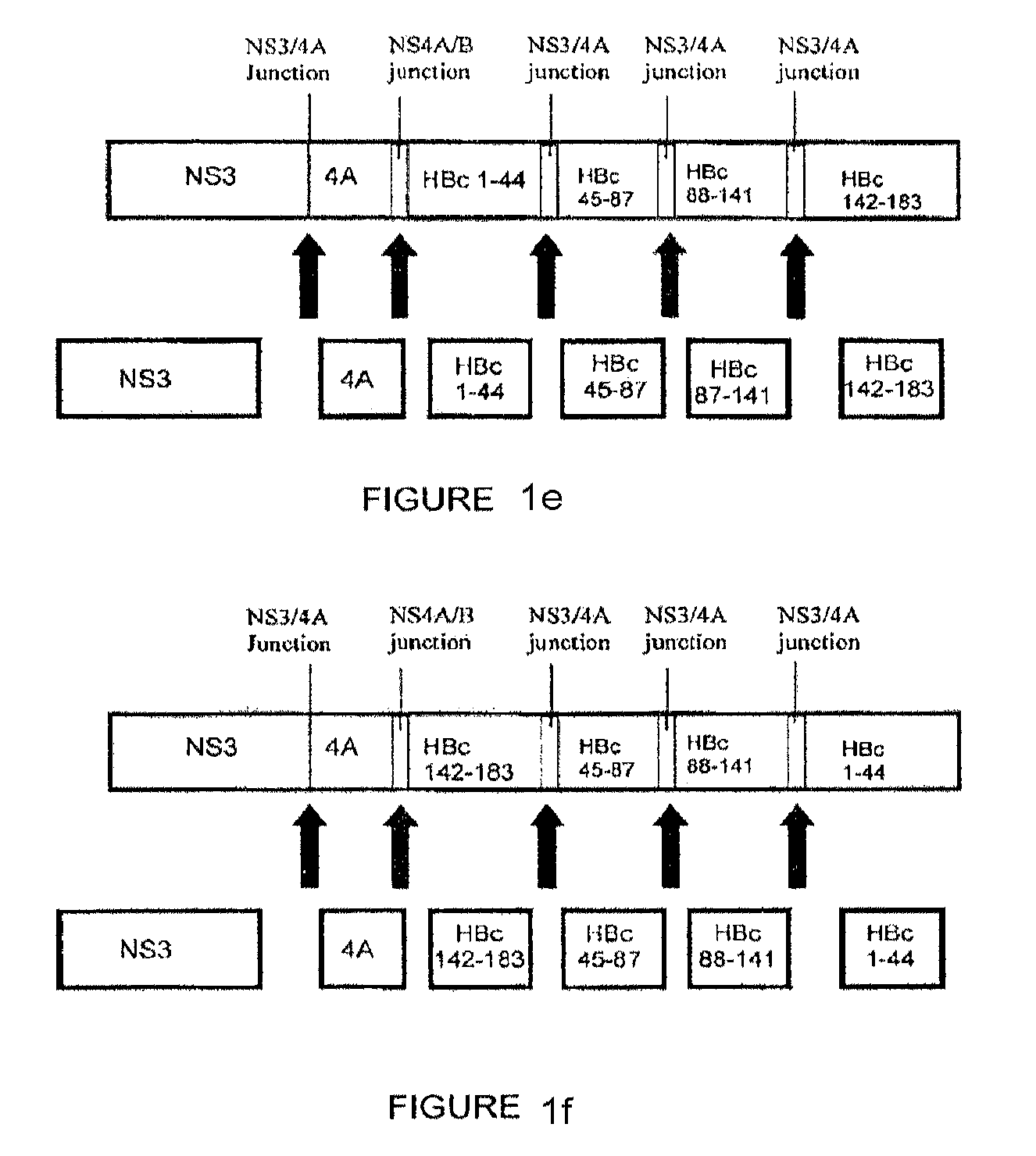
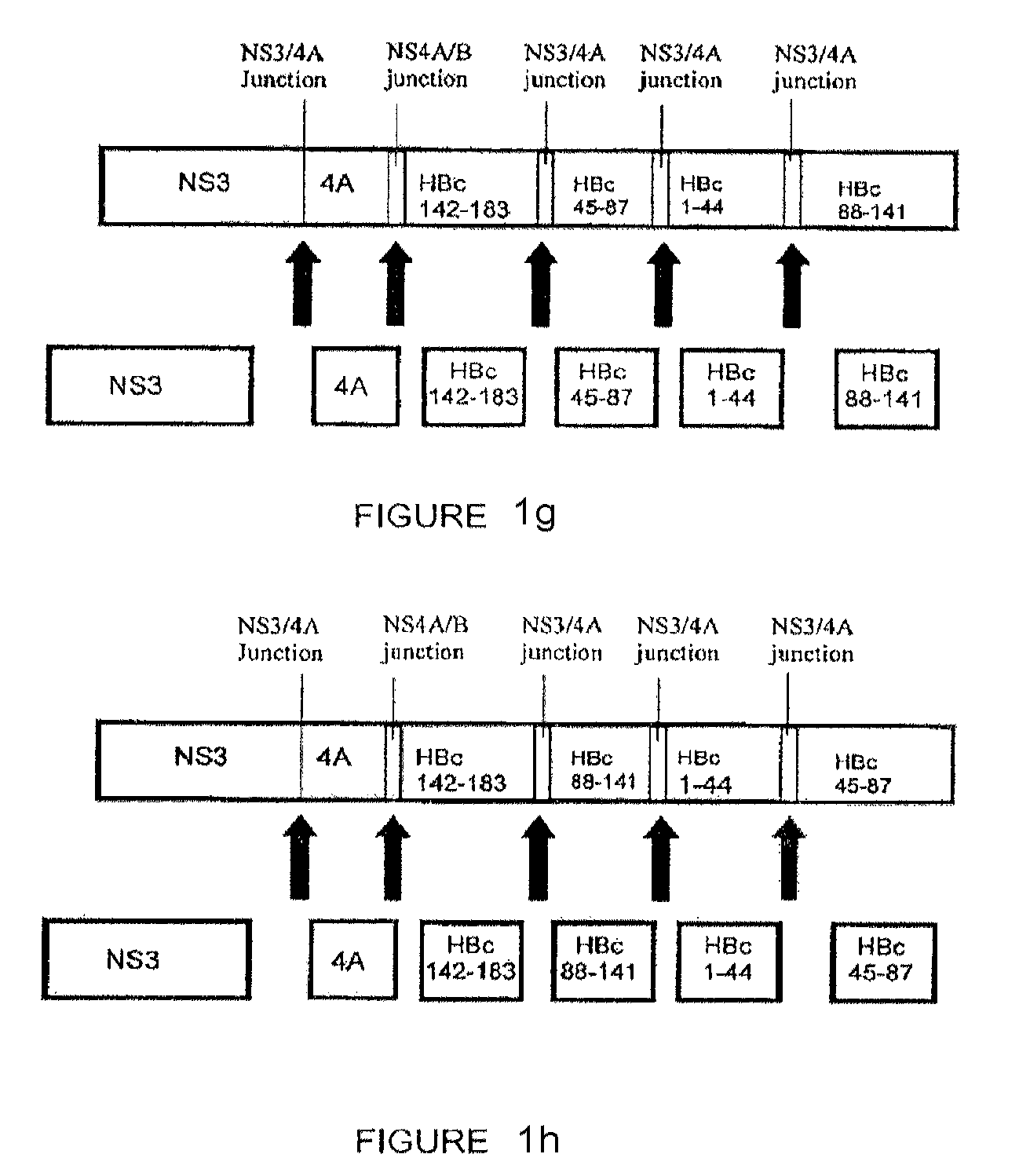
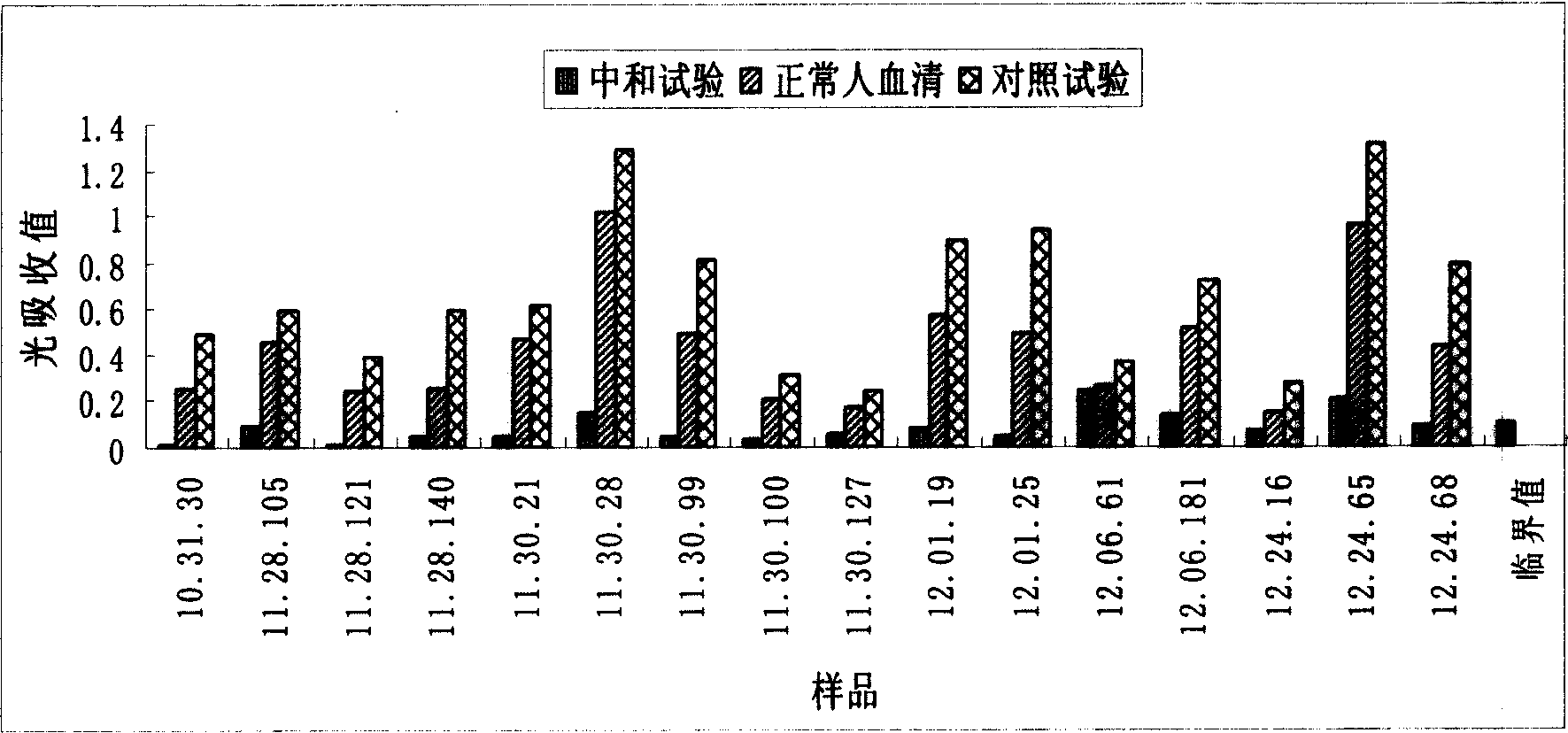
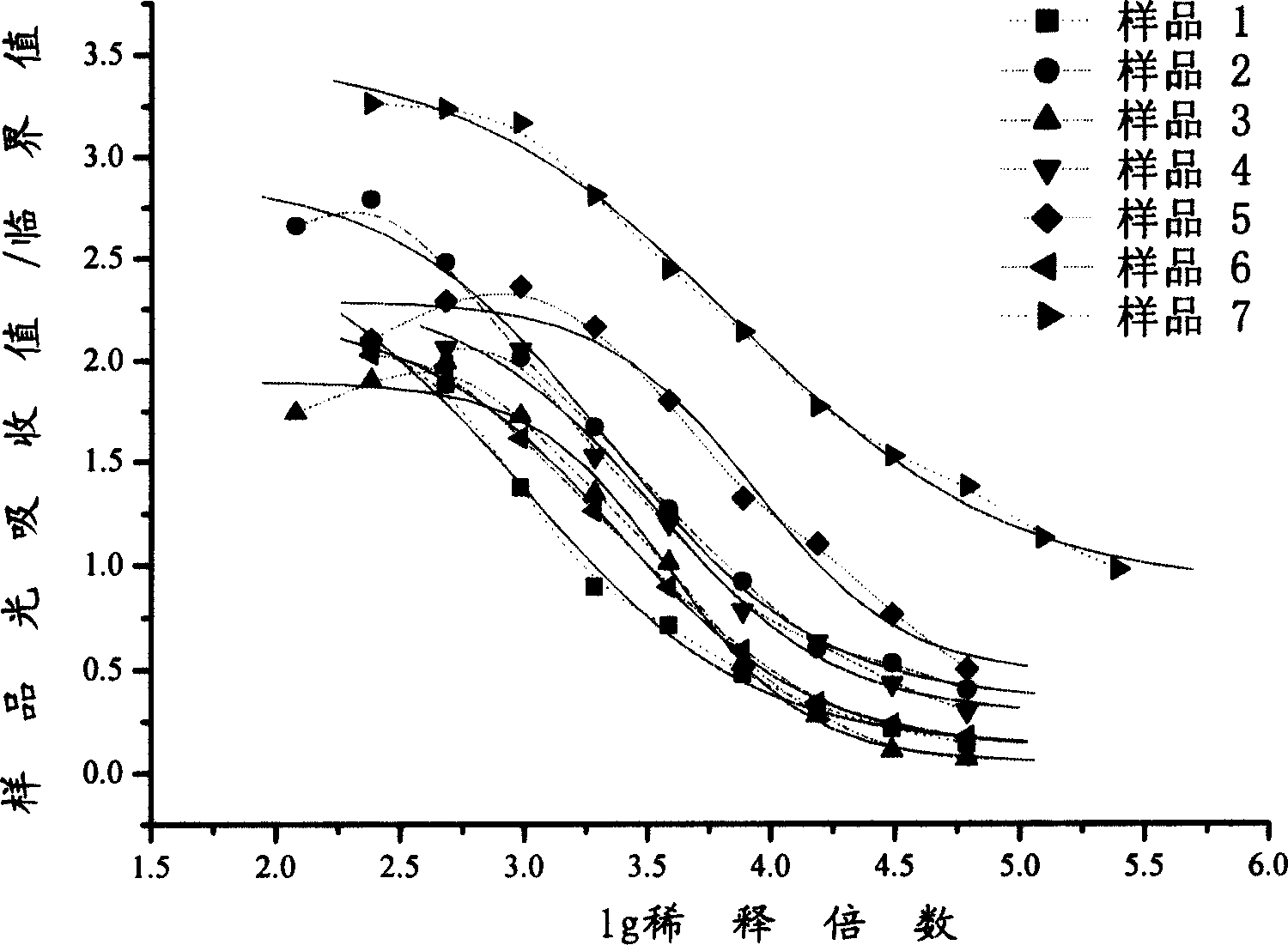
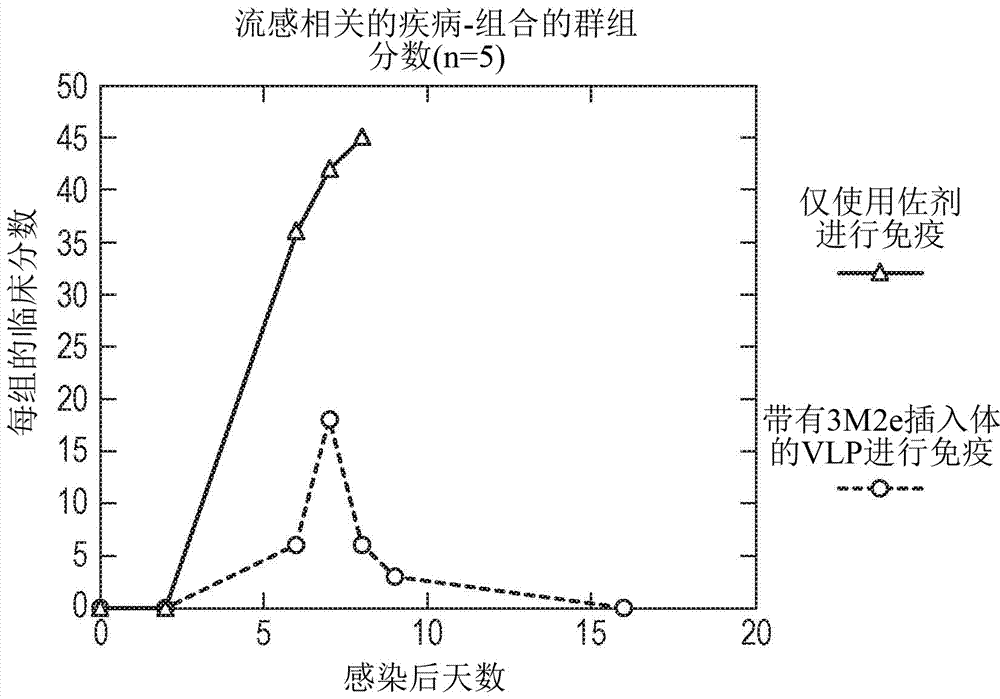
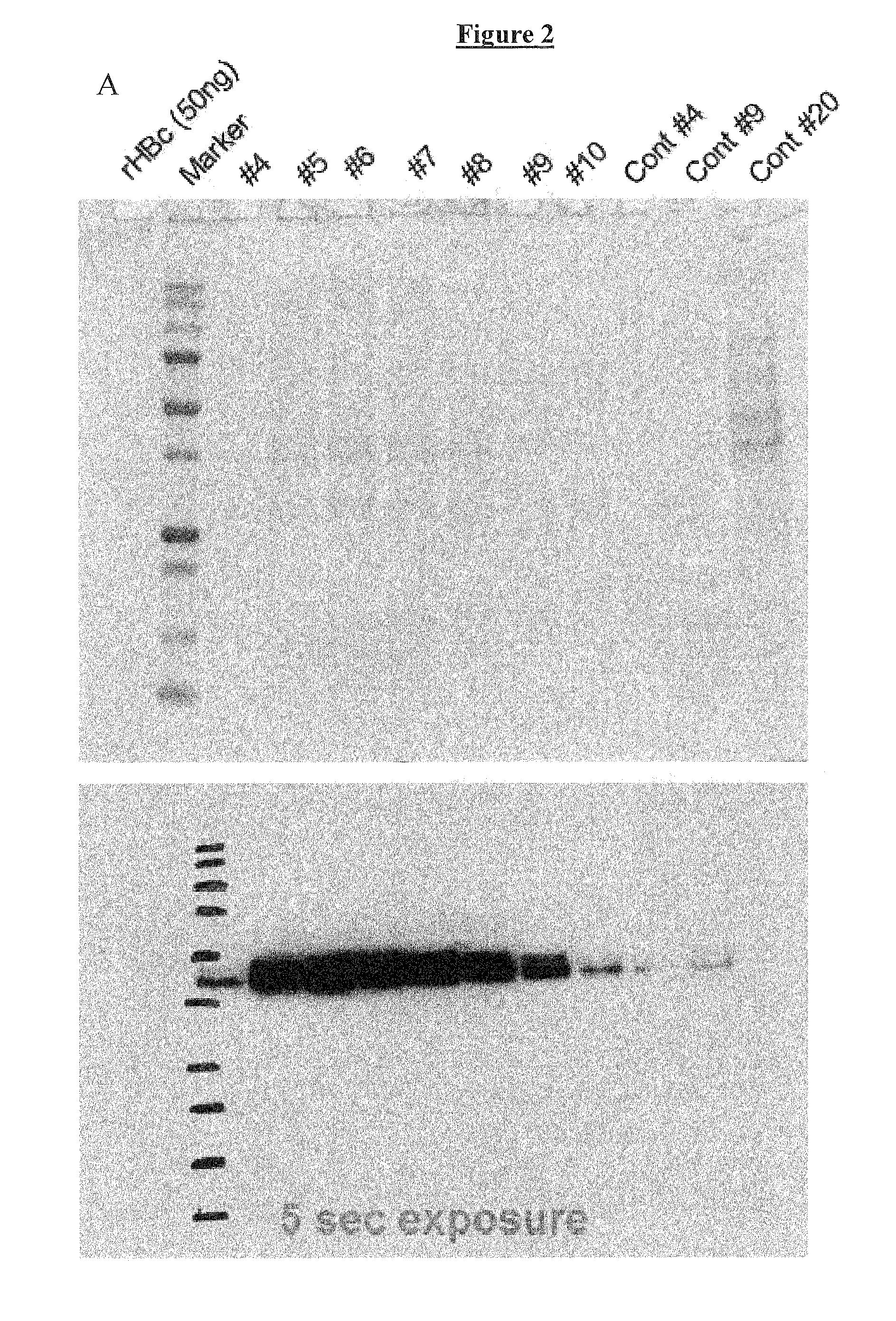
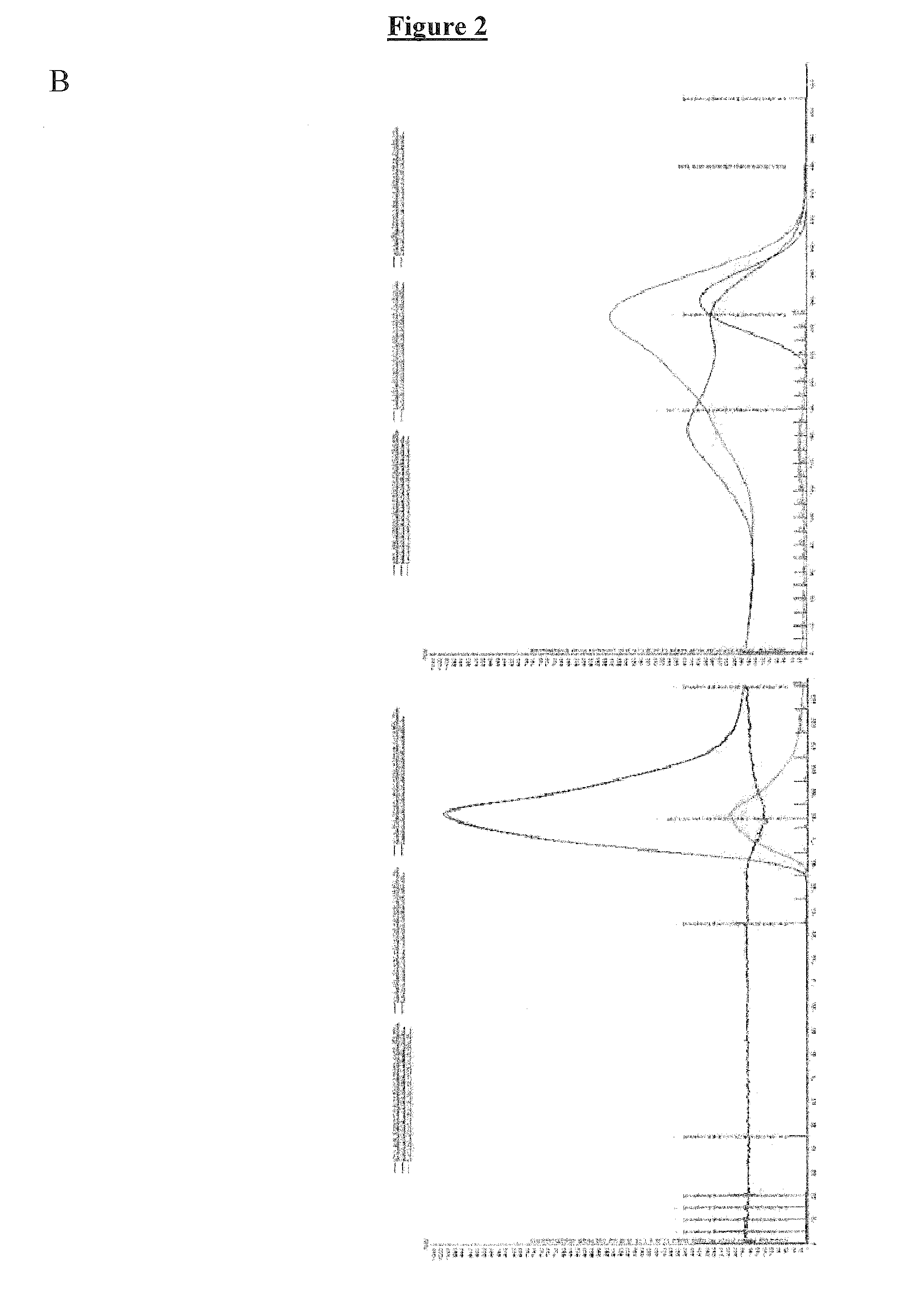
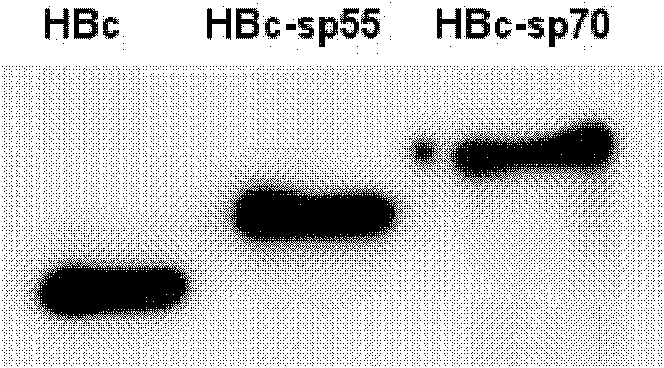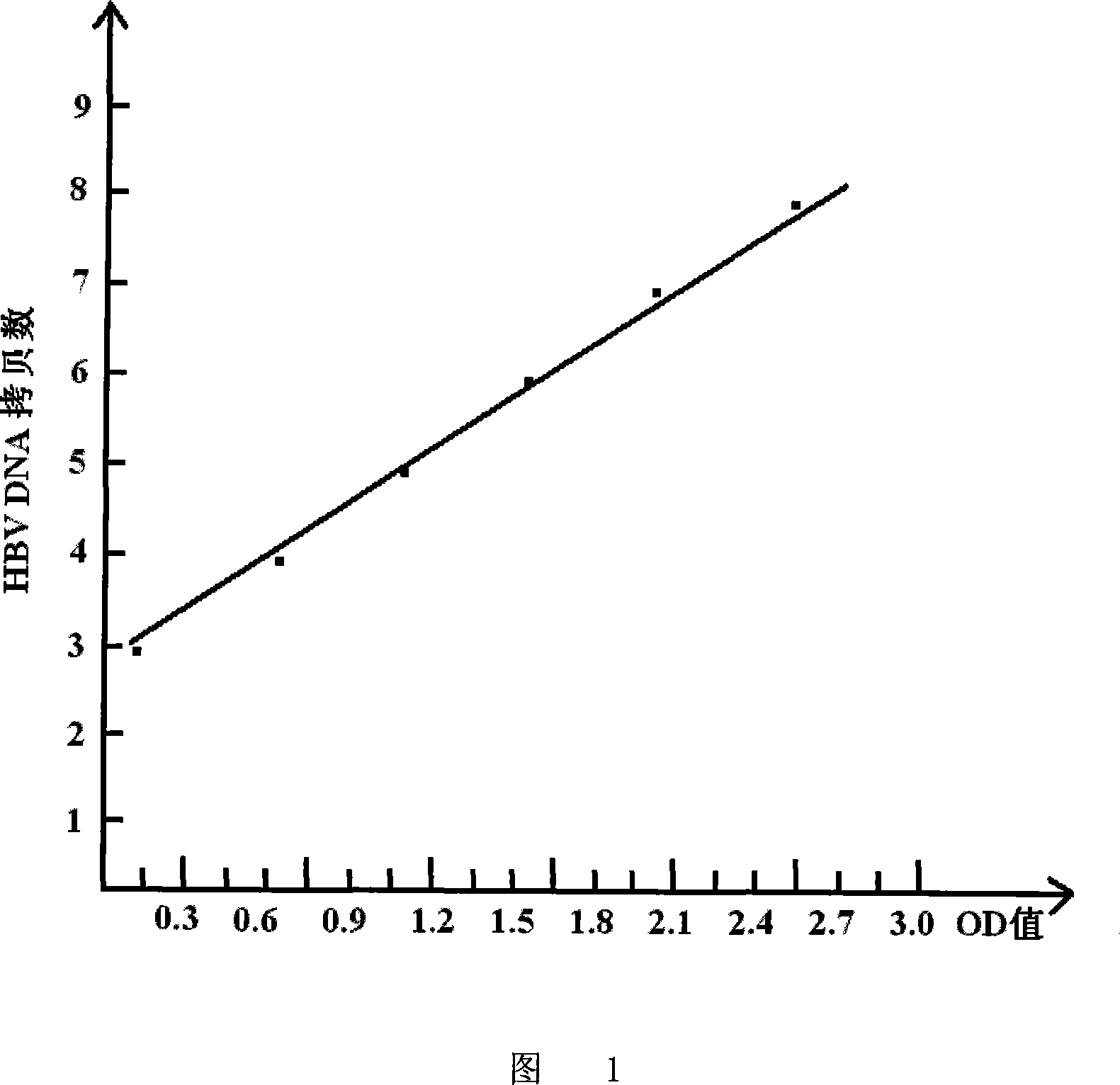Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
62 results about "Hepatitis B Core Antigens" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Use of composition of isoflavonoids from Belamcanda chinensis in preparing anti-hepatitis medicament
InactiveCN101301287ASignificant anti-HBV effectOrganic active ingredientsDigestive systemAntigenBelamcanda chinensis
The invention belongs to the medical technical field, in particular relating to an application of isoflavonoids from Belamcanda chinensis in the preparation of anti-hepatitis virus drugs. A Hepatitis B surface antigen (HBsAg) detection reagent test and a hepatitis B core antigen (HBeAg) detection reagent test are performed, and the results indicate that the isoflavonoids from the Belamcanda chinensis can remarkably suppress hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg) and has remarkable effect on preventing and treating hepatitis B viruses. Therefore, the isoflavonoids from the Belamcanda chinensis can be used to prepare medicines or health-care food used to prevent and treat the hepatitis viruses.
