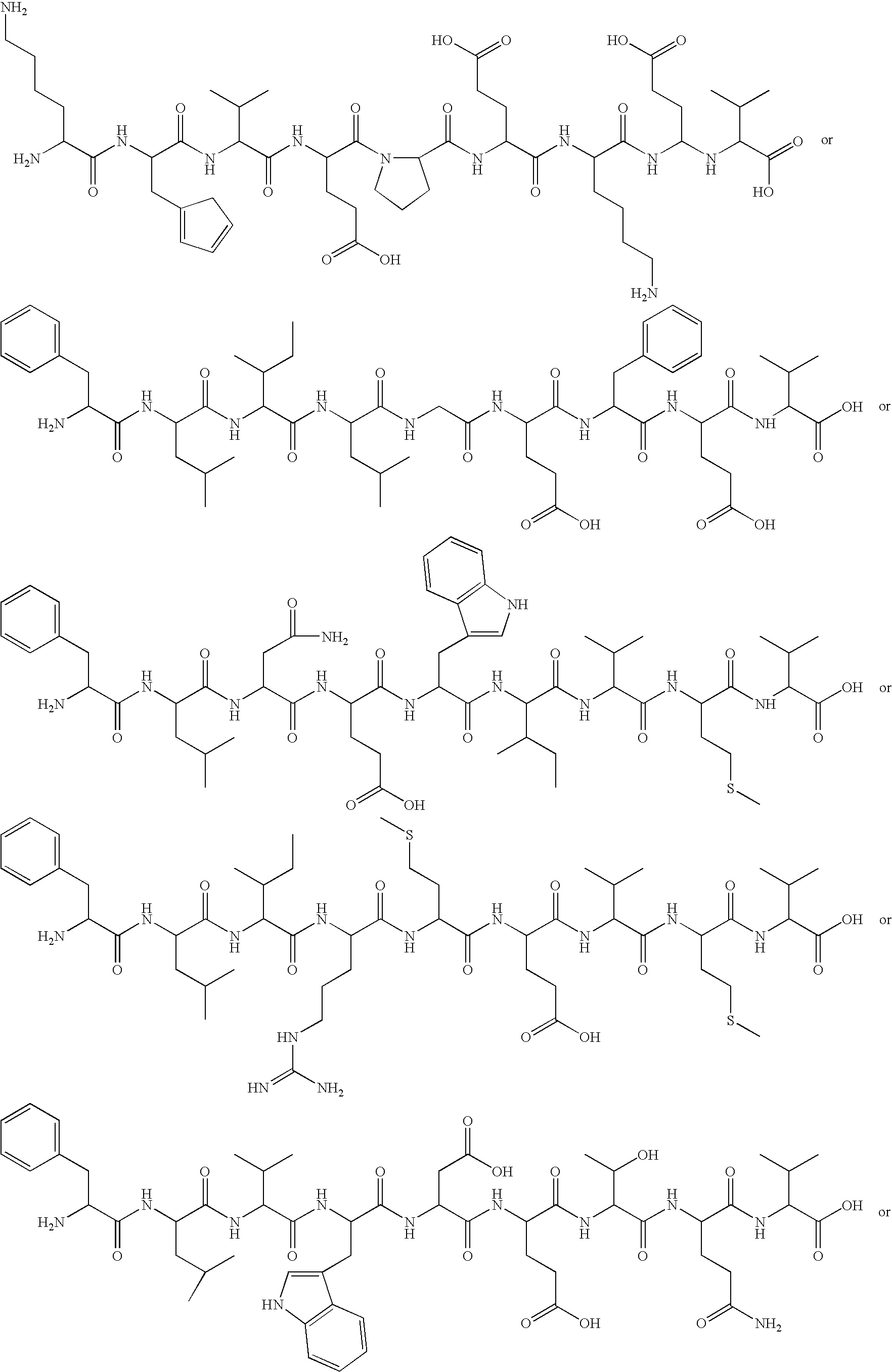
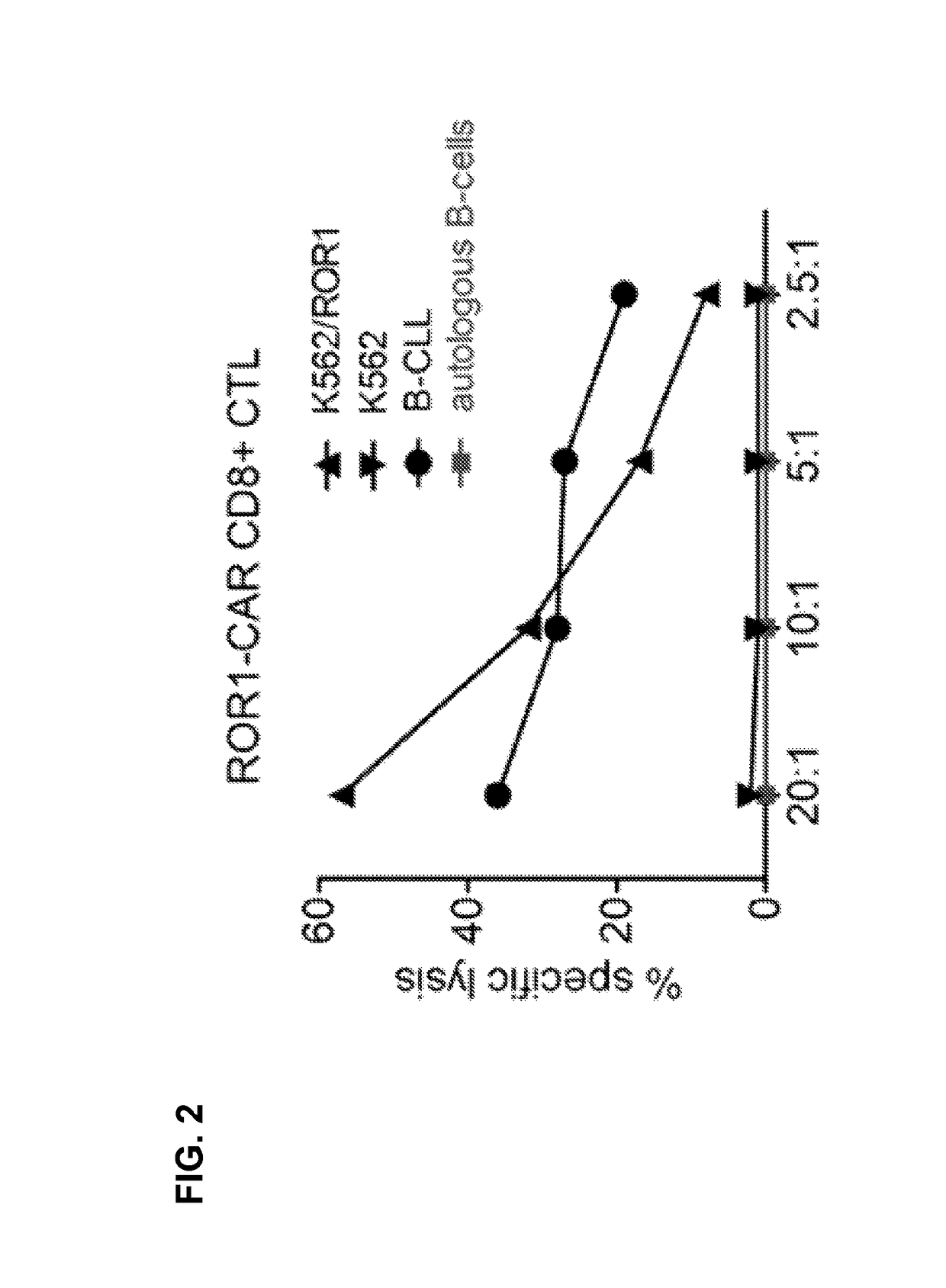
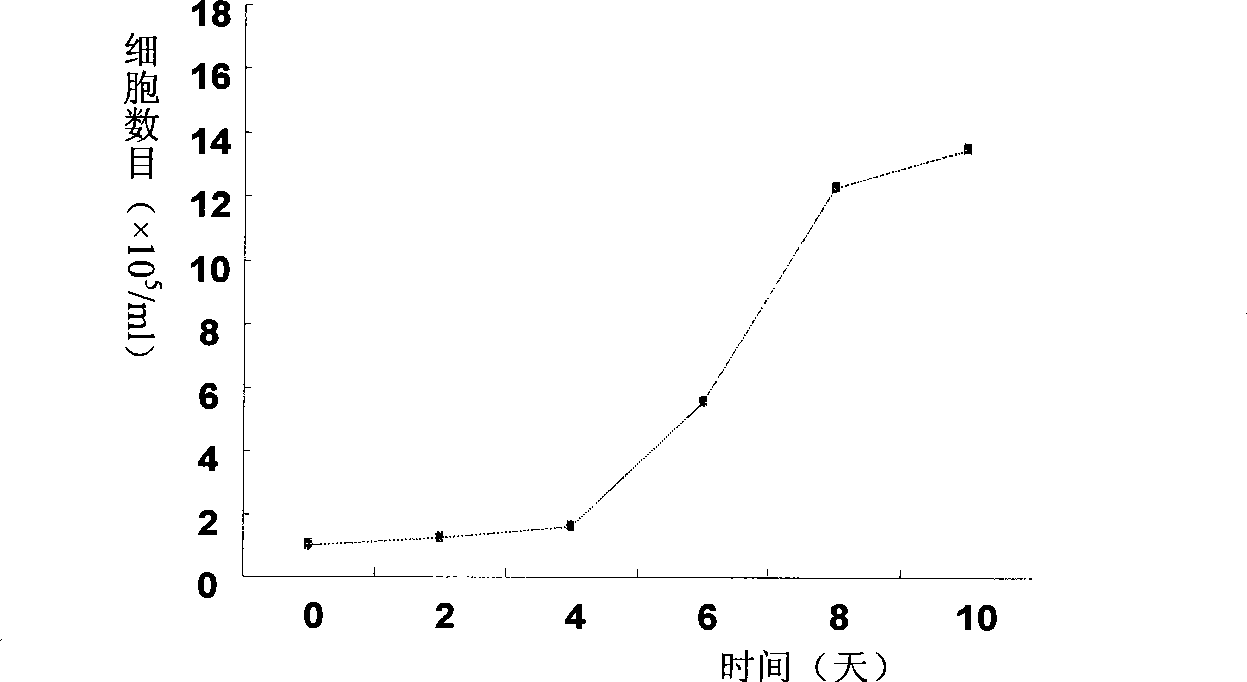
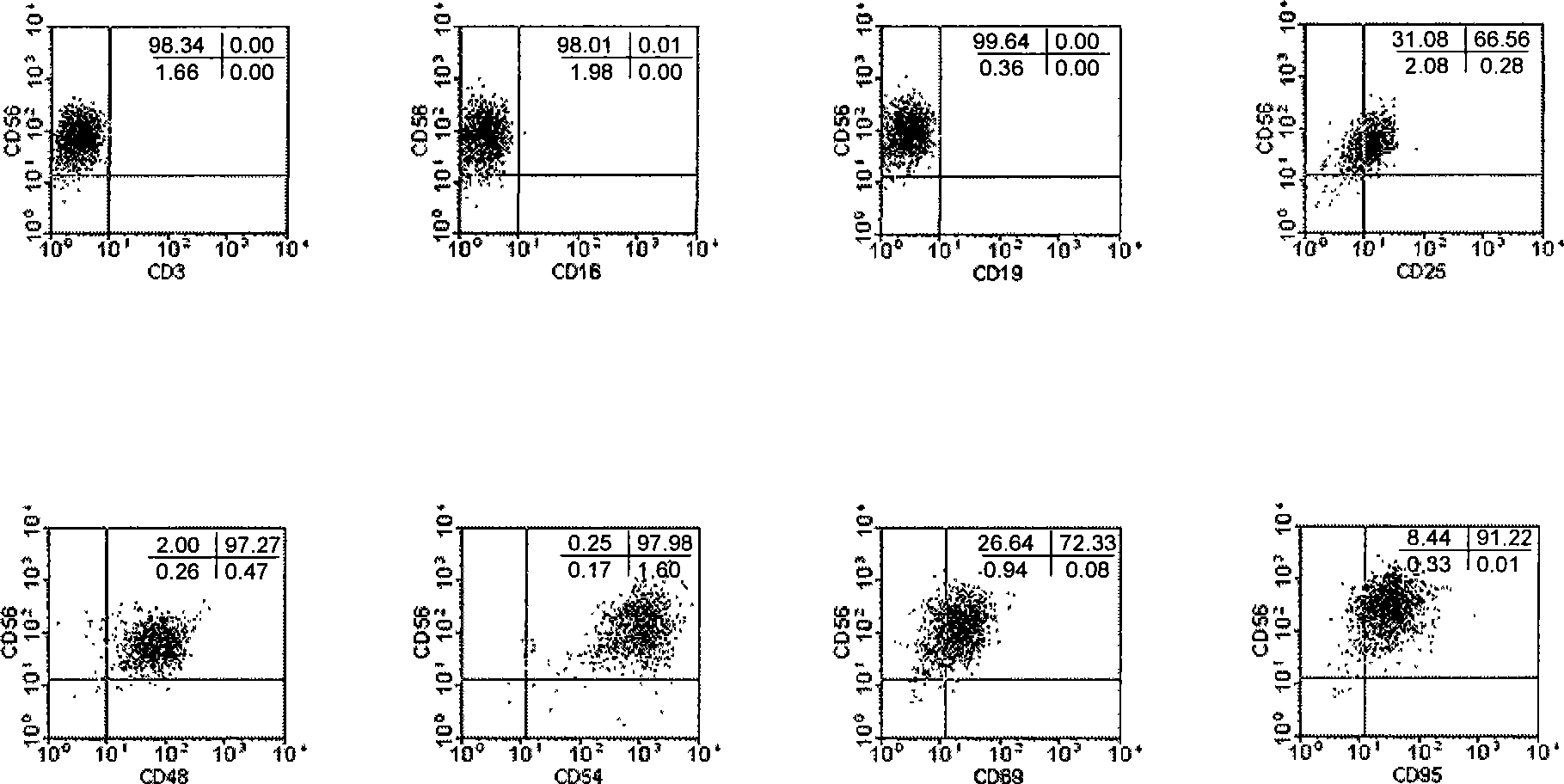
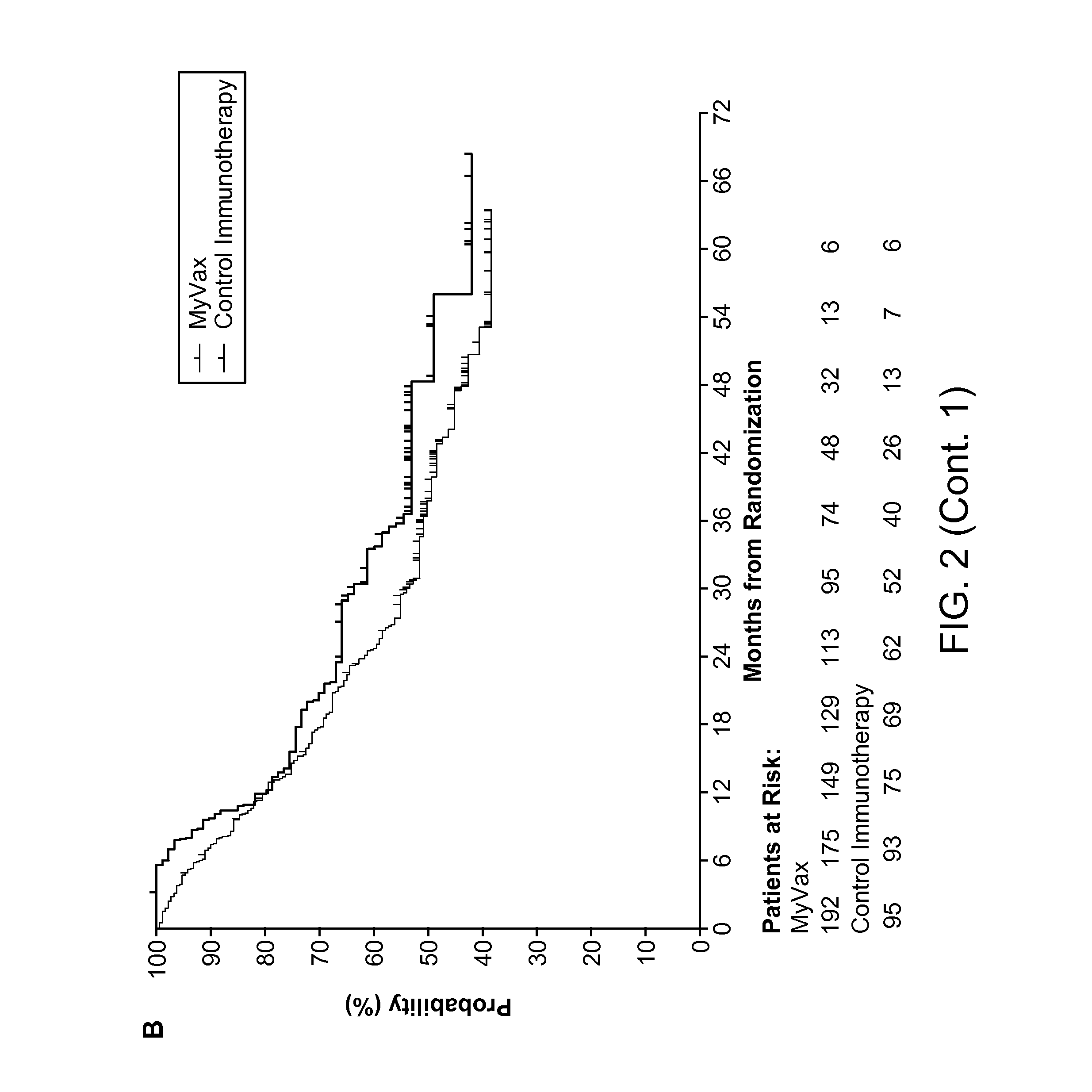
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
54 results about "Antitumor response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
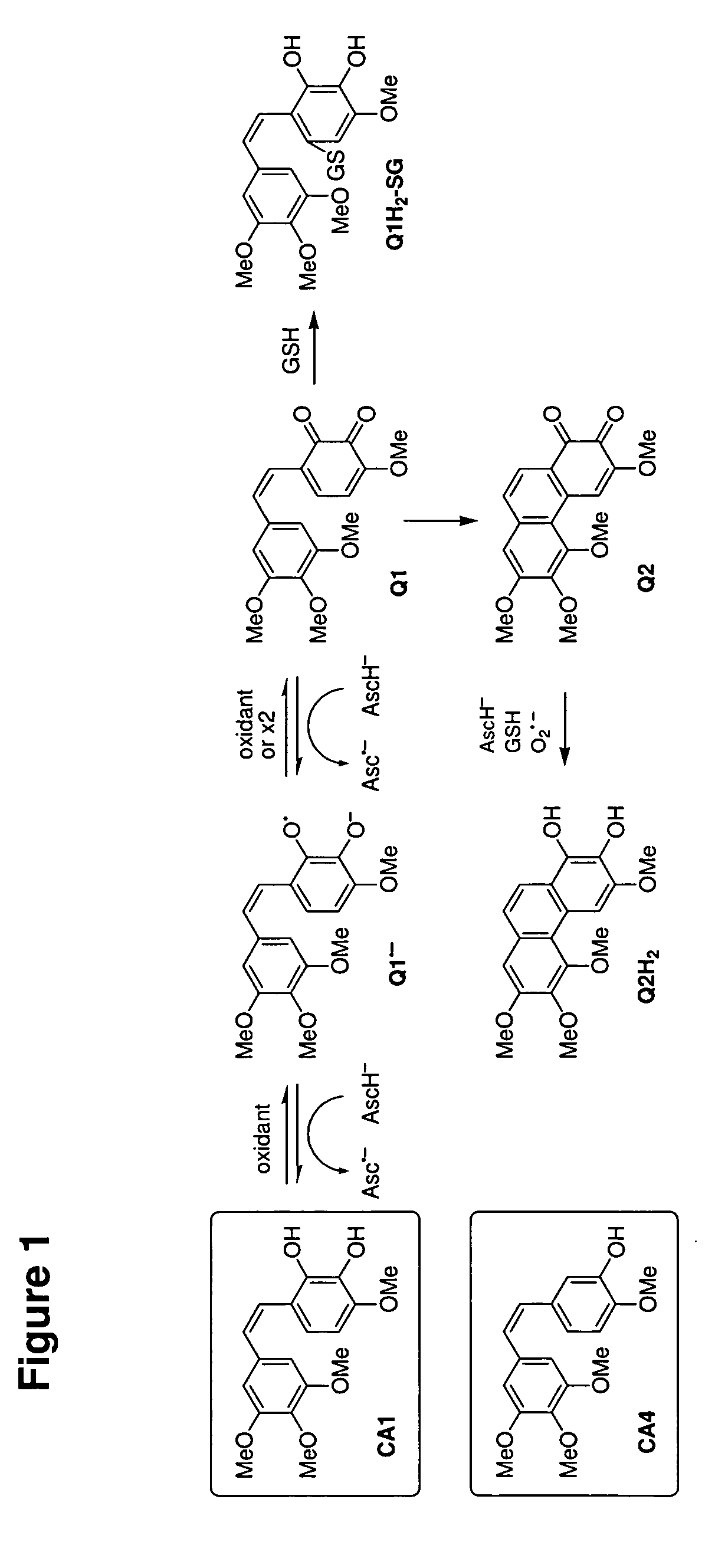
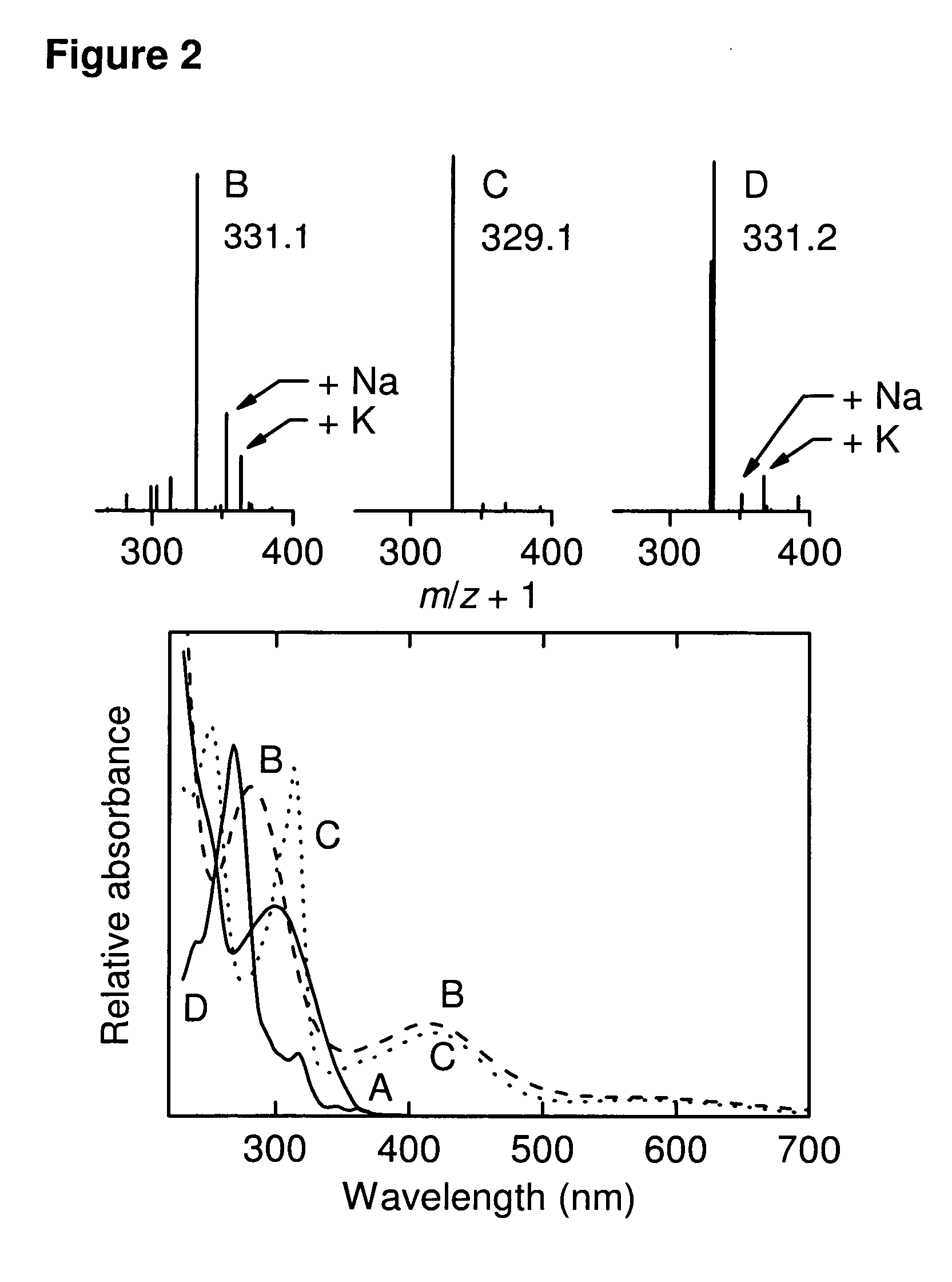
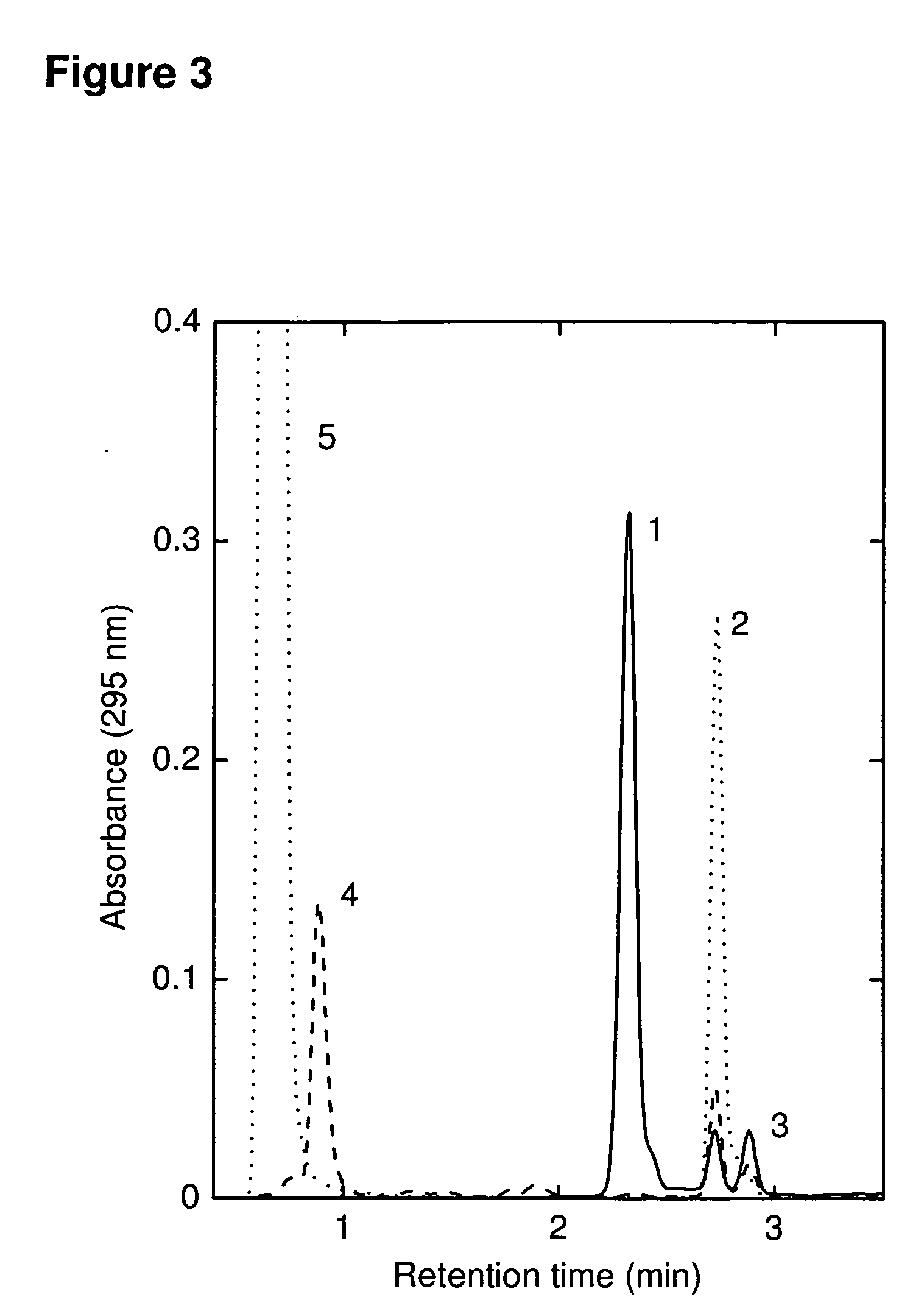
Antitumor immune responses expand over time. The antitumor immune response evolves and expands over time by constantly recognizing and remembering tumor antigens 1. The effects of immune activation are not static; instead, they can improve and deepen over time 2,3.
