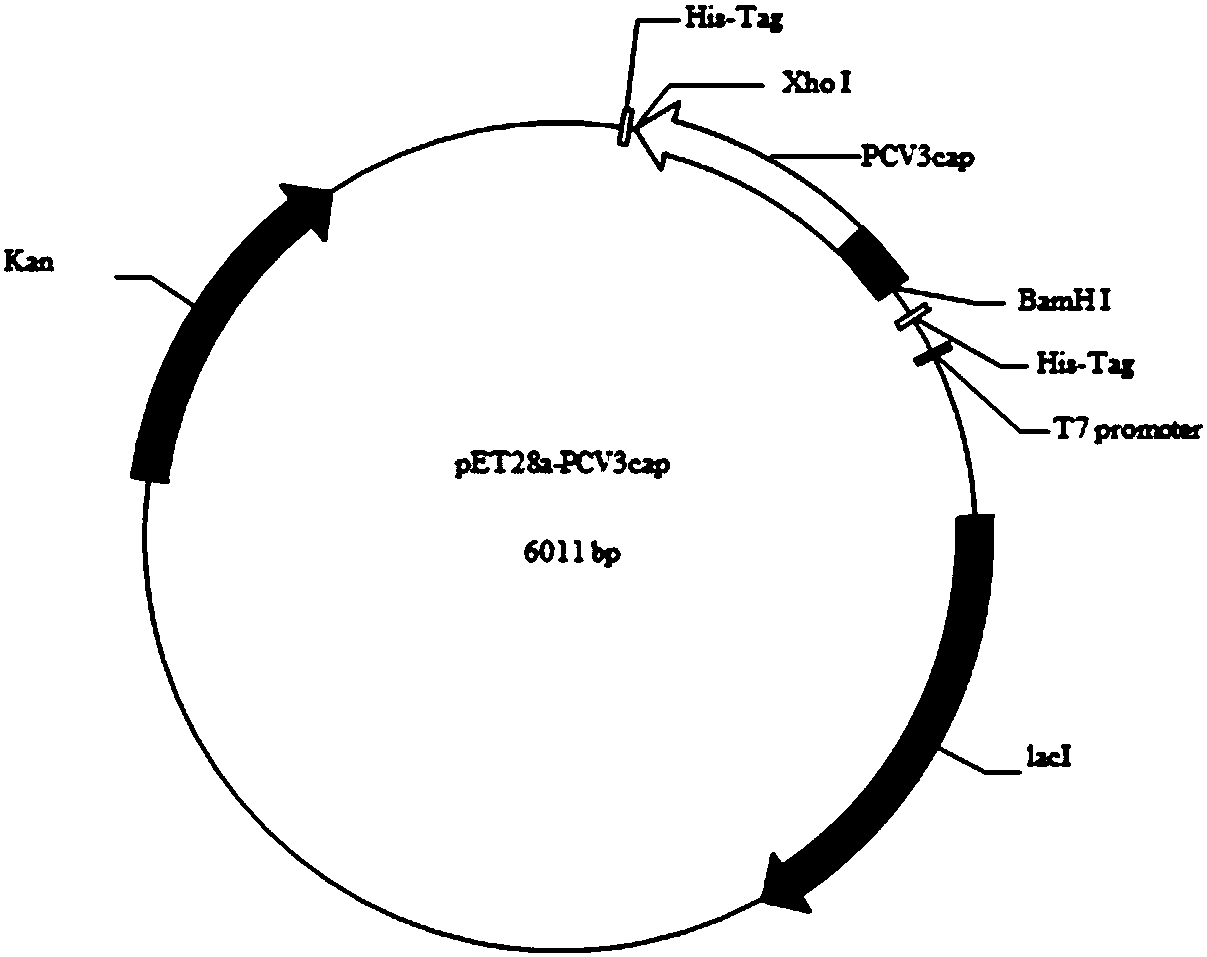
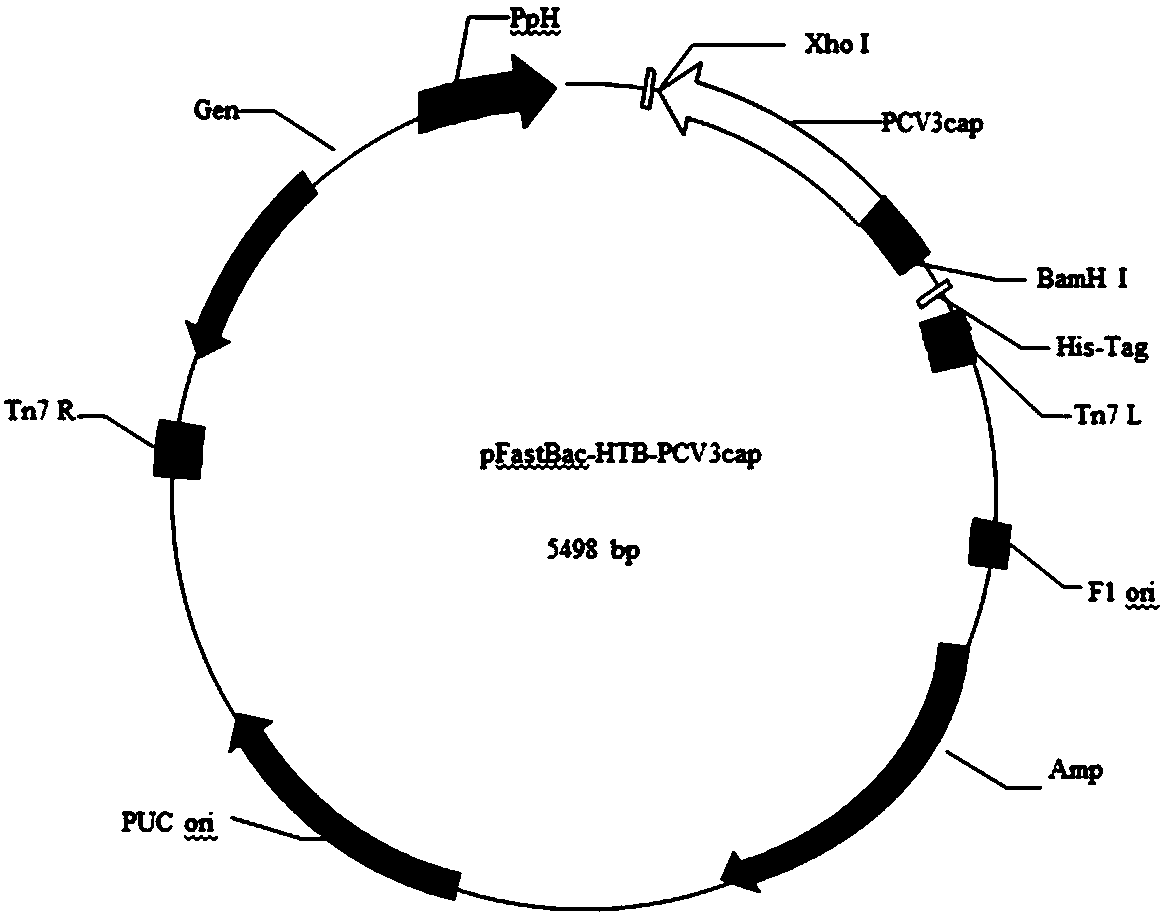
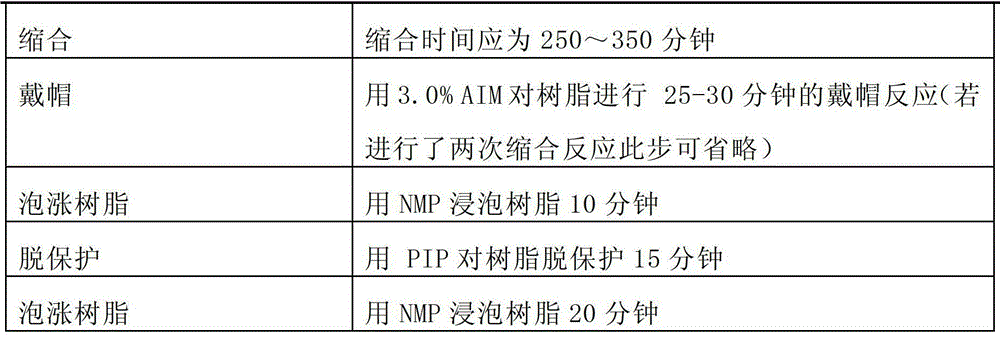
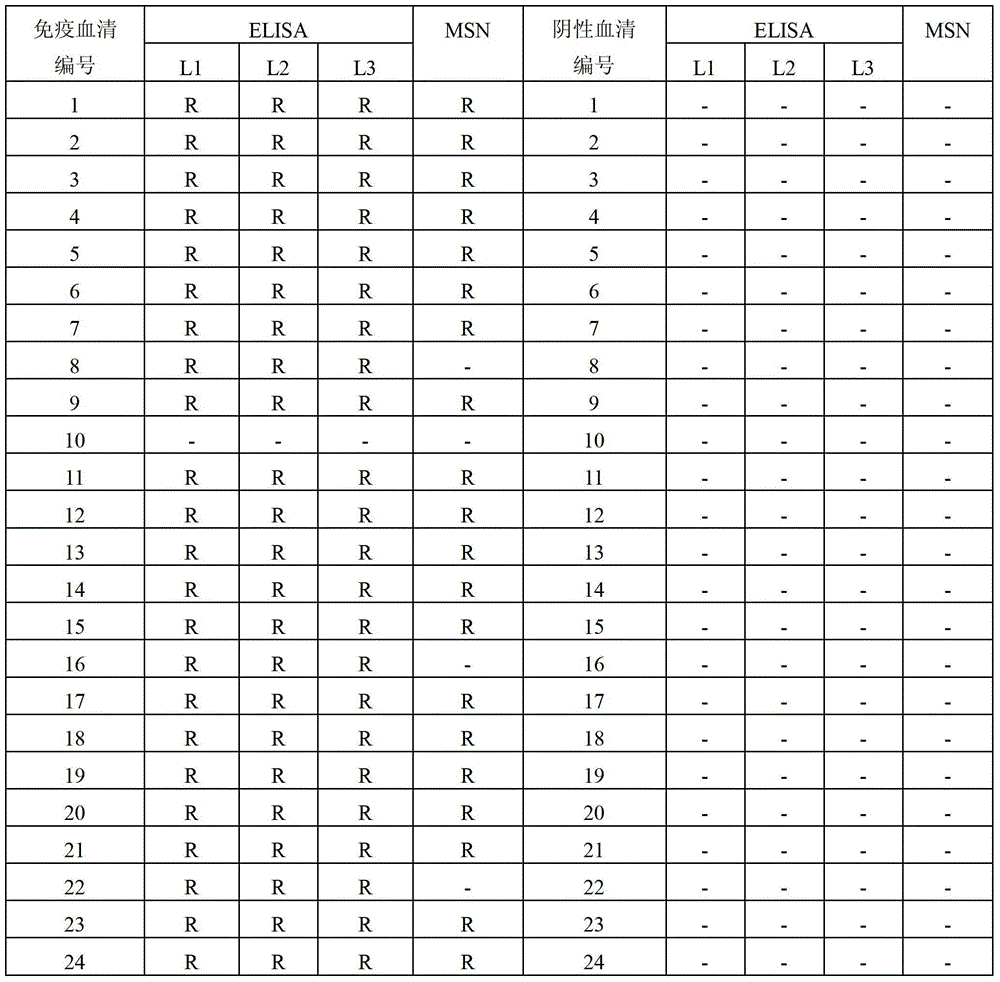
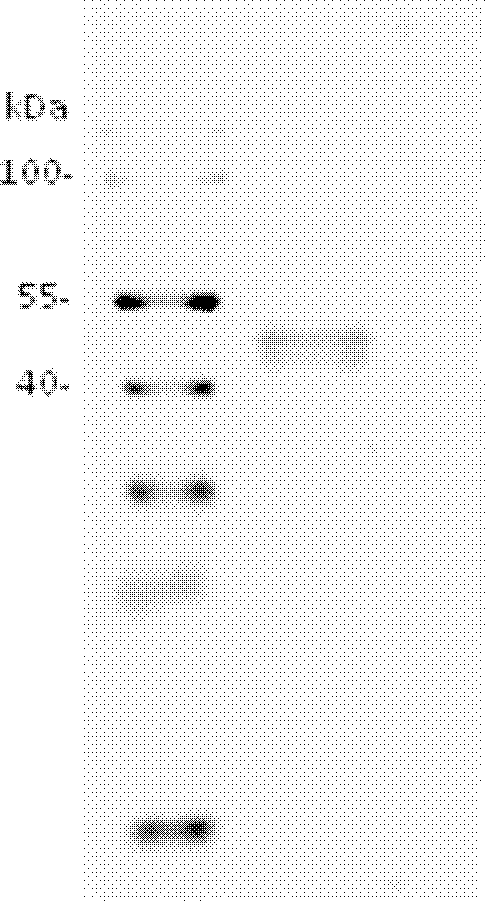Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35results about How to "High antigen purity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Genetic engineering subunit vaccine for porcine circovirus as well as preparation method and application of genetic engineering subunit vaccine
PendingCN108619503AReduce virus contentHigh antigen purityViral antigen ingredientsAntiviralsSolubilityEscherichia coli
The invention discloses a genetic engineering subunit vaccine for a porcine circovirus as well as a preparation method and application of the genetic engineering subunit vaccine. By cloning the nucleocapsid protein of the novel porcine circovirus 3 (PCV3), a PCV-Cap protein with higher purity is successfully expressed by using an escherichia coli or baculovirus expression system. The subunit vaccine for the PCV3 is successfully developed for the first time by using the PCV-Cap protein; the prepared vaccine is high in antigen purity, good in safety and strong in immunogenicity, and has no pathogenicity to pigs and other animals; the antigen has good solubility in a neutral PH buffer solution; furthermore, the preparation method is simple and low in cost, thus being suitable for large-scaleindustrial production; an effective and powerful means is provided for the prevention and control of novel PCV, and the genetic engineering subunit vaccine has a wide application prospect in the fieldof the prevention and control of the PCV3.
