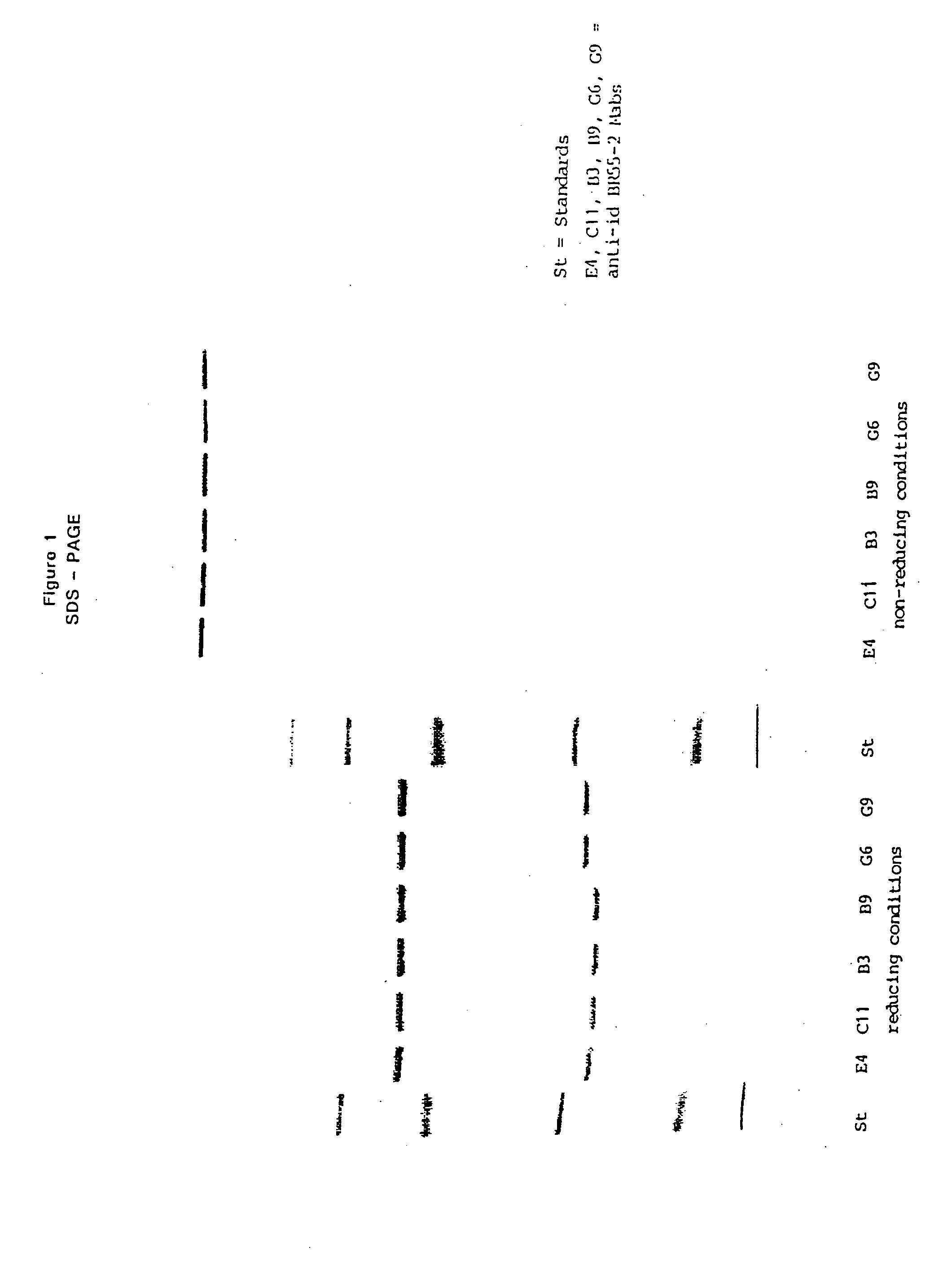
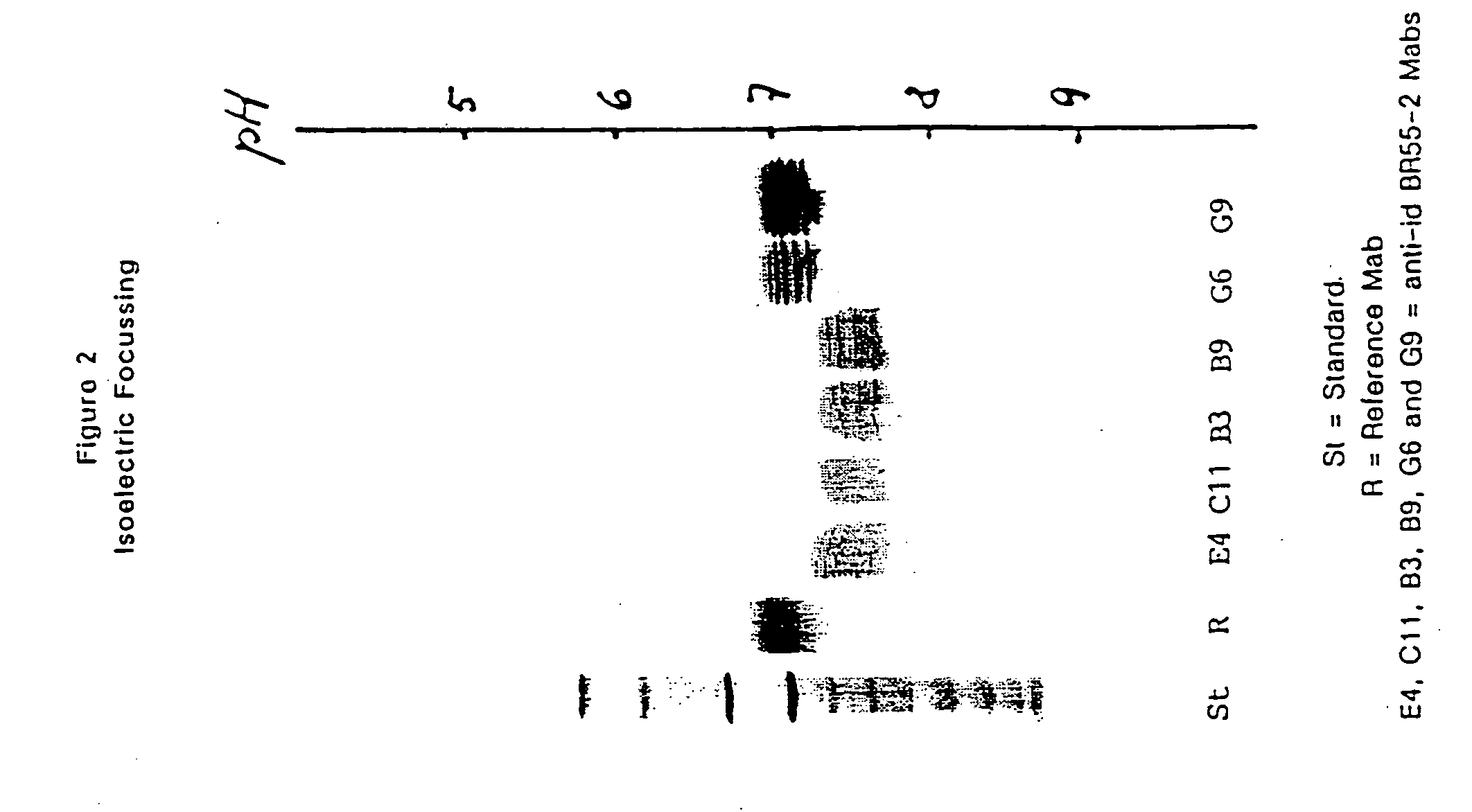
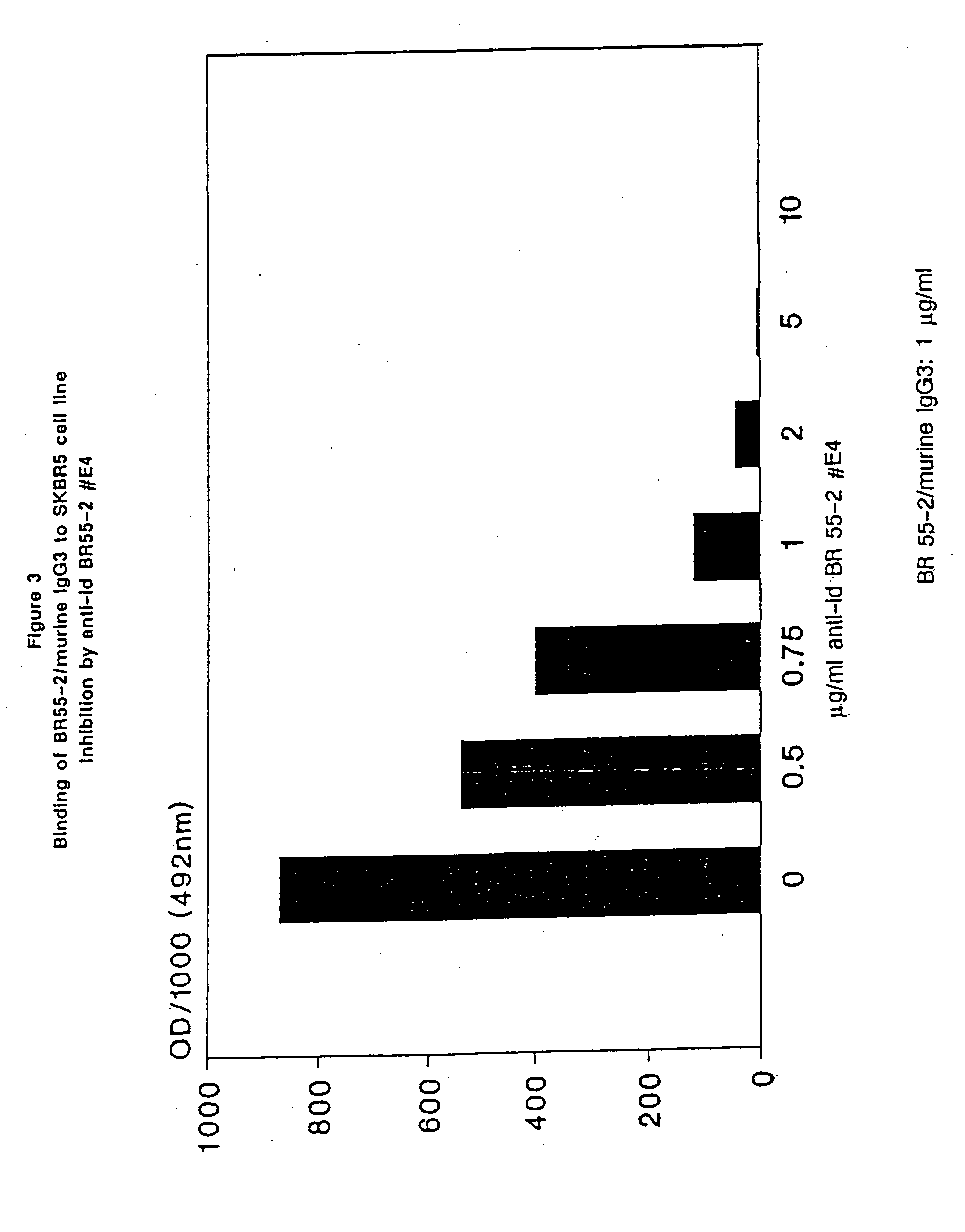
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103 results about "Idiotype" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
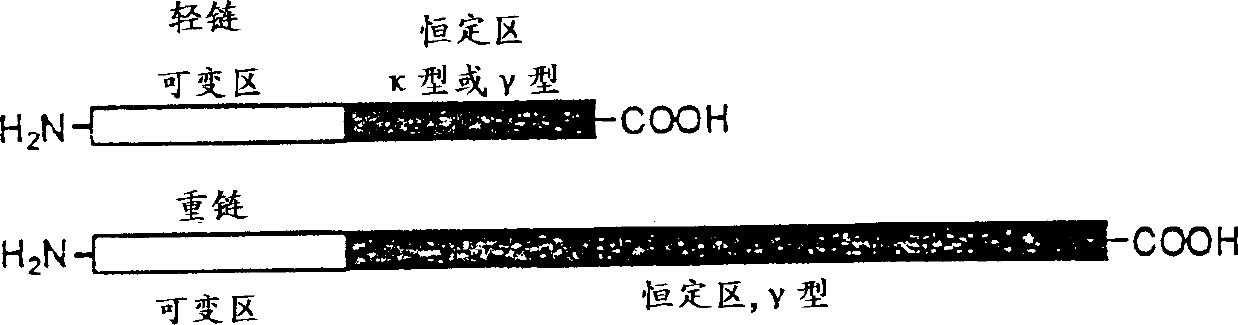
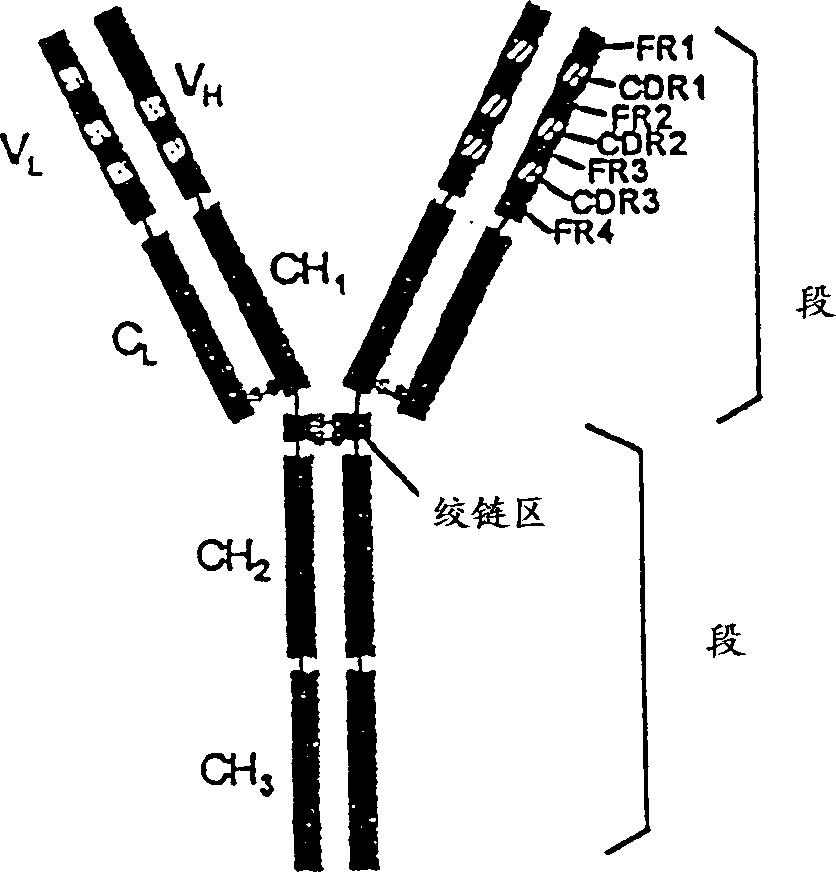
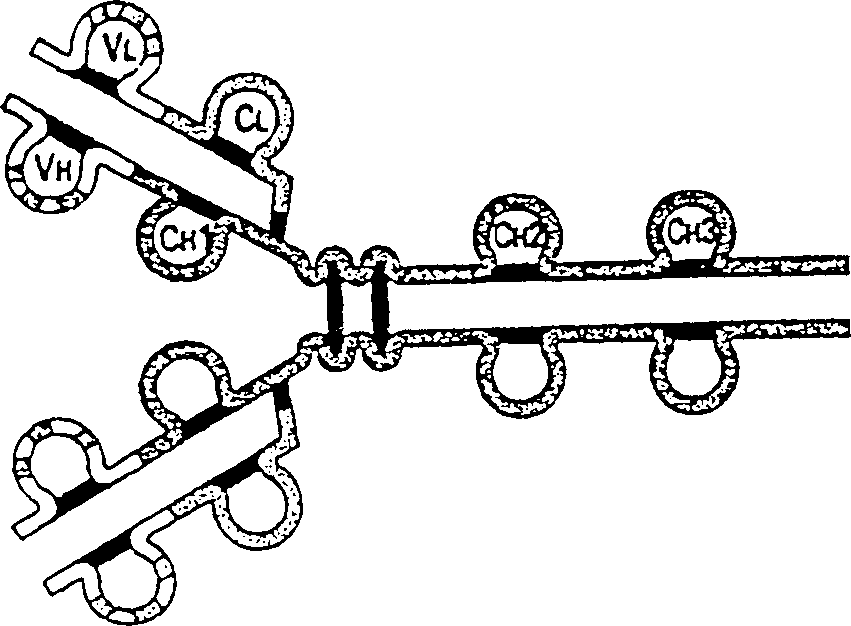
In immunology, an idiotype is a shared characteristic between a group of immunoglobulin or T cell receptor (TCR) molecules based upon the antigen binding specificity and therefore structure of their variable region. The variable region of antigen receptors of T cells (TCRs) and B cells (immunoglobulins) contain complementarity determining regions (CDRs) with unique amino acid sequences. They define the surface and properties of the variable region, determining the antigen specificity and therefore the idiotope of the molecule. Immunoglobulins or TCRs with a shared idiotope are the same idiotype. Antibody idiotype is determined by...