Structure and use of 5' phosphate oligonucleotides
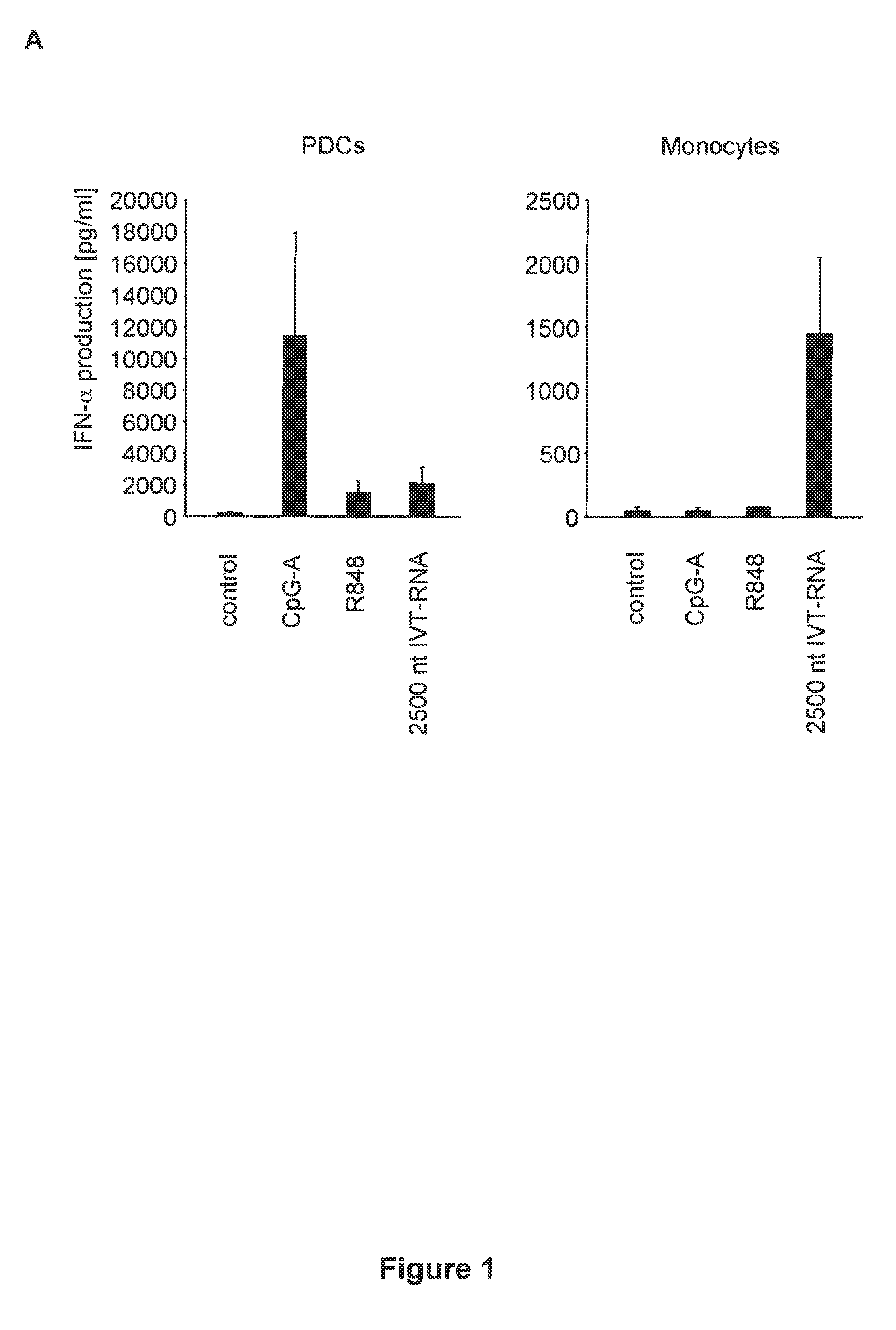
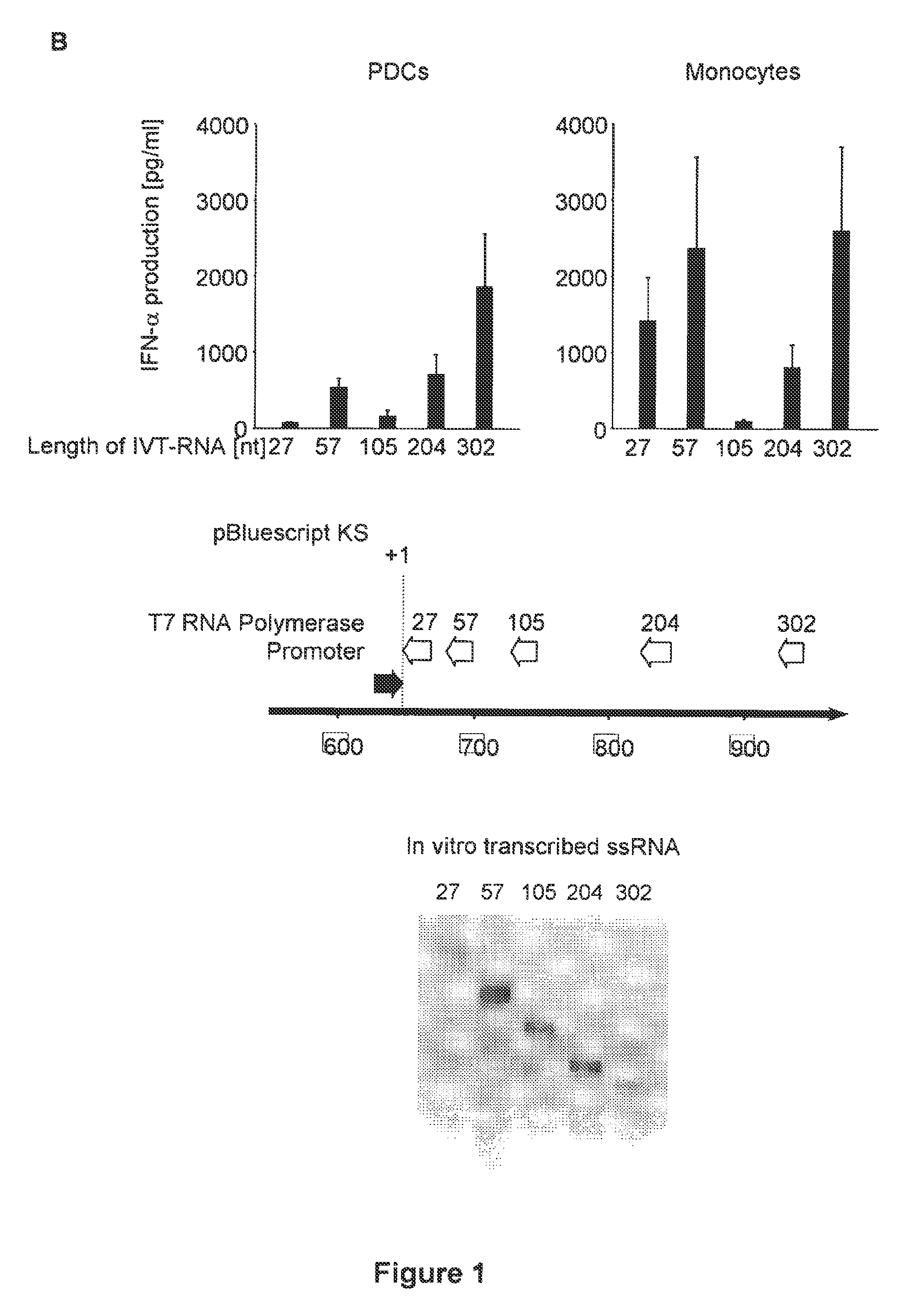
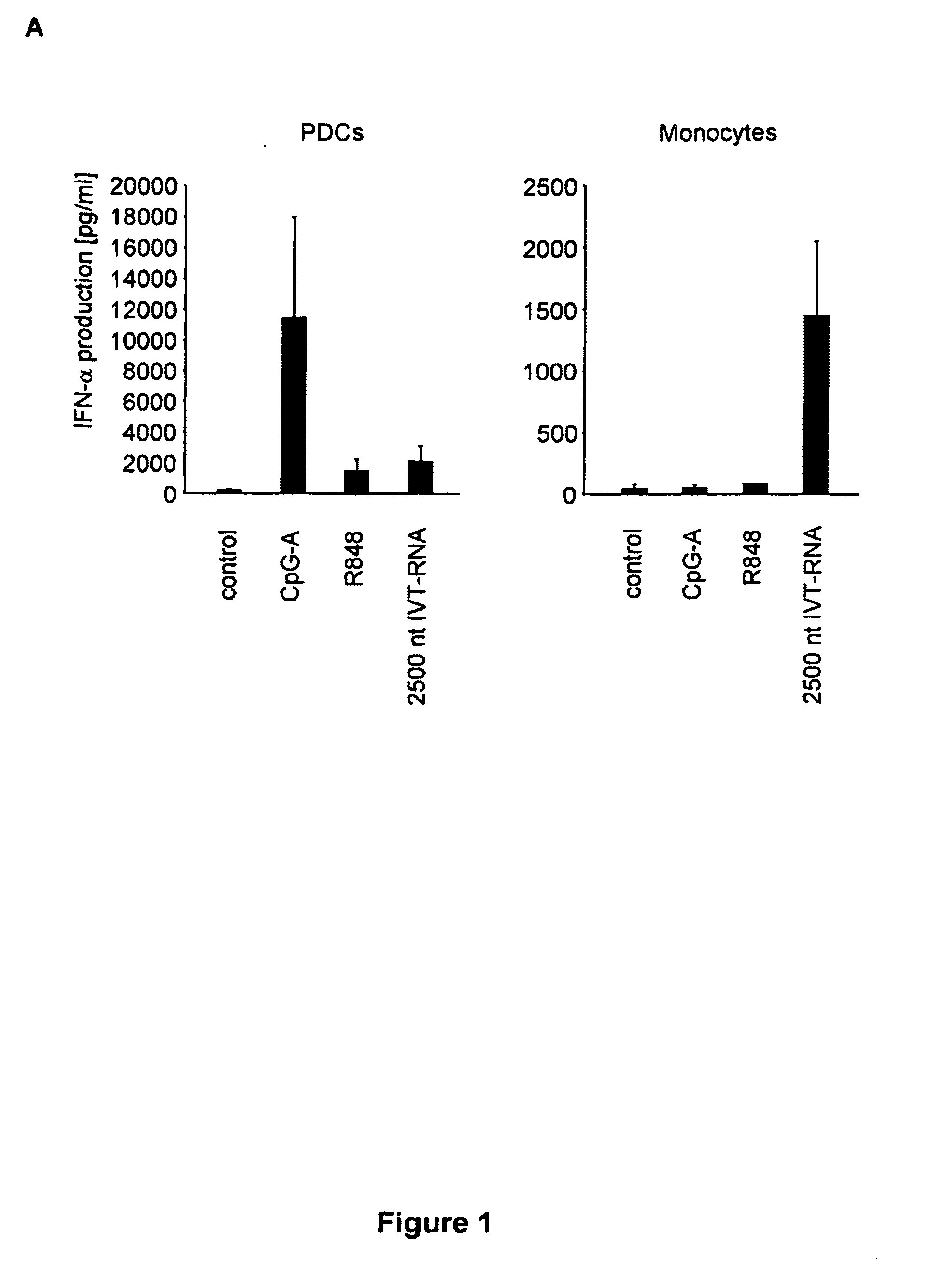
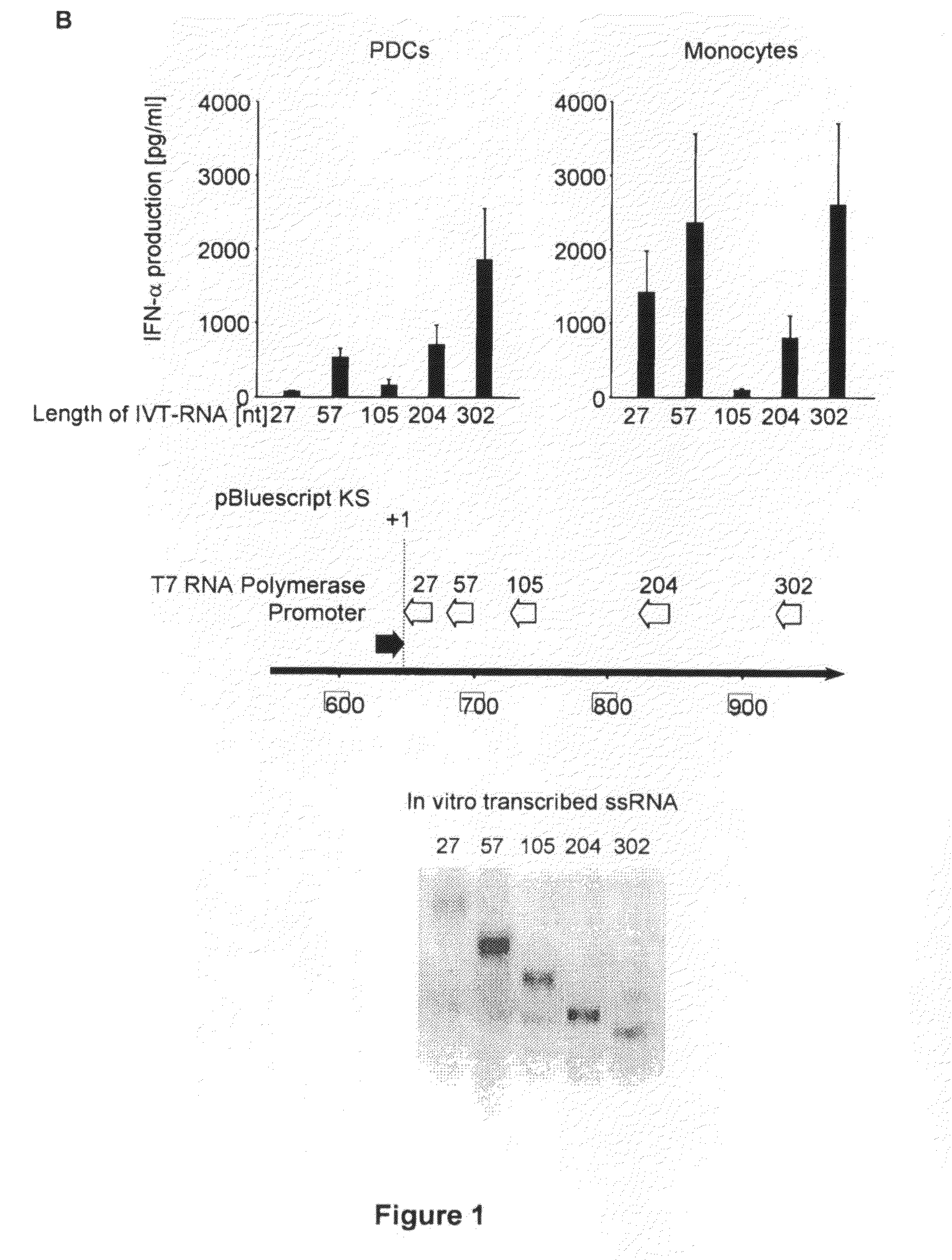
Oligonucleotides bearing free, uncapped 5′ phosphate group(s) are recognized by RIG-I, leading to the induction of type I IFN, IL-18 and IL-1β production. Bacterial RNA also induces type I IFN production. 5′ phosphate oligonucleotides and bacterial RNA can be used for inducing an anti-viral response or an anti-bacterial response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in vitro and in vivo and for treating various disorders and diseases such as viral infections, bacterial infections, parasitic infections, tumors, allergies, autoimmune diseases, immunodeficiencies and immunosuppression. Single-stranded 5′ triphosphate RNA can be used for inducing an anti-viral response, an anti-bacterial response, or an anti-tumor response, in particular, type I IFN and / or IL-18 and / or IL-1β production, in a target cell-specific manner.
