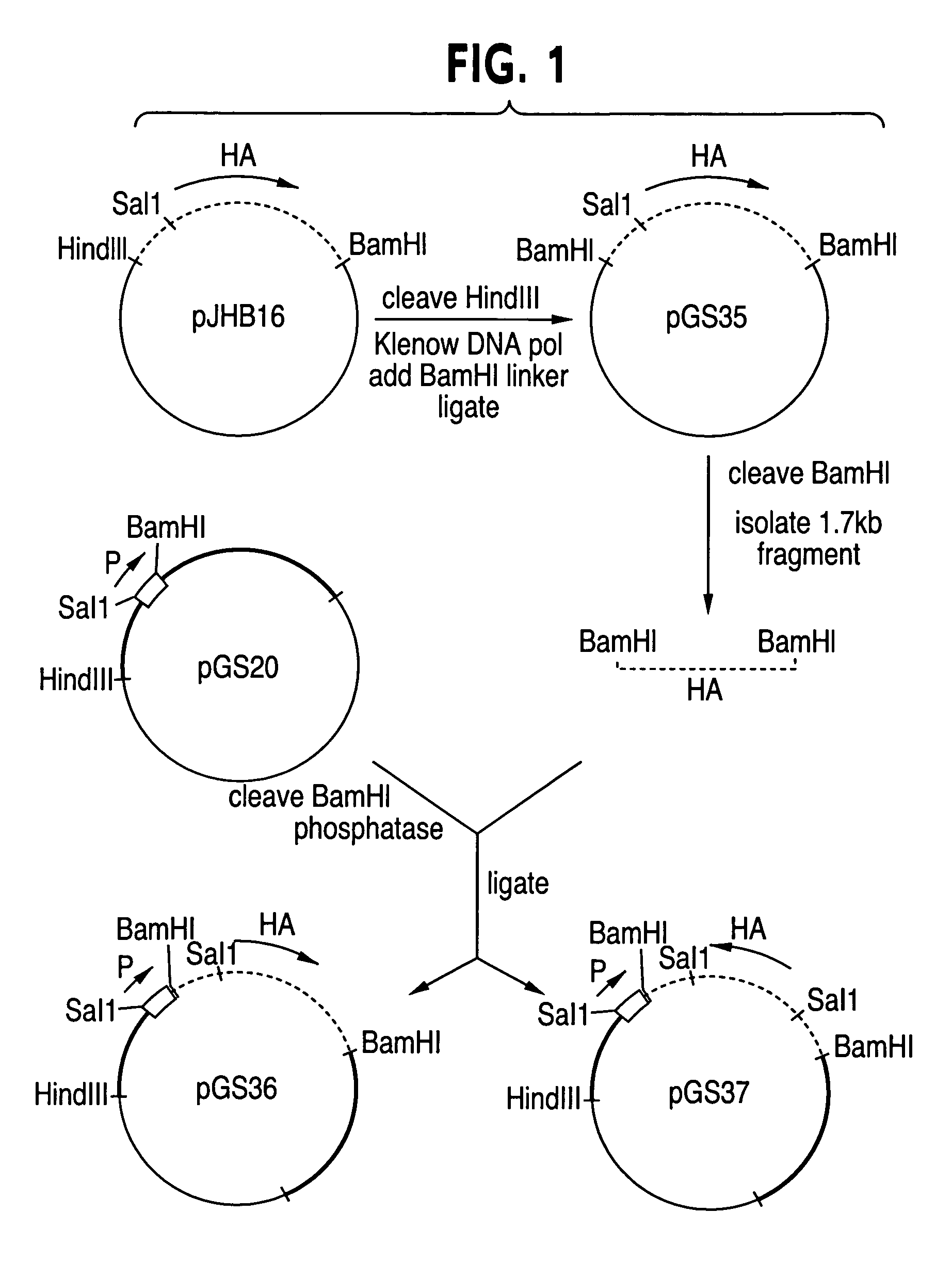
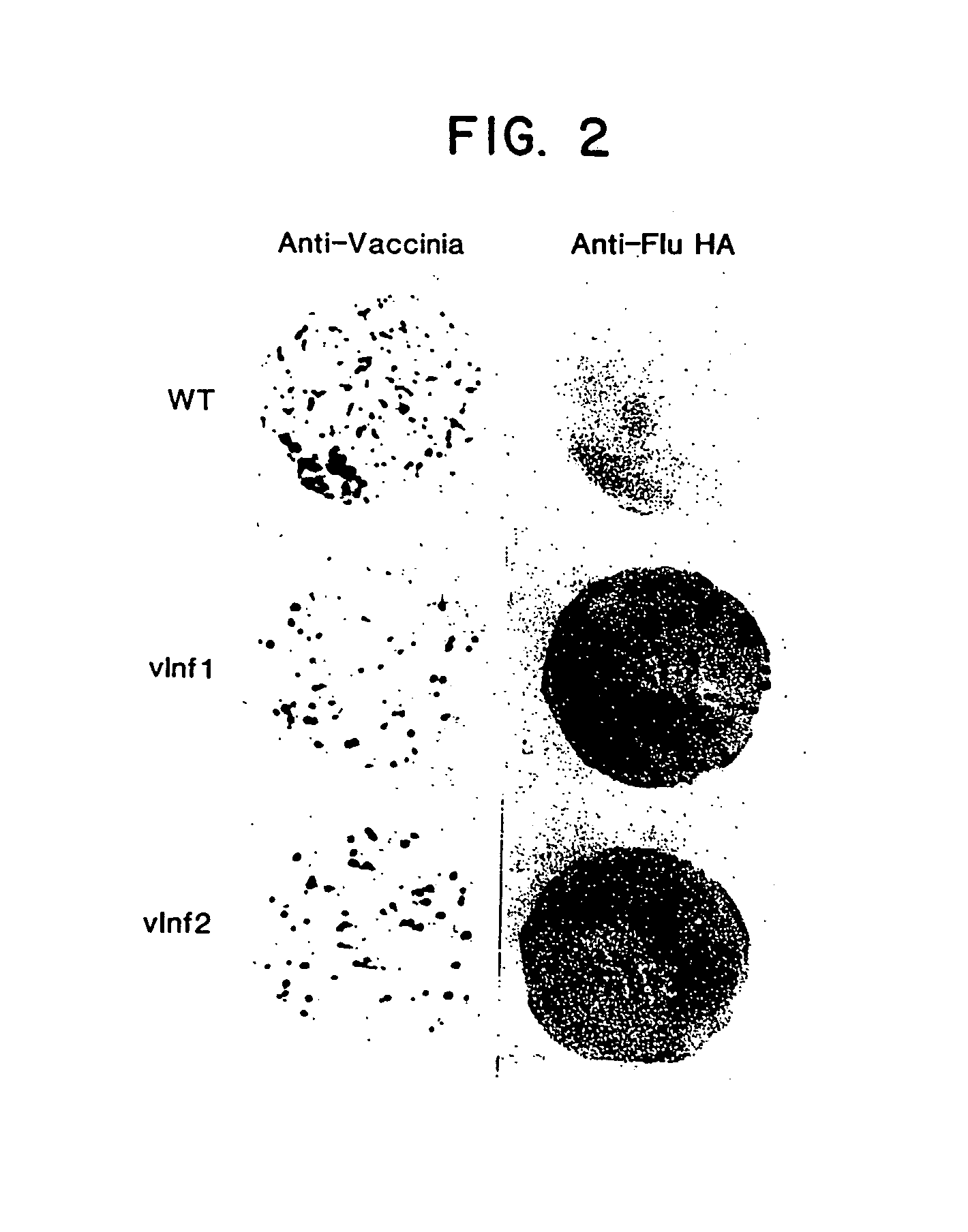
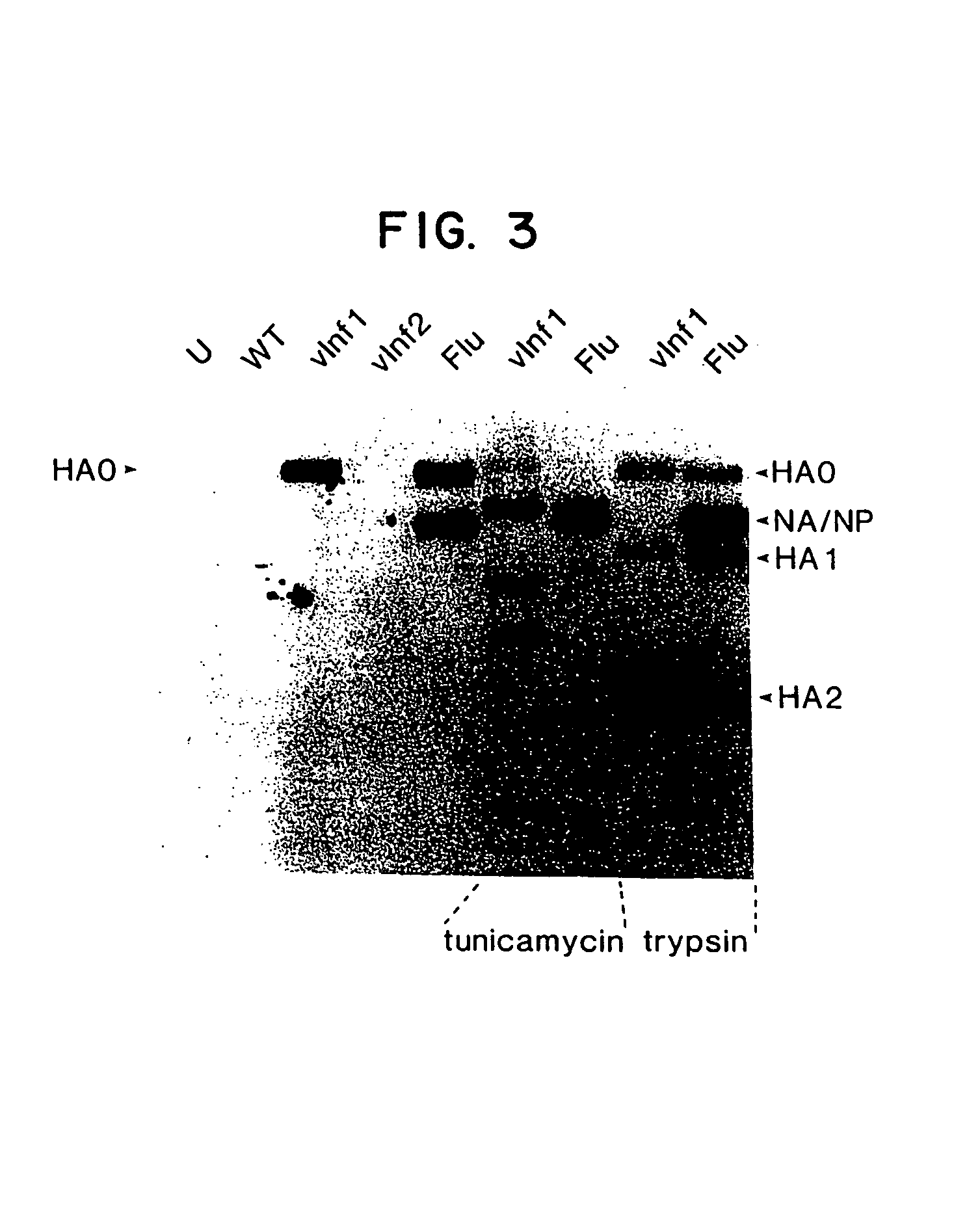
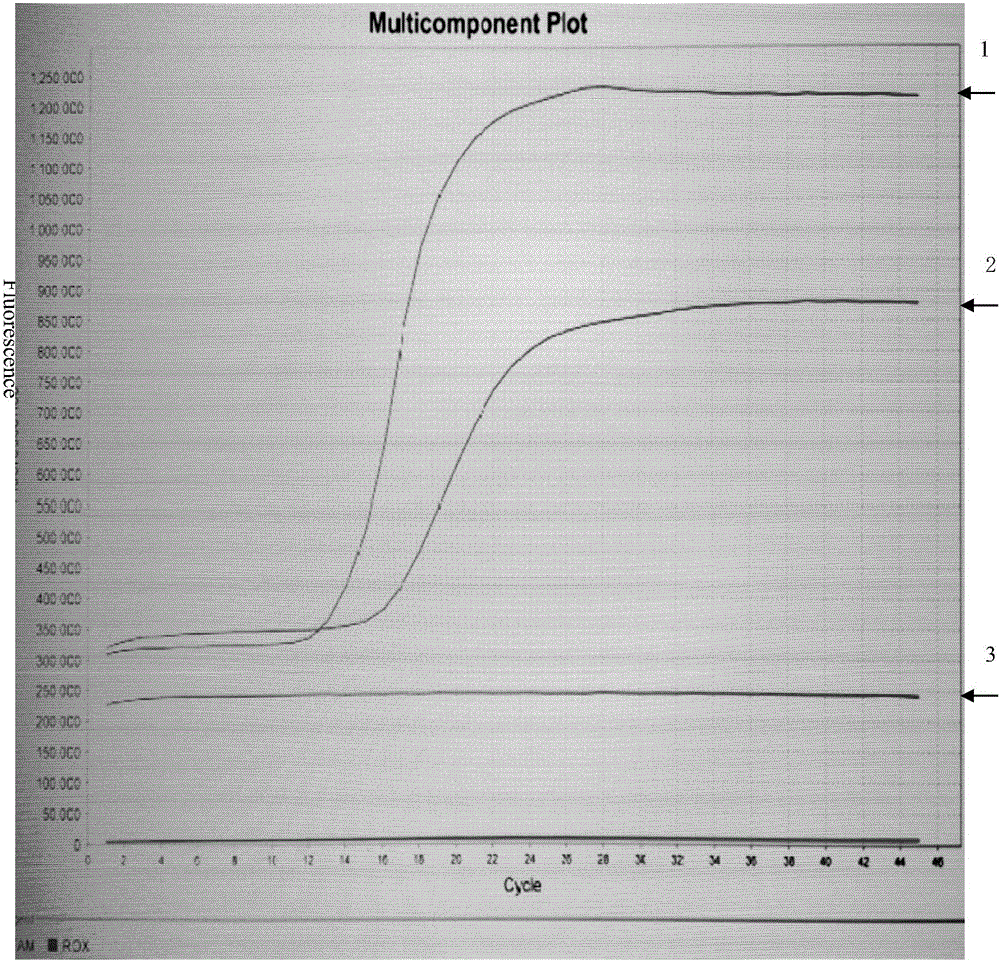
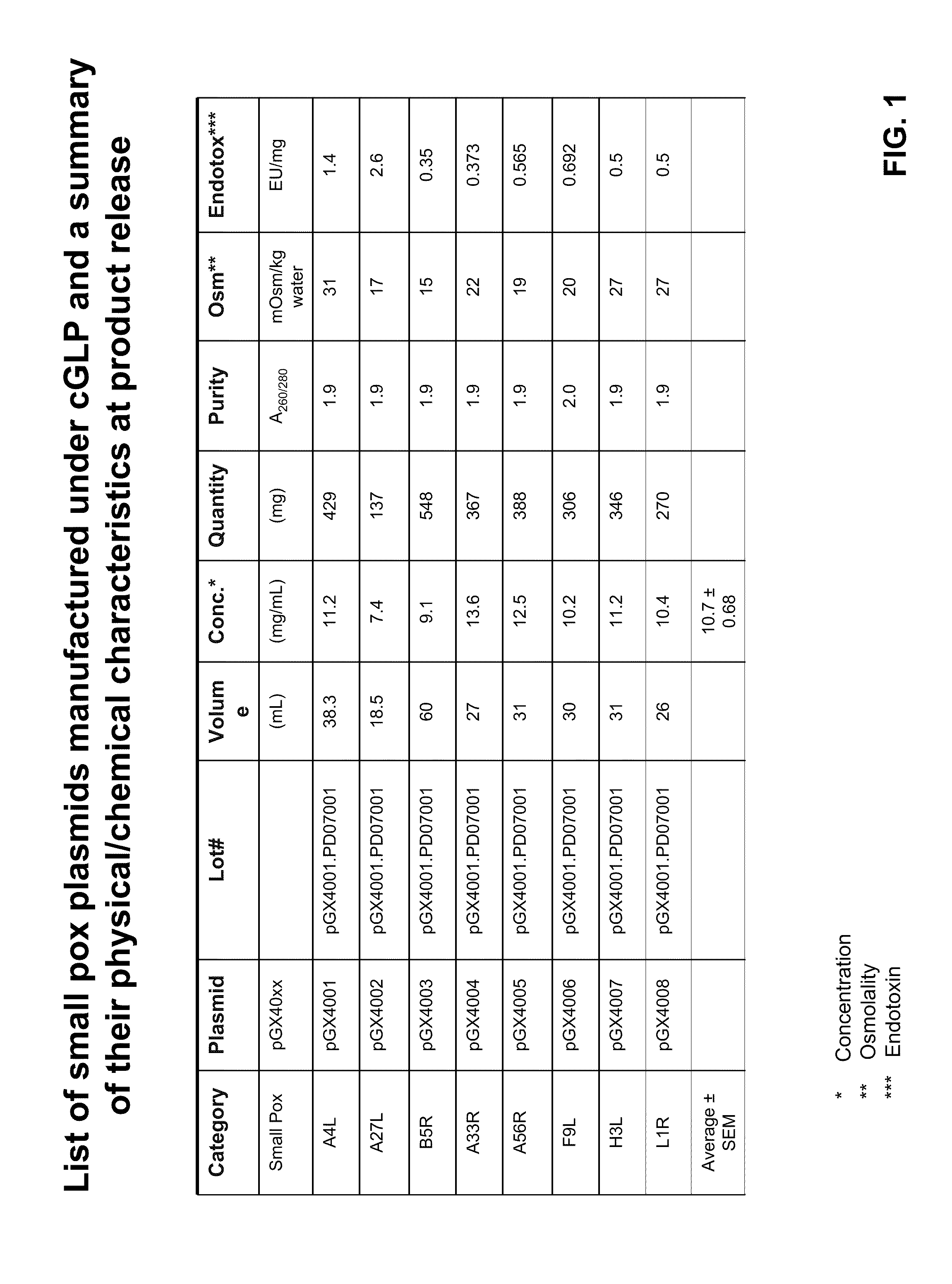
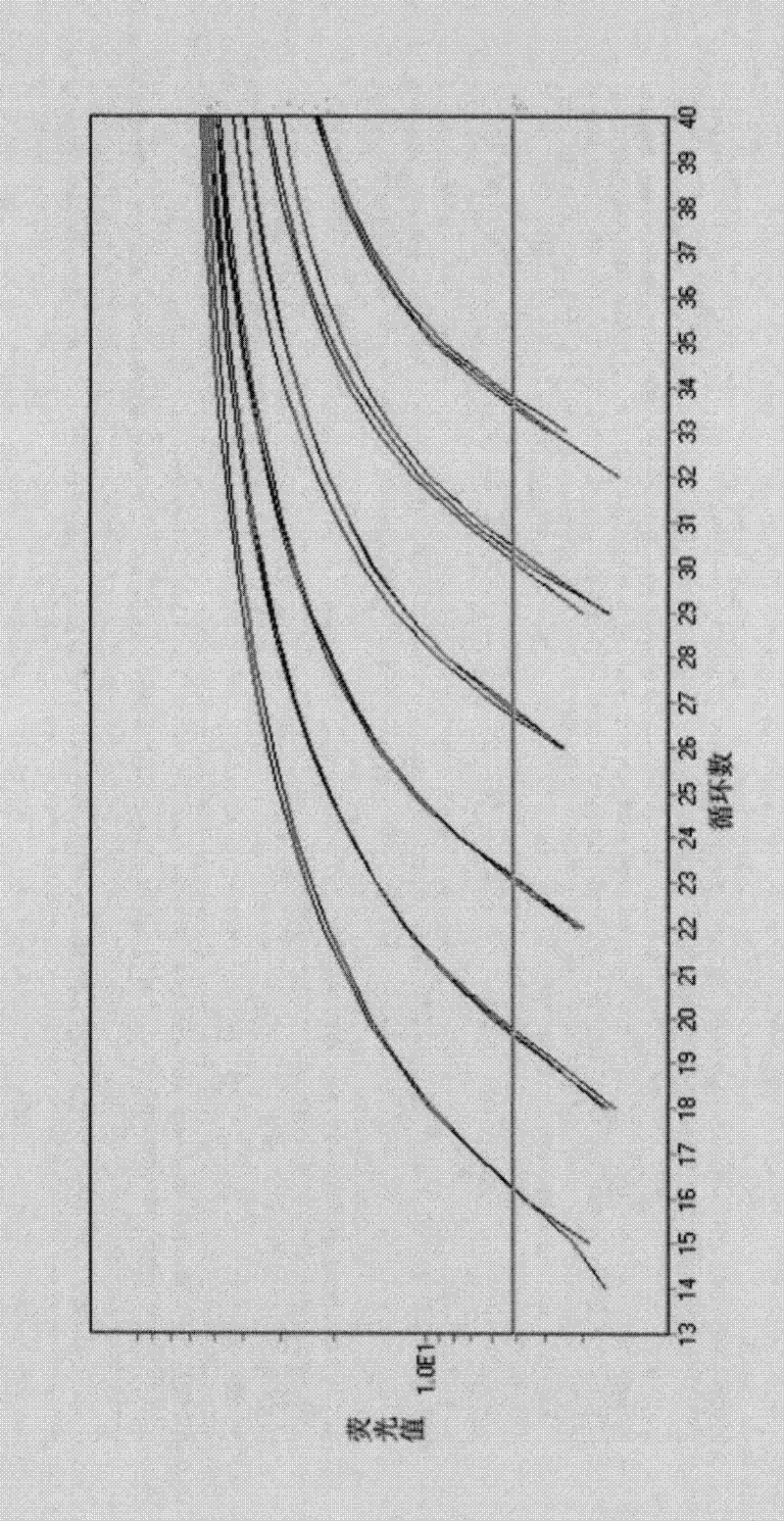
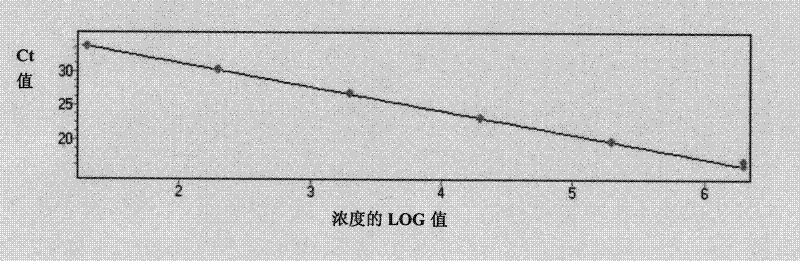
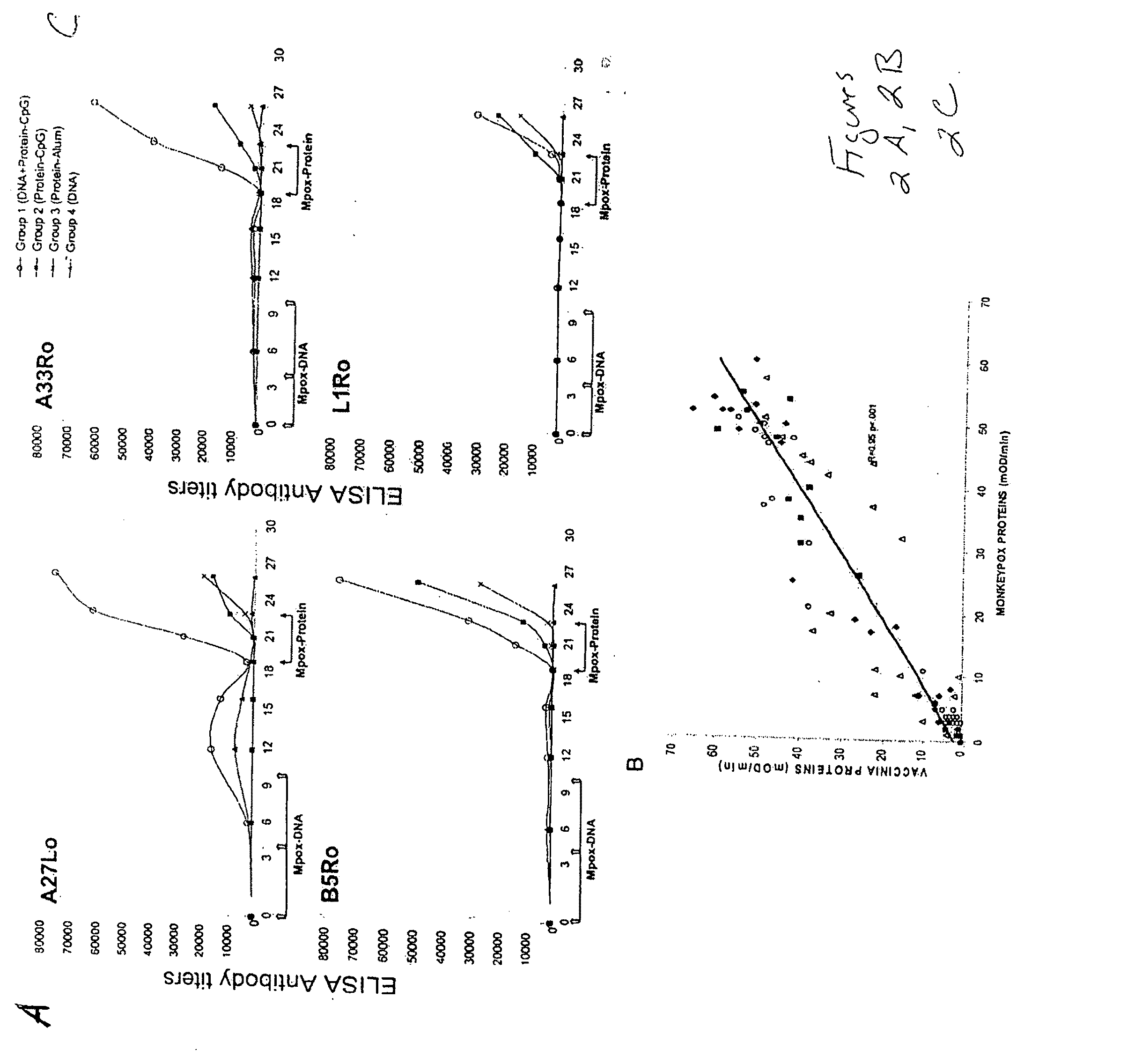
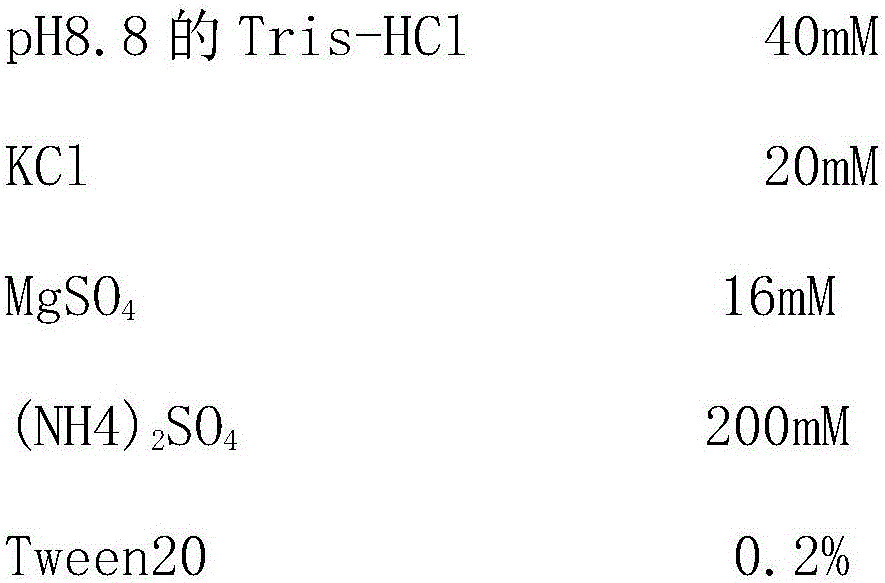Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
136 results about "Pox viruses" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
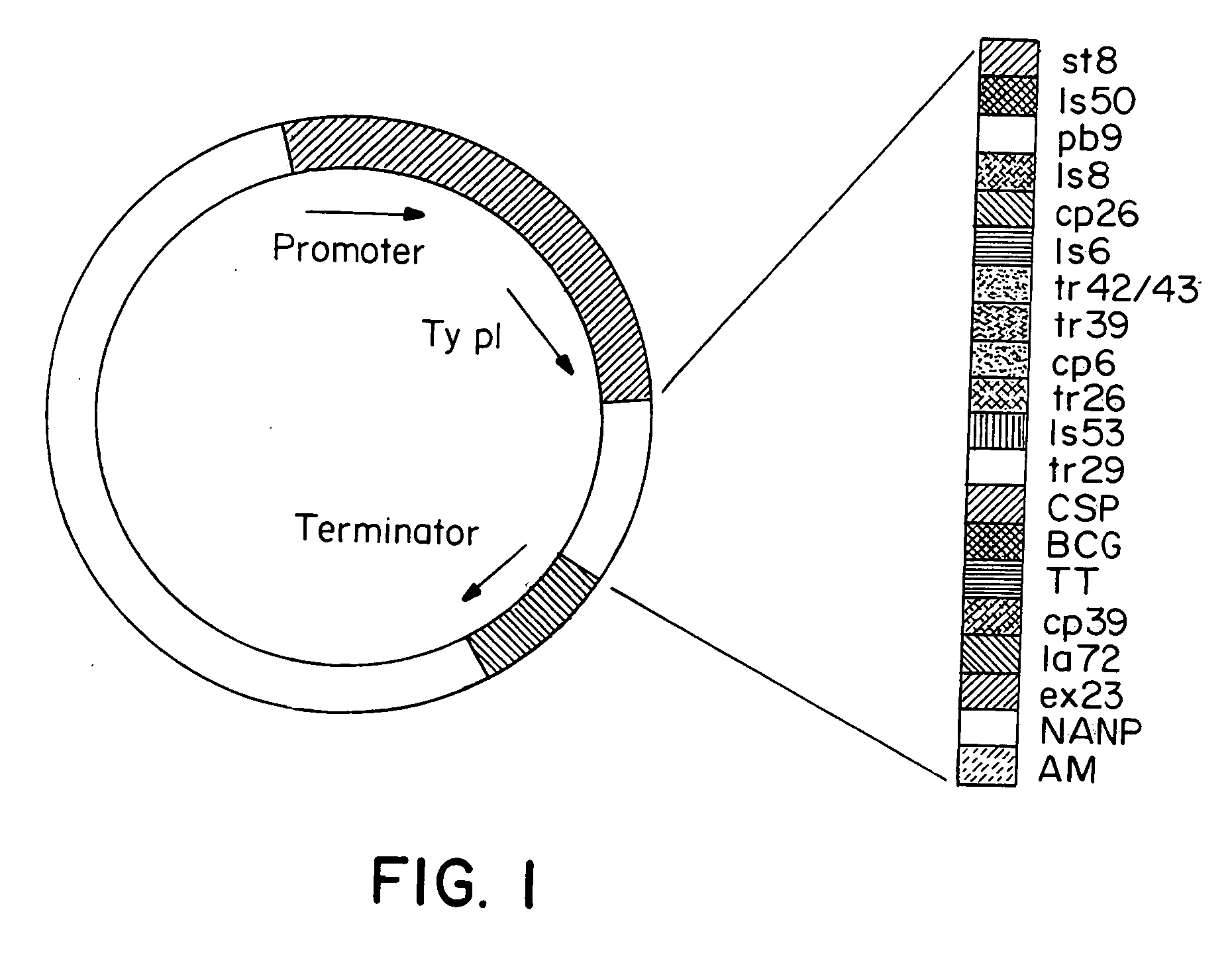
Recombinant poxviruses having foreign DNA expressed under the control of poxvirus regulatory sequences
InactiveUS6998252B1SsRNA viruses negative-senseViral antigen ingredientsTranscriptional regulationVaccinia
Recombinant poxviruses, such as vaccinia, are provided that comprises a segment comprised of (A) a first DNA sequence encoding a polypeptide that is foreign to poxvirus and (B) a poxvirus transcriptional regulatory sequence, wherein (i) said transcriptional regulatory sequence is adjacent to and exerts transcriptional control over said first DNA sequence and (ii) said segment is positioned within a nonessential genomic region of said recombinant poxvirus. Vaccines, carriers, cells, and media comprising recombinant poxviruses, and methods of immunization with recombinant poxviruses also are provided.