Owner:上海双科医药科技有限公司
Chemoluminescence immunoassay measuring kit and preparation method thereof for hepatitis B core antibody magnetic particles
InactiveCN101545906ASimple structureLow priceChemiluminescene/bioluminescenceAntigenHepatitis b core
The invention provides a chemoluminescence immunoassay measuring kit and a preparation method thereof for hepatitis B core antibody magnetic particles. The kit comprises a hepatitis B core antibody calibration sample, magnetic particles coated by a hepatitis B core antigen, an enzyme marker, a chemoluminescence substrate and a concentrated washing solution. The invention effectively utilizes a magnetic particle solid separation system and a chemoluminescence immunoassay technology to detect the hepatitis B core antibody. The kit has the advantages of simple and fast detection, high sensitivity, strong specificity, stability, and the like, can well satisfy the requirement of clinic diagnosis and is suitable to be effectively generalized and applied in industry.
Owner:CHEMCLIN DIAGNOSTICS CO LTD
Separation and purification method for recombinant hepatitis B core antigen
ActiveCN108047316AImprove thermal stabilityAggregate compact and stableVirus peptidesPeptide preparation methodsAntigenPurification methods
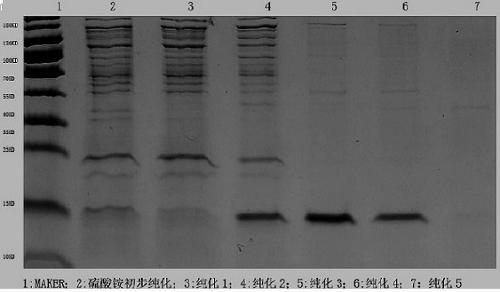
The invention relates to a separation and purification method for a recombinant hepatitis B core antigen. The method includes the steps of thermal denaturation and clarification; ammonium sulfate precipitation; ultrafiltration and concentration, washing filtering and liquid exchange; depolymerization; first-step molecular sieve chromatography; ultrafiltration and concentration, washing filtering and liquid exchange; repolymerization; second-step chromatography. By means of the method, the high-purity, low-host-residue and high-stability recombinant hepatitis B core antigen with a uniform granular structure can be obtained, that is to say, the method has the advantages that the antigen purity is high, the residue of host protein and host nucleic acid is low, antigen particles are uniform, and industrial enlargement is easy.
Owner:JIANGSU THERAVAC BIO PHARMA
Compositions and methods that enhance an immune response
InactiveUS8445663B2Improve immunityEnhance immune responseBiocideSsRNA viruses positive-senseHeterologousAdjuvant
Disclosed herein are isolated nucleic acids, compositions of isolated nucleic acids, and compositions of polypeptides that are useful for the generation, enhancement, or improvement of an immune response to a target antigen. Some embodiments of the compositions include hepatitis B core antigen (HBcAg) protein and a heterologous protein antigen. In some embodiments, an isolated nucleic acid encoding hepatitis B core antigen (HBcAg) protein and a heterologous protein antigen is disclosed. Also disclosed herein are methods of administering the composition or isolated nucleic acid to generate an immune response, where HBcAg acts as adjuvant to improve the immune response to the heterologous protein. In certain embodiments, the HBcAg is as a stork or heron hepatitis antigen.
Owner:TRIPEP
Recombinant fusion protein and use thereof
InactiveCN104341506AAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenAntigen epitope
The invention belongs to the biological medicine field, relates to recombinant fusion protein and use thereof, and in particular relates to recombinant fusion protein carrying hepatitis B virus therapeutic antigen epitopes which are inserted into hepatitis B core antigen protein particles or truncated fragments and use thereof. The recombinant fusion protein contains multiple epitope antigens of hepatitis B virus (HBV) and other immune stimulating epitope antigens and hepatitis B core antigen virus-like particles or truncated fragments thereof for preparation of chimeric antigen, the multiple antigen epitopes can be inserted into same or different sites of hepatitis B virus core antigen HBc or truncated fragments thereof in the manner of single epitope or multi epitope combination, and by combination with different adjuvants, HBV specific humoral and cellular immune functions can be strengthened.
Owner:FUDAN UNIV
ELISA measuring reagent kit for detecting hepatitis B virus kernel antigen in blood serum
ActiveCN101140285AAvoid interferenceImprove featuresMaterial analysisAntigenHepatitis B virus core Antigen
The present invention discloses an enzyme association immunity test reagent kit and its operation instruction to test hepatitis B core antigen. Wherein, the reagent kit mainly comprises a monoclonal hepatitis B core antigen prepackaged micro-perforated plate, sample processing agent and polyclone hepatitis B core antigens marked with horse radish peroxidase. During operation, a sample to be tested is firstly preprocessed with the sample processing agent and then added into the monoclonal hepatitis B core antigen prepackaged micro-perforated plate. And then, polyclone hepatitis B core antigens marked with horse radish peroxidase and zymolytes are added to colorize. Stop solution is added to stop reaction and finally the measuring result is confirmed through color comparison. The present invention can detect hepatitis B core antigen in blood serum, ensure quite high conformance ratio in aspect of HBV-DNA detecting result, achieve requirements of clinical examination, have characteristics of convenience, sensitivity and stability, supplement conventional hepatitis B virus detecting method and bring better clinical operation.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
