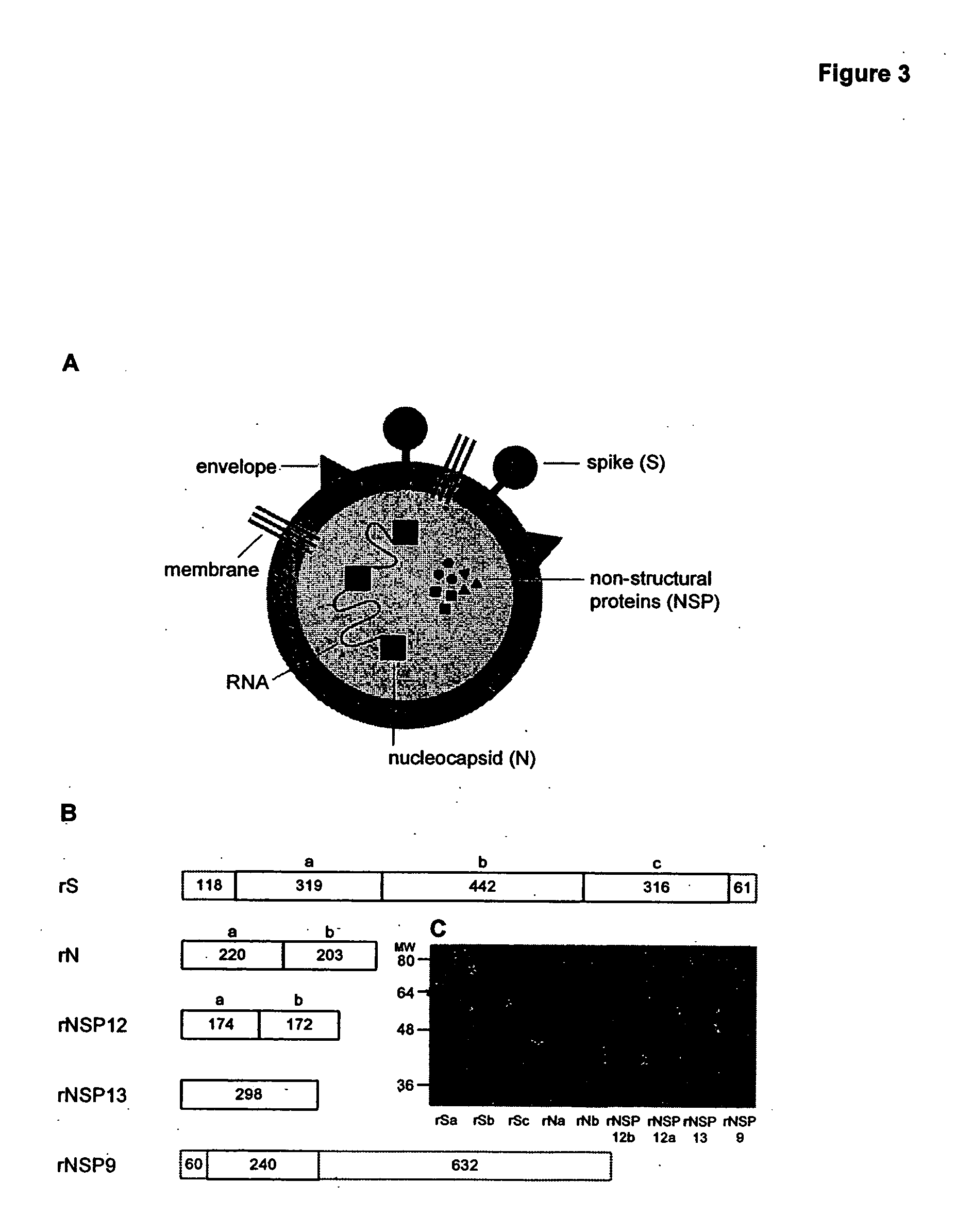
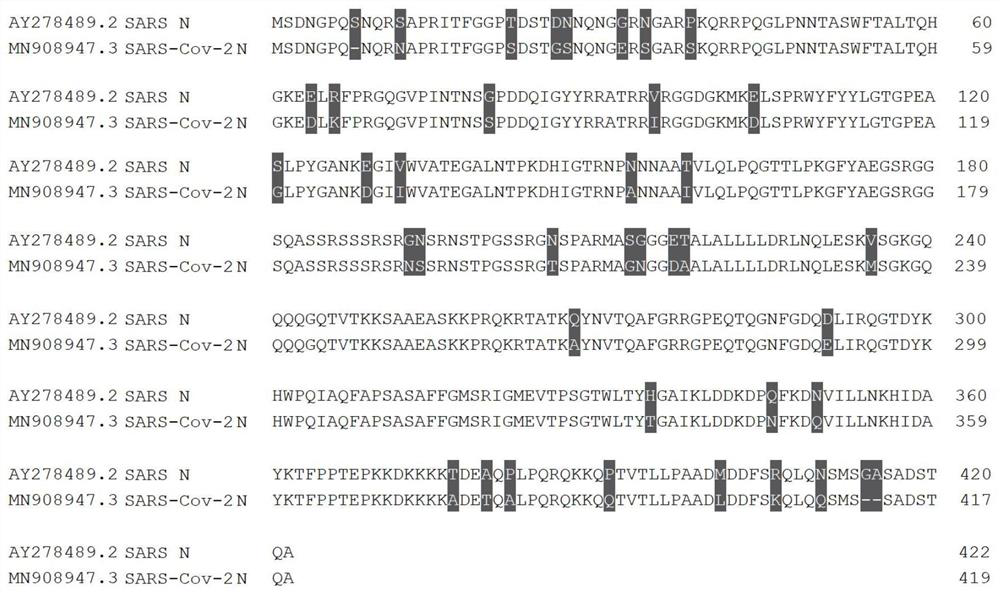
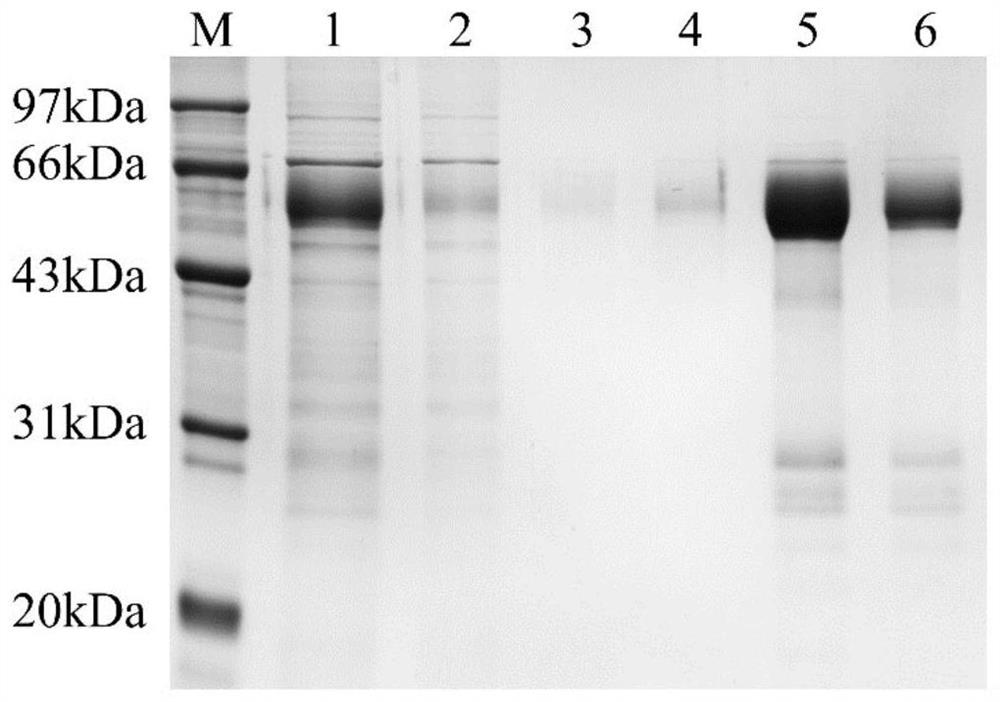
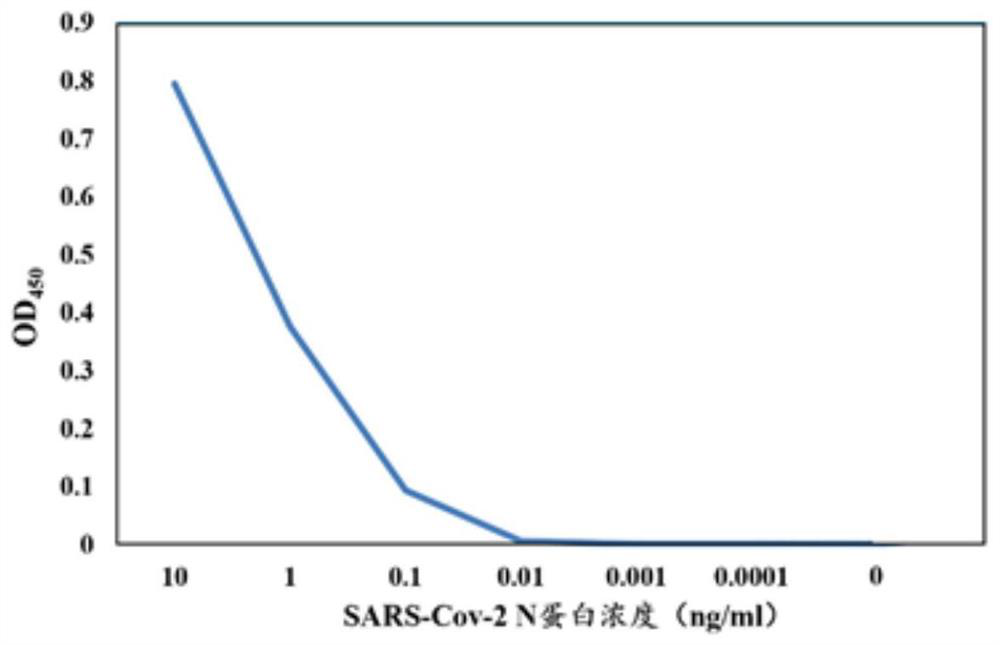
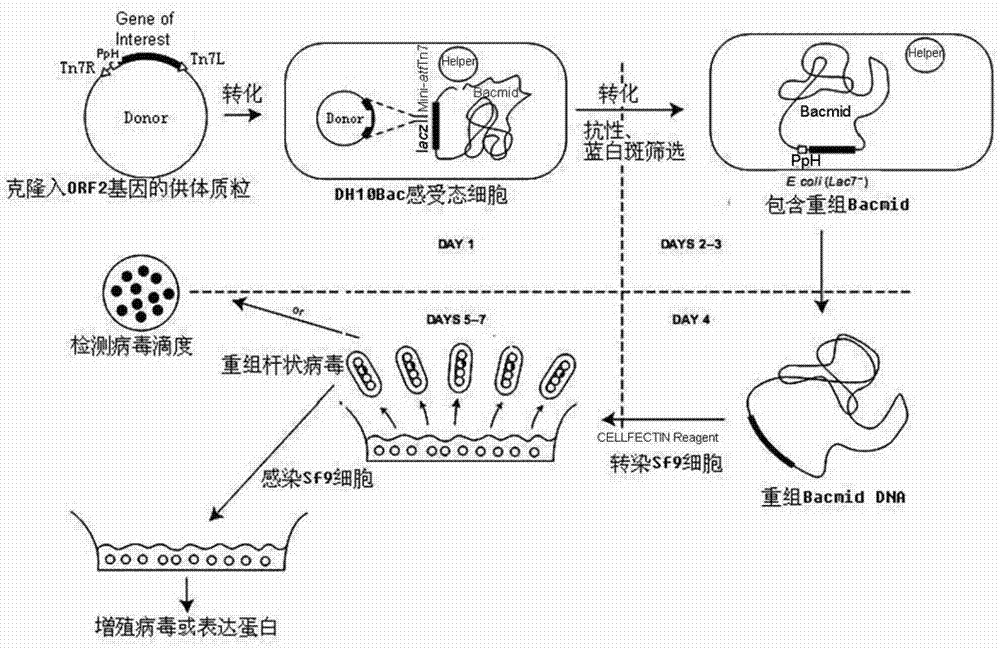
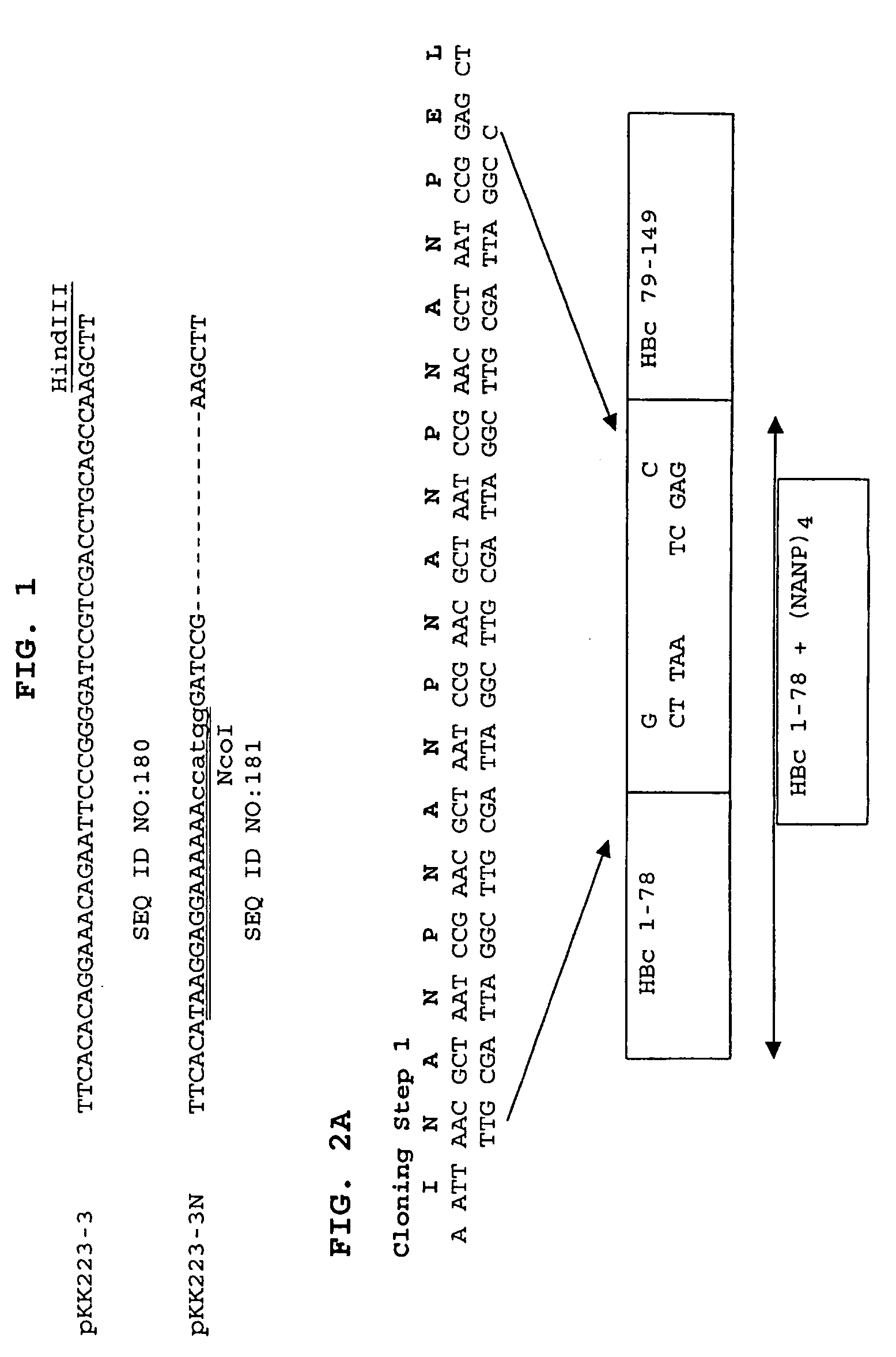
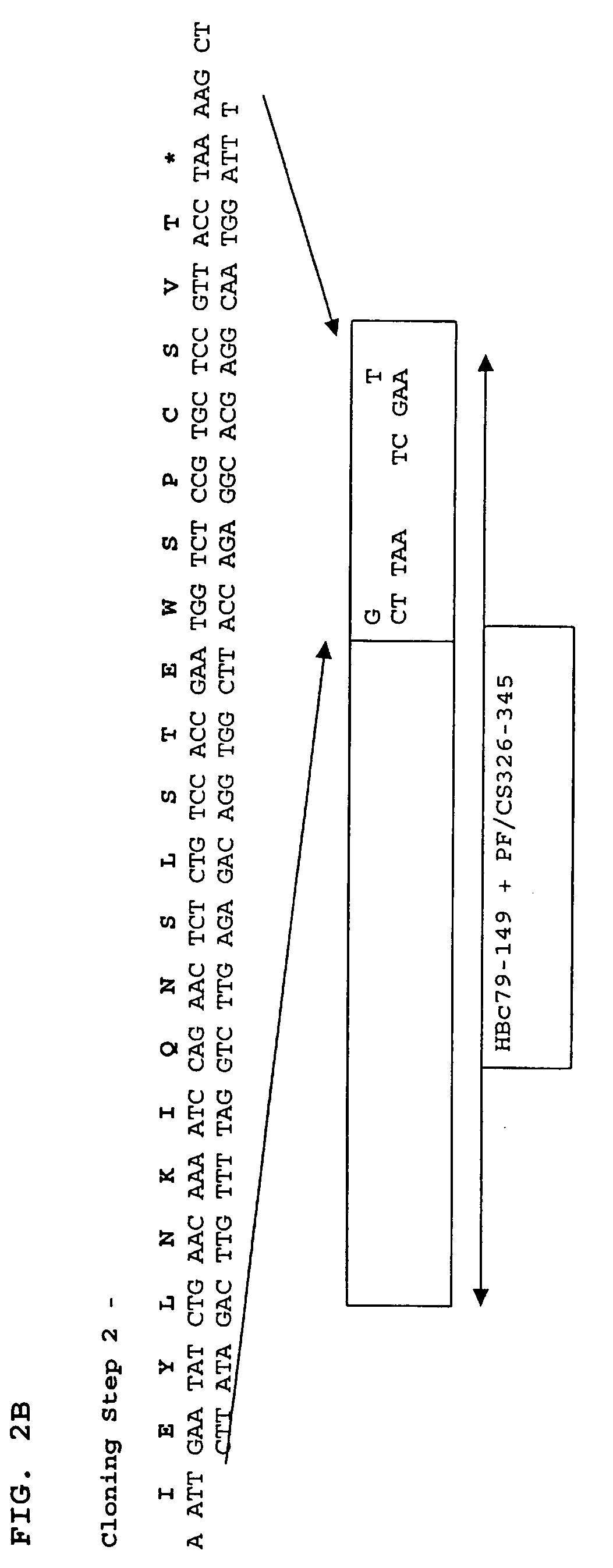
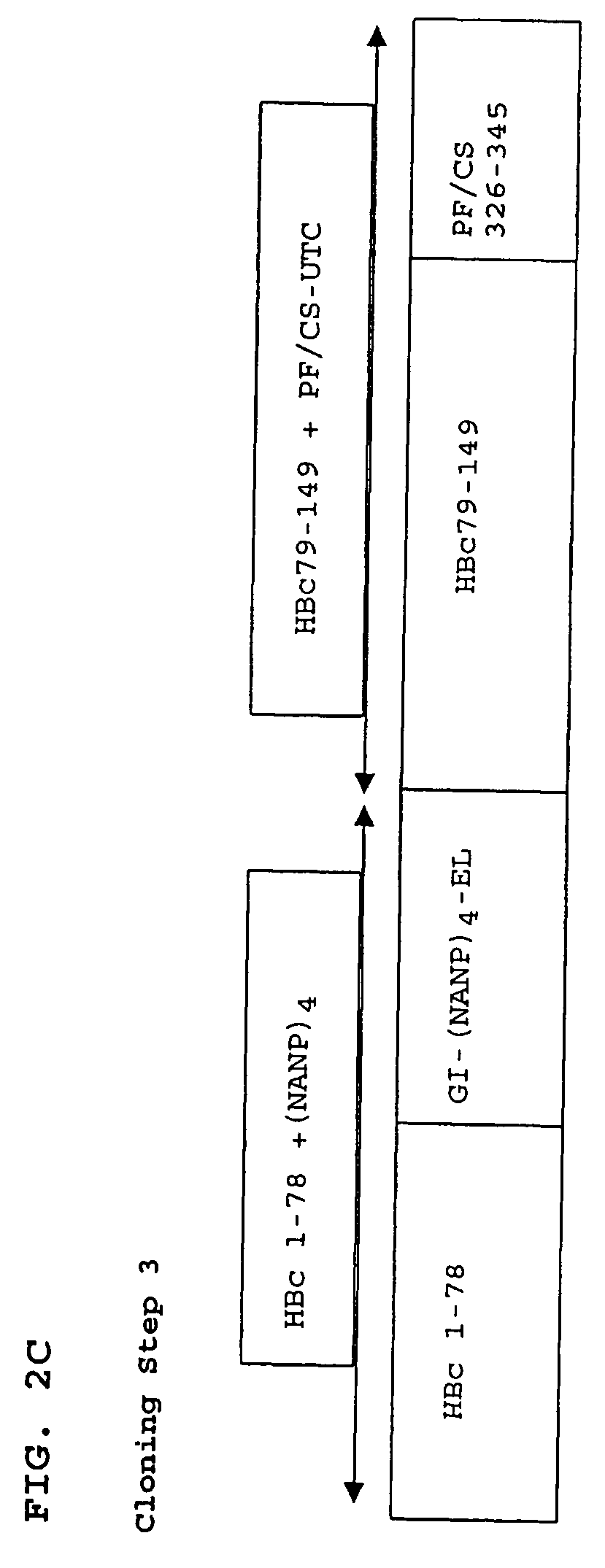
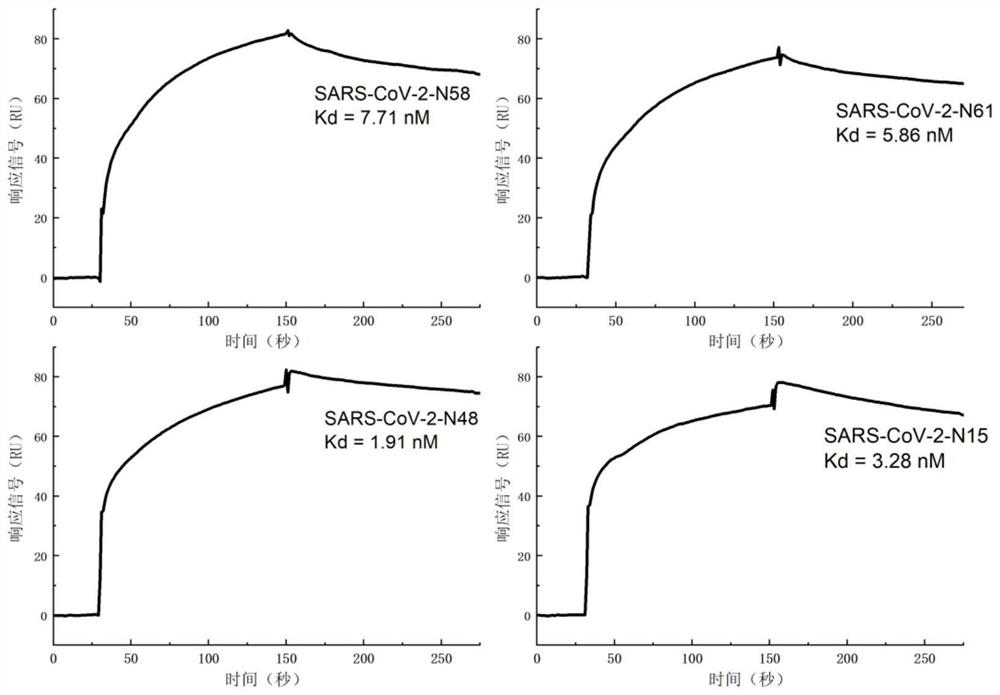
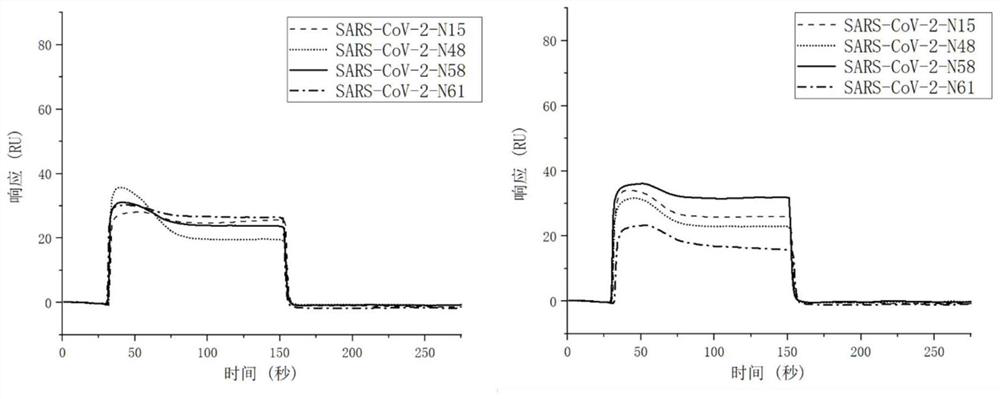
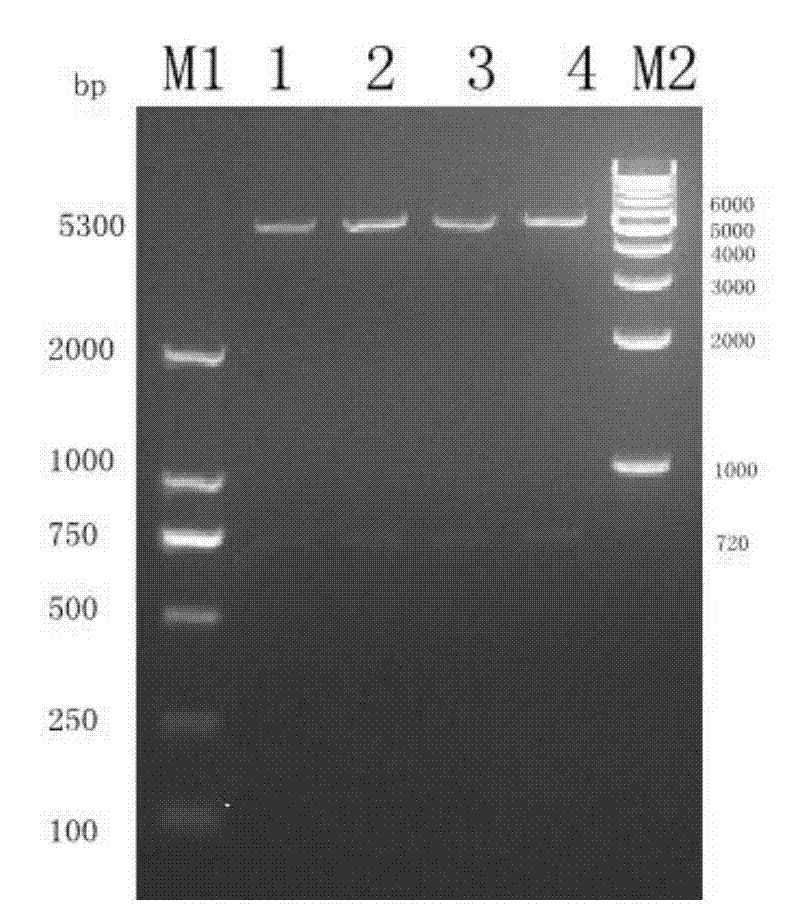
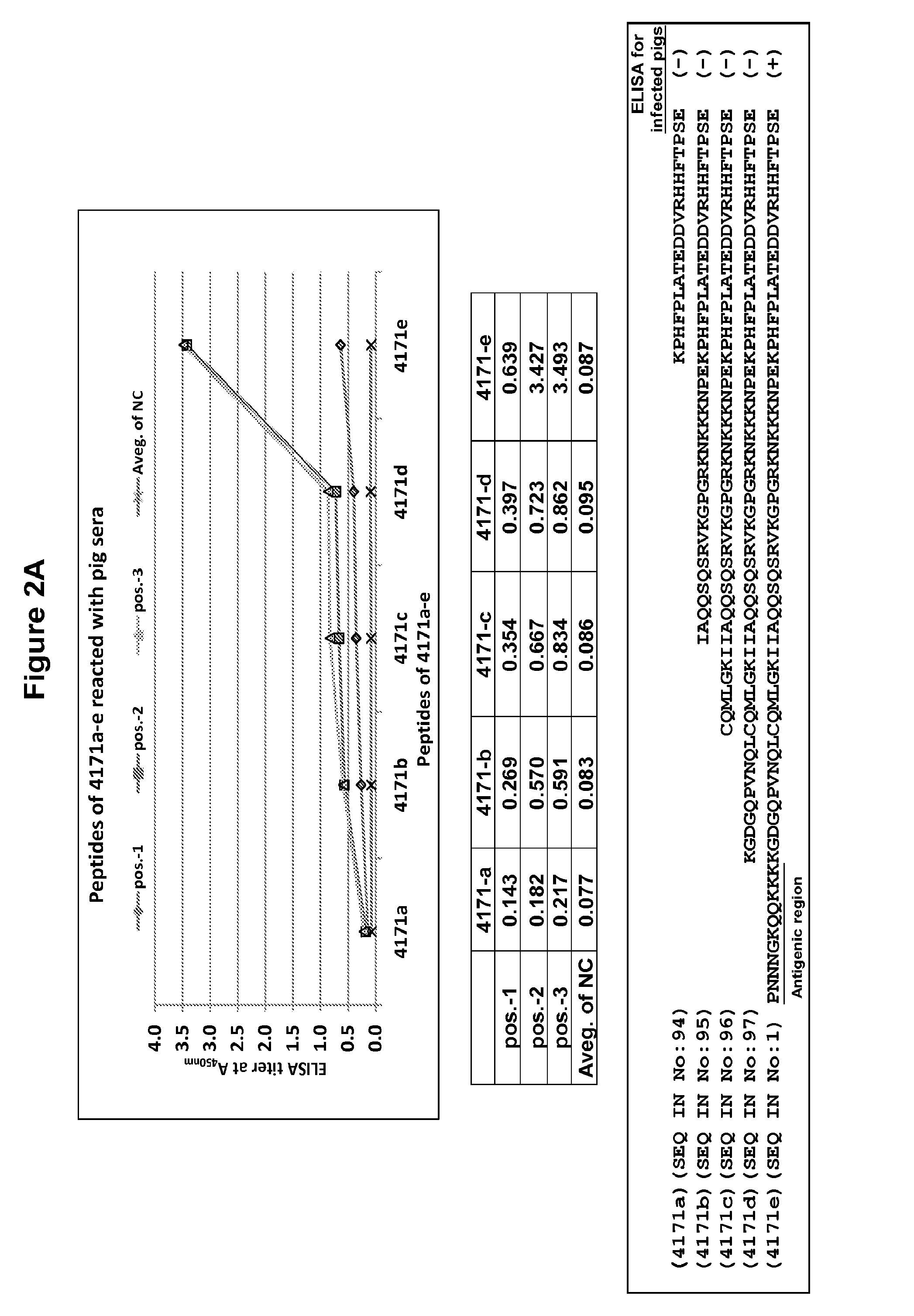
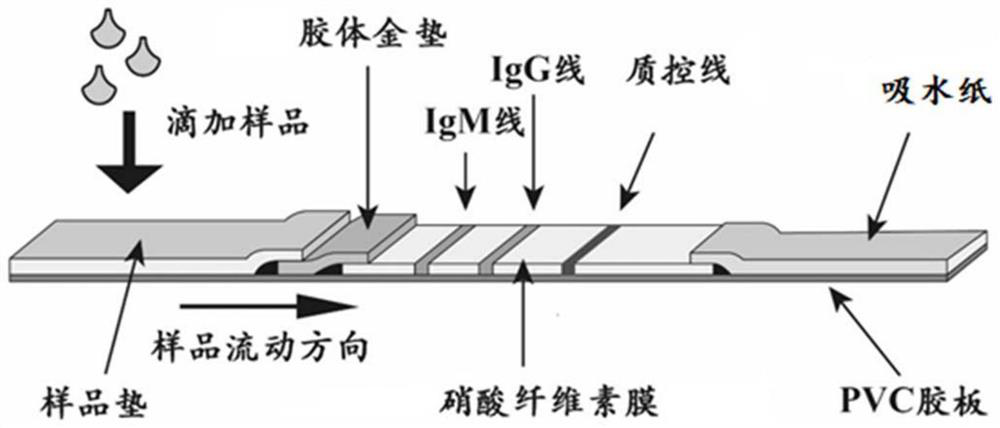
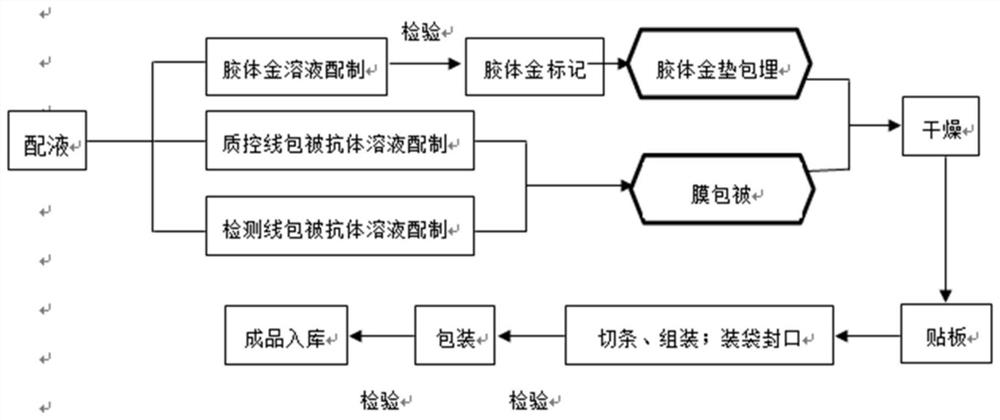
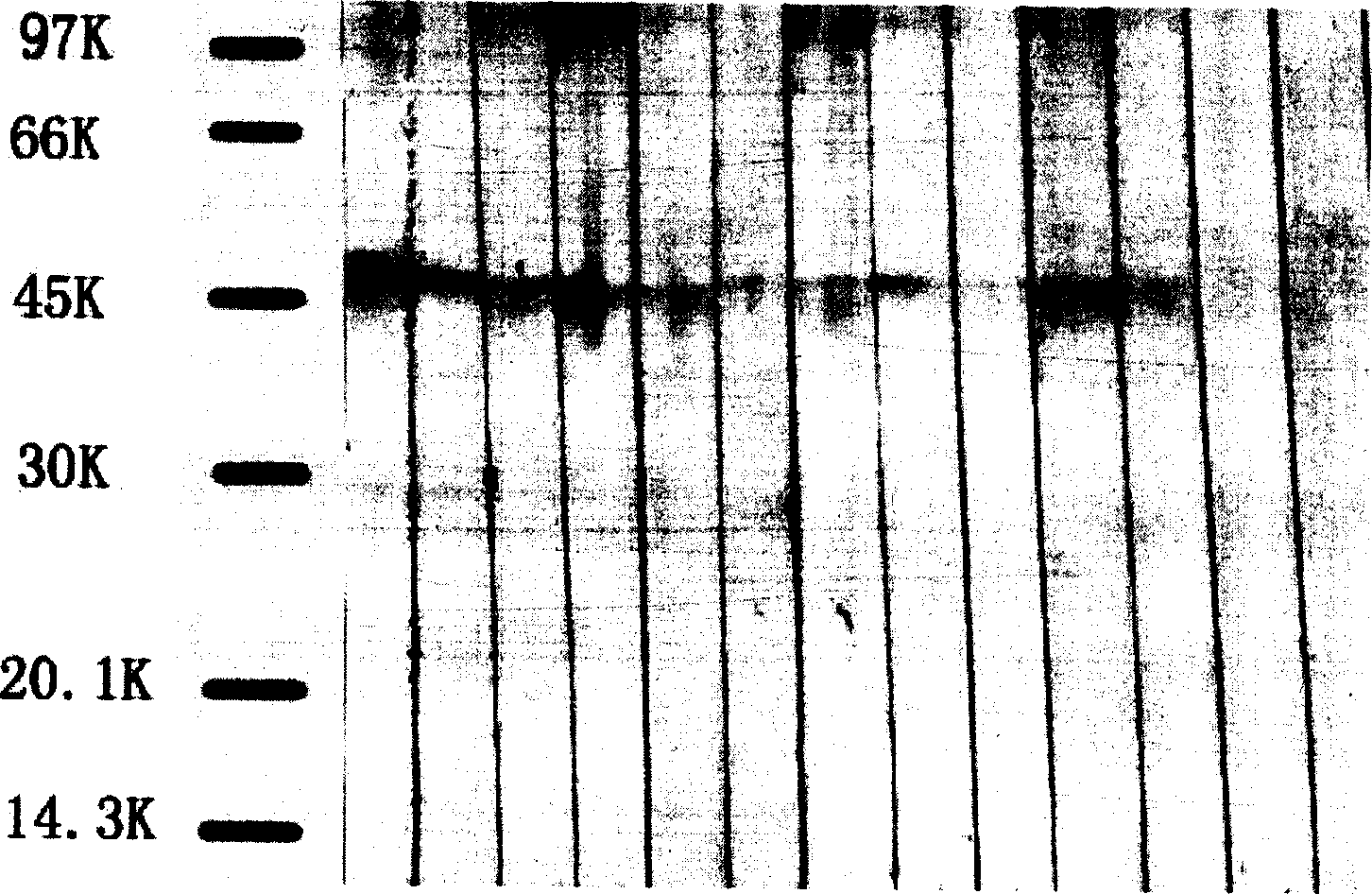
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
172 results about "Nucleocapsid Proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
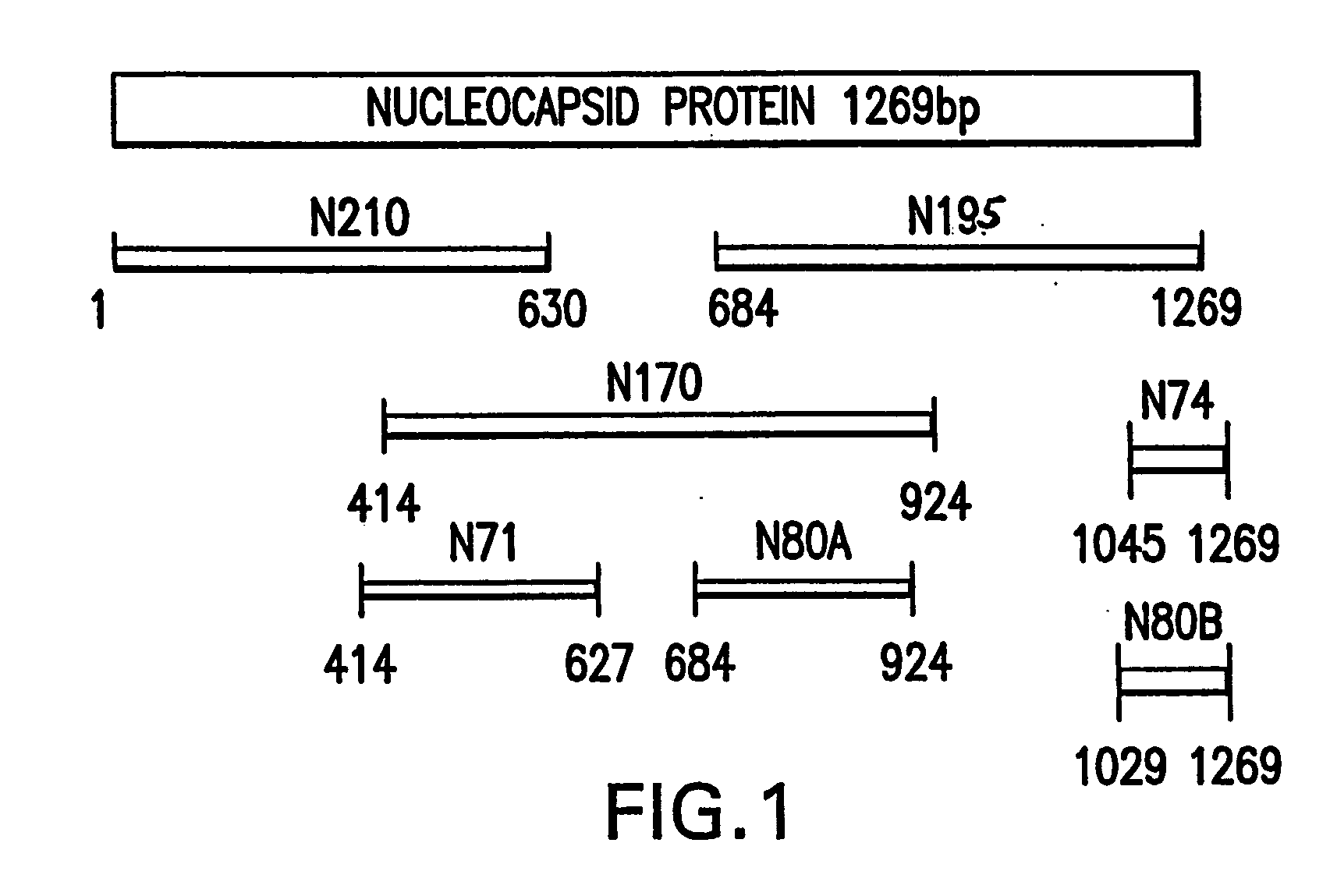