Owner:DEPT OF HEALTH & HUMAN SERVICES UNITED STATES OF AMERICA AS REPRESENTED BY THE SEC
Methods and compositions concerning poxviruses and cancer
InactiveUS20060099224A1Improve anti-tumor efficacyTreatment effectPeptide/protein ingredientsGenetic material ingredientsCancer cellAnti viral response
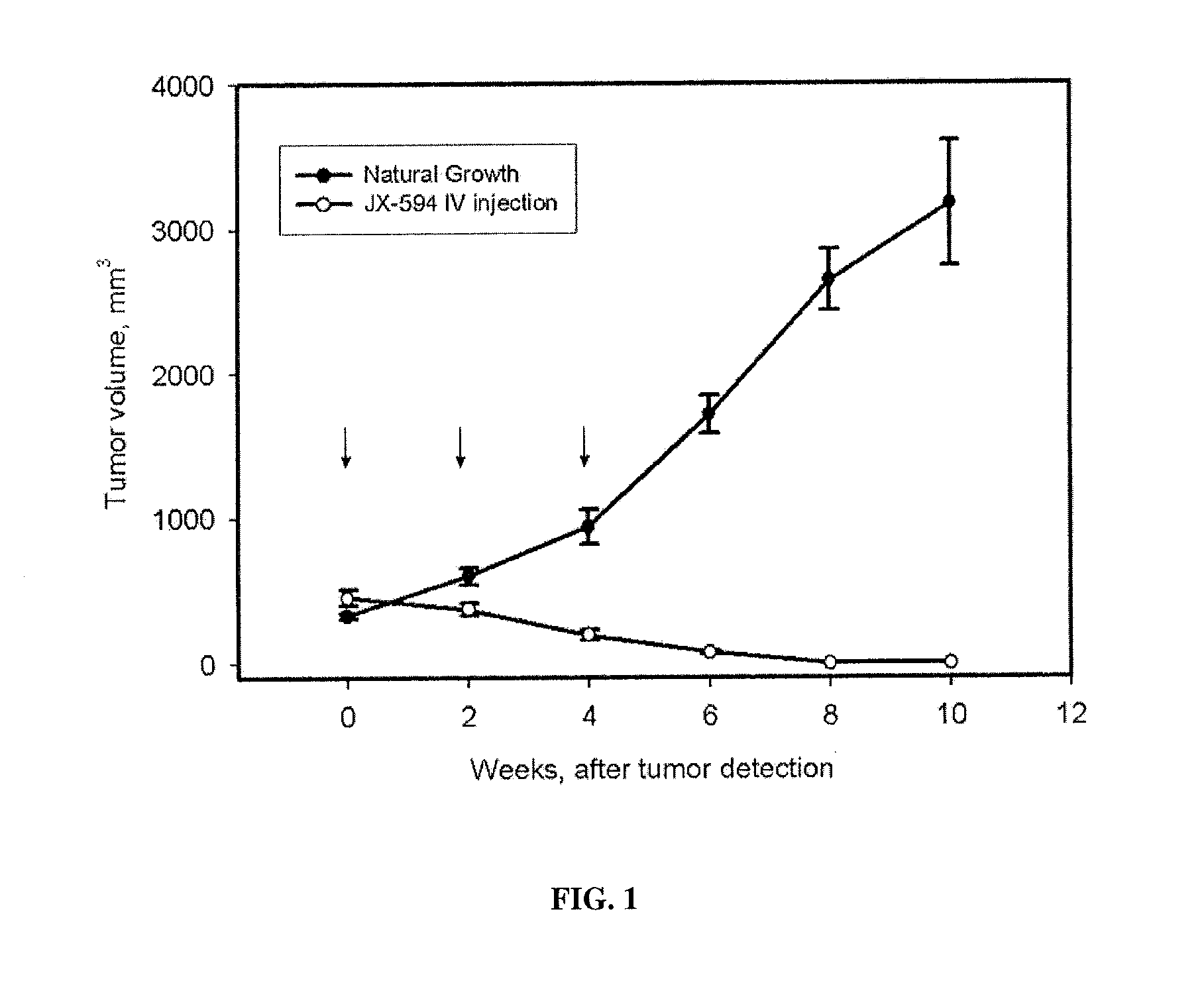
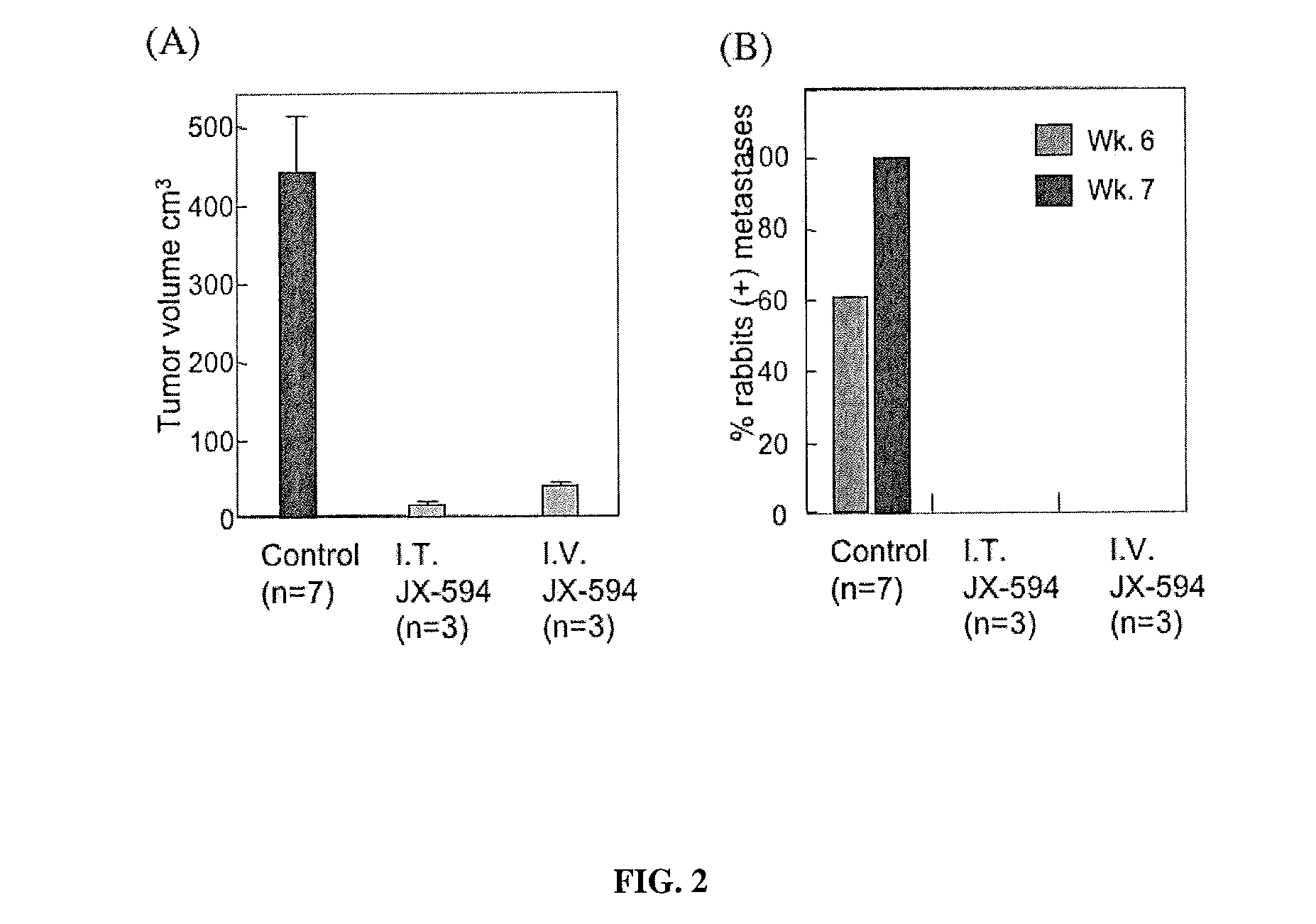
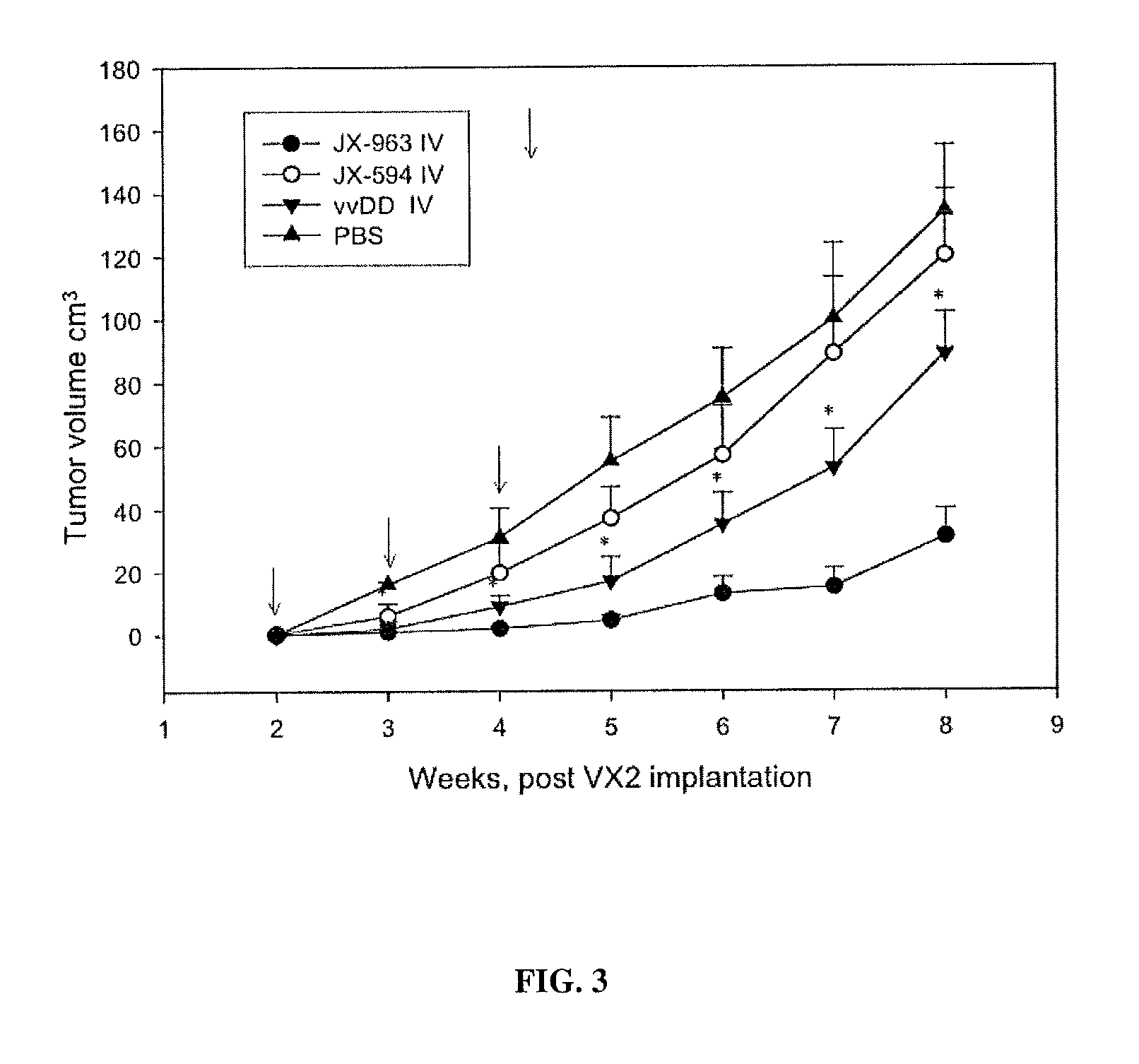
The present invention concerns methods and compositions for the treatment of cancer and cancer cells using altered poxviruses, including a vaccinia virus that has been altered to generate a more effective therapeutic agent. Such poxviruses are engineered to be attenuated or weakened in their ability to affect normal cells. In some embodiments, methods and compositions involve poxviruses that possess mutations that result in poxviruses with diminished or eliminated capability to implement an antiviral response in a host. Poxviruses with these mutations in combination with other mutations can be employed for more effective treatment of cancer.
