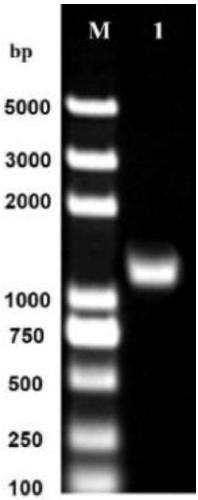
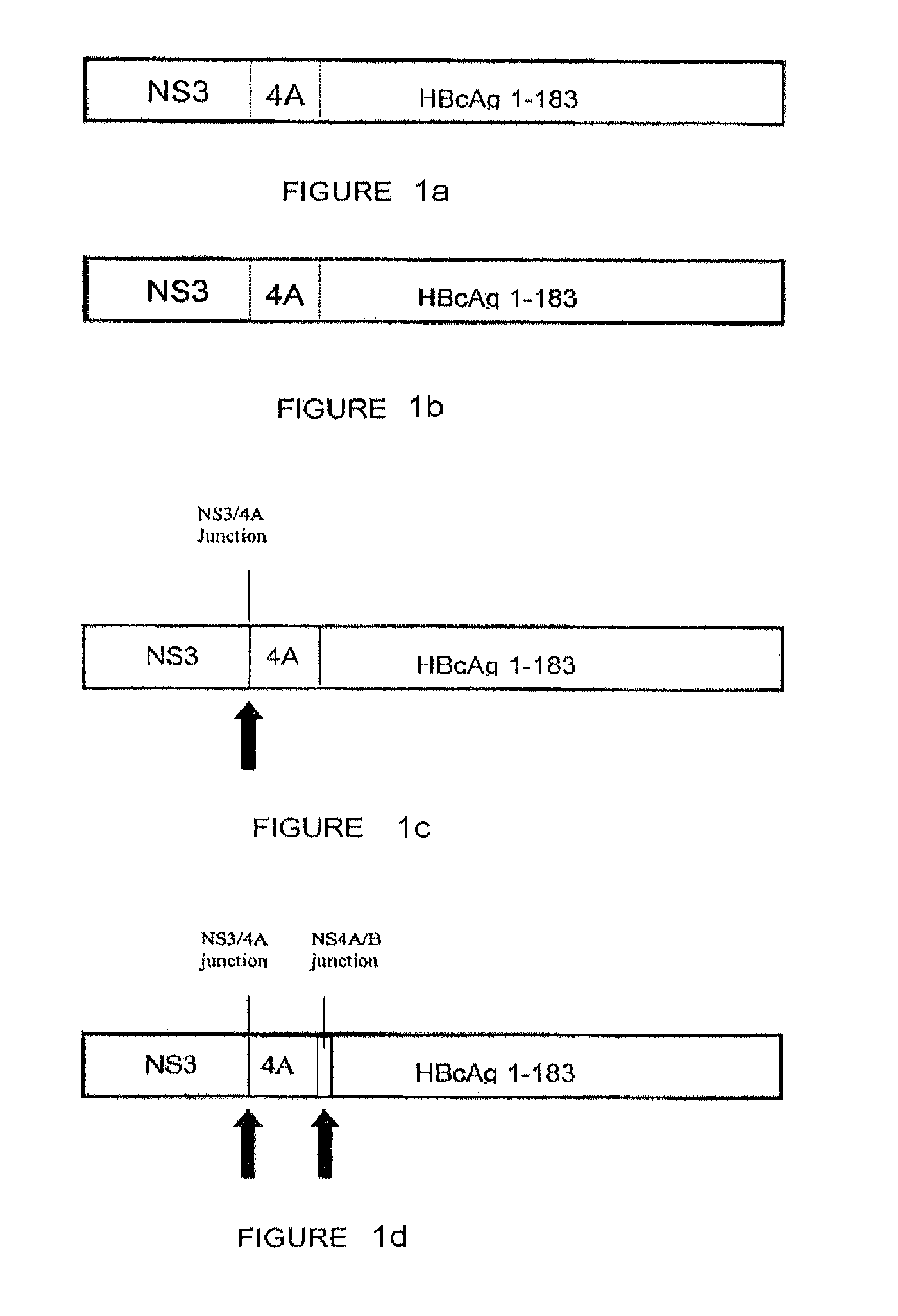
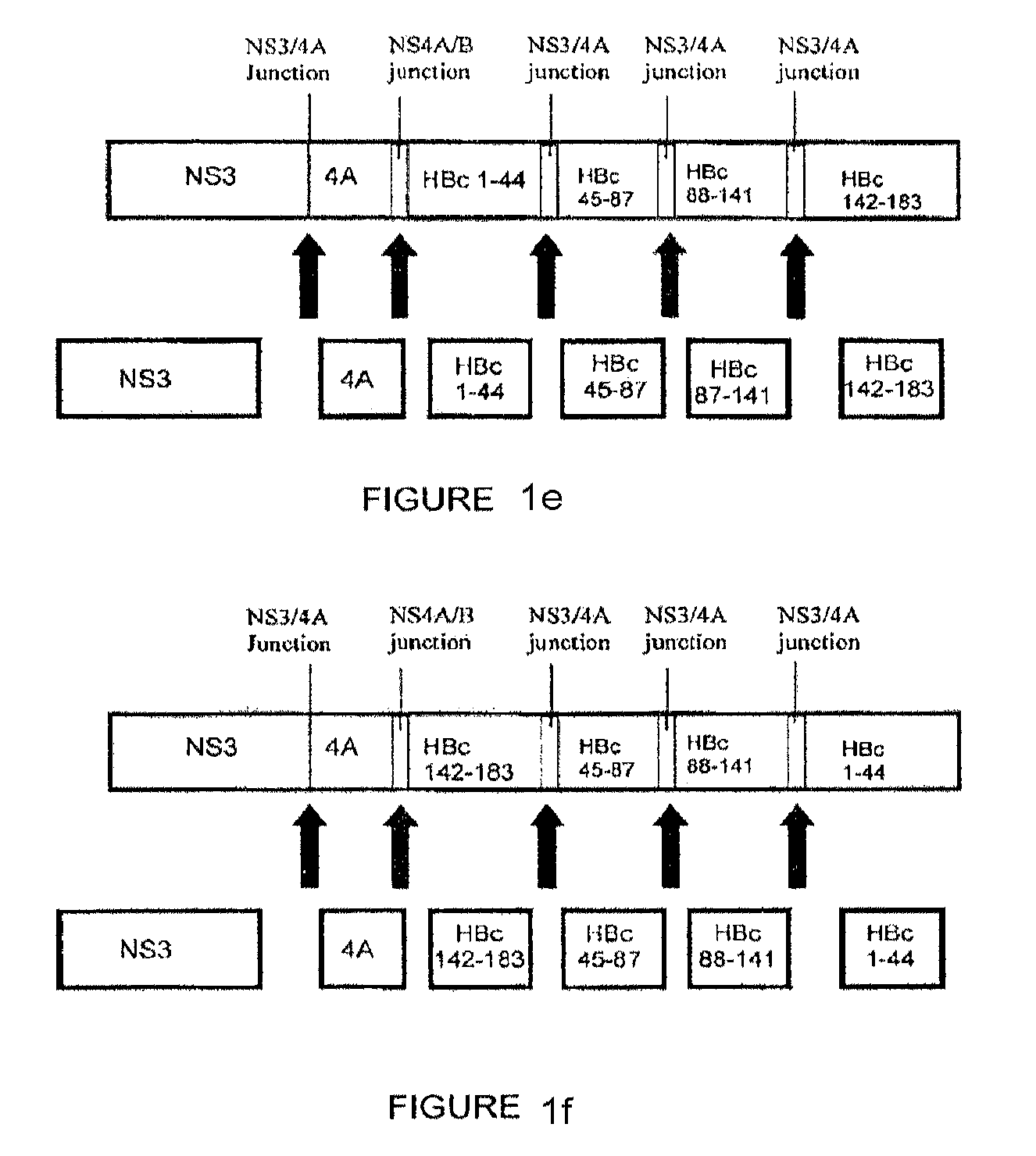
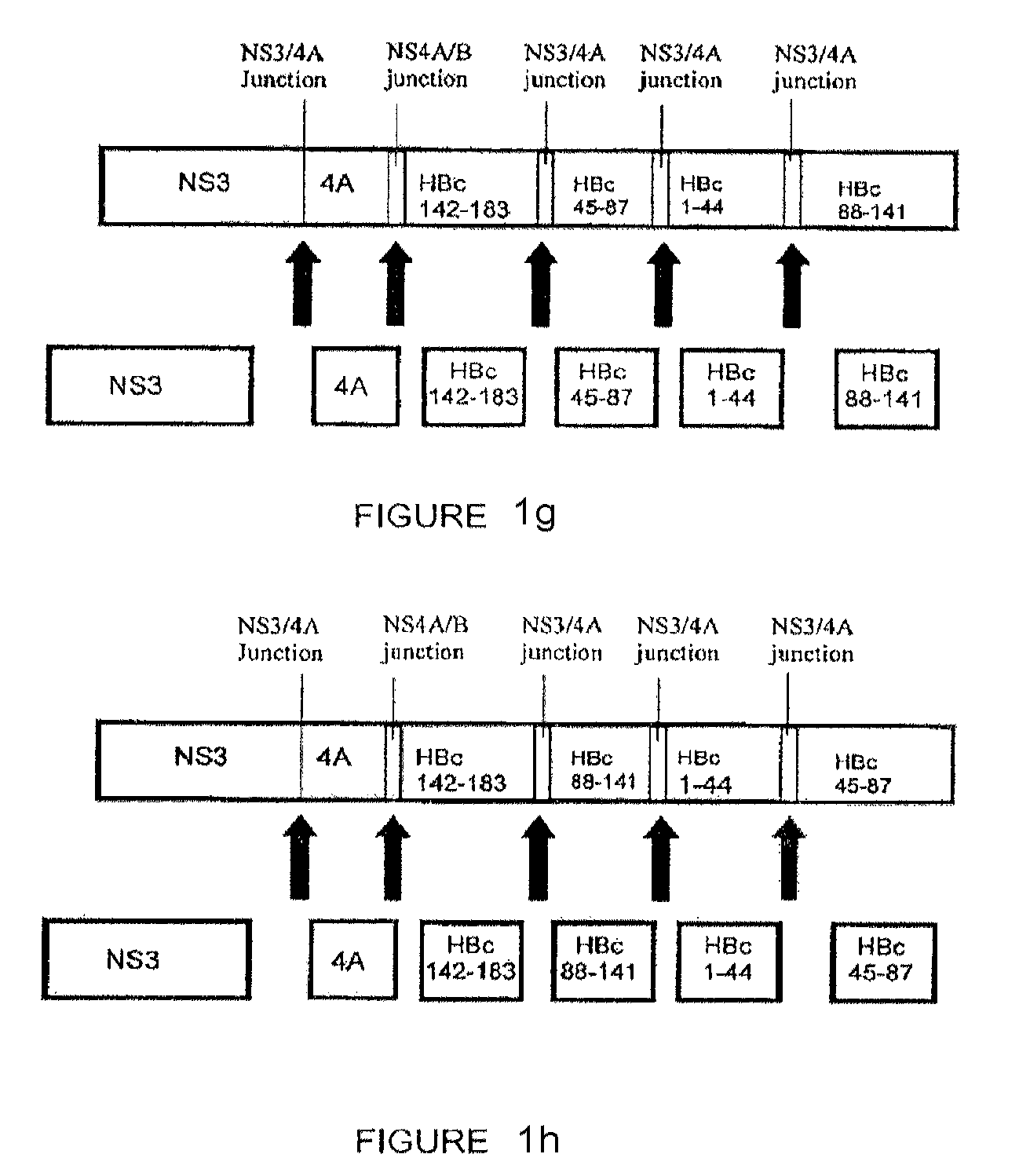
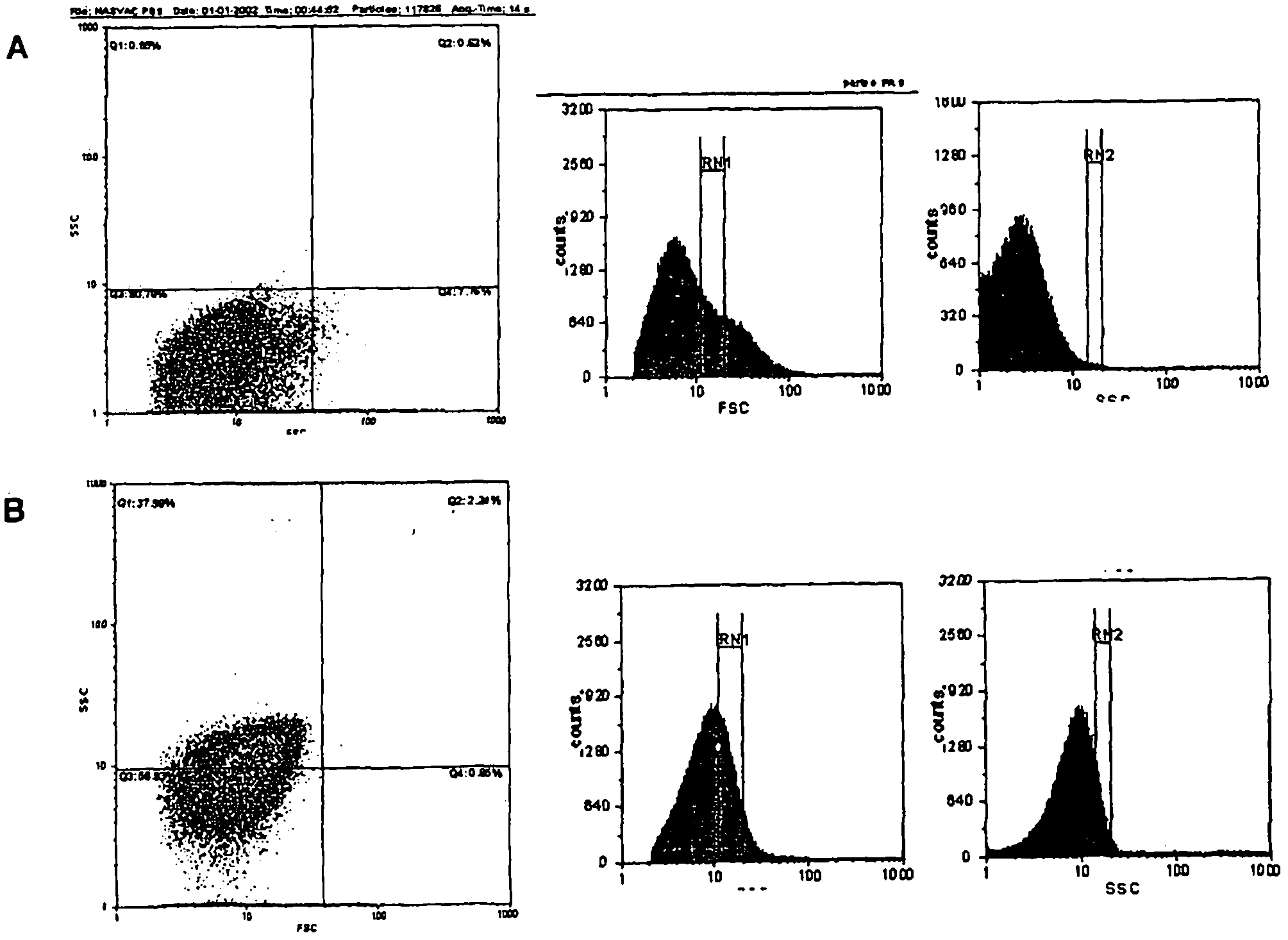
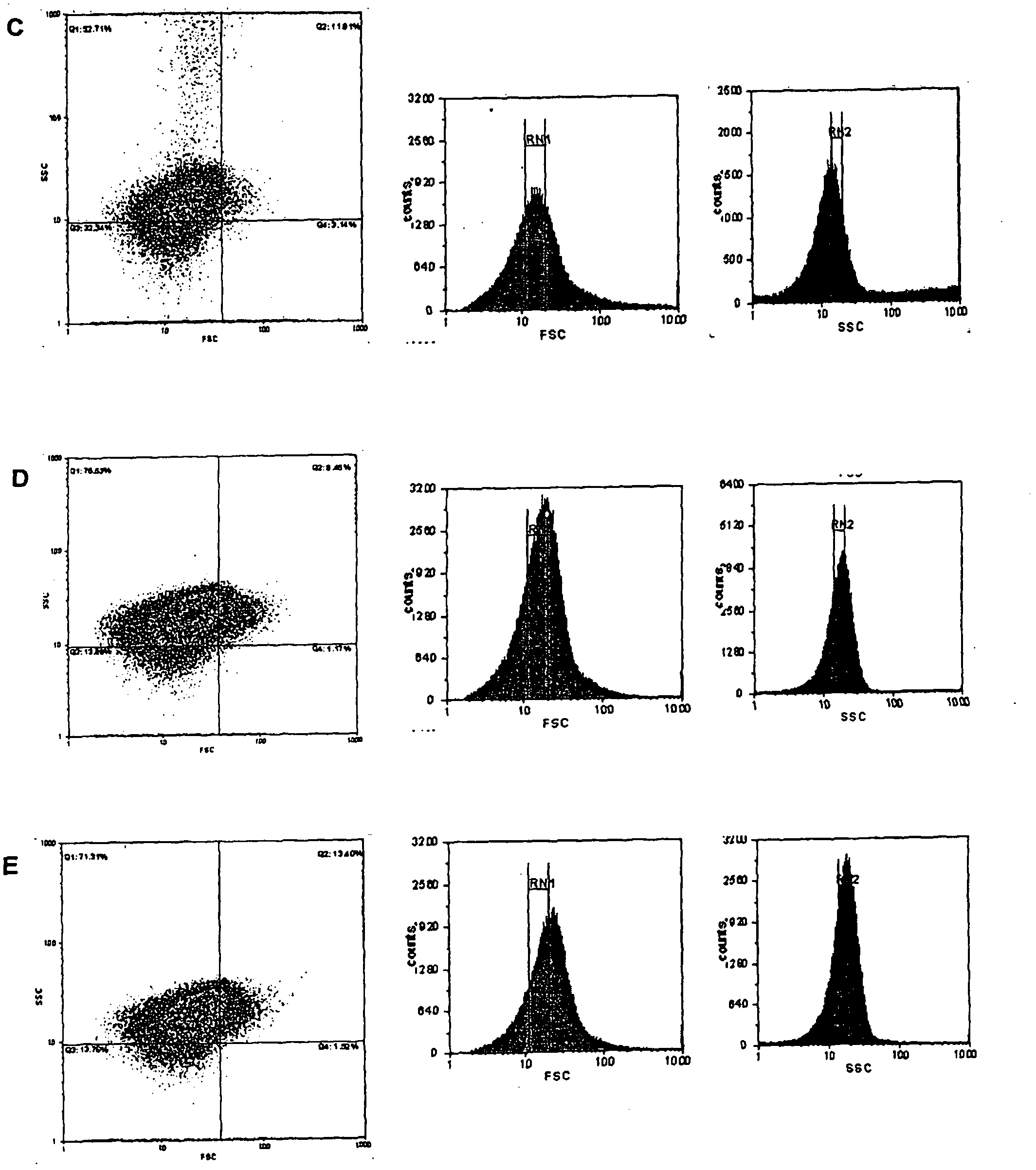
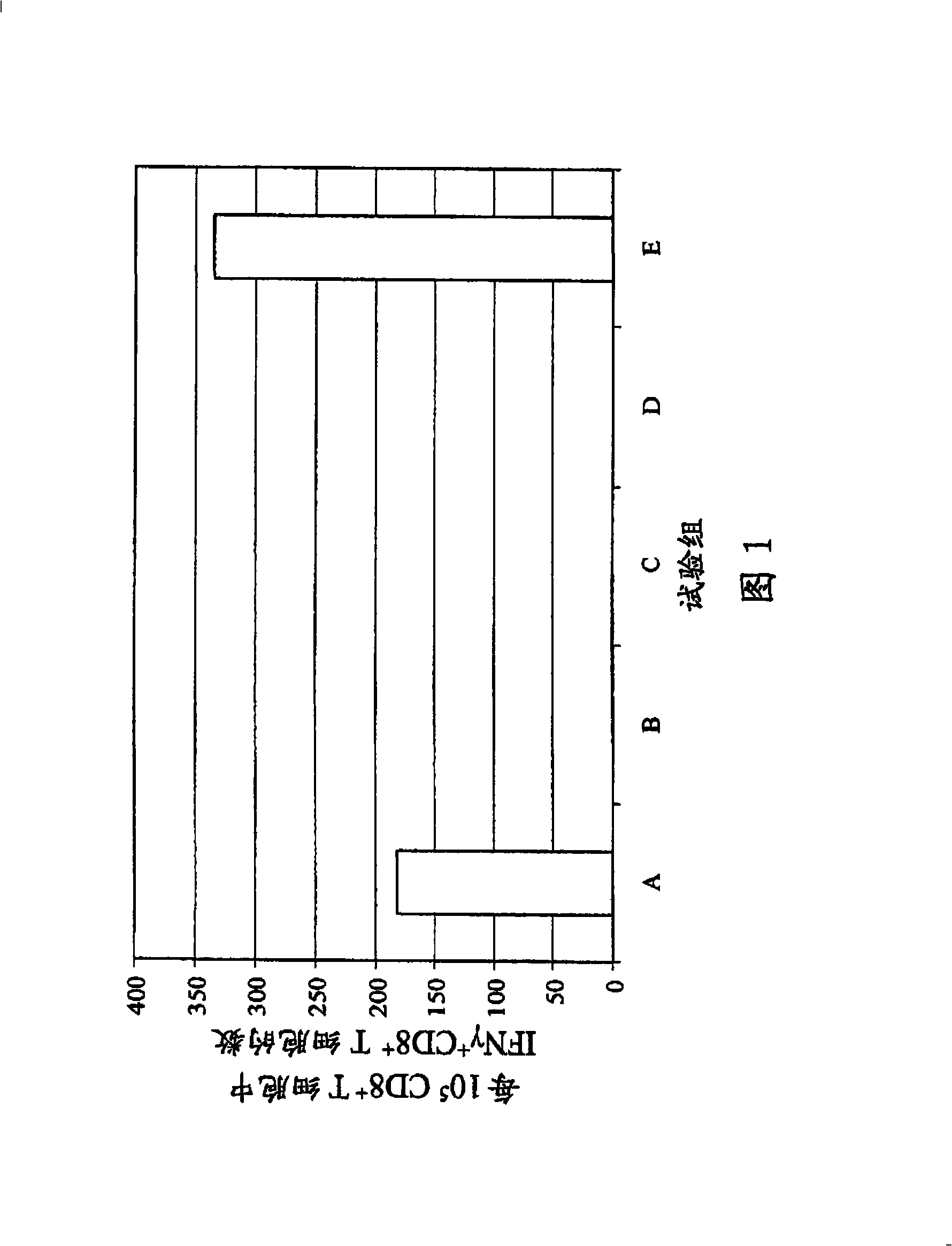
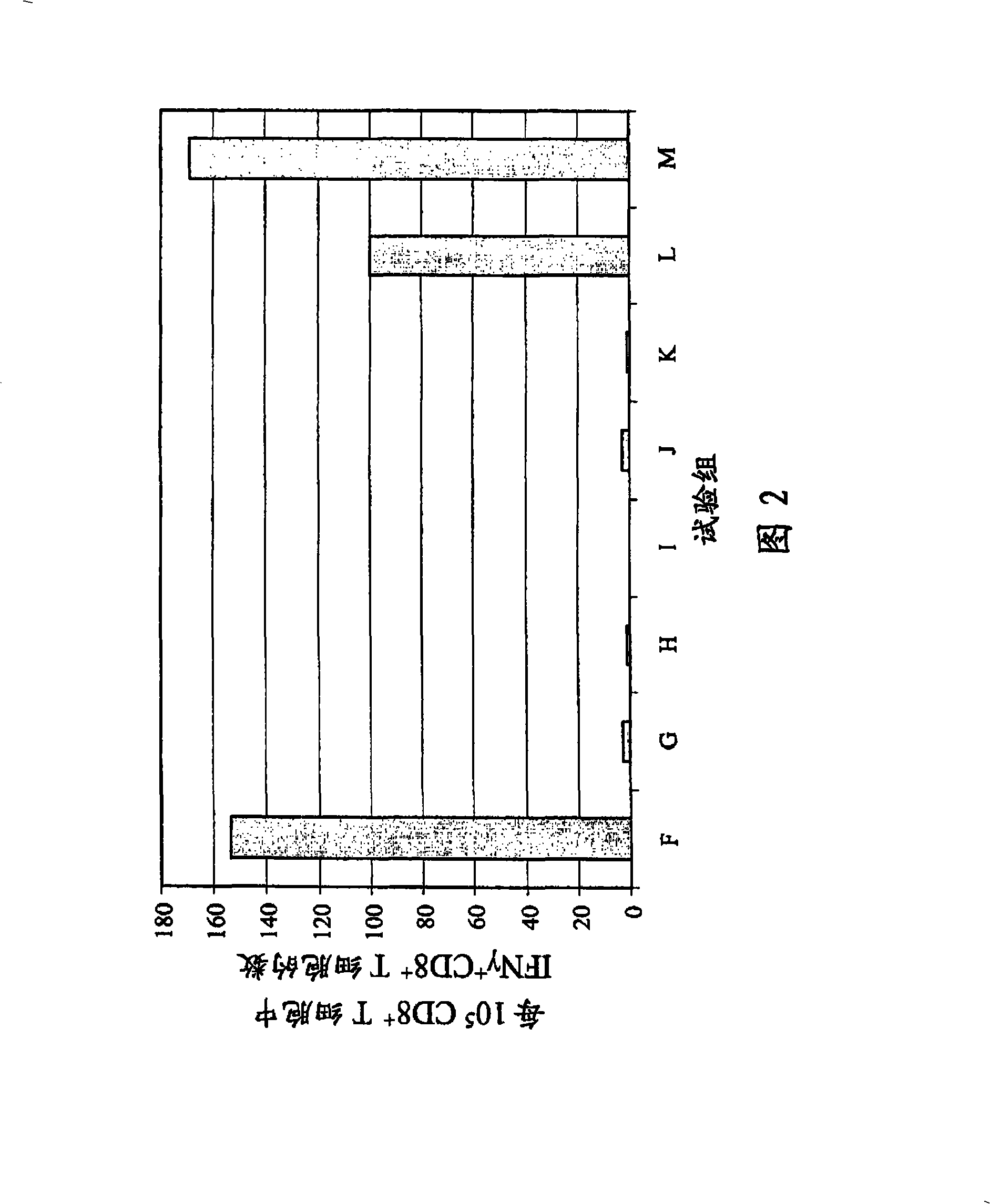
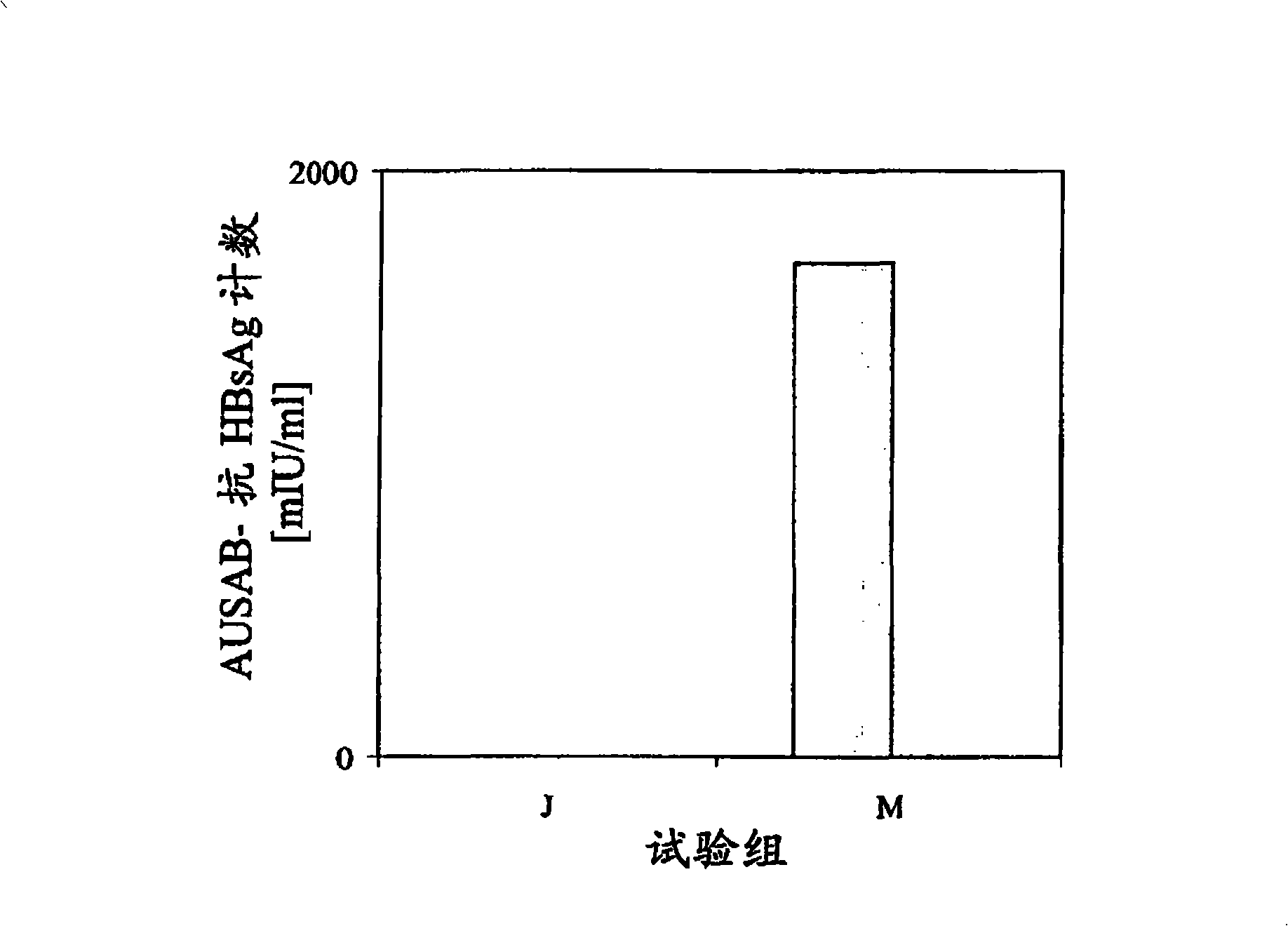
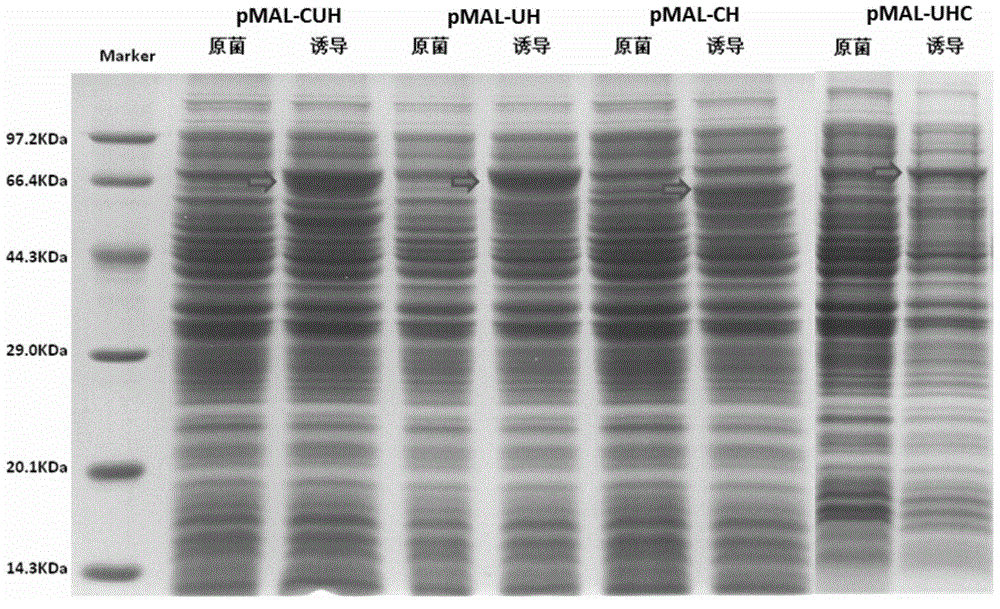
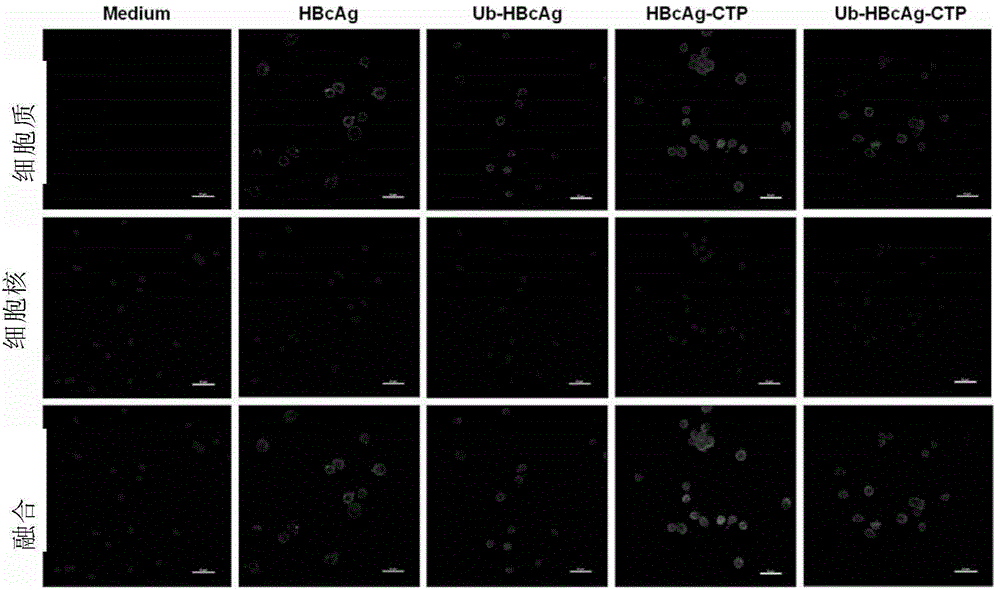
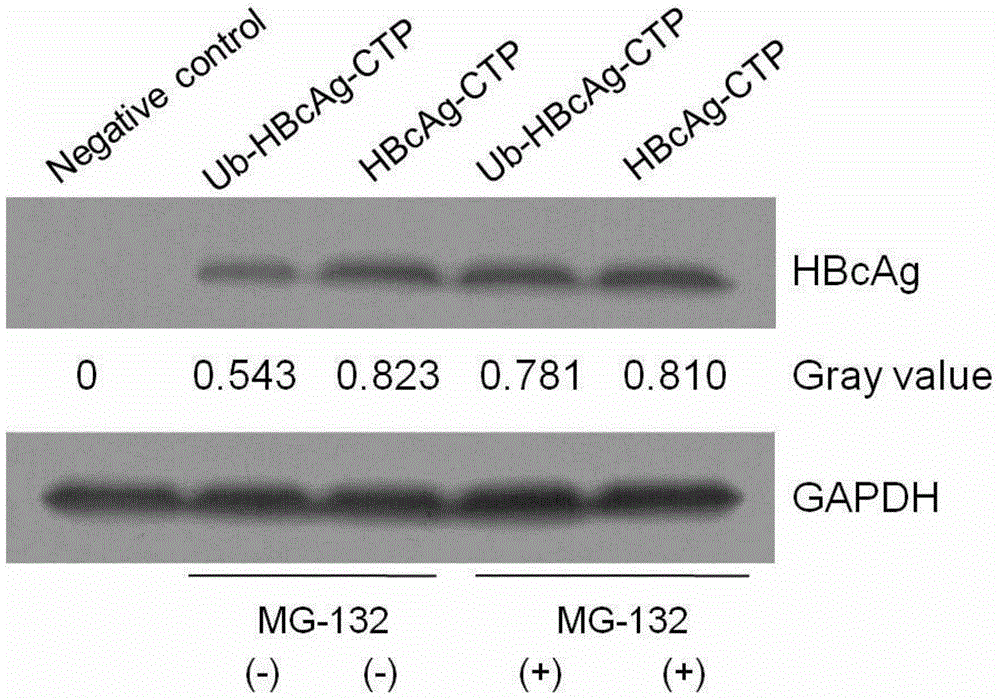
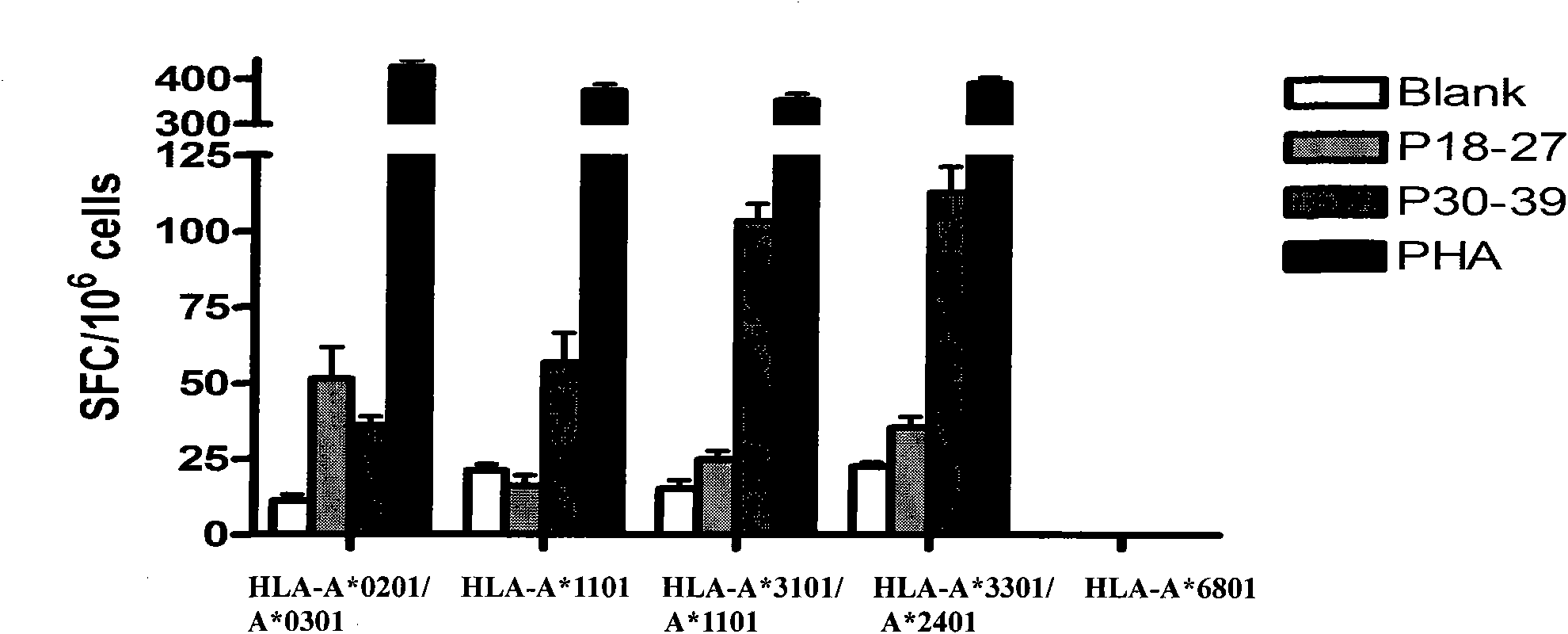
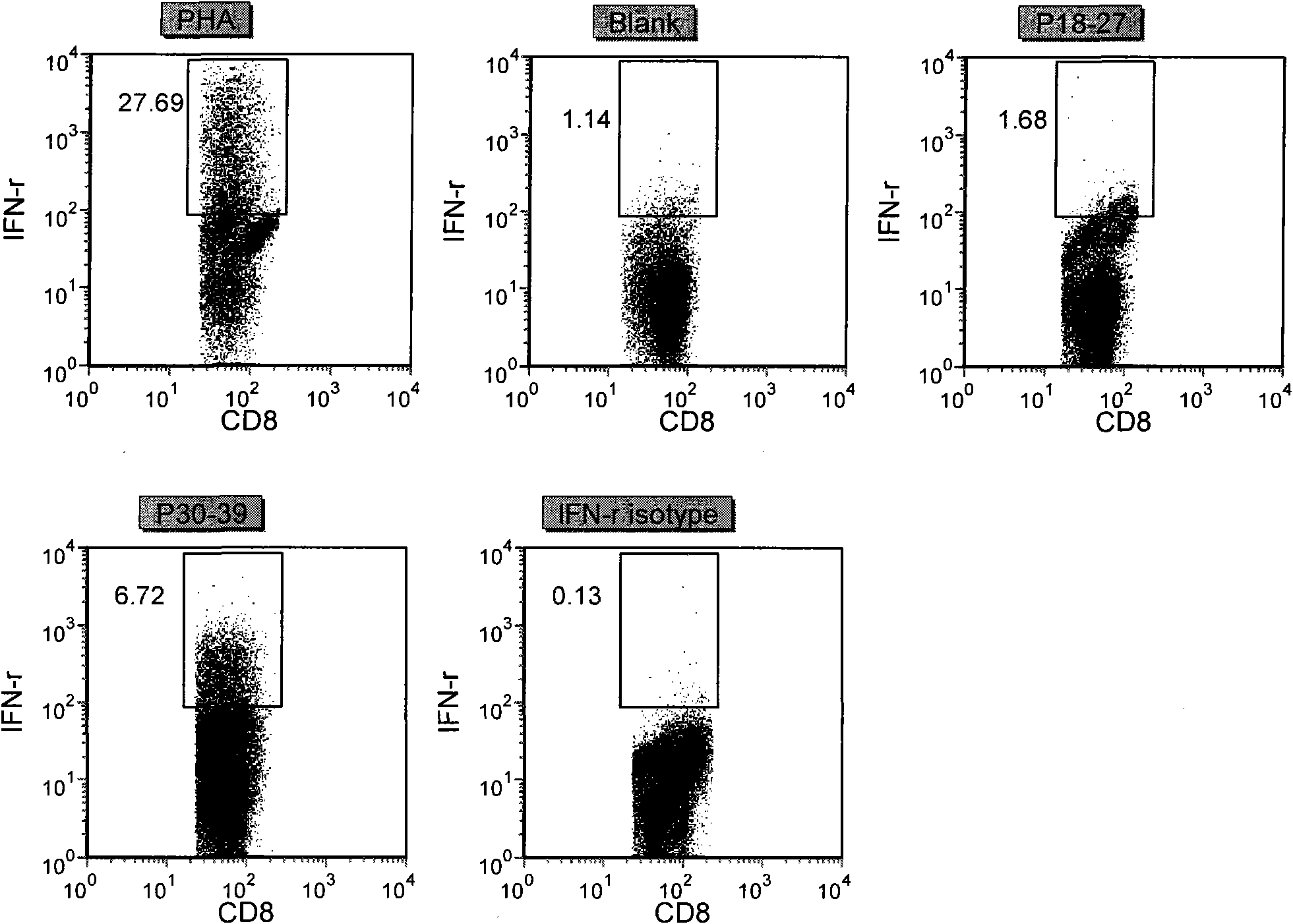
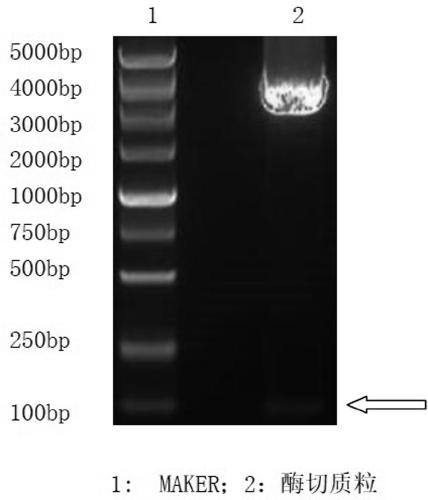
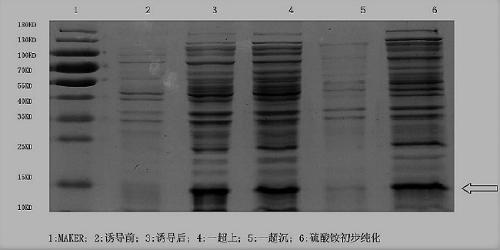
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
75 results about "HBcAg" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
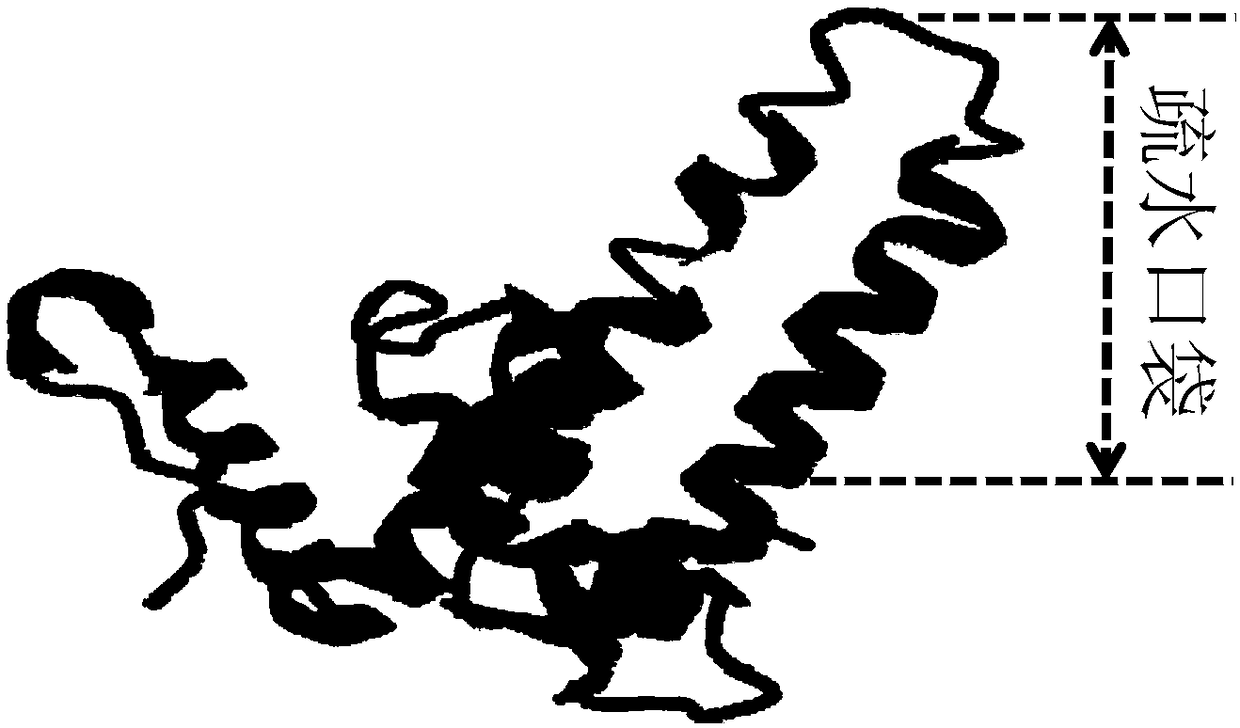
HBcAg (core antigen) is a hepatitis B viral protein. It is an indicator of active viral replication; this means the person infected with Hepatitis B can likely transmit the virus on to another person (i.e. the person is infectious).
Porcine epidemic diarrhea virus genetic engineering vaccine and preparation method thereof
ActiveCN109456412AEfficient assemblyImprove uniformityImmunoglobulins against virusesAntiviralsAntigenProtective antigen
The invention discloses a porcine epidemic diarrhea virus genetic engineering vaccine and a preparation method thereof, which belongs to the field of veterinary biological products. Core proteins of hepatitis B virus are used as a vector, a porcine epidemic diarrhea virus protective antigen is inserted between a 79 amino acid to 80 amino acid of the vector by using a molecular biology method to form the genetic engineering vaccine for preventing the porcine epidemic diarrhea virus. The preparation method of the