Owner:SOUTH CHINA AGRI UNIV
Separation and purification method for recombinant hepatitis B core antigen
ActiveCN108047316AImprove thermal stabilityAggregate compact and stableVirus peptidesPeptide preparation methodsAntigenPurification methods
The invention relates to a separation and purification method for a recombinant hepatitis B core antigen. The method includes the steps of thermal denaturation and clarification; ammonium sulfate precipitation; ultrafiltration and concentration, washing filtering and liquid exchange; depolymerization; first-step molecular sieve chromatography; ultrafiltration and concentration, washing filtering and liquid exchange; repolymerization; second-step chromatography. By means of the method, the high-purity, low-host-residue and high-stability recombinant hepatitis B core antigen with a uniform granular structure can be obtained, that is to say, the method has the advantages that the antigen purity is high, the residue of host protein and host nucleic acid is low, antigen particles are uniform, and industrial enlargement is easy.
Owner:JIANGSU THERAVAC BIO PHARMA
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249AStrong specificityReduce false positive ratePeptidesGenetic engineeringMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Galactomannan antigen and preparation method thereof
InactiveCN104945527AHigh antigen purityEliminate distractionsAspergillus fumigatus AntibodyIon exchange
The invention relates to a preparation method of a galactomannan antigen. The method comprises the steps that breaking, centrifugation, alcohol precipitation, washing and drying are carried out on a living body rich in galactomannan, and galactomannan crude extract is obtained through separation; hydrazinolysis and hydrolysis are carried out on the obtained galactomannan crude extract in sequence; an enzyme is added to a hydrolysis product for enzymolysis, and the enzyme and small molecular substances in an enzymolysis product are removed; a product is purified through an ion exchange column, an affinity column and gel chromatography, and the pure galactomannan antigen is obtained; the obtained pure galactomannan antigen is identified. The galactomannan antigen obtained through the preparation method is high in purity, the inference of galactosamine, protein, miscellaneous sugar possibly included in the galactomannan crude extract is eliminated, and the preparation method can be used for preparing aspergillus fumigatus antibodies.
Owner:DYNAMIKER BIOTECH TIANJIN
Foot and mouth disease virus-like particle vaccine and preparation method thereof
InactiveCN105126096AEasy to manufactureEasy to purifyAntiviralsGranular deliveryVaccine ProductionNeutralizing antibody
The invention discloses a foot and mouth disease virus-like particle vaccine and a preparation method thereof. By expressing P12A (three amino acid site mutation, VP1K210E, VP1E83K and VP1C134S) and serum-free suspension culture, a virus-like particle (VLP) standard similar to natural 75S FMDV is built, and VLP yield is increased. The invention further provides serum-free suspension cultured and high pH tamed insect cells, the insect cells can stably and efficiently produce FMDV VLP, the size and structure of the VLP produced by the insect cells is similar to those of the VLP produced by standard BHK-21, and the yield of the VLP produced by the insect cells is 11 times of that of the VLP produced by female parent cells Sf21. The vaccine prepared by the VLP produced by the insect cells can immunize animals to generate IgG antibody response. The IgG antibody titer, foot and mouth disease virus neutralizing antibody titer and antibody titer of immunization of the VLP vaccine produced by the insect cells are not evidently different from those of immunization of the VLP vaccine prepared by the BHK-21, so that the high-pH-adaptive insect cells are applicable to FMDV VLP vaccine production.
Owner:吕宏亮 +1
Method for detecting human tumor antigen P 185 Her-2 and its application in diagnosing tumor
InactiveCN1397803AStrong specificityImprove purification efficiencyPeptide preparation methodsRecovery/purificationNeoplasm diagnosisHuman tumor
A dual-antibody sandwith ELISA detecting method for human soluble p185HER-2 antigen features that the purified and coated P185HER-2 monoclonal antibody is bound to solid supporter, the specimen containing soluble p185HER-2, the purified standard p185HER-2 antigen and the coated antibody are incubated, and another purified and coated p185HER-2 monoclonal antibody marked by horseradish perioxidase is used as detecting antibody. It can be used for serum diagnosis of tumor.
Owner:UNIV OF SCI & TECH OF CHINA
Porcine circovirus type II gene engineering subunit vaccine, and preparation method and application thereof
ActiveCN103204942AAntigen highHigh purityBacteriaViral antigen ingredientsSite-directed mutagenesisImmunogenicity
The invention discloses a porcine circovirus type II gene engineering subunit vaccine, and a preparation method and application thereof. The porcine circovirus type II vaccine comprises a soluble fusion protein composed of a protein obtained by expressing a porcine circovirus type II capsid protein gene by Escherichia coli; and the porcine circovirus type II capsid protein gene is subjected to positioning signal zone shear and site-directed mutagenesis, and the site-directed mutagenesis comprises mutation of codon AGA or AGG to CGC. A porcine circovirus type II vaccine prepared by the technical scheme provided by the invention has high antigen purity, good safety, strong immunogenicity, and no pathogenicity to animals like pig. Furthermore, the vaccine antigen in the invention is expressed by Escherichia coli, so a preparation process is relatively simple and low-cost.
