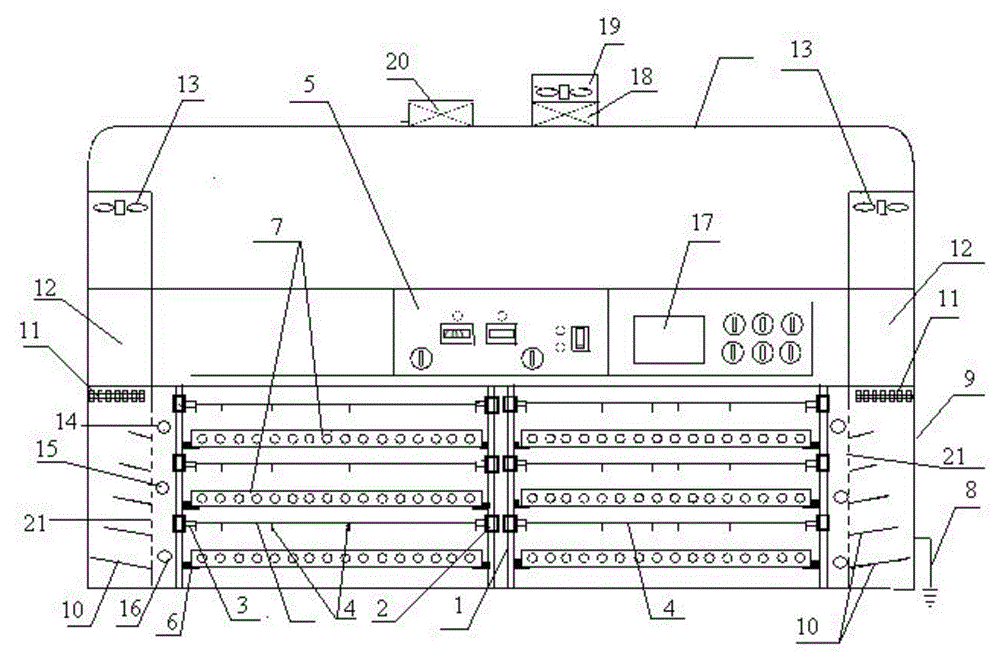
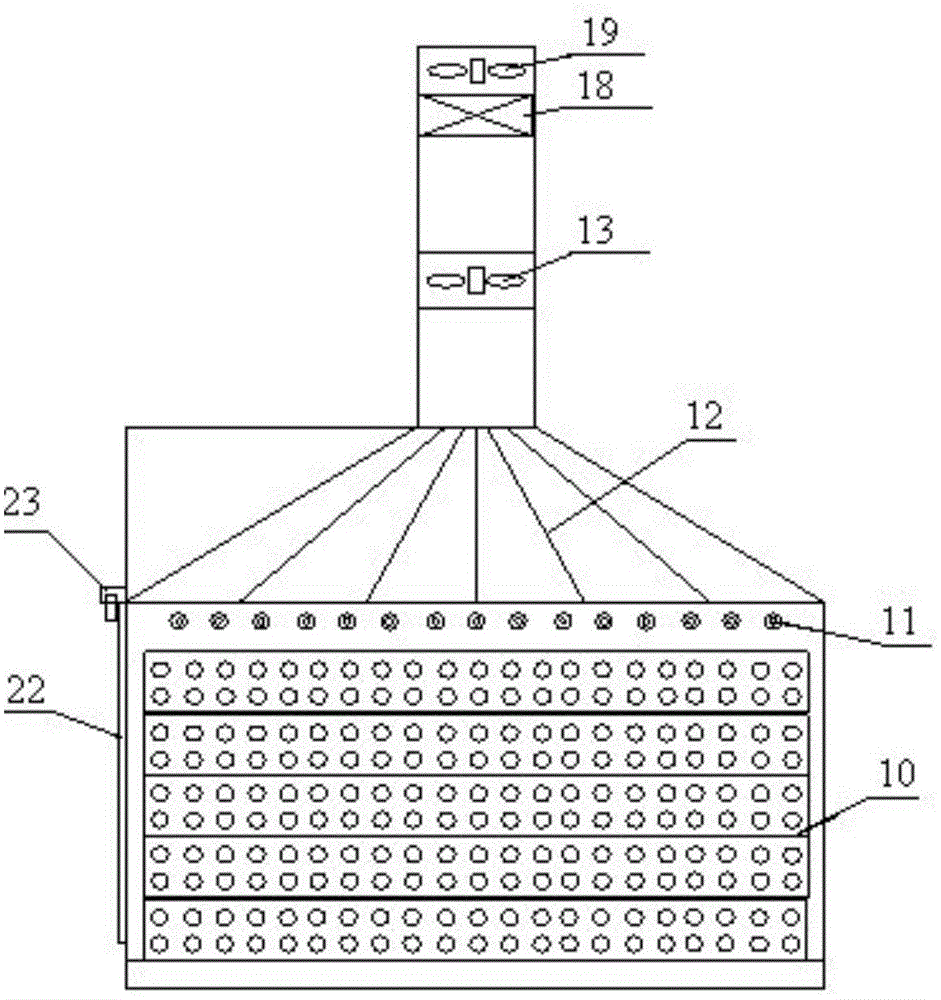
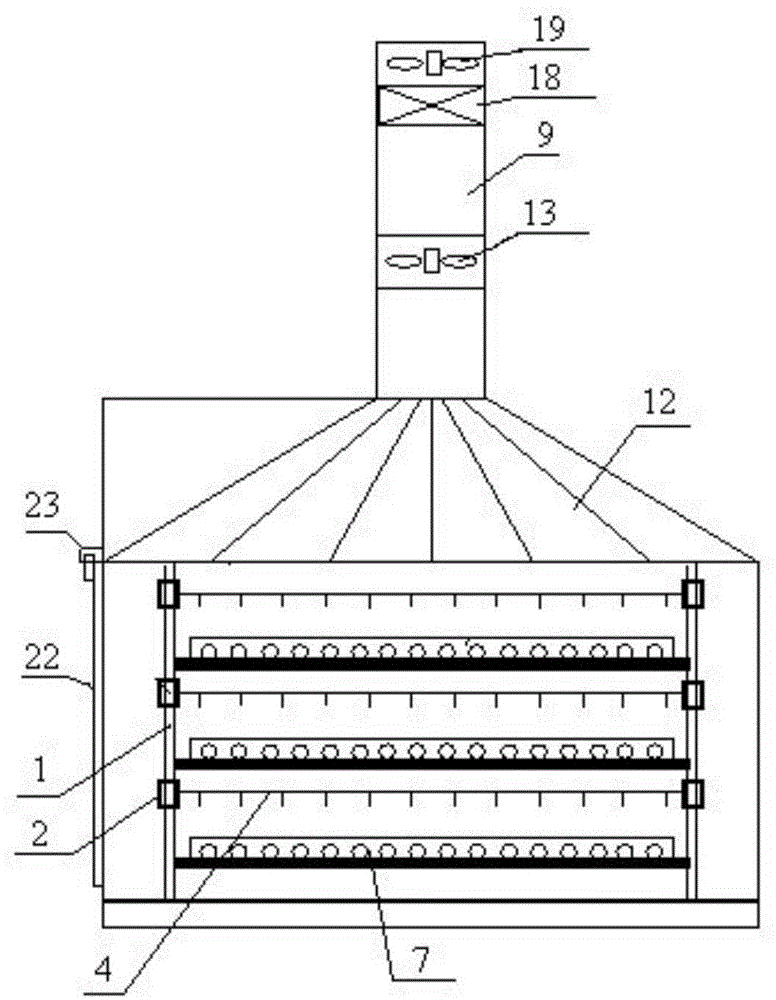
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
816 results about "Biologic Products" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Biologics are medical products that are made from living organisms or produced from substances that are generated by living things. These products are also known as biologic agents.
Nano-Chinese medicinal biological product and its preparation
A nano-class biologic product of Chinese-medicinal material for higher biological utilization rate and target nature, lower toxic by-effect and improved curative effect is prepared through preparing the nanoparticles of Chinese-medicinal materials, and preparing nano-class (50-80 nm) biologic product.
Owner:陈永丽
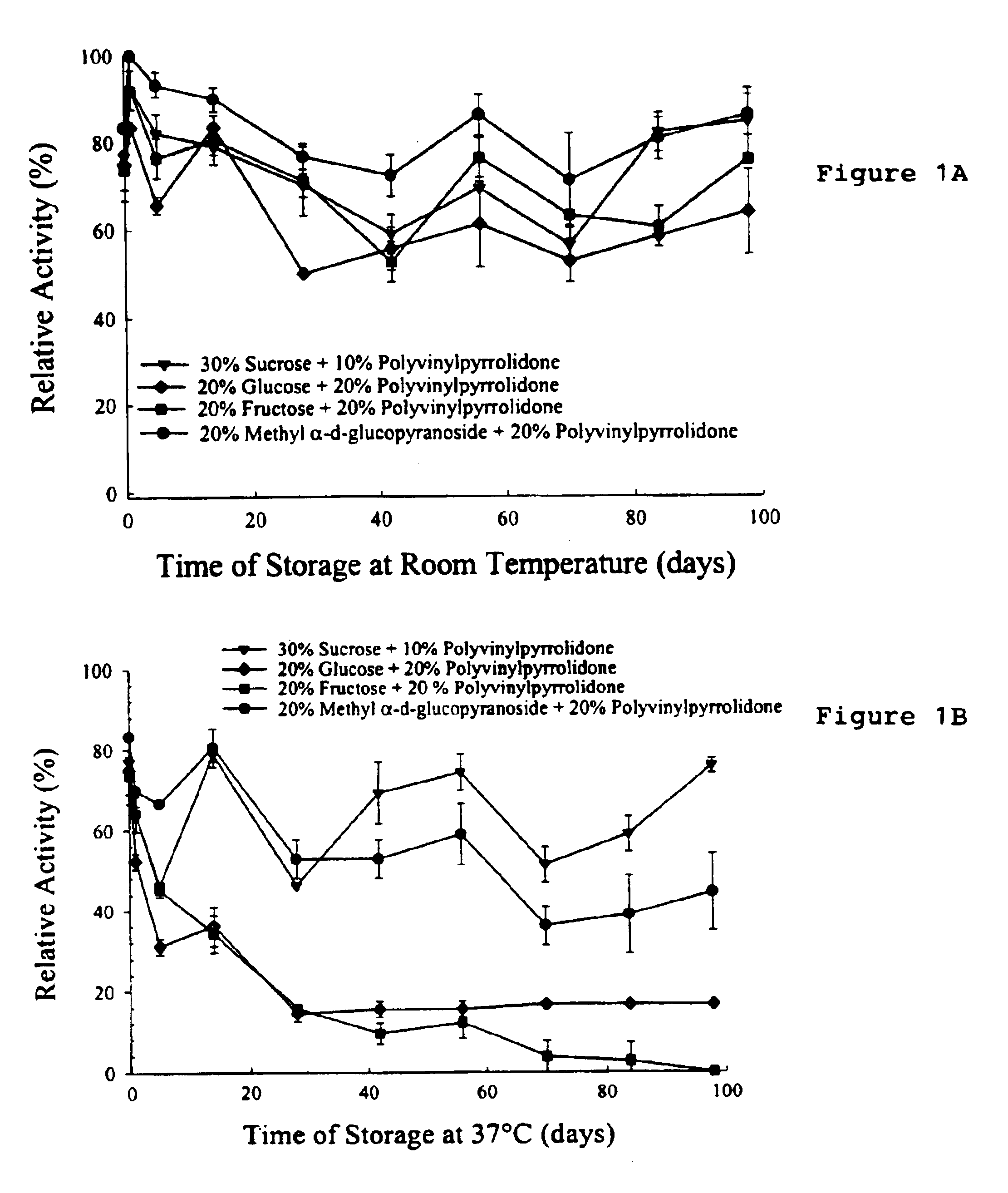
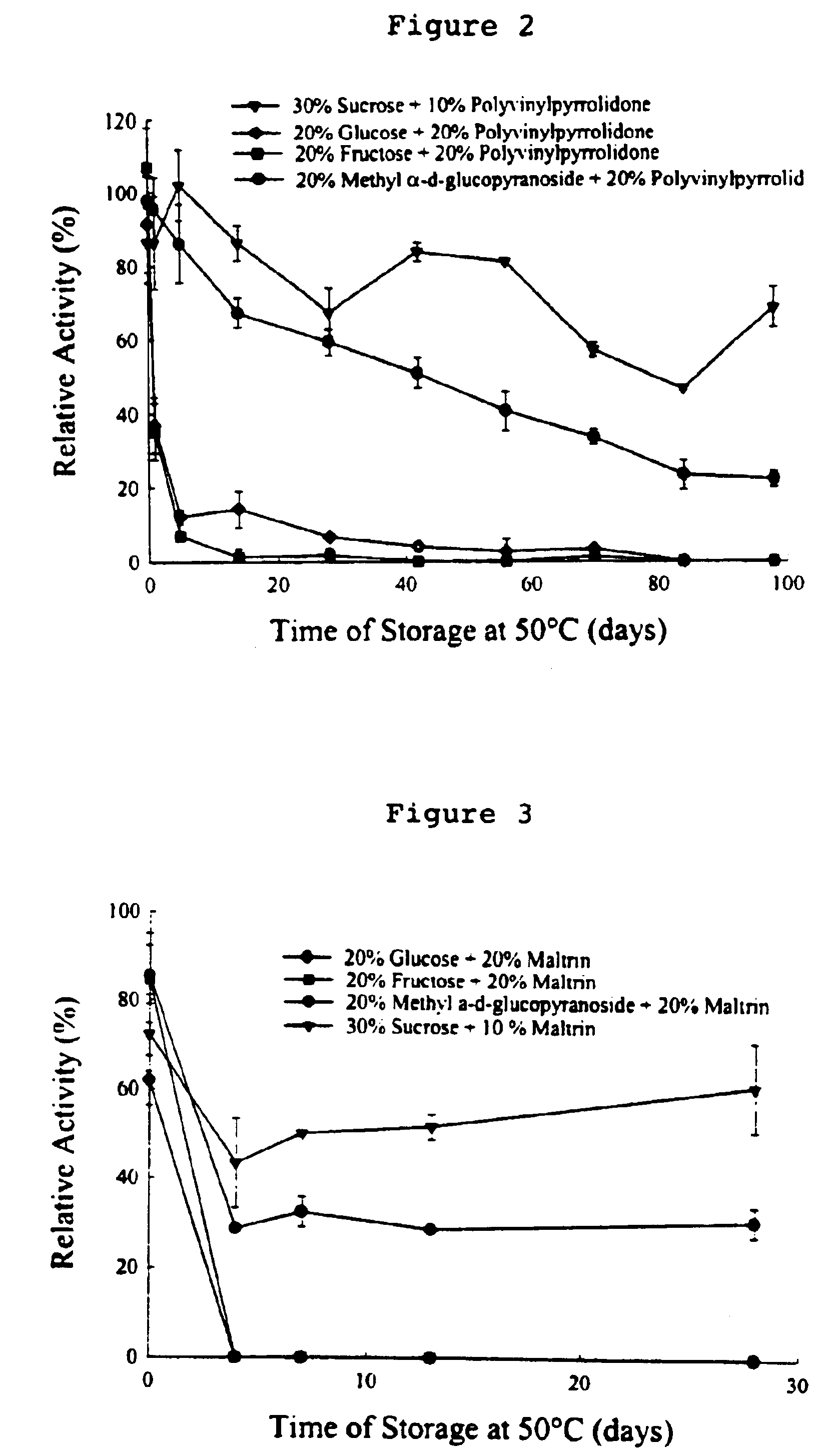
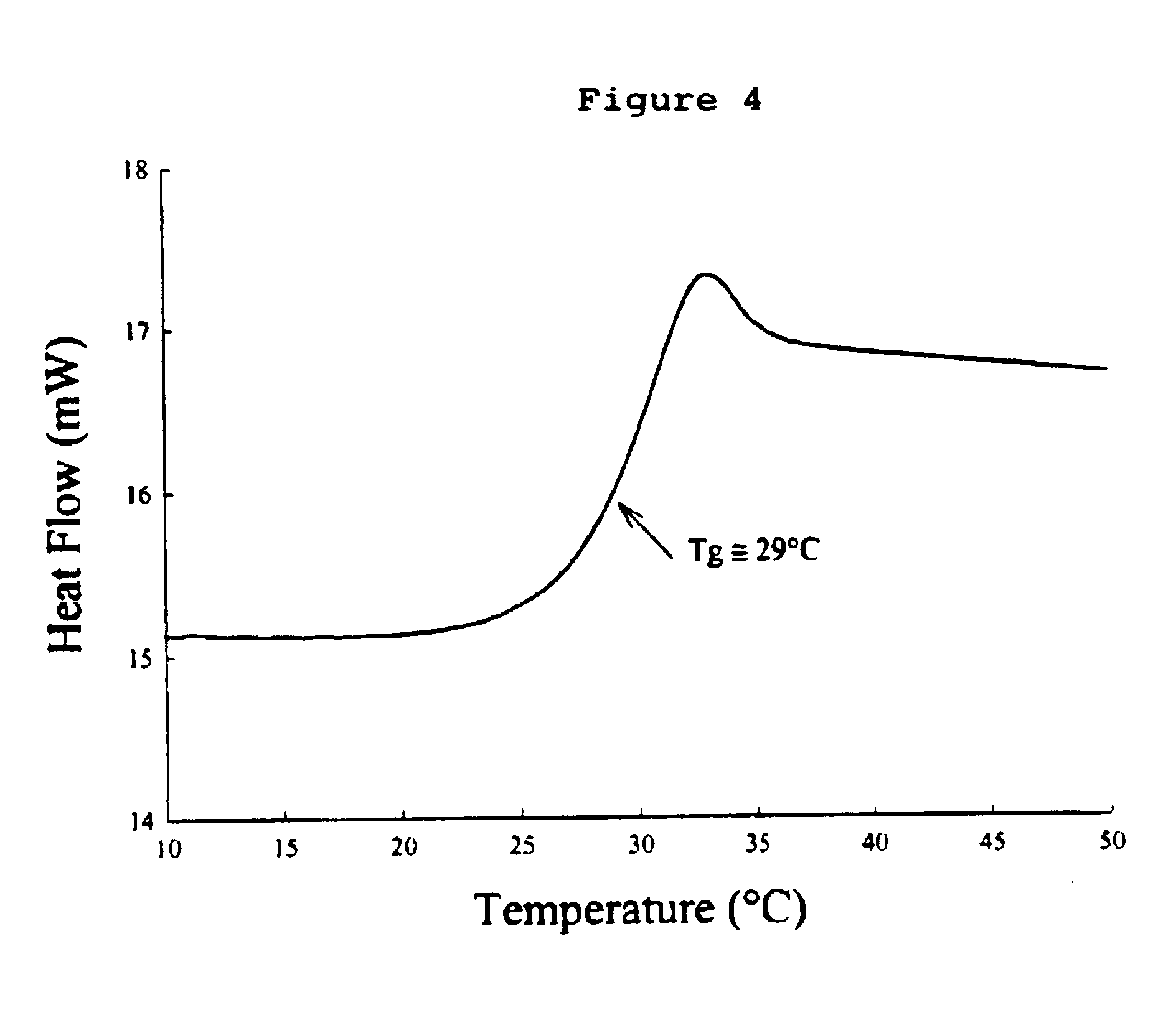
Preservation by Vaporization
ActiveUS20080229609A1Easy to controlImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
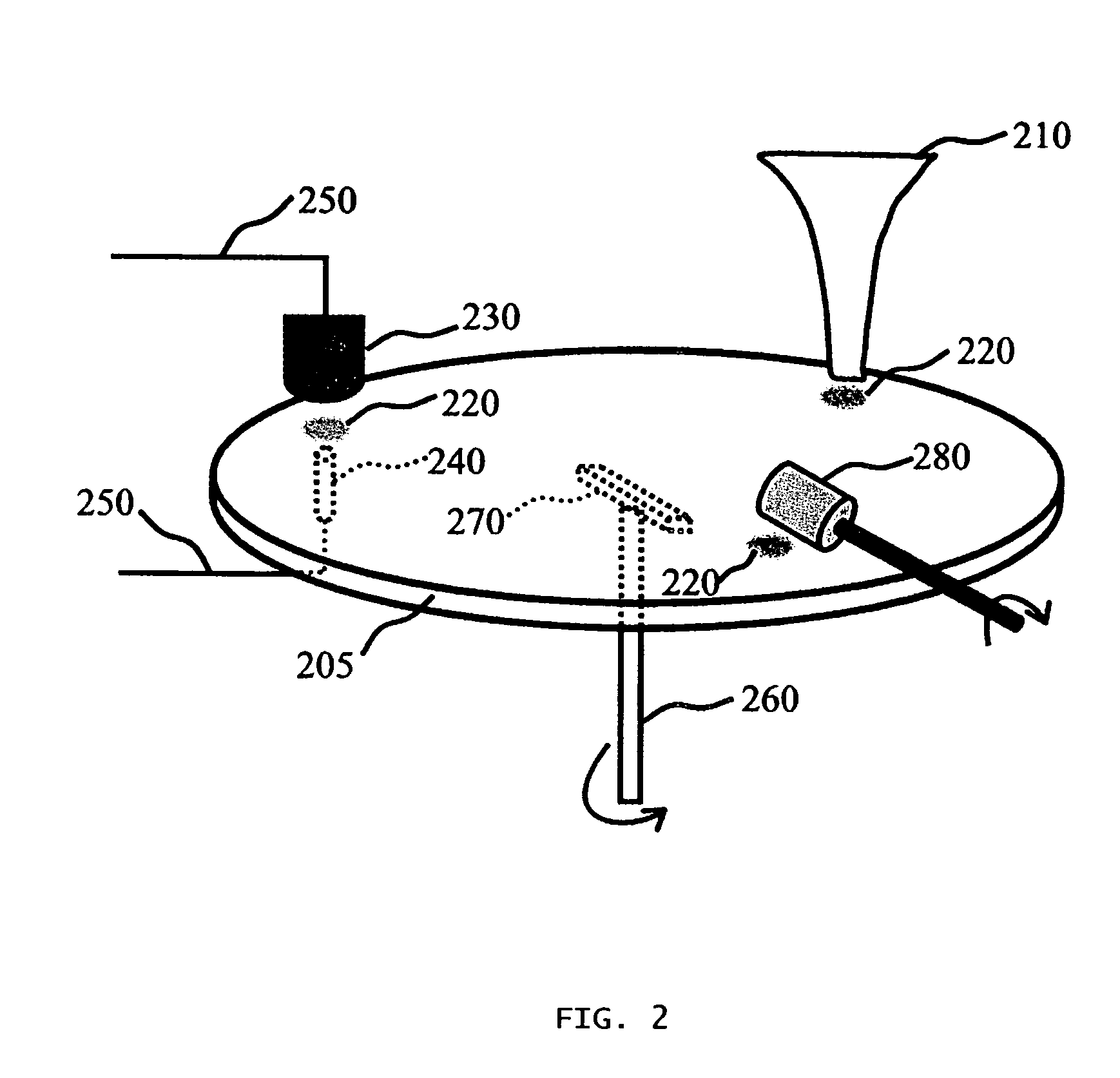
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
Formulation of preservation mixtures containing sensitive biologicals to be stabilized for ambient temperature storage by drying