vaccine is simple, a great amount of antigen proteins can be prepared, theconsumed time is short, the expression amount is high, the production cost is greatly reduced, and the mass production is facilitated. The porcine epidemic diarrhea sub-unit vaccine comprising HBcAg-PEDV-COE recombinant proteins prepared in the invention is good in immunity effect, small in immunity dose, and capable of effectively preventing the infection of the porcine epidemic diarrhea virus.
Owner:扬州优邦生物药品有限公司
Compositions and methods that enhance an immune response
InactiveUS8445663B2Improve immunityEnhance immune responseBiocideSsRNA viruses positive-senseHeterologousAdjuvant
Disclosed herein are isolated nucleic acids, compositions of isolated nucleic acids, and compositions of polypeptides that are useful for the generation, enhancement, or improvement of an immune response to a target antigen. Some embodiments of the compositions include hepatitis B core antigen (HBcAg) protein and a heterologous protein antigen. In some embodiments, an isolated nucleic acid encoding hepatitis B core antigen (HBcAg) protein and a heterologous protein antigen is disclosed. Also disclosed herein are methods of administering the composition or isolated nucleic acid to generate an immune response, where HBcAg acts as adjuvant to improve the immune response to the heterologous protein. In certain embodiments, the HBcAg is as a stork or heron hepatitis antigen.
Owner:TRIPEP
Immune enhanced gene vaccine of hepatitis B virus core antigen and preparation method thereof
InactiveCN101884793AEnhance immune responseEfficient induction of antiviral replication abilityGenetic material ingredientsDigestive systemAnti virusHepatitis B virus core Antigen