Owner:YEBIO BIOENG OF QINGDAO
Preparation method and application of African swine fever virus P11.5 protein specific polyclonal antibody
ActiveCN109836478AStrong specificityHigh affinitySerum immunoglobulinsVirus peptidesEpitopeAfrican swine fever
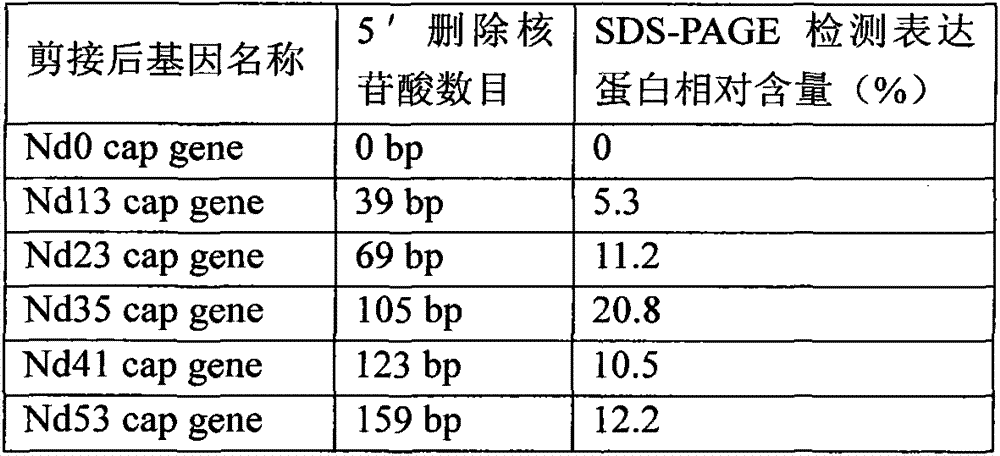
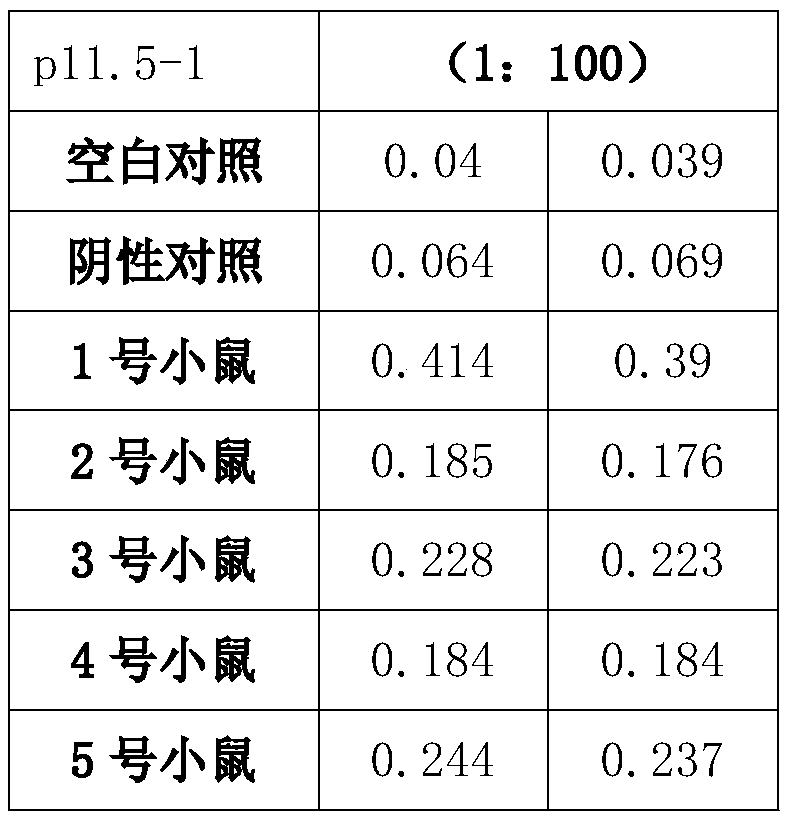
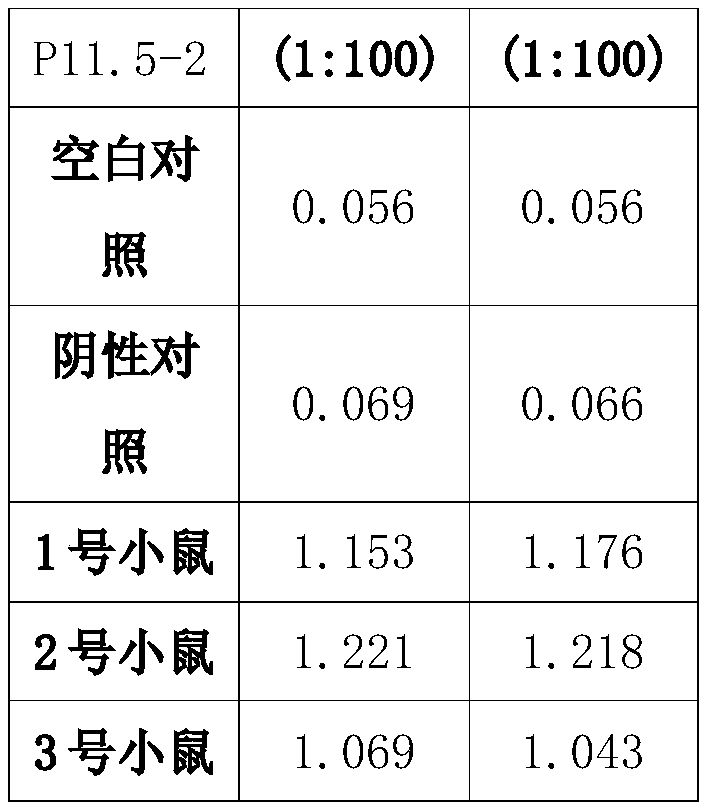
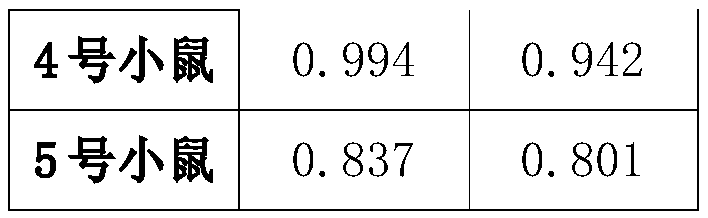
The present invention discloses a preparation method and an application of an African swine fever virus P11.5 protein specific polyclonal antibody. The method uses P11.5 polypeptide epitope immunological experimental animals of mice, rabbits, etc. to prepare the specific polyclonal antibody. The method has high antigen purity, the prepared antibody is strong in specificity and high in affinity, used antigen is small in amount, and operations are simple and convenient. The preparation method of the polyclonal antibody comprises steps of ASFV P11.5 specific antigen epitope selection, polypeptideantigen synthesis, animal immunity, antibody determination, etc. Compared with conventional methods, the preparation method is simple and rapid, and high in antigen purity, the prepared antibody is strong in specificity, and the used antigen is small in amount. The polyclonal antibody can be used for detection of African swine fever virus P11.5 protein and provides an important technical method for prevention and control of African swine fever in China.
Owner:YANGZHOU UNIV
Aviadenovirus Hexon and chicken infectious bursal disease virus VP2 fusion antigen and subunit vaccine, and preparation method of aviadenovirus Hexon and chicken infectious bursal disease virus VP2 fusion antigen and subunit vaccine
ActiveCN107488234ALow costHigh antigen purityViral antigen ingredientsAntibody mimetics/scaffoldsAntigenSubunit vaccines
The invention discloses an aviadenovirus Hexon and infectious bursal disease virus VP2 fusion antigen and subunit vaccine, and a preparation method and application of the aviadenovirus Hexon and chicken infectious bursal disease virus VP2 fusion antigen and subunit vaccine. The aviadenovirus Hexon and chicken infectious bursal disease virus VP2 fusion antigen comprises an aviadenovirus Hexon protein and an chicken infectious bursal disease virus VP2 protein. The fusion antigen has the following amino acid sequence: (1) a protein formed by an amino acid sequence as shown in SEQ ID No.1; or (2) an amino acid sequence of a functional protein with the same code and homology being 95 percent to 100 percent as the protein amino acid sequence limited by the sequence SEQ ID No.1. A protective antigen of the subunit vaccine prepared through the invention is the fusion antigen, and the fusion antigen is prepared by adopting a gene engineering fermentation method, and has the advantages of low cost, high antigen purity, good immunogenicity and high safety.