Nucleocapsid Definition. Proteins associated with nucleic acid are known as nucleoproteins, and the association of viral capsid proteins with viral nucleic acid is called a nucleocapsid. Nucleocapsid is an unit of vrial structure, consisting of a capsid with the enclosed nucleic acid; it is generally inside the cytoplasm.
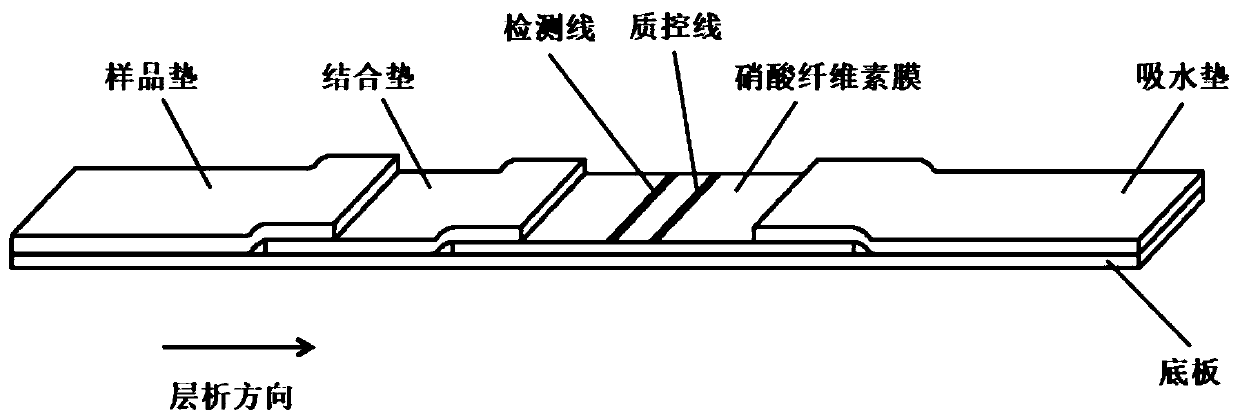
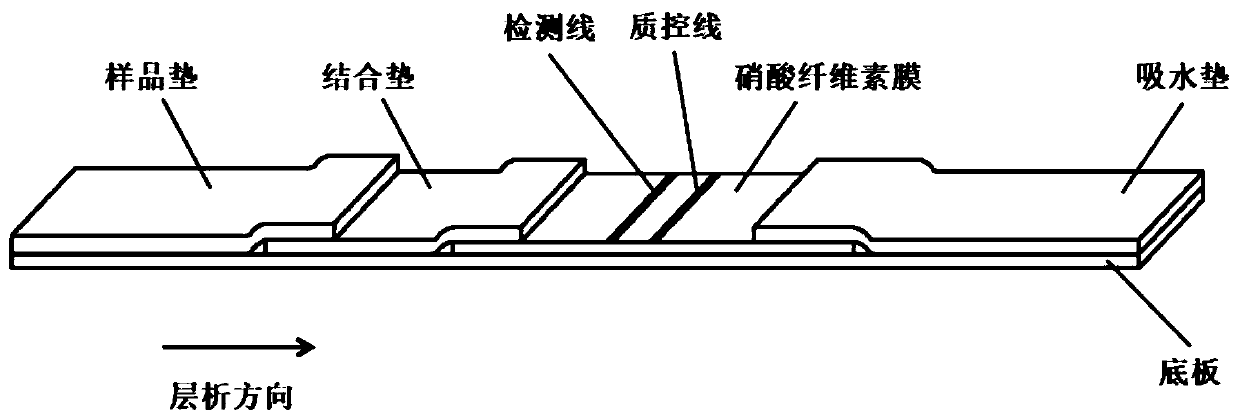
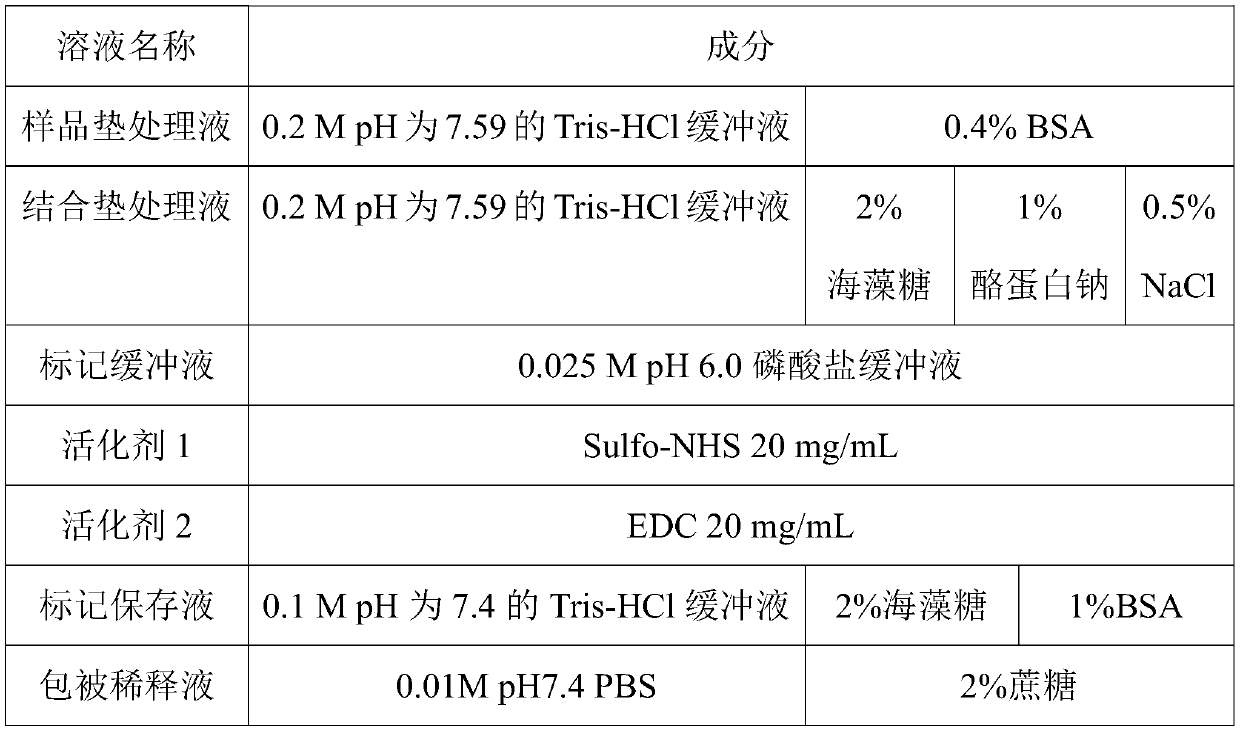
Colloidal gold kit for jointly detecting coronavirus IgM/IgG antibody, and preparation method thereof
ActiveCN111089962ASimple preparation processEasy to useMaterial analysisCoronavirus antibodyIgm antibody
The invention discloses a colloidal gold kit for jointly detecting coronavirus IgM / IgG antibody, and a preparation method thereof, and relates to the field of biological medicine. Whether anti-novel coronavirus nucleocapsid protein IgM antibody and / or anti-novel coronavirus nucleocapsid protein IgG antibody exists in human serum or plasma or not by adopting an antigen-antibody sandwich method anda colloidal gold immunochromatography method principle, the novel coronavirus nucleocapsid protein containing 6xHis mark is marked by applying colloidal gold, thereby forming gold-marked N protein tobe adsorbed on a gold-marked pad, the novel coronavirus nucleocapsid protein containing 6xHis mark is used as an indication marker, the mouse-anti-human u chain monoclonal antibody is coated on the IgM detection line of a NC membrane, the mouse-anti-human IgG monoclonal antibody is coated on the IgG detection line and the mouse-anti 6xHis monoclonal antibody is coated on a quality control line ofthe NC membrane, the qualitative detection of the anti-novel coronavirus nucleocapsid protein IgG antibody is realized, and the colloidal gold kit disclosed by the invention has the advantages of being convenient to use, high in sensitivity and short in detection time.