The invention relates to the technical field of gene engineering, in particular to an immune enhanced gene vaccine of a hepatitis B virus core antigen (HBcAg). The vaccine is an immune stimulating complex (ISCOM) vaccine consisting of HBcAg and OX40 ligand (OX40L) dual-gene co-expression recombinant eukaryotic vector, saponin Quil A, cholesterol and lecithin. The vaccine can promote immune response of specific T cells of the HBcAg and efficiently induce the anti-virus replication capacity of immune cells, and has good application prospect in the field of hepatitis B virus infection resistance. The invention also relates to a preparation method for the vaccine with simple and convenient operation and low cost.
Owner:ARMY MEDICAL UNIV
Novel coronavirus vaccine based on chimeric virus-like particles
PendingCN111620952AEnhance immune responseGood immune protectionSsRNA viruses positive-senseBacteriaCoronavirus vaccinationReceptor
Owner:SUZHOU MIDI BIOTECH CO LTD
Composition for the prophylaxis and treatment of HBV infections and HBV-mediated diseases
InactiveUS20060233832A1Easy to handleSimple preparation processAntipyreticGenetic material ingredientsAntigenGenotype
The present invention is a composition that comprises at least two hepatitis B virus surface antigens (HBsAgs), fragments thereof and / or nucleic acids encoding them, the HBsAgs differing in HBV genotype in the S region and / or pre-S1 region and the composition containing no HBV core antigen (HBcAg) or nucleic acid encoding that antigen. The present invention also includes pharmaceutical compositions, especially vaccines, comprising these compositions for the prevention and / or treatment of an HBV infection or an HBV-mediated disease. The present invention further includes a method of preparing a patient-specific medicament for the therapeutic treatment of hepatitis B.
