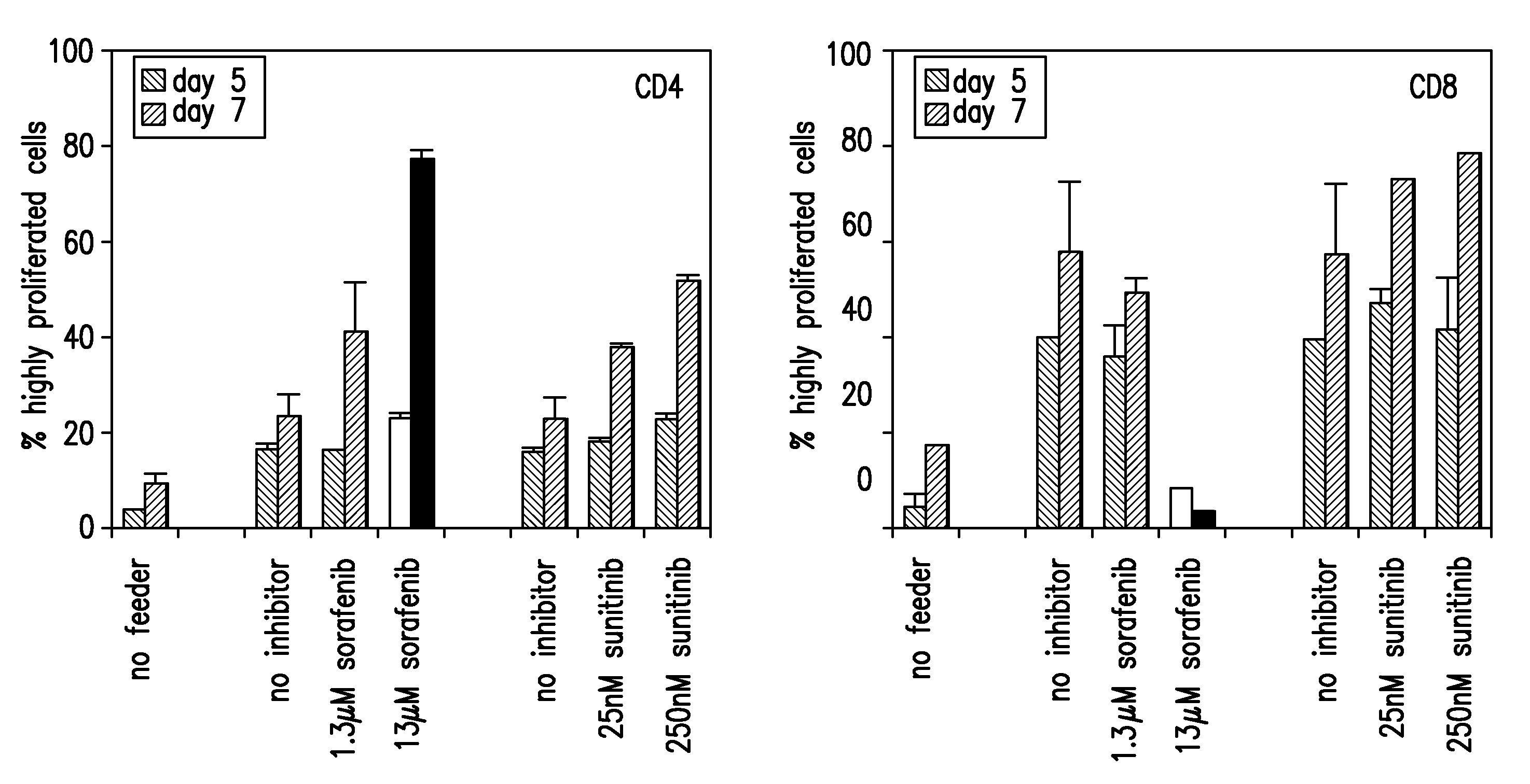
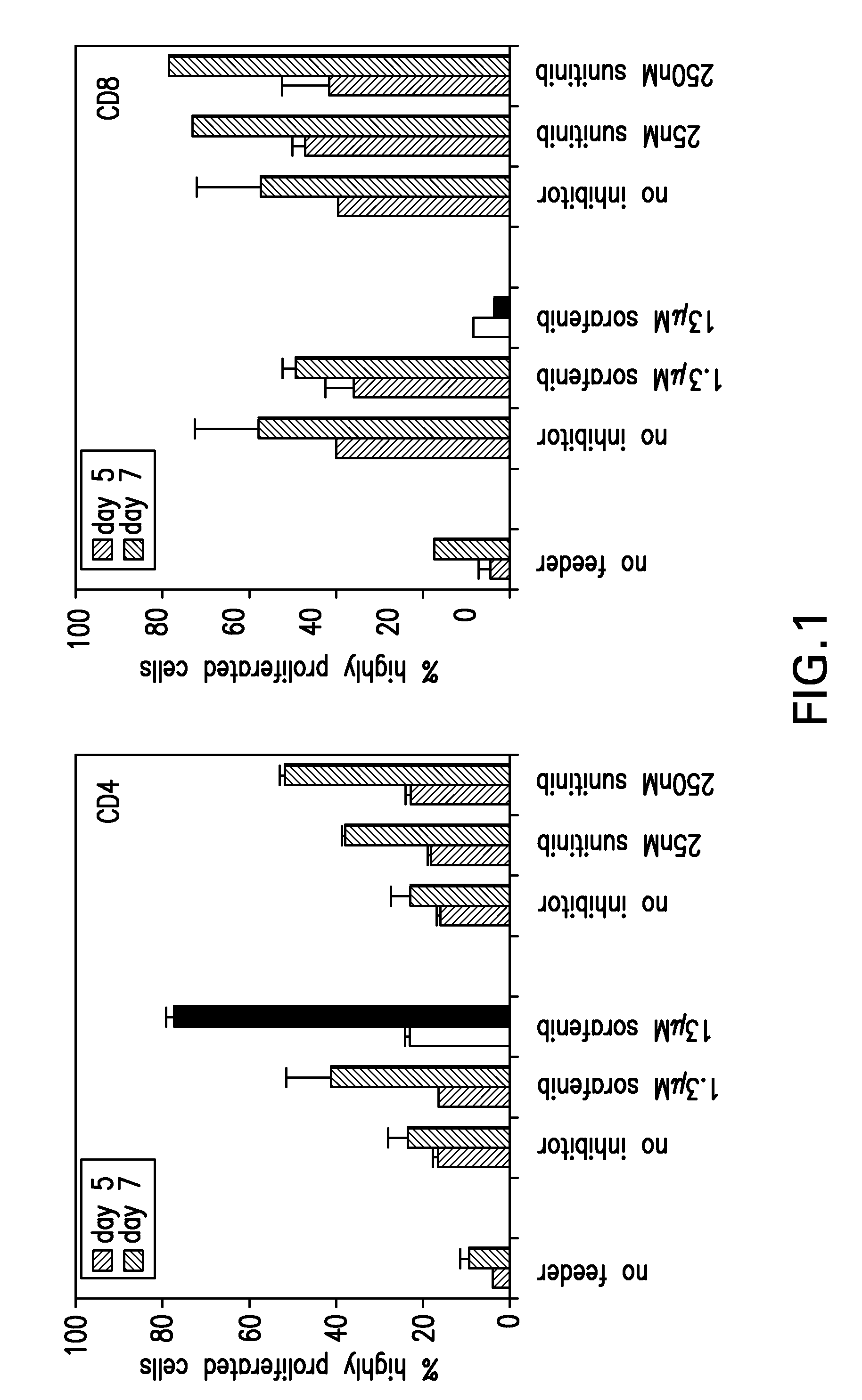
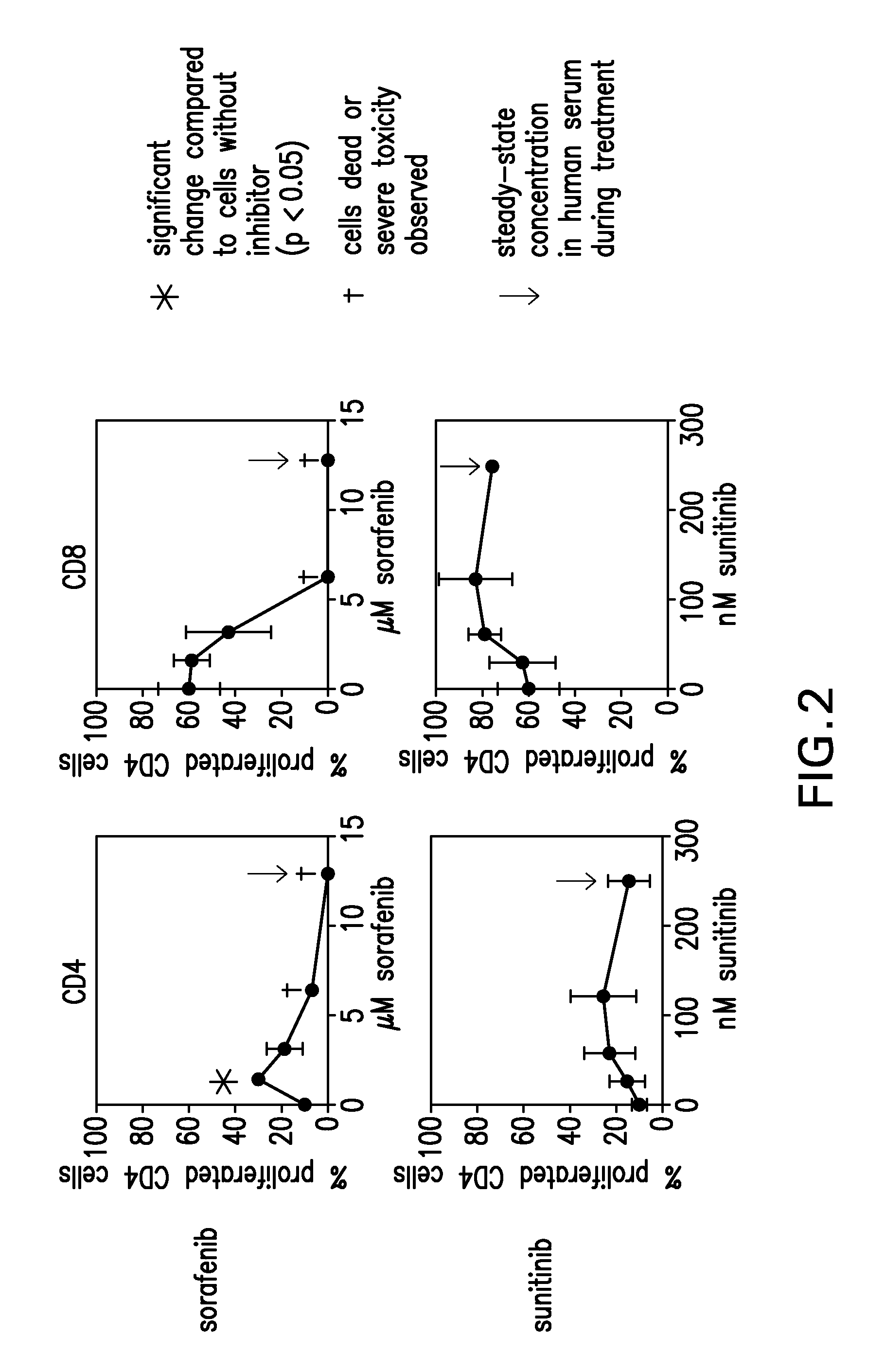
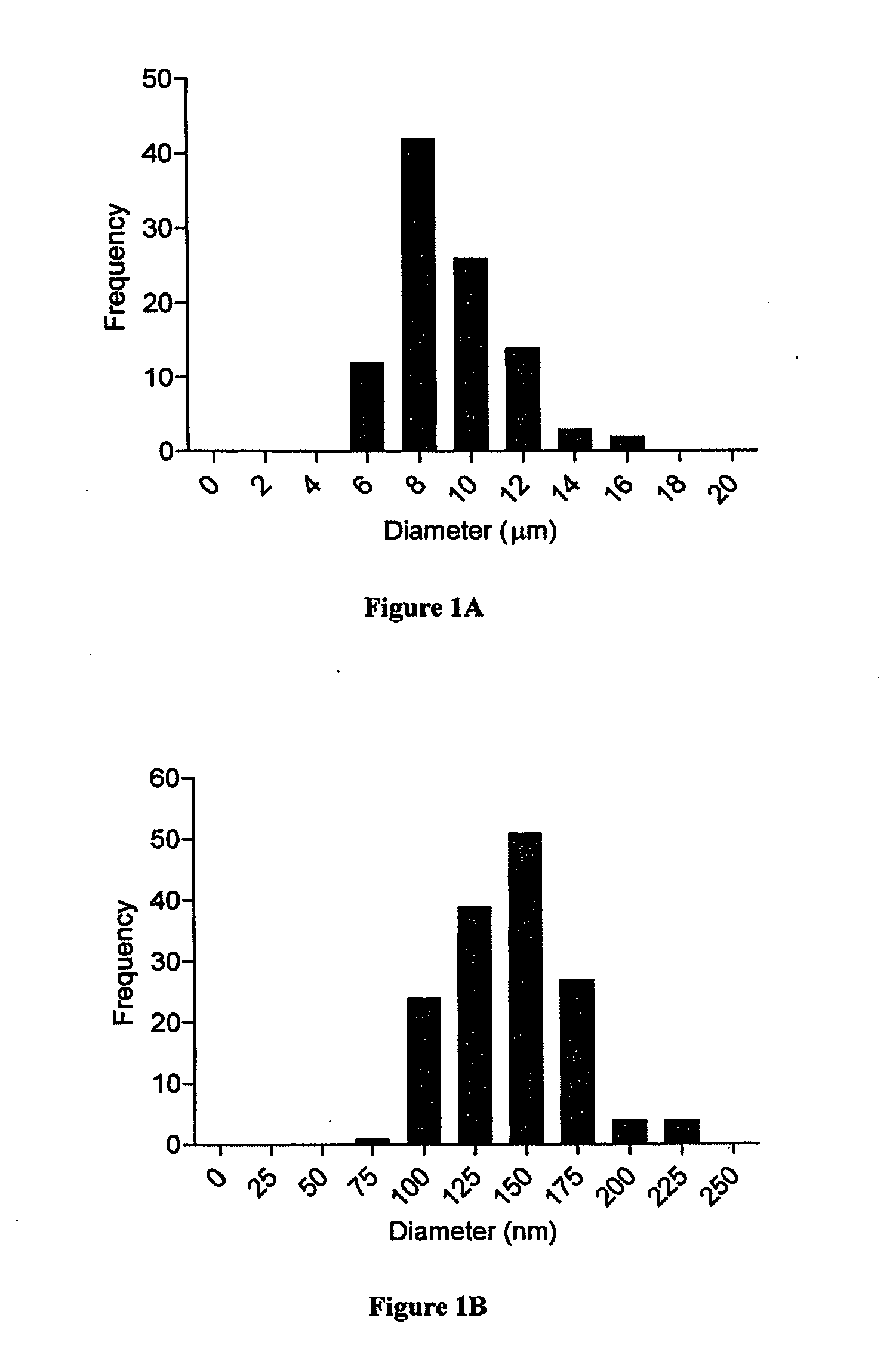
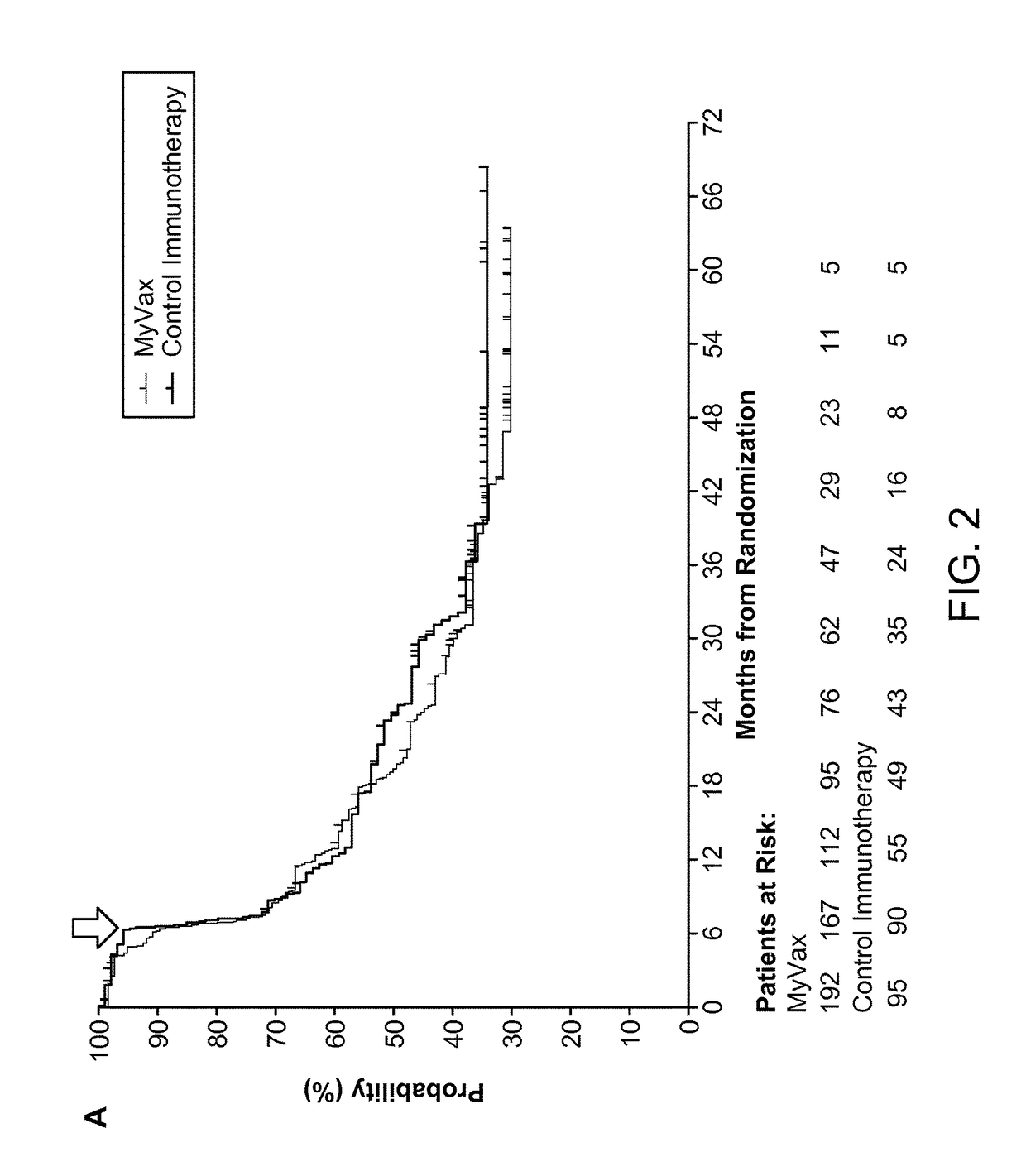
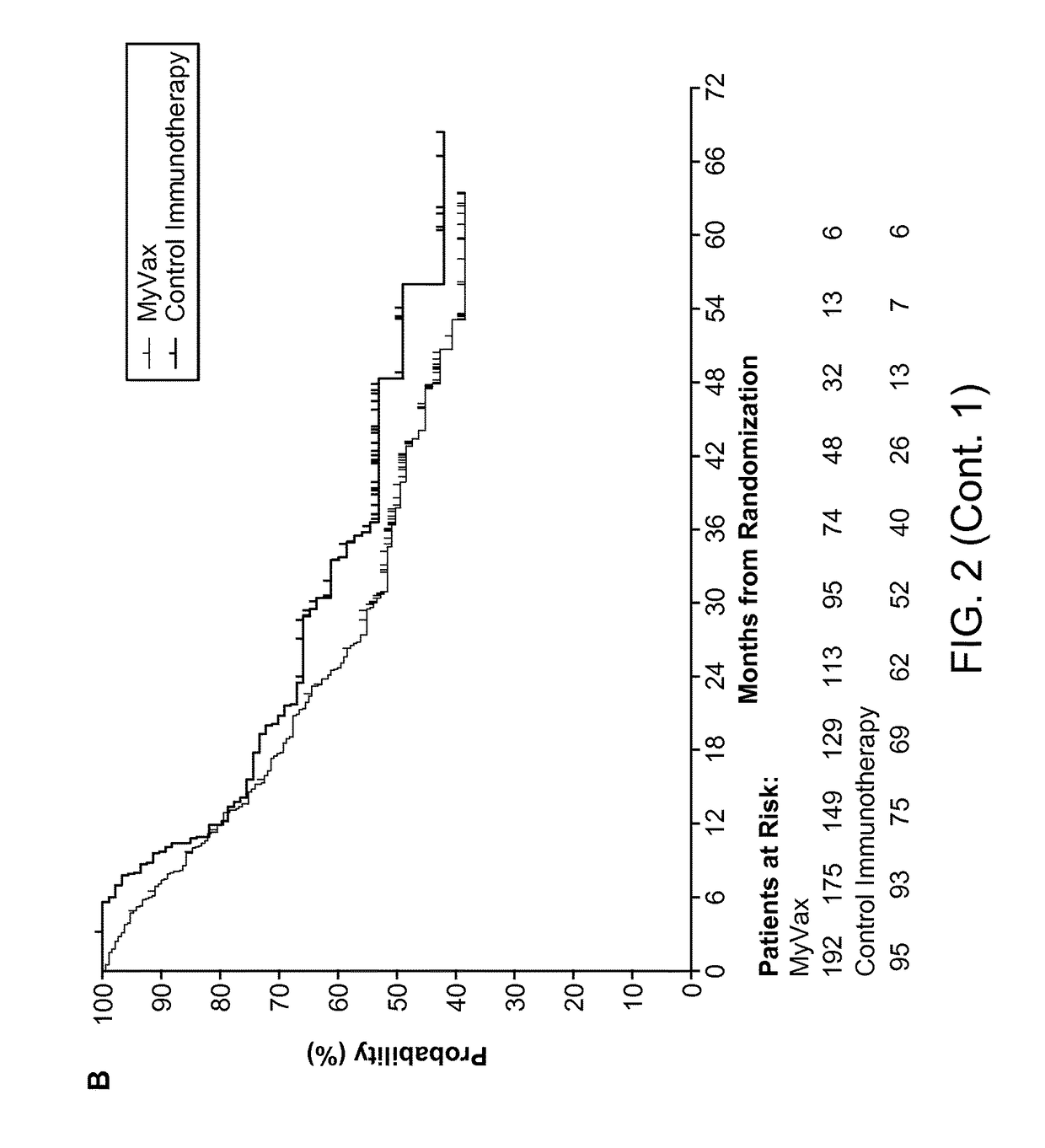
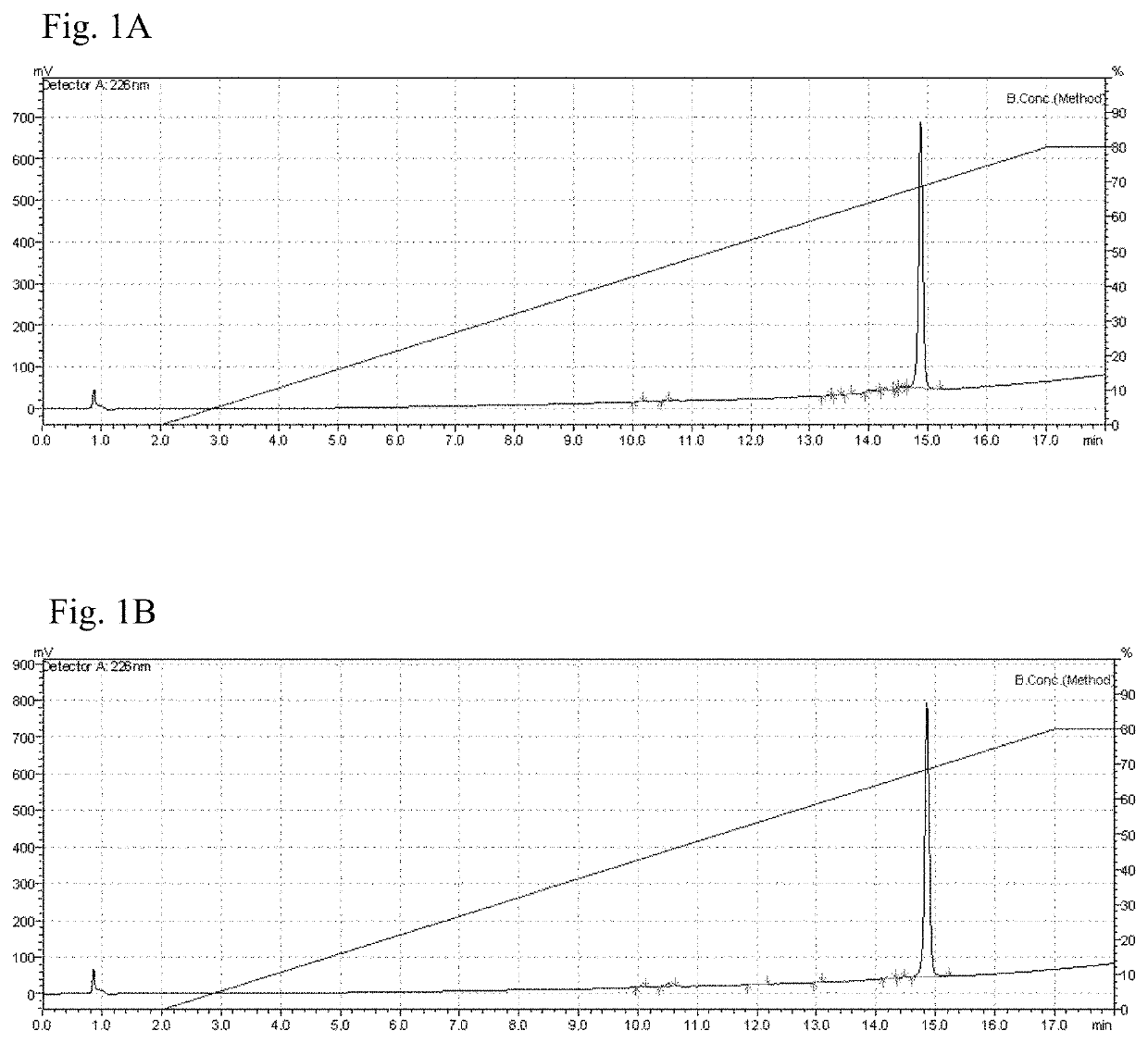
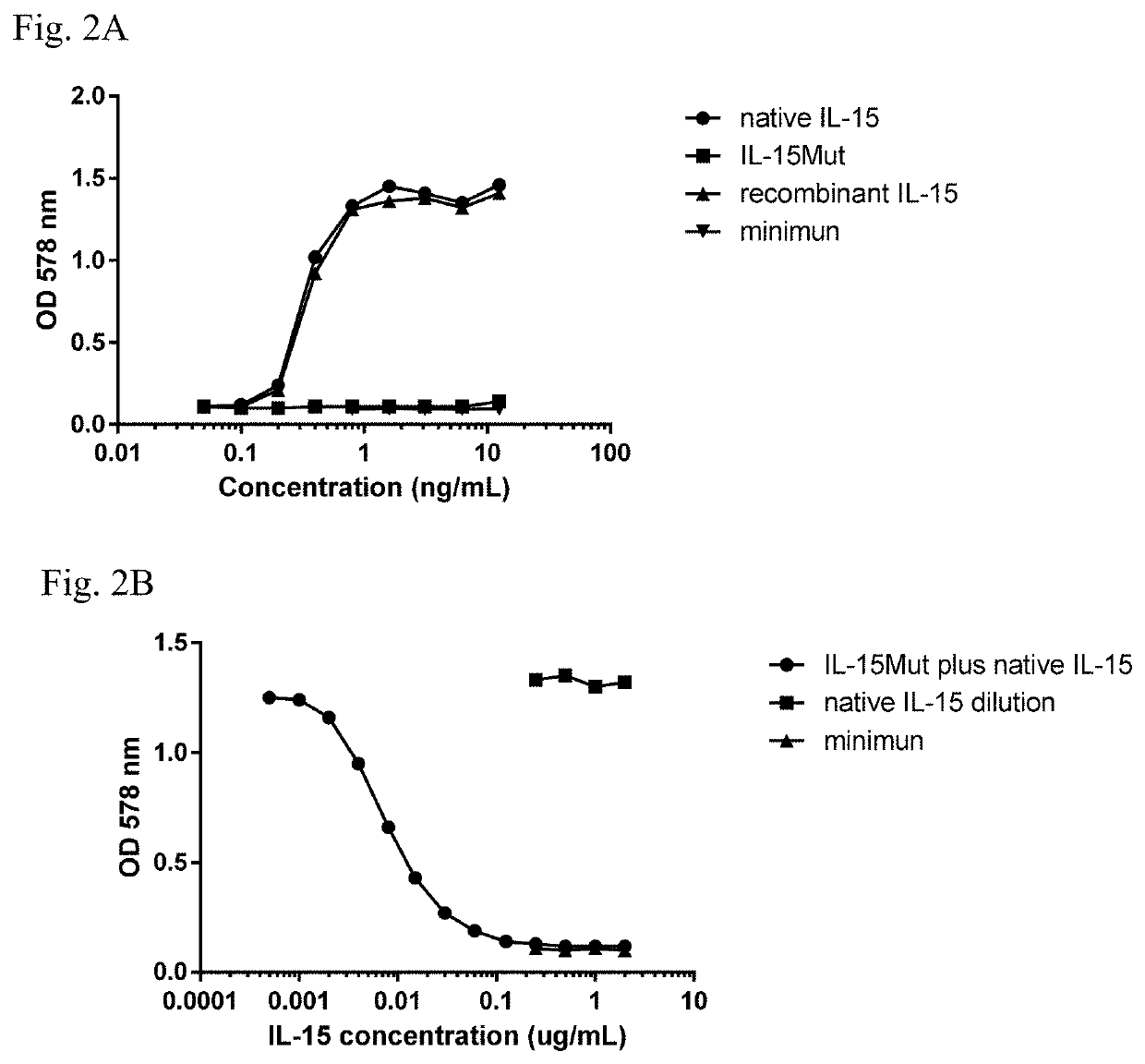
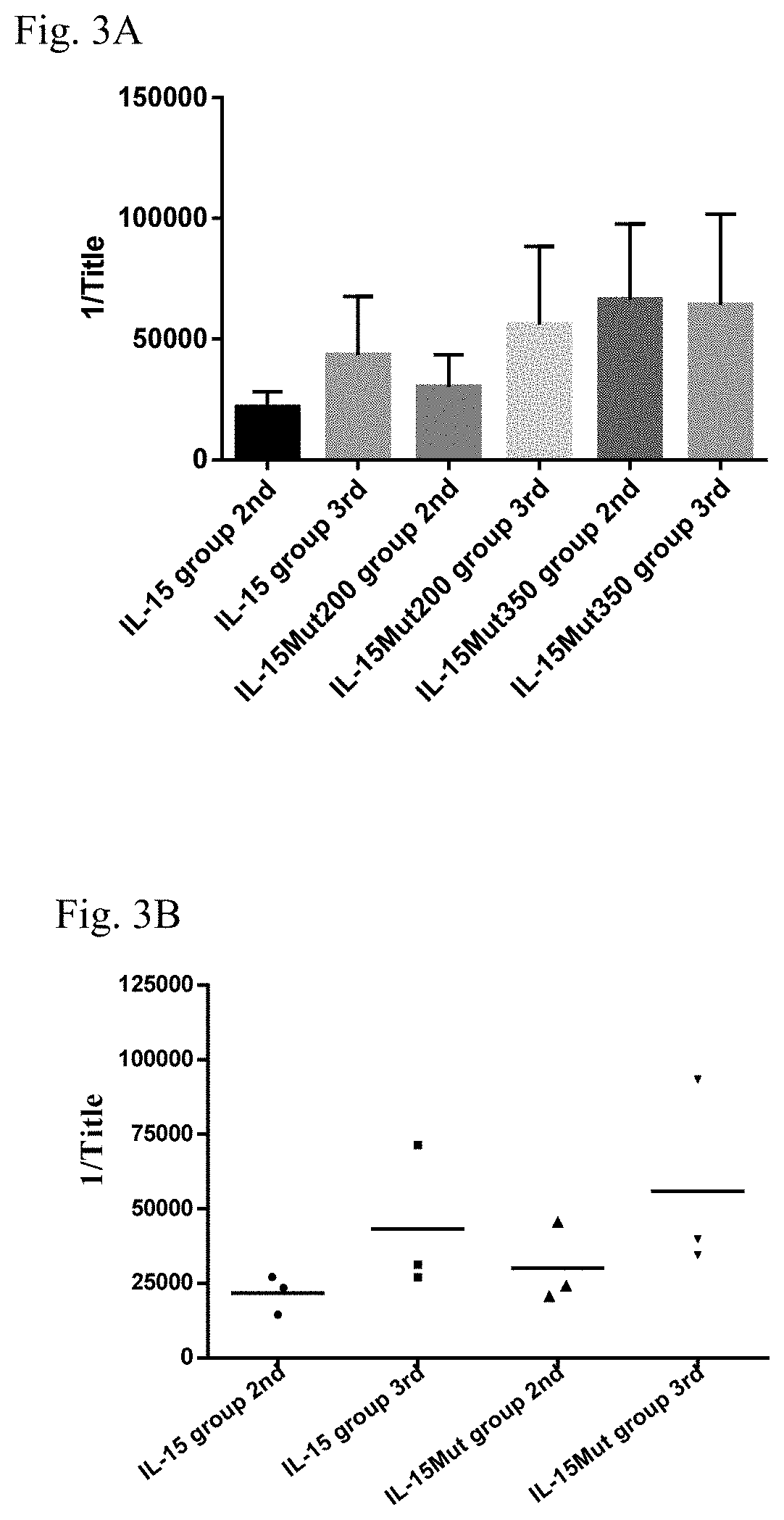
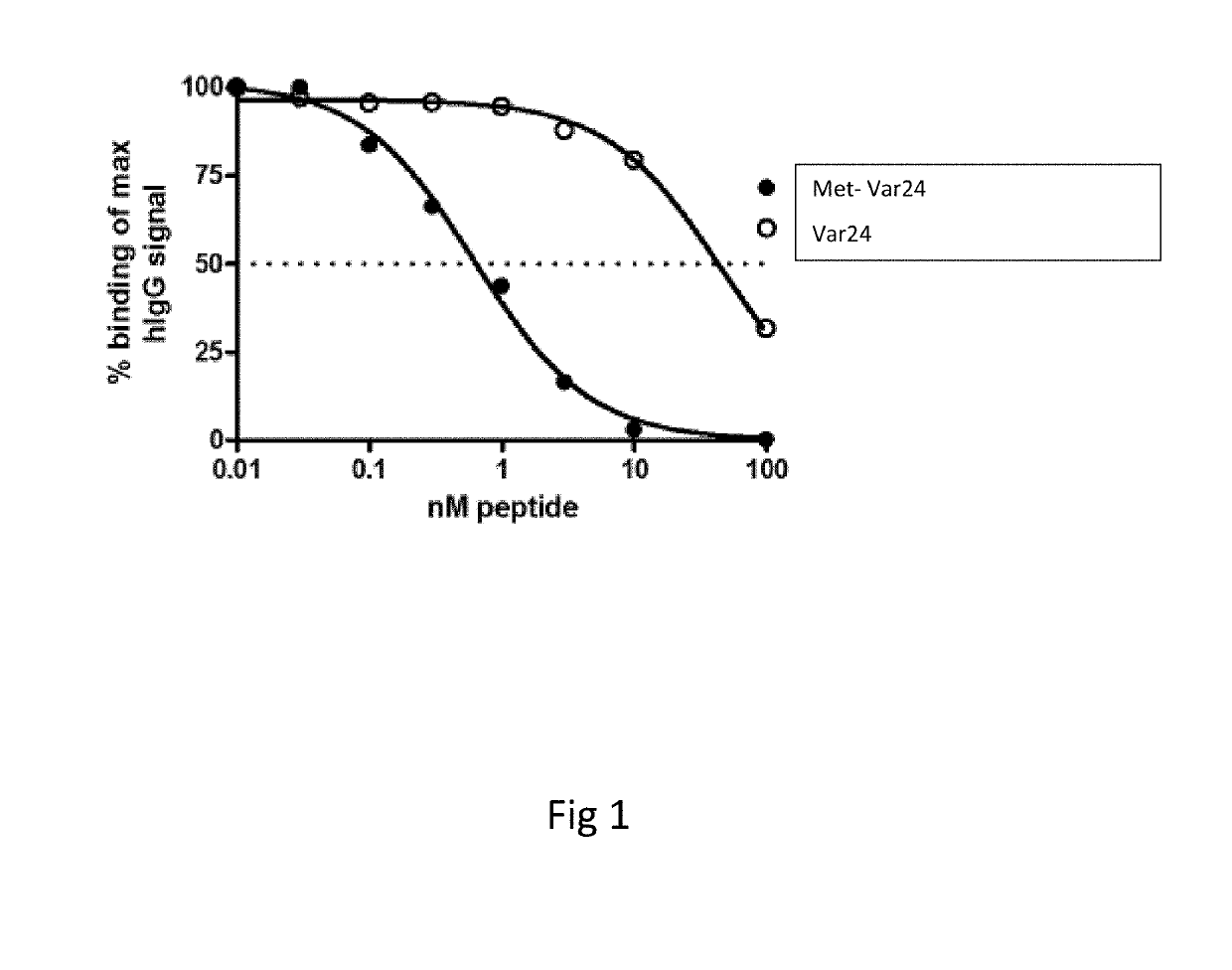
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Active immunotherapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Active immunotherapy is a type of immunotherapy that aims to stimulate the host's immune system or a specific immune response to a disease or pathogen and is most commonly used in cancer treatments. Active immunotherapy is also used for treatment of neurodegenerative disorders, such as Alzheimer's disease, Parkinson's disease, prion disease, and multiple sclerosis. Active immunotherapies induce an immune response through direct immune system stimulation, while immunotherapies that administer antibodies directly