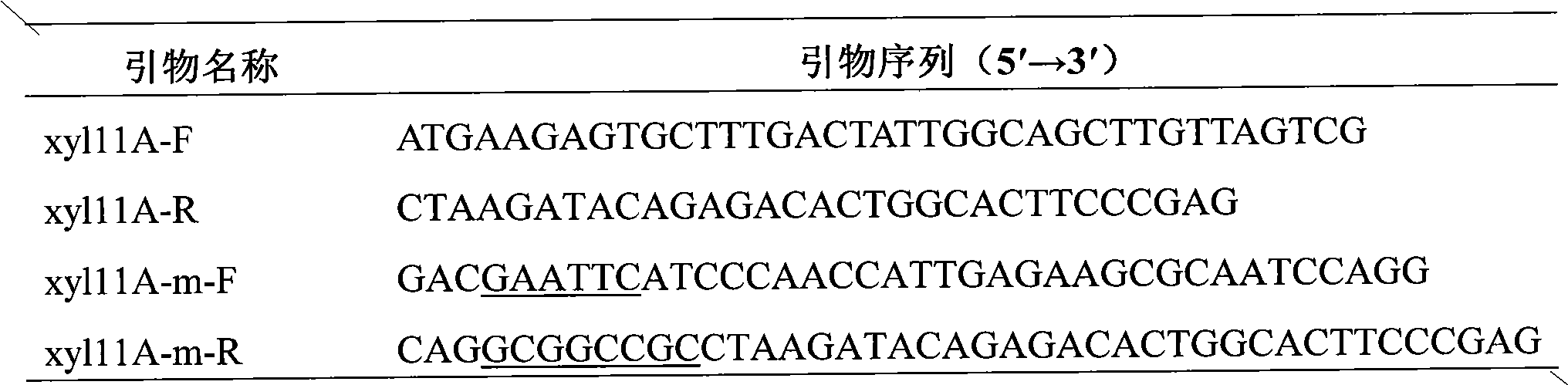
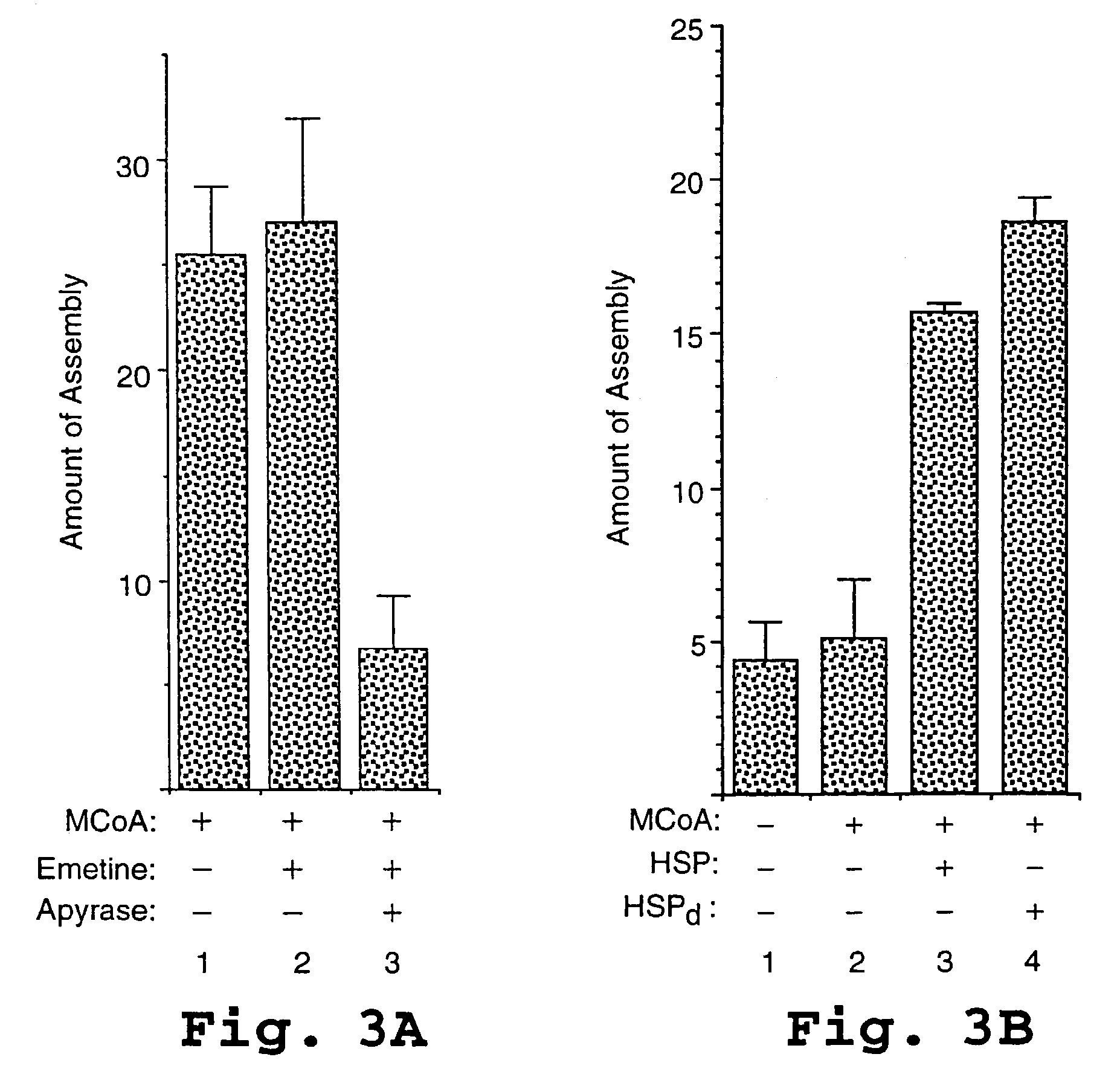
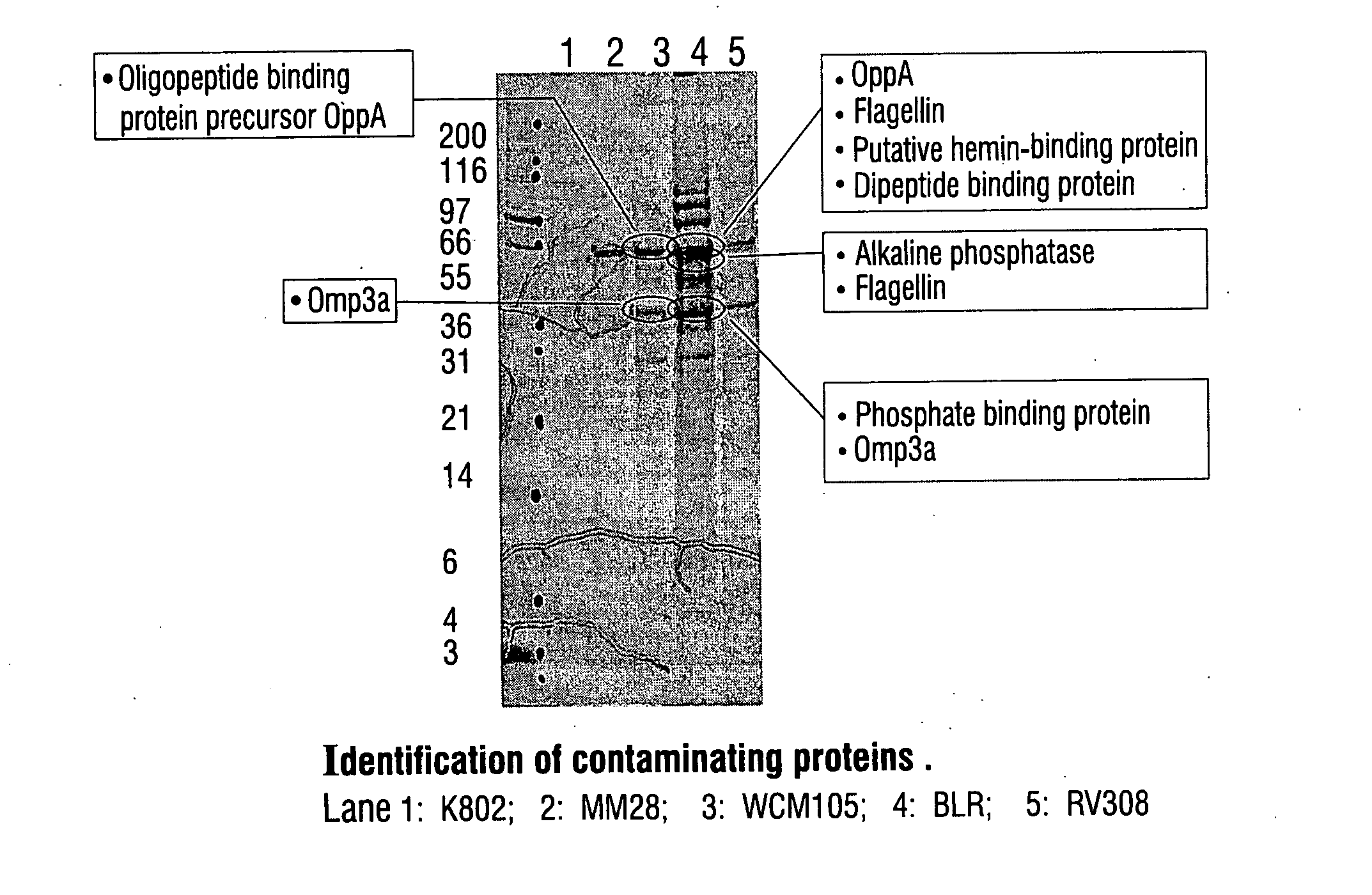
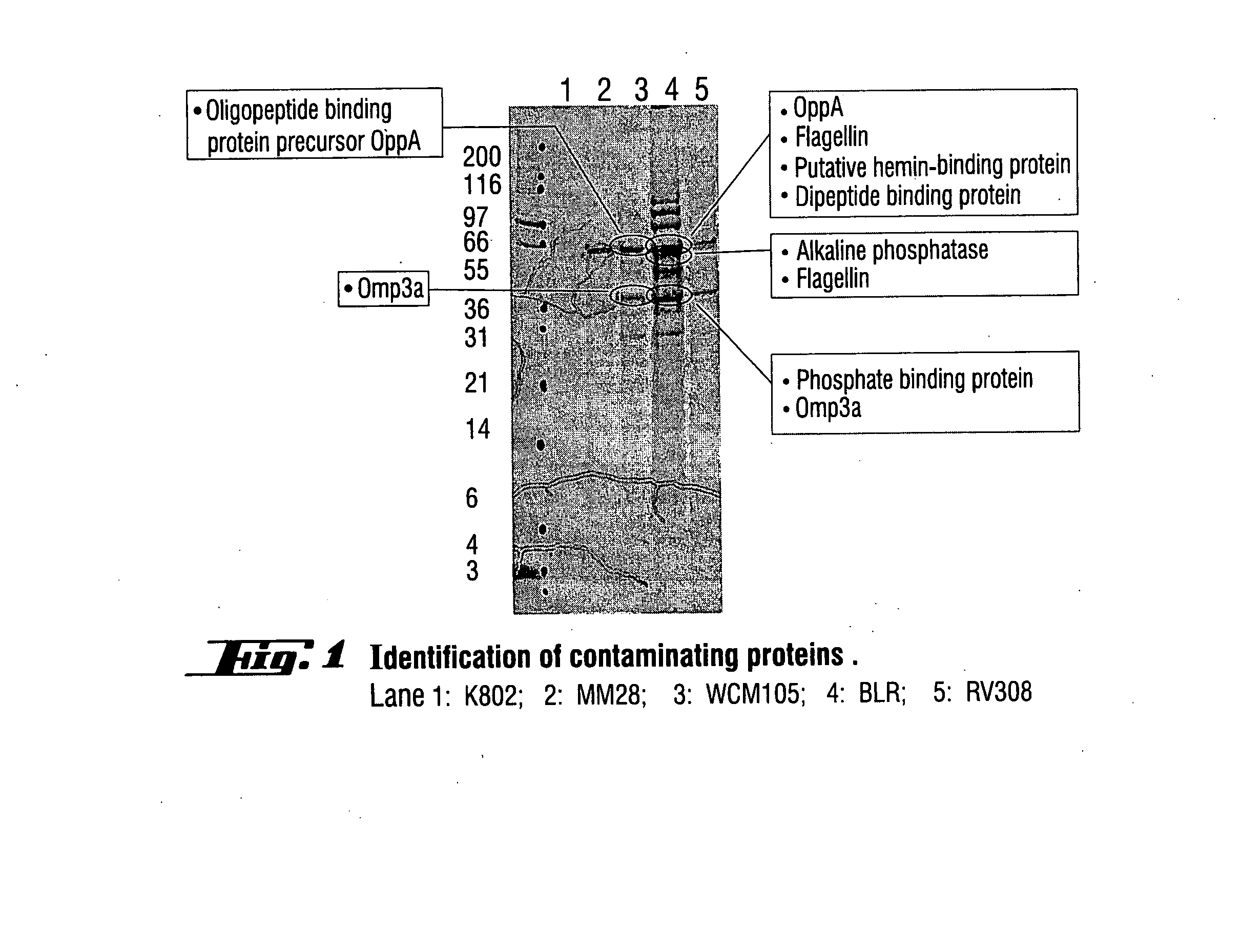
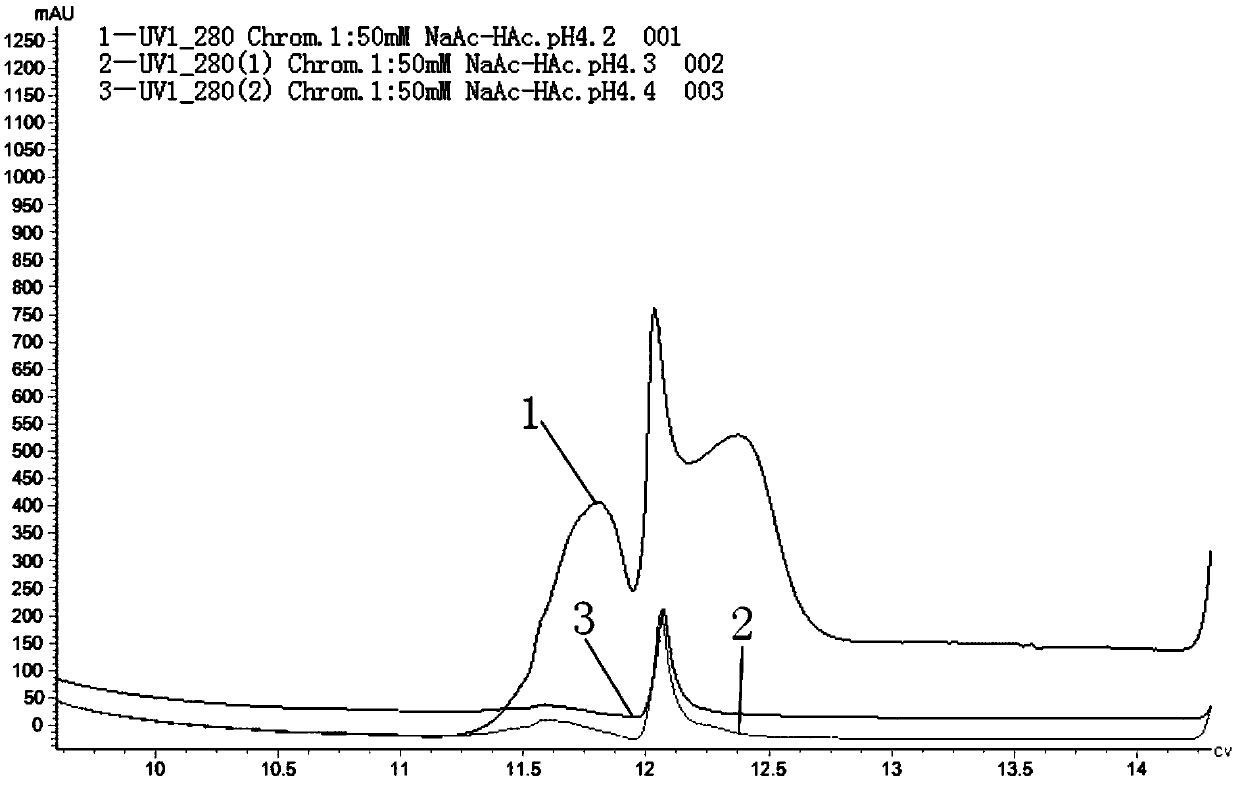
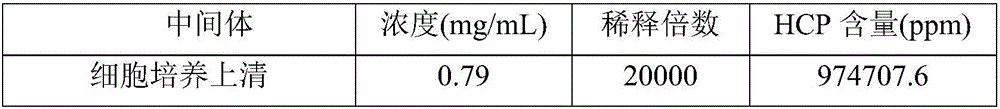Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
138 results about "Host protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
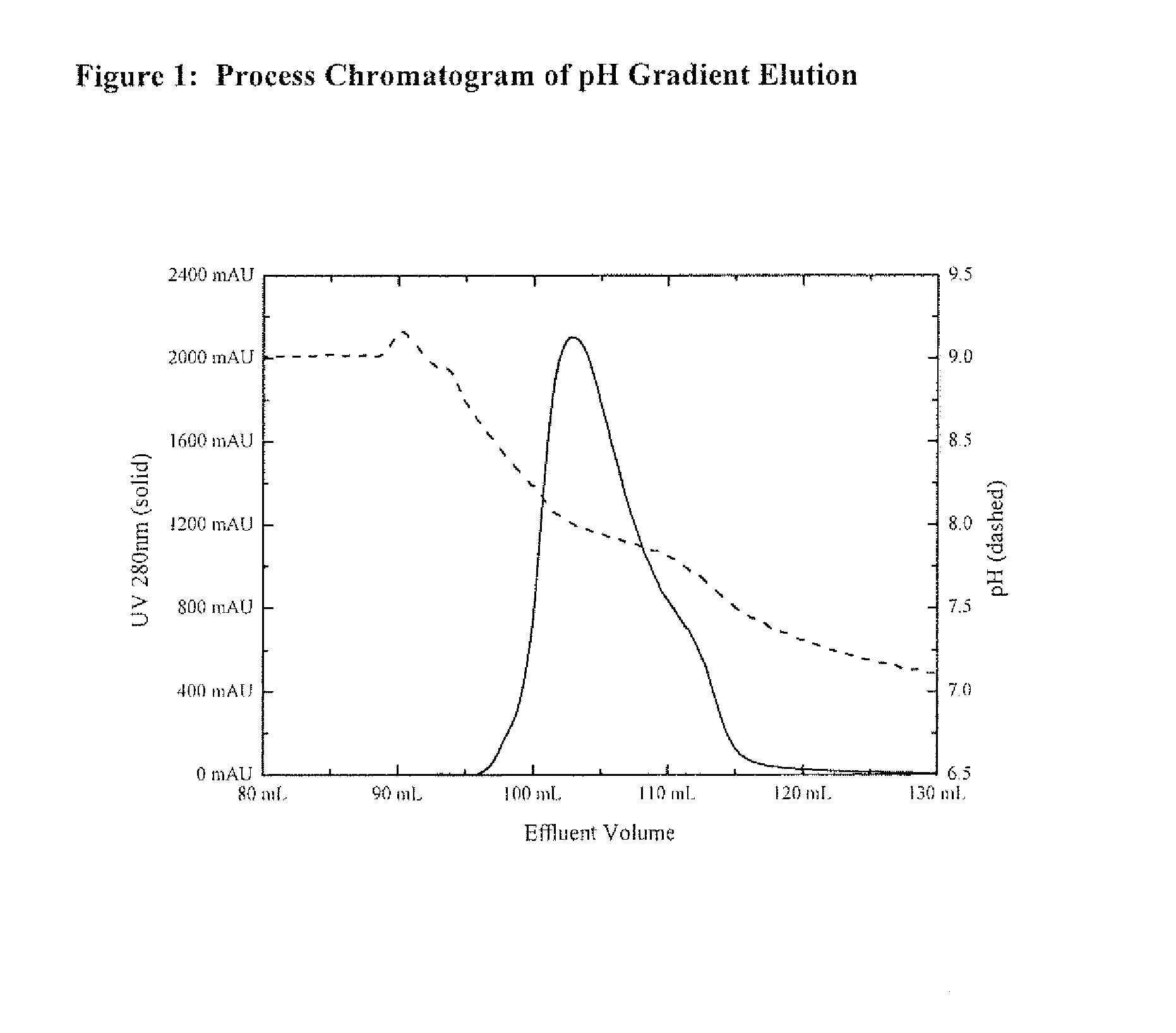
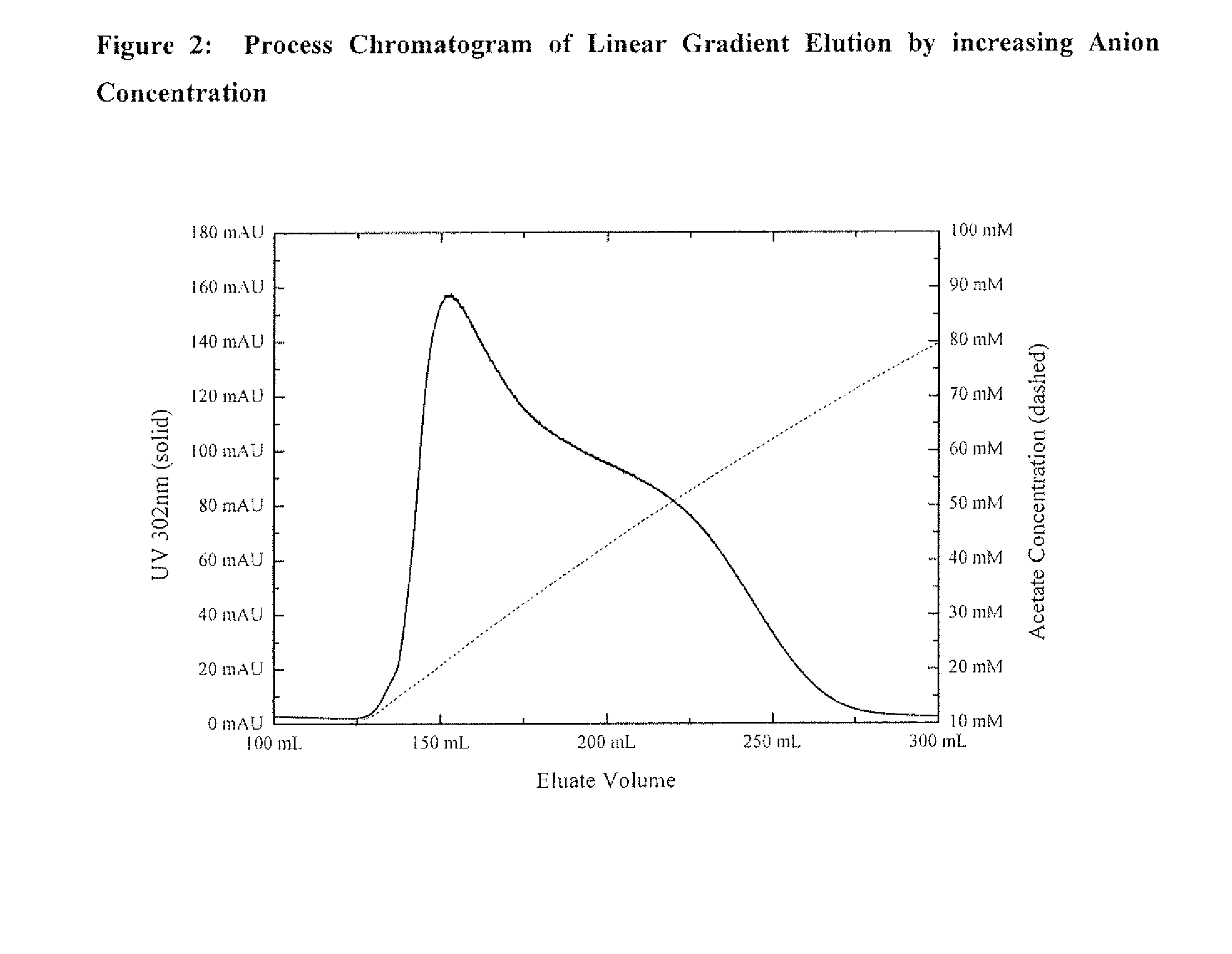
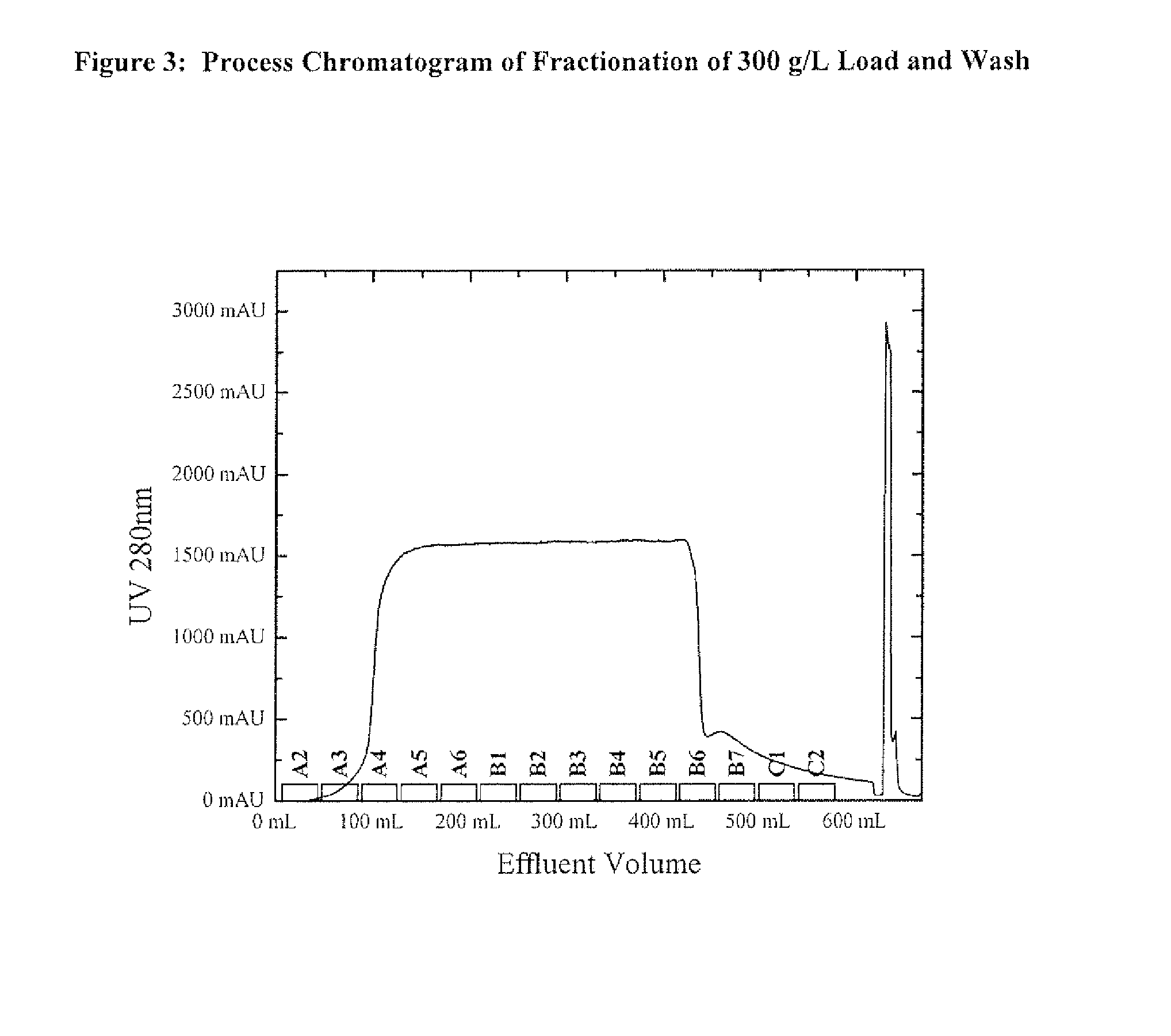
Host cell proteins (HCPs) are process-related impurities, expressed by the host cell used for production of biopharmaceutical proteins.
Protein purification methods to reduce acidic species
ActiveUS20130338344A1Reduce the amount requiredImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsPurification methodsProtein purification
The instant invention relates to the field of protein production and purification, and in particular to compositions and processes for controlling the amount of charge variants, aggregates, and fragments of a protein of interest, as well as host cell proteins, present in purified preparations by applying particular chromatography conditions during such protein purification.