Owner:JENNEREX BIOTHERAPEUTICS ULC
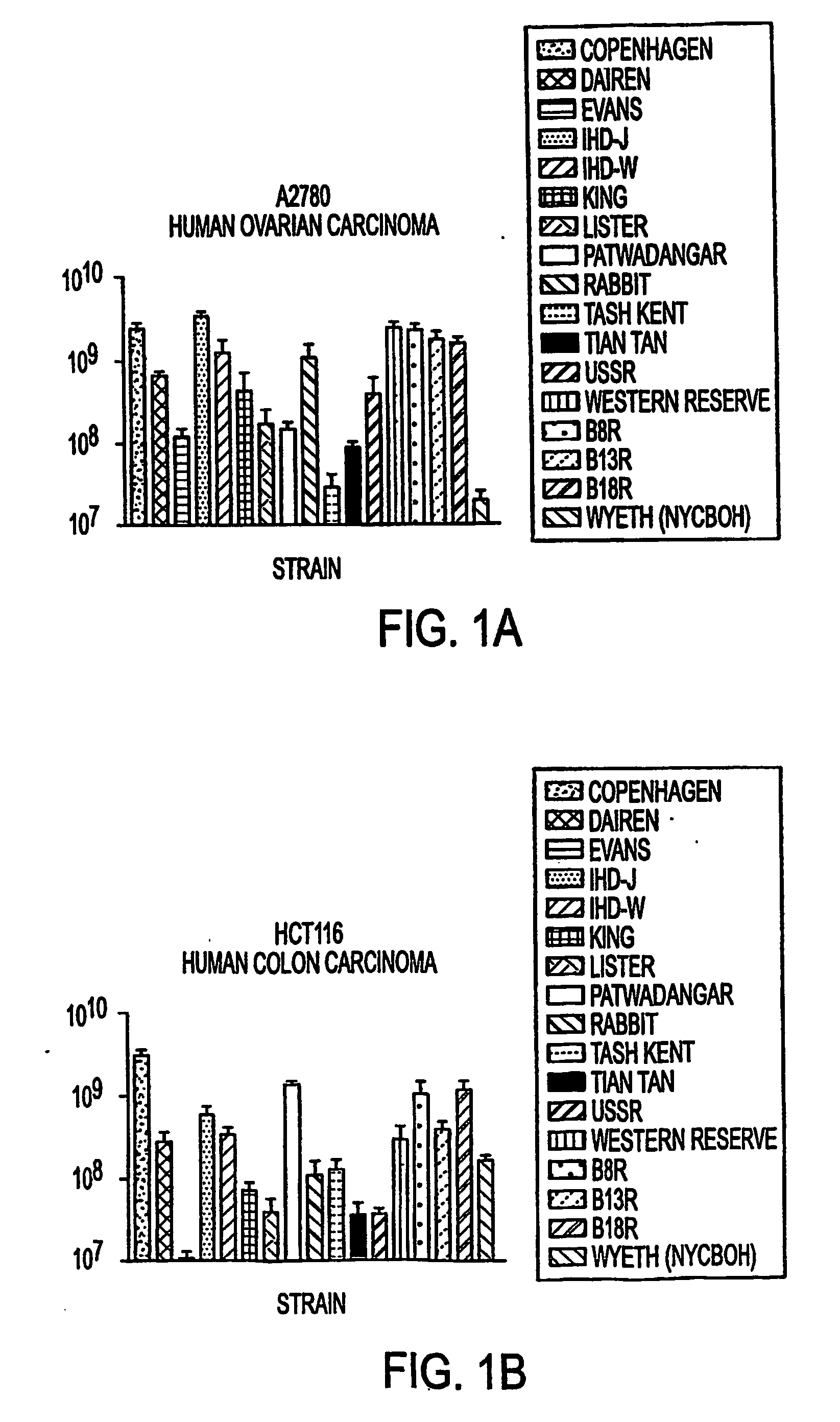
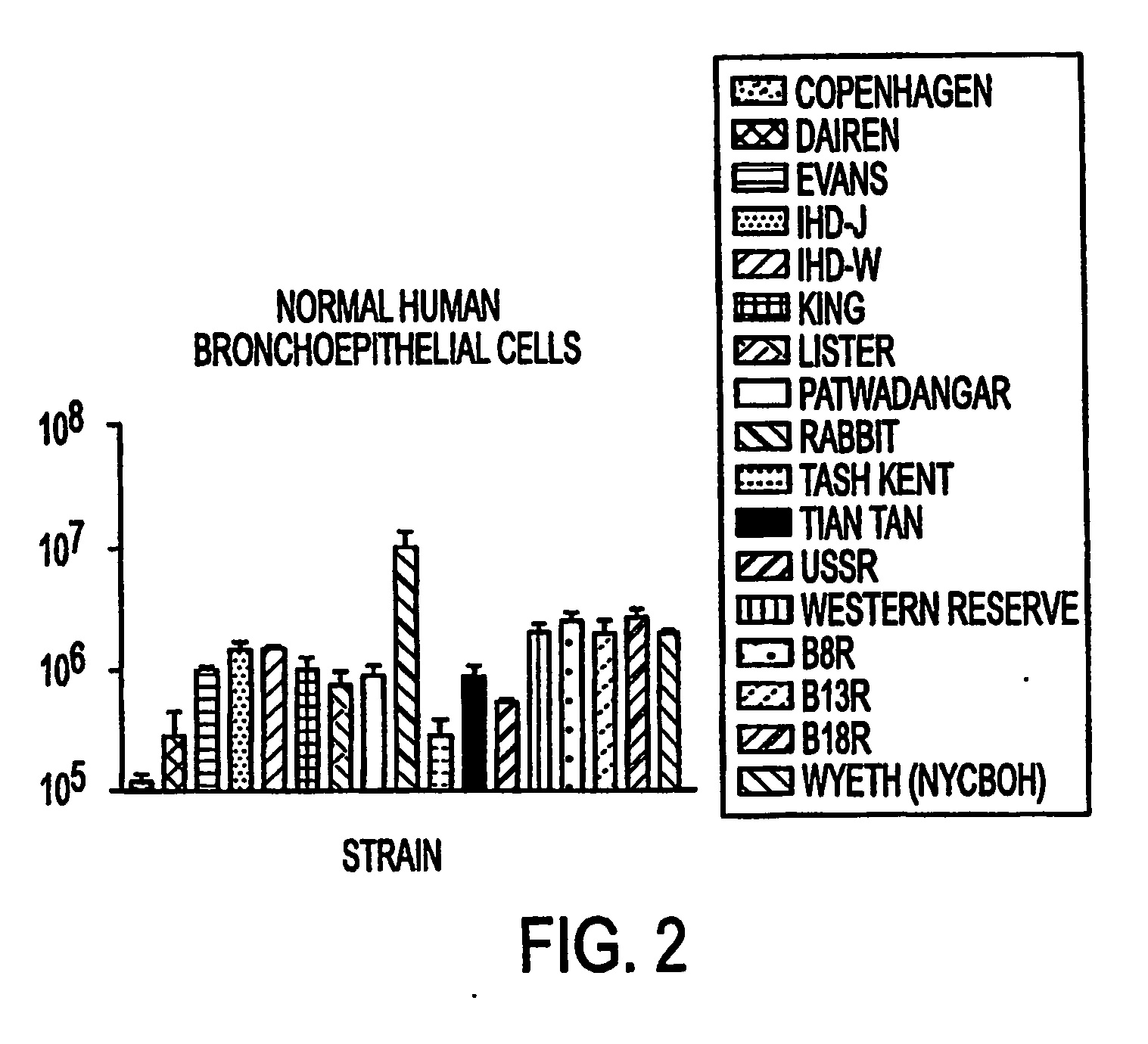
Oral smallpox vaccine production and methods to evaluate safety, efficacy, and potency of orally delivered vaccine
InactiveUS20040175398A1Efficient responseSafety and efficacyViral antigen ingredientsMicrobiological testing/measurementHuman useDiagnostic test
This invention relates to methods and systems for generating a safe and effective oral smallpox vaccine for humans using a genetically defective strain of vaccinia virus to confer immunity following oral delivery of the vaccine. This invention is one that expands on current use of vaccinia virus propagation developed for gene therapy applications, and pharmaceuticals and nutraceuticals packaging and formualtion technologies. The vaccine invention can be delivered as a live virus with the ability to express viral proteins but unable to achieve complete, lytic virus replication, or it may be derived from such a virus, contain additional immunogens, or be delivered as viral antigens. Furthermore, the invention establishes innovative methods for formulation and packaging and for preclinical testing of the vaccine invention for safety, efficacy and potency with the use of human intestinal and other test cells and diagnostic test systems and kits.
Owner:INCELLS
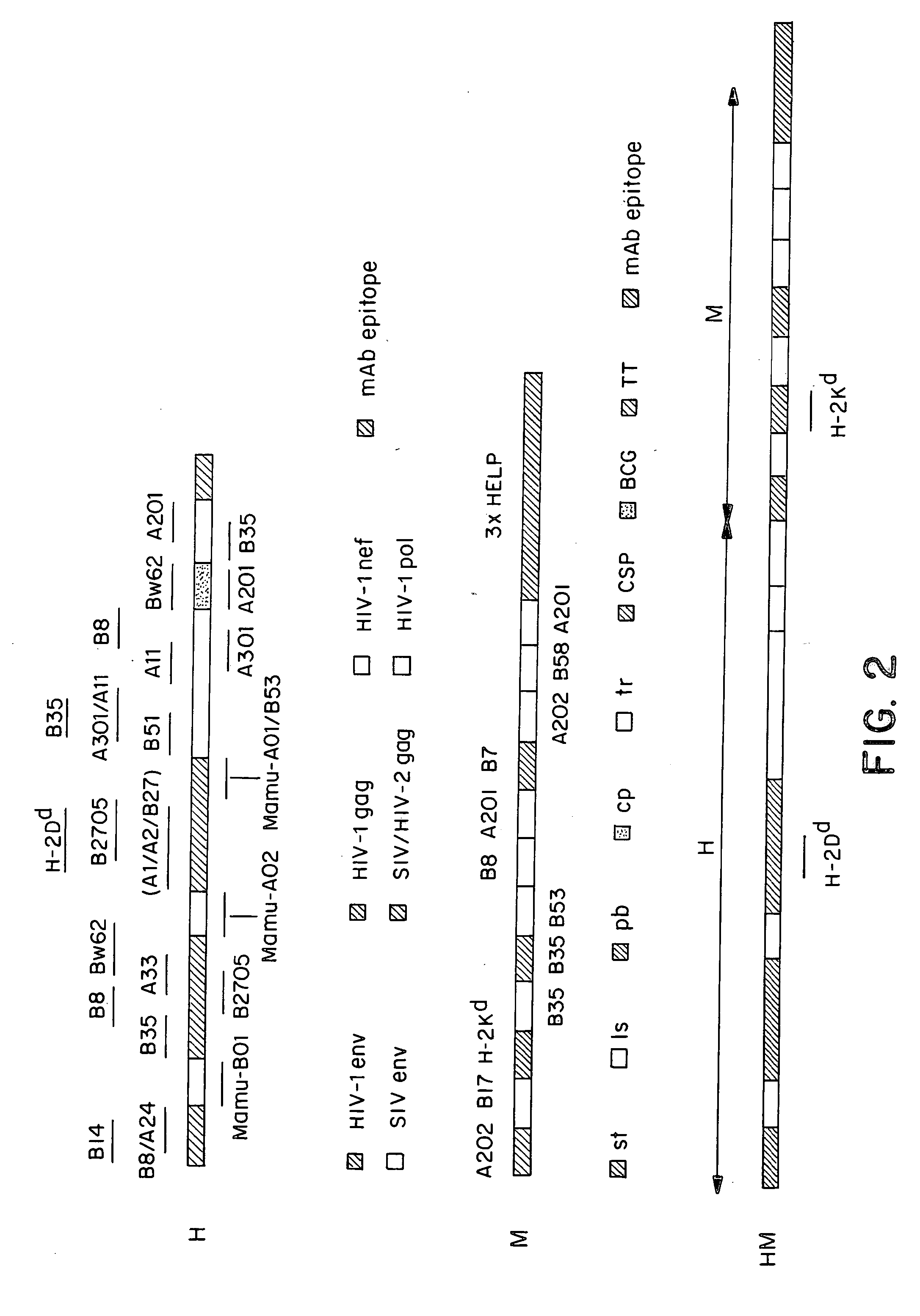
Methods and reagents for vaccination which generate a CD8 T cell immune response
New methods and reagents for vaccination are described which generate a CD8 T cell immune response against malarial and other antigens such as viral and tumour antigens. Novel vaccination regimes are described which employ a priming composition and a boosting composition, the boosting composition comprising a non-replicating or replication-impaired pox virus vector carrying at least one CD8 T cell epitope which is also present in the priming composition.