to the system are classified as passive immunotherapies. Active immunotherapies can elicit generic and specific immune responses depending on the goal of the treatment. The categories of active immunotherapy divide into...
Combination therapy using active immunotherapy
InactiveUS20090004213A1Organic active ingredientsCancer antigen ingredientsSunitinib malateMajor histocompatibility
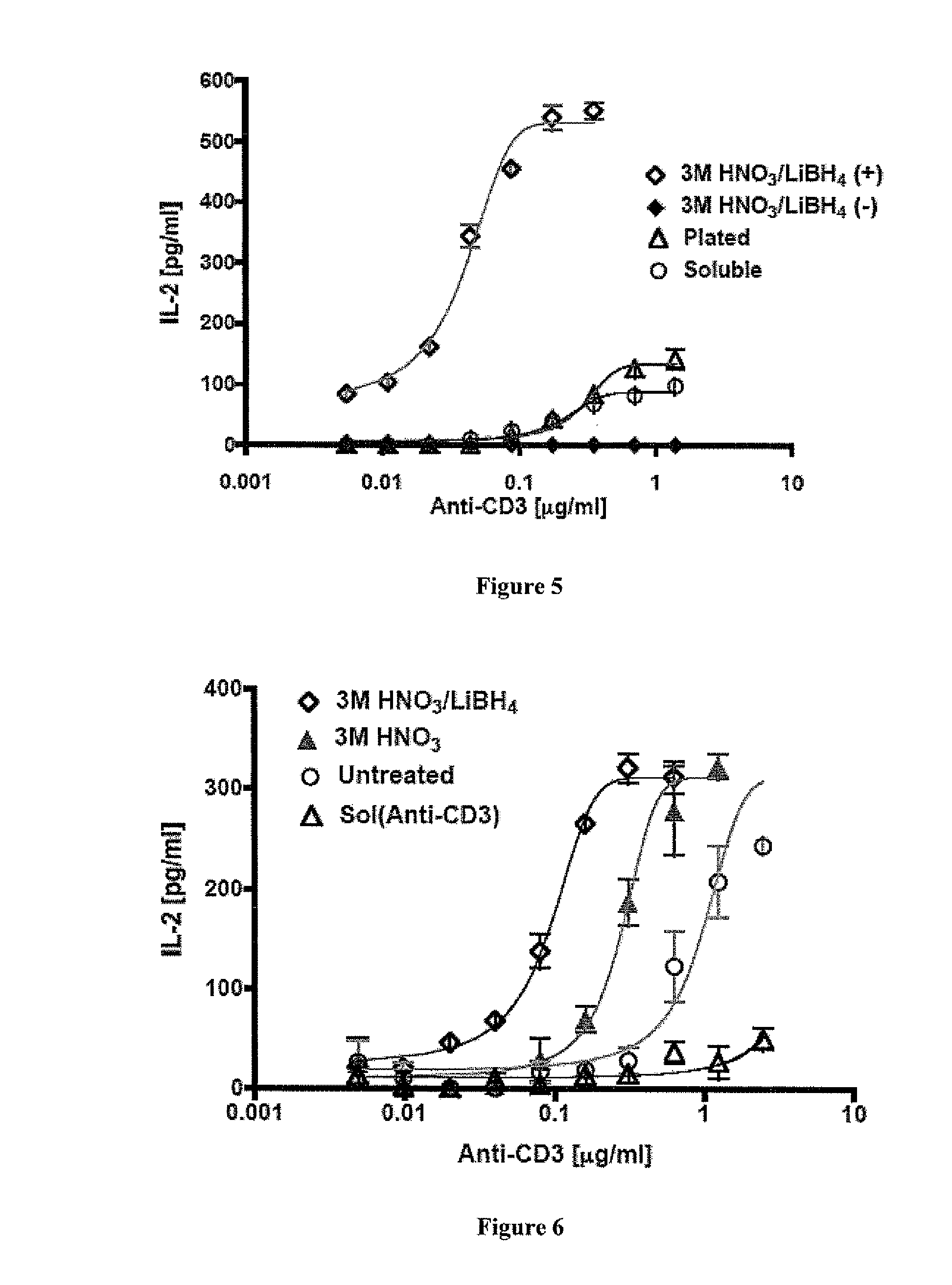
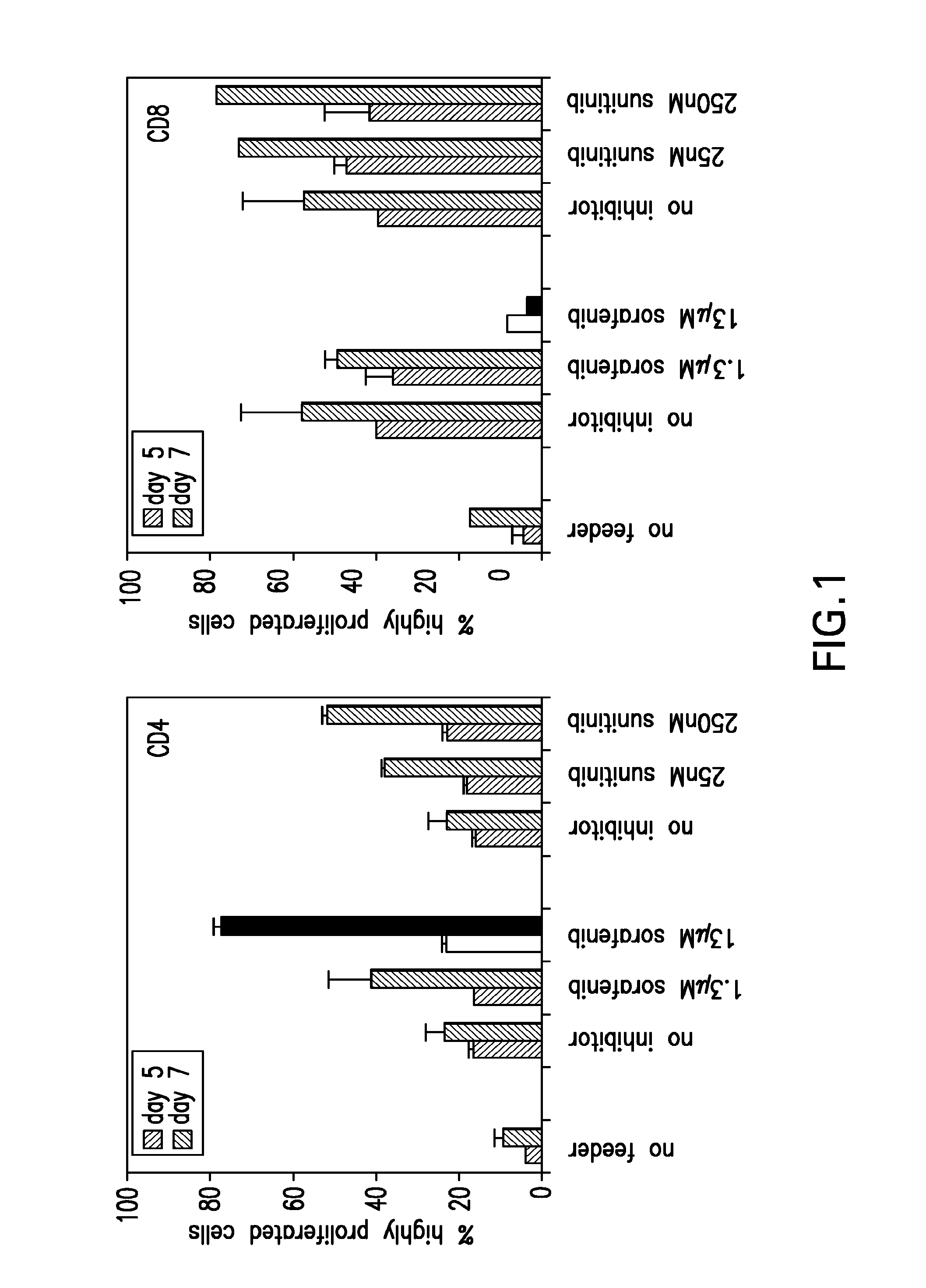
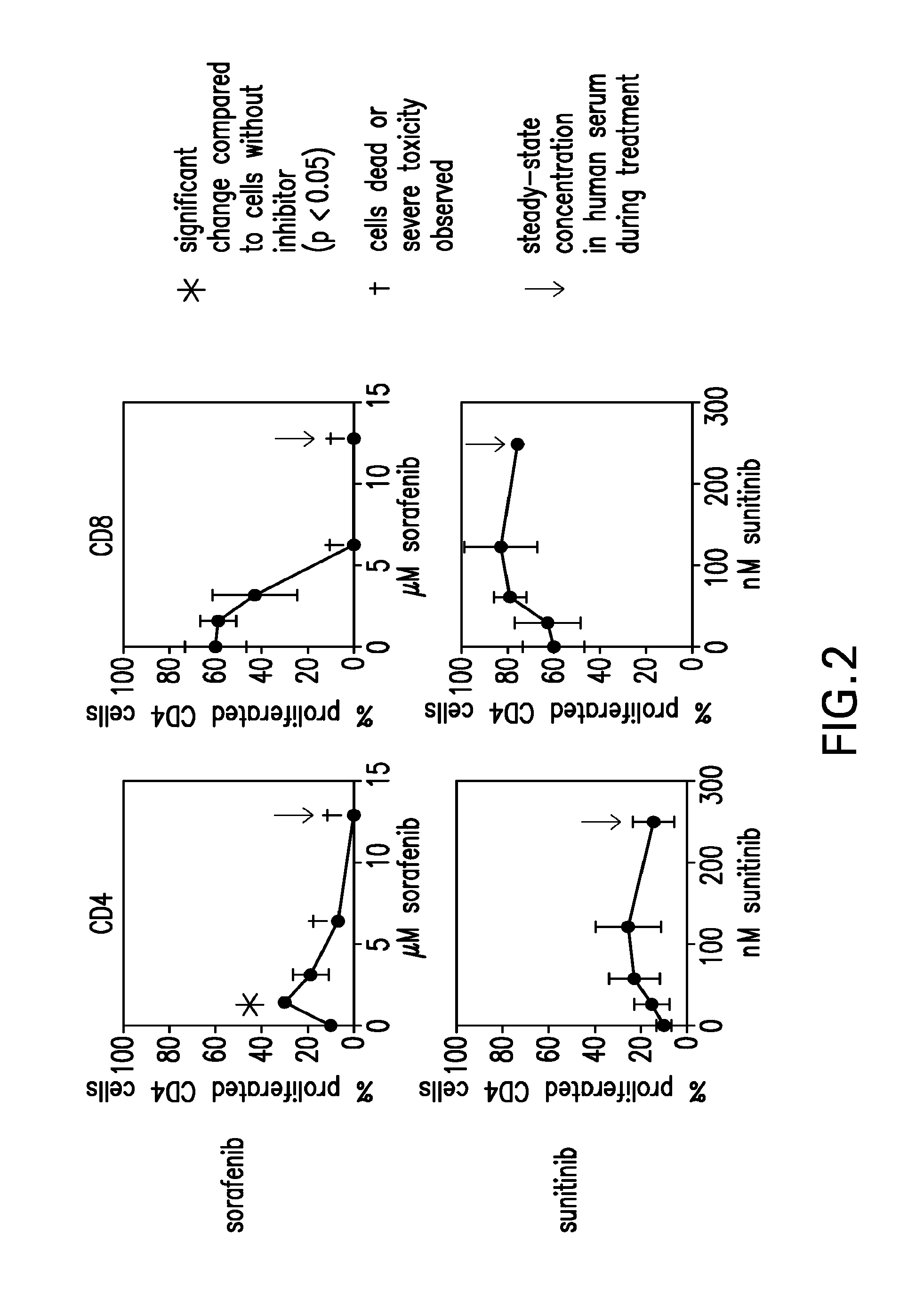
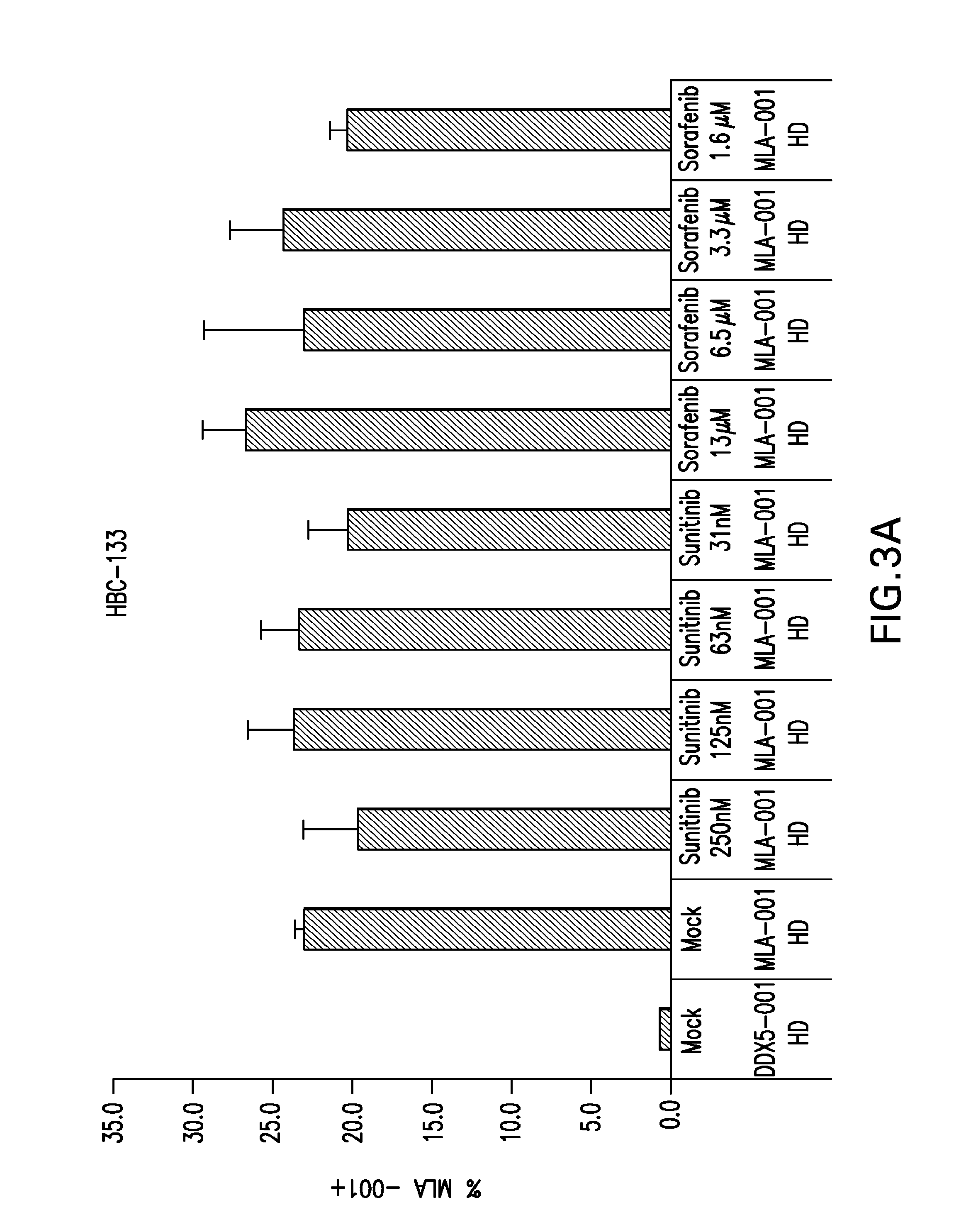
The present invention relates to methods of treating cancer in a mammal comprising administering to the mammal a combination therapy comprising a vaccine and a multi-kinase inhibitor, wherein the vaccine comprises an isolated tumor associated peptide having the ability to bind to a molecule of the human major histocompatibility complex (MHC) class-I or class-II. Preferably the multi-kinase inhibitor is sunitinib malate and / or sorafenib tosylate or a pharmaceutically acceptable salt thereof.
