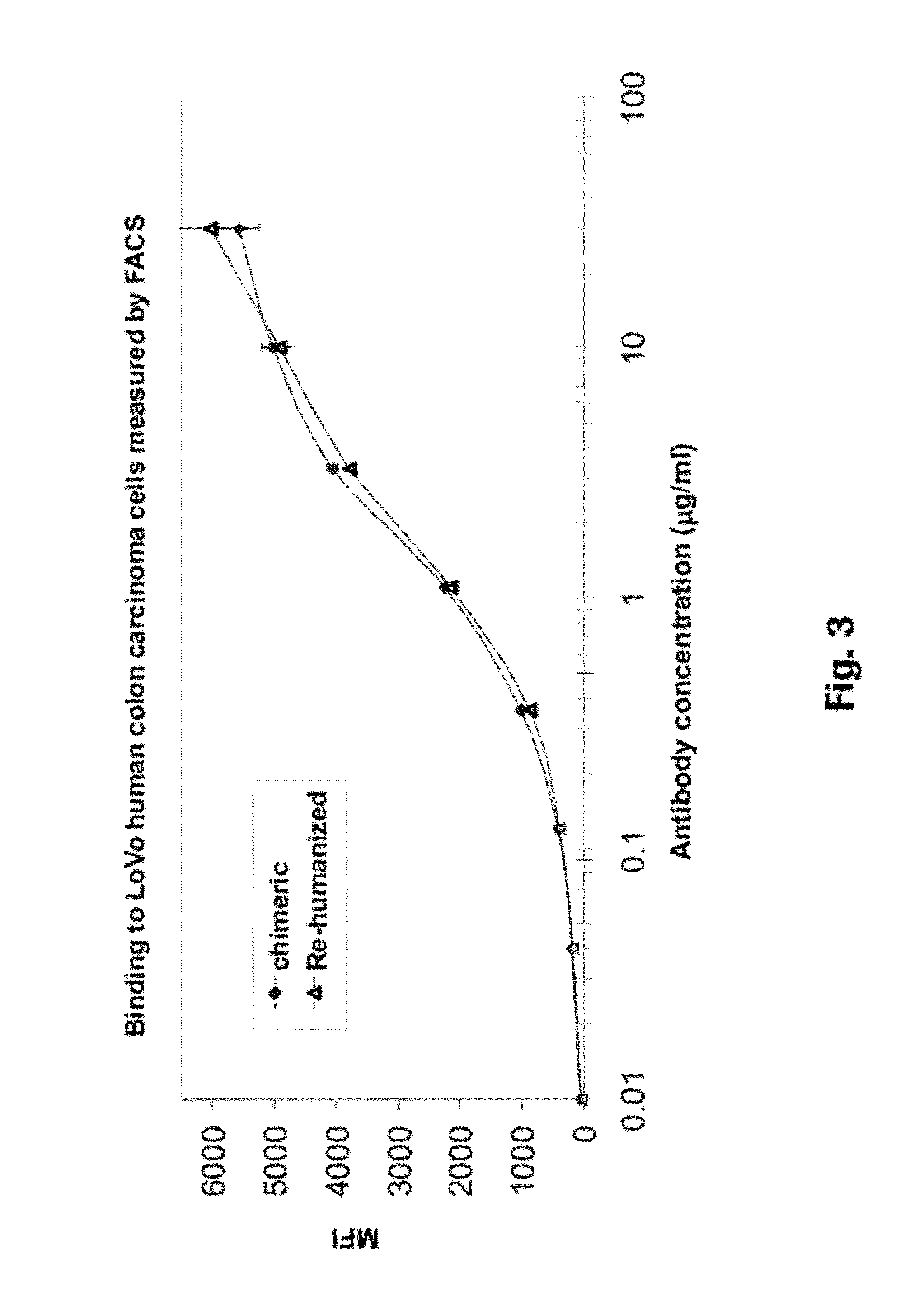
Humanization of an Anti-carcinoembryonic antigen Anti-idiotype antibody as a tumor vaccine and for targeting applications
InactiveUS20080069775A1Ultrasonic/sonic/infrasonic diagnosticsOrganic active ingredientsAntibody fragmentsAnti-CEA Antibody
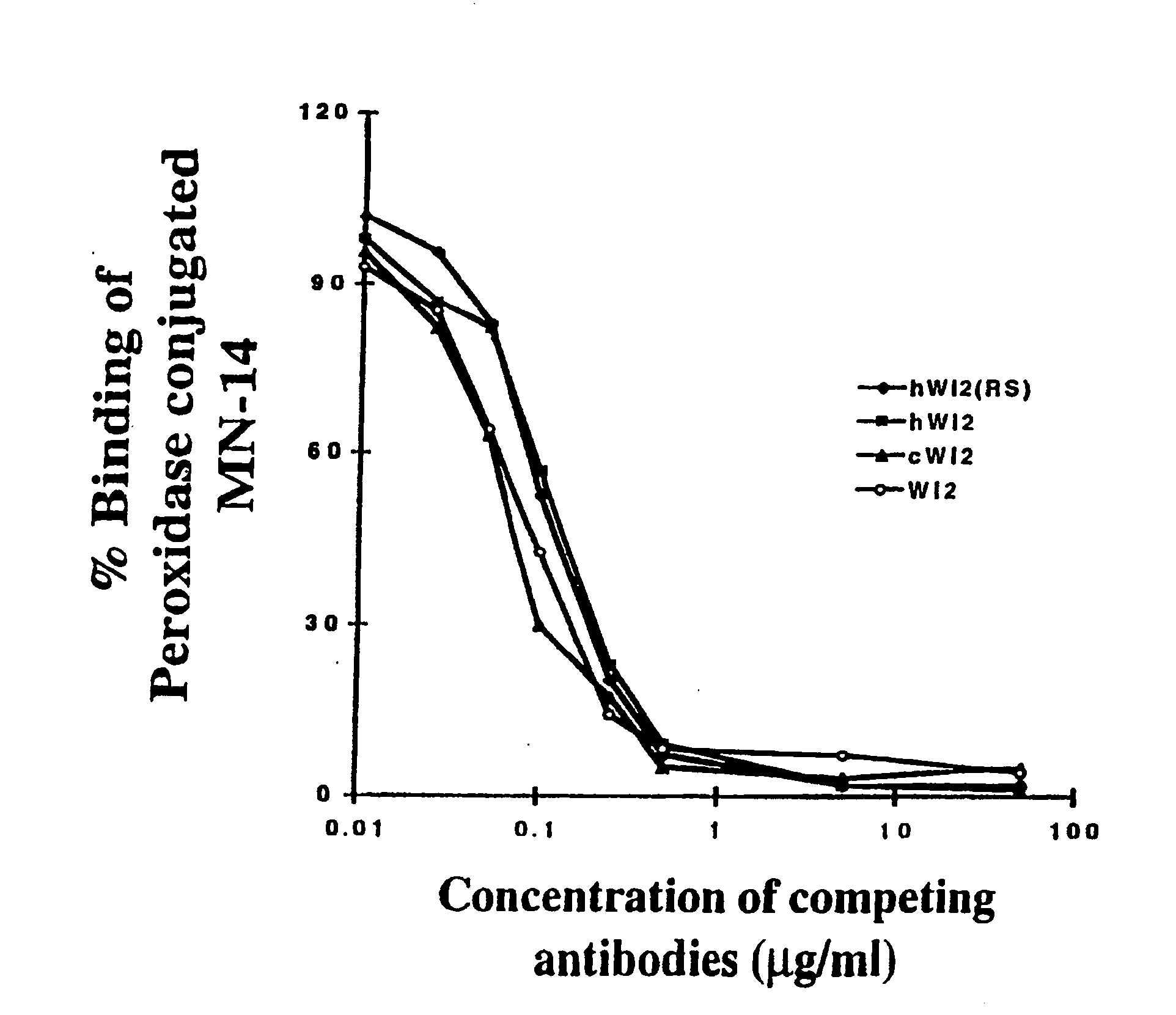
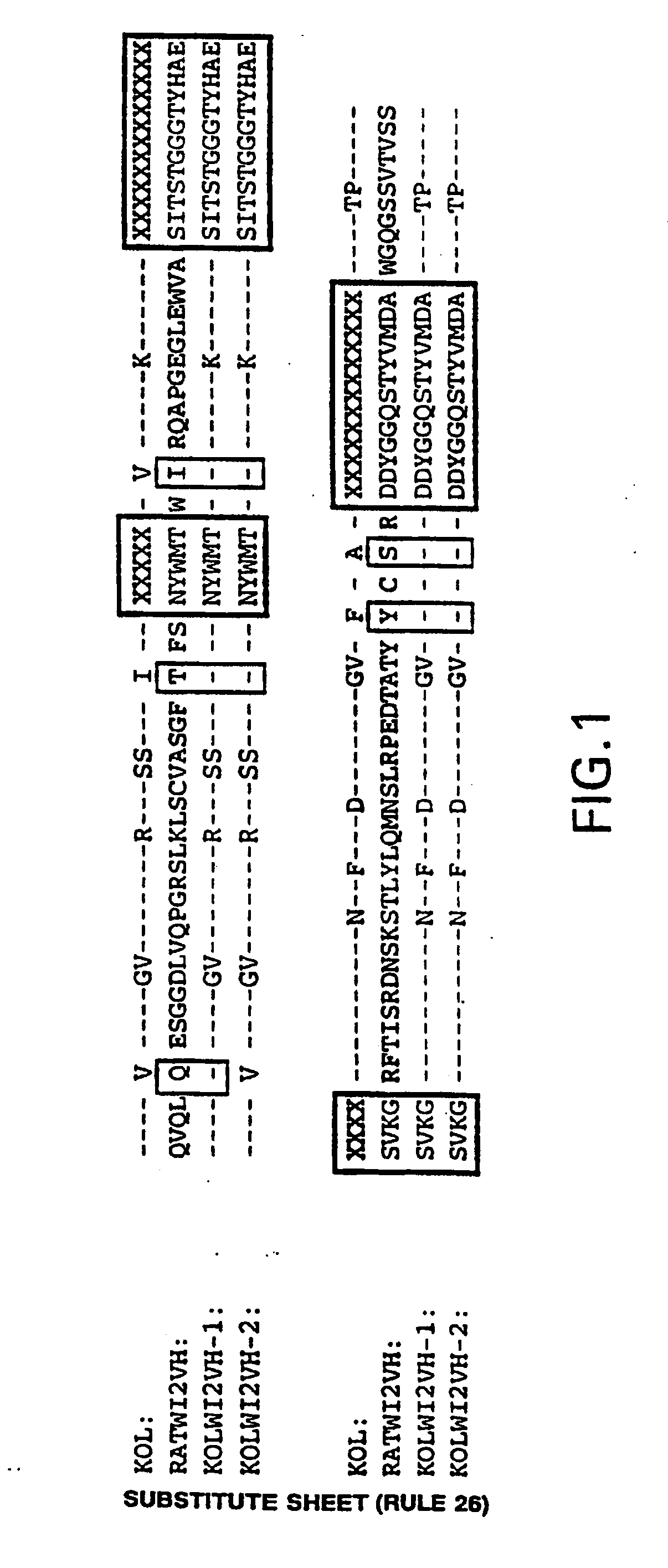
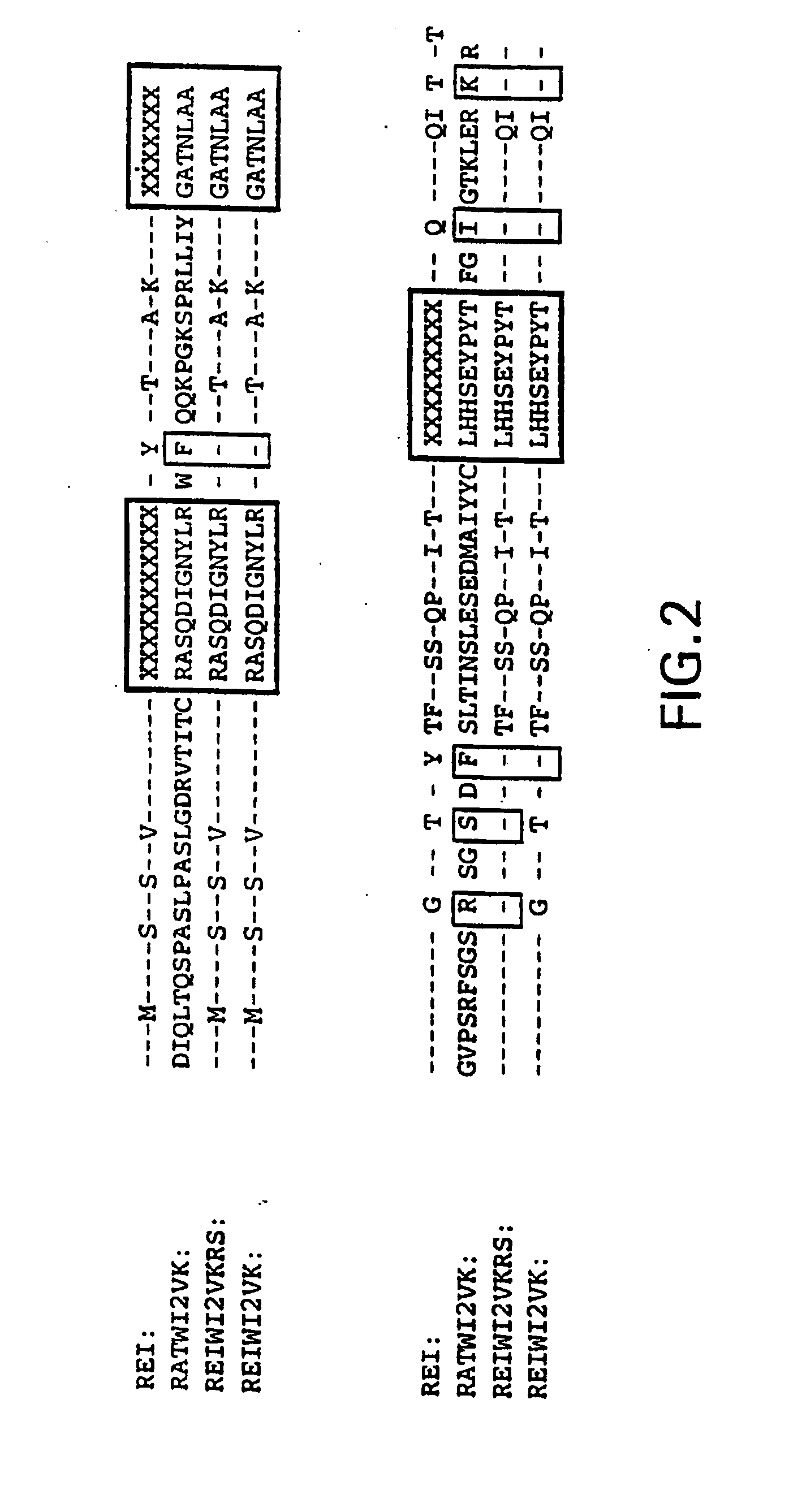
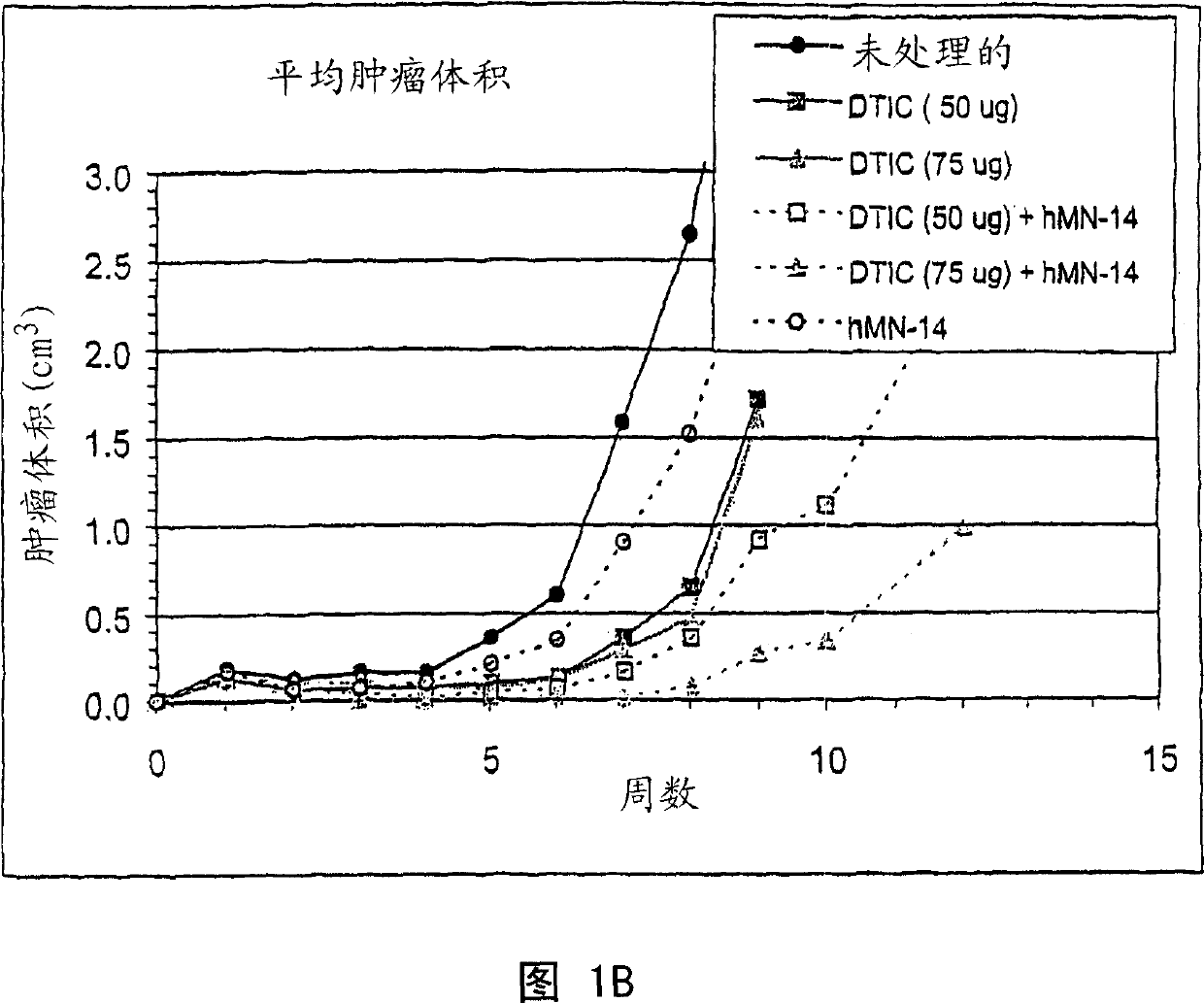
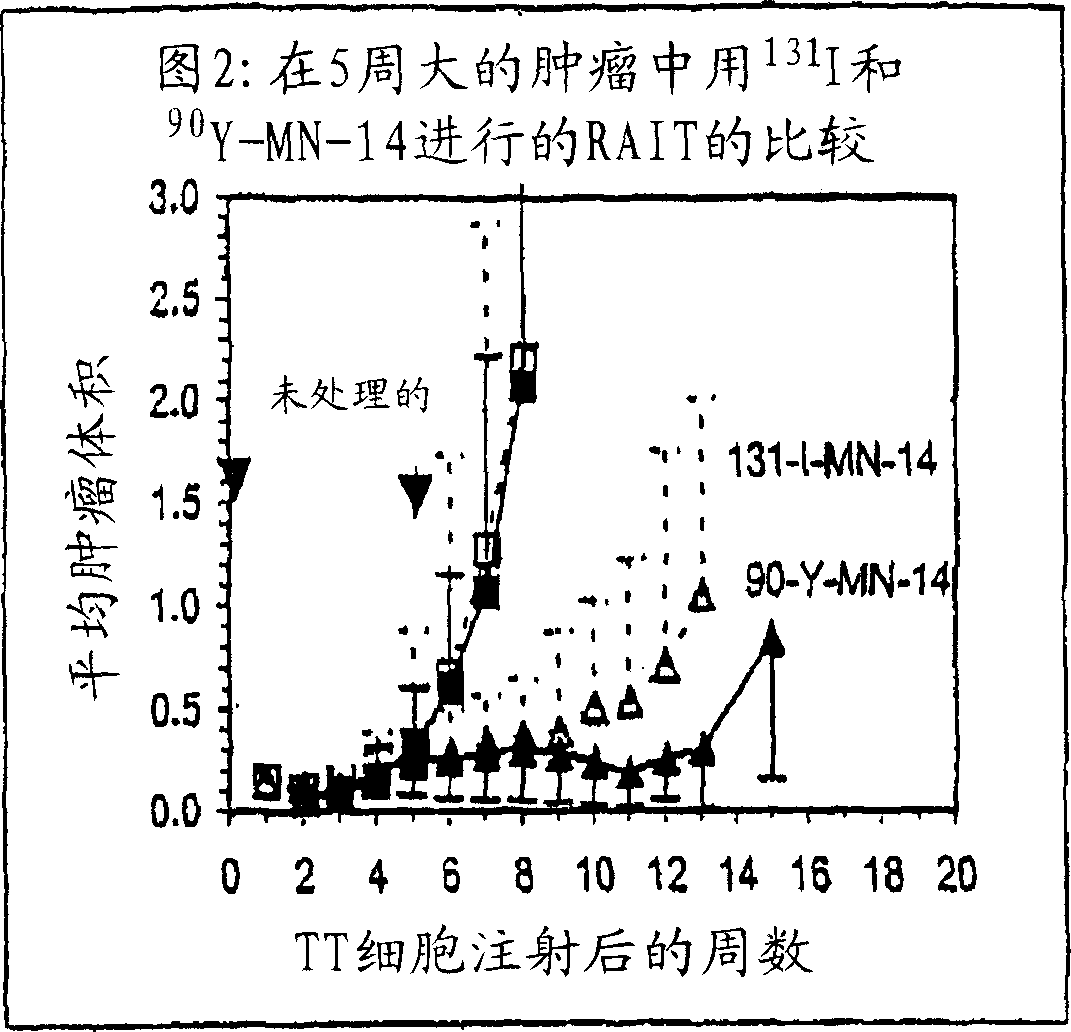
A humanized form of an anti-idiotype antibody to CEA, e.g., hWI2, has conserved immunoreactivity. The clinical benefits