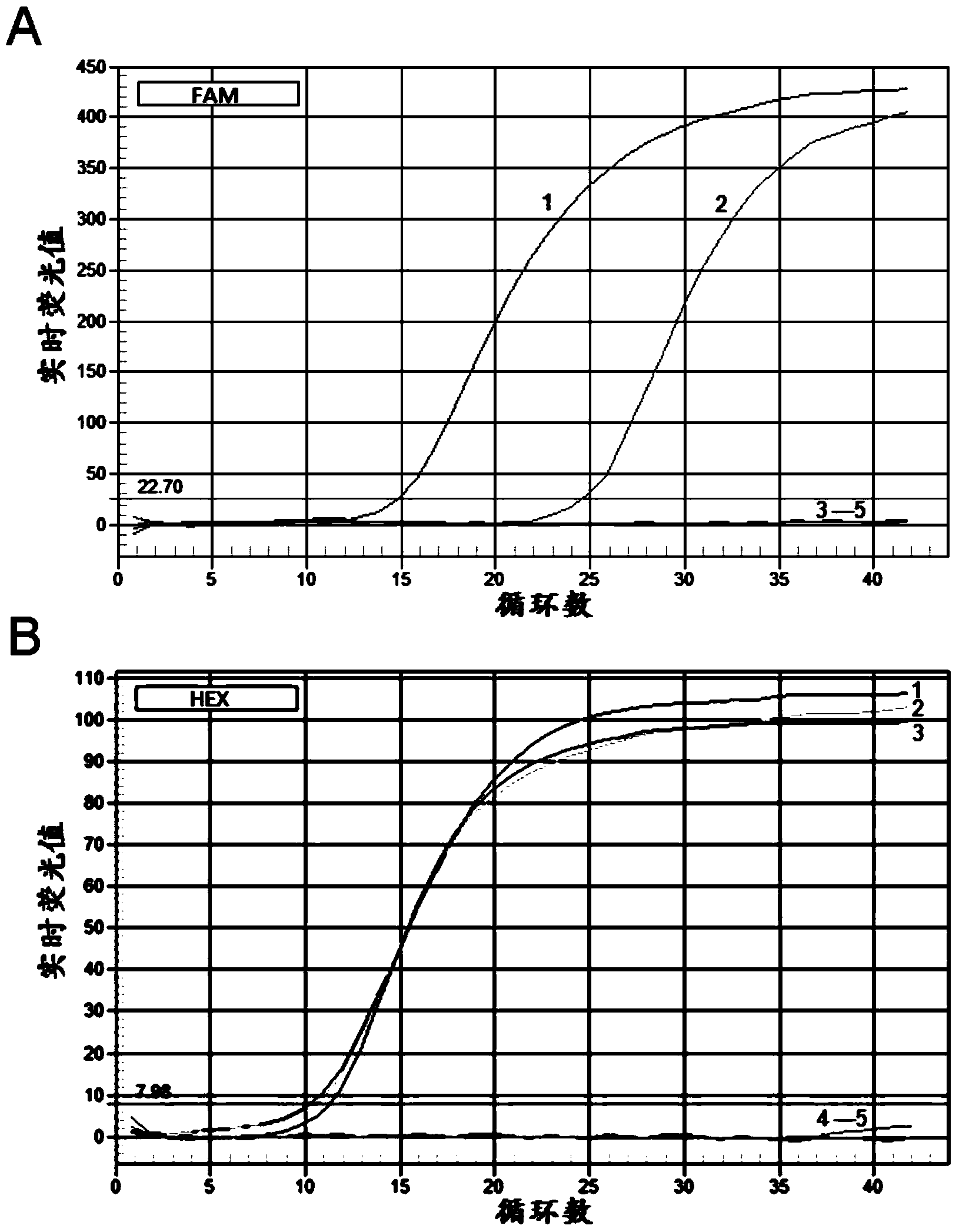
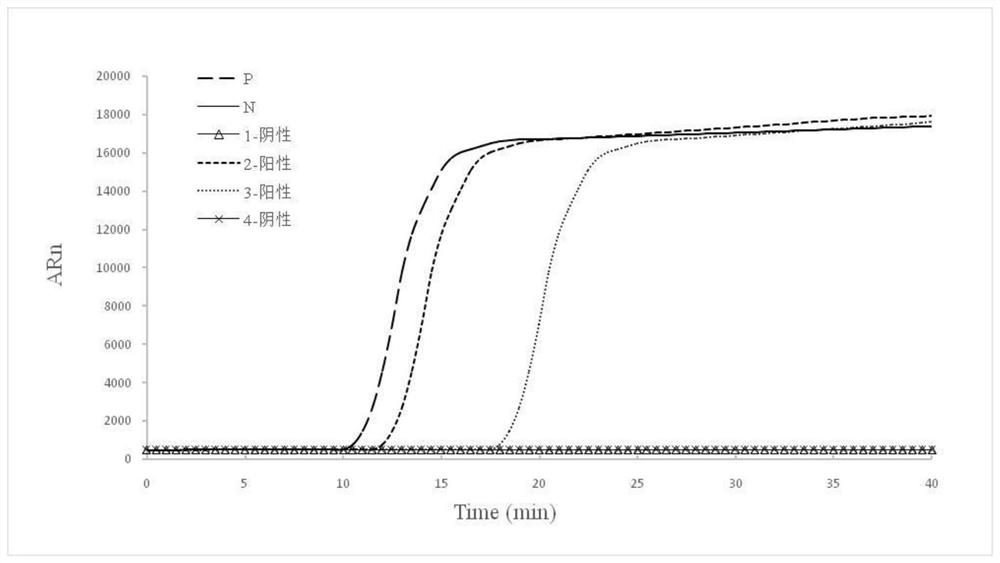
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
37 results about "Viral nucleocapsid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The complete protein-nucleic acid complex that is the packaged form of the genome in a virus particle. [ISBN:0781702534]
Recombinant akabane virus capsid protein, its preparation method and uses
InactiveCN1769294AHigh immunological activityHighly immunologically activeVirus peptidesBiological testingEscherichia coliProkaryotic expression
The invention discloses a novel recombinant Akabane virus nucleotide capsid protein, gene encoding the recombinant protein and the process for preparing the recombinant protein. The recombinant protein has the amino acid sequence represented by SEQ ID No:1, the gene encoding the recombinant Akabane virus nucleotide capsid protein has the amino acid sequence represented by SEQ ID No:2. The preparing process of the recombinant protain comprises the following steps: constructing recombinant Akabane virus nucleotide capsid protein prokaryotic expression vectors, transforming bacillus coli with the recombinant Akabane virus nucleotide capsid protein prokaryotic expression vectors, inducing recombinant nucleotide capsid protein expression with IPTG, reclaiming and purifying the expressed recombinant nucleotide capsid protein.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for marking enveloped virus nucleocapsid by using quantum dot
InactiveCN103555681AConvenient researchAchieve markupMicrobiological testing/measurementMicroorganism based processesChemical reactionCapsid
The invention relates to a method for marking enveloped virus nucleocapsid by using a quantum dot. The method is characterized in that a capsid protein sequence blended with a biotin receptor peptide sequence and a catalytic receptor peptide biotinylation enzyme sequence are simultaneously inserted in a virus whole-genome so as to obtain a recombinant virus plasmid, the biotinylation of the nucleocapsid is realized by virtue of the reproduction process of the virus plasmid in a host cell, and then the quantum dot coupled with streptavidin is used for realizing the marking of the enveloped virus nucleocapsid. The method does not needs the violent chemical reaction transformation and the marking of a virus and is beneficial to the maintenance of infection activity of the virus self; and the marking of the nucleocapsid in the enveloped virus does not affect the surface of the enveloped virus and the indentation of the surface. The method can be applied to the virus infection mechanism study and the virus-related disease treatment research.
Owner:WUHAN UNIV
Method for preparing precious metal palladium nanorod by means of cotton bollworm baculovirus nucleocapsid
ActiveCN104001931ARaw materials are easy to obtainReduce manufacturing costCotton bollwormAlkaline hydrolysis
A method for preparing a precious metal palladium nanorod by means of cotton bollworm baculovirus nucleocapsid mainly includes the steps that cotton bollworm karyotype polyhedron virus raw powder is dispersed through ultra-pure water to obtain grey polyhedrin suspension, alkaline hydrolysis liquid is added into the grey polyhedrin suspension, the mixture stands still for 15-25 min, then the pH value is adjusted to be 7 through acetic acid, chloroform is added, centrifugalization is carried out for 5-10 min at the speed of 5000-6000 r / min, supernatant fluid is sucked, centrifugalization is carried out for 1-2 h at the temperature of 4-10 DEG C and at the speed of 25000-30000 r / min, pigmentation is re-suspeneded through a PBS solution, and a cotton bollworm baculovirus nucleocapsid solution is obtained; a PdCl2 solution is added into the cotton bollworm baculovirus nucleocapsid solution and fully and evenly mixed, incubation is carried out in a shaking mode for 15-25 h at the temperature of 20-30 DEG C and at the speed of 130-170 r / min, and the precious metal palladium nanorod loaded by the cotton bollworm baculovirus nucleocapsid is obtained. The method is simple in process, environmentally friendly and efficient, industrial production is easy to achieve, and the prepared palladium nanorod is stable in appearance and uniform in size.
Owner:YANSHAN UNIV
Cell line for stably expressing schmallenberg virus nucleocapsid protein and application of cell line
ActiveCN104232587AGood passage stabilityFast growthMicroorganism based processesGenetic engineeringDiseaseFluorescence
The invention provides a BHK-21 cell line for stably expressing schmallenberg virus (SBV) nucleocapsid protein. The preservation number of the BHK-21cell line is CGMCC NO. 7616, and the cell line is named BHK-21-pLV-EGFP-SBV-N. The cellular immunity fluorescence experiment result shows that the cell line can not only have specific reaction with a monoclonal antibody 2C8 of SBV, but also can recognize positive serum of SBV infected cattle. The BHK-21 cell line for stably expressing the SBV N protein can be used for detecting schmallenberg disease and has good application prospect.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
SARS-CoV-2 detection kit
ActiveCN112964872AEasy to detectSave operating timeBiological material analysisBiological testingEpitopeProtein labeling
The invention relates to an SARS-CoV-2 detection kit. The kit comprises: (a) a solid phase conjugate. The solid-phase conjugate consists of a solid-phase carrier as well as an antigen and an antibody which coat the solid-phase carrier. The antibody is a monoclonal antibody for resisting novel coronavirus nucleocapsid N protein. The antigen comprises an N protein of recombinant SARS-CoV-2. The kit further comprises (b) an antigen marked with a chemiluminescent marker, and an antibody and / or an anti-antibody marked with a chemiluminescent marker. The antibody is a monoclonal antibody to the novel coronavirus nucleocapsid N protein, but is bound to a different epitope from the antibody in (a). The antigen comprises an N protein of recombinant SARS-CoV-2. The anti-antibody is at least one of an anti-human IgG secondary antibody, an anti-human IgA secondary antibody and an anti-human IgM secondary antibody. The anti-antibody is a monoclonal antibody or a polyclonal antibody. In (a) and (b), the N protein of the recombinant SARS-CoV-2 does not have the antigen epitope recognized by the monoclonal antibody for resisting the novel coronavirus nucleocapsid N protein.