This invention relates to formulations and methods for preserving sensitive biologicals, viruses, bacteria and eukaryotic cells by drying. More particularly, the invention relates to preservation mixtures containing viruses or cells and protectants, including a combination of a methylated monosaccharide and a disaccharide, or oligosaccharide, wherein the mixtures are adapted to stabilize these during dehydration and subsequent storage at ambient and higher temperature.
Owner:AVANT IMMUNOTHERAPEUTICS
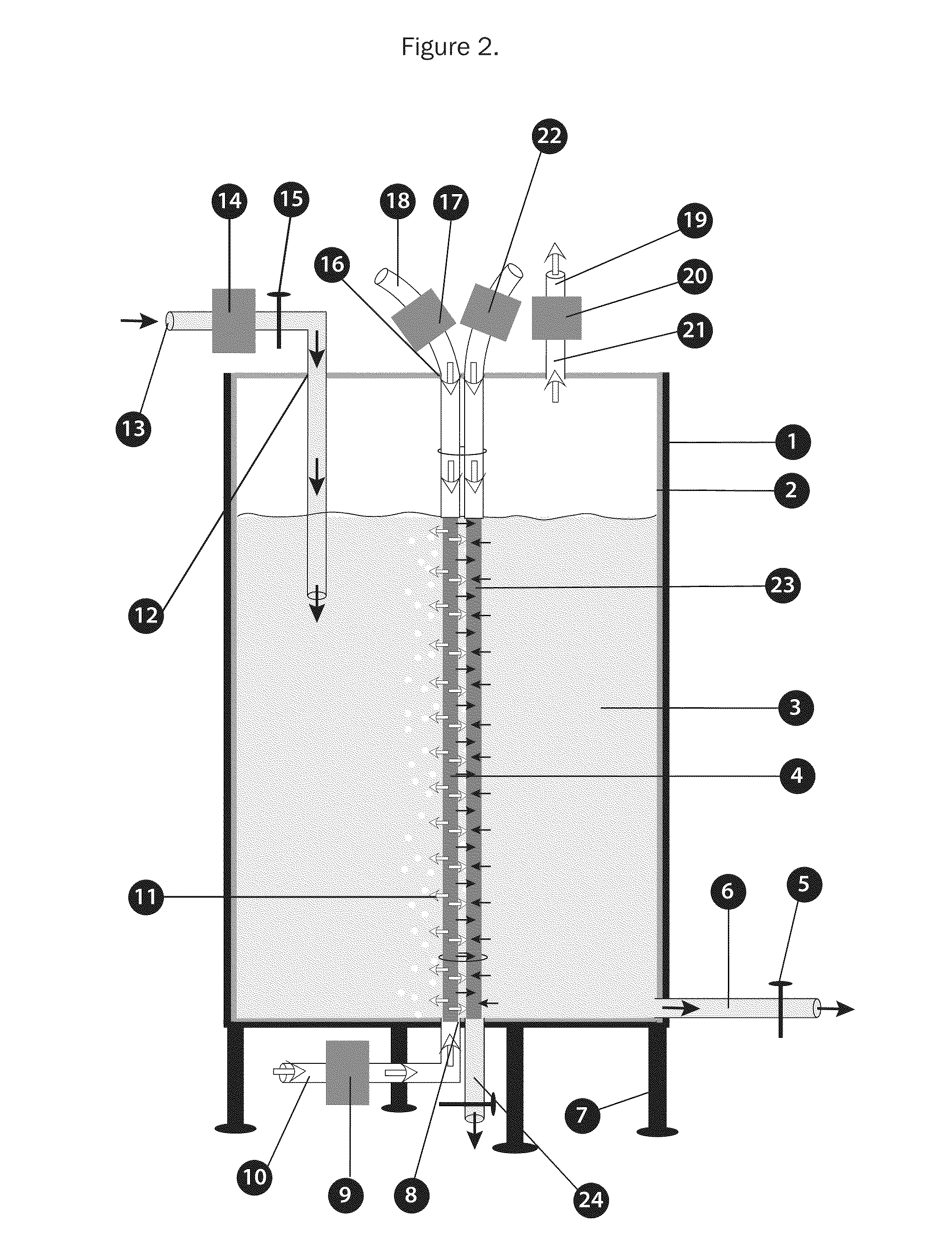
Bioreactors for fermentation and related methods
InactiveUS20110117538A1Suitable for useEvenly distributedBioreactor/fermenter combinationsBiological substance pretreatmentsFermentationBiologic Products
Bioreactors suitable for housing a predetermined volume of liquid comprising nutrient medium and biological culture comprising: (a) a container having at least one interior wall; (b) at least one nutrient medium inlet; (c) at least one liquid outlet; (d) at least one gas inlet; (e) at least one gas outlet; and (f) at least one cylindrical sparging filter attached to the at least one gas inlet, wherein the sparging filter comprises a plurality of pores along its axis which permit gas to be emitted radially from the sparging filter into the liquid, wherein the diameter of the plurality of pores does not exceed about 50 μm, and wherein the orientation of the at least one sparging filter within the container provides for immersion of the plurality of pores within the liquid and substantially uniform distribution of emitted gas throughout the liquid, and related methods of using said bioreactors to prepare various biological products.
