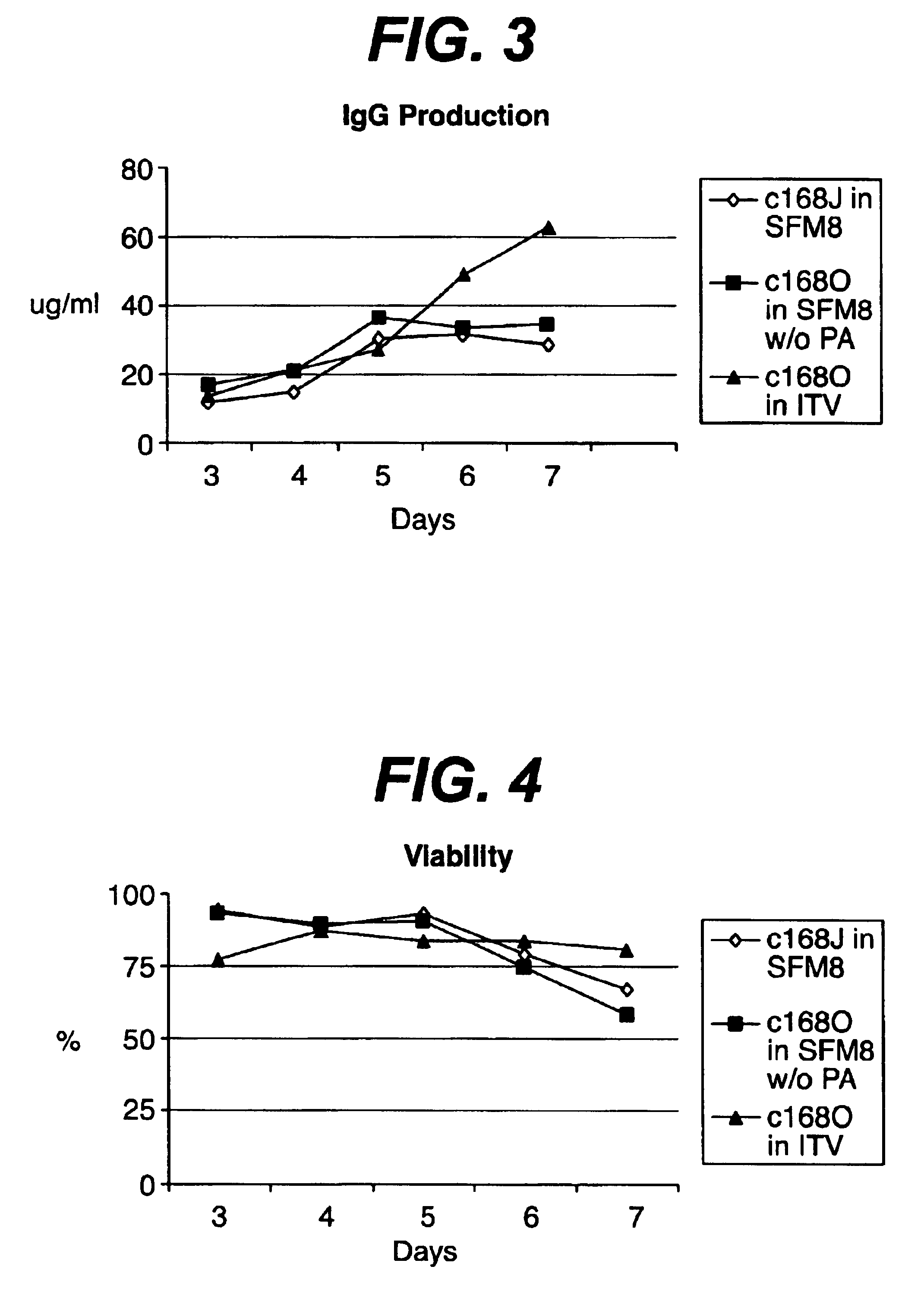
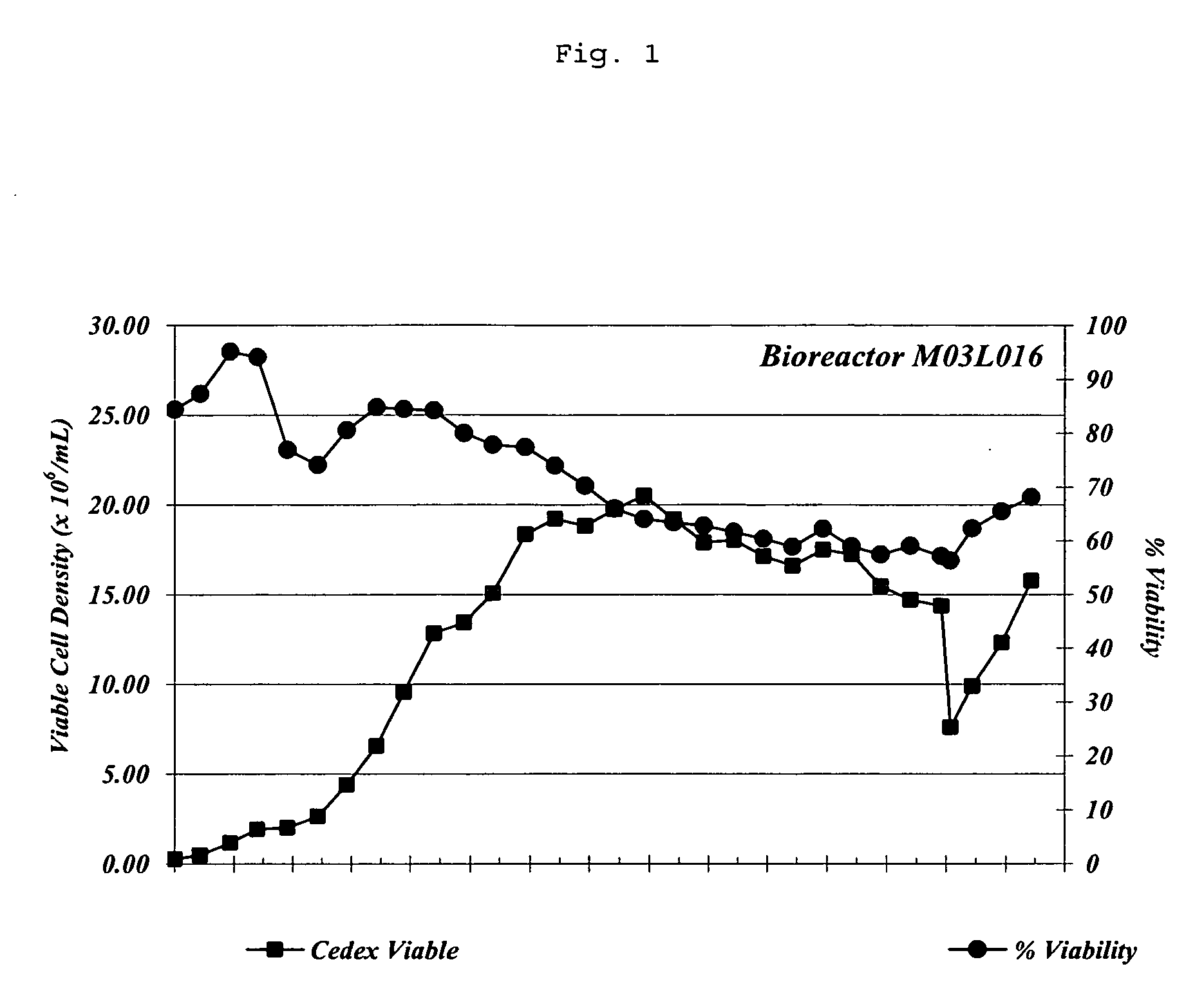
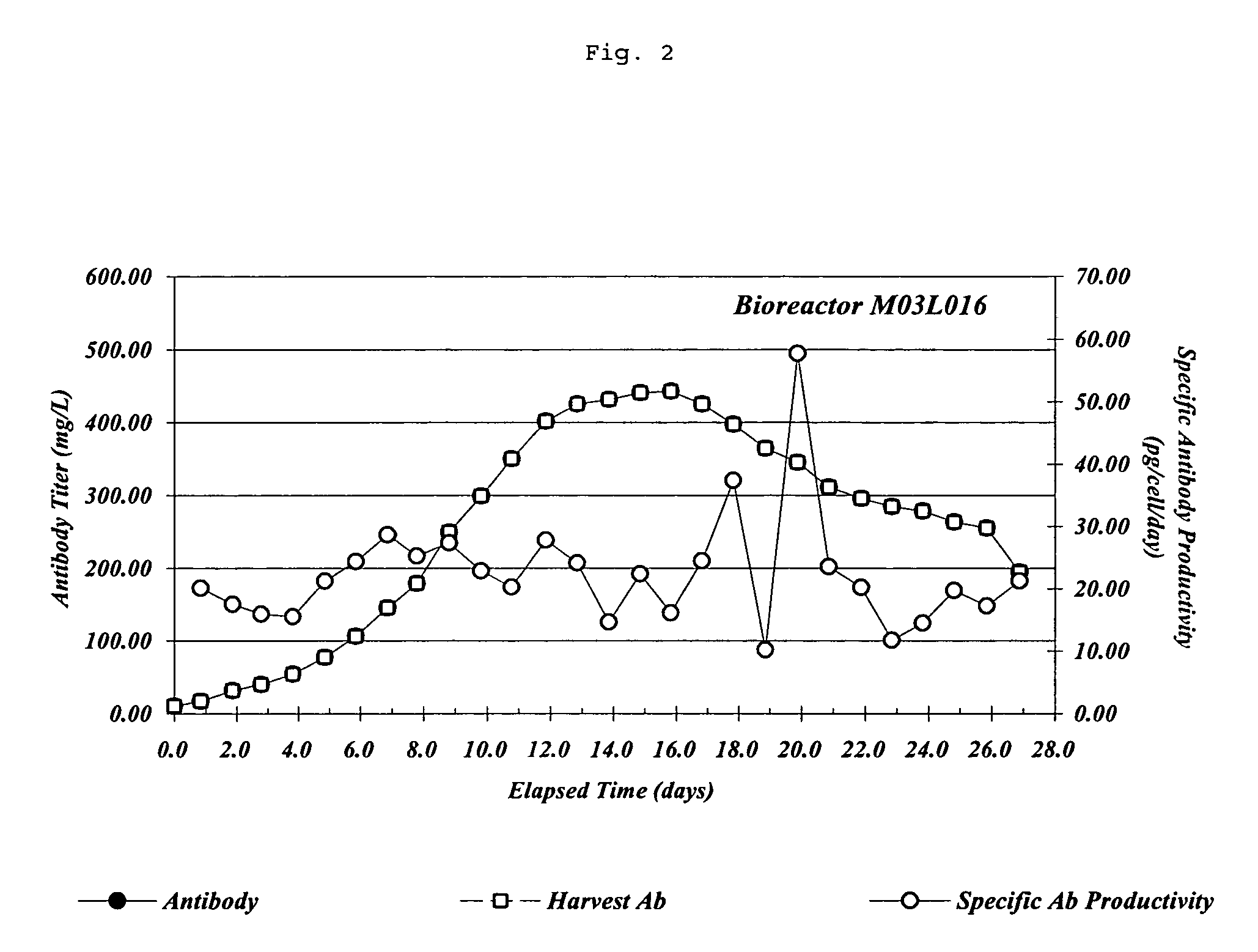
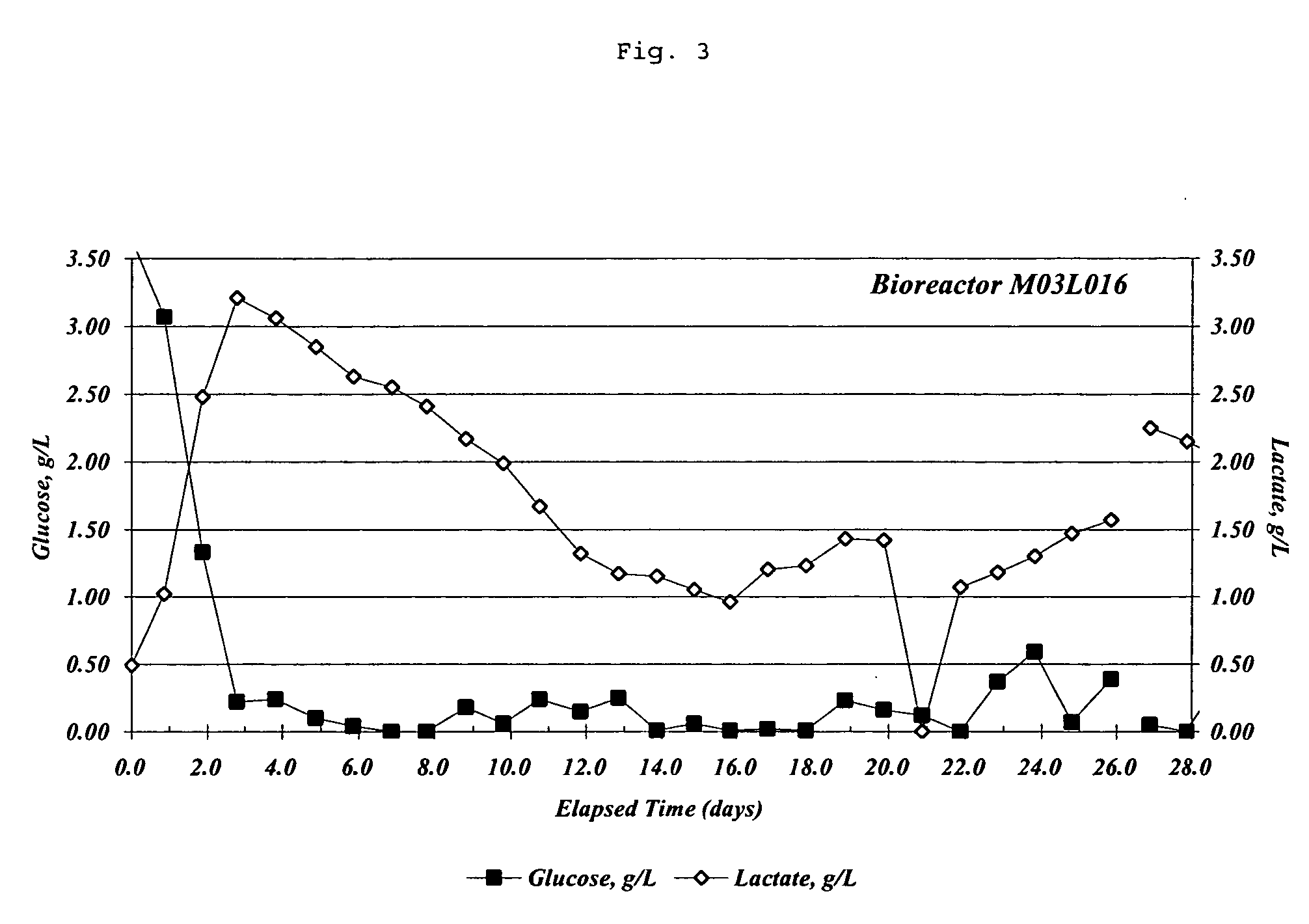
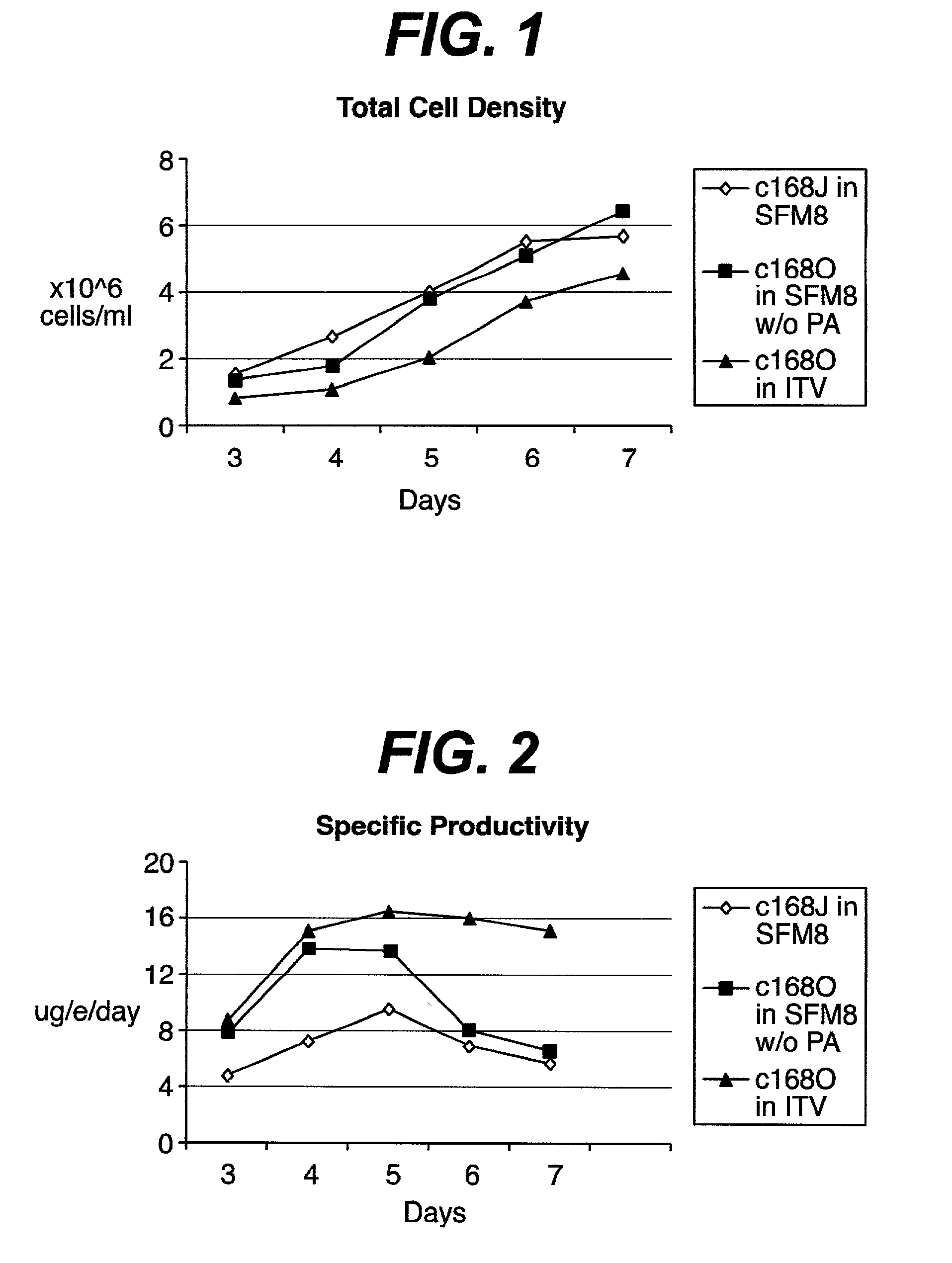
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
70 results about "Chemically defined medium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chemically defined medium is a growth medium suitable for the in vitro cell culture of human or animal cells in which all of the chemical components are known. Standard cell culture media commonly consist of a basal medium supplemented with animal serum (such as fetal bovine serum, FBS) as a source of nutrients and other ill-defined factors. The technical disadvantages to using serum include its undefined nature, batch-to-batch variability in composition, and the risk of contamination.