Owner:ABBVIE INC
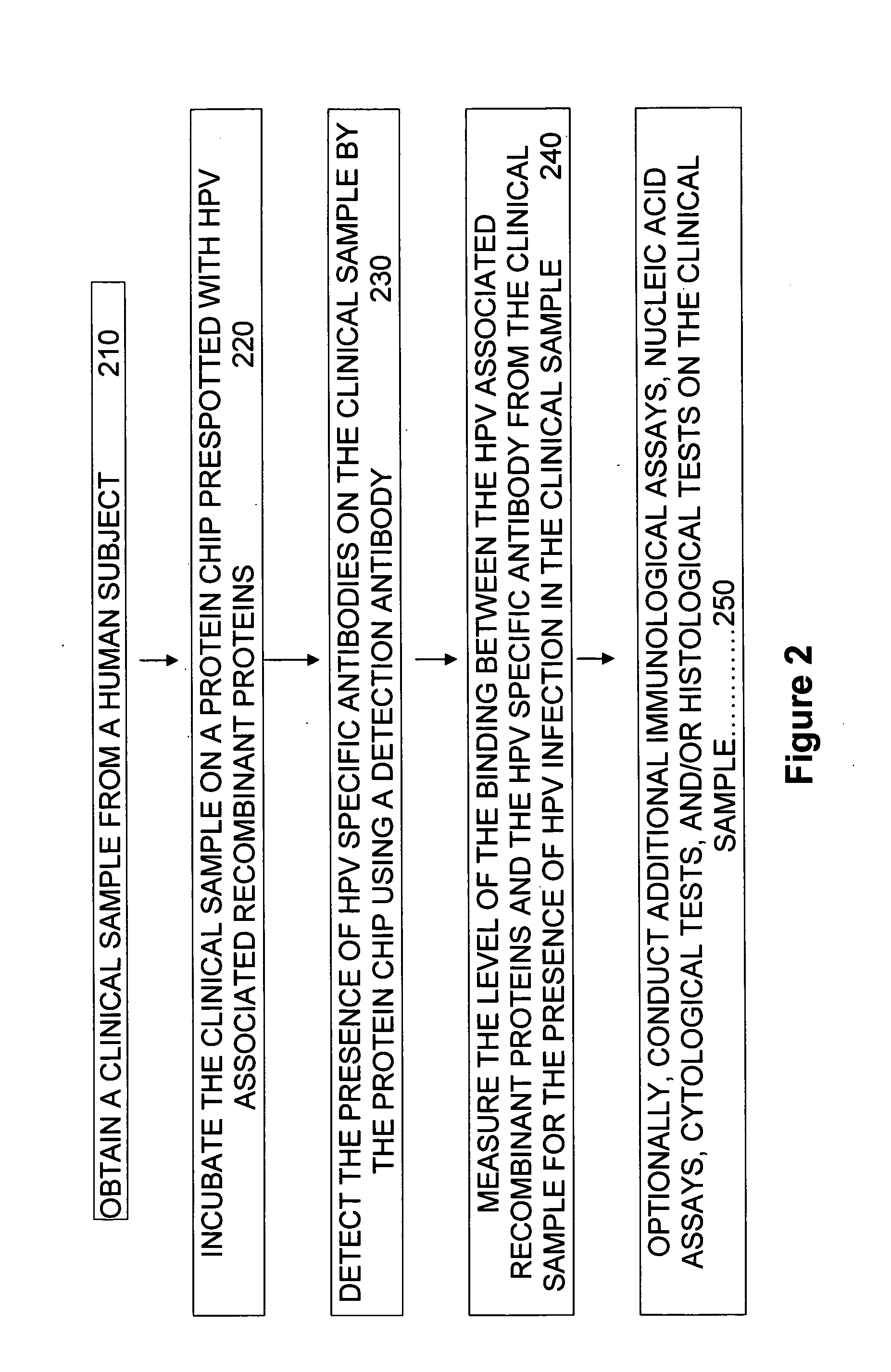
Protein chips for HPV detection
Embodiments of the invention provide methods, assays, and kits for detecting HPV infection, including infection by various HPV genotypes, early and / or late HPV-associated or HPV-specific proteins or antibodies. Detection of HPV DNAs, genomes, and / or oncoproteins by protein chips immunological assays can be used in early clinical screening for HPV infection and general diagnosis for cervical cancer and can be advantageous performed in a multiplexed test. Comparative detection of altered levels of HPV proteins and host proteins can performed in one or more assays. The polypeptides, recombinant proteins, antibodies, nucleic acids, and various detection methods thereof are particularly useful for diagnosing carcinomas of the uterine cervix and those at risk of developing cervical cancer.
Owner:HEER MEDICAL TECH DEV CO LTD
Modified beta-lactamase and method for its preparation
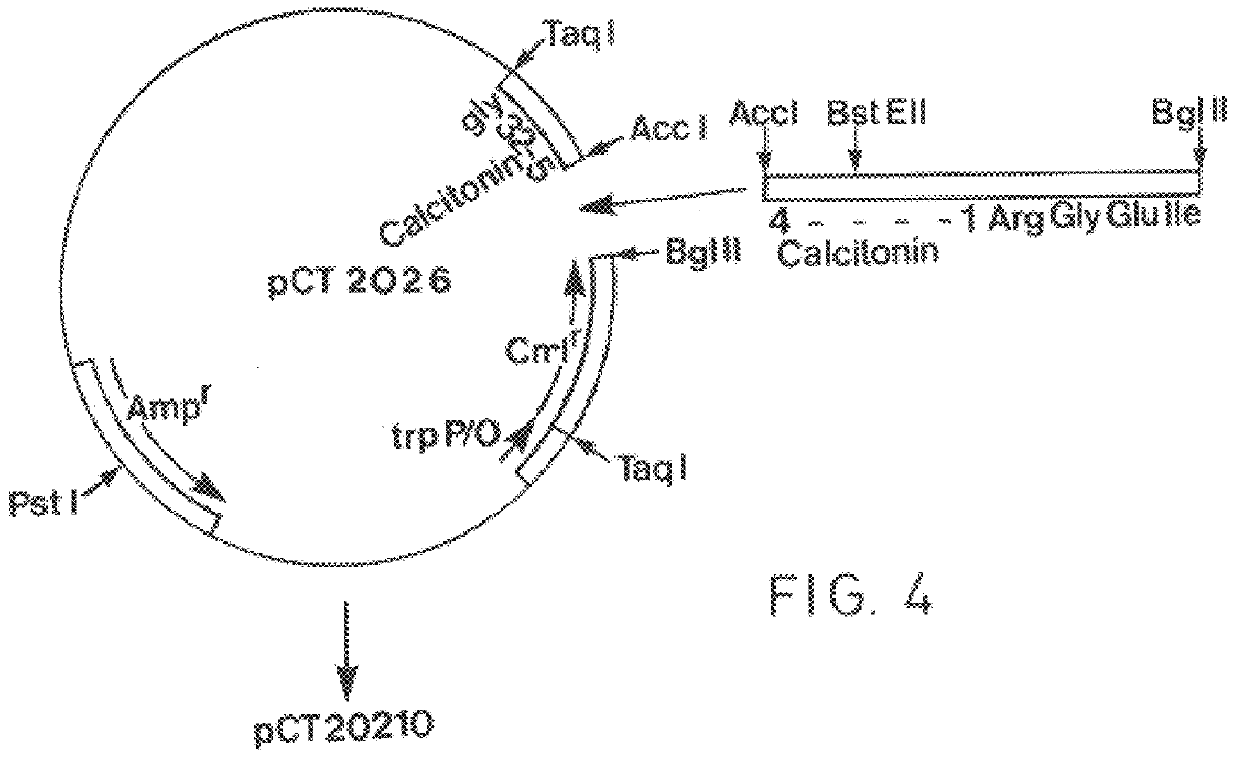
The invention relates to targeted post translational modifi-cation of metallo-beta-lactamase by truncation and inser-tion of a dipeptide at the amino terminal end to reduce amino terminal heterogeneity in a recombinant DNA pro-duction system. A protein K-T-E-ΔBL is expressed, and modified by host proteases to E-ΔBL. Appropriate nucleotide molecules, vectors and hosts are also de-scribed. E-ΔBL is useful in a pharmaceutical composition for treating antibiotic induced adverse effects in the intes-tine of patients treated with beta-lactam antibiotics.
Owner:SYNTHETIC BIOLOGICS INC
Modified beta-lactamase and method for its preparation
The invention relates to targeted post translational modification of metallo-beta-lactamase by truncation and insertion of a dipeptide at the amino terminal end to reduce amino terminal heterogeneity in a recombinant DNA production system. A protein K-T-E-ΔBL is expressed, and modified by host proteases to E-ΔBL. Appropriate nucleotide molecules, vectors and hosts are also described. E-ΔBL is useful in a pharmaceutical composition for treating antibiotic induced adverse effects in the intestine of patients treated with beta-lactam antibiotics.
Owner:SYNTHETIC BIOLOGICS INC
Recombinant fusion proteins
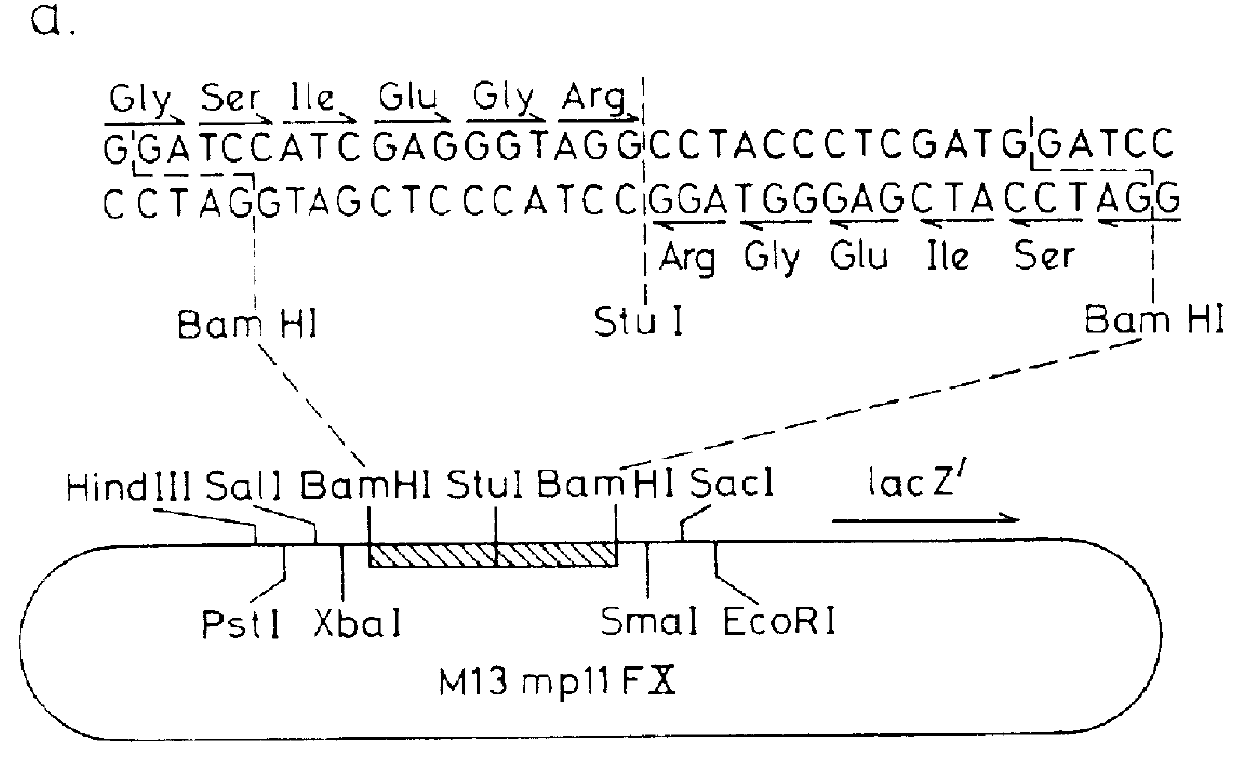
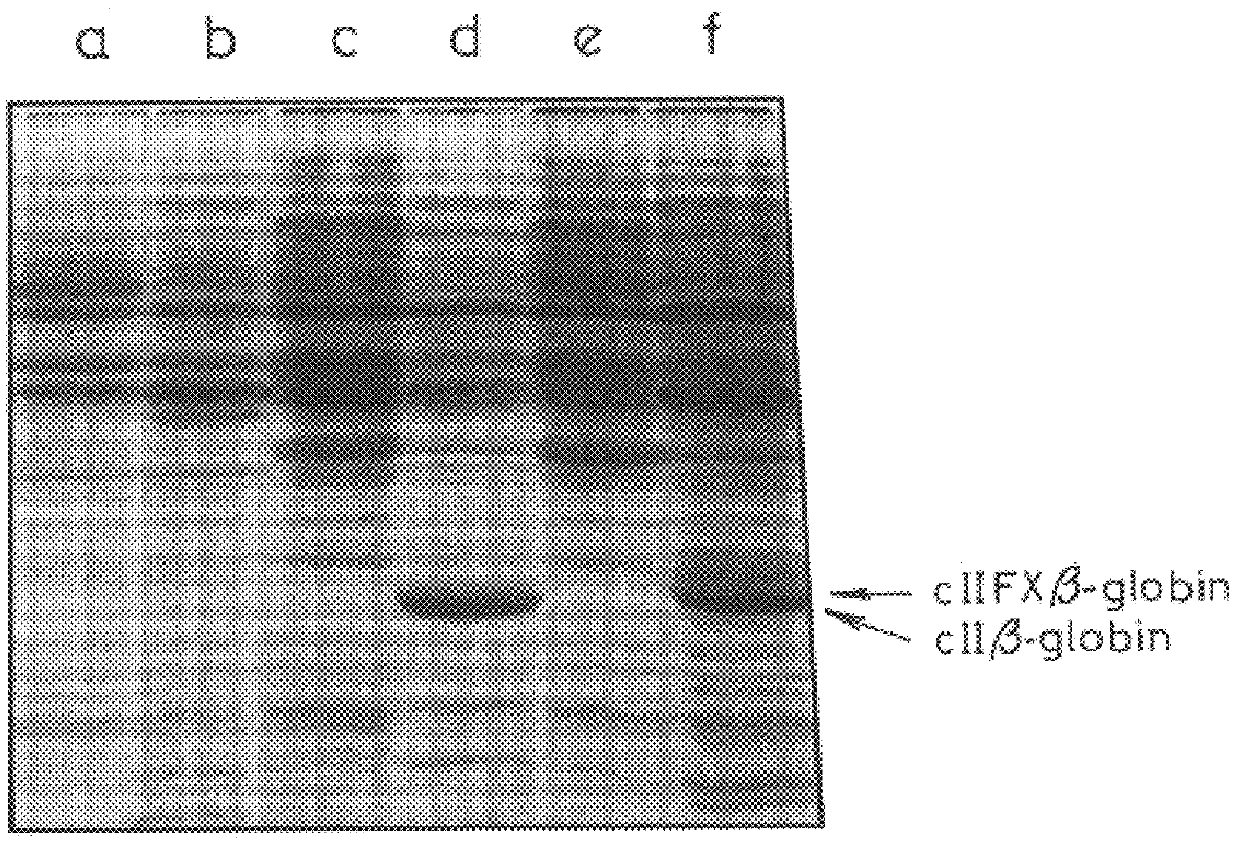
This invention provides a DNA sequence coding for a cleavage site which is specifically cleaved by blood coagulation Factor Xa, a vector containing such a sequence, and a host organism transformed with such a vector. Preferably, in the vector, the Factor Xa cleavage site coding sequence is fused at one end to a product and at its other end to an ATG codon or a sequence coding for at least part of a host protein. This invention also provides a process, for the production of a desired protein or peptide product in native form, comprising: transforming a host organism with a vector as described above; expressing the desired protein or peptide product as a fusion protein comprising the desired protein or peptide product fused to a Factor Xa cleavage site; and a cleaving the fusion protein with Factor Xa to yield the foreign gene product in native form.