Owner:SHENZHEN YHLO BIOTECH
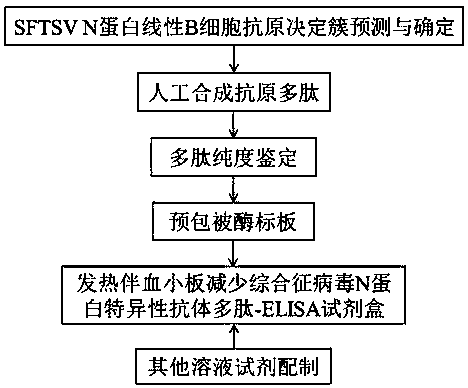
Polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting specific antibody of N (nucleocapsid) protein of SFTSV (severe fever with thrombocytopenia syndrome virus)
ActiveCN105510580AReduce peptideReduce false positivesMaterial analysisPositive controlHorse radish peroxidase
The invention relates to detection kits in the technical field of biology, in particular to a polypeptide-ELISA (enzyme-linked immunosorbent assay) kit for detecting a specific antibody of an N (nucleocapsid) protein of an SFTSV (severe fever with thrombocytopenia syndrome virus). The kit comprises an ELISA plate pre-coated with synthetic SFTSV N protein linear B-cell antigenic polypeptide, a sample diluent, negative control serum, positive control serum, an HRP (horse radish peroxidase) marked antibody, a concentrated cleaning solution, an enzyme substrate solution and a stop buffer. The kit has the advantages of high specificity, good repeatability and simplicity, convenience and rapidness in operation, and can be widely used for evaluating SFTSV infection conditions.
Owner:ZHEJIANG PUKANG BIOTECH
Standard positive reference material for rana grylio virus (RGV) detection
InactiveCN101921757AHigh purity and easy quantificationSubspecific amplificationMicrobiological testing/measurementFermentationAgricultural scienceNucleotide sequencing
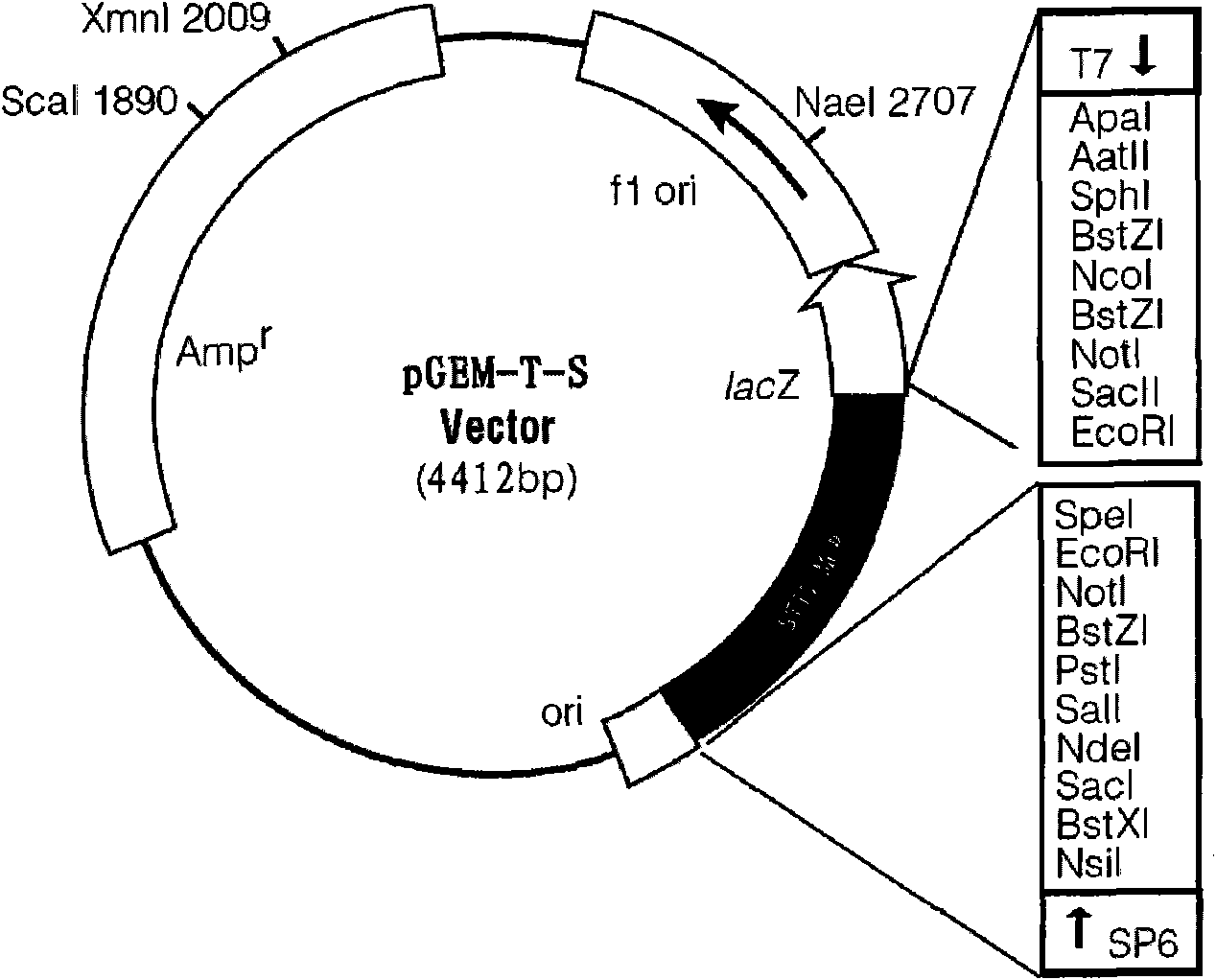
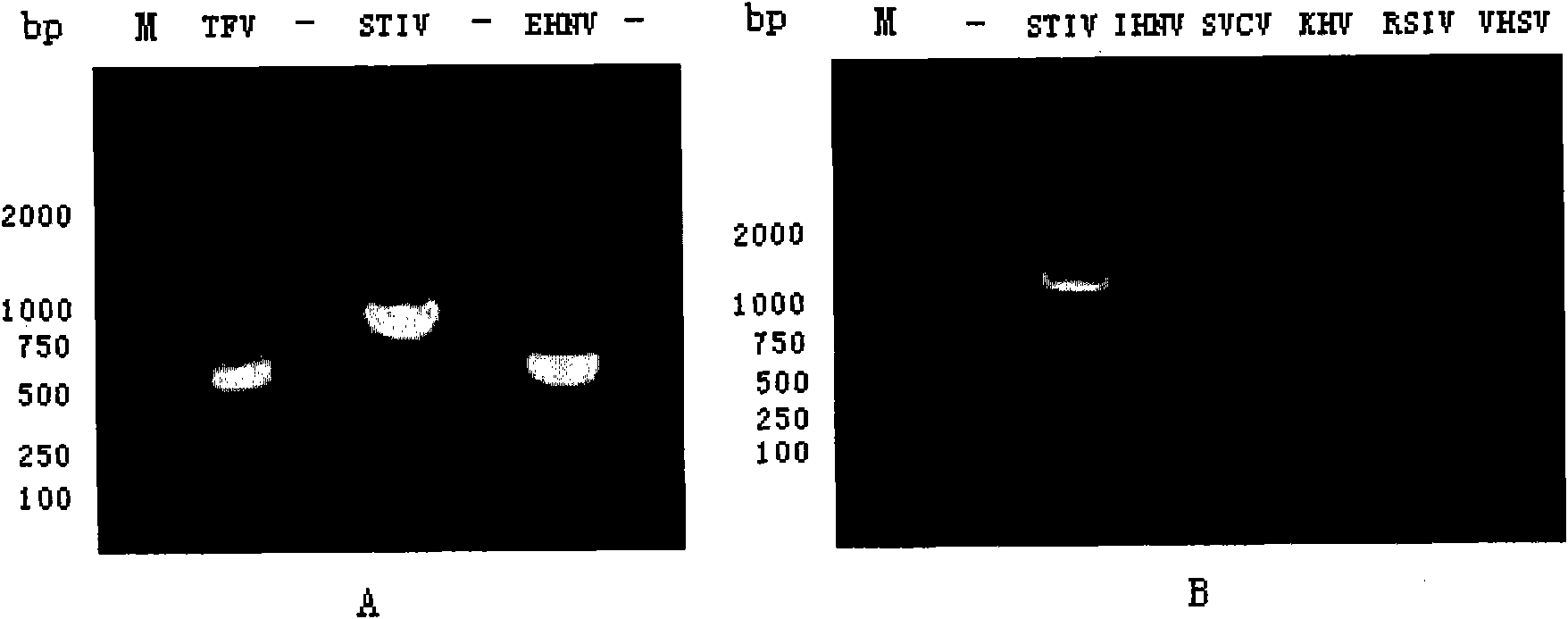
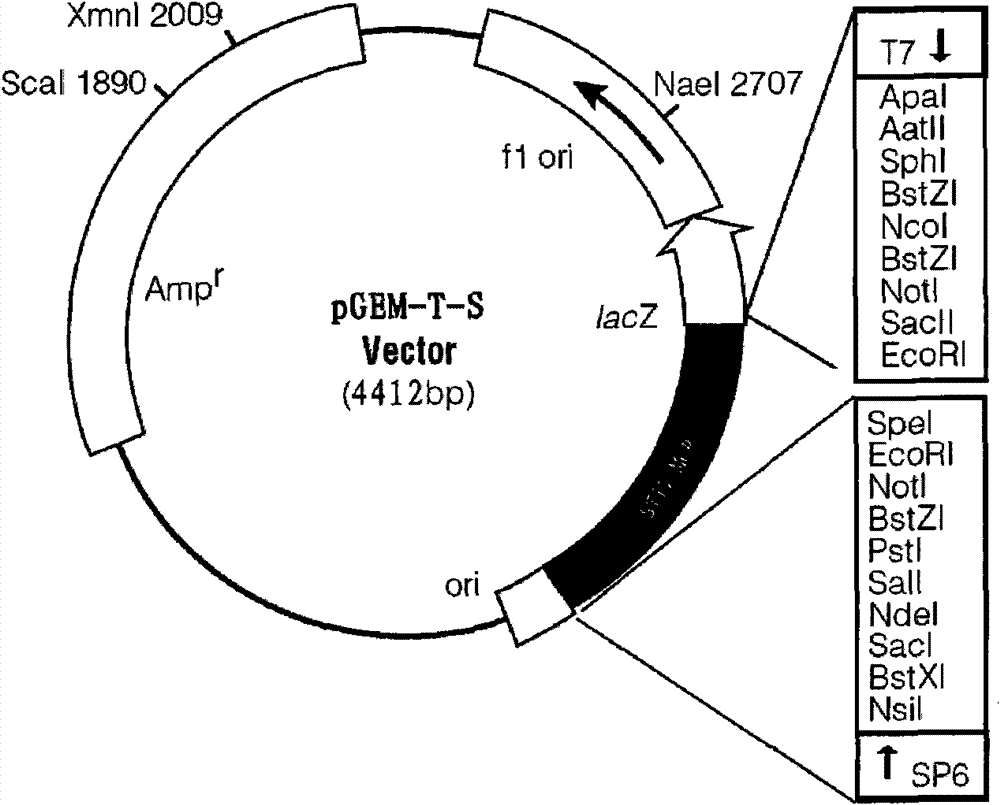
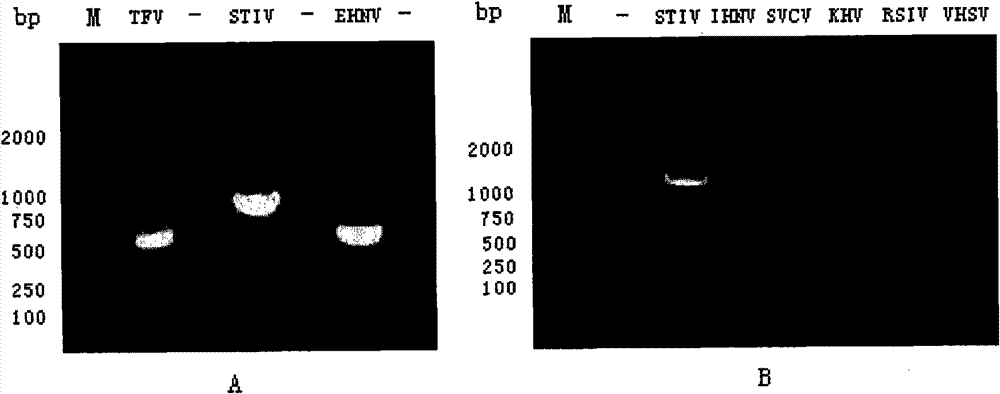
The invention discloses a standard positive reference material for rana grylio virus (RGV) detection. In the invention, a pair of primers are firstly designed, which can specifically amplify capsid protein complete genes of soft-shelled turtle iridovirus nuclears, wherein the nucleotide sequences of the primers are shown in SEQ ID.1 and SEQ ID NO.2; the capsid protein complete genes of the soft-shelled turtle iridovirus nuclears amplified by the primers have the nucleotide sequence shown in SEQ ID NO.3; and a recombinant plasmid pGEM-T-S containing the capsid protein complete genes of the soft-shelled turtle iridovirus nuclears is constructed and screened after a proper PCR reaction system and reaction conditions are found out, which can be used as the standard positive reference material for molecular biology detection on STIV MCP, EHNV MCP and TFV MCP.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Leucodermia virus rapid detecting kit, and its preparing and using method
The invention is a melanodermia virus quickly-detecting reagent box, including: a detecting device; tween-phosphate buffer liquid containing ox serum albumin; tween-phosphate buffer liquid; low osmotic liquid; gold labeled anti-melanodermia virus monoclonal antibody probe, prepared of two colloidal gold labeled monoantibodies E and D in proportion; the E and D are anti-melanodermia virus bursa membrane antibody E and anti-melanodermia virus nucleus coat antibody D secreted by two hybrid tumor cells, respectively; the conservation numbers of the two hybrid tumor cells are CCTCC-C200421 and CCTCC-C200422, respectively. As the E and D are used as detecting antibodies, they can obviously improve detecting sensitivity. The detecting reaction only takes 3 minutes without hatching and any instrument and equipment, and their detecting sensitivity is the same as that of spot immune printing method. The reagent box has the characters: simple and convenient, quick, accurate, etc.