Owner:RAJN BIOTEKH GEZELLSHAFT FJUR NOJE BIOTEKHNOLOGISHE PROTSESSE & PROD MBKH
Application of HBcAg (hepatitis B core antigen) virus-like particle serving as cancer therapeutic vaccine carrier
InactiveCN105497886AImprove the level ofViral antigen ingredientsAntineoplastic agentsHepatitis B virus core AntigenEscherichia coli
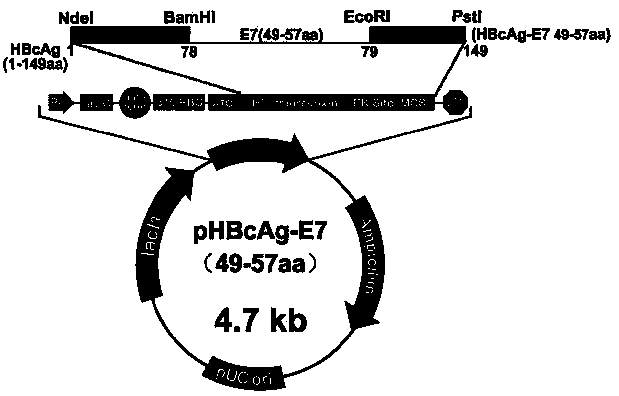
The invention relates to the field of molecular biology and immunology, in particular to an application of an HBcAg (hepatitis B core antigen) virus-like particle serving as a cervical cancer therapeutic vaccine carrier. A preparation method comprises steps as follows: an HPV16 E749-57CTLs epitope peptide fragment is selected, a DNA (deoxyribose nucleic acid ) fragment of the HPV16 E749-57CTLs epitope peptide fragment is inserted between 78 and 79 amino acids of the HBcAg through genetic recombination, an obtained recombinant plasmid pHBcAg-E749-57 is converted into Escherichia coli DH5alpha, and an HBcAg virus-like particle vaccine presenting E749-57 is obtained after induction expression and purification. After a tumor-bearing mouse is immunized with the virus-like particle vaccine, the body of the mouse can be induced to generate a higher HPV16E7 specific cellular immunologic response, and growth of tumors is remarkably inhibited.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Hepatitis B core antigen fusion proteins
InactiveUS7270821B2Enhance immune responseFungiFusion with DNA-binding domainHeterologousProtein composition
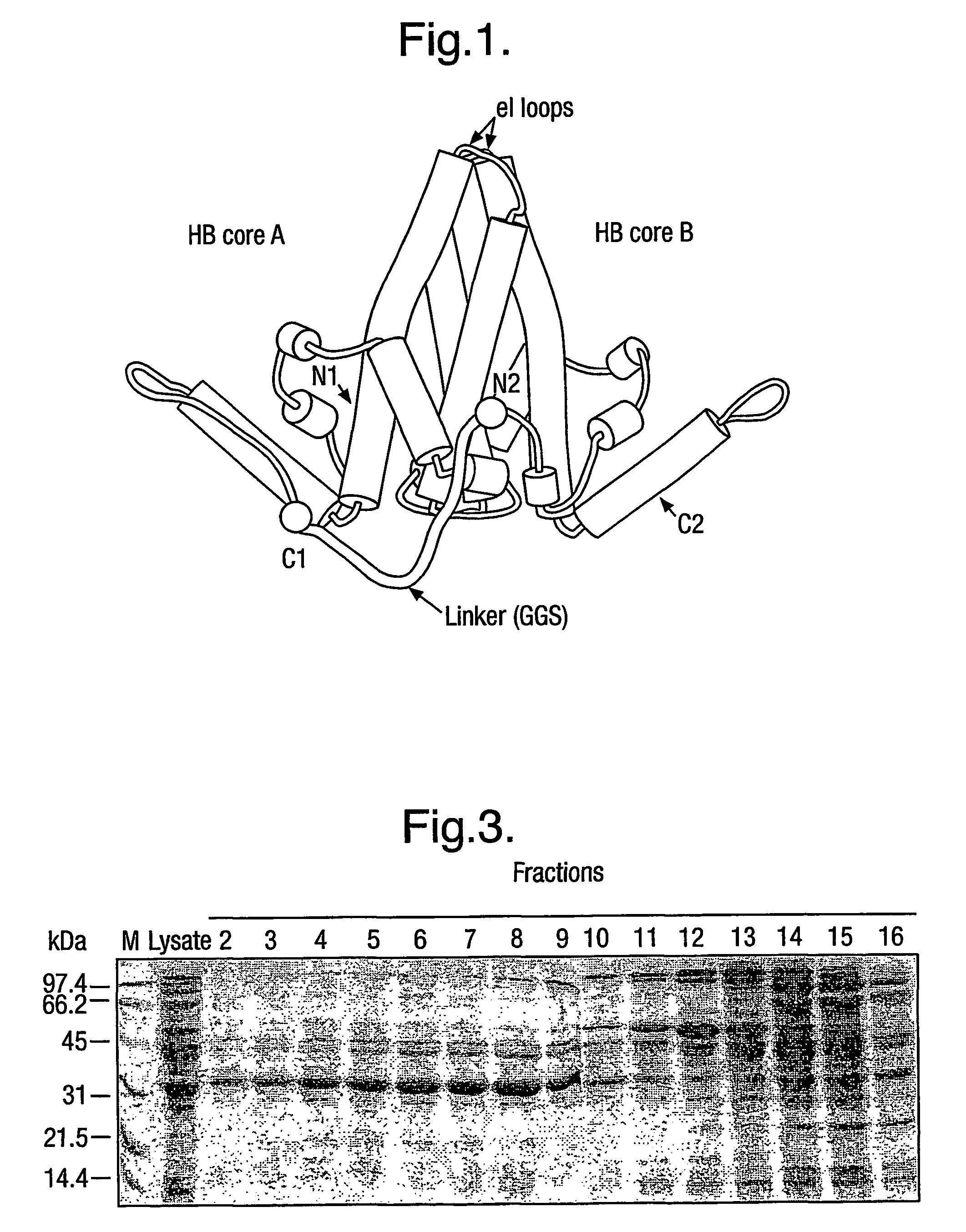
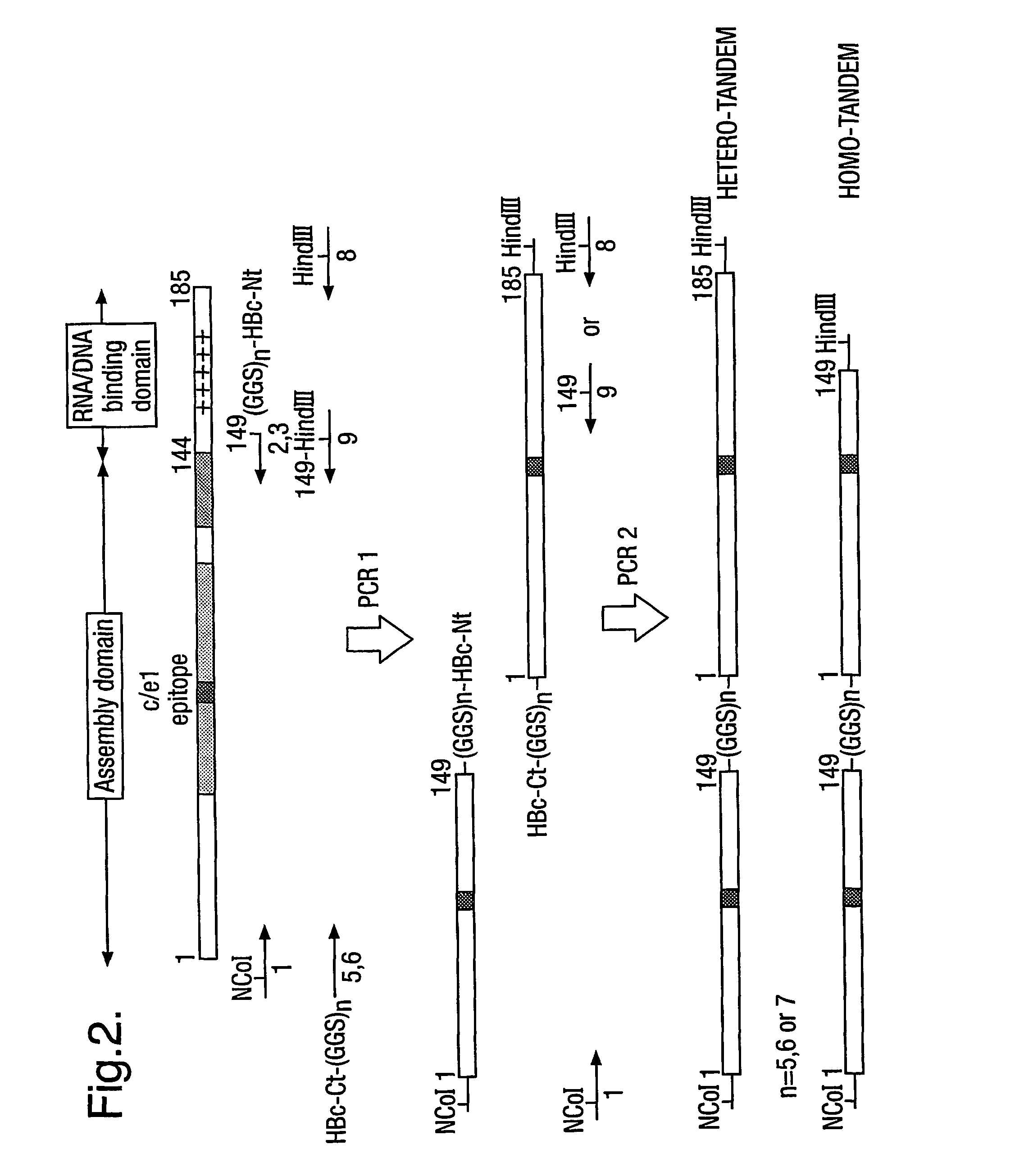
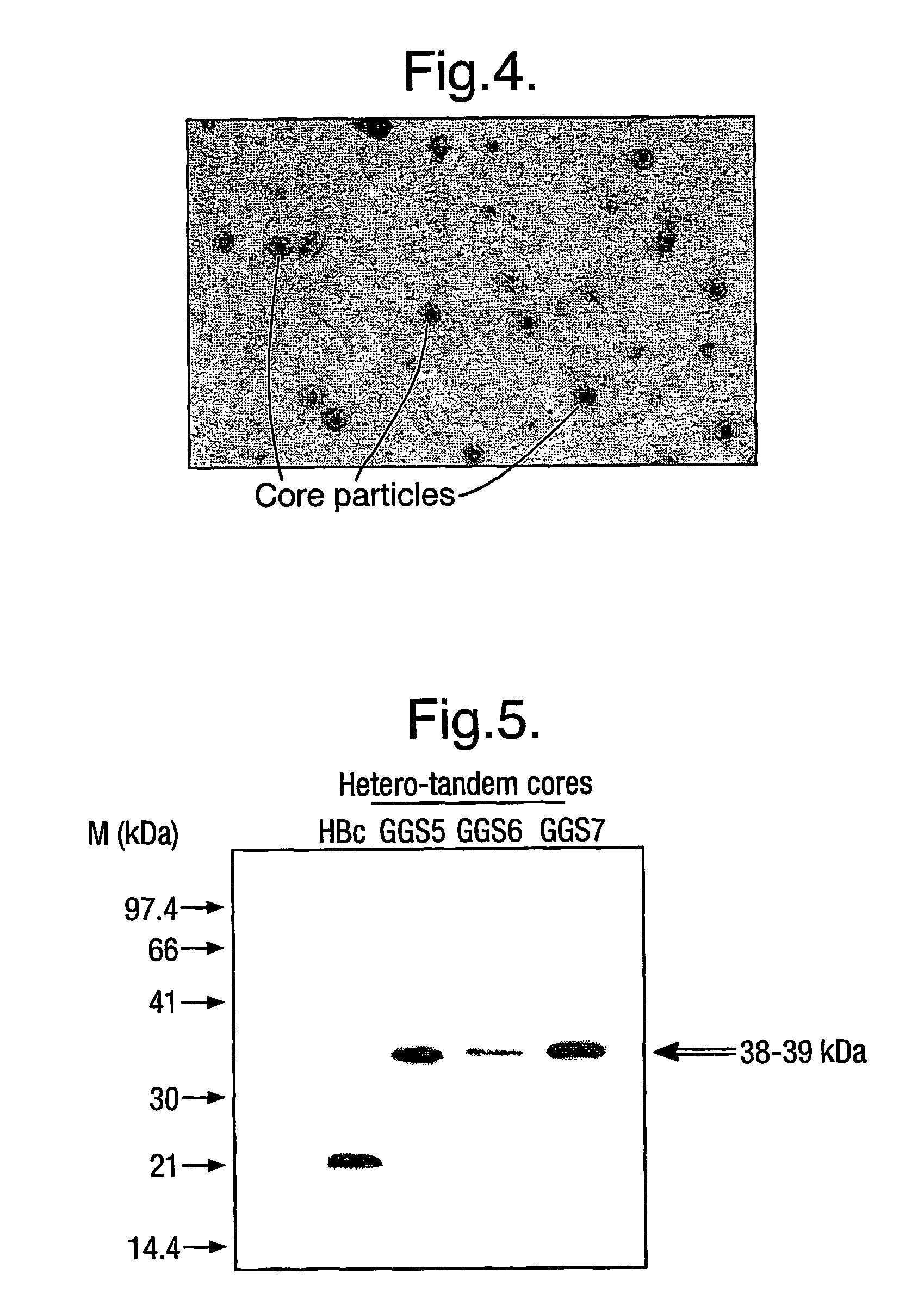
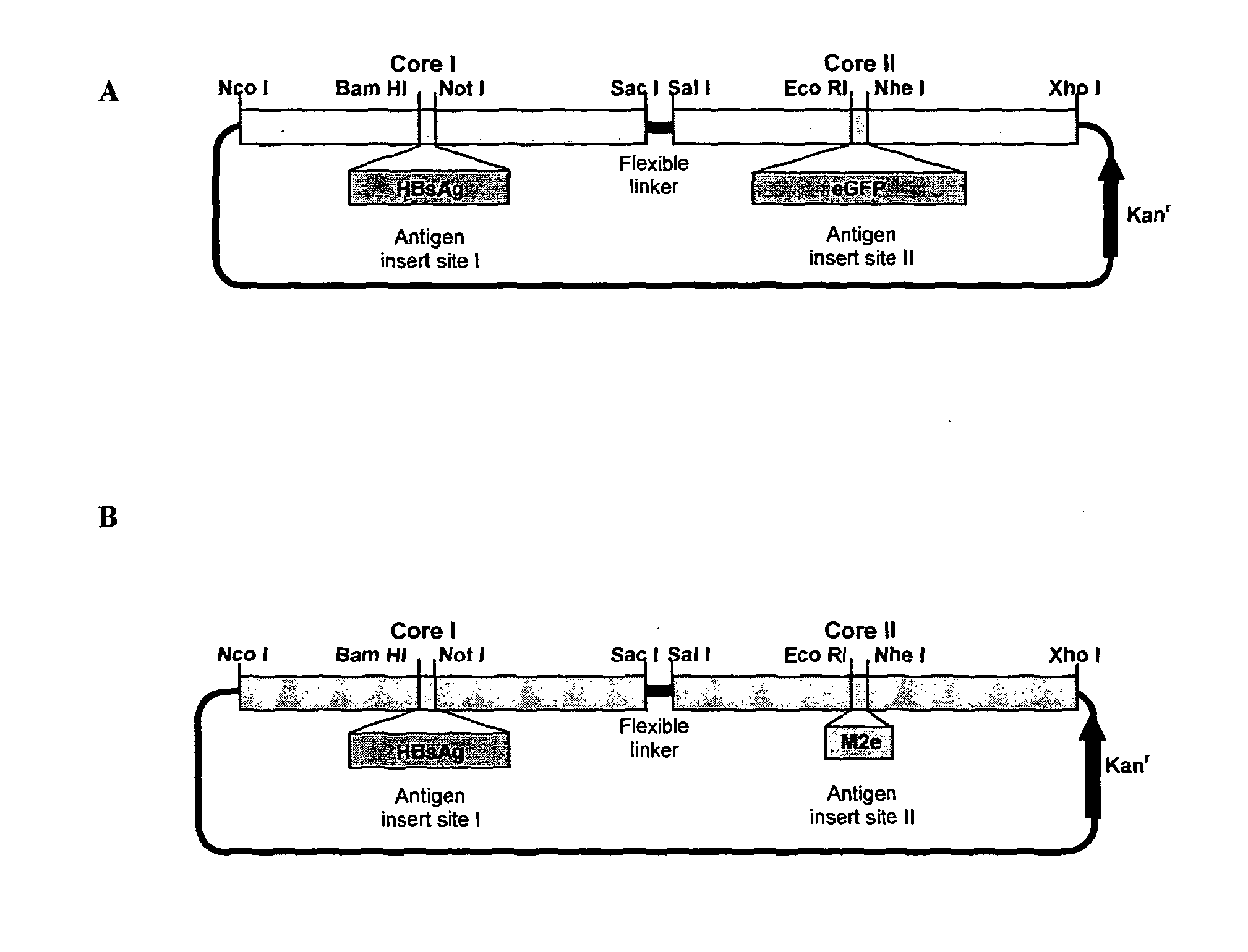
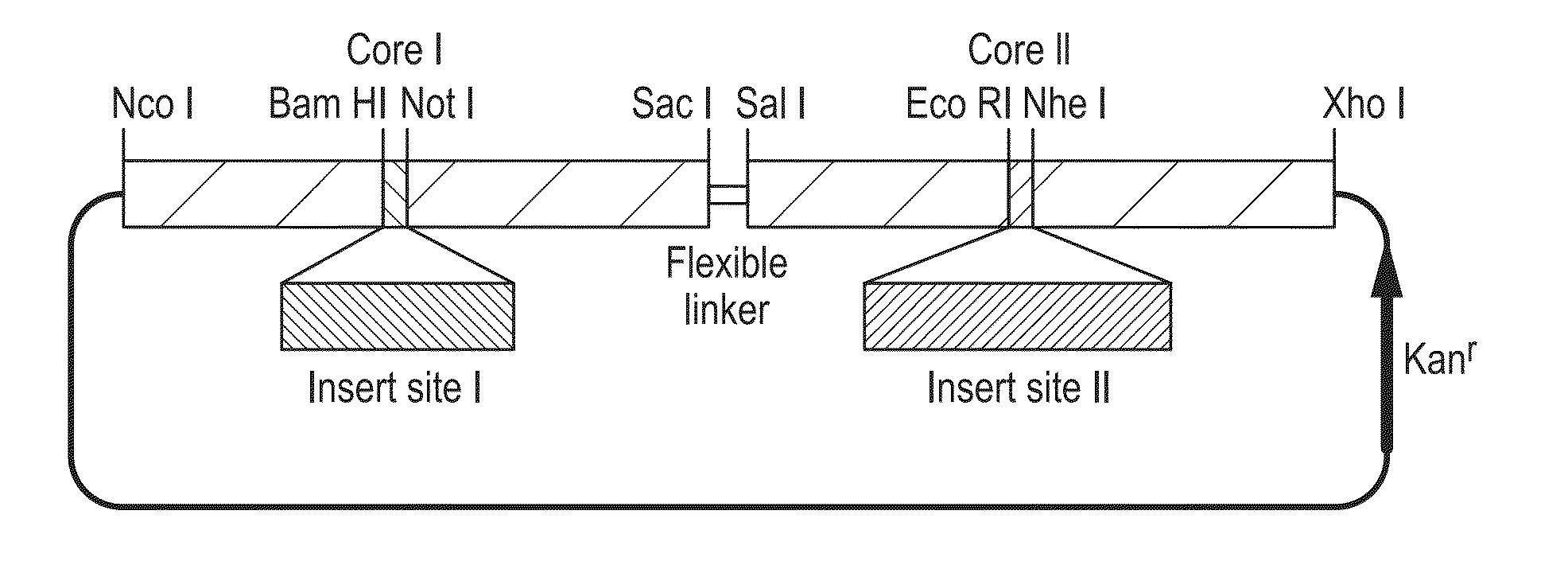
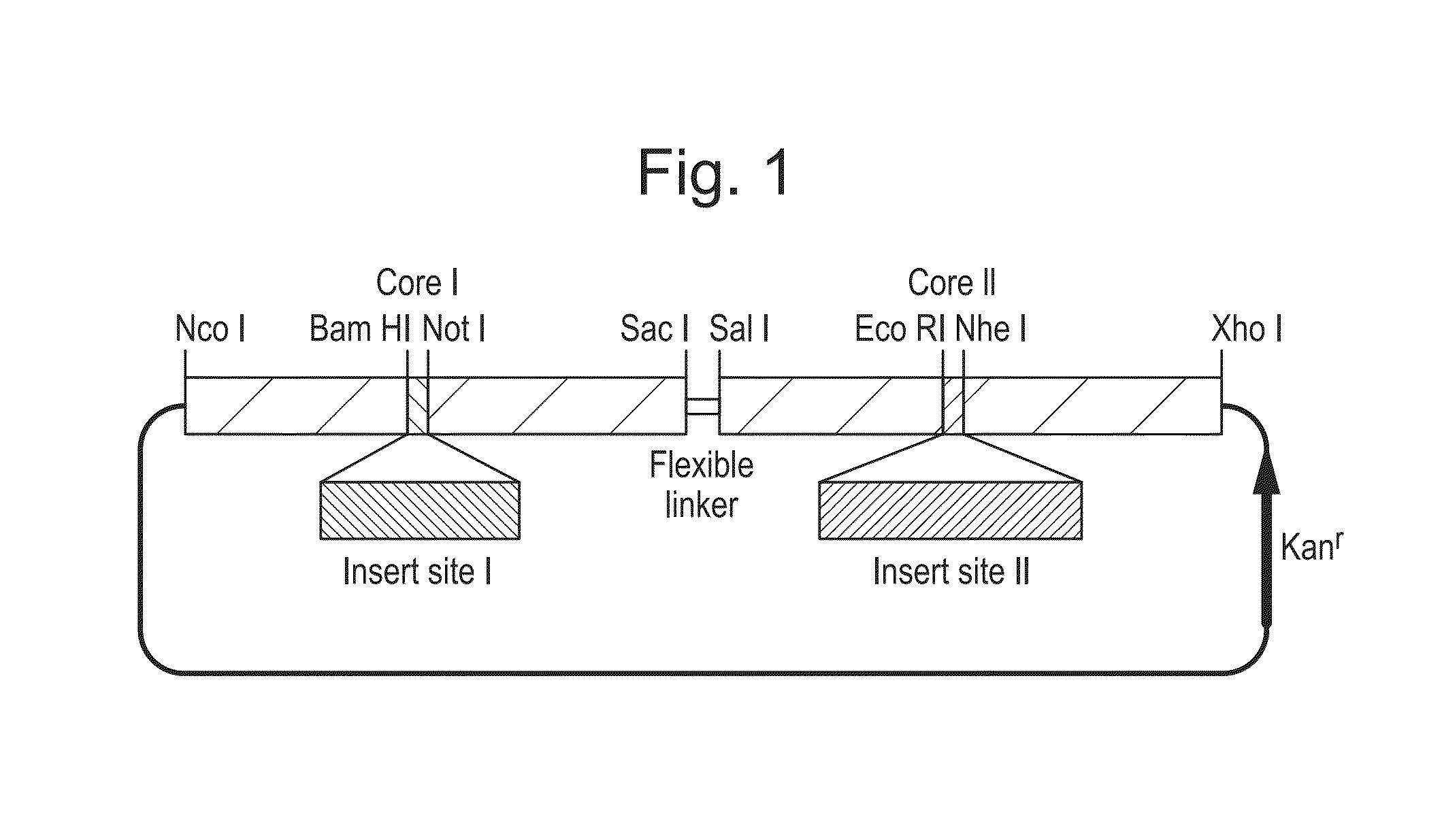
The hepatitis B virus (HBV) capsid is made up of a single species of protein called the core antigen (HBcAg) which self-assembles into particles. The particles are highly immunogenic and are able to present heterologous epitopes to the immune system when the epitopes are inserted into a surface-exposed region of the particles called the “e1 loop”. The structural building blocks of the particles are tightly associated dimers of HBcAg in which the adjacent e1 loops are closely juxtaposed. It is proposed that sequences inserted into the e1 loop are conformationally restrained in the assembled particles when presented in monomeric core protein. The invention seeks to solve this problem by covalently linking core proteins as tandem copies (e.g., as dimers) so that insertions can be made independently in each copy. This is particularly useful for insertion of large sequences into the e1 loop because it allows such sequences to be inserted into just one copy of the core protein per tandem repeat, thereby reducing potential conformational clashes in assembly. Alternatively, a different sequence may be inserted into each e1 loop of a tandem repeat, thus increasing the flexibility of HBcAg particles as an epitope delivery system.