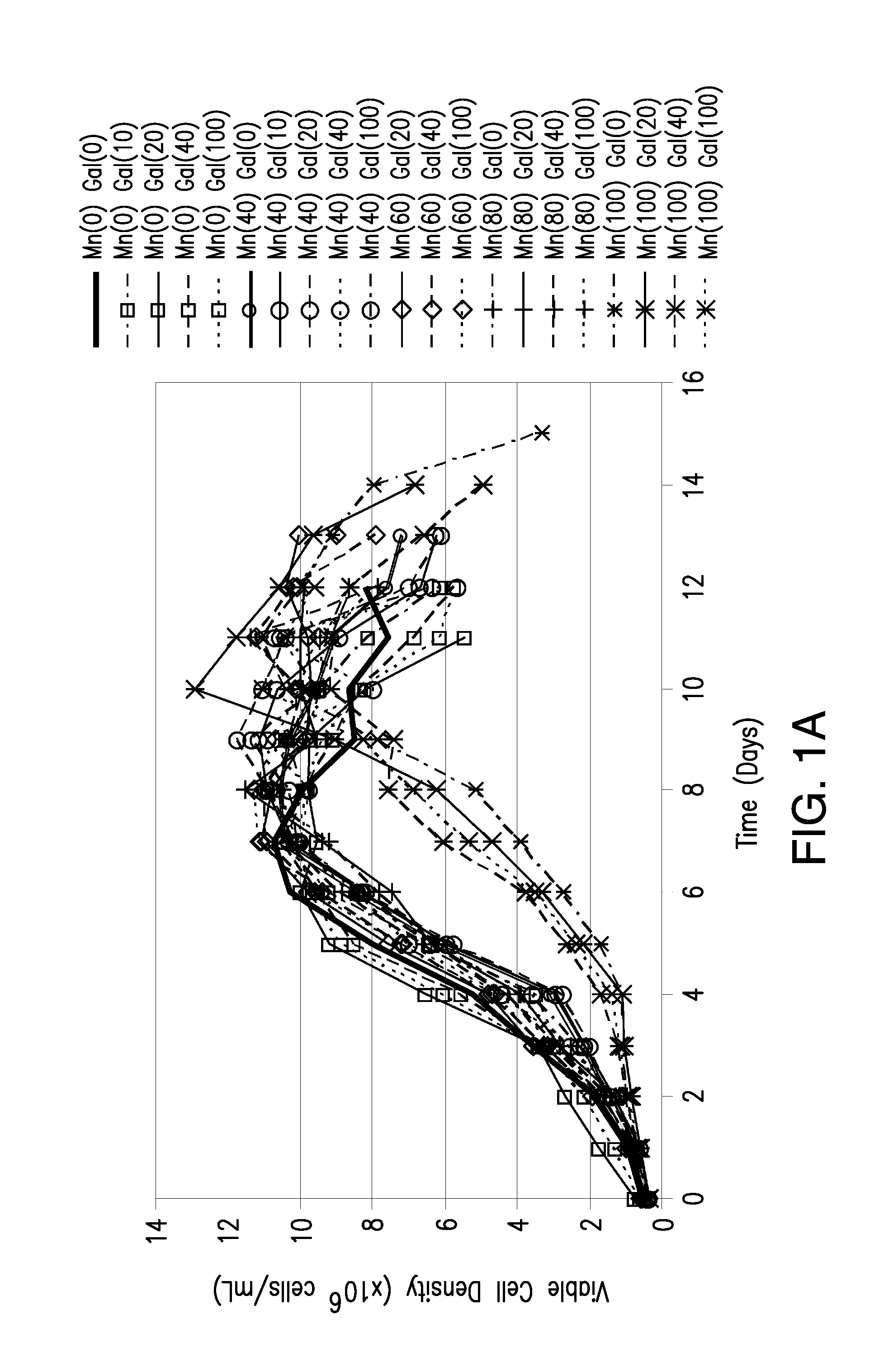
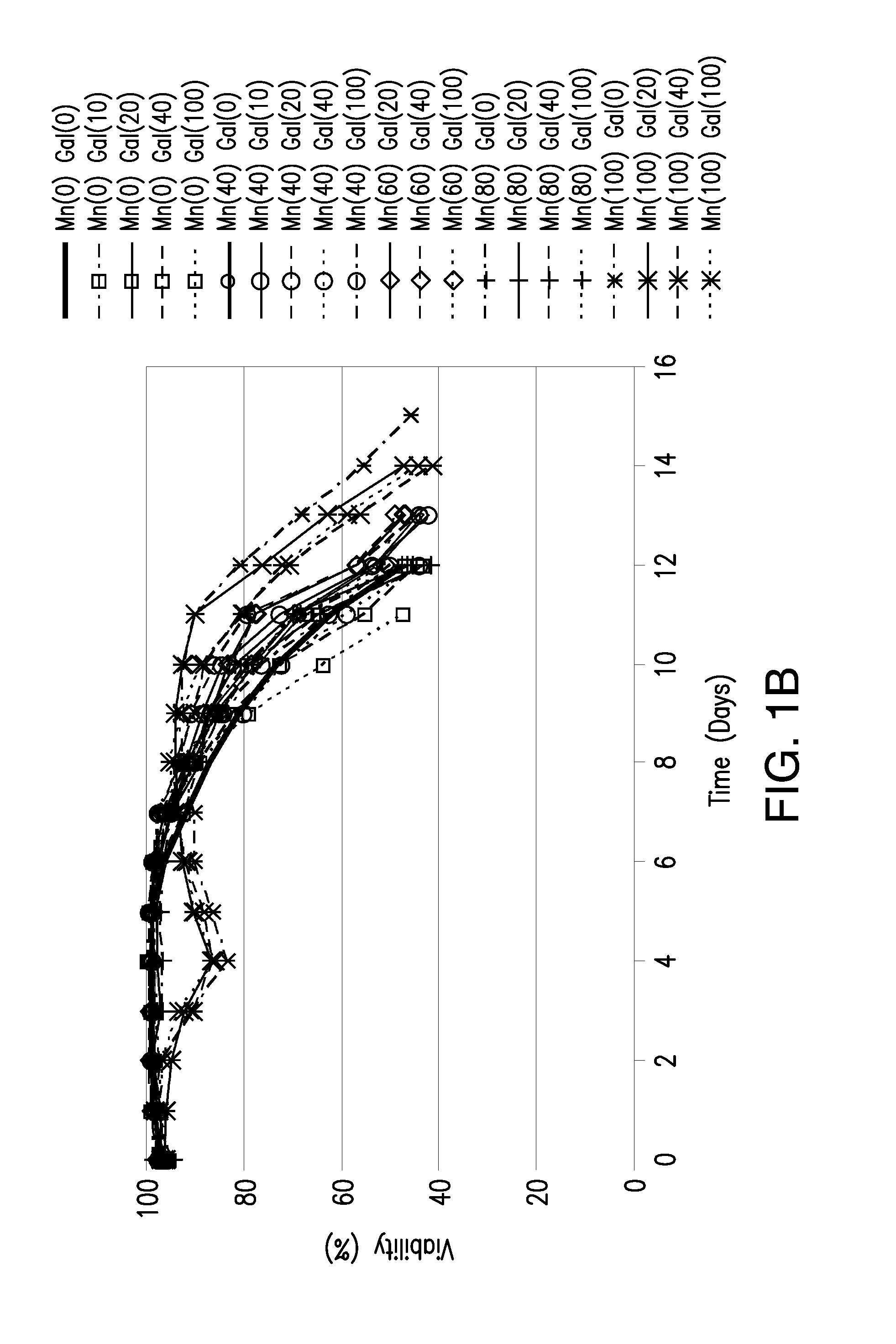
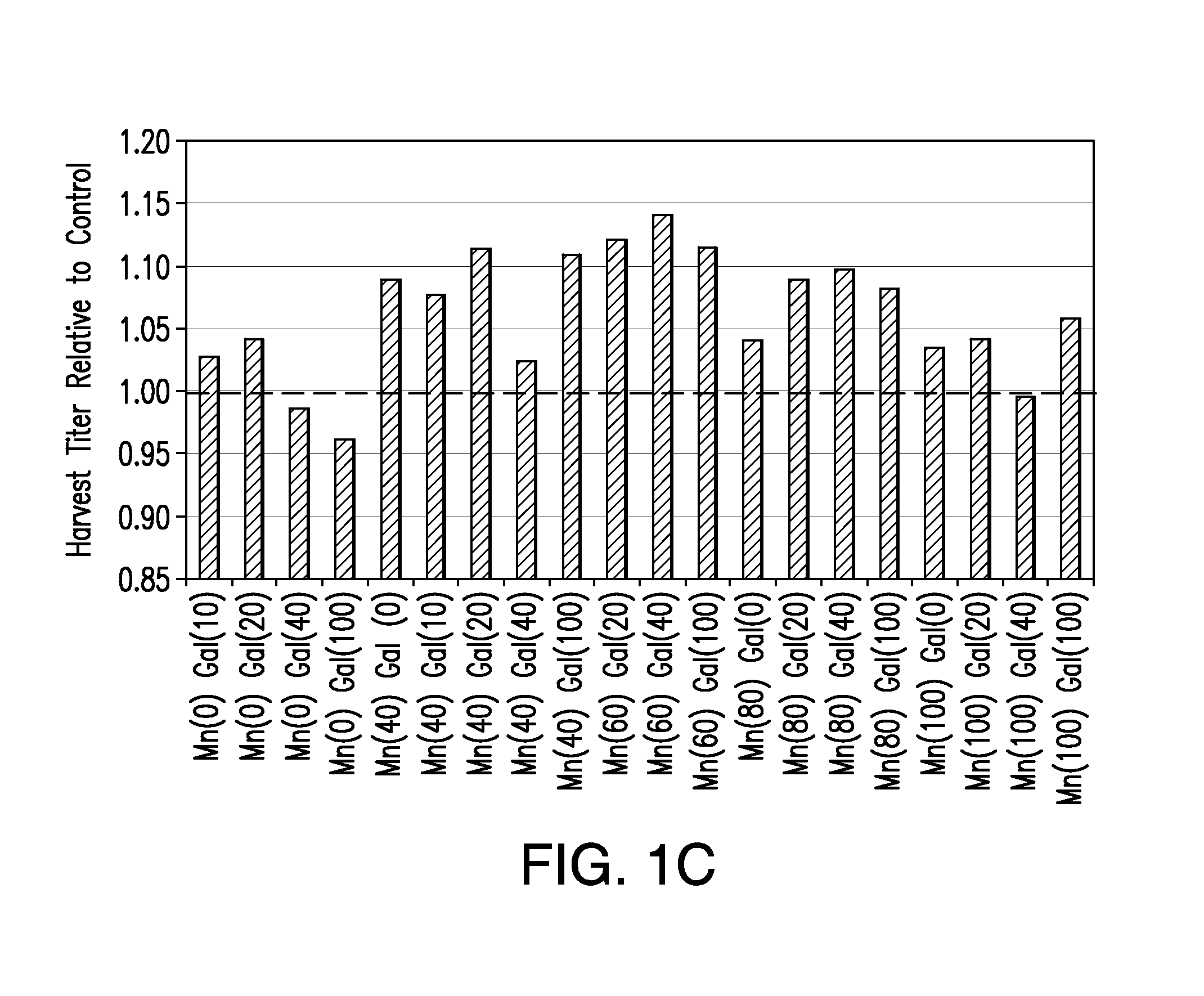
Methods for controlling the galactosylation profile of recombinantly-expressed proteins
The present invention relates to methods for modulating the glycosylation profile of recombinantly-expressed proteins. In particular, the present invention relates to methods of controlling the galactosylation profile of recombinantly-expressed proteins by supplementing production medium, e.g., a hydrolysate-based or a chemically defined medium, with manganese and / or D-galactose.