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Compositions and methods for adoptive and active immunotherapy
ActiveUS20100284965A1Easy modular assemblyHigh densityPowder deliveryBiocideControl mannerMicroparticle
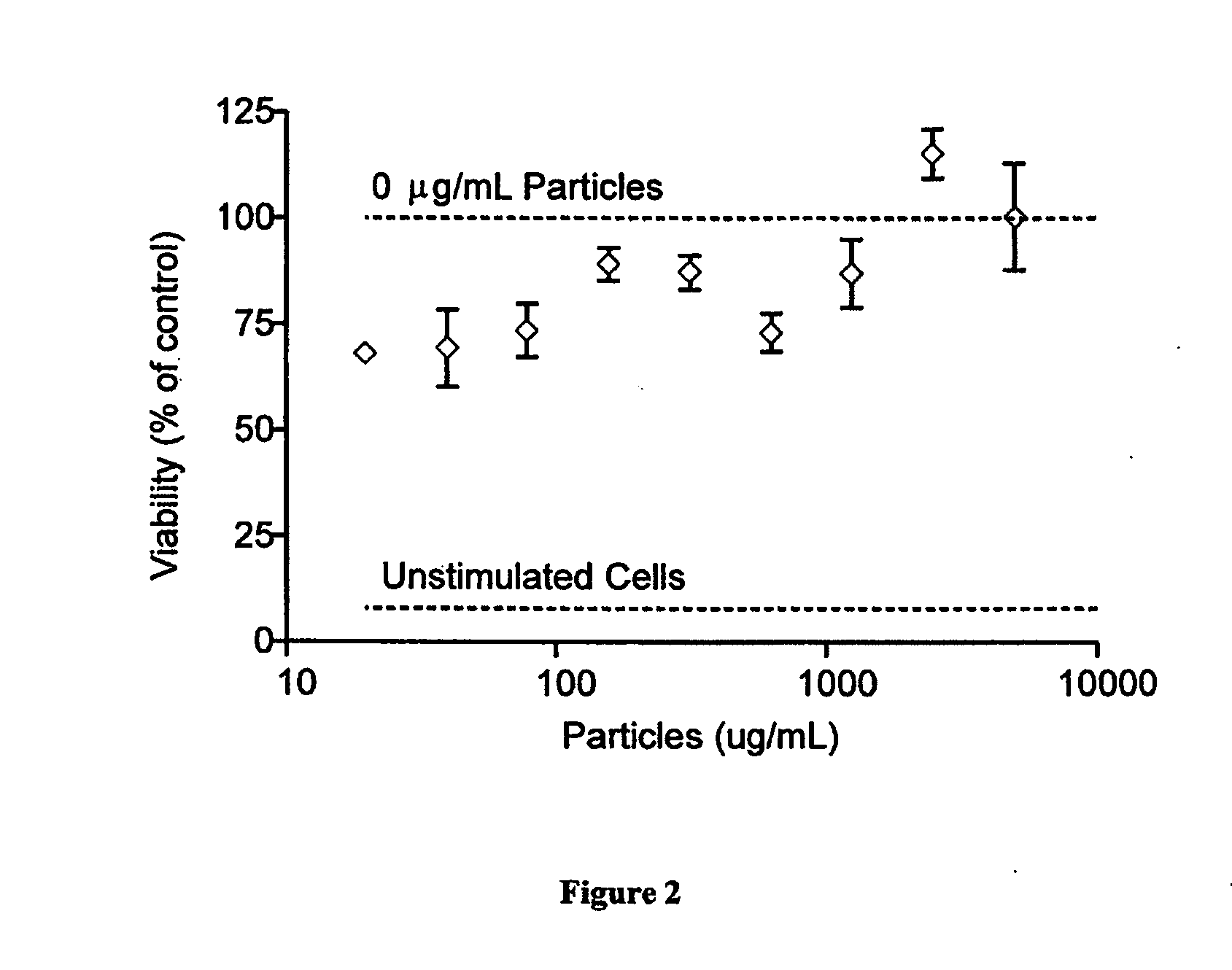
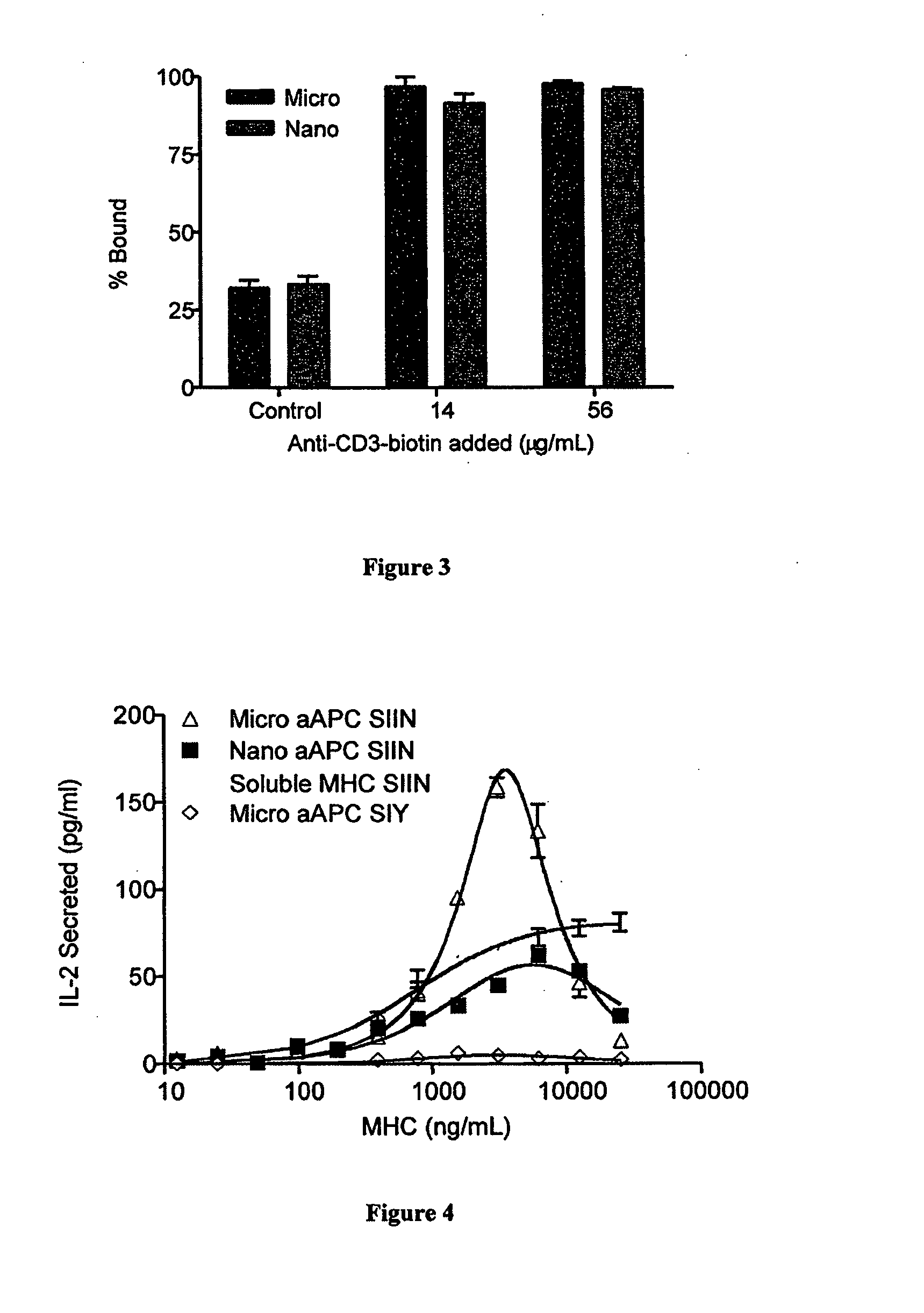
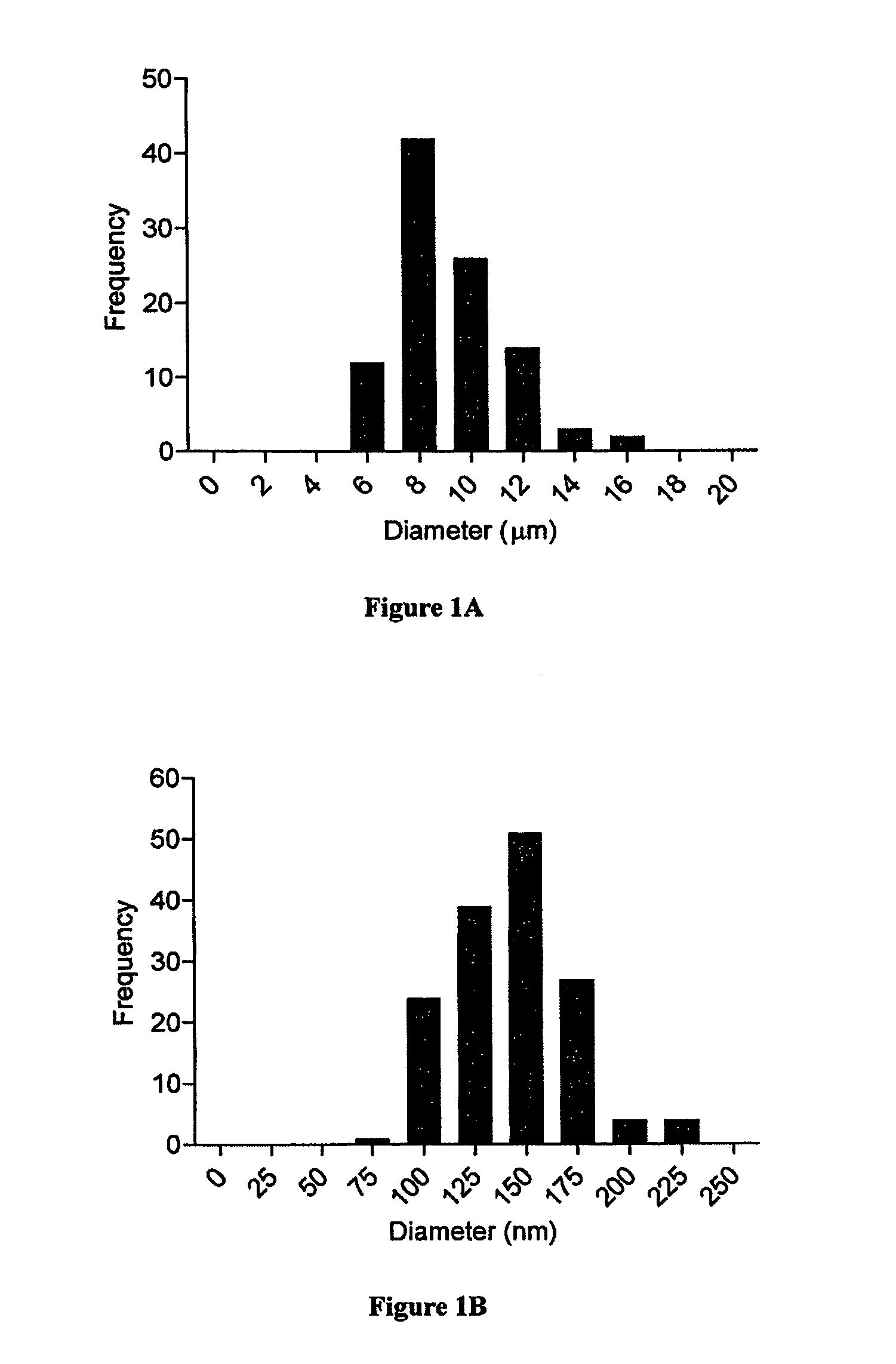
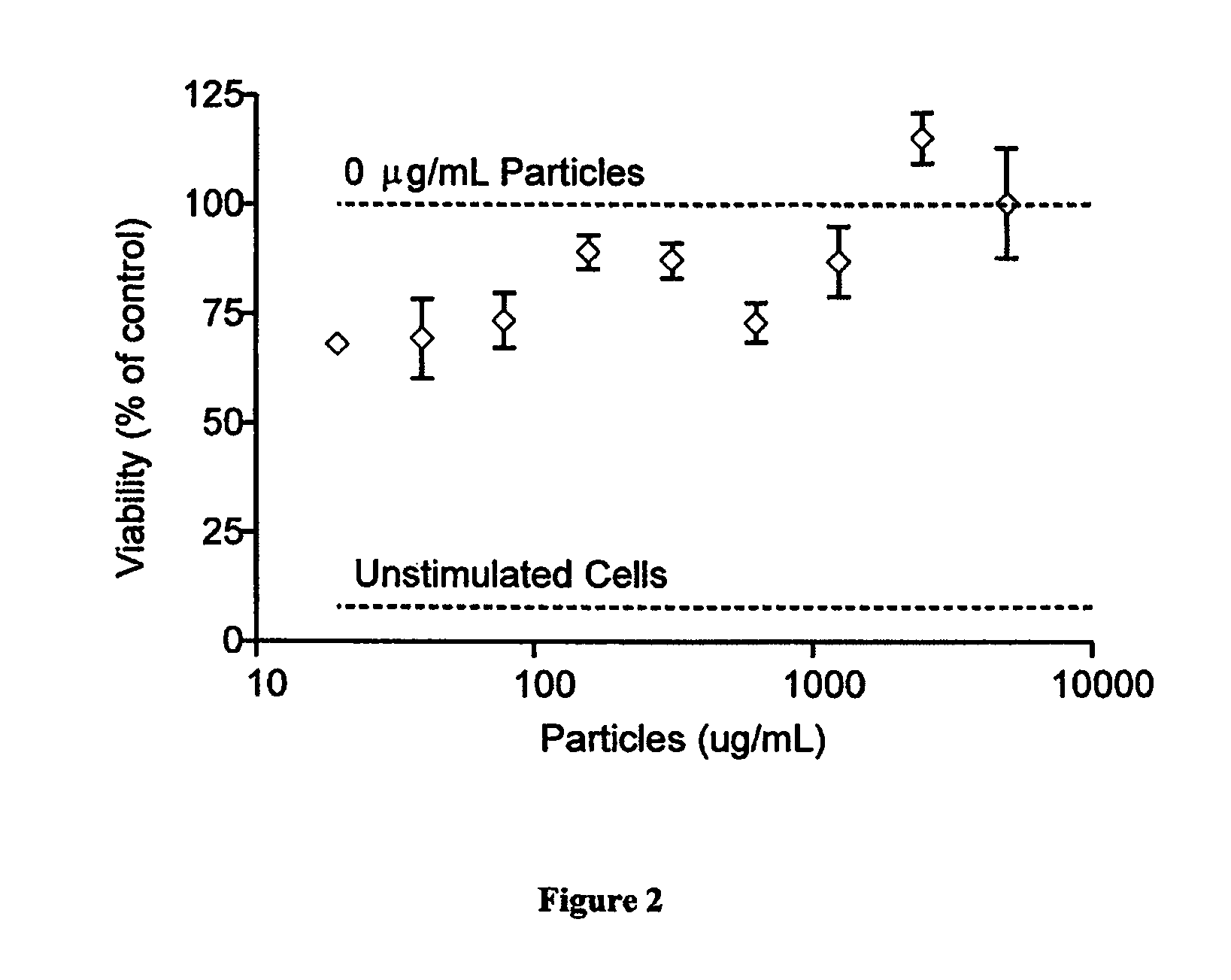
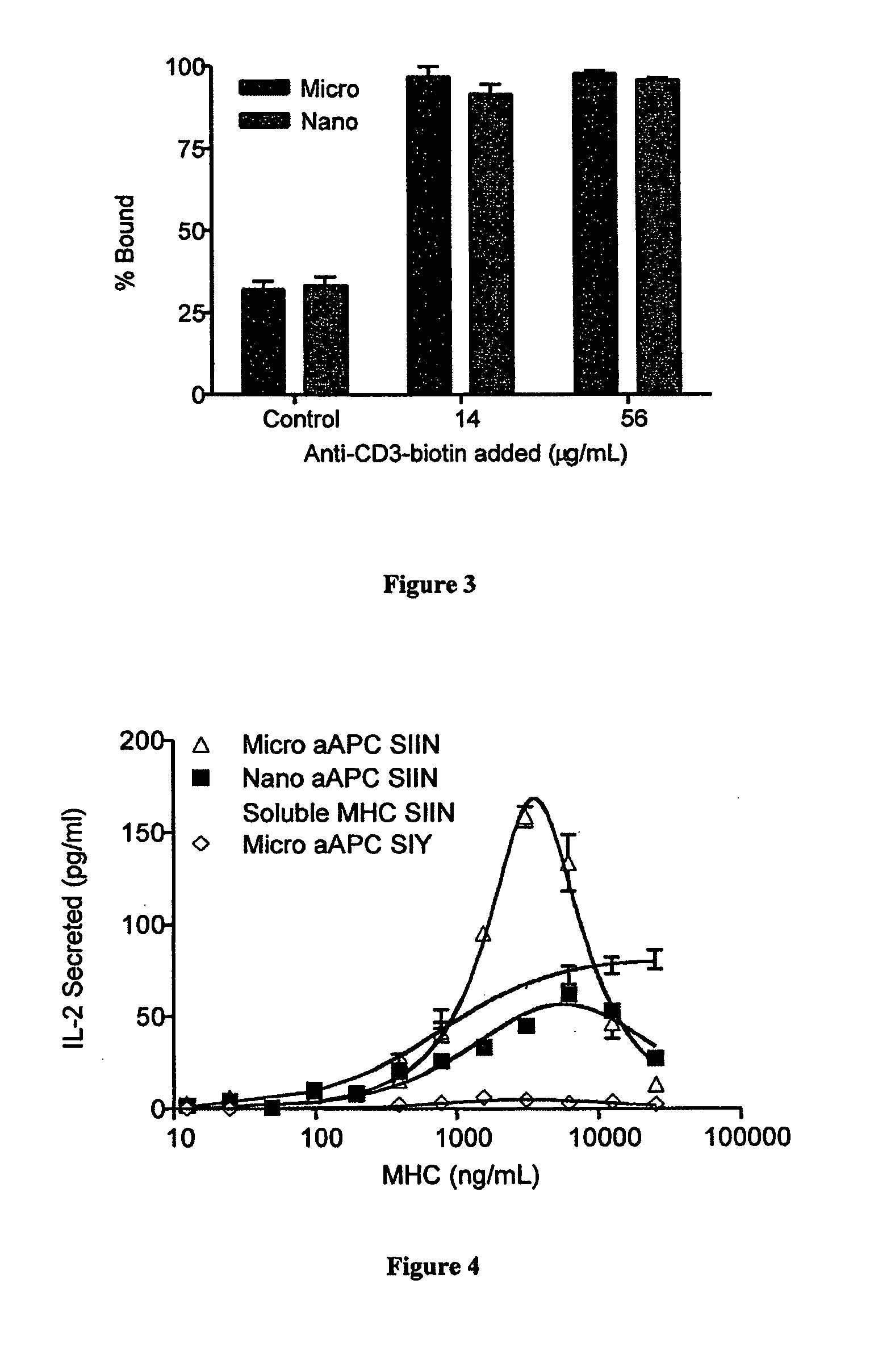
Modular aAPCs and methods of their manufacture and use are provided. The modular aAPCs are constructed from polymeric microparticles. The aAPCs include encapsulated cytokines and coupling agents which modularly couple functional elements including T cell receptor activators, co-stimulatory molecules and adhesion molecules to the particle. The ability of these aAPCs to release cytokines in a controlled manner, coupled with their modular nature and ease of ligand attachment, results in an ideal, tunable APC capable of stimulating and expanding primary T cells.