Owner:UNIV OF LEEDS INNOVATIONS
Method for constructing IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma
ActiveCN104001168AEasy to makeEfficient purificationPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsDiseaseHepatitis B virus core Antigen
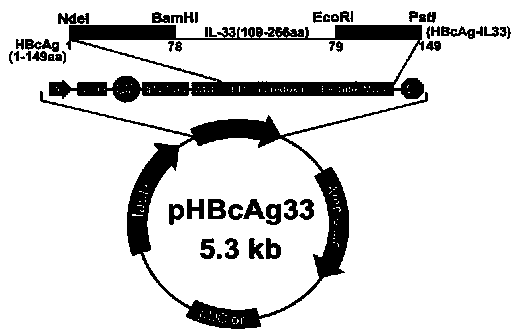
The invention provides a method for constructing an IL-33 presentation VLP (Virus-Like Particle) vaccine used in active immunotherapy of chronic asthma. The method comprises the following steps: extracting IL-33 total RNA (Ribonucleic Acid) from a mouse; performing reverse transcription to obtain IL33 total cDNA (complementary deoxyribonucleic acid); performing PCR (Polymerase Chain Reaction) amplification on the obtained total cDNA with a designed specific primer to obtain a coded IL-33 mature segment gene; inserting the gene between 78-bit amino acid and 79-bit amino acid of a hepatitis B virus core antigen HBcAg to obtain a recombinant plasmid pHBcAg33; transferring the plasmid onto escherichia coli DH5alpha or a BL21 competent cell; inducing by using IPTG (isopropyl-beta-d-thiogalactoside) and purifying to obtain the IL-33 presentation VLP vaccine. A strong neutralizing antibody which is specific to own molecules and has a durable action can be induced by inoculating the vaccine repeatedly in order to regulate and control immune response, thereby fulfilling the aim of regulating and controlling the progress of asthma.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
DC vaccine for treating chronic hepatitis B
InactiveCN1981866AImprove efficiencyImprove immunityPharmaceutical delivery mechanismAntiviralsHepatitis B virus core AntigenChronic hepatitis
A therapeutic DC vaccine for preventing and treating chronic hepatitis B is a hepatitis B specific DC vaccine carried by both HBsAg and HBcAg. The dendritic cells coming from mononuclear cells are carried by both recombinant HBV surface antigen and recombinant HBV core antigen.
Owner:解放军三〇二医院生物治疗研究中心
HBV core area resisting siRNA expression template and application
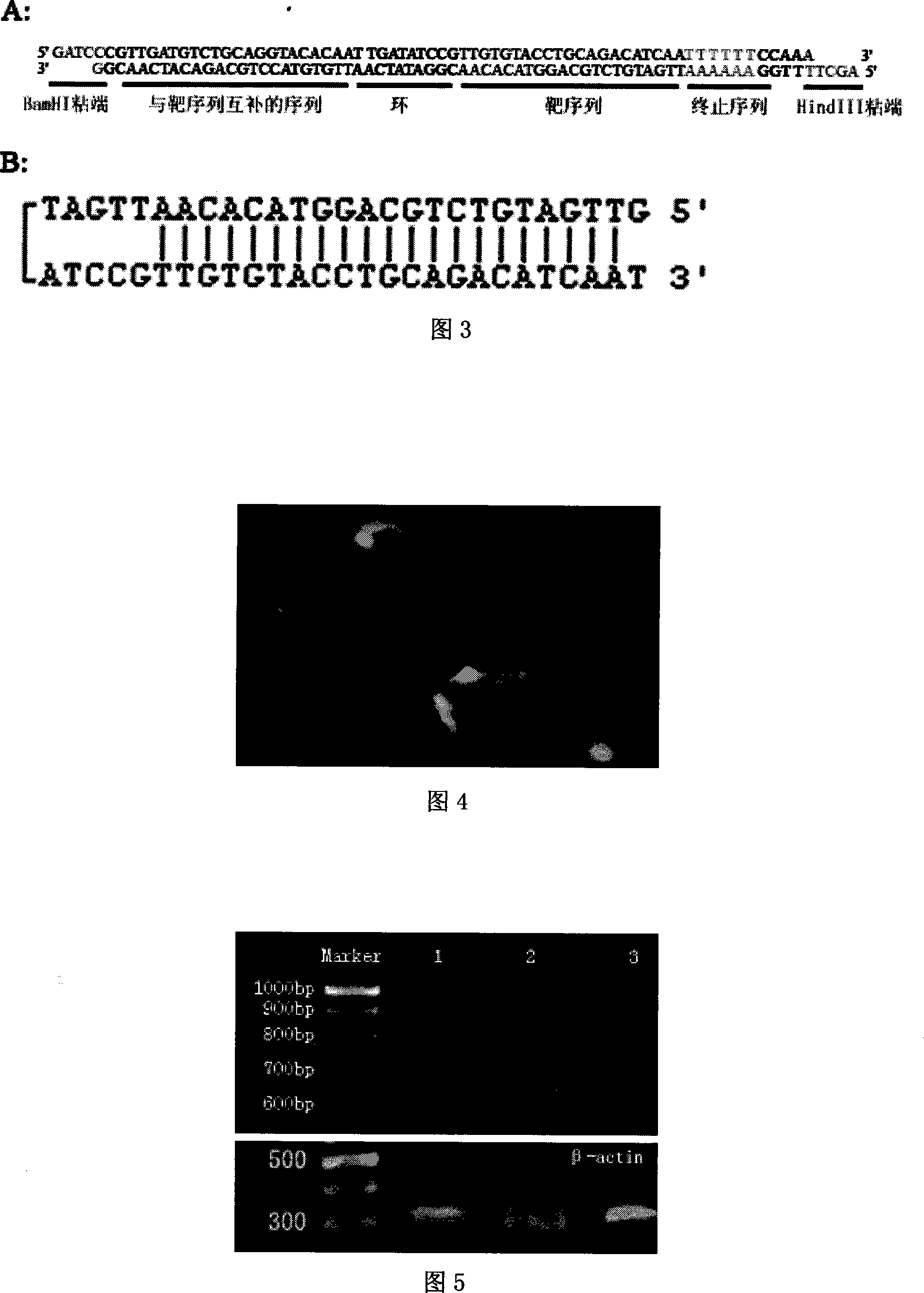
InactiveCN101235372ABroad targetingImprove import efficiencyOrganic active ingredientsAntiviralsExpression templatesGene
The invention closes HBV core region through adopting siRNA technique to combine with a nanometer technique and inhibits the expression of HBcAg, and finally inhibits the expression and copy of HBV. The invention has wide target and obvious effect, which improves gene inducing efficiency through adopting the nanometer technique.
Owner:苏先狮
Influenza vaccine
InactiveUS20130171182A1Reduce weight lossImprove survivalSsRNA viruses negative-senseBacteriaNGAL ProteinInfluenza vaccine
Owner:IQUR
Immune-enhanced hepatitis B therapeutic multivalent vaccine and preparation method thereof
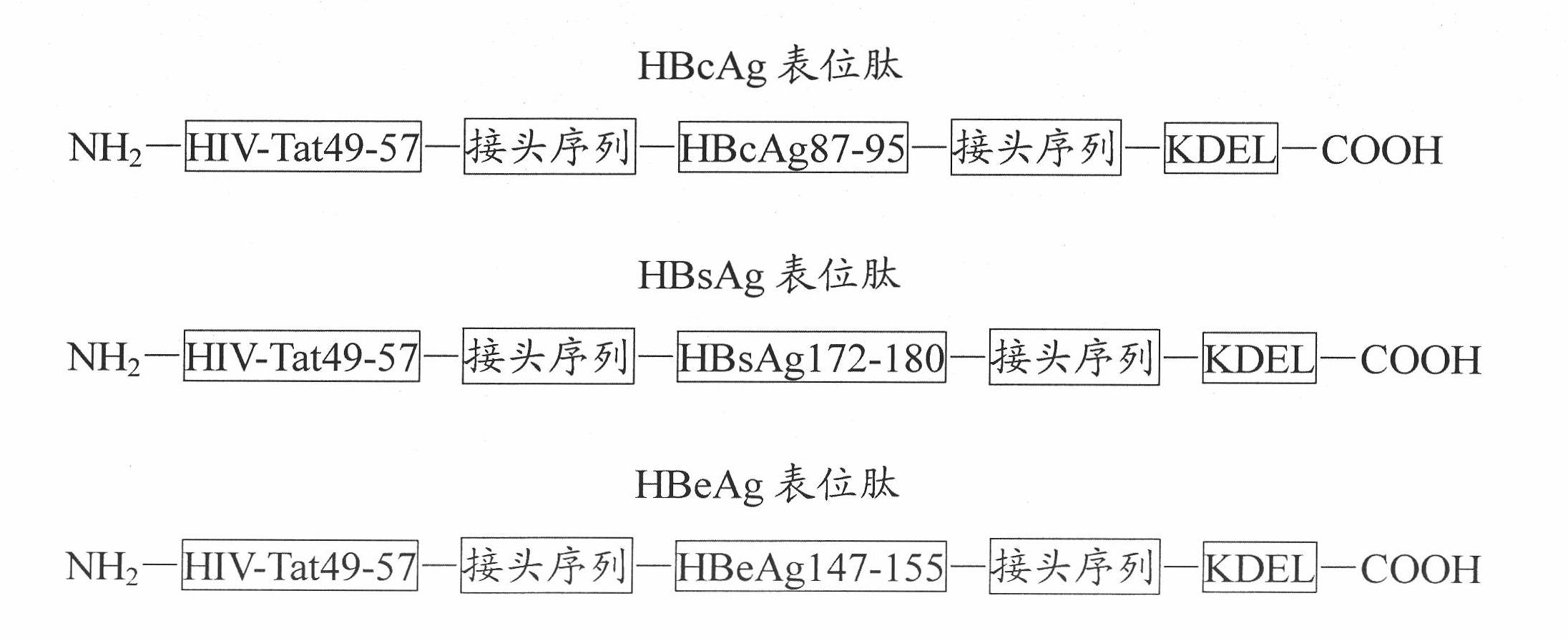
InactiveCN101920021AEasy to prepareLow costGenetic material ingredientsAntiviralsReticulum cellI antigen
The invention discloses an immune-enhanced hepatitis B therapeutic multivalent vaccine and a preparation method thereof. The vaccine is a compound of an HBcAg epitope peptide, an HBsAg epitope peptide, an HBeAg epitope peptide and an LIGHT gene recombinant expression vector, wherein the HBcAg epitope peptide, the HBsAg epitope peptide and the HBeAg epitope peptide respectively comprise epitopes HBcAg87-95 / HBsAg172-180 / HBeAg147-155, a transmembrane sequence of HIV-Tat49-57, an endoplasmic reticulum retention signal sequence KDEL and a connector sequence, the transmembrane sequence is arranged at the amino terminal, the endoplasmic reticulum retention signal sequence is arranged at the carboxyl terminal, the epitopes are connected with the transmembrane sequence or the endoplasmic reticulum retention signal sequence through the connector sequence, and the vaccine can successfully enter cells and target the endoplasmic reticulum and effectively stimulate hepatitis B virus protein epitope to enter an MHC-I antigen presentation pathway to activate CTL (Cytotoxic Lymphocyte) responses.
Owner:ARMY MEDICAL UNIV
Hepatitis B virus dendritic cell therapeutic vaccine and preparation method thereof
InactiveCN101897965AEnhance immune responseAbility to efficiently induce antiviral replicationAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenDendritic cell
The invention discloses a hepatitis B virus dendritic cell therapeutic vaccine and a preparation method thereof. The preparation method thereof includes the steps that: HBcAg-Fc digenic co-expression recombinant eukaryotic vector is constructed; the obtained digenic co-expression recombinant eukaryotic vector is added with saponin Quil A and then is added with cholesterol and lecithin, ice-bath ultrasonic processing is carried out, so that solution is fully mixed and emulsified, PBS dialysis is carried out, and the obtained micro floccus is immunostimulating complex; and the obtained immunostimulating complex activates and simulates dendritic cell, thus obtaining the cell therapeutic vaccine. The vaccine carries out high efficiency transfection on dendritic cell, the generated fusion protein targets the dendritic cell in high efficiency by virtue of Fc segment, thus activating the dendritic cell, promoting immune response of hepatitis B virus core antigen specificity T cell and inducing the antivirus replication capability of immune cell in high efficiency, so that the vaccine can be taken as hepatitis B virus therapeutic vaccine.