Owner:THERAPEUTIC PROTEINS +1
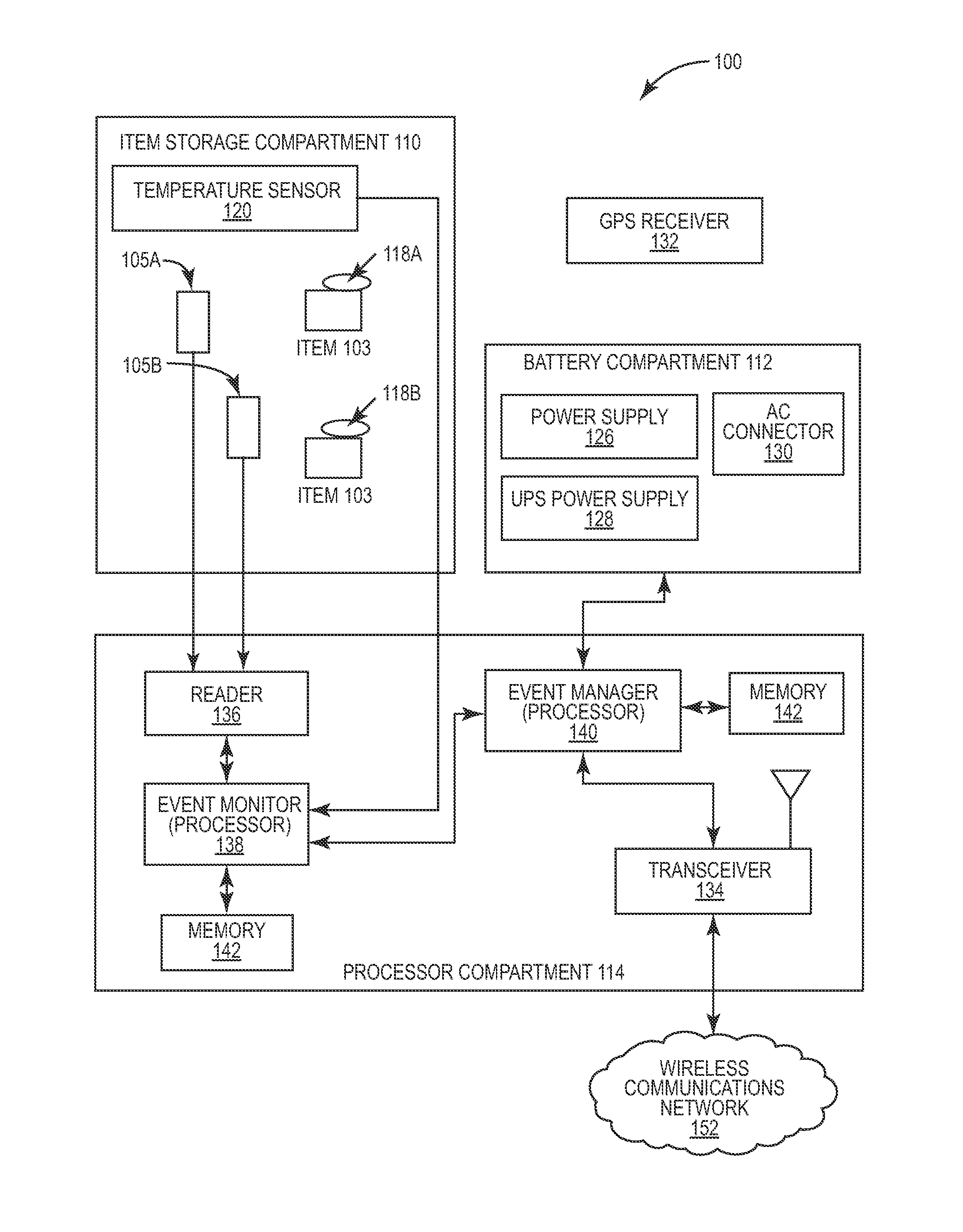
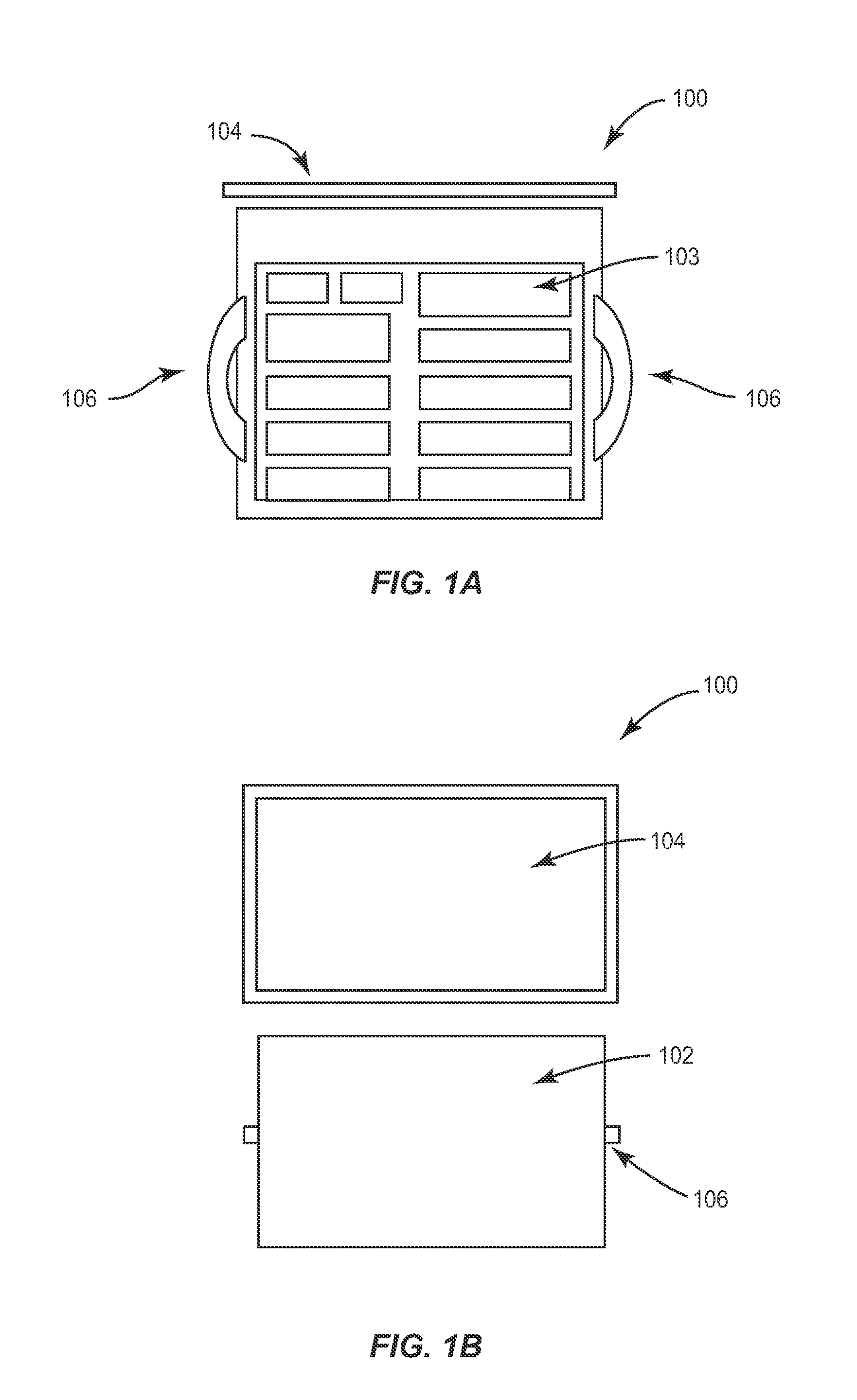
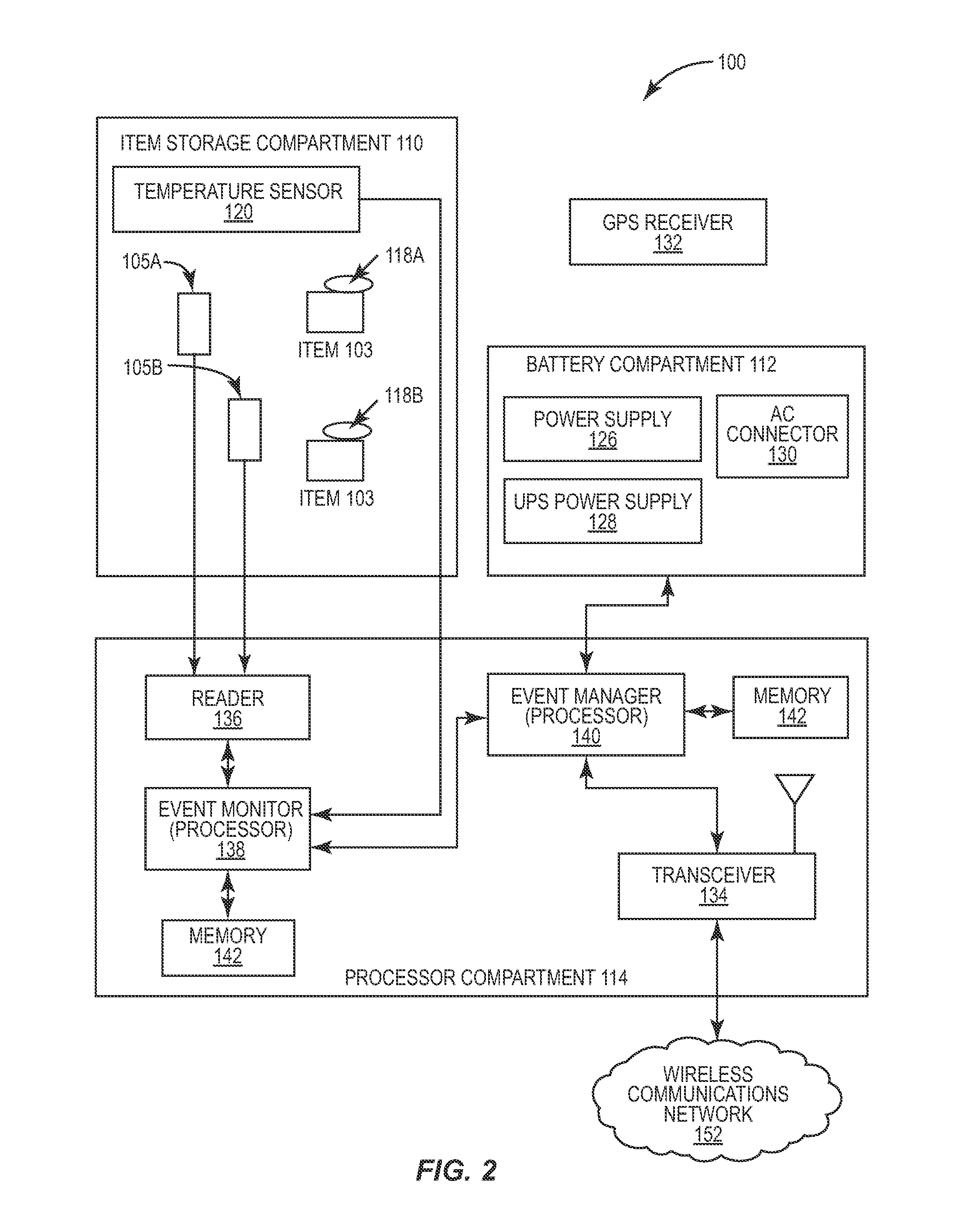
Portable RFID tagged carrier for sterile implants and biological products
ActiveUS20130106607A1Light weightVaccination/ovulation diagnosticsDead animal preservationEngineeringRadio frequency
Intelligent portable carrier device for supporting movement in product tracking and monitoring of regulated products, such as tissue and biologics. Embodiments of the invention use product identification technology, such as radio-frequency identification (RFID) tags and readers, to uniquely identify the regulated products as they are added to or removed from the intelligent portable carrier device. Embodiments of the invention may also be configured to monitor and report temperature and other environmental conditions associated with the intelligent portable carrier device.
Owner:WARSAW ORTHOPEDIC INC
Flexible manufacturing system
ActiveUS20110258837A1High capacity supplyDischarging wasteTreatment involving filtrationChemoinformaticsFlexible manufacturing systemEngineering
Owner:XOMA US
Continuous processing methods for biological products
InactiveUS20130260419A1Improve manufacturing productivityImprove efficiencyBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyMonoclonal antibody
The present invention is directed to the development of continuous processing technology for the purification of biopharmaceuticals and biological products, such as monoclonal antibodies, protein therapeutics, and vaccines. Methods for continuous processing of a biological product in a feed stream toward formulation of a purified bulk product are described.
Owner:SARTORIUS STEDIM CHROMATOGRAPHY SYST LTD
Low Aspect Ratio Staged Closure Devices, Systems, and Methods for Freeze-Drying, Storing, Reconstituting, and Administering Lyophilized Plasma
InactiveUS20140259724A1Easy to keepAvoid cross contaminationDrying solid materials without heatDrying machines with progressive movementsFreeze-dryingEngineering
The inventive device and methods described herein address the introduction of a safe and effective freeze-dried biological product, and particularly a plasma product, to a subject in need thereof. The present invention relates to a multifunctional, staged closure device, which also is described as a lyophilization container for plasma (LCP). The device and methods described herein address how to reproducibly achieve a low moisture and substantially oxygen-free atmosphere within a finally hermetically sealed biocompatible low aspect plastic vessel within a standard shelf-stoppering freeze dryer. The present inventive device and methods provide a freeze-dried plasma product that is fully traceable, preserves the constituent plasma activity, is readily prepared in a sterile fashion, is stable, ensures ease of storage and permits rapid reconstitution and delivery to a patient.