Owner:UNIVERSITY OF BONN
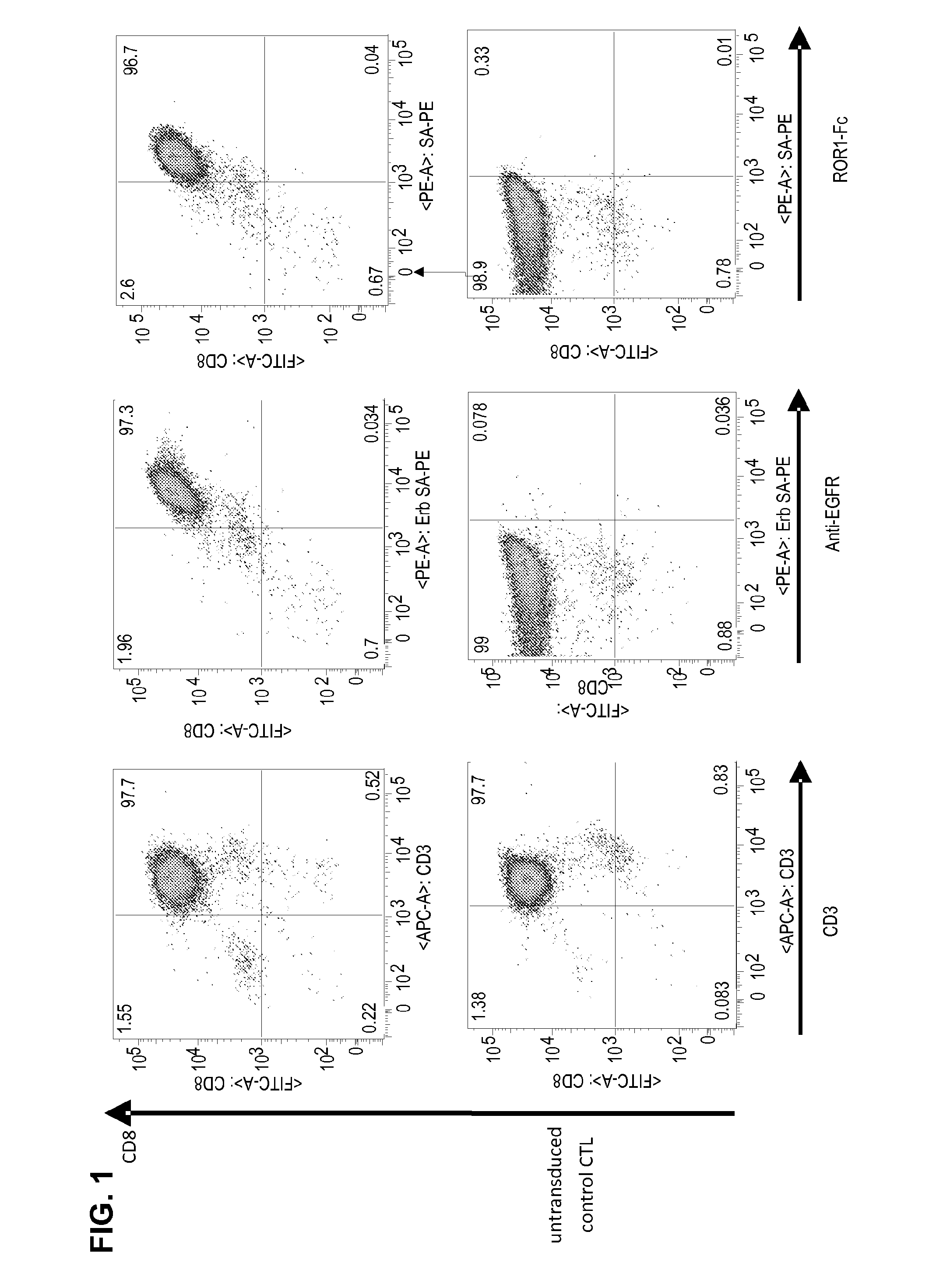
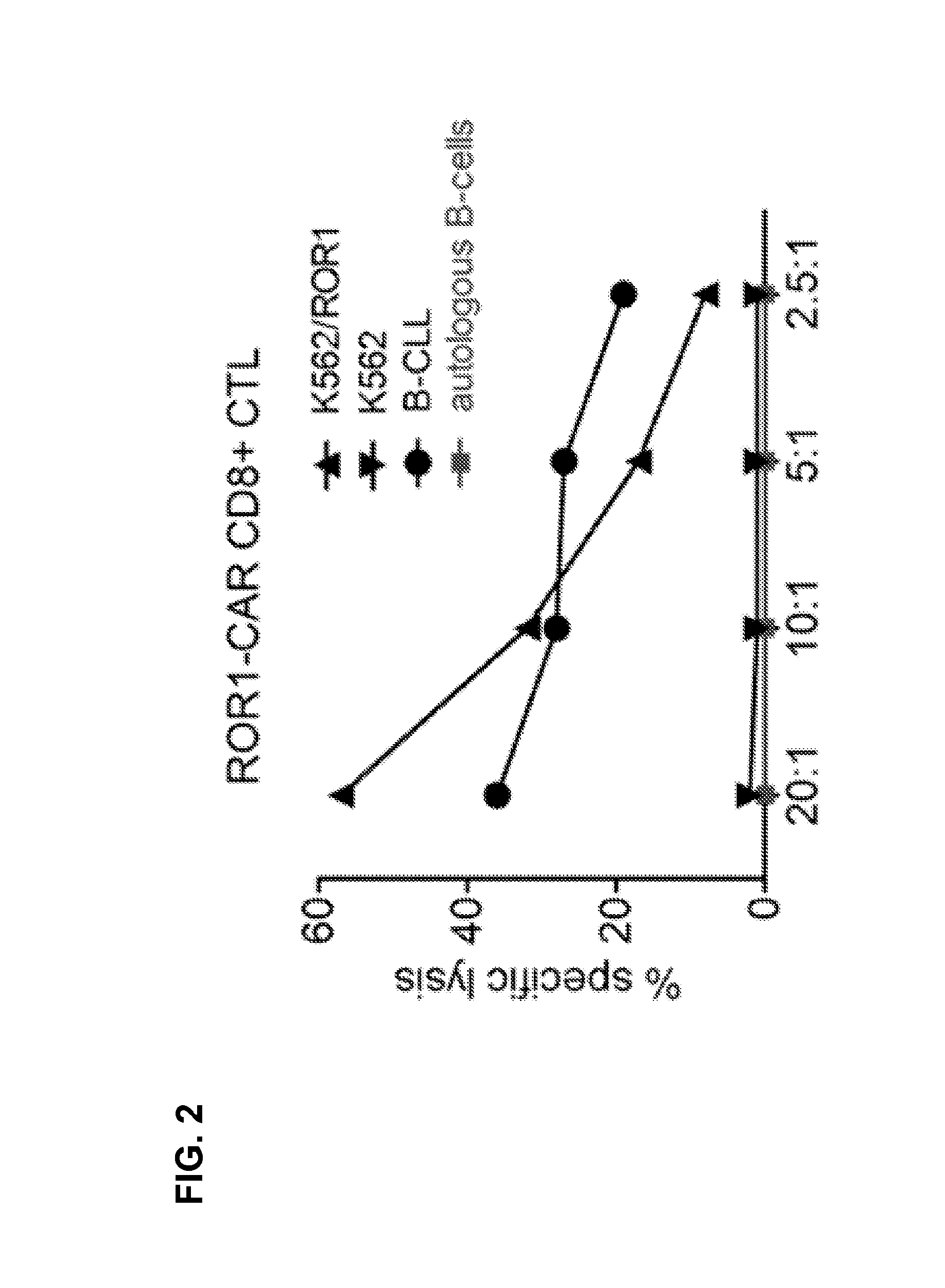
Method and compositions for cellular immunotherapy
ActiveUS20140314795A1Maintain normalEnhance immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsPharmaceutical formulationWilms' tumor
The present invention provides methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring genetically modified tumor specific CD8+ T cells in the presence of tumor-specific, subset specific genetically modified CD4+ T cells, wherein the CD4+ T cells confer and / or augment a CD8+ T cells ability to sustain anti-tumor reactivity and increase and / or maximize tumor-specific proliferation of the tumor-specific CD8+ T cells of interest. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Biomarkers for cancer treatment
ActiveUS20080131885A1Reduce healingAnalysis using chemical indicatorsSugar derivativesTumor responseWilms' tumor
The present invention provides identification of a thirty-five gene set that predicts the anticancer activity of inhibitors of the RAF / MEK / MAPK pathway, methods of qualifying cancer status in a subject, methods of identifying an anti-tumor response in a subject, methods of monitoring the efficacy of a therapeutic drug in a subject, and methods of identifying an agent useful in the treatment of a cancer based on expression of the thirty-five gene set.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Structure and use of 5'phosphate oligonucleotides
