HLA Specific human cytomegalovirus Multi-epitope adenovirus DNA Vaccine of Chinese population
A technology of human cytomegalovirus and nucleic acid vaccines, which is applied in the direction of antiviral agents, recombinant DNA technology, biochemical equipment and methods, etc., can solve the problem of difficult presentation of epitope vaccines, uneven population and ethnic spatial distribution, and inability to cause protection The problem of immune response and other problems, to overcome the effect of weak immunogenicity and strong immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0025] The present invention will be described in further detail below in conjunction with specific examples, but not as a limitation of the present invention.
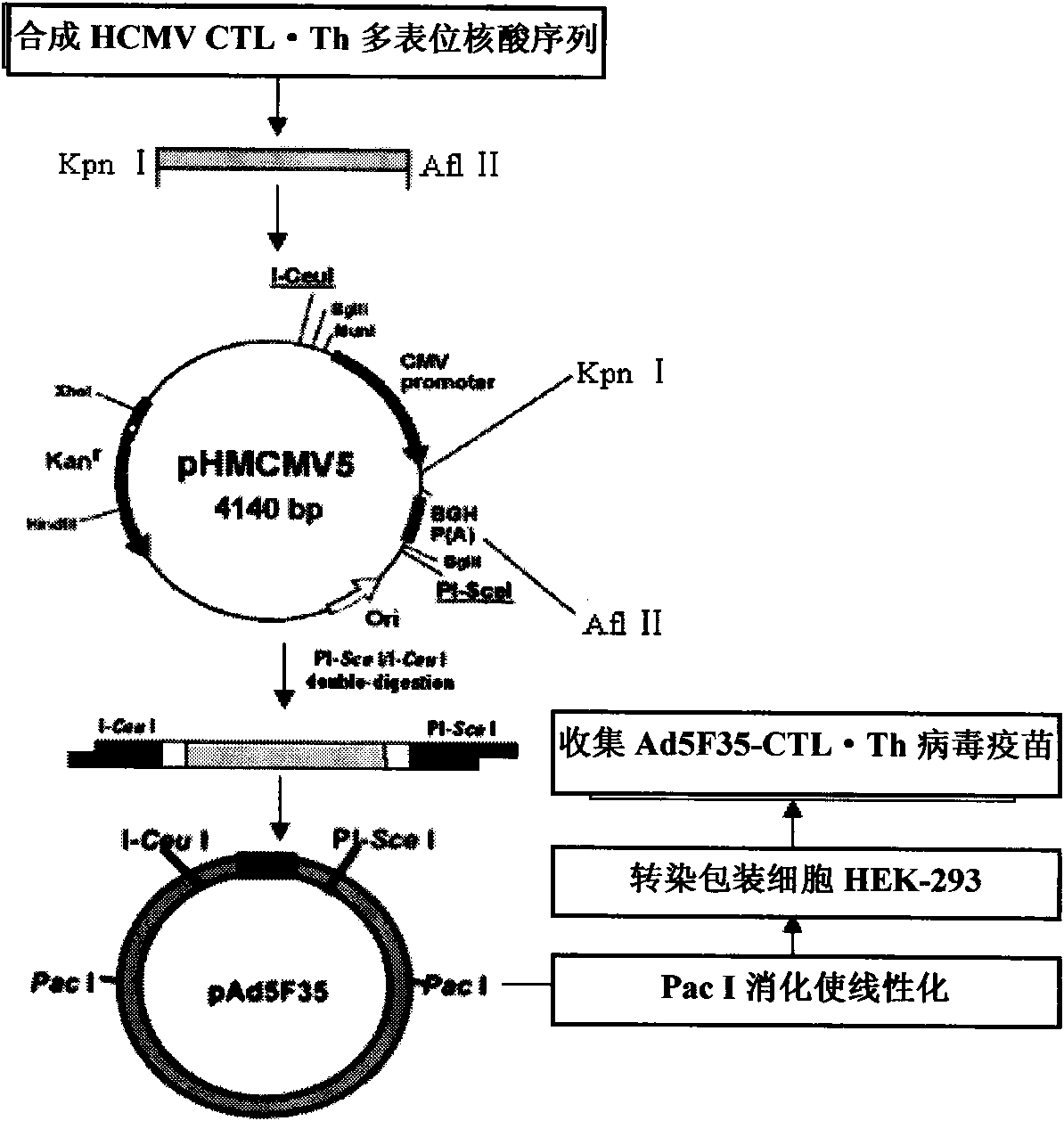
[0026] Preparation of HLA-specific human cytomegalovirus multi-epitope adenovirus nucleic acid vaccine in Chinese population