of anti-CEA antibodies are maximized by using the humanized anti-idiotype as a clearing agent for anti-CEA antibodies or antibody fragments. The humanized anti-idiotype also can be used as an immunogenic vaccine.
Owner:IMMUNOMEDICS INC
Anti-CEA antibodies
ActiveUS8642742B2Prolong survival timeMicroorganismsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen bindingAnti-CEA Antibody
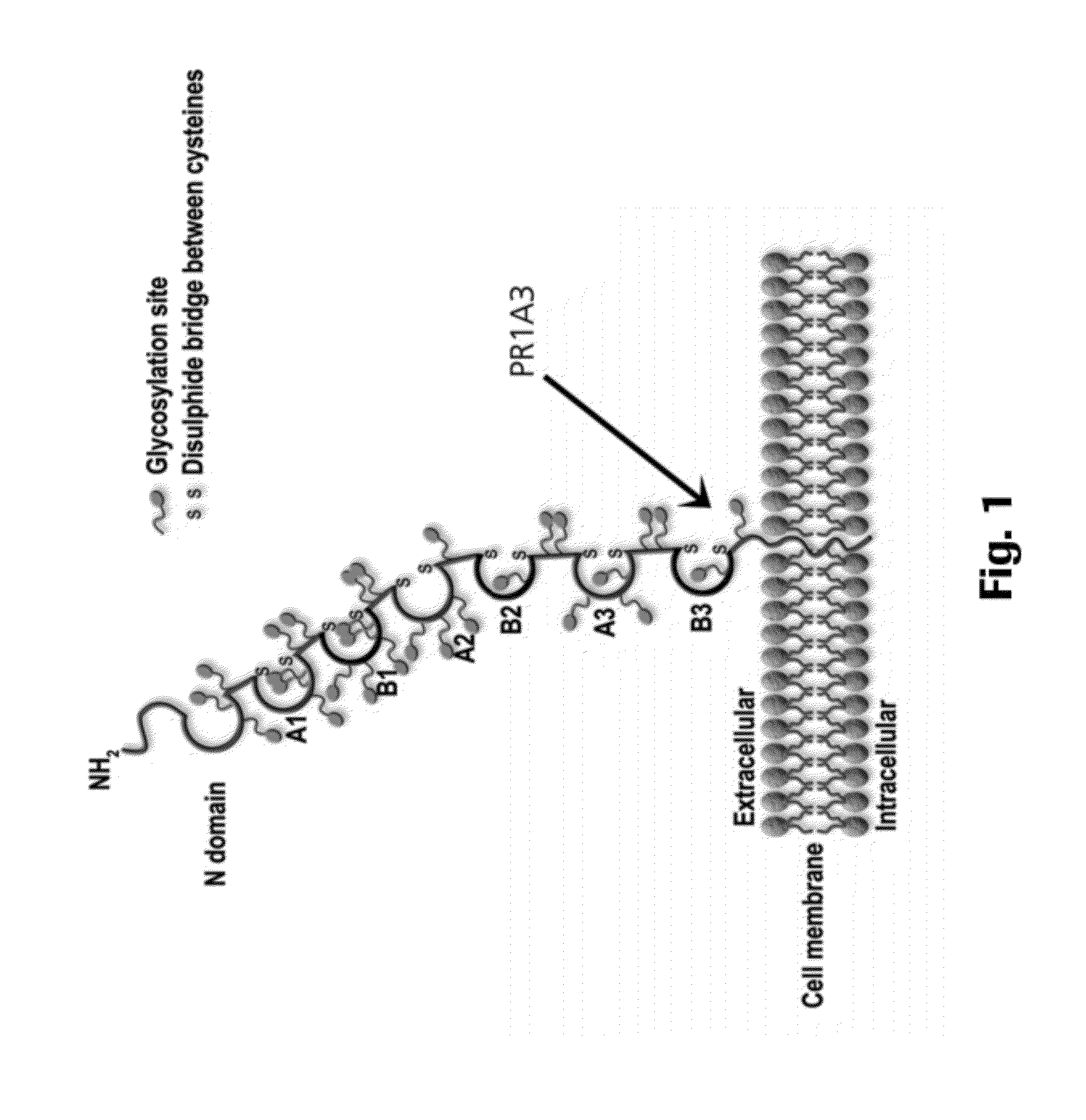
The present invention provides antigen binding molecules (ABMs) which bind membrane-bound CEA, including ABMs with improved therapeutic properties, and methods of using the same.