Owner:ARMY MEDICAL UNIV
EGFR and HER2 combined polypeptide epitope vaccine
InactiveCN102357246AHigh antibody titerLow antibody titerAntibody medical ingredientsHybrid peptidesB-Cell EpitopesEpitope vaccine
The invention discloses an EGFR and HER2 combined polypeptide epitope vaccine; two B cell epitope mimic polypeptide I and II of HER2 of the HER family, and a B cell epitope mimic polypeptide of EGFR of the HER family are inserted in a serial form between the 78th amino acid and the 79th amino acid of a hepatitis B core antigen HBcAg so as to form fusion protein which is the combined polypeptide epitope vaccine. The EGFR and HER2 combined polypeptide epitope vaccine of the invention breaks through the limitations of existing single epitope vaccines, and the combined epitope vaccine is providedwith more extensive antineoplastic activity.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
ActiveCN102199217AHighly inhibitoryEfficient removalDigestive systemAntiviralsEscherichia coliFusion Protein Expression
The invention relates to a hepatitis B virus multi-epitope fusion protein and a preparation method and application thereof. The fusion protein is obtained by inserting hepatitis B virus multi-epitope fusion peptide (with the sequence shown as SEQIDNo.1) formed by serially connecting HBsAg313-321, HBsAg335-343, Pol150-159, Pol455-463 and Padre epitopes through connecting peptide between amino acidat the 78th position and amino acid at the 79th position of a hepatitis B virus core protein; and the preparation method comprises the following steps of: constructing a hepatitis B virus multi-epitope fusion protein expression plasmid pET28-HBc-HP; performing isopropyl thiogalactoside (IPTG) inducing expression by using an Escherichia coli expression system; and purifying by using affinity chromatography. The fusion protein carries a plurality of supertype epitopes of hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) and polymerase, is viral particles, has the advantages of strong immunogenicity, wide applicable range and the like, and can be used for preparing therapeutic hepatitis B vaccines.
Owner:ARMY MEDICAL UNIV
Hepatitis B nucleic acid vaccine and construction method thereof
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
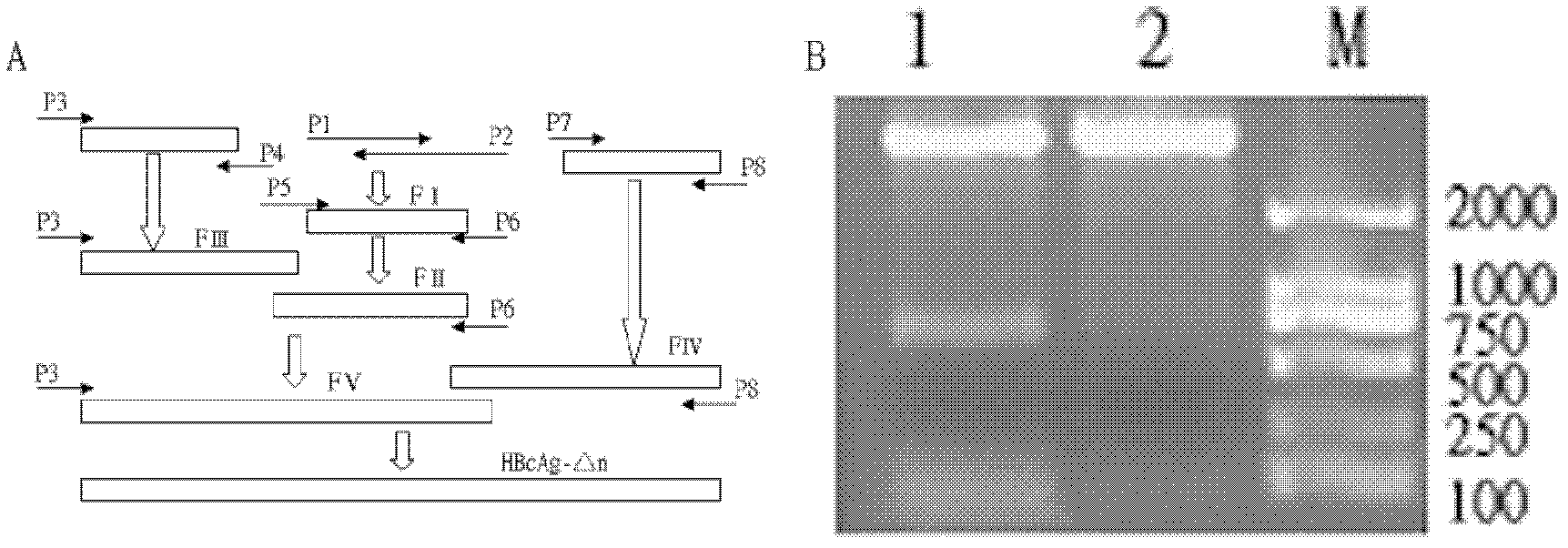
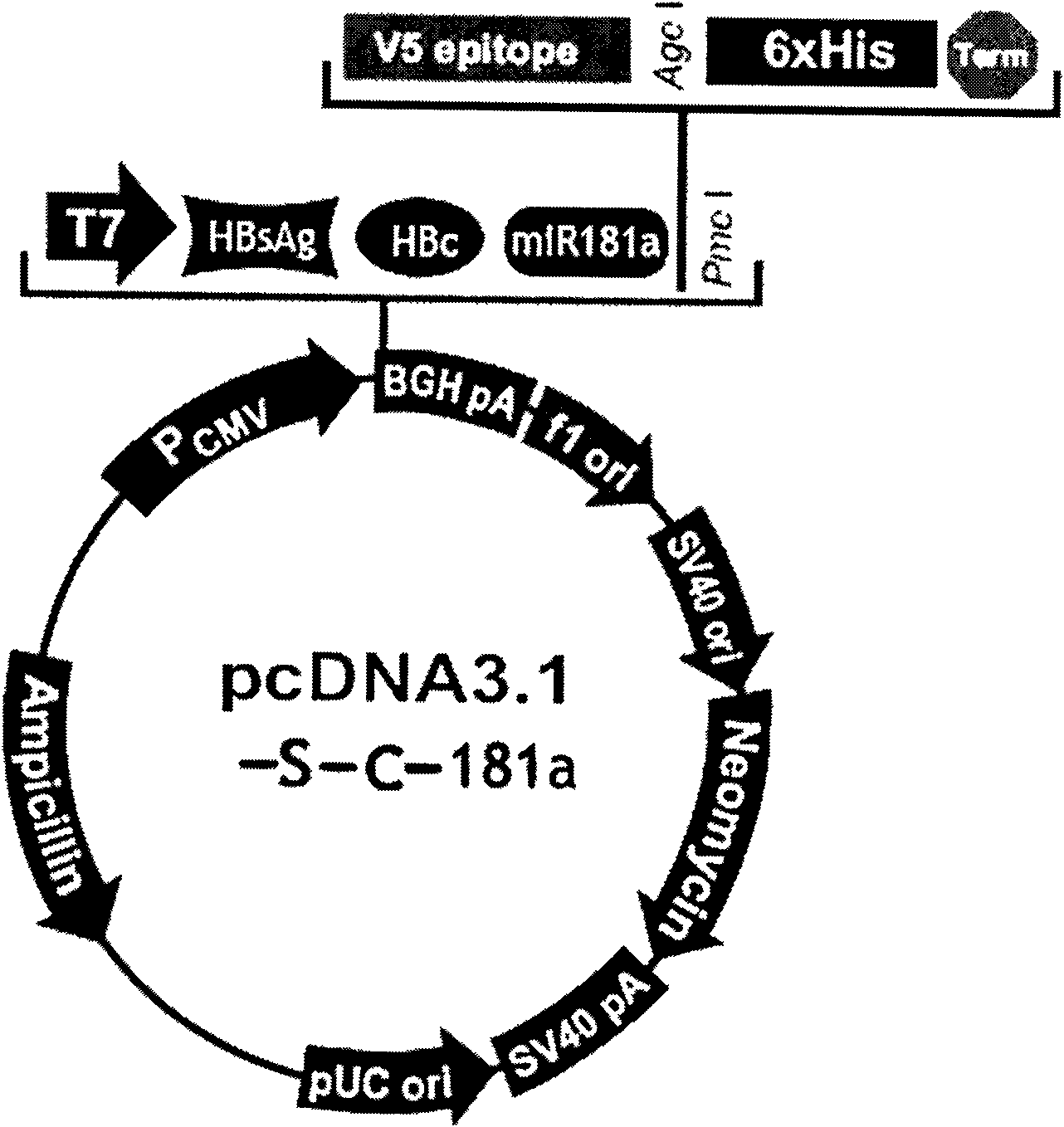
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Antibody display
InactiveUS20160122420A1High sensitivityEasy to measureVirusesBacteriaSingle-domain antibodyHepatitis B Core Antigens
Owner:ROWLANDS DAVID J +2
Antigen epitope peptide and application thereof
ActiveCN106366167AEasy to synthesizeEasy to manufactureViral antigen ingredientsVirus peptidesDendritic cellCytotoxicity
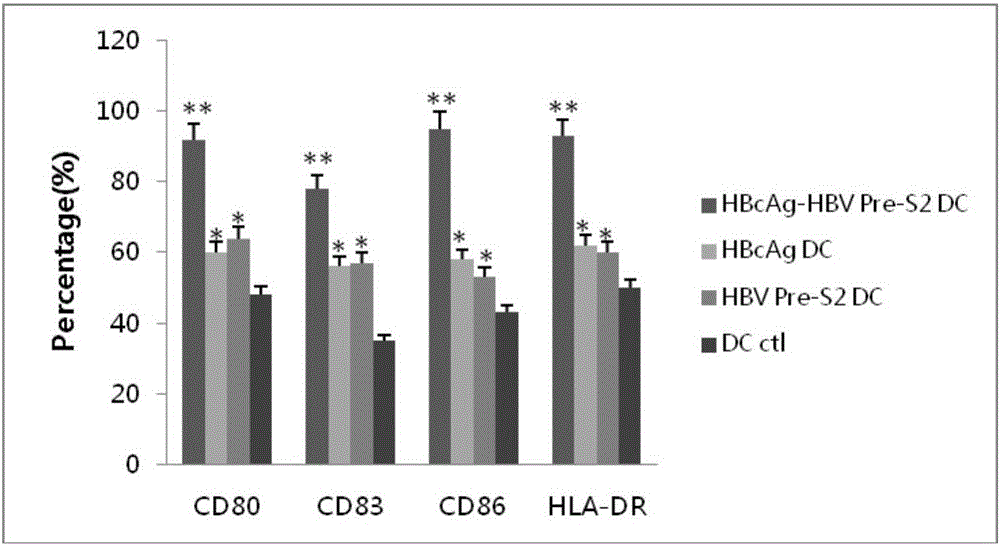
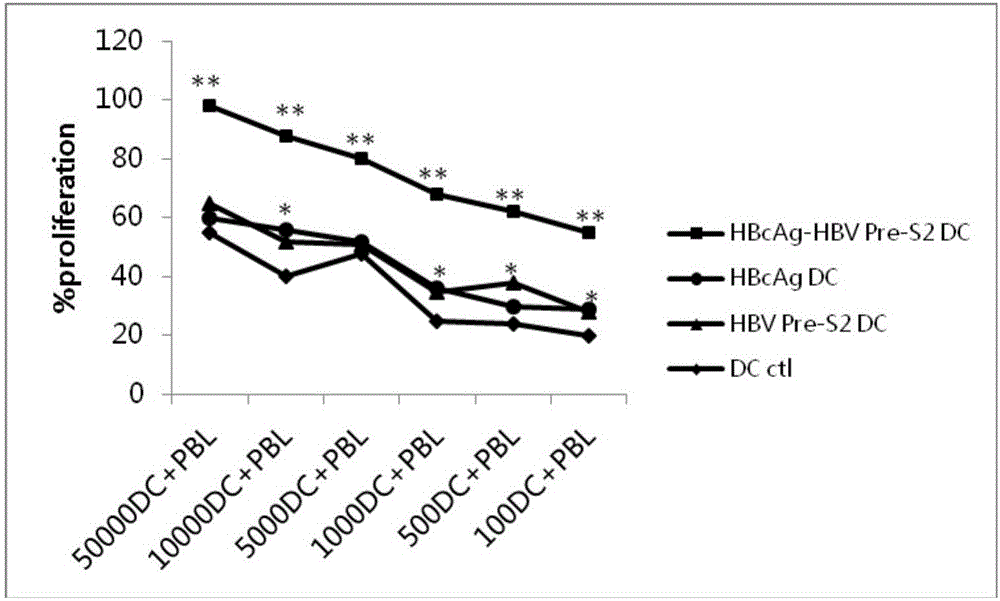
The invention relates to the technical field of genetic engineering and biomedicines, in particular to an antigen epitope peptide and application thereof. The amino acid sequence of the antigen epitope peptide provided by the invention is shown as SEQ ID NO:1; the antigen epitope peptide is obtained by connecting HBcAg (or / and HBsAg) and surface antigen epitope peptide HBV Pre-S2 in series. Experimental results show that the mature of dendritic cells can be effectively induced, the antigen presentation capacity can be effectively improved and more cell factors can be secreted by adopting the antigen epitope peptide provided by the invention; after the antigen epitope peptide is co-cultured with lymphocytes, specific cytotoxicity can be produced to kill cells, so that the tumour killing efficiency is improved; the growth of a tumour can be effectively inhibited; an anti-tumour effect is achieved; a new choice is provided for clinical treatment of hepatitis B and related liver cancer thereof.
Owner:中美赛尔生物科技(广东)有限公司
Quantum dot immunochromatography test strip for synchronous and quantitative joint inspection of HBsAg, HBeAg and HBcAb and method for synchronous and quantitative joint inspection of HBsAg, HBeAg and HBcAb
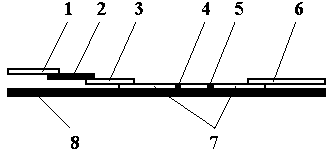
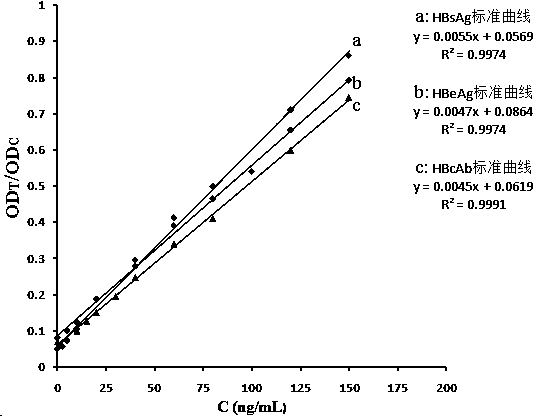
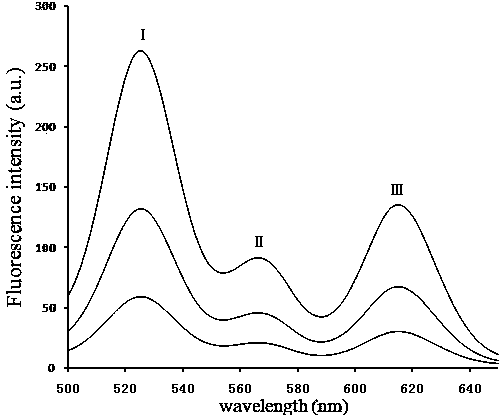
InactiveCN104297483ARapid Quantitative DetectionPrevent passageBiological testingFluorescence/phosphorescenceHematological testQuantum dot
The invention particularly relates to a quantum dot immunochromatography test strip for synchronous and quantitative joint inspection of HBsAg, HBeAg and HBcAb and a method for the synchronous and quantitative joint inspection of the HBsAg, the HBeAg and the HBcAb. A label pad (3) of the test strip is coated with a mixture of a rat anti-HBsAg monoclonal antibody, a rat anti-HBeAg monoclonal antibody and HBcAg of corresponding labels of quantum dots with different wavelengths; a T belt (4) of an analyzing membrane (7) is coated with a mixture of a rabbit anti-HBsAg antibody, a rabbit anti-HBeAg antibody and the HBcAg; and a C belt (5) of the analyzing membrane (7) is coated with a goat anti-rat antibody. An HBsAg, HBeAg and HBcAb standard curve is stored and mounted on the test strip by an electronic label. The test strip adopts a detector with a signal detection function to read the standard curve stored by the electronic label and combines corresponding fluorescence intensity of a sample to be detected, which is measured by the detector, so as to synchronously and quantitatively detect the concentrations of the HBsAg, the HBeAg and the HBcAb in a blood sample.
Owner:CHENGDU LINGYU BIOTECH
Heat shock protein 65- multiple epitope hepatitis B virus core antigen recombinant protein ú¿HSP65-HBcAgú®
InactiveCN1737147AStrong ability to kill HBV-infected cellsPeptide/protein ingredientsVirus peptidesHepatitis B virus core AntigenT lymphocyte
The invention relates to a recombinant fusion protein HSP65-HbcAg, which is fused from heat shock protein 65 and multi-epitope hepatitis B virus HbcAg, in the recombinant fusion protein, the multi-epitope hepatitis B virus HbcAg comprises 5 different antigen epitopes through interconnection by mathematical combination modes. the recombinant fusion protein can make hepatitis B virus HbcAg specific cell toxic T lymphocyte CTL eradicate the cells infected by hepatitis B virus, thus achieving the goal of treating and / or preventing hepatitis B. The invention also provides the nucleic acid sequence encoding the recombination fusion protein.