[0027] 1. Epitope prediction and determination
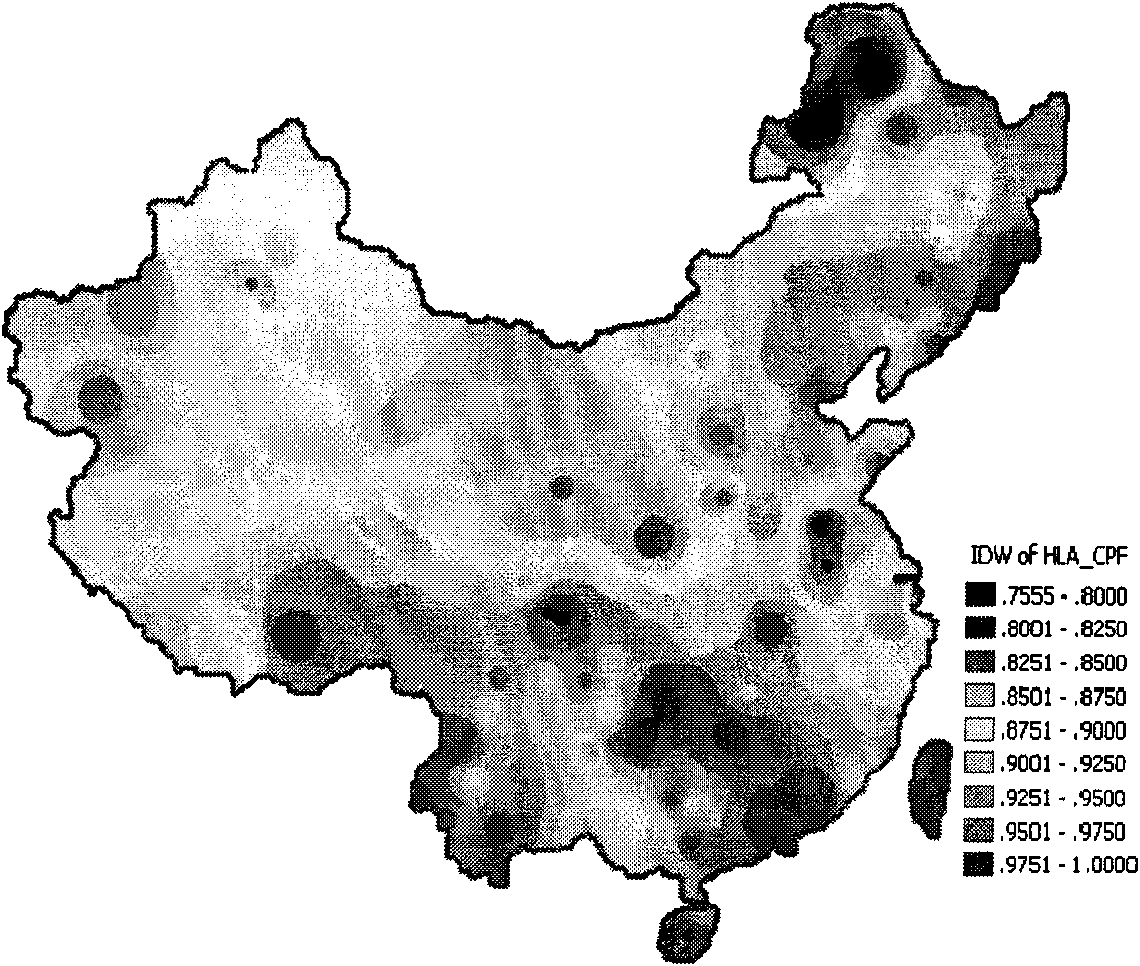
[0028] Using the Chinese HLA I cumulative phenotype frequency spatial prediction system ("Journal of Immunology" 2005, Volume 21, No. 2, P136), select 14 HLA I gene loci for combination, such as figure 1 As shown, the HLA coverage rate of the Chinese population of the HCMV multi-epitope vaccine of the present invention is as high as 92.07% (75.49-99.99%). Then select 83 epitopes in 15 kinds of antigenic proteins (pp28, pp50, pp65, pp150, pp71, gH, gB, IE-1, IE-2, US2, US3, US6, US11, UL16, UL18) of HCMV, Among them, there are 76 CTL epitopes for the above-mentioned 14 HLA I gene loci (Table 1), and 7 Th epitopes for the HLAII gene loci (Table ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| cover factor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com