Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
269 results about "Multi epitope" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Targeted multi-epitope dosage forms for induction of an immune response to antigens
InactiveUS20120070493A1Induce and enhance and cytokine productionAntibacterial agentsPowder deliveryAntigenBinding peptide
Provided herein are compositions and methods related to MHC II binding peptides. In some embodiments, the peptides are obtained or derived from a common source. In other embodiment, the peptides are obtained or derived from an infectious agent to which a subject has been repeatedly exposed.
Owner:SELECTA BIOSCI
Antigen specific multi epitope vaccines
ActiveUS20100074925A1Strong and comprehensive responseEffective immune responseTumor rejection antigen precursorsSugar derivativesMHC class IProtein target
The present invention relates to cancer vaccines composed of the signal peptide domain of tumor associated antigens or proteins. The peptide vaccines of the invention are characterized by having multiple MHC class I and class II epitopes which are highly abundant in the population. Therefore, these vaccines are likely to induce a strong, comprehensive immune response against the target proteins in the majority of the vaccinated population, and thereby induce an immune reaction against tumors expressing such target proteins. Specifically, the invention relates to peptide vaccines composed of the signal peptide domain of Mucin (MUC1), BAGE-1 or ARMET, and their use for the treatment of cancers which express Mucin (MUC1), BAGE-1 or ARMET.
Owner:VAXIL BIOTHERAPEUTICS
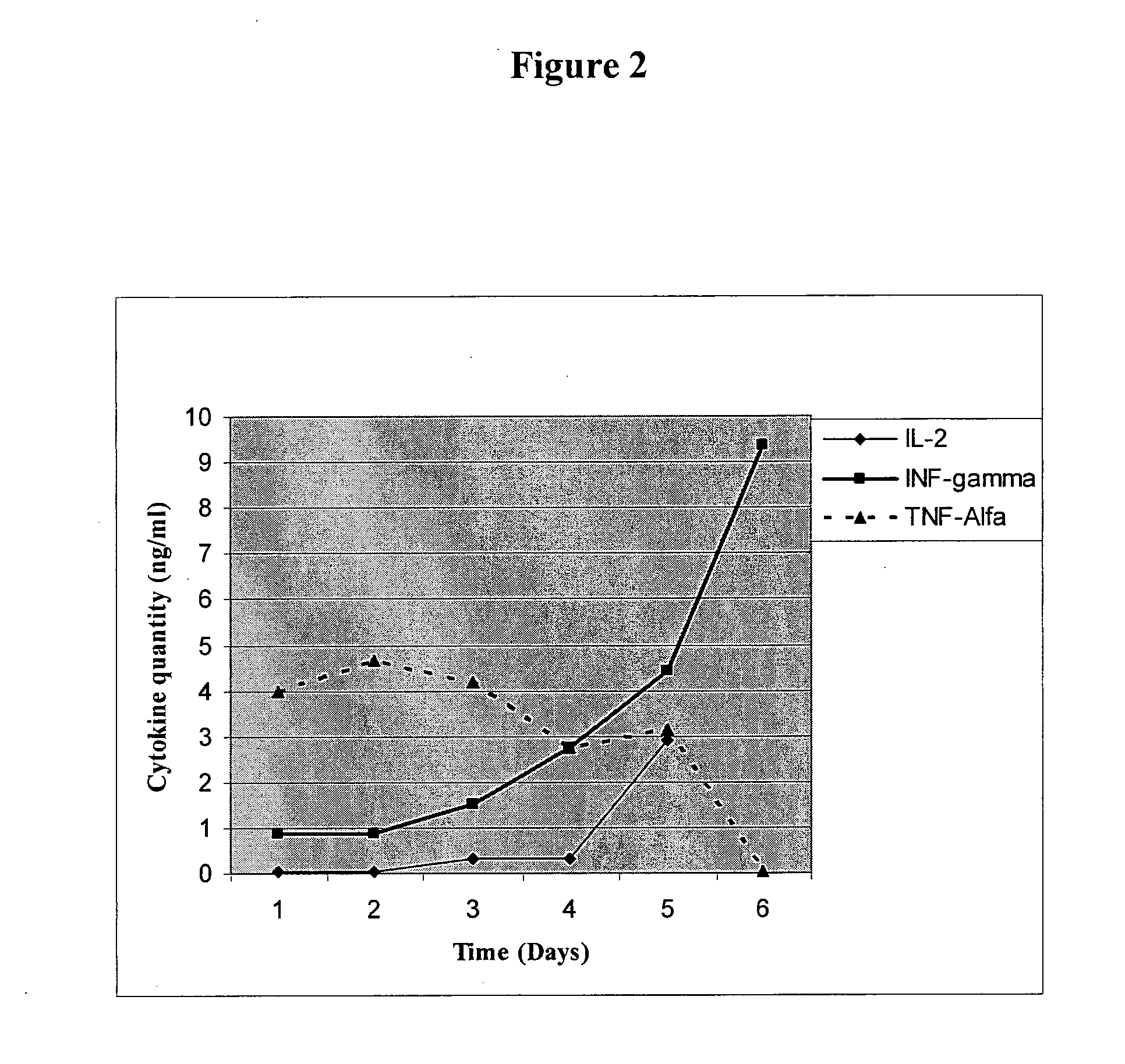
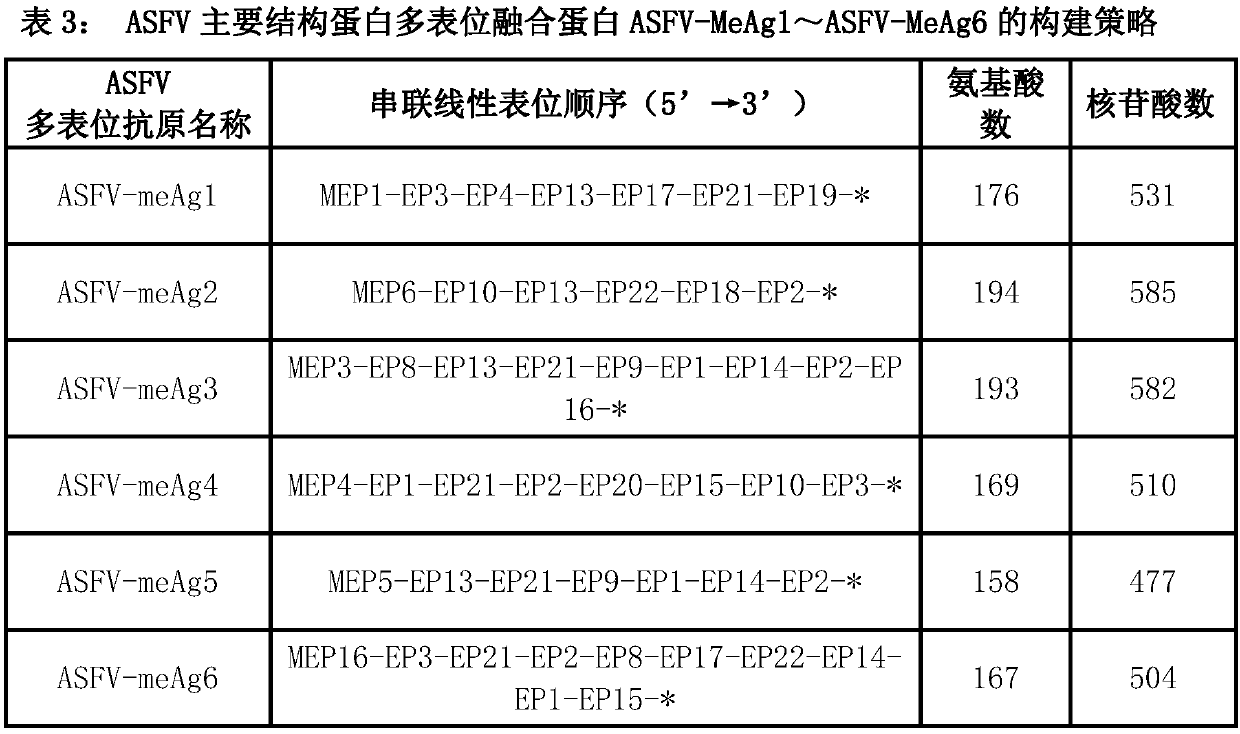
Multi-epitope fusion diagnosis antigen for African swine fever virus as well as preparation method and application thereof
InactiveCN108148138AImprove featuresIncreased sensitivityAntibody mimetics/scaffoldsVirus peptidesAntigenBacillus coli
The invention discloses a multi-epitope fusion diagnosis antigen for African swine fever virus as well as a preparation method and application thereof. An ASFV (African swine fever virus) important structural protein gene encoding amino acid sequence is analyzed, screened and recombined through bioinformatics software, a multi-epitope fusion antigen gene is built and synthesized and is expressed in bacillus coli; through screening, the recombinant multi-epitope fusion antigen ASFV-meAg6 is obtained, so that diagnosis antigen protein with strong specificity and high sensitivity is provided foran ASFV serological diagnosis method.