Owner:HEMCON MEDICAL TECH
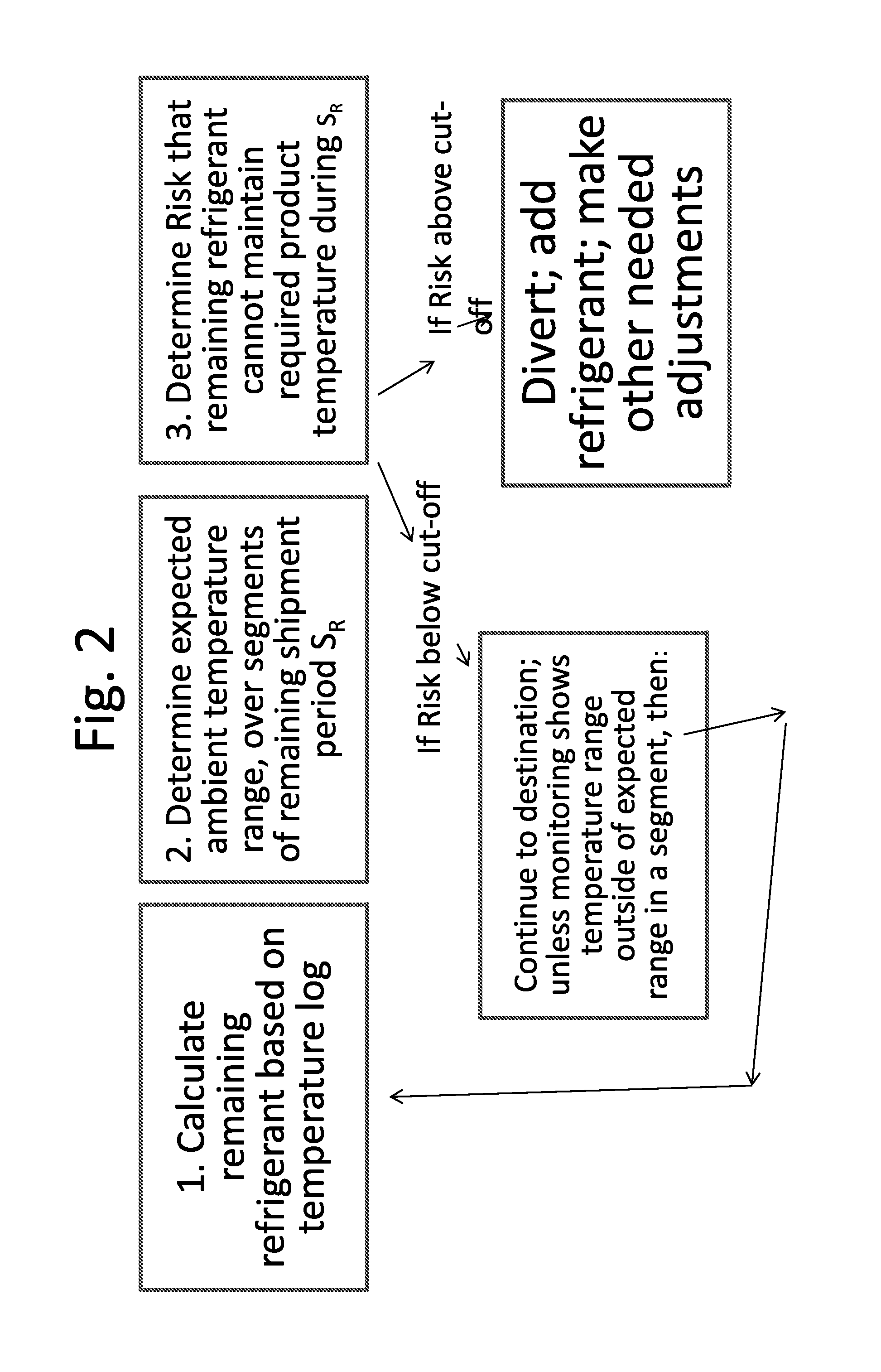
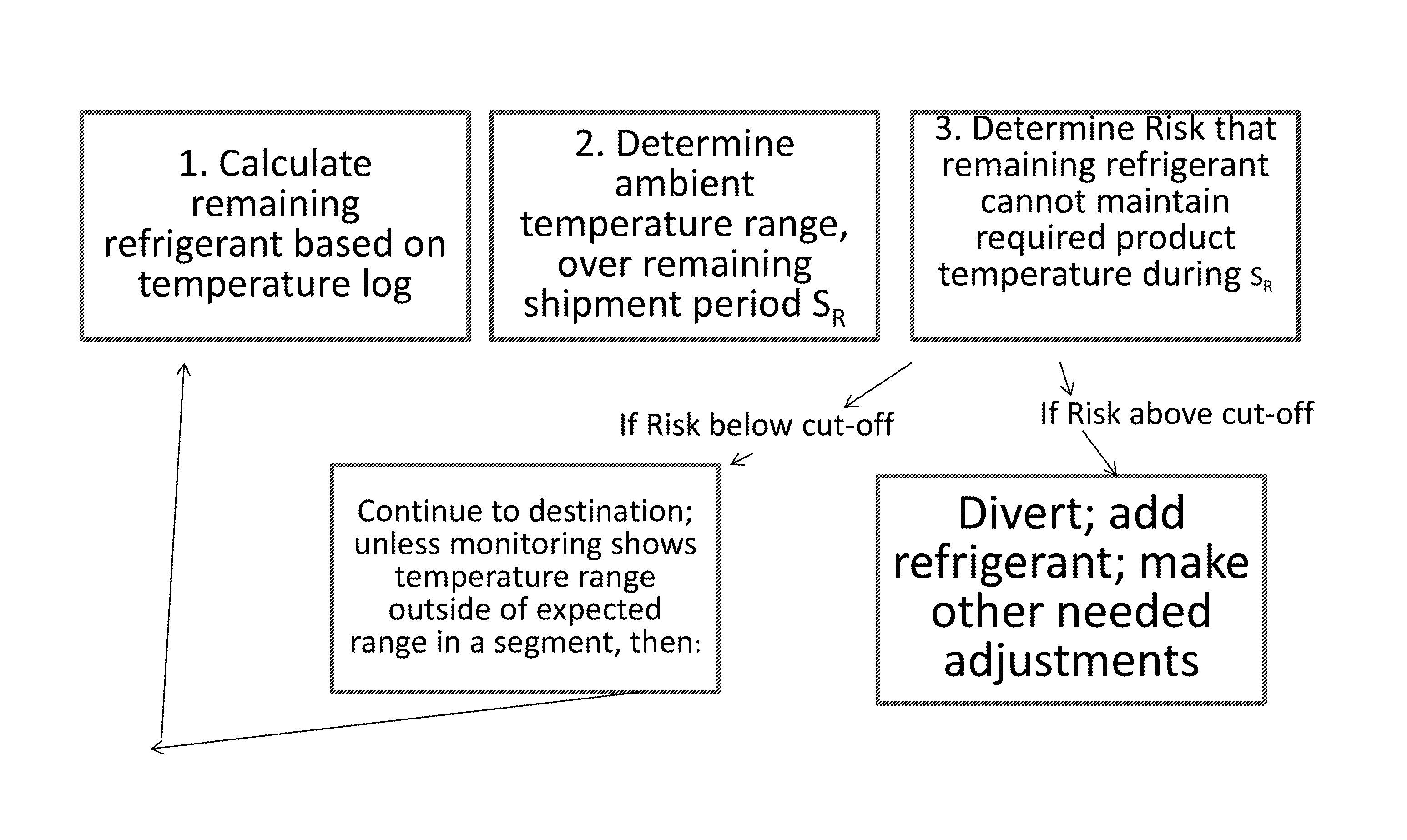
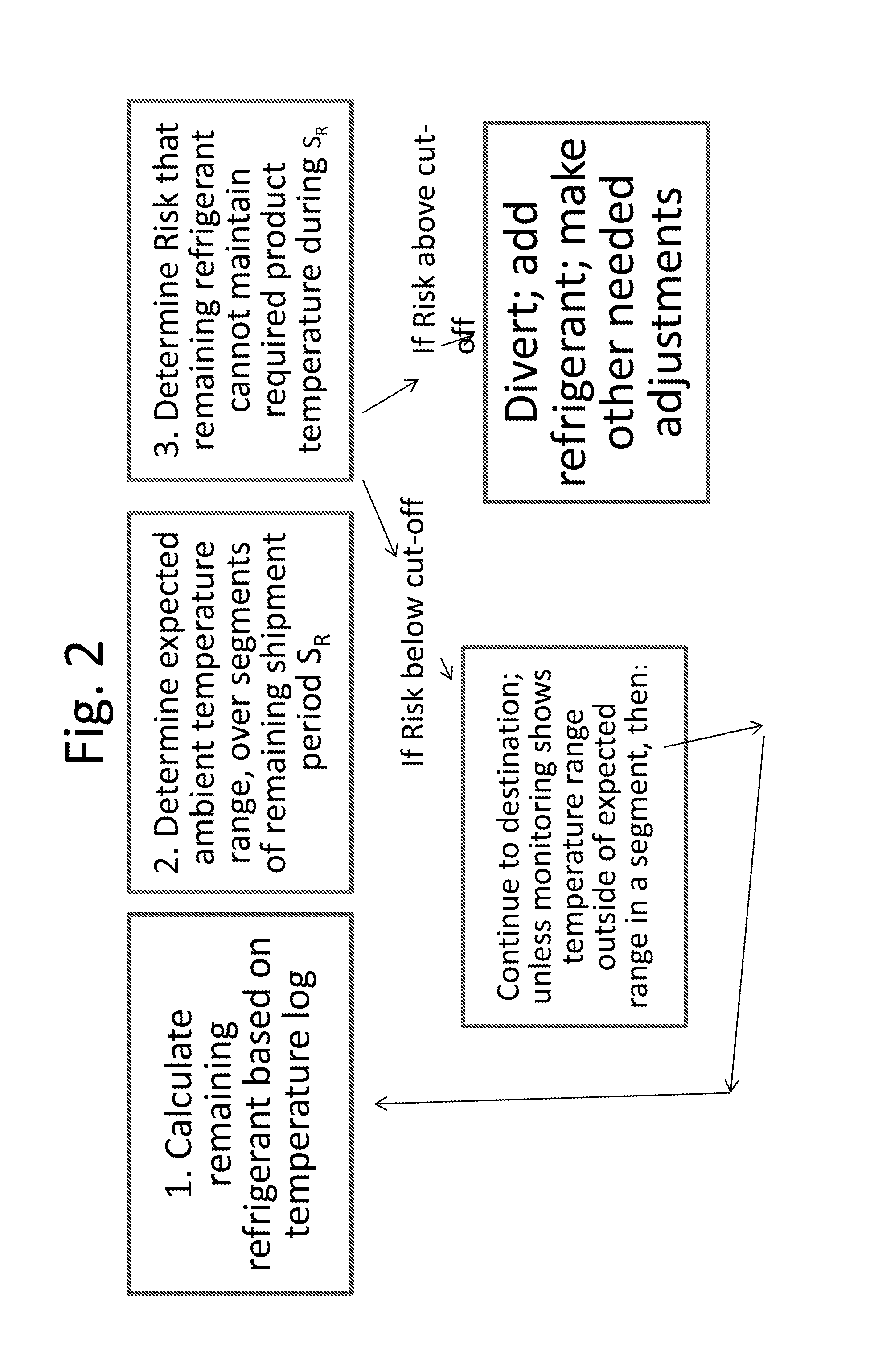
Monitoring Temperature-Sensitive Cargo with Automated Generation of Regulatory Qualification
ActiveUS20150120597A1Reduces effective lifeRetain valueThermometers using mean/integrated valuesLighting and heating apparatusMonitoring temperatureBiologic Products
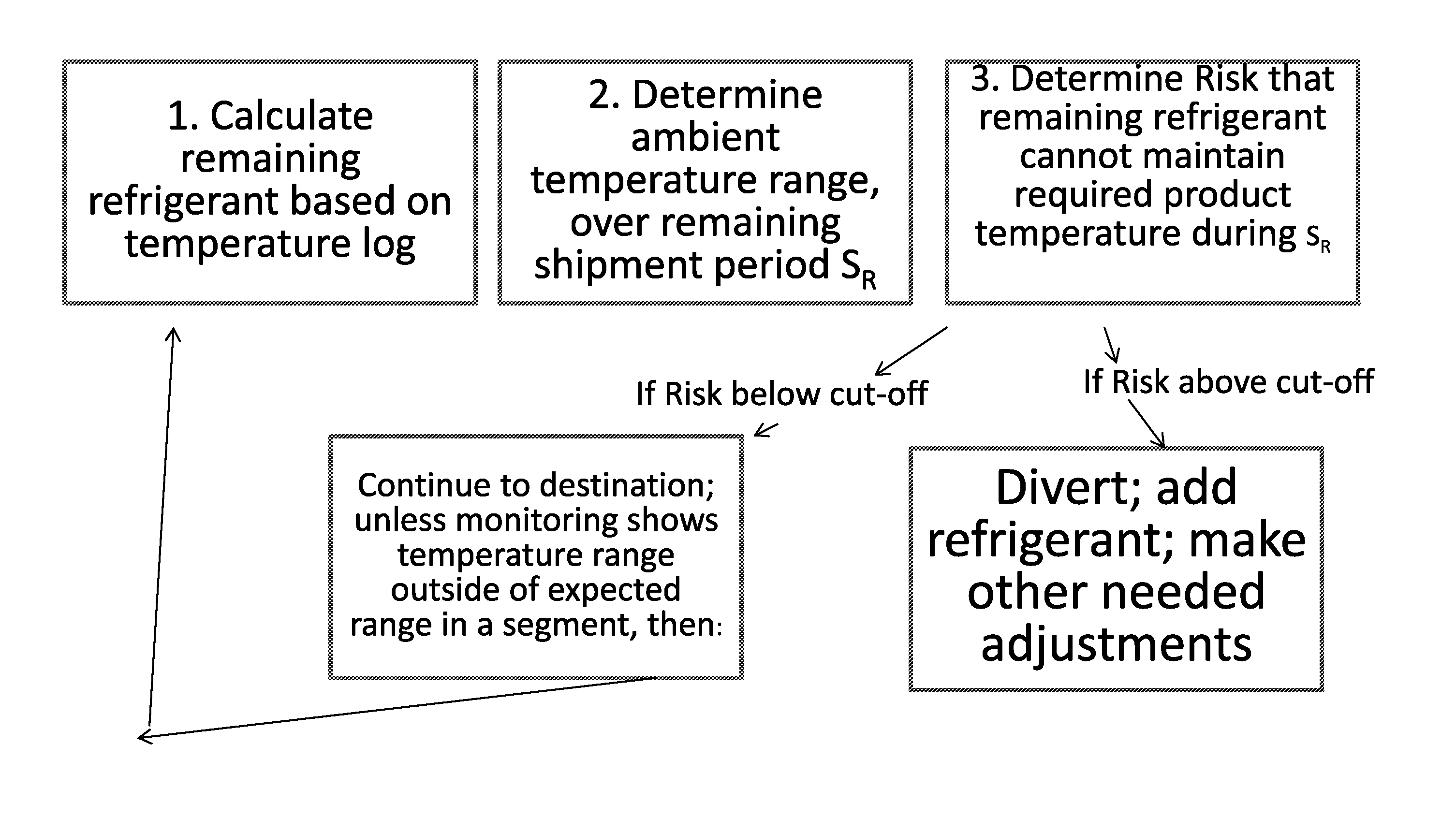
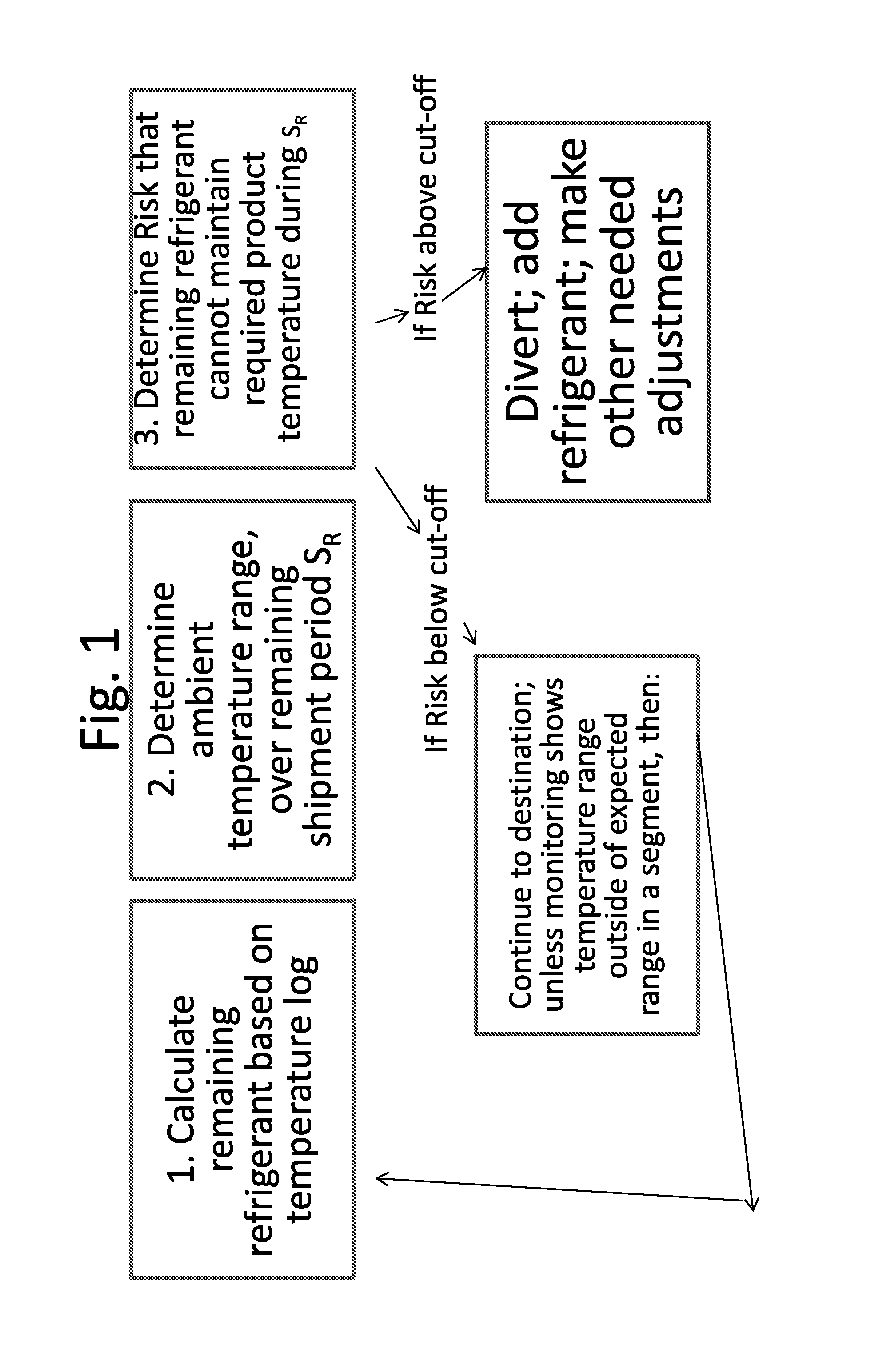
Disclosed is a process of determining, at a point during shipment, whether the solid phase refrigerant in a shipment is sufficient to preserve the shipment cargo, which is blood or other biological products, for the remaining shipment period, by: monitoring the temperatures encountered to said point and estimating the temperatures likely to be encountered during the remaining shipment period; determining the likelihood that the remaining refrigerant can maintain the shipment cargo within a specified temperature range during the remaining shipment period; and if the risk that the remaining refrigerant cannot maintain the shipment cargo within said range during the remaining shipment period is above a cut-off level, then taking action to preserve the value of the cargo.