Owner:YALE UNIV
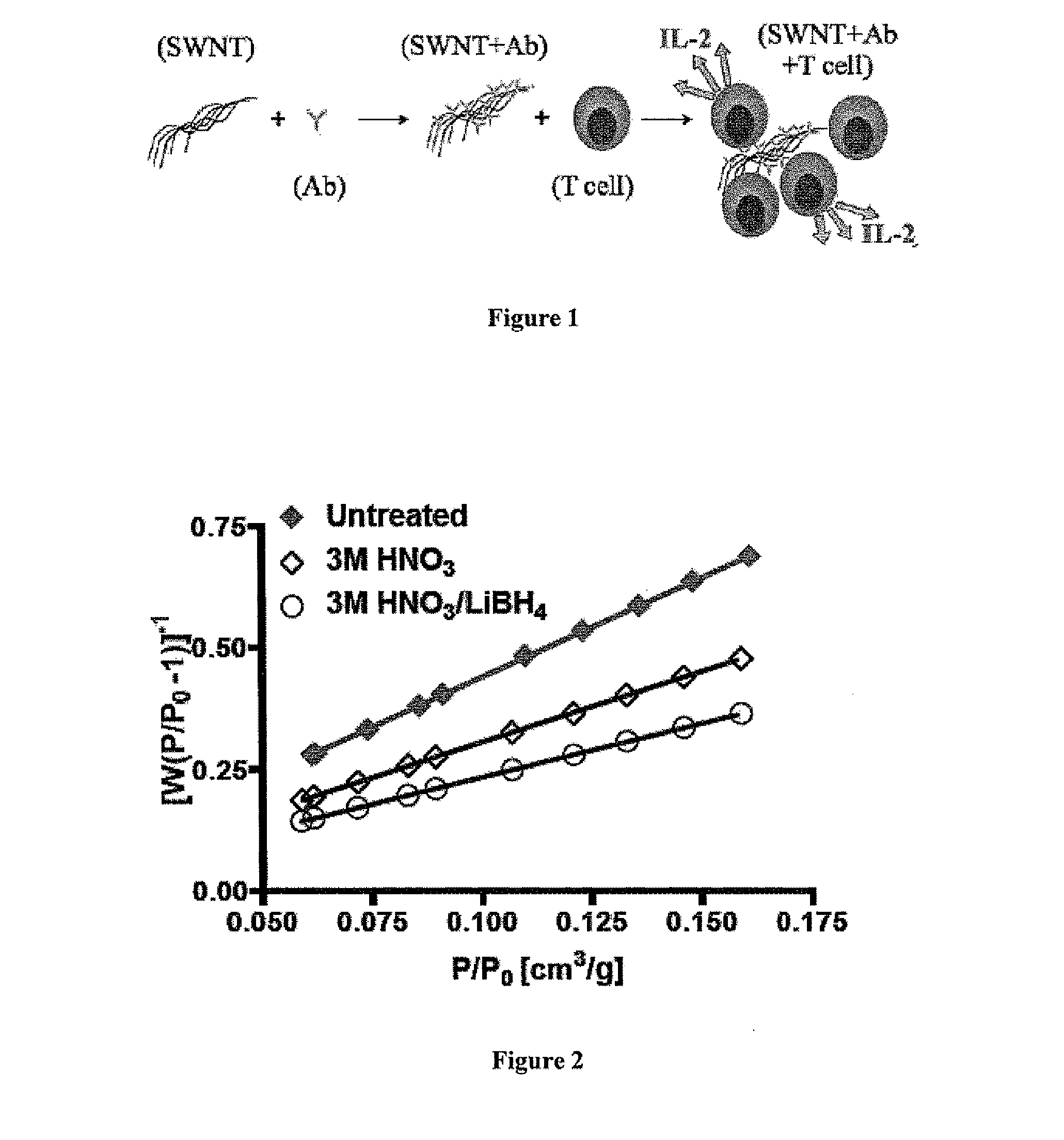
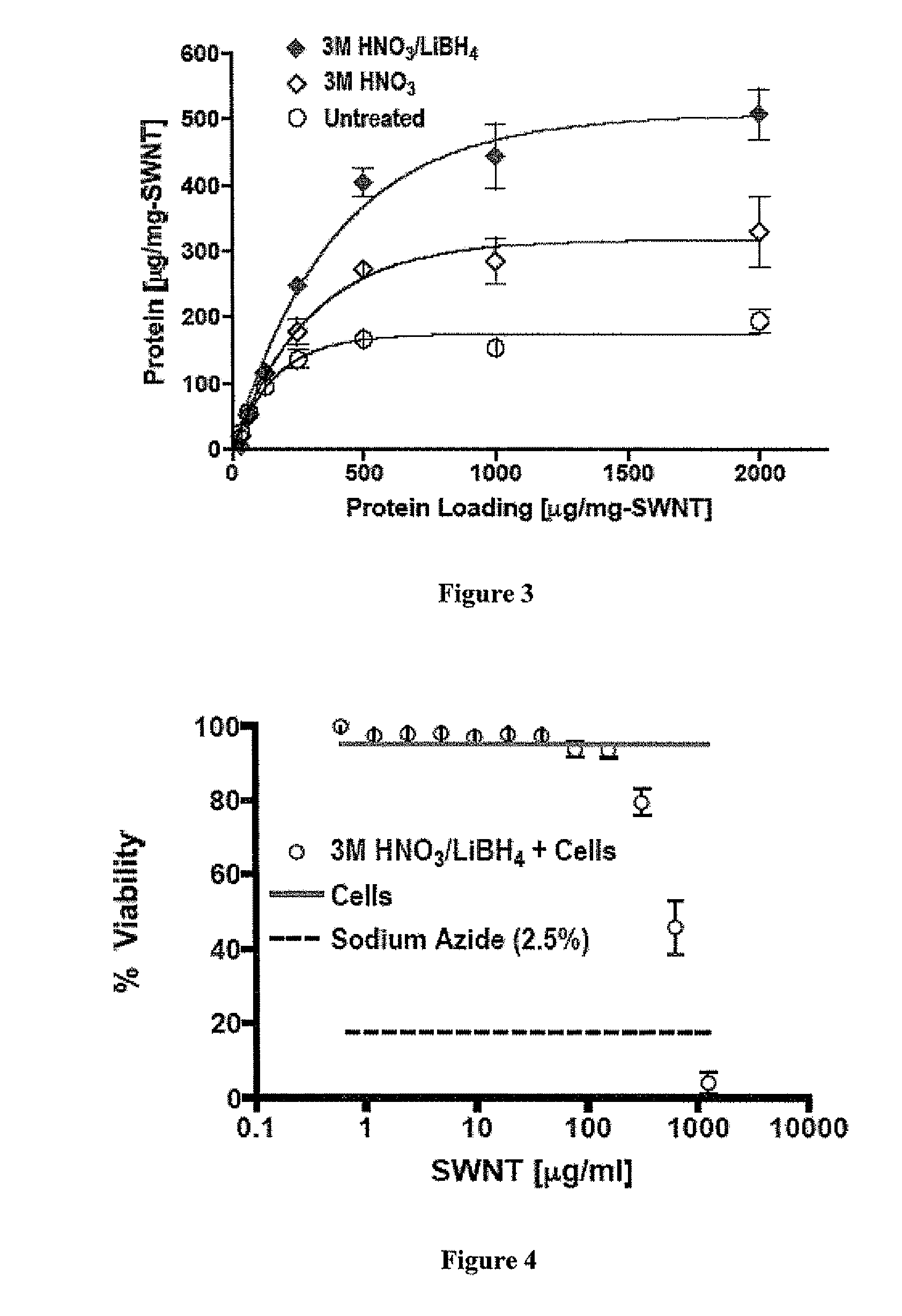
Carbon Nanotube Compositions and Methods of Use Thereof
ActiveUS20130216581A1Reduce deliveryFacilitate antigen uptakePeptide/protein ingredientsSnake antigen ingredientsAntigenCarbon nanotube
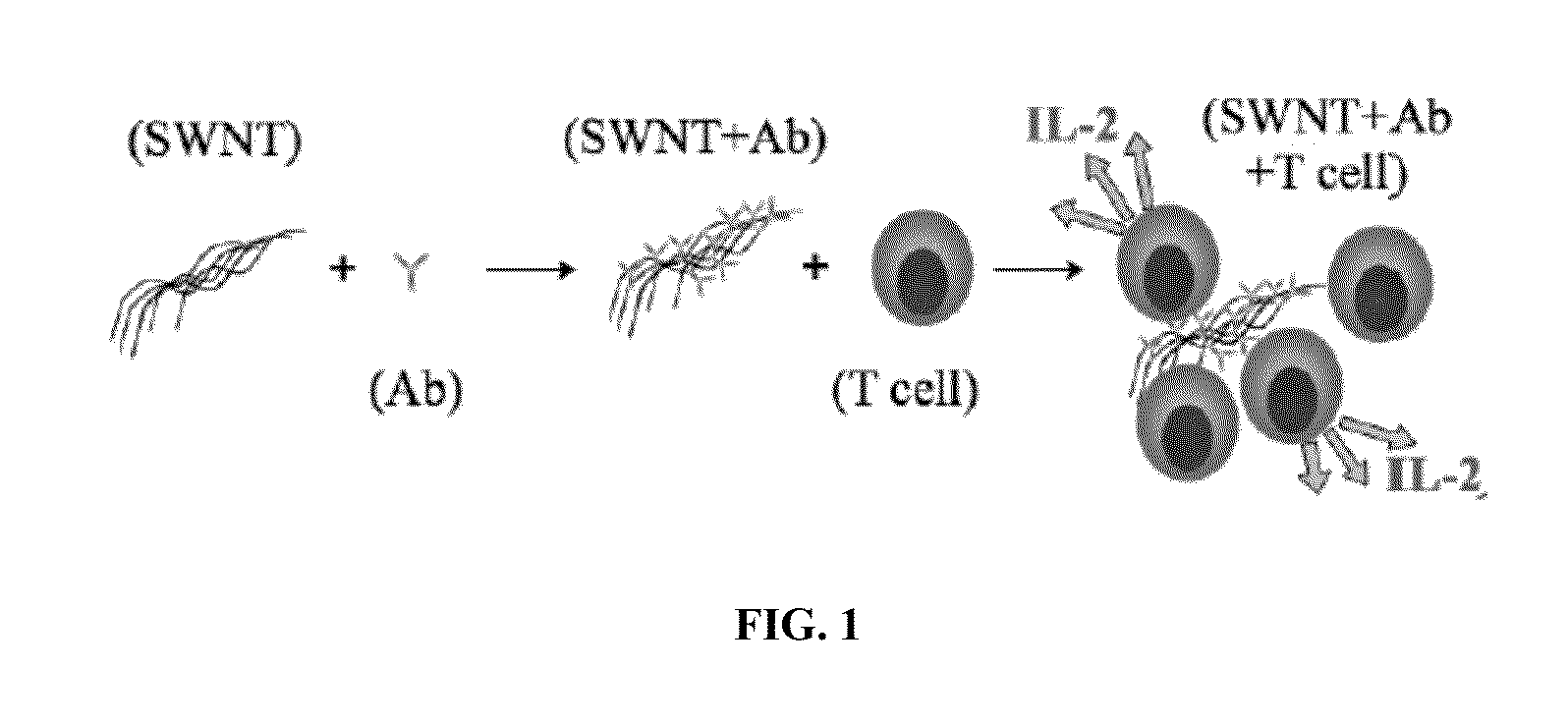
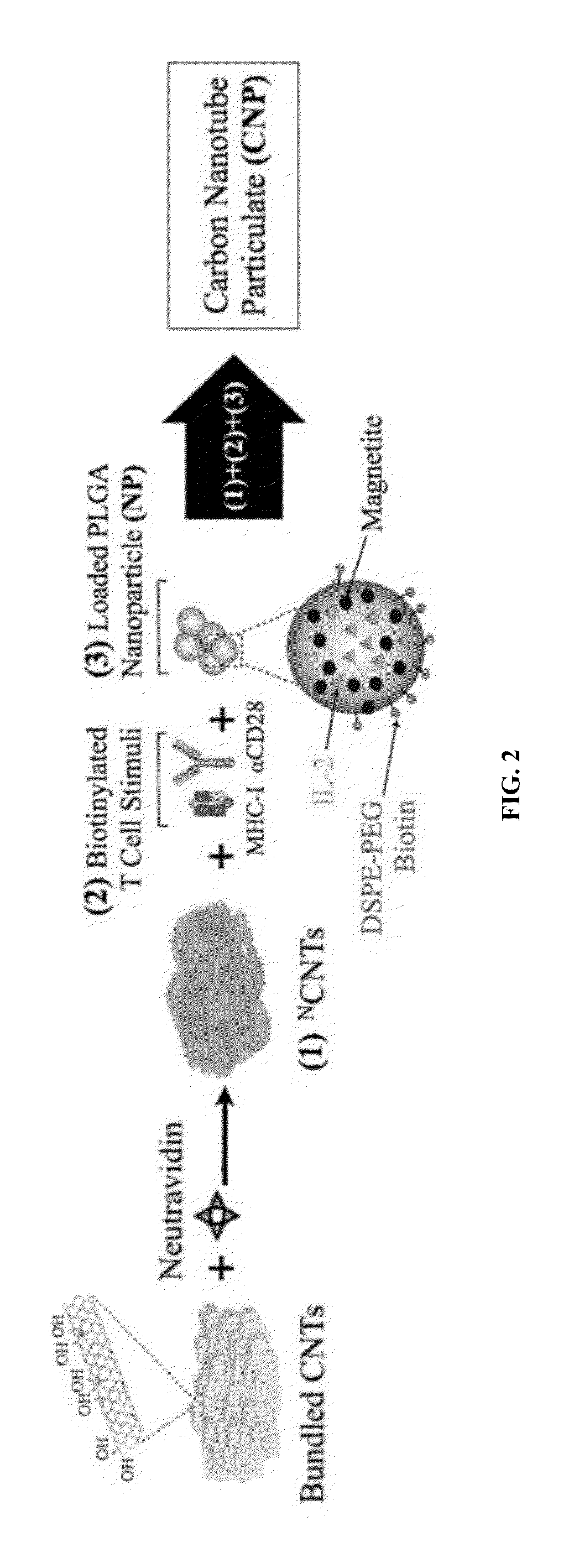
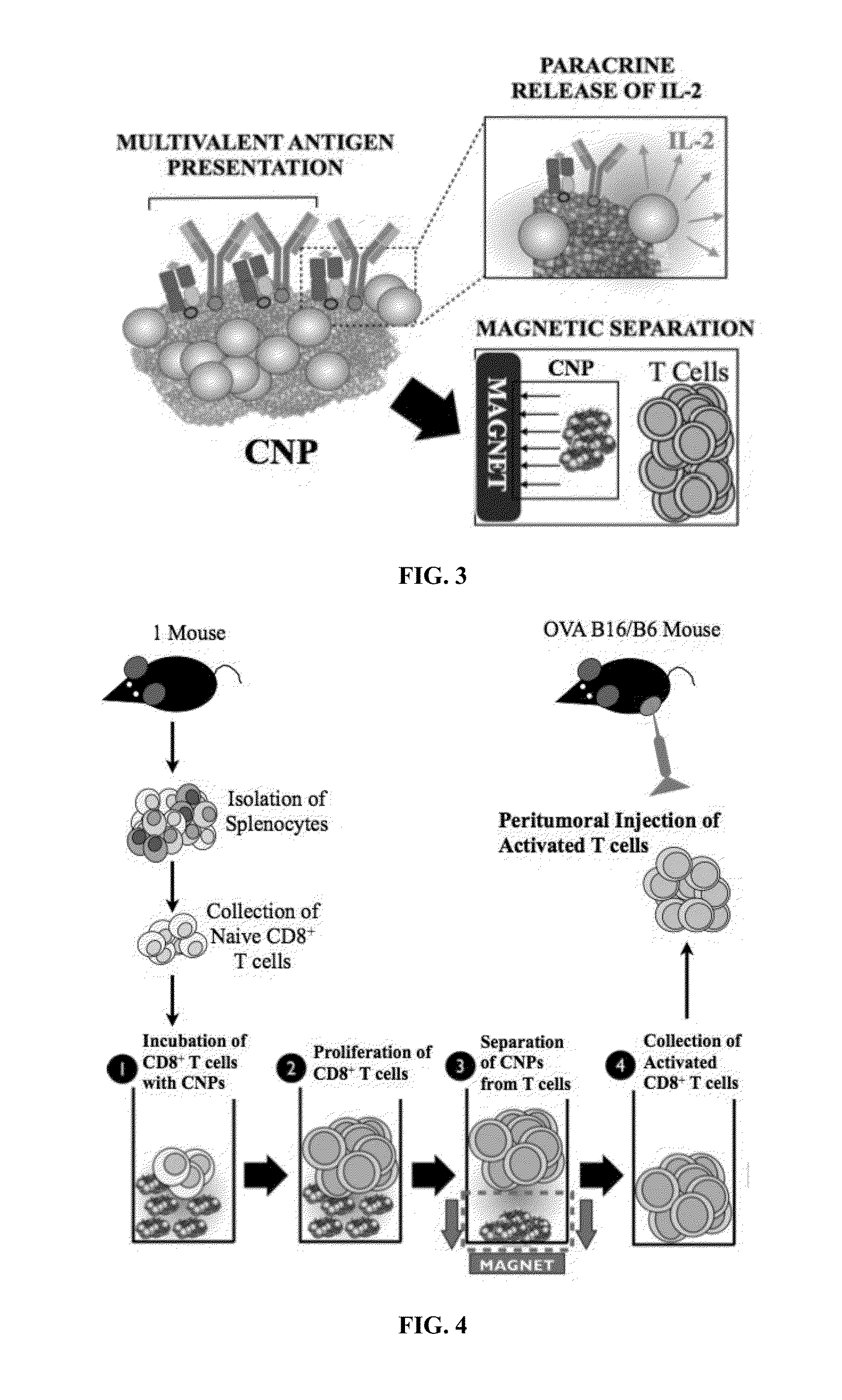
Carbon nanotube (CNT)-based compositions for activating cellular immune responses are provided. The CNTs function as high surface area scaffolds for the attachment of T cell ligands and / or antigens. The CNT compositions function as artificial antigen-presenting cells (aAPCs) or as modular vaccines. The disclosed CNT aAPCs are efficient at activating T cells and may be used to activate T cells ex vivo or in vivo for adoptive or active immunotherapy.
Owner:YALE UNIV
Carbon nanotube compositions and methods of use thereof
ActiveUS20110014217A1Reduce deliveryFacilitate antigen uptakePowder deliveryPeptide/protein ingredientsCarbon nanotubeT cell
Carbon nanotube (CNT)-based compositions for activating cellular immune responses are provided. The CNTs function as high surface area scaffolds for the attachment of T cell ligands and / or antigens. The CNT compositions function as artificial antigen-presenting cells (aAPCs) or as modular vaccines. The disclosed CNT aAPCs are efficient at activating T cells and may be used to activate T cells ex vivo or in vivo for adoptive or active immunotherapy.