Enzyme linked immunity diagnose reagent kit for HB core antigen detecting in two sandwich method and application thereof
The disclosed dual-sandwich enzyme-linked immunologic diagnosis agent box for hepatitis-B core antibody comprises: a micro-porous reaction plate composed by the hepatitis-B core antigen recombinant with purity more than 90% and 5*104~5*105u / mg titer, and the enzyme compound composed by the hepatitis-B core antigen recombinant marked by HRP. This invention has high specificity and sensitivity and convenient to qualitative or semi-qualitative detection.
Owner:SHENYANG HUIMIN BIOTECH CO LTD
Application of HBcAg (hepatitis B core antigen) virus-like particle serving as cancer therapeutic vaccine carrier
InactiveCN105497886AImprove the level ofViral antigen ingredientsAntineoplastic agentsHepatitis B virus core AntigenEscherichia coli
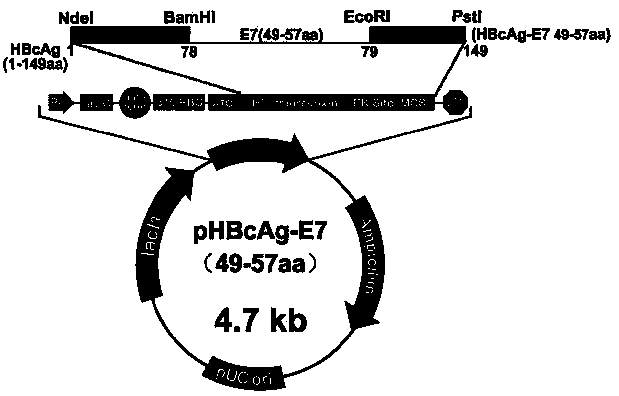
The invention relates to the field of molecular biology and immunology, in particular to an application of an HBcAg (hepatitis B core antigen) virus-like particle serving as a cervical cancer therapeutic vaccine carrier. A preparation method comprises steps as follows: an HPV16 E749-57CTLs epitope peptide fragment is selected, a DNA (deoxyribose nucleic acid ) fragment of the HPV16 E749-57CTLs epitope peptide fragment is inserted between 78 and 79 amino acids of the HBcAg through genetic recombination, an obtained recombinant plasmid pHBcAg-E749-57 is converted into Escherichia coli DH5alpha, and an HBcAg virus-like particle vaccine presenting E749-57 is obtained after induction expression and purification. After a tumor-bearing mouse is immunized with the virus-like particle vaccine, the body of the mouse can be induced to generate a higher HPV16E7 specific cellular immunologic response, and growth of tumors is remarkably inhibited.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
Hepatitis B core antigen fusion proteins
InactiveUS7270821B2Enhance immune responseFungiFusion with DNA-binding domainHeterologousProtein composition
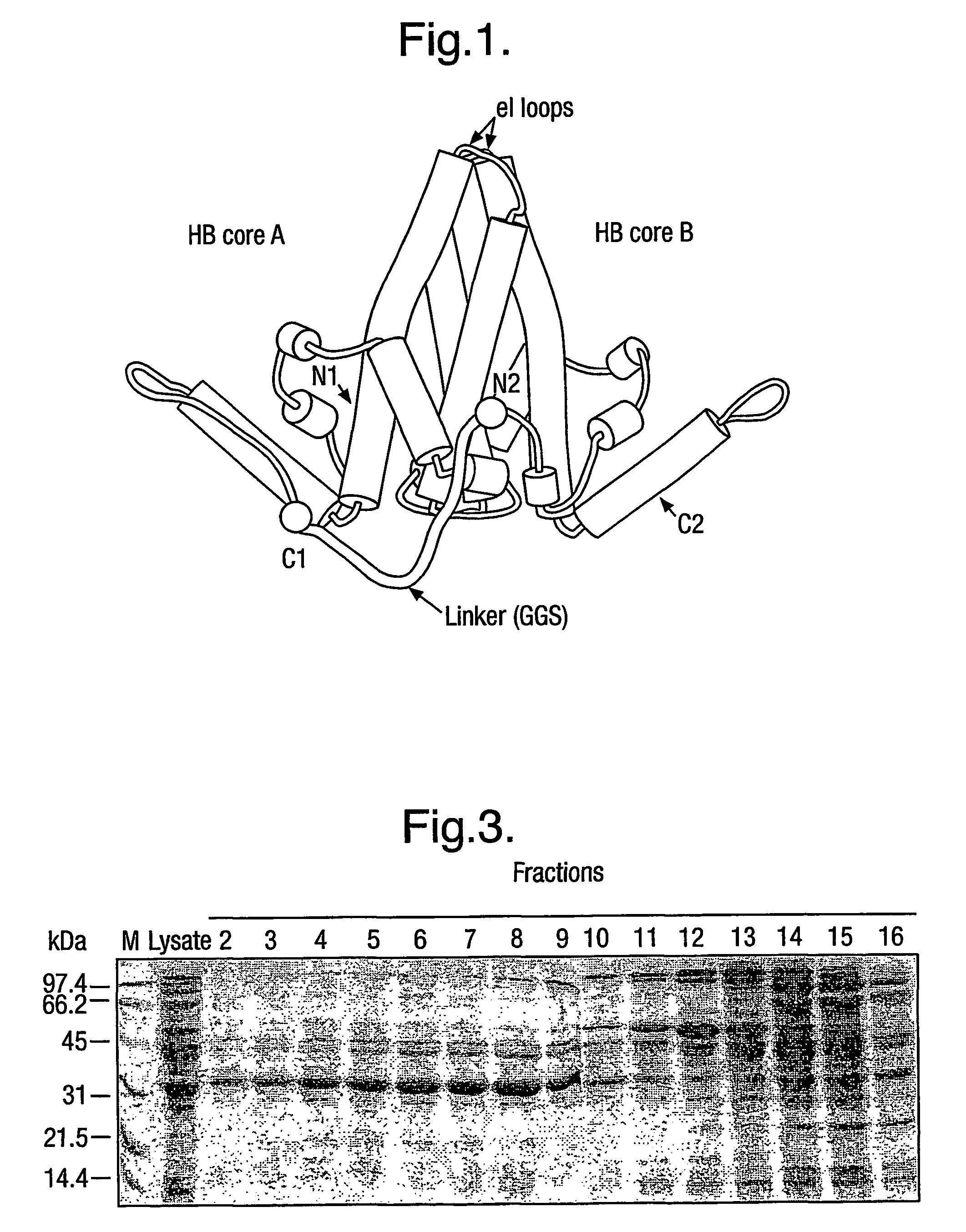
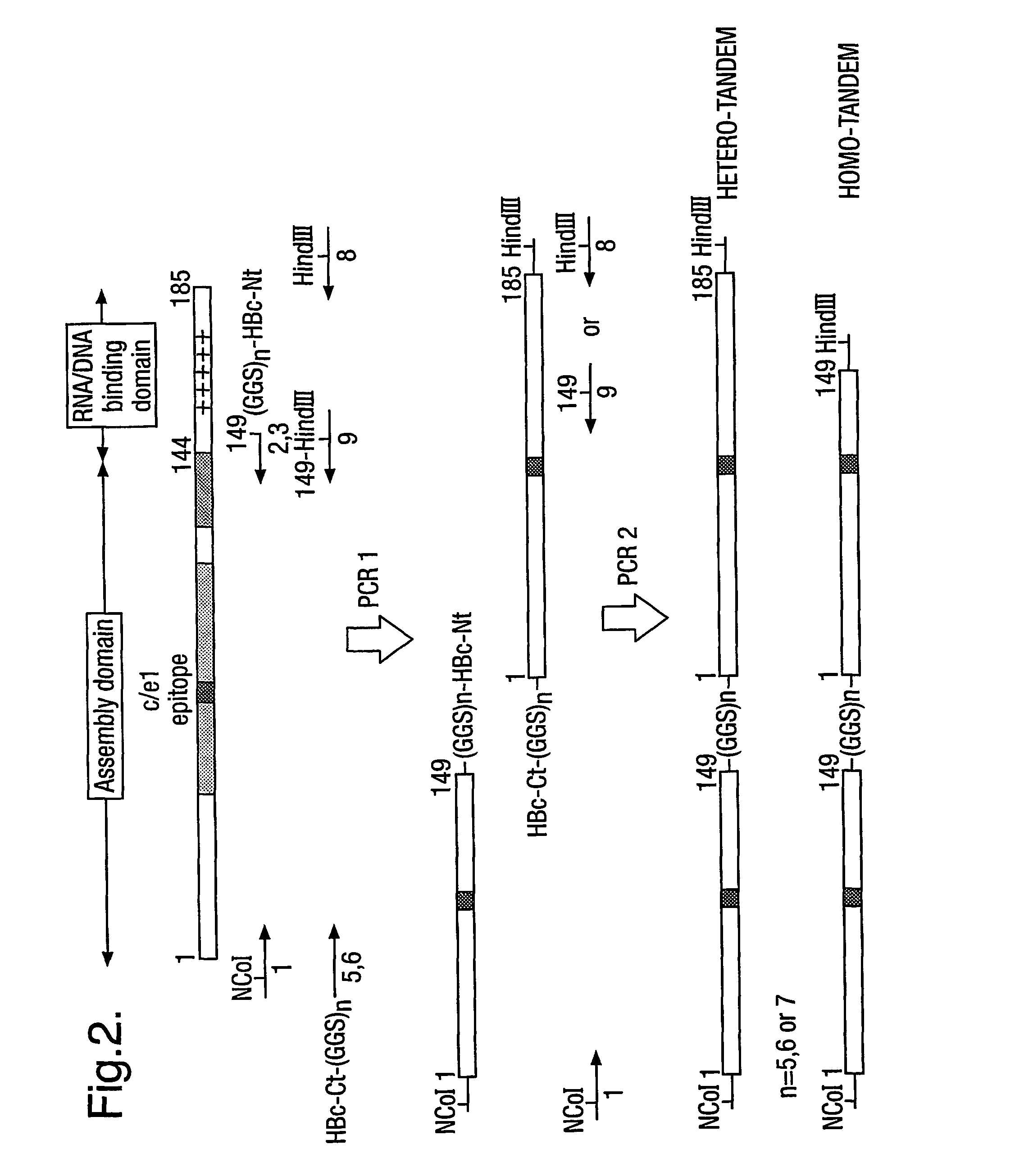
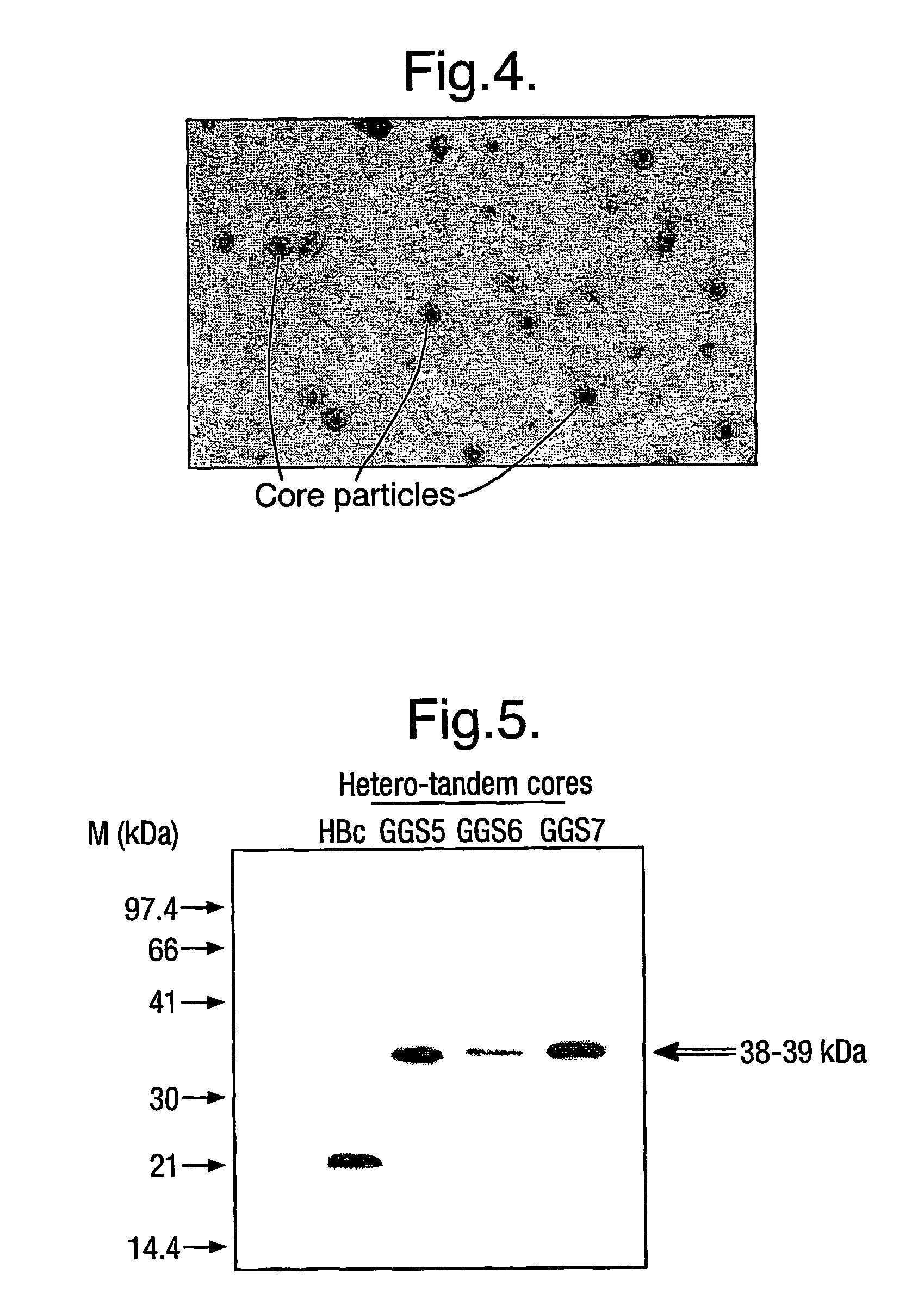
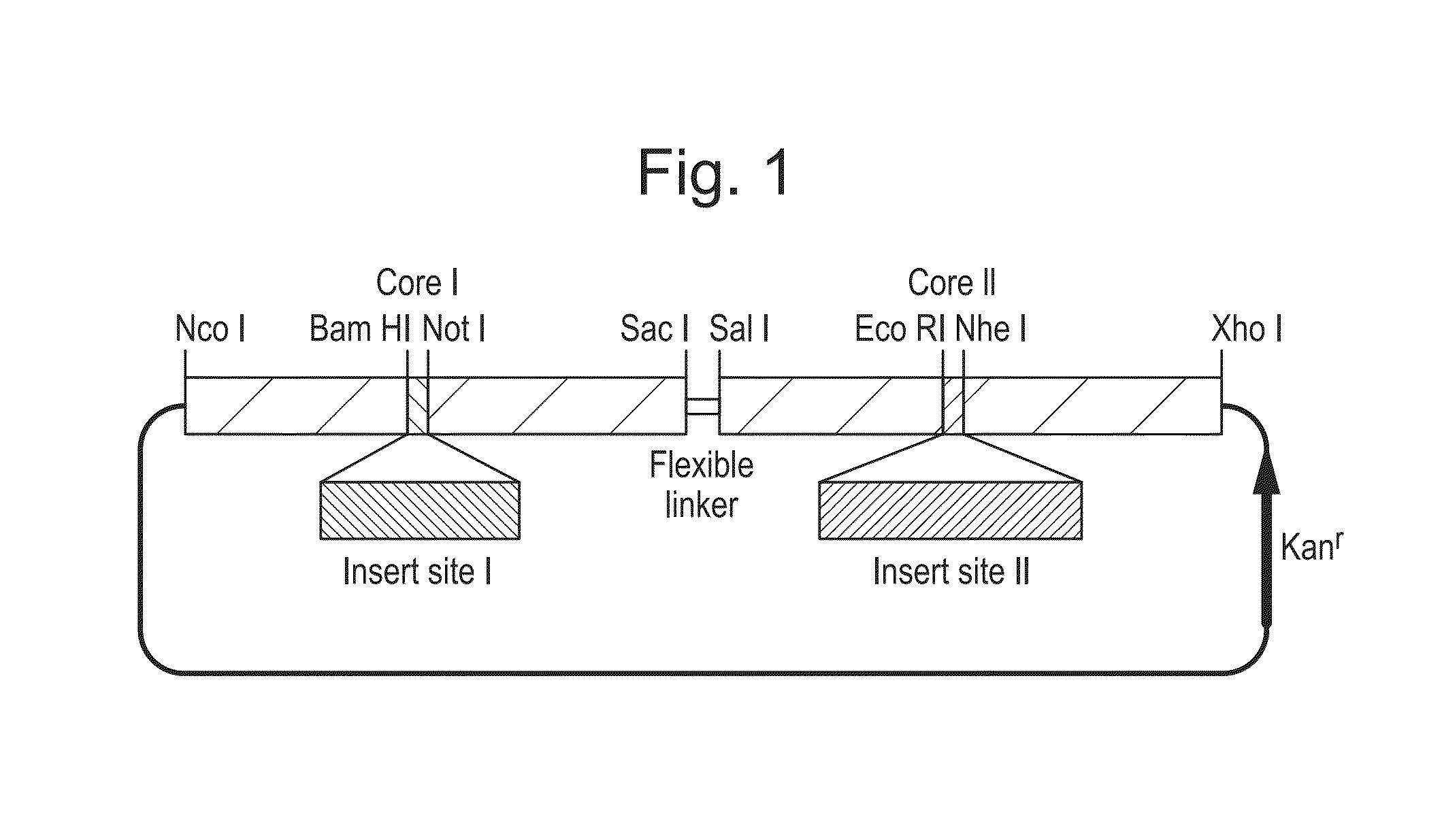
The hepatitis B virus (HBV) capsid is made up of a single species of protein called the core antigen (HBcAg) which self-assembles into particles. The particles are highly immunogenic and are able to present heterologous epitopes to the immune system when the epitopes are inserted into a surface-exposed region of the particles called the “e1 loop”. The structural building blocks of the particles are tightly associated dimers of HBcAg in which the adjacent e1 loops are closely juxtaposed. It is proposed that sequences inserted into the e1 loop are conformationally restrained in the assembled particles when presented in monomeric core protein. The invention seeks to solve this problem by covalently linking core proteins as tandem copies (e.g., as dimers) so that insertions can be made independently in each copy. This is particularly useful for insertion of large sequences into the e1 loop because it allows such sequences to be inserted into just one copy of the core protein per tandem repeat, thereby reducing potential conformational clashes in assembly. Alternatively, a different sequence may be inserted into each e1 loop of a tandem repeat, thus increasing the flexibility of HBcAg particles as an epitope delivery system.
Owner:UNIV OF LEEDS INNOVATIONS
Influenza vaccine
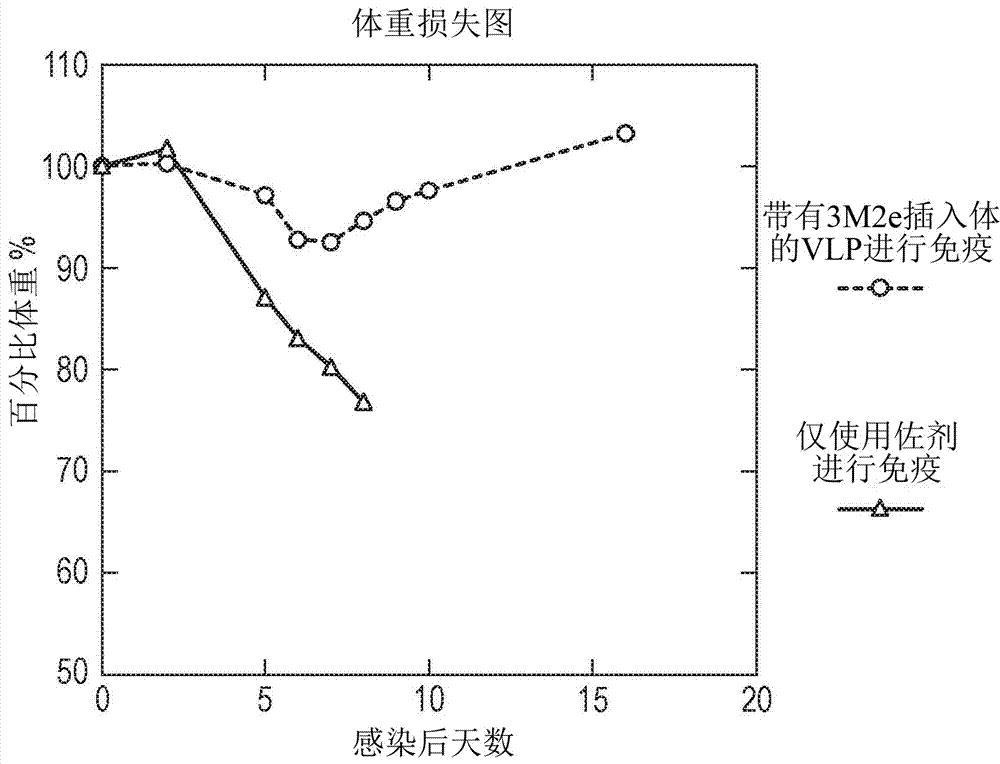
InactiveUS20130171182A1Reduce weight lossImprove survivalSsRNA viruses negative-senseBacteriaNGAL ProteinInfluenza vaccine
Owner:IQUR
Application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments
The invention discloses an application of lindley eupatorium herb in preparing anti-hepatitis B virus medicaments. Lindley eupatorium herb ethanol extract, lindley eupatorium herb flavone part, lindley eupatorium herb hemiterpene part, lindley eupatorium herb 70% ethanol water elution part and chain diterpene are prepared from lindley eupatorium herb and are subjected to hepatitis B virus (HBV) testing, and testing results indicate that the four extracts and the chain diterpene have the effect of remarkably inhibiting hepatitis B surface antigen (HBsAg) and hepatitis B core antigen (HBeAg), and have better drug activities than those of clinical medicament lamivudine (3TC) for treating hepatitis B. The lindley eupatorium herb can be used for preparing medicaments for resisting hepatitis B virus.