Owner:OCEAN UNIV OF CHINA
MIA primer, probe and kit for detecting COVID-19 and application of MIA primer and probe
PendingCN112094947AEasy to detectGuaranteed responseMicrobiological testing/measurementAgainst vector-borne diseasesPneumovirusEnvelope Gene
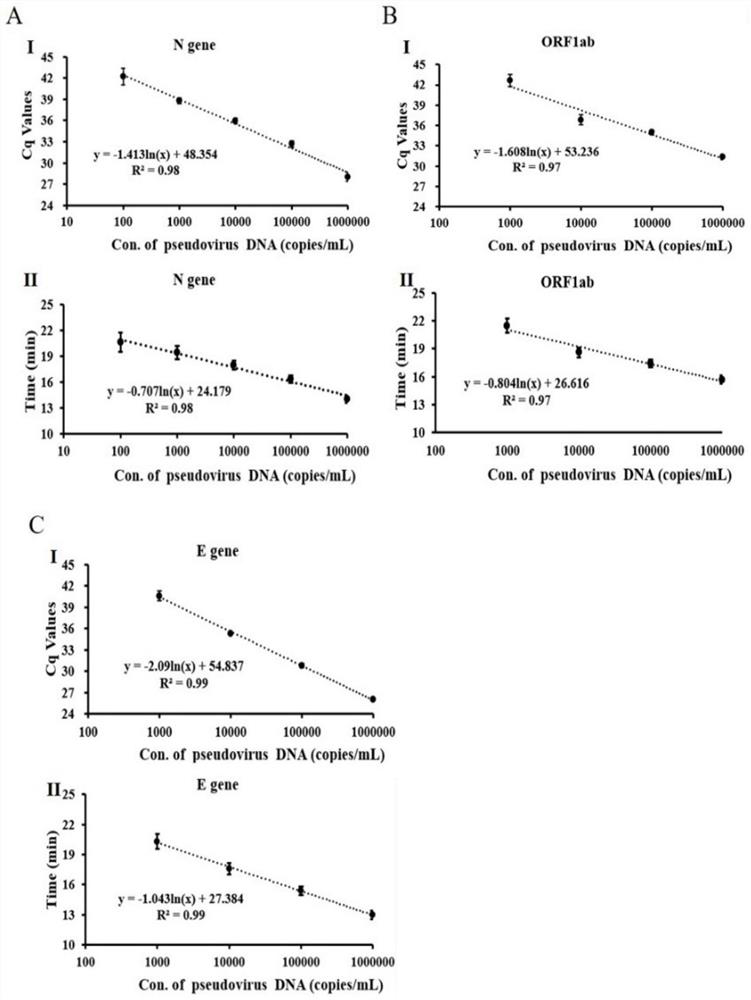
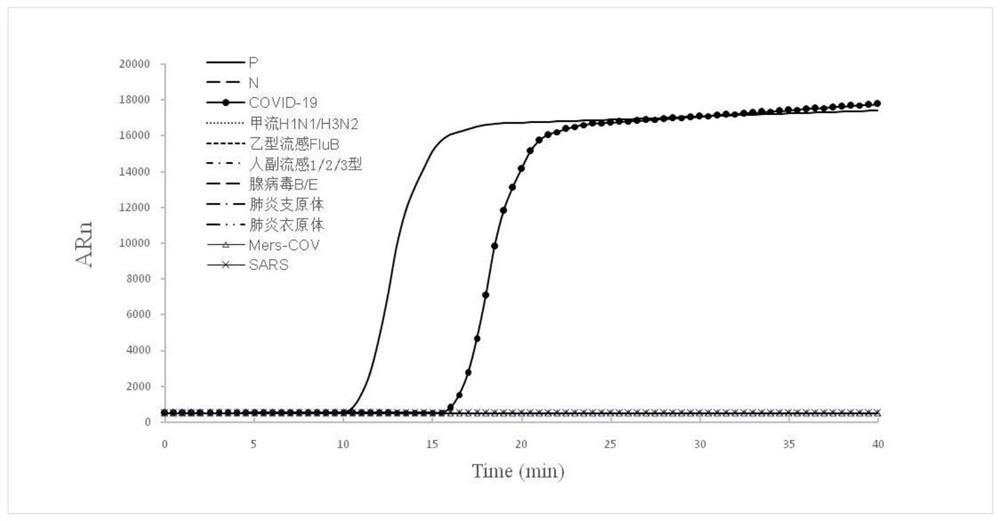
The invention discloses an MIA primer, a probe and a kit for detecting COVID-19 and an application of the MIA primer and the probe. According to the invention, the MIA primer and the probe are respectively designed based on a nucleocapsid N gene, an envelope E gene and an ORF1ab region of the COVID-19 publicized by NCBI. According to the MIA method disclosed by the invention, the detection of ORF1ab, E and N target genes of COVID-19 is realized in 3-15 minutes for the first time, and the detection sensitivity reaches 100 copies / ml (sample template concentration). The MIA method has no non-specific cross reaction with pathogen nucleic acid samples such as type A influenza, type B influenza and respiratory syncytial virus.
Owner:HANGZHOU BAOLIN BIOTECHNOLOGY CO LTD
Schmallenberg virus nucleocapsid protein monoclonal antibody, and preparation method thereof
ActiveCN103275219AHigh purityStrong specificityMicroorganism based processesImmunoglobulins against virusesAntigenEscherichia coli
The invention provides a schmallenberg virus nucleocapsid protein monoclonal antibody which is produced and secreted by hybridoma SBV-N McAb2C8 with a preservation number of CGMCC No. 7617. The preparation method of the hybridoma comprises the following steps of extracting genomic RNA of SBV, obtaining N gene sequences by RT-PCR amplification; inserting the N gene sequences into prokaryotic expression vectors; transforming into Escherichia coli; carrying out inducible expression and protein purification; immunizing an animal by using purified protein as an antigen; fusing splenocytes of the immunized animal with mouse myeloma cells to prepare the hybridoma; and screening to obtain the hybridoma capable of stably secreting the schmallenberg virus nucleocapsid protein monoclonal antibody. The monoclonal antibody provides a material basis for establishment of an ELISA detection method for schmallenberg diseases.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Compositions containing virus-like particles as immunopotentiators administered through the mucosa
InactiveUS7374766B1Adjuvant effectViral antigen ingredientsSnake antigen ingredientsWhole bodyPharmaceutical industry
The present invention is related to the branch of medicine, particularly to the new formulations of vaccine antigens.The technical objective pursued with the present invention is, precisely, the development of formulations that are able to enhance the immune response to mucosally administered antigens, minimising the number of compounds in the formulation and generating strong mucosal and systemic responses through a synergic interaction between the antigens in the formulation.These formulations enable: a) to broaden the spectrum of the anti-hepatitis B immune response, containing as main compounds HBsAg and HBcAg, b) to enhance the response against HBsAg with a viral nucleocapsid c) to generate combined vaccines through the mucosal route with HBsAg as a central antigen. Stabilizers and preservatives can be introduced.The formulations of this invention can be applied in the pharmaceutical industry as human or veterinary vaccine formulations.