Owner:CELLTECH R & D LTD
Method for effectively removing host protein in monoclonal antibody downstream purification process
ActiveCN106749660AImmunoglobulins against growth factorsPeptide preparation methodsVirus inactivationFiltration
The invention relates to a method for effectively removing a host protein in a monoclonal antibody downstream purification process. The method comprises the following steps: (1) performing deep filtration on cell culture fluid, and collecting the filtrate; (2) performing Protein A affinity chromatography on the filtrate obtained in the step (1); (3) performing virus inactivation on the sample obtained in the step (2), performing deep filtering on the sample subjected to virus inactivation, thereby effectively removing the host protein in the sample containing the monoclonal antibody. According to the method disclosed by the invention, the deep filtering after virus inactivation is adopted, the deep filtering process after Protein A affinity chromatography and virus inactivation is optimized, the content of HCP in the culture fluid supernatant collected by deep filtering can be reduced to 2.3ppm by virtue of two purification steps, namely the Protein A affinity chromatography and deep filtering after virus inactivation, and the content of the HCP can be reduced to 0.4ppm by virtue of further anion and cation exchange chromatography.
Owner:GENOR BIOPHARMA
Proteins and polypeptides from coagulase-negative staphylococci
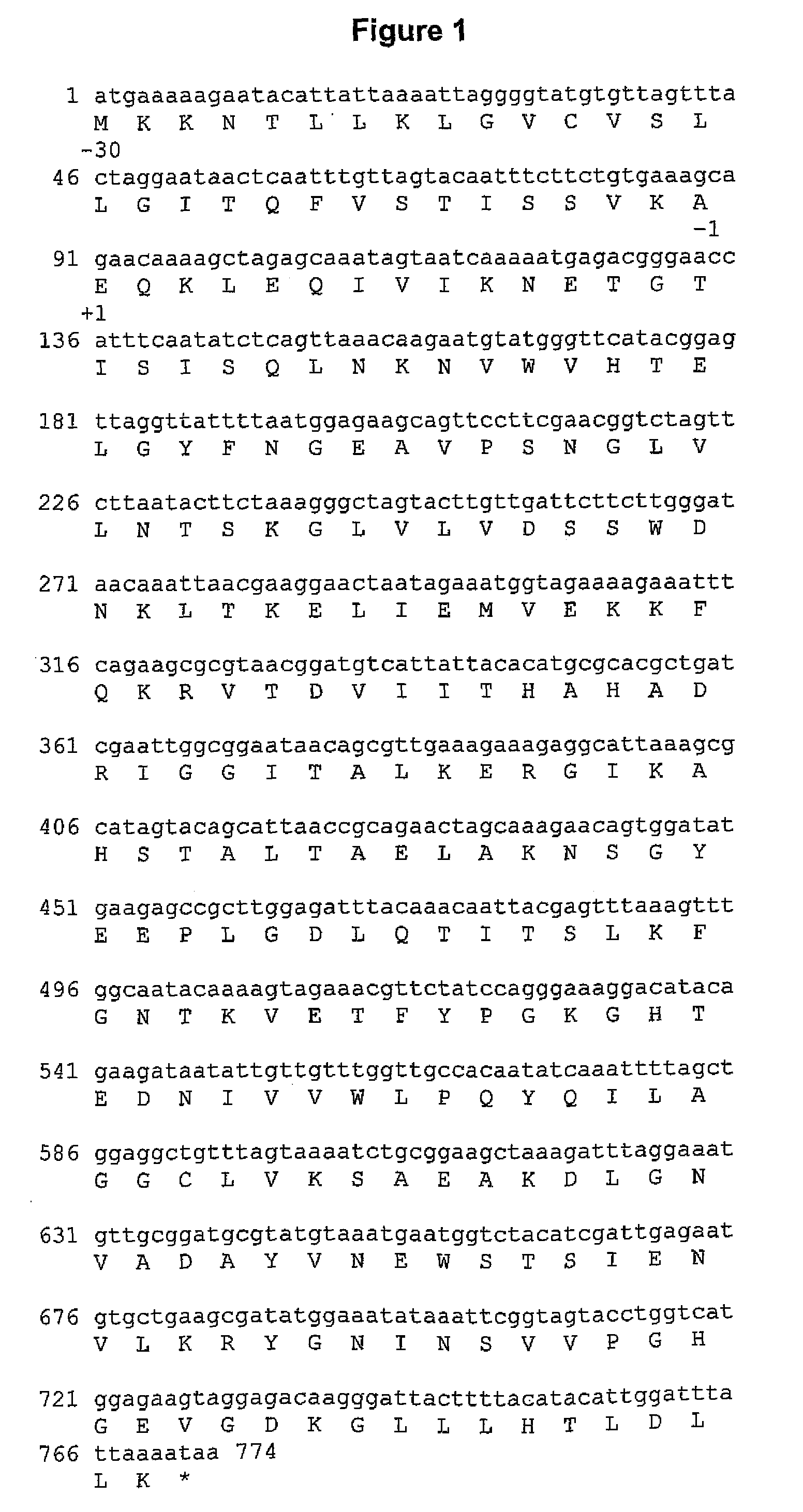
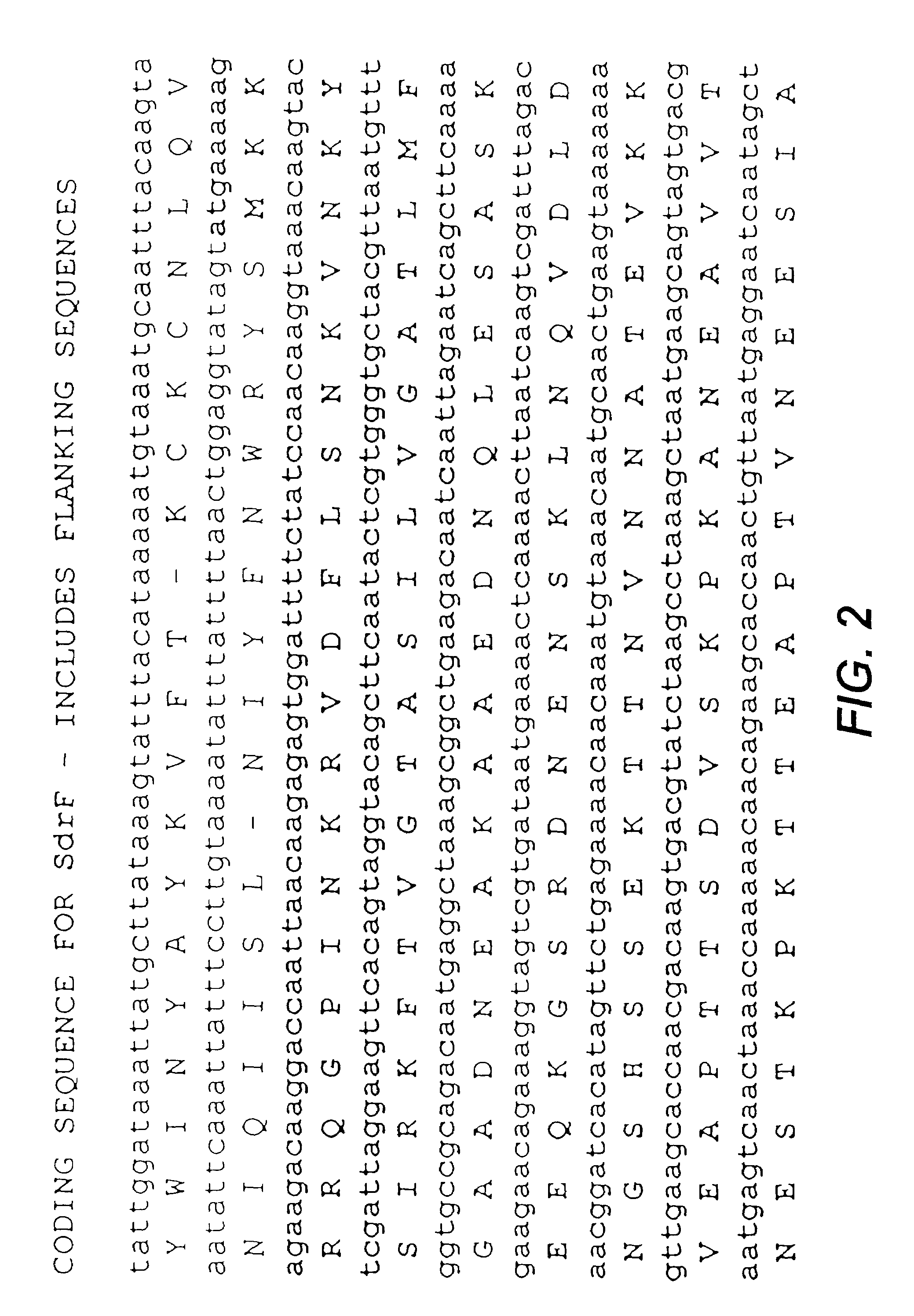
Proteins and polypeptides from coagulase-negative staphylococcal bacteria such as S. epidermidis, including proteins designated SdrF, SdrG and SdrH, and their effective fragments such as their respective A domains, are provided which are useful in the prevention and treatment of infection caused by coagulase-negative staphylococcal bacteria such as S. epidermidis. The SdrF, SdrG and SdrH proteins are cell-wall associated proteins that specifically bind host proteins and which each have a highly conserved motif of which the consensus sequence is TYTFTDYVD (SEQ ID NO: 16). The proteins and polypetides may be useful in generating antibodies for the diagnosis and treatment of coagulase-negative staphylococcal infections.
Owner:TRINITY COLLEGE DUBLIN +1
Prenylation inhibitors reduce host cell permissiveness to viral replication
InactiveUS20050085529A1Reducing permissivenessConfirming a resultant reduction in permissiveness of the cellsBiocideAnimal repellantsHMG-CoA reductaseHuman cell
Permissiveness of human cells to replication of susceptible pathogenic human viruses is reduced by treating the cells with a selective inhibitor of prenylation of a host cell protein. Target viruses, especially Flaviviridae, are predetermined to lack a CXXX box and prenylated viral protein, and to be replication-dependent on host protein prenylation. The general method comprises (a) contacting human cells subject to infection by the virus with an effective amount of a selective inhibitor of a prenylation enzyme of the cells; and (b) confirming a resultant reduction in permissiveness of the cells to replication of the virus. Targeted enzymes include prenyl biosynthetic enzyme like HMG CoA reductase farnesyl and / or geranylgeranyl transferase enzymes.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Method for extracting rabies virus
InactiveCN101270350AImprove removal efficiencyGood removal effectSsRNA viruses negative-senseMicroorganism based processesHollow fibreFiber
The invention provides a method for extracting rabies virus, and is to solve the defects that great discrepancy of quality indices of different batches occurs; removal of remaining DNA becomes difficult; the protein content of the remaining host is too high; and great side effects appear clinically when the single method of molecular sieve gel chromatography is adopted for extracting rabies virus vaccine. The essential of the invention is that a hollow fiber ultrafiltration column or ultrafiltration membrane with the molecular weight cut-off of 750KD or 500KD is used to condense and partially purify harvested liquid of virus; anion exchange chromatography or molecular sieve gel chromatography is adopted to separate and purify samples; molecular sieve gel chromatography or anion exchange chromatography is adopted to separate and purify samples got in step (2). The method for extracting rabies virus has the characteristics of great productive capacity, high product quality, excellent batch stability, being remarkably effective in removal of remaining DNA and HCP, and reducing the potential safety hazard of vaccine.
Owner:LIAONING YISHENG BIOLOGY PHARMACY
Method for effectively expressing cationic antibacterial peptides in pichia pastoris
ActiveCN101914565AHigh expressionToxic reductionFungiMicroorganism based processesPichia pastorisProteinase activity
The invention relates to the field of genetic engineering, in particular to a method for effectively expressing cationic antibacterial peptides in pichia pastoris. In the method, the cationic antibacterial peptides can be driven to be expressed by acid enzyme; a large amount of acidic amino acid of the acid enzyme can neutralize the alkaline amino acid of the cationic antibacterial peptides to keep the cationic antibacterial peptides out of the attack of host protease; the yield of antibacterial peptides can be judged directly by the activity of expressed recombinase; moreover, expressed products and the antibacterial peptides can be directly applied at the same time.