Owner:YALE UNIV
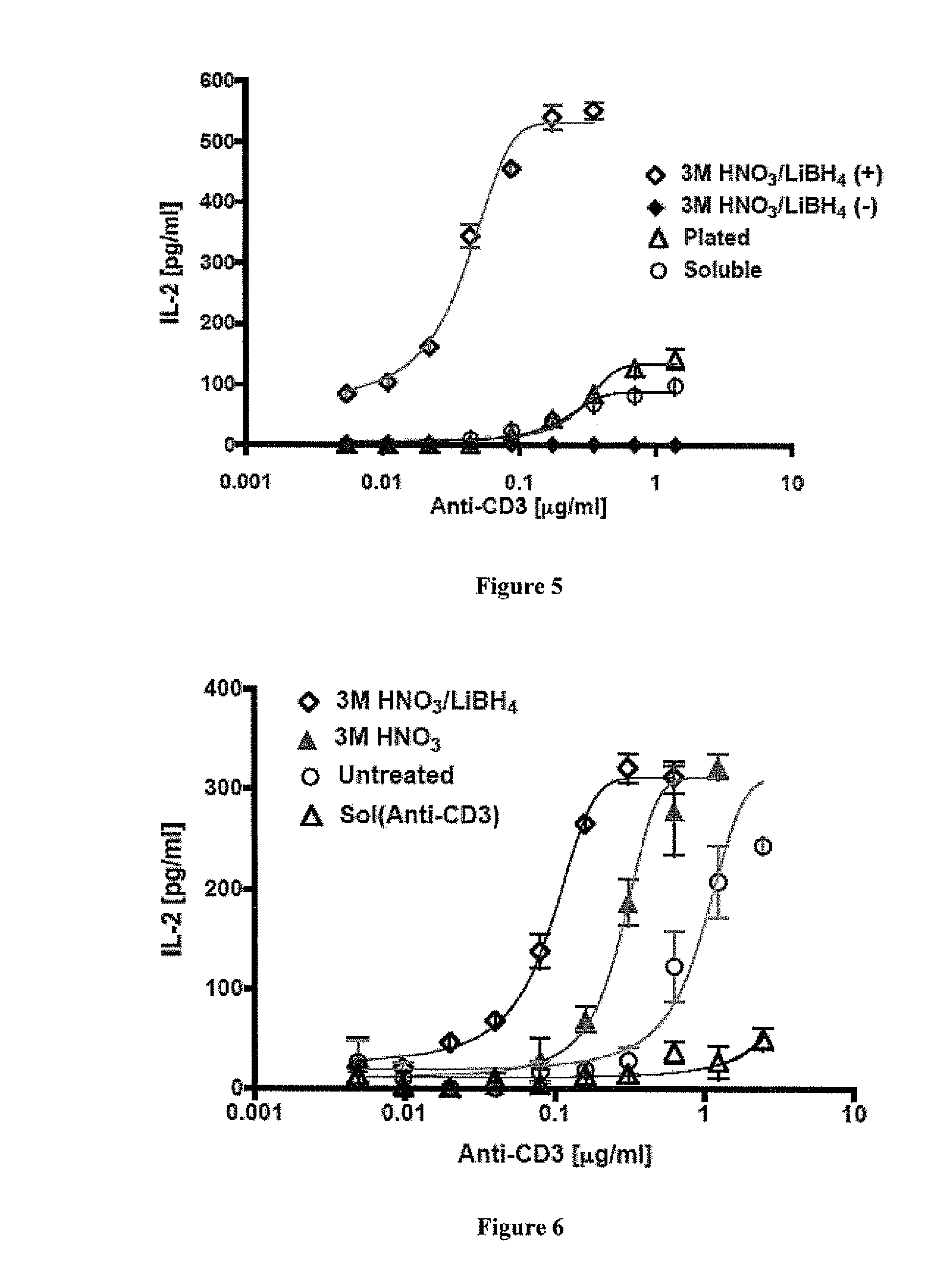
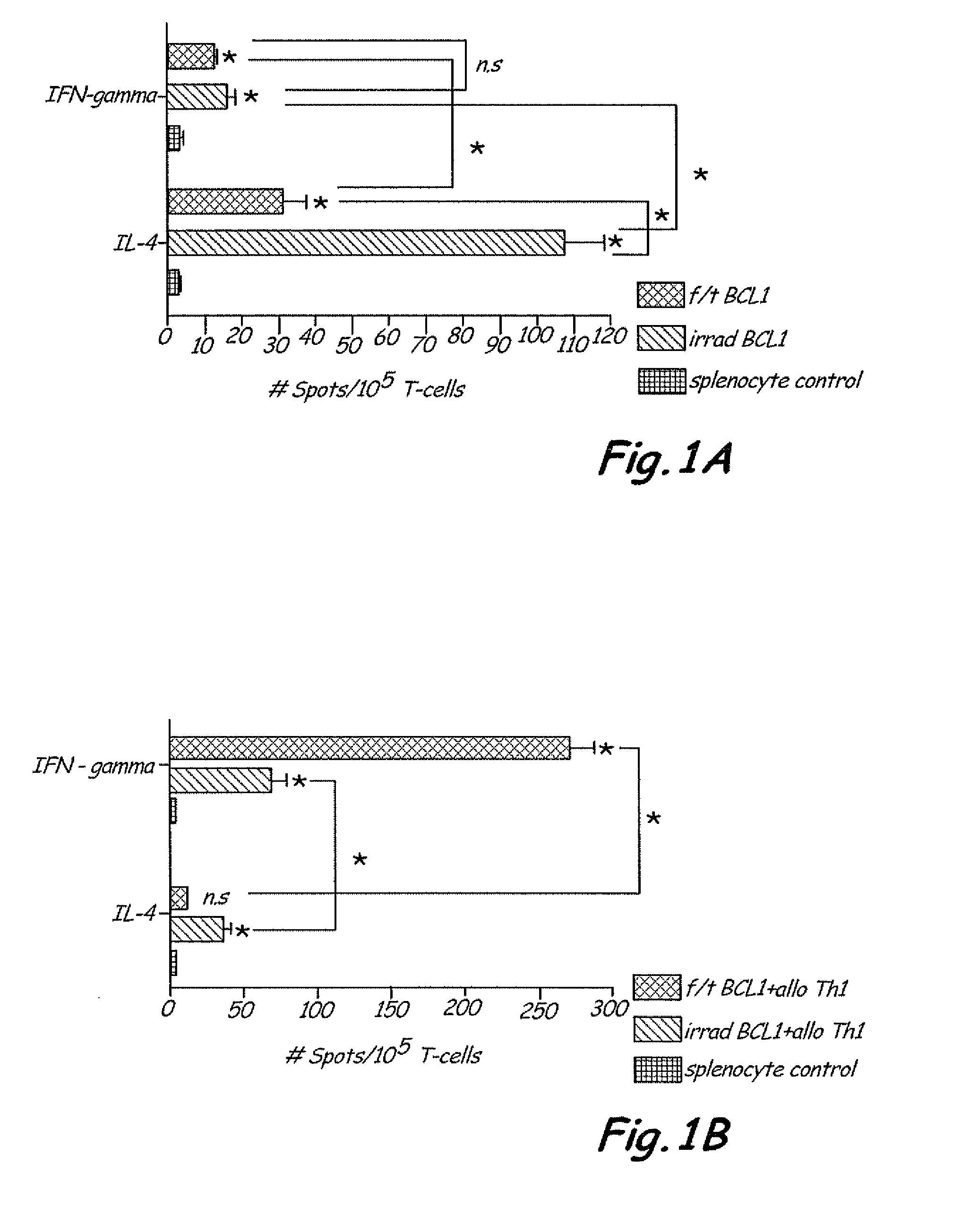
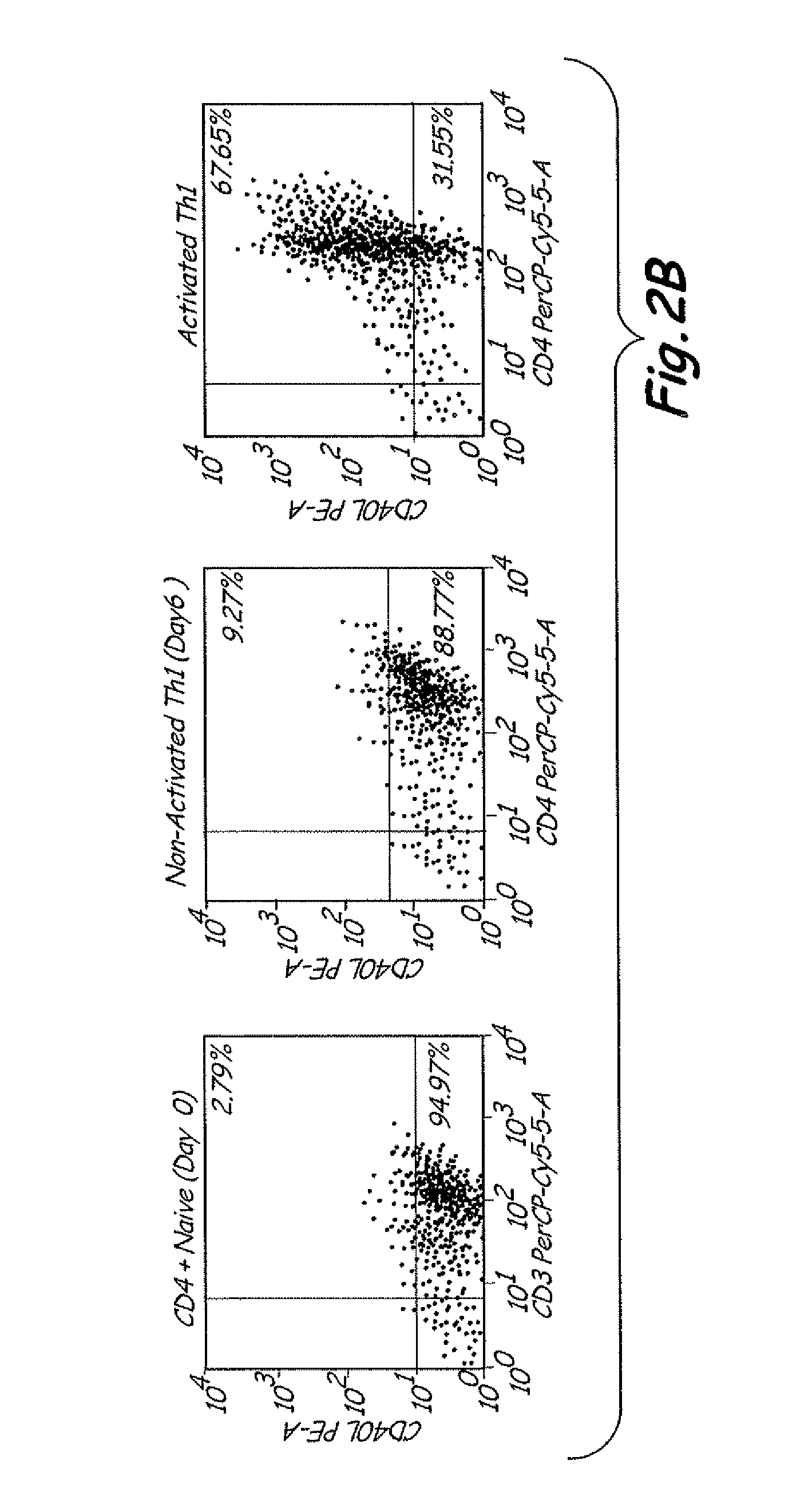
Th1 vaccination priming for active immunotherapy
ActiveUS20100086561A1Good auxiliary effectMaintaining immune healthAntibacterial agentsBiocideMRD NegativeDisease
The present invention includes vaccine compositions and methods for using these vaccine compositions in active immunotherapy. The vaccine compositions include allogeneic activated Th1 memory cells. The compositions can also include one or more disease-related antigens. The methods include administering the vaccine compositions to provide a Th1 footprint in normal individuals or patients susceptible to disease or having minimal residual disease.
Owner:MIRROR BIOLOGICS INC +1
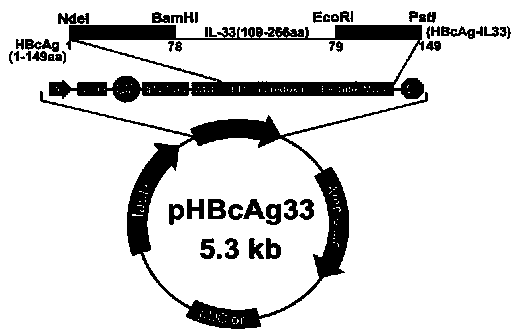
Method for constructing IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma
ActiveCN104001168AEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDiseaseHepatitis B virus core Antigen
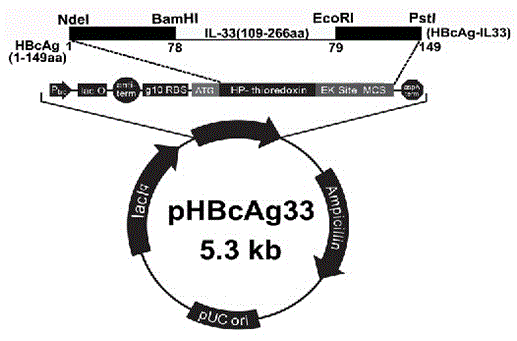
The invention provides a method for constructing an IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma. The method comprises the following steps: extracting IL-33 total RNA (Ribonucleic Acid) from a mouse; performing reverse transcription to obtain IL33 total cDNA (complementary deoxyribonucleic acid); performing PCR (Polymerase Chain Reaction) amplification on the obtained total cDNA with a designed specific primer to obtain a coded IL-33 mature segment gene; inserting the gene between 78-bit amino acid and 79-bit amino acid of a hepatitis B virus core antigen HBcAg to obtain a recombinant plasmid pHBcAg33; transferring the plasmid onto escherichia coli DH5alpha or a BL21 competent cell; inducing by using IPTG (isopropyl-beta-d-thiogalactoside) and purifying to obtain the IL-33 presentation VLP vaccine. A strong neutralizing antibody which is specific to own molecules and has a durable action can be induced by inoculating the vaccine repeatedly in order to regulate and control immune response, thereby fulfilling the aim of regulating and controlling the progress of asthma.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Carbon nanotube compositions and methods of use thereof
ActiveUS8658178B2Reduce deliveryFacilitate antigen uptakePowder deliveryPeptide/protein ingredientsCarbon nanotubeT cell
Carbon nanotube (CNT)-based compositions for activating cellular immune responses are provided. The CNTs function as high surface area scaffolds for the attachment of T cell ligands and / or antigens. The CNT compositions function as artificial antigen-presenting cells (aAPCs) or as modular vaccines. The disclosed CNT aAPCs are efficient at activating T cells and may be used to activate T cells ex vivo or in vivo for adoptive or active immunotherapy.
Owner:YALE UNIV
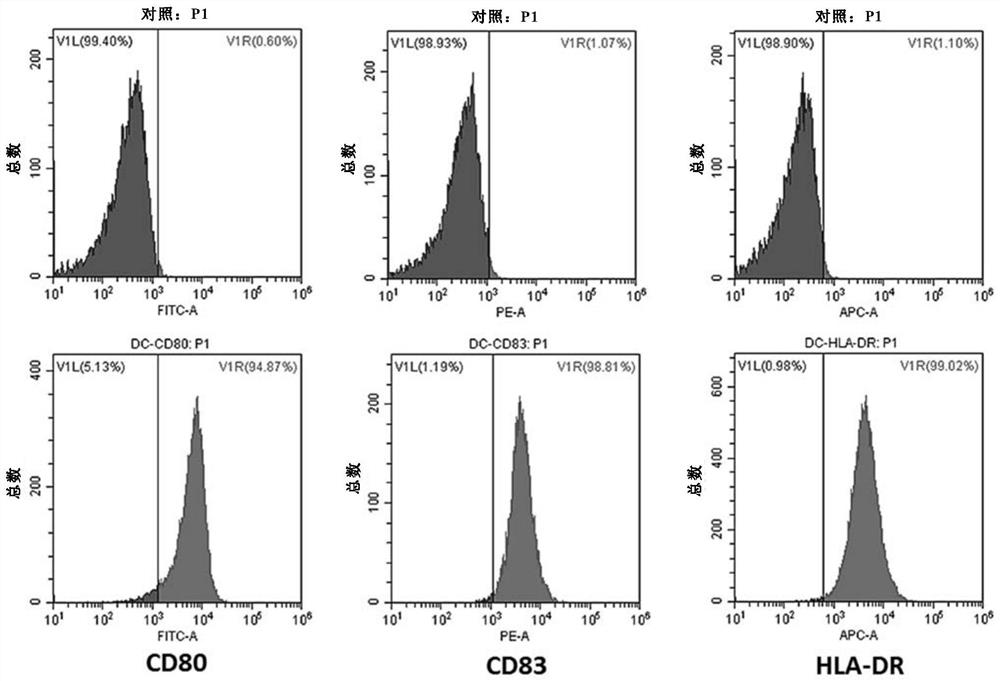
Genetically modified dendritic cell vaccine
ActiveCN109957548AEfficient DCGenetically modified cellsCancer antigen ingredientsHigh concentrationBULK ACTIVE INGREDIENT
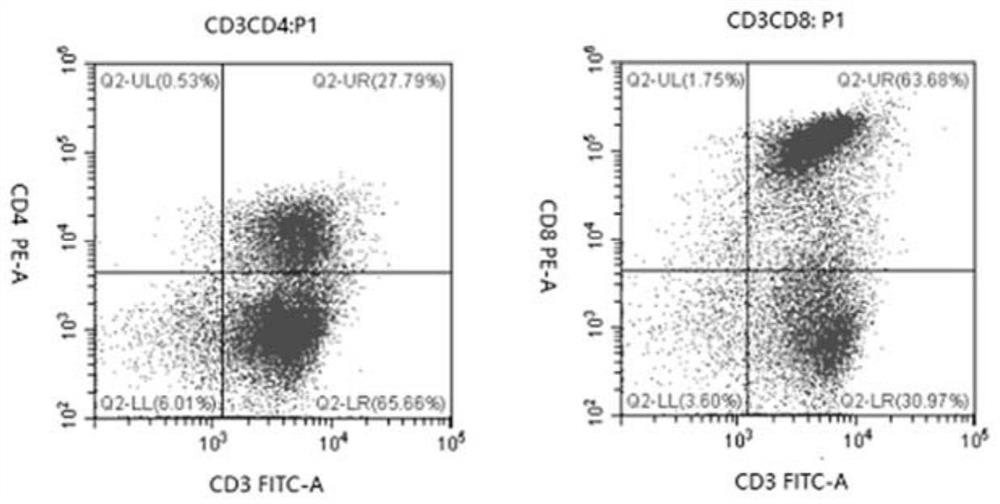
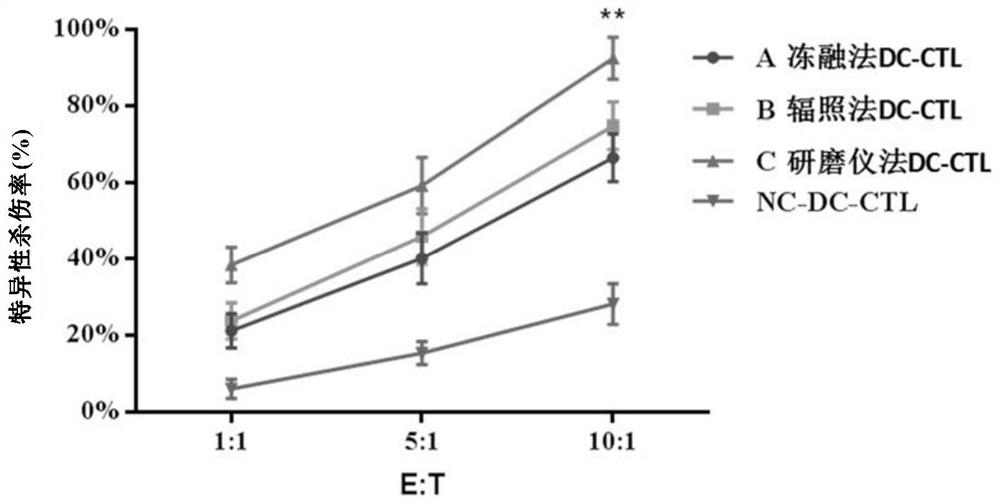
The invention relates to the fields of biotechnology and medicine, and provides a modified dendritic cell (DC); a dendritic cell is infected with MG-7Ag antigen mimic epitope tandem sequences loaded with a lentivirus vector, and a target antigen sequence is integrated into a dendritic cell genome. The invention provides a rapid culture scheme of the dendritic cell derived from a peripheral blood monocyte in vitro, and also provides a vaccine with an active ingredient of the modified dendritic cell. The vaccine is used for tumor prevention and active immunotherapy. The MG-7Ag antigen sequence-modified DC vaccine can obtain high-purity CTL cells and efficient target cell killing ability after DC-CTL co-culture, and the co-culture supernatant contains high-concentration IFN[gamma] secretion.After tumor attack, the tumorigenic volume of mice in a DC-CTL group is significantly smaller than that of mice in a control group, and the DC vaccine has great potential value in immunotherapy of MG-7Ag positive tumor.