Owner:CENT DE ING GENETICA & BIOTECNOLOGIA
SARS-CoV-2 detection kit based on sandwich method
ActiveCN112964873AEasy to detectSave operating timeBiological material analysisBiological testingEpitopeProtein labeling
The invention relates to the field of biological medicine, in particular to an SARS-CoV-2 detection kit based on a sandwich method. The kit comprises (a) a solid phase conjugate. The solid-phase conjugate consists of a solid-phase carrier as well as an antigen and an antibody which coat the solid-phase carrier. The antibody is a monoclonal antibody for resisting novel coronavirus nucleocapsid N protein. The antigen comprises an S1 subunit, an S2 subunit, an RBD, an N protein and an E protein of the SAR-CoV-2 Spike protein. The kit further comprises (b) an antigen and an antibody marked with a chemiluminescent marker. The antibody is a monoclonal antibody to the novel coronavirus nucleocapsid N protein, but is bound to a different epitope from the antibody in (a). The antigen comprises an S1 subunit, an S2 subunit, an RBD, an N protein and an E protein of the SAR-CoV-2 Spike protein.
Owner:SHENZHEN YHLO BIOTECH
SARS-CoV-2 detection kit based on indirect method
ActiveCN112964874AEasy to detectSave operating timeBiological material analysisBiological testingAntigen epitopeAntiendomysial antibodies
Owner:SHENZHEN YHLO BIOTECH
Anti-SARS-CoV-2 nucleocapsid protein monoclonal antibody as well as preparation method and application thereof
PendingCN113817052AShort detection cycleImprove throughputImmunoglobulins against virusesAntiviralsAntiendomysial antibodiesHeavy chain
The invention belongs to the field of virus detection and diagnosis, and relates to an anti-SARS-CoV-2 virus nucleocapsid protein monoclonal antibody and a preparation method and application thereof. The invention provides the anti-SARS-CoV-2 virus nucleocapsid protein monoclonal antibody and amino acid sequences of heavy chain variable regions and light chain variable regions of the monoclonal antiboy. The anti-SARS-CoV-2 virus nucleocapsid protein monoclonal antibody provided by the invention can be specifically combined with the nucleocapsid protein. The anti-SARS-CoV-2 virus nucleocapsid protein monoclonal antibody provided by the invention provides possibility and convenience for the detection and diagnosis of the SARS-CoV-2 virus.
Owner:NANJING GENSCRIPT BIOTECH CO LTD
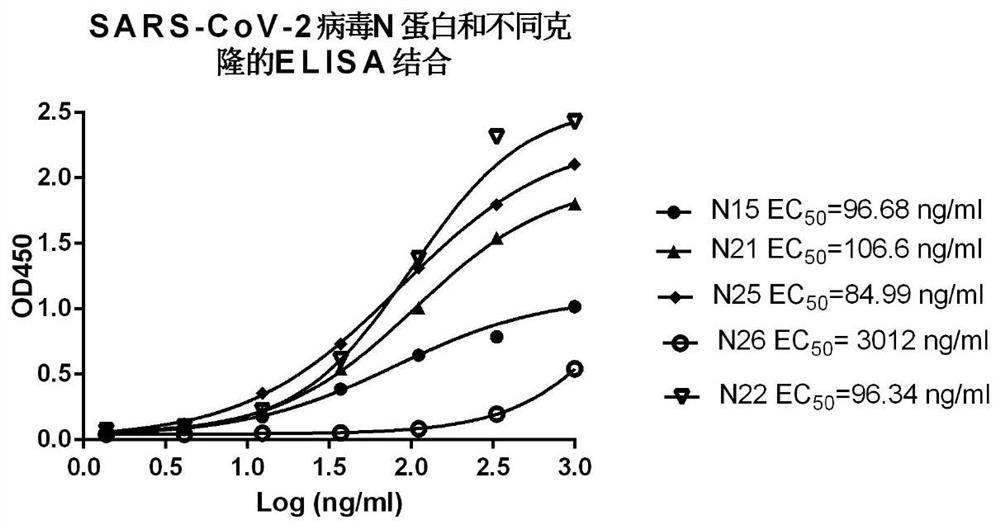
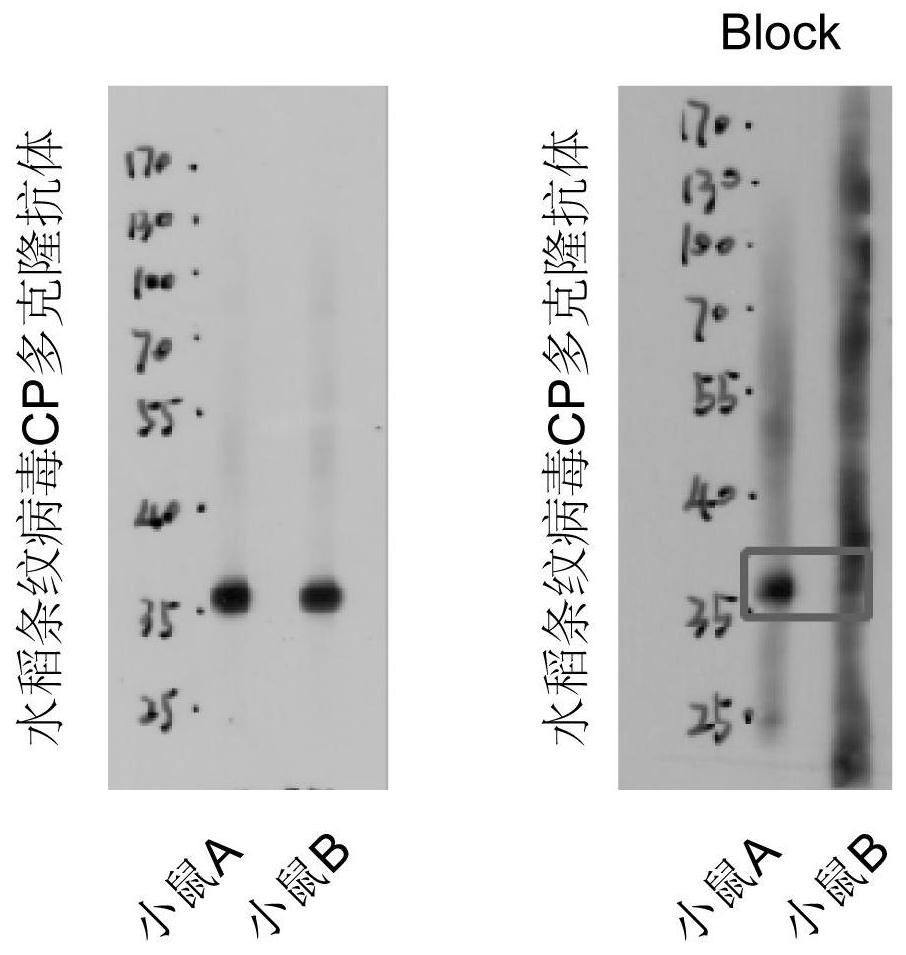
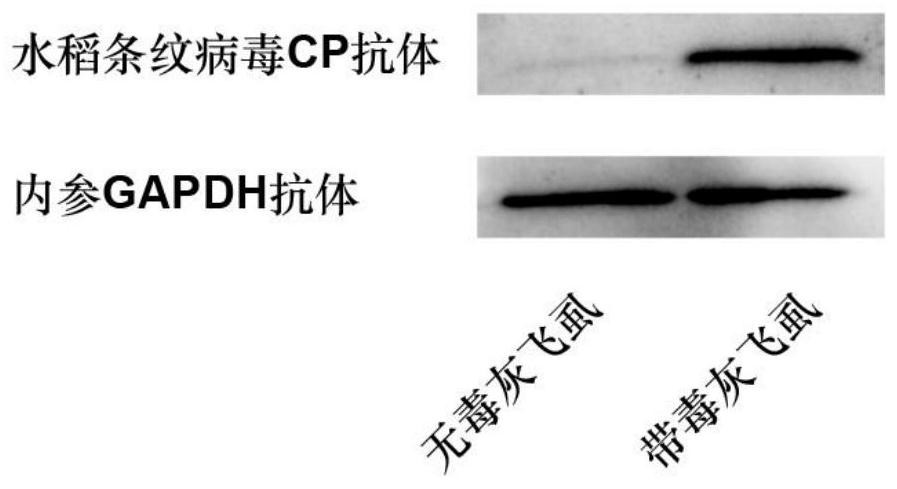
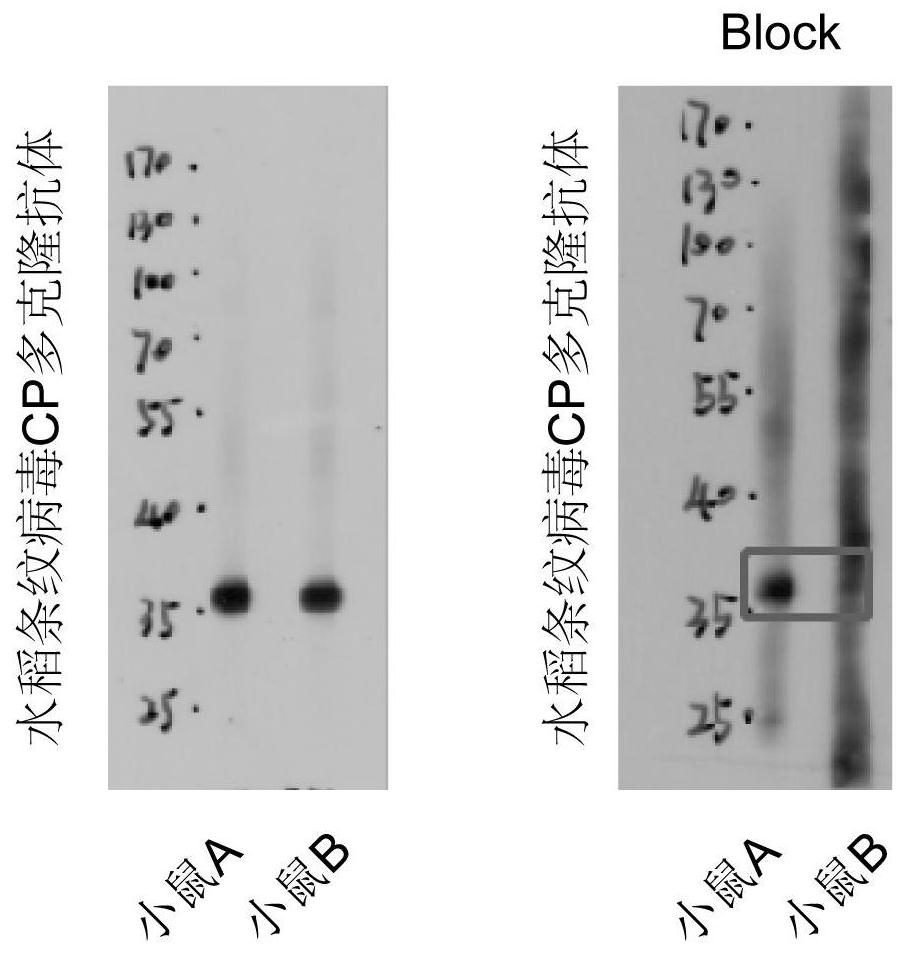
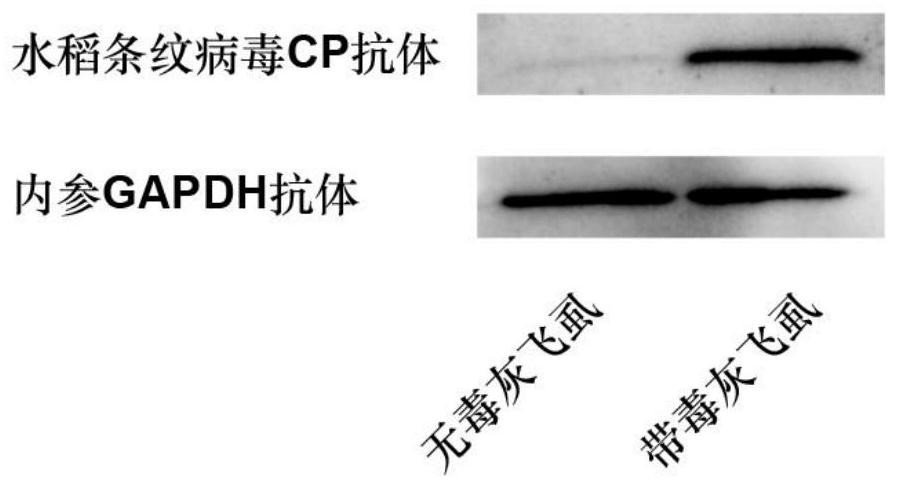
Polyclonal antibody of rice stripe virus nucleocapsid protein as well as preparation method and application thereof
ActiveCN111606979AQuick checkEfficient detectionSsRNA viruses negative-senseBacteriaDisease monitoringIMMUNE FLUORESCENCE
The invention provides a preparation method and application of a polyclonal antibody of a rice stripe virus nucleocapsid protein, and belongs to the technical field of rice stripe virus detection. Thepolyclonal antibody of the rice stripe virus nucleocapsid protein CP is obtained by immunizing animals with the rice stripe virus nucleocapsid protein CP as immunogen; and the amino acid sequence ofthe rice stripe virus nucleocapsid protein CP is as shown in SEQ ID No. 1. The polyclonal antibody of the rice stripe virus nucleocapsid protein has the characteristics of low cost, wide application range, multiple audiences, high sensitivity, and the like, and can be used for multiple biological detection experiments such as an immune spot determination method, an enzyme-linked immunosorbent assay method, a protein immunoblotting method, an immuno-fluorescence method, an immuno-electron microscopy method, and the like. By utilizing the disclosed polyclonal antibody, disease early warning is carried out through small brown rice plant hoppers at the beginning of outbreak of rice stripe disease, and multi-means disease monitoring is facilitated.