Owner:ABBVIE INC
Chemically defined medium for cultured mammalian cells
InactiveUS6900056B2Reduced regulatory concerns for proteinsAdvantageously producedCell receptors/surface-antigens/surface-determinantsGenetically modified cellsSerum free mediaChemical composition
Owner:CENTOCOR
Synthetic surfaces for culturing cells in chemically defined media
InactiveUS20090191627A1Reduce Potential ContaminationExtended shelf lifeArtificial cell constructsCell culture supports/coatingSerum free mediaCross-link
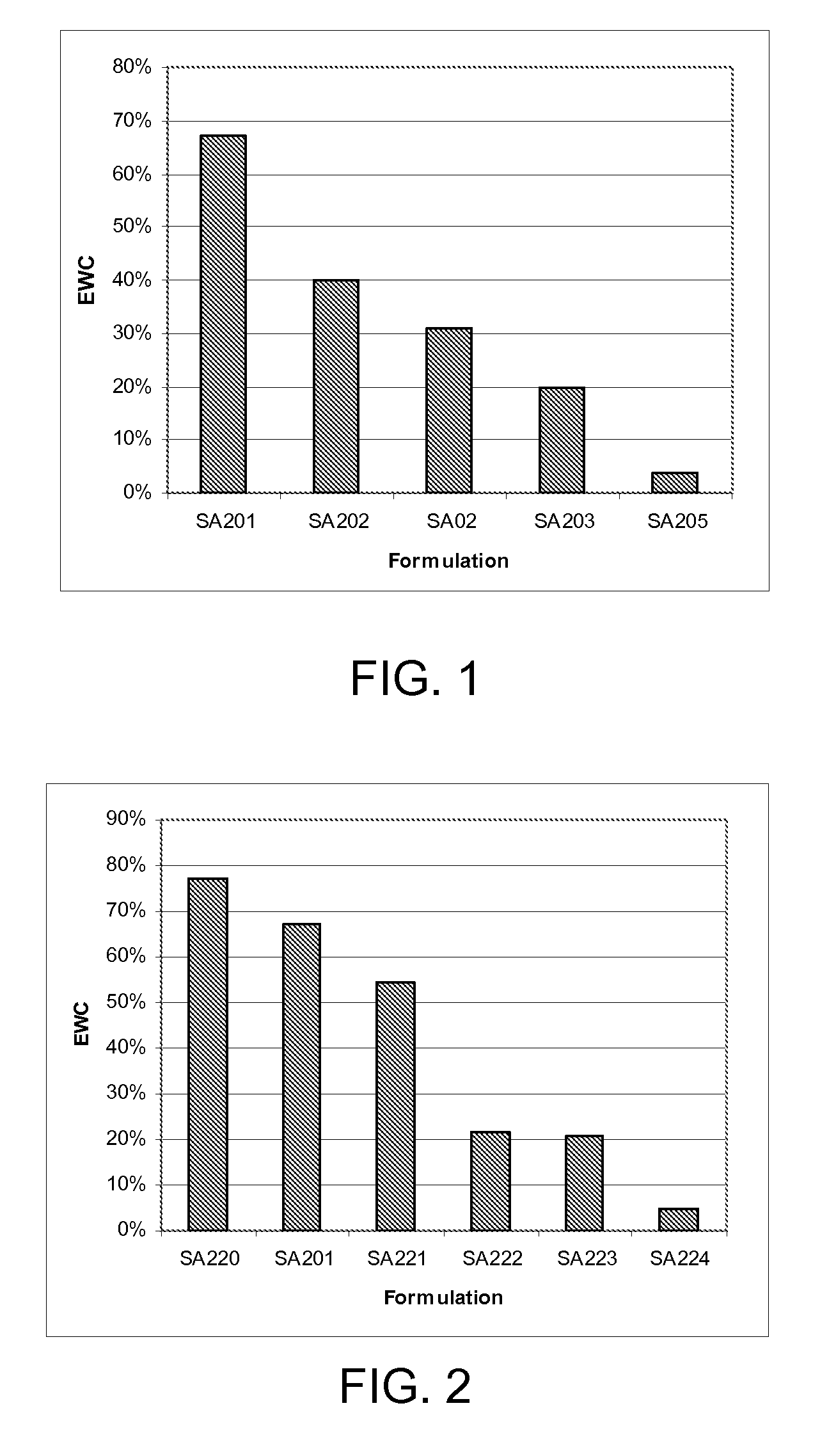
Synthetic surfaces capable of supporting culture of eukaryotic cells including stem cells and undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a polypeptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes a carboxyl group-containing (meth)acrylate monomer, a cross-linking (di- or higher-functional) (meth)acrylate monomer, and a hydrophilic monomer capable of polymerizing with the carboxyl group-containing (meth)acrylate monomer and the cross-linking (meth)acrylate monomer. The swellable (meth)acrylate layer has an equilibrium water content in water of between about 5% and about 70%. The conjugated peptide may include an RGD amino acid sequence.
Owner:GERON CORPORATION
Swellable (METH)acrylate surfaces for culturing cells in chemically defined media
InactiveUS20090191632A1Reduce Potential ContaminationExtended shelf lifeCell culture supports/coatingEmbryonic cellsSerum free mediaPolymer science
Synthetic surfaces capable of supporting culture of undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a peptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes hydroxyethyl methacrylate, 2-carboxyehylacrylate, and tetra(ethylene glycol) dimethacrylate. The conjugated peptide may include an amino acid sequence of XaanProGlnValThrArgGlyAspValPheThrMetPro, where n is an integer from 0 to 3 and where Xaa is any amino acid. Further, disclosed herein is a swellable (meth)acrylate synthetic surface which can be sterilized by gamma irradiation.
Owner:GERON CORPORATION
Culture medium used for Vero cell and cultivation method thereof
InactiveCN101182490AFast growthArtificial cell constructsVertebrate cellsSerum free mediaMicrobiology
The invention relates to the preparation and application method of a medium, which is specifically related to a medium which is used for Vero cell and a culture method of the Vero cell. The invention belongs to the biological product area. The culture method of the invention is that the adhering Vero cells are suspend and cultured in SFM serum-free medium for the domestication and adaptation; the growing pattern of the Vero cell changes from adherence growth to suspension growth; the Vero cells are further amplified in Vero amplification CHEMICALLY DEFINED MEDIUM. The method of the invention has good effects; the growing pattern of the Vero cell is transformed from adherence growth to suspension growth, which changes the growth pattern of the cell and greatly increases the yield of the cell.
Owner:天津百若克医药生物技术有限责任公司
Chemically defined media compositions
Chemically defined media compositions for the culture of eukaryotic cells are disclosed. The compositions are useful for eukaryotic cell culture in perfusion bioreactors and other vessels.
Owner:JANSSEN BIOTECH INC
Chemically defined medium for cultured mammalian cells
InactiveUS20030096402A1Protein producingReduced regulatory concerns for proteinsCell receptors/surface-antigens/surface-determinantsGenetically modified cellsSerum free mediaChemical composition
Owner:CENTOCOR
Synthetic Surfaces for Differentiating Stem Cells into Cardiomyocytes
InactiveUS20100248366A1Good reproducibilityReduce Potential ContaminationCell culture supports/coatingEmbryonic cellsSerum free mediaHydrophilic monomer
Synthetic surfaces capable of supporting culture of eukaryotic cells including stem cells and undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a polypeptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes a carboxyl group-containing (meth)acrylate monomer, a cross-linking (di- or higher-functional) (meth)acrylate monomer, and a hydrophilic monomer capable of polymerizing with the carboxyl group-containing (meth)acrylate monomer and the cross-linking (meth)acrylate monomer. The swellable (meth)acrylate layer has an equilibrium water content in water of between about 5% and about 70%. The conjugated peptide may include an RGD amino acid sequence.
Owner:ASTERIAS BIOTHERAPEUTICS INC
Synthetic Surfaces for Culturing Stem Cell Derived Cardiomyocytes
ActiveUS20090191633A1Reduce Potential ContaminationExtended shelf lifeBioreactor/fermenter combinationsBiological substance pretreatmentsSerum free mediaHeart Muscle Cell
Owner:ASTERIAS BIOTHERAPEUTICS INC
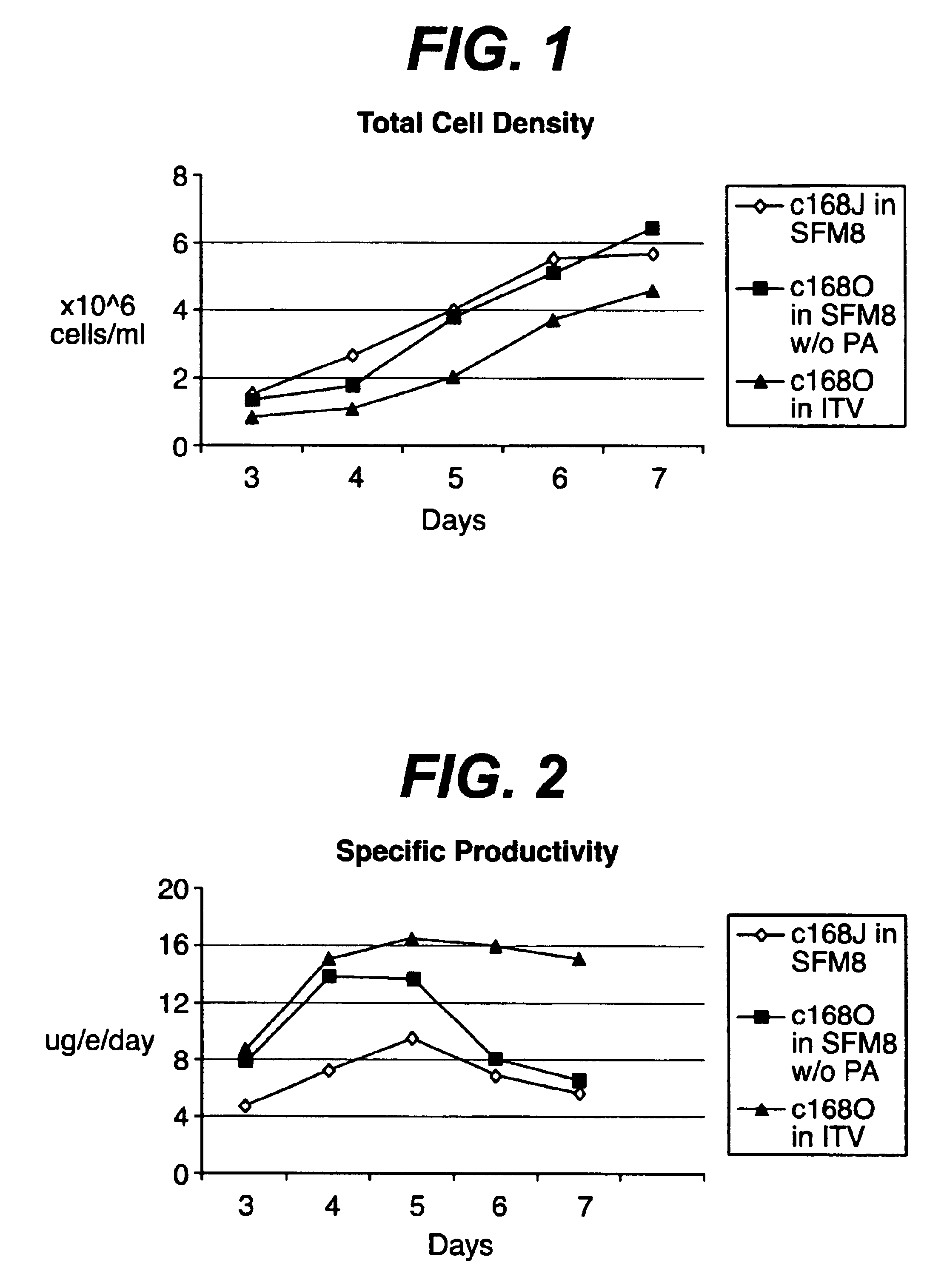
Method of producing a polypeptide or virus of interest in a continuous cell culture
ActiveUS8580554B2Increase productionHigh densityCulture processCell culture mediaSerum free mediaSerum free
Described herein is a chemostat-like continuous cell culture system that combines certain advantages of perfusion open systems and chemostat open systems to improve the culturing of mammalian cells, e.g., genetically modified cells, particularly in serum-free or chemically-defined media. The continuous culture system described herein involves culturing mammalian cells in a continuous cell culture system, which comprises a cell retention device, wherein the cell culture system has a dilution rate (D) of less than about 2 d−1, and a cell density of less than about 2×107 cell / mL. Also described herein is a method for producing a polypeptide and / or virus of interest in a continuous cell culture, the method comprising culturing mammalian cells expressing the polypeptide and / or virus of interest in a continuous cell culture system, which comprises a cell retention device, wherein the cell culture system has a dilution rate (D) of less than about 2 d−1, and a cell density of less than about 2×107 cell / mL; and recovering the polypeptide and / or virus of interest from medium of the cell culture system.