Owner:中山生物工程有限公司
Novel coronavirus pneumonia (COVID-19) serological diagnosis kit
ActiveCN111239392AHigh detection sensitivityImprove detection accuracySsRNA viruses positive-senseVirus peptidesSerodiagnosesAntigen
The invention discloses a novel coronavirus pneumonia (COVID-19) serological diagnosis kit. The kit comprises an S-IgM / IgG test strip and an N-IgM / IgG test strip, the double-antigen quadruple detection kit can be used for simultaneously detecting four indexes of an IgM / IgG antibody for resisting novel coronavirus spinous process protein S and an IgM / IgG antibody for resisting novel coronavirus nucleocapsid protein N in serum of a patient suffering from novel coronavirus pneumonia COVID-19. According to the kit, the detection sensitivity is improved through quantum dot fluorescence labeling andmultistage coupling amplification signals, the detection accuracy is improved through double-antigen quadruple detection, and the biological safety in the detection process is guaranteed by establishing a virus inactivation system. The kit is suitable for whole blood, plasma and serum detection, and can be applied to novel COVID-19 serological diagnosis.
Owner:浙江诺迦生物科技有限公司 +1
Compositions and methods for diagnosing and preventing severe acute respiratory syndrome (SARS)
InactiveUS20050112559A1Reduce usageSuitable for detectionSsRNA viruses positive-senseViral antigen ingredientsBiologic DMARDImmunologic function
The present invention relates to the fields of immunology and molecular biology and describes compositions and methods for using proteins, peptides and nucleic acids related to the SARS CoV nucleocapsid protein and the spike glycoprotein. In particular, the present invention provides immunostimulatory preparations, prophylactic pharmaceutical preparations, diagnostic assays and kits for identifying and preventing SARS infections.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Monoclonal antibody for detecting SARS-CoV-2 virus nucleocapsid protein (N protein) and application thereof
PendingCN112079920AStrong specificityBiological material analysisImmunoglobulins against virusesCapsidGene clone
The invention discloses a monoclonal antibody 64360-52D1 capable of specifically combining with SARS-Cov-2 virus structural nucleocapsid protein (N protein), a preparation method thereof, and 6 complementarity-determining regions (CDR) of heavy chain and light chain variable regions; and more specifically, the monoclonal antibody is secreted by a hybridoma strain 64360#52D1, and can specifically recognize the SARS-Cov-2 virus N protein rather than SARS virus N protein. Thus, the monoclonal antibody can be used for identifying the two coronaviruses with high similarity. The invention further provides an enzyme-linked immunosorbent assay (ELISA) and an immune colloidal gold test strip detection method for specifically detecting the SARS-Cov-2 virus N protein by preparing the 64360#52D1 antibody. The antigen of the antibody is SARS-Cov-2 virus N protein subjected to heat treatment and expressed in mammalian cells; the finally obtained antibody belongs to an IgG1 subtype; and a sequence for encoding the variable region of the antibody is obtained in a gene cloning mode.
Owner:BEIJING PROTEIN INNOVATION
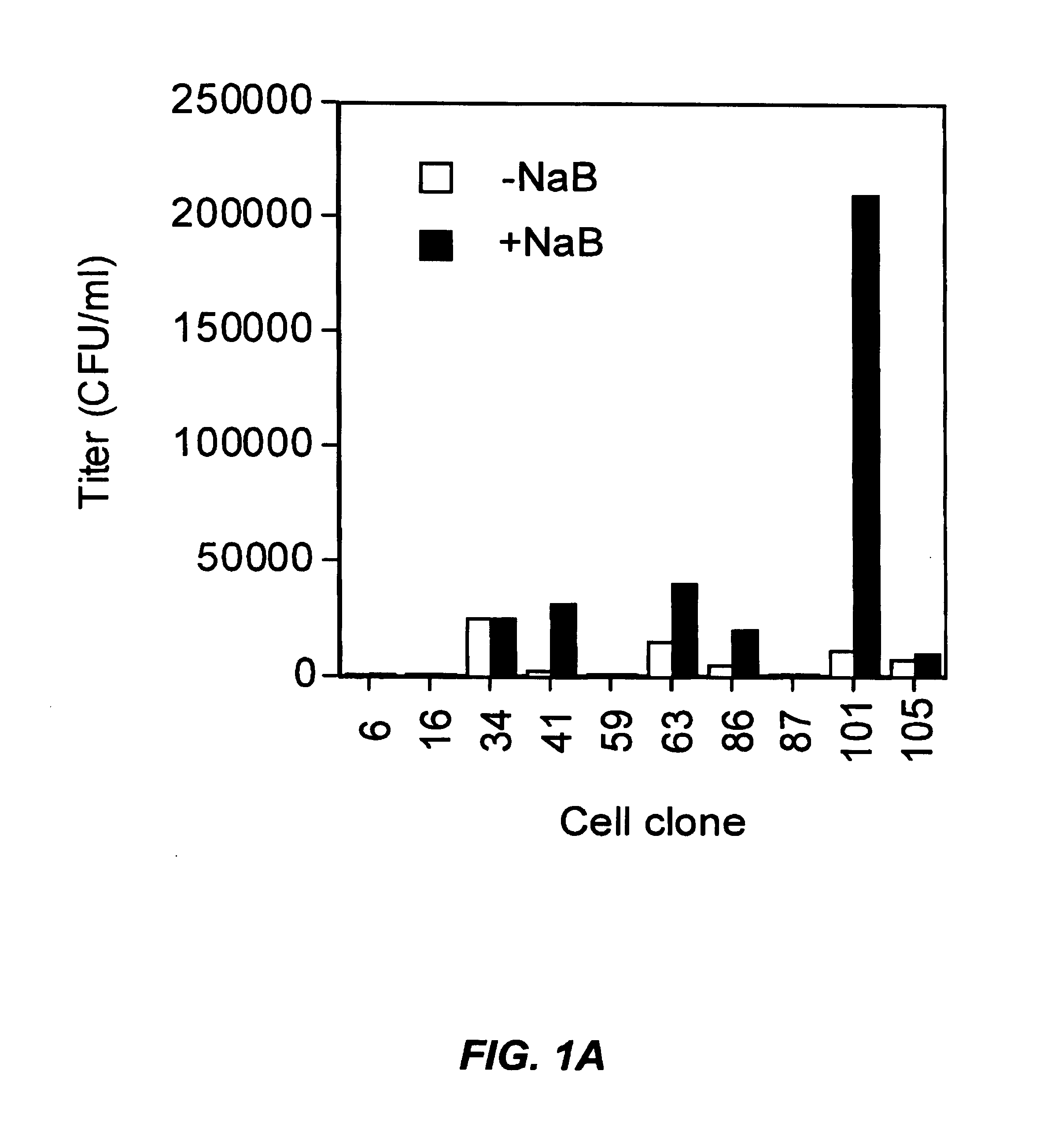
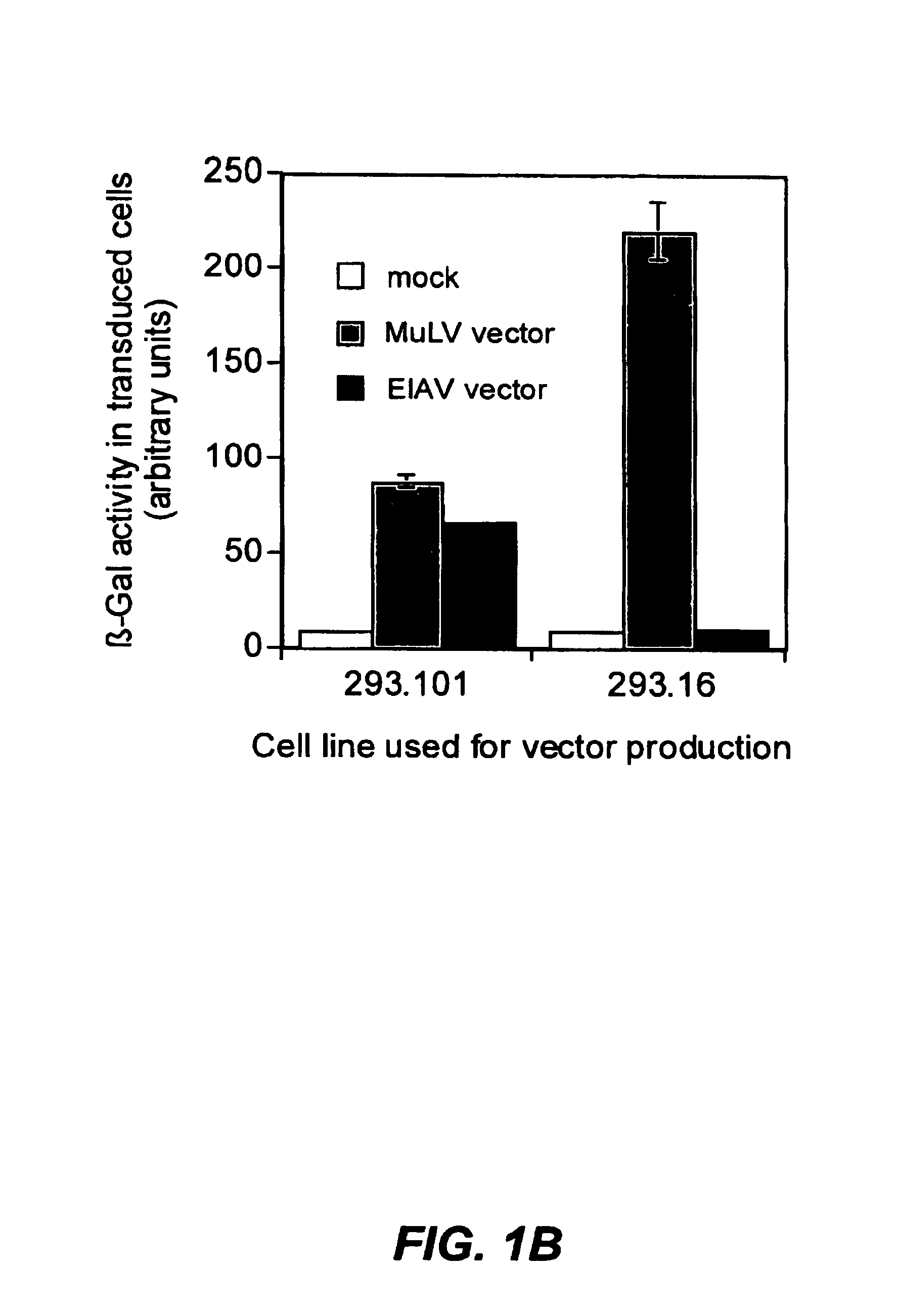
Methods for producing high titre vectors and compositions used in such methods
InactiveUS6969598B2Reduce chanceQuantity minimizationGenetic material ingredientsVirus peptidesNucleotideViral envelope
A method for producing viral vectors is described using packaging and producer cell lines is described. The producer cell comprises: (i) a first nucleotide sequence (NS) encoding a toxic viral envelope protein operably linked to a promoter; wherein the promoter is operably linked to at least one copy of a TRE; (ii) a second NS wherein the second NS comprises a sequence encoding a tetracycline modulator; (iii) a third NS encoding a retrovirus nucleocapsid protein; and (iv) a fourth NS comprising a retroviral sequence capable of being encapsidated in the nucleocapsid protein such that the retroviral vector particle titre obtainable from the producer cell is regulatable by tetracycline and an initial stimulus with sodium butyrate or functional analogues thereof.