Owner:RINGPU (BAODING) BIOLOGICAL PHARMACEUTICAL CO LTD
Porcine circovirus II antibody detection ELISA kit
InactiveCN105004856AIncreased sensitivityStrong specificityMaterial analysisEscherichia coliElisa kit
The invention provides a porcine circovirus II antibody detection ELISA kit. The kit comprises an ELISA plate coated with a purified escherichia coli-expressed porcine circovirus II Cap protein as an antigen, positive contrast serum, negative contrast serum, a sample diluent, an enzyme labeling conjugate, a washing solution, a substrate solution A, a substrate solution B and a stopping solution. An indirect ELISA method is adopted, the coated plate is coated with the prokaryotic expressed Cap protein as an antigen and antigen purity is high. The ELISA detection kit prepared from the coated plate with the antigen has high sensitivity, specificity and repeatability.
Owner:YEBIO BIOENG OF QINGDAO
Fused gene, recombinant expression vector, antigen, preparation method and application
ActiveCN110128545AFree from infectionReduce repetitive workSsRNA viruses negative-senseAntibody mimetics/scaffoldsAgricultural scienceAvian adenovirus
The invention belongs to the technical field of veterinary biological products, and discloses a fused gene. A base sequence is as shown in a sequence table SEQ ID NO: 1. An avian influenza H9 subtypeHA gene, an adenovirus type 4 fiber2 gene and a specific connecting peptide segment gene are fused to form the fused gene. An expression protein antigen corresponding to the fused gene can induce poultry to generate high-level specific antibodies, and the poultry is protected from infection of avian influenza and avian adenoviruses. Besides, the invention further discloses a recombinant expressionvector, an antigen, a preparation method and an application.
Owner:ZHAOQING DAHUANONG BIOLOGIC PHARMA +1
Preparation method of rabies inactivation vaccine for livestock and stabilizing agent contained in vaccine
ActiveCN110339351AEfficient removalAvoid it happening againSsRNA viruses negative-senseViral antigen ingredientsMembrane penetrationAntigen
The invention provides a preparation method of a rabies inactivation vaccine for livestock and a stabilizing agent contained in the vaccine. According to the preparation method, a virus concentrationprocess is that (1) a virus supernate continuously penetrates through a 300 KD membrane bag at the flow speed of 0.8-1 L / min, the virus is concentrated until the volume of the virus is 1 / 50-1 / 40 of the original volume, and coarse concentration liquid is obtained; (2) a PBS solution with the volume 10-15 times larger than that of the coarse concentration liquid is added into the coarse concentration liquid, the mixture penetrates through the 300 KD membrane bag at the flow speed of 1.8-2 L / min under the concentration pressure of 0.5-1.0 bar, under the condition that the mixture is concentrateduntil the volume of the mixture is 1 / 4-2 / 5 of the original volume of the mixture before penetration, the flow speed is adjusted to 0.8-1 L / min, and continuous concentration is conducted until the volume of the mixture is the same as the original volume of the coarse concentration liquid. According to the preparation method, by optimizing the virus concentration process, different membrane-penetration flow speeds are adopted for different antigen concentrations, in the concentration process, 30-50% of impure protein can be removed, the virus titer is kept at 90% or above, and the prepared vaccine is more stable and more efficient.
Owner:WUHAN KEQIAN BIOLOGY CO LTD
Method for preparing pig porcine reproductive and respiratory syndrome inactivated vaccine
InactiveCN106729687AHigh antigen contentImproving immunogenicityViral antigen ingredientsAntiviralsMolecular sieveAdjuvant
The invention provides a method for preparing a pig porcine reproductive and respiratory syndrome inactivated vaccine. Through steps of virus clarification, concentration, molecular sieve chromatography, inactivation, freeze-drying and the like, the method is capable of effectively ensuring that the content of impurities in antigen for preparing the vaccine is reduced to the minimum extent, so that the phenomenon that side effects are caused after the product is used can be avoided, and meanwhile the inactivation effect of the vaccine can be ensured. The PRRSV inactivated vaccine prepared by using the method is high in antigen content, high in immunogenicity, high in antigen purity, good in security, small in side effect, free of adjuvant, rapid in antibody protection generation, easy to inject and easy to absorb.
Owner:鼎正生物药业(天津)有限公司