Owner:TAKEDA PHARMA CO LTD
Suspension acclimatization and serum-free acclimatization method for HEK (human embryonic kidney)-293T cells
ActiveCN103205396AStable growthGood dispersionBlood/immune system cellsMicrobiology processesKidneyChemically defined medium
The invention relates to a suspension acclimatization and serum-free acclimatization method for HEK (human embryonic kidney)-293T cells, and belongs to the field of biotechnology. The suspension acclimatization and serum-free acclimatization method includes acclimatizing the HEK-293T cells adaptive to adherent culture so that the HEK-293T cells are adaptive to suspension culture in culture media with serum; and acclimatizing the HEK-293T cells adaptive to suspension culture in the culture media with the serum in a serum reducing mode so that the HEK-293T cells are adaptive to serum-free acclimatization. The suspension acclimatization and serum-free acclimatization method has the advantages that the growth status of the HEK-293T cells cultured by the suspension acclimatization and serum-free acclimatization method is stable, the HEK-293T cells are high in dispersibility and are completely adaptive to suspension culture in the serum-free culture media with certain chemical compositions, and batch production can be carried out.
Owner:AKESO BIOPHARMA
Method of culturing akkermansia
A method for cost-effectively and efficiently culturing Akkermansia muciniphilais is disclosed. High biomass yields can be obtained on chemically defined media. This allows for large scale production of A. muciniphila suitable for use in humans, such as for pharmaceutical or food applications. The A. muciniphila can be produced free of animal-derived products, thereby allowing a broad-range of applications.
Owner:WAGENINGEN UNIV
Chemically-defined medium, application thereof and production technology for large-scale culture of mammalian cells
ActiveCN103773732AEasy to purifyImprove stabilityArtificial cell constructsVertebrate cellsSerum free mediaHydrolysate
The invention discloses a chemically-defined medium. A disclosed DMEM / F12 formula is taken as a basis, the concentration of various components is adjusted, certain components are added or eliminated on the basis, and the chemically-defined medium is applicable to the large-scale production of various mammalian cells. The invention further discloses application of the medium in large-scale culture of mammalian cells (CHO cells, BHK cells or hybridoma cells) and a production technology for large-scale culture of mammalian cells. The chemically-defined medium provided by the invention does not contain serum or proteins, eliminates animal ingredients and vegetable protein hydrolysate used in the conventional medium, and facilitates the product purification and production batch stability, and the chemical constituents of all the components are defined, so that the product quality is guaranteed and improved; through the adoption of the medium and the production technology, provided by the invention, the cell density is improved, and the yield of target products is increased, and the overall production cost is low.
Owner:上海多宁生物科技股份有限公司
Vitro hepatic differentiation
Owner:CAMBRIDGE ENTERPRISE LTD
Fermentative production of lipids on an industrial scale using chemically defined media
InactiveUS20140342396A1Broaden the formation processIncrease probabilityFungiBacteriaBiotechnologySerum free media
Owner:DSM IP ASSETS BV
Method of Producing a Polypeptide or Virus of Interest in a Continuous Cell Culture
Described herein is a chemostat-like continuous cell culture system that combines certain advantages of perfusion open systems and chemostat open systems to improve the culturing of mammalian cells, e.g., genetically modified cells, particularly in serum-free or chemically-defined media. The continuous culture system described herein involves culturing mammalian cells in a continuous cell culture system, which comprises a cell retention device, wherein the cell culture system has a dilution rate (D) of less than about 2 d−1, and a cell density of less than about 2×107 cell / mL. Also described herein is a method for producing a polypeptide and / or virus of interest in a continuous cell culture, the method comprising culturing mammalian cells expressing the polypeptide and / or virus of interest in a continuous cell culture system, which comprises a cell retention device, wherein the cell culture system has a dilution rate (D) of less than about 2 d−1, and a cell density of less than about 2×107 cell / mL; and recovering the polypeptide and / or virus of interest from medium of the cell culture system.
Owner:TAKEDA PHARMA CO LTD
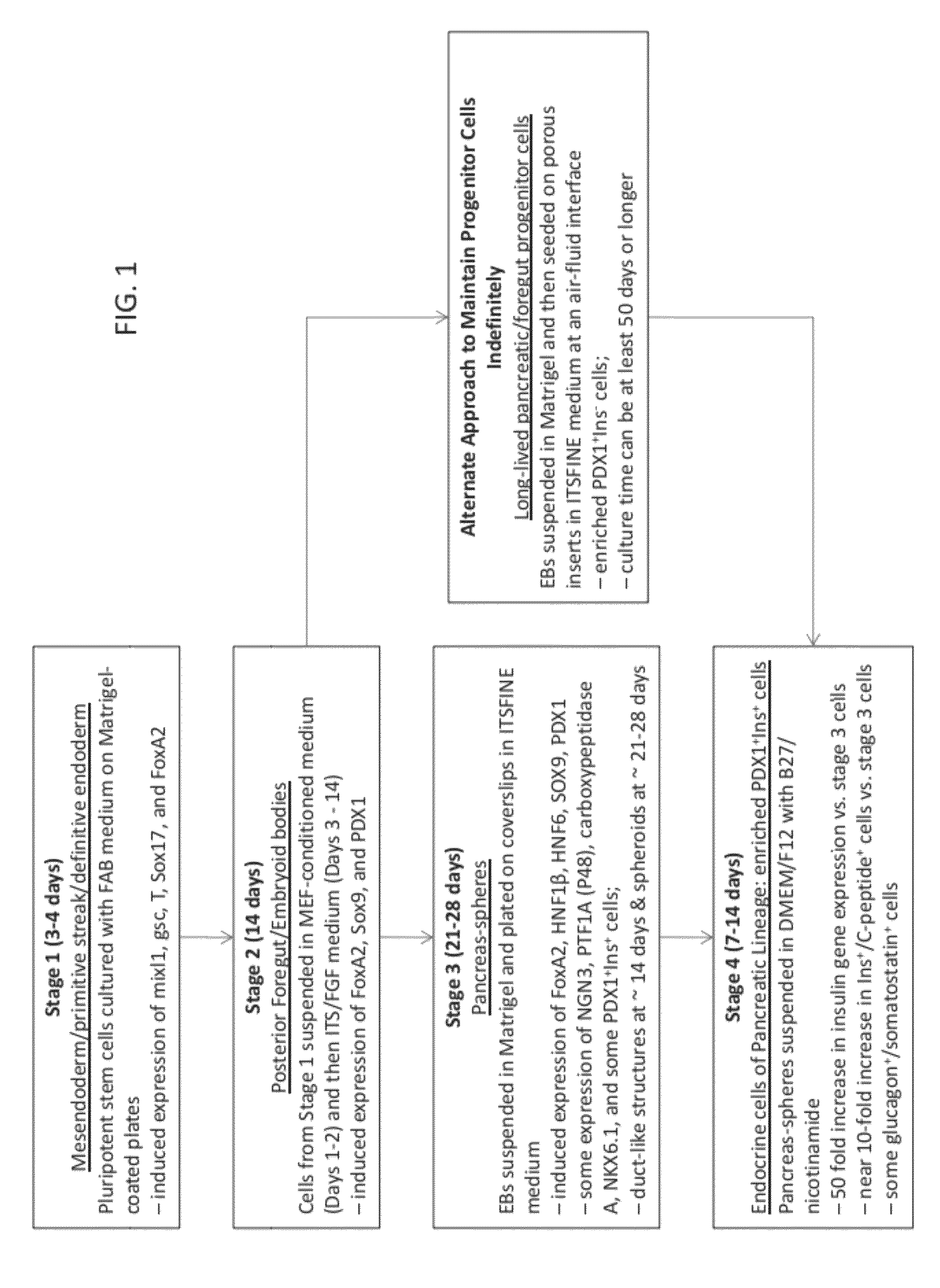
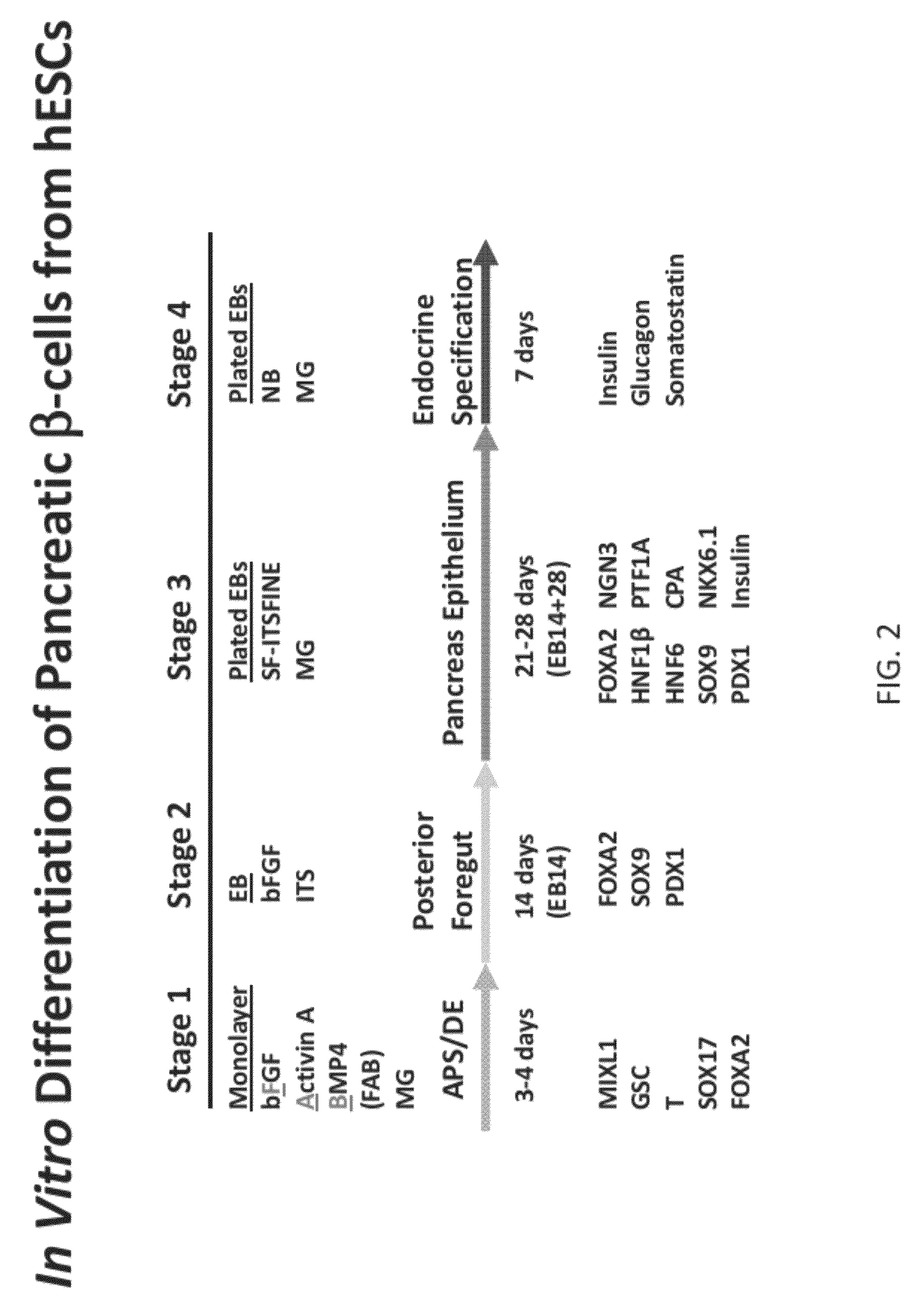
Methods and devices for differentiating pluripotent stem cells into cells of the pancreatic lineage
Methods and devices for culturing human pluripotent stem cells to produce cells of the pancreatic lineage are disclosed. The methods include steps of culturing the stem cells under conditions that induce the expression of mesendoderm / primitive streak and definitive endoderm markers in a chemically defined medium including an effective amount of i) fibroblast growth factor, ii) Activin A, and iii) bone morphogenetic protein. The methods further include the steps of culturing cells under conditions favoring the formation of at least one of intact embryoid bodies and pancreatic progenitor PDX1+Ins− cells.