Owner:YANGZHOU UNIV
Sample diluent for colloidal gold method new coronavirus antigen detection card
PendingCN114878804AIncrease exposureImprove the detection rateBiological material analysisBiological testingPhospholipaseColloidal au
Owner:山东博科快速检测技术有限公司
Double-target SARS-CoV-2 virus nucleic acid detection primer group, application and fluorescent kit
PendingCN114075611APrecise screeningNo cross reactionMicrobiological testing/measurementAgainst vector-borne diseasesFluorescenceViral nucleic acid
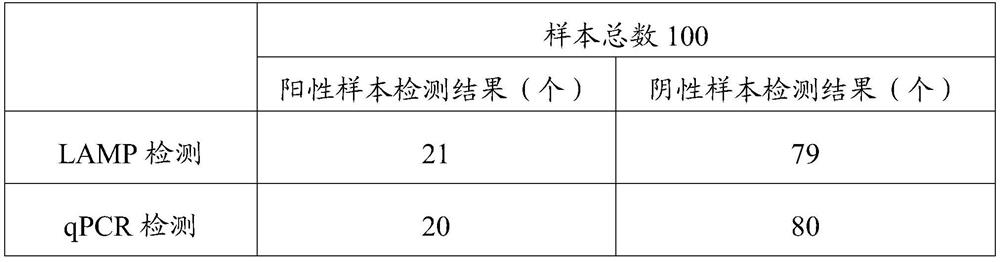
The invention discloses a double-target SARS-CoV-2 virus nucleic acid detection primer group, application and a fluorescent kit, and relates to the technical field of virus nucleic acid detection methods. The double-target specific nucleic acid detection primer group is designed for a conserved region of the SARS-CoV-2 virus by selecting double gene sequences of the SARS-CoV-2 virus nucleocapsid protein N gene and the spike protein S gene, and the SARS-CoV-2 virus can be specifically detected. The invention further provides a one-pot RT-LAMP fluorescence detection kit for detecting the SARS-CoV-2 virus, a throat swab and other samples, nucleic acid extraction is not needed, an amplification curve is observed through a fluorescence amplification instrument under the isothermal condition of 65 DEG C, the SARS-CoV-2 virus can be accurately detected within 30 minutes, and virus-result detection is directly achieved. The fluorescent kit adopts a double-primer group design, is strong in specificity, high in sensitivity, free of cross reaction, capable of rapidly and accurately screening the SARS-CoV-2 virus, simple to operate and high in safety; the detection result is visual, the judgment operation is simple, and the method has good application advantages in the field of viral nucleic acid detection.
Owner:SOUTH CHINA UNIV OF TECH
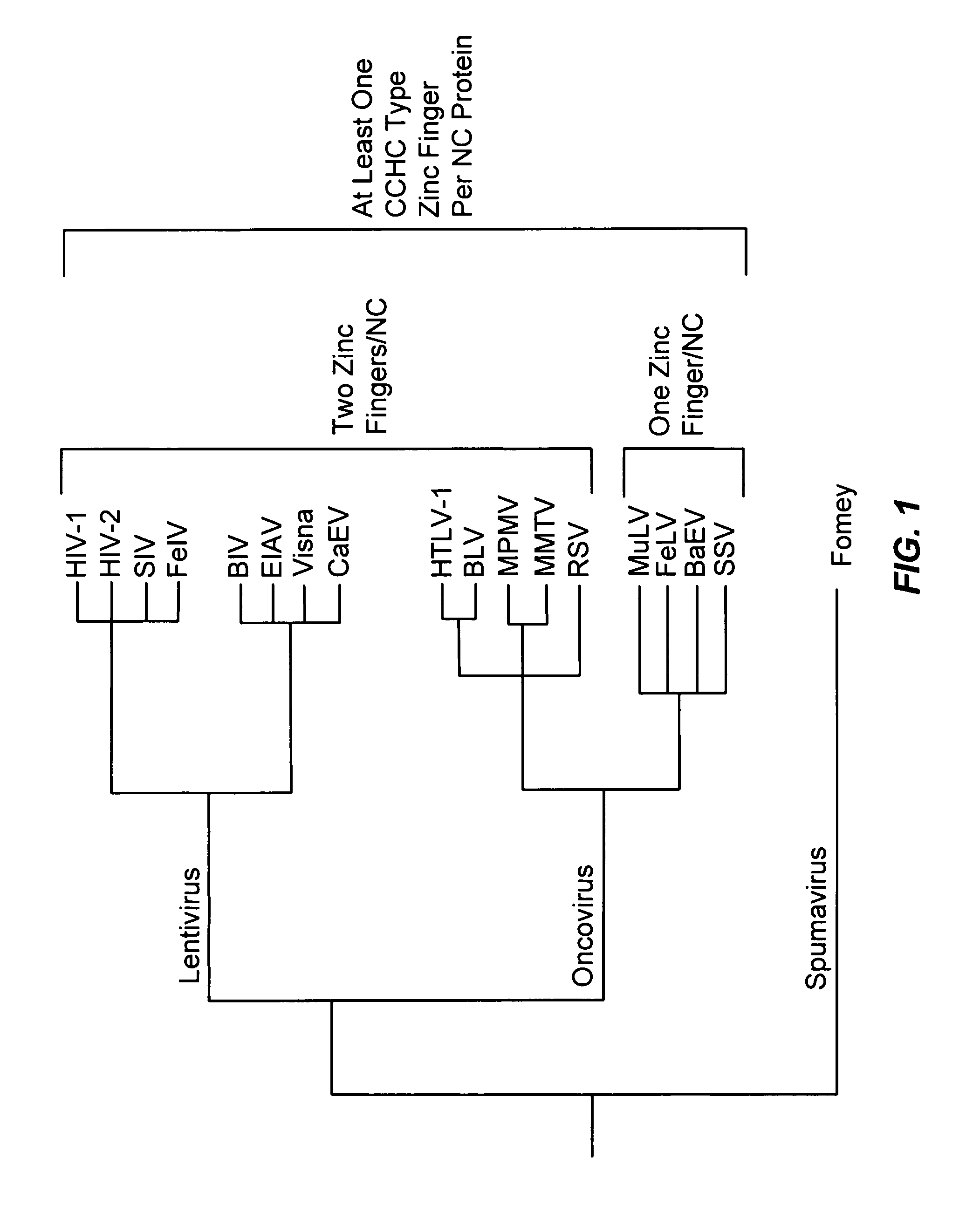
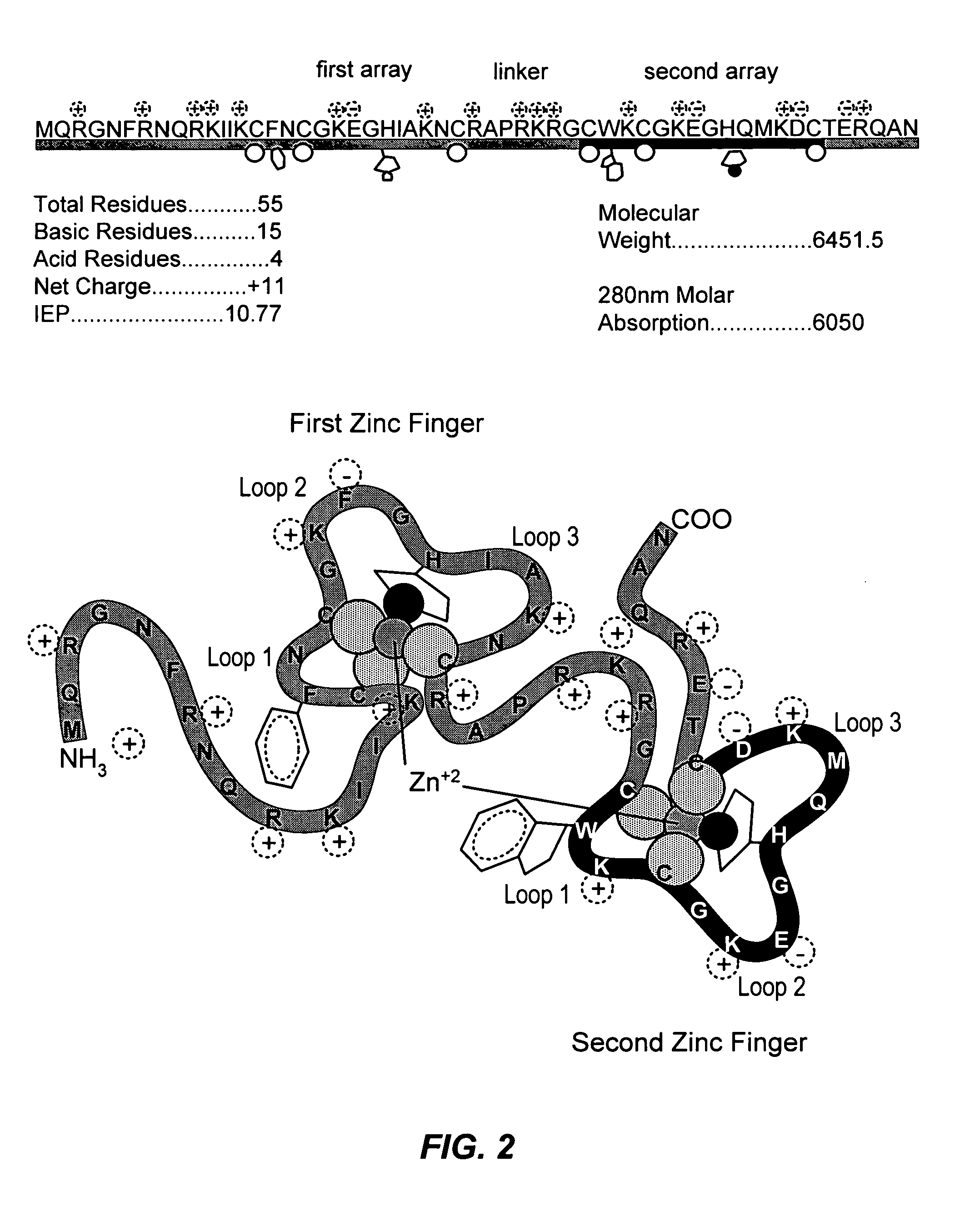
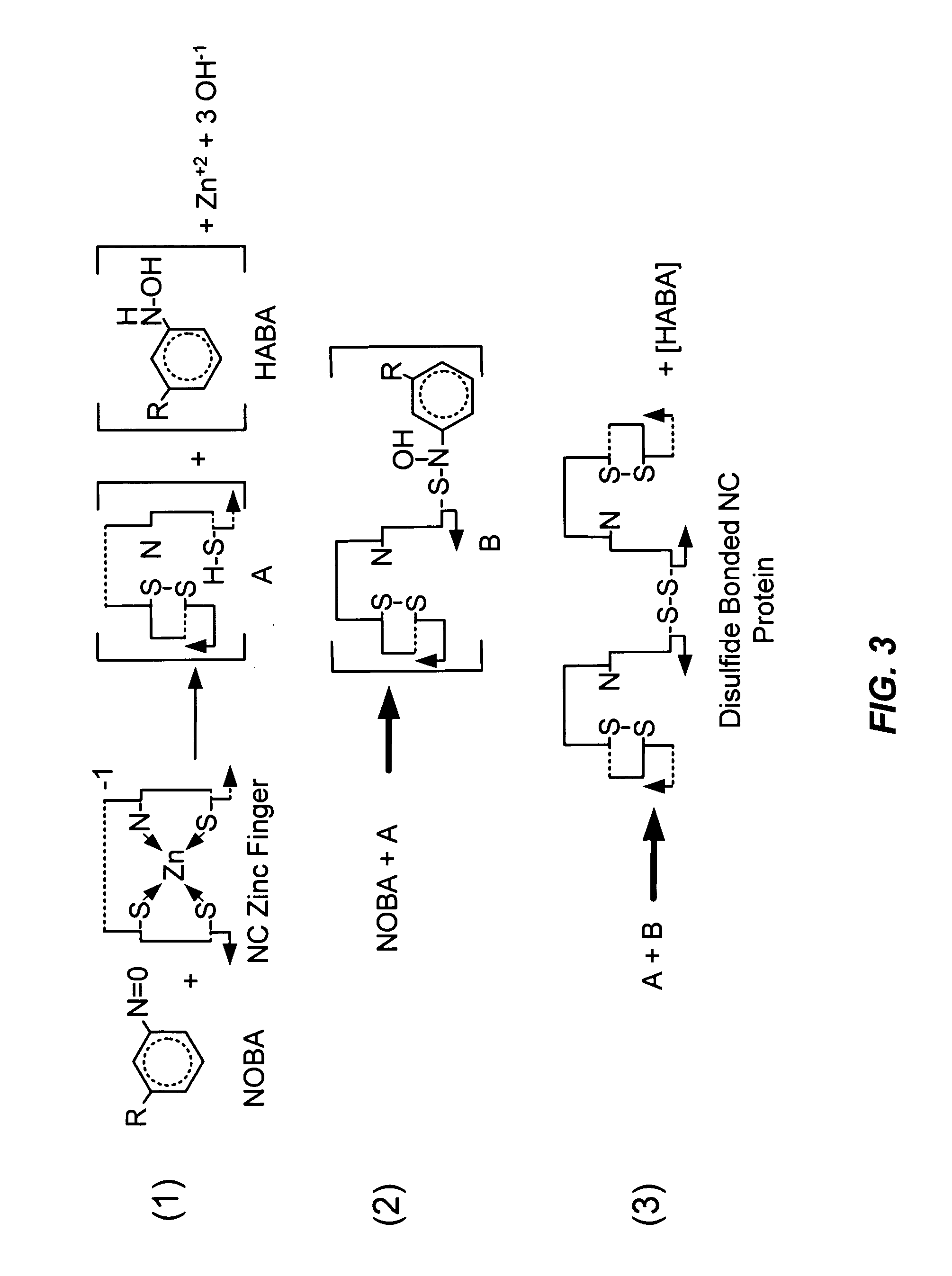
Method for identifying and using compounds that inactivate HIV-1 and other retroviruses by attacking highly conserved zinc fingers in the viral nucleocapsid protein