Owner:INST OF ANIMAL SCI OF CHINESE ACAD OF AGRI SCI
Purification method for recombinant human follicle-stimulating hormone
ActiveCN103059125ALow costHigh purityDepsipeptidesPeptide preparation methodsPurification methodsRecombinant human follicle stimulating hormone
The present invention relates to a purification method for recombinant human follicle-stimulating hormone (FSH), including anion-exchange chromatography, affinity chromatography, and gel filtration chromatography. The method is low in cost, few in steps, simple and feasible, and stable in quality; and no complicated denaturation and renaturation processes are comprised in the method, and the use of reversed phase chromatography, metal chelate chromatography or hydrophobic interaction chromatography, which have a greater impact on protein activity, is prevented. According to the method, firstly, anion exchange of flow-through mode is used for removing a large number of contaminating proteins, pigments and some residual DNA, endotoxin and host proteins, which not only protects the subsequent affinity filler, but also achieves a coarse purification and concentration enrichment; affinity media are conjugated with camel-sourced antibodies, the carrying capacity is high, and only an intact FSH molecule, instead of a single subunit, can be bound specifically, so degraded monomer subunits are better removed; and gel filtration can further remove residual DNA, endotoxin and host proteins, and can also remove protein aggregates and inadequately glycosylated FSH proteins.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Separation and purification method for recombinant hepatitis B core antigen
ActiveCN108047316AImprove thermal stabilityAggregate compact and stableVirus peptidesPeptide preparation methodsAntigenPurification methods
The invention relates to a separation and purification method for a recombinant hepatitis B core antigen. The method includes the steps of thermal denaturation and clarification; ammonium sulfate precipitation; ultrafiltration and concentration, washing filtering and liquid exchange; depolymerization; first-step molecular sieve chromatography; ultrafiltration and concentration, washing filtering and liquid exchange; repolymerization; second-step chromatography. By means of the method, the high-purity, low-host-residue and high-stability recombinant hepatitis B core antigen with a uniform granular structure can be obtained, that is to say, the method has the advantages that the antigen purity is high, the residue of host protein and host nucleic acid is low, antigen particles are uniform, and industrial enlargement is easy.
Owner:JIANGSU THERAVAC BIO PHARMA
Hybrid vegetable protein and method for obtaining same
InactiveUS20110319596A1Group 1/11 element organic compoundsProtein composition from vegetable seedsBiotechnologyGlutelin
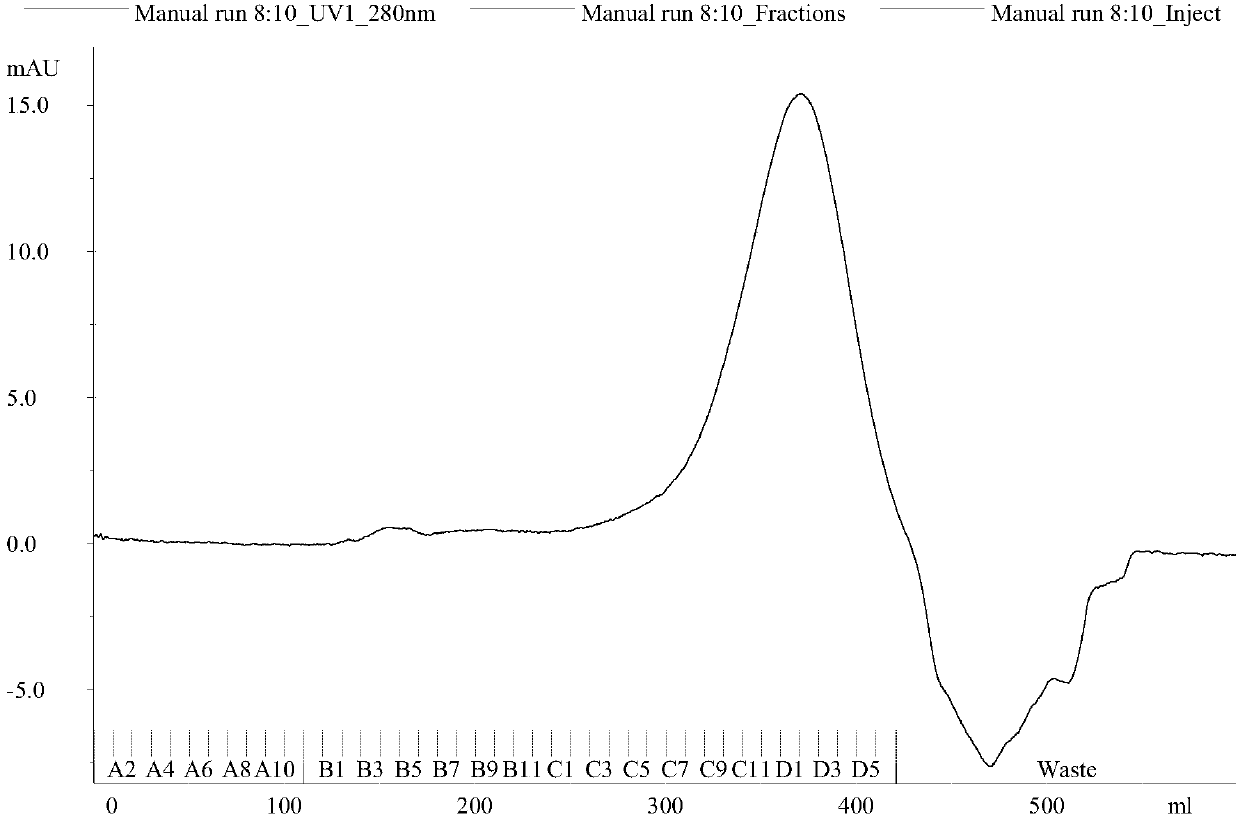
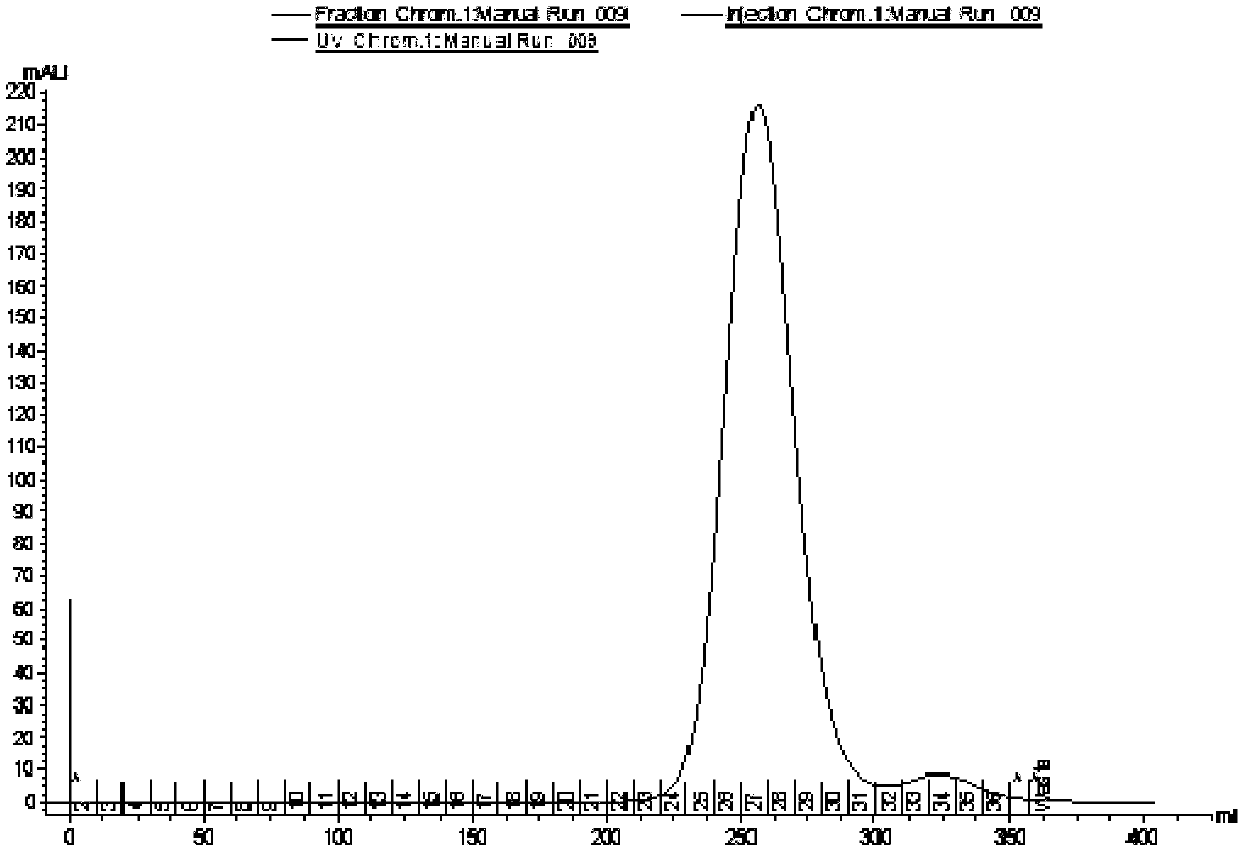
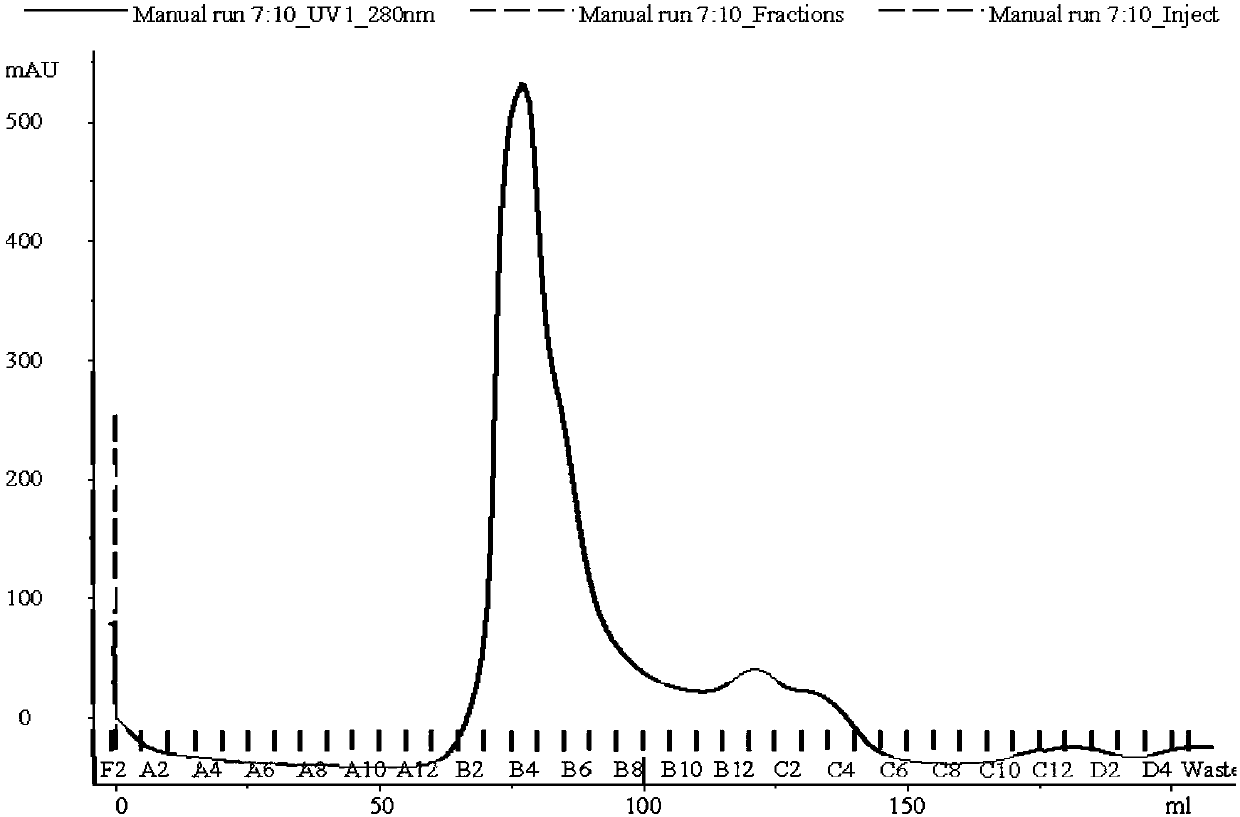
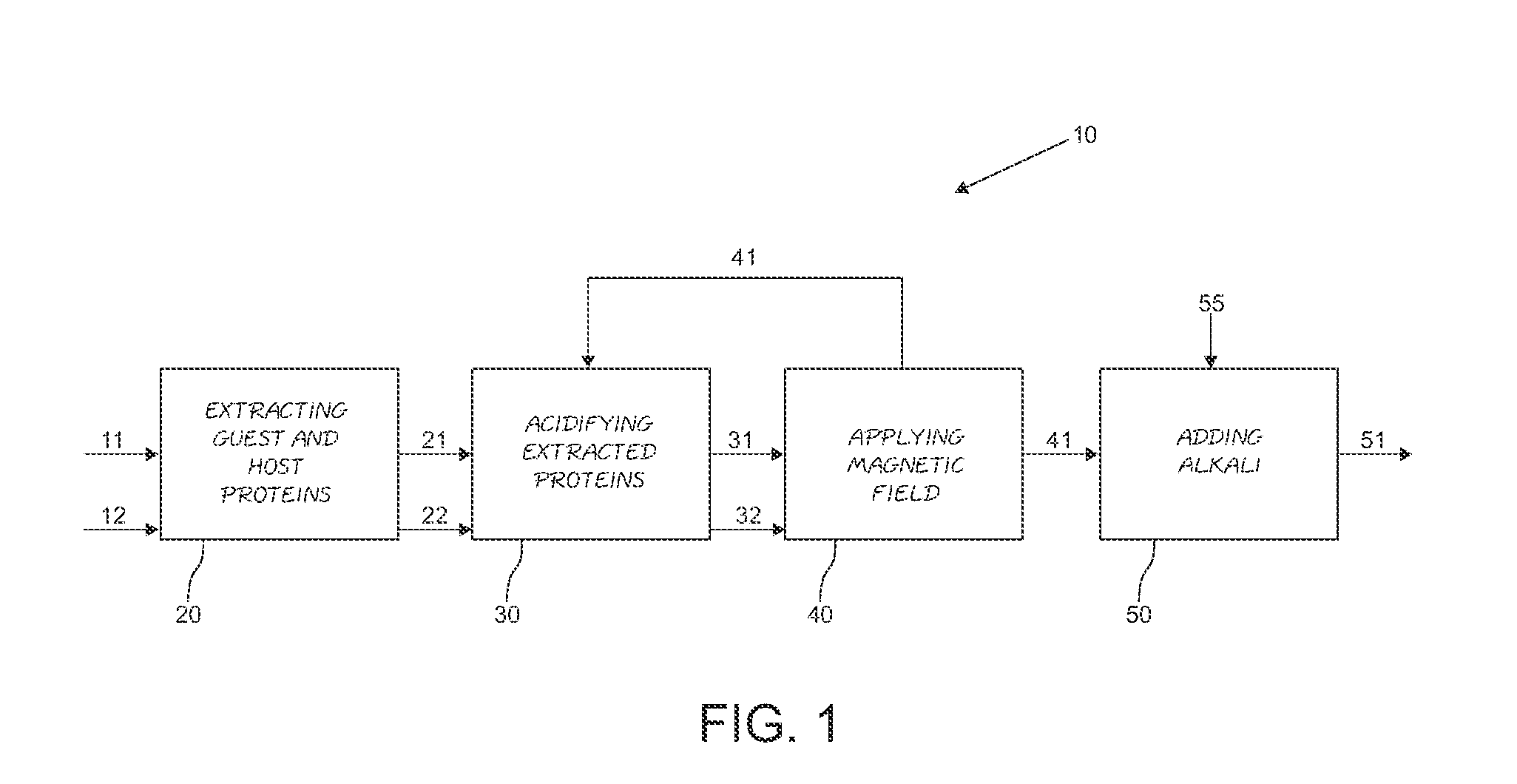
A hybrid vegetable protein is described, comprising a guest protein having the structure of prolamine and glutelin, and a host protein having the structure of globulin and albumin, obtained from vegetable grains, such as corn and soybean, respectively. Likewise, a method for obtaining said hybrid vegetable protein is described, which comprises the steps of extracting the guest and host proteins, carrying out an acidification thereof, and further applying a magnetic field to provoke their attachment, and finally adding an alkali to the attached proteins to obtain a hybrid vegetable protein at its isoelectric point. The protein thus produced has a value higher than 0.97 according to the PDCAAS rating.