Owner:SUZHOU UNIV
Application of diterpene compounds in rabdosia japonica to preparation of anti-hepatitis virus medicines
InactiveCN101548965AOrganic active ingredientsDigestive systemHepatitis B Surface AntigensGlaucocalyxin B
The invention relates to the technical field of medicine and the application of diterpene compounds, including glaucocalyxin B, glaucocalyxin C, glaucocalyxin D, glaucocalyxin E and glaucocalyxin F in rabdosia japonica, to preparation of anti-hepatitis virus medicines. The invention performs anti-hepatitis B surface antigen and hepatitis B core antigen experiments of diterpene compounds including glaucocalyxin B, glaucocalyxin C, glaucocalyxin D, glaucocalyxin E and glaucocalyxin F by a hepatitis B surface antigen (HBsAg) diagnostic kit and a hepatitis B core antigen (HBeAg) diagnostic kit. The result shows that these compounds have obvious anti-hepatitis B virus activity, thus, these compound can be used for preparation of anti-hepatitis B virus medicines.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
EGFR and HER2 combined polypeptide epitope vaccine
InactiveCN102357246AHigh antibody titerLow antibody titerAntibody medical ingredientsHybrid peptidesB-Cell EpitopesEpitope vaccine
The invention discloses an EGFR and HER2 combined polypeptide epitope vaccine; two B cell epitope mimic polypeptide I and II of HER2 of the HER family, and a B cell epitope mimic polypeptide of EGFR of the HER family are inserted in a serial form between the 78th amino acid and the 79th amino acid of a hepatitis B core antigen HBcAg so as to form fusion protein which is the combined polypeptide epitope vaccine. The EGFR and HER2 combined polypeptide epitope vaccine of the invention breaks through the limitations of existing single epitope vaccines, and the combined epitope vaccine is providedwith more extensive antineoplastic activity.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
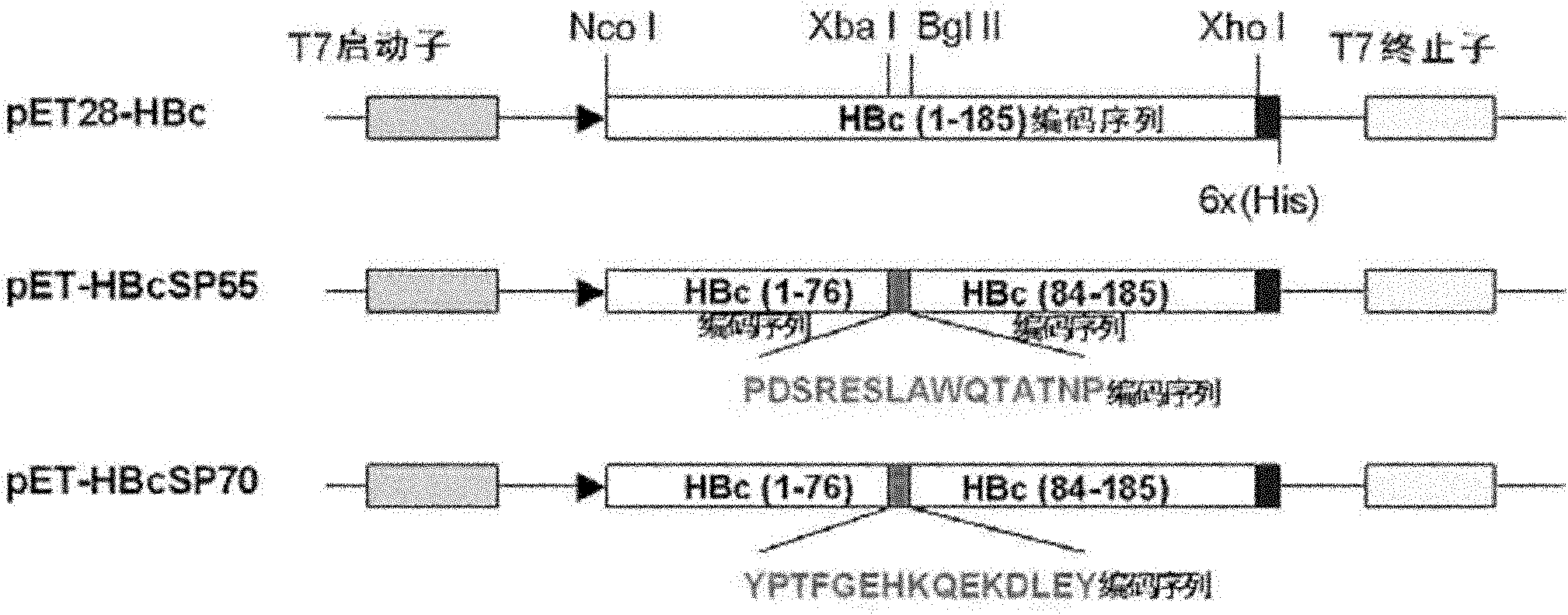
ActiveCN102199217AHighly inhibitoryEfficient removalDigestive systemAntiviralsEscherichia coliFusion Protein Expression
The invention relates to a hepatitis B virus multi-epitope fusion protein and a preparation method and application thereof. The fusion protein is obtained by inserting hepatitis B virus multi-epitope fusion peptide (with the sequence shown as SEQIDNo.1) formed by serially connecting HBsAg313-321, HBsAg335-343, Pol150-159, Pol455-463 and Padre epitopes through connecting peptide between amino acidat the 78th position and amino acid at the 79th position of a hepatitis B virus core protein; and the preparation method comprises the following steps of: constructing a hepatitis B virus multi-epitope fusion protein expression plasmid pET28-HBc-HP; performing isopropyl thiogalactoside (IPTG) inducing expression by using an Escherichia coli expression system; and purifying by using affinity chromatography. The fusion protein carries a plurality of supertype epitopes of hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) and polymerase, is viral particles, has the advantages of strong immunogenicity, wide applicable range and the like, and can be used for preparing therapeutic hepatitis B vaccines.
Owner:ARMY MEDICAL UNIV
Recombination broad-spectrum vaccine specific to Human enterovirus 71
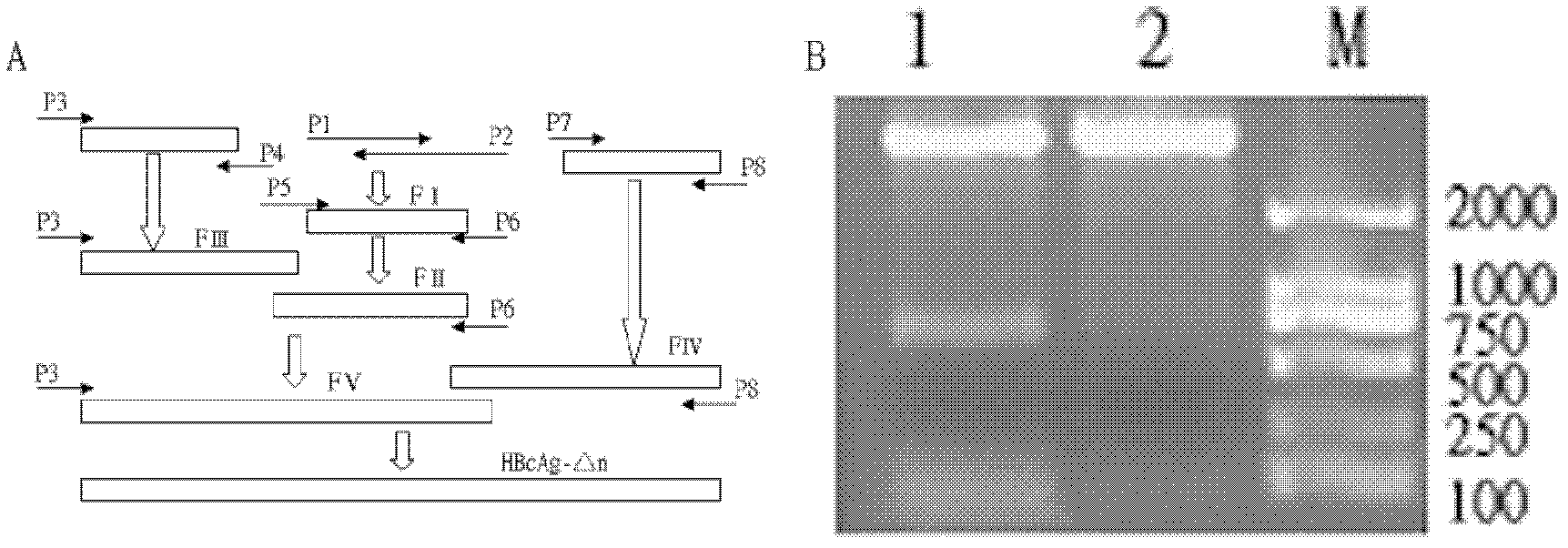
The invention relates to a recombination broad-spectrum vaccine specific to Human enterovirus 71. The invention constructs a fusion protein which comprises at least one copied peptide SP55 and / or SP70 of Human enterovirus 71 VP1 protein and a hepatitis B virus core antigen. The fusion protein can renature and assemble by self in a solubility manner after being expressed, denatured and purified, thereby forming virus-like particles with strong immunogenicity; and the virus-like particles can induce the generation of a broad-spectrum neutralizing antibody with high valence in vivo, thereby being capable of serving as the vaccine for preventing diseases related to Human enterovirus 71 infection. According to the invention, suitable carriers for forming the virus-like particles are found for antigens deriving from the enterovirus 71 VP1 protein, thereby the formed virus-like particles properly expose the antigens and increase the immunogenicity of the antigens.
Owner:INST PASTEUR OF SHANGHAI CHINESE ACADEMY OF SCI
Application of isoflavonoids compounds to preparation of antihepatitis drug
InactiveCN102247395ASignificant anti-HBV effectAntiviralsFood preparationHepatitis b e antigenIridoid Glucosides
The invention relates to an application of isoflavonoids compounds, i.e., tectoridin, iridin, irisflorentin, tectorigenin or irigenin, separated from the belamcanda chinensis of an iridaceae belamcanda plant to preparation of drugs or health-care foods for preventing and treating hepatitis, relating to the technical field of natural pharmaceutical chemistry and medicines. In the invention, HBsAg (Hepatitis B Surface Antigen) and HBeAg(Hepatitis B e Antigen) resistance experiments are carried out by adopting detection reagents of an HBsAg diagnostic reagent kit and an HBeAg diagnostic reagent kit; and an experimental result discovers that all the tectoridin, the iridin, the irisflorentin, the tectorigenin and the irigenin have an obvious hepatitis B virus resistant action, thus the tectoridin, the iridin, the irisflorentin, the tectorigenin and the irigenin can be used for preparing the drugs or health-care foods for preventing and treating hepatitis viruses.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Antibody display
InactiveUS20160122420A1High sensitivityEasy to measureVirusesBacteriaSingle-domain antibodyHepatitis B Core Antigens
Owner:ROWLANDS DAVID J +2
HBV-DNA kit for quantitative enzyme-linked measurment of heaptitis B core antigen
InactiveCN101046477ALower requirementSimple and fast operationMaterial analysisPolyclonal antibodiesDNA
The present invention discloses HBV-DNA kit for quantitative enzyme-linked immunological measurement of hepatitis B core antigen and its preparation process, and belongs to the field of molecular biology and immunological diagnosis reagent. The kit includes: fluorescent quantitative HBV-DNA serum standard, HBsAb polyclonal antibody pre-coated standard enzyme reaction strip, HBcAb polyclonal antibody pre-coated standard enzyme reaction strip, diductor, enzyme labeling core antibody, substrate developer, positive contrast, negative contrast and terminating liquid. The present invention can measure HBV-DNA content in serum specifically and accurately, and has the advantages of simple operation, high sensitivity, high specificity, high stability, etc.
Owner:BEIJING BIOKIT
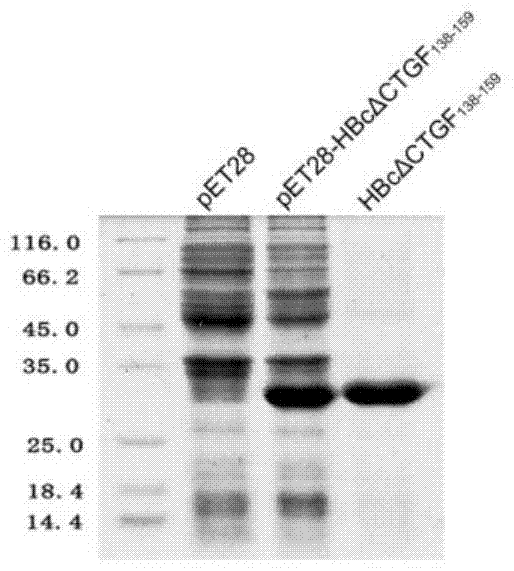
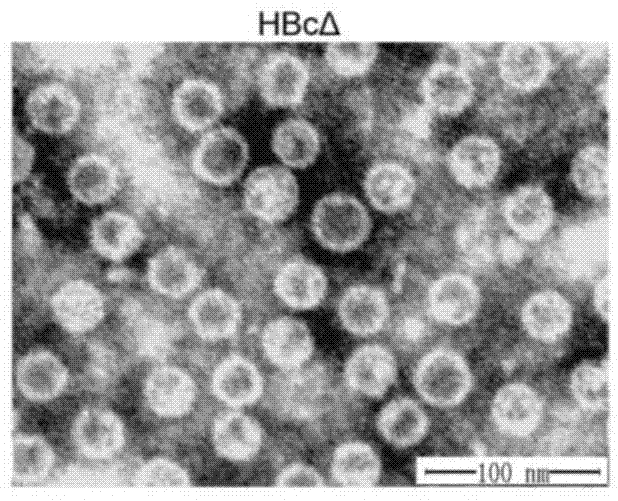
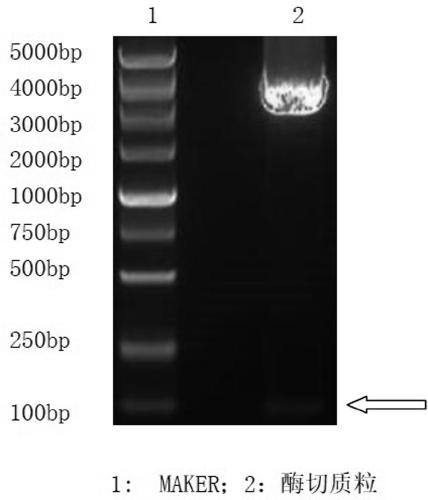
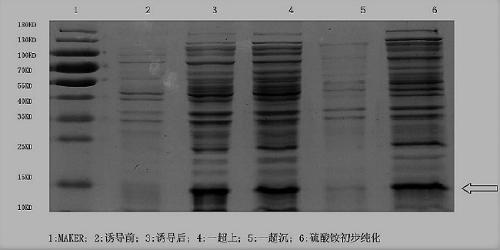
Connective tissue growth factor chimeric vaccine for treating liver fibrosis and application of connective tissue growth factor chimeric vaccine
ActiveCN104740628ADegree of inhibitory activationReduce processDigestive systemAntibody medical ingredientsHepatitis B virus core AntigenCTGF
The invention discloses a CTGF (connective tissue growth factor) chimeric vaccine for treating liver fibrosis. The antigenic epitope of the CTGF is inserted into c / e1B cell epitope of hepatitis b virus core antigen to form the CTGF chimeric vaccine which can be assembled into hepatitis B core sample particles. Under the non-adjuvant assistance, the chimeric vaccine can stimulate a body to generate high-valence anti-CTGF neutral antibody. A mouse which is immunized by the chimeric vaccine is obviously relieved in fibrosis degree generated after carbon tetrachloride induction. According to the experimental verification, the CTGF chimeric vaccine can be used for obviously restraining the activation intensity of hepatic stellate cells in the mouse liver, and also can be used for stimulating liver cells to multiply and restraining liver apoptosis. Besides, the TGF-beta 1 and PDGF level in the immunized mouse is obviously reduced, so that the process of liver fibrosis can be relieved in a facilitated manner. According to the results, the CTGF chimeric vaccine can be used for successfully restraining the carbon-tetrachloride-induced mouse liver fibrosis. Therefore, the CTGF chimeric vaccine is expected to be developed into effective means for treating the liver fibrosis.