The present invention provides several classes of compounds which can be used to inactivate retroviruses, such as HIV-1, by attacking the CCHC zinc fingers of the viral nucleocapsid protein and ejecting the zinc therefrom. In addition, kits for identifying compounds that can react with CCHC zinc fingers of the nucleocapsid proteins of a large number of different retroviruses have also been developed. The kits of the present invention describe a set of specific tests and reagents that can be used to screen and identify compounds based on their ability to react with and disrupt retroviral zinc fingers in the viral NC proteins and, in turn, inactivate the retrovirus of interest.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Application of human HOXA10 (Homeobox A10) in preparing drug for treating or preventing HBV (Hepatitis B Virus) infection
ActiveCN108853479ANo side effectsLower resistancePeptide/protein ingredientsDigestive systemAntigenSide effect
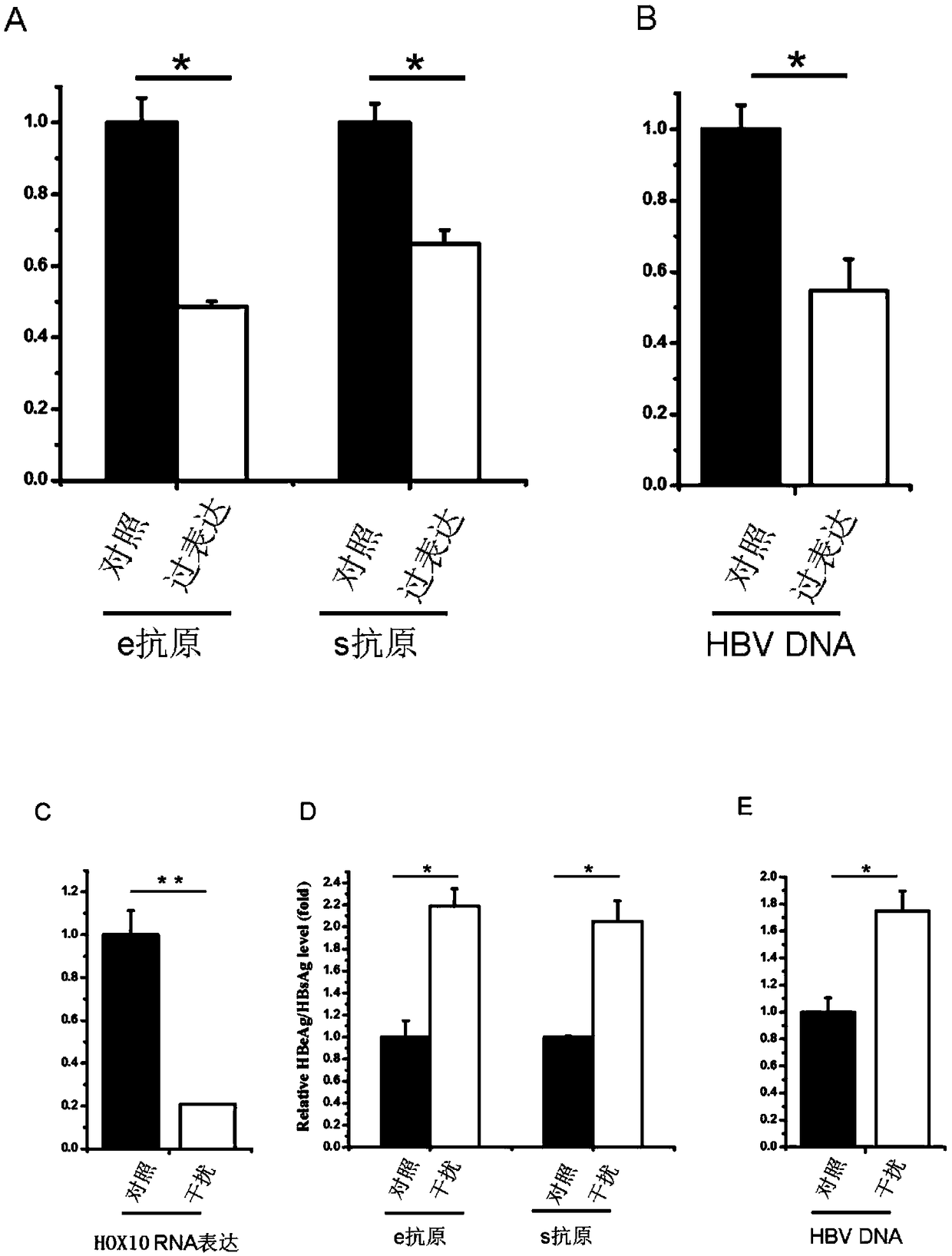
The invention discloses application of human HOXA10 (Homeobox A10) in preparing a drug for treating or preventing HBV (Hepatitis B Virus) infection. The influence of HOXA10 on HBV replication can be researched through a way of cotransferring HOXA10 overexpression plasmids or small interfering RNA (Ribonucleic Acid) and HBV 1.3-ploid plasmids of targeted HOXA10 to hepatic cells, a surface antigen and an e antigen secreted by viruses and the level of DNA (Deoxyribonucleic Acid) related to virus nucleocapsid in cells are detected, and the HBV replication in hepatic cell inhibition by the HOXA10 is displayed; the HOXA10 can affect the activity of HBV or affect the HBV infection or a replication process after infection. Therefore, through independent use or combined use with other anti-HBV drugs, the HOXA10 can play an important role during a process of preventing or treating the HBV infection; the HOXA10 is a cell factor which is constitutively expressed in a human body, and the HOXA10 hasno toxic and side effects on the human body under physiological concentration.