Owner:ROCHE GLYCART AG
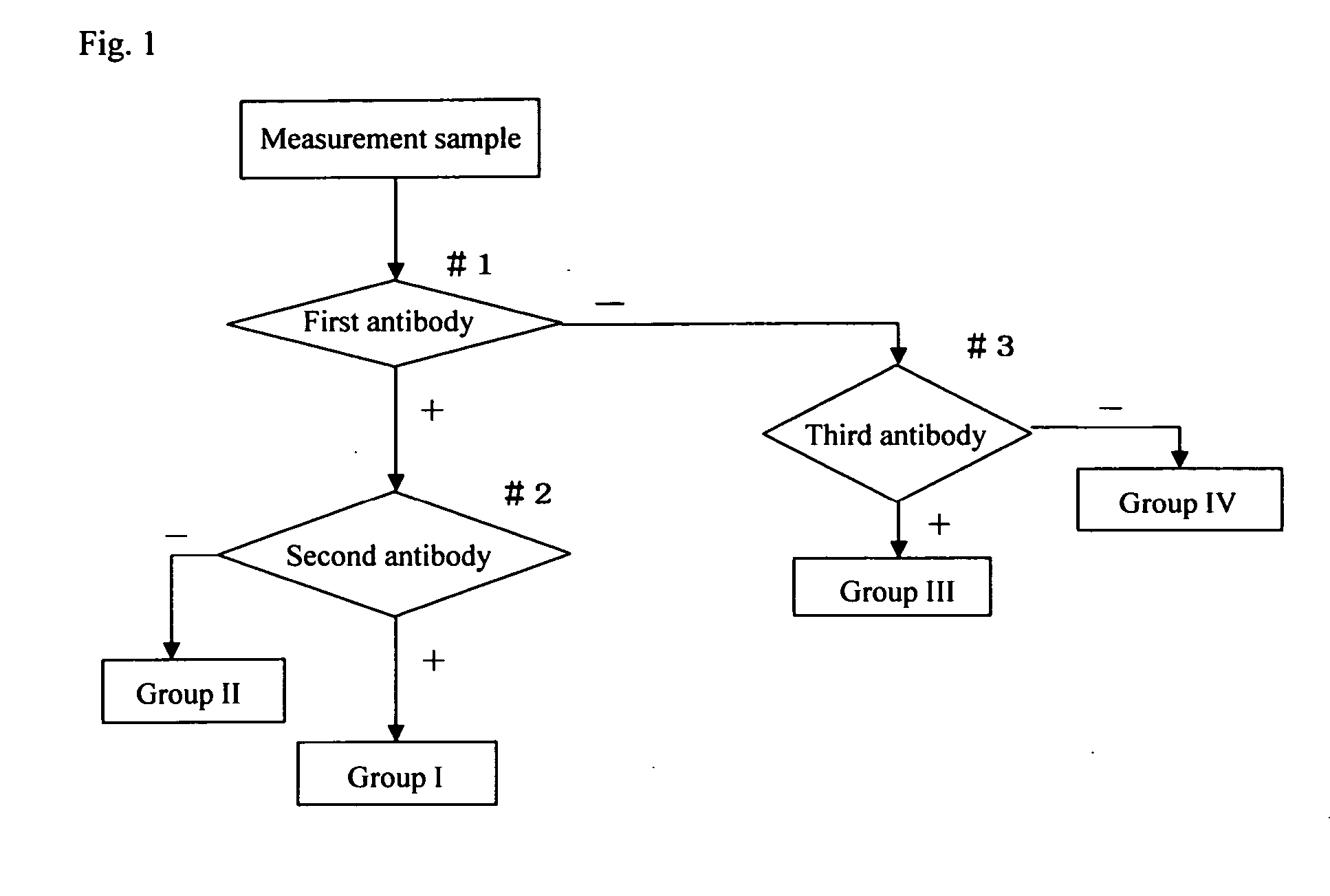
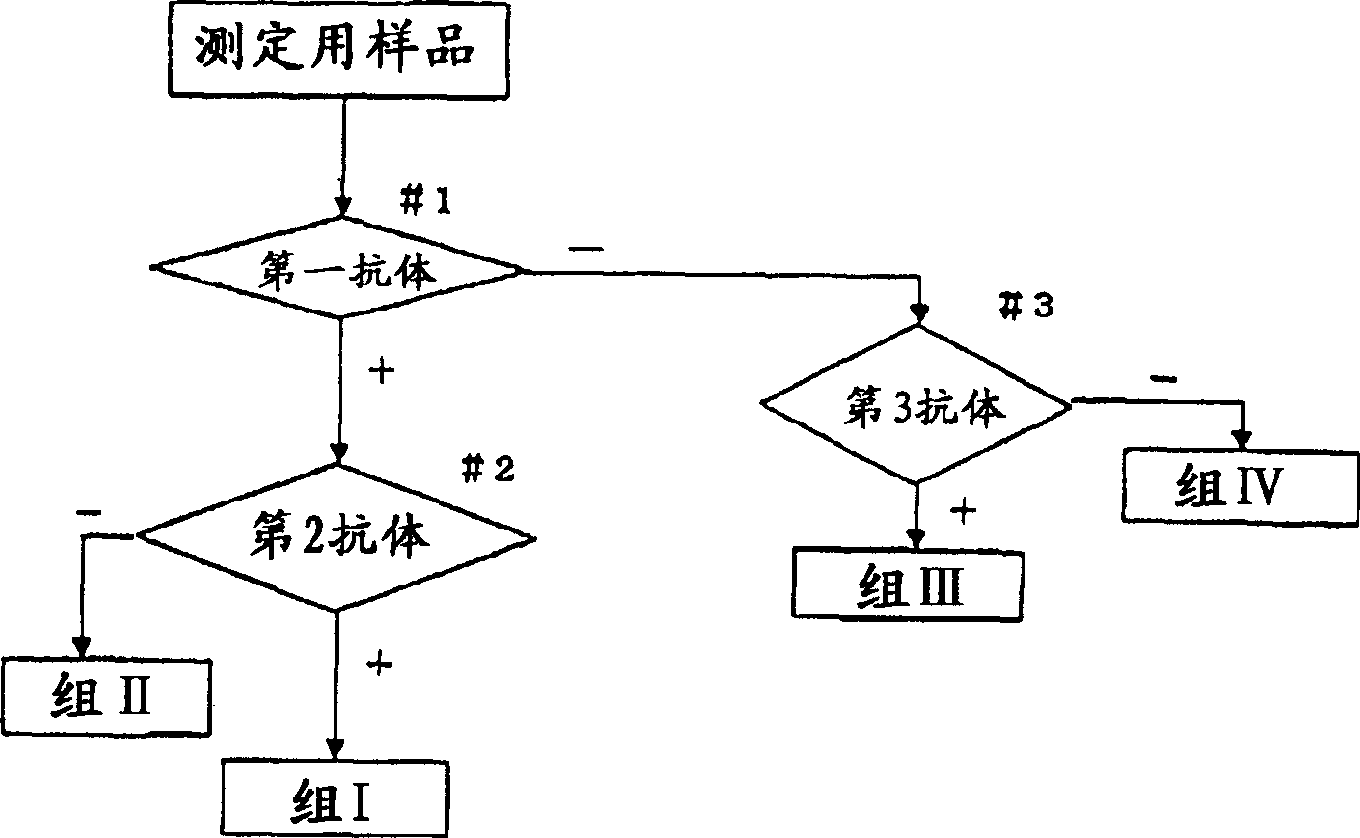
Method for screening cervical cancer
InactiveUS20050221399A1Effective diagnosisEfficient screeningBiological testingSquamous CarcinomasScreening method
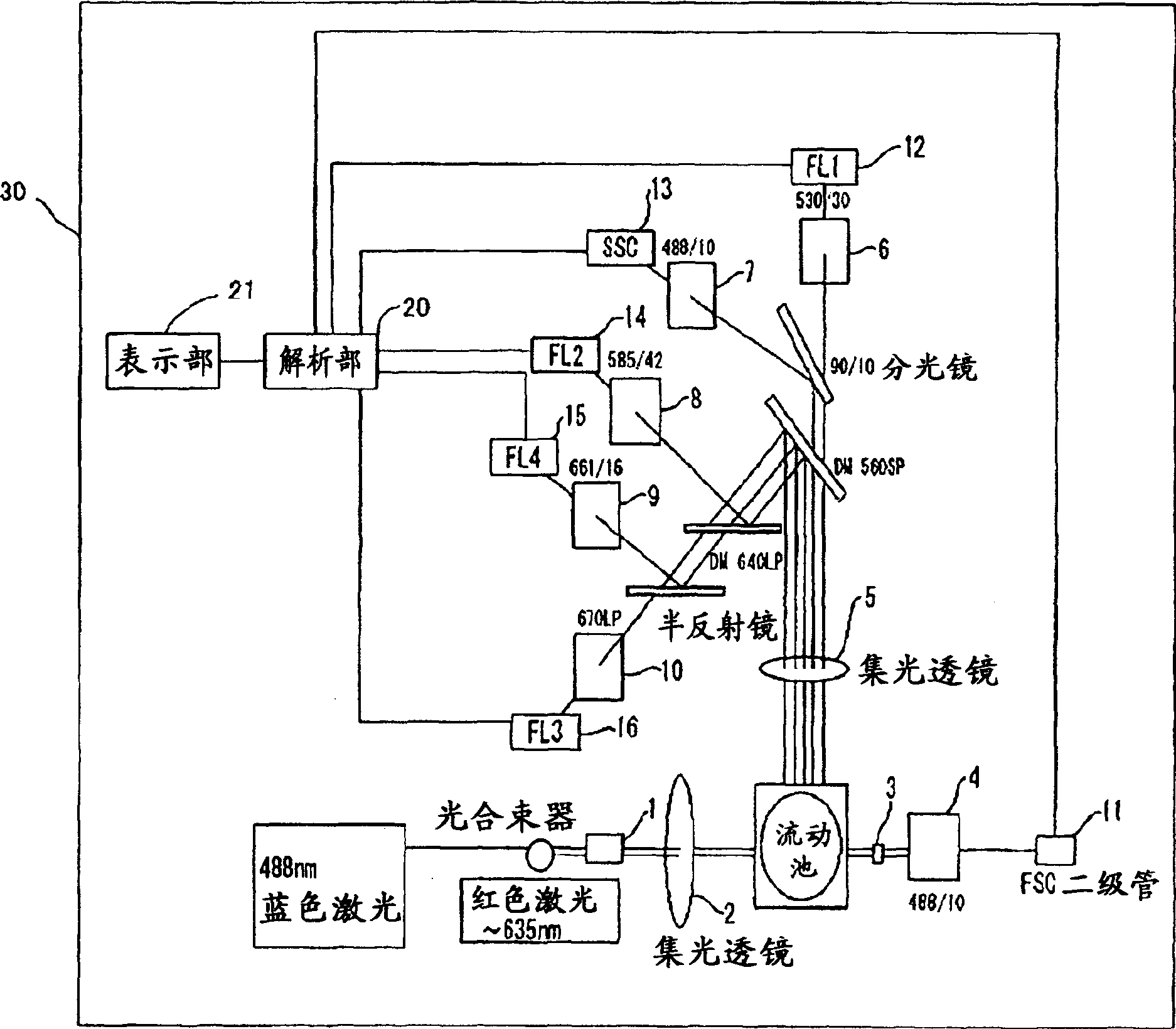
The invention provides a reagent for diagnosing cervical cancer, which can not only detect the presence or absence of cervical cancer but can also distinguish squamous cell carcinoma and adenocarcinoma from each other, a method for screening cervical cancer by using the reagent, particularly a screening method capable high speed processing by utilizing flow cytometry. The reagent comprises a first labeled antibody reacting with gland cells, a second labeled antibody reacting with adenocarcinoma cells and a third labeled antibody reacting with atypical cervical squamous cells, the antibodies being labeled with mutually distinguishable labels respectively. Preferably, at least one selected from the group consisting of MUC1 antibody, cytokeratin 7 antibody, and cytokeratin 18 antibody is used as the first labeled antibody, at least one selected from the group consisting of cytokeratin 8 antibody and HIK1083 antibody is used as the second labeled antibody, and at least one member selected from the group consisting of NMP179 antibody, p16INK4A antibody, Ki-67 antibody, p53 antibody, p21 antibody, EMA antibody, CEA antibody and MIB-1 antibody is used as the third labeled antibody.
Owner:SYSMEX CORP
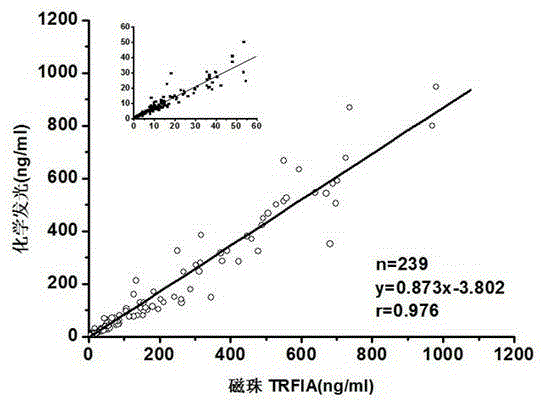
CEA TRFIA (time-resolved fluoroimmunoassay) kit based on IMB (immunomagnetic beads)
The invention discloses a CEA TRFIA (time-resolved fluoroimmunoassay) kit based on IMB (immunomagnetic beads). The kit includes a calibrator, immunomagnetic beads connected with CEA antibodies, europium labeled CEA antibodies, an assay buffer solution, a cleaning solution and an enhanced solution. Through double antibody sandwich immunoreaction, IMB-CEA-europium labeled-CEA monoclonal antibody complexes are formed, IMB which adsorb CEA and supernate are separated through magnetic seperation and washed, the enhanced solution is added, and the value is measured through a time-resolved instrument. Besides the TRFIA technology, the invention further has the advantages as follows: through IMB enrichment and full diffusion of IMB in the solution, the combination superficial area is enlarged, the reaction time is greatly shortened, and the detection sensitivity is improved. IMB and antibodies are directionally connected through chemical groups, so that the consumption of paired antibodies is greatly reduced and the detection precision is improved. The technology realizes automation easily, and overcomes the problem that the traditional micropore plate type TRFIA technology can only carry out detection after samples are accumulated to a certain number, thereby realizing real-time sample detection.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Lung cancer marker detection immunochromatographic test paper and application
InactiveCN101609096AAchieve early diagnosisIncreased sensitivityMaterial analysisAntigenWilms' tumor
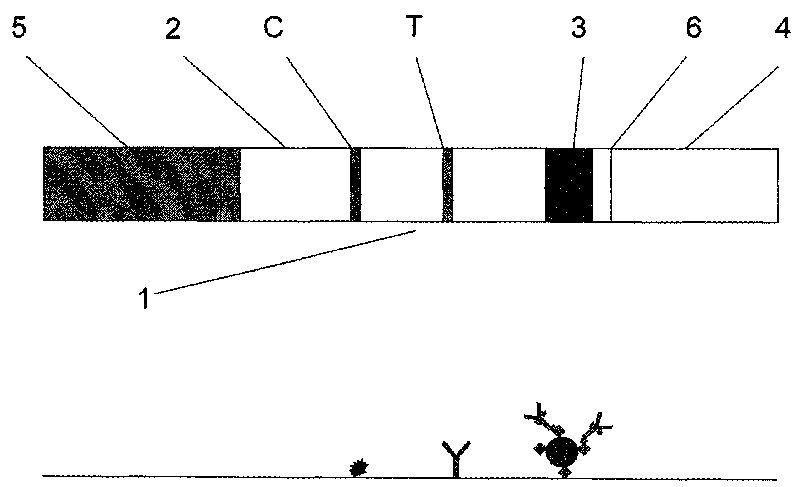
The invention belongs to the cancer detection technology, relating to a lung cancer marker detection immunochromatographic test paper and an application. The detection of a single tumour marker in the prior art can not meet the requirements of clinical early diagnosis and differential diagnosis. The invention realizes the early diagnosis of lung cancer by jointly detecting NSE and CEA in serum with a double antibody sandwich immunochromatography. The invention comprises the following preparation steps: preparing aurosol-neure NSE and aurosol-carcinoembryonic antigen CEA antibody compounds; preparing double antibody sandwich NSE and CEA detection test strips. The invention comprises the following steps in lung cancer detection: configurating series concentration NSE and CEA standard substances; preparing NSE and CEA mixed antigen solution with serum of normal people; inserting the sample pad ends of the test strips into the above concentration series standard substances respectively to observe the changes in colours of T and C lines on a NC film. The invention has the advantages of convenient detection operation, high sensitivity and precise result, and can determine lung cancer recurrence 4-12 weeks ahead.
Owner:SHANGHAI NORMAL UNIVERSITY
Method for screening cervical cancer
InactiveCN1677109AEfficient screeningChemiluminescene/bioluminescenceSurgerySquamous CarcinomasKeratin Antibody
The invention provides a reagent for diagnosing cervical cancer, which can not only detect the presence or absence of cervical cancer but can also distinguish squamous cell carcinoma and adenocarcinoma from each other, a method for screening cervical cancer by using the reagent, particularly a screening method capable high speed processing by utilizing flow cytometry. The reagent comprises a first labeled antibody reacting with gland cells, a second labeled antibody reacting with adenocarcinoma cells and a third labeled antibody reacting with atypical cervical squamous cells, the antibodies being labeled with mutually distinguishable labels respectively. Preferably, at least one selected from the group consisting of MUC1 antibody, cytokeratin 7 antibody, and cytokeratin 18 antibody is used as the first labeled antibody, at least one selected from the group consisting of cytokeratin 8 antibody and HIK1083 antibody is used as the second labeled antibody, and at least one member selected from the group consisting of NMP179 antibody, p16[INK4A] antibody, Ki-67 antibody, p53 antibody, p21 antibody, EMA antibody, CEA antibody and MIB-1 antibody is used as the third labeled antibody.