Owner:OXXON THERAPEUTICS LTD
Tecovirimat dry suspension and preparation method thereof
ActiveCN102406617AGood liquidityLess foamOrganic active ingredientsPowder deliveryChemistryHalf-life
The invention discloses a tecovirimat dry suspension and a preparation method thereof. The tecovirimat dry suspension provided by the invention comprises the following components in parts by mass: 20 to 30 parts of monoclinic crystal micro-powder of tecovirimat monohydrate, 40 to 80 parts of filler, 5 to 10 parts of suspending aid, and 1 to 5 parts of wetting agent, wherein, in the monoclinic crystal micro-powder of tecovirimat monohydrate, the space group of a monoclinic system is C2 / c, cell parameters are as follows: a is 28.724 (2), b is 10.533 (1), c is 12.902 (1) tenthmeter and beta is 112.18 (1) degrees, the cell volume V is 3614.7 (6) cubic tenthmeter, and the number Z of molecules in unit cell is 8. The dry suspension solid powder has good liquidity, can be quickly transformed into a uniform and stable suspension after being mixed with water, has less foam, low deposition speed and excellent redispersibility, and is convenient for administration of patients. Animal experimentsprove that: the preparation has long half-life period, high blood concentration and strong mousepox virus resisting activity.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Methods and Compositions Concerning Poxviruses and Cancer
InactiveUS20090004723A1Less effectImprove clearanceBiocidePeptide/protein ingredientsCancer cellAnti viral response
The present invention concerns methods and compositions for the treatment of cancer and cancer cells using altered poxviruses, including a vaccinia virus that has been altered to generate a more effective therapeutic agent. Such poxviruses are engineered to be attenuated or weakened in their ability to affect normal cells. In some embodiments, methods and compositions involve poxviruses that possess mutations that result in poxviruses with diminished or eliminated capability to implement an antiviral response in a host. Poxviruses with these mutations in combination with other mutations can be employed for more effective treatment of cancer.
Owner:SILLAJEN BIOTHERAPEUTICS
Identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes
The present invention relates to the identification of gene sequences and proteins involved in vaccinia virus dominant T cell epitopes. Two vaccinia virus CD8+ T cell epitopes restricted by the most common human MHC class I allele, HLA-A0201 have been identified. Both epitopes are highly conserved in vaccinia and variola viruses. The induction of the T cell responses following primary vaccination is demonstrated by the kinetics of epitope specific CD8+ T cells in 3 HLA-A0201 individuals. This information will be useful for the design and analyses of the immunogenicity of experimental vaccinia vaccines, and for basic studies of human T cell memory.
Owner:UNIV OF MASSACHUSETTS MEDICAL SCHOOL
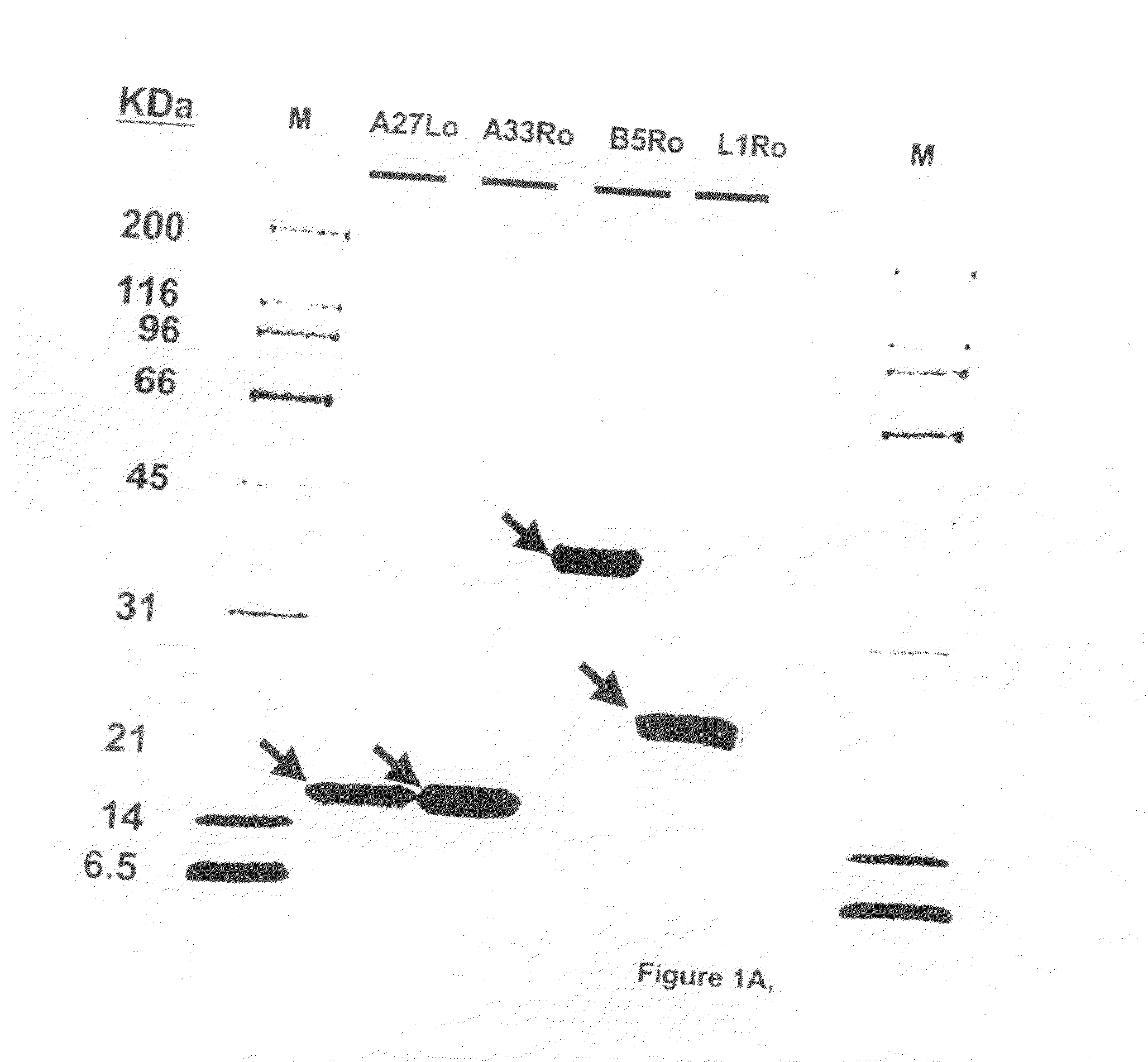
Protein vaccines against poxviruses
InactiveUS20100196491A1High degreeIncreased cross-reactivityPowder deliveryViral antigen ingredientsOpen reading frameMonkeypox
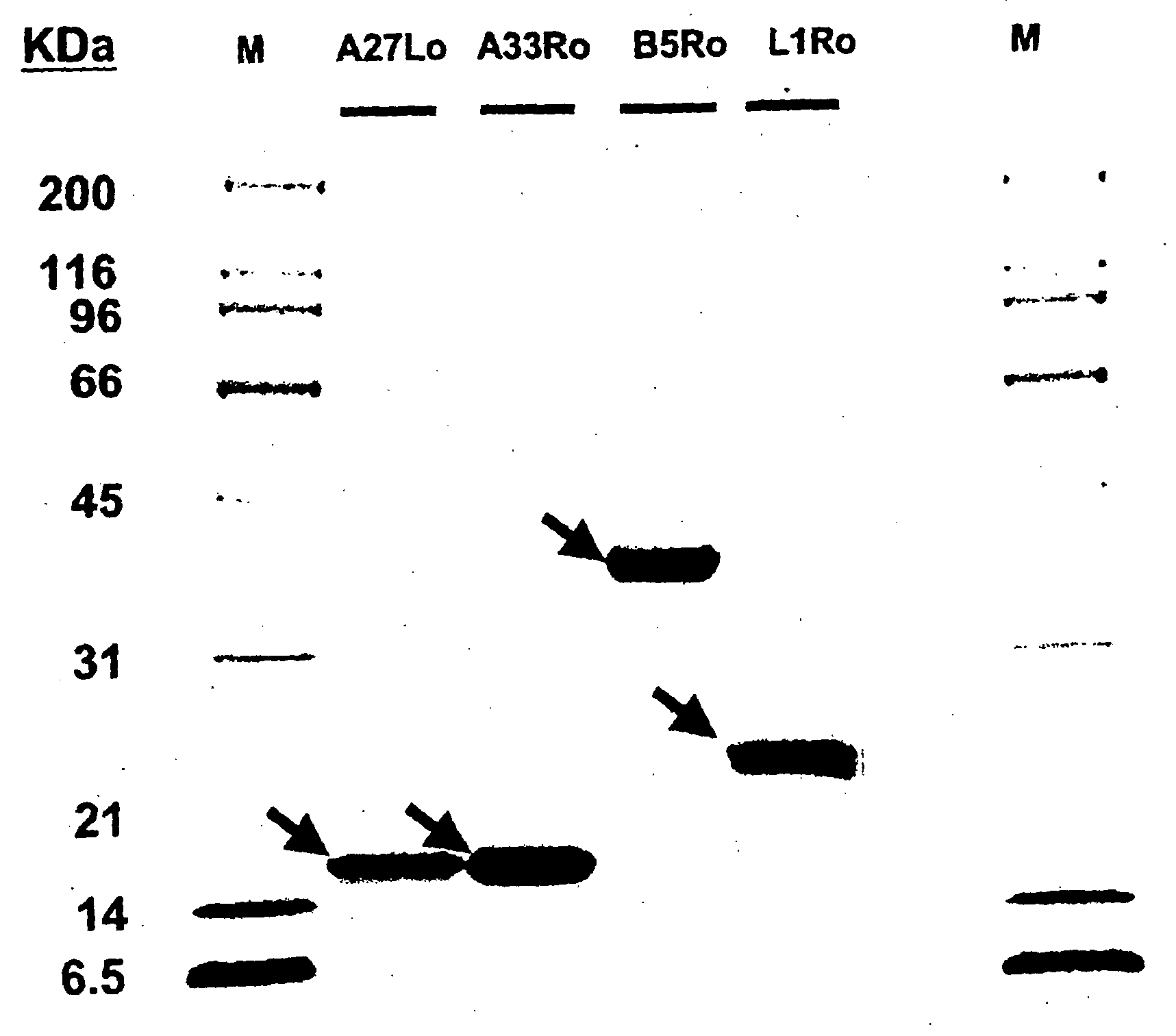
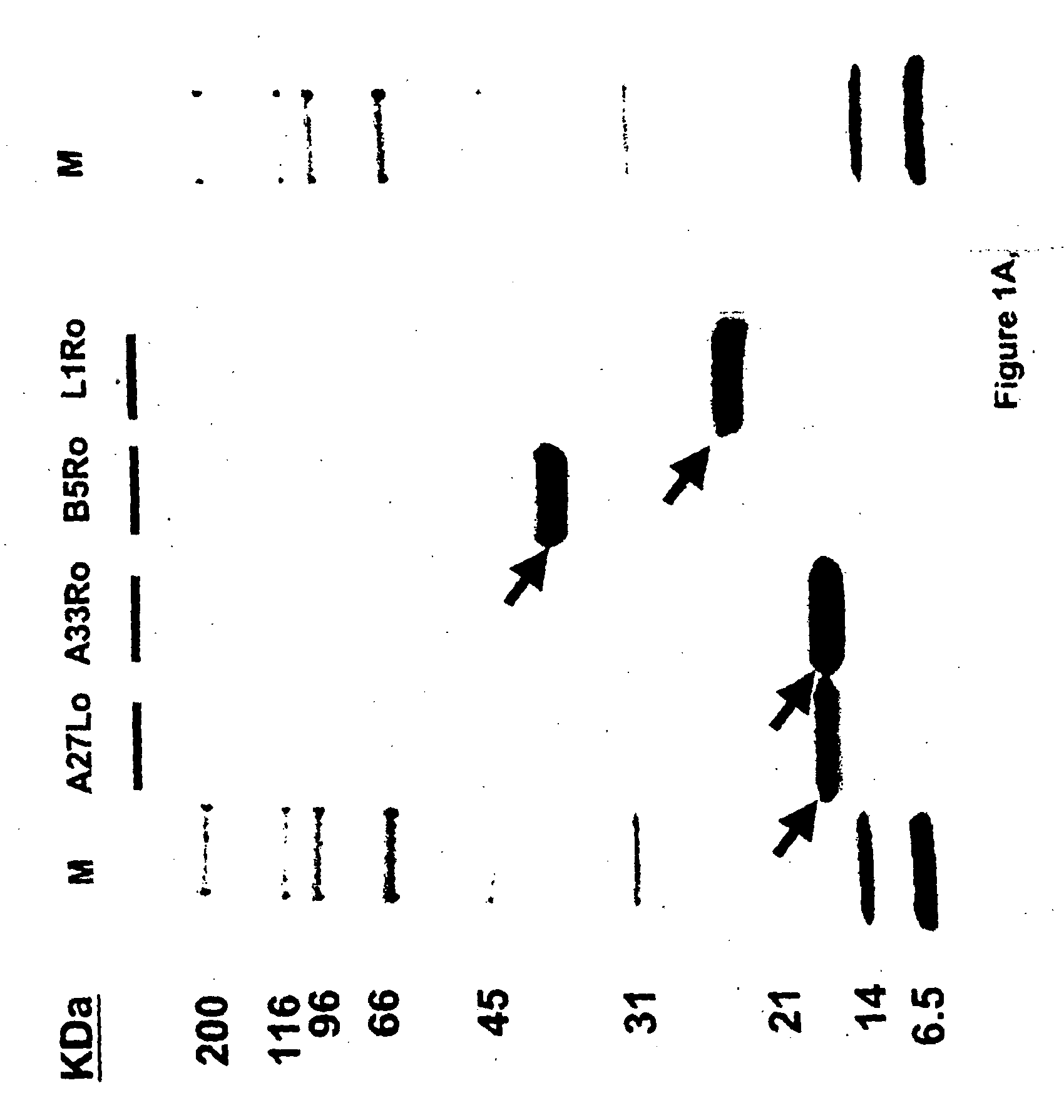
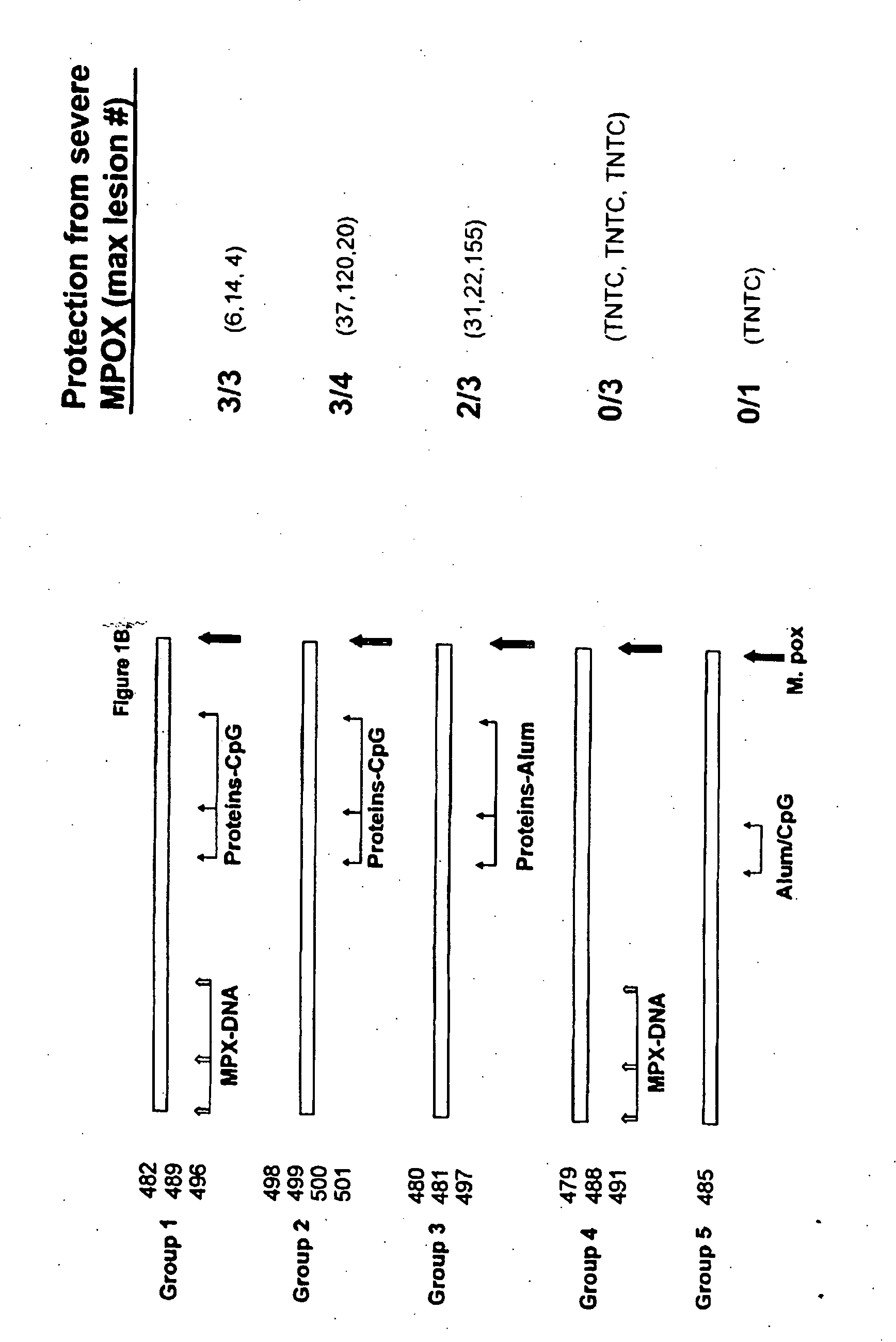
The invention described here entails a protein vaccine against poxviruses which contains at least two purified recombinant monkeypox virus proteins or peptides. The proteins or peptides are encoded by the open reading frames of the monkeypox ortholog genes M1R, A35R, A29L B6R, and orthologs of these proteins or peptides having 90% identity. The invention also entails a vaccine protocol against poxvirus whereby a vaccine is vaccinated with a first vaccine made up of a nucleic acid vaccine of three or more poxvirus virus genes, and subsequently vaccinated with at least one other booster vaccine made up of two or more poxvirus virus proteins.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Oncolytic vaccinia virus cancer therapy
Owner:SILLAJEN BIOTHERAPEUTICS
Vaccine
The present invention relates to a fowlpox virus genome which has modifications in one or more wild-type FPV genes. The present invention also relates to a viral particle comprising such a genome and its use to deliver a nucleotide of interest (NOI) to a target cell. The present invention also relates to vaccination methods, particularly a method which comprises administering a priming composition (which comprises a first non-replicating viral vector) and a boosting composition (which comprises a second non-replicating viral vector) to a subject to treat and / or prevent a disease.
Owner:ISIS INNOVATION LTD
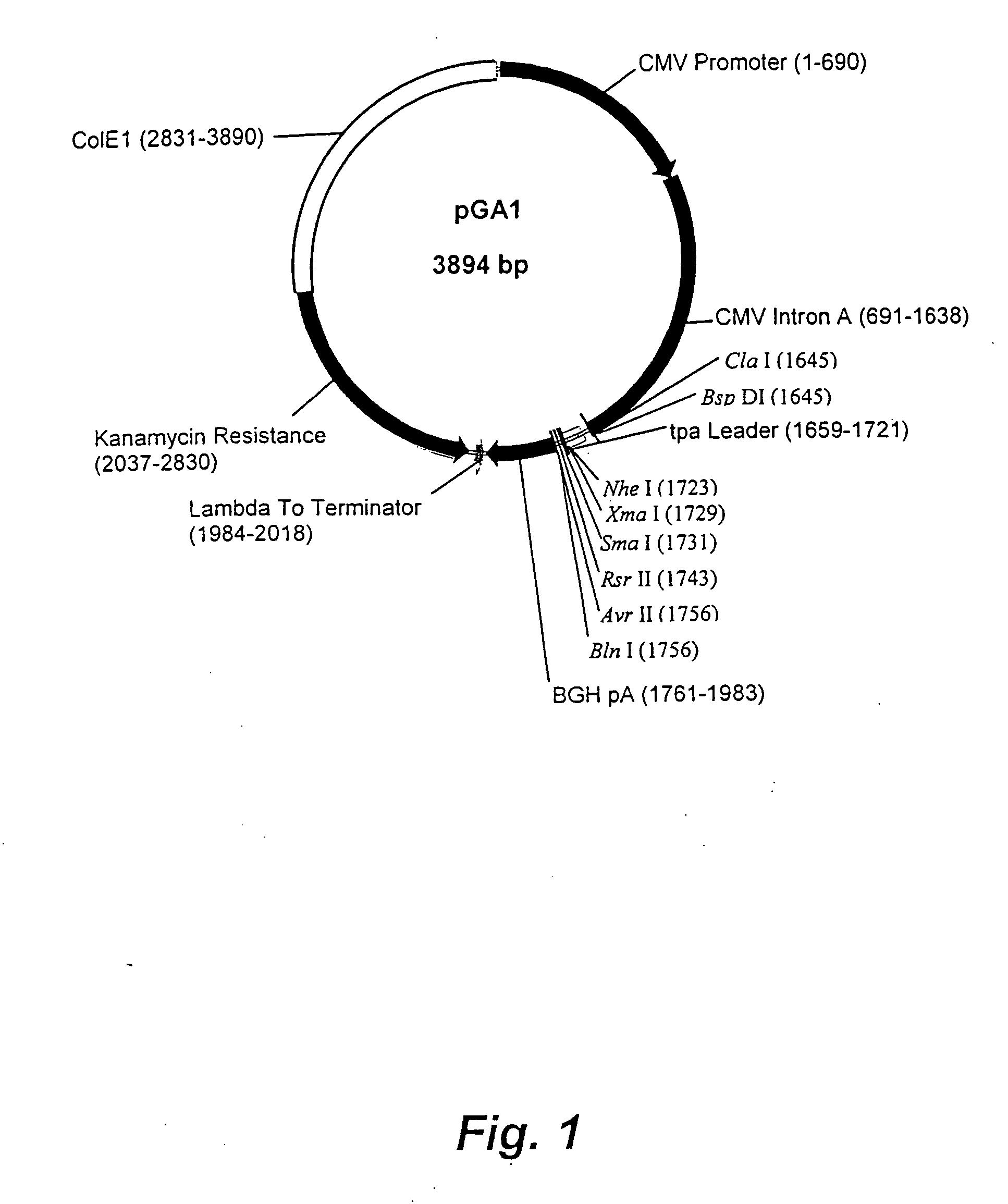
DNA expression vectors and methods of use