Owner:上海尚泰生物技术有限公司
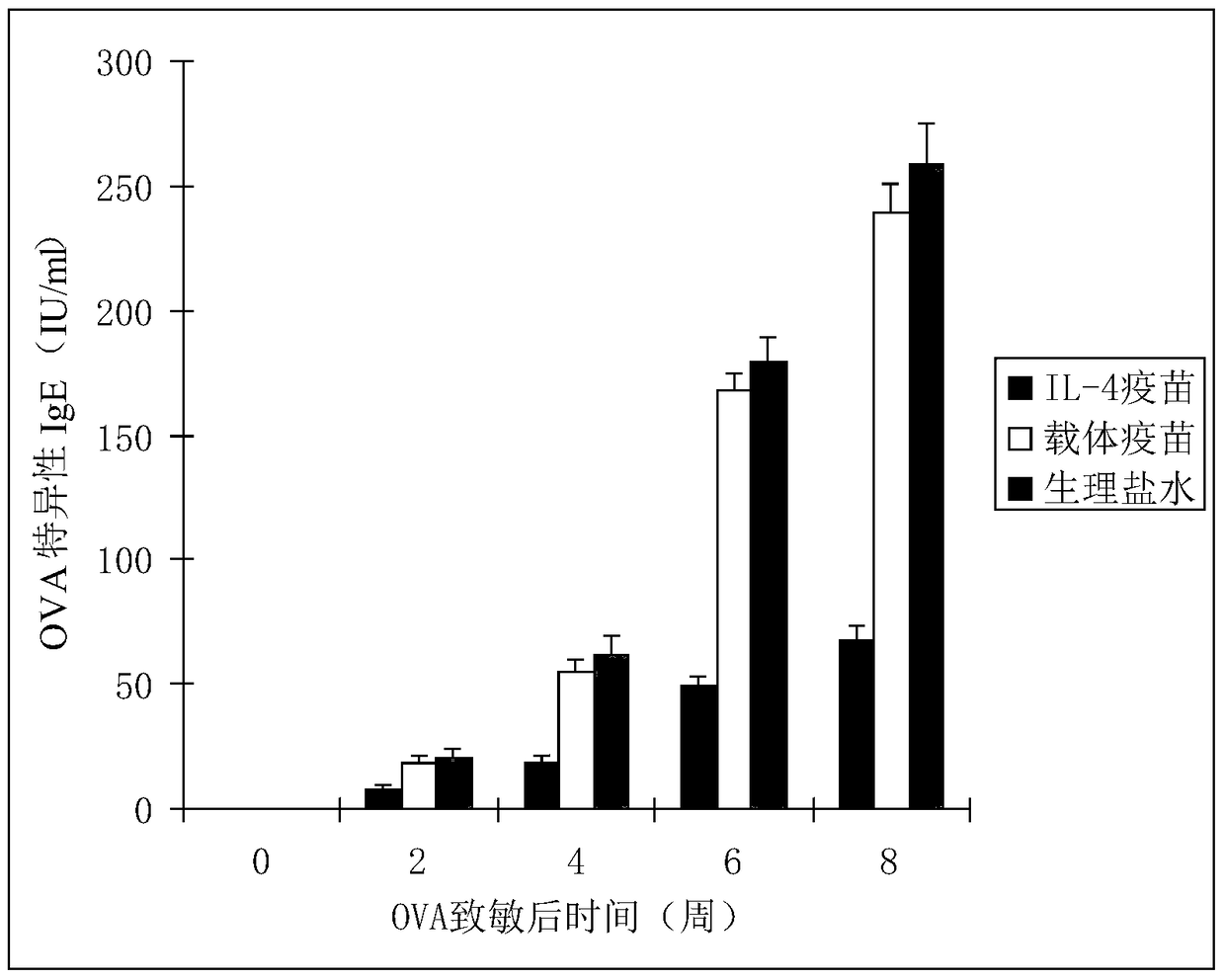
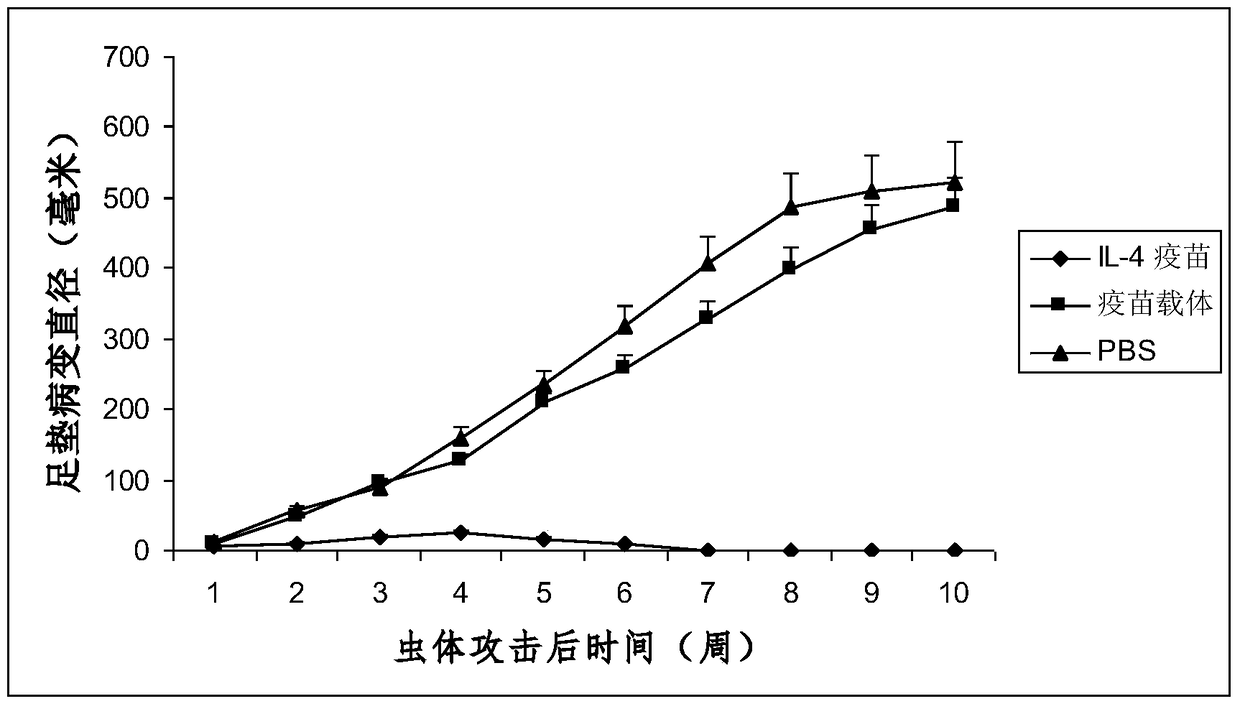
Interleukin-4 Therapeutic Vaccines for the Treatment of Human or Animal Immune-Related Diseases
ActiveCN102266551AProne to allergic reactionsFew applicationsGenetic material ingredientsImmunological disordersDiseaseActive immunization
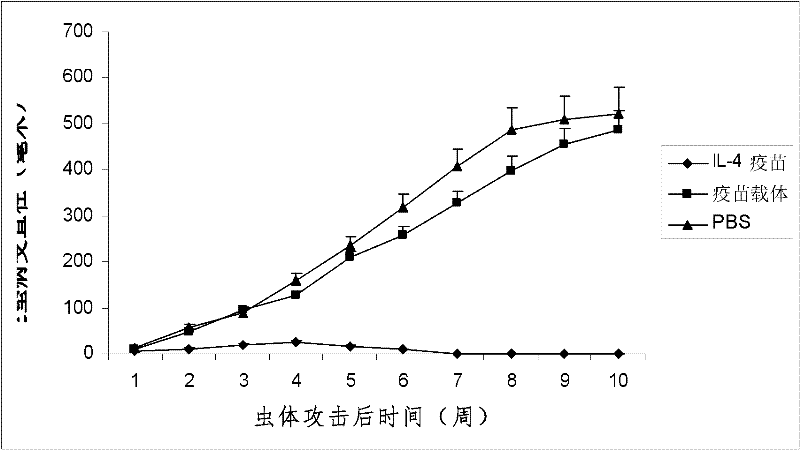
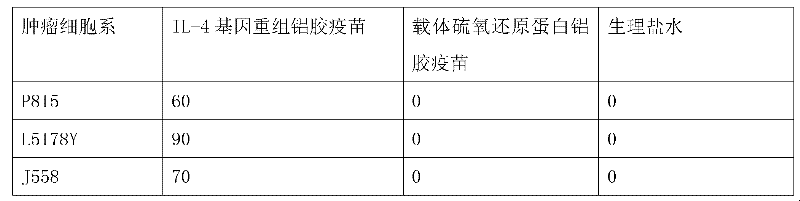
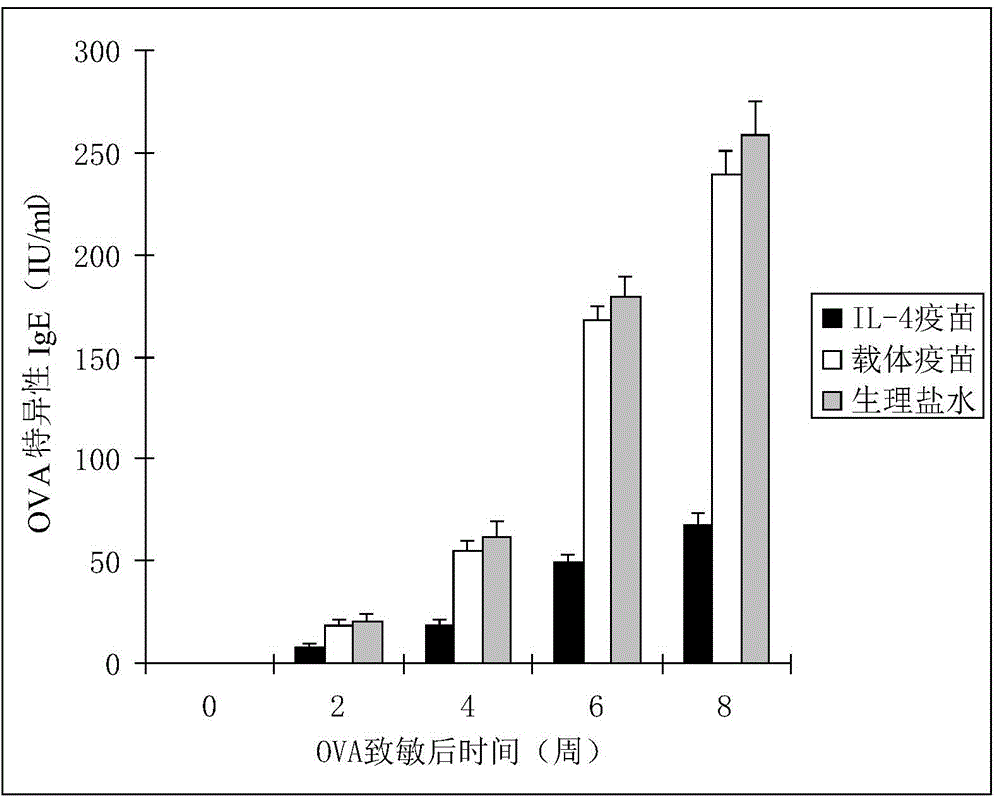
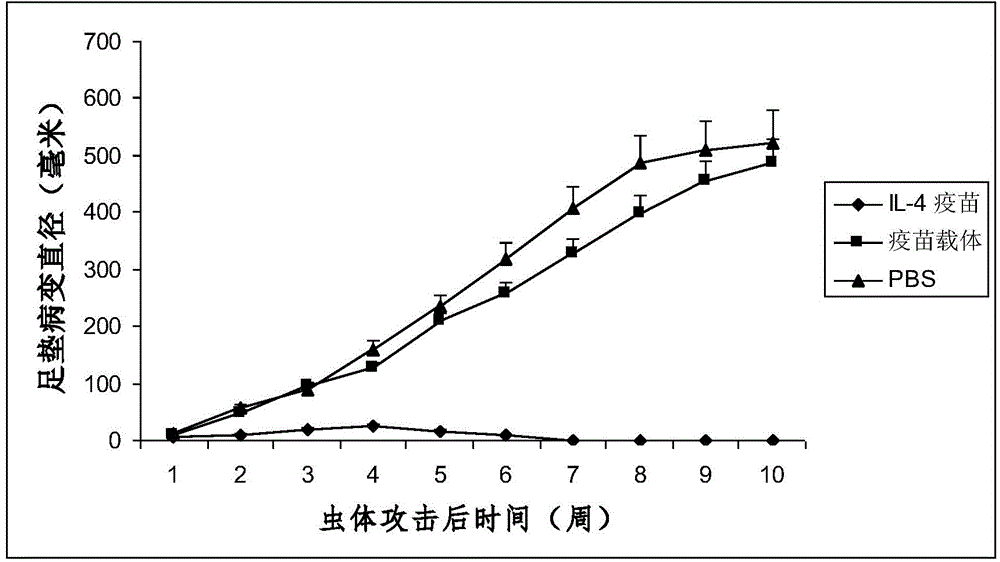
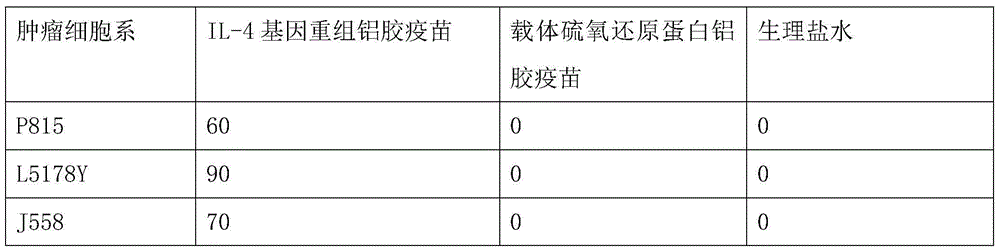
The invention discloses an interleukin-4 therapeutic vaccine for treating immune related diseases of humans or animals. The interleukin-4 therapeutic vaccine is a protein vaccine or coupling protein vaccine of any form prepared by taking the natural or artificially synthesized complete protein or protein fragment of interleukin-4 as an antigen; or the interleukin-4 therapeutic vaccine is a gene vaccine or fusion gene vaccine of any form prepared by taking the complete gene or gene fragment of interleukin-4 as the antigen gene or the major antigen gene. The IL (interleukin)-4 vaccine is used for performing active immunotherapy on a host, generally, the effective time lasts for about 2-3 months by immunization for the first time, the effective therapeutic time lasts for about half a year by secondary immunization, and recovery can be achieved by 1-3 times of immunotherapy. Compared with direct application of anti-IL-4 antibody for treatment, the invention has the characteristics of few times of application, low dose and the like, thereby greatly reducing the therapeutic cost, and also greatly reducing the possibility of generating allergic reaction.
Owner:潍坊康奥思生物技术有限公司
Vaccine composition containing transforming growth factor alpha (TGFalpha). it use in malignant diseases therapy
InactiveUS20030054011A1Prevent proliferationAvoid resistanceBacteriaPeptide/protein ingredientsVaccinationImmunologic function
The present invention relates to the field of immunology and human medicine, in particular with a vaccine preparation able to provoke an immune-castration of self-TGFalpha. The object of this invention is to obtain a vaccine composition for the active immunotherapy of malignant tumors that depend of TGFalpha for its growth. As well as for the treatment of other TGFalpha depend diseases. Another important object of this invention is to obtain a vaccine composition containing a combination of TGFalpha with other EGF-R ligands, such as epidermal growth factor (EGF), able to inhibit the proliferation of tumors whose progression depends on these factors. In that way would be avoided the resistance generated by tumors vaccines containing each molecule for separate, developing tumorigenic phenotype that not depend on the growth factor used in the vaccination. These vaccine preparations are able to inhibit the proliferation of tumors with the characteristics mentioned before, and in this way to be useful in the treatment of malignant neoplasias. Therefore, the invention is also related with the field of specific active immunotherapy of cancer.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Humanized breast cancer antigen and antibody thereof
InactiveCN101962405AHigh expression positive rateStrong specificityCell receptors/surface-antigens/surface-determinantsBacteriaNormal cellPolyclonal antibodies
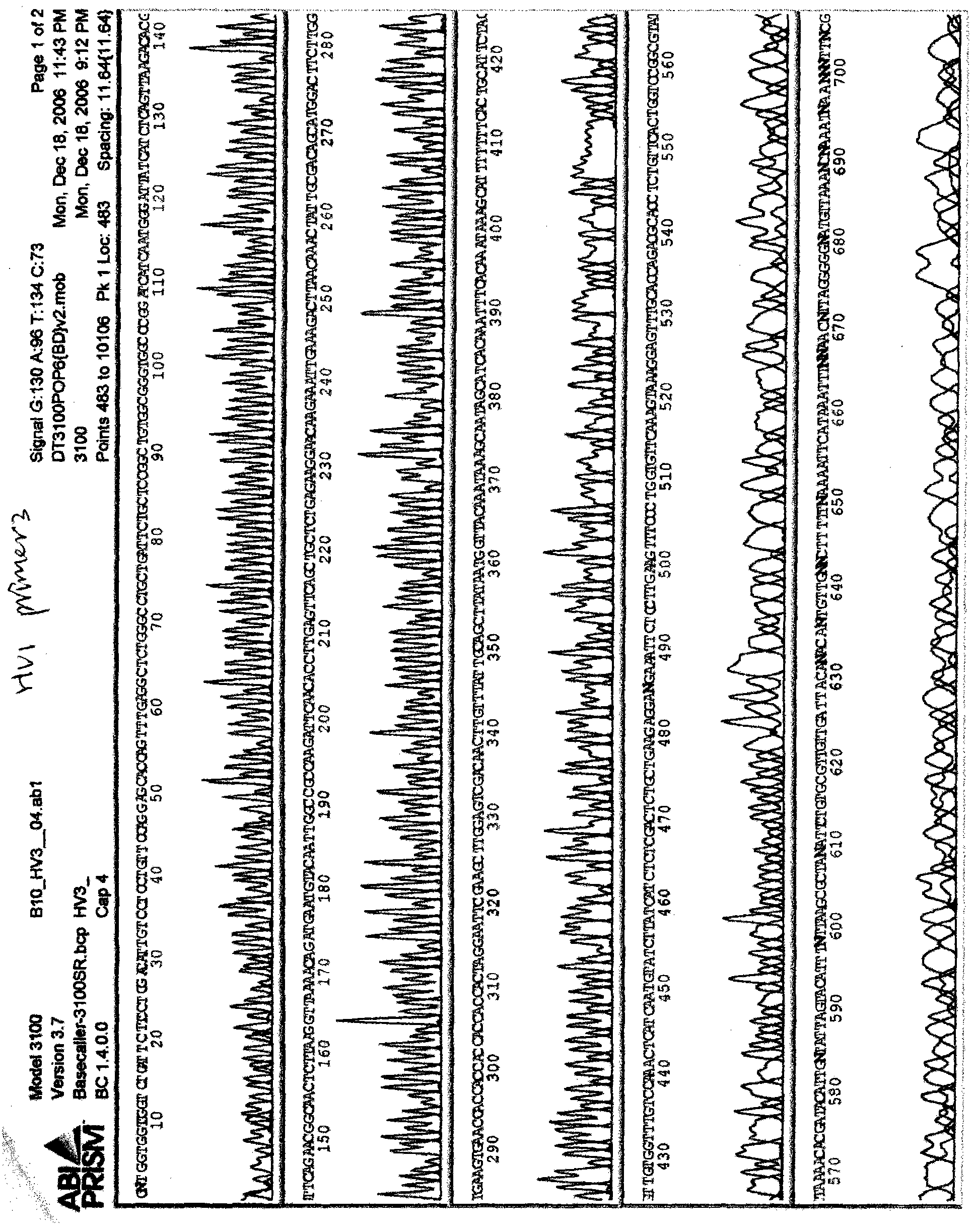
The invention discloses a humanized breast cancer antigen and an antibody thereof. The amino acid residue sequence of the antigen is shown in a sequence list 1. The antigen is a natural antigen or a recombinant protein antigen, which is mainly expressed in cancer cells of the human breast cancer, but is not expressed in human normal cells; and the antigen can be used as a target point of human breast cancer active immunotherapy or prevention and as a target point of various targeted immunological therapies. The invention also provides an antibody resistant to the humanized breast cancer antigen. The antibody is a polyclonal antibody, a mouse monoclonal antibody or antibody segment, and derivates of the antibodies, wherein the derivates comprise the antibody crosslinked with nuclide, the antibody crosslinked with chemical medicaments, the antibody crosslinked with liposome, the antibody fused with a medicament preemzyme, the antibody fused with other effector molecules and the like. The invention also provides application of the antibody and derivates resistant to the humanized breast cancer antigen Hv1 in the preparation of medicaments for diagnosing, treating and preventing human breast cancer.
Owner:NANKAI UNIV
Method of Predicting Responsiveness of B Cell Lineage Malignancies to Active Immunotherapy
InactiveUS20140220562A1Microbiological testing/measurementDisease diagnosisTumor responseActive immunization
Predictive biomarkers identify those patients suffering from immunoglobulin positive (Ig+) B lineage malignancies that are responsive to active immunotherapy, where the active immunotherapy comprises vaccination with a tumor-specific idiotype-immunogen. It is shown herein that patient responsiveness to the idiotype-immunogen is dependent upon the sequence of the immunogen, where an immunogen having a low number of tyrosine residues in the CDR1 (herein termed CDR1-Y10) regions of one or both of the immunogen heavy and light chains is predictive of a positive anti-tumor response, while a high number of CDR1 tyrosine residues (herein termed CDR1-Yhi) is predictive of a low anti tumor response.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Carbon nanotube compositions and methods of use thereof
InactiveUS20160213761A1Reduce deliveryFacilitate antigen uptakePeptide/protein ingredientsNanomedicineCarbon nanotubeT cell
Carbon nanotube (CNT)-based compositions for activating cellular immune responses are provided. The CNTs function as high surface area scaffolds for the attachment of T cell ligands and / or antigens. The CNT compositions function as artificial antigen-presenting cells (aAPCs) or as modular vaccines. The disclosed CNT aAPCs are efficient at activating T cells and may be used to activate T cells ex vivo or in vivo for adoptive or active immunotherapy.