Curing method for pathologic syndrome and medicinal preparation
A method of treating a pathological syndrome includes administration of an activated form of ultra-low doses of antibodies to an antigen, wherein said activated form is obtained by repeated consecutive dilution combined with external impact, and the antigen is a substance or a pharmaceutical agent exerting influence upon the mechanisms of formation of this particular pathological syndrome. Pharmaceutical agent for treating a pathological syndrome contains activated form of ultra-low doses of monoclonal, polyclonal or natural antibodies to an antigen, wherein said activated form is prepared by means of repeated consecutive dilution and external treatment, predominantly based on homeopathic technology, and said antigen is a substance or a drug acting as a direct cause of the pathological syndrome or involved in regulation of mechanisms of its formation. At that, activated forms of ultra-low doses of antibodies are raised against antigens of exogenous or endogenous origin, against autologous antigens, fetal antigens; anti-idiotypic antibodies are used too.
Owner:EPSHTEIN OLEG I
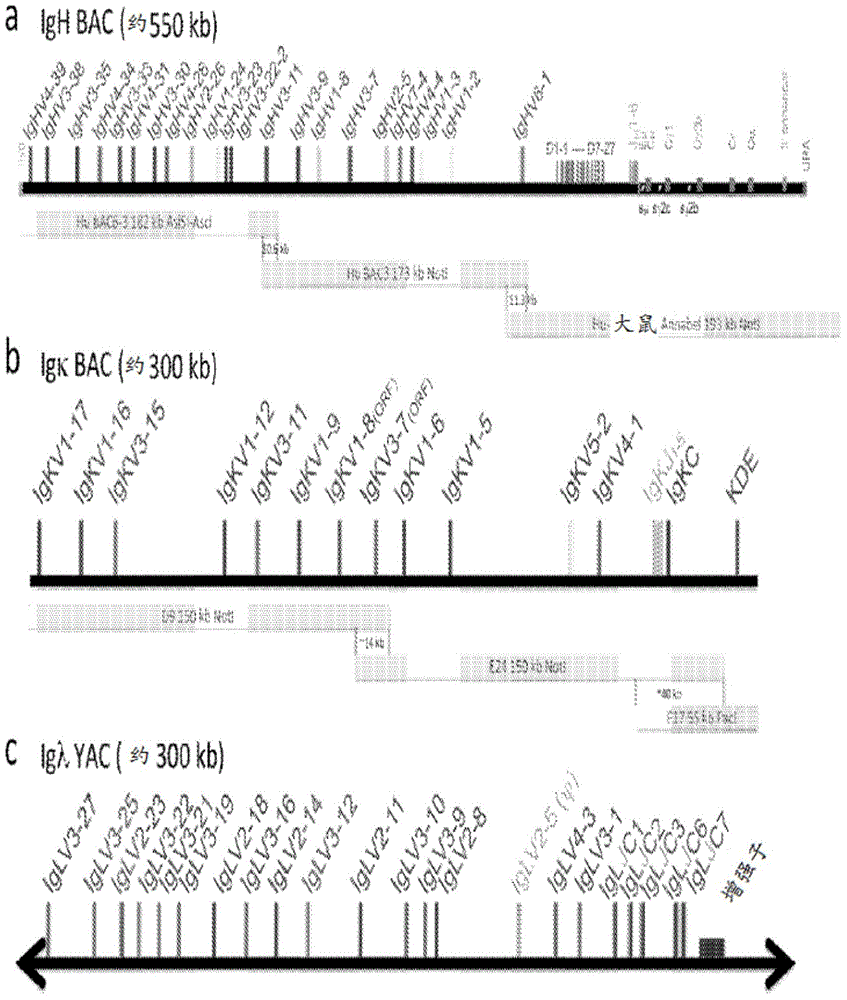
Compositions and methods for inhibiting endogenous immunoglobulin genes and producing transgenic human idiotype antibodies
ActiveUS20090098134A1Efficiently deletingHydrolasesGenetically modified cellsAlpha globulinTransgene
Owner:OMNIAB OPERATIONS INC
Purified antigen for Alzheimer's disease and methods of obtaining and using same
The invention relates, among other things, a preparation comprising Alzheimer's disease antigen (A68), as well as methods of obtaining this purified antigen, and methods of using this purified antigen, for instance, for diagnosing Alzheimer's disease and for detecting human autoantibodies to the Alzheimer disease antigen. The antigen preparation according to the invention is purified in that it is substantially free of immunoglobulin G. The invention further relates to methods of making Alzheimer disease antigens that can be used instead of or along with the A68 antigen preparation (e.g., for diagnosing AD), such as recombinant human tau, tau isolated from various species including human, and phosphorylated recombinant human tau or isolated tau, as well as A68 anti-idiotypic antibodies.
Owner:MOLECULAR GERIATRICS
Assay for antibodies
InactiveUS20060099662A1Reduce non-specific stickingReduce backgroundBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsHuman patientElisa method
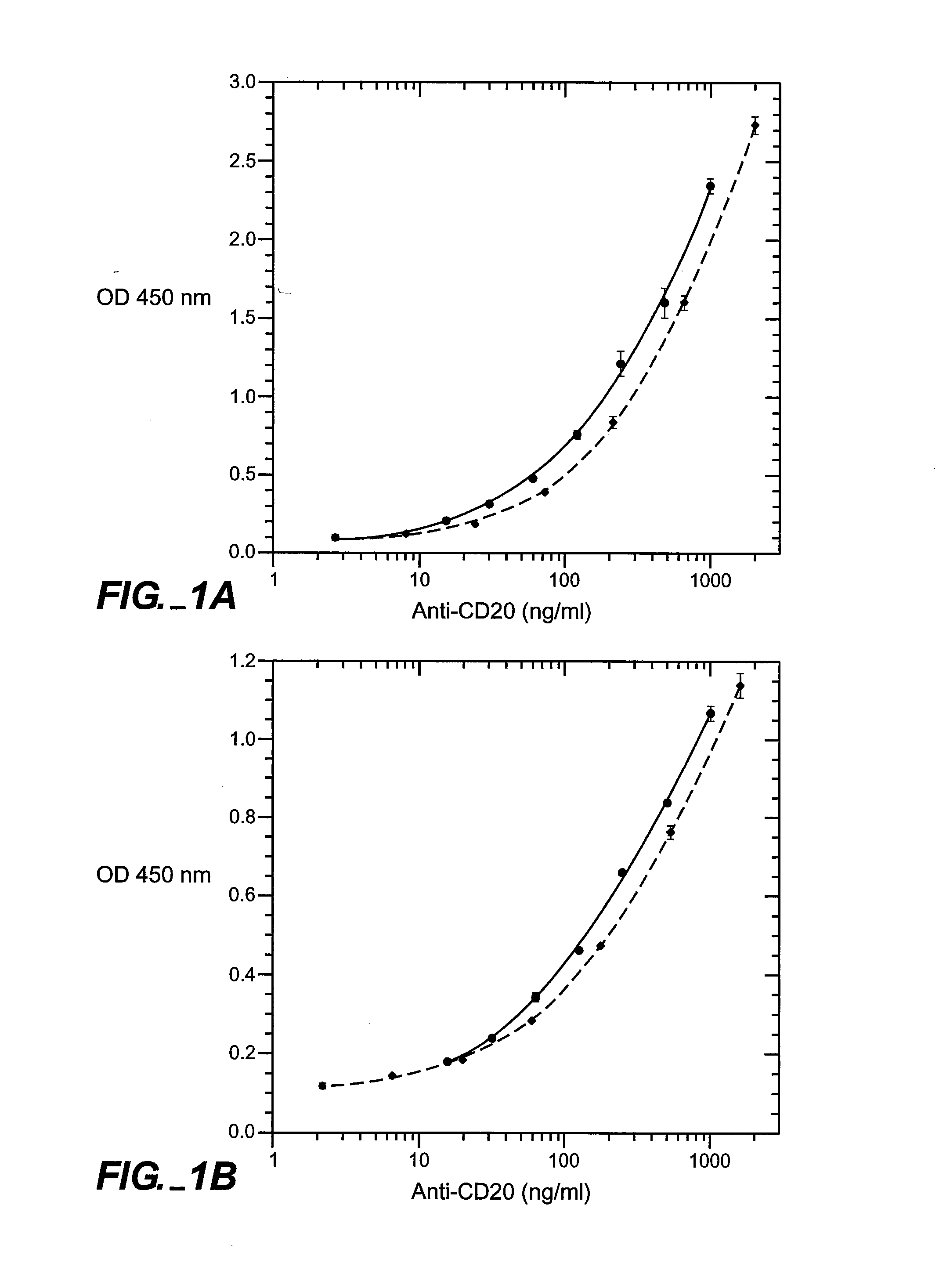
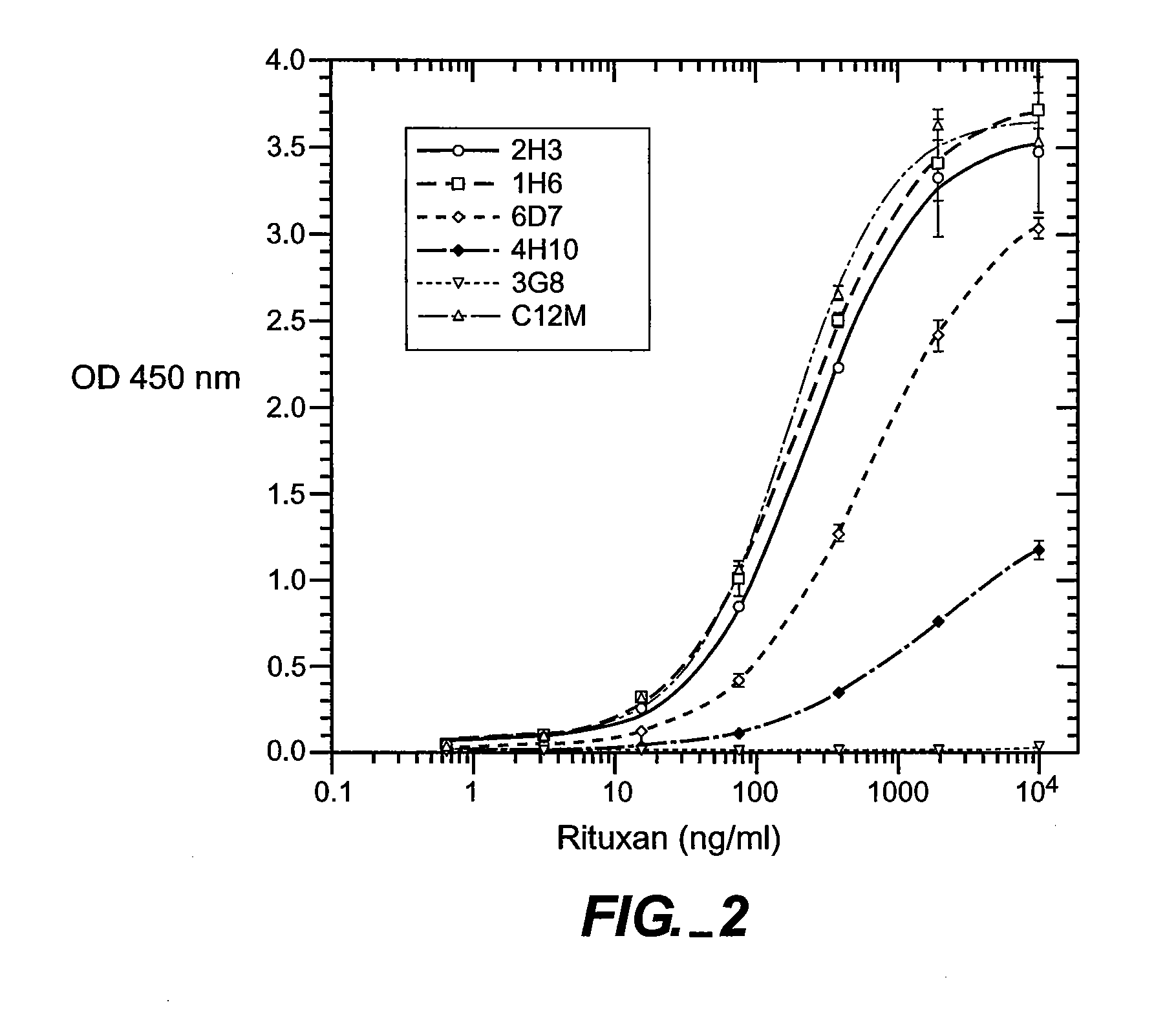
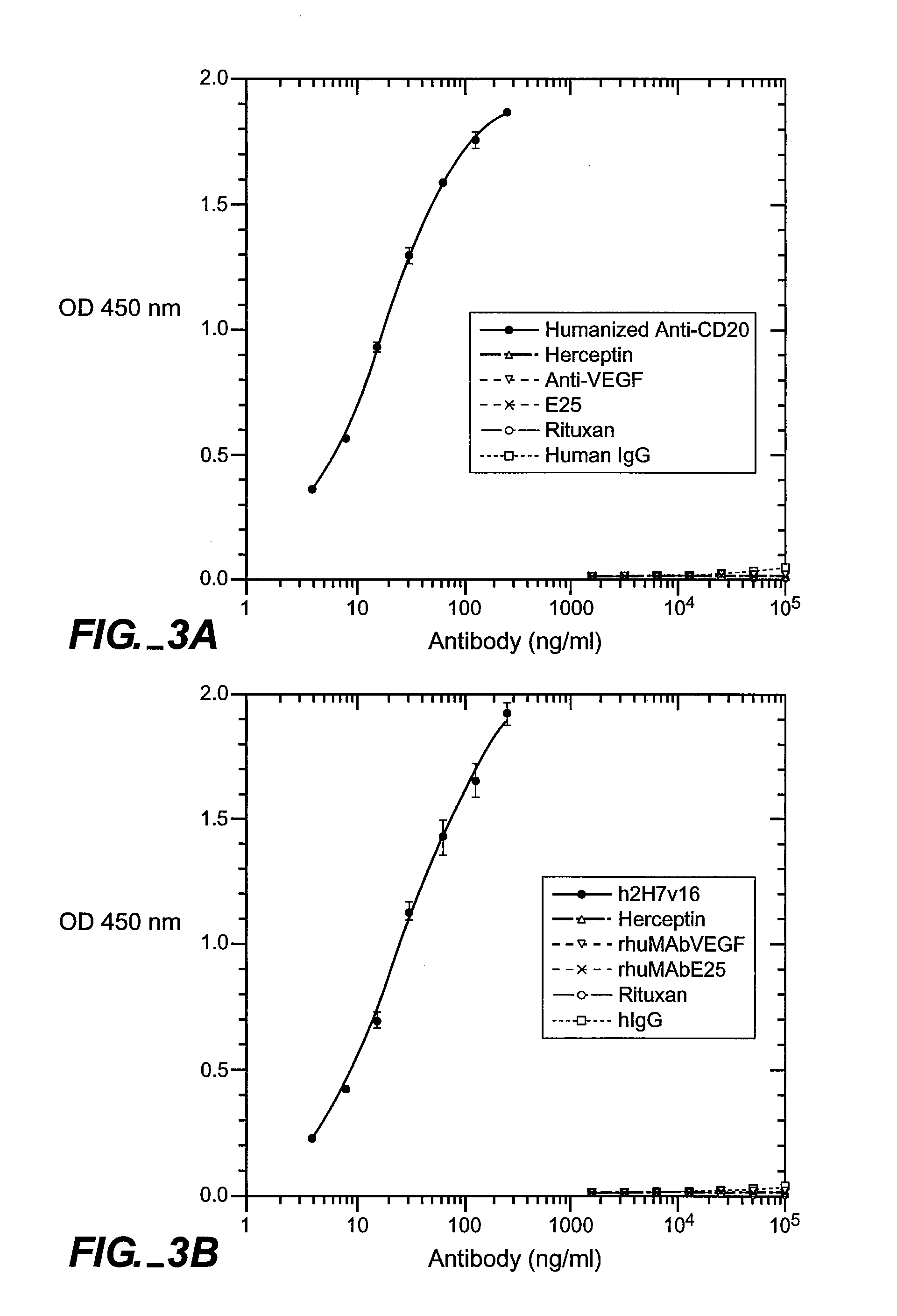
The presence and quantity of an antibody of interest in a patient's bloodstream or other biological sample can serve as an important clinical or other analytical or diagnostic tool. ELISA methods, and kits for such assays, as well as anti-idiotypic antibodies and hybridomas producing them, are developed to detect levels of the antibody in biological samples, which are from, for example, animal models and human patients.