The present invention relates to novel plasmid constructs useful for the delivery of DNA vaccines. The present invention provides novel plasmids having a transcription cassette capable of directing the expression of a vaccine nucleic acid insert encoding immunogens derived from any pathogen, including fungi, bacteria and viruses. The present invention, however, is particularly useful for inducing in a patient an immune response against pathogenic viruses such as HIV, measles or influenza. Immunodeficiency virus vaccine inserts of the present invention express non-infectious HIV virus-like particles (VLP) bearing multiple viral epitopes. VLPs allow presentation of the epitopes to multiple histocompatability types, thereby reducing the possibility of the targeted virus escaping the immune response. Also described are methods for immunizing a patient by delivery of a novel plasmid of the present invention to the patient for expression of the vaccine insert therein. Optionally, the immunization protocol may include a booster vaccination that may be a live vector vaccine such as a recombinant pox virus or modified vaccinia Arbora vector. The booster live vaccine vector includes a transcription cassette expressing the same vaccine insert as the primary immunizing vector.
Owner:EMORY UNIVERSITY
Highly Attenuated Pox Virus Strains, Method for the Production Thereof and the Use Thereof as Paramunity Inducers or For Producing Vector Vaccines
The present invention relates to highly attenuated animal smallpox viral strains and to the use thereof as paramunity inducers or for producing vector vaccines. As a result of the high attenuation process, the claimed animal smallpox strains lose their virulent and immunising properties. The invention also relates to a method for producing such highly attenuated pox virus strains and the use thereof for inducing paramunity, i.e. for activating the non-specific immune system in mammals and humans or for producing vector vaccines for specific immunisation with the positive side-effect of paramunisation. The claimed highly attenuated animal smallpox viruses are thus suitable for preventing and treating diseases associated with an immune deficiency. Preferred embodiments relate to highly attenuated orthopox—(e.g. camel smallpox viruses), leporipox—(e.g. myxoma viruses), avipox-, parapox- and other orthopox viral strains, such as MVA, which have excellent paramunisation properties and in which the immunising properties have been lost.
Owner:MAYR ANTON
Colloidal gold test strip based on goat pox virus and preparation method thereof
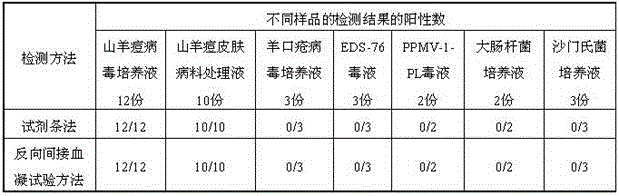
The invention relates to a colloidal gold test strip based on a goat pox virus. The colloidal gold test strip comprises a PVC (Polyvinyl Chloride) rubber plate, a nitrocellulose membrane, a colloidal gold mat, a sample mat and absorbent paper, wherein the sample mat adheres to one end of the PVC rubber plate; the absorbent paper adheres to the other end of the PVC rubber plate; the colloidal gold mat and nitrocellulose membrane adhere to the middle part of the PVC rubber plate in sequence; the sample mat adheres to one end of the golden standard mat; the nitrocellulose membrane adheres to the other end of the colloidal gold mat; the nitrocellulose membrane adheres to the absorbent paper; the colloidal gold mat is coated with an anti-GTPV-P32 protein monoclonal antibody marked with colloidal gold; the nitrocellulose membrane is provided with a detection line which is linearly coated with an anti-GTPV-ORF122 protein monoclonal antibody at the concentration of 1mg / mL, and a quality control line which is linearly coated with a goat anti-mouse IgG (Intravenous Gamma Globulin) antibody at the concentration of 1mg / mL in sequence along the flow direction of a sample. Meanwhile, the invention further discloses a preparation method of the diagnosis strip. The test strip has the characteristics of high specificity, high stability, easiness in operation and rapid detection, and is suitable for field detection at the occurrence of animal epidemic diseases.
Owner:GANSU ANIMAL HUSBANDRY & VETERINARY MEDICINE INST
Use of a varicellovirus tap-inhibitor for the induction of tumor-or virus-specific immunity against teipp
InactiveUS20100092435A1Reduce transportationEffective blockingBiocidePeptide/protein ingredientsAntigenAbnormal tissue growth
The present invention provides a novel approach to the modulation of the immune response, directing it towards specific antigens, away from antigens against which no response is desired. The invention is based on the use of viral immune evasion proteins, such as UL49.5, which block antigen presentation to CD8+ T cells. The viral immune evasion proteins are used for: 1) the induction of tumor-specific or virus-specific immunity in cases where a conventional immune response is absent due to antigen processing defects; 2) the induction of empty MHC class I molecules at the cell surface that can be loaded with peptides of a desired specificity; 3) the inhibition of unwanted immune responses against transplanted tissues or organs, e.g. against islets of Langerhans in type 1 diabetes or allogeneic stem cells, or against self antigens in the case of autoimmunity.
