Immune-enhanced hepatitis B therapeutic multivalent vaccine and preparation method thereof
An immune-enhancing, multivalent vaccine technology, applied in the field of multivalent vaccines, can solve the problems of weak immunogenicity, short half-life, and difficult APC uptake, and achieve the effects of inhibiting replication, increasing activity, and improving development and application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] Hereinafter, preferred embodiments of the present invention will be described in detail with reference to the accompanying drawings. The experimental method that does not indicate specific conditions in the preferred embodiment is usually according to conventional conditions, such as described in the Molecular Cloning Experiment Guide (Third Edition, J. Sambrook et al., translated by Huang Peitang, etc., Science Press, 2002) conditions, or as recommended by the manufacturer.
[0030] 1. Preparation of immunoenhanced hepatitis B therapeutic multivalent vaccine
[0031] 1. Design and synthesis of HBcAg epitope peptide, HBsAg epitope peptide and HBeAg epitope peptide
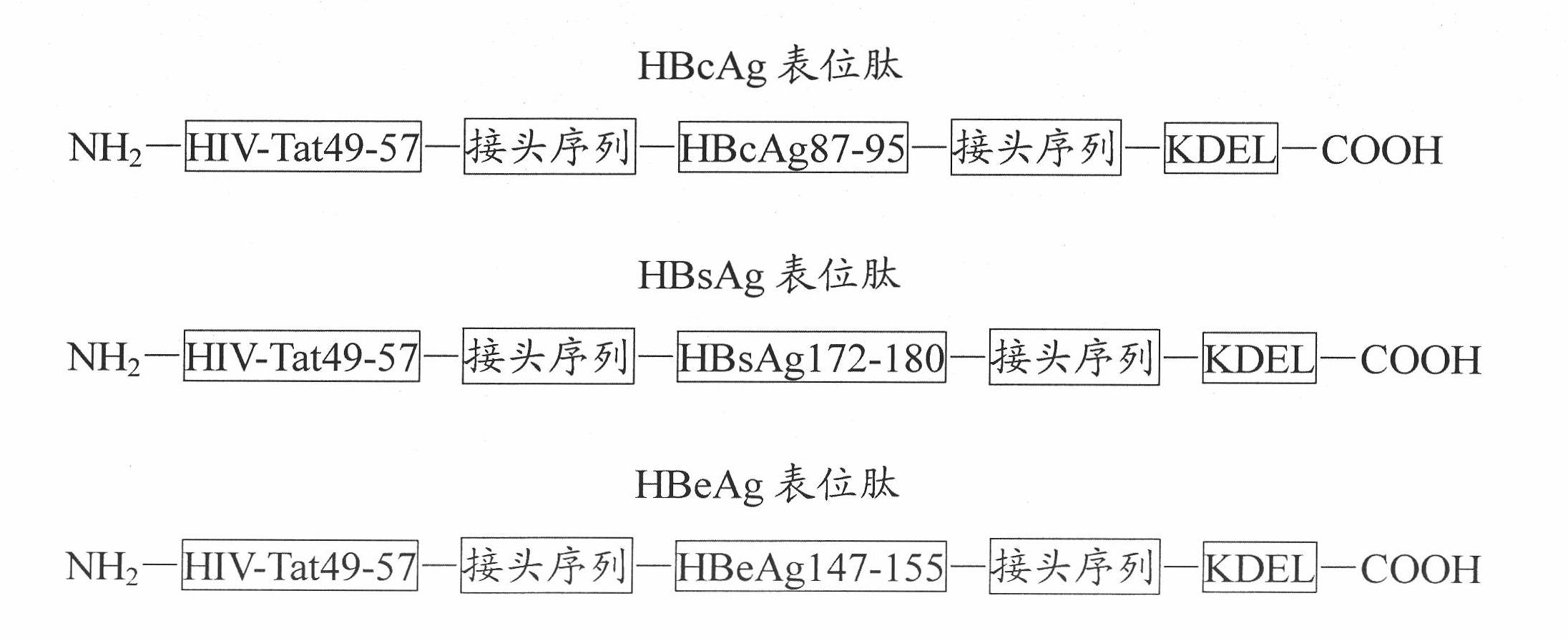
[0032] The primary structures of HBcAg epitope peptide, HBsAg epitope peptide and HBeAg epitope peptide designed by the present invention are as follows: figure 1 As shown, each epitope is connected with the transmembrane sequence or the endoplasmic reticulum retention signal sequence with a linker sequence,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com