Owner:SYSMEX CORP
Nanometer manetic powder for magnetothermical therapy and anti-CEA antibody target medicine
InactiveCN1772300APenetratingGood biocompatibilityPowder deliveryInorganic non-active ingredientsHysteresisSide effect
The present invention relates to target medicine of nanometer magnetic powder and anti-CEA antibody for magentothermical therapy on colon cancer, stomach cancer, liver cancer, etc. The present invention features that nanometer magnetic powder and antibody in the weight ratio of 1 to 0.0001-0.20 are coupled via mixing in solution to prepare the nanometer target medicine. The target medicine has grain size one 5-1000 nm, nanometer magnetic powder with one or several antibodies coupled to the surface, and bioactivity. The target medicine is injection with medicine dispersed into nanometer grains, and is targeted and concentrated to the cancer focus. Under the action of the AC magnetic field of the magentothermical therapy instrument, the target medicine produces hysteresis heat effect of 10-7000 W / gFe to heat the cancer focus to 42-95 deg.c and kill cancer cell. The target medicine has biodegrading function and is used in the magentothermical treatment of cancer without toxic side effect.
Owner:南京伍盛纳米科技有限公司
Humanized Anti-ceacam5 antibody and uses thereof
InactiveUS20150125386A1Overcome tumorImprove targetingImmunoglobulins against animals/humansRadioactive preparation carriersDrug conjugationWhole body
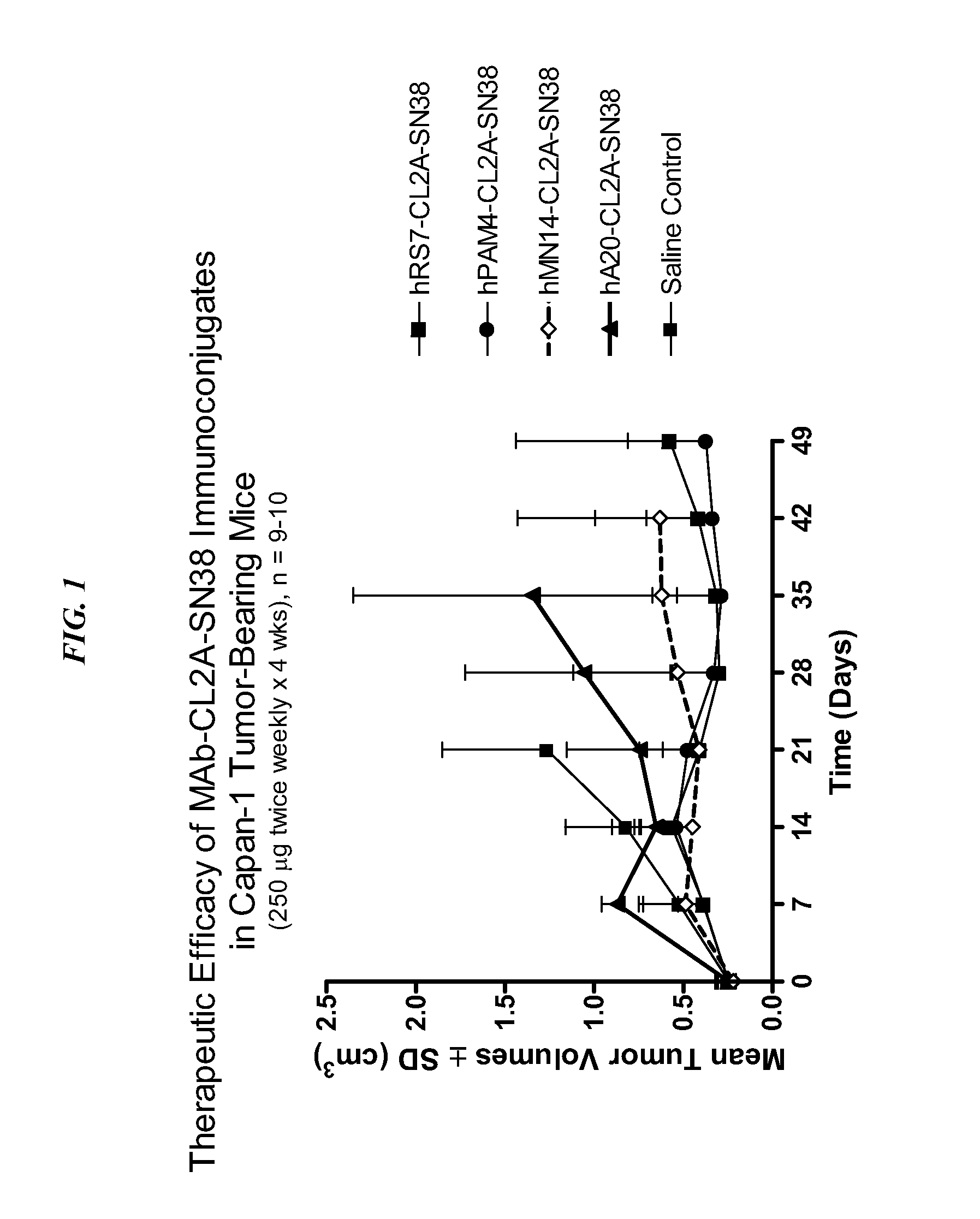
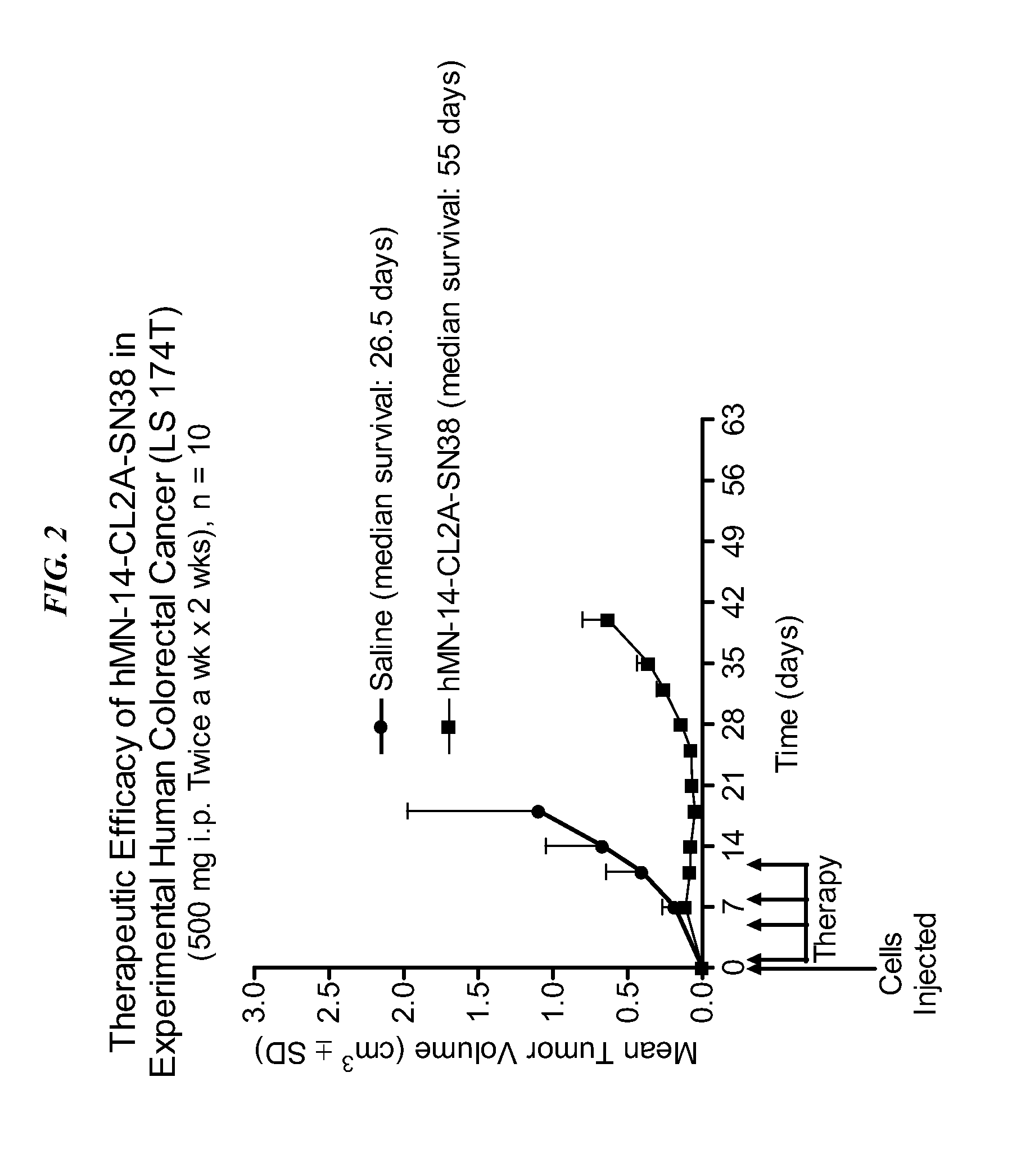
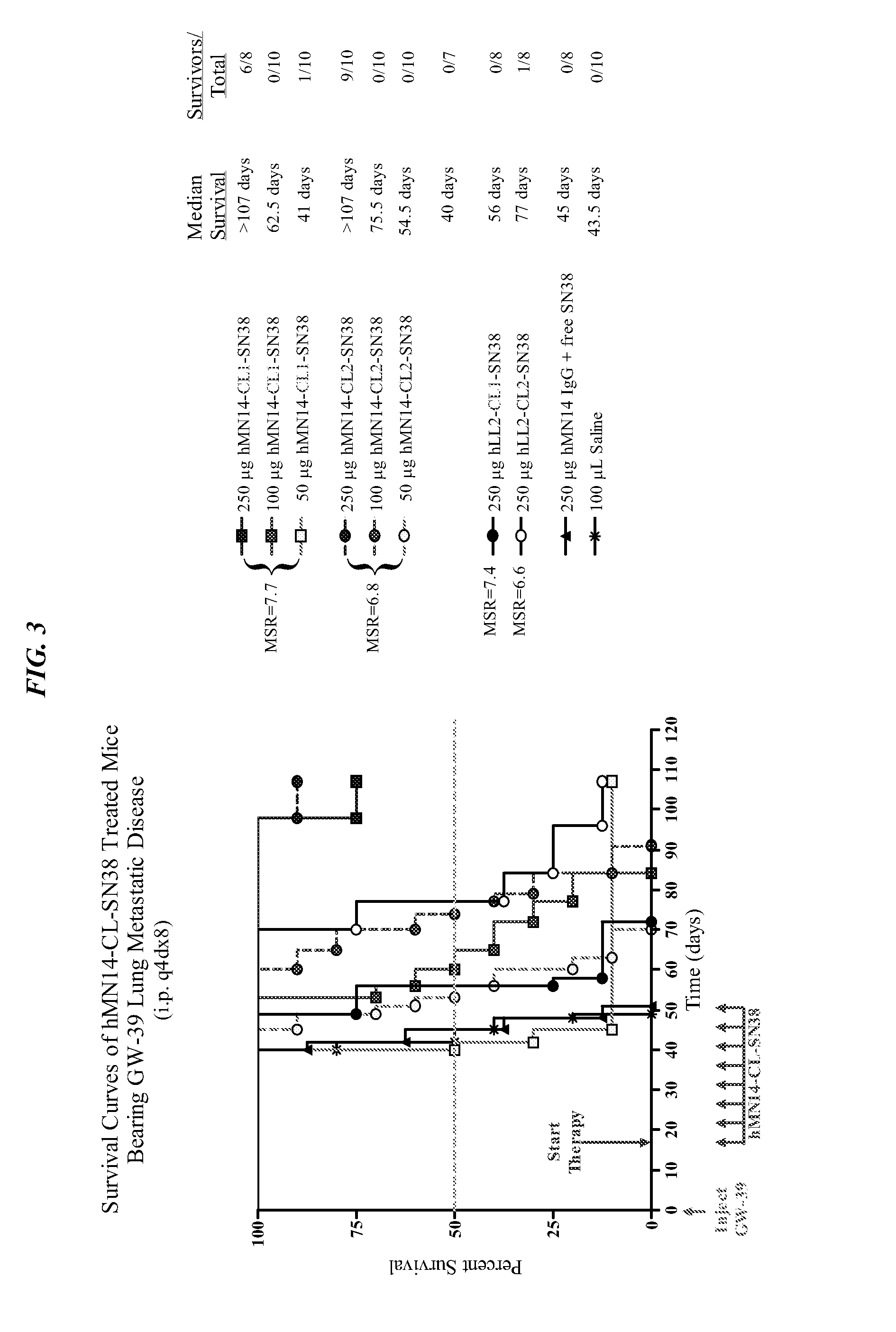
The present invention concerns compositions and methods of use of a humanized Class III anti-CEA antibody, comprising the heavy and light amino acid sequences SEQ ID NO:1 and SEQ ID NO:2. The antibody is effective to treat CEACAM5-expressing tumors, either alone or in combination with one or more therapeutic agents. Drug conjugated Class III anti-CEA antibodies, such as SN-38 or P2PDox immunoconjugates, are particularly efficacious. Surprisingly, the antibody-drug conjugates (ADCs) exhibit high anti-cancer efficacy, while exhibiting low levels of systemic toxicity that are readily treated with standard amelioration techniques. Antibodies and / or immunoconjugates comprising the amino acid sequences SEQ ID NO:1 and SEQ ID NO:2 are surprisingly efficacious for therapy of solid tumors, even when the tumor has proven resistant to standard anti-cancer therapies.