Owner:WISCONSIN ALUMNI RES FOUND
Serum-free cell freezing medium
InactiveCN108450458AEasy to prepareLow toxicityDead animal preservationCell phenotypeSerum free media
Owner:成都菱祐生物科技有限公司
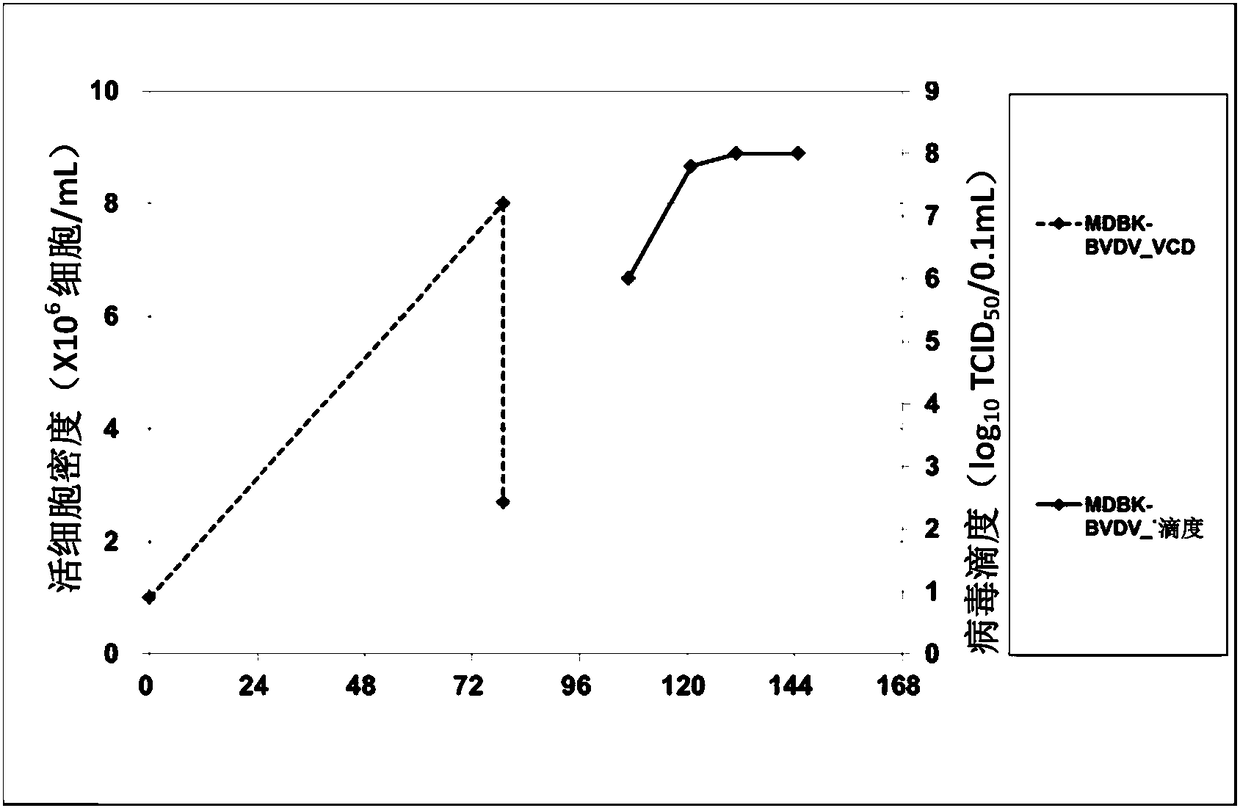
MDBK acclimation suspension method and two-stage virus production process
PendingCN108570454AHigh densityTo achieve growthSsRNA viruses positive-senseViral antigen ingredientsCytopathic effectSerum free
The invention relates to an MDBK cell acclimation suspension method and a two-stage virus production process. According to the MDBK cell acclimation suspension method, adherent MDBK cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension MDBK cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carried out or virus inoculation is performed before dilution. The suspension MDBK cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virusproduction process is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical ornot and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
Chemically defined media compositions
Chemically defined media compositions for the culture of eukaryotic cells are disclosed. The compositions are useful for eukaryotic cell culture in perfusion bioreactors and other vessels.
Owner:JANSSEN BIOTECH INC
Synthetic surfaces for culturing stem cell derived cardiomyocytes
ActiveUS8241907B2Good reproducibilityReduce Potential ContaminationBioreactor/fermenter combinationsBiological substance pretreatmentsChemical compositionCardiac muscle
Owner:ASTERIAS BIOTHERAPEUTICS INC
PK15 cell acclimation suspension method and two-stage virus production process
PendingCN108570445ATo achieve growthTo achieve the role of replication and proliferationArtificial cell constructsViruses/bacteriophagesCytopathic effectChemical composition
The invention relates to a PK15 cell acclimation suspension method and a two-stage virus production process. According to the PK15 cell acclimation suspension method, PK15 cells are acclimated to adapt to serum-free suspension culture and used as host cells for producing viruses which can have cytopathic effect or sensitivity on suspension PK15 cells by two-stage culture virus inoculation; when the cells grow to 2.0-20.0*10<6> cells / mL, diluting with a production culture medium by 1-5 times is performed to reach a density range of 1.0-10.0*10<6> cells / mL, and then virus inoculation is carriedout or virus inoculation is performed before dilution. The suspension PK15 cells are adaptive to suspension growth in the serum-free chemical-defined culture medium, and the two-stage virus productionprocess is simple and easy in amplification; liquid changing is avoided, and high culture medium utilization efficiency is achieved; production and growth culture media can be identical or not and can be mixed proportionally to realize cell secondary growth and viral expression promotion.