Owner:THE FIRST AFFILIATED HOSPITAL OF MEDICAL COLLEGE OF XIAN JIAOTONG UNIV
Subunit vaccine based on prokaryotic expression of norovirus antigenic epitope and preparation method of subunit vaccine
ActiveCN109589406AStrong specificityHigh sensitivitySsRNA viruses positive-senseViral antigen ingredientsOrganismSpecific antibody
The invention relates to the field of biomedicine, in particular to a preparation method of a subunit vaccine based on prokaryotic expression of a norovirus antigenic epitope. The invention provides the prokaryotic expressed subunit vaccine which is used for stimulating a body to produce a specific antibody against norovirus, and is characterized in that the norovirus recombinant antigen sequenceis as shown in SEQ ID NO: 1, source: NCBI Reference Sequence: NC_029646.1, a neutralizing epitope of human norovirus GII type: 105 bp, and an expression vector: a modified hepatitis B core antigen (HBcAg) gene cloned into a pThioHisA expression vector.
Owner:INST OF MEDICAL BIOLOGY CHINESE ACAD OF MEDICAL SCI
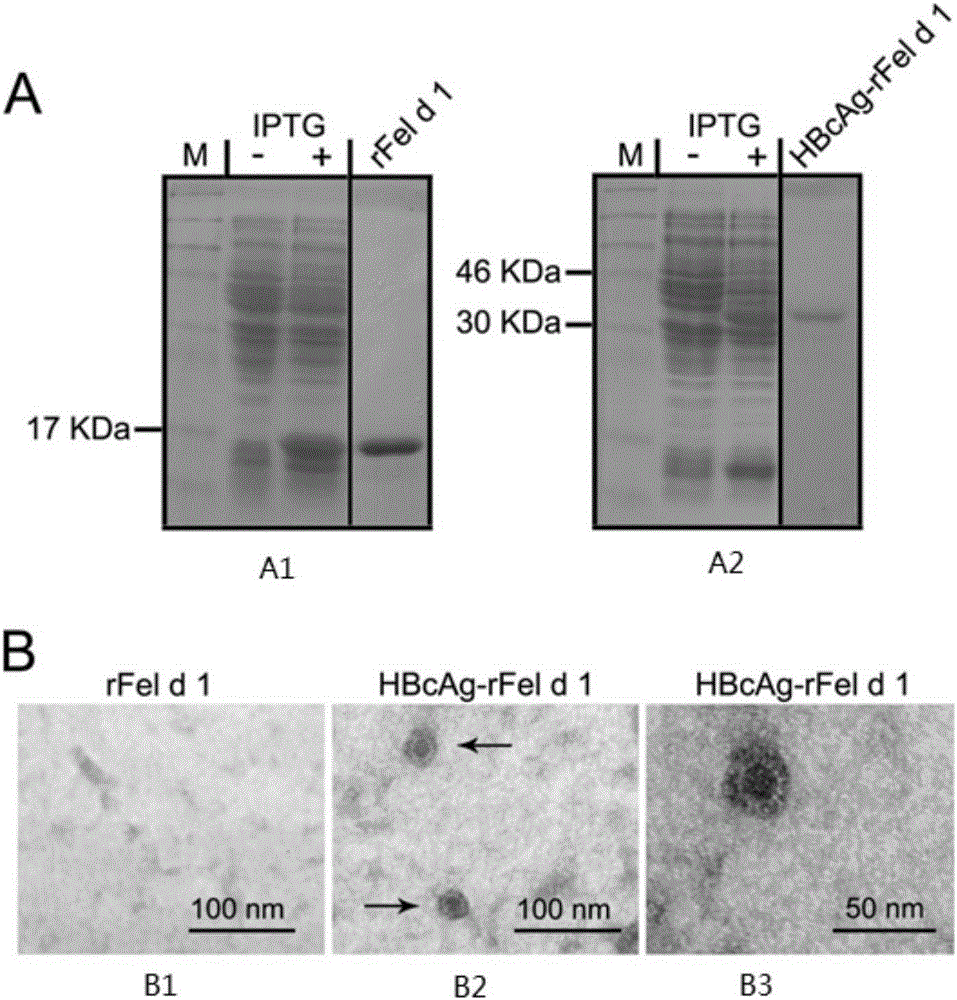
Immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, expression vector of immunity-enhanced type virus-like particle and preparation and application of mmunity-enhanced type virus-like particle
InactiveCN105219742AImproving immunogenicityHigh detection sensitivityInactivation/attenuationAllergen ingredientsHepatitis B virus core AntigenADAMTS Proteins
The invention provides an immunity-enhanced type virus-like particle for presenting recombinant cat sensitinogen rFel d 1 protein, an expression vector of the immunity-enhanced type virus-like particle and preparation and application of the immunity-enhanced type virus-like particle. The protein subunit forming the virus-like particle is fused protein of hepatitis B core antigen and polypeptides with the amino acid sequence of SEQ ID NO:1. The amino acid sequence of the fused protein is formed by inserting the sequence SEQ ID NO:1 of polypeptides between the 78th to 81st amino acid of hepatitis B core antigen and replacing the 79th to 80th amino acid of hepatitis B core antigen. By means of HBcAg-fFel d 1 fused protein, the immunogenicity of fFel d 1 can be remarkably improved, and therefore the detection sensitivity of fFel d 1 recombined and expressed in vitro in application of in-vitro diagnosis cat allergies is improved.
Owner:HAINAN UNIVERSITY
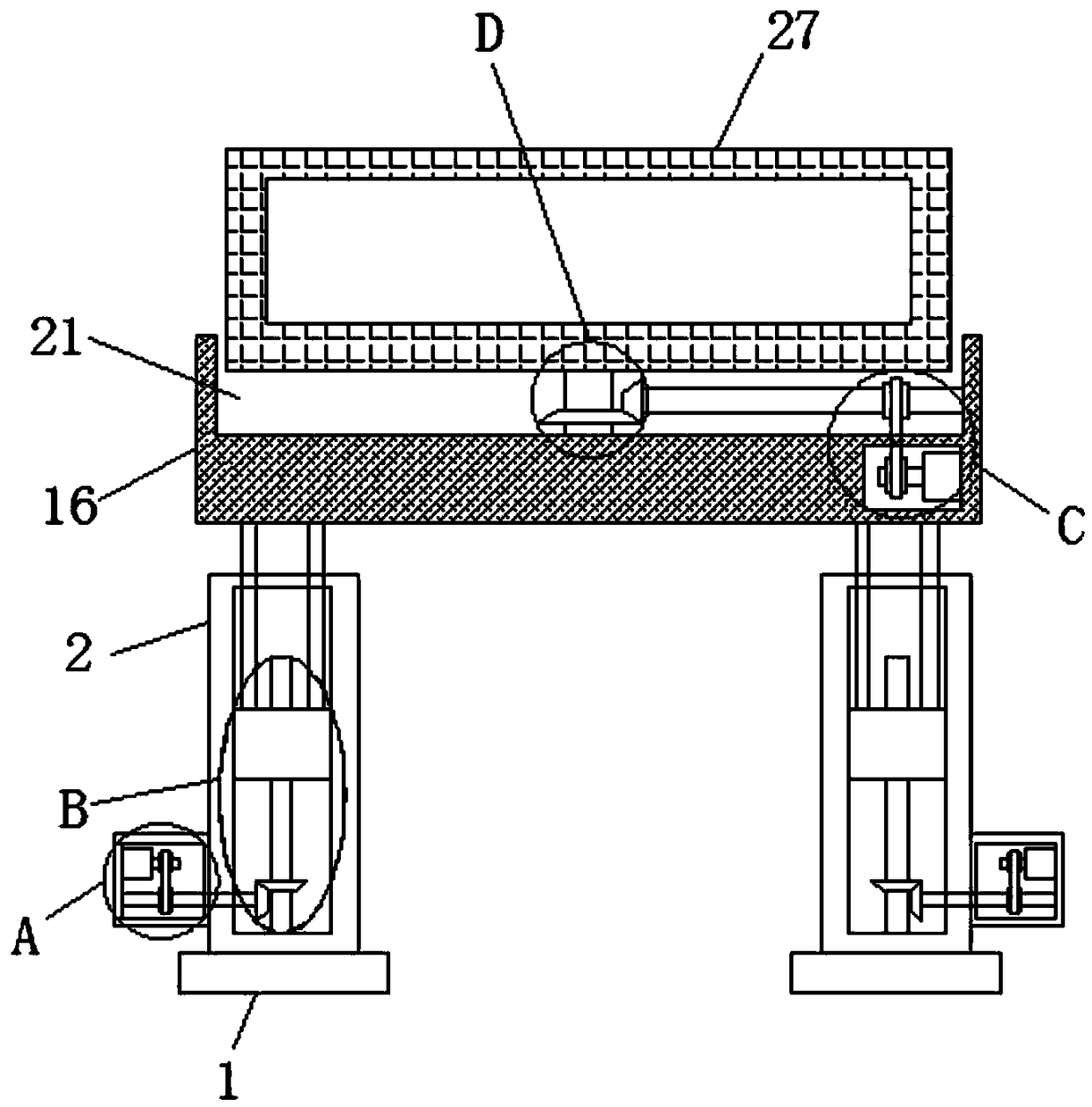
Simple device for directly detecting hepatitis B core antigen
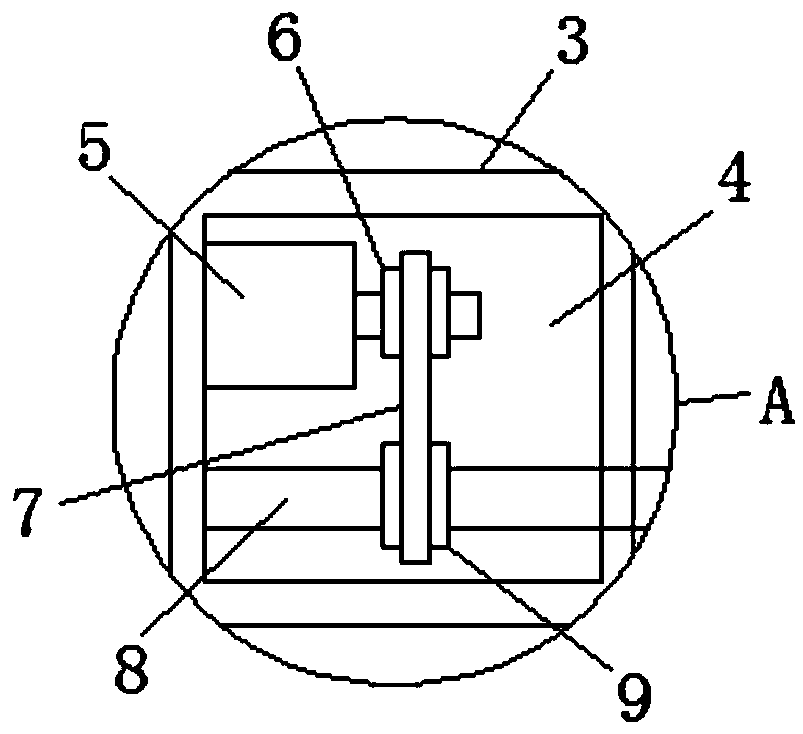
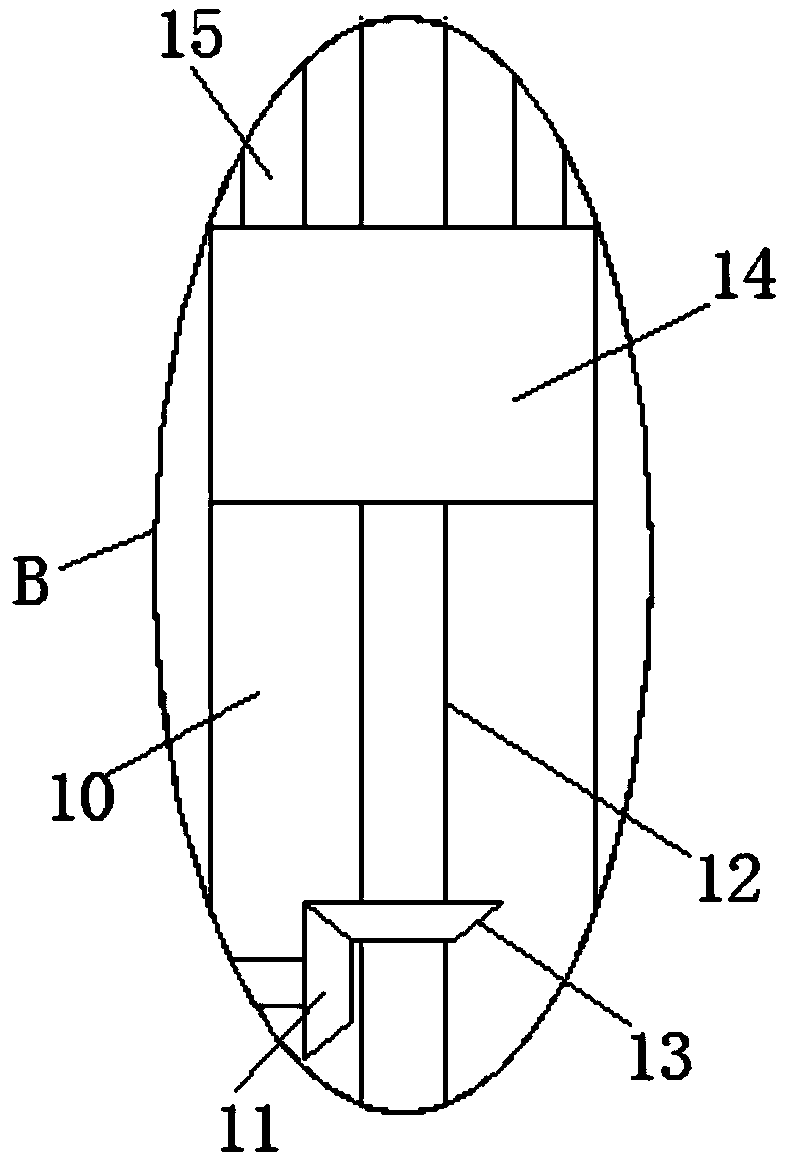
InactiveCN109373123AEasy to liftEasy to adjust the angleStands/trestlesBiological testingSprocketEngineering
The invention discloses a simple device for directly detecting a hepatitis B core antigen. The simple device comprises two bases, installing bases are welded to the tops of the two bases, and fixing bases are welded to the sides, far away from each other, of the two installing bases; the two fixing bases are each provided with a rotating cavity, and first motors are fixedly installed on the innerwalls of the sides, far away from each other, of the two rotating cavities; first chain wheels are welded to output shafts of the two first motors, and the two first chain wheels each mesh with a first chain; rotating rods are rotatably installed on the inner walls of the sides, far away from each other, of the two rotating cavities, second chain wheels are welded to the two rotating rods separately, and the two second chain wheels are in transmission connection with the two first chain wheels through the two first chains; and the two installing bases are each provided with a sliding cavity, and the ends, close to each other, of the two rotating rods extend into the two sliding cavities separately. According to the simple device, simple device body lifting and angle adjusting are convenient, the structure is simple, and use is convenient.