Owner:IND NUTRIGRAINS S A DE
Identification and use of antiviral compounds that inhibit interaction of host cell proteins and viral proteins required for viral replication
The present invention relates to the identification of host cell proteins that interact with viral proteins required for virus replication, and high throughput assays to identify compounds that interfere with the specific interaction between the viral and host cell protein. Interfering compounds that inhibit viral replication can be used therapeutically to treat viral infection.The invention is based, in part, on the Applicants' discovery of novel interactions between proteins of the influenza virus and a human host cell proteins. One of these host cell proteins, referred to herein as NPI-1, interacts with influenza virus protein NP, and may be an accessory protein required for replication of influenza virus. Another of these host cell proteins, referred to herein as NS1I-1, interacts with influenza virus protein NS1. Compounds that interfere with the binding of the host cell and viral proteins, and inhibit viral replication can be useful for treating viral infection in vivo.
Owner:MT SINAI SCHOOL OF MEDICINE
HIV capsid assembly-associated compositions and method
InactiveUS7348134B2Inhibiting HIV capsid formationInhibition formationSsRNA viruses positive-senseMicrobiological testing/measurementCell freeScreening method
A cell-free method for translation and assembly of retroviral, particularly HIV, capsid and capsid intermediates is disclosed. Also disclosed are novel HIV capsid assembly intermediates and novel host proteins which bind to such assembly intermediates. The invention also includes a screening method for compounds that alter retrovirus capsid assembly, and a method of treating HIV using compounds which inhibit the HIV capsid assembly pathway.
Owner:RGT UNIV OF CALIFORNIA
Purification method of anti-VEGF (Vascular Endothelial Growth Facto) type monoclonal antibody
InactiveCN106279412AImmunoglobulins against growth factorsPeptide preparation methodsPurification methodsProtein insertion
The invention relates to the field of protein purification and in particular relates to a purification method of an anti-VEGF (Vascular Endothelial Growth Facto) type monoclonal antibody. The purification method comprises: firstly, capturing by utilizing a composite material chromatography to separate the antibody and almost components in a harvesting solution; furthermore, carrying out fine purification by utilizing hydroxyapatite chromatography, so as to further remove host cell pollutants, aggregates and the like, wherein a composite chromatography material is a composite medium with ion exchange effect and hydrophobic effect. According to the purification method of the anti-VEGF type monoclonal antibody, the content of host cell protein (HCP), DNA (Deoxyribonucleic Acid), polymers and acidic peaks is reduced, so that the aim of remarkably improving the purity of the antibody is realized; the purification method is simple in structure and relatively low in cost.
Owner:SUNSHINE LAKE PHARM CO LTD
Microorganism strain for producing recombinant proteins
A microorganism strain comprises a gene coding for a recombinant protein and a mutated gene coding for a host protein that is not a protease. The recombinant protein is secreted during a fermentation and the mutated gene coding for the host protein has been mutated so as to cause reduced expression of the host protein compared to the wild-type gene on which the mutated gene is based.
Owner:WACKER CHEM GMBH
Novel HIV Targets
InactiveUS20090221679A1Organic active ingredientsMicrobiological testing/measurementCellular proteinsNucleic acid
Using a method to measure the effect of downregulation of certain cellular proteins on HIV integration, host proteins implicated in HIV infection were identified. The identified proteins and encoding nucleic acids provide targets for inhibiting HIV infection and for evaluating the ability of compounds to inhibit HIV infection. Compounds inhibiting HIV infection include compounds targeting identified proteins and compounds targeting nucleic acids encoding the proteins.
Owner:MERCK SHARP & DOHME CORP
Affinity purification process capable of removing host cell protein content
ActiveCN107793469ARemarkable effect in removing HCPEffective control of contentPeptide preparation methodsImmunoglobulinsElutionBuffer solution
The invention provides a novel affinity purification process capable of effectively lowering host cell protein (HCP) content. The affinity purification process specifically includes the steps of firstly, processing affinity filler, and performing sample loading; secondly, using a buffer solution with low pH (3.9-5.6) and an elution buffer solution 2 using sodium chloride, calcium chloride and / or arginine hydrochloric acid as additives to elute a chromatographic column; thirdly, using different pH elution buffer solutions to elute a product, and collecting the product. The affinity purificationprocess has the advantages that materials used by the affinity purification process are evident in HCP removing effect, wide in application range and widely applicable to the affinity purification process of antibody protein, and accordingly technical support is provided for the effective control of HCP content in antibody medicine.
Owner:WUXI BIOLOGICS CO LTD
Rapid purification method for recombinant pyrolytic enzyme
InactiveCN107794254AHigh thermal denaturation removal rateHigh purification and concentration efficiencyMicroorganism based processesLyasesThermal denaturationPurification methods