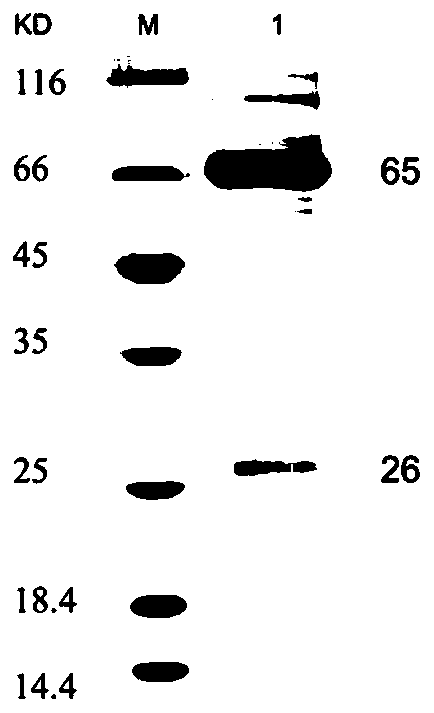
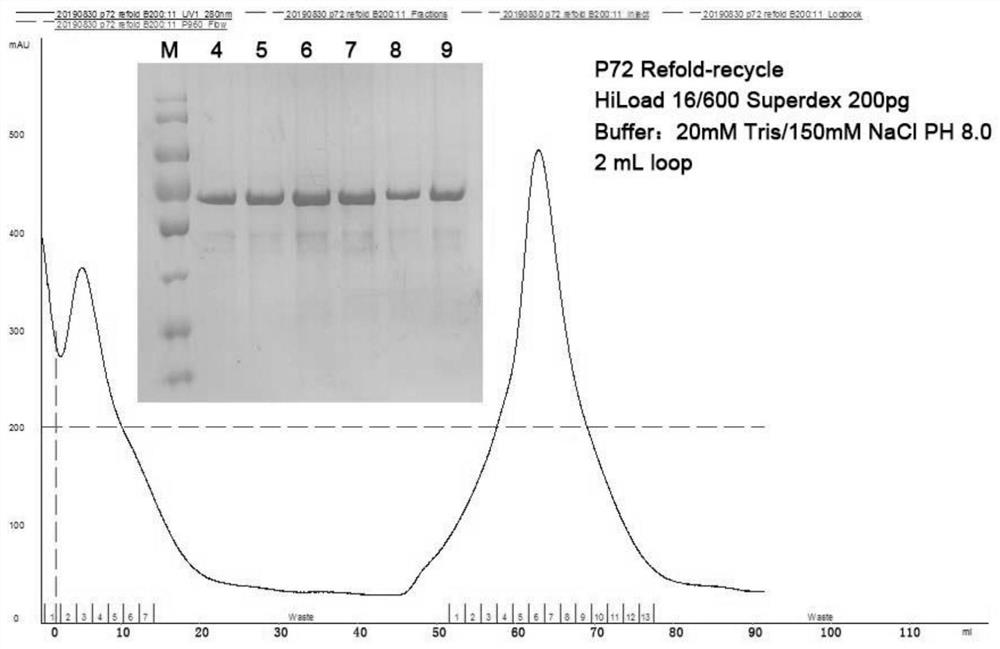
Double-antibody sandwich ELISA based on African swine fever virus p72 gene and application of double-antibody sandwich ELISA
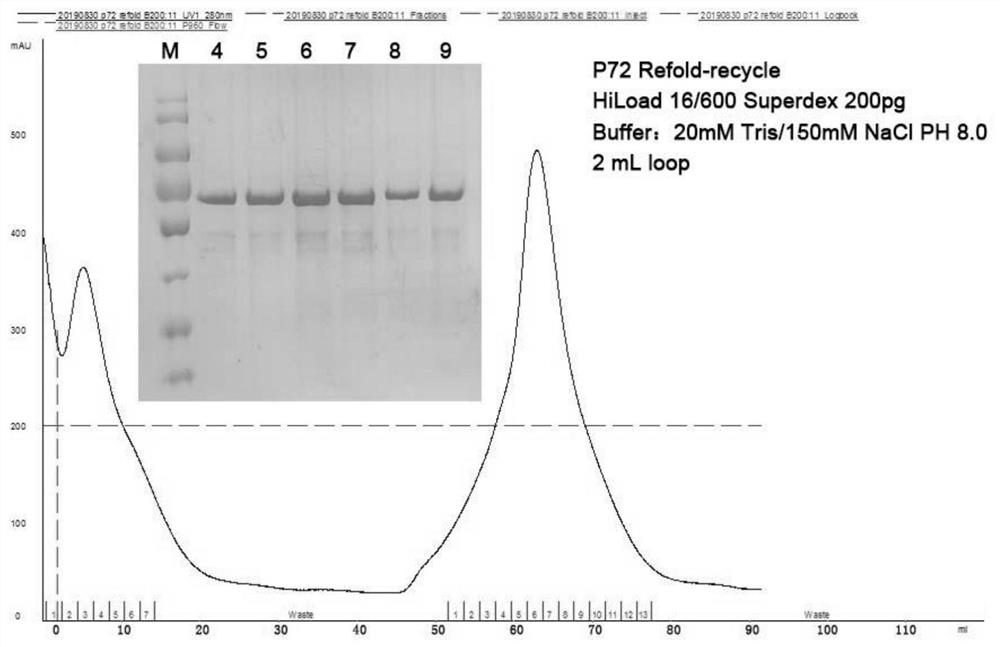
ActiveCN113150124AHigh antigen purityImproving immunogenicityImmunoglobulins against virusesBiological testingClassical swine fever virus CSFVConjugated protein
The invention discloses an antigen conjugated protein specifically conjugated with an African swine fever virus p72 protein, an antibody or active fragment and a double-antibody sandwich ELISA antigen detection kit for the African swine fever virus p72 protein containing the antigen conjugated protein, the antibody or the active fragment and application of the double-antibody sandwich ELISA antigen detection kit. The kit disclosed by the invention is coated with an ELISA plate, positive control and negative control of a monoclonal antibody ASFV-p72-5G8, an HRP-labeled detection antibody ASFV-p72-12F6, a sample diluent, color developing liquid and washing liquid. In which, the antibody ASFV-p72-5G8 and the antibody ASFV-p72-12F6 are secreted from hybridoma cell strains ASFV-p72-5G8 and ASFV-p72-12F6 separately. According to the kit disclosed by the invention, a double-antibody sandwich ELISA method is established by taking 5G8 as a capturing antibody and taking 12F6-coupled HRP as a detection antibody, can be used for specifically detecting ASFV, is simple and convenient in operation, high in sensitivity and good in specificity and provides a novel detecting means for clinical diagnosis and epidemiological survey on ASFV infection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Goat contagious pleuropneumonia subunit vaccine as well as preparation method and application thereof
ActiveCN112870341AReduce spreadReduce the risk of contaminationAntibacterial agentsBacterial antigen ingredientsImmune effectsAdjuvant
Owner:LANZHOU INST OF VETERINARY SCI CHINESE ACAD OF AGRI SCI
Preparation and application of virus-like particle based on African swine fever virus P30 and P72 proteins
PendingCN113755513AGood immune protectionImprove securityViral antigen ingredientsAntibody mimetics/scaffoldsEscherichia coliGenetic engineering
The invention discloses preparation and application of a recombinant virus-like particle for surface display of African swine fever virus P30 and P72 proteins, a SpyTag tag protein is displayed on the surface of T7 bacteriophage through a T7 bacteriophage display technology, and therefore, engineered bacteriophage (T7-ST) is constructed. Meanwhile, through an escherichia coli prokaryotic expression system, a P30-Spycatcher fusion protein and a P72-Spycatcher fusion protein can be expressed and purified. In a proper buffer system, the T7-ST, the P30-SC fusion protein and the P72-SC fusion protein are mixed and incubated respectively, and finally, the recombinant VLP particle which carries out the surface play of the P30-SC protein and the P72-SC protein is obtained. The novel genetic engineering vaccine prepared by VLP has the characteristics of an outstanding immune protection effect, good safety, high antigen purity and the like, and multiple protection can be provided for the organism.
Owner:TIANJIN UNIV
Reagent and method for in-vitro diagnosis of hepatitis B virus antibody
ActiveCN113358878AHigh antigen purityStrong specificityChemiluminescene/bioluminescenceBiological testingAntigenHorse radish peroxidase
The invention discloses a reagent for hepatitis B virus antibody in-vitro diagnosis, the reagent comprises a solid phase antigen, an enzyme-labeled antigen and a chromogenic substrate, the solid phase antigen comprises a solid phase carrier and an antigen coated on the surface of the solid phase carrier, the enzyme-labeled antigen comprises an enzyme for labeling the antigen and an enzyme-labeled antigen, the antigen is hepatitis B antigen recombinant protein, the antigens in the solid-phase antigen and the enzyme-labeled antigen are the same hepatitis B antigen recombinant protein, the solid-phase carrier comprises magnetic microspheres, the enzyme comprises horse radish peroxidase, and the chromogenic substrate comprises TMB. The invention also discloses a method for preparing the reagent and a method for identifying the hepatitis B virus antibody by using the reagent. The reagent is used for detecting the hepatitis B virus antibody, has the advantages of good specificity, high sensitivity, accurate result, good repeatability and strong clinical applicability, and can accurately reflect the content index of the hepatitis B virus antibody in a human body.