Owner:IMMUNOMEDICS INC
Anti-idiotype anti-cea antibody molecules and its use as cancer vaccine
InactiveUS20050222392A1Stimulate immune responseHigh activityHybrid immunoglobulinsPeptide/protein ingredientsMHC class IVaccination
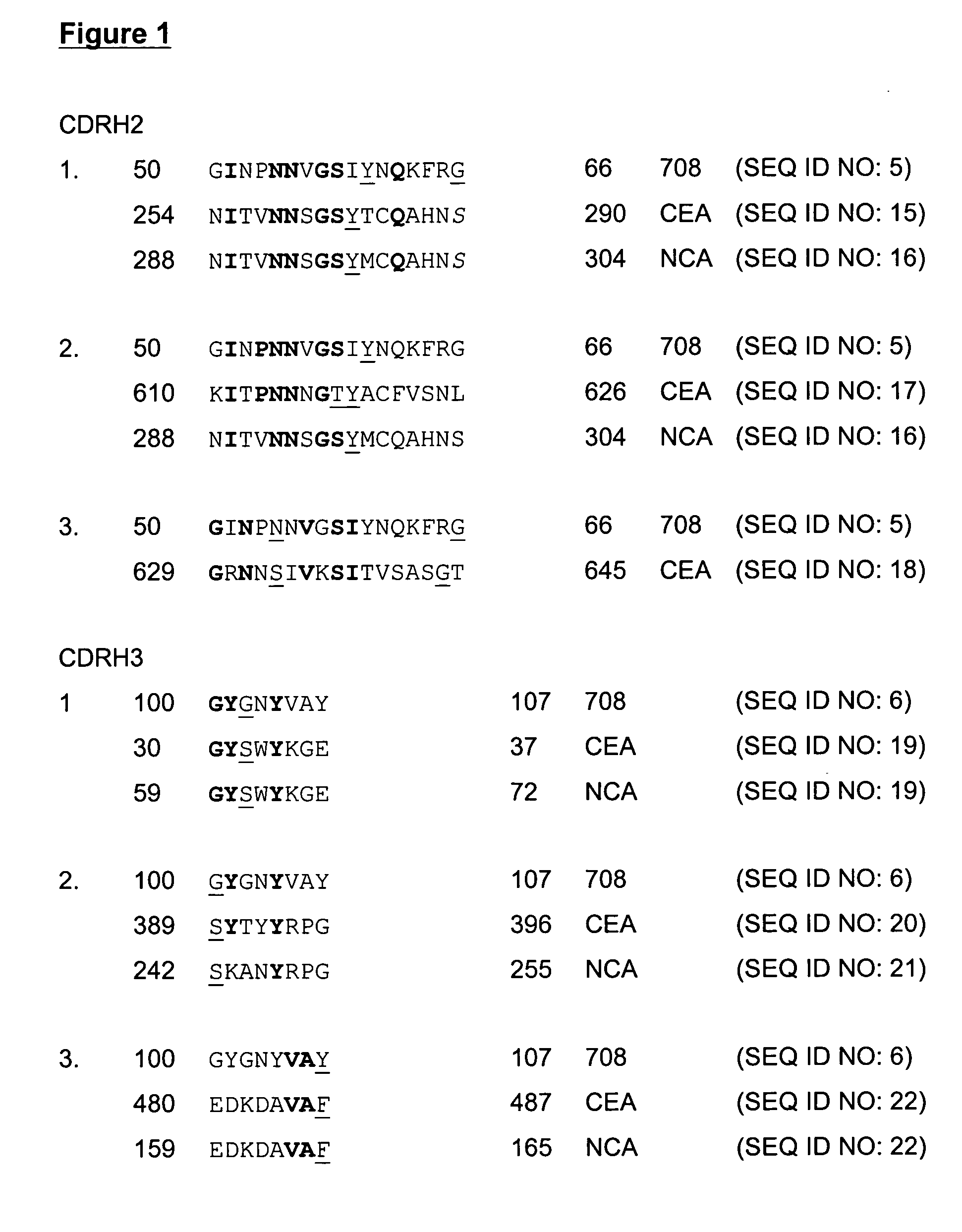
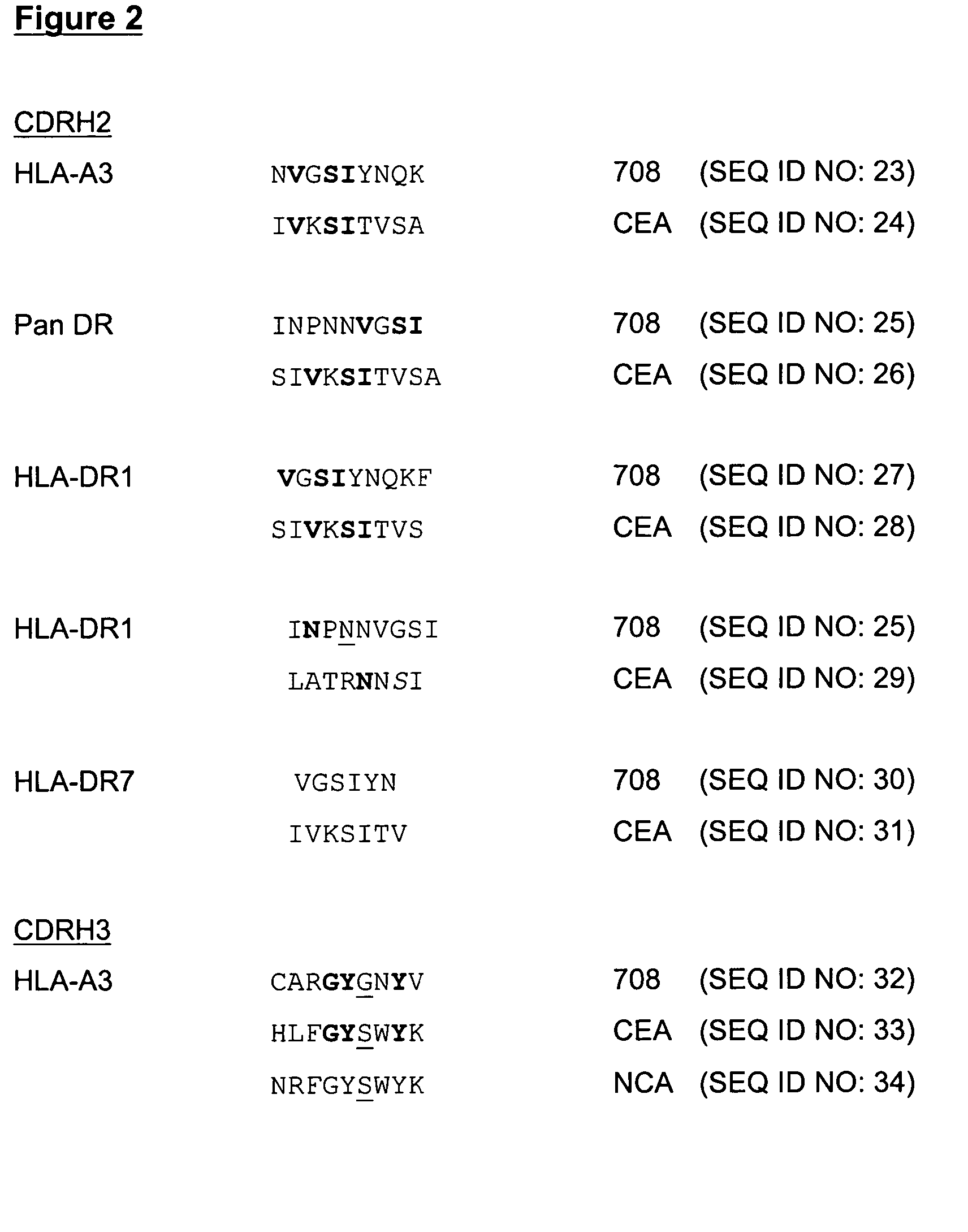
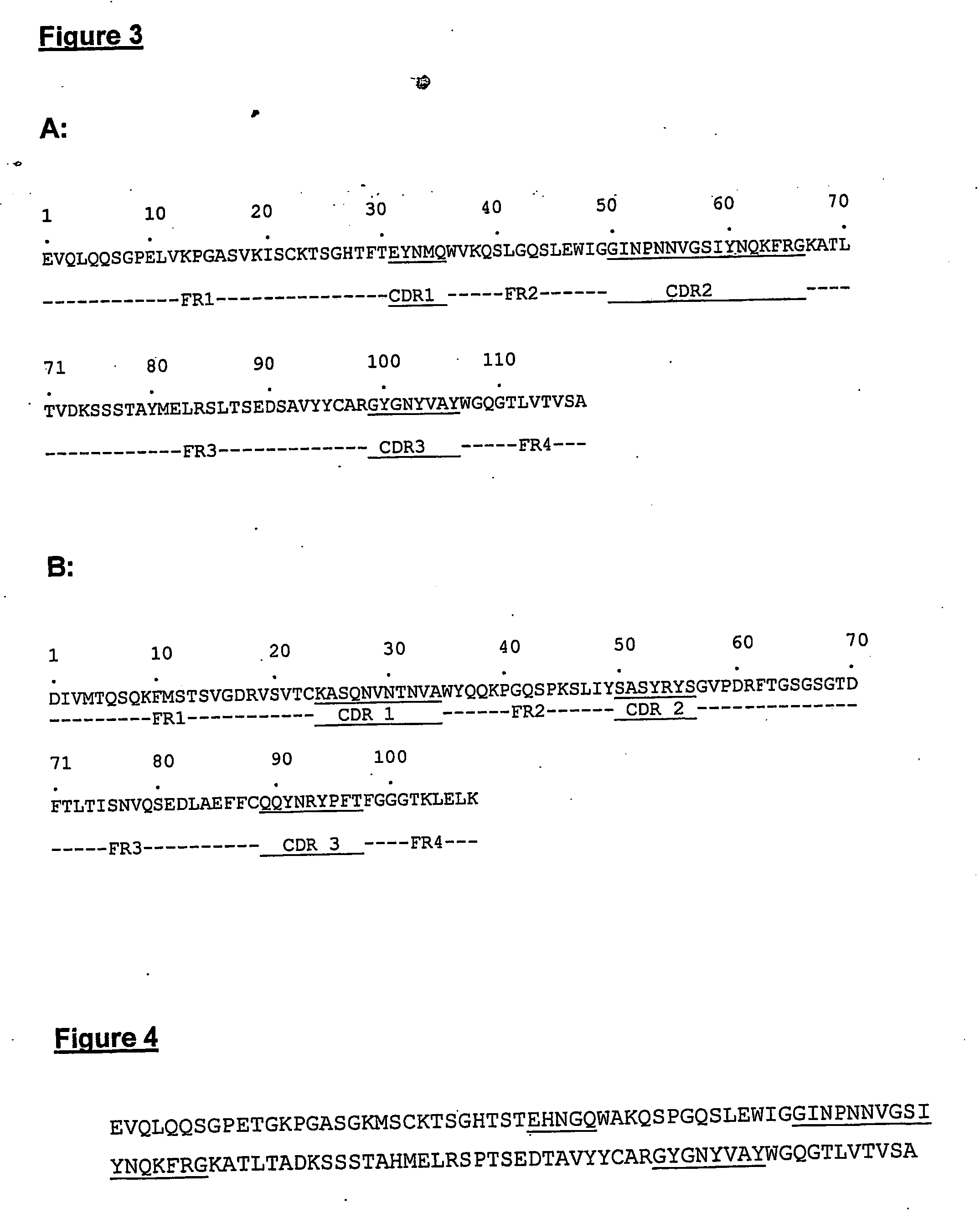
The present invention provides molecules, preferably designed immunoglubulins, suitable for use as an anti-idiotype vaccine to CEA positive tumours. The molecules induce both an MHC class I and MHC class II mediated immune response to the CEA bearing tumour cells for an efficient and sustained host anti-tumour response. The present invention provides modified versions of anti-idiotype anti-CEA antibodies, preferably mouse antibody 708, with improved vaccination properties. The modifications are related to the introduction of sequences tracts deriving from e.g. CEA, CD55 antigen and CEA cancer-specific MHC epitopes into the variable regions of said antibody molecules.
Owner:CARTER GRAHAM +1
Anti-cea antibodies
ActiveUS20120251529A1Prolong survival timeFungiImmunoglobulins against cell receptors/antigens/surface-determinantsAntiendomysial antibodiesAntigen binding
The present invention provides antigen binding molecules (ABMs) which bind membrane-bound CEA, including ABMs with improved therapeutic properties, and methods of using the same.
Owner:ROCHE GLYCART AG
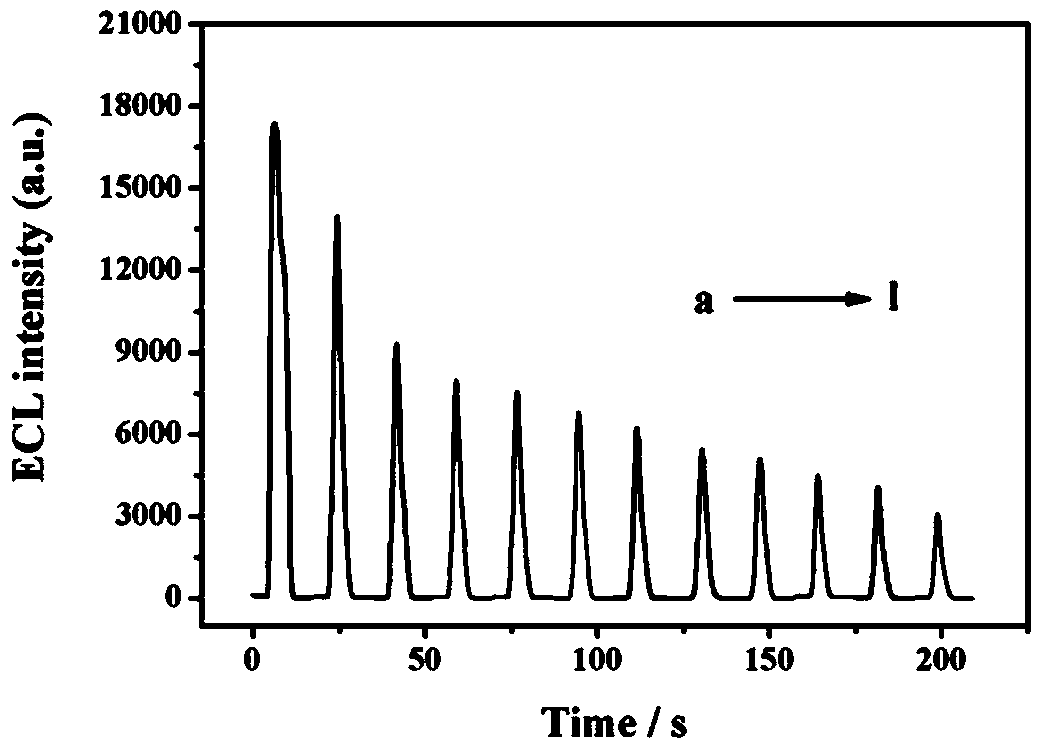
Method for preparing electrochemical luminescence sensor made of nano-composites
ActiveCN105115961AGood biocompatibilityLarge specific surface areaChemiluminescene/bioluminescenceNano compositesLuminol
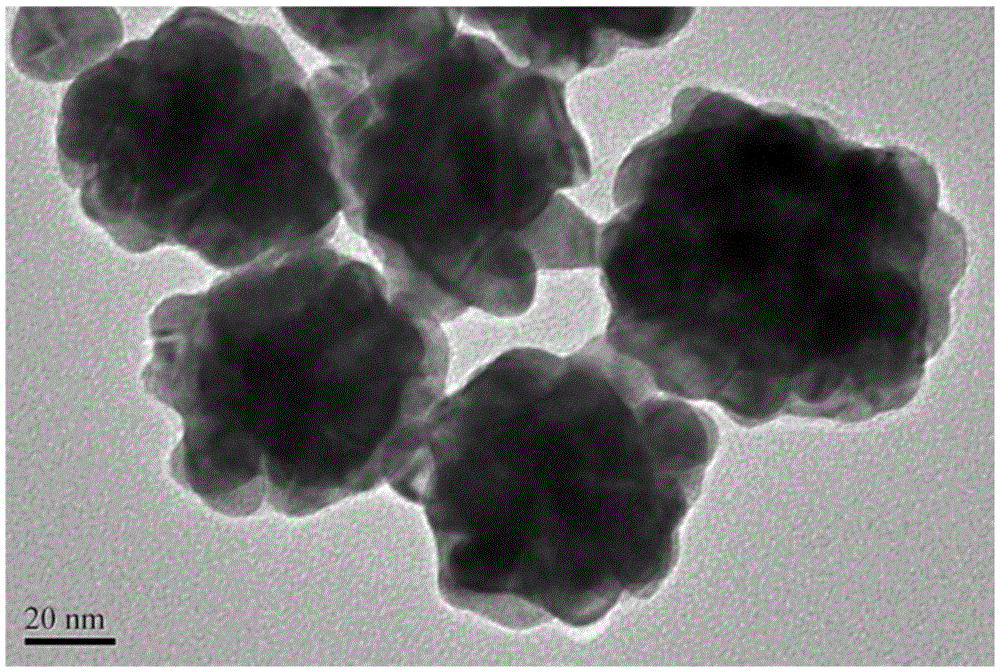
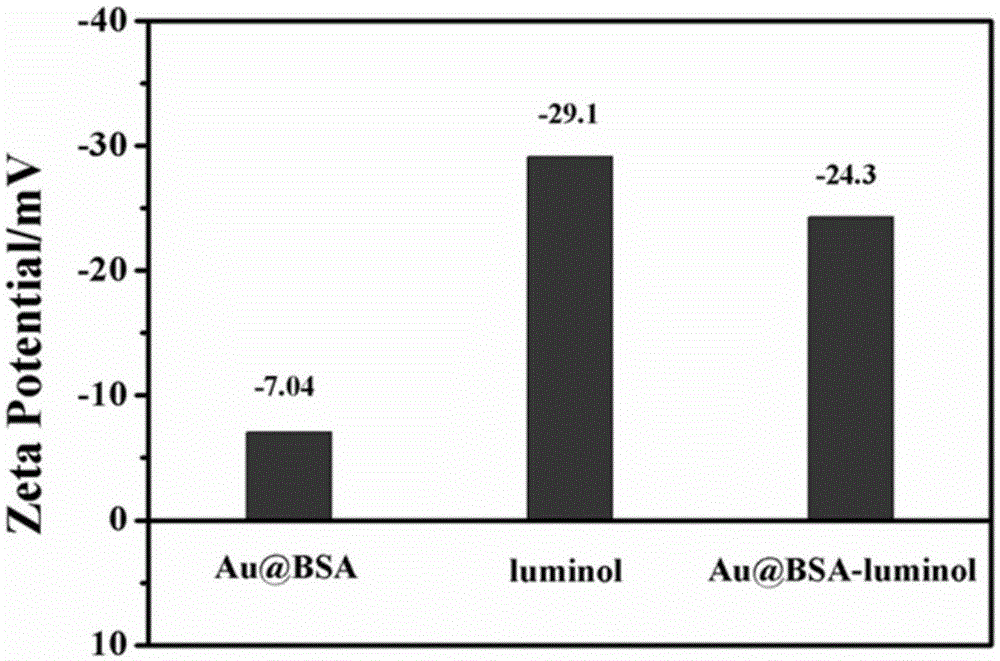
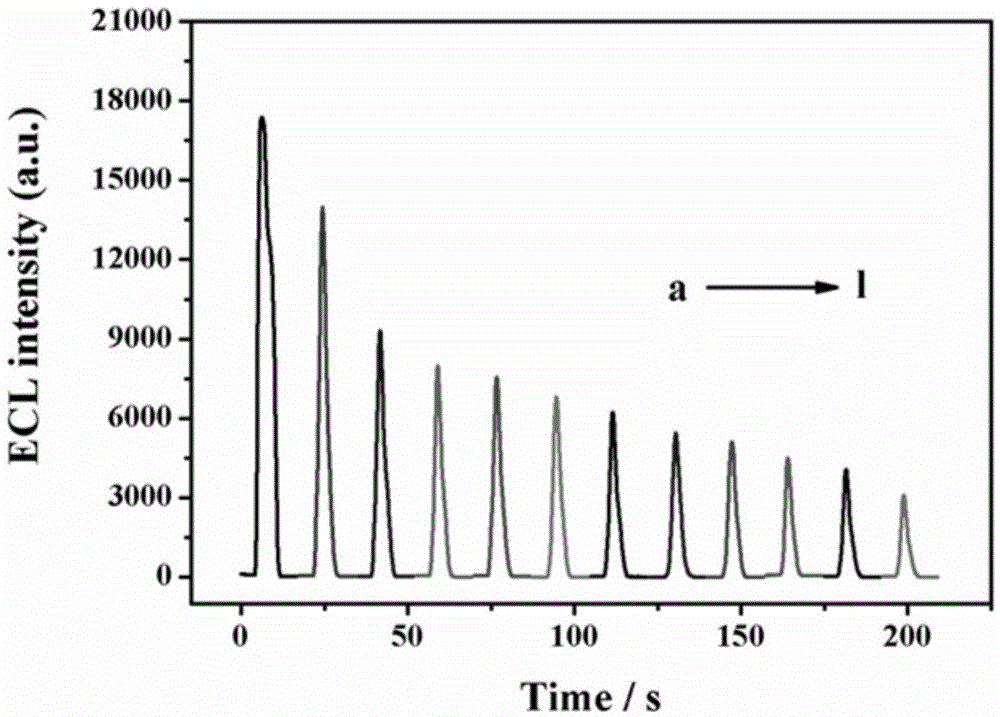
The invention relates to a method for preparing an electrochemical luminescence sensor made of nano-composites. The method includes the three steps that Au-and-BSA nano-materials are prepared; luminol is crosslinked to the surfaces of the Au-and-BSA nano-materials through glutaraldehyde, carboxyl on the surfaces of the Au-and-BSA nano-materials is activated to be connected with CEA antibodies to obtain luminol-Au-and-BSA-anti-CEAs; a gold nano-layer is electrodeposited on the surface of a naked glassy carbon electrode serving as a working electrode with an electro-deposition method, the obtained luminol-Au-and-BSA-anti-CEAs are connected to the surface of the electrode, and the electrochemical luminescence immunosensor with the targeting effect on CEAs is obtained. The method is rapid, easy and convenient, and the constructed immunosensor has the advantages of being good in biocompatibility, high in specificity and sensitivity, wide in detection range and the like.
Owner:SHANGHAI NORMAL UNIVERSITY
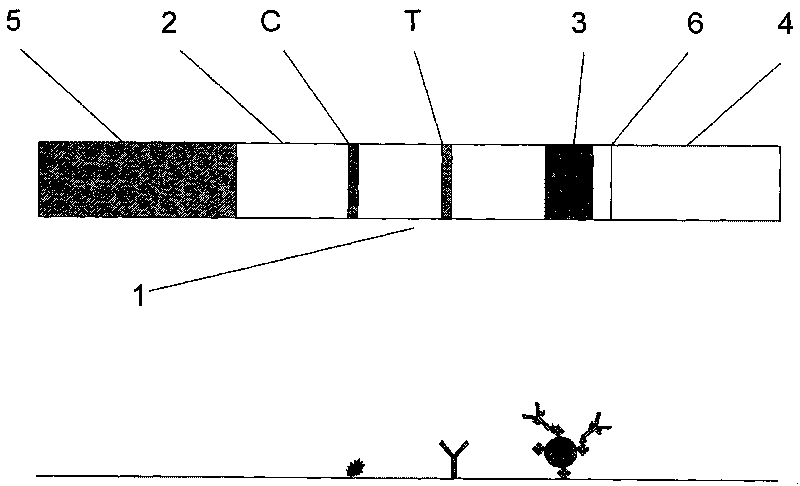
Magnetic immuno-chromatographic test paper strip for quantitatively detecting carcinoembryonic antigen in blood and preparation method thereof
InactiveCN101762700ARealize single-serving wide-range quantitative detectionClinically convenientMaterial analysisMedicineQuantitative Result
The invention relates to a magnetic immuno-chromatographic test paper strip for quantitatively detecting carcinoembryonic antigen in blood and a preparation method thereof. A coating film, a magnet particle pad combined with CEA antibodies, a sample pad and a water absorbing pad are sequentially and alternately stuck on a base plate in a staggering way at intervals of 2mm, and then the upper layer is covered by a transparent plastic sealing film to form a test paper strip. A CEA antibody detection line and a quality control line are pre-coated on the coating film. The invention introduces the magnetic immuno-chromatographic technology and the biotin-aviden system into the quantitative detection of CEA in blood, and has the advantages that the detection sensitivity is greatly improved, the accurate quantitative results can be given, the labor strength of the operators is reduced, and the clinicians can rapidly and accurately diagnose the illness of patients.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Method for preparing carcinoembryonic antigen working electrode for screen printing electrode
InactiveCN101923092AReach renewableMaterial analysis by electric/magnetic meansMagnetite NanoparticlesMicroparticle
The invention discloses a method for preparing a carcinoembryonic antigen working electrode for a screen printing electrode. The method comprises the following steps of: mixing a CEA antibody with solution of gold magnetic nanoparticles, performing sealing treatment by coating the CEA antibody on the surfaces of the gold magnetic nanoparticles, sealing and fixing the coated CEA antibody, and separating the uncoated CEA antibody so as to prepare gold magnetic nanoparticle-CEA antibody solution; and forming a Nafion film on the surface of a working electrode, and adsorbing the prepared gold magnetic nanoparticle-CEA antibody on the Nafion film so as to prepare the carcinoembryonic antigen working electrode of a cation-exchange membrane of gold magnetic nanoparticle-CEA antibody-polytetrafluoroethylene. The method for preparing the carcinoembryonic antigen working electrode for the screen printing electrode has the advantages of no need of purifying the detected serum sample, no need of adding an electronic mediator during use and regeneration on the surface of the electrode after use each time.
Owner:NINGBO UNIV
Antybody therapy
InactiveCN1878562APeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseMonoclonal antibody
The present invention provides a composition comprising naked humanized, chimeric and human anti-CEA monoclonal antibody and a therapeutic agent, which is useful for the treatement of CEA expressing cancers and other diseases, and methods of treatment using this composition.
Owner:IMMUNOMEDICS INC
Detection method of CEA (carcinoembryonic antigen) concentration
InactiveCN106771204AIncrease loadFacilitates signal amplificationMaterial electrochemical variablesAptamerSodium molybdate