Owner:BEIJING HYDVAX BIOTECH
Compositions containing virus-like particles as immunopotentiators administered through the mucosa
InactiveUS7374766B1Adjuvant effectViral antigen ingredientsSnake antigen ingredientsWhole bodyPharmaceutical industry
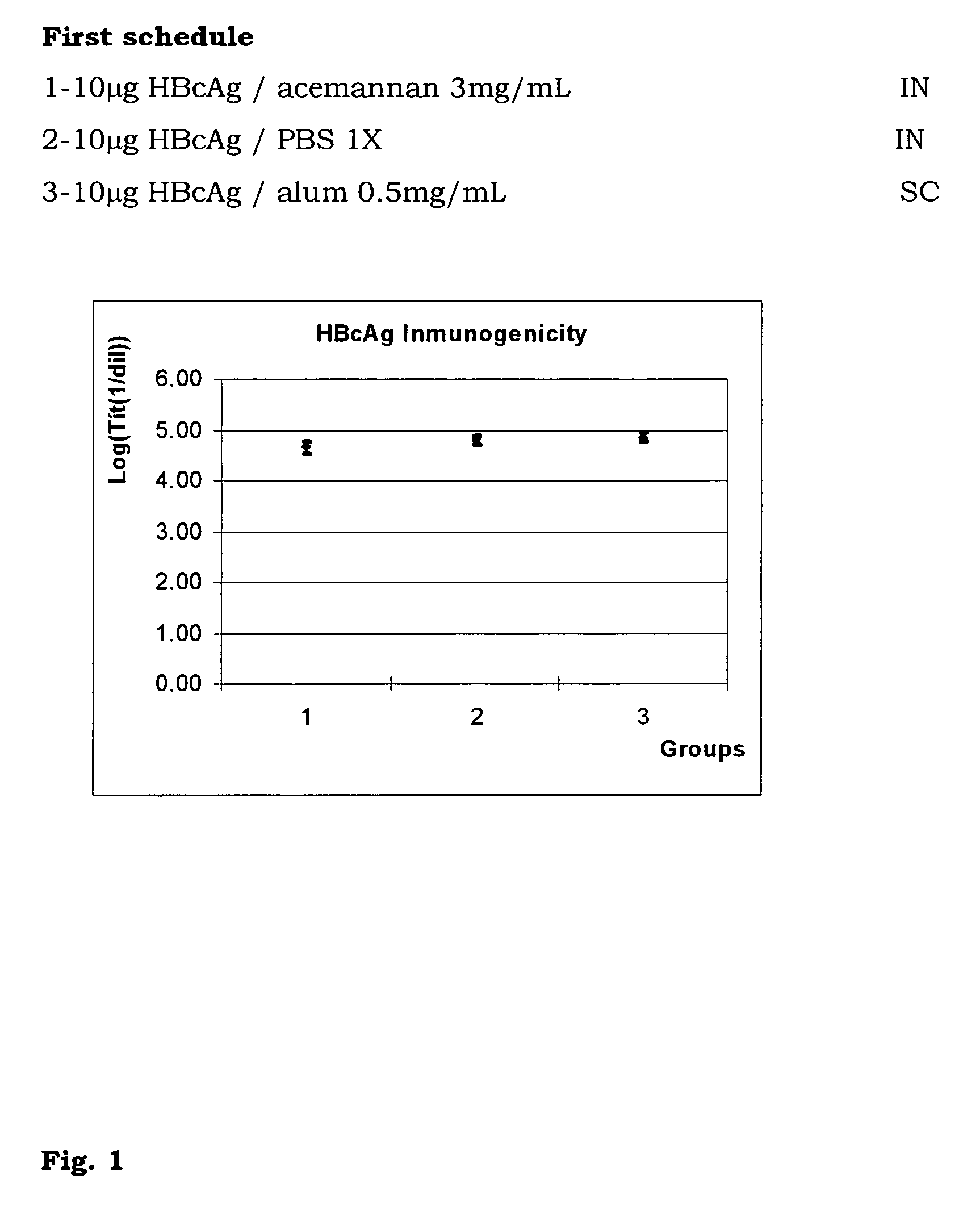
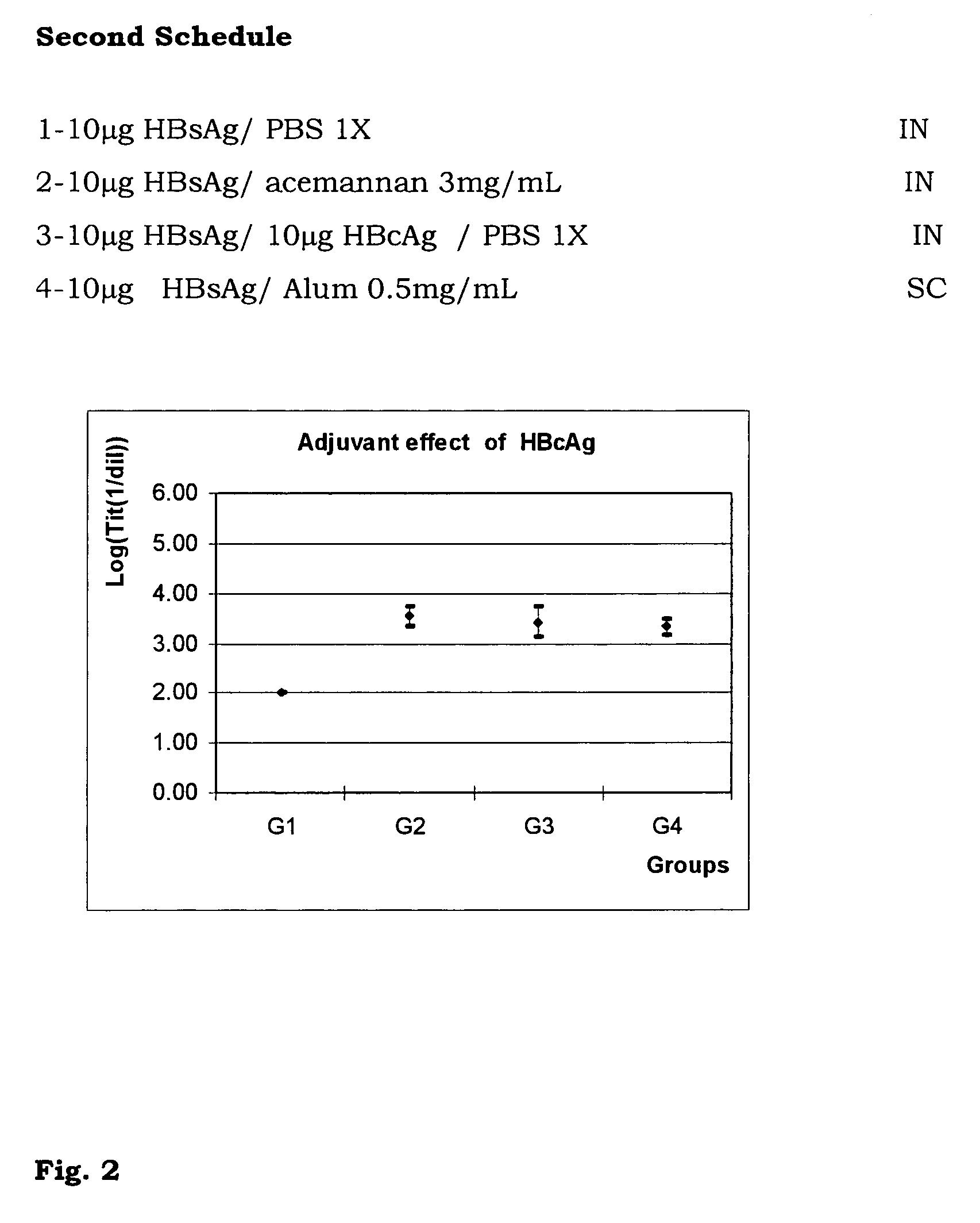
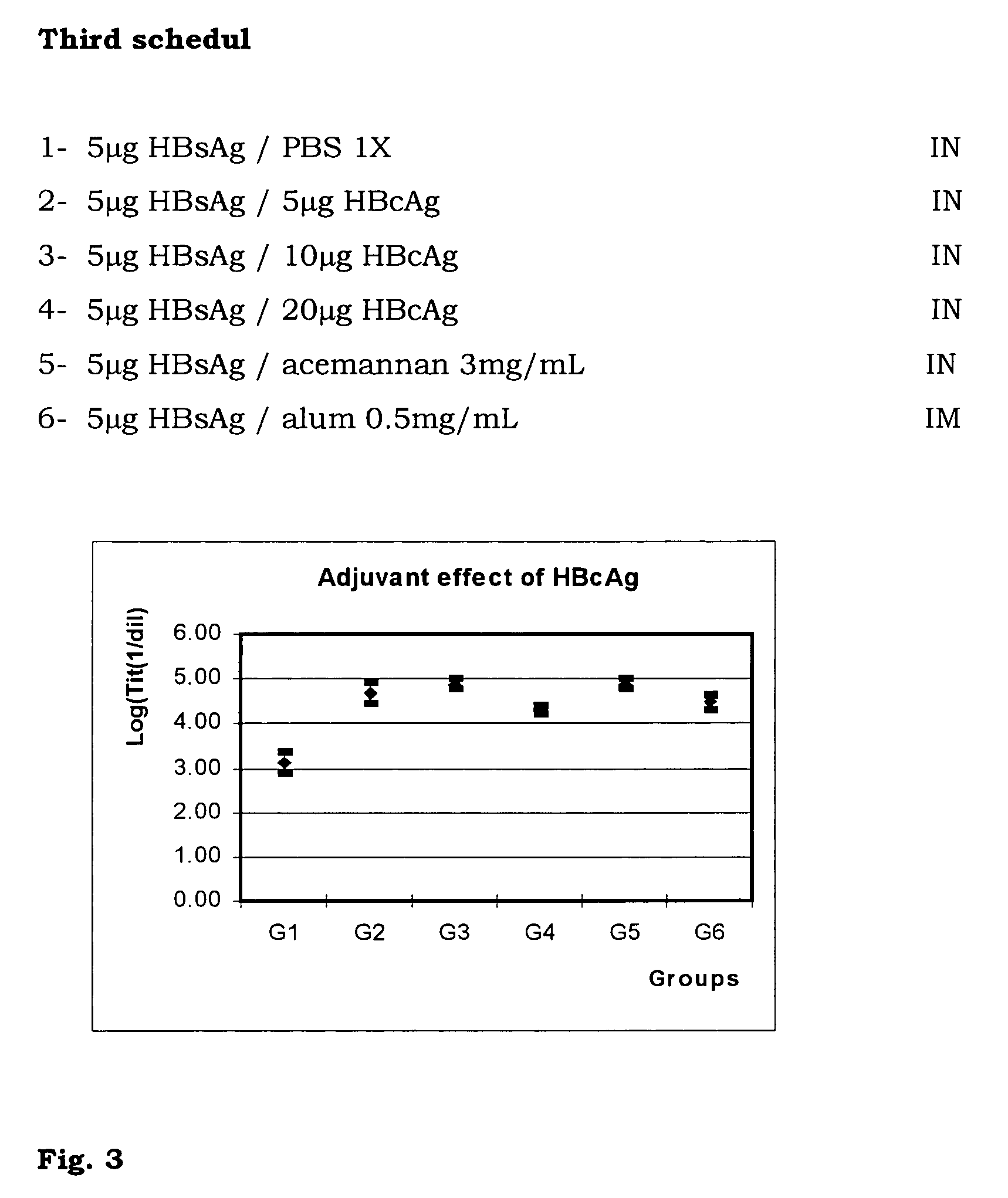
The present invention is related to the branch of medicine, particularly to the new formulations of vaccine antigens.The technical objective pursued with the present invention is, precisely, the development of formulations that are able to enhance the immune response to mucosally administered antigens, minimising the number of compounds in the formulation and generating strong mucosal and systemic responses through a synergic interaction between the antigens in the formulation.These formulations enable: a) to broaden the spectrum of the anti-hepatitis B immune response, containing as main compounds HBsAg and HBcAg, b) to enhance the response against HBsAg with a viral nucleocapsid c) to generate combined vaccines through the mucosal route with HBsAg as a central antigen. Stabilizers and preservatives can be introduced.The formulations of this invention can be applied in the pharmaceutical industry as human or veterinary vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Hepatitis B virus antigen formulation for cell stimulation followed by therapeutic immunization
The present invention relates to the field of therapeutic immunization, specifically with the use of a novel hepatitis B virus (HBV) antigen formulation for cell stimulation. The formulation is formed by HBV surface antigen (HBsAg) and nucleocapsid antigen (HBcAg) precipitates in suspension. The formulation contains said antigens as precipitates in suspension as particles with sizes less than 500 nm and greater than 500 nm, in a mixture in which the proportion between the particles of the sizes mentioned is between 50% - 50% and 80% - 20%, respectively. The selection of a range of particle sizes allows the levels of stimulation of various cell types to be maximized. In addition, a description is given of a method for cell stimulation using said formulation and the subsequent passive immunization of patients with chronic hepatitis B, based on the maximum in vivo or in vitro stimulation of heterologous or autologous cells (dendritic cells, B cells and macrophages). The cells stimulated with this formulation are transferred to patients with a chronic HBV infection.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
Vaccines comprising truncated HBC core protein plus saponin-based adjuvants
InactiveCN101309931ACan control the immune responseSmall preparation complexityAntibacterial agentsSsRNA viruses positive-senseAntigenDisease
The present invention relates to a composition, comprising: i) an HBcAg<1-x>, wherein x is a whole number from the range from 100 to 160, a fragment of this antigen, a variant of this antigen or of the fragment of this antigen or at least two thereof, ii) an adjuvant comprising a saponin or a saponin derivative or a mixture of at least two thereof, and optionally iii) an HBsAg, a fragment from the antigen, a variant of this antigen or of the fragment of this antigen or at least two thereof. The invention also relates to a process for production of a composition, the composition obtainable by this process, a pharmaceutical formulation, the use of the composition or of the pharmaceutical formulation for treatment and / or prevention of HBV infections and HBV-mediated diseases, the use of the composition or of the pharmaceutical formulation for production of a pharmaceutical for treatment and / or for prevention of HBV-infections and HBV-mediated diseases as well as a process for treatment and / or for prevention of HBV-infections and HBV-mediated diseases.
Owner:RAJN BIOTEKH GEZELLSHAFT FJUR NOJE BIOTEKHNOLOGISHE PROTSESSE & PROD MBKH
Fusion protein related to HBV, preparing method thereof and application thereof
ActiveCN105367662AHigh activityHigh expressionGenetic material ingredientsAntiviralsProkaryotic expressionGenetic engineering
The invention provides a fusion protein related to HBV, a preparing method thereof and an application thereof. Particularly, a prokaryotic expression carrier related to HbcAg is built and expressed through the genetic engineering technology, and the obtained fusion protein can remarkably induce an HBV-specificity CTL reaction in vitro and vivo.