The invention discloses a rapid purification method for recombinant pyrolytic enzyme. According to the method, escherichia coli is used as an expression host of recombinant pyrolytic enzyme, the recombinant pyrolytic enzyme is subjected to inducible expression in host cells of escherichia coli, thalli are collected centrifugally, and escherichia coli is broken by heating to release expressed pyrolytic enzyme; meanwhile, host protein of escherichia coli is denatured, devitalized and precipitated at high temperature, which is favorable for purifying pyrolytic enzyme resisting to thermal denaturation; further, high-purity pyrolytic enzyme is obtained rapidly through the process of bacteria debris catching and protein concentration of pyrolytic enzyme through ultra-filtration. The rapid purification method is easy to operate; the escherichia coli protein thermal denaturation removal rate is high; the purification concentration efficiency is high; the method is adaptable to industrial production and marketing promotion and application.
Owner:KUNMING UNIV OF SCI & TECH
Antivirals
InactiveUS20100272706A1Inhibit and decrease viral infectionBiocideOrganic active ingredientsSelect agentGene silencing
This invention provides methods for inhibiting or treating infection by viruses, in particular pox viruses by modulating a kinase, in particular by inhibiting a host cell kinase, involved in mediating viral infection. Methods to identify, validate, and classify the cellular proteins required by viruses during infection of host cells in order to select agents which can inhibit viral infection are described herein. Using a systems biology approach the virus / host cell interaction is studied from initial attachment of the incoming virus to the cell surface, to entry, transcription, replication, biosynthesis, and assembly of progeny particles. The method employs a siRNA screening platform and uses gene silencing to map the ‘viral infectome’—a compilation of cellular proteins that the virus needs to establish infection and drive the infectious cycle. Charting the infectome provides information on the viral biology by the identification of host cell proteins involved in viral infection and allows the development of novel anti-viral drugs that prevent the viruses from establishing productive infection in cells.
Owner:MERCER JASON +4
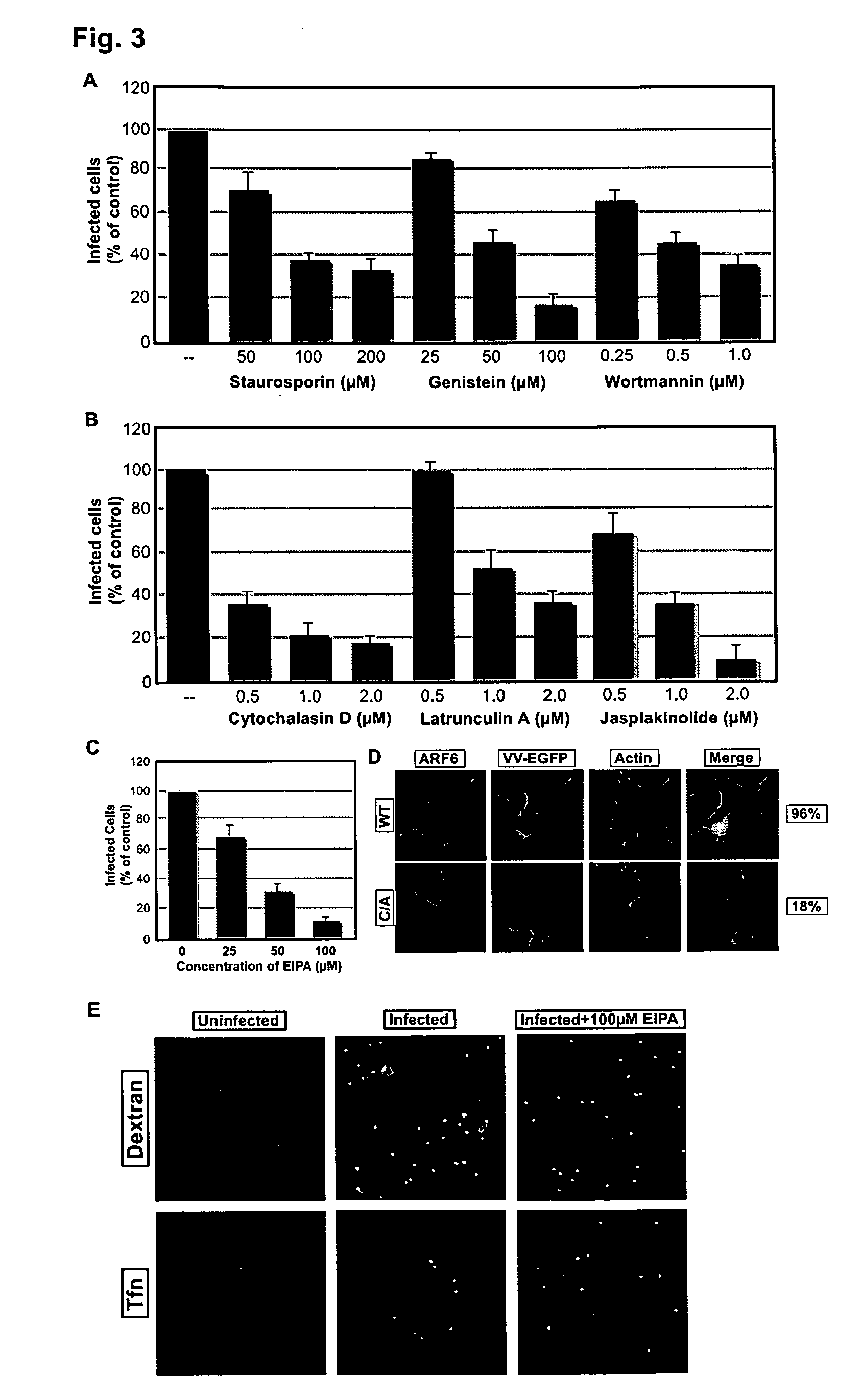
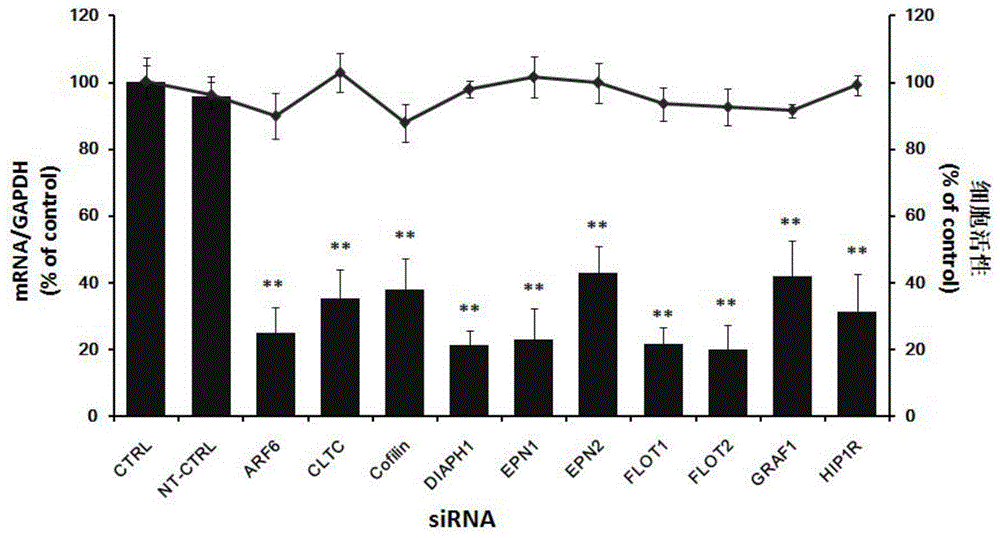
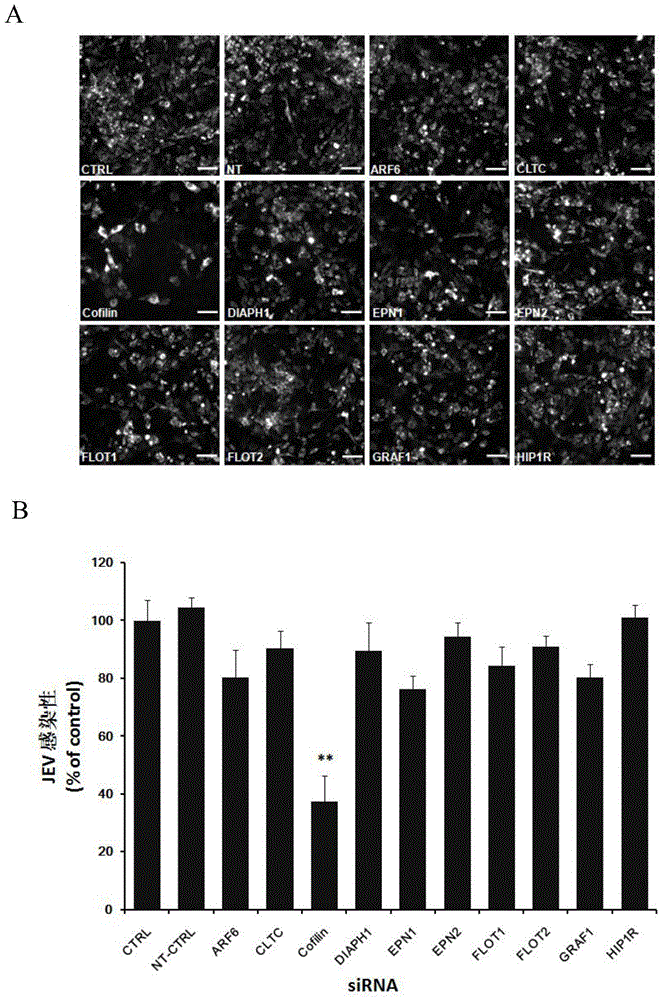
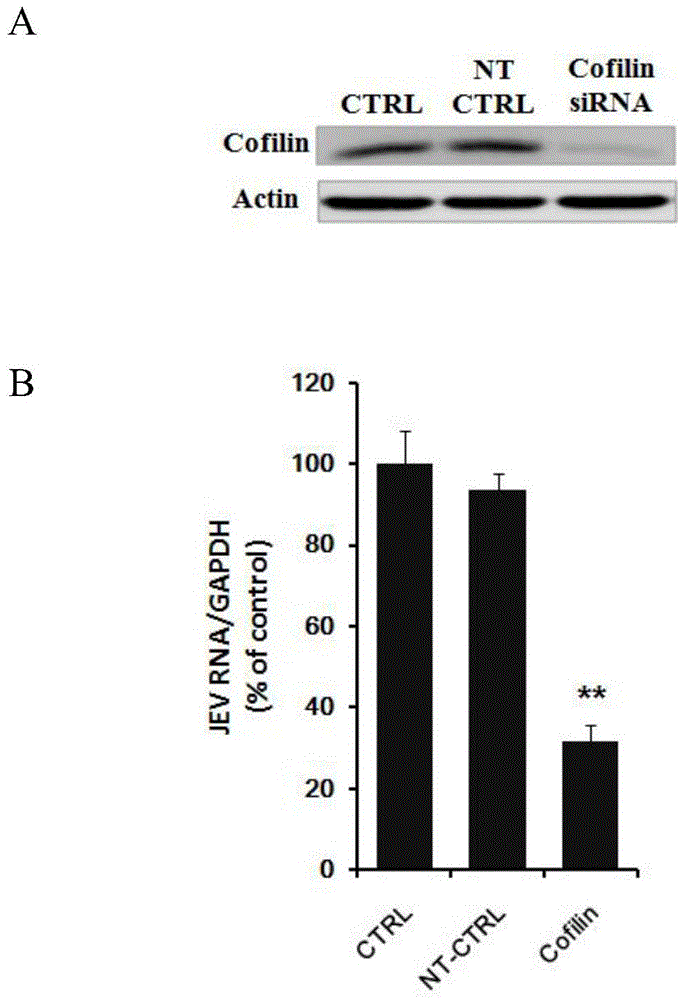
Application of Cofilin in preventing and treating Japanese encephalitis virus infection
The invention relates to the technical field of biomedicine, in particular to a novel target resistant to Japanese encephalitis virus (JEV) infection and application. Human neuroblastoma cells (SK-N-SH) are taken as target cells, RNA technology is adopted to reduce expression of host protein of the target cells to find host factors capable of effectively inhibiting JEV-infected human neuroblastoma cells (SK-N-SH) so as to protect functions of a nerve system and prevent virus from infecting a central nerve system to cause inflammation. The invention provides application of Cofilin in preventing and treating JEV infection, and experiments find that Cofilin plays an important role in JEV-infected SK-N-SH, and JEV infection can be inhibited remarkably by reducing expression of Cofilin. The invention further provides application of Cofilin in preparing drug for preventing or treating JEV infection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Method for producing recombinant human-like collagen and host cell protein by using pichia pastoris
ActiveCN112724242AHigh purityReduce manufacturing costConnective tissue peptidesFungiPichia pastorisUltrafiltration
The invention discloses a method for producing recombinant human-like collagen and host cell protein by using pichia pastoris, wherein the method comprises the steps: carrying out methanol induction on a strain Pichia pastoris-col3-6 in the fermentation culture process, and carrying out centrifugation, filtration sterilization, ultrafiltration desalination concentration, ion exchange chromatography, ultrafiltration desalination and freeze-drying on the obtained fermentation liquor to obtain the recombinant human-like collagen and the host cell protein. High yield, high yield and high purity of fermentation production of the recombinant human-like collagen are maintained, and meanwhile, the problem of high cost of industrial large-scale production of the recombinant human-like collagen expressed by pichia pastoris is solved.
Owner:西安德诺海思医疗科技有限公司
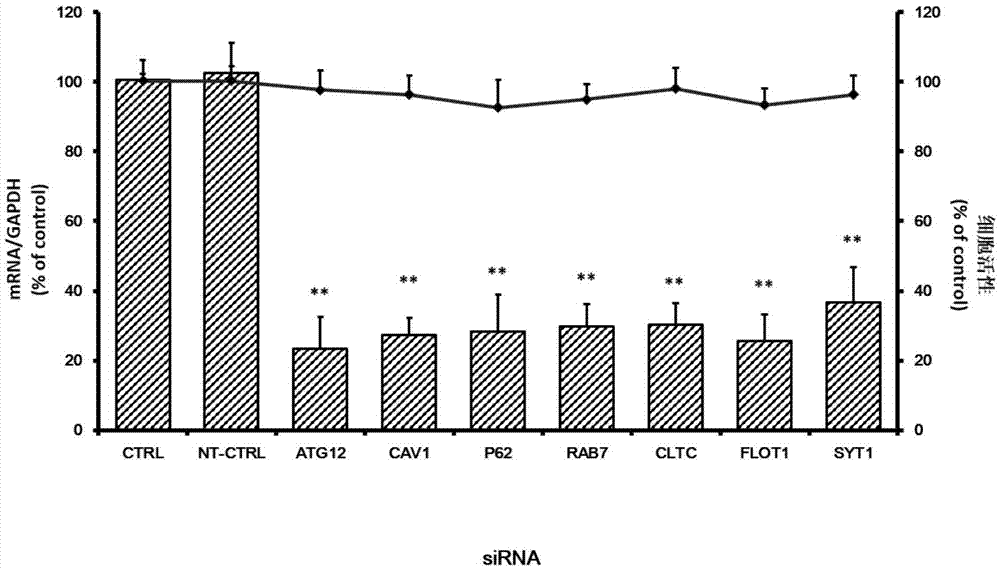
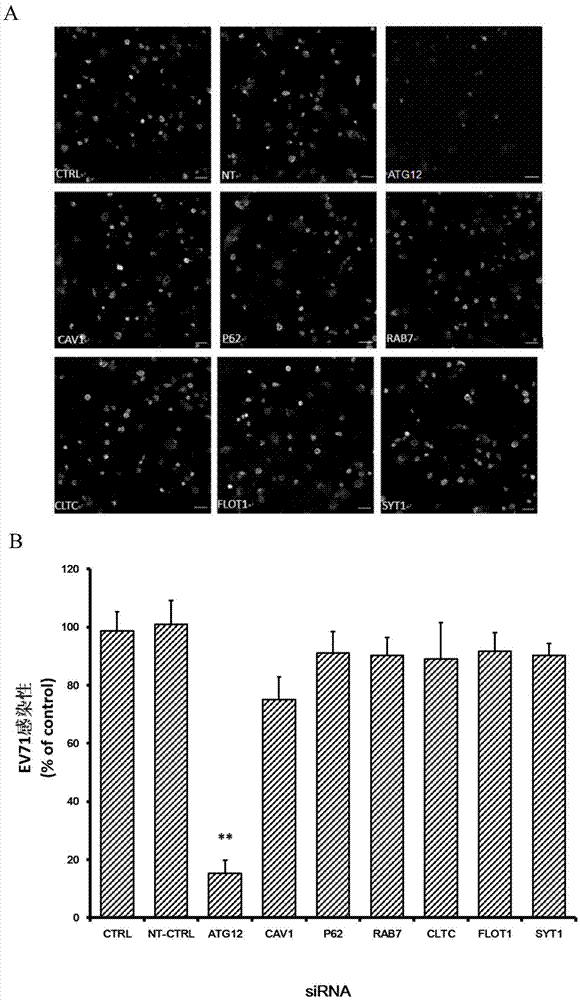
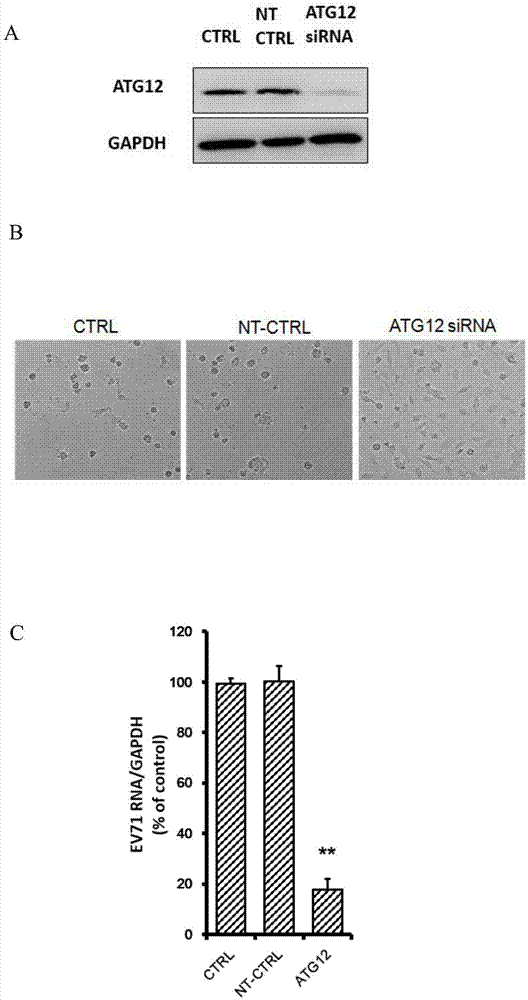
Applications of autophagy-related protein 12 in preventing and treating enterovirus 71 infection
The invention belongs to the technical field of biomedicine, and relates to a novel target used for preventing enterovirus 71 infection, and applications thereof. According to the invention, Human Brain Microvascular Endothelial Cell (HBMEC) is taken as the target cell, and RNA interference technology is adopted for down-regulation of expression of target cell host protein so as to search host factors capable of inhibiting EV71 infection of HBMEC effectively, protect the functions of the blood brain barrier, and prevent central nervous system infection caused by penetrating of the blood brain barrier by virus. It is fond by experiments that the autophagy-related protein 12 (ATG12) possesses significant importance in infection of HBMEC by EV71, and down-regulation of expression of ATG12, and is capable of inhibiting EV71 infection obviously. The invention also provides applications of ATG12 in preparing drugs used for preventing or treating EV71 infection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Methods and formulations for targeting infectious agents bearing host cell proteins
A formulation is disclosed for the treatment of diseases caused by an infectious agent which acquires host membranes protein during its life cycle. The formulation is a targeting pharmaceutical composition. It comprises a ligand capable of binding the host membrane proteins coupled to a lipid-comprising vesicle, which may comprise or not a drug effective in the treatment of the disease. Specific liposomes bearing anti-HLA-DR or anti-CD4 antibodies comprising or not antiviral drugs, namely anti-HIV drugs, are disclosed and claimed. A method of formulation as well as a method of using the formulation in the treatment of a disease are also disclosed.