Owner:INTEGREON GLOBAL INC
Serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture
ActiveCN101760442ASupports adherent growthReduce the burden of separation and purification in the later stageVertebrate cellsArtificial cell constructsLipid formationSerum free media
The invention relates to the culture medium research and development technical field of modern biological technology and provides a serum-free medium for MDCK cell large-scale adherent culture and single-cell suspension culture, which comprises 21 amino acids, 6 vitamins, 8 salts, 8 lipids, 4 trace elements, 2 buffers, 1 protein hydrolysate, 1 acid-base indicator and 6 other additives. The serum-free medium can be prepared by the conventional preparation method, and an application method thereof is the conventional method. The serum-free medium has the beneficial effects that: the serum-free medium does not contain serum, has clear components, is beneficial for separating and purifying the product and improves the product quality; the serum-free medium supports long-term subculture of MDCK cells and does not require long-term and complex adaptation process; and the serum-free medium can well support the adherent growth and single-cell suspension growth of the MDCK cells, has clear components and easy preparation and utilization, and is suitable for mass production of biological products.
Owner:EAST CHINA UNIV OF SCI & TECH
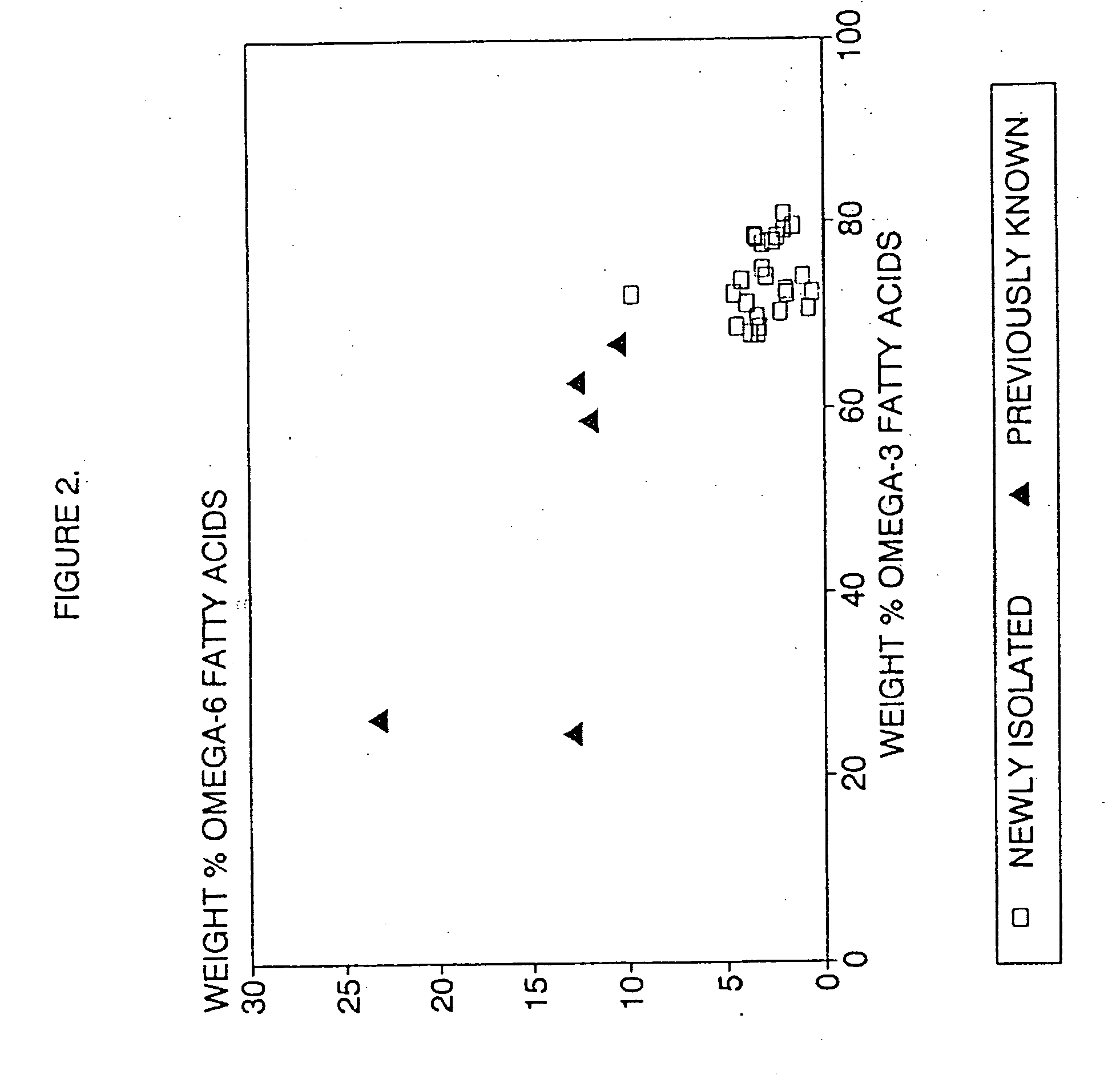
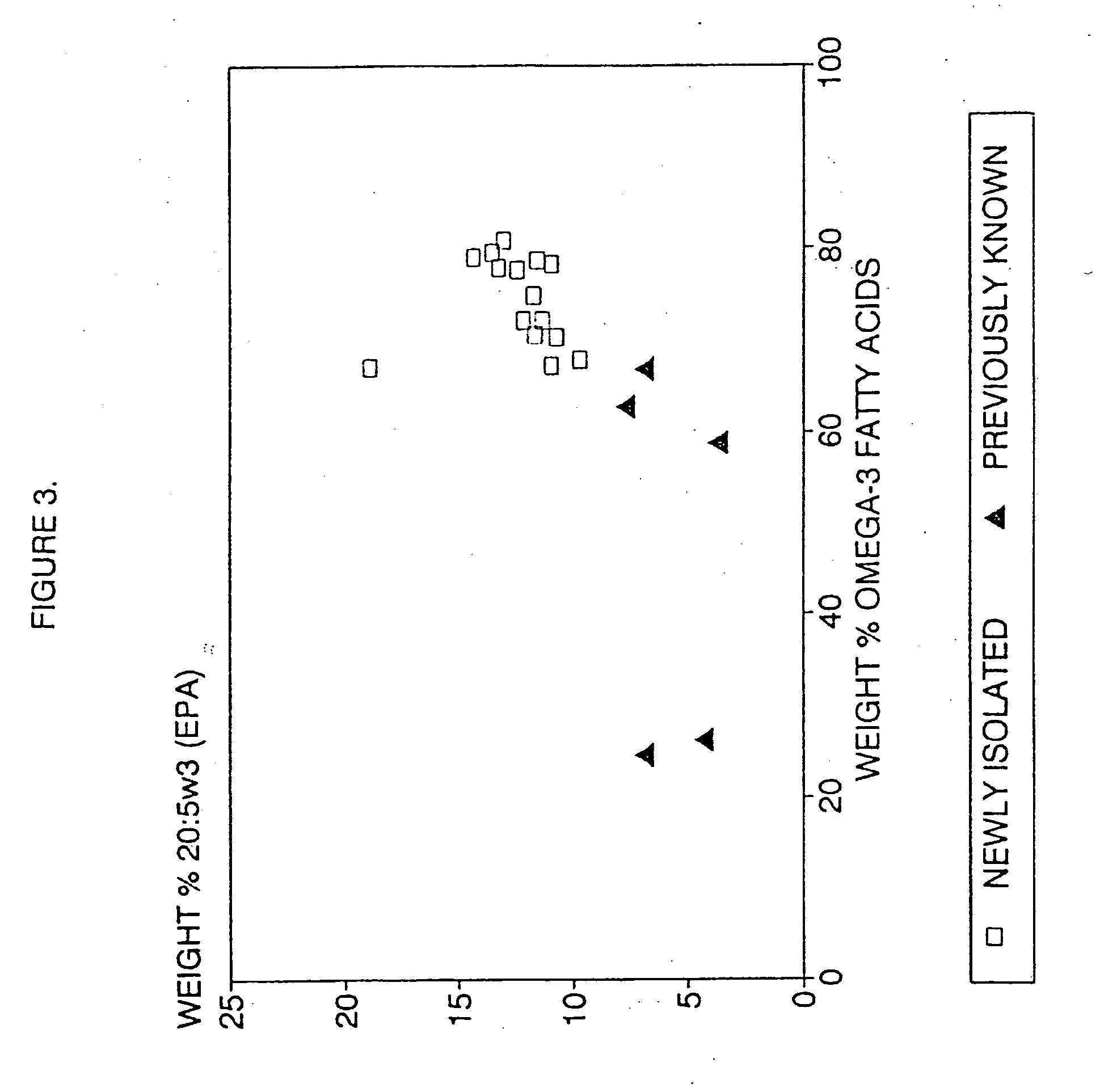
Process for the heterotrophic production of microbial products with high concentrations of omega-3 highly unsaturated fatty acids
InactiveUS20060188969A1Prevent degradationIncrease concentrationFermentationHigh concentrationLipid formation
Owner:DSM IP ASSETS BV
Devices for continuous sampling of airborne particles using a regenerative surface
InactiveUS7578973B2Good removal effectEasy to disassembleFixed microstructural devicesVolume/mass flow measurementBuilding automationBiologic Products
Airborne particles are impacted on a collection surface, analyzed, and then the collection surface is regenerated. Thus, the same collection surface can be used in numerous cycles. The analysis can be focused on one or more properties of interest, such as the concentration of airborne biologicals. Sensors based on regenerative collection surfaces may be incorporated in many networks for applications such as building automation.
Owner:FLIR DETECTION
Veterinarian virus kind biological product heat resisting freeze drying protective agent and its preparation technique
A freeze-dried high-temp. resistant protecting agent for virus-type biologic products for veterinary medicine is prepared from several components through proportionally mixing. It features that each of its components is sterilized separately. For the components which can be sterilized under high temp., they are dissolved in distilled water according to the proportion of formulation and are sterilized at 116 deg.C for 30-40 min; and for those which do not resist the high temp., they are also dissolved in distilled water according the proportion of formulation and are filtered with 0.22 micron pore diameter filter membrane to remove bacteria; and then the two parts are mixed to obtain the freeze-dried high-temp. resistant, protecting agent. The mentioned two parts are added to the virus antigen liquid according to a proportion of 1:1 and after packaging, they are freeze-dried. The product can be stored at 2-8 deg.C for 24 months.
Owner:卫广森
High-voltage electric field and hot air combined drying device
ActiveCN103983091AImprove retentionGood appearanceDrying gas arrangementsDrying chambers/containersThermal instabilityOperability
The invention discloses a high-voltage electric field and hot air combined drying device. The high-voltage electric field and hot air combined drying device comprises a box body, a high-voltage electric field drying system, a hot air circulation drying system and a monitoring control system, wherein the high-voltage electric field drying system, the hot air circulation drying system and the monitoring control system are arranged on the box body. According to the high-voltage electric field and hot air combined drying device provided by the invention, the structural design is reasonable, the operability is strong, and the use is convenient; compared with traditional hot air drying, the drying efficiency is high, the drying temperature is low, the drying time can be shortened by 50% than the drying time of drying by pure hot air under the same temperature, the drying energy consumption can be reduced by 51.9%, the high-voltage electric field and hot air combined drying device is particularly suitable for drying materials containing thermal-instability active components, for example, drying the materials of traditional Chinese medicine, medicinal slices, drug, agricultural products, food, biological products and the like, the appearance quality after the drying is good, the reservation degree of the active components is high, and the application range is very wide.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Preservation by vaporization
ActiveUS9469835B2Maximize potencyMaximize viabilityBioreactor/fermenter combinationsBiological substance pretreatmentsIndustrial scaleVacuum chamber
Significant research is being done to develop and improve delivery mechanisms for biopharmaceuticals and vaccines, including pulmonary (inhalation), nasal, transdermal, and oral alternatives. Market projections indicate that the delivery of proteins and vaccines by inhalation and oral formulation has become and will continue to be increasingly important. These delivery mechanisms, to be effective, will require better stabilization of the biologicals so that they can maintain potency and effectiveness at ambient temperatures for extended periods of time. The novel Preservation by Vaporization (PBV) Technology described herein provides cost-effective and efficient industrial scale stabilization of proteins, viruses, bacteria, and other sensitive biologicals, thereby allowing a production of products that are not possible to be produced by existing methods. The suggested new PBV process comprises primary drying under vacuum from a partially frozen state (i.e. slush) at near subzero temperatures followed by stability drying at elevated temperatures (i.e., above 40 degrees Celsius). The new suggested method can be performed aseptically in unit doze format (in vials) and / or in bulk format (in trays, bags, or other containers). The drying can be performed as a continuous load process in a manifold vacuum dryer comprising a plurality (e.g., 30) of vacuum chambers attached to a condenser during the drying.
Owner:UNIVERSAL STABILIZATION TECH INC
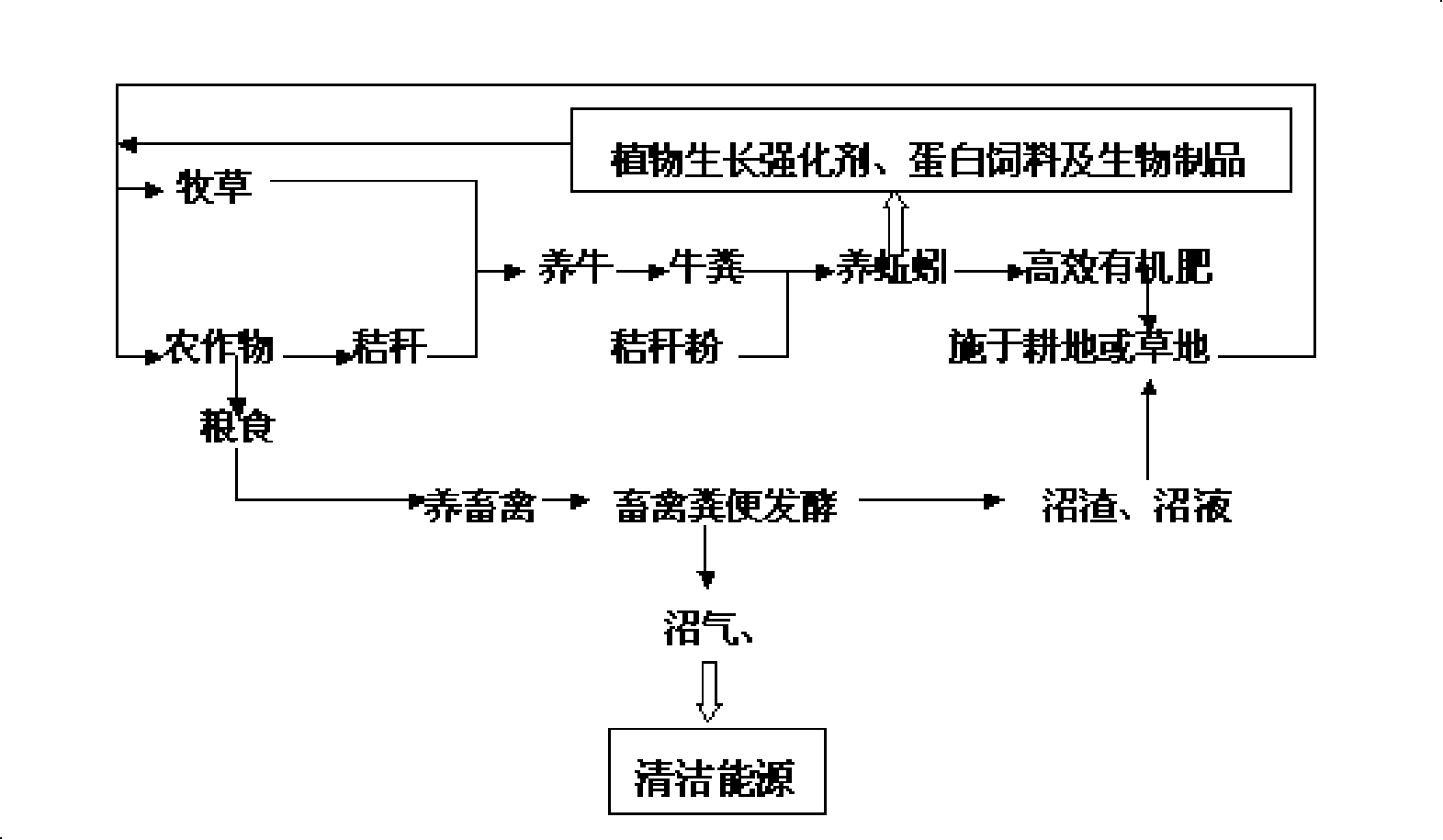
High-efficiency organic circulating agricultural production system
InactiveCN101233836AReasonable useImprove utilizationAnthropod material medical ingredientsFood processingFecesAgricultural science
The invention discloses an organic recycling agriculture production system with high efficiency, which uses food obtained from planted crops for feeding livestock, manure produced by which is fermented so as to produce biogas, biogas residue and biogas slurry, wherein, the biogas can be used as clean energy, while the biogas residue and biogas slurry are applied to cultivated lands or grass lands; the planted forage and crop stalks can be used for raising cattle or earthworm after grinded together with cattle manure; the earthworm can be extracted to produce plant growth enhancer, protein feed and biologic products; the earthworm manure can be used as highly effective organic fertilizer to be applied to farm lands or grasslands. The organic recycling agriculture production system of the invention abides by the theory of agriculture circular economy, utilizes nature resource fully and reasonably, promotes resource regeneration, forms virtuous circle of multi-grade utilization of material and energy, and thus is a modern agriculture technique with high quality, high yielding, high efficiency, low consumption and environment protection as well.