Owner:广东龙帆生物科技有限公司
Standard positive reference material for rana grylio virus (RGV) detection
InactiveCN101921757BHigh purity and easy quantificationSubspecific amplificationMicrobiological testing/measurementFermentationAgricultural scienceNucleotide sequencing
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
A kind of polyclonal antibody of rice stripe virus nucleocapsid protein and its preparation method and application
ActiveCN111606979BQuick checkEfficient detectionSsRNA viruses negative-senseBacteriaDisease monitoringWestern blot
The invention provides a method for preparing a polyclonal antibody to rice stripe virus nucleocapsid protein and its application, and belongs to the technical field of rice stripe virus detection. The polyclonal antibody to rice stripe virus nucleocapsid protein CP is based on rice stripe virus The nucleocapsid protein CP is obtained by immunizing animals with an immunogen; the amino acid sequence of the rice stripe virus nucleocapsid protein CP is shown in SEQ ID No.1. The polyclonal antibody of the rice stripe virus nucleocapsid protein of the present invention has the characteristics of low cost, wide application, multiple audiences, high sensitivity, etc., and can be used in immunospot assay, enzyme-linked immunosorbent assay, western blot, immuno A number of biological detection experiments such as fluorescence method and immunoelectron microscope method. Using the polyclonal antibody of the present invention, the early warning of the disease is carried out by the striatellus striatellus at the beginning of the outbreak of the rice stripe leaf blight, which is beneficial to the multi-means disease monitoring.
Owner:YANGZHOU UNIV
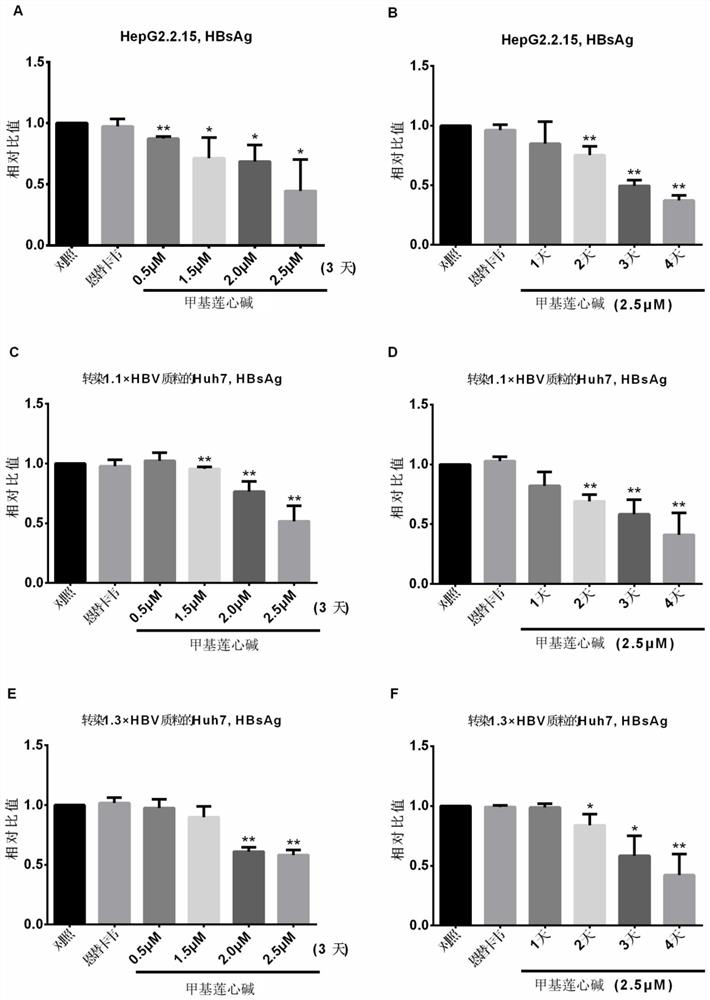
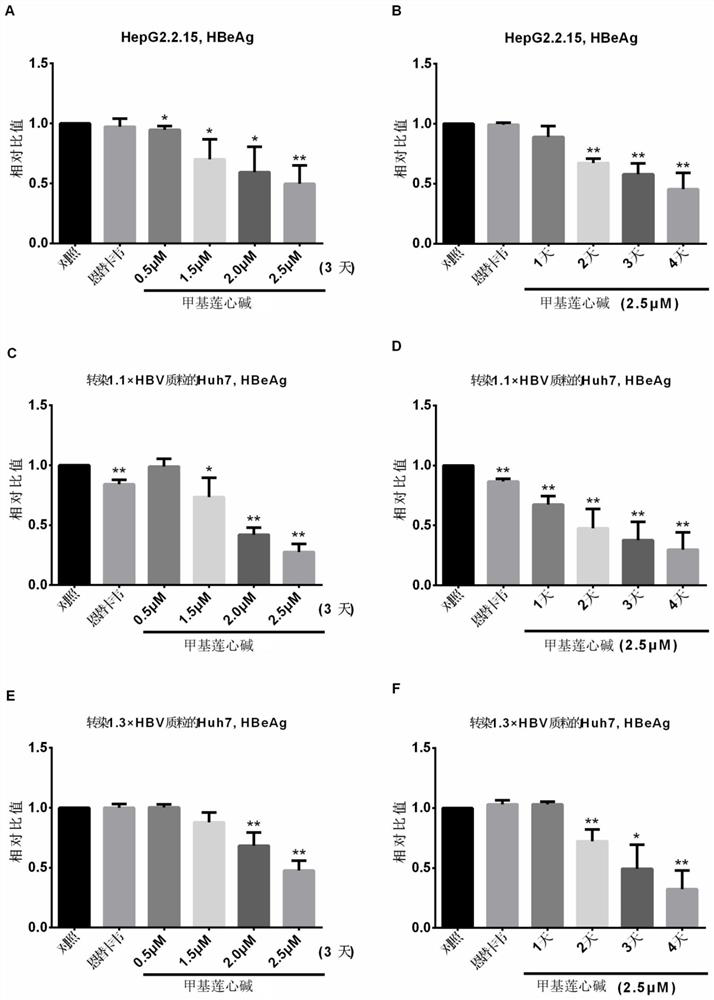
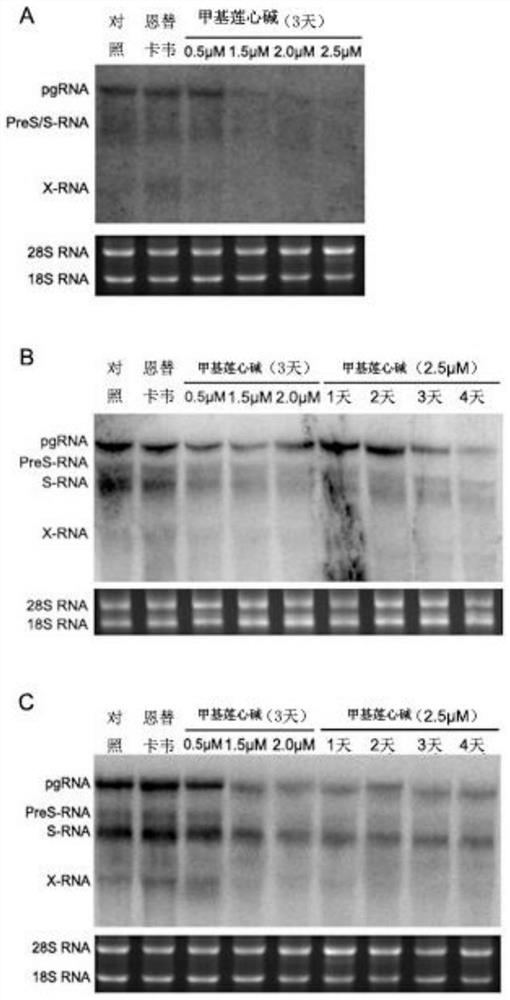
Application of neferine in preparation of anti-hepatitis B virus drug
PendingCN114796220AEasy to getLow costOrganic active ingredientsAntiviralsAntigenHepatitis B immunization
According to the application of the neferine in preparation of the anti-hepatitis B virus medicine, the neferine targets hepatitis B virus RNA and interferes with synthesis of the hepatitis B virus RNA, so that transcription of pgRNA, preS / S RNA and X RNA of the virus is reduced, reverse transcription synthesis of cDNA in a virus nucleocapsid in a cell and synthesis of virus protein are reduced, and therefore, the anti-hepatitis B virus medicine is obtained. Therefore, the synthesis and secretion of antigens HBsAg and HBeAg are reduced. According to the application disclosed by the invention, an in-vitro cell model is applied, the neferine is found to have an inhibiting effect on the HBV for the first time, and a new clue is provided for subsequent in-depth research and clinical medication of resisting the HBV.
Owner:康珞生物科技(武汉)有限公司
Method for preparing noble metal palladium nanorods by utilizing the nucleocapsid of cotton bollworm baculovirus
ActiveCN104001931BRaw materials are easy to obtainReduce manufacturing costCotton bollwormAlkaline hydrolysis
A method for preparing a precious metal palladium nanorod by means of cotton bollworm baculovirus nucleocapsid mainly includes the steps that cotton bollworm karyotype polyhedron virus raw powder is dispersed through ultra-pure water to obtain grey polyhedrin suspension, alkaline hydrolysis liquid is added into the grey polyhedrin suspension, the mixture stands still for 15-25 min, then the pH value is adjusted to be 7 through acetic acid, chloroform is added, centrifugalization is carried out for 5-10 min at the speed of 5000-6000 r / min, supernatant fluid is sucked, centrifugalization is carried out for 1-2 h at the temperature of 4-10 DEG C and at the speed of 25000-30000 r / min, pigmentation is re-suspeneded through a PBS solution, and a cotton bollworm baculovirus nucleocapsid solution is obtained; a PdCl2 solution is added into the cotton bollworm baculovirus nucleocapsid solution and fully and evenly mixed, incubation is carried out in a shaking mode for 15-25 h at the temperature of 20-30 DEG C and at the speed of 130-170 r / min, and the precious metal palladium nanorod loaded by the cotton bollworm baculovirus nucleocapsid is obtained. The method is simple in process, environmentally friendly and efficient, industrial production is easy to achieve, and the prepared palladium nanorod is stable in appearance and uniform in size.
Owner:YANSHAN UNIV
An essential element for nucleocapsid assembly and its application
The invention relates to the assembling field of virus nucleocapsid, particularly to a baculovirus nucleocapsid assembling necessary element and an application thereof in nucleocapsid assembling, establishment of a carrier comprising a target nucleotide sequence, and the like. The nucleocapsid assembling necessary element disclosed by the invention comprises a 45-65 map unit positioned in a viral genome of a hepatitis A baculovirus, preferably, a DNA sequence in the position of a 47-61 map unit. An NAE sequence plays an essential role in the nucleocapsid assembling process. The baculovirus has wide application prospect in the gene transduction field, so that the NAE sequence found in the invention can improve a baculovirus gene-transduced carrier, so that the establishment process is simpler and more convenient, the target DNA is higher in capacity, and higher biosecurity is achieved; and therefore, the nucleocapsid assembling necessary element is applicable to a required gene transduction operation, and requirements of clinical application, such as gene therapy and the like, can be better satisfied.