Owner:YALE UNIV
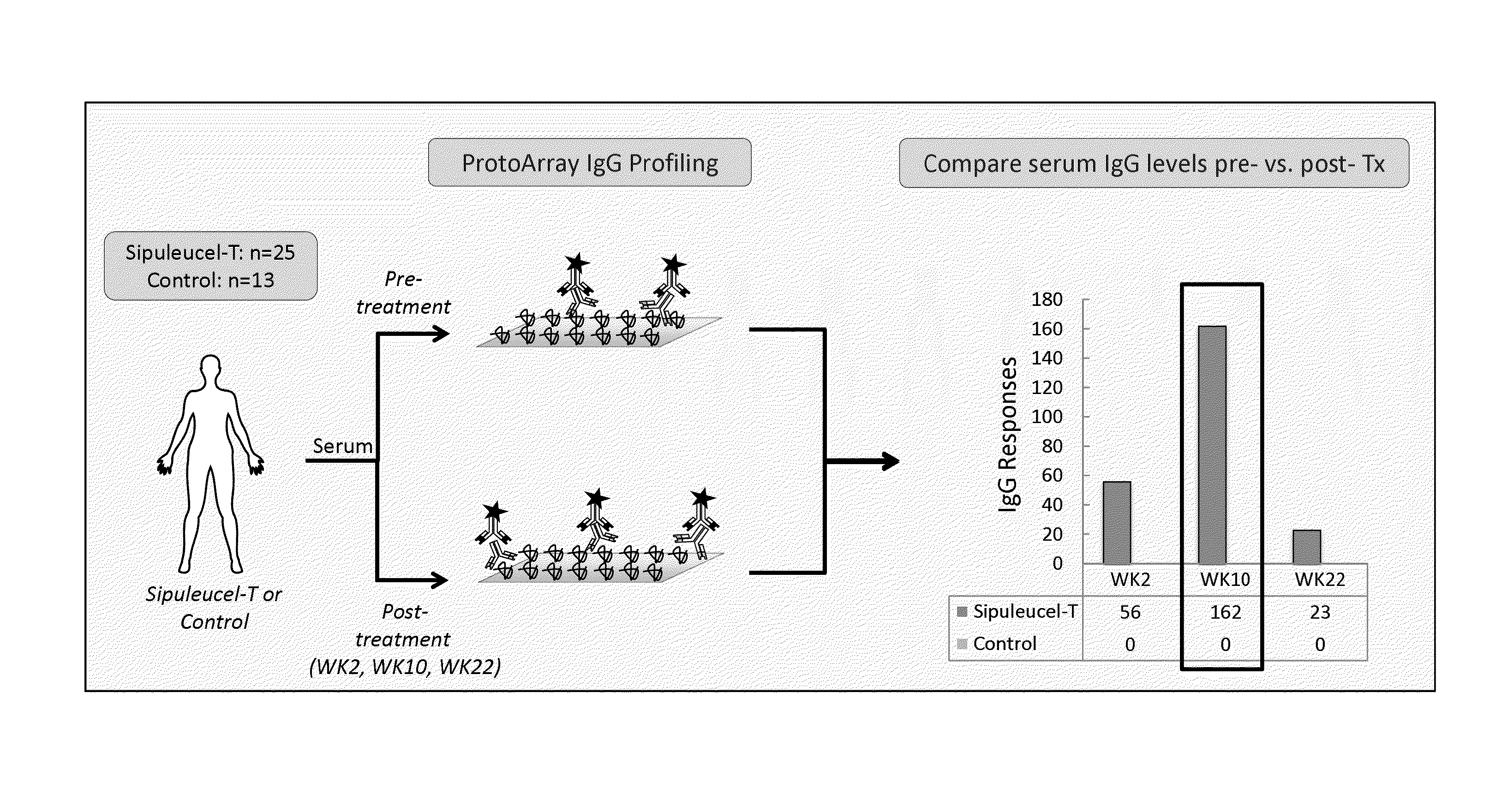
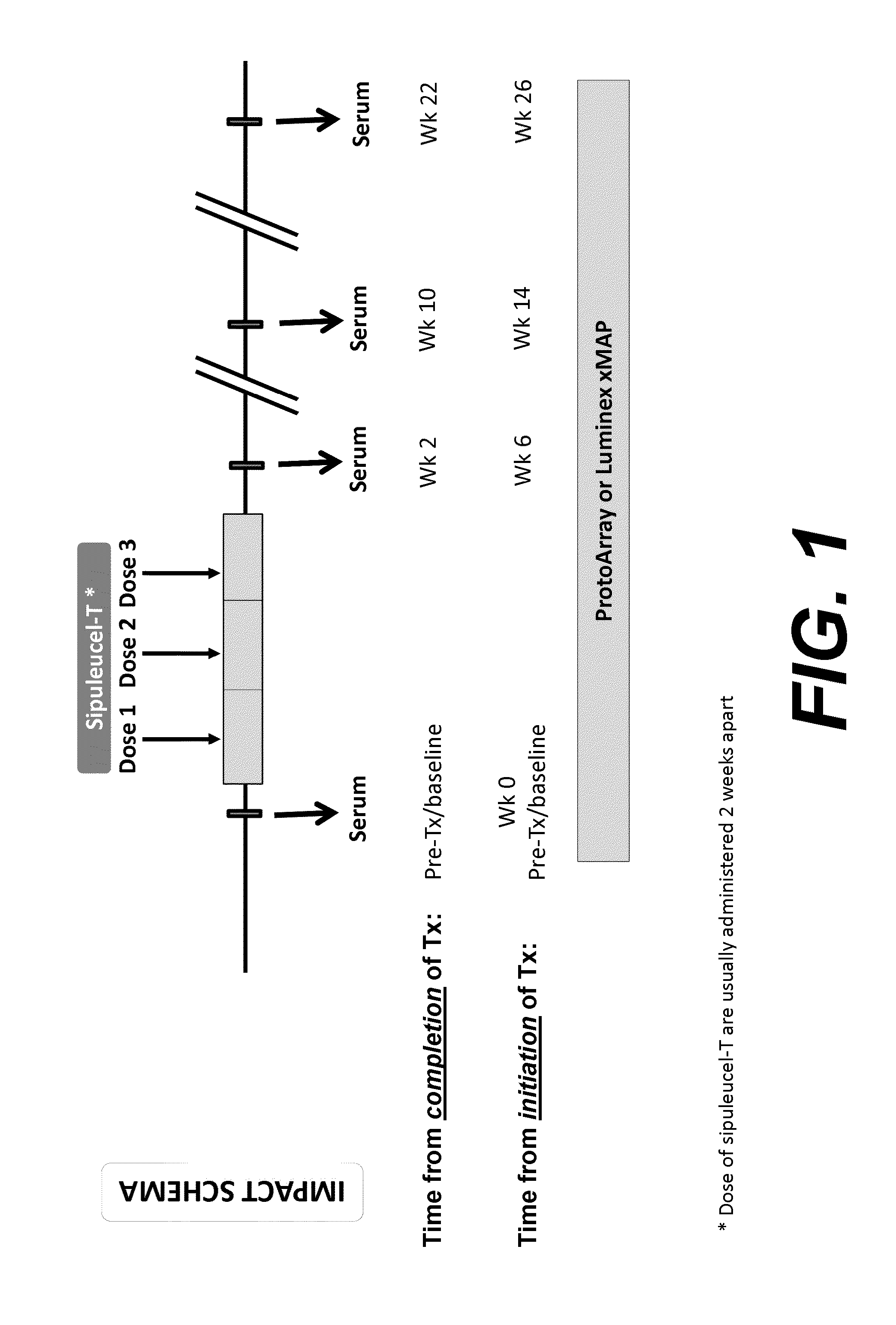
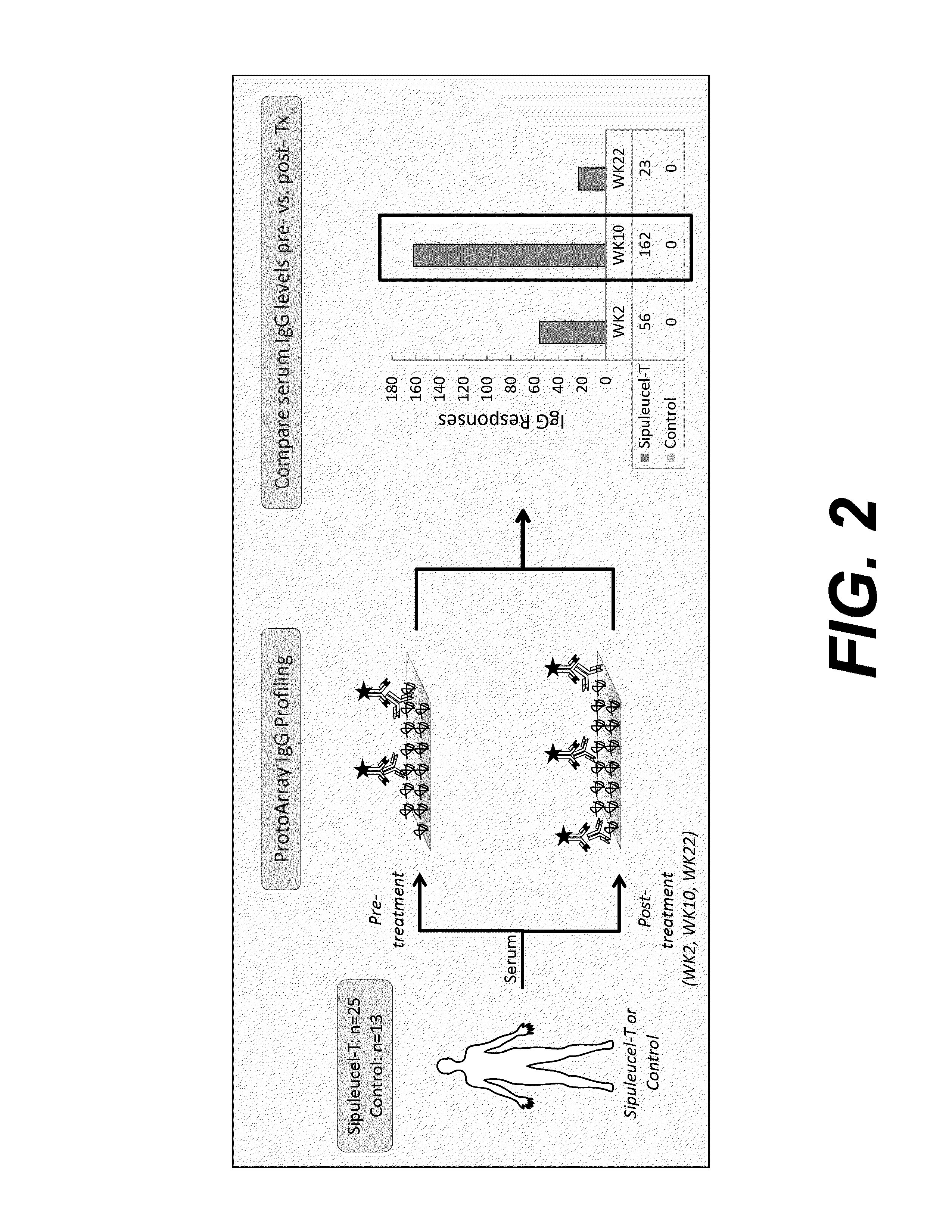
Humoral immune response against tumor antigens after treatment with a cancer antigen specific active immunotherapy and its association with improved clinical outcome
ActiveUS20150064210A1Improve survivalRaise antibody levelsMedical simulationCompound screeningAbnormal tissue growthCellular antigens
Compositions and methods are provided herein for predicting therapeutic outcome by measuring patient response to cellular antigen specific active immunotherapy (CASAI) using predetermined biomarkers.
Owner:DENDREON PHARMA LLC
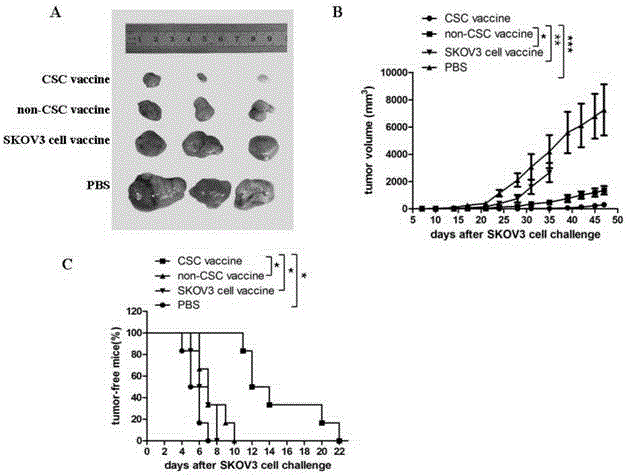
Ovarian cancer stem cell vaccine and preparation method thereof
InactiveCN105219731AHigh activityHigh tumorigenicityTumor/cancer cellsAntibody medical ingredientsCancer cellNatural Killer Cell Inhibitory Receptors
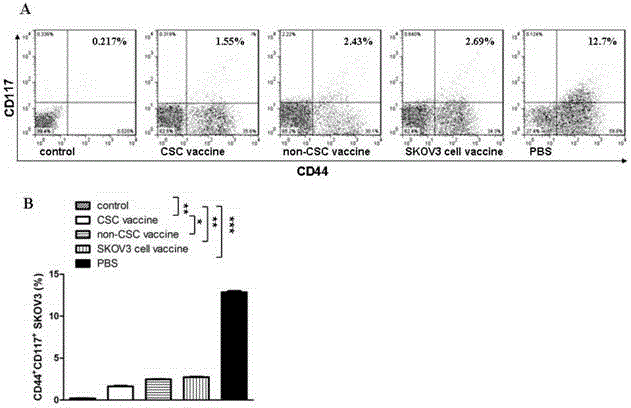
The invention relates to the field of molecular biology and discloses an ovarian cancer stem cell vaccine and a preparation method and application thereof. Cancer stem cells are insensitive to radiotherapy and chemotherapy, which is the root of cancer metastasis and recurrence. Aiming at active immunotherapy of the cancer stem cells, satisfactory therapeutic effect can be gained only on the condition that cancer 'seed' cells, namely the cancer stem cells are eliminated. The ovarian cancer stem cell CD117+CD44+ vaccine is capable of improving blood serum IFN (interferon)-gamma level, lowering TGF (transforming growth factor)-beta expression, enhancing NK cell activity, decreasing the number of ovarian cancer stem cells CD117+CD44+ remarkably and inhibiting cancer growth in animal experiments.
Owner:SOUTHEAST UNIV +1
Therapeutic interleukin-4 vaccine capable of treating human or animal chronic tuberculosis
InactiveCN104623647AProne to allergic reactionsFew applicationsAntibacterial agentsAntibody medical ingredientsIntact proteinConjugated protein
The invention discloses a therapeutic interleukin-4 vaccine capable of treating human or animal chronic tuberculosis. The therapeutic interleukin-4 vaccine is an any-form protein vaccine or conjugated protein vaccine prepared by taking a natural or artificially-synthesized intact protein or protein fragment of interleukin-4 as an antigen or an any-form gene vaccine or fused gene vaccine prepared by taking an intact gene or gene fragment of interleukin-4 as an antigen gene or main antigen gene. According to the invention, a host is subjected to active immunotherapy by using an IL-4 vaccine, generally, the effective time of the first immunity can be up to about 2-3 months, the effective treatment time of the second immunity can be up to about 6 months, and the rehabilitation aim can be achieved through carrying out immunotherapy for 1-3 times. Compared with a method for treating human or animal chronic tuberculosis by directly applying an IL-4 antibody, the therapeutic interleukin-4 vaccine has the characteristics of low application frequency, small dosage and the like, so that not only is the treatment cost greatly reduced, but also the possibility for generating anaphylactic reaction is greatly reduced.
Owner:刘永庆
Modified dendritic cell and vaccine containing the same
ActiveCN102787097BSimple preparation processGood effect of active immunotherapyGenetic material ingredientsAntibody medical ingredientsDendritic cellVirus type
Owner:广东省医学医疗有限公司
Method of predicting responsiveness of B cell lineage malignancies to active immunotherapy
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Tumorized human umbilical vein endothelial cell vaccine and its application in anticancer
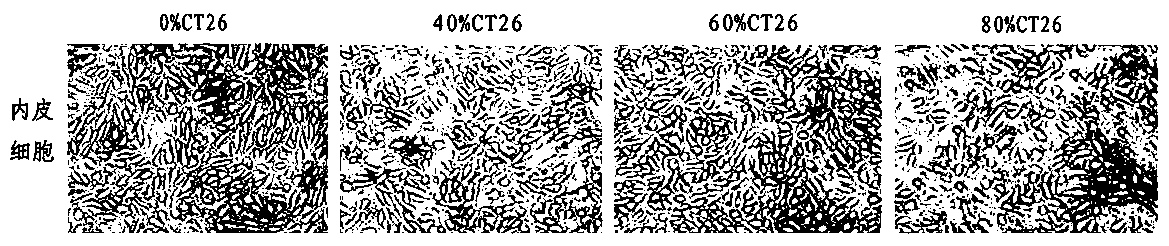
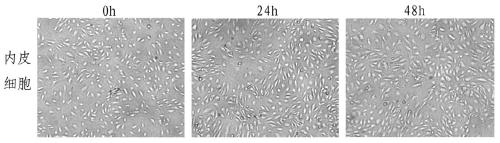
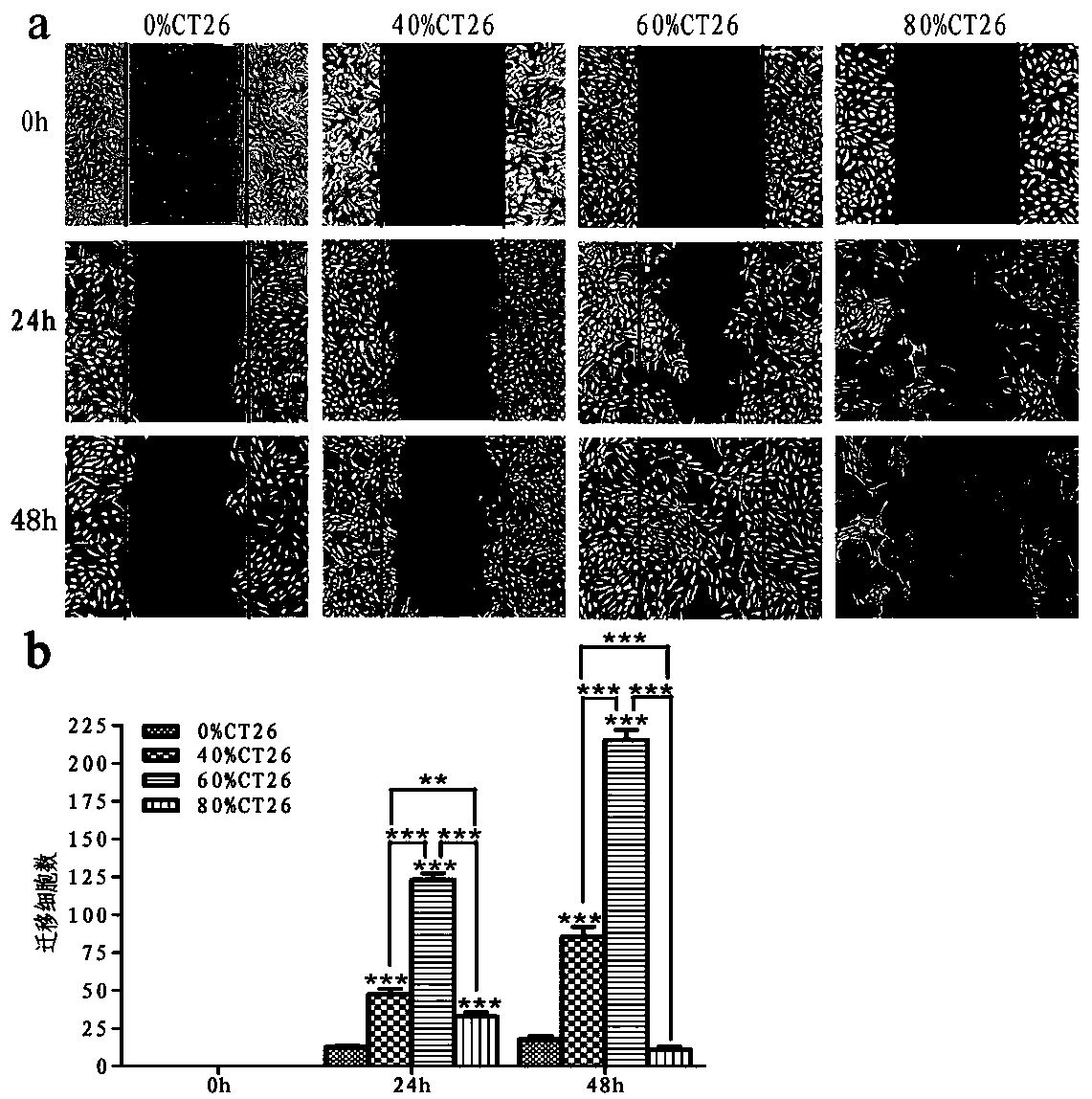
ActiveCN108379566BSolve hard-to-get puzzlesImprove migration abilityCancer antigen ingredientsAntineoplastic agentsActive immunizationTumor vessel
The invention belongs to the field of technical methods for preventing tumors, and in particular relates to a tumor microenvironment induced cancerous human umbilical vein endothelial cell vaccine andan application thereof in resisting angiogenesis of colon carcinoma. The vaccine is prepared by the following steps: collecting supernatant of CT26 cells, inducing HUVEC cells via the CT26 cell supernatant, collecting the HUVEC cells, immobilizing glutaraldehyde and the like. According to the invention, tumor vessel endothelial cells function as a target in tumor immnuotherapy, belonging to active immunotherapy. The cancerous HUVEC vaccine is prepared through inducing by an in-vitro simulated tumor microenvironment. Based upon preliminary experimental results, it is indicated that the prepared cancerous HUVEC vaccine can cause a more effective action in inhibiting tumor angiogenesis, and subsequently, an effect of resisting colon carcinoma growth can be developed; therefore, a relativelygood application effect can be achieved; and meanwhile, a new possibility is also provided for the application of a treatment method based on resisting tumor angiogenesis.
Owner:ZHENGZHOU UNIV
Construction method of vlp vaccine presenting il-33 for active immunotherapy of chronic asthma
ActiveCN104001168BEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsHepatitis B virus core AntigenEscherichia coli
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
A gene-modified dendritic cell vaccine
ActiveCN109957548BEfficient DCGenetically modified cellsCancer antigen ingredientsPeripheral blood mononuclear cellActive immunization
The present invention relates to the fields of biotechnology and medicine, and provides a modified dendritic cell (dendritic cell, DC). The dendritic cell is infected with a tandem sequence of MG-7Ag antigen mimetic epitopes loaded with a lentiviral vector, so that the target antigen sequence Integrating into the dendritic cell genome, the present invention provides a protocol for rapidly culturing dendritic cells derived from peripheral blood mononuclear cells in vitro, and the present invention also provides a vaccine whose active ingredient is the modified dendritic cells. The vaccine is used for prevention and active immunotherapy against tumors. The MG-7Ag antigen sequence modified DC vaccine of the present invention has the ability to obtain high-purity CTL cells and high-efficiency target cell killing ability after DC-CTL co-culture, and the co-culture supernatant contains a high concentration of IFNγ secretion. After tumor challenge, the tumor volume of mice in the DC-CTL group was significantly smaller than that in the control group. This DC vaccine has great potential value in the immunotherapy of MG-7Ag-positive tumors.
Owner:上海尚泰生物技术有限公司
Combination therapy using active immunotherapy
ActiveUS20150023993A1Organic active ingredientsCancer antigen ingredientsSunitinib malateMajor histocompatibility
Owner:IMMATICS BIOTECHNOLOGIES GMBH
Dendritic cell vaccine sensitized by A2B5 + glioma cells
InactiveCN114209820ACell dissociation methodsTumor rejection antigen precursorsImmunocompetenceActive immunization
The invention provides a dendritic cell vaccine sensitized by an A2B5 < + > brain glioma stem cell-like antigen. Specifically, the invention also provides a dendritic cell targeting A2B5 positive tumor, a cytotoxic T lymphocyte, a cell preparation and a preparation method and application thereof. The dendritic cell vaccine can be used for tumor prevention and active immunotherapy, has good safety, high purity, efficient killing ability and strong specificity, and can enhance the immunocompetence of patients, improve prognosis and prevent tumor recurrence to a certain extent.
Owner:上海尚泰生物技术有限公司
Th1 vaccination priming for active immunotherapy
The present invention includes vaccine compositions and methods for using these vaccine compositions in active immunotherapy. The vaccine compositions include allogeneic activated Th1 memory cells. The compositions can also include one or more disease-related antigens. The methods include administering the vaccine compositions to provide a Th1 footprint in normal individuals or patients susceptible to disease or having minimal residual disease.