Owner:GENENTECH INC
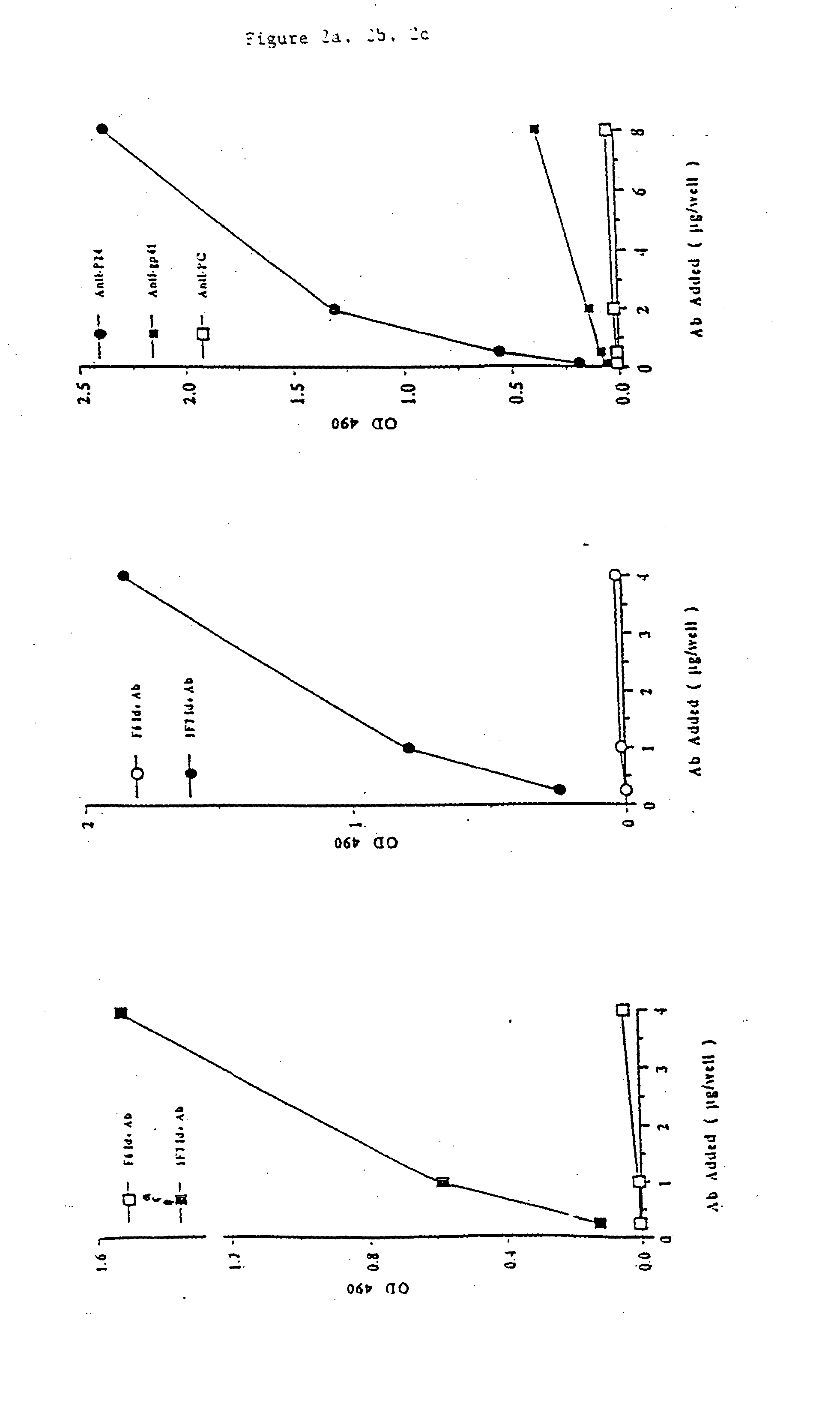
Anti-idiotypic antibody inducing hiv-1 neutralizing antibodies
The invention relates to Ab2-type anti-idiotypic antibodies and fragments thereof which mimic HVI-1 epitopes that are otherwise cryptic to the immune system and which antibodies or fragments thereof are directed against potently neutralizing anti-HIV-1 antibodies. The invention further relates to a hybridoma cell line 3H6 expressing the anti-idiotypic antibody and to pharmaceutical compositions containing the antibody or fragment thereof. The invention also relates to HIV-1 neutralizing Ab3-type antibodies elicited upon administration of the Ab2-type anti-idiotypic antibody or fragment thereof and to pharmaceutical compositions containing them. The invention also relates to the use of the present antibodies or fragments thereof as screening tools or as diagnostic or therapeutic agents.
Owner:POLYMUN SCIENC IMMUNOLOGISCHE
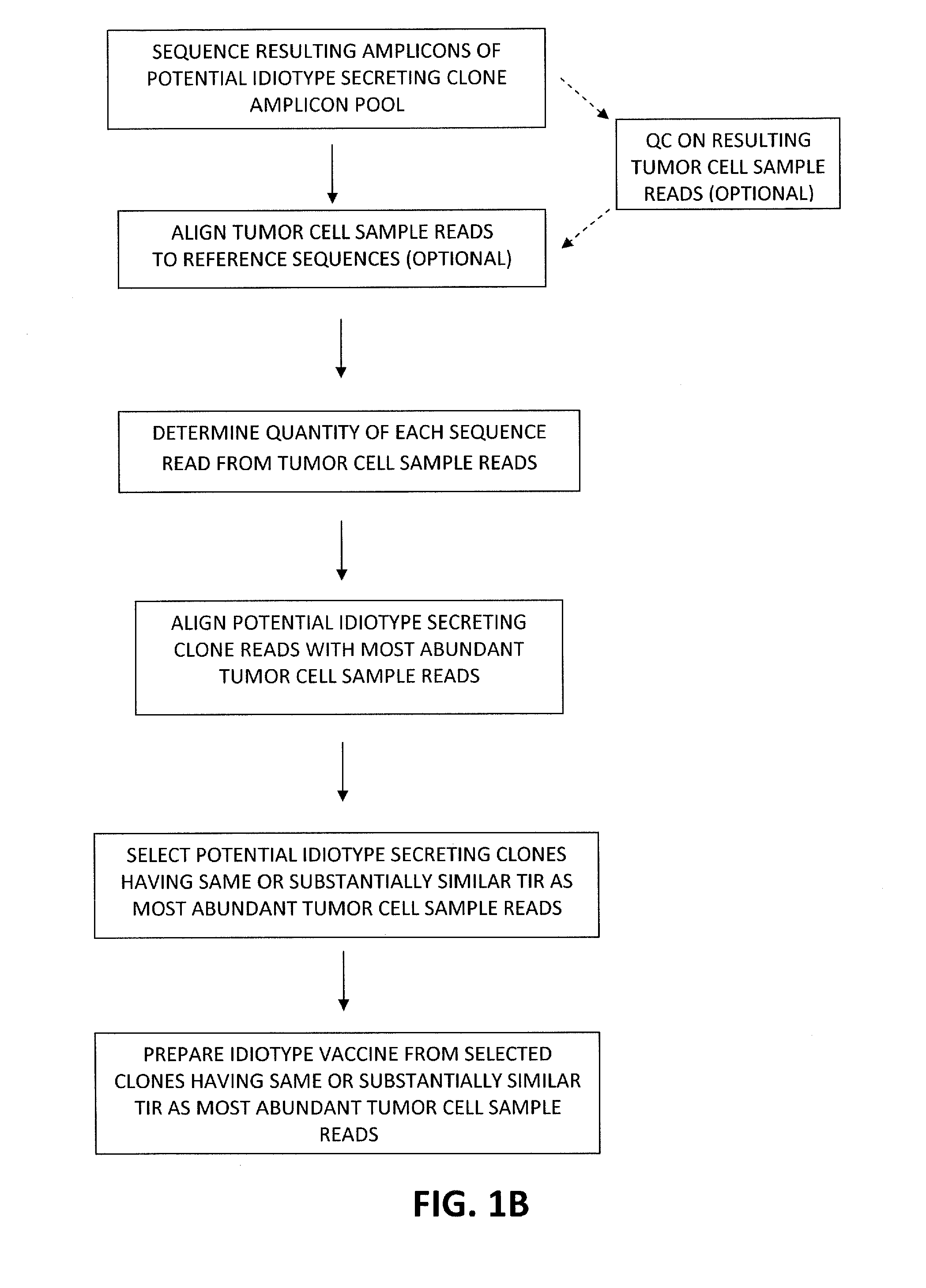
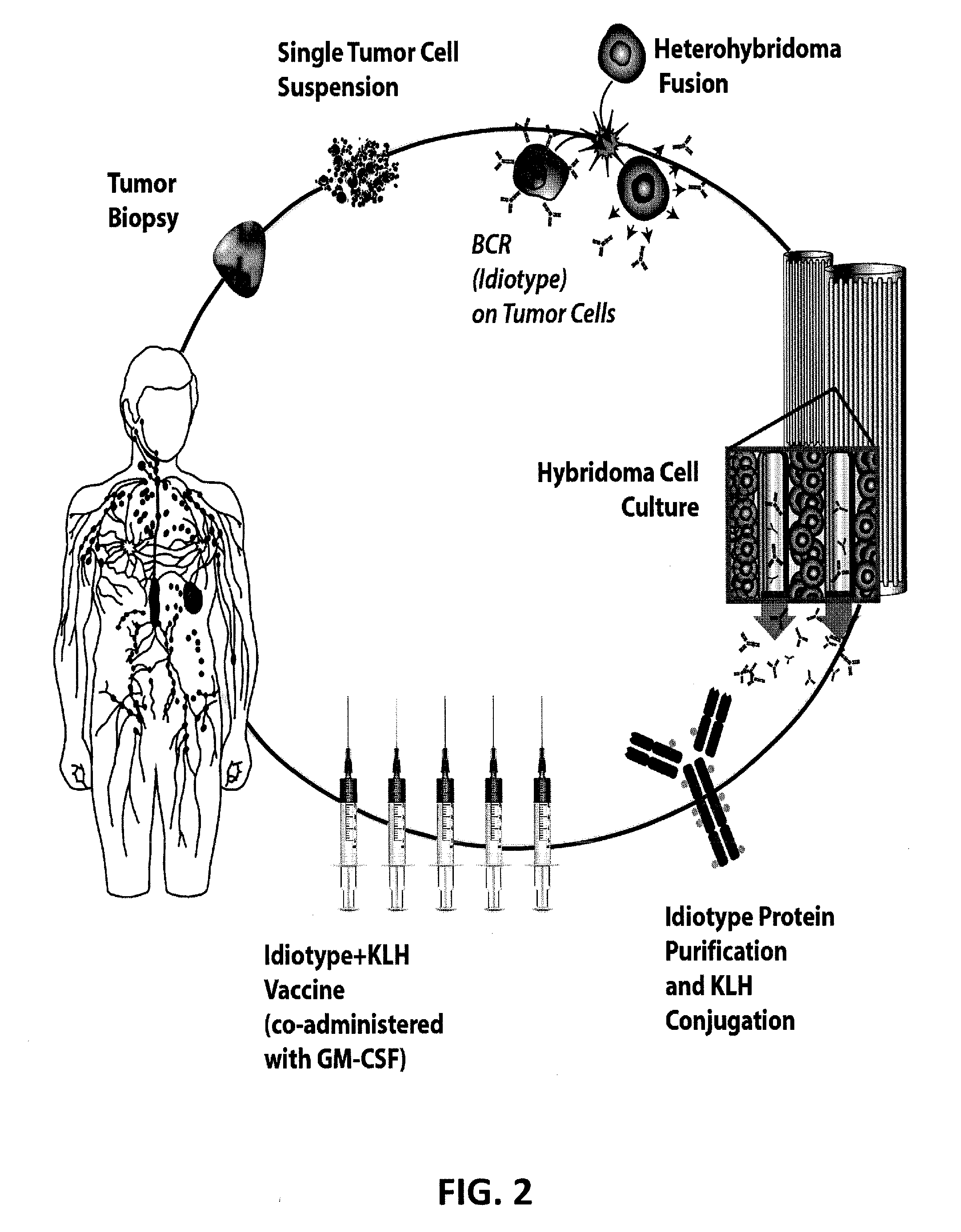
Methods for producing high-fidelity autologous idiotype vaccines
ActiveUS20150259749A1Maintain fidelityMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsMalignancyB cell
The present invention concerns methods for selecting and producing idiotype vaccines, and in particular methods for selecting and producing an idiotype vaccine for treatment of a B-cell derived malignancy in a subject based on the clonal profile (clonotype) of the malignancy; a method for producing an updated idiotype vaccine matched to a B-cell derived malignancy exhibiting a shifting clonal profile; and the high-fidelity idiotype vaccines produced using the methods. The invention also includes idiotype vaccines produced using the described methods and methods of treating B-cell derived malignancies using the produced vaccines.
Owner:BIOVEST INT
Compositions and Methods for Inhibiting Endogenous Immunoglobulin Genes and Producing Transgenic Human Idiotype Antibodies
Owner:OMNIAB INC
Anti-idiotypic antibodies neutralizing the inhibitory activity of an inhibitory antibody directed against the c1 domain of factor viii
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the C1 domain of Factor VIII, as well as to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as medicament, and more particularly to the use thereof for manufacturing a medicament intended for the treatment of haemophilia A.