Owner:OXFORD BIOMEDICA (UK) LTD +1
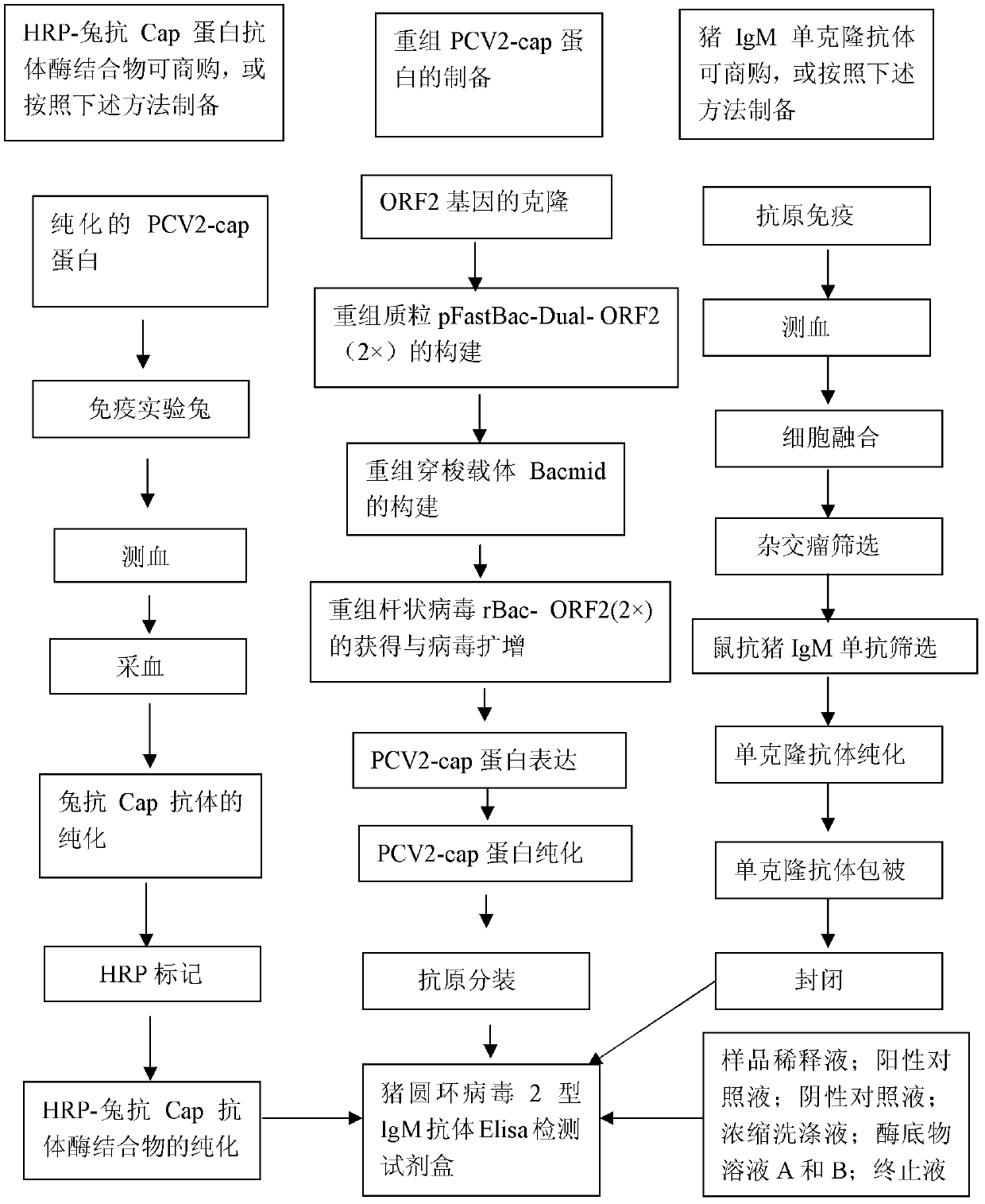
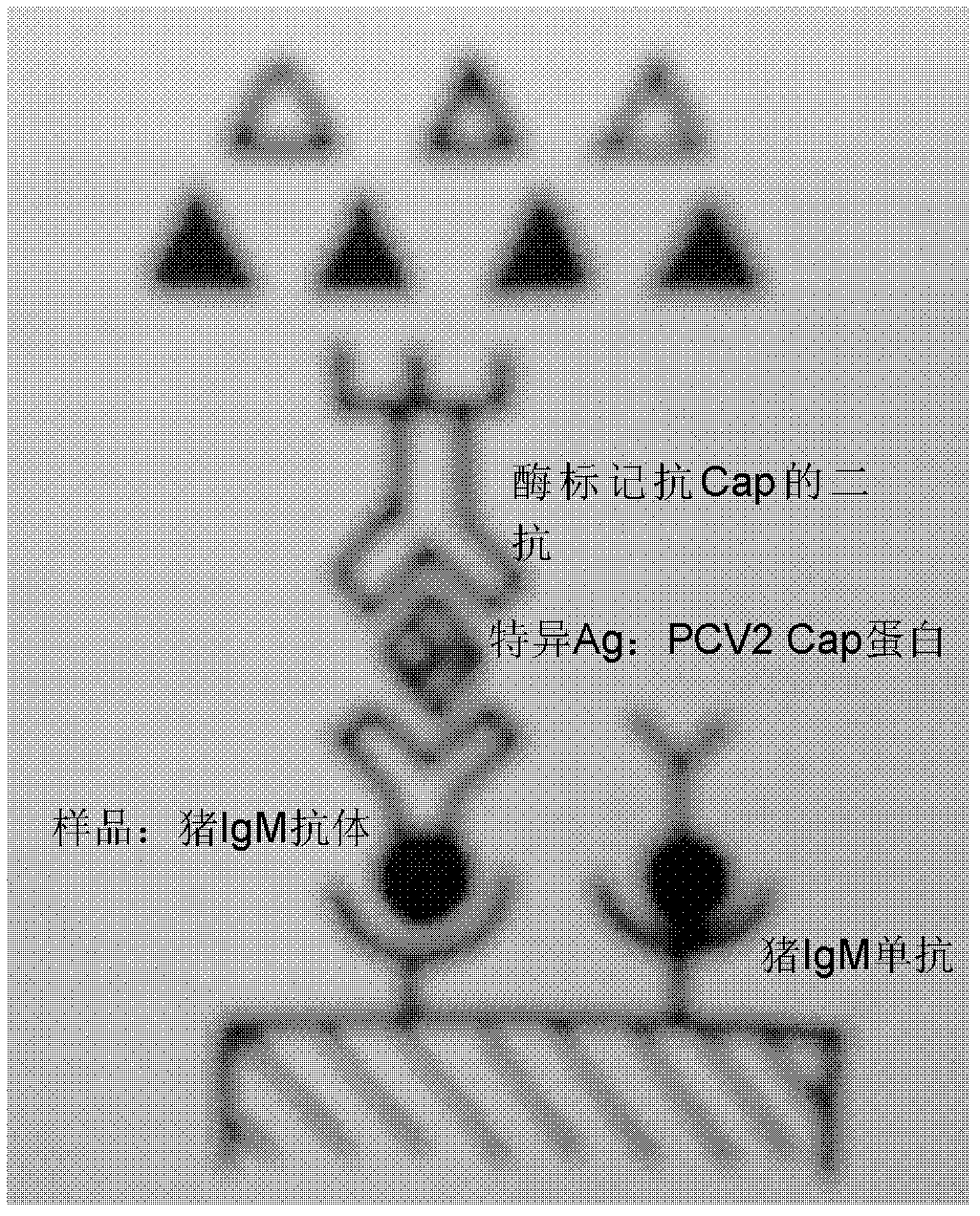
PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as preparation method and applications thereof
ActiveCN103033622AImprove expression efficiencyImproving immunogenicityMicroorganism based processesGenetic engineeringSorbentMonoclonal antibody
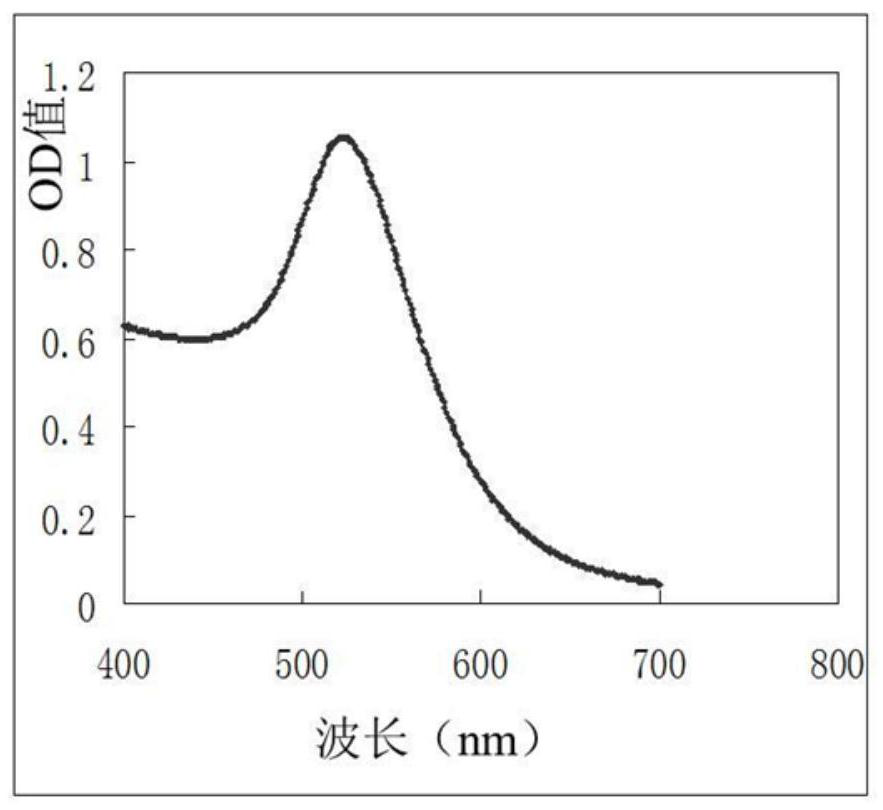
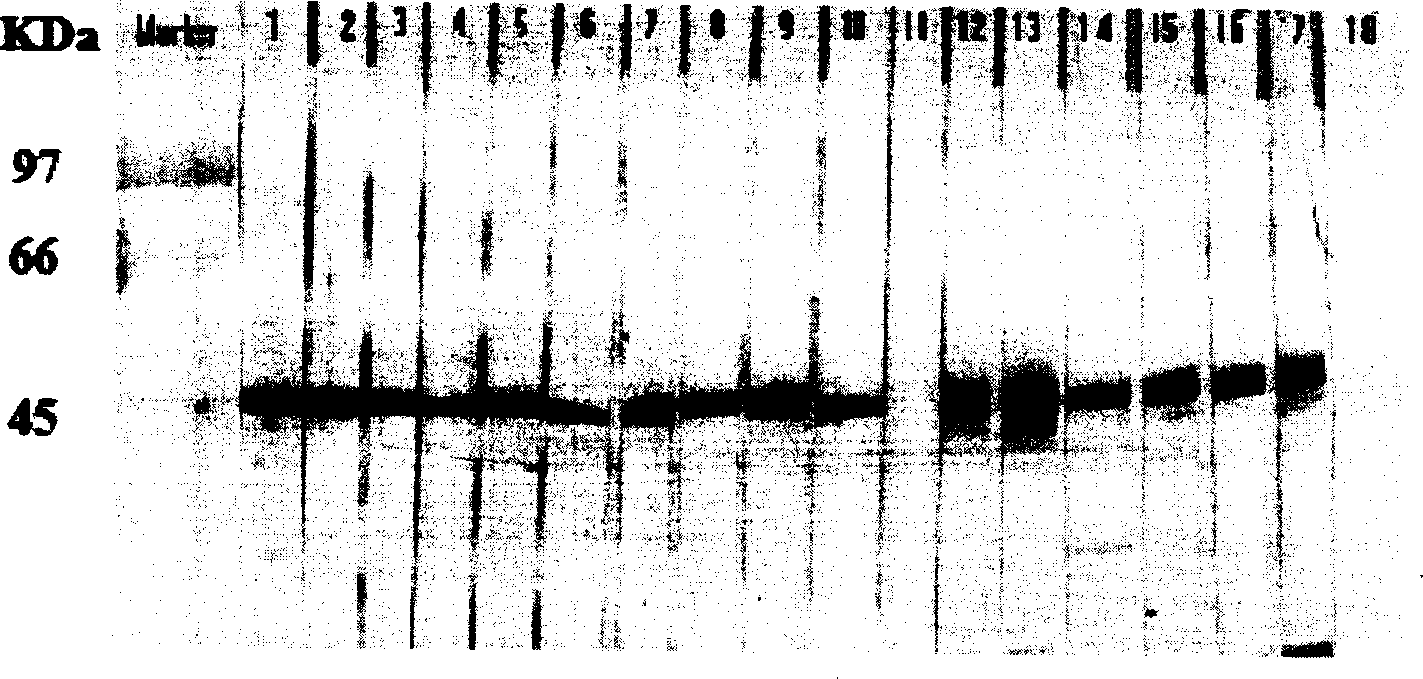
The invention relates to a PCV2 (porcine circovirus 2) ELISA (enzyme-linked immuno sorbent assay) antigen detection kit as well as a preparation method and applications thereof, wherein the detection kit comprises an elisa plate of a polyclonal antibody of peridium anti-PCV2-Cap (nucleocapsid) protein, seal liquids, sample diluent, an antigen standard product, a second antibody of a monoclonal antibody of HRP marked anti-PCV2-Cap protein, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen standard product is purified reconstructed PCV2-Cap protein. The specificity of the kit provided by the invention achieves 100%, and the sensitivity is as high as 4ng / ml, and the kit can be used for swinery PCV2 antigen detection and PCV2 vaccine product quantitative detection.
Owner:WUHAN CHOPPER BIOLOGY
Malaria immunogen and vaccine
InactiveUS20050208068A1High antibody titersEasy to prepareAntibody mimetics/scaffoldsVirus peptidesHepatitis B immunizationMalaria
A chimeric, carboxy-terminal truncated hepatitis B virus nucleocapsid protein (HBc) is disclosed that contains an immunogen for inducing the production of antibodies to malarial proteins. An immunogenic malarial epitope is expressed between residues 78 and 79 of the HBc immunogenic loop sequence. The chimer preferably contains a malaria-specific T cell epitope and is preferably engineered for both enhanced stability of self-assembled particles and enhanced yield of those chimeric particles. Methods of making and using the chimers are also disclosed.
Owner:MILICH DAVID +1
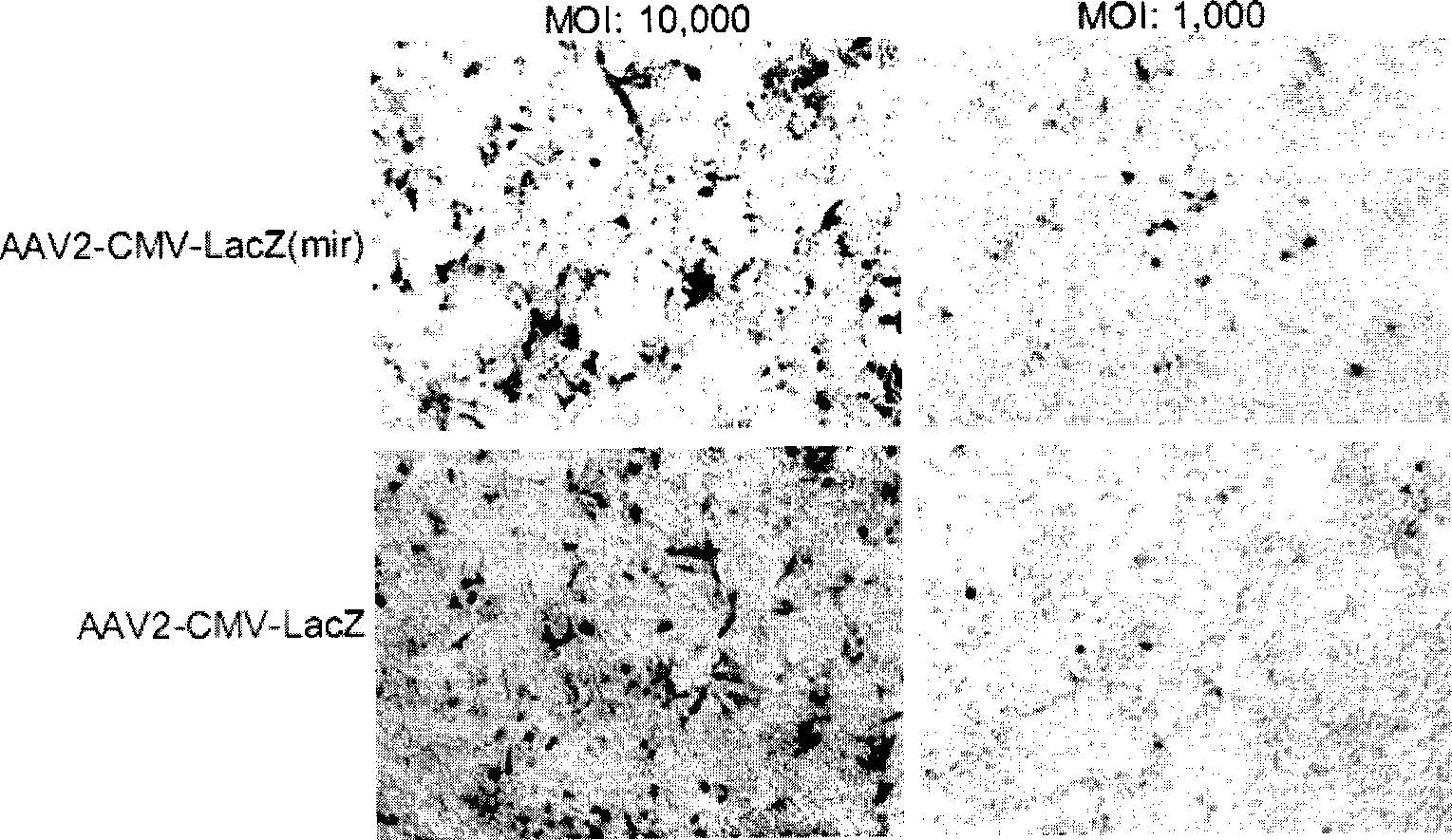
New type cell special gene HAAVmir containing microRNA combined sequence for gene treating
InactiveCN101532024ADoes not affect appearanceDoes not affect productivityGenetic material ingredientsMicroorganism based processesMicroRNACell strain
Owner:许瑞安 +2
Diagnostics for sars virus
InactiveUS20070092938A1SsRNA viruses positive-senseMicrobiological testing/measurementDiseaseEpitope
This invention relates to Severe Acute Respiratory Syndrome associated coronavirus (SARS virus) isolated and recombinant proteins, in particular the nucleocapsid (N) protein and spike (S) protein, as well as fragments thereof and their use in the diagnosis, treatment and prevention of Severe Acute Respiratory Syndrome (SARS). The proteins and fragments carry epitopes that are specific for the SARS virus. Thus, detection methods based on these proteins or fragments as well as the monoclonal antibodies against these proteins or fragments are specific for the SARS virus.
Owner:TEMASEK LIFE SCIENCES LABORATORY
Recombination virus particles for expressing 2-typed porcine circovirus nucleocapsid protein Cap gene
InactiveCN101289658ANon-pathogenicImprove replication efficiencyViral antigen ingredientsAntiviralsAntigenShuttle vector
The invention relates to construction and application of recombinant nucleocapsids of Cap genes of porcine circovirus expression type 2 nucleocapsid protein, belonging to the genetic engineering bacterin field. C-terminal gene fragments of porcine parvovirus (PPV) VP2 genes are cloned into a type 5 adenovirus shuttle vector of the human beings, and recombinant adenovirus rAd-deltaVP2 is obtained; deltaVP2 proteins are expressed successfully and highly efficiently and can be self-assembled into the nucleocapsids [PPV:VLPs]; the PPV VP2 nucleocapsids are used as antigen transport vectors and 165 to 200 sites of amino acid (deltaCap) genes of the porcine circovirus type 2 (PCV2) nucleocapsid proteins (Cap) are embedded into an N-terminal (deltaVP2) of the PPV VP2, and then recombinant adenovirus rAd-deltaCap-deltaVP2 is obtained; embedded VP2 (deltaCap-deltaVP2) proteins are expressed successfully and highly efficiently and can be self-assembled into nucleocapsids [PPV:VLP(PCV2)]. The invention also relates to application of the recombinant virus and recombinant PPV VP2 nucleocapsids of the expression Cap genes of the recombinant virus in the aspects of bacterin immunity and so on.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Fluorescent quantitative PCR primers and kit for detecting novel SADSCoV (swine acute diarrhea syndrome coronavirus)
InactiveCN107630109AAccurate identificationRapid identificationMicrobiological testing/measurementAgainst vector-borne diseasesAcute diarrheaConserved sequence
The invention belongs to the technical field of biological detection and discloses fluorescent quantitative PCR primers and a kit for detecting novel SADSCoV (swine acute diarrhea syndrome coronavirus). The specific primers and probe are designed according to a nucleocapsid protein gene of a conserved sequence of the SADSCoV, and sequences of the primers and the probe are shown as SEQ ID NO:4-6; the SADSCoV can be amplified specifically by the primers and the probe, for real-time fluorescent quantification, fluorescent data can be acquired by monitoring specific binding condition of the probewith PCR amplified fragments in a PCR process in real time, and the SADSCoV is identified according to Cq value. The SADSCoV can be identified rapidly after PCR amplification with the method, the method is high in accuracy and specificity and good in repeatability and can identify the SADSCoV accurately, rapidly and efficiently, and popularization and application in clinical practice are facilitated.