Owner:SHIHEZI UNIVERSITY
Multi-epitopic vaccine
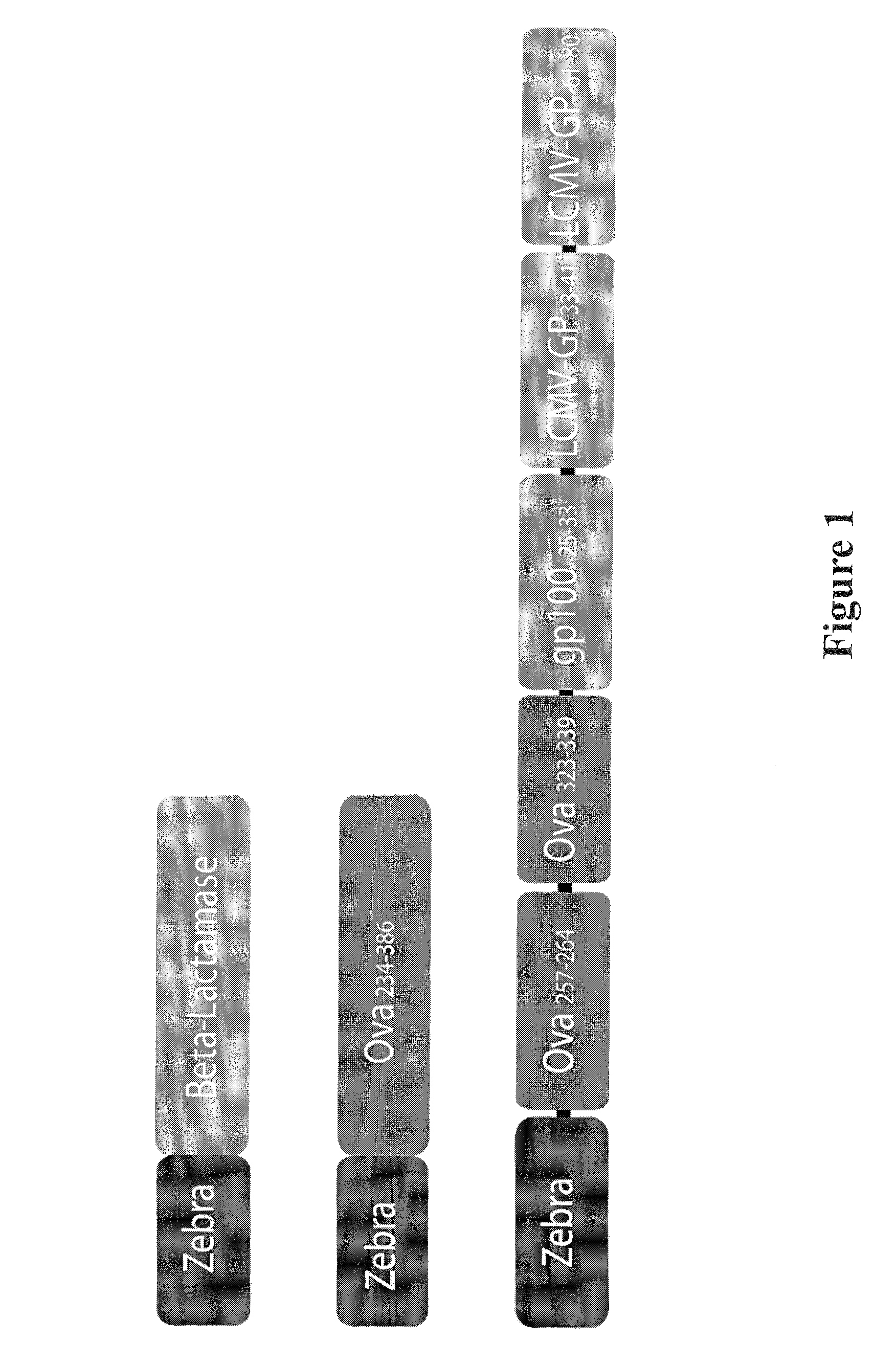
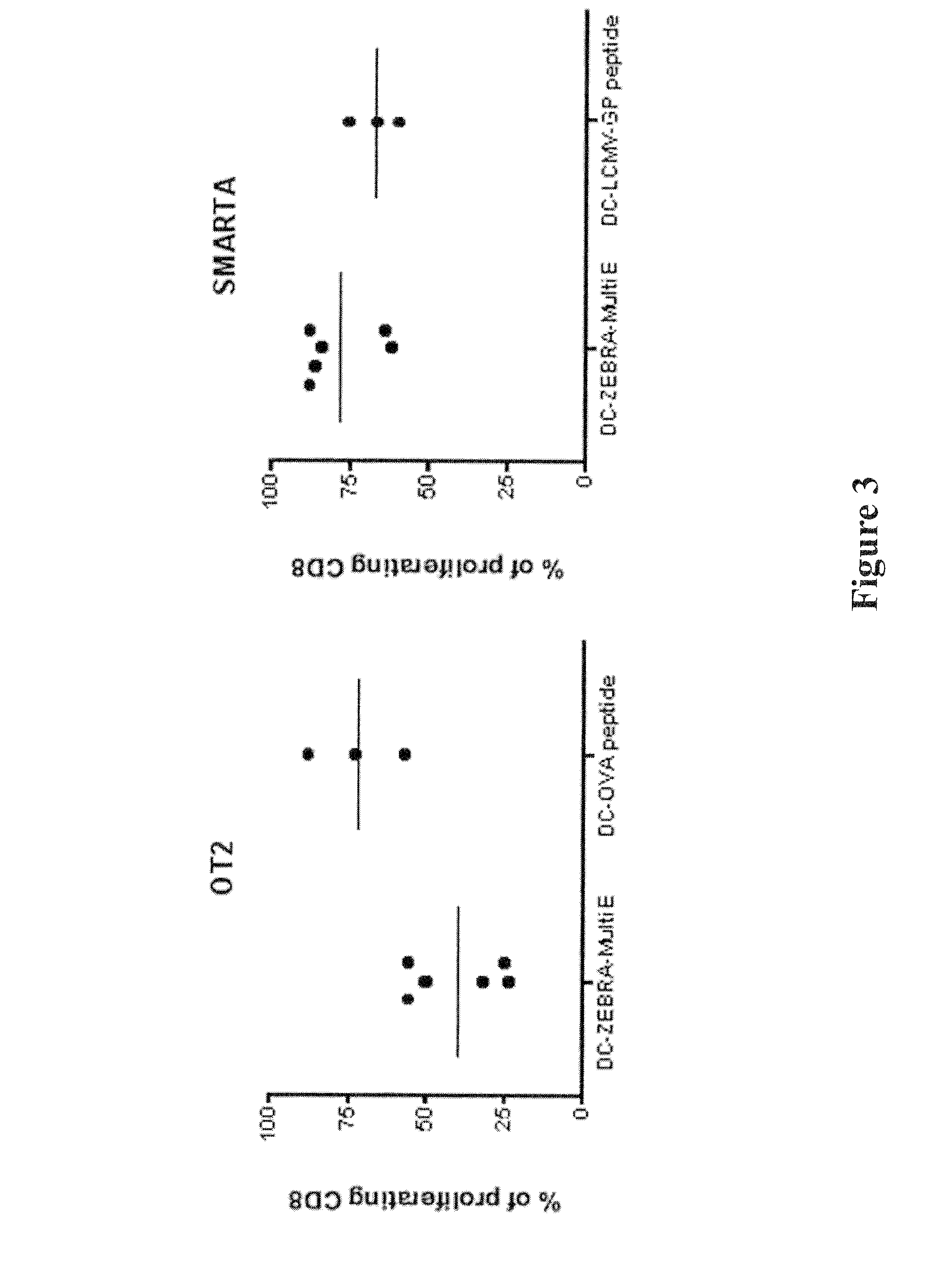
The present invention relates to isolated polypeptides comprising: (i) a protein transduction domain consisting of ZEBRA or a fragment thereof that retains the capacity of internalization, (ii) at least one CD4+ epitope; and (iii) at least one CD8+ epitope. It also relates to antigen presenting cells loaded with said polypeptides, and the use thereof in immunotherapy including prevention and / or treatment of cancers or infectious diseases.
Owner:UNIVERSITY OF GENEVA +1
Multiple epitope fusion protein
InactiveUS7056658B2Easily purifyReduce maskSsRNA viruses positive-senseAntibody mimetics/scaffoldsAmino acidImmunology
Multiple epitope fusion proteins and immunoassays using the same are disclosed. The multiple epitope fusion proteins are encompassed by the general structural formula (A)x−(B)y−C2 which represents a linear amino acid sequence, wherein B is an amino acid sequence of an epitope or cluster of epitopes and each B contains at least five and not more than 1,000 amino acids, y is an integer of 2 or more, A and C are each independently an amino acid sequence of an epitope or cluster of epitopes not adjacent to B in nature and x and z are each independently an integer of 0 or more wherein at least one of x and z is 1 or more.
Owner:GRIFOLS WORLDWIDE OPERATIONS
O-type foot-and-mouth disease virus multi-epitope mucous membrane immunization vaccine and use
This invention relates to a fusion protein used for preventing aftosa, its preparation method and application. This fusion protein contains O type foot-and-mouth disease virus main cytomembrane protein VP1 epitope, colibacillus thermolability toxin B subunit, thymus derived cell epitope and purification label.
Owner:GUANGZHOU PUTAI BIOTECH
Pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof
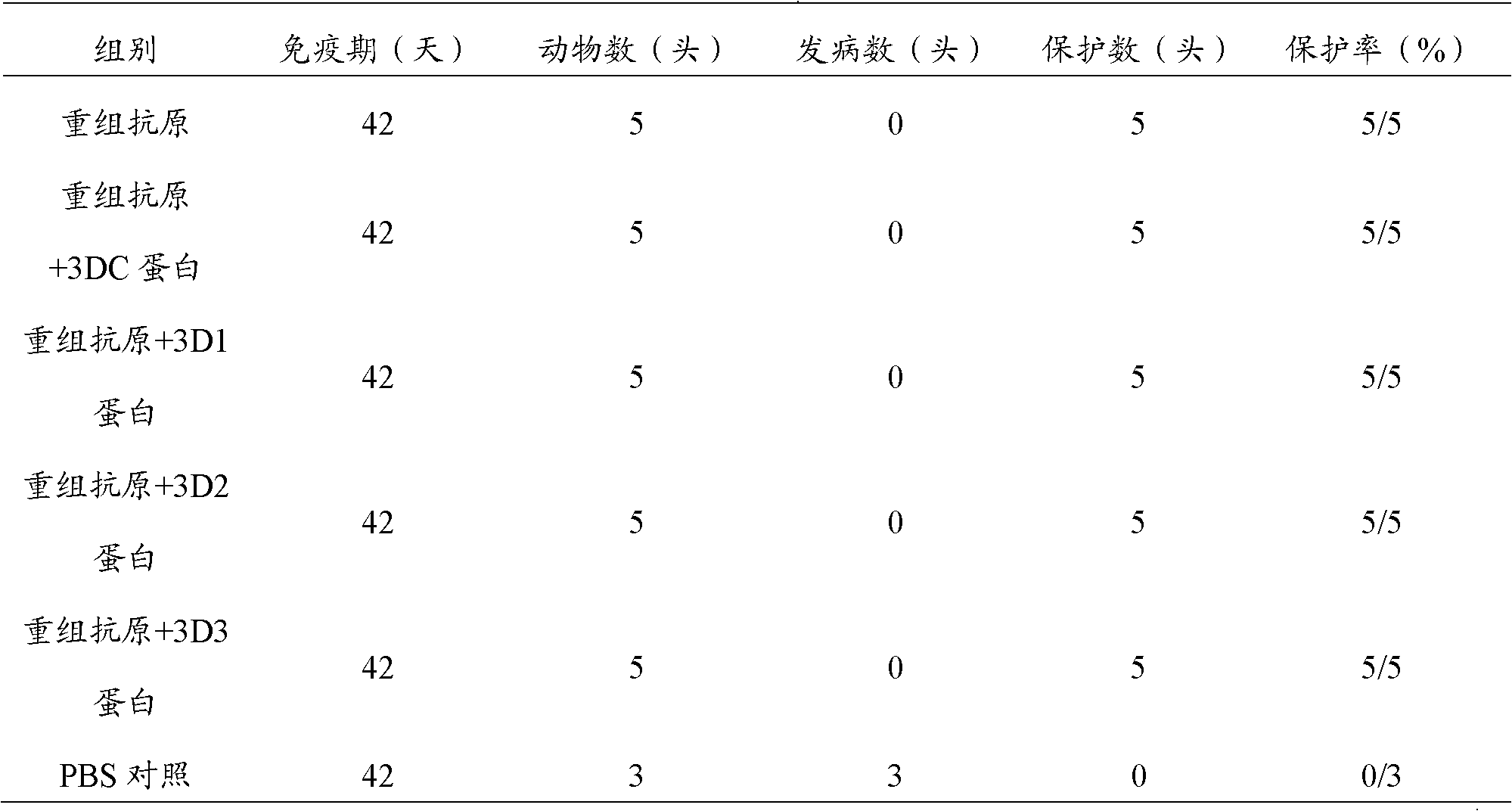
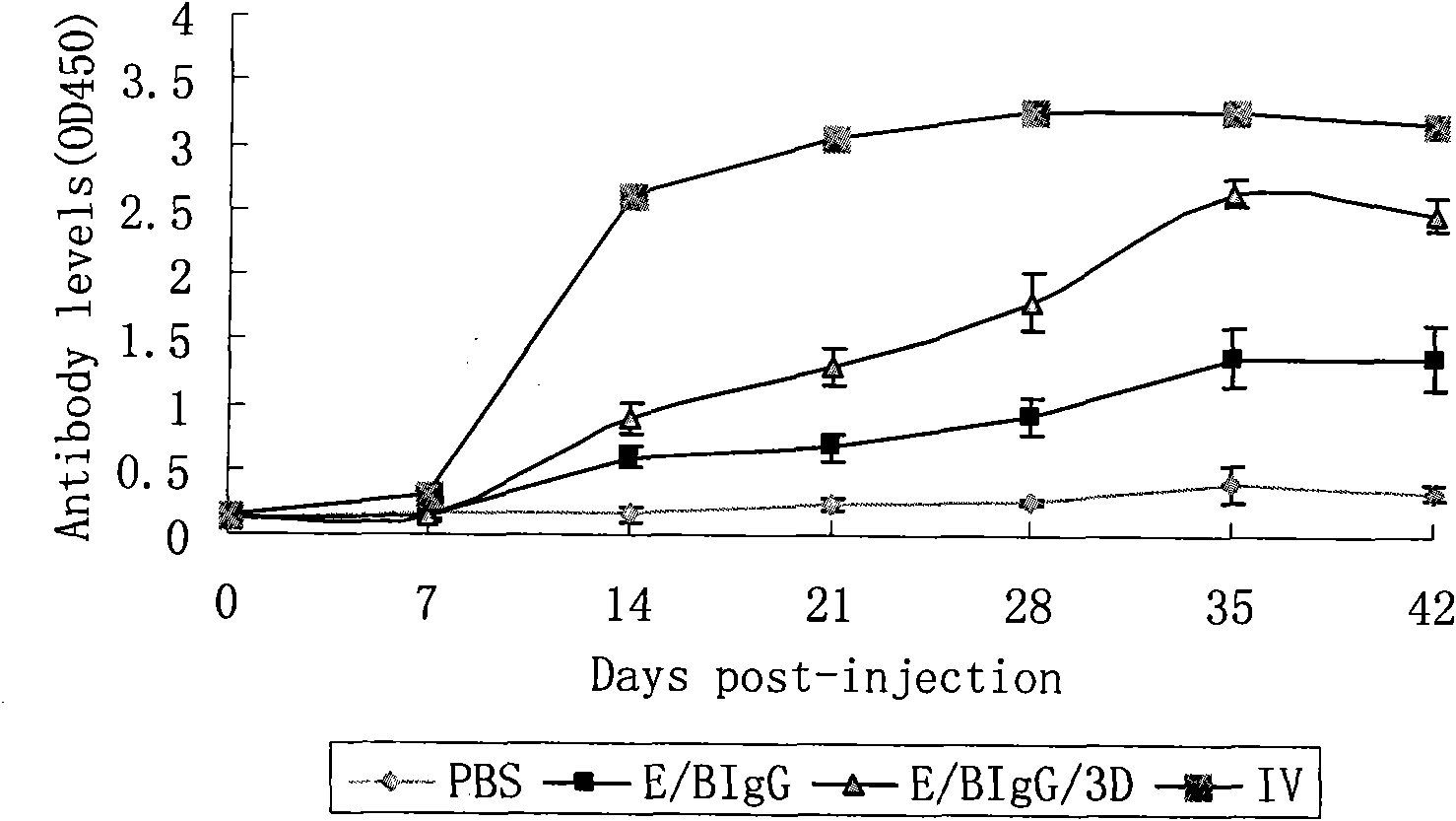
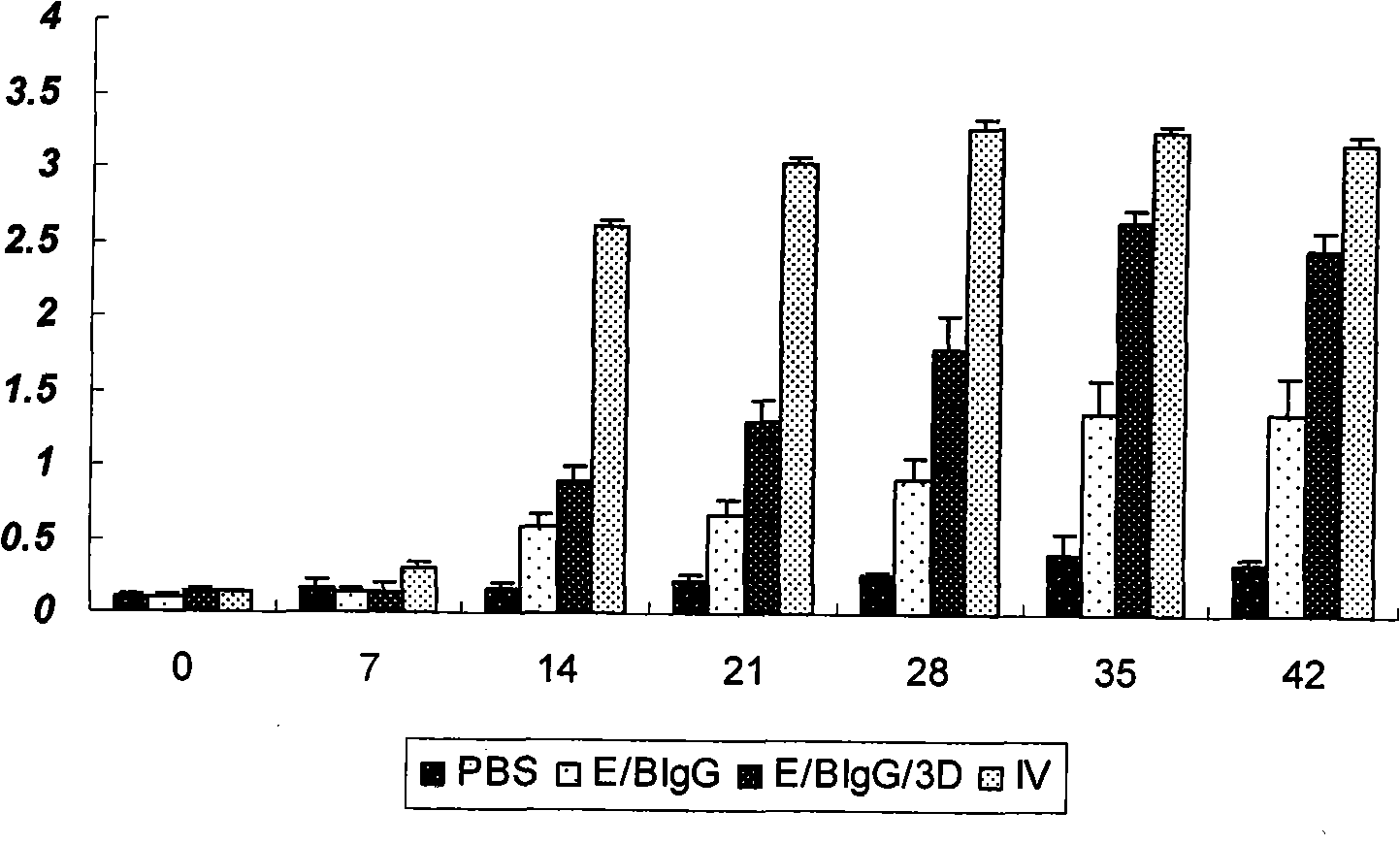
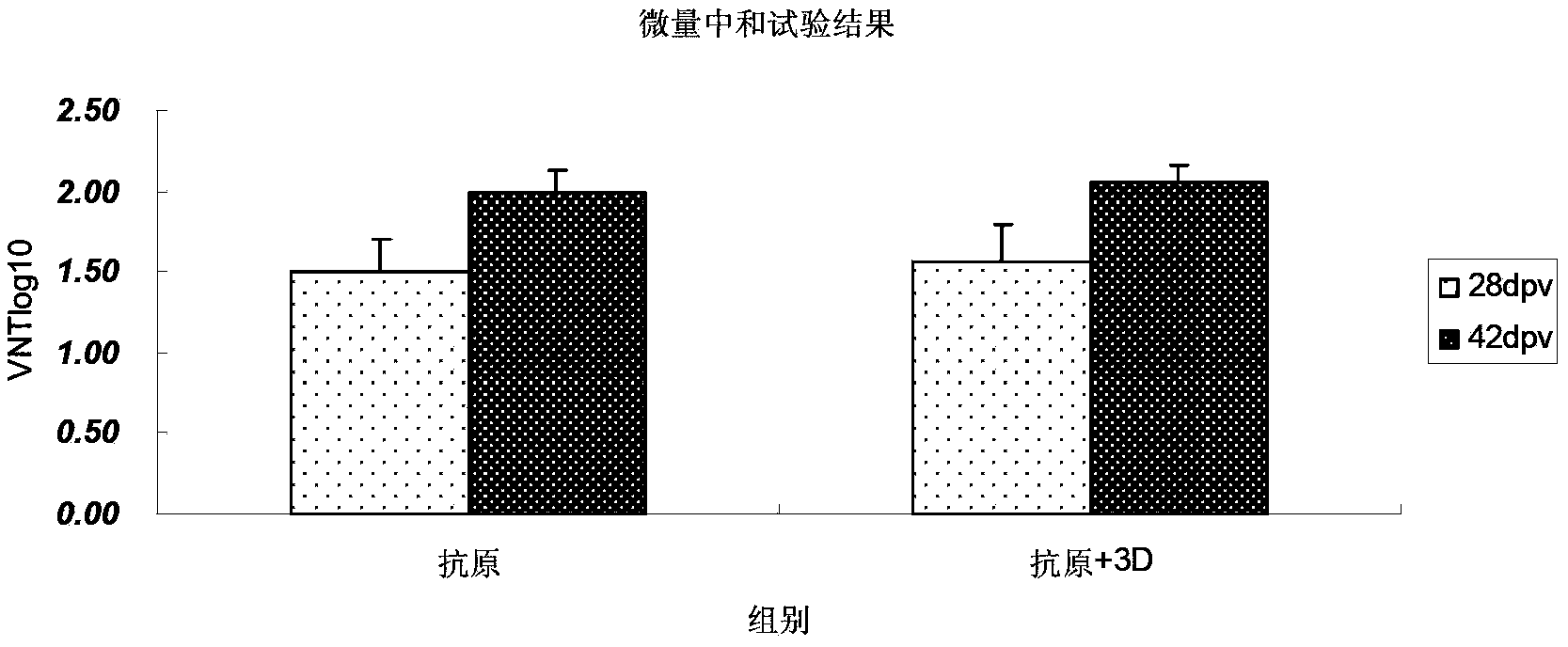
The invention discloses a pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and application thereof and belongs to the field of biological vaccines. The pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen adopts a strategy of an antigenized antibody, after main antigen epitopes of a plurality of strains of pig foot-and-mouth disease virus O-type are connected