Owner:SUN YAT SEN UNIV
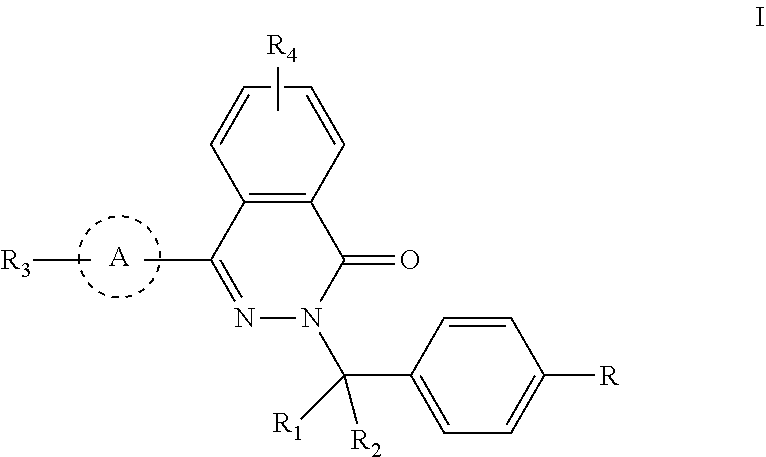
Phthalazinone compound, method for preparation thereof, pharmaceutical composition thereof, and use thereof
ActiveUS20200181109A1High activityLess growth toxicityOrganic active ingredientsOrganic chemistryAnti-inhibitorMedicine
Provided are a phthalazinone compound, method for preparation thereof, pharmaceutical composition thereof, and a use thereof; the structure of said phthalazinone compound is as represented by formula I; the compound of said formula I is targeted to a viral nucleocapsid; it can inhibit the replication of a virus by means of interference of the viral nucleocapsid, has a potent activity for inhibiting HBV DNA replication and a good liver targeting, can stably exist and enrich in the liver, and is a new and effective anti-HBV inhibitor.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
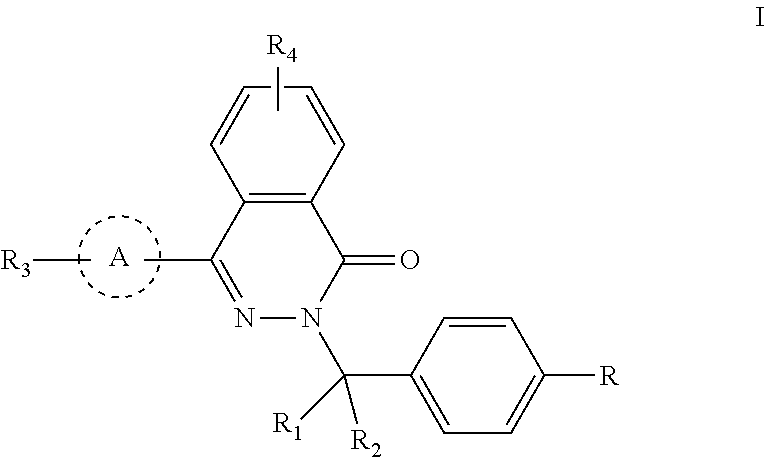
Phthalazinone compound, method for preparation thereof, pharmaceutical composition thereof, and use thereof
ActiveUS11040952B2High activityLess growth toxicityOrganic active ingredientsOrganic chemistryAnti-inhibitorChemical compound
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Cell line stably expressing Schmallenberg virus nucleocapsid protein and its application
ActiveCN104232587BGood passage stabilityFast growthMicroorganism based processesFermentationDiseaseImmunofluorescence
The present invention provides a BHK-21 cell line stably expressing Schmallenberg virus (SBV) nucleocapsid protein, its preservation number is CGMCC No.7616, named BHK-21-pLV-EGFP-SBV-N . The results of cell immunofluorescence test showed that the cell line not only reacted specifically with SBV monoclonal antibody 2C8, but also recognized the positive serum of SBV-infected cattle. The BHK‑21 cell line stably expressing SBV N protein can be used for the detection of Schmallenberg disease and has a good application prospect.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
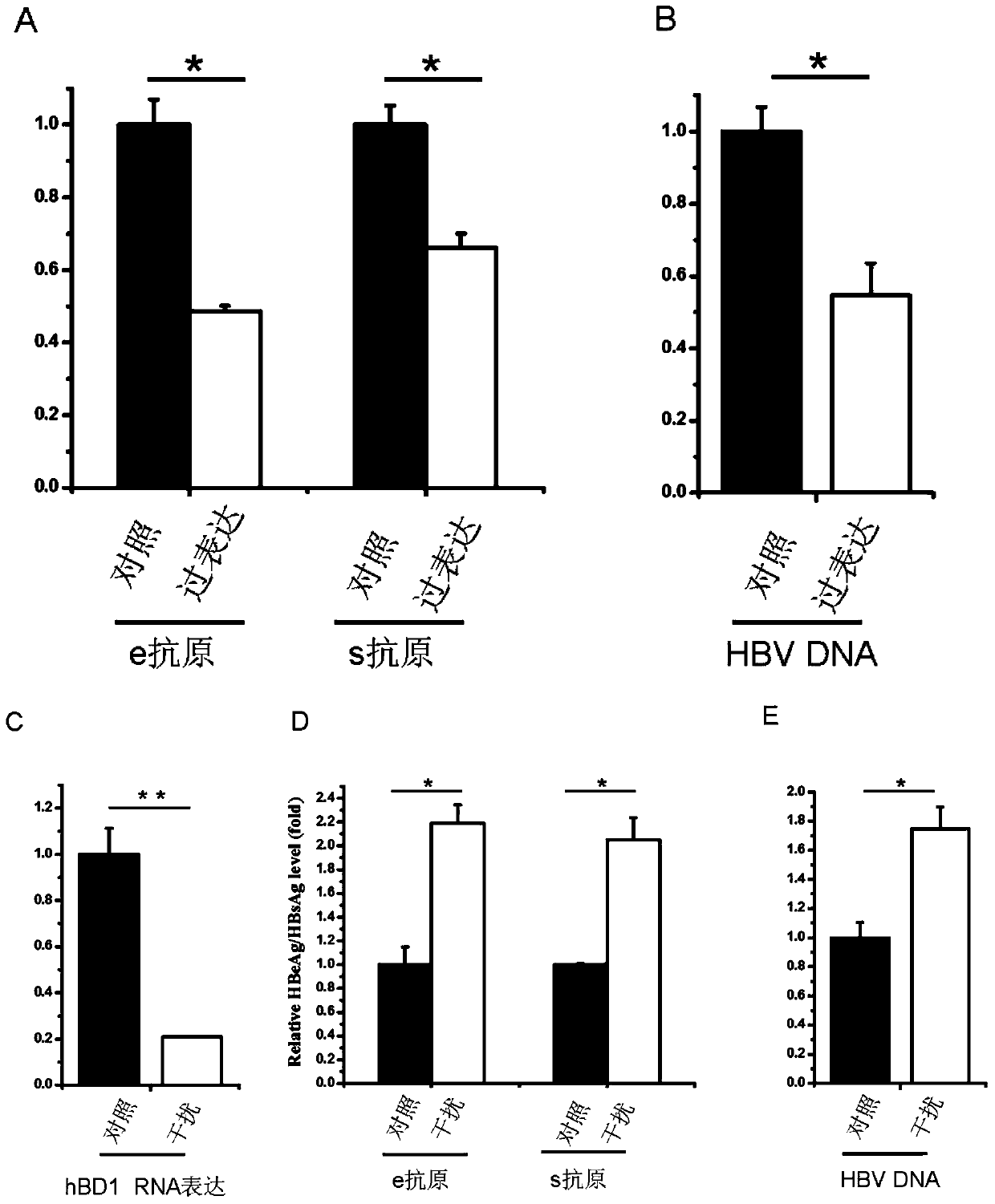
Application of a kind of human β-defensin-1 in the preparation of medicine for treating or preventing hepatitis B virus infection
ActiveCN105031613BNo side effectsLower resistancePeptide/protein ingredientsAntiviralsAntigenSide effect
The invention discloses the application of β-defensin-1 in the preparation of medicines for treating or preventing hepatitis B virus infection. Study on the effect of β-defensin-1 on hepatitis B virus by co-transfection of β-defensin-1 overexpression plasmid or small interfering RNA targeting β-defensin-1 and HBV1.3 ploidy plasmid into liver cells Effects on replication, the surface antigen and e-antigen secreted by the virus were examined, as well as the levels of intracellular viral nucleocapsid-associated DNA, showing that β‑defensin‑1 inhibits HBV replication in hepatocytes. β-defensin-1 may affect the activity of HBV or affect the replication process of HBV infection or after infection. Therefore, by using alone or in combination with other anti-HBV drugs, β-defensin-1 can play an important role in the prevention or treatment of hepatitis B virus infection. Human β‑defensin‑1 is an antimicrobial polypeptide constitutively expressed in the human body, and has no toxic side effects on the human body at physiological concentrations.
Owner:广东龙帆生物科技有限公司
Nucleotide sequence of porcine TT virus I CJNY strain genome
The invention discloses a complete nucleotide sequence of a porcine TT virus I CJNY strain and belongs to the field of biotechnology. The length of the sequence is 2,879 bases. The nucleotide homology of three strains of reported TT virus I (GQ120664, AY823990 AND AB076001) with complete genome sequences and the porcine TT virus I CJNY strain is between 68.1 and 86.7 percent. The most different point is that a virus nucleocapsid protein encoded by the sequence only has 415 amino acids which are greatly reduced when compared with those of the other three strains. The sequences can be used for research and development of specific detection technology and is used for research of virus biological characteristics by construction of infectious molecular clone.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method for preparing novel coronavirus nucleocapsid protein by using HEK293 cells
ActiveCN112410374AHigh purityImprove accuracySsRNA viruses positive-senseViral antigen ingredientsEscherichia coliTGE VACCINE
The invention provides a method for preparing novel coronavirus nucleocapsid protein by using HEK293 cells. The method comprises the following steps: 1) constructing a recombinant expression vector ofthe novel coronavirus nucleocapsid protein (N protein); 2) transfecting HEK293 cells by using the recombinant expression vector; and 3) culturing the cells in vitro, and separating and purifying theN protein from the culture supernatant. A large amount of new coronavirus N protein can be obtained in a short time by utilizing an HEK293 expression system, and N protein with the purity of 98% or above can be obtained through a one-step affinity chromatography method. Compared with escherichia coli, the N protein prepared by adopting the HEK293 expression system has great advantages in the aspects of antibody binding activity and novel coronavirus antibody colloidal gold detection, and the protein space conformation of the N protein prepared by adopting the HEK293 expression system is closeto the protein expression conformation of a virus N gene in a host body; the invention has higher accuracy of immunodiagnosis and antibody preparation, and has a wide prospect in preparation of diagnostic reagents and vaccines.
Owner:ACROBIOSYSTEMS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com