Owner:GUIZHOU JILONG ECOLOGICAL TECH
Culture medium used for Vero cell and cultivation method thereof
InactiveCN101182490AFast growthArtificial cell constructsVertebrate cellsSerum free mediaMicrobiology
The invention relates to the preparation and application method of a medium, which is specifically related to a medium which is used for Vero cell and a culture method of the Vero cell. The invention belongs to the biological product area. The culture method of the invention is that the adhering Vero cells are suspend and cultured in SFM serum-free medium for the domestication and adaptation; the growing pattern of the Vero cell changes from adherence growth to suspension growth; the Vero cells are further amplified in Vero amplification CHEMICALLY DEFINED MEDIUM. The method of the invention has good effects; the growing pattern of the Vero cell is transformed from adherence growth to suspension growth, which changes the growth pattern of the cell and greatly increases the yield of the cell.
Owner:天津百若克医药生物技术有限责任公司
Method for producing edible fungi flavoring powder
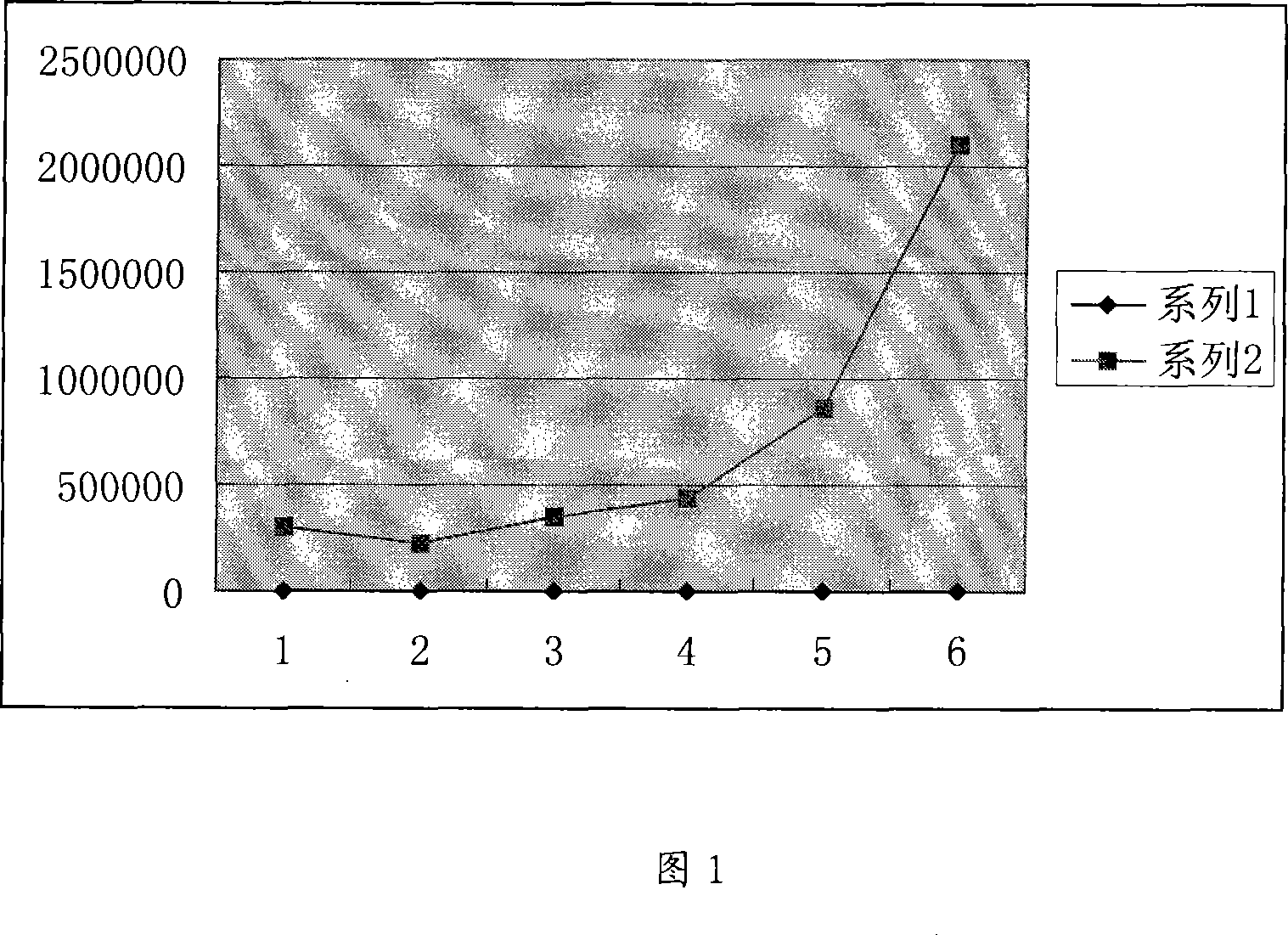
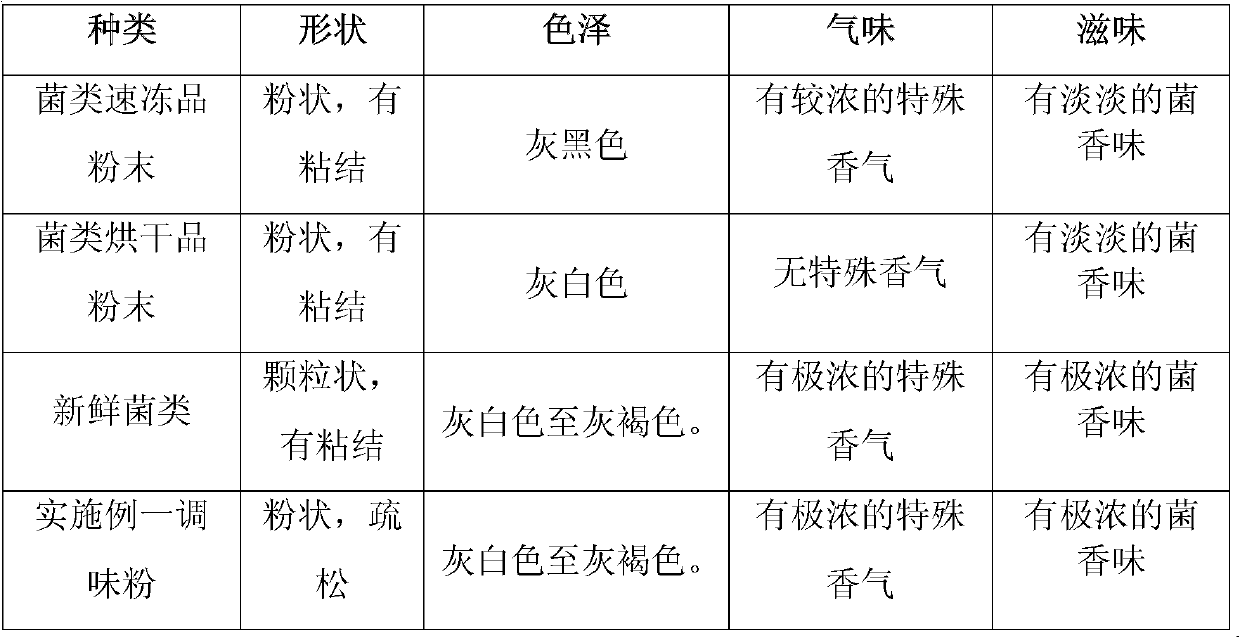
The invention discloses a method for producing edible fungi flavoring powder, and belongs to the field of novel biological products. For solving the technical problems, the invention provides the method for producing the edible fungi flavoring powder for keeping the nutritional and functional components of fungi and keeping original flavor and form. The method comprises the following steps of: a, raw material treatment: cleaning and slicing at least one of fresh tricholoma matsutake, toadstool, bolete, sarcodon quel, hericium erinaceus, lentinus edodes, pleurotus cornucopiae, pleurotus eryngii, cantharellus cibarius, termitomyces albuminosus, agrocybe cylindracea, lactarius deliciosus and russulaceae and fresh truffle, and drying the surface moisture in air; b, freezing; c, vacuum drying; d, resolving and drying; e, crushing; and f, sterile packing. The flavoring powder produced by using the method can keep original flavor and form, the nutritional and functional components are not lost, the indexes of the stored flavoring powder are in accordance with the regulation of edible fungi health standard GB7096-2003, and the water content index of the flavoring powder is lower than 1 / 3 of the national standard.
Owner:PANZHIHUA CHENFENG FORESTRY
Decomposition maturing agent for fast decomposition-maturing straw
InactiveCN101139561ASimple structureHigh organic contentBio-organic fraction processingFungiBacillus licheniformisDecomposition
The invention discloses a farm fertilizer produced by biologic technology, in particular a decomposing agent for rapidly decomposing straws at low cost, the mixture ratio of which is aerobic bacillus subtilis Cohn 20%, Bacillus licheniformis 10%, aspergillus flavus 20%, chaetomiaceae 20%, absidia corymbifera 20%, and saccharomycetes 10%. The production procedures are: extracting different beneficial decomposed bacteria from organics from different environments, purifying, rejuvenating, breeding and culturing; inoculating the cultured bacterial strains into disinfected solid culture medium; fermenting for 4-5 days under 25-50 DEG C, airing dry so that the residual moisture in the bacterial strains is 25%; mixing, agitating evenly and packing the aired solid bacterial strains into solid decomposing agent. As the invention is made by using a unique optimization and combination of aerobic bacterial group and a high and new technology and production process, the invention is a pure biologic product, is free from smell and harm for making organic fertilizer by effectively decomposing wastes in the countryside, and the fermenting speed is high, the cost is low, and the source of raw material is wide. The invention has improved the soil structure and reduces environmental pollution.
Owner:李海泉
Animal Bifidobacterium and use method thereof
ActiveCN102191192AGood synergistic growthGood recombinationMilk preparationBacteriaEscherichia coliMicrobiological culture
The invention relates to an animal Bifidobacterium and a use method thereof. The animal Bifidobacterium is the Bifidobactrium amimalis Subsp Lactis B174, the CGMCC No. is 4182, the conservation date is September 16, 2010 and the conservation unit is the China General Microbiological Culture Collection Center. The Bifidobactrium amimalis Subsp Lactis B174 can colonize in human body and resist the gastric acid and cholate of human body. Meanwhile, the strain has better inhibitory action on Escherichia coli and can survive for 30 days at the room temperature. The strain and other lactic acid bacteria have better synergic action for growth; the strain has better combination effect with lactic acid bacteria; the product has better flavor, can be used to prepare fermented yoghurt, animal feed additives and antibacterial solution and can be widely used in the fields of other foods and biological products.
Owner:BEIJING BOJINYUAN BIOTECH
Method and Apparatus to Perform Limited Two Dimensional Separation of Proteins and Other Biologicals
ActiveUS20090194419A1Easy to separateSolve the separation problemSludge treatmentVolume/mass flow measurementElectricityElectrophoresis
A method and apparatus are provided for performing capillary isoelectric focusing followed by mobilization of the focused zones by induced hydrodynamic flow or chemical mobilization. These two dimensions of separation are integrated with real-time whole-channel electrophoresis detection and automatic sample injection to achieve a separation resolution superior to that obtainable using known orthogonal capillary two dimensional arrangements.
Owner:PROTEINSIMPLE
Devices and methods for biomaterial production
ActiveUS20050014245A1Avoid excessive fragmentationIndependent controlBioreactor/fermenter combinationsBiological substance pretreatmentsCellular componentUltrafiltration
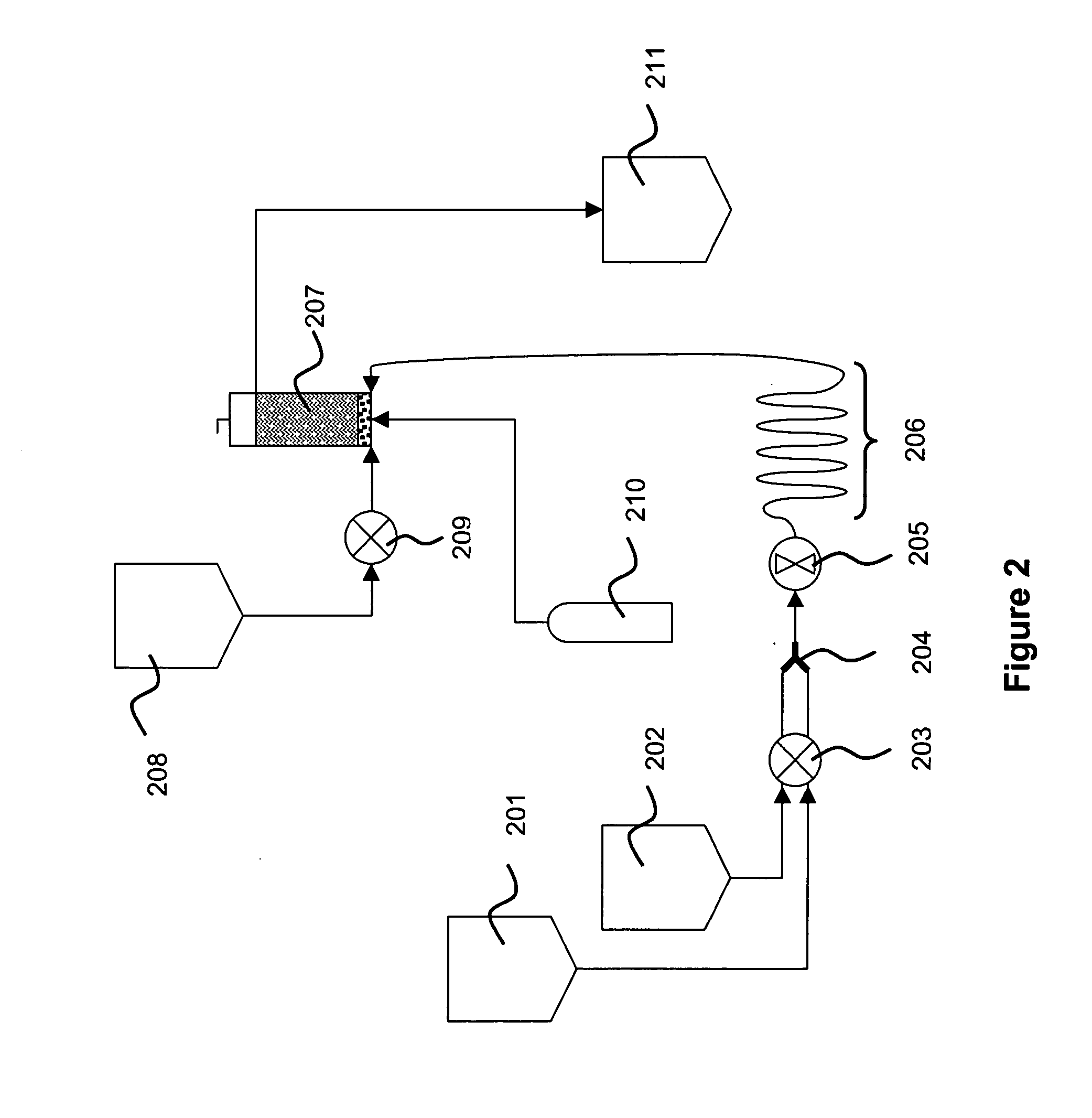
An apparatus and a method for isolating a biologic product, such as plasmid DNA, from cells. The method involves lysing cells in a controlled manner separate insoluble components from a fluid lysate containing cellular components of interest, followed by membrane chromatographic techniques to purify the cellular components of interest. The process utilizes a unique lysis apparatus, ion exchange and, optionally, hydrophobic interaction chromatography membranes in cartridge form, and ultrafiltration. The process can be applied to any biologic product extracted from a cellular source. The process uses a lysis apparatus, including a high shear, low residence-time mixer for advantageously mixing a cell suspension with a lysis solution, a hold time that denatures impurities, and an air-sparging bubble mixer that gently yet thoroughly mixes lysed cells with a neutralization / precipitation buffer and floats compacted precipitated cellular material.