Owner:LFB BIOTECH
Method of treating a pathological syndrome and a pharmaceutical agent
A method of treating a pathological syndrome includes administration of an activated form of ultra-low doses of antibodies to an antigen, wherein said activated form is obtained by repeated consecutive dilution combined with external impact, and the antigen is a substance or a pharmaceutical agent exerting influence upon the mechanisms of formation of this particular pathological syndrome. Pharmaceutical agent for treating a pathological syndrome contains activated form of ultra-low doses of monoclonal, polyclonal or natural antibodies to an antigen, wherein said activated form is prepared by means of repeated consecutive dilution and external treatment, predominantly based on homeopathic technology, and said antigen is a substance or a drug acting as a direct cause of the pathological syndrome or involved in regulation of mechanisms of its formation. At that, activated forms of ultra-low doses of antibodies are raised against antigens of exogenous or endogenous origin, against autologous antigens, fetal antigens; anti-idiotypic antibodies are used too.
Owner:EPSHTEIN OLEG I
Anti-idiotypic antibody and its use in diagnosis and therapy in HIV-related disease
The present invention provides an anti-idiotypic antibody having specific reactivity with an idiotope common to more than one type of anti-HIV-1 antibody, and having no specific reactivity with non-HIV-1 antibodies. The present invention provides methods of diagnosis, monitoring and treatment of HIV-related diseases through the use of this antibody or related compounds.
Owner:RAPID MEDICAL DIAGNOSTICS CORP
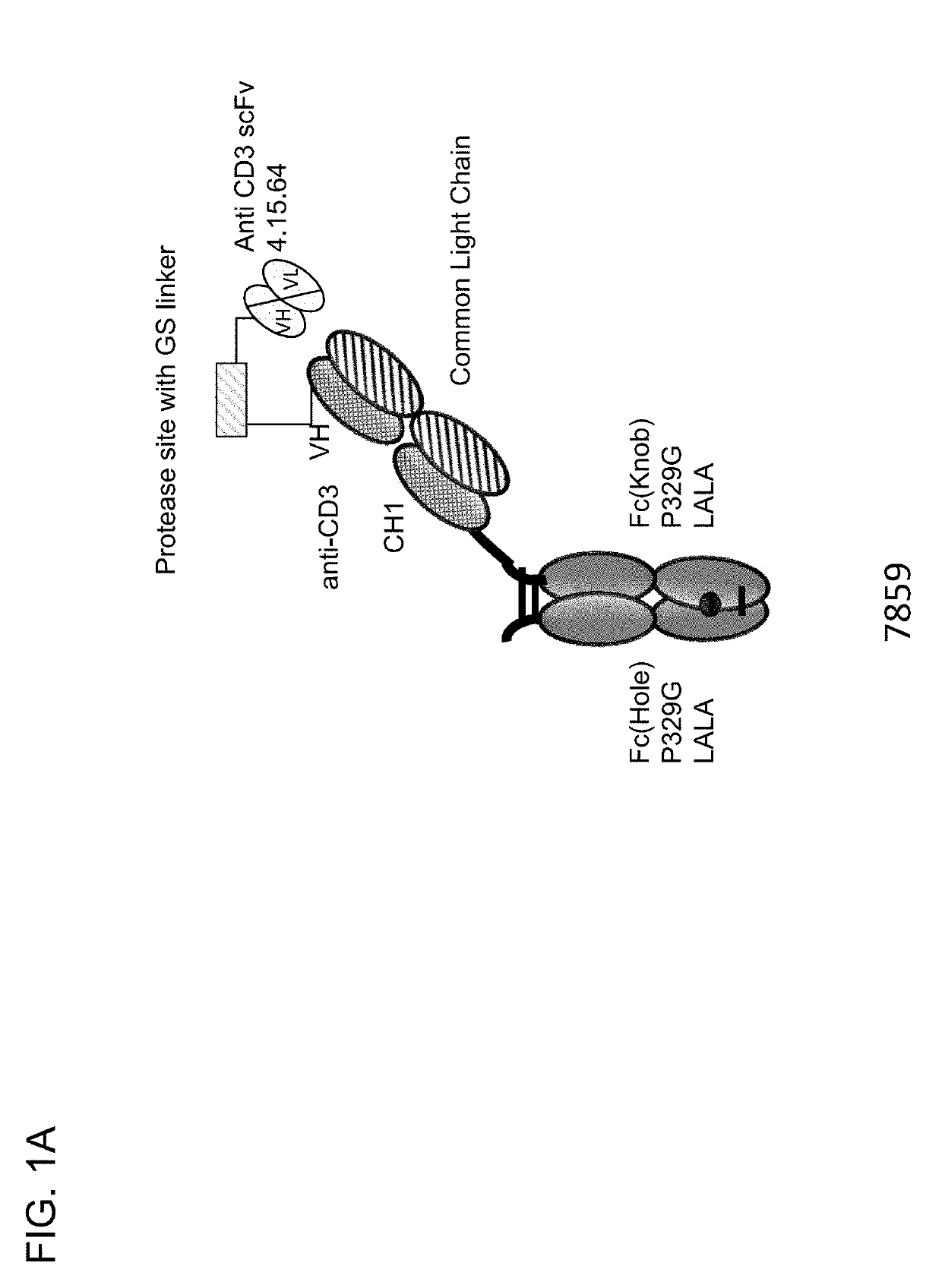
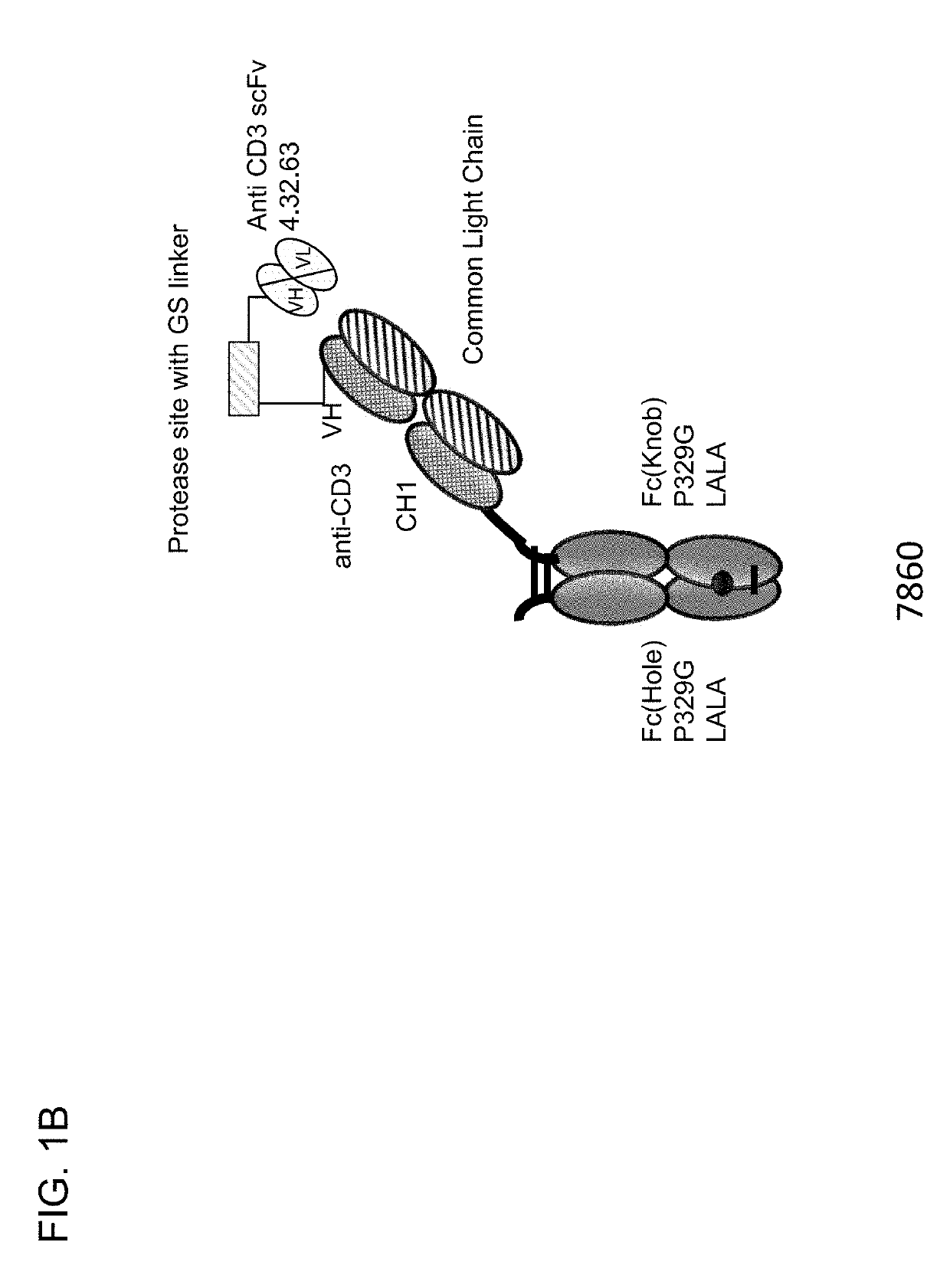
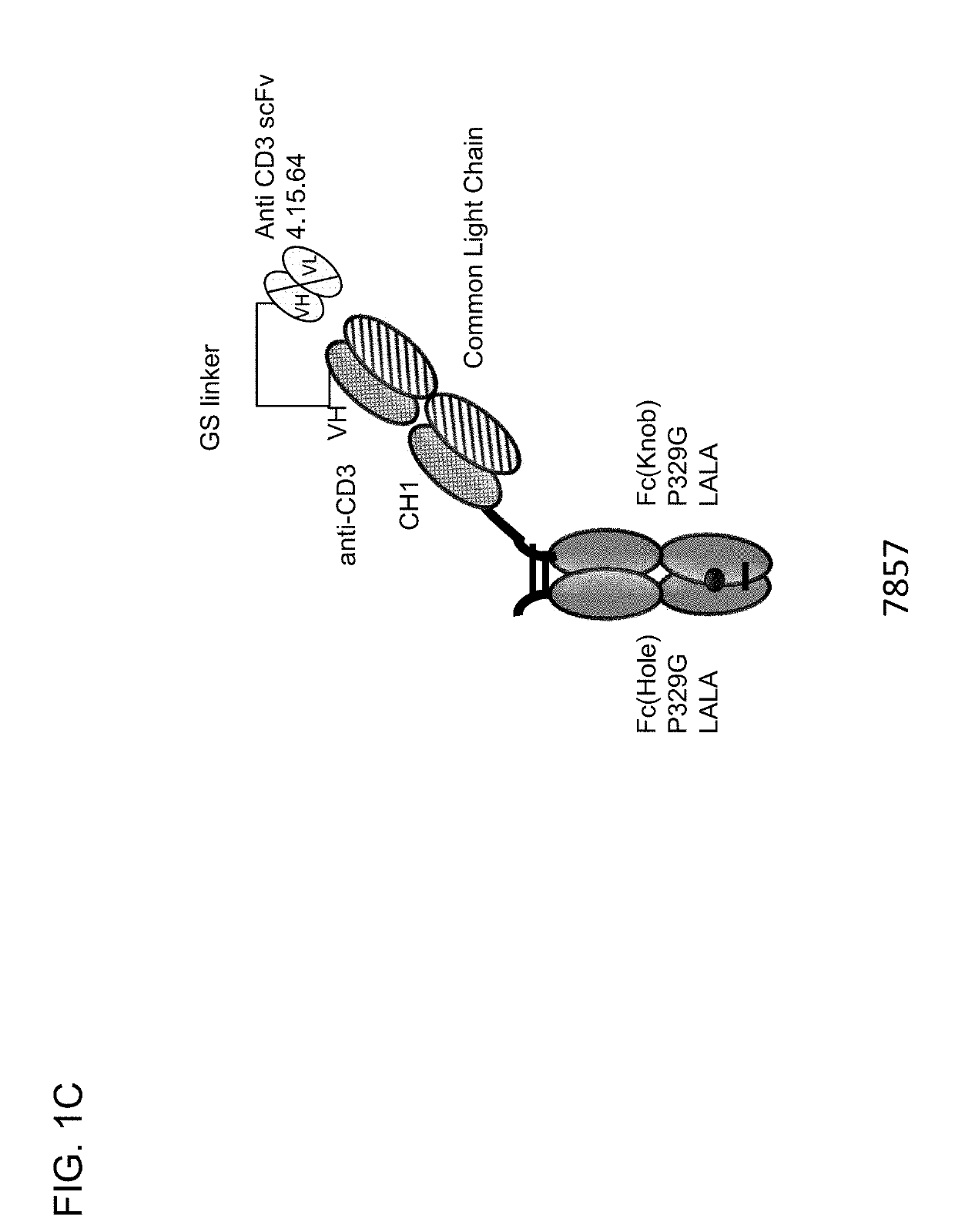
Protease-activated t cell bispecific molecules
ActiveUS20190119383A1Low toxicityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsActivation cellsT cell
The present invention generally relates to novel protease-activatable T cell activating bispecific molecules and idiotype-specific polypeptides. The present invention also relates to polynucleotides encoding such protease-activatable T cell activating bispecific molecules and idiotype-specific polypeptides, and vectors and host cells comprising such polynucleotides. The invention further relates to methods for producing the protease-activatable T cell activating bispecific molecules and idiotype-specific polypeptides of the invention, and to methods of using these protease-activatable T cell activating bispecific molecules and idiotype-specific polypeptides in the treatment of disease.
