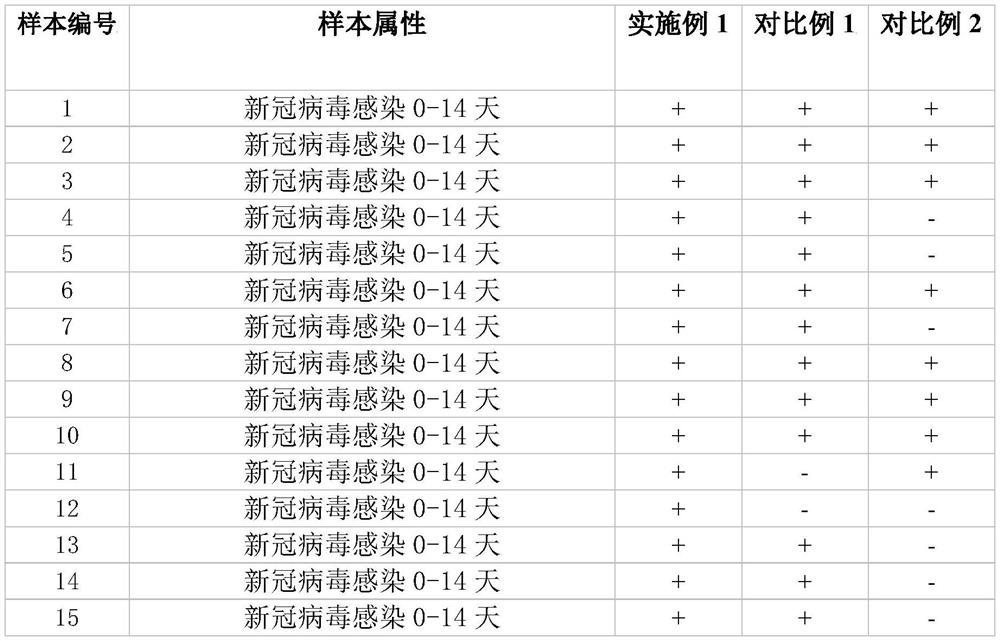
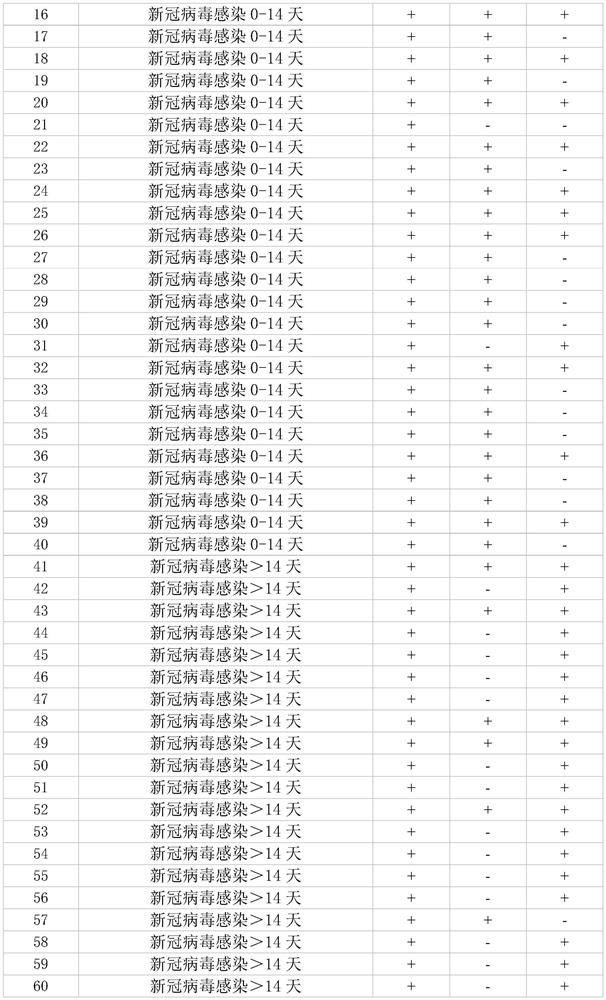
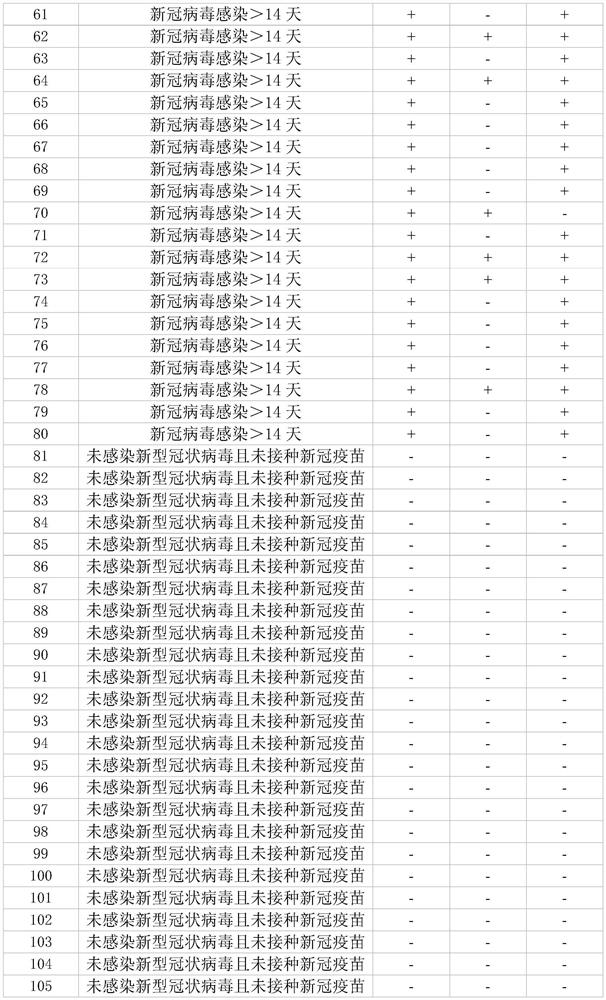
SARS-CoV-2 detection kit
A detection kit and kit technology, applied in the field of biomedicine, can solve problems such as unsatisfactory antibody sensitivity and limited sampling methods, and achieve the effects of saving operating time and cost, shortening the diagnostic window period, and preventing missed detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] This kit is mainly divided into calibrator and detection reagent, among which, the detection reagent consists of 3 parts:
[0097] 1.1R1 magnetic bead components:
[0098] Composed of 7 kinds of paramagnetic magnetic beads and magnetic bead diluent, the combination of magnetic bead coating protein is as follows:
[0099] ① Anti-new coronavirus N protein mouse monoclonal antibody;
[0100] ② SARS-CoV-2 N protein;
[0101] ③SARS-CoV-2 Spike protein S1 subunit;
[0102] ④SARS-CoV-2 Spike protein S2 subunit;
[0103] ⑤ SARS-CoV-2 Spike protein S1 subunit receptor binding domain (Receptor Binding Domian, RBD) protein;
[0104] ⑥SARS-CoV-2 M protein;
[0105] ⑦ SARS-CoV-2 E protein;
[0106] Among them, the anti-viral N protein mouse monoclonal antibody can be extracted from mouse ascites or hybridoma cells in vitro, and can be a variety of monoclonal antibodies against different immune sites of different N proteins; S1, S2, RBD, M, and E proteins can be extracted natu...
Embodiment 2
[0122] This kit is mainly divided into calibrator and detection reagent, among which, the detection reagent consists of 3 parts:
[0123] 1.1R1 magnetic bead components:
[0124] Composed of 7 kinds of paramagnetic magnetic beads and magnetic bead diluent, the combination of magnetic bead coating protein is as follows:
[0125] ① Anti-new coronavirus N protein mouse monoclonal antibody;
[0126] ② SARS-CoV-2 N protein;
[0127] ③SARS-CoV-2 Spike protein S1 subunit;
[0128] ④SARS-CoV-2 Spike protein S2 subunit;
[0129] ⑤ SARS-CoV-2 Spike protein S1 subunit receptor binding domain (Receptor Binding Domian, RBD) protein;
[0130] ⑥SARS-CoV-2 M protein;
[0131] ⑦ SARS-CoV-2 E protein;
[0132] Among them, the anti-viral N protein mouse monoclonal antibody can be extracted from mouse ascites or hybridoma cells in vitro, and can be a variety of monoclonal antibodies against different immune sites of different N proteins; S1, S2, RBD, M, and E proteins can be extracted natu...
Embodiment 3
[0148] This kit is mainly divided into calibrator and detection reagent, among which, the detection reagent consists of 3 parts:
[0149] 1.1R1 magnetic bead components:
[0150] Composed of 5 kinds of paramagnetic magnetic beads and magnetic bead diluent, the combination of magnetic bead coating protein is as follows:
[0151] ① Anti-new coronavirus N protein mouse monoclonal antibody, which can form a paired antibody with the monoclonal antibody in the R1 magnetic bead component to detect the N protein antigen.
[0152] ② SARS-CoV-2 N protein;
[0153] ③SARS-CoV-2 Spike protein S1 subunit;
[0154] ④SARS-CoV-2 Spike protein S2 subunit;
[0155] ⑤ SARS-CoV-2 Spike protein S1 subunit receptor binding domain (Receptor Binding Domian, RBD) protein;
[0156] ① and ② have no specific binding.
[0157] Among them, the anti-viral N protein mouse monoclonal antibody can be extracted from mouse ascites or hybridoma cells in vitro, and can be a variety of monoclonal antibodies aga...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com