Owner:PUBLIEKRECHTELIJKE RECHTSPERSOON ACADEMISCH ZIEKENHUIS LEIDEN H O D N LEIDS UNIVIR MEDISCH CENT
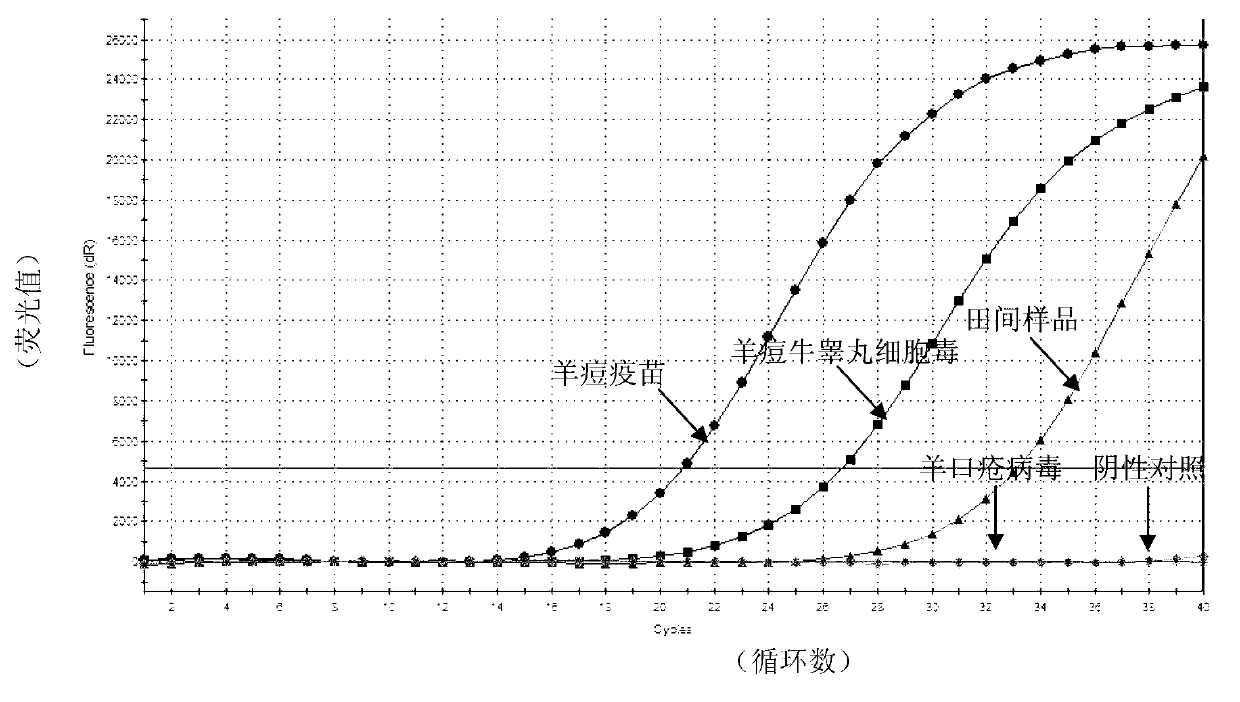
Real-time fluorescent RPA kit and test strip RPA kit for rapidly detecting capripoxvirus and application thereof
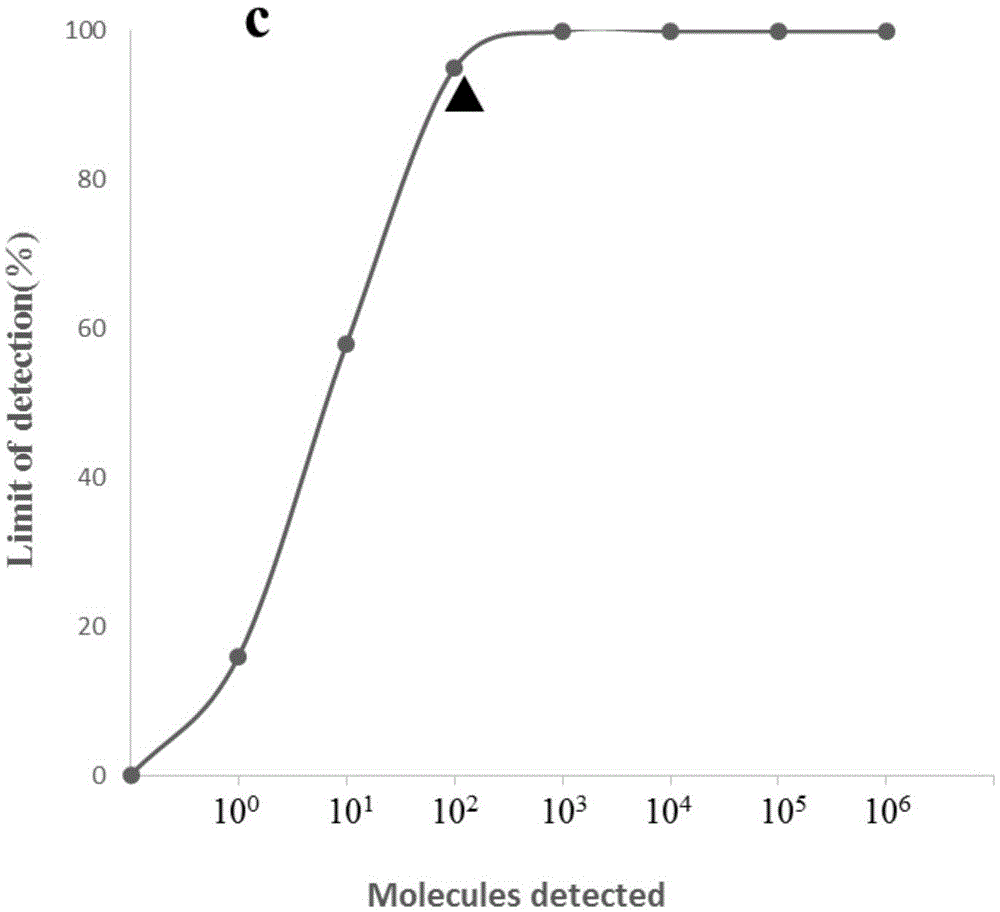
ActiveCN105567873AShorten test timeSimple methodMicrobiological testing/measurementMicroorganism based processesCapripoxvirusPox viruses
The invention discloses a real-time fluorescent RPA kit for rapidly detecting capripoxvirus and application thereof, and further discloses a test strip RPA kit for rapidly detecting the capripoxvirus and application thereof. The two kits comprise primers shown as SEQ ID NO.1 and SEQ ID NO.2 respectively and respective probes, and modified bases and detection platforms of the primers and the probes are different although the primers and the probes aim at a same target sequence. Experiments prove that the sensibility of the real-time fluorescent RPA kit and the sensibility of the test strip RPA kit are both 102 copies per response, and both of the two kits only can specially detect a sheep pox virus and a goat pox virus. A capripoxvirus suspected sample is detected by the two kits separately, and results show that the detection results of the two kits both have the very high conformity with that of qPCR. Therefore, the two kits both can rapidly, efficiently and sensitively detect the sheep pox virus or the goat pox virus, and an effective technological means is supplied to differentiation and diagnosis of the sheep pox virus or the goat pox virus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Nipah Virus Vaccines
ActiveUS20100278862A1SsRNA viruses negative-senseViral antigen ingredientsViral glycoproteinPox viruses
The present invention relates to recombinant anti-Nipah virus vaccines and the administration of such vaccines to animals, advantageously pigs. Advantageously, the anti-Nipah virus vaccine may comprise a recombinant avipox virus containing a Nipah virus glycoprotein gene. The invention encompasses methods of vaccinating animals, advantageously pigs, by administration of anti-Nipah virus vaccines that may comprise a recombinant avipox virus that may contain a Nipah virus glycoprotein gene.
Owner:MERIAL INC
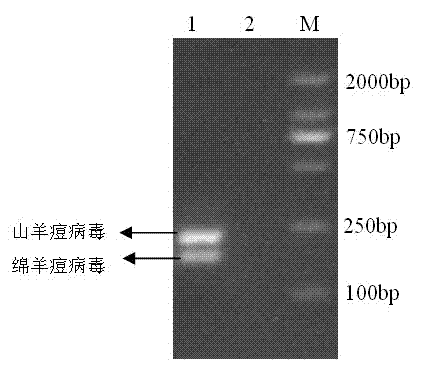
Goat pox virus and sheep pox virus dual-PCR (Polymerase Chain Reaction) detection kit and detection method
ActiveCN102367492AEasy to identifyPromote amplificationMicrobiological testing/measurementMicroorganism based processesDuplex pcrPcr method
The invention discloses a goat pox virus and sheep pox virus dual-PCR (Polymerase Chain Reaction) detection kit and a dual-PCR method for rapidly identifying and detecting goat pox virus and sheep pox virus. One pair of PCR primers is respectively designed according to genome sequences of the goat pox virus and the sheep pox virus, and corresponding specific fragments can be obtained through amplification, thus detection and identification of the goat pox virus and the sheep pox virus are realized. By using the dual-PCR technology, the goat pox virus and the sheep pox virus can be simultaneously detected by using a single tube, thus detection cost and workload of goat pox and sheep pox are reduced, and identification detection of the goat pox and the sheep pox is realized. According to the invention, a dual-PCR method with the advantages of strong specificity, high sensitivity, time and labor saving and easiness in observing results is established, and the advantages of convenience for operation, strong specificity, high sensitivity, high repeatability and no complex post-treatment are achieved.
Owner:重庆海关技术中心 +1
Pigeon pox resisting Chinese medicinal composition and method for preparing oral liquid thereof
ActiveCN101530481AReduce or eliminate residueConducive to pollution-free productionPharmaceutical delivery mechanismAntiviralsForsythiaPigeon pox
The invention discloses a pigeon pox resisting Chinese medicinal composition, which comprises the following components in portion by weight: 25 to 45 portions of root of kudzuvine, 25 to 45 portions of forsythia, 20 to 35 portions of cimicifuga foetida, 20 to 35 portions of glabrous greenbrier rhizome, 20 to 35 portions of dianthus superbus, 15 to 30 portions of honeysuckle, and 15 to 30 portions of liquorice. The invention also discloses a method for preparing oral liquid of the Chinese medicinal composition for resisting the pigeon pox. The Chinese medicament replaced for western medicine is used for treating virus infection of the pigeon pox, and can reduce or eliminate medicament residues in poultry and eggs so that the Chinese medicinal composition is favorable for pollution-free production of animal products, and can be widely applied to pox virus infection of various birds comprising pigeons; after the pigeons suffer from the pox virus infection, the product can treat, so remarkable curative effect can be obtained, and the healing rate reaches more than 95 percent; and in particular for the produced smallpox scabs, the smallpox scabs can fall and heal after medication for one week and do not relapse.
Owner:江苏欧克动物药业有限公司
Broad Spectrum Inhibitors of the Post Proline Cleaving Enzymes for Treatment of Hepatitis C Virus Infections
InactiveUS20150202218A1Increase secretionBiocidePeptide/protein ingredientsEcho virusesCOXSACKIE A VIRUS
Disclosed are methods of treating, inhibiting, or preventing a viral infection in a mammal in need thereof by administering a therapeutically or prophylactically effective amount of an inhibitor of FAP, an inhibitor of DPPIV, an inhibitor of DPP8, or an inhibitor of DPP9. The inhibitor may act as both an inhibitor of DPPIV and an inhibitor of DPP8 / 9. The viral infection includes, but is not limited to, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, Polio virus, Coxsackie A virus, Coxsackie B virus, Rhino virus, respiratory syncytial virus, dengue virus, equine infectious anemia virus, Echo virus, small pox virus, Ebola virus, and West Nile virus.
Owner:TRUSTEES OF TUFTS COLLEGE TUFTS UNIV
Kit used for detecting sheep pox virus and detection method thereof
ActiveCN103276111AStrong specificityHigh sensitivityMicrobiological testing/measurementMicroorganism based processes3-deoxyriboseA-DNA
The invention provides a detection method used for detecting sheep pox virus, wherein real-time fluorescent quantitative PCR (polymerase chain reaction) detection is carried out on a DNA (deoxyribose nucleic acid) template of a sample by designing a pair of primers and one probe; and the invention also provides a kit used for detecting the sheep pox virus by applying the detection method. The kit used for detecting the sheep pox virus designs two specific primers aiming at P32 and one probe, real-time quantitative PCR detection is carried out on sheep pox virus Taqman, and a real-time quantitative method established aiming at the gene has high specificity and good stability; the kit used for detecting the sheep pox virus is easy to operate, a user only needs to add DNA of a sample to be inspected into a reaction tube, a detection result can be obtained according to an amplification curve, time and labour are saved, cost is lower, and time is shortened to 1-2 hours from the original 3-4 hours; and the kit used for detecting the sheep pox virus has higher sensitivity compared with common PCR and can be used for detecting the sheep pox virus with low content.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
LAMP technology-based primer, kit and detection method for detecting Plum pox virus
InactiveCN105671210AHigh detection sensitivityIncrease the lengthMicrobiological testing/measurementDNA/RNA fragmentationPox virusesWeakness
The invention discloses primers, a kit and a detection method for detecting plum pox virus based on LAMP technology. The primers include: forward outer primer F3: 5'-CCTTTGATTTCTACGAGATGAC-3'; reverse outer primer B3: 5'-ACCACTACACTCCCCTC-3 '; forward internal primer FIP: 5'-TCCAAGCCAAATAAACGATTTTGAAGTGAGGCACATATTCAAATGAAGG-3'; wherein said forward internal primer FIP comprises F1c and F2; reverse internal primer BIP, wherein said reverse internal primer BIP comprises B1c and B2; loop Primers: LF: 5'-CATTTCTCAATGCTGCTG-3'; LB: 5'-ACCGCTGGTGATGTTAA-3'. The purpose of the present invention is to overcome the deficiencies in the prior art, to provide a quick, easy, efficient, high accuracy, strong specificity, high sensitivity, primers, kits, Method for detection of plum pox virus.