Owner:MIRROR BIOLOGICS INC +1
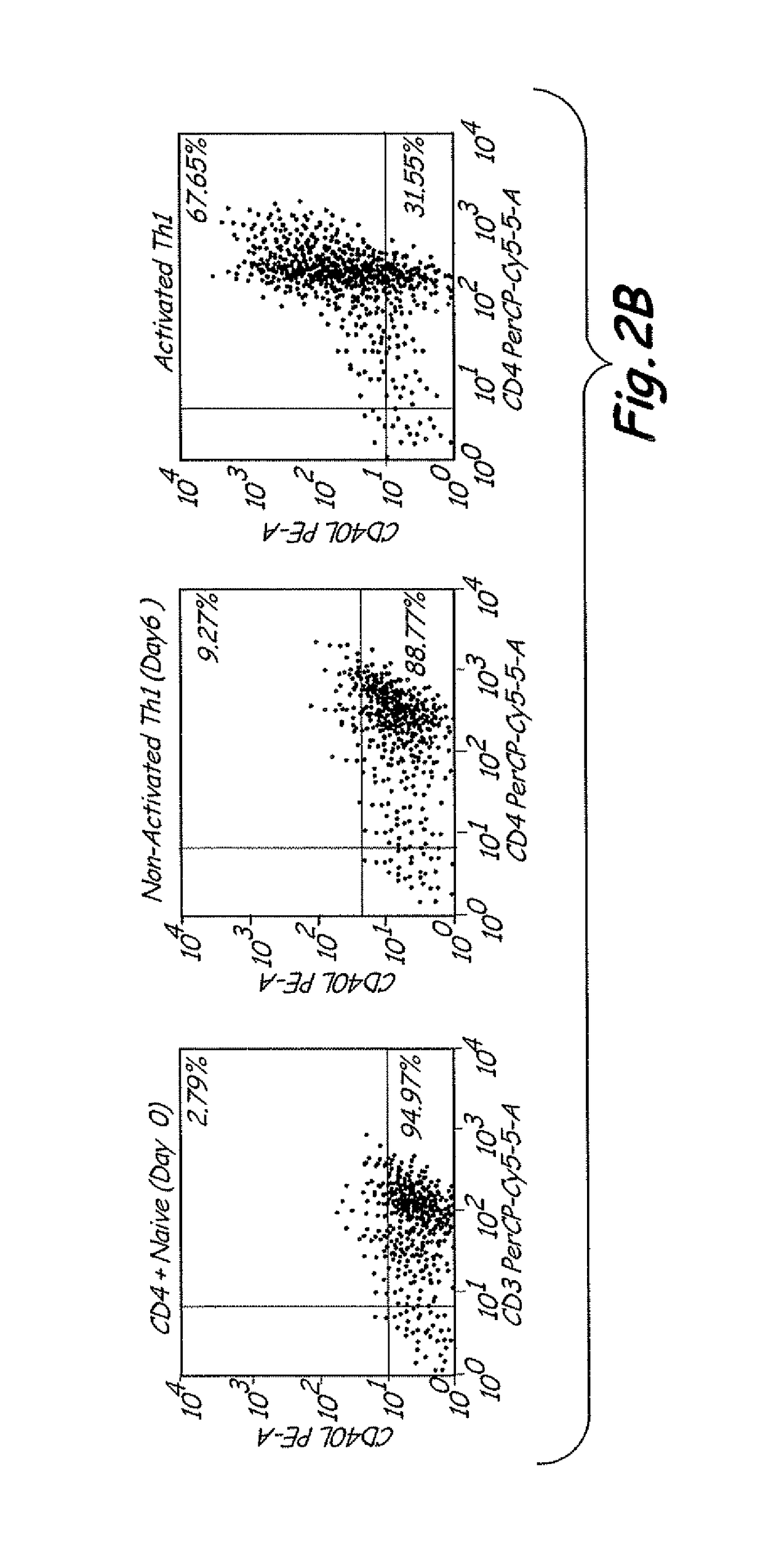
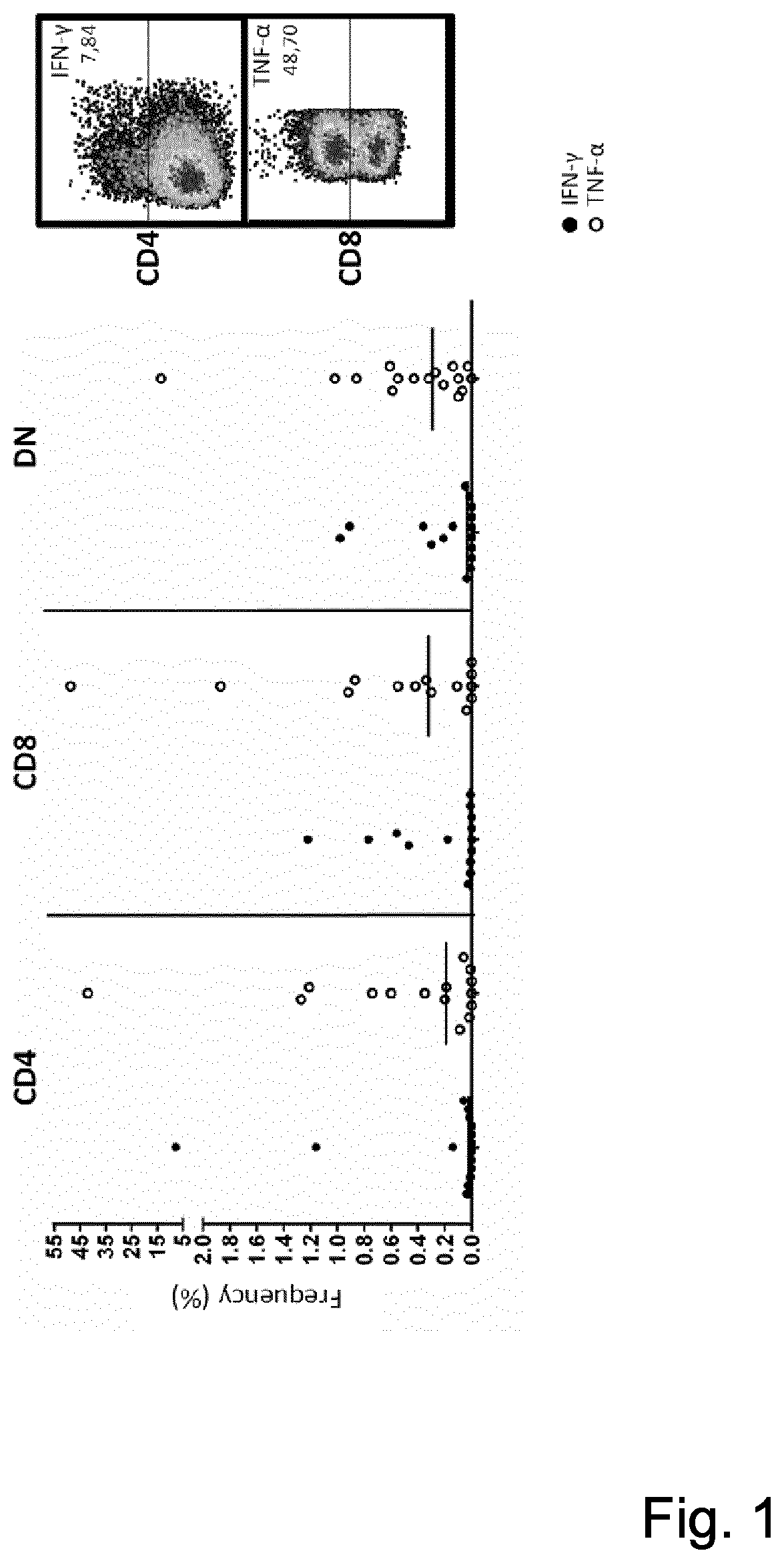
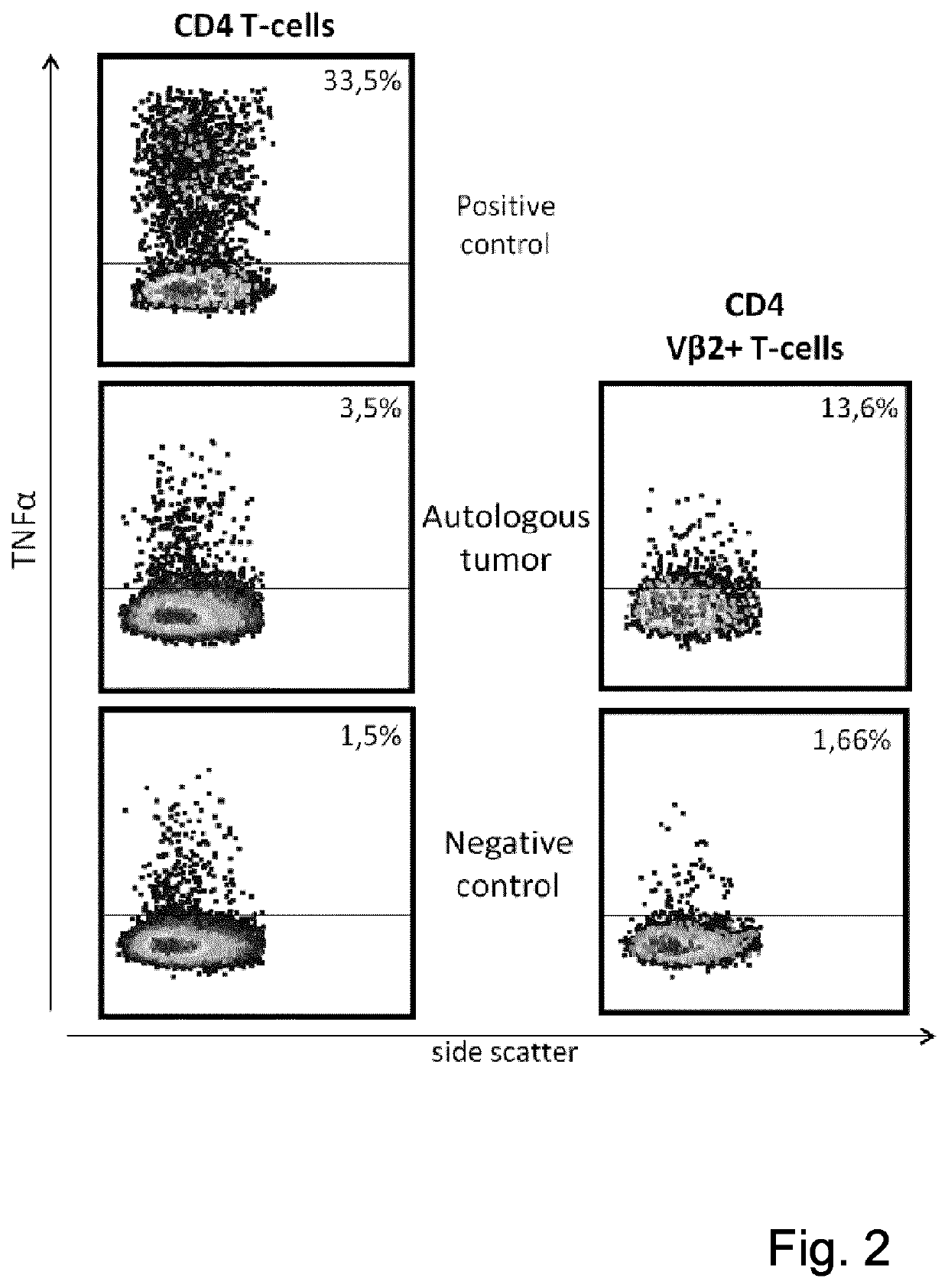
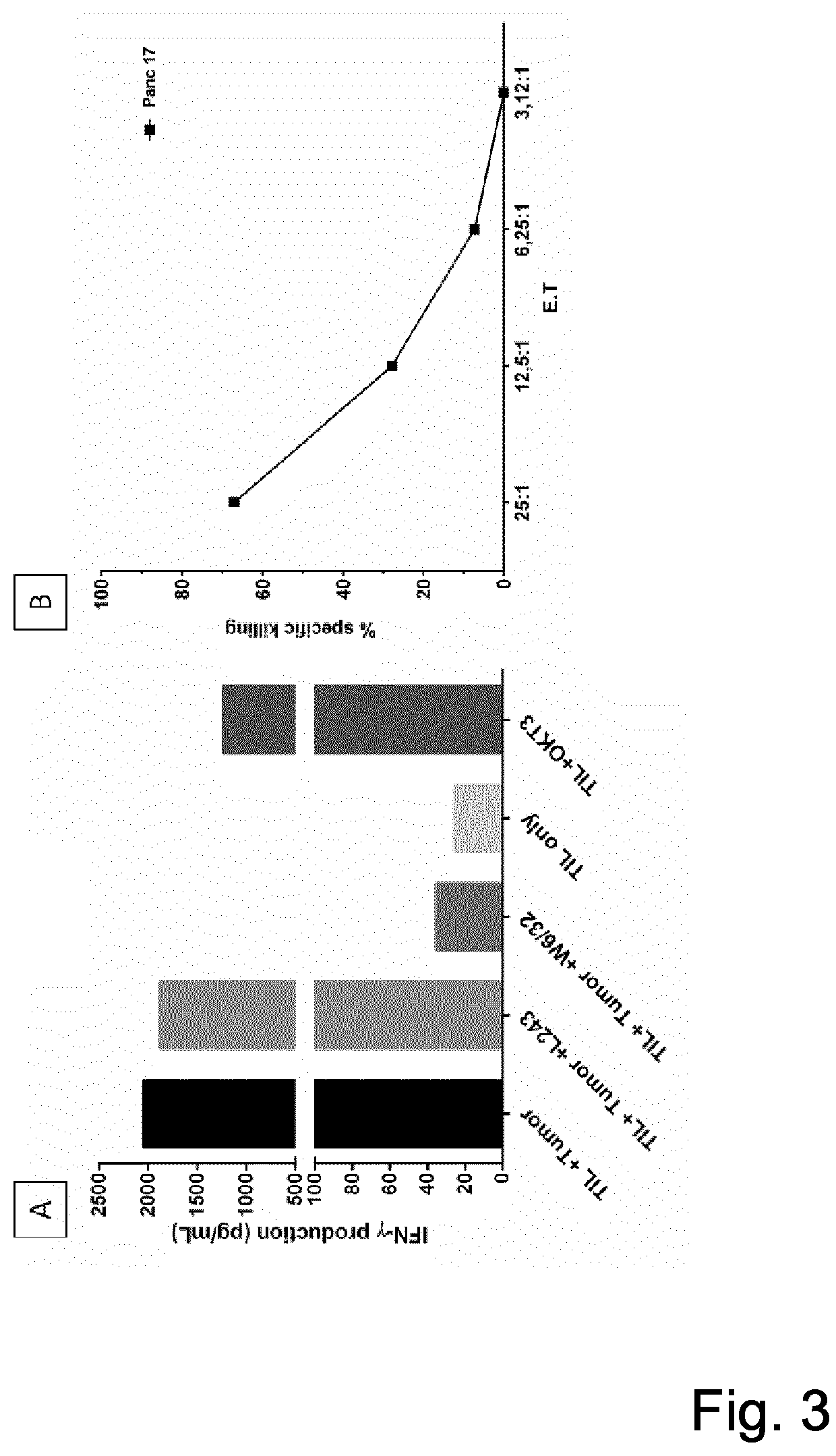
T-cell receptor sequences for active immunotherapy
InactiveUS20200368277A1Immunoglobulin superfamilyPeptide/protein ingredientsCellular receptorCell biology
The present invention provides a protein, in particular a TCR comprising a Vβ amino acid sequence with a sequence identity of at 90%, to a sequence selected from SEQ ID NO: 1 to 27, a T-cell comprising the TCR a method of selecting a T-cell product for use in active immunotherapy based on the identification of an Vβ amino acid sequence with a sequence identity of at 90%, to a sequence selected from SEQ ID NO: 1 to 27 in a T-cell product.
Owner:POLYBIOCEPT GMBH
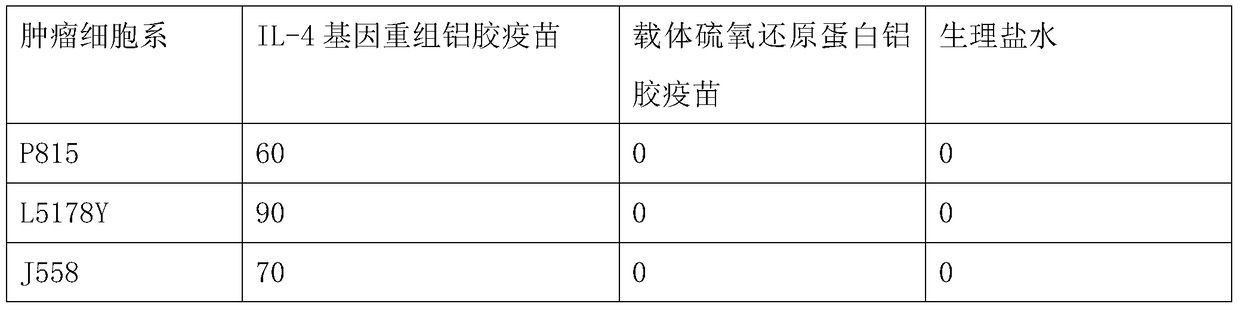
Vaccine composition comprising a mutant of human interleukin-15
Vaccine composition containing a mutant of the human Interleukin-15 (IL-15). The use of said mutant polypeptide of human IL-15 to manufacture a medicament for the active immunotherapy of diseases associated with IL-15 overexpression, including some autoimmune diseases and hematological malignancies. Administration to an individual that needs it of a therapeutically effective amount of the vaccine, that comprises the mutant polypeptide of the human IL-15 of the invention, constitutes a method for the therapy of IL-15 over-expressing related diseases.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
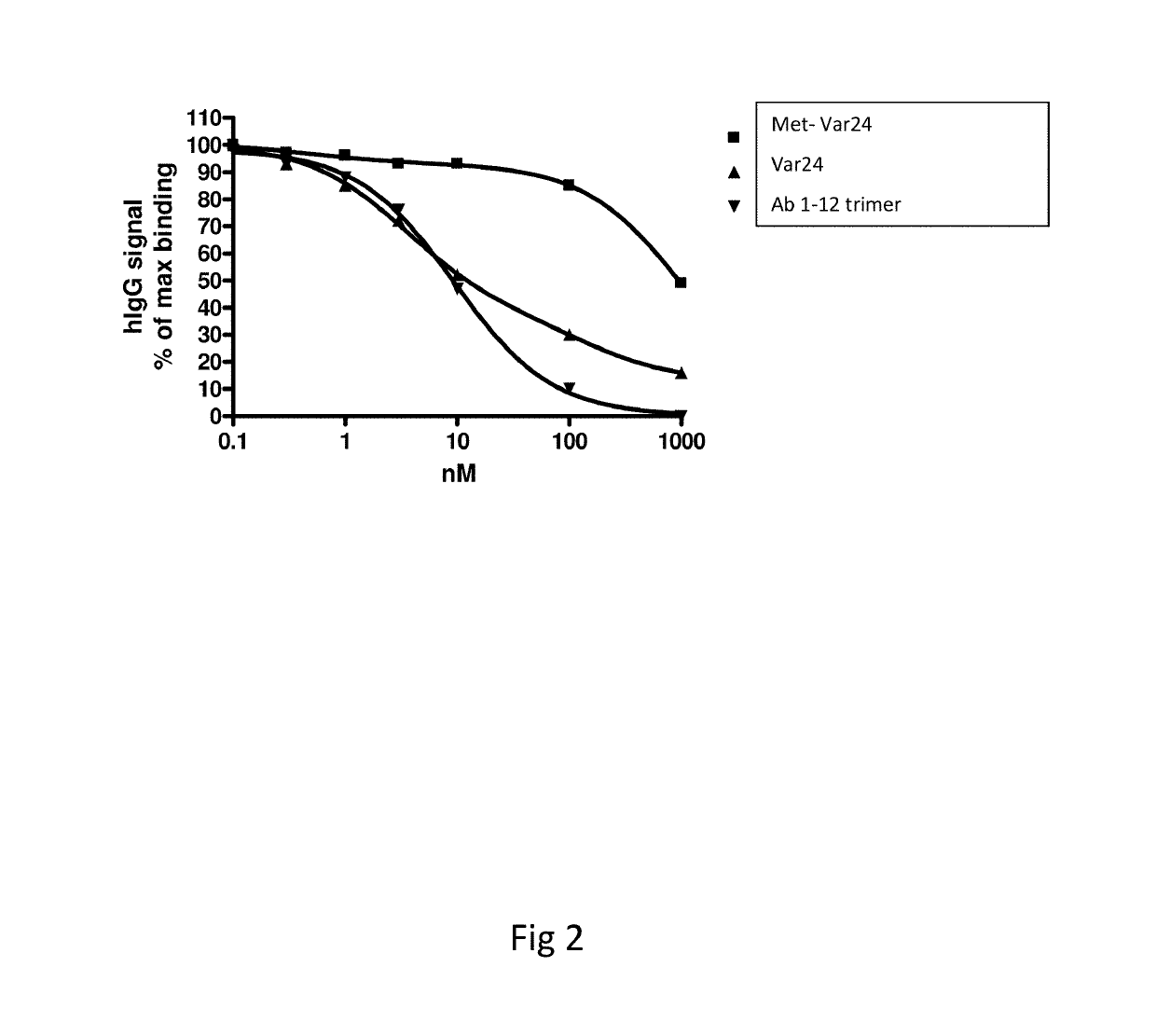
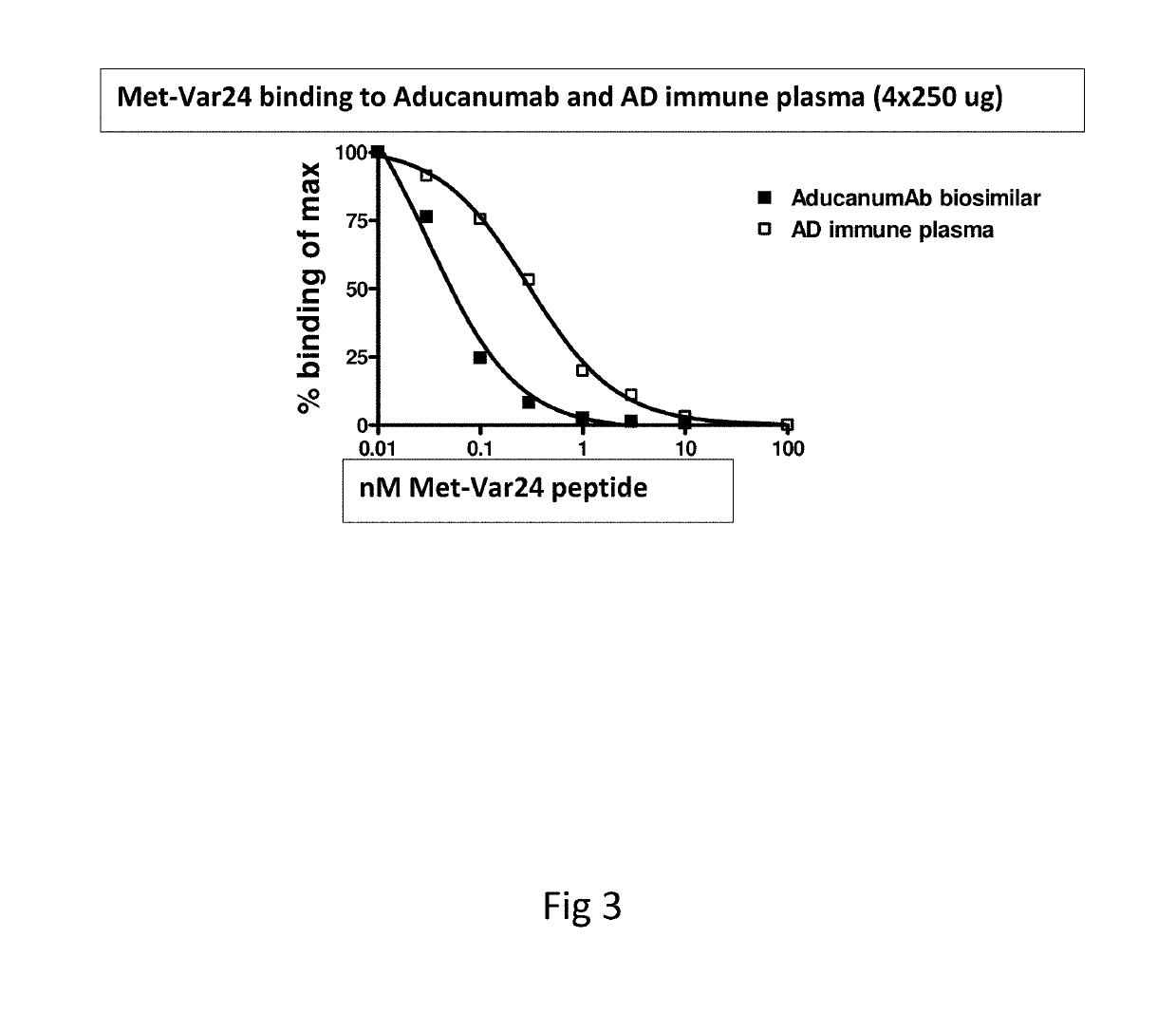
Abeta variants, assay, method and treatment of alzheimer's disease
InactiveUS20190275125A1Effective monitoringAss qualityBacterial antigen ingredientsNervous disorderDisease patientImmune therapy
The present invention relates to a new abeta immunogen variants that enables efficient treatment of Alzheimer's Disease patients by raising specific antibodies against oligimeric and toxic abeta deposits in the brain of Alzheimer's patients. The invention also relates to an assay that enables efficient treatment of Alzheimer's Disease patients by assessing and monitoring the titre response to active immune therapy, as well as treatment and identification of specific subpopulations of Alzheimer Disease patients
Owner:H LUNDBECK AS
Interleukin-4 therapeutic vaccine that can be used to treat human or animal tumor diseases
ActiveCN104623688BProne to allergic reactionsFew applicationsGenetic material ingredientsAntiinfectivesDiseaseIntact protein
The invention discloses a therapeutic interleukin-4 vaccine capable of treating human or animal tumor diseases. The therapeutic interleukin-4 vaccine is an any-form protein vaccine or conjugated protein vaccine prepared by taking a natural or artificially-synthesized intact protein or protein fragment of interleukin-4 as an antigen or an any-form gene vaccine or fused gene vaccine prepared by taking an intact gene or gene fragment of interleukin-4 as an antigen gene or main antigen gene. According to the invention, a host is subjected to active immunotherapy by using an IL-4 vaccine, generally, the effective time of the first immunity can be up to about 2-3 months, the effective treatment time of the second immunity can be up to about 6 months, and the rehabilitation aim can be achieved through carrying out immunotherapy for 1-3 times. Compared with a method for treating human or animal tumor diseases by directly applying an IL-4 antibody, the therapeutic interleukin-4 vaccine has the characteristics of low application frequency, small dosage and the like, so that not only is the treatment cost greatly reduced, but also the possibility for generating anaphylactic reaction is greatly reduced.
Owner:济南翰宝生物技术有限公司
Vaccine composition comprising a mutant of human interleukin-15
Vaccine composition containing a mutant of the human Interleukin-15 (IL-15). The use of said mutant polypeptide of human IL-15 to manufacture a medicament for the active immunotherapy of diseases associated with IL-15 overexpression, including some autoimmune diseases and hematological malignancies. Administration to an individual that needs it of a therapeutically effective amount of the vaccine, that comprises the mutant polypeptide of the human IL-15 of the invention, constitutes a method for the therapy of IL-15 over-expressing related diseases.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com