The invention discloses a detection method of the CEA (carcinoembryonic antigen) concentration. According to the method, CEA aptamer modified gold nanometer rods are used as electrochemical probes; firstly, a CEA antibody-CEA-aptamer / gold nanometer rod three-layer sandwich type structure is firstly built; then, on the basis of a principle that phosphate radicals on the aptamers can take a reaction with sodium molybdate to generate oxidation-reduction current; the electrochemical method detection of the CEA concentration is realized. The method has the advantages of high selectivity, high sensitivity, low detection limit, wide detection range and the like.
Owner:CENT SOUTH UNIV
Immunochromatographic test paper strip and preparation method and application thereof
InactiveCN105527440ARealize multiple quantitative detectionRealize quantitative detectionBiological material analysisImmunofluorescenceQuality control
The present invention discloses an immunochromatographic test paper strip which includes a sample pad, a conjugate pad, a nitrocellulose membrane, testing lines, a quality control line, a water absorbent pad and a backing plate; the conjugate pad is loaded with two immunofluorescence probes using quantum dots as markers, and the quantum dots are respectively coupled with a NSE antibody and a CEA antibody to form anti-NSE immunofluorescence and anti-CEA immunofluorescence probes; and the number of the testing lines is two, one testing line includes the NSE antibody, and the other testing line includes the CEA antibody. The immunochromatographic test paper strip is simple and fast in detection, intuitive in test results, capable of qualitatively, quantitatively even semi-quantitatively detecting, and low in price, can simultaneously detect two or more tumor markers, and achieves multiple detection of the tumor markers by use of the quantum dot test paper strip for detection.
Owner:SHANGHAI JIAO TONG UNIV
Magnetic immunochromatographic test strip capable of quantitatively detecting CEA and CA19-9 in blood simultaneously and preparation method
InactiveCN108020666ARealize quantitative detection of single servingHigh precisionMaterial analysisMedicineQuality control
The invention discloses a magnetic immunochromatographic test strip capable of quantitatively detecting CEA and CA19-9 in blood simultaneously and a preparation method. The test strip is prepared by successively binding a sample pad, a combining pad, a nitrocellulose membrane and water absorbing paper to a bottom plate of the test strip and 2mm of adjacent parts are alternately overlaid one another, wherein the nitrocellulose membrane is pre-coated with a detection line of a CEA antibody and a CA19-9 antibody and a quality control line of a rabbit antimouse IgG antibody. Compared with existingtumor marker detection technologies, the magnetic immunochromatographic technology is creatively introduced into combined quantitative detection of CEA and CA19-9 to achieve microscale quantitative analysis of CEA and CA19-9, so that the detecting sensitivity is improved greatly; and based on double positive CEA and CA19-9, the occurrence rate and transfer rate of tumor can be further judged to improve early stage diagnostic rate of occurrence and transfer of colon cancer to achieve the purpose of immediate detection and quick diagnosis, and the test strip is simple to operate and low in cost, and can be widely popularized.
Owner:FUZHOU UNIV
Kit for quantitatively detecting CEA (Carcino-Embryonic Antigen) as well as preparation method and application method of kit
The invention discloses a kit for quantitatively detecting a CEA (Carcino-Embryonic Antigen). The kit comprises following reagents: a biological functional magnetic nano composite particle coupled with a CEA antibody, an enzyme-labeled CEA antibody and a chemiluminiscence substrate, wherein the biological functional magnetic nano composite particle coupled with the CEA antibody is formed by compounding a nano-grade magnetic nucleus with a high molecular material containing various functional groups. According to the kit for quantitatively detecting the CEA, a nano magnetic bead separation technology, a chemiluminiscence technology and an enzyme immunoassay technology are combined together; the kit has the advantages of high sensitivity, wide linearity range, high specificity, rapidness in reaction and the like.
Owner:INST OF ELECTRONICS CHINESE ACAD OF SCI
Carcinoembryonic antigen detection kit and preparation method thereof
The invention relates to the technical field of immunodetection and in particular relates to a carcinoembryonic antigen (CEA) detection kit and a preparation method thereof. The detection kit comprises a magnetic microsphere suspension solution coated with a CEA antibody, a CEA calibration product, a CEA quality control product, a CEA tracer working solution, a concentrated washing solution, a substrate solution and a specimen dilution solution. The kit has the advantages of accurate detection result, good stability, strong specificity, high sensitivity, wide linear range, short detection timeand convenience for utilization and is suitable for being popularized and applied.
Owner:山东康华生物医疗科技股份有限公司
Visual multicolor detection kit forantigen-antibody reaction and using method of kit
ActiveCN105067803AGet rid of dependenceFast readoutBiological testingAlkaline waterMonoclonal antibody
The invention belongs to a field of immunization analysis and diagnosis technology, and concretely relates to a visual multicolor detection kit for antigen-antibody reaction and a using method of kit. According to the invention, the kit comprises CEA antibodies labeled with grapheme modified by methyl red; NSE antibodies labeled with grapheme modified by phenolphthalein;Cyfra21-1 antibodies labeled with grapheme modified by thymolphthalein; a 96-well plate coated with a monoclonal antibody mixture containing CEA, NSE and Cyfra21-1 antibody; alkaline water whose pH value is 12.0; and a PBST buffersolution. The kit provided by the invention can be used for simultaneous multicolor detection of CEA, NSE and Cyfra21-1 antigen. The kit provided by the invention has the advantages of simple operation, high efficiency and low cost, and is convenient to be used in the places short of resource and less developed areas.