Owner:上海健士拜生物科技有限公司 +2
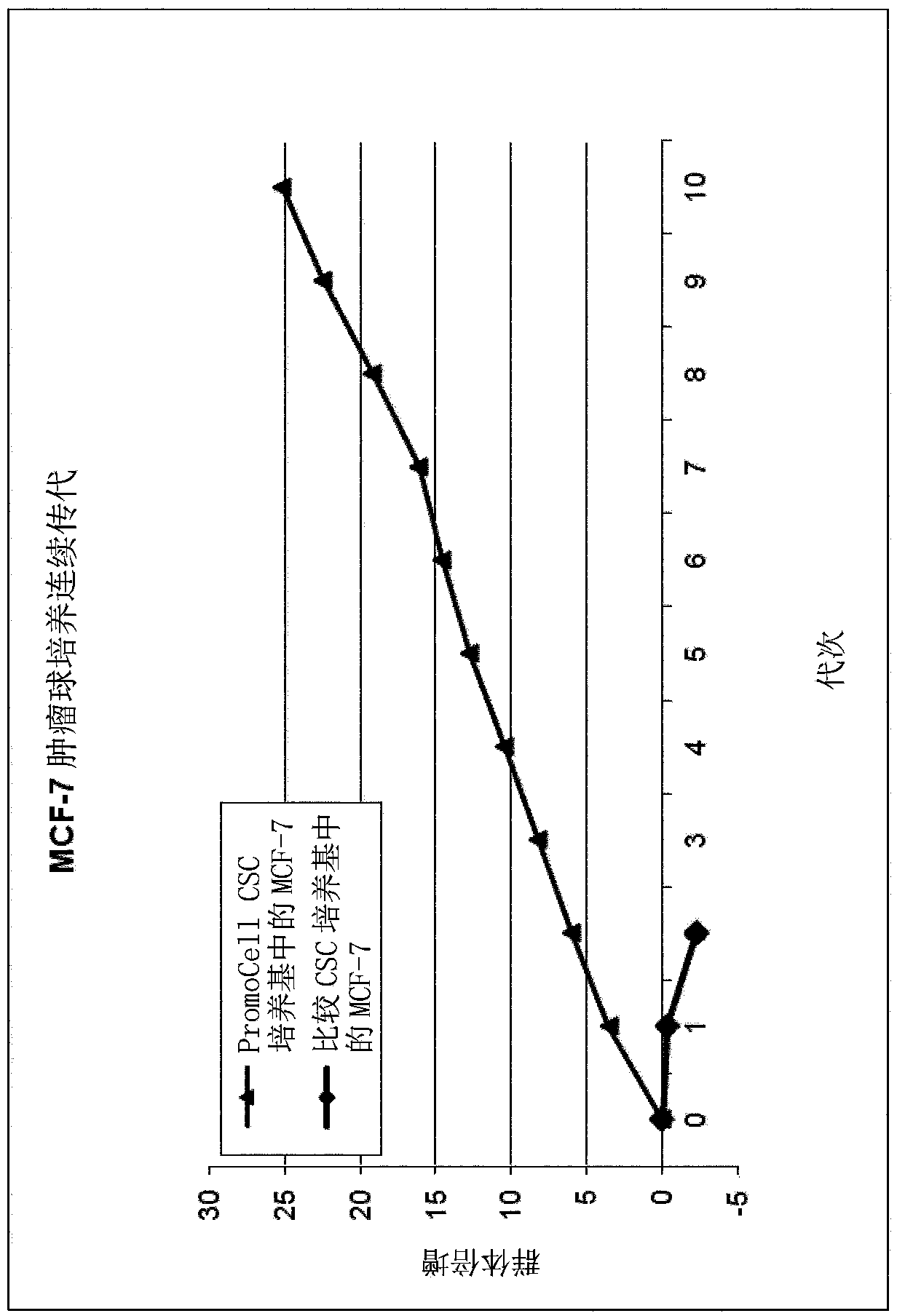
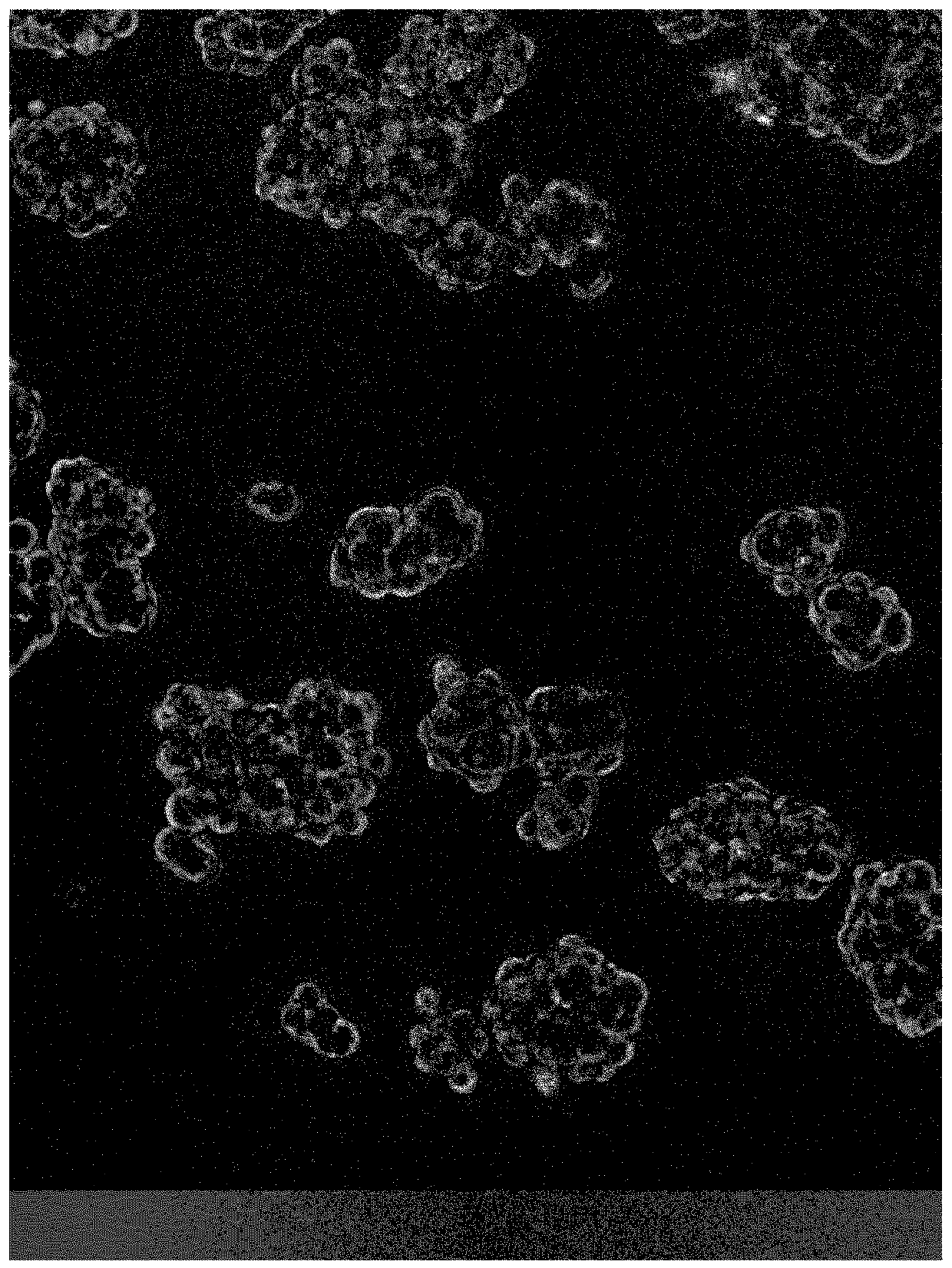
Chemically defined medium for the culture of cancer stem cell (CSC) containing cell populations
The present invention relates to a chemically defined medium for eukaryotic cell culture, comprising water, at least one carbon source, one or more vitamins, one or more salts, one or more growth factors, one or more fatty acids, one or more buffer components, selenium and one or more further trace elements and its use in the culture of cancer stem cells, in particular tumorsphere culture of cancer stem cells.
Owner:普乐思尔有限公司
Synthetic surfaces for culturing stem cell derived cardiomyocytes
InactiveUS20120276626A1Good reproducibilityReduce Potential ContaminationBioreactor/fermenter combinationsBiological substance pretreatmentsChemical compositionCardiac muscle
Owner:ASTERIAS BIOTHERAPEUTICS INC
Conversion of somatic cells to induced reprogrammed neural stem cells (irnscs)
InactiveUS20140051171A1Reduce riskLimited regeneration capacityNervous disorderGenetically modified cellsSerum free mediaReprogramming
This application relates to a method for converting somatic cells to Neural Stem Cells (NSCs). Moreover this application relates to a method for converting human fibroblasts, keratinocytes or adipocytes to neural stem cells based on linked steps of genes transduction and chemically defined medium induction.
Owner:F HOFFMANN LA ROCHE INC
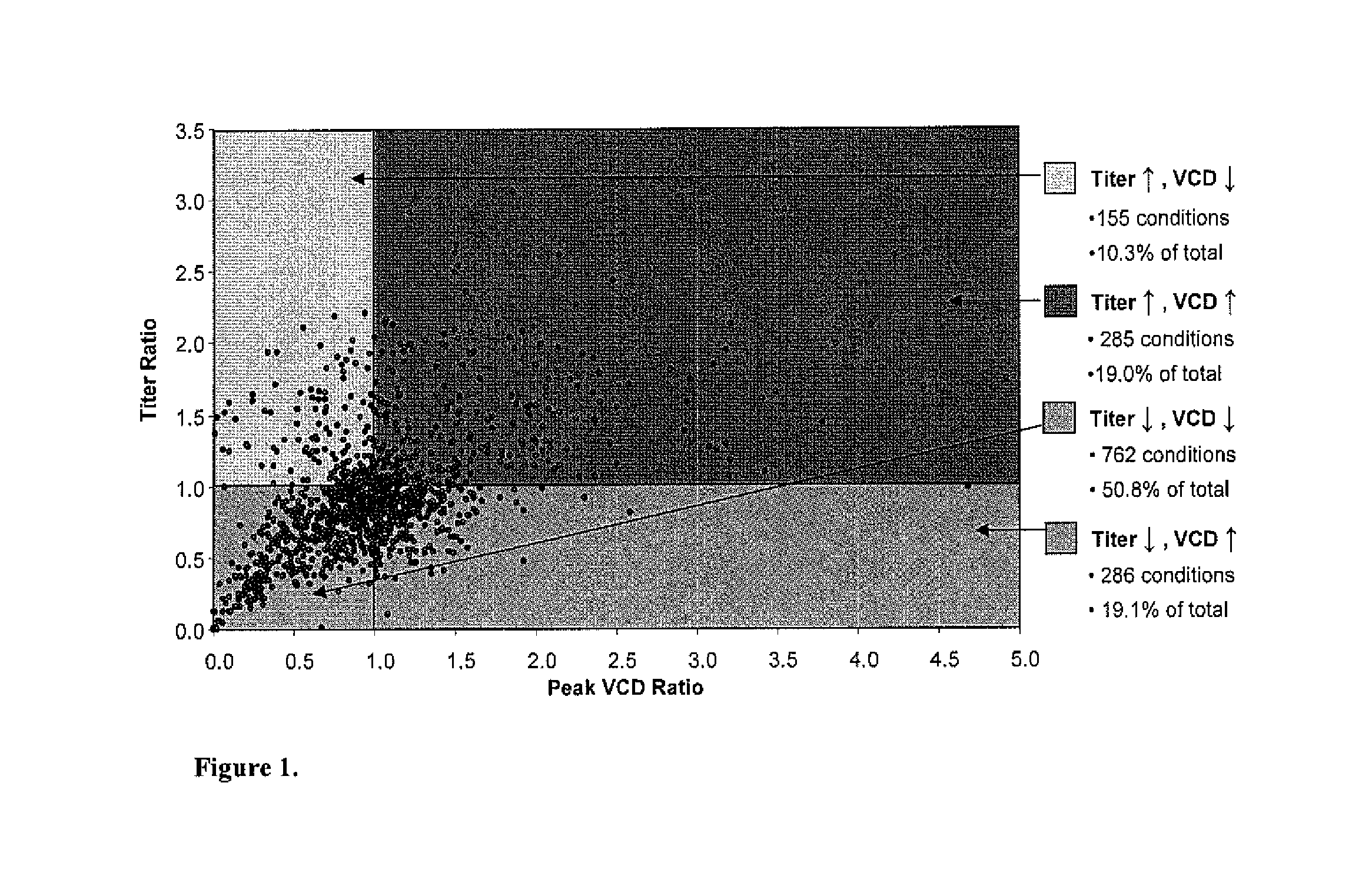
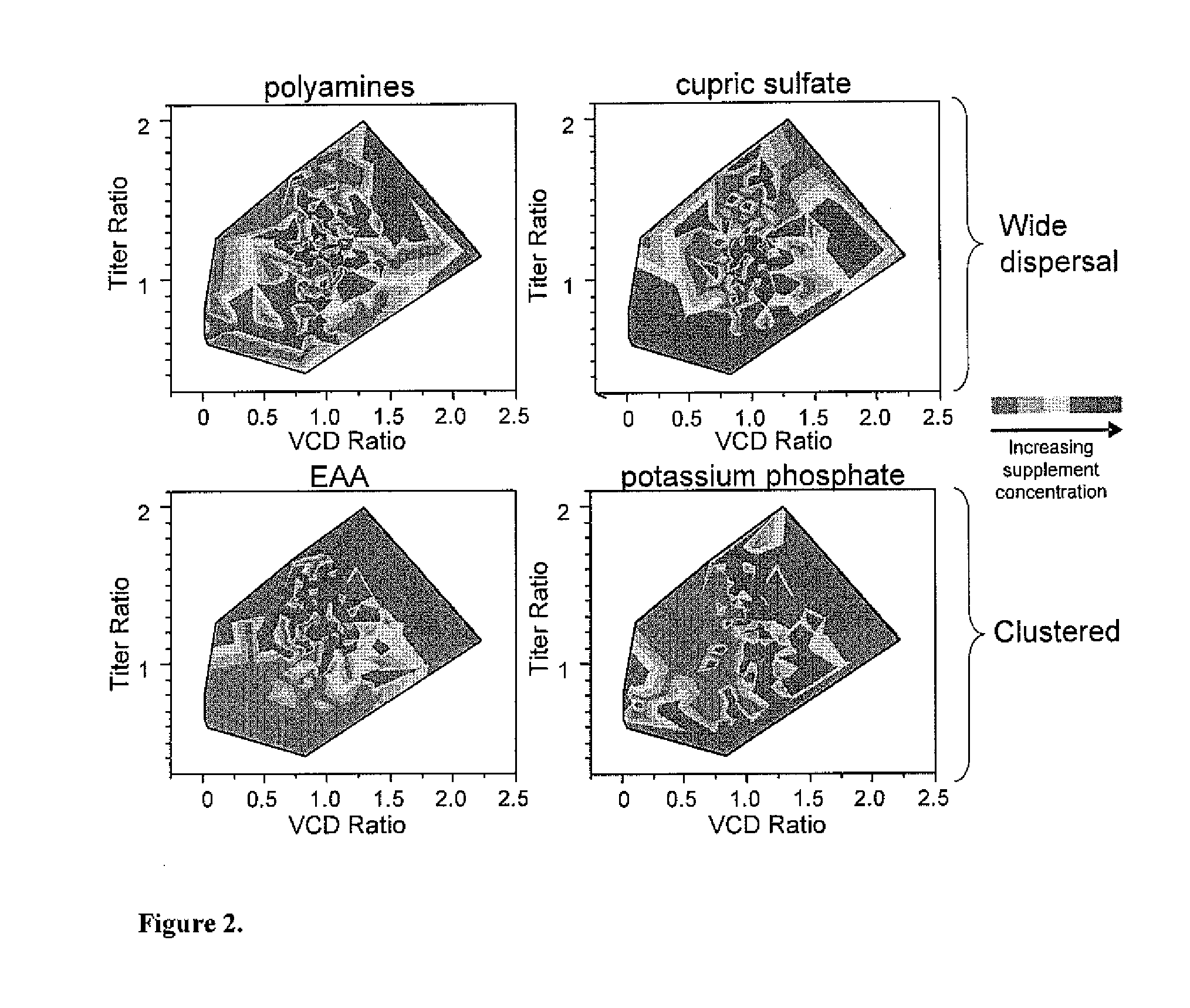
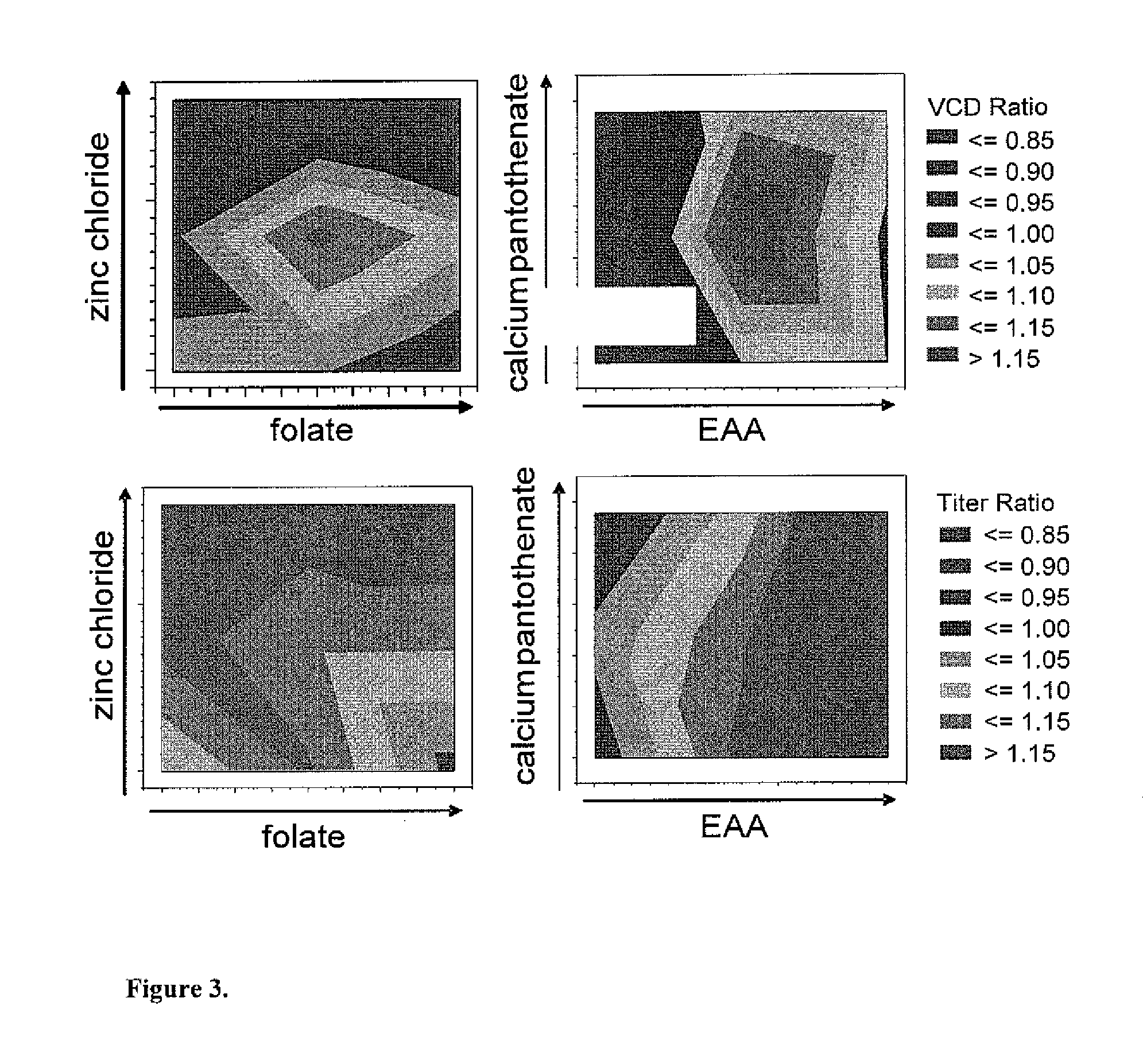
Efficient and effective supplement screening for the development of chemically defined media in cell culture
InactiveUS20120129727A1Increasing cell culture performanceImprove performanceMicroorganismsMicrobiological testing/measurementSerum free mediaBioproducts
The present invention relates to methods of selecting and developing a chemically defined media (“CDM”) for use in the manufacture of biological products. In particular, the present invention is directed to screening methods to determine cell culture technique media supplement blends with enhanced performance characteristics. The present invention is also directed to identifying CDM supplement blends that demonstrate significant increases in harvest titer and / or viable cell density.
Owner:ABBVIE INC
Method for inducing human embryonic stem cells to differentiate to neuroderm
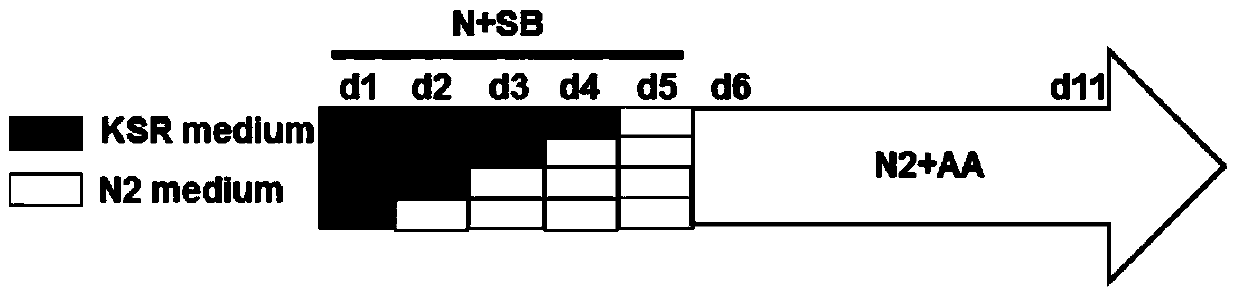
ActiveCN104195102AUniform sizeMaintain high density cultureEmbryonic cellsVitamin CDirected differentiation