InactiveUS20100178272A1Antibacterial agentsOrganic active ingredientsImmunologic disordersAutoimmune condition
Owner:KLINISCHE PHARMAKOLOGIE
Cancer immunotherapy
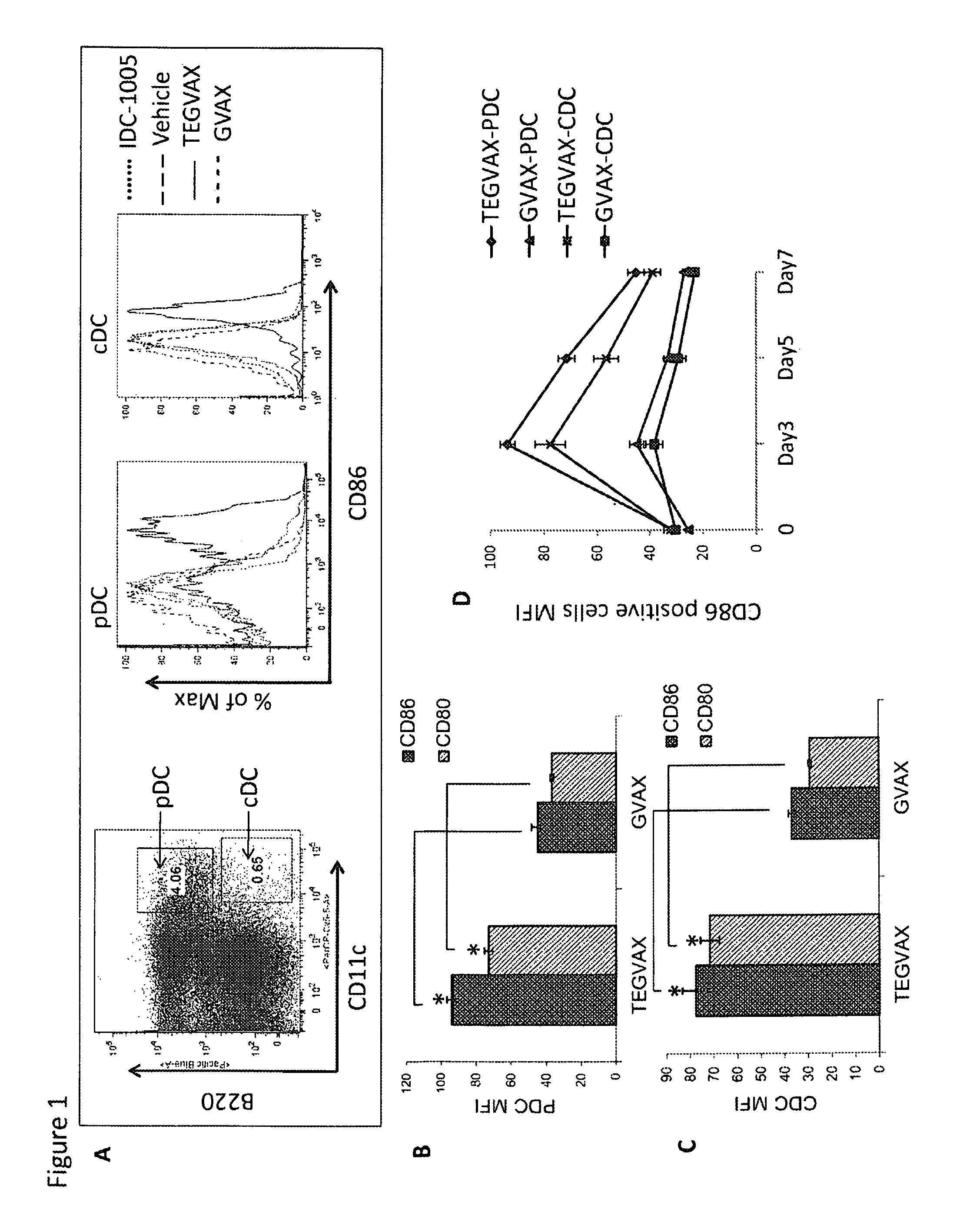
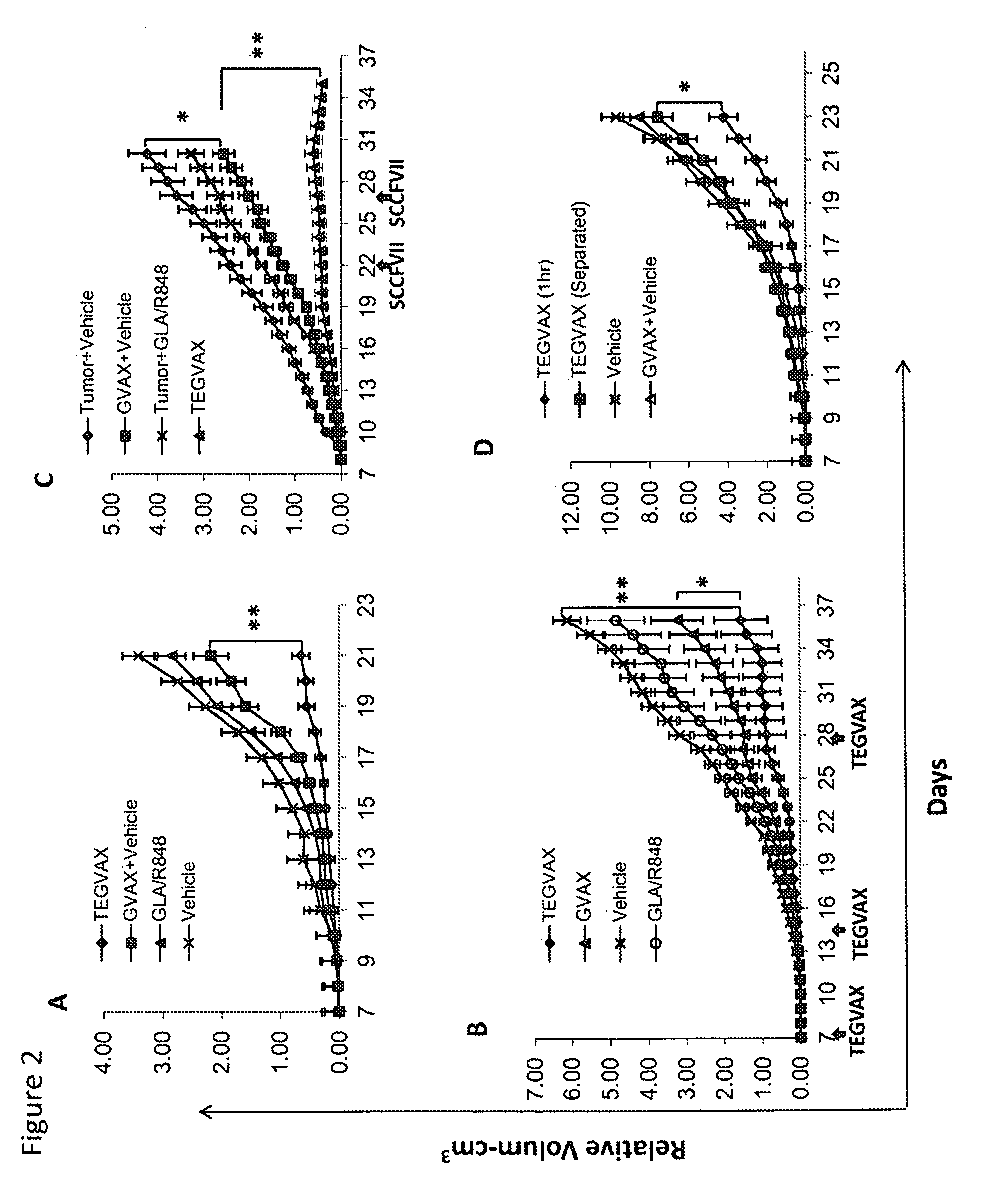
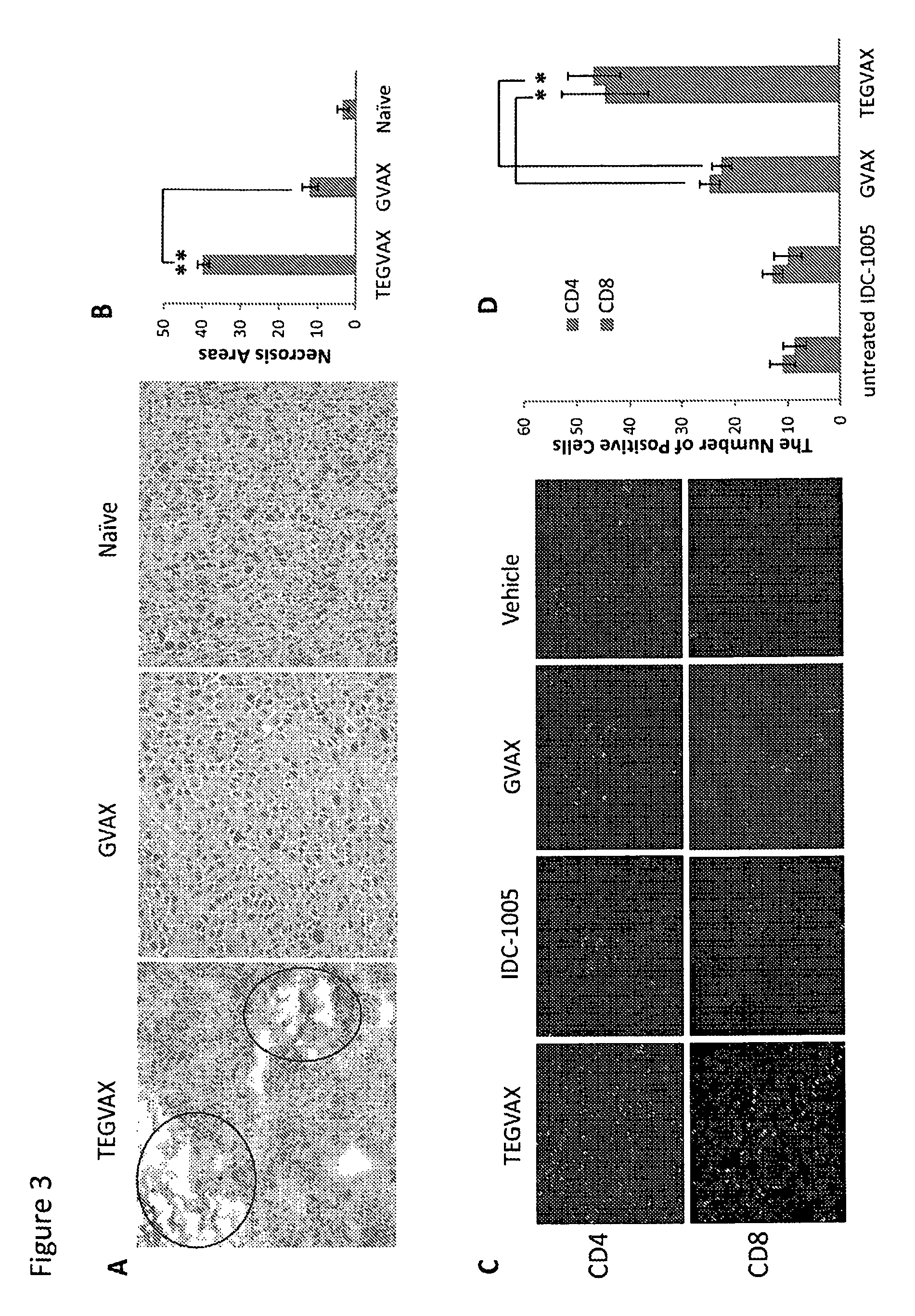
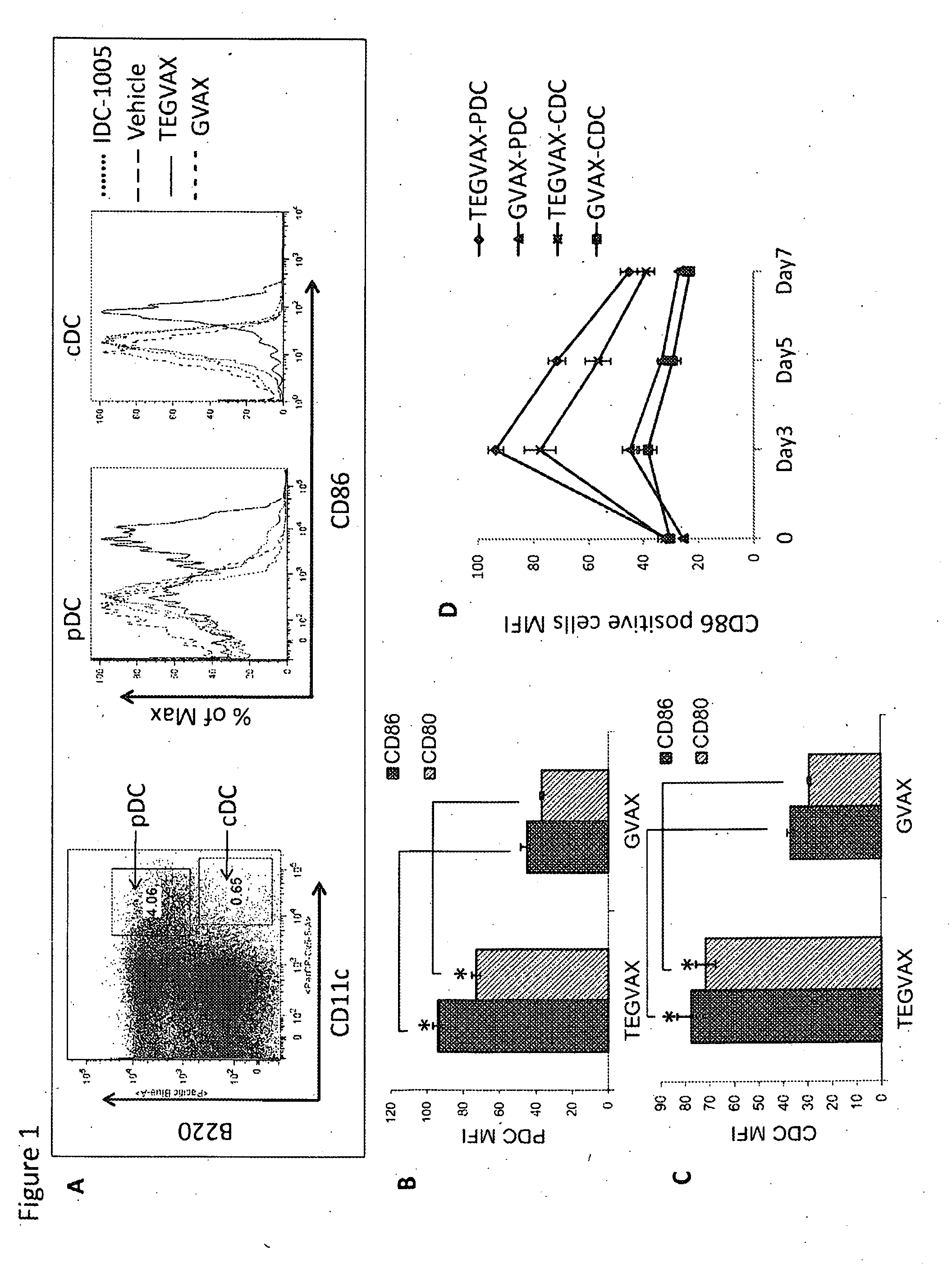
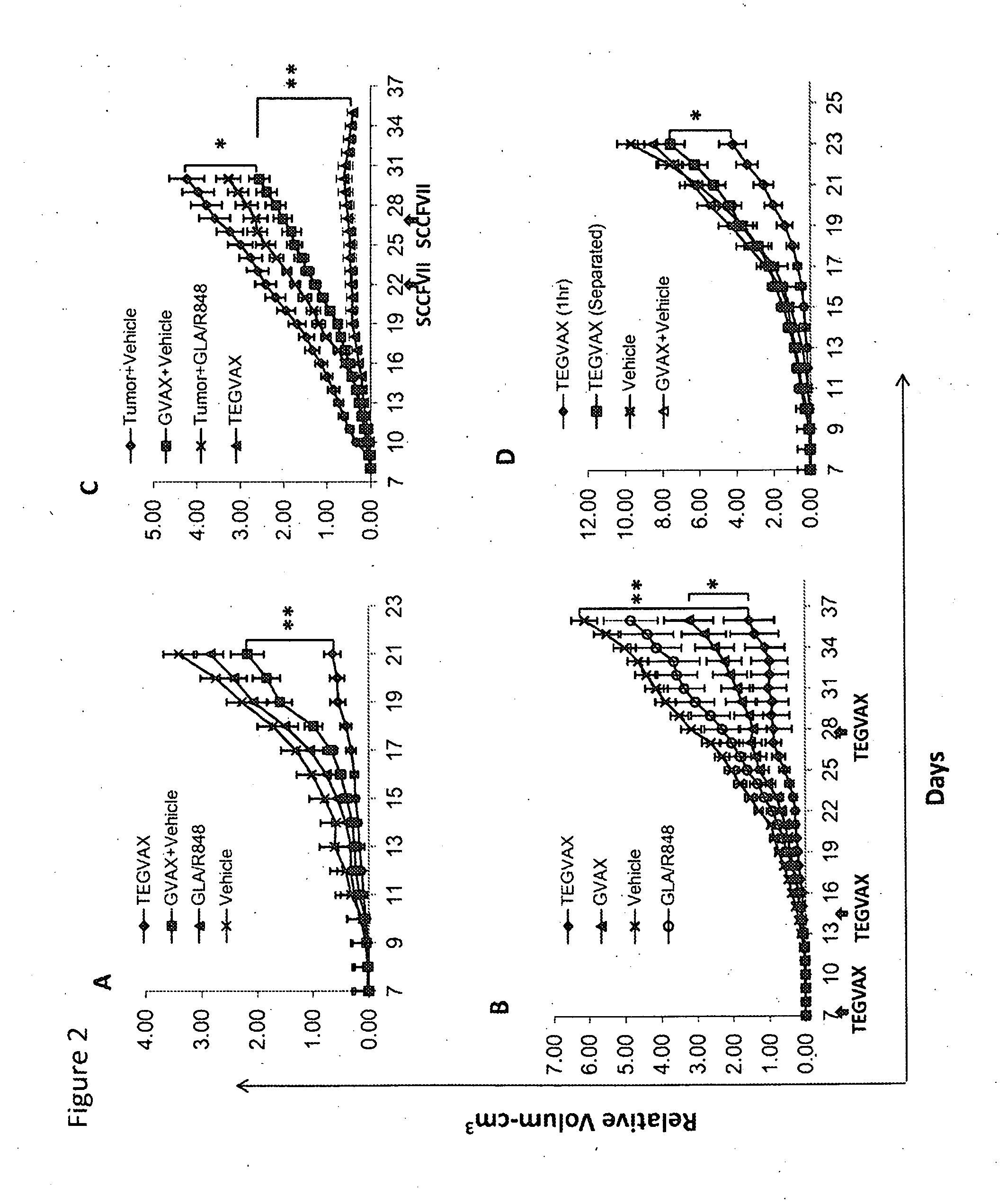
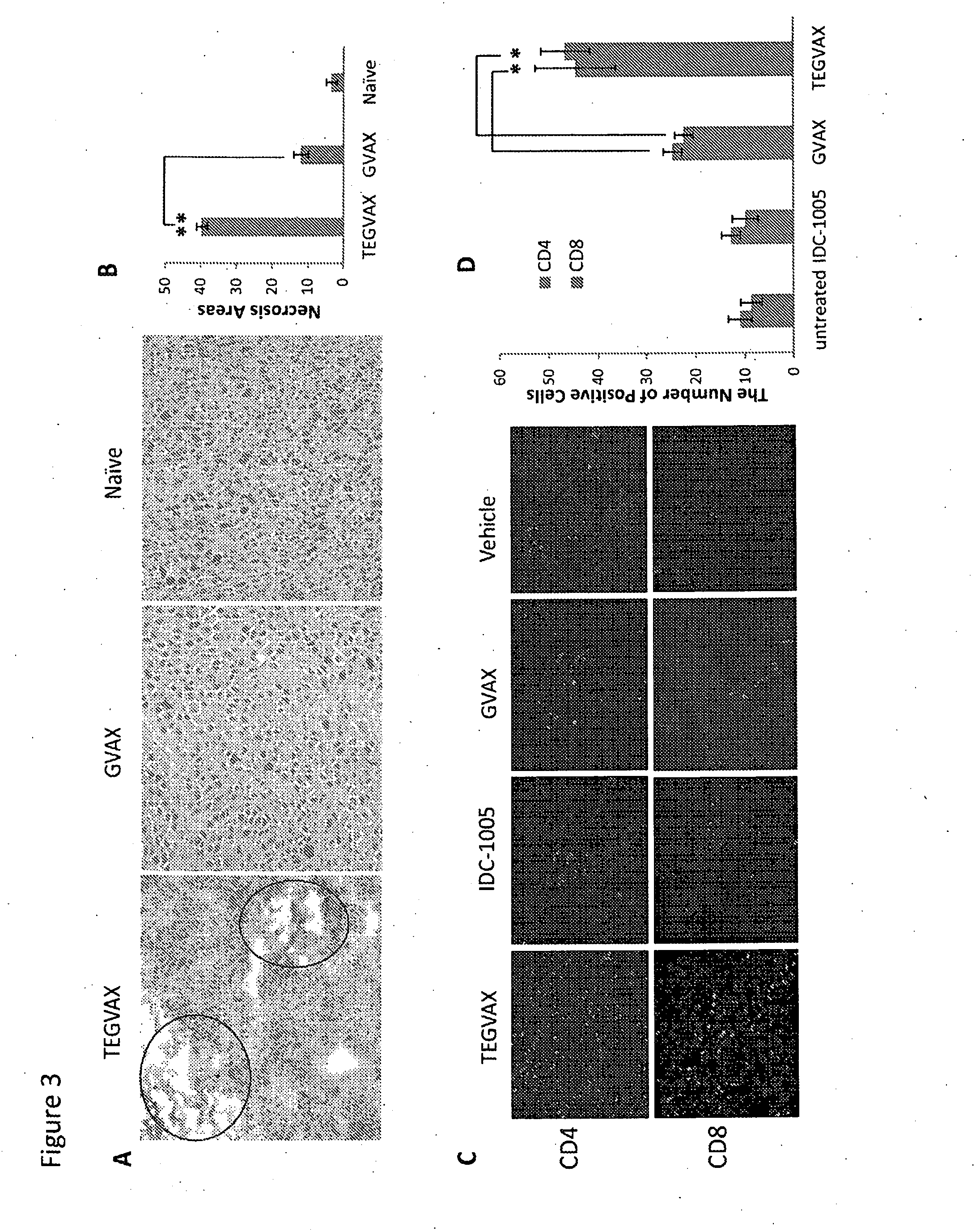
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Anti-idiotype anti-cea antibody molecules and its use as cancer vaccine
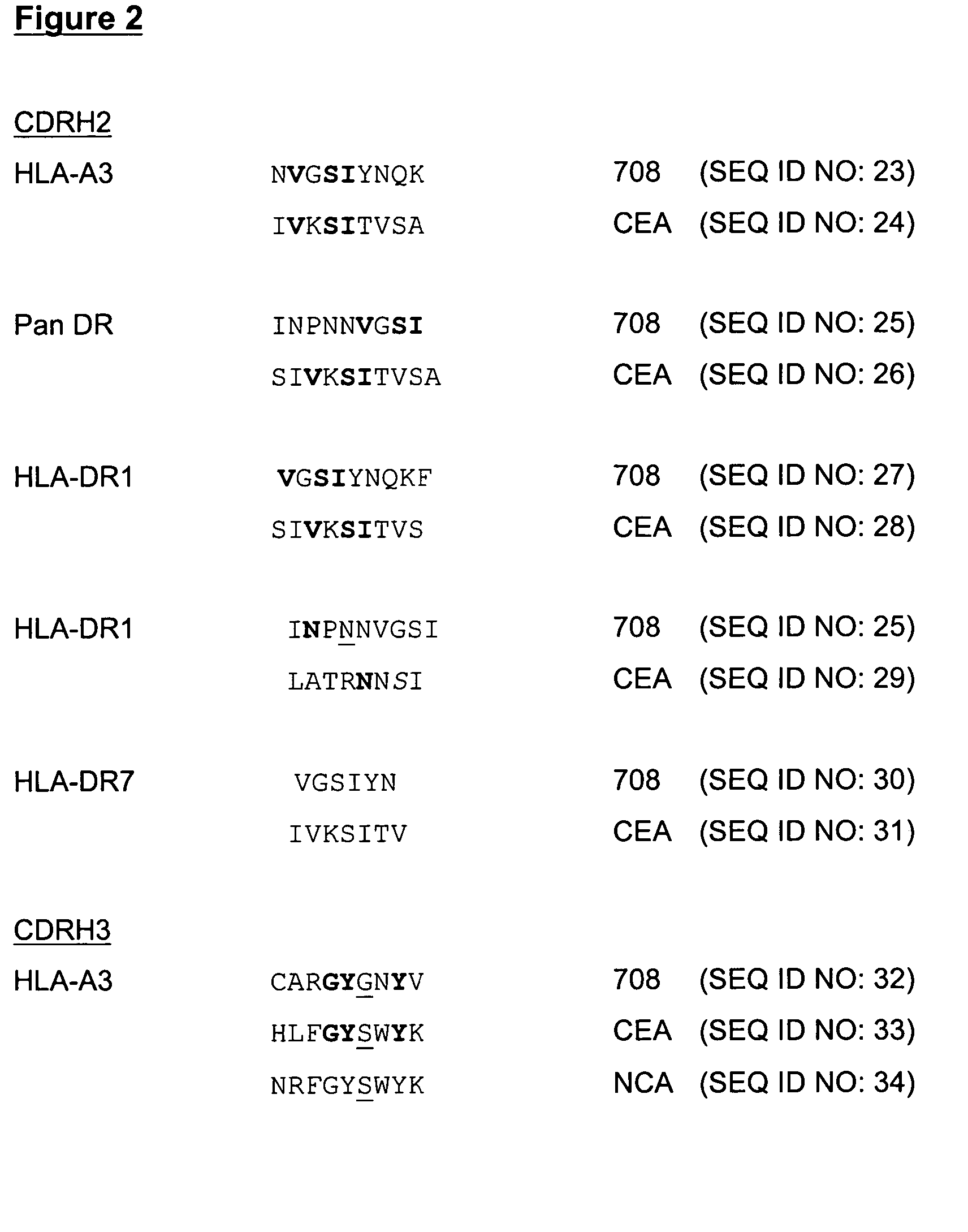
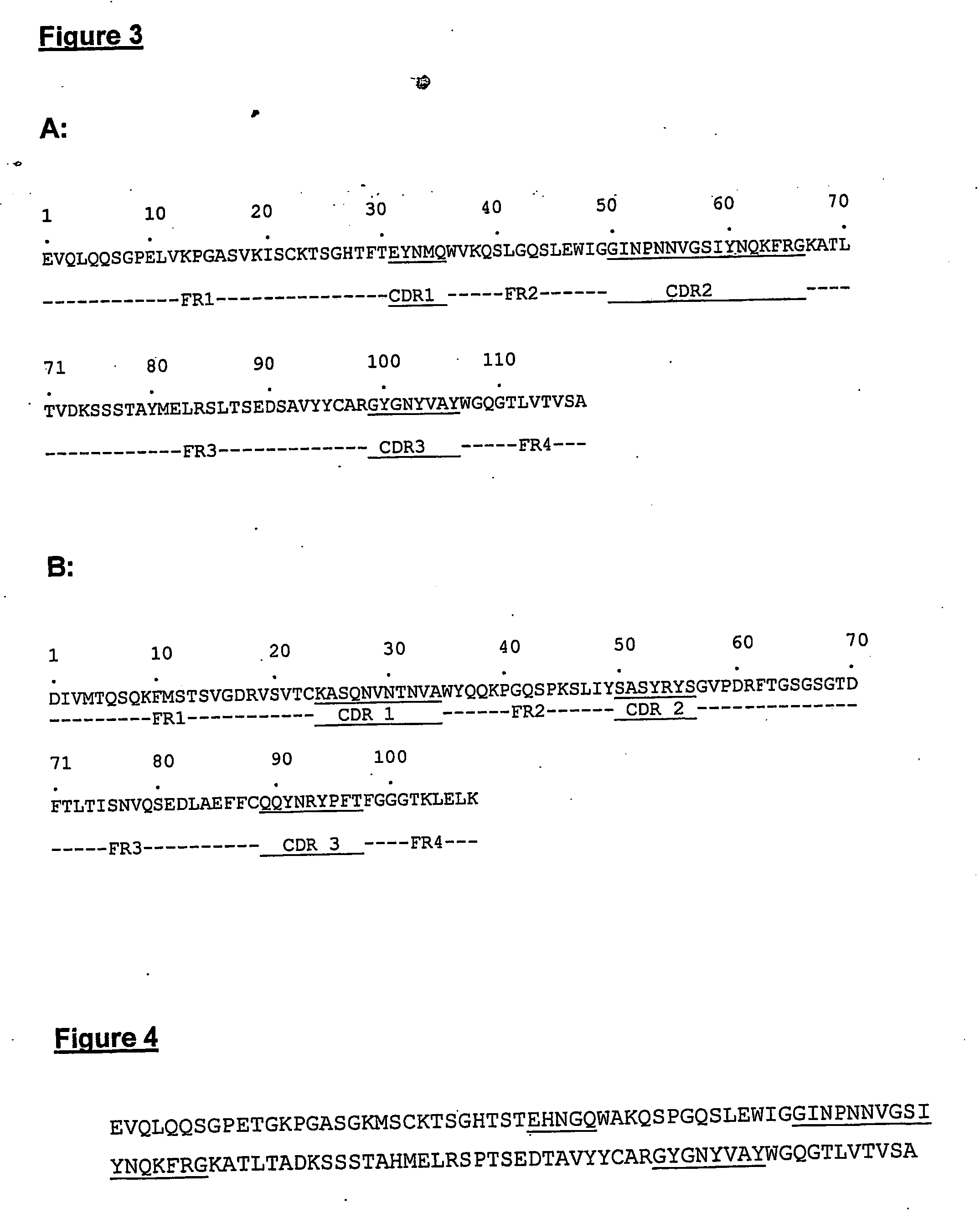
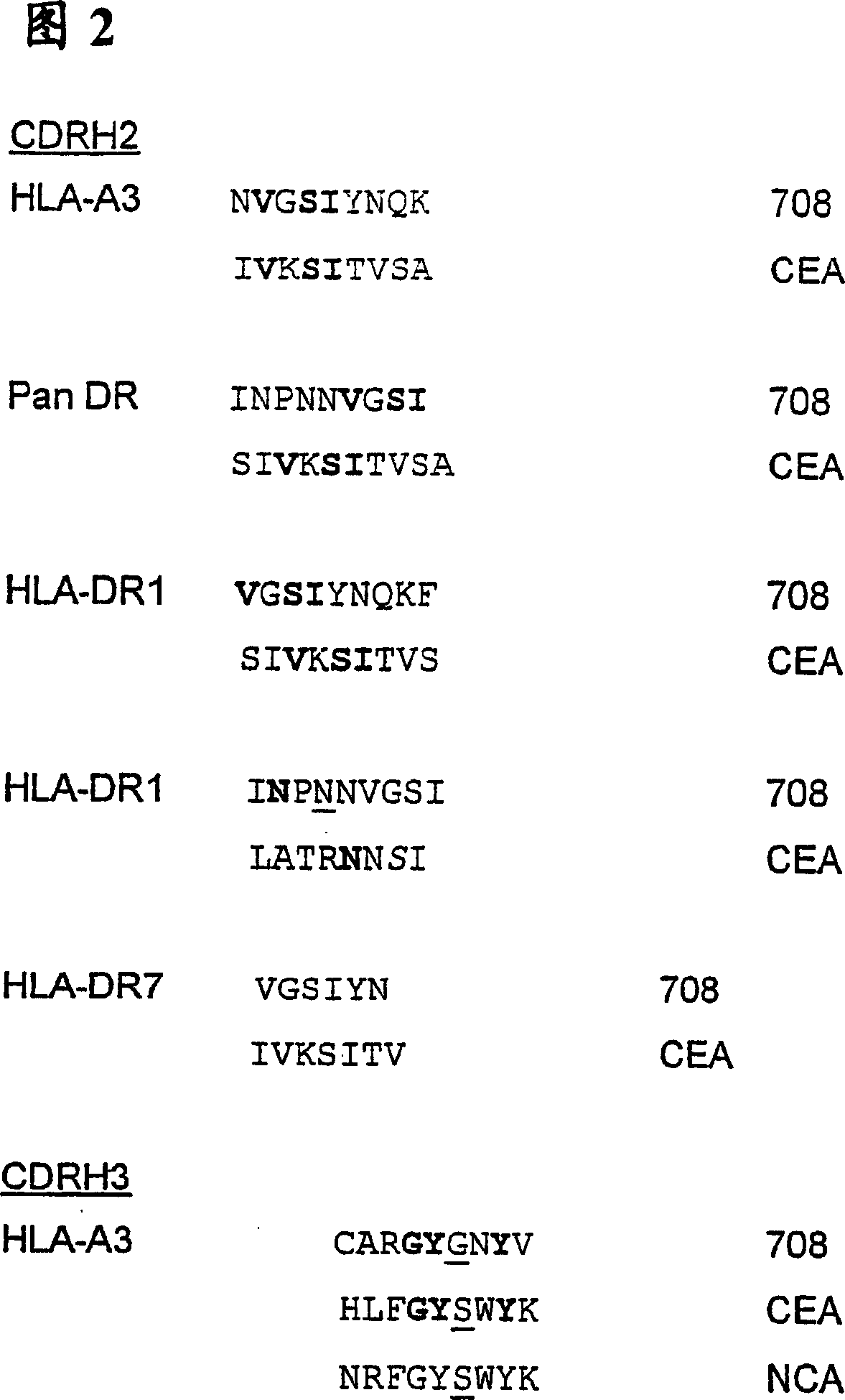
InactiveUS20050222392A1Stimulate immune responseHigh activityHybrid immunoglobulinsPeptide/protein ingredientsMHC class IVaccination
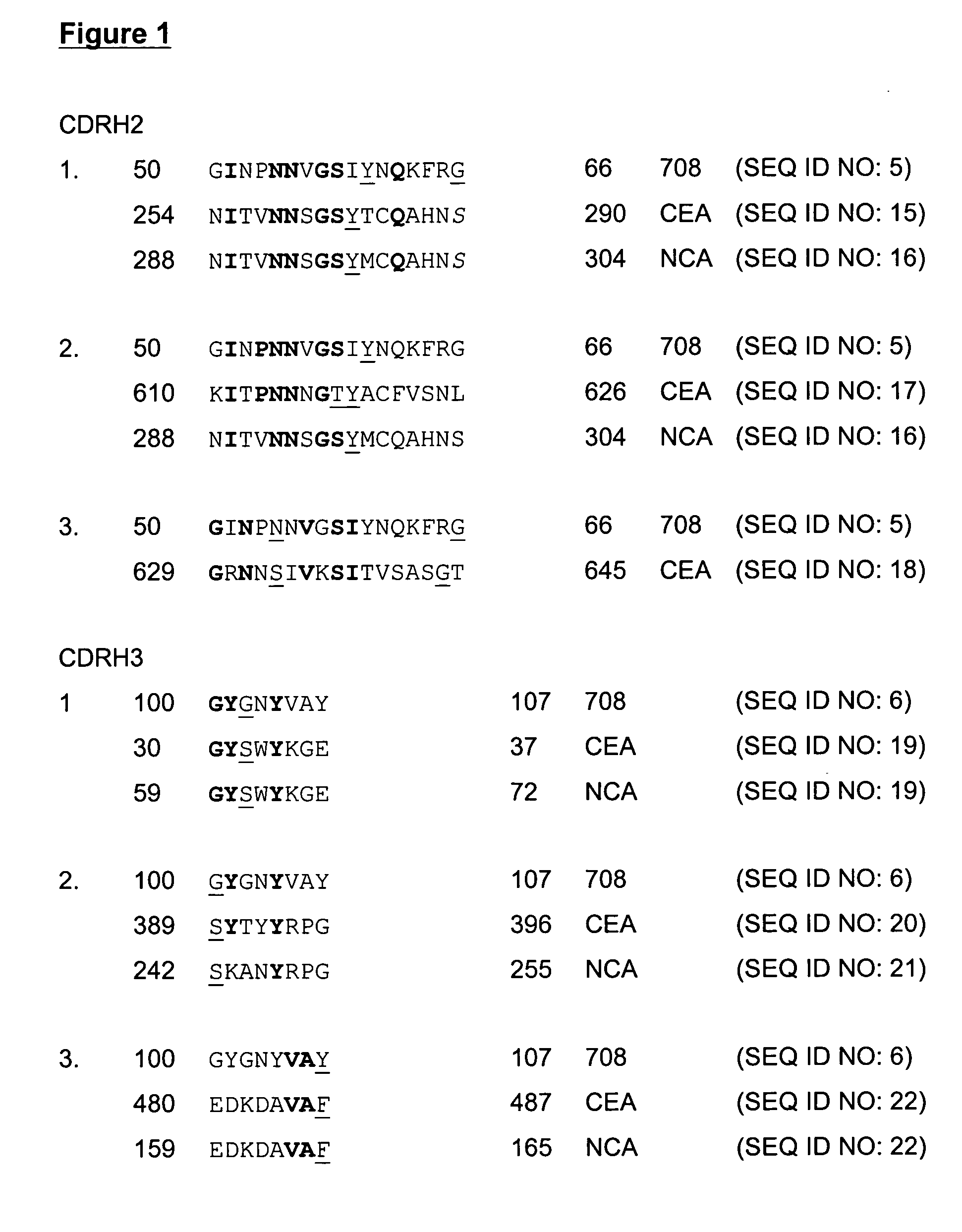
The present invention provides molecules, preferably designed immunoglubulins, suitable for use as an anti-idiotype vaccine to CEA positive tumours. The molecules induce both an MHC class I and MHC class II mediated immune response to the CEA bearing tumour cells for an efficient and sustained host anti-tumour response. The present invention provides modified versions of anti-idiotype anti-CEA antibodies, preferably mouse antibody 708, with improved vaccination properties. The modifications are related to the introduction of sequences tracts deriving from e.g. CEA, CD55 antigen and CEA cancer-specific MHC epitopes into the variable regions of said antibody molecules.