in series reasonably, the plurality of strains of pig foot-and-mouth disease virus O-type are coupled with a pig intravenous gamma globulin (IgG) heavy chain constant region to construct the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen, and after ration through a Bio-Rad protein ration kit, the pig foot-and-mouth disease virus O-type broad spectrum multi-epitope recombination antigen and recombination foot-and-mouth disease virus 3D protein are matched to prepare the vaccines. Animal immunity testing results show that the vaccines can stimulate an organism to generate high-titer protective antibodies when the vaccines are used independently or matched with the recombination foot-and-mouth disease virus 3D protein to be used, an antibody level is higher than a national standard, and good application prospects are achieved.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Schistosoma japonicum recombinant multi-epitope antigens, method for expressing and purifying same and application thereof
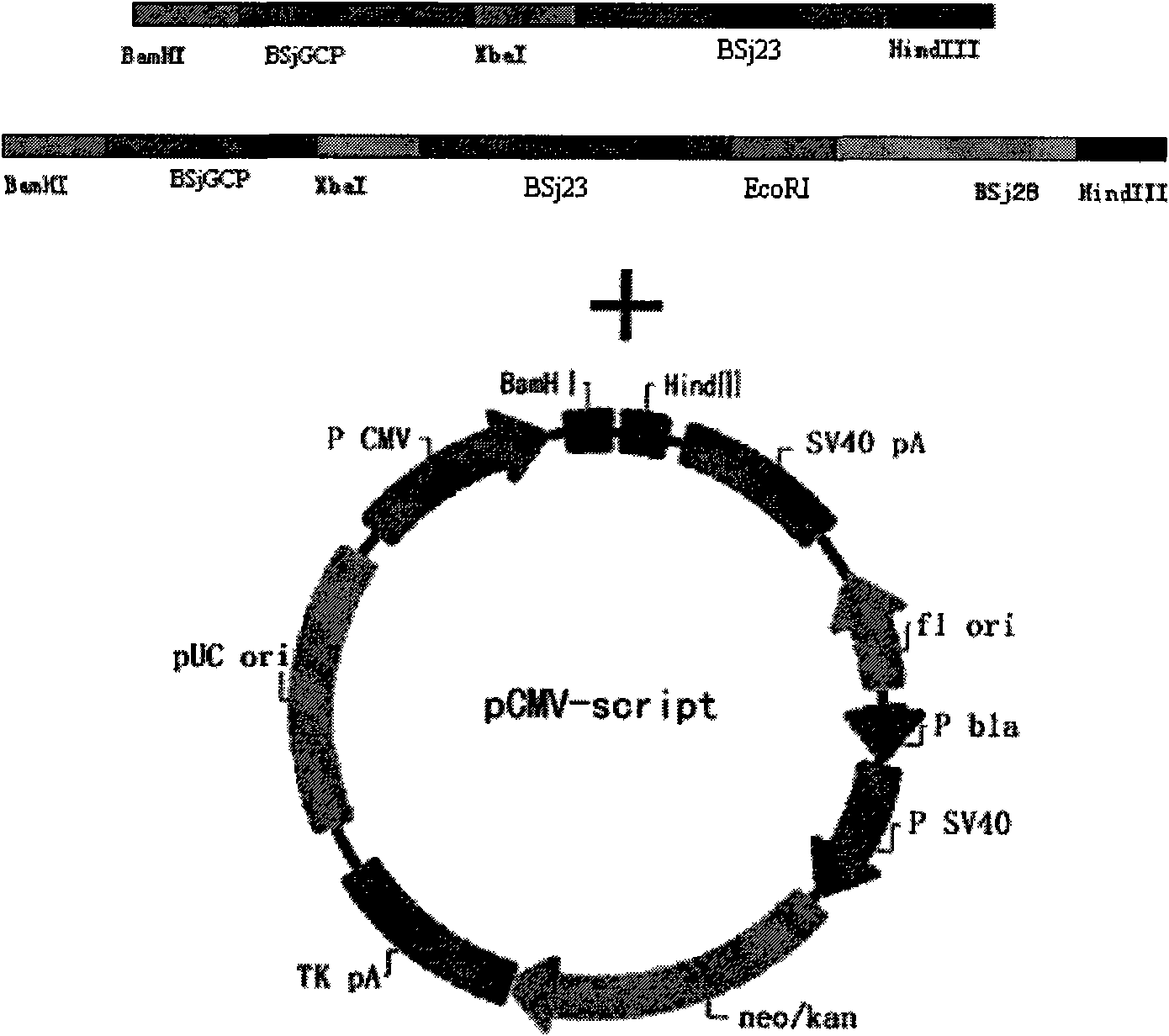
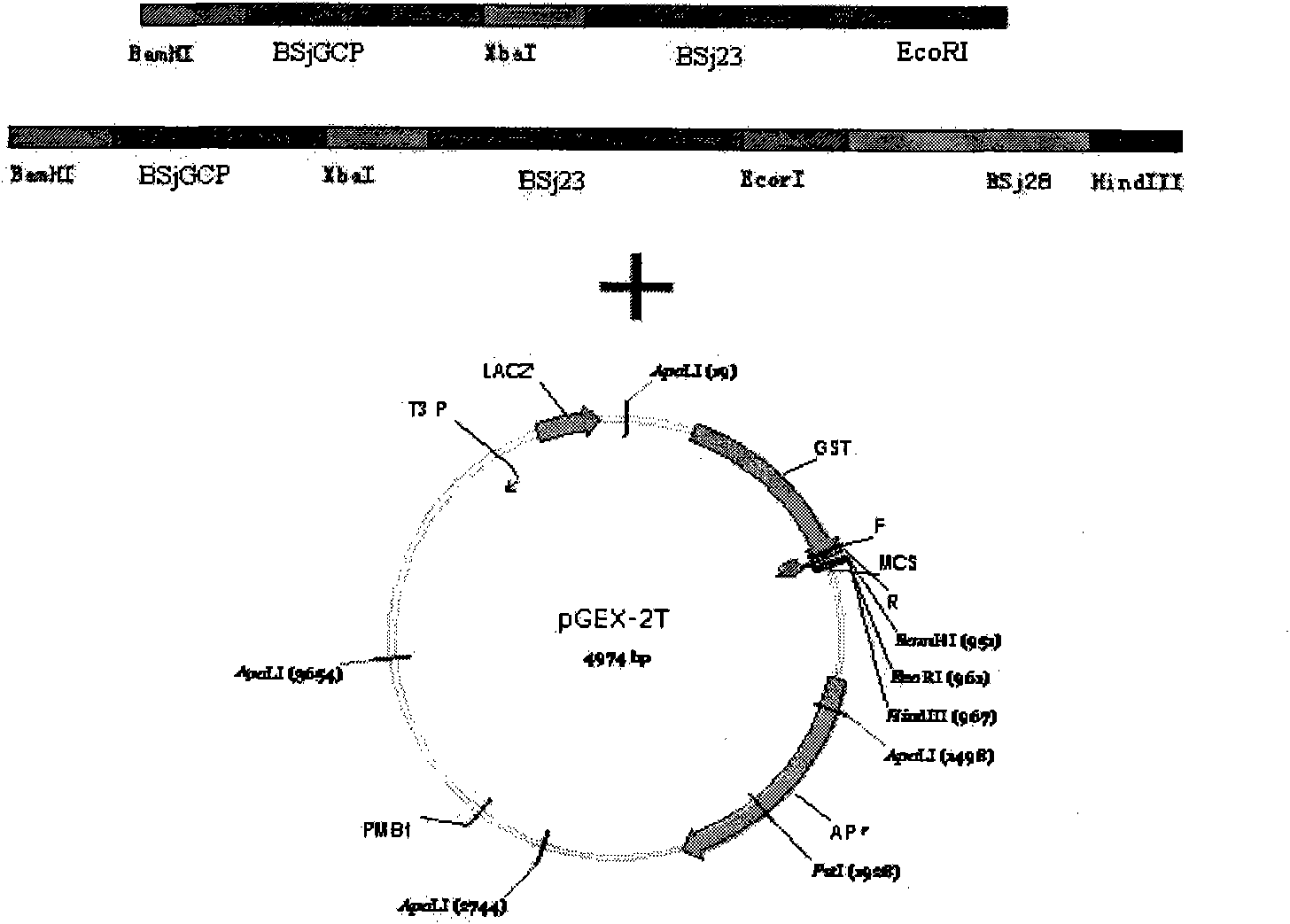
The invention discloses gene orders of schistosoma japonicum recombinant multi-epitope antigens BSjGCP-BSj23 and BSjGCP-BSj23-BSj28, a method for expressing and purifying the same, and application thereof in preparing schistosomiasis japonica immunity prevention vaccines and diagnostic reagents. Recombinant multi-epitope nucleic acid vaccines pCMV-BSjGCP-BSj23 and pCMV-BSjGCP-BSj23-BSj28 obtain 14.76 percent and 64.95 percent of worm reduction rates respectively in Kunming mice. The recombinant multi-epitope antigens pGEX-BSjGCP-BSj23 and pGEX-BSjGCP-BSj23-BSj28 obtain 15.7 percent and 57.99 percent of worm reduction rates in immunizing BalB / c mice, and obtain 91.0 percent and 89.9 percent of sensitivities as well as 97.8 percent and 93.4 percent of specificities respectively as diagnostic antigens.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
IgA (Immunoglobulin A) antibody detection reagent kit (colloidal gold method) for EB (Epstein-Barr) viruses and preparation method thereof