Owner:深圳市辰纳生物科技有限公司
J-subgroup avian leukosis virus (ALV) antibody quick detection test strip and preparation method and application thereof
The invention relates to a J-subgroup avian leukosis virus (ALV) antibody quick detection test strip and a preparation method and application thereof. The ALV-J envelope protein gp85 specific epitopeserves as an antigen to detect an ALV-J specific antibody in serum; the specific polypeptide epitope serves as an antigen to be spray-painted on an NC film, and a detection line of the test strip is made; and a monoclonal mouse anti-chicken IgG antibody is selected as a gold-labelled antibody, and a prepared colloidal gold-antibody coupled composite is sprayed on a gold-labelled pad of the test strip. The antigen in the serum of ALV-J artificially infected chickens can be detected through the test strip; and the test strip can be used for evaluating whether ALV-J infection exists in a chickengroup or not, and is easy and convenient to prepare, low in cost, wide in application and high in economic benefit.
Owner:YANGZHOU UNIV
A kind of eukaryotic expressed African swine fever virus p72 antigen and application thereof
ActiveCN113150079BHigh antigen purityImproving immunogenicityVirus peptidesImmunoglobulins against virusesAntigenAfrican swine fever virus Antigen
The invention discloses a eukaryotic expressed African swine fever virus p72 antigen and application thereof. The invention also discloses a detection kit comprising the p72 protein. The kit of the invention can specifically detect ASFV, has high sensitivity and good specificity, and provides a new detection method for clinical diagnosis and epidemiological investigation of ASFV infection.
Owner:CHINESE ACAD OF INSPECTION & QUARANTINE
Porcine circovirus type Ⅱ genetic engineering subunit vaccine and its preparation method and application
ActiveCN103204942BHigh antigen purityImprove securityBacteriaViral antigen ingredientsEscherichia coliVaccine antigen
The invention discloses a porcine circovirus type II gene engineering subunit vaccine, and a preparation method and application thereof. The porcine circovirus type II vaccine comprises a soluble fusion protein composed of a protein obtained by expressing a porcine circovirus type II capsid protein gene by Escherichia coli; and the porcine circovirus type II capsid protein gene is subjected to positioning signal zone shear and site-directed mutagenesis, and the site-directed mutagenesis comprises mutation of codon AGA or AGG to CGC. A porcine circovirus type II vaccine prepared by the technical scheme provided by the invention has high antigen purity, good safety, strong immunogenicity, and no pathogenicity to animals like pig. Furthermore, the vaccine antigen in the invention is expressed by Escherichia coli, so a preparation process is relatively simple and low-cost.
Owner:YEBIO BIOENG OF QINGDAO
Separation and purification method of recombinant hepatitis B core antigen
ActiveCN108047316BImprove thermal stabilityAggregate compact and stableVirus peptidesPeptide preparation methodsAntigenMolecular sieve
The invention relates to a separation and purification method of recombinant hepatitis B core antigen, which comprises heat denaturation and clarification, ammonium sulfate precipitation, ultrafiltration and concentration, diafiltration and liquid replacement, depolymerization, the first step of molecular sieve chromatography, ultrafiltration and concentration, diafiltration And the steps of changing liquid, repolymerization and second step molecular sieve chromatography. The method can obtain recombinant hepatitis B core antigen with high purity, low host residue, uniform particle structure, and high stability, that is, the method has the characteristics of high antigen purity, low host protein and host nucleic acid residue, uniform antigen particles, and easy industrial scale-up.
Owner:JIANGSU THERAVAC BIO PHARMA CO LTD
Porcine reproductive and respiratory syndrome virus ultrafiltration purification system
PendingCN114618304AHigh antigen purityReduce stress responseMembranesSsRNA viruses positive-senseUltrafiltrationAntigen
The invention relates to the technical field of virus reproduction, in particular to a porcine reproductive and respiratory syndrome virus ultrafiltration purification system which comprises a first concentration tank, a second concentration tank and a third concentration tank which are sequentially connected through pipelines. A first ultrafiltration membrane bag is arranged between the first concentration tank and the second concentration tank, a second ultrafiltration membrane bag is arranged between the second concentration tank and the third concentration tank, a first ultrafiltration membrane and a second ultrafiltration membrane are installed in the first ultrafiltration membrane bag and the second ultrafiltration membrane bag respectively, the aperture of the first ultrafiltration membrane is larger than the molecular weight of target viruses, and the aperture of the second ultrafiltration membrane is larger than the molecular weight of the target viruses. The aperture of the second ultrafiltration membrane is smaller than the molecular weight of a target virus. According to the porcine reproductive and respiratory syndrome virus ultrafiltration purification system disclosed by the invention, by arranging a three-stage filtration purification system, invalid proteins in vaccines can be removed by 95% or above, the antigen purity can be effectively improved, and the stress reaction of animals to the vaccines can be reduced.
Owner:浙江美保龙生物技术有限公司
A kind of porcine circovirus type ii genetic engineering subunit vaccine and its application
ActiveCN106478783BImprove securityNot pathogenicBacteriaViral antigen ingredientsEscherichia coliEngineered genetic
The invention discloses a porcine circovirus type 2 recombinant CAP protein and a subunit vaccine of the recombinant CAP protein. The invention provides a recombinant CAP protein of porcine circovirus type 2. The C-terminus of the Cap protein is fused with 8 histidine tags, and its amino acid sequence is shown in SEQ ID No.1. Compared with the inactivated vaccine of PCV2 currently used, the porcine circovirus type 2 Cap protein subunit vaccine expressed by recombinant Escherichia coli of the present invention has a relatively simple preparation process and lower cost, so it has a wider scope in the prevention and treatment of PCV2. application prospects.
Owner:兆丰华生物科技(南京)有限公司北京生物医药科技中心 +3
An Inactivated Vaccine Produced Using Attenuated Poliomyelitis Strain
ActiveCN105396129BEfficient separationImproving immunogenicityViral antigen ingredientsAntiviralsGradient centrifugationPoliomyelitis virus
The invention provides inactivated vaccine produced through poliomyelitis attenuated strains and belongs to the field of preparation of biological products. According to the poliomyelitis inactivated vaccine, a traditional method that the inactivated vaccine is prepared from wild strains is changed, and the attenuated strains Sabin are adopted for producing the poliomyelitis inactivated vaccine. Due to the fact that I type virus liquid, II type virus liquid and III type virus liquid of the poliomyelitis strains Sabin are ultra-filtered, concentrated and subjected to sucrose density gradient centrifugation for ultra-filtration sucrose removing, sterilization and filtration, purified poliomyelitis virus liquid is obtained, solid virus particles and hollow virus particles in the poliomyelitis virus liquid can be effectively separated, impurity protein can be effectively removed, the content of the solid virus particles in centrifugal products is greatly increased, and the poliomyelitis inactivated vaccine which is good in immunogenicity, low in impurity content and high in safety and stability is prepared from the virus liquid.