Owner:SOUTH CHINA AGRI UNIV
Genetic engineering subunit vaccine for porcine circovirus as well as preparation method and application of genetic engineering subunit vaccine
PendingCN108619503AReduce virus contentHigh antigen purityViral antigen ingredientsAntiviralsSolubilityEscherichia coli
The invention discloses a genetic engineering subunit vaccine for a porcine circovirus as well as a preparation method and application of the genetic engineering subunit vaccine. By cloning the nucleocapsid protein of the novel porcine circovirus 3 (PCV3), a PCV-Cap protein with higher purity is successfully expressed by using an escherichia coli or baculovirus expression system. The subunit vaccine for the PCV3 is successfully developed for the first time by using the PCV-Cap protein; the prepared vaccine is high in antigen purity, good in safety and strong in immunogenicity, and has no pathogenicity to pigs and other animals; the antigen has good solubility in a neutral PH buffer solution; furthermore, the preparation method is simple and low in cost, thus being suitable for large-scaleindustrial production; an effective and powerful means is provided for the prevention and control of novel PCV, and the genetic engineering subunit vaccine has a wide application prospect in the fieldof the prevention and control of the PCV3.
Owner:SOUTH CHINA AGRI UNIV
PCV2 virus-like particles as well as preparation method thereof and splitting and VLP assembly buffer liquor
ActiveCN104073473AHigh expressionImprove solubilityInactivation/attenuationMicroorganism based processesEscherichia coliPorcine circovirus
The invention discloses PCV2 virus-like particles as well as a preparation method thereof and splitting and VLP assembly buffer liquor. Based on an autonomous optimization design, a PCV2 nucleocapsid protein gene which is suitable for efficiently expressing in a prokaryotic expression system is artificially synthesized, a full-length gene sequence of the PCV2 nucleocapsid protein gene is expressed by an escherichia coli prokaryotic expression system, and the virus-like particles are efficiently and autonomously assembled by utilizing soluble nucleocapsid protein of the full-length gene sequence under a special condition. An innovation point of the invention is that PCV2VLPs are obtained by utilizing the prokaryotic expression system instead of adopting the conventional method for obtaining VLPs through an eukaryotic expression system; the method is low in cost, simple and efficient, and suitable for large-scale industrial application; moreover, an innovative buffer liquor formula integrating double functions, which not only can promote thallus splitting, but also can be suitable for self-assembling of VLPs, is also applied; besides, the PCV2 virus-like particles obtained in the invention are very highly similar with wild type virus in outline and good in immunogenicity, and can be applied to developing a subunit vaccine and a drug delivery carrier with utilization potentiality of porcine circovirus.
Owner:湖南派智生物科技有限公司
Nucleic acid aptamer combined with nucleocapsid protein of novel coronavirus SARS-CoV-2 and application of nucleic acid aptamer
ActiveCN111748558ASmall molecular weightChemically stableSsRNA viruses positive-senseVirus peptidesAptamerA-DNA
The invention discloses a nucleic acid aptamer specifically combined with nucleocapsid protein of novel coronavirus SARS-CoV-2. The sequence of the nucleic acid aptamer comprises at least one of the following nucleotide sequences: A, any one DNA sequence as shown in SEQ ID No. 1-2; B, a DNA sequence which has more than 60% of homology with any DNA sequence shown as SEQ ID No. 1-2; C, a DNA sequence which is hybridized with any one DNA sequence shown as SEQ ID No. 1-2 under strict conditions; D, an RNA sequence transcribed by any one DNA sequence shown as SEQ ID No. 1-2; wherein the nucleotidesequences can be specifically combined with the nucleocapsid protein of the novel coronavirus SARS-CoV-2. The invention further discloses conjugates and derivatives of the aptamer and an application of the conjugates and the derivatives.
Owner:安徽省昂普拓迈生物科技有限责任公司
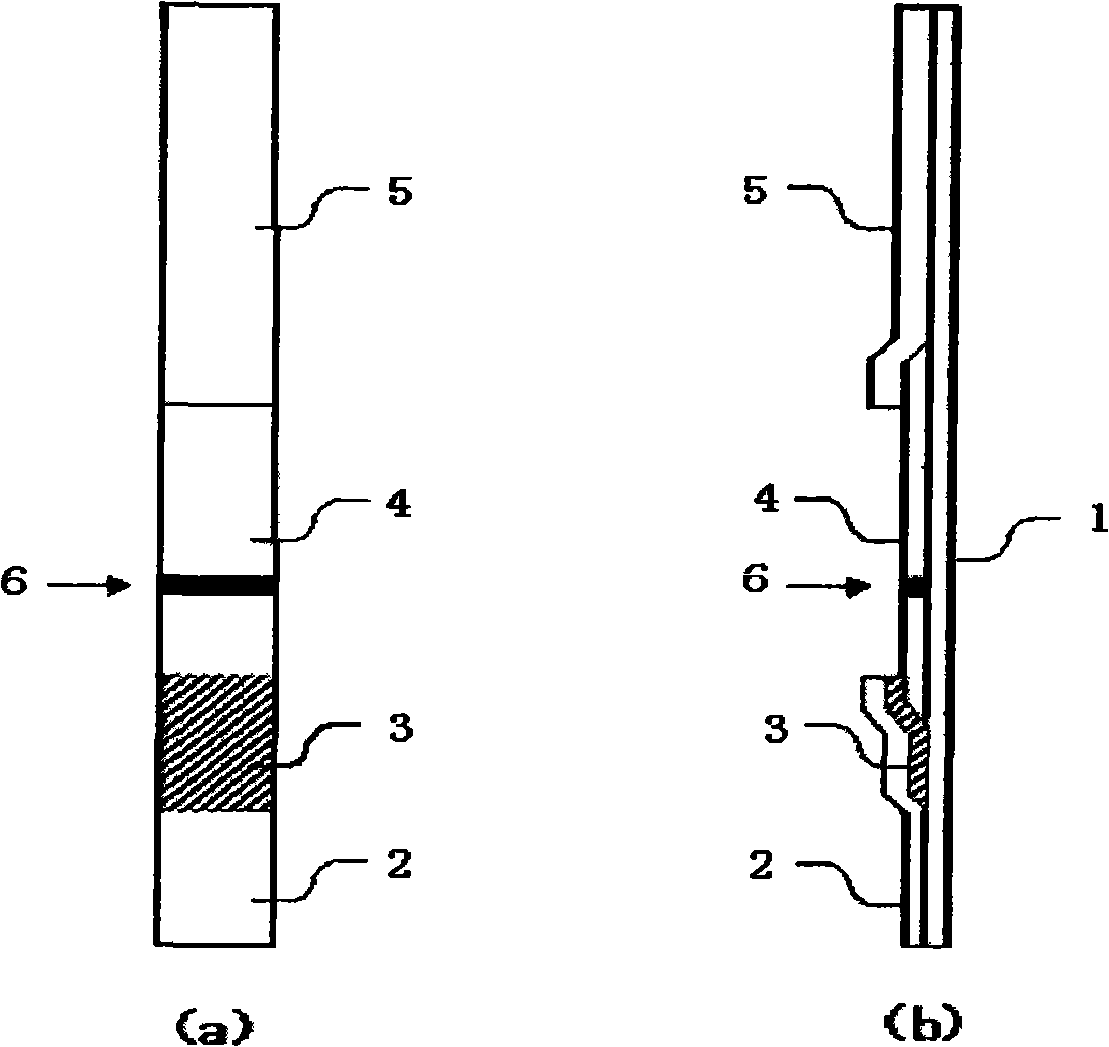
Kidney syndrome blooding diagnosis test paper strip, preparing method and detection reagent kit thereof
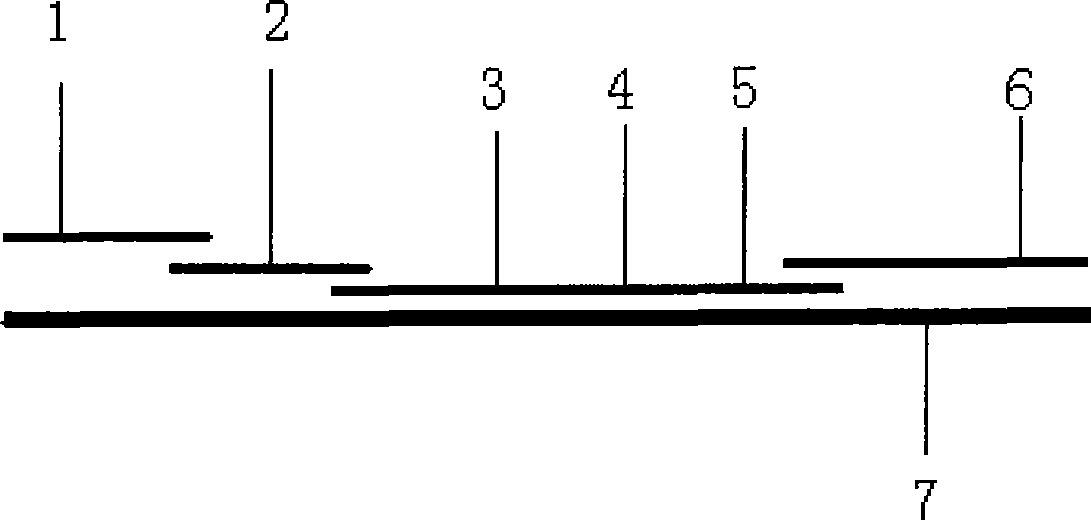
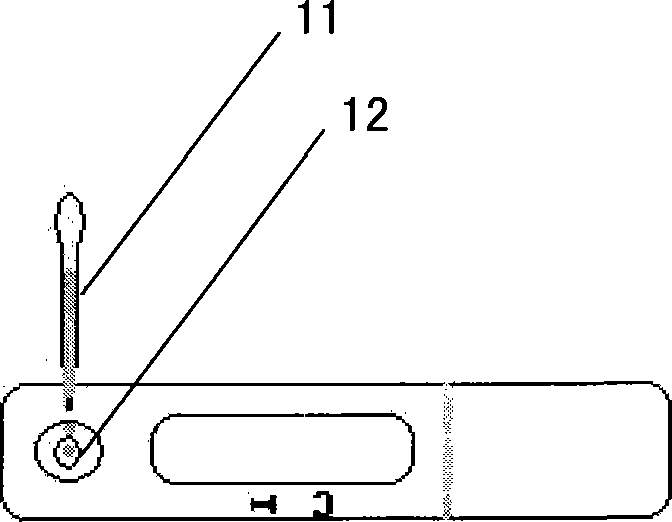
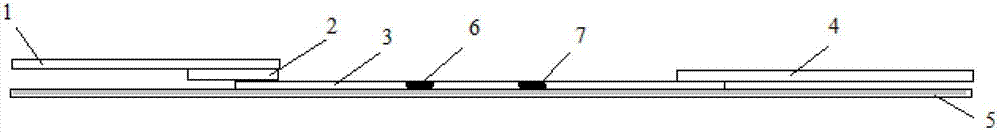
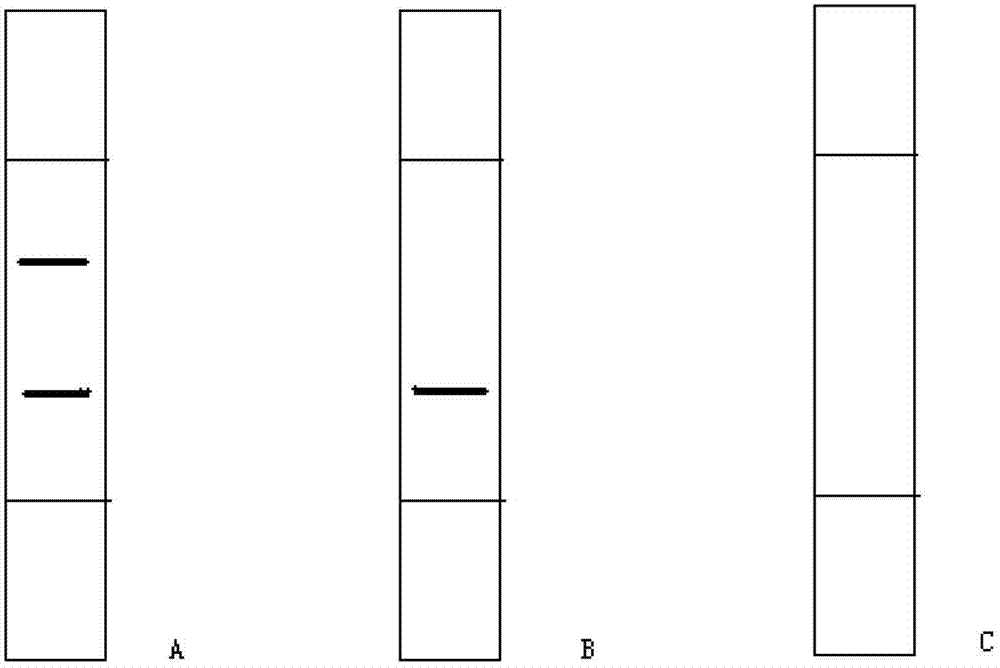
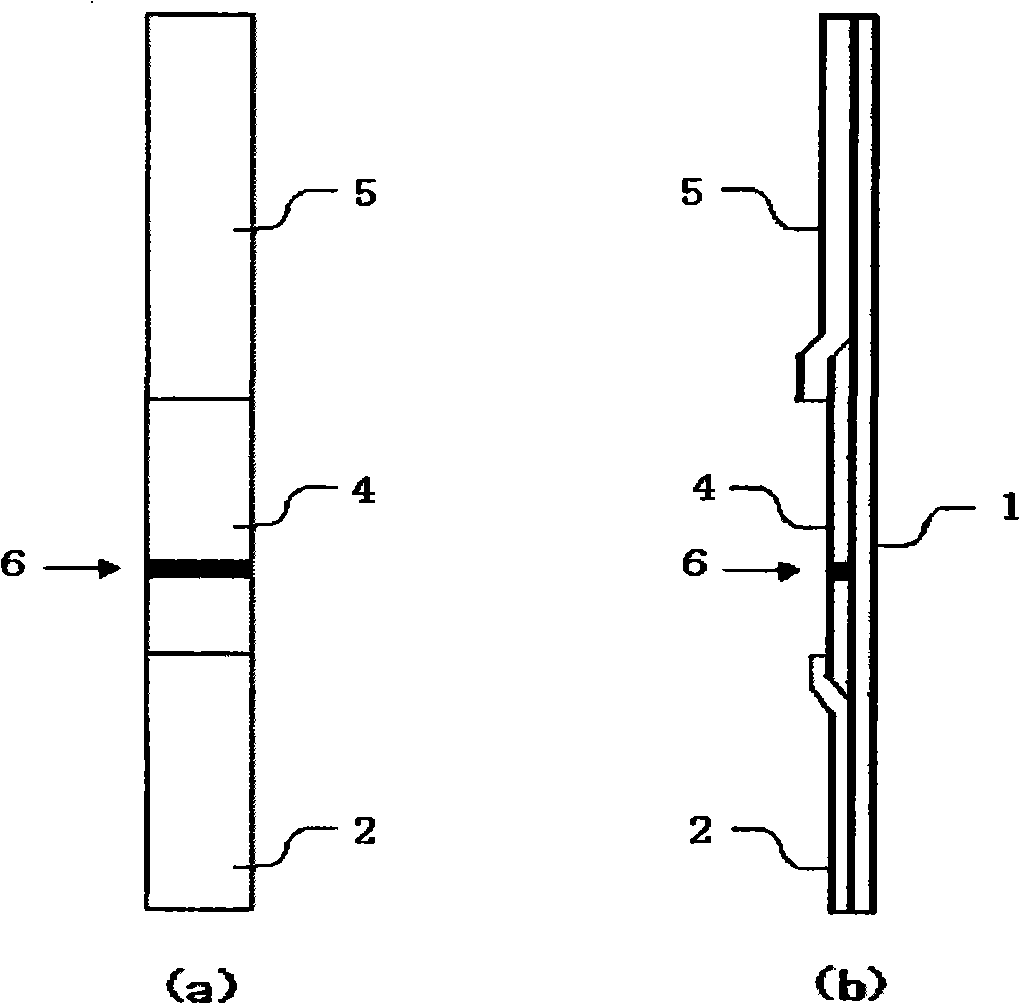
The invention provides a diagnostic strip for hemorrhagic fever with renal syndrome, and a preparation method and a detection reagent kit thereof. The diagnostic strip is a colloidal gold immunochromatographic strip which is formed by that a sample absorption pad (1), a colloidal gold bonding pad (2), a pyroxylin film (5) and a water absorption pad (6) are sequentially stuck on a PVC soleplate (7) in a mutual lapping way. The diagnostic strip is characterized in that the colloidal gold bonding pad (2) is composed of glass-fiber membranes of goat anti-human Mu-chain immune gold complexes containing colloidal gold markers, a detection area (3) and a quality control area (4) are arranged on the pyroxylin film (5), the position of the detection area (3) is coated with hantavirus nucleocapsid protein antigens, and the position of the control area (4) is coated with rabbit anti-goat IgG antibodies. The diagnostic strip has good specificity, sensitivity and repeatability, can realize the early and rapid diagnosis of HFRS, and is easy to carry; and the operation is simple, the cost is low, and the response is fast. The invention is easy to be popularized in primary medical units.