The invention relates to an IgA (Immunoglobulin A) antibody detection reagent kit (a colloidal gold method) for EB (Epstein-Barr) viruses and a preparation method thereof. The reagent kit comprises recombination antigen EB-NA1 coated by a nitrocellulose membrane detection line, a goat-anti-mouse IgG antibody coated on a quality control line and a mouse-anti-human IGA monoclonal antibody marked by colloidal gold and coated on a gold mark pad. The preparation method comprises the steps of: preparing a reaction membrane and a mouse-anti-human IGA monoclonal antibody gold combo pad, cutting and assembling to prepare the product. The invention has the advantages that: the IgA antibody detection reagent kit for the EB viruses has the characteristics of fast, simple and convenient detection, and high accuracy and sensitivity; the integrated operation time only requires 20 minutes to judge and read results; the colloidal gold is used for fast detecting test paper; a multi-epitope recombination antigen is used as a raw material; the method has the characteristics of simple and convenient operation, low cost, good specificity, high sensitivity, single portion detection and easy popularization; and the detection and control effect to the EB viruses is obvious.
Owner:北京中检安泰诊断科技有限公司
Mycoplasma bovis diagnosis reagent and its application
InactiveCN103172752AReduce manufacturing costSimple and fast operationBiological testingHybrid peptidesMycoplasma antibodySpecific antibody
The invention relates to the diagnostic medicine of animals, especially relates to a mycoplasma bovis diagnosis technology, and concretely relates to a multi-epitope fusion antigen having an amino acid sequence represented by SEQ ID NO:1 or SEQ ID NO:2, and its application in the preparation of a mycoplasma bovis diagnosis reagent. The diagnosis reagent can be used as a solid phase vector coating antigen of an indirect ELISA kit and is combined with its specific antibody, a horseradish peroxidase coupled anti-cattle IgG antibody is added and incubated, and a color development reaction is carried out, and the color development degree is proportional to the amount of the anti-mycoplasma bovis antibody in a sample to be measured. The technology has the advantages of simple operation, no need of complex equipment, low technical requirements on the laboratorial conditions and experiment personals, low detection cost, and suitableness for the large-scale development in the basic level and the culture farm; the multi-epitope fusion antigen has a low making cost and is suitable for large-scale application; and has the advantages of high sensitivity and specificity, small batch difference, and high detection result consistence because of the adoption of multi-epitope as a target.