Owner:陈定虎
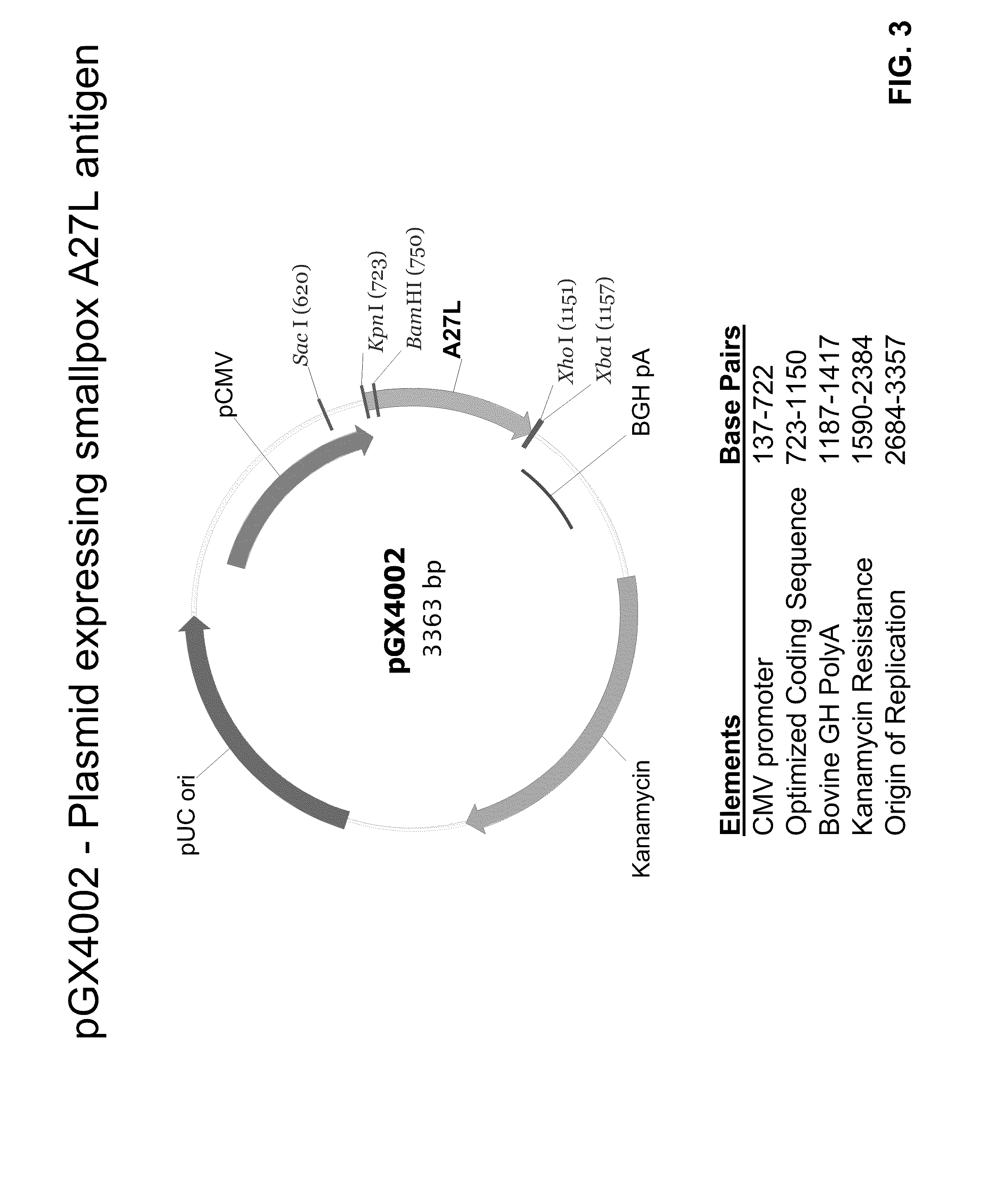
Smallpox DNA vaccine and the antigens therein that elicit an immune response
The present invention relates to DNA vaccines that are capable of generating a protective immune response in mammals against a pox virus, and comprises at least one DNA plasmid capable of expressing a plurality of VACV MV antigens, and at least one DNA plasmid capable of expressing a plurality of VACV EV antigens. Also, the present invention relates to methods of inducing a protective immune response in a mammal to pox virus, including a neutralizing antibody response, comprising: injecting into tissue of said mammal said DNA vaccine.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
RAA primer, probe and method for detecting knopvelsiekte virus
ActiveCN110592286AAccurate detectionNo cross reactionMicrobiological testing/measurementDNA/RNA fragmentationBovine parainfluenza virusBovine Viral Diarrhea Viruses
The invention discloses a primer, a probe and a method for detecting knopvelsiekte virus by a RAA fluorescence method. The primer and the probe are suitable for the detection by the RAA fluorescence method, can accurately detect knopvelsiekte virus plasmids without cross reaction with mycoplasma, bovine infectious rhinotracheitis virus, bovine viral diarrhea virus, bovine parainfluenza virus, bovine respiratory syncytial virus, goat pox virus and sheep pox virus, and have a specificity of 100%. The method is fast and easy to achieve high throughput, and can reduce time and cost for detection.The method for rapid detection of DNA of the knopvelsiekte virus based on the RAA fluorescence method has high sensitivity reaching 10 copies / reaction.
Owner:CHINA ANIMAL HEALTH & EPIDEMIOLOGY CENT +1
Multiplex real-time fluorescent polymerase chain reaction (PCR) primer for simultaneously detecting mouse adenovirus and poxvirus muris mouse pox virus, probe as well as reagent kit thereof
ActiveCN102399907AImprove quality controlLow environmental requirementsMicrobiological testing/measurementMicroorganism based processesMouse adenovirusPox viruses
Owner:STAIDSON (BEIJING) BIOPHARMACEUTICALS CO LTD
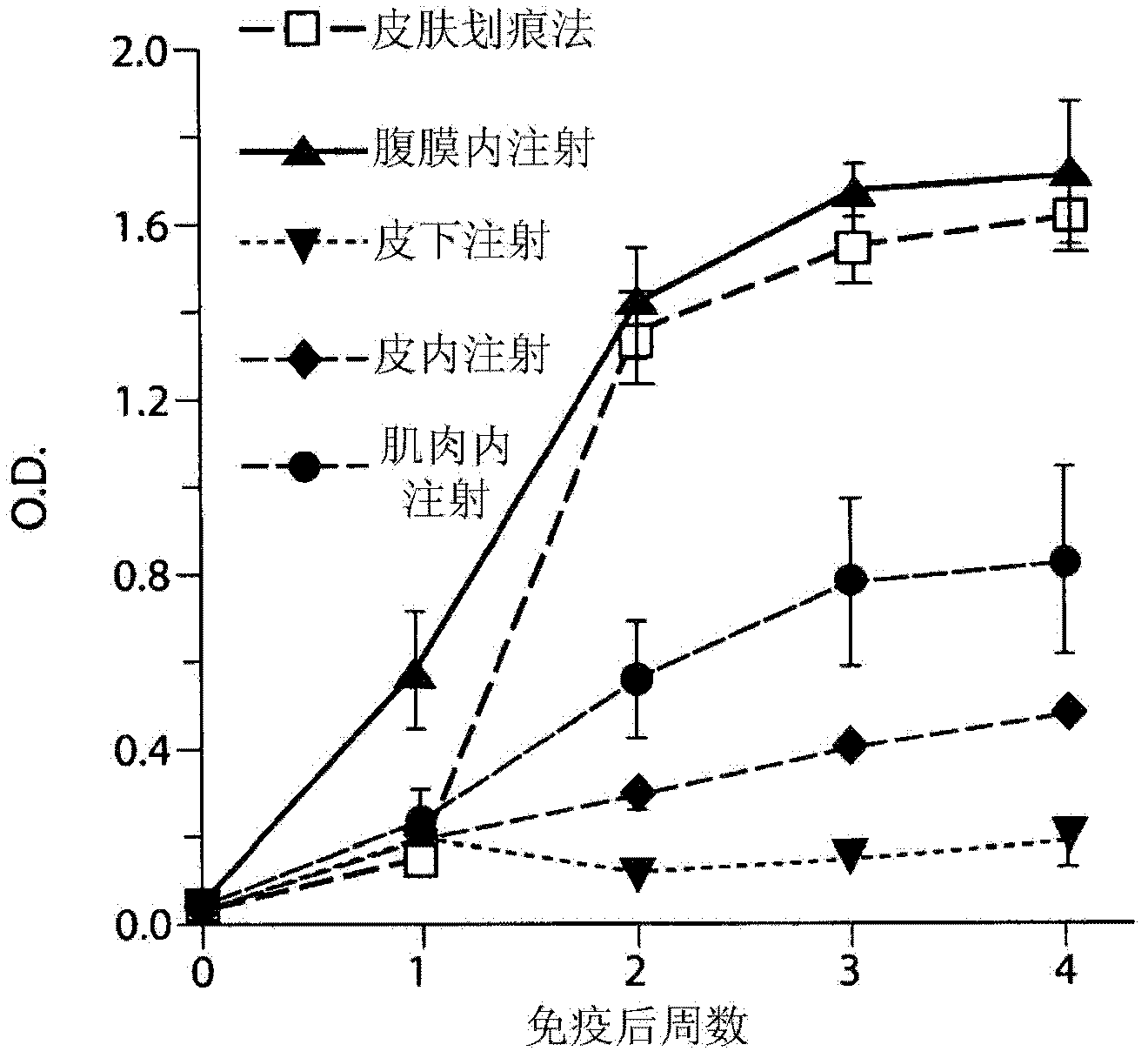
Vaccination with poxvirus vectors by mechanical disruption of the epidermis
The present invention provides methods of stimulating an immune response to an antigen in an individual comprising administering to an individual in need thereof a live, modified and / or recombinant replication-attenuated or non-replicating poxvirus comprising sufficient to stimulate the An immune responsive amount of antigen wherein the virus is administered by mechanical disruption of the epidermis. The present invention also provides a kit comprising said virus and an epidermis disrupting device.
Owner:创雷克斯公司
Primers for detecting plum pox viruses as well as kit and detection method
The invention discloses primers for detecting plum pox viruses as well as a kit and a detection method, wherein the primers comprise the forward direction outer primer F3: 5'-GATGATGGATGGGGAAACA-3', the reverse direction outer primer B3: 5'-AGGCTGTAGTCTGTCAGG-3', the forward direction inner primer FIP: 5'-GCCATAATTTGTCTAAAAGTGGGTT-CAAGTGGAGTATCCAATAAAGC-3', and the reverse direction inner primer BIP: 5'-ACATTTCAGTAACGTGGCTGAA-GCTGAATCCCATACCTTGG-3'; the kit comprises the primers and the detection method adopts the primers; with adoption of the primers and the detection method, the plum pox virus detection is high in speed, simple and convenient, efficient, high in accuracy, strong in specificity and high in sensitivity.
Owner:陈定虎
Protein vaccines against poxviruses
InactiveUS20110081368A1Effective protectionEasy to optimizeViral antigen ingredientsPharmaceutical delivery mechanismOpen reading frameVirus Protein
The invention described here entails a protein vaccine against poxviruses which contains at least two purified recombinant monkeypox virus proteins or peptides. The proteins or peptides are encoded by the open reading frames of the monkeypox ortholog genes M1R, A35R, A29L B6R, and orthologs of these proteins or peptides having 90% identity. The invention also entails a vaccine protocol against poxvirus whereby a vaccine is vaccinated with a first vaccine made up of a nucleic acid vaccine of three or more poxvirus virus genes, and subsequently vaccinated with at least one other booster vaccine made up of two or more poxvirus virus proteins.
Owner:HOOPER JAY W +1
Lamb testis support cell immortalized cell line and establishment method and application thereof
ActiveCN109837250AExtend your lifeEfficient ProliferationMicroorganism based processesViruses/bacteriophagesCell divisionTesticle
The invention discloses a lamb testis support cell immortalized cell line and an establishment method and application thereof. The lamb testis support cell immortalized cell line is named a lamb testis support cell immortalized cell line hTERT-LSC with a collection number CCTCC NO: C2018202. Tests find that the hTERT-LSC cell line can realize continuous passage for more than 60 generations, and the growth and proliferation characteristics are not changed. The cell line is relatively sensitive to inoculation of the sheep pox virus, can efficiently proliferate the sheep pox virus, and can ensurethe uniformity and stability of the virus. The titer of the sheep pox virus proliferated by the cell line is equivalent to that of the lamb testis support cell LSC, and the cell division rate is higher than that of the LSC and can reach 1:3. Furthermore, by utilizing the immortalized cell line for preparing a vaccine, the production process is simplified, the production cycle is shortened, the production cost is reduced, and simultaneously, the quality stability of a prepared sheep pox virus vaccine is also ensured. Therefore, the invention provides a new technical means for large-scale production of the sheep pox virus vaccine.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
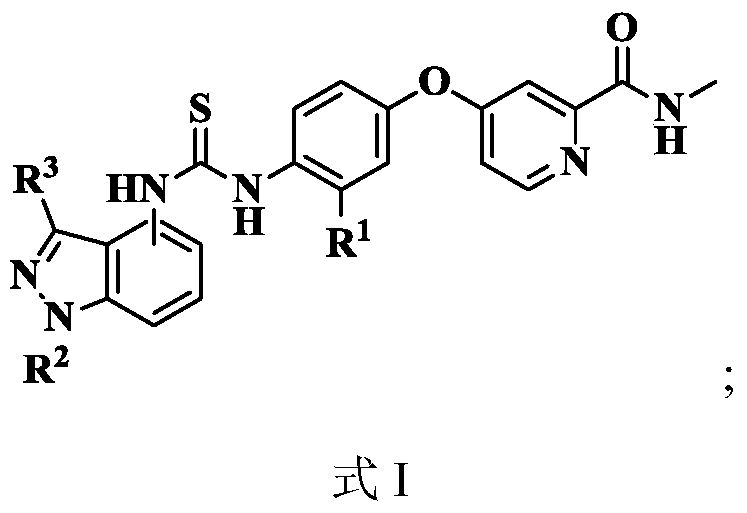
N-indazole substituted thiourea derivatives and preparation method and application thereof
InactiveCN105753841AImproves antioxidant activityImprove clearanceOrganic active ingredientsOrganic chemistryDPPHThiourea
The invention discloses N-indazole substituted thiourea derivatives and a preparation method and application thereof and belongs to the technical field of chemical medicine.The thiourea derivatives are a series of compounds simultaneously containing the 1H-indazole ring structure and the asymmetrical thiourea structure, and the compounds are not reported in the literature.The bioactivity test result analysis of the thiourea derivatives show that the compounds are good in antioxidant activity, the average scavenging rate is above 80%, the scavenging rate of the compound 12b, the compound 12c, and the compound 12d and the compound 12h is higher than 90%, the scavenging activity IC50 of the compound 12h on DPPH is 0.14mg / mL; part of the target compounds has certain inhibition activity on herpes viruses, vaccinia viruses, reoviruses, Coxsackie viruses, Feline coronary herpes viruses, HIV viruses and the like, and the compound 12c and the compound 12n are high in antivirus activity; the synthesized compounds hopefully have new bioactivity which is not expounded, and a certain material basis is provided for the development of new medicine.
Owner:XI AN JIAOTONG UNIV
Immortalized avian cell lines and use thereof
InactiveUS20110212488A1Easy maintenanceImprove stabilityAntibacterial agentsNervous disorderFlaviviridaeVaccinia
The present invention relates to specific immortalized avian cell lines expressing telomerase reverse transcriptase (TERT), and exhibiting distinct biologics production patterns. More particularly, the present invention relates to immortalized avian cell line capable of either amplifying Flaviviridae but not capable of amplifying Vaccinia virus strain Copenhagen (W—COP) nor Modified Vaccinia virus Ankara (MVA), or capable of amplifying both Flaviviridae and Poxyiridae. The invention further relates to the use of said immortalized avian cell lines and related methods for producing biologics, including viruses and proteins.
Owner:TRANSGENE SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com