Owner:秦小清
Method for preparing chimeric monoclonal antibody capable of neutralizing EV71 (enterovirus 71)
The invention relates to a method for preparing a chimeric monoclonal antibody capable of neutralizing EV71 (enterovirus 71). The chimeric monoclonal antibody for neutralizing EV71 or a fragment of the chimeric monoclonal antibody is characterized in that a heavy chain variable region of the chimeric monoclonal antibody or the fragment of the chimeric monoclonal antibody has an amino acid sequence shown in SEQ ID NO:1; a light chain variable region has an amino acid sequence shown in SEQ ID NO:2. EV71 VP4 (viral protein 4) protein is fused into an HBcAg (hepatitis B core antigen) virus-like particle to be prepared into fusion protein, and the fusion protein is taken as an antigen to endow a Balb / c mouse with immunity. A phage display antibody library technique is used for screening to obtain the monoclonal antibody capable of specifically neutralizing EV71 VP4 protein. The invention provides the method for preparing a potential monoclonal antibody medicine with broad spectrum neutralization activity and for preventing and treating a hand foot mouth disease.
Owner:BEIJING UNIV OF TECH
Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
ActiveCN102199217BEfficient removalImproving immunogenicityDigestive systemAntiviralsEscherichia coliFusion Protein Expression
The invention relates to a hepatitis B virus multi-epitope fusion protein and a preparation method and application thereof. The fusion protein is obtained by inserting hepatitis B virus multi-epitope fusion peptide (with the sequence shown as SEQIDNo.1) formed by serially connecting HBsAg313-321, HBsAg335-343, Pol150-159, Pol455-463 and Padre epitopes through connecting peptide between amino acid at the 78th position and amino acid at the 79th position of a hepatitis B virus core protein; and the preparation method comprises the following steps of: constructing a hepatitis B virus multi-epitope fusion protein expression plasmid pET28-HBc-HP; performing isopropyl thiogalactoside (IPTG) inducing expression by using an Escherichia coli expression system; and purifying by using affinity chromatography. The fusion protein carries a plurality of supertype epitopes of hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) and polymerase, is viral particles, has the advantages of strong immunogenicity, wide applicable range and the like, and can be used for preparing therapeutic hepatitis B vaccines.
Owner:ARMY MEDICAL UNIV
Expression purification method for HBcAg
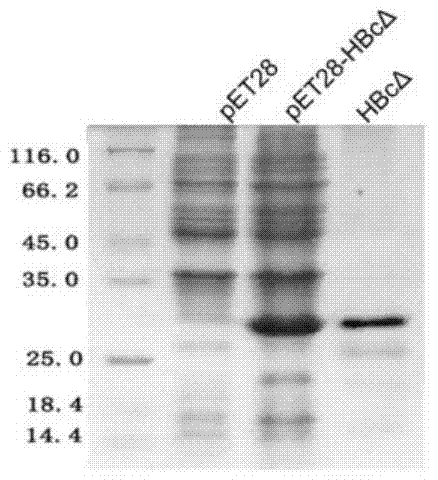
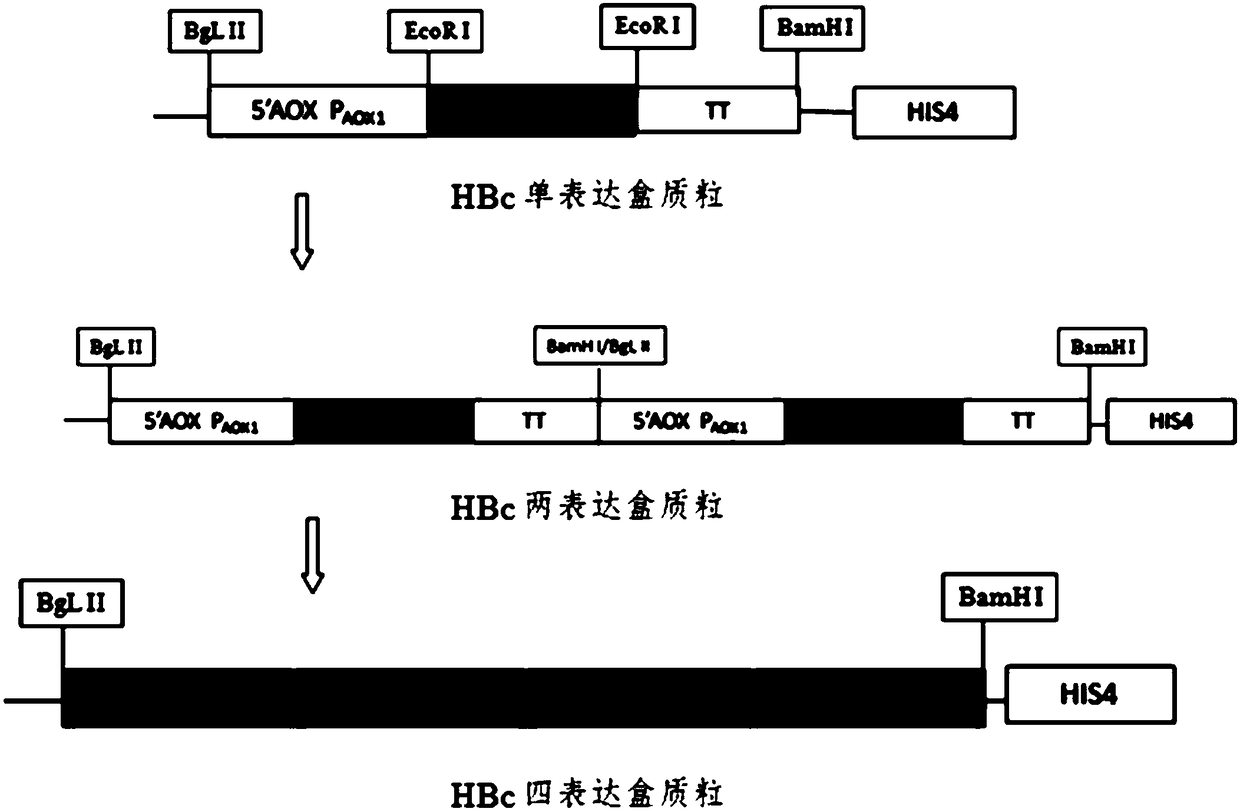
InactiveCN108659107AHigh yieldHigh purityVirus peptidesPeptide preparation methodsHepatitis B virus core AntigenPurification methods
The invention relates to the field of genetic engineering and in particular relates to an expression purification method for hepatitis B core antigen (HBcAg). The expression purification method comprises the following steps: carrying out linearization on a yeast expression vector with a nucleotide sequence for expressing HBcAg, and transinfecting competence yeast so as to obtain a transformant; carrying out amplified culture and induced expression on the transformant, crushing a cell, centrifuging, and collecting supernate; carrying out fractional precipitation and anion exchange chromatographyon the supernate with ammonium sulfate, and carrying out ultrafiltration dialysis, thereby obtaining a purified HbcAg protein. The HBcAg obtained by using the method is high in yield, high in purity,simple in step and high in activity, and a high-specificity diagnosis raw material can be provided for an in-vitro diagnosis reagent.
Owner:GETEIN BIOTECH
Vaccines based on hepatitis b core antigens
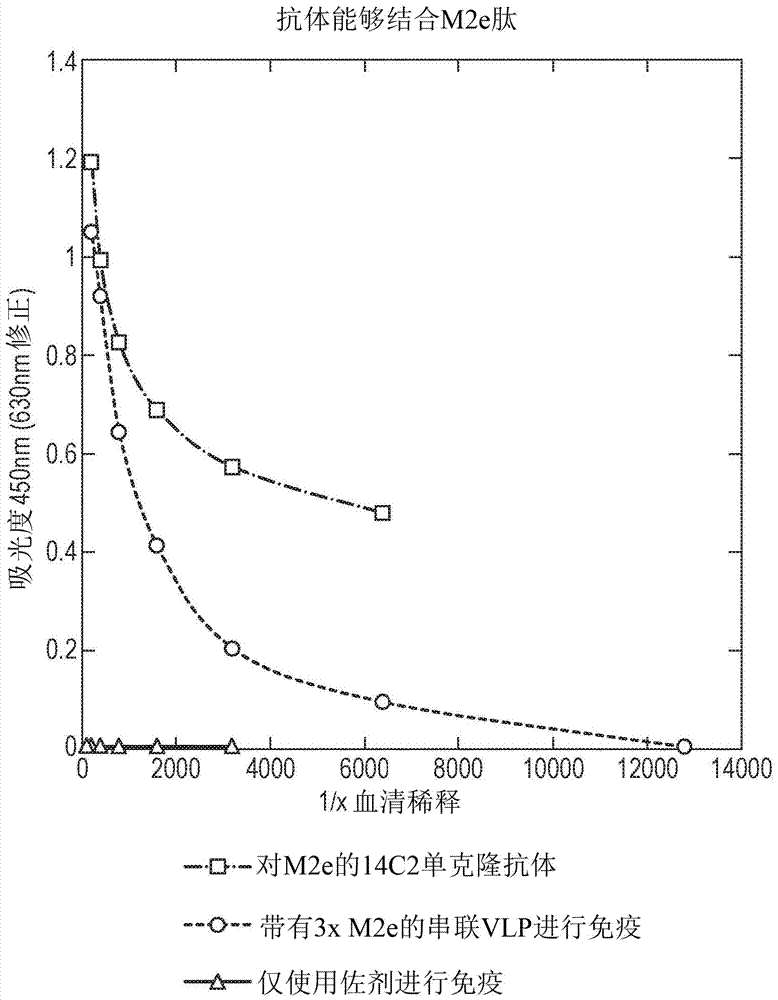
InactiveCN107207620ASsRNA viruses negative-senseAntibody mimetics/scaffoldsHemagglutininInfluenza virus A hemagglutinin
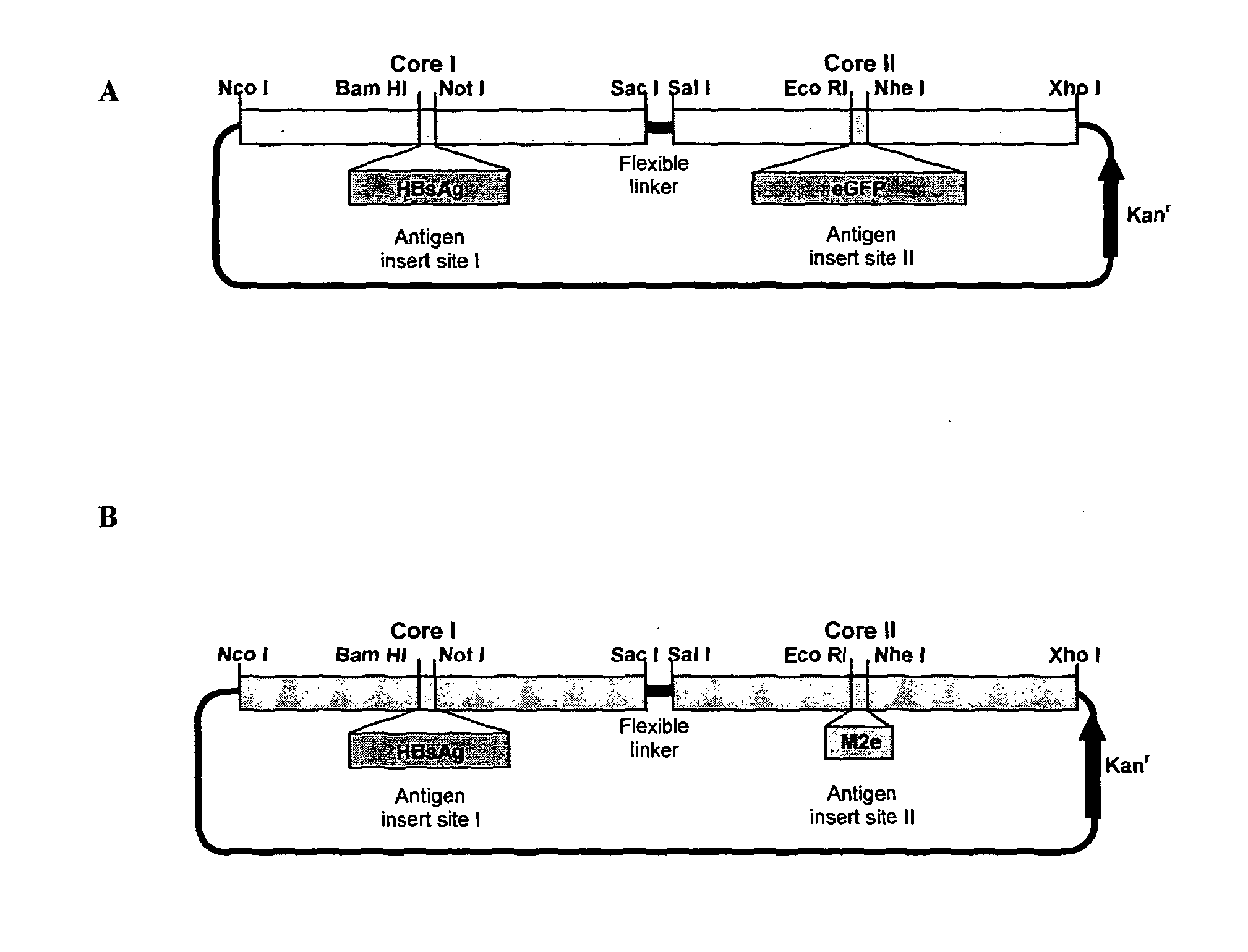
The invention provides a protein comprising hepatitis B core antigen (HBcAg) and influenza virus A surface polypeptide M2 or an immunogenic fragment thereof. The invention also provides a protein comprising hepatitis B core antigen (HBcAg) and influenza virus hemagglutinin (HA) or an immunogenic fragment thereof. The invention also provides particles formed from the proteins, nucleic acid molecules encoding the proteins, processes for producing the proteins, pharmaceutical compositions containing the proteins and use of the proteins to induce an immune response in a subject.