Owner:重庆市动物疫病预防控制中心 +1
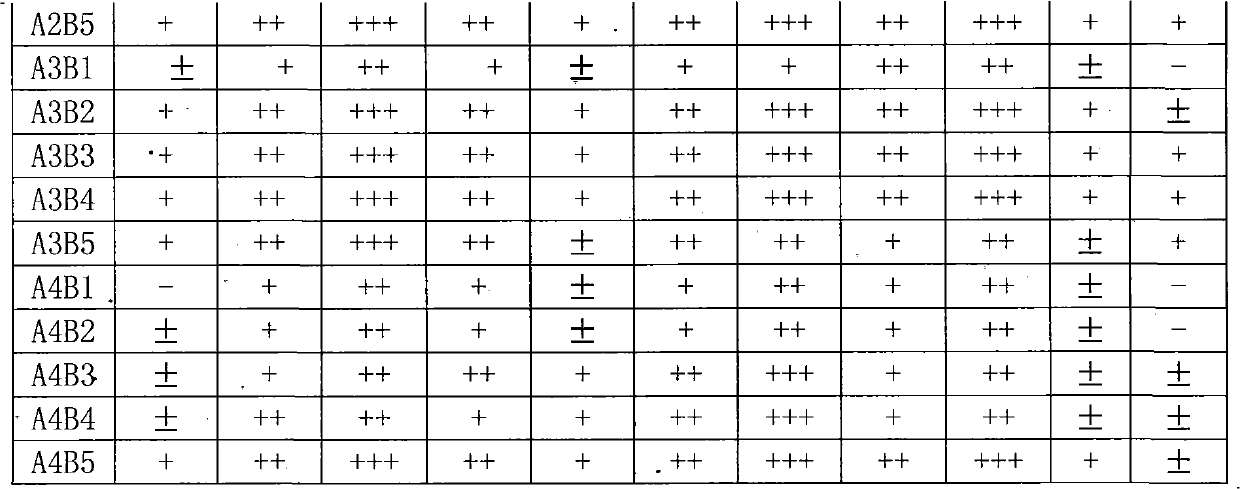
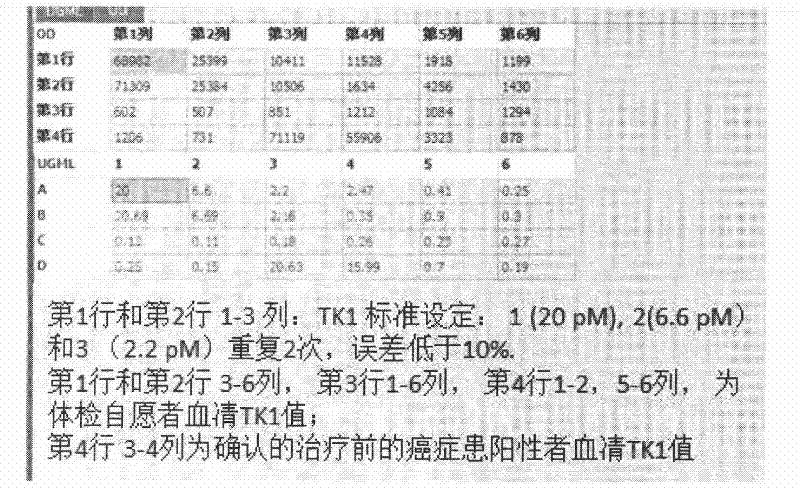
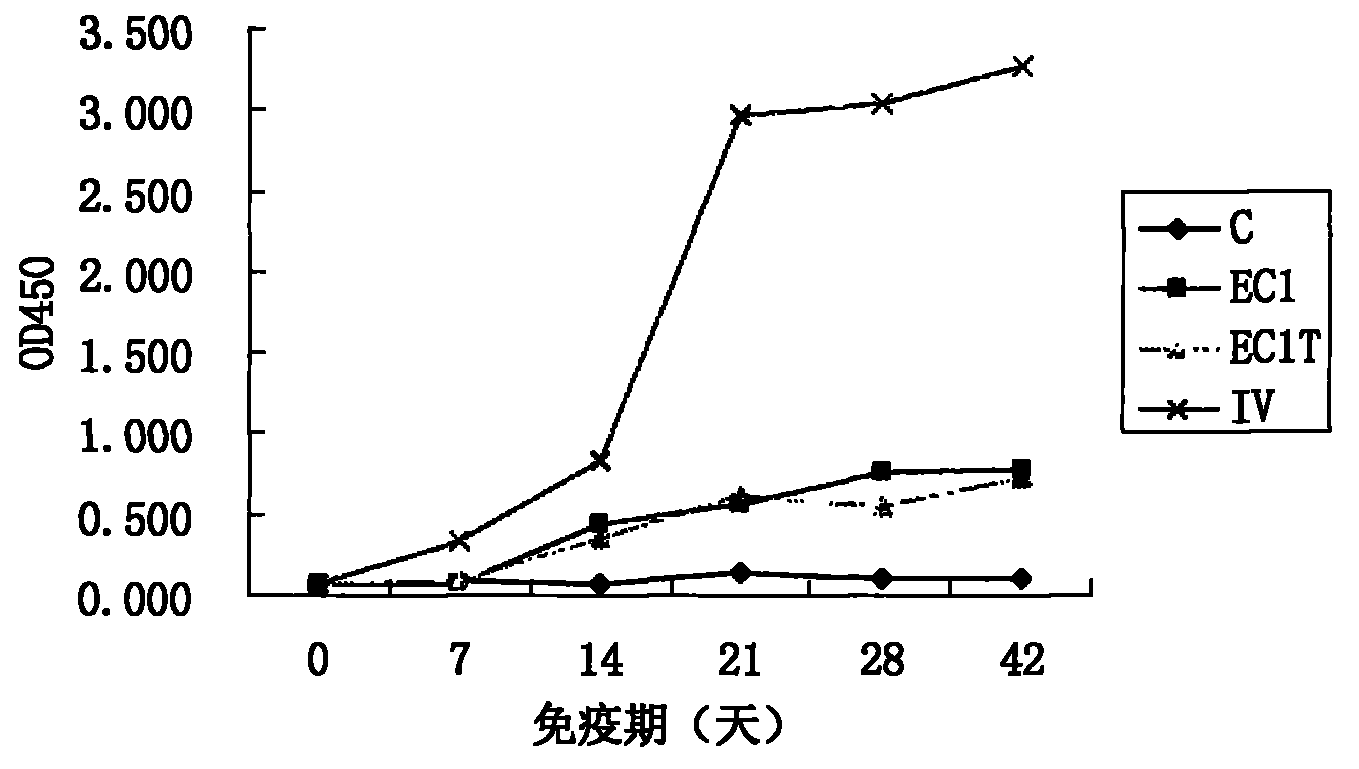
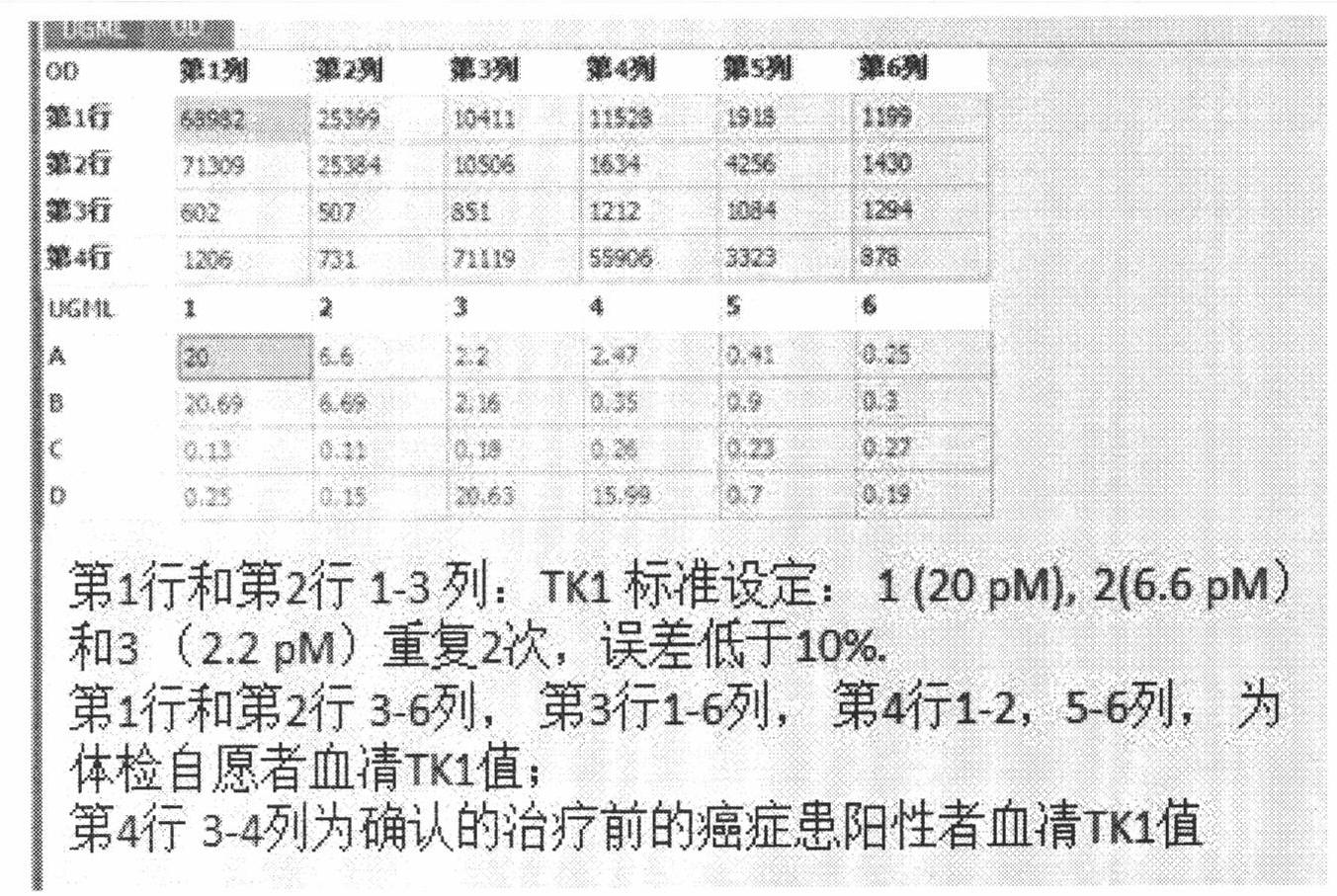
Preparation of multi-epitope TK-1 antibody, and application of multi-epitope TK-1 antibody in evaluating treatment effect on tumor patient
ActiveCN102516390AHigh purityIncrease productionEgg immunoglobulinsTransferasesAntigenTreatment effect
The invention provides a high-specificity high-sensitivity coordinated compound anti-human-TK1 antibody prepared from an antigenic determinant composed of human cervical cancer cell TK1 monomer N-terminal 23 peptide, C-terminal 20 peptide and C-terminal 28 peptide, and application thereof in tumor diagnosis. The antigenic determinant contains the following amino acid sequences: N-terminal 23 peptide (3-25): CINLPTVLPGSPSKTRGQIQVIL, C-terminal 20 peptide (206-225) CPVPGKPGEAVAARKLFAPQ, and C-terminal 28 peptide (198-225) AGPDNKENCPVPGKPGEAVAARKLFAPQ. The invention also relates to a method for preparing the antibody prepared from the antigen. The antibody kit provided by the invention has the characteristics of high sensitivity, high specificity, low cost and the like. The treatment effect on the tumor patient is evaluated by an enhanced chemiluminescent point blotting detection method, immunohistochemical detection and the detection kit.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and preparation method thereof
ActiveCN101775399AImproving immunogenicityFacilitate presentationGenetic material ingredientsVirus peptidesAdjuvantRecombinant vaccines
The invention discloses an Asia1 type multi-epitope recombinant vaccine of bovine foot-and-mouth disease viruses and a preparation method thereof. The recombinant vaccine comprises the following components: proteins coded by foot-and-mouth disease virus multi-epitope genes and the fusion genes of carrier proteins, and foot-and-mouth disease virus 3D proteins. The preparation method comprises the following steps: diluting the proteins expressed by the foot-and-mouth disease virus multi-epitope genes and the fusion genes of the carrier proteins and the foot-and-mouth disease virus 3D proteins, mixing the diluted proteins uniformly, adding an adjuvant into the mixture to emulsify the mixture. Animal models and animal immune effect experiments show that the bovine Asia1 epitope recombinant vaccine can make comprehensive immune protective response, can induce injected and immunized bovine and guinea pigs to generate high level neutralizing antibodies, and can also induce cell immune response, so the recombinant vaccine can effectively protect animals against the virulent attack of the foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Sheep aphthovirus Asial type multi-epitope recombinant vaccine and preparation method thereof
ActiveCN101864434AComprehensive immune efficiencyComprehensive evaluation of immune efficiencyGenetic material ingredientsVirus peptidesAdjuvantAdditive ingredient
The invention discloses a sheep aphthovirus Asial type multi-epitope recombinant vaccine and a preparation method thereof. The recombinant vaccine comprises the following ingredients: a protein encoded by aphthovirus multi-epitope genes and carrier protein fusion genes, and an aphthovirus 3D protein. The preparation method comprises the following steps: respectively diluting the protein encoded by the aphthovirus multi-epitope genes and the carrier protein fusion genes and the aphthovirus 3D protein; then, uniformly mixing the diluted proteins; adding auxiliary agents into the mixture; carrying out emulsification; and obtaining the sheep aphthovirus Asia1 type multi-epitope recombinant vaccine. Animal model and animal immune efficiency experiments show that the sheep Asial epitope recombinant vaccine of the invention can generate overall immunoprotection reaction, injected immune sheep can be induced to generate high-level neutralizing antibodies, and can also induce the cellullar immunologic response. The recombinant vaccine of the invention can effectively protect animals from the virulent strain attack of the aphthovirus.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation of multi-epitope TK1 antibody and application of multi-epitope TK1 antibody to evaluation on recurrence risk and prognosis of tumor patient at early stage
ActiveCN102432683ATrue reflection of proliferationReflect proliferationEgg immunoglobulinsTransferasesAntigenCvd risk
The invention provides a high-specificity and high-sensitivity coordination combination anti-human TK1 antibody prepared from an antigenic determinant consisting of 23 peptide at an N end, 20 peptide at a C end and 28 peptide at the C end of human cervical cancer cells, and application thereof to tumor diagnosis. The antigenic determinant comprises the following amino acid sequences: 1) the 23 peptide (3-25) at the N end: CINLPTVLPGSPSKTRGQIQVIL: 2) the 20 peptide (206-225) at the C end: CPVPGKPGEAVAARKLFAPQ; and 3) the 28 peptide at the C end: AGPDNKENCPVPGKPGEAVAARKLFAPQ. The invention simultaneously provides a method applying the antigen for preparing the antibody. An antibody kit provided by the invention has the characteristics of high sensitivity, high specificity, low cost and the like, tumor rehabilitation populations are periodically monitored by an enhanced chemiluminescence dot blot detection method, immunohistochemistry detection and the detection kit, and the recurrence and transfer risk and the prognosis of the tumor rehabilitation people are evaluated.