Owner:SINOVAC BIOTECH
A sheep echinococcosis antibody ELISA detection kit and its preparation method and application
ActiveCN111896744BPreserve immunogenicityAvoid interferenceBiological material analysisBiological testingPeptide antigenEscherichia coli
The invention discloses an ELISA detection kit for sheep echinococcosis antibody and its preparation method and application, belonging to the technical field of biological detection. Sheep Echinococcus Antibody ELISA Kit includes EG95 Polypeptide Antigen Coated Microtiter Plate, Diluent, Washing Solution, Blocking Solution, Positive Control Solution, Negative Control Solution, Enzyme Conjugate, Substrate Chromogenic Solution, Stop Solution and Serum Dilution plate. The sheep echinococcosis antibody ELISA detection kit of the present invention adopts the Echinococcus granulosus EG95 protein B cell epitope-specific polypeptide antigen as the coating antigen, and has a simple and convenient production method and high antigen purity. It avoids the complex purification steps and impurity protein interference produced by E. coli fermentation, and is suitable for the detection of a large number of serum samples. It has the advantages of economy and convenience in animal disease detection and epidemiological investigation, and the inspection cost is low, which is conducive to popularization and application. It has broad application prospects in the detection of sheep hydatid (hydatid) EG95 protein antibody.
Owner:CHONGQING AULEON BIOLOGICALS
Application of gram-positive bacterium expression system in expression of clostridium septium toxin, preparation method of clostridium septium alpha toxin and vaccine
ActiveCN111944838AAchieve secretory expressionHigh purityAntibacterial agentsBacterial antigen ingredientsClostridial toxinAlpha-toxin
The invention provides application of a gram-positive bacterium expression system in expression of clostridium septium toxin, a preparation method of clostridium septium alpha toxin and a vaccine, andrelates to the technical field of biology. Extracellular secretion expression of the clostridium septium toxin is realized by expressing the clostridium septium toxin by adopting a gram-positive bacterium expression system, and supernate is collected centrifugally, so that a target protein with high expression quantity and relatively good purity can be obtained, and the production cost of a target protein purification and endotoxin removal process is reduced.
Owner:天康制药股份有限公司
Specific antigen polypeptide for reticuloendotheliosis virus of poultry and application
InactiveCN109627296AEasy to detectEasy to manufactureVirus peptidesImmunoglobulins against virusesEpitopeAntibody reactivity
The invention relates to a specific antigen polypeptide for a reticuloendotheliosis virus of poultry and application of the polypeptide. The amino acid sequence of the specific antigen polypeptide isSEQ ID No.5; the specific antigen polypeptide is coded by envelope protein gp90 gene of the virus, and has strong reactogenicity, the epitope of the antigen has good antibody reactivity, and can be used for specifically detecting the antibody of the REV, and the specific antigen polypeptide has the application value of clinical detection. The antibody for specifically resisting the REV can be prepared by utilizing a polypeptide antigen immune animal.
Owner:YANGZHOU UNIV
Specific B cell epitope polypeptide of NS1 protein of encephalitis B virus and use thereof
ActiveCN102206249BStrong specificityReduce false positive ratePeptidesFermentationMolecular ImmunologyScreening method
The invention discloses the sequence of a specific B cell epitope polypeptide of an NS1 protein of encephalitis B virus and the use of the epitope polypeptide in the diagnosis of encephalitis B virus and a screening method of specific B cell epitope polypeptide of NS1 protein of encephalitis B virus, and belongs to the field of molecular immunology. The amino sequence of the epitope polypeptide is represented by SEQ ID No.1 or SEQ ID No.2. The specific B cell epitope synthetic polypeptide of the JEV NS1 protein, the coupling antigen of the synthetic epitope polypeptide and the epitope fusion expression protein can be used for specifically detecting a JEV NS1 protein antibody generated in body which is immunized and infected. The epitope polypeptide can be used for diagnosis of JEV infection, evaluation on immunity effect and identification and diagnosis of immunity of inactivated vaccine and natural infection.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Method for detecting human tumor antigen P185 Her-2 and its application in diagnosing tumor
InactiveCN1232636CStrong specificityImprove purification efficiencyPeptide preparation methodsRecovery/purificationAbnormal tissue growthHuman tumor
Soluble p185 of the present invention HER-2 The double-antibody sandwich ELISA detection method for antigen is characterized in that the purified p185 prepared by surface epitope embedding method HER-2 The monoclonal antibody is used as the coating antibody to bind to the solid phase support, respectively will contain soluble p185 HER-2 Samples and purified p185 of known concentration HER-2 Standard antigens were incubated with coated antibodies, followed by another purified, horseradish peroxidase-tagged p185 prepared by surface epitope embedding HER-2 Monoclonal antibody is used as a sandwich ELISA detection method for detecting antibodies. The purified p185 HER-2 The standard antigen was anti-p185 prepared by surface epitope embedding method HER-2 Affinity bead pairs covalently crosslinked with Sepharose 4B-ProteinG monoclonal antibodies to the extracellular region containing p185 HER-2 Antigen samples are obtained by affinity chromatography; the detection method of the present invention has the ability to detect soluble p185 HER-2 It has the advantages of strong specificity of antigen and high purity of standard soluble antigen; it can be used for tumor serum diagnosis.
Owner:UNIV OF SCI & TECH OF CHINA
Foot and mouth disease virus structural protein antibody enzyme-linked immunosorbent assay kit
ActiveCN103408641BStrong specificityHigh sensitivityVirus peptidesBiological material analysisEpitopeStructural protein
The invention discloses a foot and mouth disease virus structural protein antibody enzyme-linked immunosorbent assay kit. The kit comprises an enzyme-linked reaction plate coated by foot and mouth disease virus structural protein VP1 antigenic epitope polypeptides, and an enzyme-labeled antibody. The foot and mouth disease virus structural protein VP1 antigenic epitope polypeptides are a polypeptide represented by the sequence 1 in the sequence list and a polypeptide represented by the sequence 2 in the sequence list. According to the kit, the reaction plate coated by chemically synthesized VP1 antigenic peptide, antigen dose is low, sensitivity is high, and specificity is high. With the kit, whether foot and mouth disease virus infection exists can be highly efficiently detected. The kit provided by the invention has good specificity, high sensitivity, high efficiency, and good market prospect.
Owner:CHINA ANIMAL HUSBANDRY IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com