Owner:XIEHE HOSPITAL ATTACHED TO TONGJI MEDICAL COLLEGE HUAZHONG SCI & TECH UNIV
Porcine circovirus-like particle, and vaccine and preparation method thereof
InactiveCN103436499ASafe infectionEffective infectionViral antigen ingredientsInactivation/attenuationAdjuvantStructural protein
The invention discloses a porcine circovirus-like particle, a vaccine and a preparation method thereof. The virus-like particle is composed of a main structural protein-nucleocapsid protein of 2-type porcine circovirus, can excite cell and humoral immune response, can be used as a virus-like particle vaccine (VLP vaccine) to immune different fauna and can safely and effectively prevent PCV-2 infections after being used. The VLP can be made into injections, nose drops and drinking preparations by adding adjuvants or not. An ideal vaccine is provided for security of different populations of sows, piglets, fattening pigs and for effective immune prevention and control of PCV-2 infections.
Owner:CHONGQING AULEON BIOLOGICALS
Test strip for quickly detecting porcine circovirus 2 (PCV2) antibody by adopting colloidal gold
ActiveCN102735680AObvious superiorityStrong specificityMaterial analysis by observing effect on chemical indicatorMonoclonal antibodyColloid
The invention discloses a test strip for quickly detecting a porcine circovirus 2 (PCV2) antibody by adopting colloidal gold. The test strip comprises a support layer, and a sample loading pad, a gold labelled antibody release pad, a detection layer and an absorption layer which are arranged on the support layer in sequence, wherein a colloidal gold labelled PCV2 nucleocapsid protein is embedded in the gold labelled antibody release pad; a detection strip and a quality control strip are arranged on the detection layer; a PCV2 nucleocapsid protein is immobilized on the detection strip; and a monoclonal antibody of the PCV2 nucleocapsid protein is immobilized on the quality control strip. The test strip has the following advantages: the test strip has strong specificity, the probability of false positive occurrence is greatly reduced, and the specificity and sensitivity of the test strip are ensured; the test strip has high safety and avoids operation of the virus in the detection process; the test strip is simple, convenient and quick to operate and does not need professional technicians for operation and professional instruments for auxiliary detection; and the detection result is intuitive and is easy to judge, and the detection time is only 10 minutes (the detection speed is extremely high).
Owner:兆丰华生物科技(南京)有限公司 +2
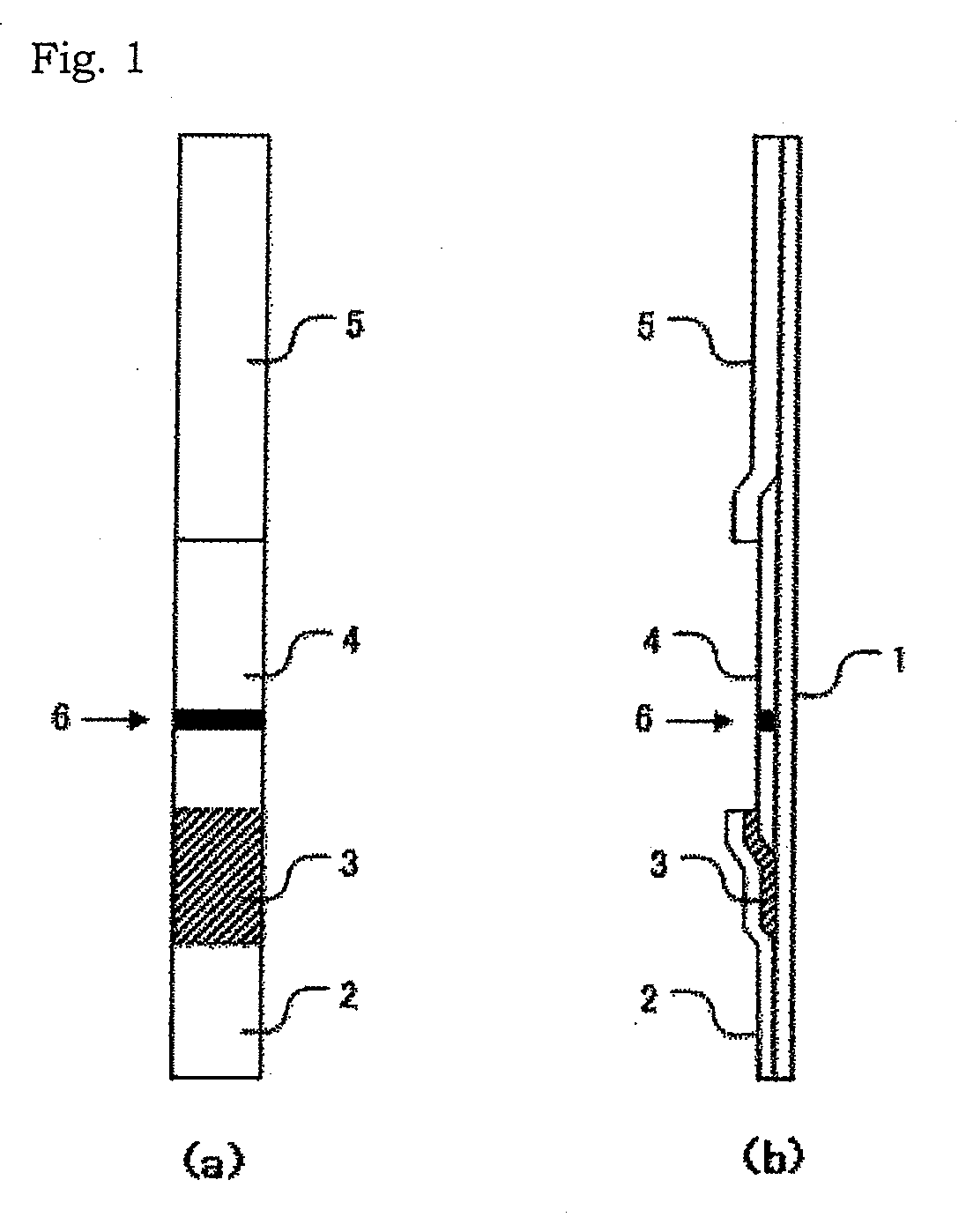
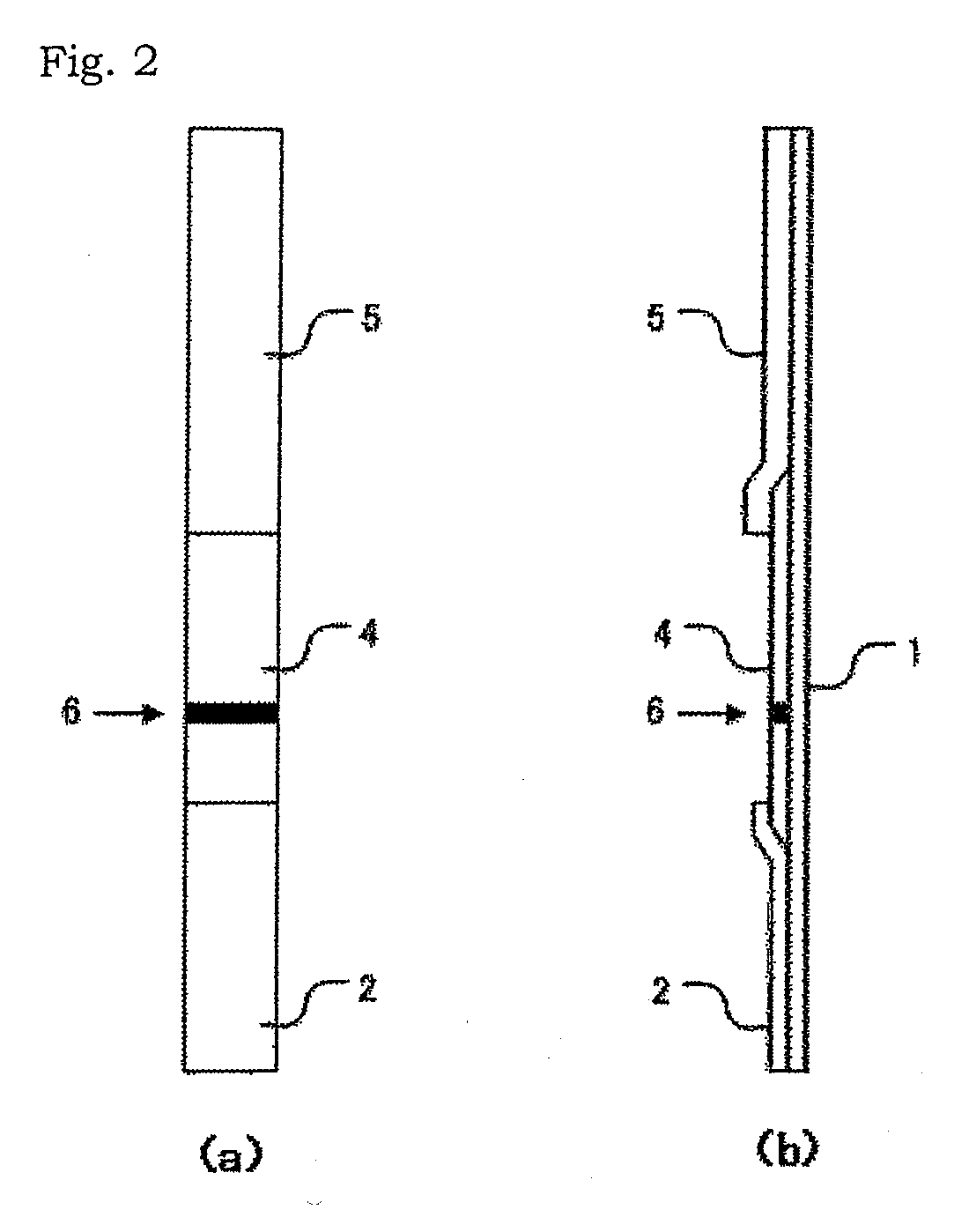
Method for measurement of sars virus nucleocapsid protein, reagent kit for the measurement, test device, monoclonal antibody directed against sars virus nucleocapsid protein, and hybridoma capable of producing the monoclonal antibody
InactiveUS20090280507A1High sensitivityEasy to detectAnimal cellsSsRNA viruses positive-senseEpitopeMonoclonal antibody
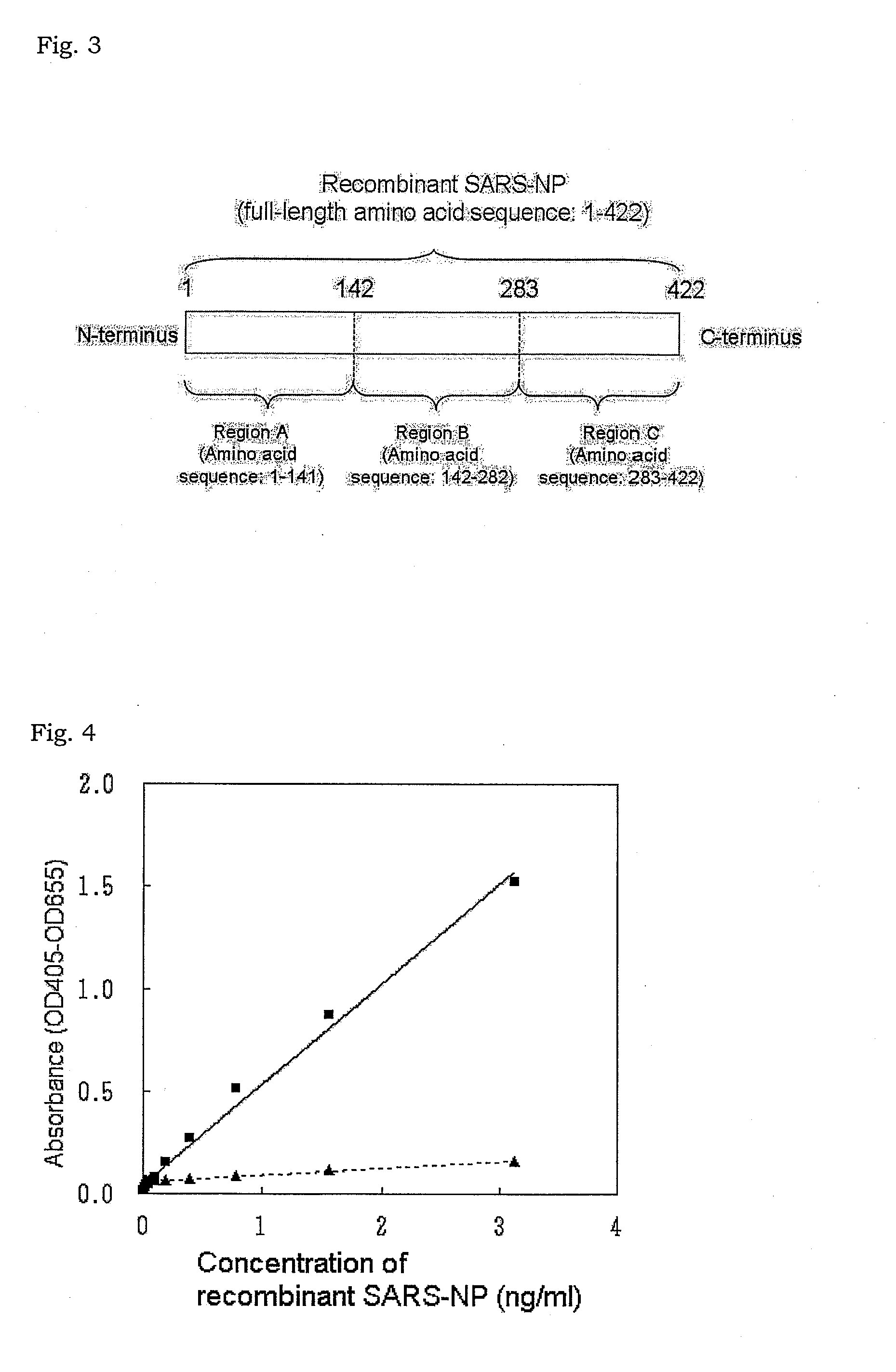
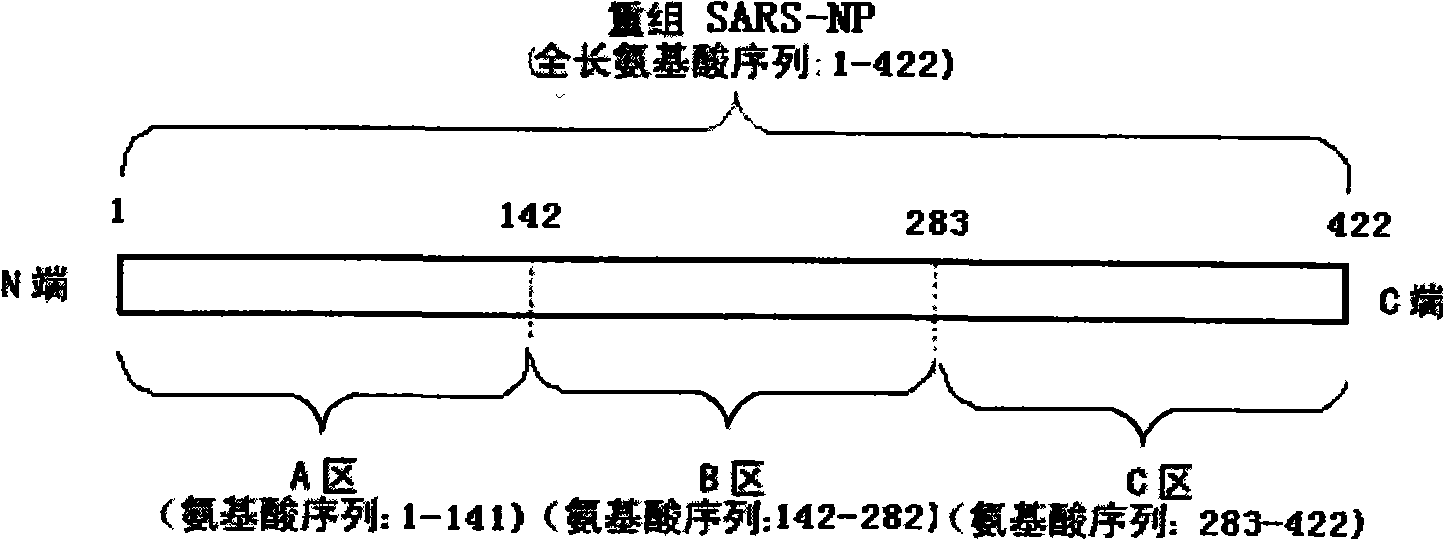
The present invention provides a method for measuring SARS virus nucleocapsid protein (SARS-NP) using first and second antibodies both binding specifically to SARS-NP, wherein the first or second antibody is an antibody recognizing an epitope located in a region (Region C) of amino acid 283 to 422 from the N-terminus of the amino acid sequence of SARS-NP.