Owner:CARTER GRAHAM +1
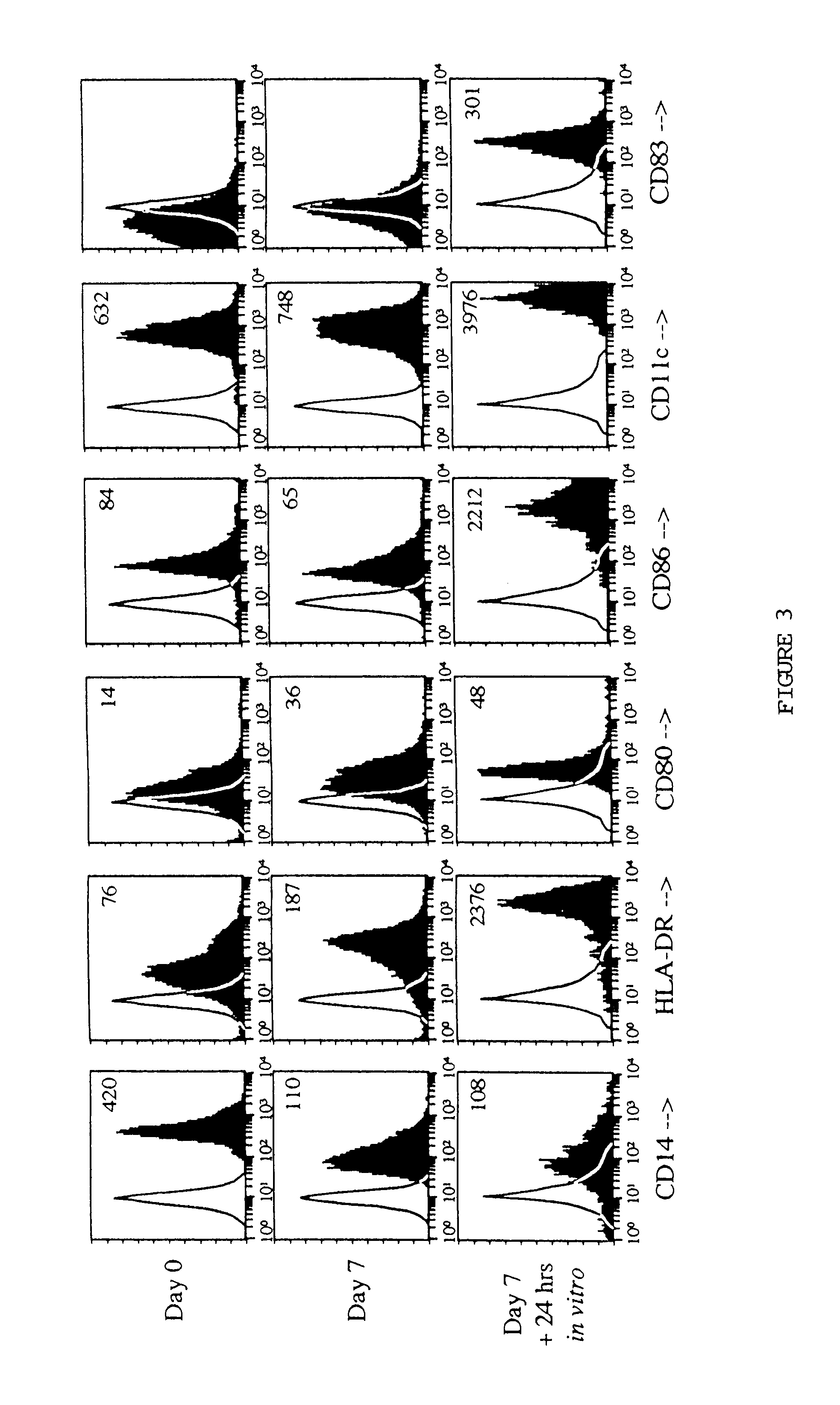
Methods for enhancing antigen-presenting cells and anti-tumor responses in a human patient
InactiveUS6838081B1Improve developmentReduce tumor burdenBiocidePeptide/protein ingredientsTumor responseCo administration
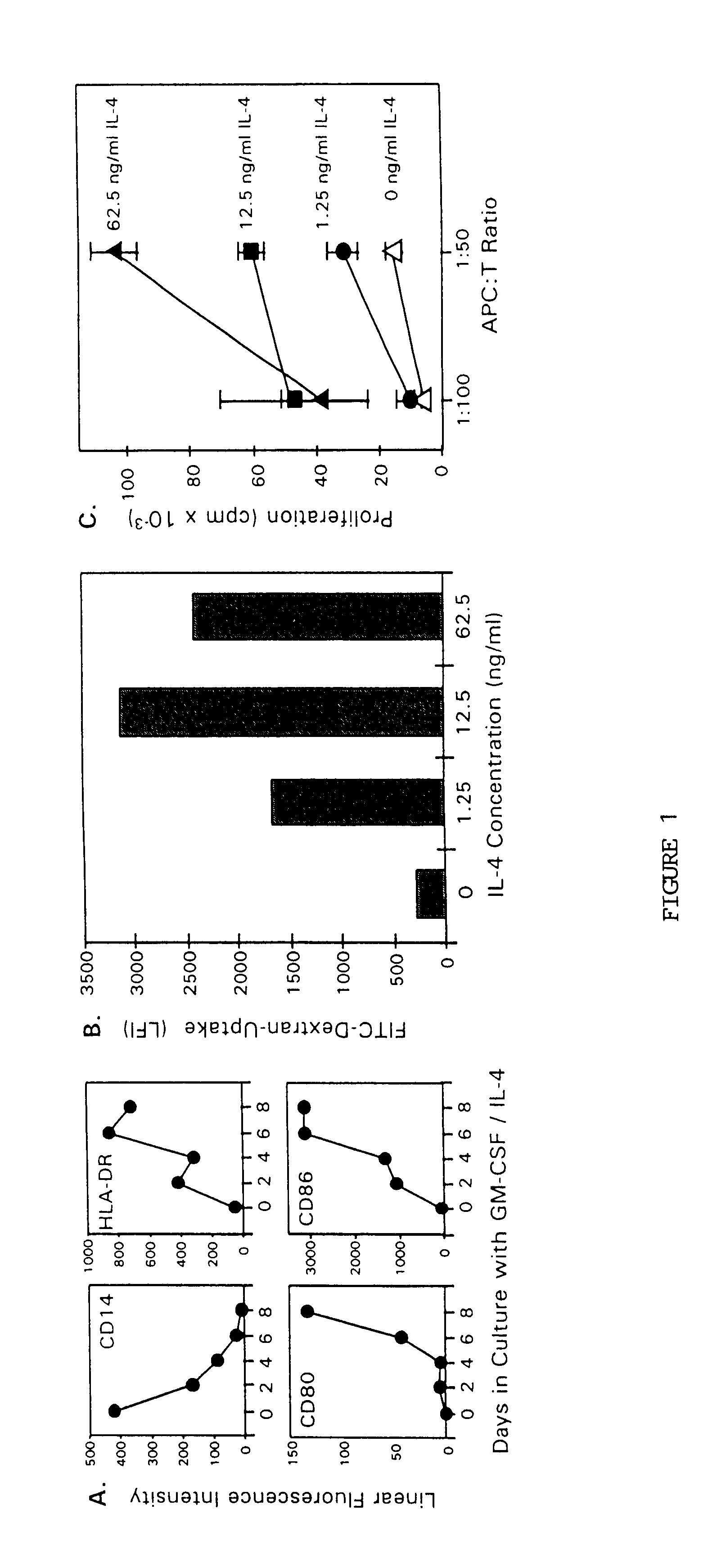
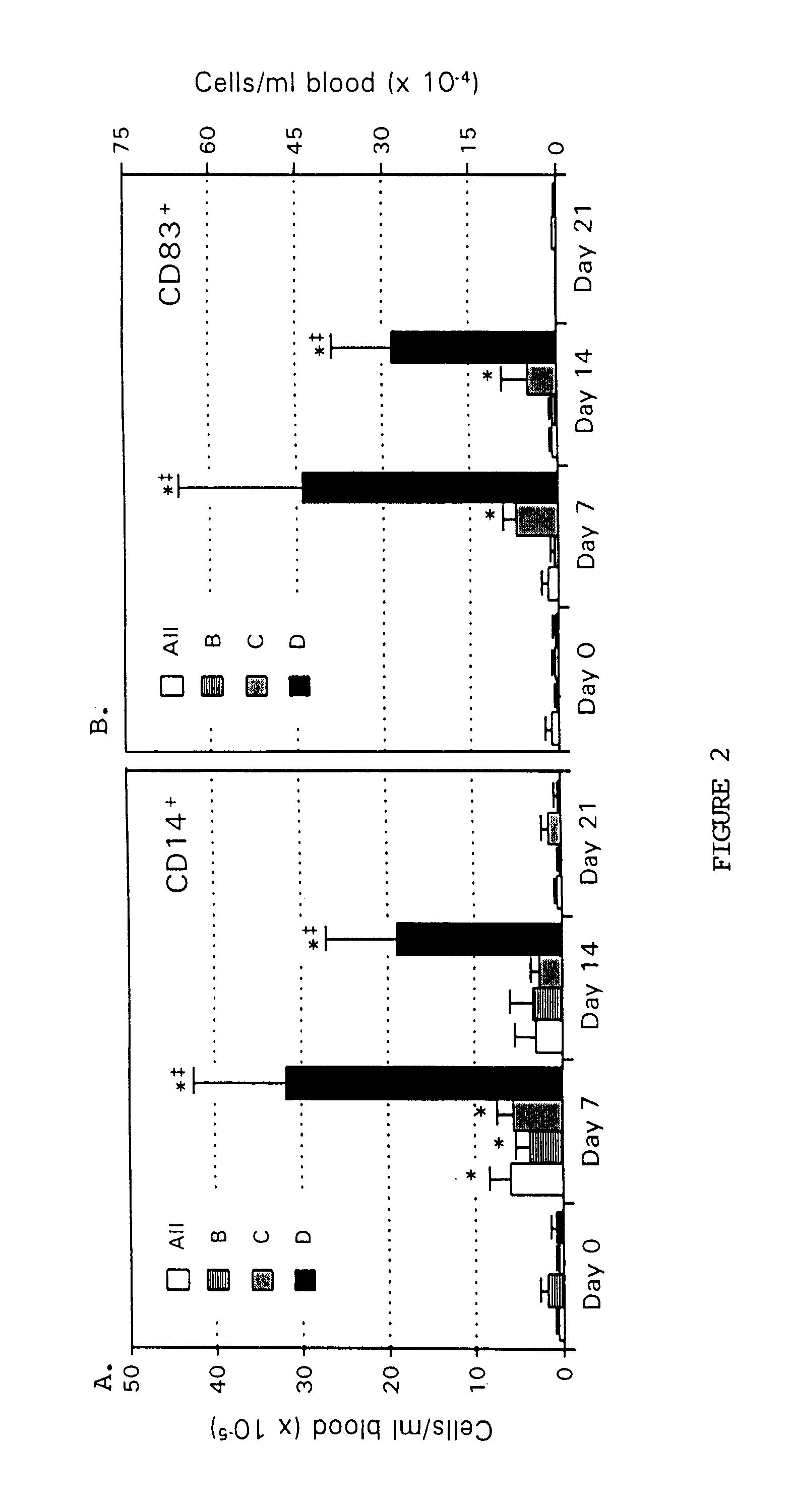
The present invention provides methods for enhancing the development of APC from precursor cells by administering a combination of GM-CSF and IL-4. The precursor cells include: cells contained in peripheral blood, CD14+ cells and precursors in bone marrow. Thus, administration of GM-CSF and IL-4 can be used as a form of cytokine immunotherapy. One embodiment of the present invention involves systemic administration of GM-CSF and IL-4. In this embodiment, APC are required to directly access tumor antigens as they exist in vivo within the patient. A further embodiment of the present invention involves co-administration of a tumor-associated or tumor-specific antigen, with GM-CSF and IL-4, to induce antigen-specific immunity mediated by APC. Yet another embodiment of the present invention describes systemic administration of GM-CSF and IL-4 to achieve reduced tumor burden.
Owner:RGT UNIV OF CALIFORNIA
Cancer immunotherapy
InactiveUS20140341978A1Skin cancer vaccineMammal material medical ingredientsAbnormal tissue growthTumor response
We formulated multiple TLR agonists into GVAX (lethally irradiated tumor cell vaccines engineered to secrete GM-CSF). Specifically, GLA and R848, TLR4 and TLR7 / 8 agonists found to be safe in patients, were formulated with GVAX (TEGVAX—for TLR agonists enhanced GVAX), and this formulation was effective in producing anti-tumor responses in 3 different preclinical models, including palpable B16. These anti-tumor responses were correlated with increased CD4 and CD8 T-cells that can secrete IFNγ circulating in the tumor microenvironment as well as significantly higher level of p15E specific CTL mediated cell killing in mice treated with TEGVAX in comparison to controls. When combined with anti-PD-1 antibody, TEGVAX was able to induce regression of established B16 tumors.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
System and methods for deriving gene signature biomarkers of response to pd-1 antagonists
ActiveUS20180327848A1Enabling detectionHealth-index calculationMicrobiological testing/measurementTumor responseBiomarker (petroleum)
A gene expression platform, which is a combination of a set of genes that are correlated with response to a PD-1 antagonist in multiple tumor types and a normalization gene set, is disclosed. A method and system of using the gene expression platform to derive gene signature biomarkers of anti-tumor response to a PD-1 antagonist and to test patient samples for predictive gene signature biomarkers are also disclosed.
Owner:MERCK SHARP & DOHME LLC
Prostatic cancer vaccine
InactiveUS20060024316A1Eliminate the problemAntibody ingredientsCancer antigen ingredientsAntigenBULK ACTIVE INGREDIENT
Vaccines capable of eliciting an immune antitumor response for prostate tumors are disclosed. The active ingredient in such vaccines is selected from the group consisting of at least one antigen over-represented in the prostate gland or an immunologically effective portion thereof; an expression system capable of generating in situ said antigen or portion; a naked DNA encoding such antigen and portion; and an anti-idiotypic antibody or fragment thereof which mimics said antigen or portion.
Owner:SPITLER LYNN E +1
Method of preparation and composition of a water soluble extract of the bioactive component of the plant species uncaria for enhancing immune, anti-inflammatory, anti-tumor and DNA repair processes of warm blooded animals
InactiveUS20050176825A1Inhibit productionIncrease heightBiocideHydroxy compound active ingredientsDiseaseFluorescence
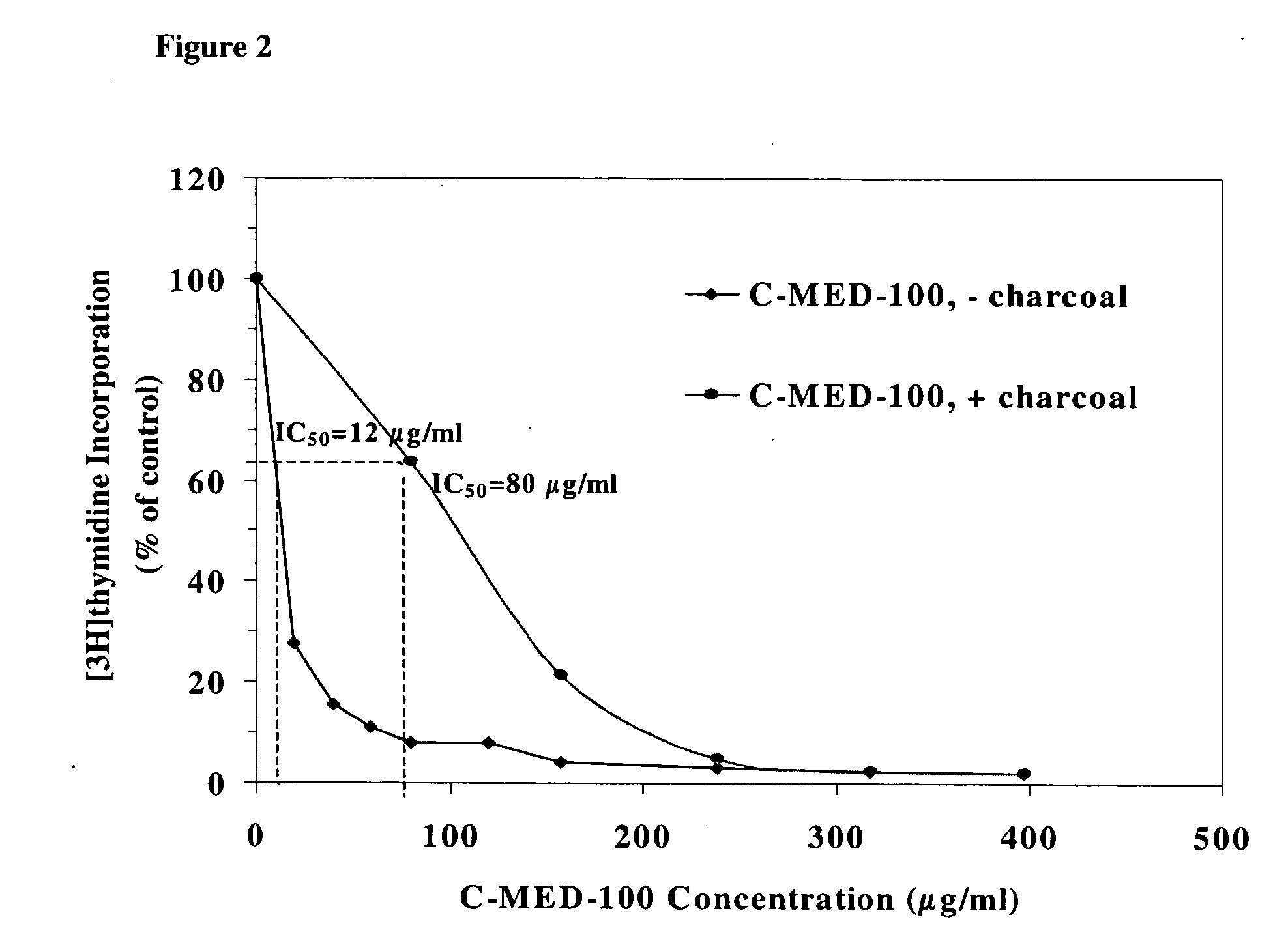
A method for isolating the bioactive component of the water-soluble extract of Uncaria tomentosa known as C-MED-100®, comprising (i) precipitating the spray drying carrier from C-MED-100®; (ii) using the resulting C-MED-100® to obtain a spotting mixture for thin layer chromatography (TLC); (iii) spotting the C-MED-100® spotting mixture on pre-run TLC plates and eluting the plates to obtain the fluorescing band with Rf=0.2-0.3; (iv) scraping off the Rf=0.2-0.3 band, eluting it in ammonia and freeze drying the eluted band to form a powder; and (v) extracting the powder with methanol to remove solubilized silica gel, concentrating the methanol solution and crystalizing the concentrated solution to obtain the bioactive component. The isolated bioactive component in vitro is a quinic acid analog, preferably quinic acid lactone. By contrast, the isolated bioactive component in vivo is quinic acid, whether as free acid or as a quinic acid salt, including quinic acid ammonium salt. A pharmaceutical composition comprising a pharmaceutically effective amount of the bioactive component and a nontoxic inert carrier or diluent. The bioactive component may be used to enhance immune competency, treat disorders associated with the immune system, inhibit the inflammatory response, treat disorders associated with the inflammatory response, enhance the anti-tumor response, and treat disorders associated with the response to tumor formation and growth, all in mammals.