Owner:NANJING UNIV
Anti-idiotype anti-CEA antibody molecules and its use as cancer vaccine
The present invention provides molecules, preferably designed immunoglubulins, suitable for use as an anti-idiotype vaccine to CEA positive tumours. The molecules induce both an MHC class I and MHC class II mediated immune response to the CEA bearing tumour cells for an efficient and sustained host anti-tumour response. The present invention provides modified versions of anti-idiotype anti-CEA antibodies, preferably mouse antibody 708, with improved vaccination properties. The modifications are related to the introduction of sequences tracts deriving from e.g. CEA, CD55 antigen and CEA cancer-specific MHC epitopes into the variable regions of said antibody molecules.
Owner:MERCK PATENT GMBH
Antibody for resisting anti-cancer embryo antigen as well as preparation method and application thereof
ActiveCN110862456AIncrease lethalityImmunoglobulins against cell receptors/antigens/surface-determinantsBlood/immune system cellsComplementarity determining regionSingle-Chain Antibodies
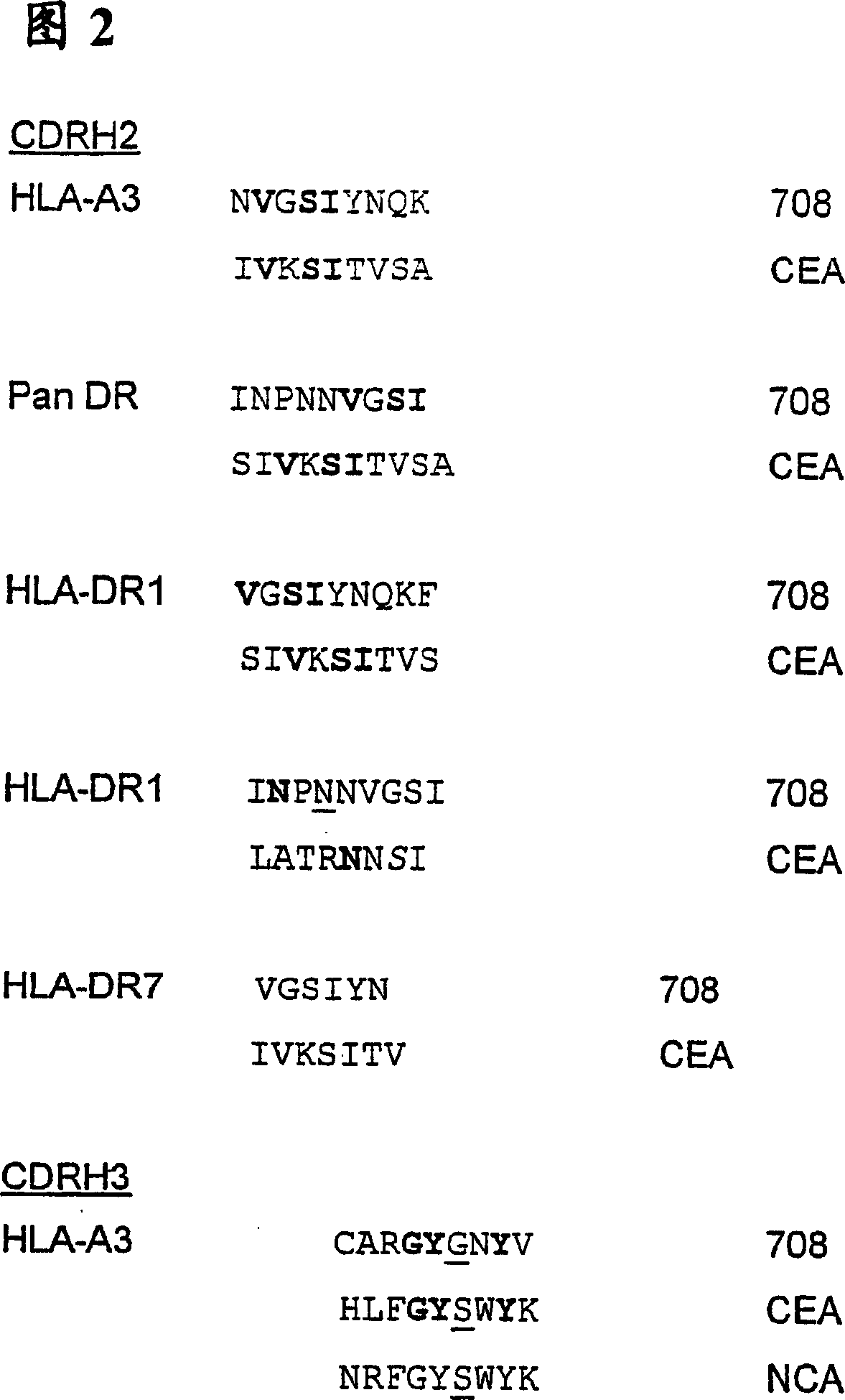
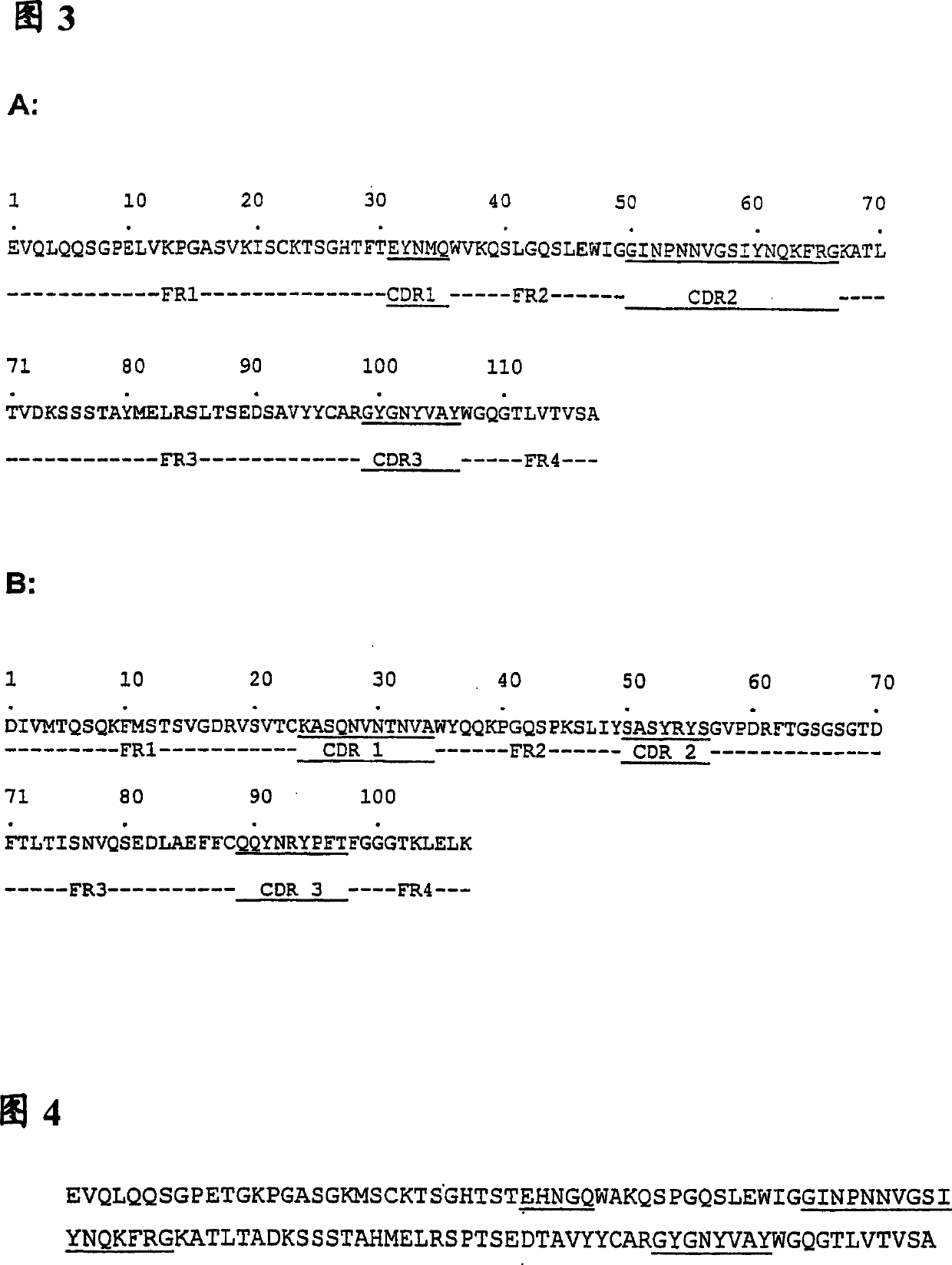
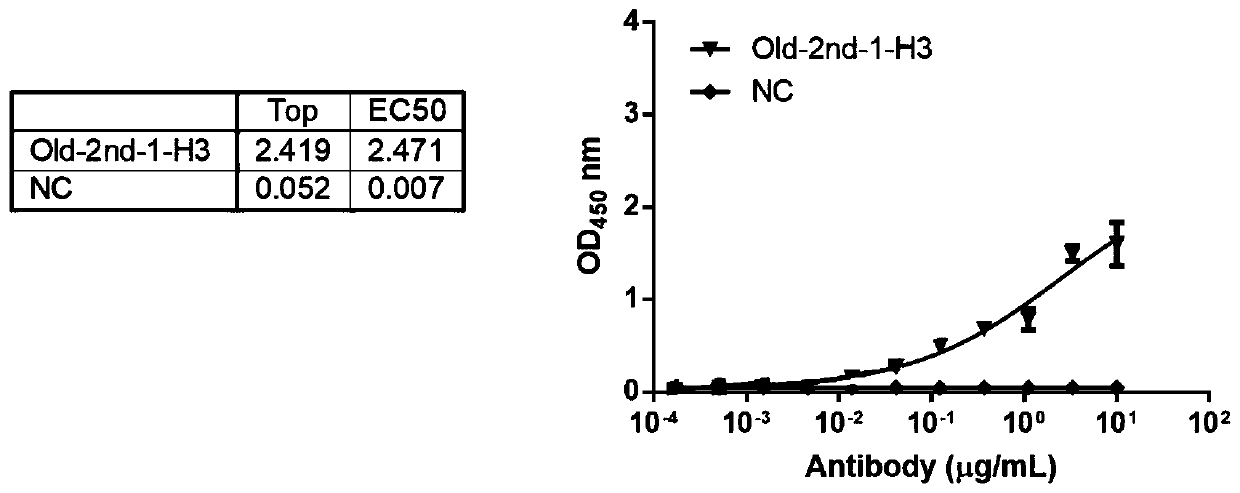
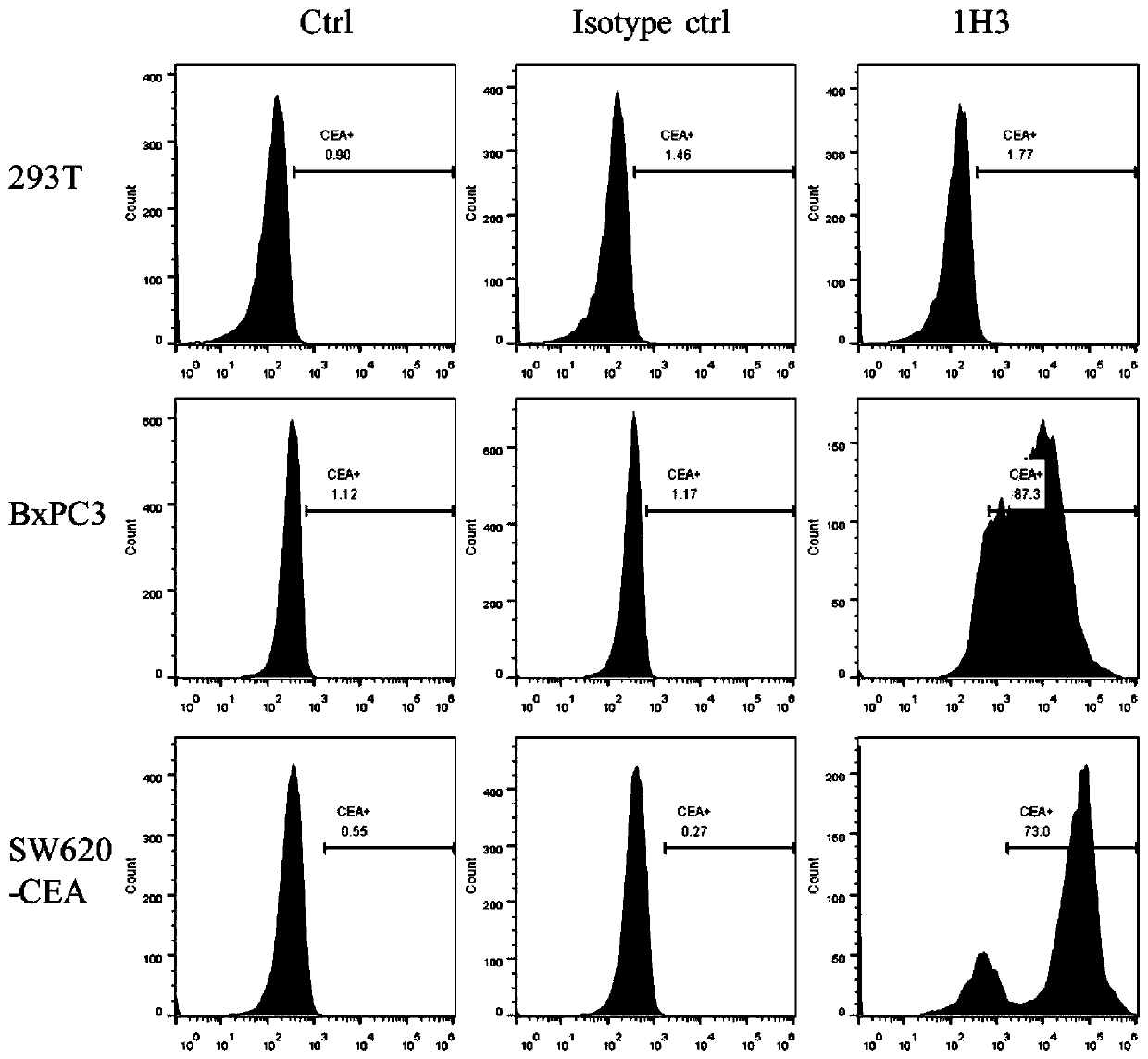
The invention provides an anti-CEA antibody. The anti-CEA antibody comprises a heavy chain variable region and a light chain variable region, wherein a complementary determining region of the heavy chain variable region comprises CDR-H1 with an amino acid sequence shown as SEQ ID No. 1, CDR-H2 with an amino acid sequence shown as SEQ ID No. 2 and CDR-H3 with an amino acid sequence shown as SEQ IDNo. 3; a complementation determining region of the light chain variable region comprises CDR-L1 with an amino acid sequence shown as SEQ ID No. 4, CDR-L2 with an amino acid sequence shown as SEQ ID No. 5 and CDR-L3 with an amino acid sequence shown as SEQ ID No. 6. The CEA scFv is screened by an inventor of the invention by utilizing a phage display technology, so that the CEA-resistant single-chain antibody with high affinity is obtained. An anti-CEA chimeric antigen receptor gene is introduced into T cells through a genetic engineering method to prepare CEA CAR-T, so that the CEA CAR-T cellsspecifically recognize digestive tract tumor cells expressing CEA and kill the digestive tract tumor cells, and the anti-tumor effect of the CEA CAR-T cells is achieved.