Owner:SHENZHEN HUARUI TONGKANG BIOTECHNOLOGICAL
Multimeric multiepitope influenza vaccines
ActiveUS20110182974A1Improved ability to induce cellularHigh densitySsRNA viruses negative-senseSugar derivativesProtective immunityInfluenza vaccine
The present invention relates to multimeric multi-epitope peptide-based vaccines. In particular, the present invention relates to the use of multimeric multi-epitope peptide-based vaccines eliciting protective immunity to influenza.
Owner:BIONDVAX PHARMA
Recombinant fusion protein and use thereof
InactiveCN104341506AAntiviralsAntibody medical ingredientsHepatitis B virus core AntigenAntigen epitope
The invention belongs to the biological medicine field, relates to recombinant fusion protein and use thereof, and in particular relates to recombinant fusion protein carrying hepatitis B virus therapeutic antigen epitopes which are inserted into hepatitis B core antigen protein particles or truncated fragments and use thereof. The recombinant fusion protein contains multiple epitope antigens of hepatitis B virus (HBV) and other immune stimulating epitope antigens and hepatitis B core antigen virus-like particles or truncated fragments thereof for preparation of chimeric antigen, the multiple antigen epitopes can be inserted into same or different sites of hepatitis B virus core antigen HBc or truncated fragments thereof in the manner of single epitope or multi epitope combination, and by combination with different adjuvants, HBV specific humoral and cellular immune functions can be strengthened.
Owner:FUDAN UNIV
Specific antibody of campylobacter jejuni specific multi-epitope artificial polypeptide and coated immunomagnetic beads and application thereof
InactiveCN102532283AImprove the detection rateWill not affect the physiologyMicrobiological testing/measurementImmunoglobulins against bacteriaNucleotideSpecific antibody
The invention discloses a specific antibody of a campylobacter jejuni specific multi-epitope artificial polypeptide and coated immunomagnetic beads and an application thereof. The amino acid sequence of the artificial polypeptide prepared after tandem expression of a campylobacter jejuni specific surface protein multi-antigen epitope is SEQ ID No.1. A nucleotide sequence for encoding the campylobacter jejuni specific surface protein multi-antigen epitope is SEQ ID No.2. A kit for enriching campylobacter jejuni comprises immunomagnetic beads containing a specific antibody coated with the campylobacter jejuni specific multi-epitope artificial polypeptide. By applying the immunomagnetic beads or the kit, campylobacter jejuni can be enriched from a sample pre-enrichment liquid, and the sensitivity and detectable rate of subsequent separate culturing or PCR (Polymerase Chain Reaction) detection are increased greatly; and the specific antibody has the advantages of high specificity, high sensitivity, easiness, convenience, rapidness and suitability for large-scale sample detection.
Owner:ANIMAL AND PLANT & FOOD DETECTION CENTER JIANGSU ENTRY EXIT INSPECTION AND QUARANTINE BUREAU
Multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses)
The invention discloses a multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses), relating to the technical field of virology and immunology. The multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine is characterized in that two CTL epitopes (namely, a NS4B (1793-1801) SMMAFSAAL and a P7 (774-782) AAWYIKGRL) are used for constructing recombinant adenoviruses, then the recombinant adenoviruses are used for infecting human dendritic cells so as to prepare a multi-epitope DC vaccine. Detection results indicate that the multi-epitope peptide-loaded DC (dendritic cell) therapeutic vaccine for HCV (hepatitis C viruses) disclosed by the invention has an immunogenicity.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Method for preparing bivalent vaccine of newcastle disease virus La Sota strain and infectious bronchitis virus N-S multi-epitope protein
InactiveCN104548088AGood immune effectReduce adverse reactionsViral antigen ingredientsAntiviralsAntibody levelInfectivity
The invention provides a preparation method of a bivalent vaccine by taking newcastle disease virus La Sota strain and infectious bronchitis virus N-S multi-epitope protein as antigen. To immunize 21-day-old chickens, the bivalent vaccine prepared by the method disclosed by the invention can be used for simultaneously preventing newcastle disease and nephrotropic infectious bronchitis virus infection, and the antibody level of the bivalent vaccine is higher than that of respectively immunizing by single vaccine, so that immunological stress is effectively relieved and immunity time is reduced.
Owner:TIANJIN RINGPU BIO TECH
HPV polyepitope constructs and uses thereof
Owner:EPIMMUNE
Vaccine for preventing and curing tumor
InactiveCN101920009AGenetic material ingredientsAntibody medical ingredientsReverse transcriptaseNucleotide
The invention belongs to vaccine and preparation thereof, in particular to a vaccine for preventing and curing tumor and preparation thereof. The vaccine is prepared by steps of: choosing a telomerase reverse transcriptase epitope sequence, serially connecting nucleotides the telomerase reverse transcriptase with multi-epitope, preparing the DNA vaccine of the telomerase reverse transcriptase with multi-epitope, and preparing the multi-epitope vaccine of the telomerase reverse transcriptase with multi-epitope. The invention overcomes the shortcomings that a single peptide vaccine only has a clinical effect in several tumor patients. After the vaccine has an immune body, the epitope specific CD4+T cells and the CD8+T cells can be activated. Under the assistance of the Th1 cell factor excreted from the CD4+T cells, the CD8+CTL can kill the tumor cell presenting TERT so as to prevent and cure tumor.
Owner:HEBEI MEDICAL UNIVERSITY
Epitope minimum motif peptide of P1, VP2 and VP4 structural proteins in type O foot and mouth disease virus (FMDV) strain (O/BY/CHA/2010) and application of epitope minimum motif peptide
The invention belongs to the technical field of biological medicine and biological detection and particularly relates to an epitope minimum motif peptide of P1, VP2 and VP4 structural proteins in a type O foot and mouth disease virus (FMDV) strain (O / BY / CHA / 2010) and application of the epitope. Five linear epitope minimum motif peptides provided by the invention can be used as candidate epitope peptides for research and development of a novel recombinant multi-epitope peptide vaccine against type O FMDV and have amino acid sequences as shown in SEQ. ID NO.1-SEQ. ID NO.5. The invention also provides a candidate epitope for research of a detection antigen of a high-specificity and high-sensitivity recombinant multi-epitope peptide for diagnosing prevalence of the FMDV and the candidate epitope has amino acid sequences as shown in the SEQ. ID NO.6-SEQ. ID NO.10.
Owner:FUDAN UNIV
HIV composite multi-epitope DNA vaccine and application thereof
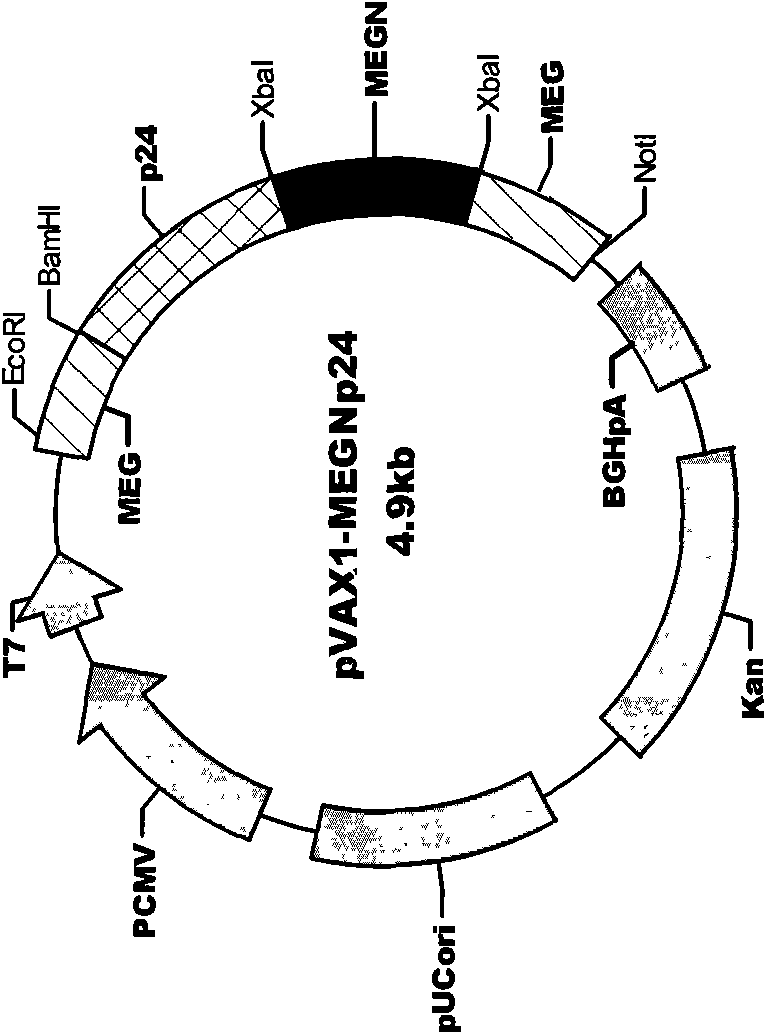
InactiveCN101579528AExtensive cross-reactivityShow feasibilityGenetic material ingredientsAntiviralsCtl epitopeVaccination
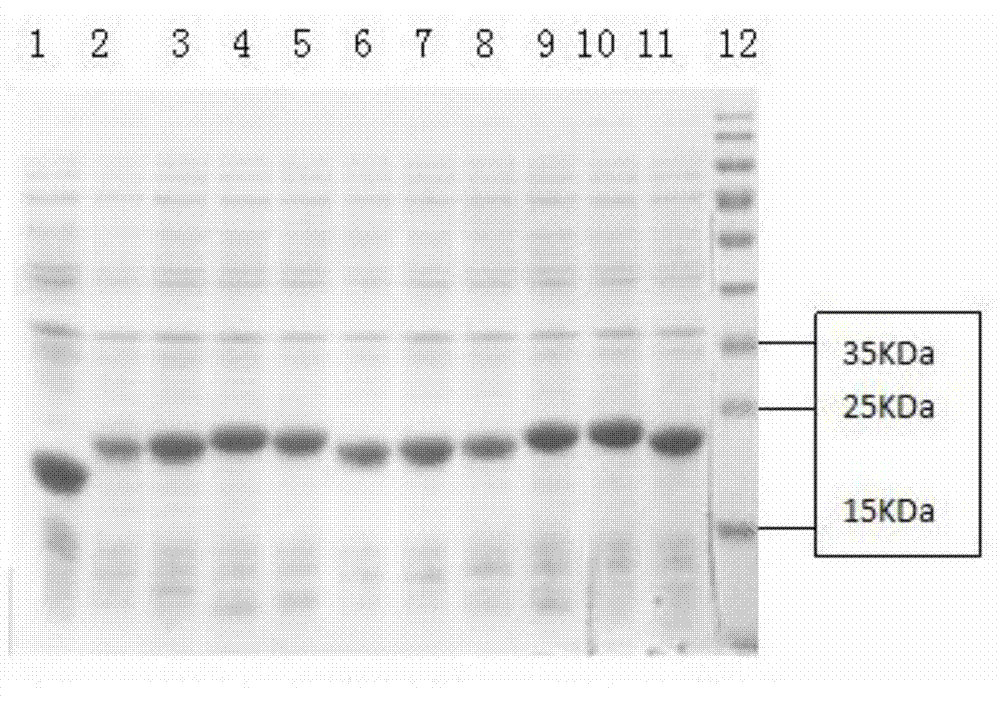
The invention discloses an HIV composite multi-epitope DNA vaccine which takes HIV-1 capsid protein p24 as a vector backbone molecule and ER signal peptide as a homing sequence, and contains twenty-six highly conservative immunodominant epitopes in an HIV genome; wherein, the HIV composite multi-epitope DNA vaccine comprises twenty-nine epitopes which are three neutralizing antibody epitopes, twenty-three CTL epitopes, one HIV-1 isolate common antibody epitope, one MHC nonrestrictive T helper lymphocyte epitope and one spasmotoxin B cell epitope. The vaccine can be used for vaccination of healthy people and immunization therapy of people who are infected by AIDS virus, thus having double effects of prevention and treatment.