Owner:INFECTIO RECHERCHE INC
Applications of hepatocyte growth factor-regulated tyropsine kinasesubstrate in preparation of medicines preventing enterovirus 71-type infection
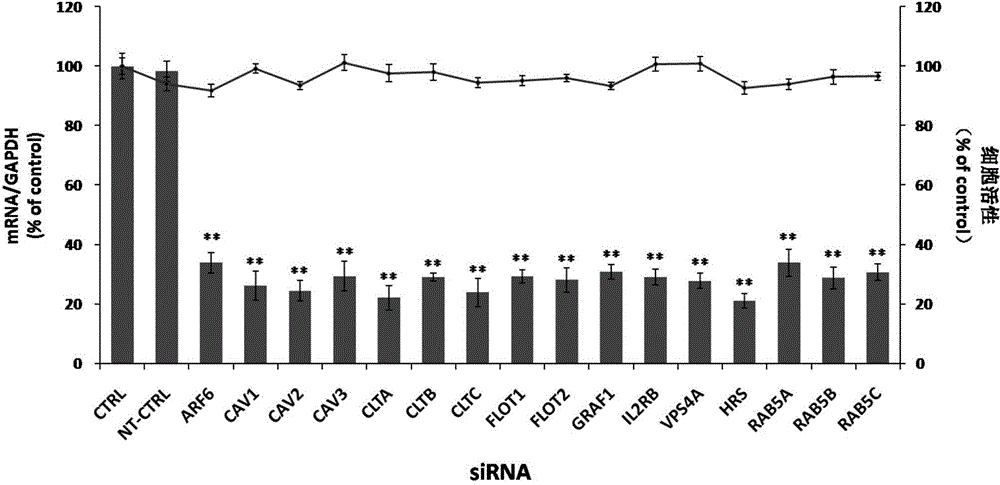
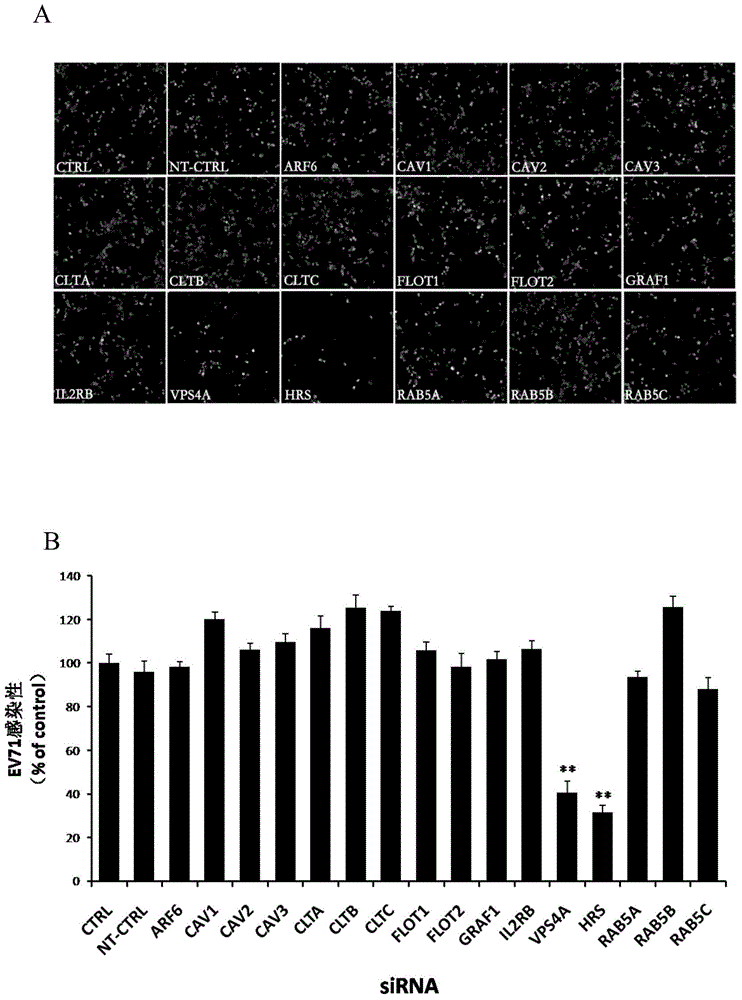
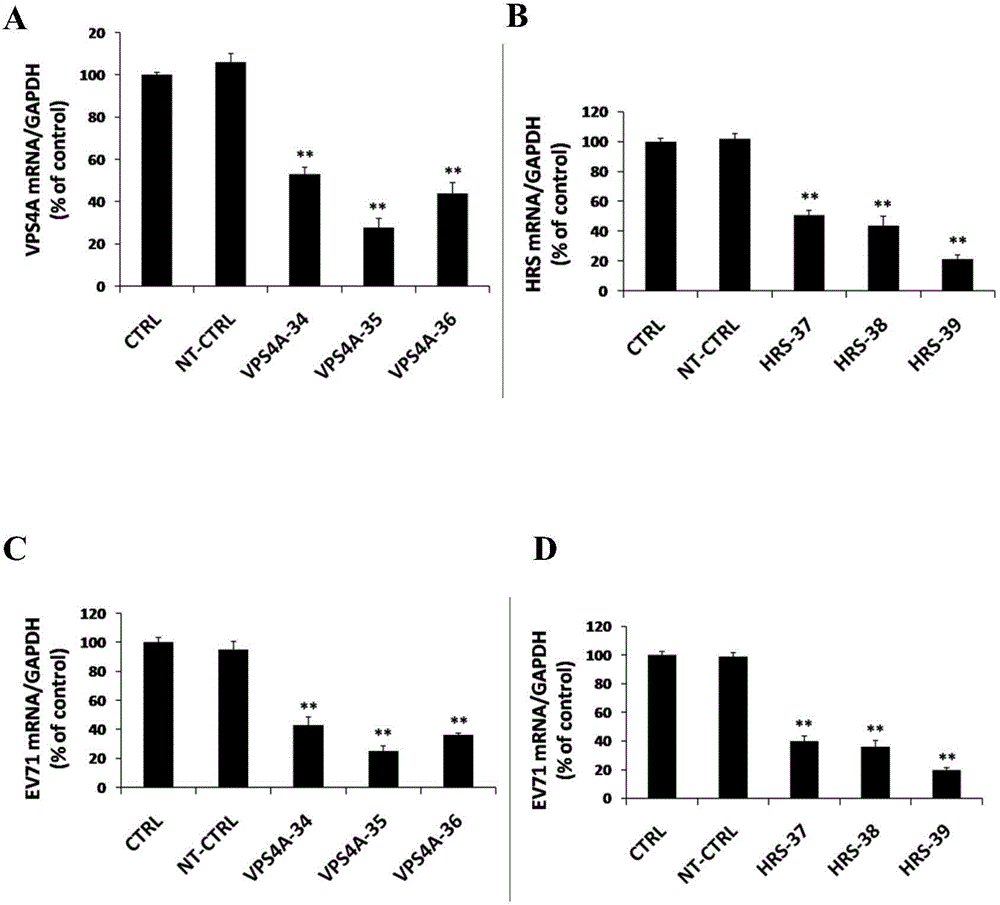
The invention relates to the biomedical technology field, and provides a new target spot for resisting enterovirus 71-type infection and applications. Human colon cancer cells (Caco-2) are employed as target cells, the RNA interference technology is employed to reduce expression of the target cell host proteins, a host factor capable of inhibiting EV71 infection of human colon cancer cells (Caco-2) effectively is sought and the purpose of cutting off EV71 infection from the source (intestinal tract) effectively is achieved. Experiments show that hepatocyte growth factor-regulated tyropsine kinasesubstrate (HRS) plays an important role in inhibiting EV71 infection of Caco-2, the HRS expression is reduced, and EV71 infection can be inhibited obviously. Applications of HRS in preparation of medicines preventing or treating enterovirus 71-type infection are provided.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Anti-TNF-alpha monoclonal antibody chromatographic method
InactiveCN105837687AImmunoglobulins against cytokines/lymphokines/interferonsPeptide preparation methodsMonoclonal antibodyIon exchange
The invention relates to an anti-TNF-alpha monoclonal antibody chromatographic method, and belongs to the field of biotechnology. In particular, the method for removing host proteins and multimers during a process of purification of a TNF-alpha monoclonal antibody is disclosed. An ion exchange and hydrophobic composite chromatographic filler is used, a low-pH buffer solution is used for removing contaminants, and then a lower-pH elution buffer solution is used for eluting the target antibody. The method can remove a part of the multimers s and most of the host proteins, significantly improves the purity of the antibody, is cheaper in price, has no protein A falling off, is simple and convenient, has no need for adjusting the sample pH value and electric conductance, and is suitable for process amplification and industrial production.
Owner:SUNSHINE LAKE PHARM CO LTD
Method for detecting specific recombinant human insulin escherichia coli residual host protein
The invention relates to a method for detecting specific recombinant human insulin escherichia coli residual host protein, which comprises the steps of taking total host protein prepared by ultrafiltration of escherichia coli fermentation liquid integrating pZRHi-1 plasmid of a human insulin gene as an antigen, stimulating a rabbit to generate an antibody, removing a human insulin antibody by affinity chromatography fixed with human insulin to prepare a specific antibody, labeling the specific antibody with horse radish peroxidase, and then detecting the residual host protein for a sample to be detected by a double antibody sandwich method. The method is high in sensitivity and specificity, and good in repeatability, and can effectively control RHI (recombinant human insulin) product quality.
Owner:TONGHUA DONGBAO PHARMA
Method for purifying slow virus
InactiveCN104371982AMeet production needsResidue reductionMicroorganism based processesRecovery/purificationPurification methodsFiltration
The invention relates to a method for purifying a slow virus. The method comprises the following steps: (S10) providing a cell culture containing the slow virus; (S20) carrying out centrifugal concentration on the cell culture, and filtering to obtain virus suspension; (S30) purifying the virus suspension by virtue of anion exchange chromatography; and (S40) purifying the virus suspension by virtue of gel filtration chromatography. A process for purifying the slow virus is easy to amplify, can be used for processing large amounts of virus liquid produced by cell factories or bioreactors on a large scale, can meet production requirements of slow virus liquid, is stable and is good in repeatability; furthermore, the purified slow virus liquid prepared by virtue of the method is not polluted by exogenous factors, is low in residues of cell host protein and host DNA, good in safety, and high in purity and titer and can completely meet requirements of clinic gene therapy.
Owner:武汉维诺赛生物技术有限公司
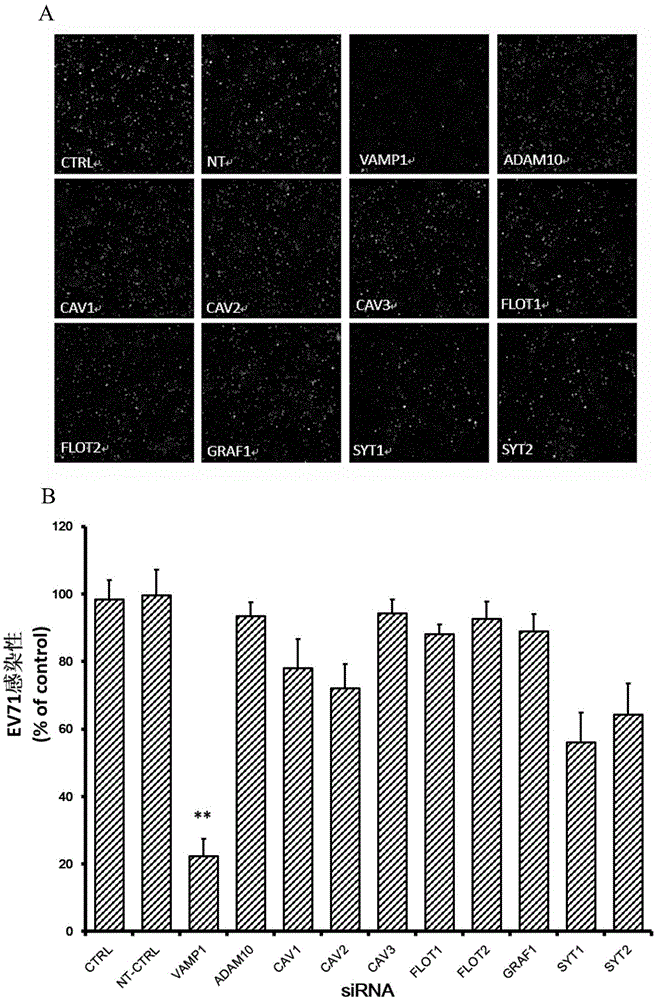
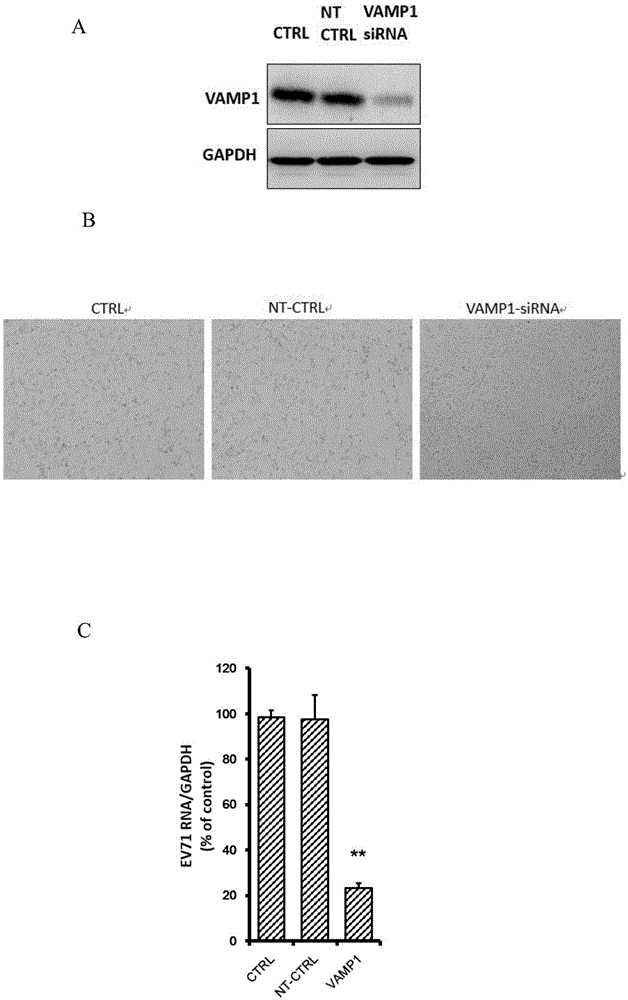
Applications of vesicle-associated membrane protein 1 in prevention and treatment of enterovirus 71 infection
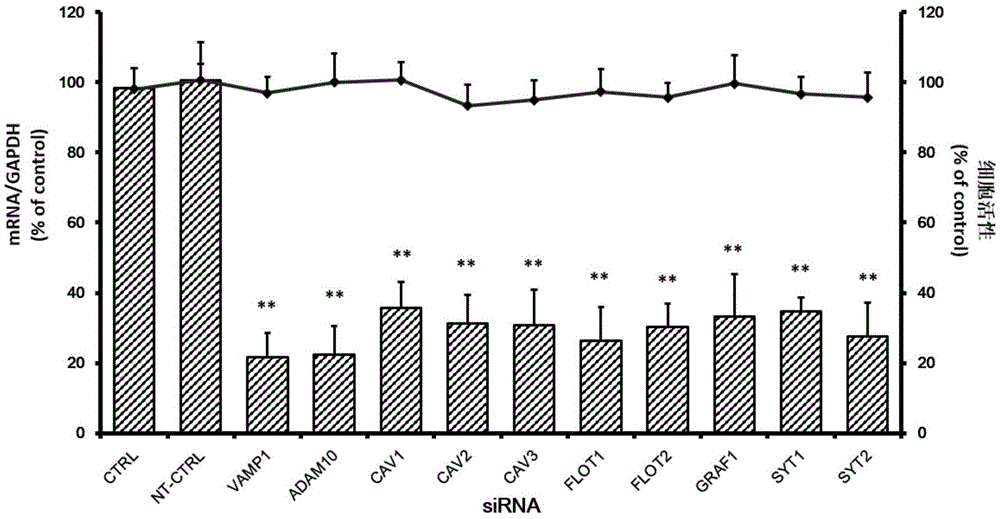
The invention relates to the technical field of biomedicine, and particularly relates to a novel target preventing enterovirus 71 infection and applications thereof. Through adopting a human neuroblastoma cell (SH-SY5Y) as a target cell, and reducing expression of target cell host protein by adopting an RNA interference technique, a host factor effectively inhibiting EV71 infection of the human neuroblastoma cell (SH-SY5Y) is searched so as to prevent central nervous system virus infection and reducing damage to the nerve system. Experiments show that vesicle-associated membrane protein 1 (called as VAMP1 for short) plays an important role in EV71 infection of the human neuroblastoma cell (SH-SY5Y) and EV71 infection can be obviously inhibited by reducing expression of the VAMP1. The invention provides applications of the VAMP1 in preparation of medicines preventing and treating the enterovirus 71 infection.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com