Owner:HUADAO SHANGHAI BIOPHARMA CO LTD
Preparation method and detection method of carcino-embryonic antigen electrochemical immunosensor
InactiveCN106770568ANo pollution in the processWill not bring inMaterial analysis by electric/magnetic meansElectrochemical responseChemical reaction
The invention belongs to the field of immunosensor, and more specifically discloses a preparation method and a detection method of a carcino-embryonic antigen (CEA) electrochemical immunosensor. The preparation method comprises following steps: poly o-phenylenediamine nanoparticles are dispersed in water via ultrasonic oscillation, and an obtained poly o-phenylenediamine nanoparticle solution is stored at room temperature; a glassy carbon electrode is modified with glutaraldehyde, and the glassy carbon electrode is immersed in a mixed solution composed of a HRP labeled CEA antibody solution and the poly o-phenylenediamine nanoparticle solution so as to obtain a poly o-phenylenediamine nanoparticle / HRP labeled CEA antibody modified electrode; solutions with different concentrations are prepared from CEA standard samples, and are taken as test solutions; an analyte is subjected to differential pulse voltammetry scanning in a H2O2 and oxygen-free PBS mixed solution; electrochemical reaction is carried out so as to establish the quantitative relationship of electrochemical current strength with the CEA solution concentration. The carcino-embryonic antigen electrochemical immunosensor is high in sensitivity; results are accurate and reliable; cost is low; detection is rapid; the carcino-embryonic antigen electrochemical immunosensor is convenient to use; and the preparation process is extremely simple.
Owner:CHONGQING UNIV OF ARTS & SCI
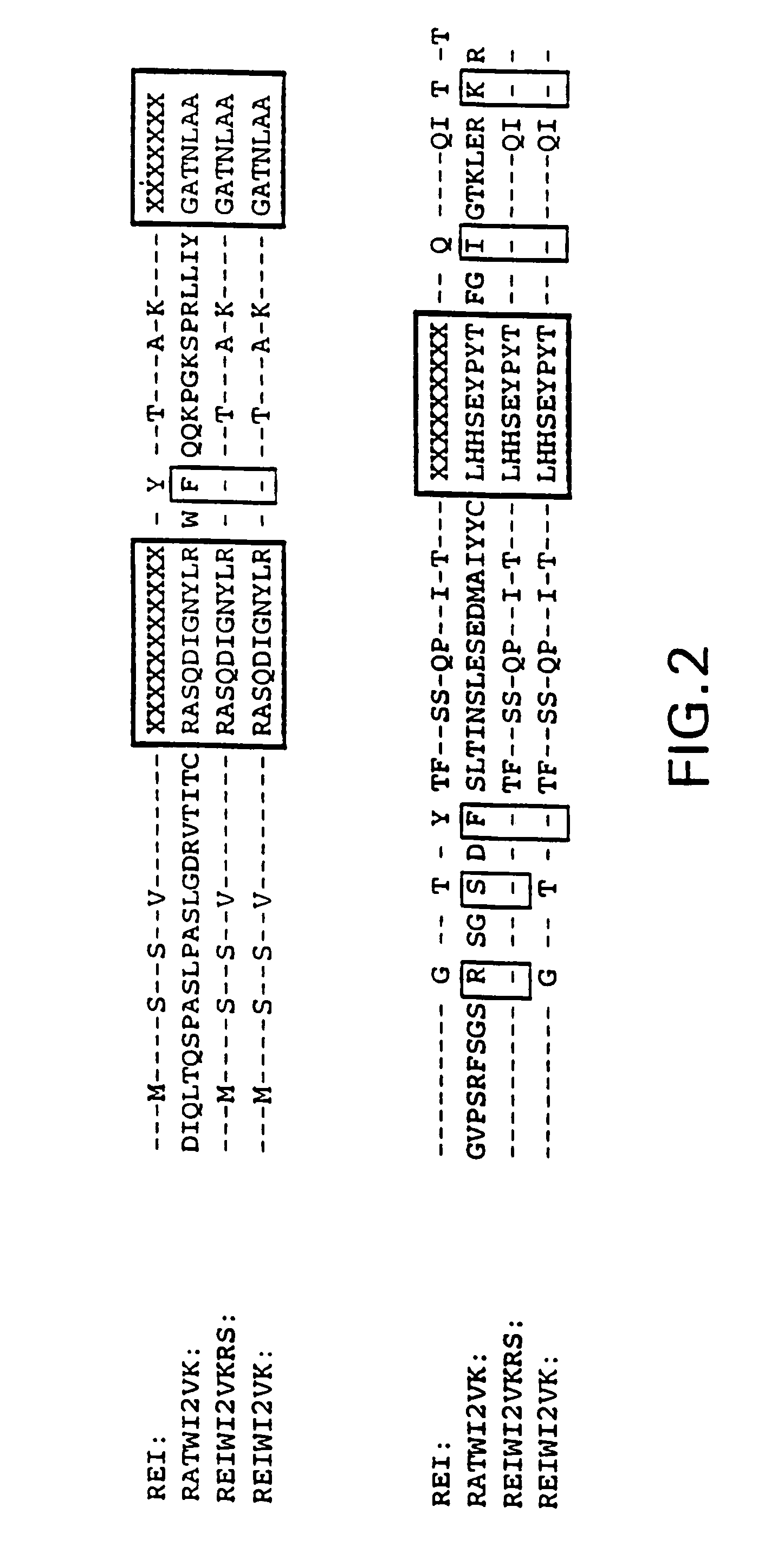
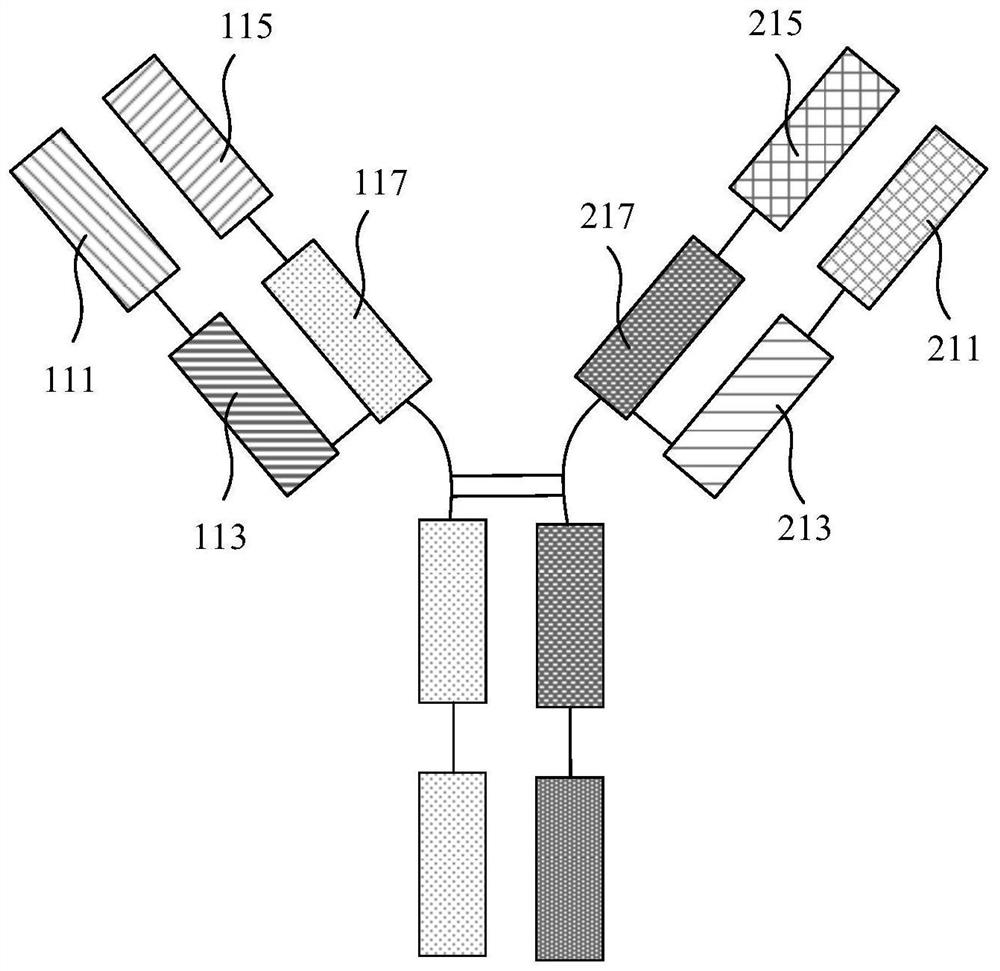
Anti-CEA antibody, application of anti-CEA antibody, cell capable of secreting anti-CEA antibody and preparation method of cell
ActiveCN111748039AHybrid immunoglobulinsGenetically modified cellsHeavy chainAntiendomysial antibodies
The invention relates to an anti-CEA antibody and an application thereof, a cell capable of secreting the anti-CEA antibody and a preparation method of the cell. The anti-CEA antibody comprises a first structural domain and a second structural domain, the first structural domain comprises a first light chain variable region and a first heavy chain variable region, the amino acid sequence of the first light chain variable region is as shown in SEQ ID No. 1, and the amino acid sequence of the first heavy chain variable region is as shown in SEQ ID No. 2; the second structural domain comprises asecond light chain variable region and a second heavy chain variable region, the amino acid sequence of the second light chain variable region is shown as SEQ ID No.3, and the amino acid sequence of the second heavy chain variable region is shown as SEQ ID No.4. The specificity of the anti-CEA antibody is relatively good.
Owner:SHENZHEN YHLO BIOTECH +2
CEA TRFIA (time-resolved fluoroimmunoassay) kit based on IMB (immunomagnetic beads)
The invention discloses a CEA TRFIA (time-resolved fluoroimmunoassay) kit based on IMB (immunomagnetic beads). The kit includes a calibrator, immunomagnetic beads connected with CEA antibodies, europium labeled CEA antibodies, an assay buffer solution, a cleaning solution and an enhanced solution. Through double antibody sandwich immunoreaction, IMB-CEA-europium labeled-CEA monoclonal antibody complexes are formed, IMB which adsorb CEA and supernate are separated through magnetic seperation and washed, the enhanced solution is added, and the value is measured through a time-resolved instrument. Besides the TRFIA technology, the invention further has the advantages as follows: through IMB enrichment and full diffusion of IMB in the solution, the combination superficial area is enlarged, the reaction time is greatly shortened, and the detection sensitivity is improved. IMB and antibodies are directionally connected through chemical groups, so that the consumption of paired antibodies is greatly reduced and the detection precision is improved. The technology realizes automation easily, and overcomes the problem that the traditional micropore plate type TRFIA technology can only carry out detection after samples are accumulated to a certain number, thereby realizing real-time sample detection.
Owner:GUANGZHOU BIOKEY HEALTH TECH CO LTD
Preparation method of an electrochemiluminescence sensor of a nanocomposite material
ActiveCN105115961BGood biocompatibilityLarge specific surface areaChemiluminescene/bioluminescenceCross-linkAntigen
The invention relates to a preparation method of an electrochemiluminescence sensor of nano-composite material, which includes preparing Au@BSA nano-material; using glutaraldehyde to cross-link luminol to the surface of Au@BSA nano-material, and activating carboxyl groups on the surface of Au@BSA Connect the CEA antibody to obtain luminol‑Au@BSA‑anti‑CEA; use the electrodeposition method to electrodeposit a gold nanolayer on the surface of the bare glassy carbon electrode of the working electrode, and connect the obtained composite material luminol‑Au@BSA‑anti‑CEA to Three steps to obtain an electrochemiluminescence immunosensor with targeting effect on the antigen CEA on the surface of the electrode. The invention is fast and simple, and the constructed immune sensor has the advantages of good biocompatibility, strong specificity, high sensitivity and wide detection range.
Owner:SHANGHAI NORMAL UNIVERSITY
Preparation method of lung cancer marker detection immunochromatographic test paper
Owner:SHANGHAI NORMAL UNIVERSITY
Humanization of an anti-carcinoembryonic antigen anti-idiotype antibody as a tumor vaccine and for targeting applications
A humanized form of an anti-idiotype antibody to CEA, e.g., hWI2, has conserved immunoreactivity. The clinical benefits of anti-CEA antibodies are maximized by using the humanized anti-idiotype as a clearing agent for anti-CEA antibodies or antibody fragments. The humanized anti-idiotype also can be used as an immunogenic vaccine.
Owner:IMMUNOMEDICS INC
Anti-cea antibody and application thereof, cell capable of secreting anti-cea antibody and preparation method thereof
ActiveCN111748039BHybrid immunoglobulinsGenetically modified cellsHeavy chainAntiendomysial antibodies
The invention relates to an anti-CEA antibody and its application, a cell capable of secreting an anti-CEA antibody and a preparation method thereof. The anti-CEA antibody includes a first structural domain and a second structural domain. The first structural domain includes a first light chain variable region and a first heavy chain variable region. The amino acid sequence of the first light chain variable region is as SEQ ID No. 1, the amino acid sequence of the first heavy chain variable region is shown in SEQ ID No.2; the second structural domain includes the second light chain variable region and the second heavy chain variable region, and the second light chain variable region The amino acid sequence of the region is shown in SEQ ID No.3, and the amino acid sequence of the second heavy chain variable region is shown in SEQ ID No.4. The specificity of the above-mentioned anti-CEA antibody is better.
Owner:SHENZHEN YHLO BIOTECH +2
Mass spectrometry kit and method for screening and prognosis evaluation of lung cancer
PendingCN114217059AImprove screening efficiencyImprove detection preparation efficiencyMaterial analysis by electric/magnetic meansImmunoglobulins against cell receptors/antigens/surface-determinantsMass Spectrometry-Mass SpectrometryPolystyrene particle
The invention discloses a mass spectrometry kit for lung cancer screening and prognosis evaluation, which comprises a CEA antibody, a CA125 antibody, an SCC antibody, an NSE antibody, a Pro-GRP antibody, a CYFRA21-1 antibody and a solid phase carrier, the CEA antibody, the CA125 antibody, the SCC antibody, the NSE antibody, the Pro-GRP antibody and the CYFRA21-1 antibody are respectively fixed on the solid phase carrier, and the solid phase carrier is fixed on the solid phase carrier. The CEA antibody, the CA125 antibody, the SCC antibody, the NSE antibody, the Pro-GRP antibody and the CYFRA21-1 antibody are combined on the solid-phase carrier in a non-covalent connection manner, and the solid-phase carrier is polystyrene particles coated with protein A. The lung cancer detection kit disclosed by the invention is higher in specificity, better in early-stage lung cancer screening effect and suitable for screening all-stage lung cancer patients.
Owner:辽宁省肿瘤医院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com