Owner:OPTIGENEX INC
Combinations of growth-and hormone-regulating factors for the treatment of neoplasia
InactiveUS20060193853A1Reducing tumor massSynergic effectPeptide/protein ingredientsAntibody ingredientsAbnormal tissue growthTumor response
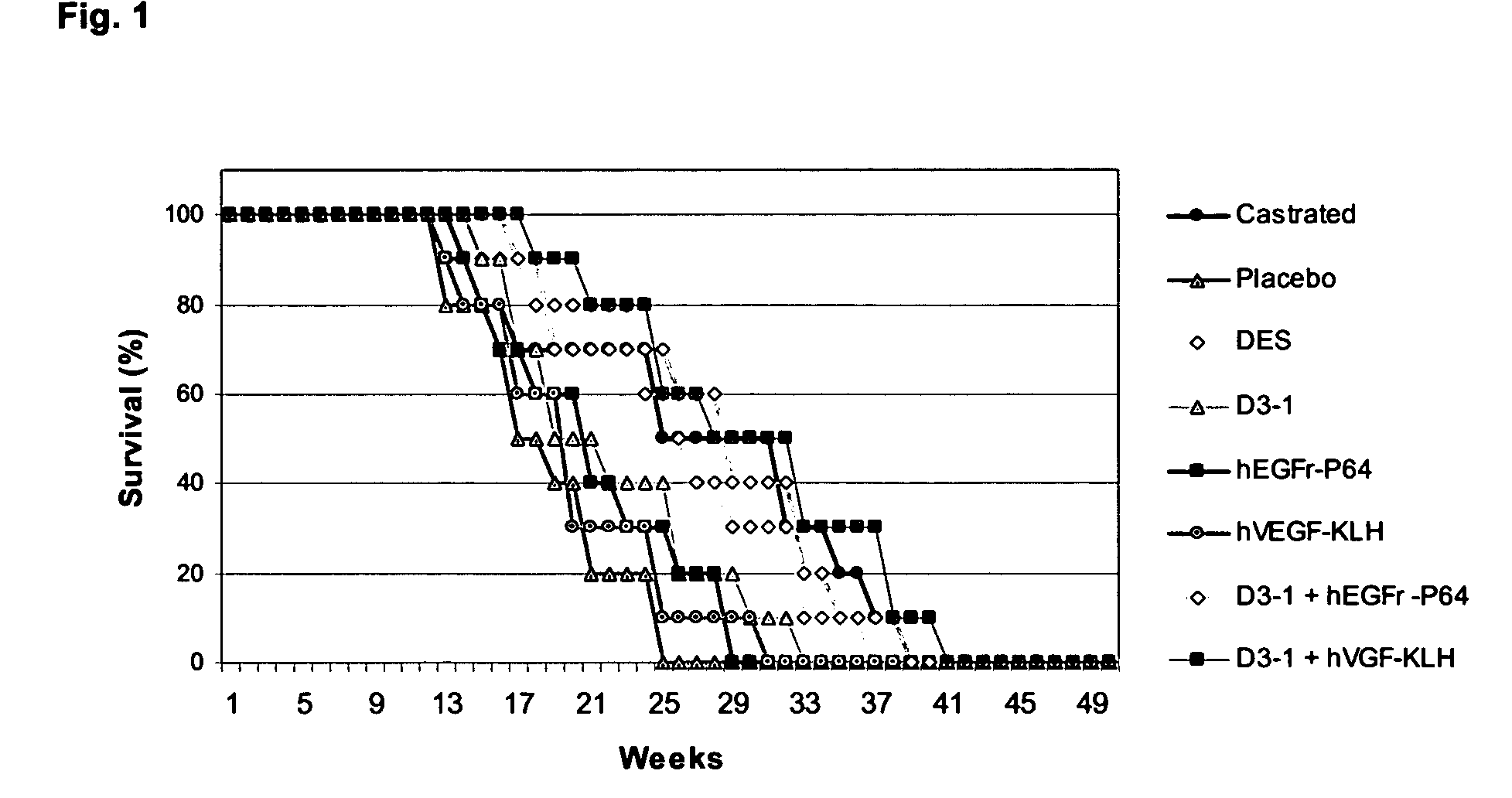
The invention relates to the field of immunology, endocrinology and oncology and, in particular, the generation of a combined immune response to determined growth factors and hormones. A synergic effect, outlined herein, between growth regulating factors (EGF, TGF and VEGF) and hormones involved in the sexual hormones release cascade or reproduction (GnRH, LH, FSH) stimulates the anti-tumor response which is expressed as a reduction in the tumor mass and an increase in the survival time.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
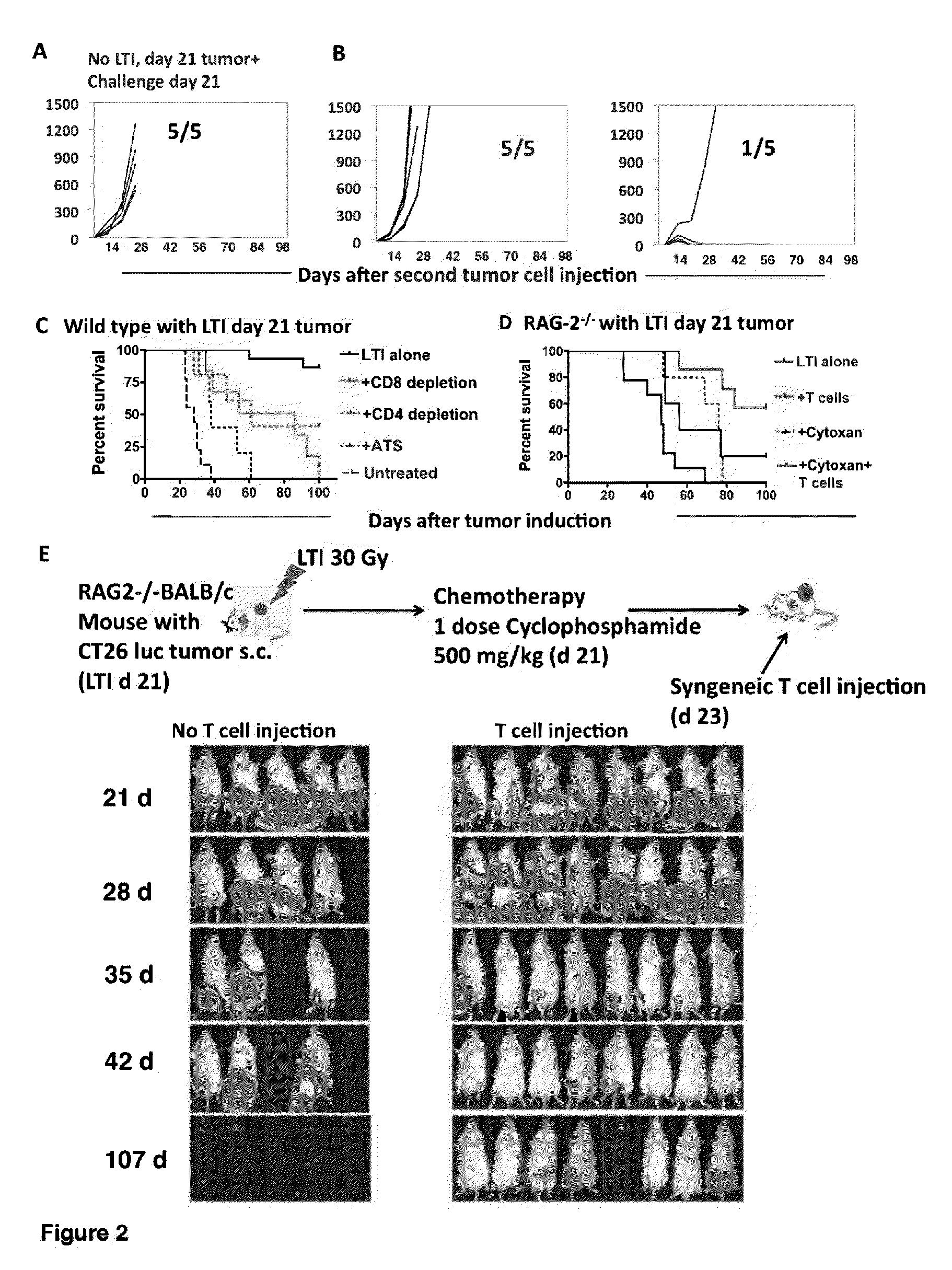
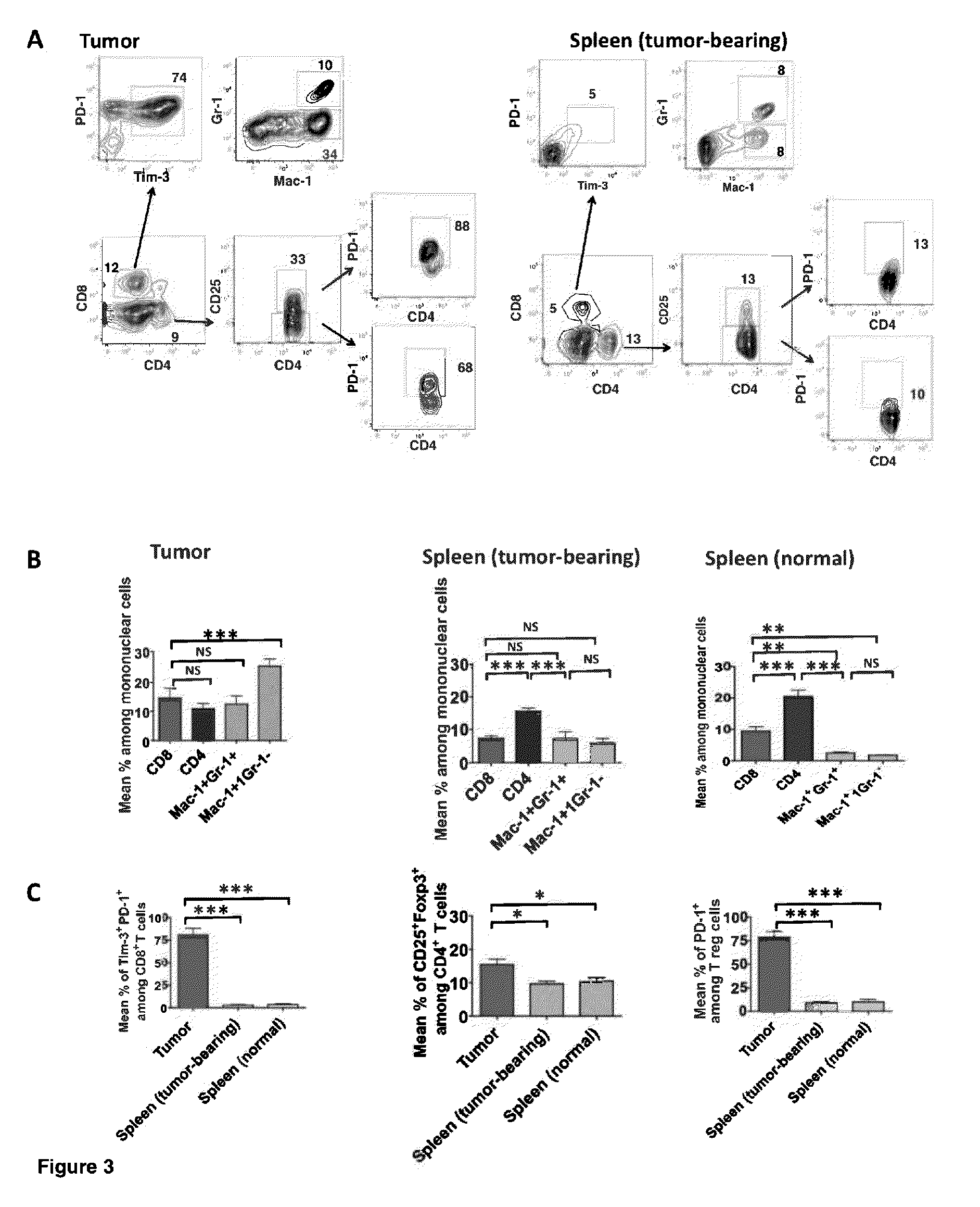
Anti-tumor T cell immunity induced by high dose radiation
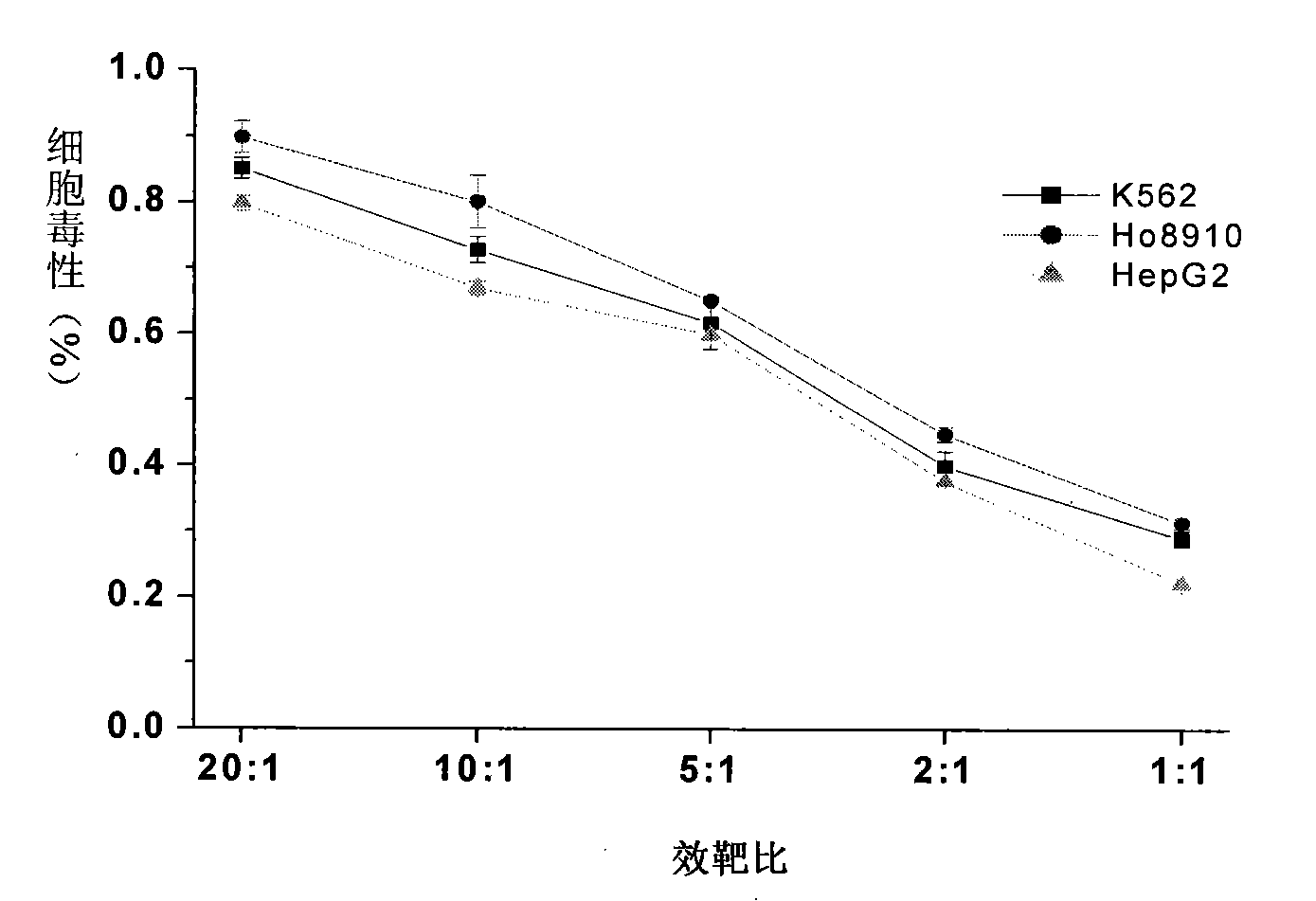
ActiveUS9114157B2Growth inhibitionMammal material medical ingredientsBlood/immune system cellsTumor responseCancer therapy
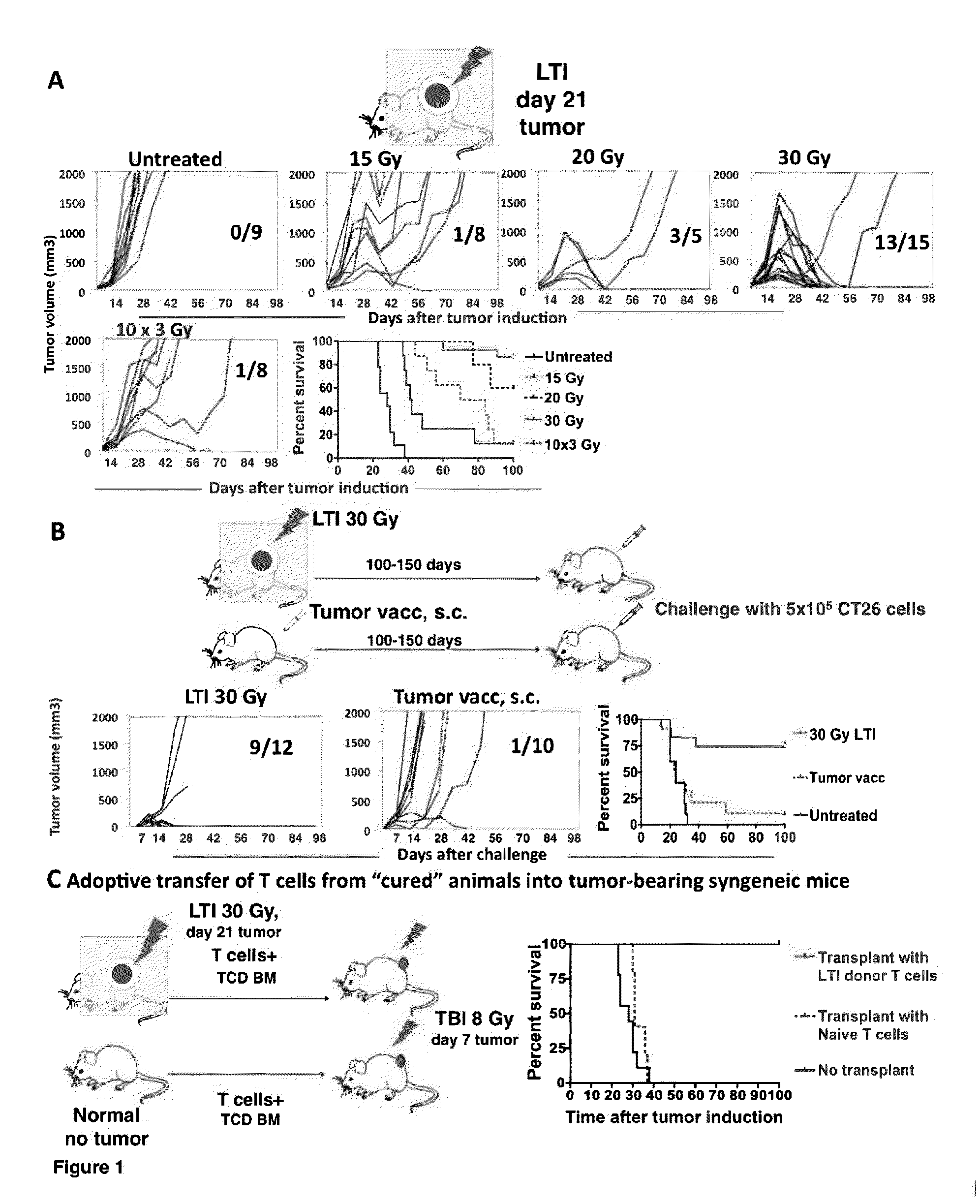
Cancer treatment is provided, by irradiating an individual with a localized, high single dose or short course of doses at a primary tumor site; collecting T cells from the individual after a period of time sufficient activation of an anti-tumor response; treating the individual with an effective dose of dose of chemotherapy; and reintroducing the T cell population back to the individual.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
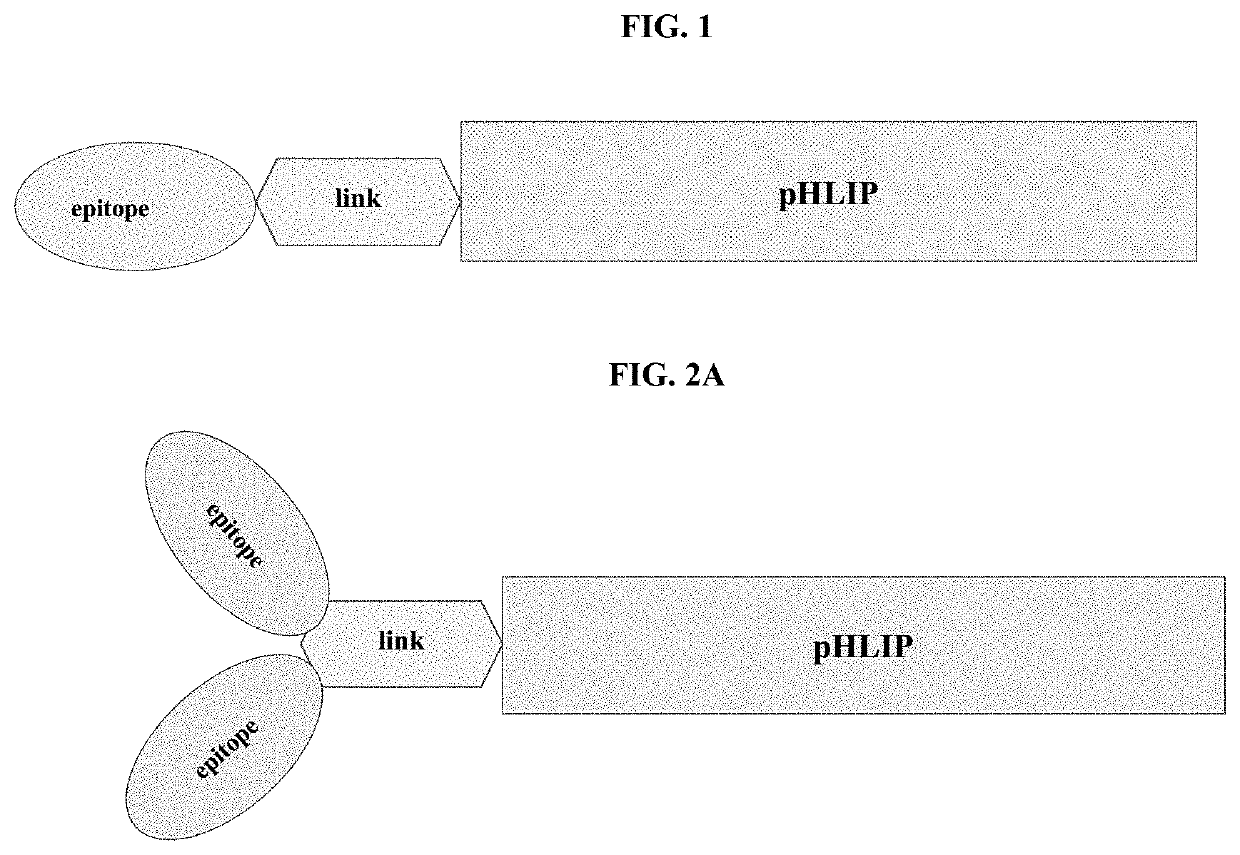
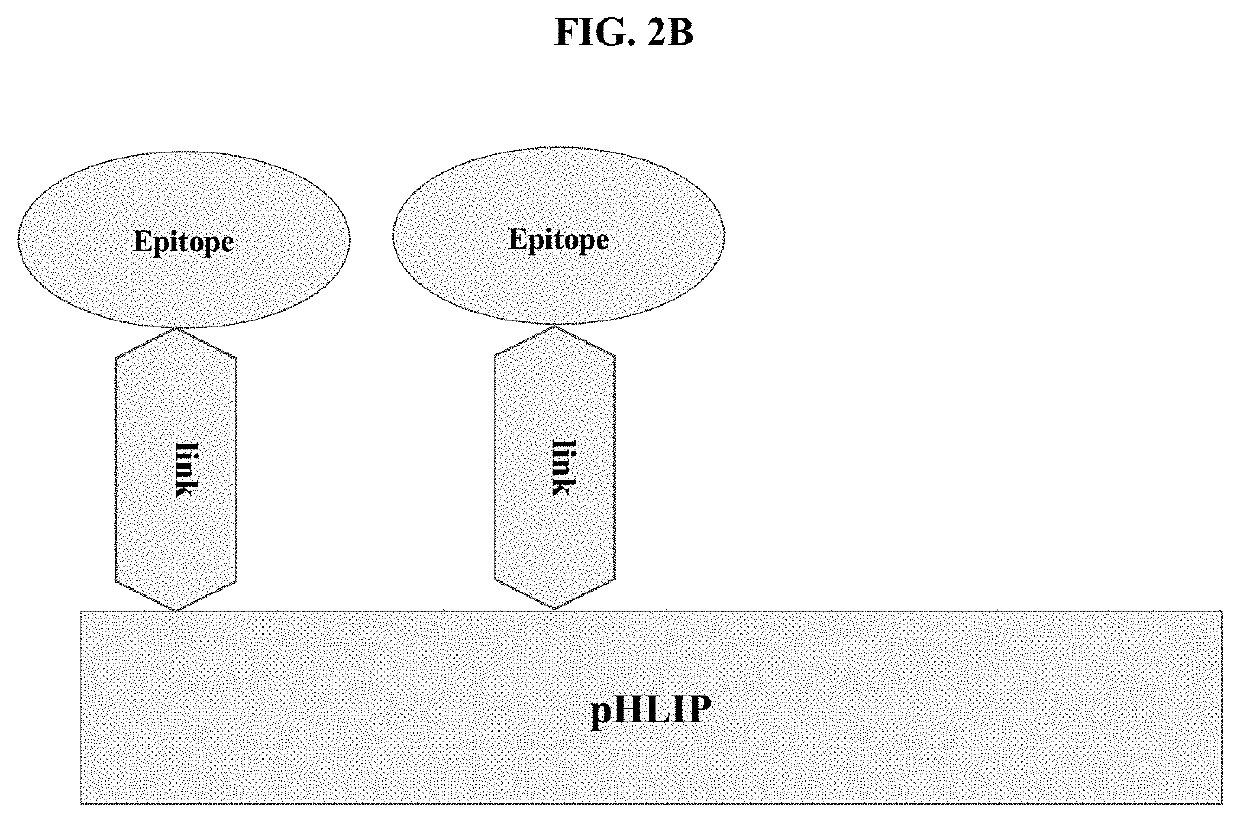
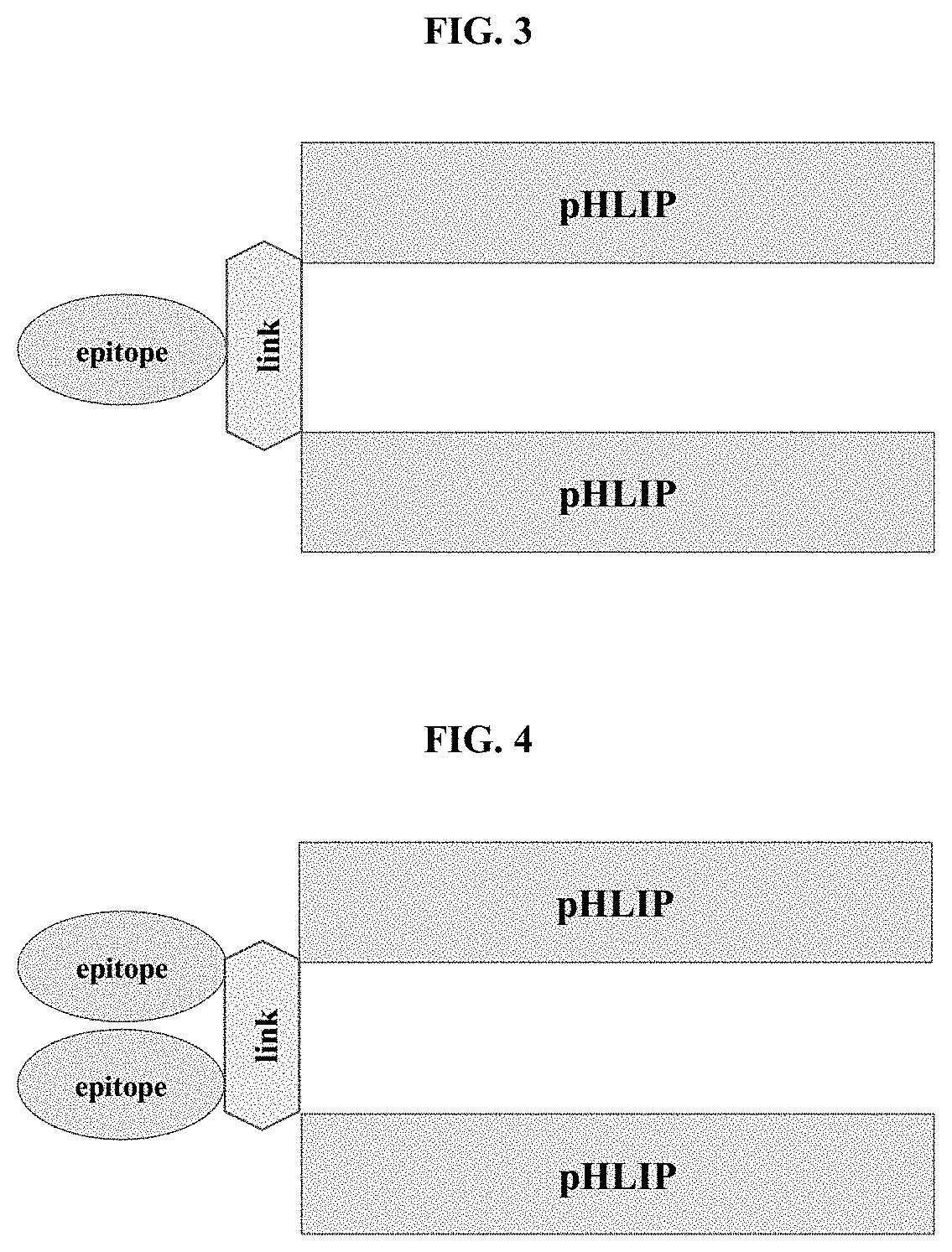
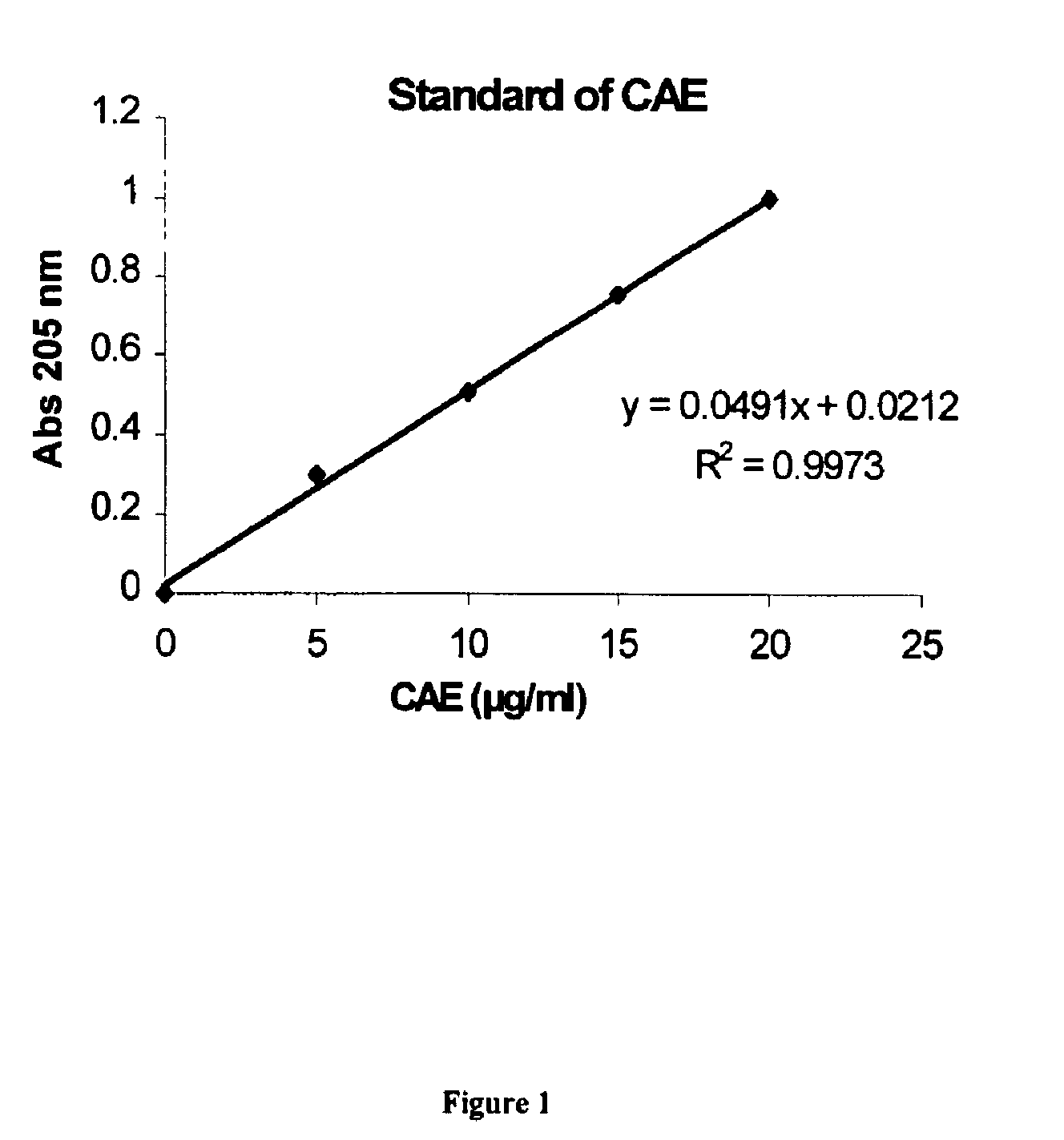
pHLIP® peptide-mediated epitope tethering at cell surfaces
PendingUS20200246420A1Enhances the recruitment of cellsEffective combinationPeptide/protein ingredientsPharmaceutical non-active ingredientsEpitopeTumor response
The invention features methods and compositions for eliciting an anti-tumor response in a subject comprising administering to the subject a pHLIP® construct comprising an antibody recruiting molecule linked to one or more pHLIP® peptides by a non-cleavable linker compound. The construct increases the amount of the antibody recruiting molecule on the surface of a diseased cell.