Owner:F HOFFMANN LA ROCHE & CO AG
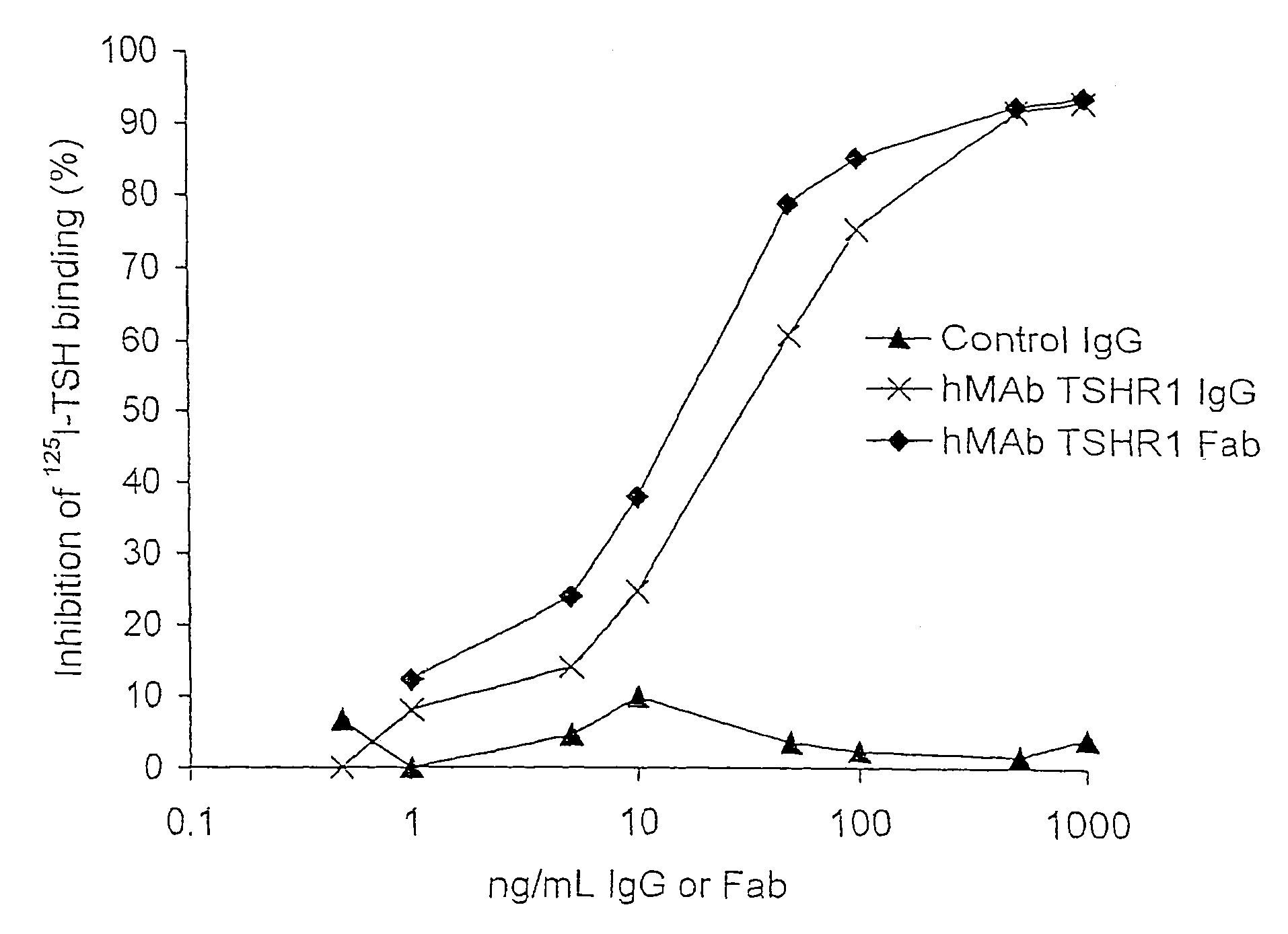
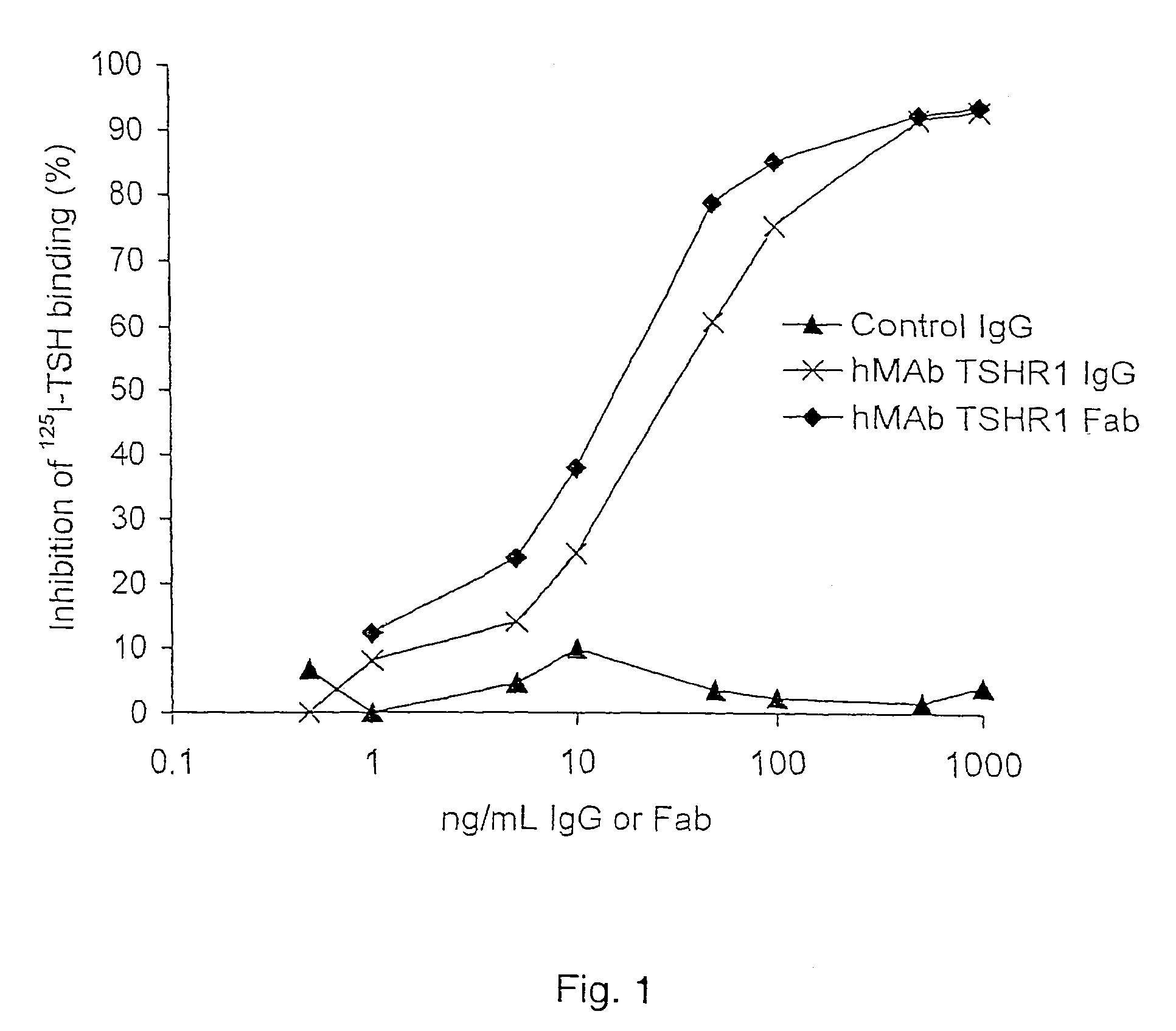
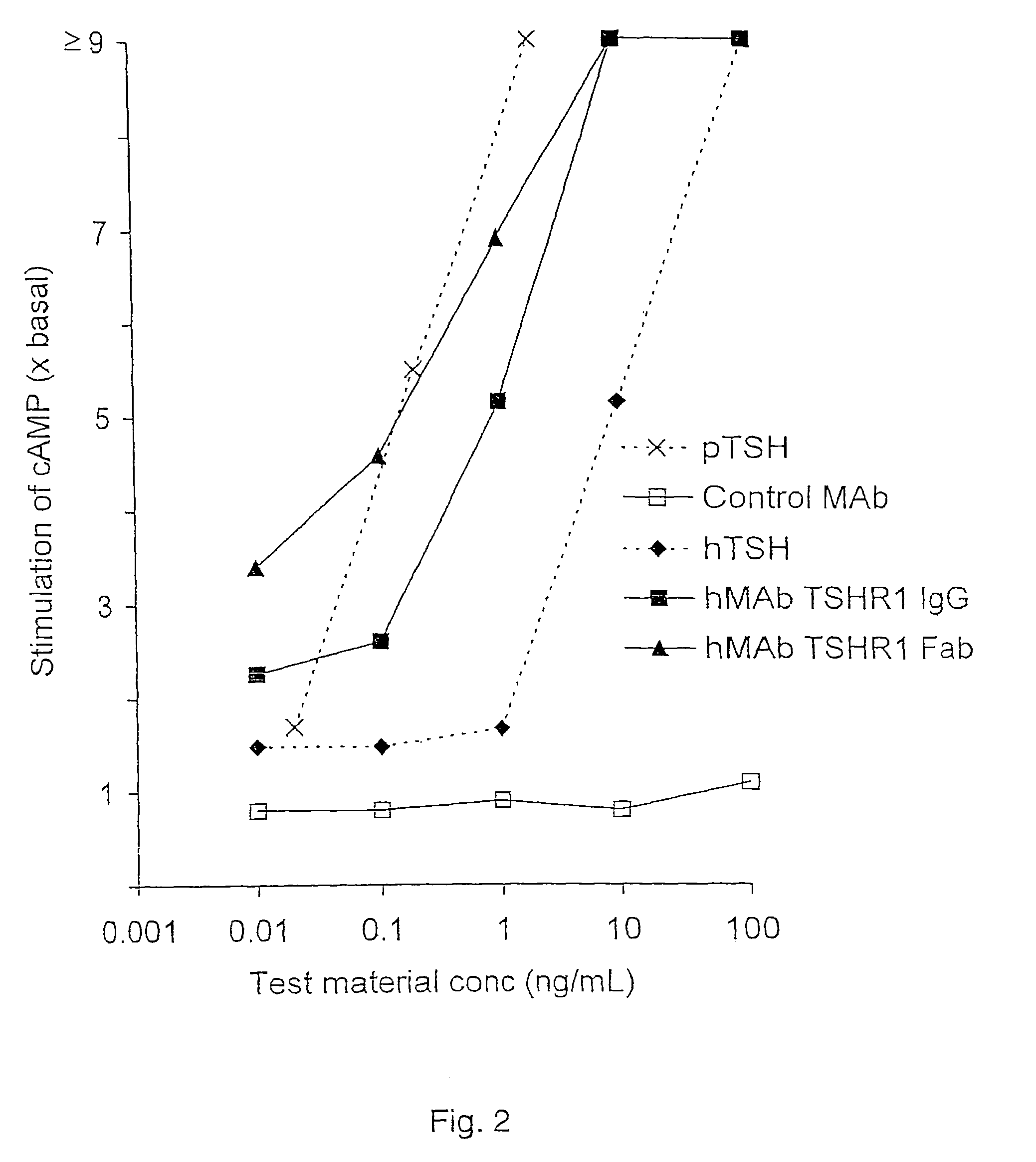
Binding partners for the thyrotropin receptor and uses thereof
ActiveUS8110664B2Prolong lifeSenses disorderPeptide/protein ingredientsAnti-idiotypic antibodiesThyrotropin receptor
A binding partner for the TSH receptor, which binding partner comprises, or is derived from, a human monoclonal or recombinant antibody, or one or more fragments thereof, reactive with the TSH receptor, uses thereof, methods of diagnosis and therapy employing the same, and anti-idiotypic antibodies thereto.
Owner:R S R
Anti-idiotypic antibody neutralizing the inhibitor activity of a factor viii inhibitor antibody
InactiveUS20070065425A1Reduce antibody immunogenicityImprove efficiencyAnimal cellsSugar derivativesFactor VIII inhibitorAnti-idiotypic antibodies
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the domain A2 of Factor VIII, and to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as drug, and more particularly, to its use for the manufacturing of a drug to be used for the treatment of haemophilia A.
Owner:LFB BIOTECH
Assay for Antibodies
InactiveUS20080176257A9Reduce non-specific sticking and backgroundImprove throughputAnimal cellsBiological material analysisHuman patientElisa method
The presence and quantity of an antibody of interest in a patient's bloodstream or other biological sample can serve as an important clinical or other analytical or diagnostic tool. ELISA methods, and kits for such assays, as well as anti-idiotypic antibodies and hybridomas producing them, are developed to detect levels of the antibody in biological samples, which are from, for example, animal models and human patients.
Owner:GENENTECH INC
Envelope protein VP28 idiotype monoclonal antibody against shrimp white spot syndrome virus (WSSV) and preparation method thereof
InactiveCN101691403APrevention and Control of Shrimp WSSV DiseaseImmunoglobulins against virusesAntiviralsAntigenBinding site
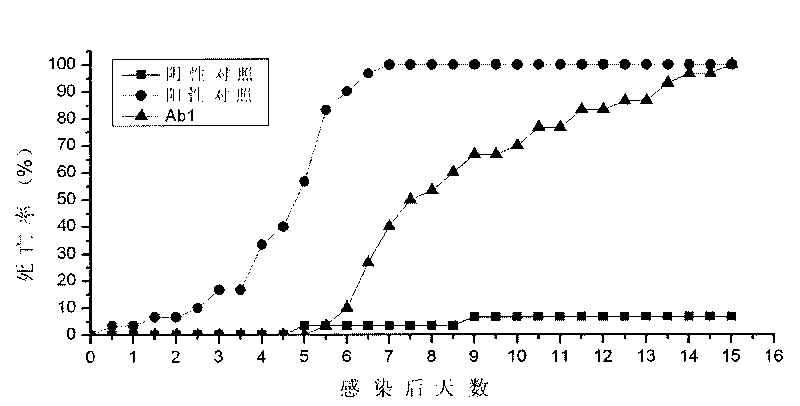
The invention discloses an envelope protein VP28 idiotype monoclonal antibody against shrimp white spot syndrome virus (WSSV) and a preparation method thereof. The antibody is secreted by a hybridoma cells with the collection number of CCTCC-CT200938, is prepared by taking anti-WSSV-VP28 monoclonal antibody (Ab1) as antigen, can bind with anti-WSSV-VP28 antibody of hare, and has the capability of competing with WSSV to bind with the anti-WSSV-VP28 antibody of the hare. The anti-WSSV-VP28 idiotype monoclonal antibody (Ab3) prepared by taking the antibody as antigen can bind with the WSSV, the binding site of the anti-WSSV-VP28 idiotype monoclonal antibody (Ab3) and the WSSV is located on an envelope, and the Ab3 can neutralize WSSV infection and has Ab1 properties. In the invention, the idiotype antibody is applied in the research of WSSV for the first time; a screening system is established, which uses an indirect enzyme-linked immunosorbent assay (ELISA) method and a competitive enzyme-linked immunosorbent assay (ELISA) method for detection; the fact that Ab3 has properties of Ab1 is proved by adopting an indirect immnnofluotesent method (IIF), a gold labeling immunoelectron microscopic method and crayfish in vivo neutralization tests, thus proving that the monoclonal antibody in the invention has the property to simulate original antigen WSSV-VP28.