Owner:VGXI
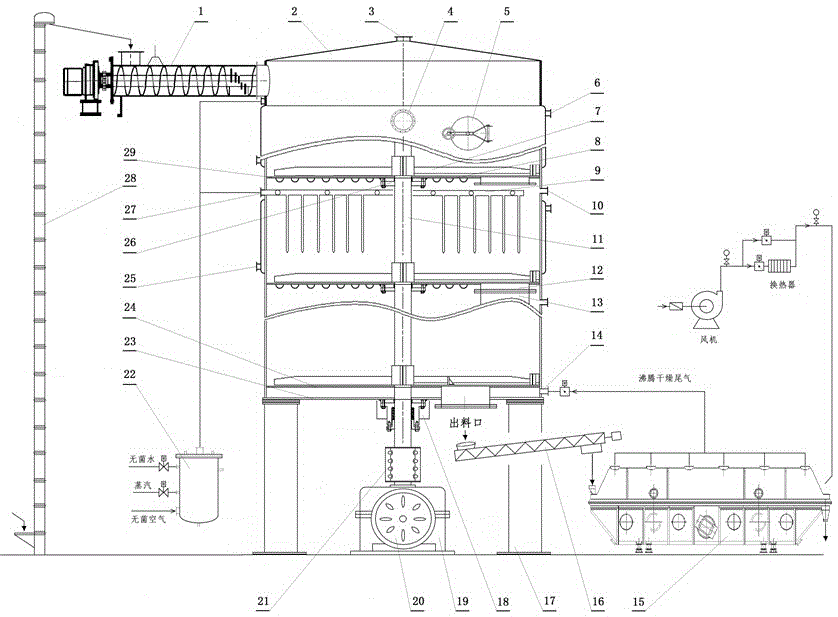
Full-automatic control multifunctional solid-state fermentation tank
ActiveCN106701563ARealize mechanized controlRealize temperature controlBioreactor/fermenter combinationsBiological substance pretreatmentsAutomatic controlControl system
The invention discloses a full-automatic control multifunctional solid-state fermentation tank, comprising a round vertical fermentation tank main body and a fermentation control system, wherein a loading auger is arranged at one side of the upper end of the fermentation tank main body; multiple clapboards are arranged in the fermentation tank main body; a high-material-bed material level automatic control device is arranged in each fermentation layer; a sterile air distribution system is arranged in each fermentation layer and is connected with a sterile air generation tank positioned at the outer side of the fermentation tank main body; a main shaft is rotationally arranged in the fermentation tank main body; and the upper ends of the clapboards are provided with stirring blades. The full-automatic control multifunctional solid-state fermentation tank disclosed by the invention integrates eight functions including dry material mixing, watering material mixing, steam sterilization, inoculation, automatic fermentation control, low-temperature drying, raw material crushing, automatic discharging and finished product smashing, and enables all operations of solid-state fermentation to be completed in a multifunctional biological solid-state fermentation tank; and by virtue of the equipment disclosed by the invention, the fermentation and production preparation processes of solid biological products can be continuously completed.
Owner:郑州良源分析仪器有限公司
Drum-type vacuum pulsing temperature-variable drying method and apparatus
InactiveCN101261073AKeep drySolve delivery difficultiesDrying using combination processesDrying solid materials with heatControl systemChemical products
The invention discloses a drum-type vacuum pulsing transformation temperature drying method and a device, comprising a sealed circular roller, a heating circulating system, a pulsing vacuum system connected with the roller and a control system, wherein, the vacuum degree of the pulsing vacuum system connected with the roller varies with the material arranging from 0.097 MPa to 0 MPa, the heating temperature of the heating circulating system varies with the state of the material. The device has good heat transmitting performance, preserves the nutrient content and the active material of the material as well as the color and luster of the material well, greatly raises the drying speed, shortens the drying time, reduces the drying energy consumption and lowers the drying cost. The drum-type vacuum pulsing transformation temperature drying method and the device are suitable for drying the foods, the biological products, the drugs and the chemical products with good fluidness, particularly for drying the products thermally sensitive; especially, the effect for drying the foods and the biotechnology products with high added value is better.
Owner:CHINA AGRI UNIV
Manufacturing method of ice bag for preserving biological products
ActiveCN103194177AImprove cooling effectImprove bindingHeat-exchange elementsFreezing thawingDissolution
The invention discloses a manufacturing method of an ice bag for preserving biological products. The manufacturing method comprises the following steps: 1) adding a thickener into hot water or boiling water, and fully stirring till dissolution; 2) adding high molecular water-absorbing resin into a solution in the step 1) while hot, stirring, then standing to carry out full reaction and imbibition on the high molecular water-absorbing resin and the thickener, and forming a gelatinous solid, wherein the reaction time is 5-30minutes; and 3) bagging the gelatinous solid in the step 2), cooling, and then placing into a cold storage, a refrigerating cabinet or a refrigerator at the temperature of -10 DEG C to -20 DEG C for freezing for above 12hours. The invention provides a simple, convenient and high-efficient manufacturing method of a high molecular cold storage agent, namely the manufacturing method of the ice bag for preserving the biological products. The prepared cold storage agent has the advantages of high repeatability, great freezing-thawing phase change latent heat, long cold storage time and fast water absorption speed, and furthermore, the cost of the process is low; and by changing the added thickener, the different phase change temperatures can be obtained for meeting various different application requirements.
Owner:浙江赛灵特医药科技有限公司
Biological alarm
ActiveUS7591980B2Easy to cleanImprove collection efficiencyRadiation pyrometryParticle separator tubesEngineeringBuilding automation
Airborne particles are impacted on a collection surface, analyzed, and then the collection surface is regenerated. Thus, the same collection surface can be used in numerous cycles. The analysis can be focused on one or more properties of interest, such as the concentration of airborne biologicals. Sensors based on regenerative collection surfaces may be incorporated in many networks for applications such as building automation.
