Structure and use of 5'phosphate oligonucleotides
a technology of phosphate oligonucleotides and oligonucleotides, which is applied in the field of structure and use of 5'phosphate oligonucleotides, can solve the problems of reduced bone marrow function, high production cost, and broad side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
examples
Material and Methods
examples 1-10
Cell Culture
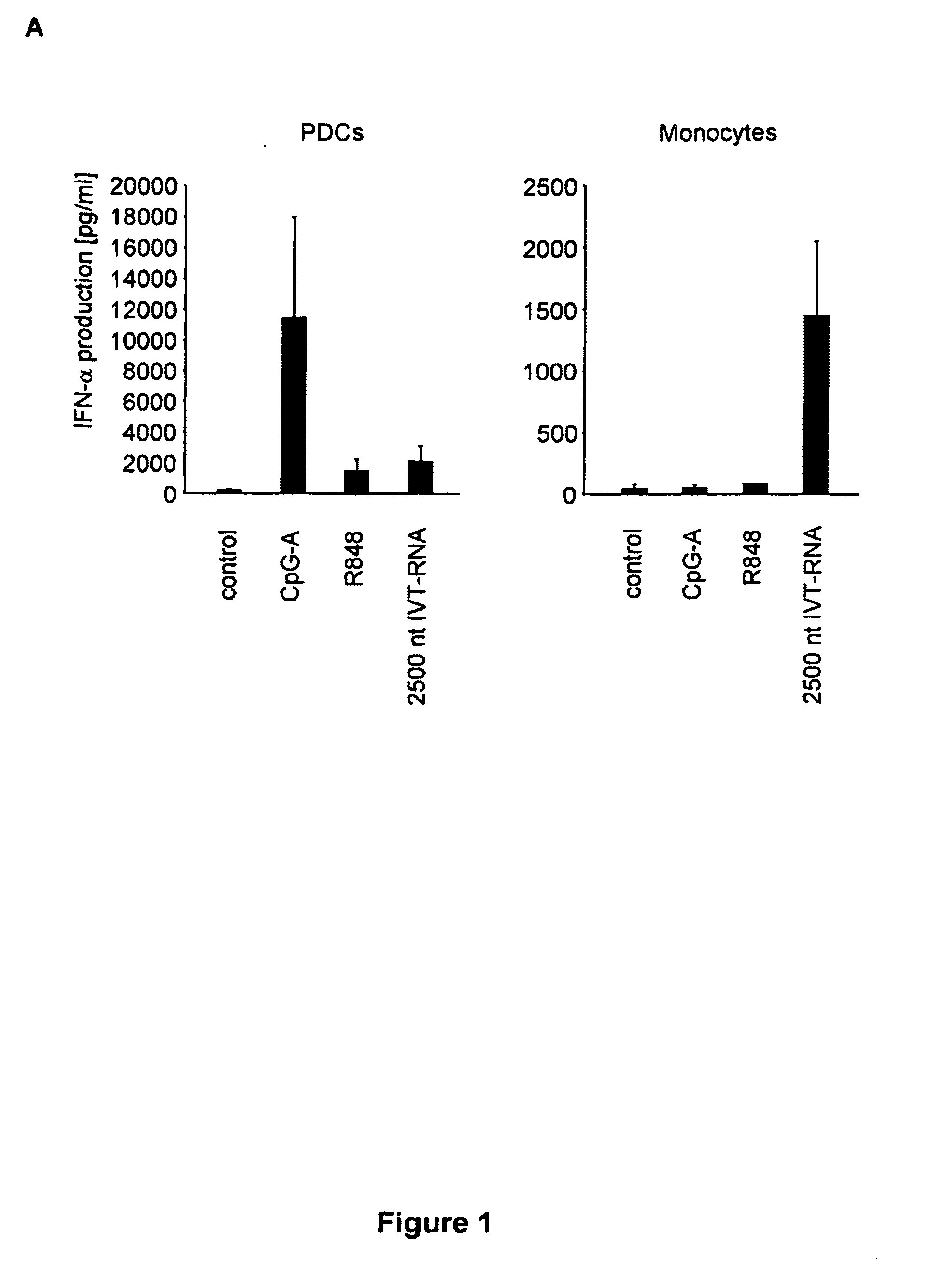
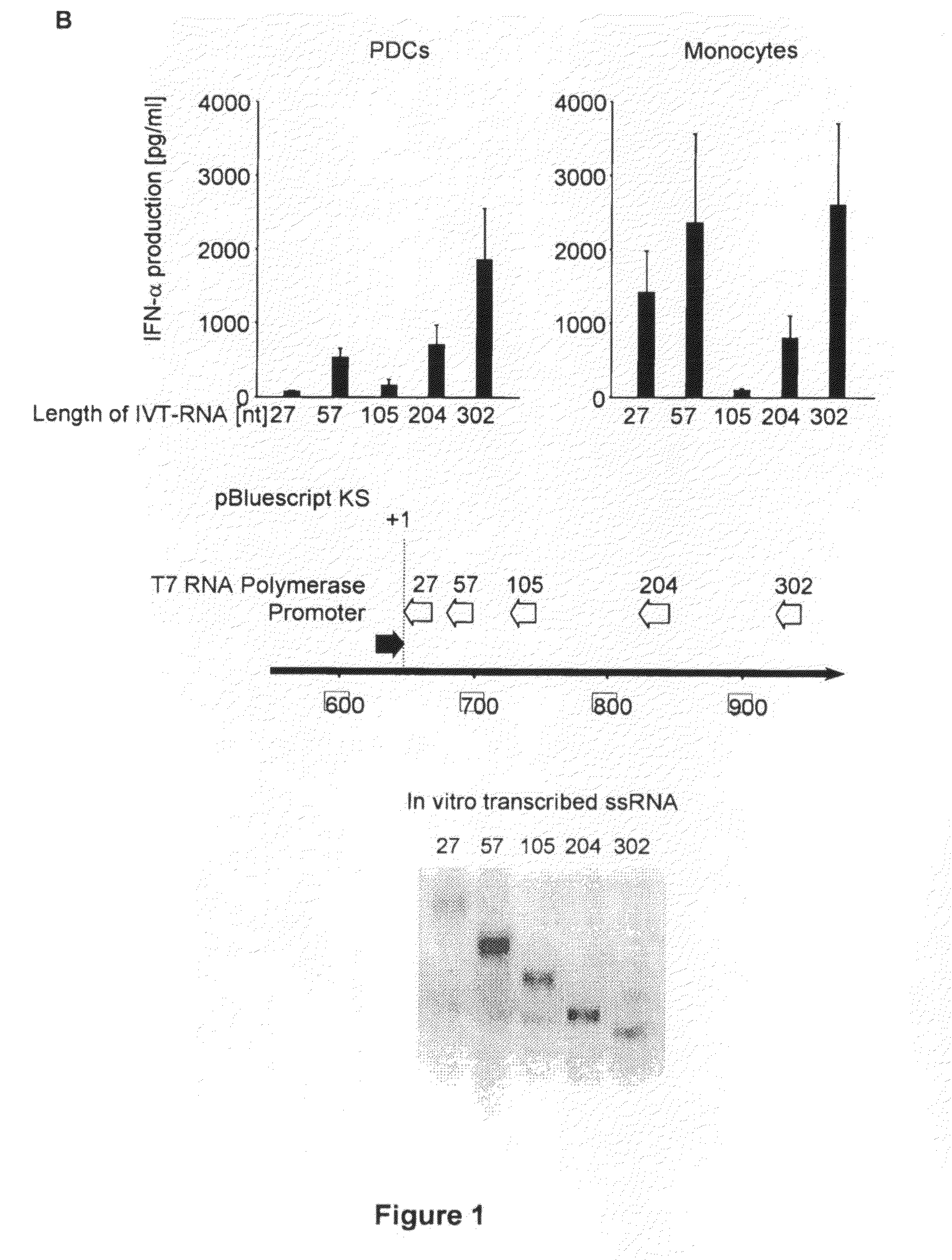
[0412]Human PBMC were prepared from whole blood donated by young healthy donors by Ficoll-Hypaque density gradient centrifugation (Biochrom, Berlin, Germany). PDC were isolated by MACS using the blood dendritic cell Ag (BCDA)-4 dendritic cell isolation kit from Miltenyi Biotec (Bergisch-Gladbach, Germany). Briefly, PDC were labelled with anti-BDCA-4 Ab coupled to colloidal paramagnetic microbeads and passed through a magnetic separation column twice (LS column, then MS column; Miltenyi Biotec). The purity of isolated PDC (lineage-negative, MHC-II-positive and CD123-positive cells) was above 95%. Before isolation of monocytes, PDC were depleted by MACS (LD column; Miltenyi Biotec) and then monocytes were isolated using the monocyte isolation kit II (Miltenyi Biotec). Murine bone marrow-derived conventional dendritic cells were generated by incubating pooled bone marrow cells in the presence of murine GM-CSF (10 ng / ml; R&D Systems, Minneapolis, Minn.). After 7 days, these ...