Owner:OCEAN UNIV OF CHINA
Toxin-related antibodies with antimicrobial and antiviral activity
InactiveUS7722876B2Improve the immunityStable maintenanceAntibacterial agentsAntimycoticsMicroorganismYeast
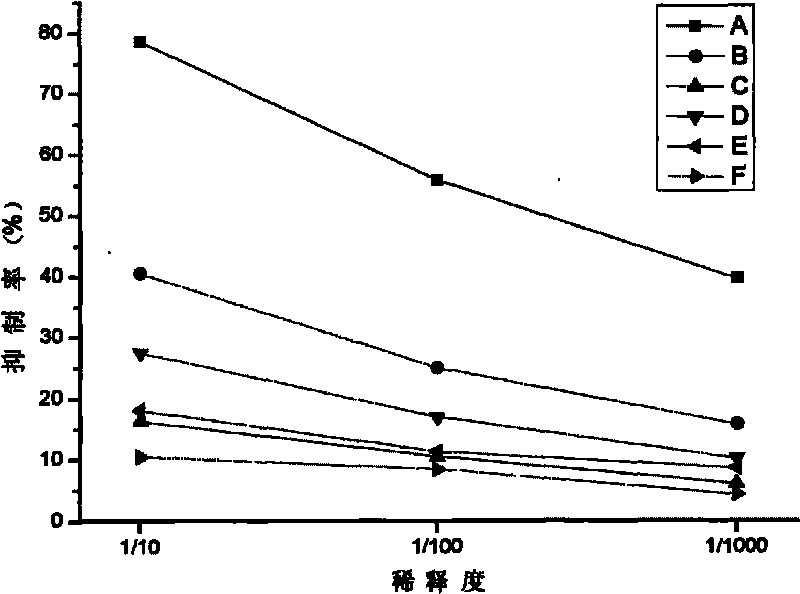
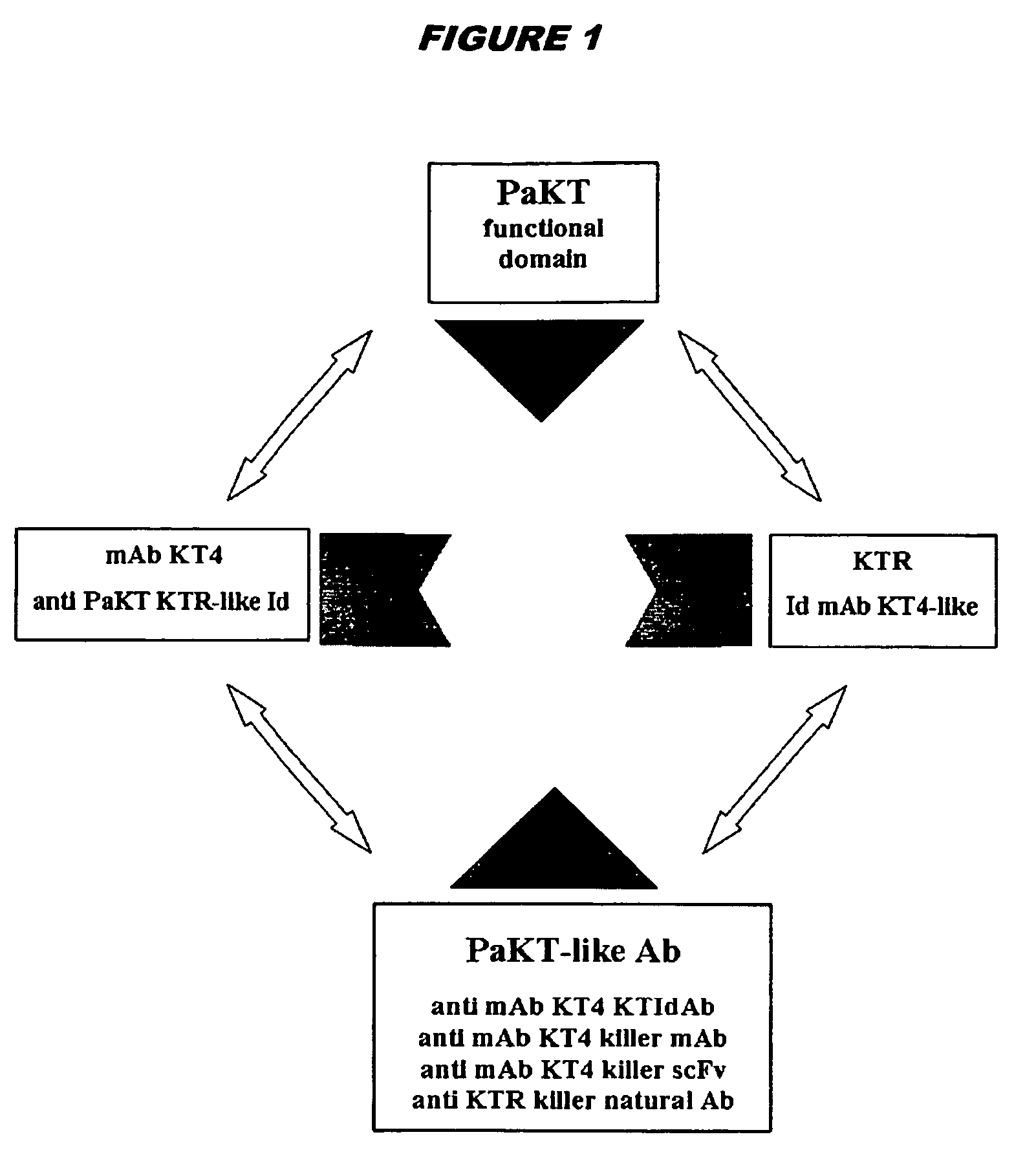
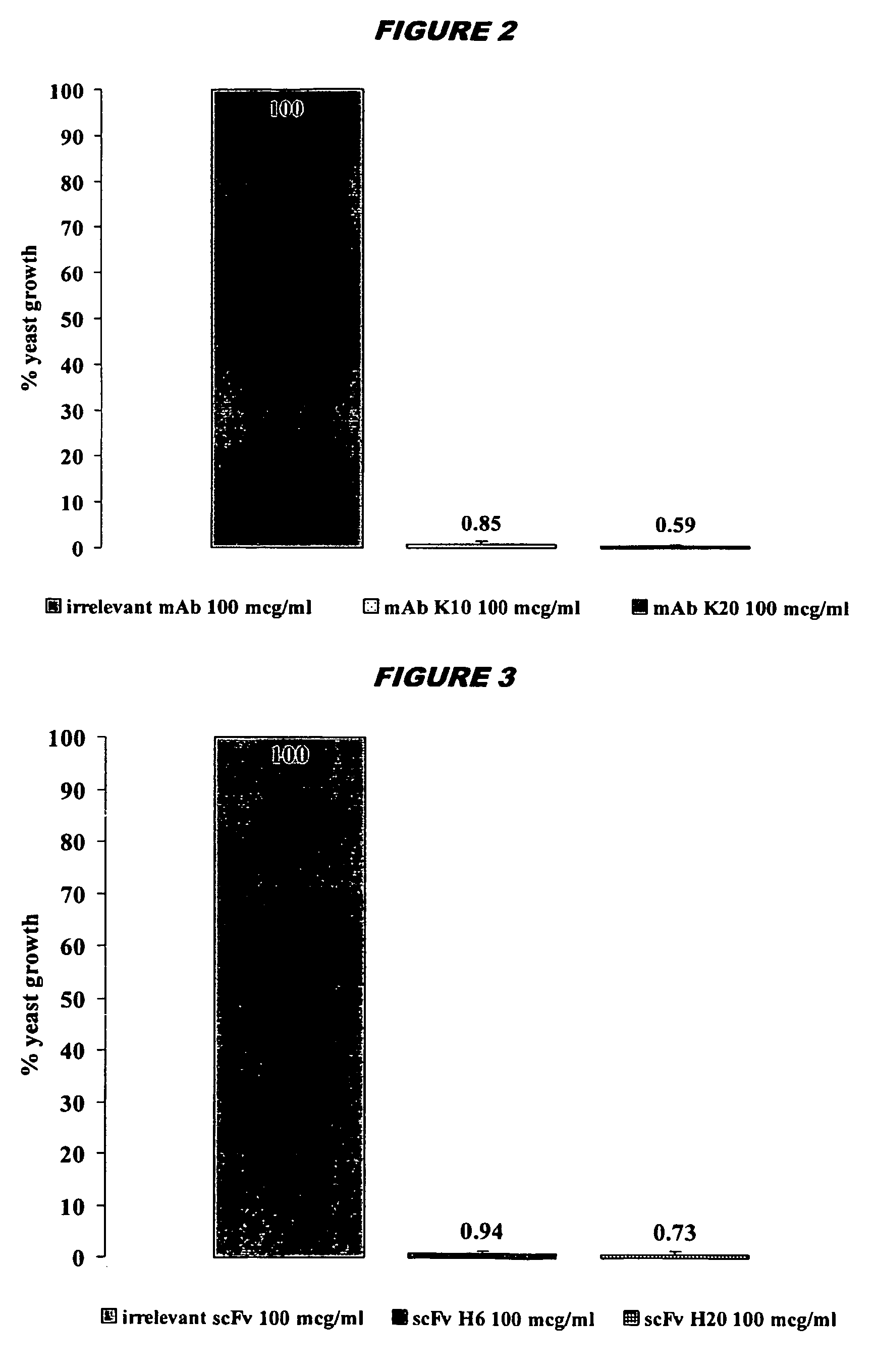
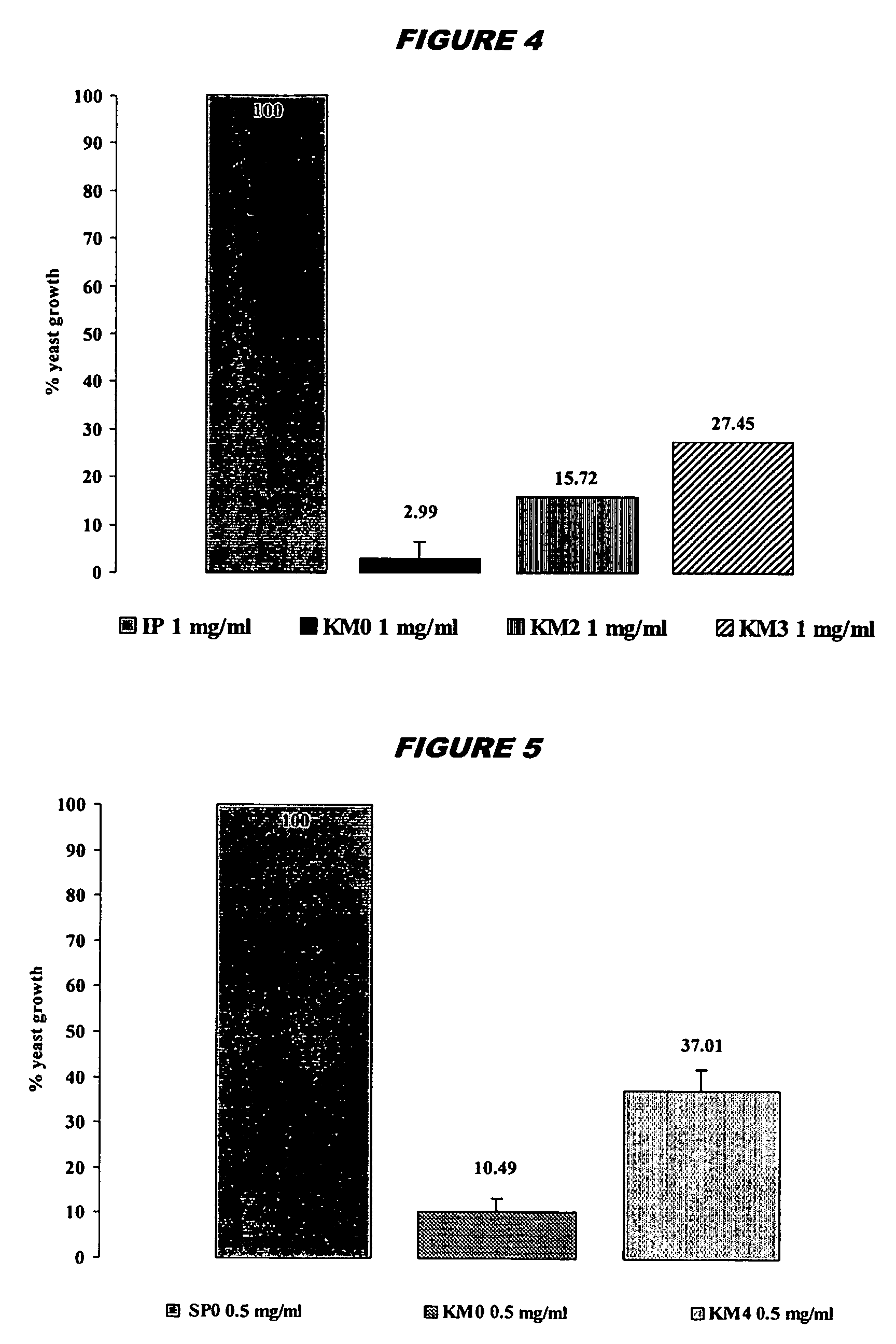
Anti-idiotypic antibodies which recognise the idiotope of an antibody specific for a yeast killer toxin possess microbicidal activity. Fragments (e.g. decapeptides) of these anti-idiotypic antibodies, particularly those comprising CDR residues, also show microbicidal activity, as do peptides having 5 the same sequence but composed of D-amino acids, or including amino acid substitutions. Peptidomimetics of these microbicidal polypeptides are also provided. Antiviral activity is also seen.
Owner:INST SUPERIORE DI SANITA +2
Combination therapy and antibody panels
InactiveUS20070134248A1High sequence similarityAntibody ingredientsImmunoglobulinsSurface ImmunoglobulinImmunogenicity
The present invention provides combination immunotherapy for Non-Hodgkin's Lymphoma. In one embodiment, the combination immunotherapy first provides for the administration of a monoclonal antibody directed to a non-idotypic portion of a lymphoma cell surface immunoglobulin (e.g. a framework region of a variable region). The combination immunotherapy next provides for the administration of an immunogenic composition comprising at least a portion of the same lymphoma cell surface immunoglobulin, whether an idiotypic portion or non-idiotypic portion.
Owner:GENITOPE CORP
Human-derived insecticidal protein and preparation method and application thereof
ActiveCN104926940AAccurate Simulation CalculationsAvoid blindnessBiocideImmunoglobulins against bacteriaSingle-Chain AntibodiesNucleotide
The invention discloses human-derived insecticidal protein. The coded nucleotide sequence of the human-derived insecticidal protein is as shown in SEQ ID NO.5, and the amino acid sequence of the human-derived insecticidal protein as shown in SEQ ID NO.6. Orthomutation is conducted on anti-Cry1B toxin idiotype single-chain antibody C7 to form a saturation mutagenesis library, then the saturation mutagenesis library is screened through a phage display technology, and the human-derived insecticidal protein is obtained; the affinity with insects BBMV of the human-derived insecticidal protein is remarkably higher than that of antibody C7 without mutation, the human-derived insecticidal protein can replace Cry1B toxin effectively to be used for biological control over pests, the human-derived insecticidal protein and C7 belong to a humanized antibody, and the human-derived insecticidal protein does less harm to the human body when used as biological pesticide.
Owner:JIANGSU ACAD OF AGRI SCI
Immune responses to fusion proteins
InactiveUS6936464B1Improve aspectEnhance vaccine efficacyPolypeptide with localisation/targeting motifAntibody mimetics/scaffoldsFusion Protein ExpressionIn vivo
The invention relates to a nucleic acid construct for delivery into living cells in vivo for inducing an immune response in a patient to an idiotypic determinant present on a malignant B cell in the patient; the construct directing the expression of a fusion protein, said fusion protein comprising the idiotypic determinant and at least one T helper cell epitope from tetanus toxin. The invention further relates to a method of making the nucleic acid construct, a method of treating a patient, and to a composition comprising the nucleic acid construct.
Owner:CANCER RES TECH LTD
Idiotypic vaccine
InactiveUS20060159693A1Immunoglobulins against cell receptors/antigens/surface-determinantsCancer antigen ingredientsMedicinePeptide
The present invention relates to idiotypic vaccine compositions for use in inducing immunity to p53. The invention preferably relates to a vaccine composition comprising a pharmaceutically acceptable carrier and at least one peptide, wherein the at least one peptide is selected from the group consisting of X1-LLQALKH-Y1, X2-FIRKAYGAATAYAASKKG-Y2 and X3-MQGLQTPYT-Y3 in which X1, X2, X3, Y1, Y2 and Y3 are independently either absent or an amino acid sequence of preferably less than 10 amino acids which provides a framework for the specified peptide.
Owner:WARD ROBYN
Prostatic cancer vaccine
InactiveUS20060024316A1Eliminate the problemAntibody ingredientsCancer antigen ingredientsAntigenBULK ACTIVE INGREDIENT
Vaccines capable of eliciting an immune antitumor response for prostate tumors are disclosed. The active ingredient in such vaccines is selected from the group consisting of at least one antigen over-represented in the prostate gland or an immunologically effective portion thereof; an expression system capable of generating in situ said antigen or portion; a naked DNA encoding such antigen and portion; and an anti-idiotypic antibody or fragment thereof which mimics said antigen or portion.