Owner:FLIR DETECTION
Zooblast culture medium dry powder composition, culture medium composition and preparation method thereof
The invention provides a dry powder composition for a zooblast culture medium. Compared with the prior dry powder composition for the zooblast culture medium, the dry powder composition provided by the invention reduces the serum dosage of animals by introduction of recombinant protein or animal origin compositions, the dry powder composition for the zooblast culture medium can be matched with low-content animal serum for use without additional introduction of protein compositions (such as the recombinant protein, plant protein or the animal origin compositions including cytokines and so on), and has the same or better function on promoting the growth of zooblast compared with high-content animal serum, so that the dry powder composition for the zooblast culture medium does not generate side effects along with the protein compositions, and has good safety and low cost. Cells cultured by the dry powder composition for the zooblast culture medium have small difference for different batches, low cost and good safety, are favorable for separating downstream products of the cells, and are suitable to be used for virus hosts, expression vectors and so on of biological products and other biological products.
Owner:BEIJING SKYWING TECH CO LTD
Method for continuously separating and purifying valine in fermentation liquor by using simulated moving bed chromatography
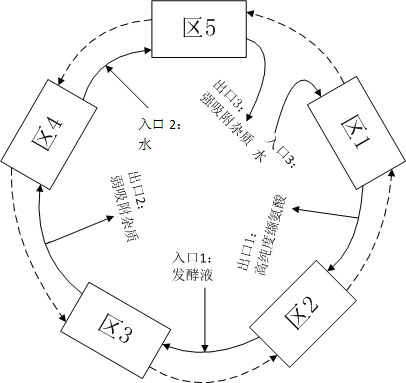
InactiveCN101948399AHigh purityFully cleanedOrganic compound preparationSolid sorbent liquid separationSimulated moving bedMoving bed
The invention discloses a method for continuously separating and purifying valine in fermentation liquor by using simulated moving bed chromatography, and belongs to the technical field of biological product processing. In the method, a simulated moving bed chromatography separating device suitable for the composition characteristic of valine fermentation liquor is designed, water is taken as a mobile phase, and the previously invented resin special for valine separation (disclosed in the Chinese patent 200910231735.4) is taken as a stationary phase. The method can completely remove impurities of alanine and leucine from the valine fermentation liquor, achieves product purity of over 99.7 percent to meet the medicinal standard, is suitable for continuous production, has higher recovery rate and production strength, and overcomes the defects of low purity, low efficiency, high pollution and the like in the conventional valine separation and purification process.
Owner:JIANGNAN UNIV
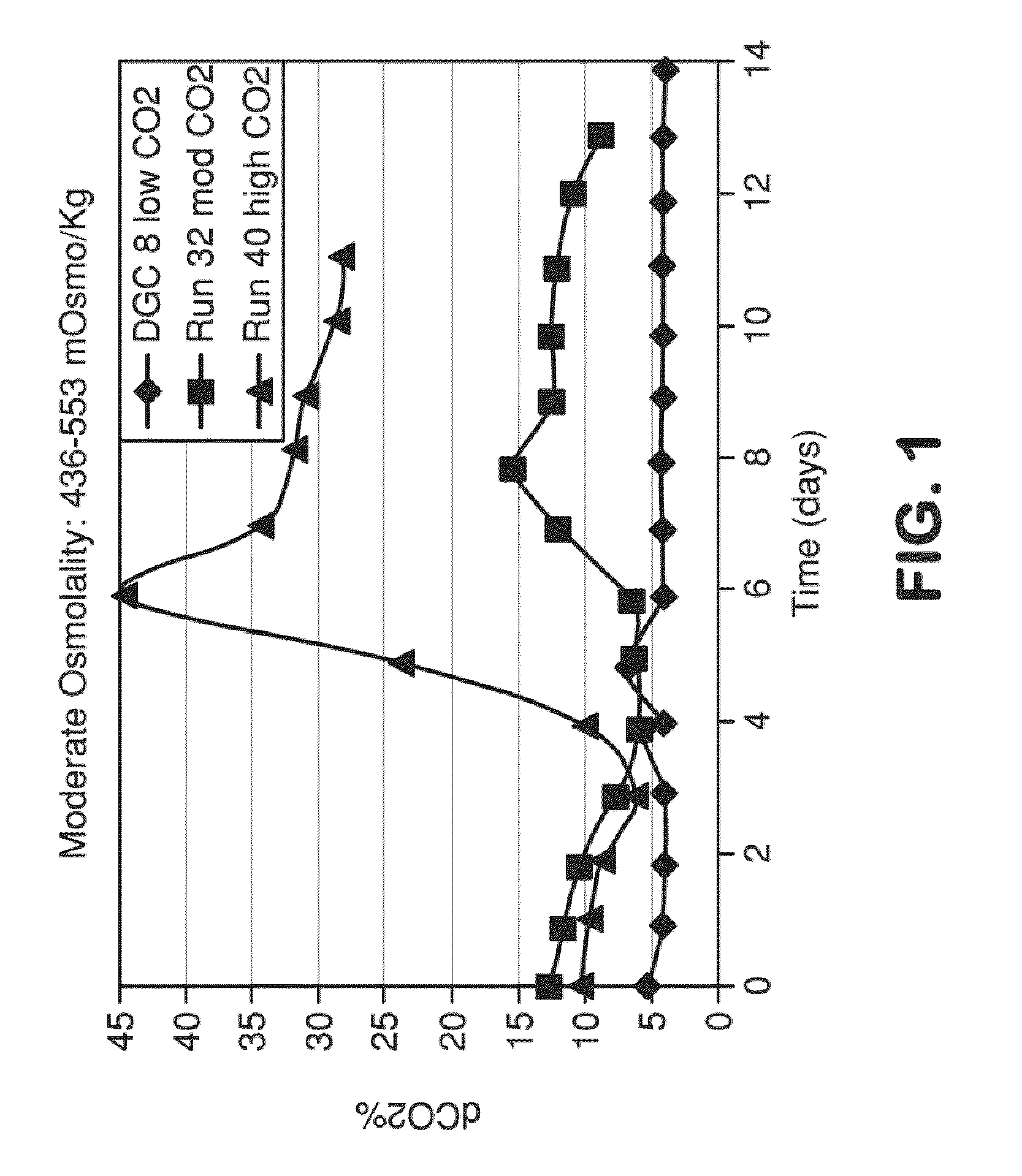
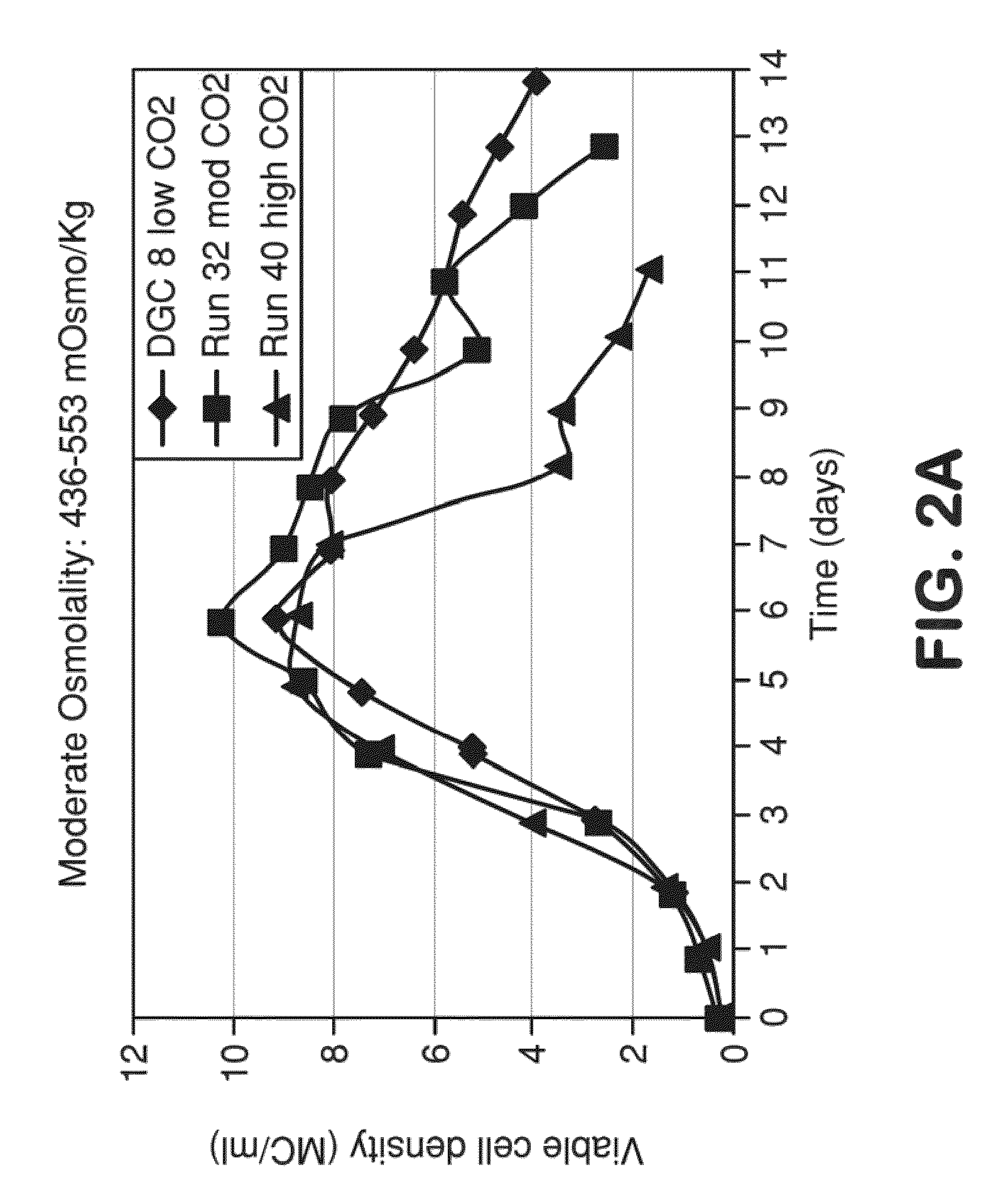
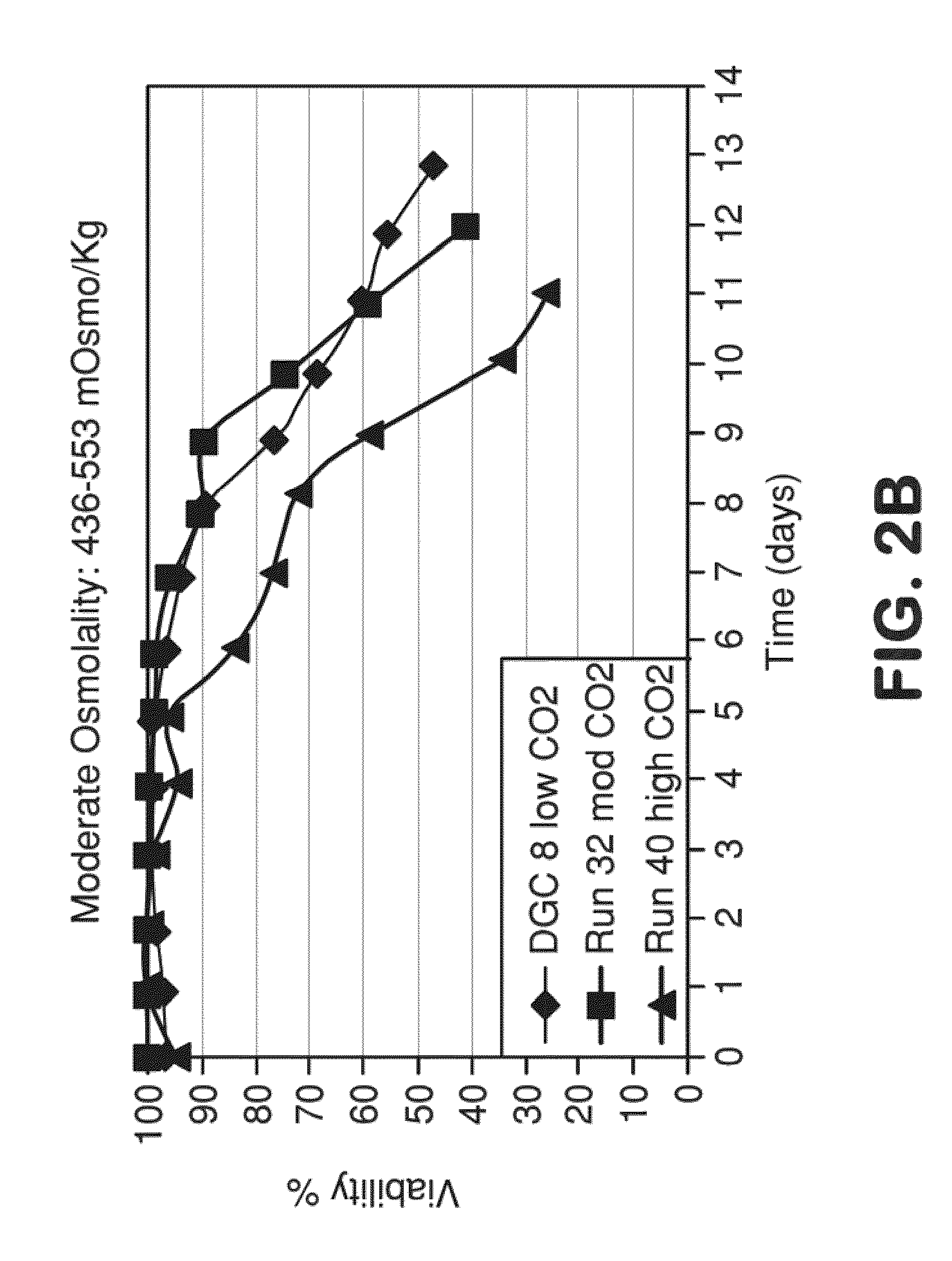
METHOD FOR CONTROLLING pH, OSMOLALITY AND DISSOLVED CARBON DIOXIDE LEVELS IN A MAMMALIAN CELL CULTURE PROCESS TO ENHANCE CELL VIABILITY AND BIOLOGIC PRODUCT YIELD
ActiveUS20100184147A1Facilitate communicationImprove production yieldBioreactor/fermenter combinationsBiological substance pretreatmentsBiotechnologyPh control
Methods for controlling the level of dissolved carbon dioxide and limiting osmolality in a mammalian cell culture process to enhance cell growth, viability and density, and increase biologic product concentration and yield are provided. Such control of the level of dissolved carbon dioxide and pH as well as the resulting ability to limit osmolality in a mammalian cell culture process is achieved by adopting alternative pH control strategies and CO2 stripping techniques during a mammalian cell culture process. Such pH control techniques and carbon dioxide stripping occur without foam and with little or no damage to the mammalian cells.
Owner:PRAXAIR TECH INC
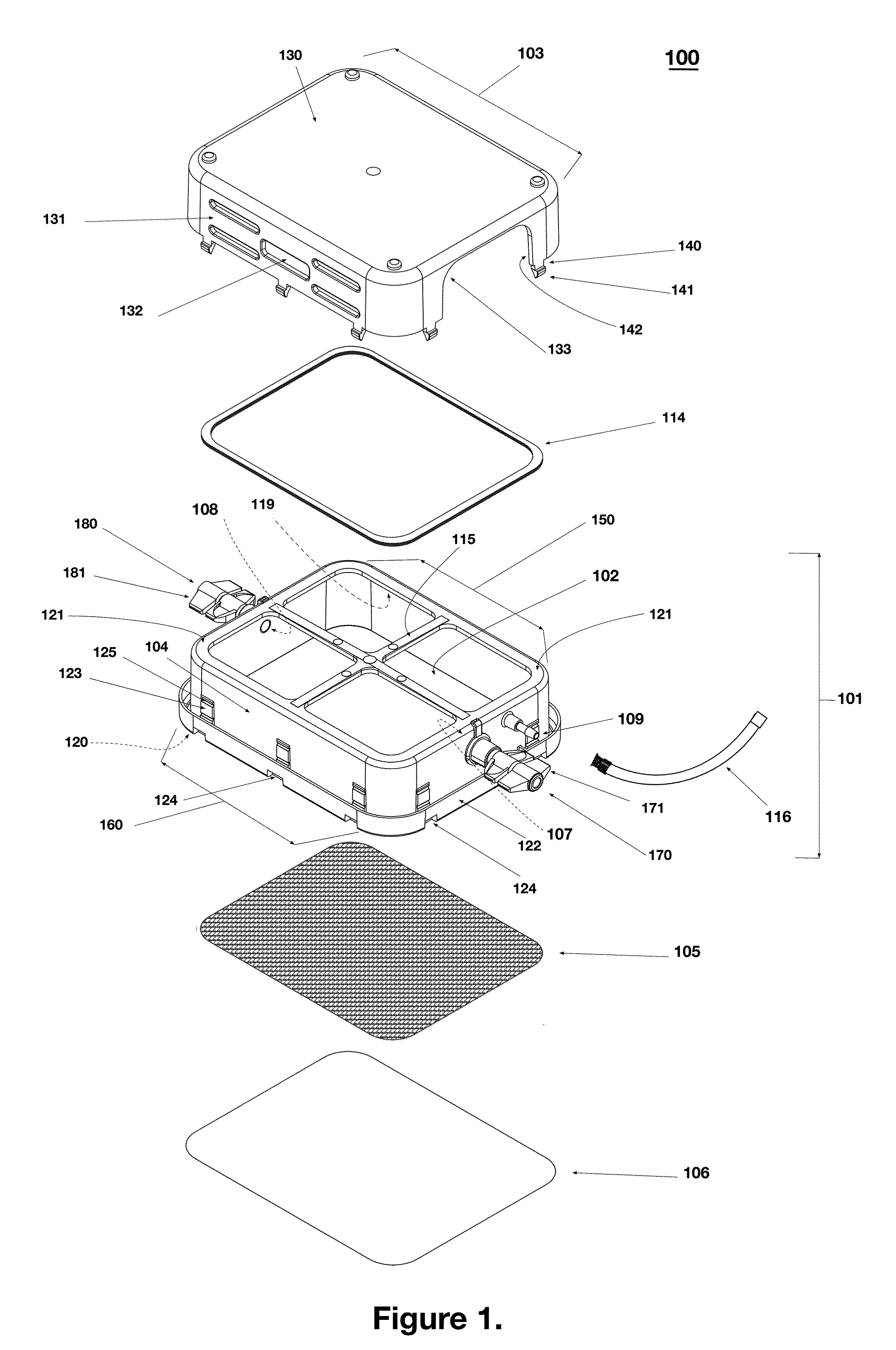
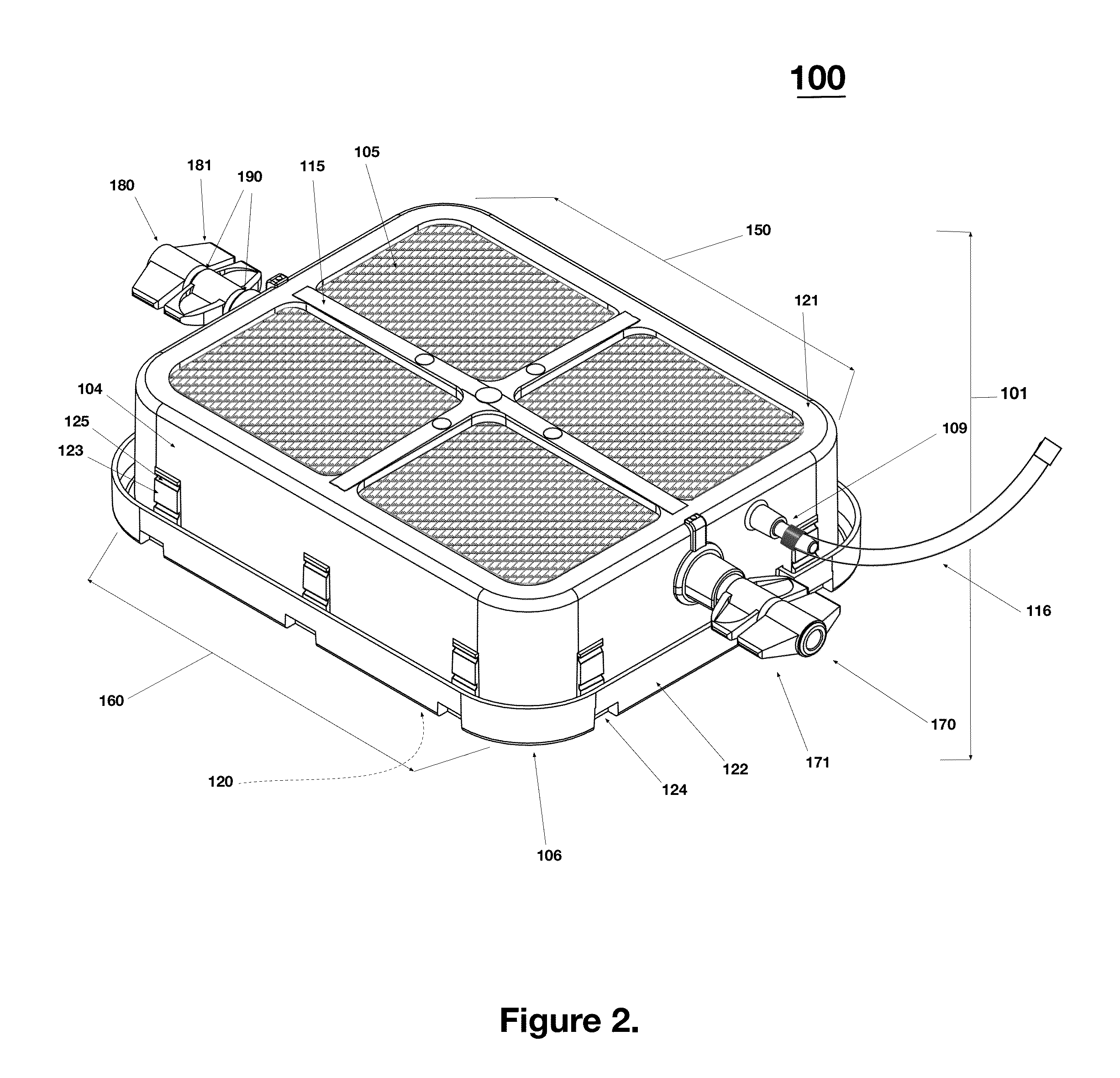
Monitoring shipment of biological products to determine remaining refrigerant quantity
ActiveUS8696151B1Reduces effective lifeRetain valueTemperatue controlContainer filling methodsRefrigerantBiologic Products
Disclosed is a process of determining, at a point during shipment, whether the solid phase refrigerant in a shipment is sufficient to preserve the shipment cargo, which is blood or other biological products, for the remaining shipment period, by: monitoring the temperatures encountered to said point and estimating the temperatures likely to be encountered during the remaining shipment period; determining the likelihood that the remaining refrigerant can maintain the shipment cargo within a specified temperature range during the remaining shipment period; and if the risk that the remaining refrigerant cannot maintain the shipment cargo within said range during the remaining shipment period is above a cut-off level, then taking action to preserve the value of the cargo.
Owner:INTEGREON GLOBAL INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com