Owner:SHANGHAI SIXTH PEOPLES HOSPITAL
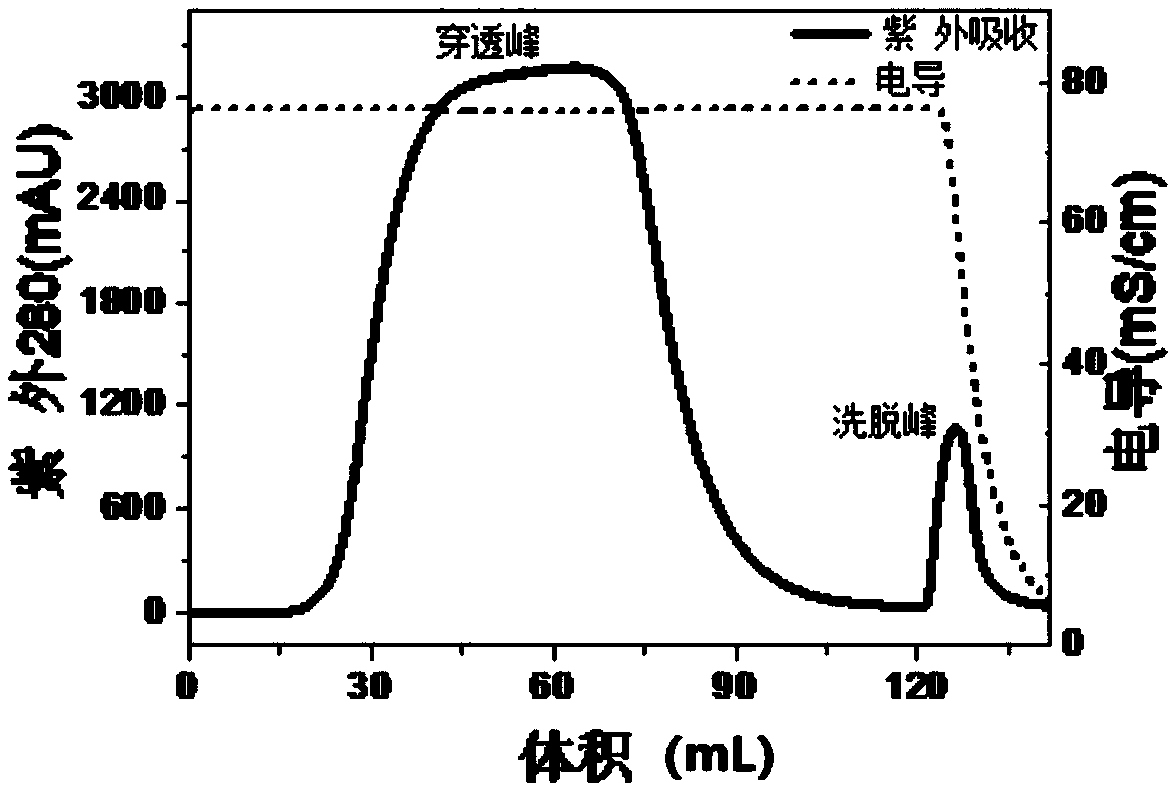
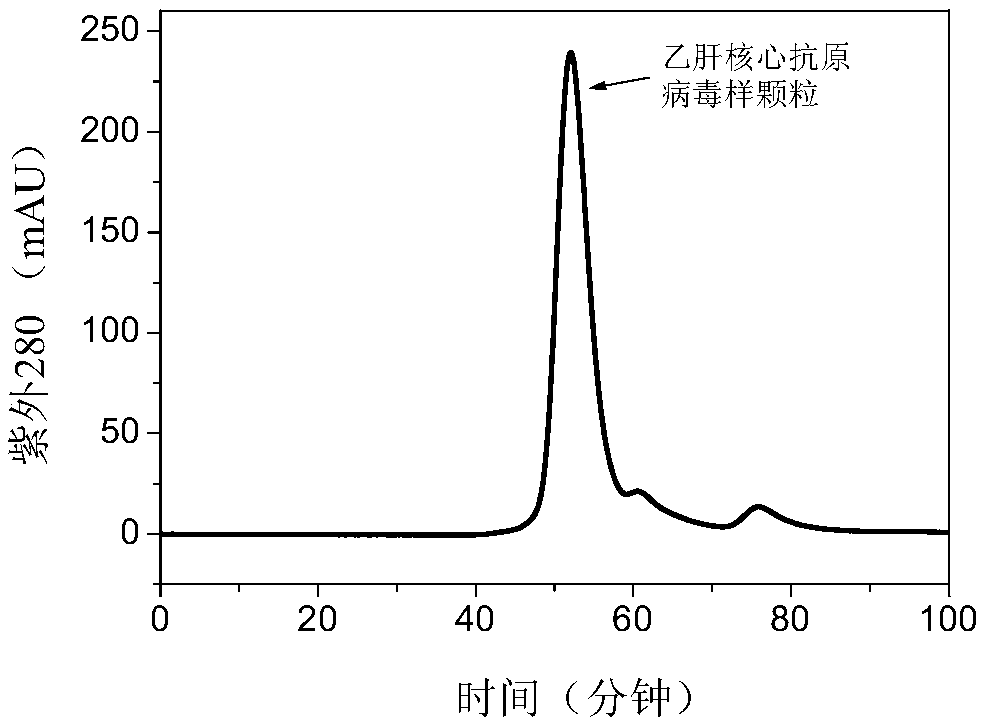
Purification method of HBcAg (hepatitis B virus core antigen)-VLP or HBcAg-VLP derivative
InactiveCN109134621AImprove stabilityTo achieve the purpose of purification and preparationBacteriaVirus peptidesHepatitis B virus core AntigenPurification methods
The invention provides a purification method for HBcAg (hepatitis B virus core antigen)-VLP or an HBcAg-VLP derivative. The method comprises the following steps: treating a loading solution containingHBcAg-VLP and a loading solution containing the HBcAg-VLP derivative by hydrophobic interaction chromatographic packing, and purified HBcAg or the purified HBcAg-VLP derivative is obtained. Accordingto the purification method, the problems of numerous steps, low yield, expensive consumables and the like of existing methods are solved, purification can be achieved by only one-step hydrophobic chromatography; when a gel filtration refining method is not used for treatment, the yield can reach up to 90% or higher, purity can reach up to about 86%, and the purification effect is greatly improved; if the purification method is supplemented with refining and purification methods such as further gel filtration, the purity can reach 99% or higher. The purification method has good application prospect and very high application value.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +1
Immune dominant HLA-A3 super-type restrictive CTL epitope of hepatitis B virus core antigen and identification method and application thereof
InactiveCN101979405AImproving immunogenicityStrong specificityMicrobiological testing/measurementDigestive systemHepatitis B virus core AntigenCtl epitope
The invention relates to an antigen epitope, in particular to an immune dominant HLA-A3 (human leukocyte antigen A3) super-type restrictive CTL (cytotoxic T lymphocyte) epitope of a hepatitis B virus core antigen (HBcAg) and an identification method for the super-type CTL epitope. The super-type CTL epitope consists of the following amino acid sequence: Leu-Leu-Asp-Thr-Ala-Ser-Ala-Leu-Tyr-Arg. The method is also suitable for identification of other antigen super-type CTL epitopes, and can provide a useful tool for design and research of therapeutic polypeptide vaccines. The invention also relates to application of the super-type CTL epitope in preparing hepatitis B therapeutic polypeptide vaccines, and provides a new strategy and a new method for efficient research of the hepatitis B therapeutic polypeptide vaccines. The method is expected to break through hepatitis B immune tolerance, reconstruct the immune function of cells and efficiently inhibit and clear hepatitis B viruses.
Owner:ARMY MEDICAL UNIV
Subunit vaccine based on prokaryotic expression of norovirus antigenic epitope and preparation method of subunit vaccine
ActiveCN109589406AStrong specificityHigh sensitivitySsRNA viruses positive-senseViral antigen ingredientsOrganismSpecific antibody
The invention relates to the field of biomedicine, in particular to a preparation method of a subunit vaccine based on prokaryotic expression of a norovirus antigenic epitope. The invention provides the prokaryotic expressed subunit vaccine which is used for stimulating a body to produce a specific antibody against norovirus, and is characterized in that the norovirus recombinant antigen sequenceis as shown in SEQ ID NO: 1, source: NCBI Reference Sequence: NC_029646.1, a neutralizing epitope of human norovirus GII type: 105 bp, and an expression vector: a modified hepatitis B core antigen (HBcAg) gene cloned into a pThioHisA expression vector.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
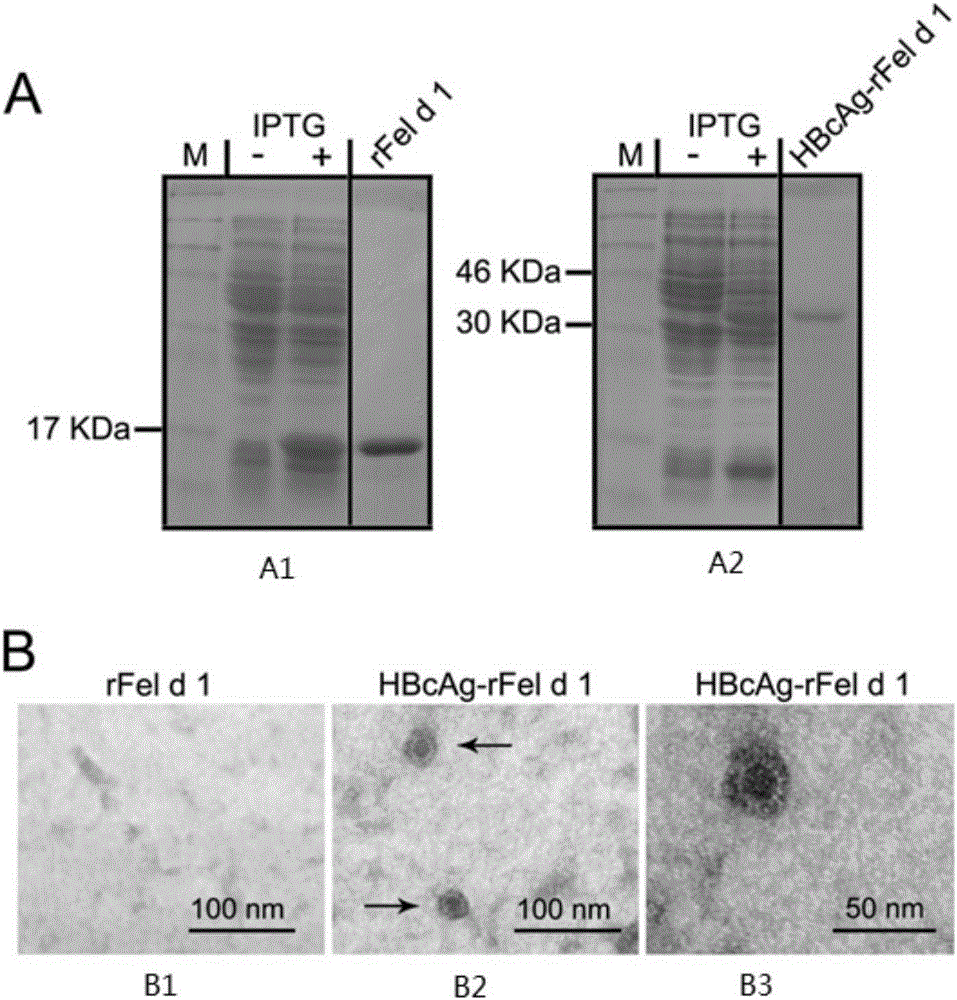
Immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, expression vector of immunity-enhanced type virus-like particle and preparation and application of mmunity-enhanced type virus-like particle
InactiveCN105219742AImproving immunogenicityHigh detection sensitivityInactivation/attenuationAllergen ingredientsHepatitis B virus core AntigenADAMTS Proteins
The invention provides an immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, an expression vector of the immunity-enhanced type virus-like particle and preparation and application of the immunity-enhanced type virus-like particle. The protein subunit forming the virus-like particle is fused protein of hepatitis B core antigen and polypeptides with the amino acid sequence of SEQ ID NO:1. The amino acid sequence of the fused protein is formed by inserting the sequence SEQ ID NO:1 of polypeptides between the 78th to 81st amino acid of hepatitis B core antigen and replacing the 79th to 80th amino acid of hepatitis B core antigen. By means of HBcAg-fFel d 1 fused protein, the immunogenicity of fFel d 1 can be remarkably improved, and therefore the detection sensitivity of fFel d 1 recombined and expressed in vitro in application of in-vitro diagnosis cat allergies is improved.
Owner:HAINAN UNIVERSITY
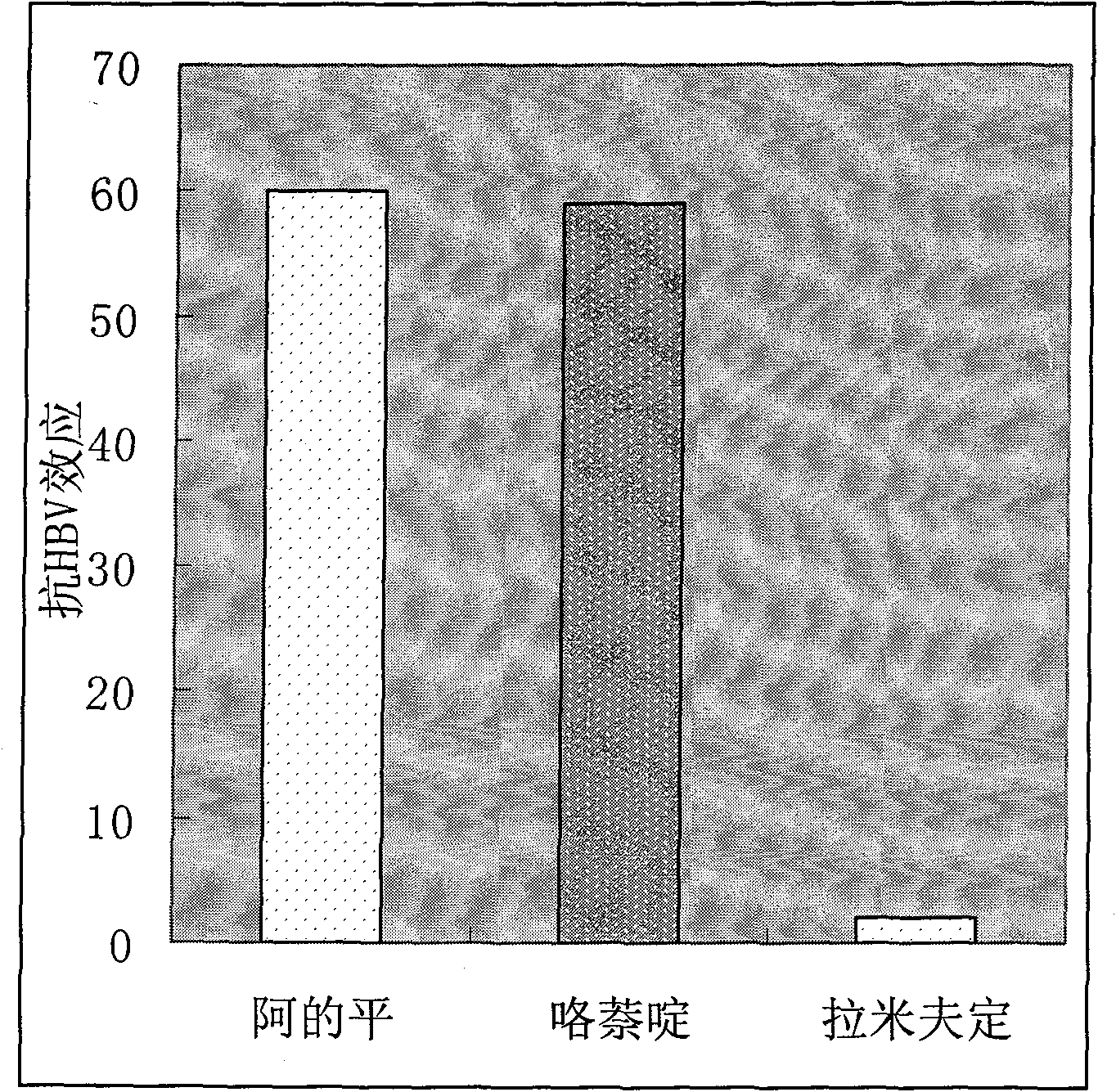
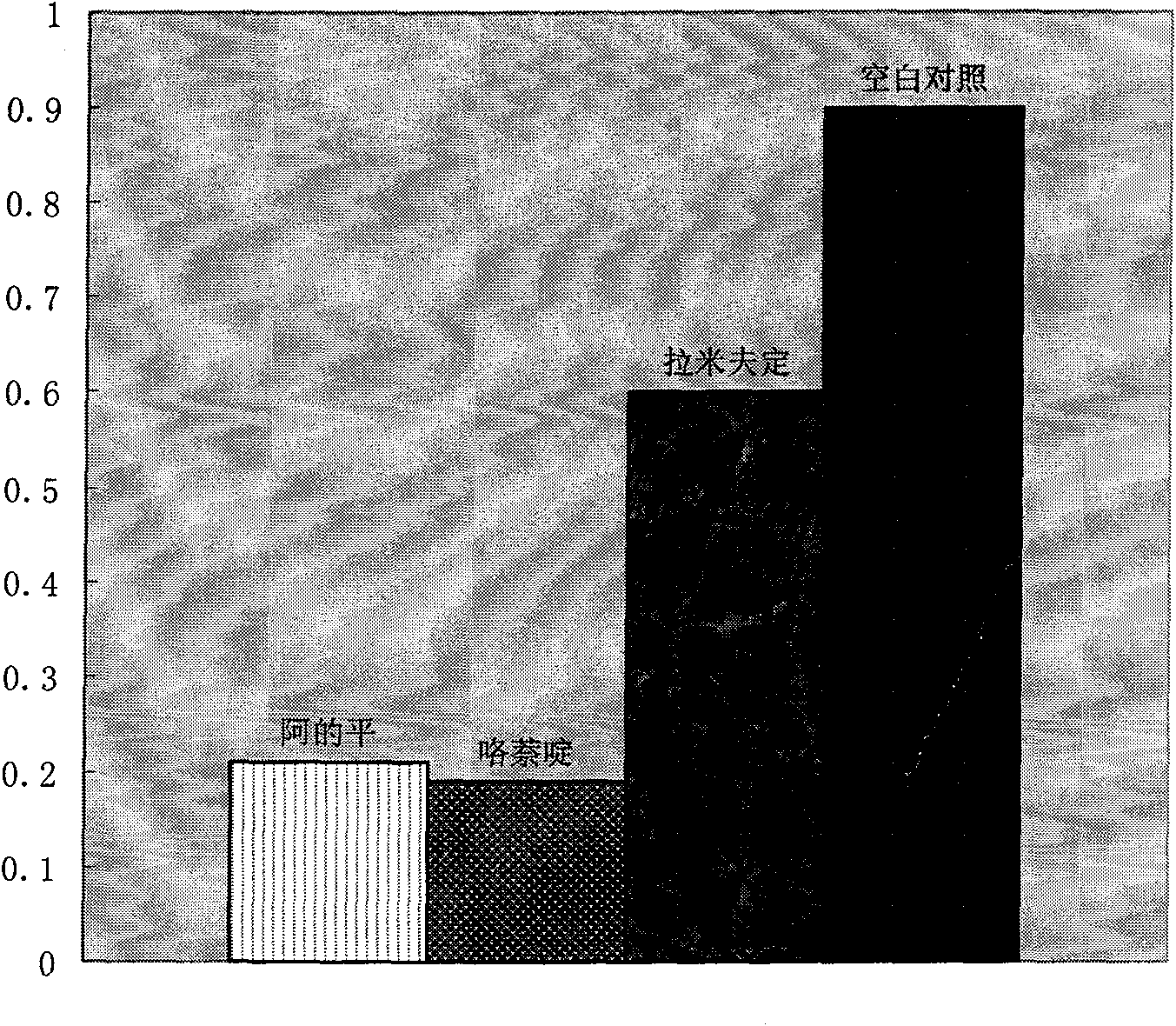
Therapeutical effect of atabrine and substitutes thereof on hepatitis B
InactiveCN101856355ALower titerInhibition killOrganic active ingredientsAntiviralsAntigenNorthern blot
Owner:蔡荣 +3
Method for preparing chimeric monoclonal antibody capable of neutralizing EV71 (enterovirus 71)
The invention relates to a method for preparing a chimeric monoclonal antibody capable of neutralizing EV71 (enterovirus 71). The chimeric monoclonal antibody for neutralizing EV71 or a fragment of the chimeric monoclonal antibody is characterized in that a heavy chain variable region of the chimeric monoclonal antibody or the fragment of the chimeric monoclonal antibody has an amino acid sequence shown in SEQ ID NO:1; a light chain variable region has an amino acid sequence shown in SEQ ID NO:2. EV71 VP4 (viral protein 4) protein is fused into an HBcAg (hepatitis B core antigen) virus-like particle to be prepared into fusion protein, and the fusion protein is taken as an antigen to endow a Balb / c mouse with immunity. A phage display antibody library technique is used for screening to obtain the monoclonal antibody capable of specifically neutralizing EV71 VP4 protein. The invention provides the method for preparing a potential monoclonal antibody medicine with broad spectrum neutralization activity and for preventing and treating a hand foot mouth disease.
Owner:BEIJING UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com