examples 11-16
Media and Reagents
[0424]RPMI 1640 (Biochrom) supplemented with 10% (v / v) heat-inactivated FCS (Invitrogen Life Technologies), 3 mM L-glutamine, 0.01 M HEPES, 100 U / ml penicillin, and 100 μg / ml streptomycin (all from Sigma-Aldrich) and Dulbecco's modified Eagle's medium (PAN, Aidenbach, Germany) supplemented with 10% fetal calf serum (FCS), 3 mM L-glutamine, 100 U / ml penicillin and 100 μg / ml streptomycin was used. CpG ODNs (Coley Pharmaceutical Group) show small letters, phosphorothioate (PT) linkage and capital letters, phosphodiester (PD) linkage 3′ of the base; CpG-A-ODN 2216 (5′-ggGGGACGATCGTCgggggG-3′), CpG-B ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′). Polyinosinic:polycytidylic acid (poly(I:C)) was purchased from Sigma-Aldrich. For depletion of NK cells and CD8 T cells, the IL-2 receptor-β chain-specific mAb TMβ1 and mAb RmCD8-2 were used as described (kind gift of Ralph Mocikat, GSF-Institut für Molekulare Immunologie, Munich, Germany). Recombinant murine IFNβ was purchased at Euro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
| Structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com