The invention discloses a method for inducing human embryonic stem cells to differentiate to neuroderm. The method comprises the following steps that H9 cells are kept to passage in a cell cluster state, wherein the cell clusters are uniform in size, and high-density culture of the cell clusters can be kept; based on this, two inducing factors of SB431542 and NOGGIN are introduced, a knock out serumreplacer (KSR) culture medium and an N2 culture medium which are determined according to chemical components are used in matched manner according to different proportions at different culture times; and ascorbic acid (vitamin C) is added into the N2 culture medium at induced differentiation later period, so that HESC can be induced within shorter time to differentiate directionally to form an NE cell for expressing a PAX6 protein.
Owner:ANHUI MEDICAL UNIV
Host cell lines for production of antibody constant region with enhanced effector function
InactiveCN101627111AReduce or prevent growthHigh activityAntibody ingredientsTissue cultureSerum free mediaChemical composition
Host cell lines for biopharmaceutical production of antibodies, antibody fragments or antibody-derived fusion proteins are selected as having the capability of inducing improved cellular effector functions, e.g., Fc-medicated effector functions. The host cells are derived from the rat myeloma cell line YB2 / 0 and are adapted to growth in chemically-defined medium.
Owner:CENTOCOR
Method for differentiation of pluripotent stem cells into cardiomyocytes
This application relates to a method for differentiating pluripotent stem cells (PSCs) into cardiomyocytes. Moreover this application relates to a method for differentiating human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) into defined cardiomyocytes based on linked steps of chemically defined medium inductions.
Owner:F HOFFMANN LA ROCHE & CO AG
Swellable (meth)acrylate surfaces for culturing cells in chemically defined media
InactiveUS8329469B2Reduce Potential ContaminationExtended shelf lifeCell culture supports/coatingEmbryonic cellsSerum free mediaMeth-
Synthetic surfaces capable of supporting culture of undifferentiated human embryonic stem cells in a chemically defined medium include a swellable (meth)acrylate layer and a peptide conjugated to the swellable (meth)acrylate layer. The swellable (meth)acrylate layer may be formed by polymerizing monomers in a composition that includes hydroxyethyl methacrylate, 2-carboxyehylacrylate, and tetra(ethylene glycol) dimethacrylate. The conjugated peptide may include an amino acid sequence of XaanProGlnValThrArgGlyAspValPheThrMetPro, where n is an integer from 0 to 3 and where Xaa is any amino acid. Further, disclosed herein is a swellable (meth)acrylate synthetic surface which can be sterilized by gamma irradiation.
Owner:GERON CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com