Owner:IQUR
Vaccines based on hepatitis b core antigens
The invention provides a protein comprising hepatitis B core antigen (HBcAg) with a sugar attached to an e1 loop. The protein may comprise a first and a second copy of HBcAg in tandem, wherein one or both copies of HBcAg has a sugar attached to the e1 loop. The first copy may have a sugar attached to the e1 loop and the second copy may comprise a peptide epitope in the e1 loop. The protein may be used to induce an immune response against the sugar and hence act as a vaccine.
Owner:IQUR
Recombinant plasmid vaccine for treating hepatitis B and composition thereof
ActiveCN102233136AImprove immunityGenetic material ingredientsDigestive systemProtein targetAdjuvant
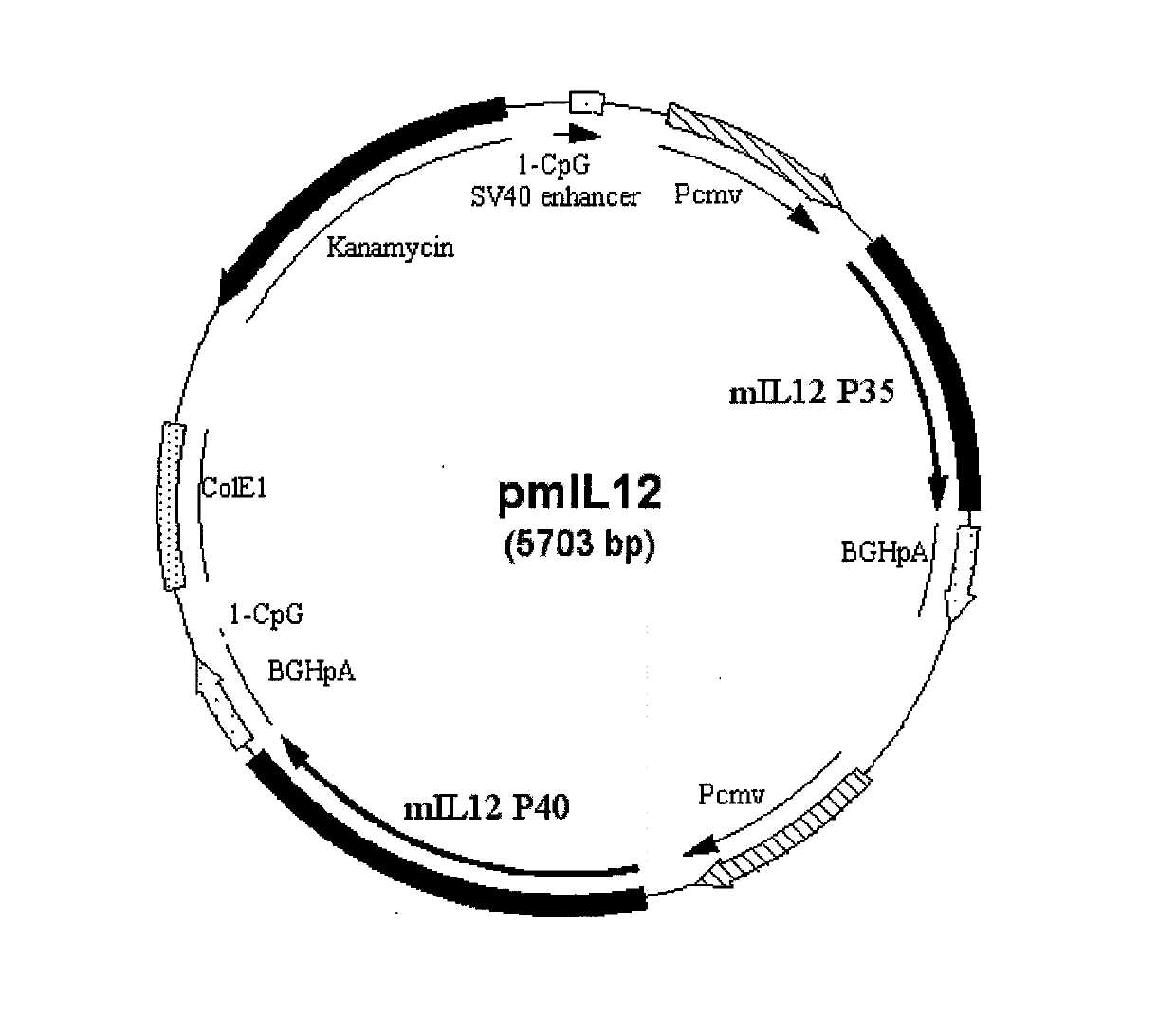
The invention relates to a recombinant plasmid DNA (deoxyribonucleic acid) vaccine which simultaneously carries a hepatitis B surface antigen protein-coding gene and a hepatitis B core antigen protein-coding gene. The constructed recombinant plasmid DNA vaccine comprises two target protein expression cassettes, wherein one target protein expression cassette is an optimized target protein expression cassette, and the two genes are separated by one target protein expression cassette. The invention also relates to a double plasmid DNA vaccine which comprises the recombinant plasmid DNA vaccine provided by the invention and a recombinant interleukin 12 adjuvant plasmid. The recombinant plasmid DNA vaccine and the composition thereof constructed by the invention can be used for preparing medicaments for preventing and treating hepatitis B.
Owner:BEIJING KAWIN TECH SHARE HLDG
Vaccines based on hepatitis B core antigens
The invention provides a protein comprising hepatitis B core antigen (HBcAg) with a sugar attached to an e1 loop. The protein may comprise a first and a second copy of HBcAg in tandem, wherein one or both copies of HBcAg has a sugar attached to the e1 loop. The first copy may have a sugar attached to the e1 loop and the second copy may comprise a peptide epitope in the e1 loop. The protein may be used to induce an immune response against the sugar and hence act as a vaccine.
Owner:IQUR
HBV-infected mouse model construction method and application
ActiveCN105943560AEasy to set upEasy to prepareCompounds screening/testingBacteria material medical ingredientsHigh pressureHepatitis B Core Antigens
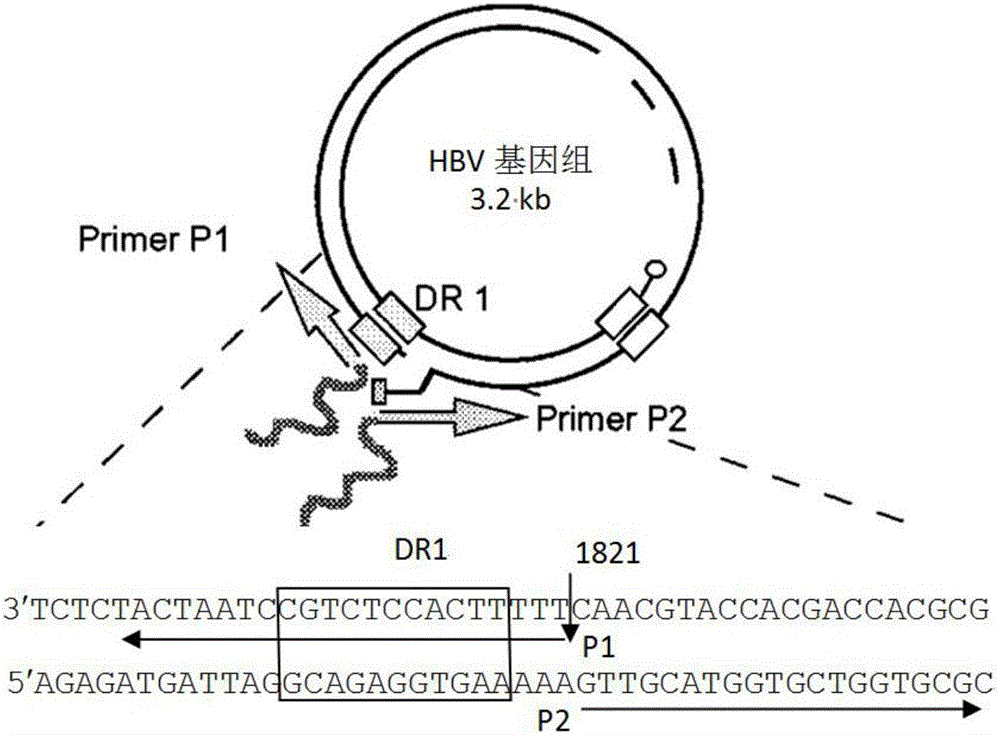
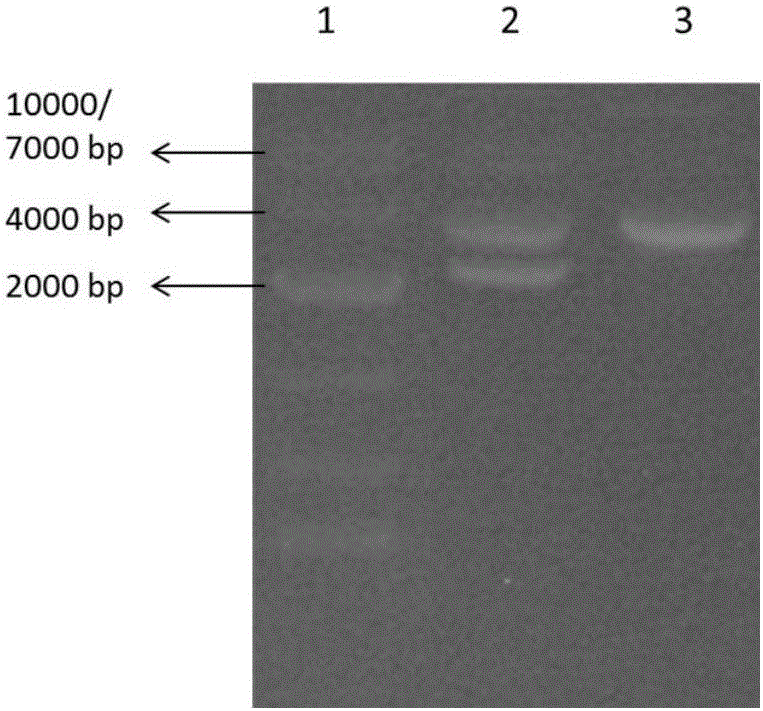
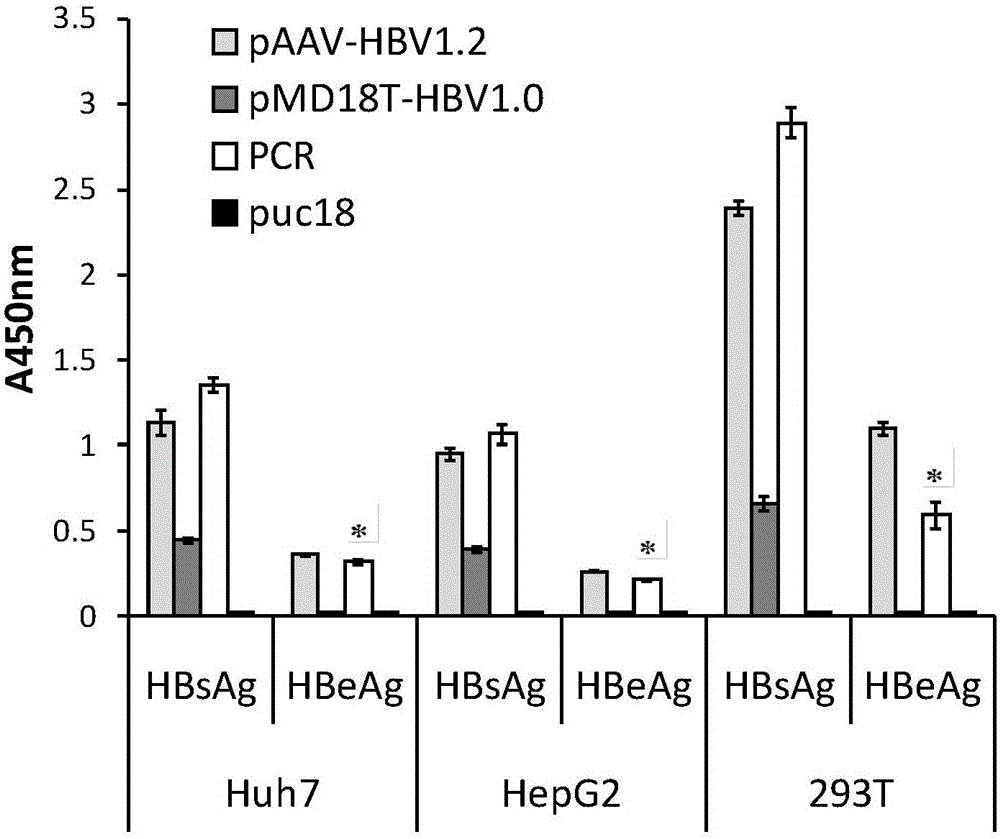
The invention relates to the technical field of medicine and provides a hepatitis B virus (HBV)-infected mouse model construction method. According to the method, a polymerase chain reaction (PCR) is adopted to amplify an HBV single-copy linear genome, a PCR product transfects Huh7 cells, HepG2 cells and 293T cells, and hepatitis b surface antigen (HBsAg) and hepatitis B e antigen (HBeAg) in supernatant of culture cells are detected through an enzyme-linked immunosorbent assay (ELISA); the PCR product is injected to a C57BL / 6J mouse through a tail vein high-pressure hydrodynamic method, so that hepatitis b core antigen (HBcAG) in mouse liver tissue is detected in an immunohistochemistry mode, and the HBsAg and the HBeAG in serum of the mouse are detected in an ELISA mode. By means of the method, a novel HBV-infected mouse model is constructed, the model is simple in preparation method, and the HBC genome similar to the HBV cccDNA exists in the liver tissue. A novel tool is provided for research on an HBV cccDNA degradation mechanism, medicine removal, HBV biological characteristics in a serum sample of a hepatitis B patient and the like.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com