Owner:UNIV OF RHODE ISLAND BOARD OF TRUSTEES +1
Method of preparation and composition of a water soluble extract of the bioactive component of the plant species uncaria for enhancing immune, Anti-inflammatory, Anti-tumor and DNA repair processes of warm blooded animals
InactiveUS6964784B2Enhance the immune competency of a mammalInhibit productionBiocideHydroxy compound active ingredientsMammalFreeze-drying
A method for isolating the bioactive component of the water-soluble extract of Uncaria tomentosa known as C-MED-100®, comprising (i) precipitating the spray drying carrier from C-MED-100®; (ii) using the resulting C-MED-100® to obtain a spotting mixture for thin layer chromatography (TLC); (iii) spotting the C-MED-100® spotting mixture on pre-run TLC plates and eluting the plates to obtain the fluorescing band with Rf=0.2-0.3; (iv) scraping off the Rf=0.2-0.3 band, eluting it in ammonia and freeze drying the eluted band to form a powder; and (v) extracting the powder with methanol to remove solubilized silica gel, concentrating the methanol solution and crystalizing the concentrated solution to obtain the bioactive component. The isolated bioactive component is a quinic acid analog, preferably quinic acid lactone. A pharmaceutical composition comprising a pharmaceutically effective amount of the bioactive component and a nontoxic inert carrier or diluent. The bioactive component may be used to enhance immune competency, treat disorders associated with the immune system, inhibit the inflammatory response, treat disorders associated with the inflammatory response, enhance the anti-tumor response, and treat disorders associated with the response to tumor formation and growth, all in mammals.
Owner:OPTIGENEX INC +1
Immunoglobulin construct containing tumor- specific p53bp2 sequenes for eliciting an anti-tumor response
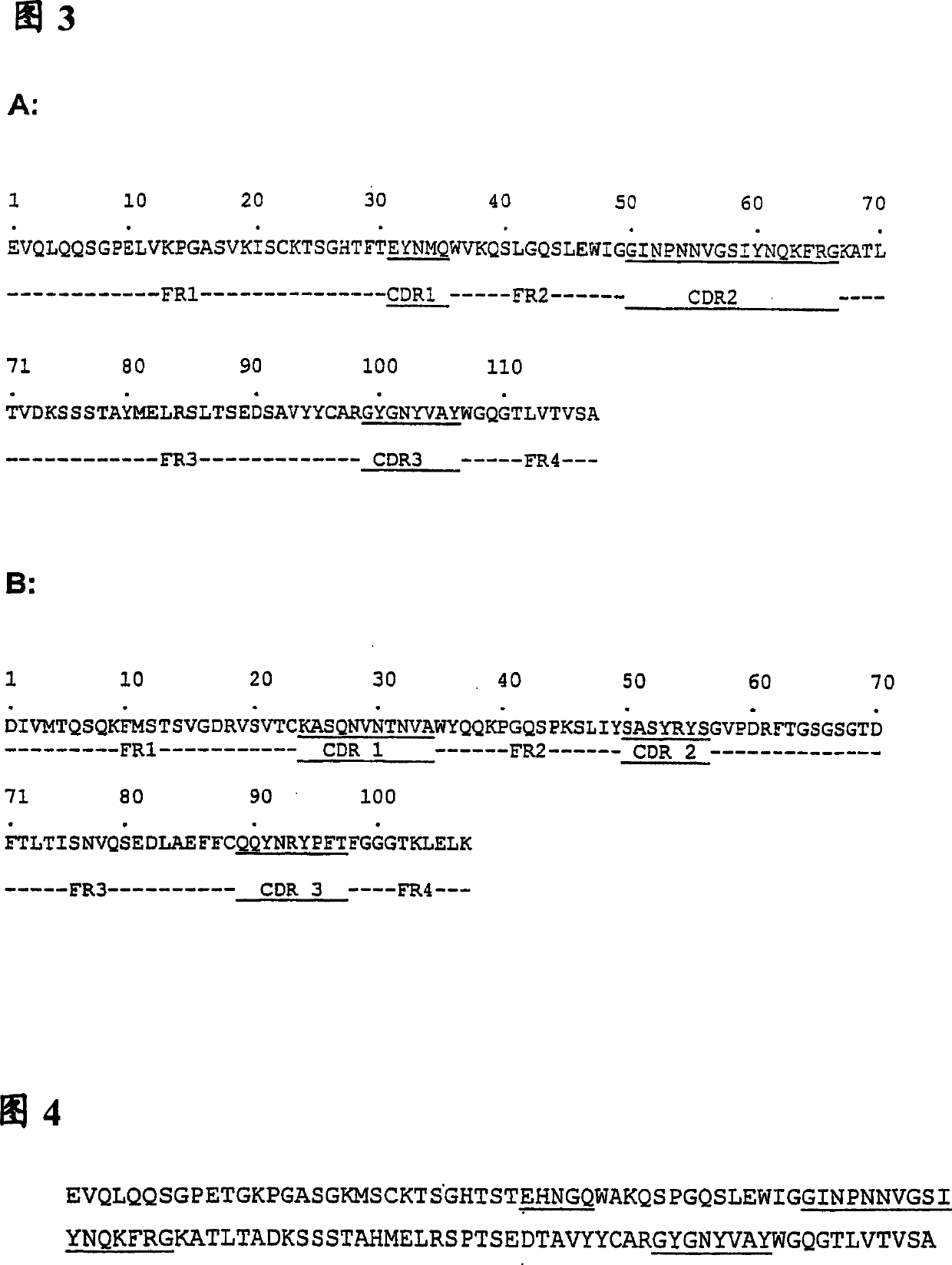
The present invention provides immunoglobulins specific for p53BP2 ligand polypeptides. In a preferred embodiment, the present invention provides a variant of an immunoglobulin variable domain, wherein the immunoglobulin variable domain contains (A) at least one CDR region and (B) framework regions flanking the CDR, and the variant includes: (a) the CDR region having added or substituted therein at least one binding sequence, and (b) the flaking framework regions, wherein the binding sequence is heterologous to the CDR and the binding sequence is derived from a human ligand having immunogenic properties relevant to human lung cancer. In a preferred embodiment, the binding sequence is an antigenic sequence. In a further preferred embodiment, the variant contains a variable domain lacking an intrachain disulfide bond.
Owner:EURO-CELTIQUE SA
Method and compositions for cellular immunotherapy
ActiveUS9987308B2Maintain normalEnhance immune responsePeptide/protein ingredientsAntibody mimetics/scaffoldsPharmaceutical formulationWilms' tumor
The present invention provides methods and compositions to confer and / or augment immune responses mediated by cellular immunotherapy, such as by adoptively transferring genetically modified tumor specific CD8+ T cells in the presence of tumor-specific, subset specific genetically modified CD4+ T cells, wherein the CD4+ T cells confer and / or augment a CD8+ T cells ability to sustain anti-tumor reactivity and increase and / or maximize tumor-specific proliferation of the tumor-specific CD8+ T cells of interest. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Natural killer cell and cultivation method thereof
ActiveCN101481678AQuality improvementUniform qualityUnknown materialsBlood/immune system cellsCell phenotypeAbnormal tissue growth
The invention discloses a natural killer cell and a culturing method thereof. The natural killer cell is a human natural killer cell NKG cell of which the preservation number is CGMCC No. 2901. The NKG cell has strong lethality to tumor cells, strong survival ability in vivo, stable quality and strong tumor-resistant reaction. Cells cultured by the method have fast growing speed, large quantity, even quality, high and stable survival rate. The preparation method has easy operation, controllable quality, high yield and low cost, and realizes mass, scale, homogeneous and streamlined production for cell treating products like medicament. The method for culturing NKG cells in a large scale can ensure cell phenotype, purity, activity, cell density, culturing volume and the like to achieve clinical treatment and ensure that the NKG cells can be applied to clinical treatment for malignant tumors and injection for 1 to 5 patients at the same time. Therefore, the cells, the method for preparing cells and the preparation method for cell liquid preparation have wide application value in treating malignant tumors.
Owner:UNIV OF SCI & TECH OF CHINA
Method of Predicting Responsiveness of B Cell Lineage Malignancies to Active Immunotherapy
InactiveUS20140220562A1Microbiological testing/measurementDisease diagnosisTumor responseActive immunization
Predictive biomarkers identify those patients suffering from immunoglobulin positive (Ig+) B lineage malignancies that are responsive to active immunotherapy, where the active immunotherapy comprises vaccination with a tumor-specific idiotype-immunogen. It is shown herein that patient responsiveness to the idiotype-immunogen is dependent upon the sequence of the immunogen, where an immunogen having a low number of tyrosine residues in the CDR1 (herein termed CDR1-Y10) regions of one or both of the immunogen heavy and light chains is predictive of a positive anti-tumor response, while a high number of CDR1 tyrosine residues (herein termed CDR1-Yhi) is predictive of a low anti tumor response.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Biomarkers for cancer treatment
The present invention provides identification of a thirty-five gene set that predicts the anticancer activity of inhibitors of the RAF / MEK / MAPK pathway, methods of qualifying cancer status in a subject, methods of identifying an anti-tumor response in a subject, methods of monitoring the efficacy of a therapeutic drug in a subject, and methods of identifying an agent useful in the treatment of a cancer based on expression of the thirty-five gene set.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
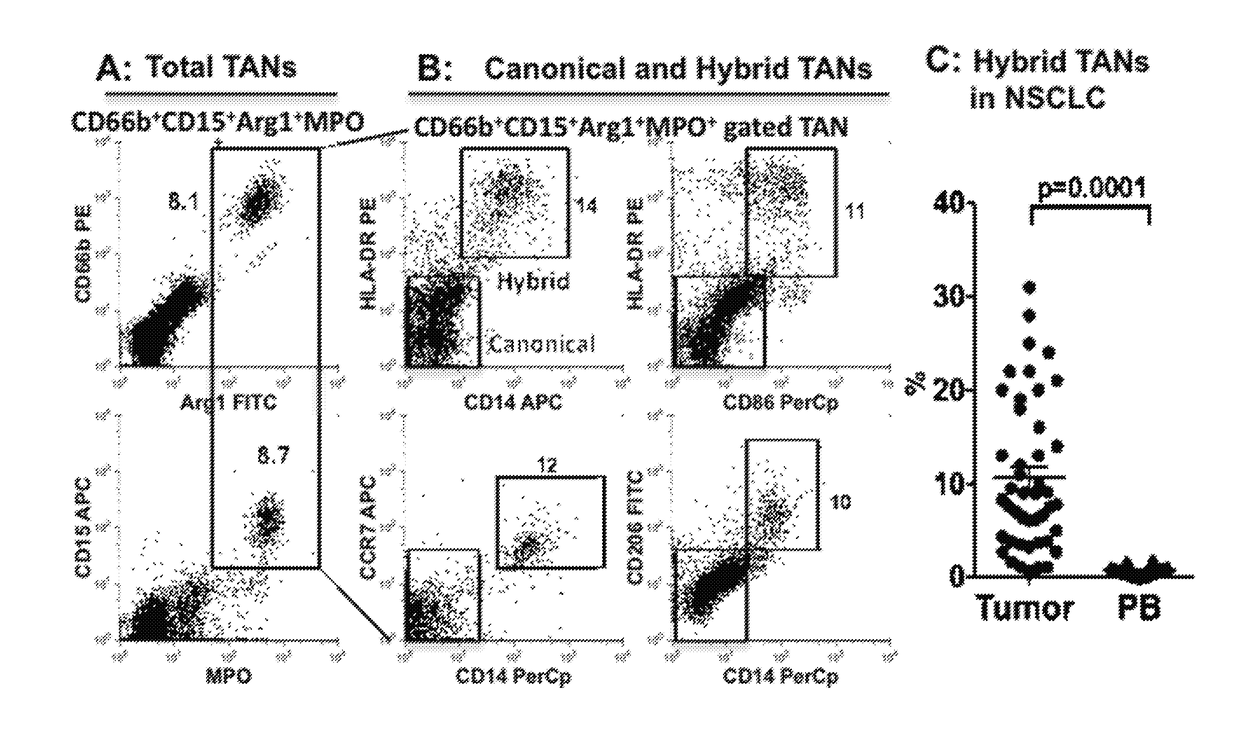
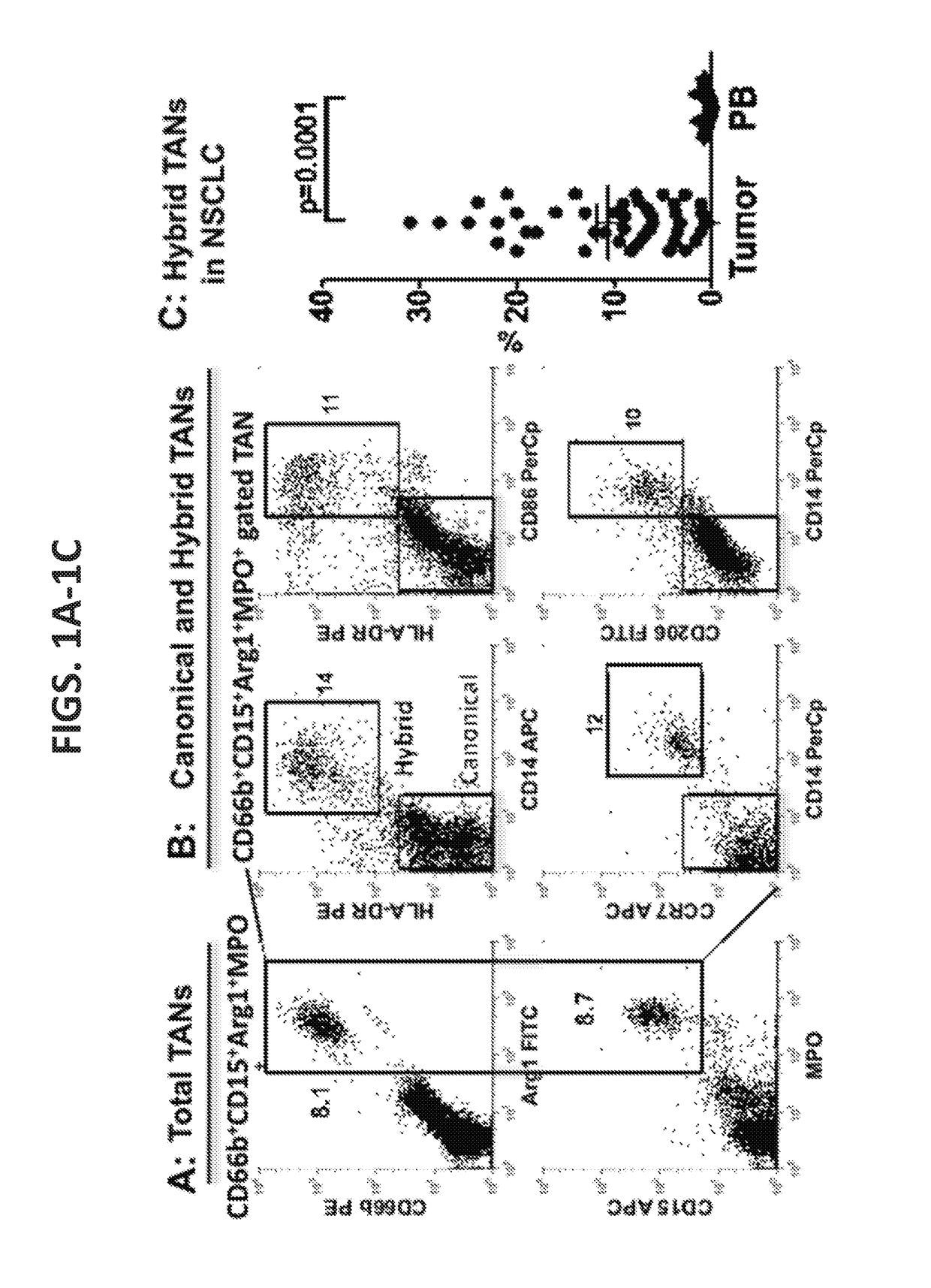
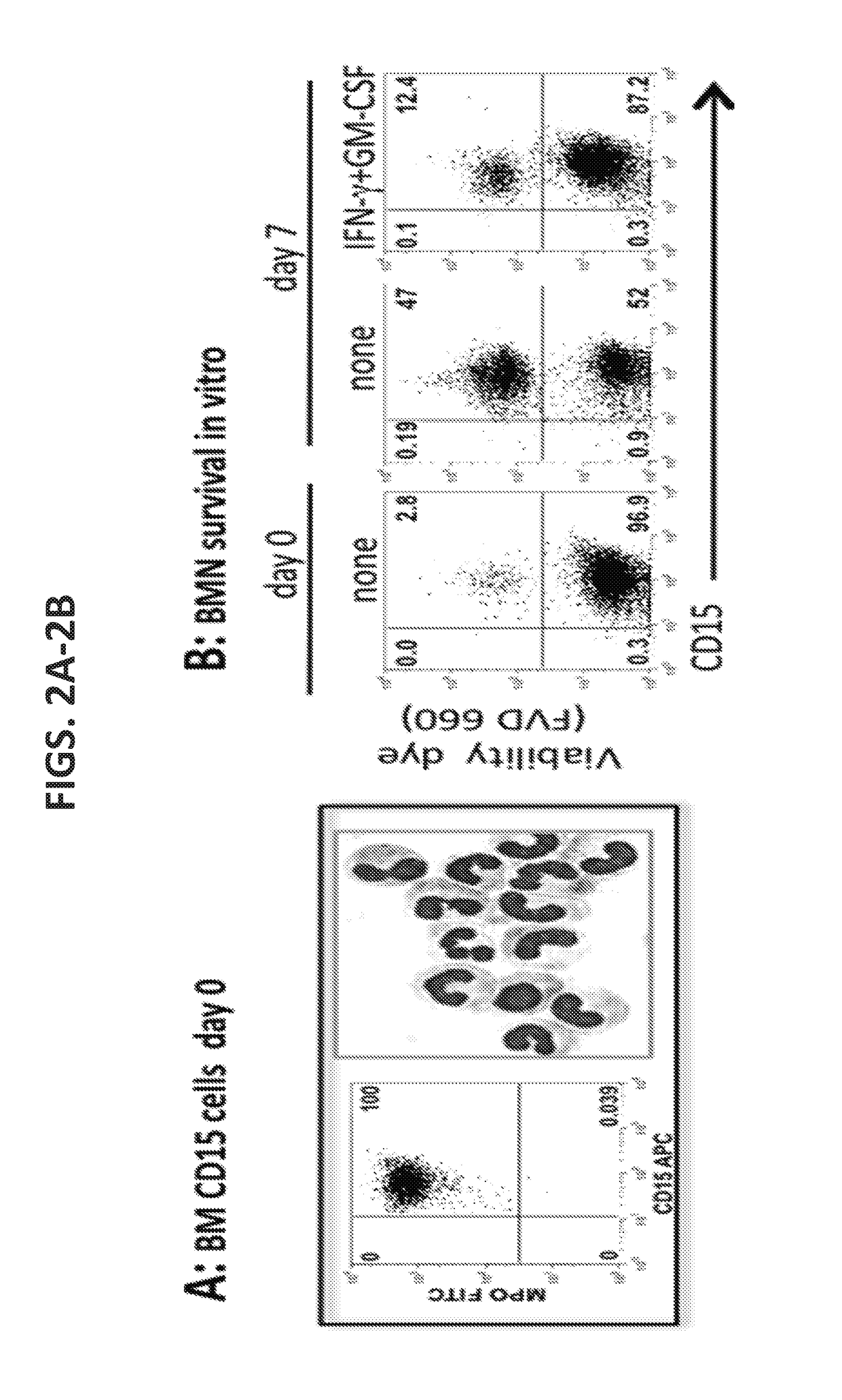
Compositions and methods of enhancing Anti-tumor response using hybrid neutrophils
InactiveUS20180250336A1Good curative effectOrganic active ingredientsPeptide/protein ingredientsTumor responseCD15
The present invention relates to compositions and methods that provide novel anti-tumor therapies in cancer. In one aspect, the present invention features a hybrid neutrophil in a non-naturally occurring container, wherein the hybrid neutrophil expresses at least one neutrophil associated molecule selected from the group consisting of: Arg1, MPO, CD66b, and CD15, and at least one antigen-presenting cell (APC) associated molecule selected from the group consisting of: CD14, HLA-DR, CD32, CD64, and CD89. In another aspect, the present invention features methods of generating a hybrid neutrophil. In still another aspect, the present invention features methods of inhibiting tumor growth in a subject, treating a tumor in a subject, and increasing efficacy of an antibody against a tumor in a subject. The methods comprise (a) administering to the subject an effective amount of an anti-tumor antibody and (b) administering to or generating in the subject an effective amount of a hybrid neutrophil.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Compositions and Methods With Enhanced Therapeutic Activity
InactiveUS20090137687A1Prevent proliferationDecreased blood flowBiocideOrganic chemistryQuinoneTumor response
This invention relates to novel tricyclic quinone and catechol compositions, compositions containing prodrugs of tricyclic quinone and catechol compositions, and methods of use for the treatment of solid tumor cancers and other vascular proliferative disorders. In certain aspects, the compositions of the invention are capable of generating both a vascular targeting effect and tumor cell cytotoxicity (e.g., by oxidative stress) in order to achieve an enhanced anti-tumor response in a patient.