Owner:MILITARY VETERINARY RES INST PLA MILITARY MEDICAL ACAD OF SCI
Vaccine of recombined albumen for preventing and treating infection of human C type hepatitis virus and its usage
A recombinant protein vaccine which is a fusion protein of BCG vaccine's heat shock protein 65 and the core antigen of multi-epitope hepatitis-C virus, its amino acid sequence and nucleotide sequencefor coding it, the expression carrier containing said nucleotide sequence, the host cell containing said expression carrier, the preparing process of said recombinant protein vaccine, the vaccine containing said recombinant protein for preventing and treating hepatitis C, and a method for detecting the activity of specifically killing T, lymphocytes by the hepatitis C induced by said vaccine and its cell model are disclosed.
Owner:BEIJING HYDVAX BIOTECH
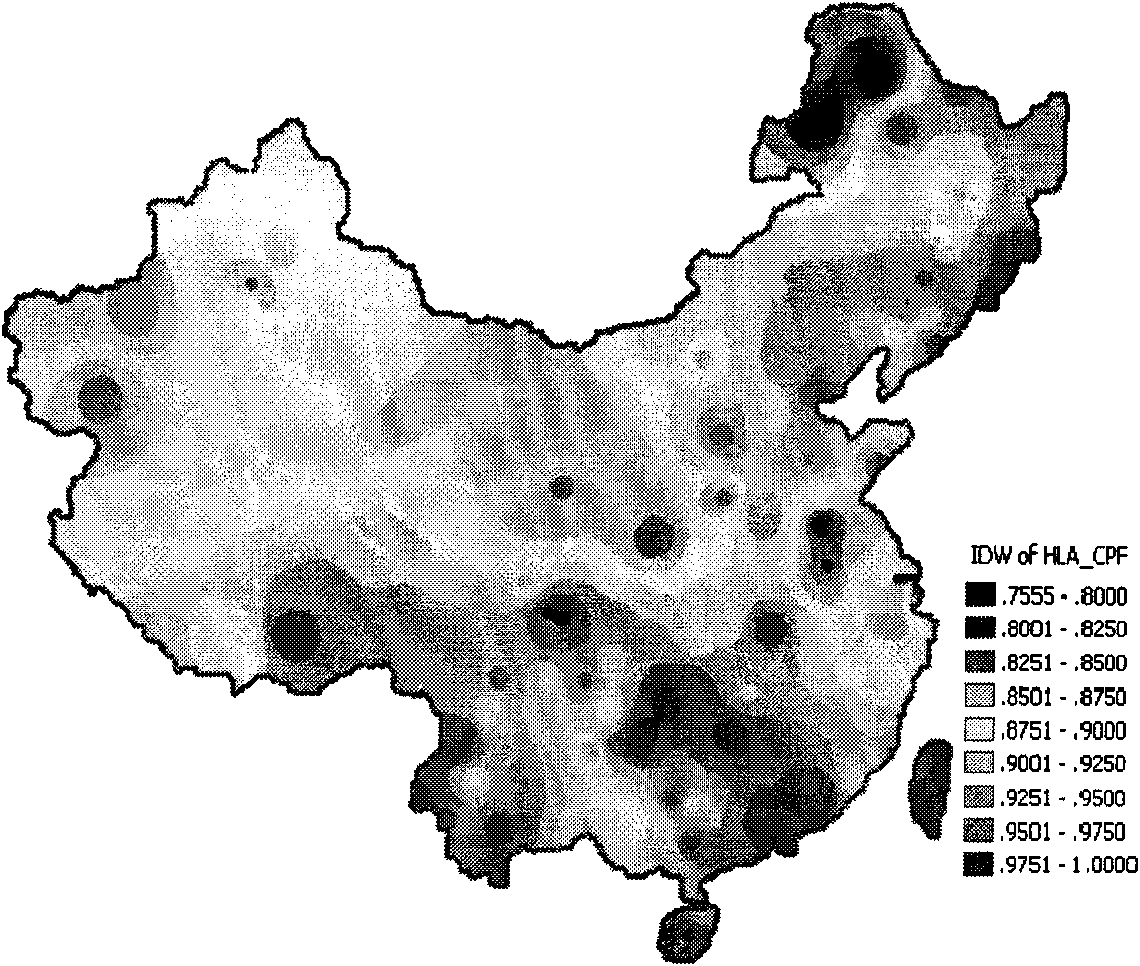
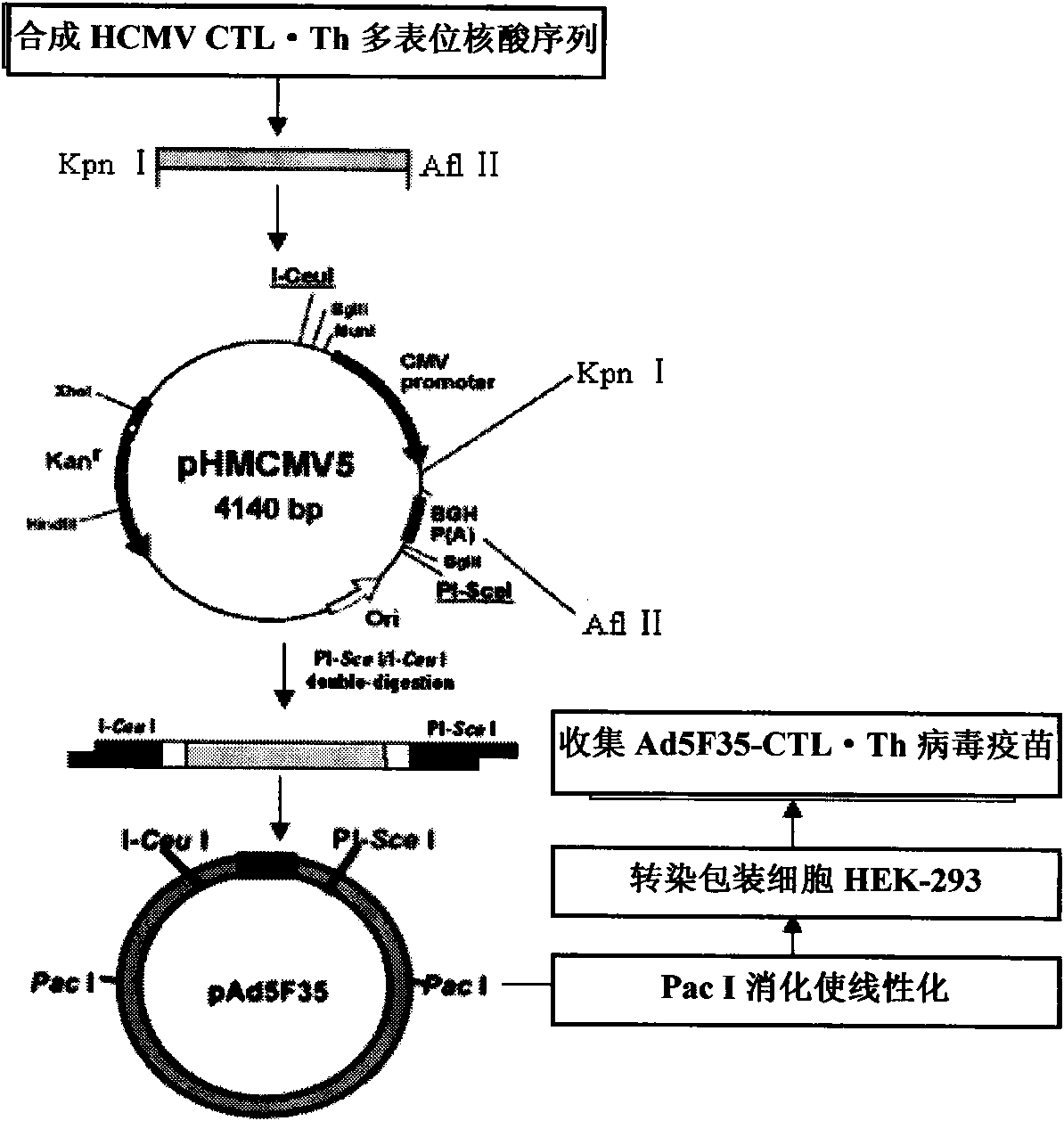
HLA Specific human cytomegalovirus Multi-epitope adenovirus DNA Vaccine of Chinese population
InactiveCN101579527AEfficient secretionImprove immunityGenetic material ingredientsMicroorganism based processesDiseaseNucleotide
The present invention discloses an HLA Specific human cytomegalovirus Multi-epitope adenovirus DNA Vaccine of Chinese population, which consists of human cytomegalovirus tandem sequence CTL*Th and adenovirus expression vector pAd5F35, wherein the human cytomegalovirus tandem sequence CTL*Th comprises a nucleotide sequence represented by SEQ ID No.1. The nucleotide sequence comprises the nucleotide sequences of 83 antigen epitopes of 15 encoding proteins of human cytomegalovirus. Furthermore, the sequence covers 14 HLA I allelomorphic gene sites and 7 HLA II allelomorphic gene sites. The coverage percent of the HCMV tandem epitope DNA vaccine representing excellent HLA is 92.07 percent in average, and is used for preventing the forming of HCMV disease in the steps of adhering, duplicating and re-activating of the virus. The in vetro experiment shows that the vaccine of the invention has excellent immune effect and overcomes the defects of inferior immunogenicity and limited immune range of the prior HCMV vaccine.