Owner:SPITLER LYNN E +1
Rapid Generation of Anti-Idiotypic Antibodies
ActiveUS20130330323A1Reduces and eliminates side effectReduce adverse effectsMuscular disorderAntibody ingredientsIn vivoAnti-idiotypic antibodies
Owner:BIOGEN MA INC
Anti-idiotypic nano antibody based zearalenone green immunoassay method
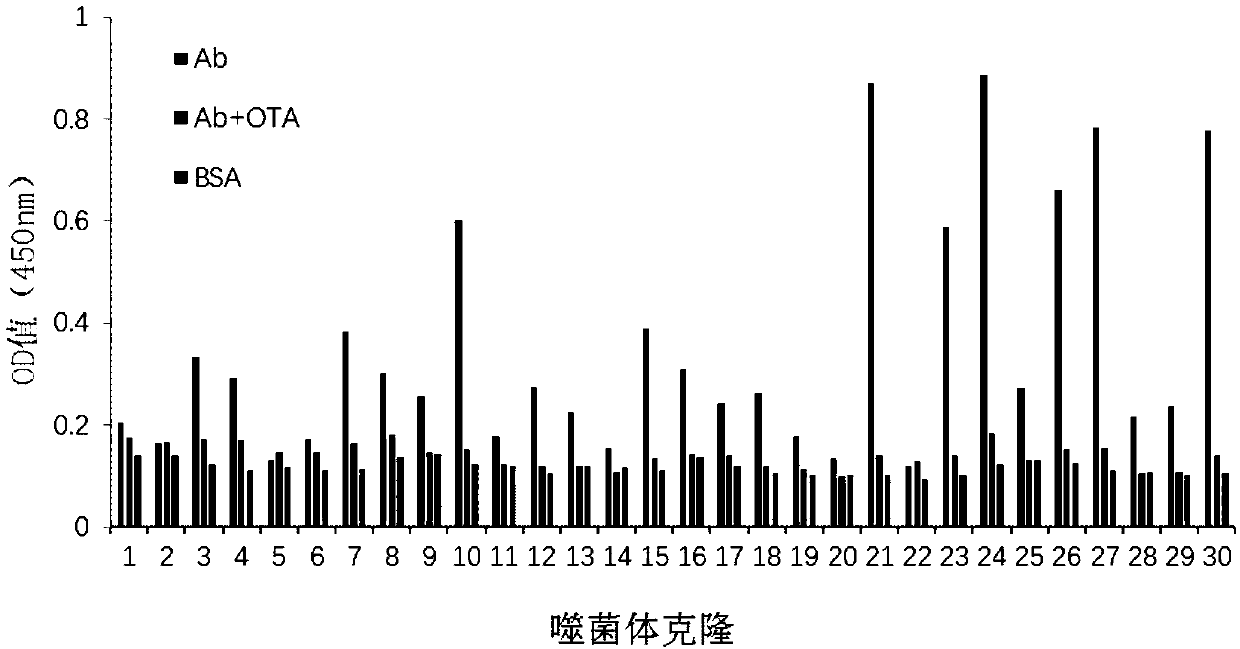
InactiveCN107602701AStrong specificityIncrease library capacityImmunoglobulinsBiological testingWhite blood cellTotal rna
Belonging to the field of molecular biology, the invention in particular relates to an anti-idiotypic nano antibody based zearalenone green immunoassay method. According to the invention, the zearalenone monoclonal antibody 2D3 is employed to immunize alpaca, and the leukocyte total RNA is extracted to construct a phage displayed nano antibody library; the 2D3 antibody is adopted as the target, four rounds of affinity enrichment panning are conducted to lower the coating concentration and competitive elution concentration round by round, and a positive phage zearalenone anti-idiotypic nano antibody with good specificity can be obtained. The invention employs the zearalenone anti-idiotypic nano antibody substitute zearalenone standard ELISA method to determine naturally polluted corn and feed samples, and performs comparison with the HPLC detection result, the two methods have good correlation, and R2 is 0.997, thus proving that the zearalenone anti-idiotypic nano antibody substitute standard method has accurate and reliable result and is an effective and feasible green immunoassay method.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Polynucleotides encoding rodent antibodies with human idiotypes and animals comprising same
ActiveCN104994729AImmunoglobulins against animals/humansVector-based foreign material introductionNucleotideMicrobiology
Owner:欧莫诺艾比公司
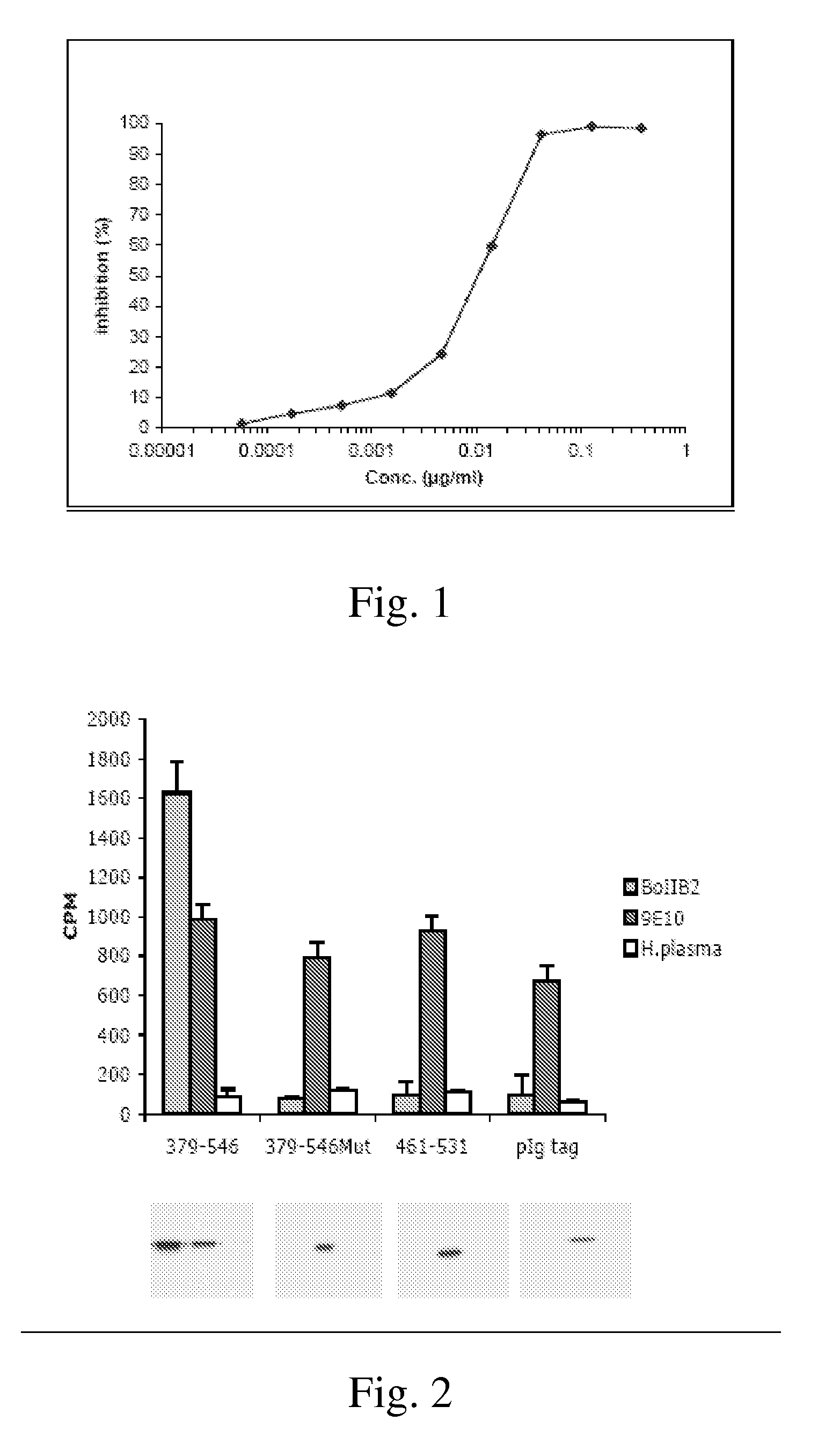
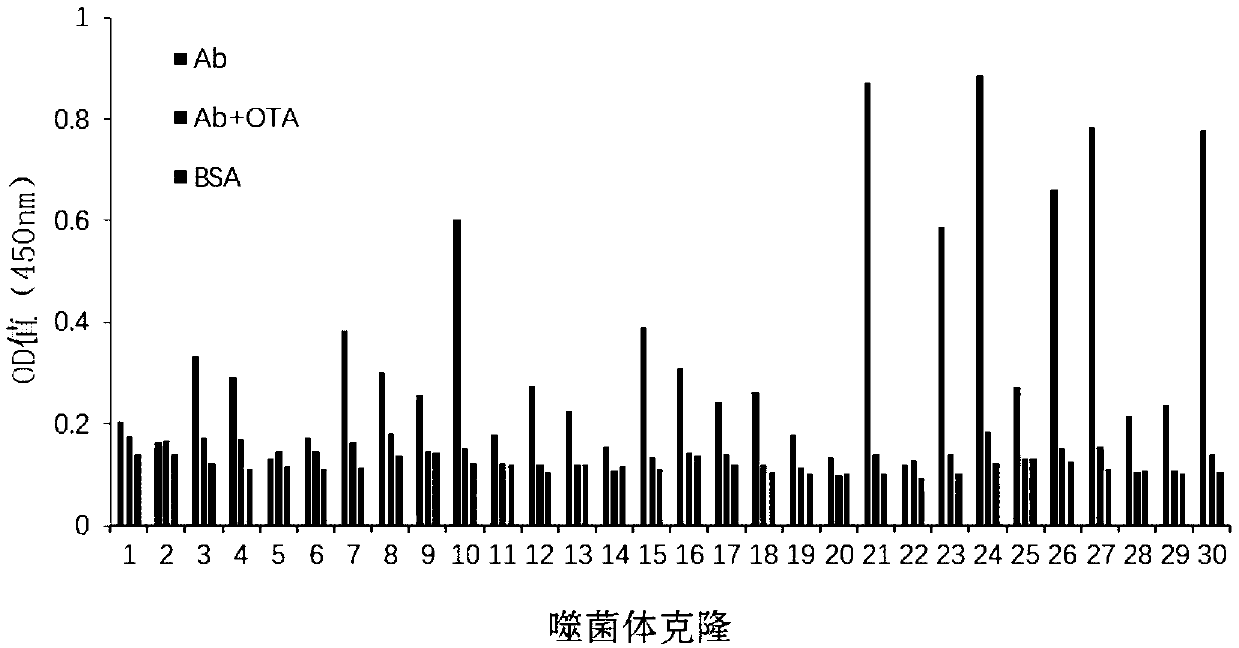
Application of ochratoxin A anti-idiotypic nano-antibody used as ochratoxin A standard substitute
ActiveCN109535256AStrong specificityImprove accuracyImmunoglobulinsGenetic engineeringOchratoxin AAnti-idiotypic antibodies
The invention belongs to the field of molecular biology, and particularly relates to an application of an ochratoxin A anti-idiotypic nano-antibody used as a ochratoxin A standard substitute. The present invention obtains the ochratoxin A anti-idiotypic nano-antibody with an amino acid sequence shown in SEQ ID NO: 1. According to ELISA standard curves of standard ochratoxin A and an ochratoxin A anti-idiotypic antibody, under the same inhibition ratio, corresponding relationship curves of the concentration of the ochratoxin A and the concentration of the ochratoxin A anti-idiotypic antibody are established. Besides, the results are subjected to exponential regression analysis to obtain a corresponding relationship between the the ochratoxin A and the ochratoxin A anti-idiotypic antibody. The corresponding relationship can be applied to the detection and analysis of ochratoxin A in agricultural products. The immunodetection method that the ochratoxin A anti-idiotypic nano-antibody replaces the standard ochratoxin A has accurate and reliable results, and is an effective and feasible method for green immunoassay.
Owner:深圳市金阅检测科技有限责任公司 +1
Anti-idiotypic antibody neutralizing the inhibitor activity of a factor VIII inhibitor antibody
InactiveUS8071094B2Reduce antibody immunogenicityImprove efficiencySugar derivativesAntibody ingredientsFactor VIII inhibitorAnti-idiotypic antibodies
The present invention is related to a monoclonal anti-idiotypic antibody directed against a Factor VIII inhibitor antibody binding to the domain A2 of Factor VIII, and to a cell line producing this monoclonal anti-idiotypic antibody, to the use of this monoclonal anti-idiotypic antibody as drug, and more particularly, to its use for the manufacturing of a drug to be used for the treatment of haemophilia A.