Owner:OXIGENE +1
Anti-idiotype anti-CEA antibody molecules and its use as cancer vaccine
The present invention provides molecules, preferably designed immunoglubulins, suitable for use as an anti-idiotype vaccine to CEA positive tumours. The molecules induce both an MHC class I and MHC class II mediated immune response to the CEA bearing tumour cells for an efficient and sustained host anti-tumour response. The present invention provides modified versions of anti-idiotype anti-CEA antibodies, preferably mouse antibody 708, with improved vaccination properties. The modifications are related to the introduction of sequences tracts deriving from e.g. CEA, CD55 antigen and CEA cancer-specific MHC epitopes into the variable regions of said antibody molecules.
Owner:MERCK PATENT GMBH
Natural killer cell and cultivation method thereof
ActiveCN101481678BQuality improvementUniform qualityArtificial cell constructsBlood/immune system cellsCell phenotypeCultured cell
The invention discloses a natural killer cell and a culturing method thereof. The natural killer cell is a human natural killer cell NKG cell of which the preservation number is CGMCC No. 2901. The NKG cell has strong lethality to tumor cells, strong survival ability in vivo, stable quality and strong tumor-resistant reaction. Cells cultured by the method have fast growing speed, large quantity, even quality, high and stable survival rate. The preparation method has easy operation, controllable quality, high yield and low cost, and realizes mass, scale, homogeneous and streamlined production for cell treating products like medicament. The method for culturing NKG cells in a large scale can ensure cell phenotype, purity, activity, cell density, culturing volume and the like to achieve clinical treatment and ensure that the NKG cells can be applied to clinical treatment for malignant tumors and injection for 1 to 5 patients at the same time. Therefore, the cells, the method for preparing cells and the preparation method for cell liquid preparation have wide application value in treating malignant tumors.
Owner:UNIV OF SCI & TECH OF CHINA
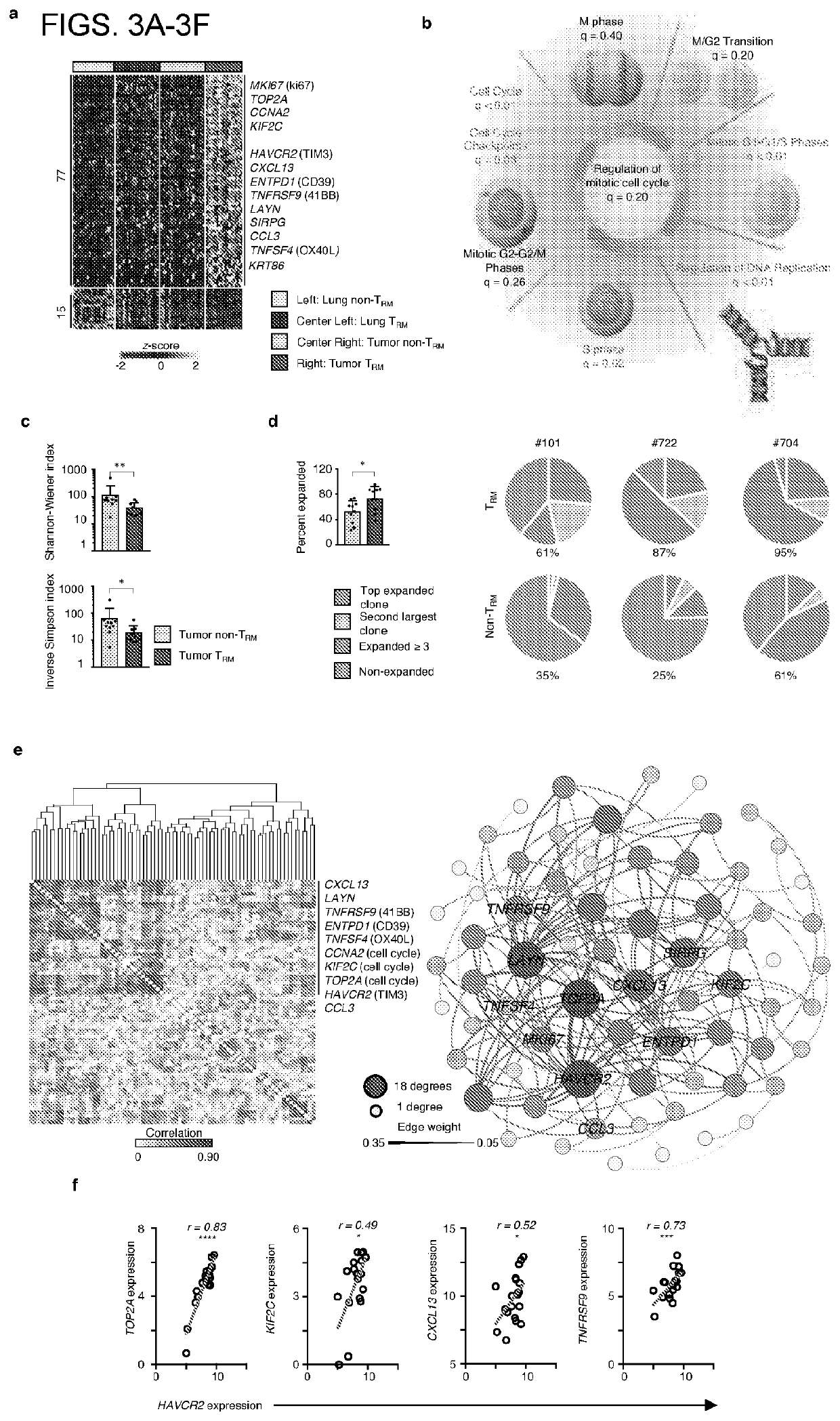
Tissue resident memory cell profiles, and uses thereof
PendingUS20210015866A1Positive prognosisIncrease probabilityOrganic active ingredientsMicrobiological testing/measurementAntigenTumor response
This disclosure provides methods of treating cancer or eliciting an anti-tumor response in a subject by administering an effective amount of a population of T-cells that exhibits higher or lower than baseline expression of one or more genes. In other aspects, methods are provided to diagnose cancer and determine prognosis of cancer patients. Also provided are methods to identify the antigens or antigen receptors associated with the isolated and / or purified cell populations that elicit a more positive prognosis.
Owner:UNIV OF SOUTHAMPTON +1
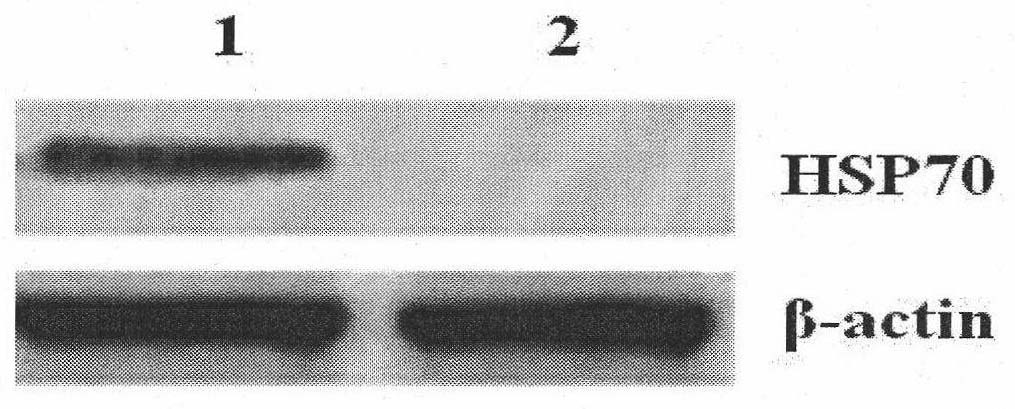
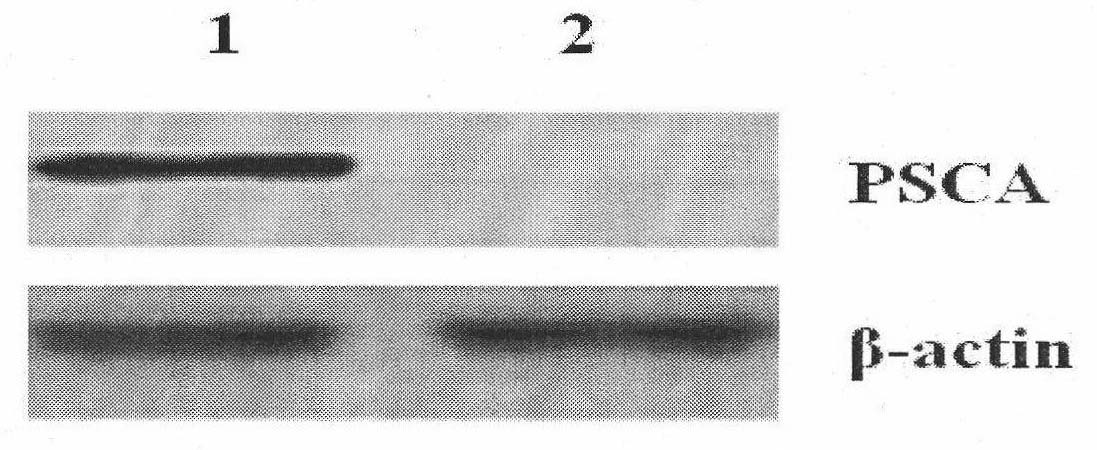
Compound of prostate stem cell antigen and heat shock protein and preparation method and application thereof
InactiveCN101912603AStructural ConservationStructurally highly conservedUrinary disorderMacromolecular non-active ingredientsAdjuvantTreatment field
Owner:ARMY MEDICAL UNIV
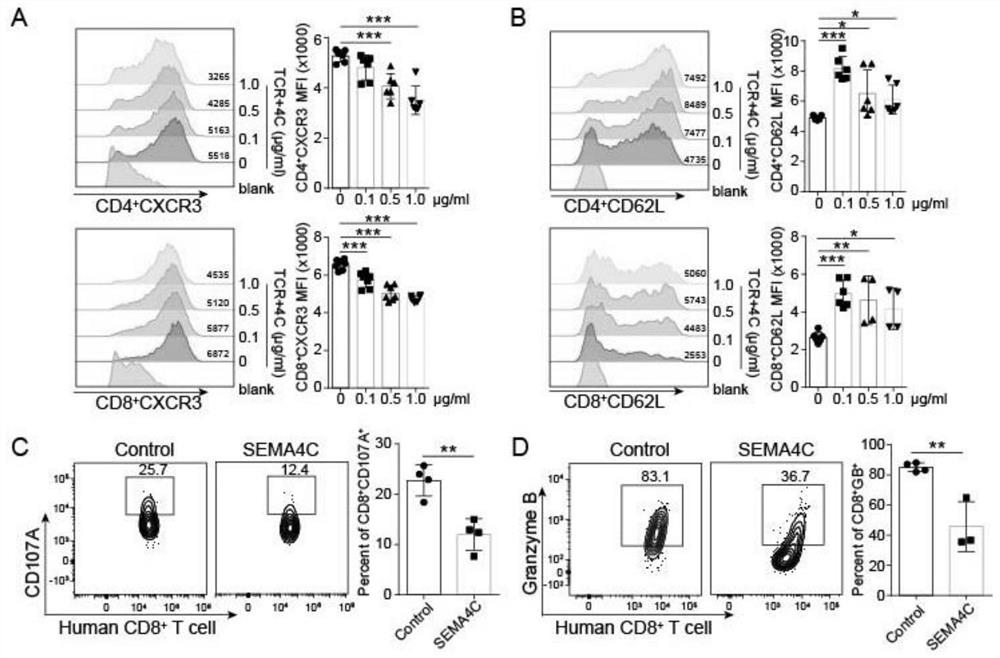
Application of SEMA4C in preparation of antitumor drugs
PendingCN112843255ARecovery functionGrowth inhibitionAntineoplastic agentsGene therapyLymph vessel formationAntitumor response
The invention belongs to the technical field of biological medicines, and particularly relates to application of SEMA4C in preparation of antitumor drugs. Based on research on breast cancer, SEMA4C with high breast cancer tissue specificity expression is found, and the SEMA4C with high expression participates in tumor metastasis invasion and tumor proliferation related to lymphatic vessel formation of breast cancer. However, the development of neutralizing antibodies in the aspects of immunoregulation, participation in anti-tumor reaction and medicines has not been studied and reported. According to the application, the interaction mode of SEMA4C and T cells is proved, a neutralizing antibody capable of blocking combination of SEMA4C and T cells is screened out, and an in-vitro co-culture experiment result shows that anti-SEMA4C can recover chemotactic and anti-tumor functions of the T cells; and in-vivo experiments find that anti-SEMA4C can significantly inhibit tumor growth, and the tumor inhibition effect is obviously better than that of anti-PD-1, which indicates that the SEMA4C neutralizing antibody has great potential in the anti-tumor aspect.
Owner:TONGJI HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI TECH +1
Process for preparing xenomal or autogenous tumor vaccine of liposome
InactiveCN1141975COvercoming limited difficultiesLasting effectAntibody medical ingredientsAntineoplastic agentsFreeze thawingLipid formation
A process for preparing xenogenic or autologous tumor vaccine of liposome includes such steps as taking the fresh specimen of human tumor, preparing suspension of tumor cells; freeze-thaw step to break off and deactivate tumor cells; n-butanol suspending to remove lipid; centrifugal step to remove karyon; lowry's test to test qualification of supernatant; and coating with liposome as carrier and wrapping with adjuvant to make vaccine. Its advantages include advanced and efficient extraction process, no toxic by-effect, and durable and slow release.
Owner:广州市肿瘤医院 +1
Methods and artificial tumors for validating an antitumor immune response
InactiveUS20190285615A1Microbiological testing/measurementAntibody ingredientsAbnormal tissue growthWilms' tumor
The invention provides methods for validating an antitumor response by growing and maintaining artificial 3D tumors under conditions that replicate the microenvironment of the naturally occurring parent tumor as found, in situ, in a subject diagnosed with a tumor or cancer. The artificial 3D tumors are obtained from the subject and once established are tested for susceptibility to proposed anticancer treatments, such as treatments personalized to the subject.
Owner:NANT HLDG IP LLC +1
Compositions and methods for enhancing the efficacy of cancer therapy
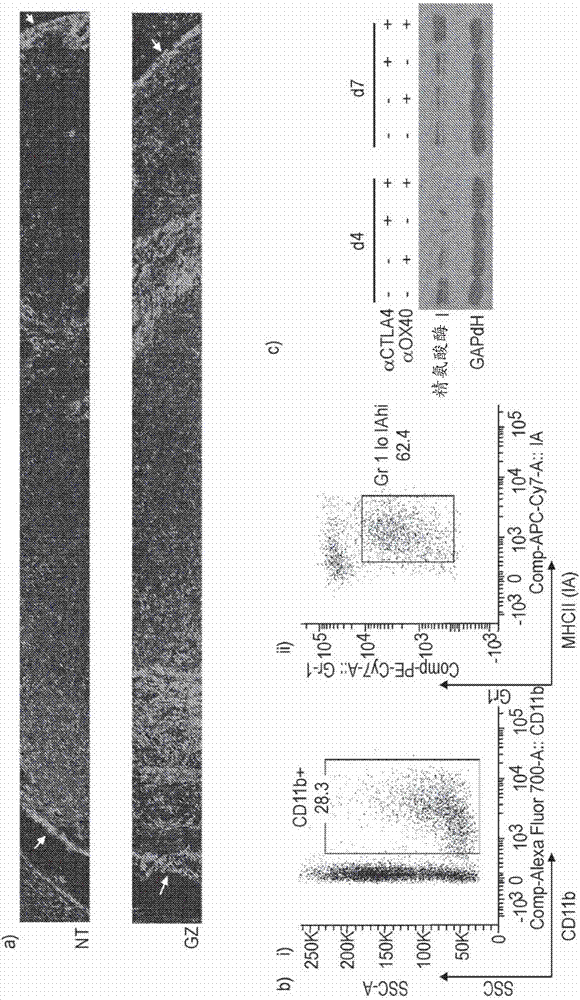
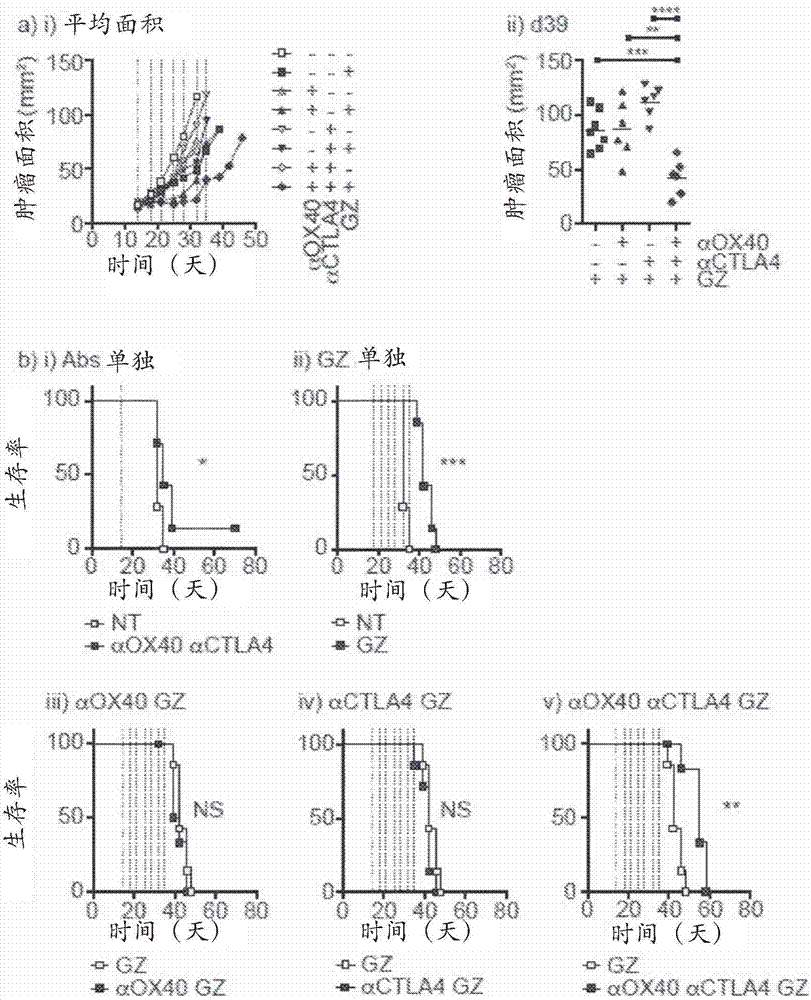
The present invention features compositions and methods for enhancing an anti-tumor response by administering an OX40 agonist (e.g., an anti-OX40 antibody) and / or an anti-CTLA4 antibody (e.g., a CTLA4-blocking antibody) in combination with a cancer therapy.
Owner:PROVIDENCE HEALTH & SERVICESOREGON
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com