Owner:SYSMEX CORP
Nucleic acid aptamer binding to novel coronavirus (SARS-CoV-2) nucleocapsid protein and use thereof
ActiveCN113151282ASmall molecular weightChemically stableOrganic active ingredientsSsRNA viruses positive-senseAptamerChemical synthesis
The invention relates to a nucleic acid aptamer binding to novel coronavirus (SARS-CoV-2) nucleocapsid protein and use thereof. The nucleic acid aptamer specifically comprises or consists of a nucleotide sequence shown in any one of SEQ ID NO: 1-4. The nucleic acid aptamer and the derivative thereof have the advantages of being rapid in chemical synthesis, small in molecular weight, stable, easy to store and label and the like, and can be used for detection, diagnosis, imaging, treatment and the like.
Owner:UNIV OF SCI & TECH OF CHINA
Method for preparing porcine circovirus 2 open reading frame 2 (ORF2) protein
InactiveCN102994518AAchieving soluble expressionAvoid sex changeVirus peptidesPeptide preparation methodsEscherichia coliAntigen
The invention discloses a method for preparing a porcine circovirus 2 open reading frame 2 (ORF2) protein. A Cap gene of an encoding porcine circovirus 2 (PCV2) nucleocapsid protein is connected to a pET-28a(+) plasmid to build an expression plasmid pET-28-Cap, and a BL21(DE3) competent cell is converted to obtain a genetic engineering strain pET-28-Cap / BL21. Induction expression and detection prove that the recombinant protein has good reactionogenicity; furthermore, the protein can be used for diagnosing antigen or preparing subunit vaccines; and the product can be expressed in Escherichia coli in a soluble manner, the expression quantity is large and a complicated process of albuminous degeneration repeatability is avoided, and the loss and production cost of the protein are lowered.
Owner:SHANDONG SINDER TECH
Novel coronavirus antibody detection kit based on magnetic particle chemiluminescence
ActiveCN112229994AReduce False Positive FactorsStrong specificityChemiluminescene/bioluminescenceImmunoassaysAcridineCoronavirus antibody
The invention provides a novel coronavirus antibody detection kit based on magnetic particle chemiluminescence. The detection kit comprises streptavidin magnetic particles, a biotin-labeled novel coronavirus antigen, an acridine sulfonamide-labeled secondary antibody, a sample diluent and a quality control material, wherein the biotin-labeled novel coronavirus antigen comprises a recombinant nucleocapsid protein and a recombinant spinous process protein S1. A to-be-detected sample, the biotin-labeled antigen and the streptavidin magnetic particles are mixed, incubated and washed, then the acridine sulfonamide-labeled antibody is added, a magnetic particle-streptavidin-biotin-antigen-novel coronavirus antibody-secondary antibody compound is formed, and then the luminous intensity is detected to achieve qualitative analysis of the to-be-detected sample.
Owner:DYNAMIKER BIOTECH TIANJIN
Test strip for detecting human novel coronavirus IgG antibody, kit and preparation method of test strip
ActiveCN111537747AHigh sensitivityHigh photochemical stabilityBiological testingImmunoassaysCoronavirus antibodyCapsid
The invention provides a test strip for detecting a human novel coronavirus IgG antibody, a kit and a preparation method of the test strip. A nitrocellulose membrane of the test strip is provided witha detection line and a quality control line; the detection line is coated with novel coronavirus nucleocapsid protein and S1 protein, and the quality control line is coated with a chicken IgY antibody; the combination pad of the test strip is coated with a quantum dot labeled goat anti-human IgG antibody and a quantum dot labeled goat anti-chicken IgY antibody. According to the test strip provided by the invention, the sensitivity and specificity of human novel coronavirus IgG antibody detection can be improved, misdiagnosis caused by a false positive result is prevented, and when the test strip is used for repeatedly detecting the same sample, the obtained T / C value is relatively close; and meanwhile, the test strip is simple in use method and can realize on-site rapid detection.
Owner:BIOISLAND LAB +2
Test strip and kit for detecting human novel coronavirus IgM antibody and preparation method thereof
ActiveCN111537748AHigh sensitivityStrong specificityBiological testingImmunoassaysCoronavirus antibodyIgm antibody
The invention provides a test strip and a kit for detecting a human novel coronavirus IgM antibody and a preparation method of the test strip and the kit. A nitrocellulose membrane of the test strip is provided with a detection line and a quality control line; the detection line is coated with novel coronavirus nucleocapsid protein and S1 protein, and the quality control line is coated with a chicken IgY antibody; the combination pad of the test strip is coated with a quantum dot labeled goat anti-human IgM antibody and a quantum dot labeled goat anti-chicken IgY antibody. According to the test strip provided by the invention, novel coronavirus N protein and S1 protein are simultaneously coated on the detection line; the goat anti-human IgM antibody and the goat anti-chicken IgY antibody are labeled by using quantum dots; the human IgM antibody is a serum specific antibody firstly appearing after being infected with novel coronavirus; and the obtained test strip can be used for effectively and accurately detecting patients infected with the novel coronavirus at the early stage, and has important significance for preventing further diffusion of the virus.
Owner:BIOISLAND LAB +2
Method for determination of SARS virus nucleocapsid protein, reagent kit for the determination, test device, monoclonal antibody directed against SARS virus nucleocapsid protein, and hybridoma capable
Disclosed is a method for determination of SARS virus nucleocapsid protein (SARS-NP) using a first antibody and a second antibody both capable of binding specifically to SARS-NP, wherein the first or second antibody can recognize an epitope present in a region lying between the 283th nucleotide and the 422th nucleotide to the N-terminus in the amino acid sequence for SARS-NP (region C).
Owner:SYSMEX CORP
Porcine circovirus 2 type ELISA antibody detection kit
InactiveCN104725490AIncreased sensitivityStrong specificityVirus peptidesBiological testingSerum igePositive control
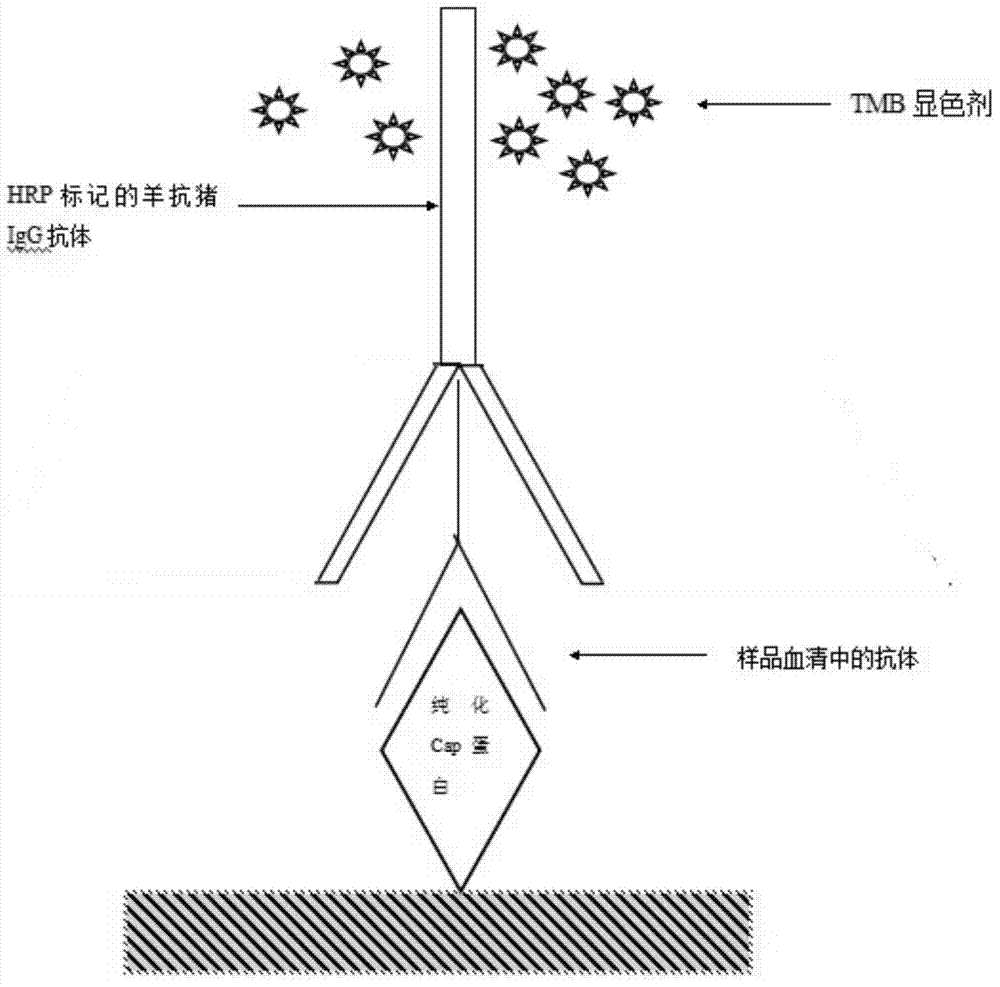
The invention discloses a preparation method of a porcine circovirus 2 type (PVC2) total-length Cap protein and an ELISA antibody detection kit taking the Cap protein as an antigen. The ELISA antibody detection kit contains an elisa plate coated with the porcine circovirus 2 type nucleocapsid protein, and also contains sample diluent, a confining liquid, a washing liquid, an enzyme conjugate, enzyme substrate solution, stop solution and negative and positive control serum. The ELISA antibody detection kit has strong specificity and high sensitivity when being used for detecting PCV2 and can be popularized and applied in a large scale.
Owner:INST OF ANIMAL SCI & VETERINARY HUBEI ACADEMY OF AGRI SCI
Synthetic peptide-based marker vaccine and diagnostic system for effective control of porcine reproductive and respiratory syndrome (PRRS)
ActiveUS20140335118A1Enhance their respective immunogenicityRisk minimizationAntibacterial agentsSsRNA viruses positive-senseHelper epitopeMonitoring and control
A peptide-based marker vaccine against Porcine Reproductive and Respiratory Syndrome (PRRS) and a set of immunodiagnostic tests for the prevention, monitoring and control of Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) are disclosed. Vaccine formulations according to various embodiments of the invention contain a mixture of peptides derived from PRRSV GP2, GP3, GP4, or GP5 proteins; each peptide individually contains a B cell PRRSV neutralizing / receptor binding epitope which is individually linked to an artificial T helper epitope for enhancement of the respective peptide's immunogenicity; and which can be supplemented with a mixture of peptides representing the T helper epitopes derived from the PRRSV GP4, GP5, M and Nucleocapsid proteins to provide cell mediated immunity. Such viral peptide compositions are prepared in an acceptable delivery system as vaccine formulations and can provide cross protection of PRRSV antibody free pigs from infection upon PRRSV challenge.
Owner:UNITED BIOMEDICAL INC
Novel coronavirus IgG and IgM antibody detection kit and preparation method and application thereof
InactiveCN111751527ARealize detectionStrong specificityBiological testingImmunoassaysNitrocelluloseIgm antibody
The invention provides a novel coronavirus IgG and IgM antibody detection kit and a preparation method and application thereof. A test strip in the kit comprises a bottom plate, and a sample pad, a colloidal gold pad, a nitrocellulose membrane and absorbent paper which are sequentially lapped and adhered on the bottom plate, the colloidal gold pad is coated with a colloidal gold labeled nucleocapsid protein, a colloidal gold labeled spinous process protein and a colloidal gold labeled rabbit IgG antibody compound; an IgG antibody detection line and an IgM antibody detection line are arranged on the nitrocellulose membrane. According to the invention, a capture method colloidal gold immunochromatography technology is adopted to realize rapid, sensitive and accurate detection of IgG antibodies and / or IgM antibodies.
Owner:DYNAMIKER BIOTECH TIANJIN
ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for porcine circovirus type 2 IgM antibody and preparation method of ELISA detection kit
InactiveCN103308688AOvercoming defects such as low sensitivityHigh sensitivityBiological testingIgm antibodyHorseradish peroxidase
The invention relates to an ELISA (Enzyme-Linked Immunosorbent Assay) detection kit for a porcine circovirus type 2 IgM antibody as well as a preparation method and application of the ELISA detection kit. The ELISA detection kit for the porcine circovirus type 2 IgM antibody comprises an ELISA plate for coating a pig IgM monoclonal antibody, a confining liquid, a sample diluent, an antigen for detection, an enzyme conjugate, a concentrated washing liquid, an enzyme substrate solution A, an enzyme substrate solution B and a stop solution, wherein the antigen for detection is a purified porcine circovirus type 2 nucleocapsid protein (PCV2-Cap protein) antigen, and the enzyme conjugate is a horse radish peroxidase-antiCap protein antibody enzyme conjugate. The kit provided by the invention can realize 100% of specificity expression, has the high sensitivity of 1:800, and can be used for the early diagnosis of PCV2 infection of a swinery.
Owner:WUHAN CHOPPER BIOLOGY
SARS coronavirus nucleocapsid protein monoclonal antibody, hybridoma for producing the same, detection agent containing the same and use thereof
InactiveCN1557838ANot reactiveEasy to detectImmunoglobulins against virusesFused cellsSARS coronavirusMonoclonal antibody
The present invention discloses the specific monocloneal antibody of SARS-CoV nuclear capsid protein, hybrid tumor producing the antibody, reagent containing the monocloneal antibody and reagent kit therewith. The monocloneal antibody is secreted and produced with the cell line including hybrid tumor 1E8A11 of preservation number of CCTCC-C200401, hybrid tumor 1E8A17 of preservation number of CCTCC-C200402, hybrid tumor 10E4A4 of preservation number of CCTCC-C200403, and hybrid tumor 14A3A3A19 of preservation number of CCTCC-C200404. The reagent kit established with the monocloneal antibody may be used in early diagnosis of SARS-CoV infection and has the features of simplicity, convenience, fastness, high sensitivity, powerful specificity, etc.
Owner:第一军医大学珠江医院
Kit for detecting porcine circovirus type 2
ActiveCN105717293AMicroorganism based processesImmunoglobulins against virusesAnimals diseasesPorcine circovirus
The invention relates to the field of detection of animal diseases, especially to monoclonal antibodies against the nucleocapsid protein of the porcine circovirus type 2 and a kit including the monoclonal antibody. The monoclonal kit comprises a monoclonal antibody secreted by a hybridoma cell line CCTCC No. C2014199 and / or a monoclonal antibody secreted by a hybridoma cell line CCTCC No. C2014198. The invention also provides the monoclonal antibody secreted by the hybridoma cell line CCTCC No. C2014199 and the monoclonal antibody secreted by the hybridoma cell line CCTCC No. C2014198, and provides the hybridoma cell line CCTCC No. C2014199 and the hybridoma cell line CCTCC No. C2014198.
Owner:LUOYANG PULIKE WANTAI BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com