Owner:LFB BIOTECH
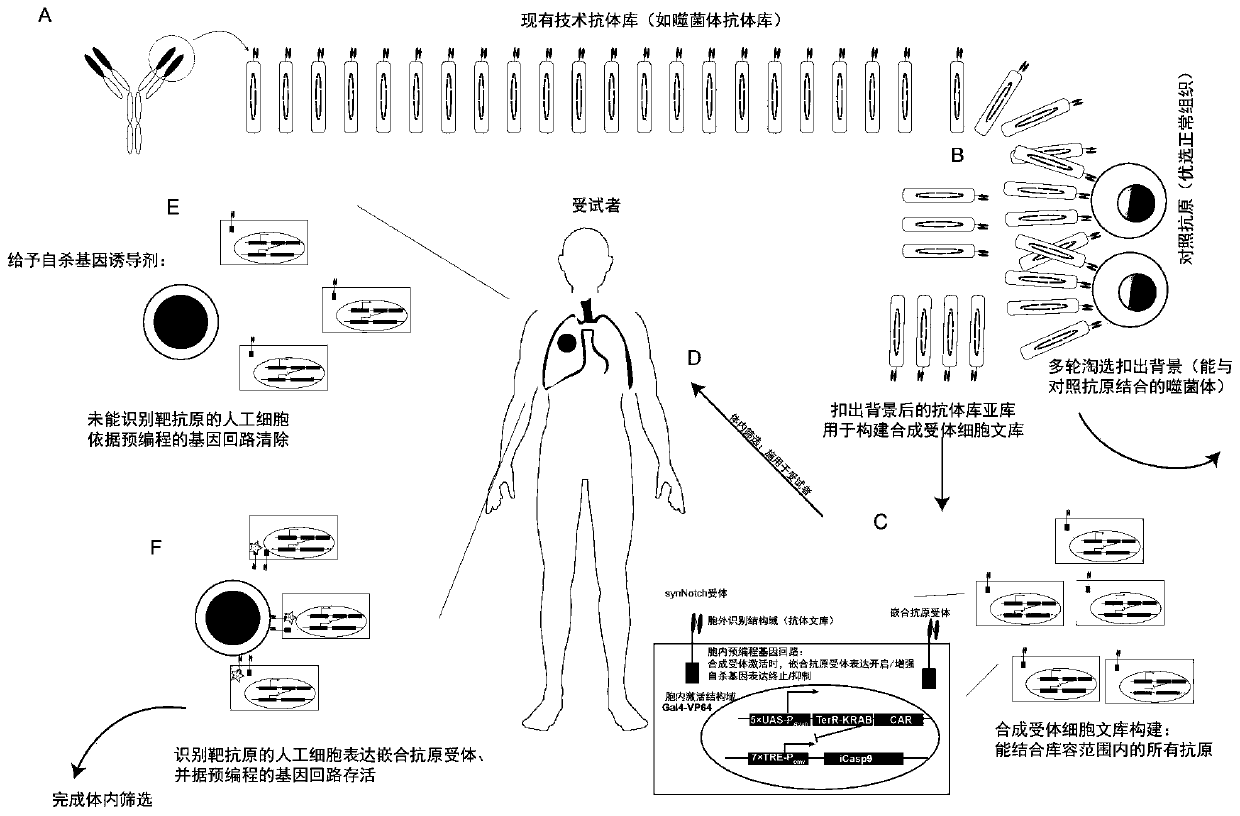
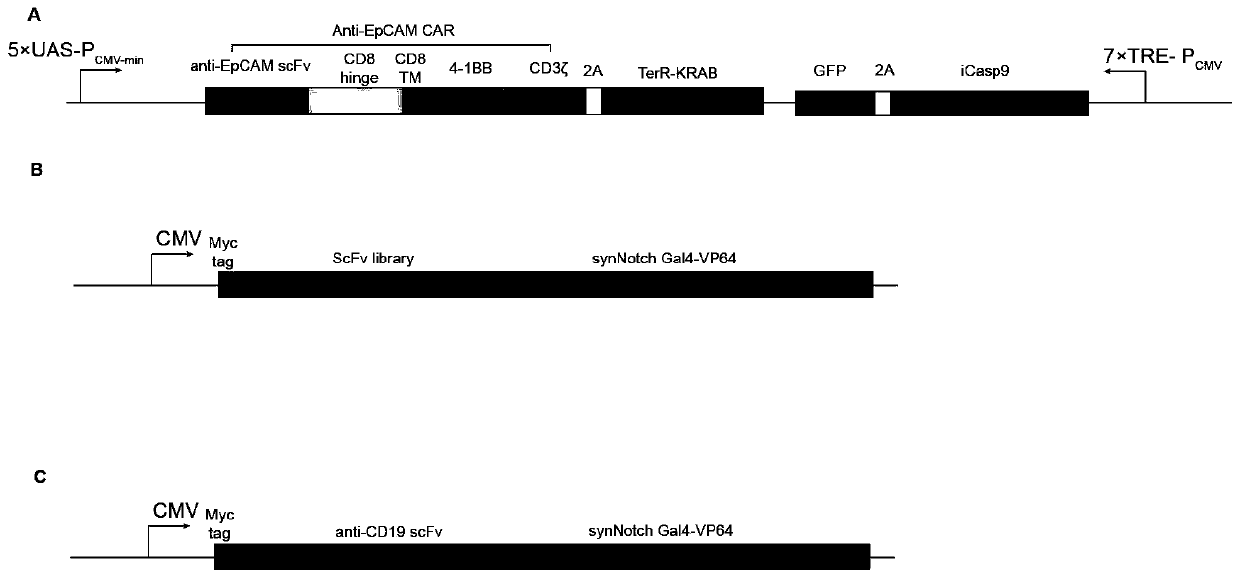
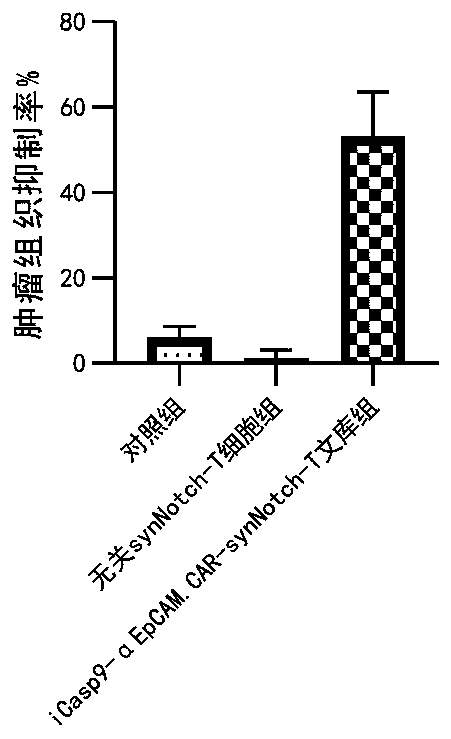
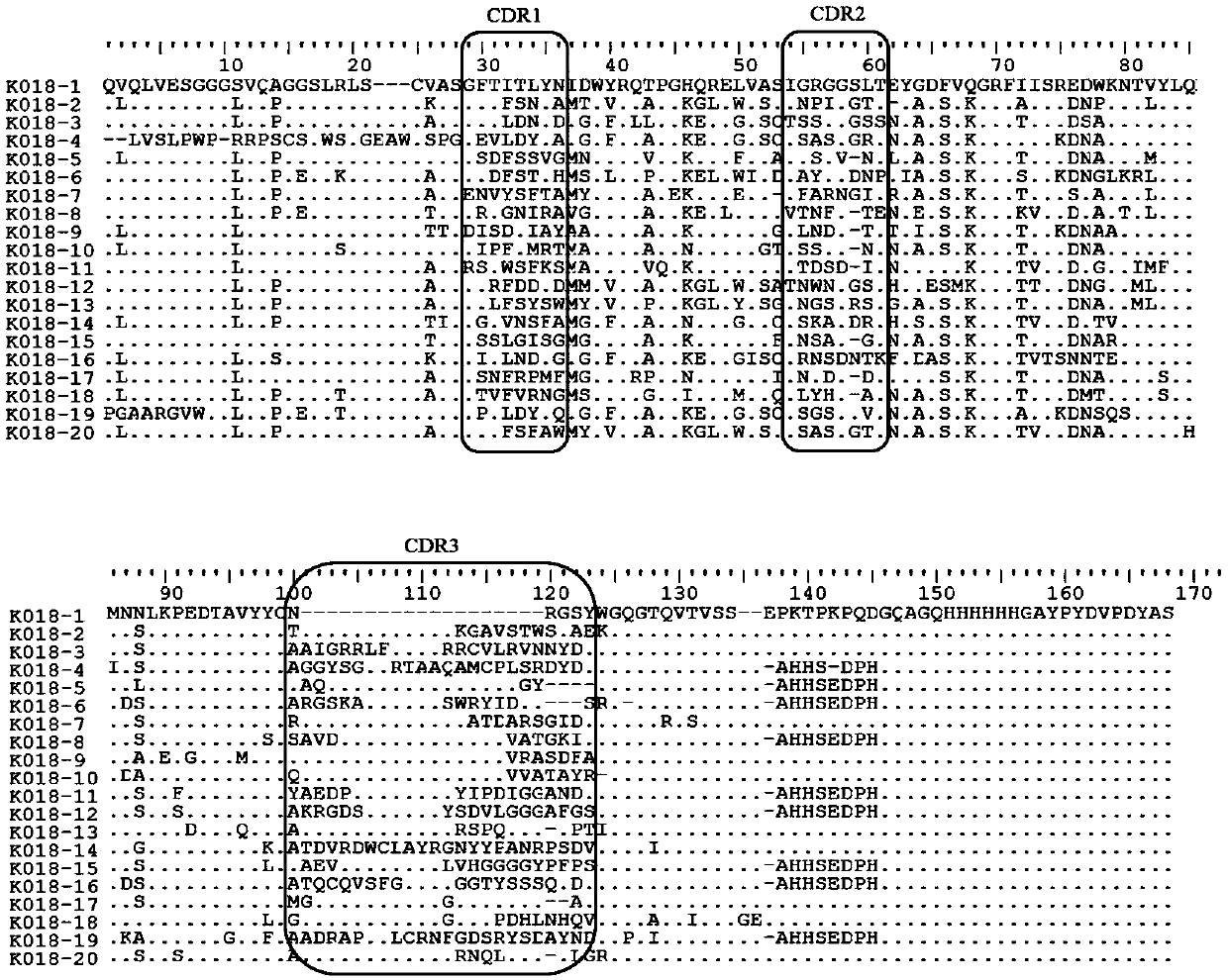
Carrier assembly carrying gene element combination, recipient cell library, preparation and screening methods and application
The invention provides a carrier assembly carrying a gene element combination, a recipient cell library, a preparation and screening method and an application, the recipient cell library is formed byfusing cells and the carrier assembly, and the carrier assembly at least carries three gene elements, respectively a plurality of first gene elements encoding one or more idiotypic synNotch receptors,respectively; a second gene element carrying one or more gene loops; a third gene element encoding one or more idiotypic chimeric antigen receptors. Wherein, when the first genetic element encodes one idiotypic synNotch receptor, the third genetic element must encode at least three idiotypic chimeric antigen receptors, and when the third genetic element encodes one idiotypic chimeric antigen receptor, the first genetic element must encode three idiotypic synNotch receptors. The gene loop is pre-programmed, a regulatory homeopathic factor is combined with a transcription factor, upon activation of the synNotch receptor encoded by the first gene element, the chimeric antigen receptor encoded by the third gene element is controllably expressed.
Owner:PHARCHOICE THERAPEUTICS INC
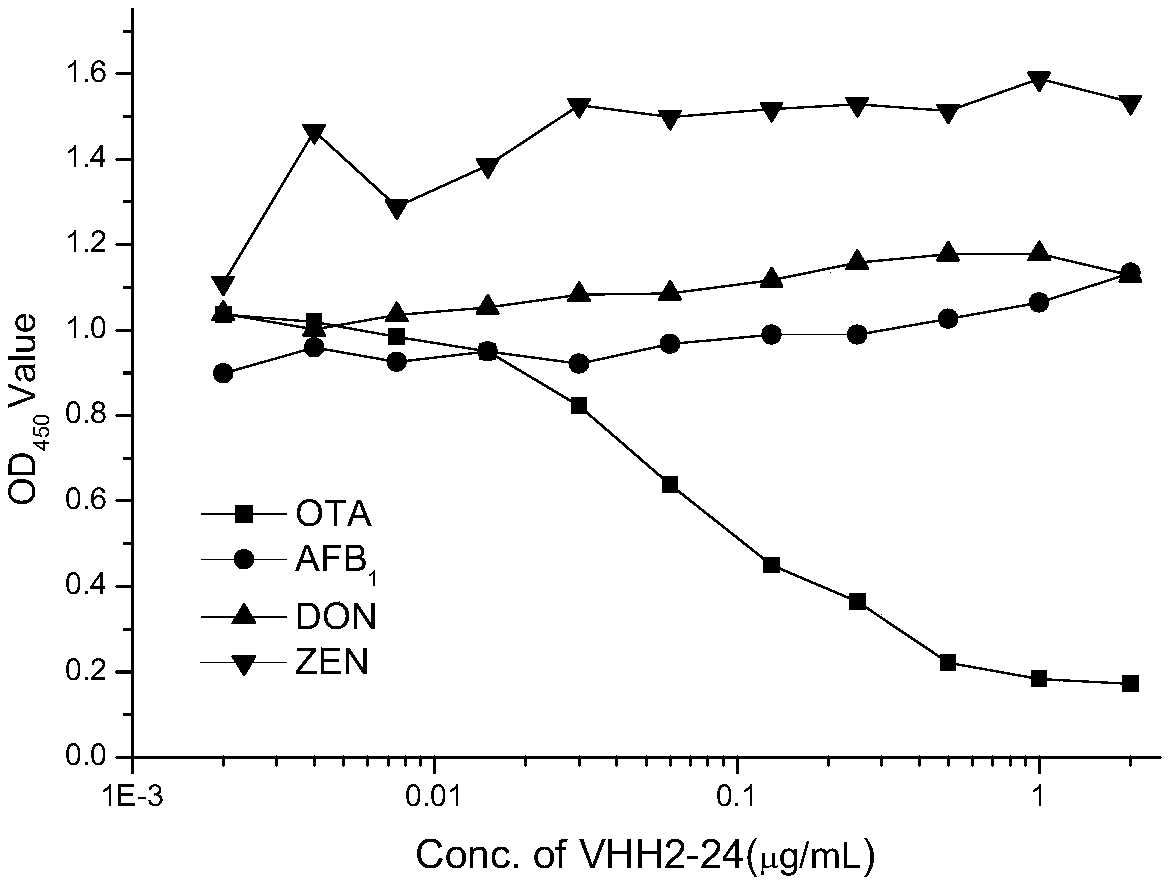
Ochratoxin A anti-idiotypic nano-antibody and preparation method thereof
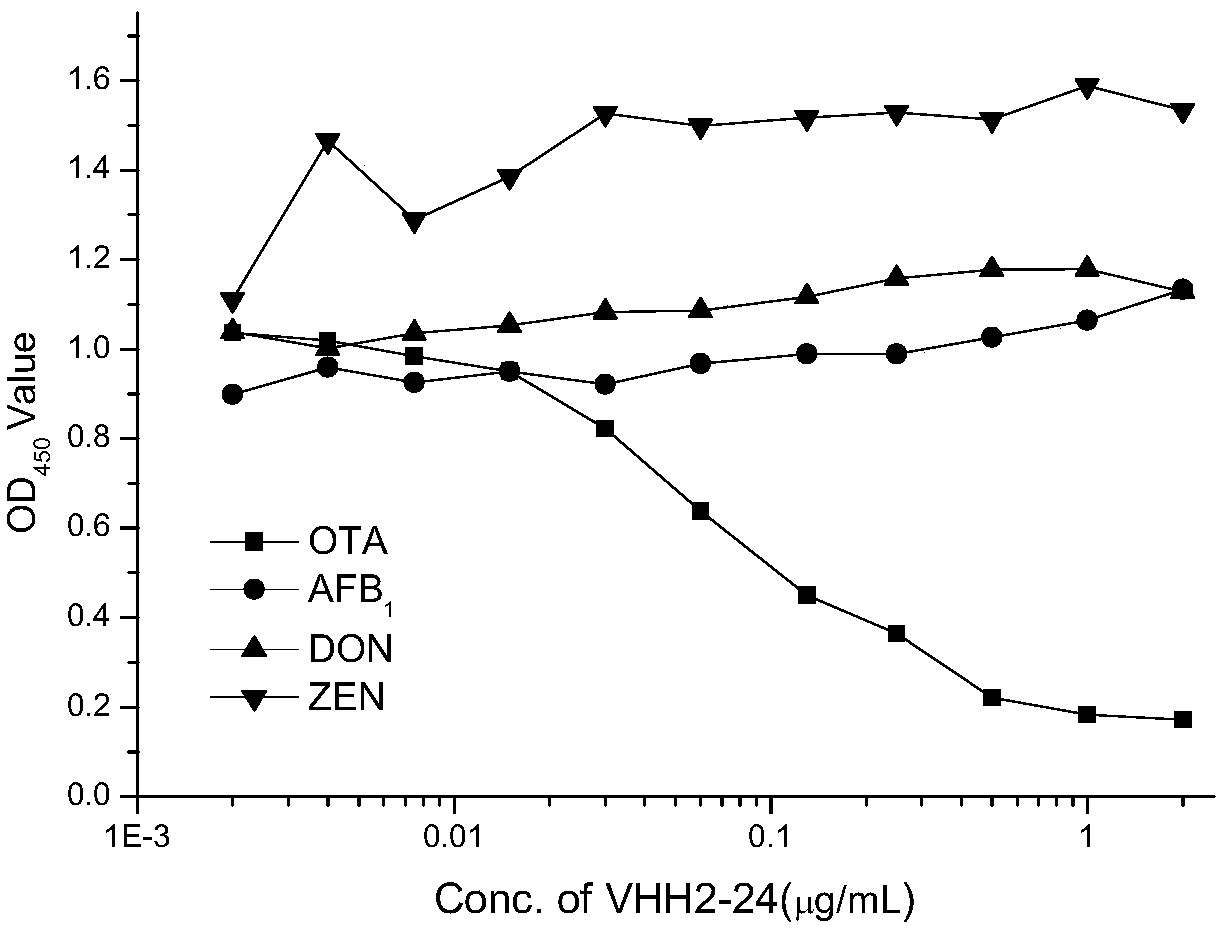
InactiveCN109535251AReduce manufacturing costStrong specificityImmunoglobulins against fungi/algae/lichensVector-based foreign material introductionWhite blood cellTotal rna
The invention belongs to the field of molecular biology, in particular to relates to an ochratoxin A anti-idiotypic nano-antibody and a preparation method thereof, wherein the amino acid sequence of ochratoxin A anti-idiotypic nano-antibody is shown in SED ID No:1. The antibody uses ochratoxin A monoclonal antibody to immunize alpaca, and constructs a bacteriophage display nanometer antibody library by extracting the total RNA of its white blood cells, and the library capacity is high and the diversity is good; taking ochratoxin A monoclonal antibody as the target, three round of affinity enrichment and eluting are used to reduce the coating concentration and competitive eluting concentration one by one, and the positive bacteriophage ochratoxin A anti-idiotypic nano-antibody gene is transformed into the expressed strain; after induction, purification and identification, the protein form nano-antibody VHH2-24. With excellent performance is obtained.
Owner:INST OF OIL CROPS RES CHINESE ACAD OF AGRI SCI
Modified antibodies of induced anti-idiotype reaction enhancement
The present invention relates to the modification of immunoglobulin molecules in which one or more cysteine residues in the variable region which form intrachain disulfide bonds are replaced by amino acids which do not contain a sulfhydryl group and thus do not form disulfide bonds. The ability of these modified immunoglobulin molecules to induce an anti-idiotypic response is enhanced. The present invention further provides a method for treating or preventing tumors and / or infectious diseases using the modified immunoglobulin of the present invention.
Owner:欧洲细胞技术有限公司
Monoclonal antibodies and their use
InactiveUS20050163768A1Immunoglobulins against virusesImmunoglobulins against cell receptors/antigens/surface-determinantsAnti-idiotypic antibodiesLung cancer
Owner:ECKERT HELMUT +4
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com