Owner:SHANDONG UNIV QILU HOSPITAL
Bovine A-type foot-and-mouth disease multi-epitope vaccine, and preparation method and application thereof
The invention discloses a bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine, and a preparation method and an application thereof, and belongs to the field of veterinary vaccine research. The preparation method comprises the following steps: carrying out reasonable serial connection on the dominant antigen epitopes of bovine A-type foot-and-mouth disease virus representative strains once pandemic in China by adopting an antigenized antibody strategy and a brand new reverse vaccinology technology and strategy, coupling with a bovine IgG immunostimulatory fragment (IgG heavy chain constant region), cloning into a prokaryotic expression vector to construct a recombinant expression vector, transforming Escherichia coli cells to express a recombinant antigen, purifying by adopting Ni-NAT column chromatography, quantifying by a Bio-Rad protein quantification kit, and preparing the vaccine individually or combining with a recombinant foot-and-mouth disease virus 3D protein. A result of animal immune experiments shows that the recombinant protein or combined vaccine can stimulate the body to produce a protective antibody with high titer and also can protect immune animals against a virus attack, so the bovine A-type foot-and-mouth disease multi-epitope recombinant vaccine has a good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Mycoplasma hyopneumoniae multi-epitope mucosal vaccine
The invention relates to preparation and application of a mycoplasma hyopneumoniae multi-epitope mucosal vaccine. A mycoplasma hyopneumoniae membrane protein, an adhesive protein P97, a lipoprotein P65, a specific membrane protein P46, a B cell epitope, a Th epitope, a CTL epitope and a cholera toxin subunit B are taken as a vaccine frame structure, a pRSETA carrier is cloned in through flexible linker connection, then Escherichia coli is transformed, and fermentation, purification and preparation technologies are carried out, so that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine with ideal immunogenicity is obtained. A self-made mucosal adjuvant is used in a preparation process, so that production and using processes of the vaccine are simpler and more convenient. Animal experiments show that the mycoplasma hyopneumoniae multi-epitope mucosal vaccine not only has good safety but also can stimulate effective mucosal immunity, humoral immunity and cellular immune reactions.
Owner:QINGDAO MINGQIN BIOLOGICAL TECH CO LTD
Type A foot-and-mouth disease virus sIgA antibody ELISA kit and application thereof
ActiveCN109187993AQuick checkAccurate detectionBiological material analysisBiological testingPositive controlElisa kit
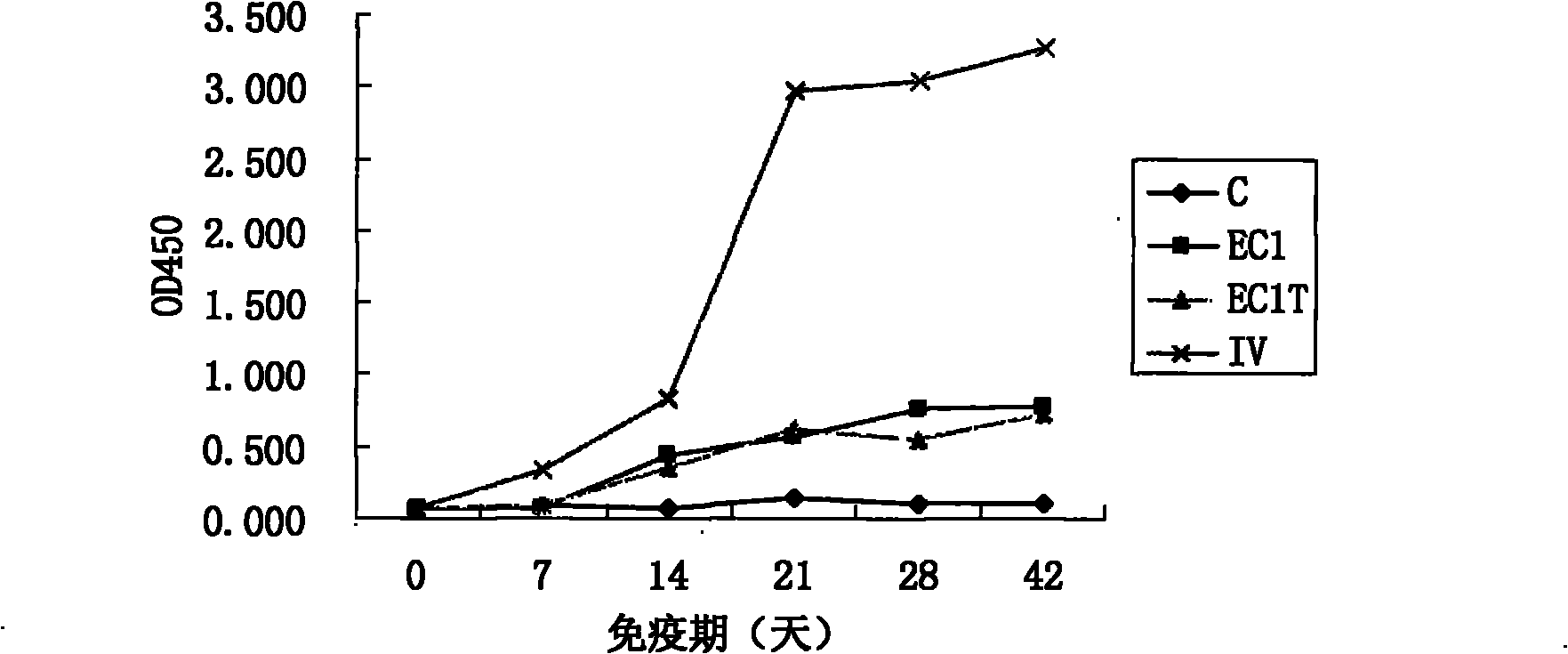
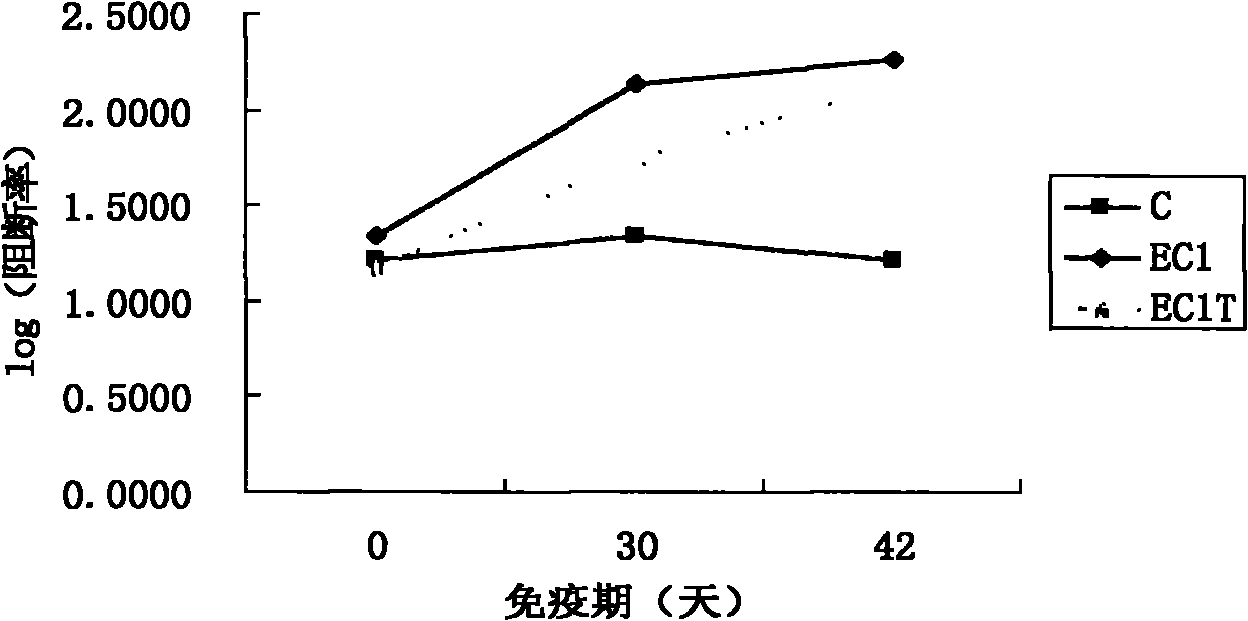
The invention discloses a type A foot-and-mouth disease virus sIgA antibody ELISA kit and application thereof. The kit comprises an enzyme-labeled reaction plate coated with a type A foot-and-mouth disease virus broad-spectrum multi-epitope recombinant antigen, a 100x concentrated enzyme-labeled antibody, an enzyme-labeled antibody diluent, a sample diluent, a washing concentrate, a developing solution, a stop solution, a positive control sample and a negative control sample. The type A foot-and-mouth disease virus broad-spectrum multi-epitope recombinant antigen consists of dominant antigen epitopes (TB / A) of three separated representative type A foot-and-mouth disease strains of China, so that the detection sensitivity and specificity of the kit are improved, and the kit is applied to detection of infection by different type A foot-and-mouth disease viruses. The kit is suitable for detecting sIgA antibodies in mucosal secretions of three susceptible animals, i.e., pigs, cattle and sheep, and is significant for prevention and control of propagation and infection of type A foot-and-mouth disease viruses.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Bovine A-type foot-and-mouth disease broad-spectrum multi-epitope vaccine, and preparation method and application thereof
ActiveCN104119441AResist attackGenetic material ingredientsAntiviralsAntigen epitopeEscherichia coli
The invention discloses a bovine A-type foot-and-mouth disease broad-spectrum multi-epitope recombinant vaccine, and a preparation method and an application thereof, and belongs to the field of veterinary vaccine research. The preparation method comprises the following steps: carrying out reasonable serial connection on the major antigen epitopes of pandemic in China and bovine A-type foot-and-mouth disease virus representative strains recently pandemic in countries bordering China by adopting a brand new reverse vaccinology idea and strategy, coupling with a bovine IgG heavy chain constant region, cloning into a prokaryotic expression vector to construct a recombinant expression vector, transforming Escherichia coli cells to express a recombinant antigen, purifying by adopting Ni-NAT column chromatography, quantifying by a Bio-Rad protein quantification kit, and preparing the vaccine individually or combining with a recombinant foot-and-mouth disease virus 3D protein. A result of animal immune experiments shows that the multi-epitope antigen or 3D protein combined vaccine can stimulate the body to produce a protective antibody with high titer and also can protect immune animals against a virus attack, so the bovine A-type foot-and-mouth disease broad-spectrum multi-epitope recombinant vaccine has a good application prospect.
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
B-cell antigenic multi-epitope peptide linked in tandem in OmpU of vibrio mimicus, making method and application thereof
InactiveCN101747416AEasy accessAccurately obtainedAntibacterial agentsMicroorganism based processesDiseaseMolecular Immunology
The present invention relates to an antigenic B-cell multi-epitope peptide linked in tandem in the outer membrane protein(Omp) U gene of vibrio mimicus, a making method and an application thereof, which belong to the field of molecular immunology. The B-cell multi-epitope peptide linked in tandem can induce fish to make protective immunity response to vibrio mimicus infection. In the present invention, the amino acid sequence of the antigenic B-cell multi-epitope peptide linked in tandem in the OmpU is shown in SEQIDNO:10. The B-cell multi-epitope peptide linked in tandem in the OmpU of vibrio mimicus is made by a genetic engineering technique. The verification of immune blotting analysis, specific antibody detection and immune animal protective experiments shows that the peptide can elicit efficient and specific protective humoral immunity response to vibrio mimicus infection. The B-cell multi-epitope peptide linked in tandem can be used for the immune diagnosis and the immune prevention and treatment of aquatic animal ascitic diseases. The present invention has high social benefit and economic benefit.
Owner:ANHUI AGRICULTURAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com